Bi-doped directional spin-polarised electron injection modulates MBene surface d–d coupling to promote nitrogen reduction kinetics
Kun
Cheng
,
Shaobin
Li
 *,
Qingyu
Cheng
,
Li
Zhang
*,
Yufeng
Jiang
,
Fengbo
Li
and
Xiaoqing
Lv
*,
Qingyu
Cheng
,
Li
Zhang
*,
Yufeng
Jiang
,
Fengbo
Li
and
Xiaoqing
Lv
Key Laboratory of Polymeric Composite Materials of Heilongjiang Province, College of Materials Science and Engineering, Qiqihar University, Qiqihar 161006, China. E-mail: shaobinli1985@qqhru.edu.cn; 03241@qqhru.edu.cn
First published on 28th November 2025
Abstract
The electrocatalytic nitrogen reduction reaction (NRR) holds great promise in the field of artificial ammonia synthesis. Nevertheless, the difficulty of N2 adsorption and dissociation as well as the competitive hydrogen evolution reaction (HER) has presented intractable problems. We provide a unique method to construct a large number of crystalline–amorphous heterostructures by uniformly growing amorphous Bi-doped FeOOH quantum dots (QDs) on MBene, changing the spin state of Fe 3d orbitals, directionally injecting electrons toward Mo atoms on the surface of MBene via d–d hybridised orbitals, and enhancing the synthesis of ammonia. The Bi-FeOOH/MBene composite gave an ammonia yield of 35.96 μg h−1 mg−1 and FE of 38.28% at −0.4 V vs. RHE. Density functional theory (DFT) calculations identified Mo atoms as the main active sites of the NRR. The doping of Bi atoms effectively modulated the d-band center of Mo to change the electronic structure of the active sites at the heterostructures and enhanced the interactions and electron transport capacity between Mo atoms and *N2, which directly promoted the dissociation and activation HER of N2. In addition, the introduction of Bi atoms significantly dispersed the Bi-FeOOH QDs and inhibited the HER. In situ Fourier transform infrared (in situ FTIR) spectroscopy reveals that the electrocatalytic NRR process on the surface of the catalyst follows the distal-associative pathway in the associative pathway.
Green foundation1. This work presents a novel Bi-FeOOH/MBene catalyst that enables efficient electrocatalytic ammonia synthesis from renewable electricity under ambient conditions, offering an eco-friendly alternative to the energy-intensive conventional process.2. We developed Bi-FeOOH/MBene catalysts to further improve the efficiency of the electrocatalytic nitrogen reduction reaction. The highest ammonia yield of 35.96 μg h−1 mg−1 and faradaic efficiency of 38.28% were obtained. The performance exceeds that of some of the current cutting-edge studies. 3. Future research will focus on the direct, efficient connection of eNRR electrolysers to photovoltaic modules or wind power systems to mitigate the problems of intermittency and fluctuation of renewable energy, enabling the production of “green power” and “green ammonia”. |
1. Introduction
Nitrogen (N2), as the most abundant gas in the atmosphere, is the central carrier of the nitrogen cycle in nature.1,2 However, due to its extremely high N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N triple bond energy (941 kJ mol−1) and lack of dipole moment, nitrogen molecules exhibit extreme chemical inertness at room temperature and pressure, making it difficult to directly convert them into nitrogen-containing compounds (e.g., NH3) with high added value.3,4 Currently, industrial ammonia synthesis relies heavily on the conventional Haber–Bosch process, which reacts nitrogen with hydrogen to produce ammonia under high-temperature (400–500 °C) and high-pressure (15–25 MPa) conditions with the assistance of iron-based catalysts.5,6 Although the method provides human society with more than 50% of the feedstock for nitrogen fertilisers, its high energy consumption (about 1–2% of global energy consumption), dependence on fossil fuels for hydrogen production, and large carbon dioxide emissions (about 1.9 tonnes of CO2 per tonne of ammonia) are in serious contradiction with respect to the goal of sustainable development.7 Therefore, the development of novel green and low-energy nitrogen fixation technologies has become a major challenge in energy chemistry and catalysis science.
N triple bond energy (941 kJ mol−1) and lack of dipole moment, nitrogen molecules exhibit extreme chemical inertness at room temperature and pressure, making it difficult to directly convert them into nitrogen-containing compounds (e.g., NH3) with high added value.3,4 Currently, industrial ammonia synthesis relies heavily on the conventional Haber–Bosch process, which reacts nitrogen with hydrogen to produce ammonia under high-temperature (400–500 °C) and high-pressure (15–25 MPa) conditions with the assistance of iron-based catalysts.5,6 Although the method provides human society with more than 50% of the feedstock for nitrogen fertilisers, its high energy consumption (about 1–2% of global energy consumption), dependence on fossil fuels for hydrogen production, and large carbon dioxide emissions (about 1.9 tonnes of CO2 per tonne of ammonia) are in serious contradiction with respect to the goal of sustainable development.7 Therefore, the development of novel green and low-energy nitrogen fixation technologies has become a major challenge in energy chemistry and catalysis science.
In recent years, the electrocatalytic NRR has attracted much attention due to its potential to drive the conversion of nitrogen to ammonia directly from water and renewable electricity under mild conditions.8,9 By rationally designing the catalyst and optimising the reaction interface, the electrocatalytic NRR can achieve “zero-carbon” ammonia synthesis, and be combined with renewable energy sources, such as wind and solar, to build distributed ammonia systems. In addition, the technology can provide a new pathway for renewable energy storage by converting excess electricity into ammonia fuel, which can be easily stored and transported.10,11 When the NRR system is coupled with renewable energy and the catalyst possesses a sufficiently long service life, its net environmental benefits over the entire lifecycle will far exceed those of the Haber–Bosch process. Concurrently, the potential for distributed, small-scale ammonia production via electrocatalytic NRR technology can significantly reduce the environmental burden associated with long-distance ammonia transportation. However, the electrocatalytic NRR still faces several scientific challenges. First, the adsorption and activation of nitrogen molecules on the catalyst surface are inefficient, resulting in slow reaction kinetics. Second, the competitive HER dominates in most aqueous electrolytes, severely reducing the faradaic efficiency and ammonia yield of the NRR. Last, the identification of the active sites of the catalysts, the resolution of the reaction pathways, and the enhancement of the stability of the catalysts under real-life conditions still need to be explored in depth.12,13
To address the above challenges, researchers have made significant progress in the areas of catalyst design. For example, transition metal-based catalysts (e.g., Fe, Mo, Ru) exhibit excellent activity in promoting N2 activation due to their unique adsorption capacity for nitrogen.14–16 In addition, high-throughput screening methods combining theoretical calculations and machine learning have provided new ideas for the rational design of catalysts.17 Nevertheless, the practical application of the electrocatalytic NRR is still limited by the low ammonia yield, insufficient selectivity, and long-term stability, and the development of highly efficient and low-cost NRR catalysts is urgently needed.
MBene (M is transition metal, B is elemental boron) is a class of two-dimensional materials composed of transition metals and elemental boron with a graphene-like layered structure and a large specific surface area, which enables them to exhibit excellent performance in many catalytic reactions.18,19 In the electrocatalytic NRR, MBene, as a novel catalytic material, has attracted much attention in recent years. MBene not only provides abundant active sites, but also optimises the reaction performance of nitrogen reduction by modulating its electronic structure and surface properties. At the same time, MBene materials have good chemical stability and corrosion resistance, especially within acidic or alkaline environments, and can maintain catalytic activity for a long time.20 Their conductivity is similar to that of metallic materials, providing efficient electron transfer channels and promoting electron exchange in the electrocatalytic reaction, and is important for improving the NRR reaction rate and catalytic efficiency.21 Therefore, MBene materials are considered as one of the more promising catalysts for electrocatalytic nitrogen reduction. Qi et al. proposed a series of two-dimensional transition metal boride (MB) catalysts by first-principles calculations, and found that the metal-electronic energy-band structure and the electronic properties of MoB contributed to the NRR catalytic activity, and a large number of active sites accelerated the NRR reaction, while inhibiting the HER process. The MoB has a limiting overpotential of 0.68 V, which is promising for nitrogen fixation applications.22 Guo et al. found that MoB not only has good intrinsic activity for the NRR, but also has excellent ability to inhibit the competitive HER through extensive density functional theory calculations. In particular, once oxidised, the MBene can catalyse the NRR through a self-activation process, reducing O*/OH* to H2O* under reaction conditions that favour the electroreduction of N2.23 In the actual electrocatalytic NRR process, although MBene possesses a two-dimensional structure, the density of available catalytic sites on its surface is relatively low, resulting in its poor NRR activity. Therefore, it is necessary to explore reasonable methods to reset the electronic structure of the MBene surface.
The ordered structure of crystalline materials confers high electrical conductivity and mechanical stability, while the disordered nature of amorphous materials exposes abundant defects and irregularly arranged active sites, the synergistic coupling of which can break through the upper limit of the performance of single-phase materials.24 The atomic arrangement mismatch and charge redistribution at the heterogeneous interface can not only induce the built-in electric field and accelerate electron transfer, but also modulate the adsorption behaviour of the active sites through the strain effect, thus simultaneously enhancing the nitrogen activation efficiency and suppressing the HER.25,26 In addition, the dynamic surface properties of the amorphous phase are complemented by the structural rigidity of the crystalline phase, which provides a guarantee of the catalyst's durability in the long-term reaction.27,28 Based on this, the extensive construction and in-depth investigation of the constitutive relationship and dynamic evolution mechanism of the crystalline–amorphous heterogeneous interface are expected to open up new paths for the design of high-efficiency NRR catalysts.
In the work, MBene modified by amorphous Bi-FeOOH QDs was developed as a novel electrocatalytic NRR catalyst by a simple room-temperature synthesis process. A large number of amorphous–crystalline heterostructures on the surface of the fabricated Bi-FeOOH/MBene reset the electronic structure around the Mo atoms on the MBene surface. The incorporation of Bi atoms significantly reduces the propensity of FeOOH QDs to aggregate and promotes the development of additional heterostructures. The modification further adjusts the electronic configuration at the interfaces and improves the efficiency of electron transfer across them. DFT calculations show that the Mo atoms at the heterostructures of the MBene surfaces are the main active sites. The introduction of Bi atoms changes the spin state of the Fe 3d orbitals, enhances the d–d orbital hybridisation between the interfaces, and provides electrons to the Mo active site, changing the electronic structure of the active site and enhancing the interaction between the Mo site and *N2. The d-band center of Mo atoms on the surface of Bi-FeOOH/MBene is closer to the Fermi energy level than that of FeOOH/MBene, which suggests that the Mo active site of the former is more likely to bond with the N2 molecule and reaction intermediates and promotes the dissociation and activation of N2. Benefiting from these advantages, Bi-FeOOH/MBene furnished an ammonia yield of 35.96 μg h−1 mg−1 and a FE of 38.28%, and exhibited excellent stability. Thos study offers novel insights into the development of surface electronic structures and the design of heterostructures at the quantum dot level for MBene-based catalysts, while also presenting a new direction for the application of MBene in the field of the NRR.
2. Experimental section
MoAlB (1 g) was weighed and slowly added to 30 mL of 10 wt% NaOH solution and stirred for 24 h. The resulting precipitate was dispersed in 1 M NaOH solution and ultrasonicated for 30 min and then centrifuged several times in deionised water to wash the precipitate to pH ≈ 7. Next, the resulting precipitate was dispersed ultrasonically in 30 mL of deionised water. Then, 25 mL of tetramethylammonium hydroxide (TMAOH), ascorbic acid (0.5 g), and H2O2 (1 mL) were added to the dispersion, which was stirred for 30 min, and then loaded into a 100 mL Teflon autoclave and reacted for 24 h at 140 °C. The reaction was completed by centrifugation to remove unreacted TMAOH and the resulting precipitate was dispersed in isopropanol and sonicated for 1 h. The product was finally collected by centrifugation and dried under vacuum at 60 °C to give MBene. MBene (0.15 g) was ultrasonically dispersed in 40 mL of anhydrous ethanol, and then FeCl3·6H2O (0.0231 g), BiCl3 (0.0014 g), and NH4HCO3 (0.0203 g) were added to the solution and stirred vigorously for 8 h. Finally, the precipitate was collected by centrifugation and dried under vacuum at 40 °C to obtain Bi-FeOOH/MBene. FeOOH/MBene was prepared similarly to Bi-FeOOH/MBene, except that FeCl3·6H2O was added in the amount of 0.0243 g, and BiCl3 was not added. MBene was not added in the preparation of Bi-FeOOH/MBene and FeOOH/MBene, resulting in Bi-FeOOH and FeOOH.In terms of green improvement potential, this method represents a preliminary laboratory-scale exploration involving multi-step processing and the use of certain non-green reagents. Compared to the high-temperature and high-pressure reaction conditions required by the conventional Haber–Bosch process, the maximum temperature (140 °C) and ambient pressure conditions employed in this catalyst synthesis represent a significant moderation. Relative to traditional hydrofluoric acid etching methods, this work offers enhanced safety, along with reduced difficulty in waste liquid recovery and lower pollution levels. Future research will focus on process optimisation. By refining reactant ratios, solvent quantities, and reaction times, we aim to enhance yield and reduce waste liquid discharge while maintaining catalyst performance. Additionally, the potential for solvent recycling will be examined. Alternatively, the development of a one-pot synthesis approach may significantly streamline the procedure and decrease energy requirements. Moreover, the substitution of TMAOH with more environmentally benign intercalating agents, such as choline-based ionic liquids, will be investigated.
3. Results and discussion
3.1. Structural characteristics of the catalysts
The preparation flow of Bi-FeOOH/MBene is shown in Fig. 1a. First, we used the alkali etching and intercalation method to obtain MBene by etching MoAlB in NaOH solution and then intercalating the obtained powder in tetramethylammonium hydroxide (TMAOH) solution. Subsequently, MBene was homogeneously dispersed in anhydrous ethanol, and Bi(NO3)3·5H2O and FeCl3·6H2O were added with vigorous stirring to ultimately obtain Bi-FeOOH/MBene (detailed synthetic methods for MBene, FeOOH QDs, Bi-FeOOH QDs, FeOOH/MBene and Bi-FeOOH/MBene can be found in the SI). The crystal structure information of the catalytic materials was acquired by recording X-ray diffraction (XRD) patterns. The XRD patterns of MoAlB and MBene showed that MBene retained the crystal characteristics of MoAlB after etching away the Al layer via alkali (Fig. 1b).29 The (0k0) crystal plane of MBene is shifted to a certain extent compared with that of MoAlB, and some of the other diffraction peaks are broadened and missing, which suggests that the grains in the (0k0) direction lack long-range ordering and MBene was successfully prepared.30The XRD patterns of FeOOH QDs and Bi-FeOOH QDs do not show obvious spectral peaks, indicating that their atomic arrangements lack long-range order and that they are amorphous (Fig. 1c).31 FeOOH QDs and Bi-FeOOH QDs have similar spectral peaks to MBene when they are complexed with MBene. Because the QDs are small in size and do not completely cover the MBene surface, the XRD spectral peaks of FeOOH/MBene and Bi-FeOOH/MBene are similar to MBene. The morphology of the catalytic materials was observed by scanning electron microscopy (SEM). MoAlB was transformed from a dense and smooth bulk to a two-dimensionally stacked layered structure after etching (Fig. 1d and e). Transmission electron microscopy (TEM) of FeOOH QDs in the amorphous state revealed that FeOOH QDs are highly prone to agglomerate into small spheres with diameters of 40–60 nm, which greatly restricts their application in the electrocatalytic NRR (Fig. S1a and b). For Bi-FeOOH QDs, on the other hand, the small amount of doping of Bi enables the QDs to become more dispersed and causes their irregular morphology (Fig. S1c). Because the introduction of heteroatoms disturbs the atomic arrangement in the amorphous material, the interaction force in the local region becomes more dispersed and thus reduces the phenomenon of agglomeration. Moreover, the atomic radius of Bi atoms is much larger than that of Fe atoms, which renders the chemical bonding between Bi atoms and the surrounding atoms much less strong than that of Fe atoms, and the presence of Bi heteroatoms hinders the mutual attraction of the edge atoms of the neighbouring amorphous QDs. Neither FeOOH QDs nor Bi-FeOOH QDs show obvious morphological changes after forming composites with MBene (Fig. 1f and g). Energy dispersive spectroscopy (EDS) of the Bi-FeOOH QDs revealed a uniform distribution of Mo, B, O, Fe, and Bi, where the content of Bi was low, suggesting that Bi was introduced in the form of a dopant (Fig. S2). The TEM image of Bi-FeOOH/MBene shows its multilayer stacked morphology (Fig. 1h). From the high-resolution transmission electron microscopy (HRTEM) image of Bi-FeOOH/MBene, it can be seen that the lattice fringe attributed to the (220) crystalline plane of MBene is 0.157 nm (Fig. 1i).32 Very small irregular amorphous Bi-FeOOH QDs are attached to the surface of MBene, and a large number of heterostructures are formed between the Bi-FeOOH QDs and MBene. This large number of heterostructures plays a significant role in tuning the electronic structure of the MBene surface. The diffraction dot pattern attributed to MBene can be observed in the selected area electron diffraction (SAED) image of Bi-FeOOH/MBene. Due to the presence of amorphous Bi-FeOOH QDs on the surface of MBene, the diffraction dot matrix of MBene appears to be less bright (Fig. 1j). All the above characterisation studies proved that Bi was successfully doped into the amorphous FeOOH QDs and that Bi-FeOOH/MBene was successfully synthesised.
The valence states of the elements on the catalyst surface were investigated by X-ray photoelectron spectroscopy (XPS). The survey spectrum following XPS of Bi-FeOOH/MBene revealed characteristic peaks attributed to Bi, Fe, Mo, O, and B, which were in agreement with the results of EDS (Fig. S3). The fine mapping of elemental Bi showed strong peak splitting, which indicated that Bi had been successfully doped into the catalytic material (Fig. S4). The spectral profile of B 1s showed two characteristic peaks at 192.5 and 188.7 eV corresponding to the oxidation states of B and Mo–B in MBene, respectively (Fig. S5).33 This indicated that most of the B on the Bi-FeOOH/MBene surface is in the oxidation state of B. The Mo 3d spectrum of MBene reveals that elemental Mo is coexists in the form of multiple valence states on the MBene surface, with the characteristic peaks at 233.0 and 236.1 eV attributed to Mo6+, and the occurrence of the higher valence states attributed to the unavoidable oxidised state of the material in contact with air (Fig. 2a).34 In contrast, the complexation of FeOOH QDs with MBene resulted in the transformation of all Mo species from their low-valent state into the high-valent Mo6+ state, suggesting that the solution environment promoting Fe3+ hydrolysis is also capable of driving the oxidation of Mo atoms. Compared with MBene, the Mo6+ characteristic peaks (232.9 and 236.0 eV) in FeOOH/MBene are shifted by 0.1 eV towards low binding energy, which is due to the interfacial effect arising from the complexation between FeOOH QDs and MBene.35–37 After the introduction of Bi atoms, the characteristic peaks of Mo6+ in Bi-FeOOH/MBene (232.8 and 235.9 eV) were again shifted toward the direction of low binding energy by 0.1 eV, indicating that the doping of Bi atoms can effectively change the electronic structure of the atoms at the heterointerfaces. The Fe 2p spectrum of the FeOOH QDs revealed the presence of characteristic peaks at 711.2 and 724.8 eV attributed to FeOOH, indicating that FeOOH QDs were successfully synthesised (Fig. 2b).38,39 After the complexation of FeOOH QDs and MBene, the characteristic peaks of FeOOH (711.4 and 725.0 eV) were shifted towards higher binding energies by 0.2 eV, which was attributed to the effect of the heterointerfaces formed. After the introduction of Bi atoms, the peaks (711.7 and 725.3 eV) are again shifted by 0.3 eV towards higher binding energy, which is because the electronegativity of Bi atoms (2.02) is higher than the electronegativity of Fe atoms (1.83), and the Bi atoms are more attractive to the electrons, which causes the neighbouring Fe atoms to lose some of their electrons. The O 1s spectra of Bi-FeOOH/MBene, FeOOH/MBene and FeOOH QDs show similar O 1s spectra (Fig. 2c).40,41 Both of the characteristic O 1s spectral peaks associated with Fe atoms (Fe–O–Fe and Fe–O–H) showed a certain degree of shifting towards higher binding energies with the formation of heterogeneous interfaces and the doping of Bi atoms. All these results indicate that the constructed heterogeneous interfaces can trigger the redistribution of electrons between the interfaces, optimize the electronic structure of the active sites, and accelerate the electron transfer rate between the interfaces. Meanwhile, the doping of Bi atoms will further change the electronic distribution state of atoms between the interfaces, which will effectively enhance the mass transfer rate between the heterogeneous interfaces and promote the adsorption and activation of N2 at the active sites.
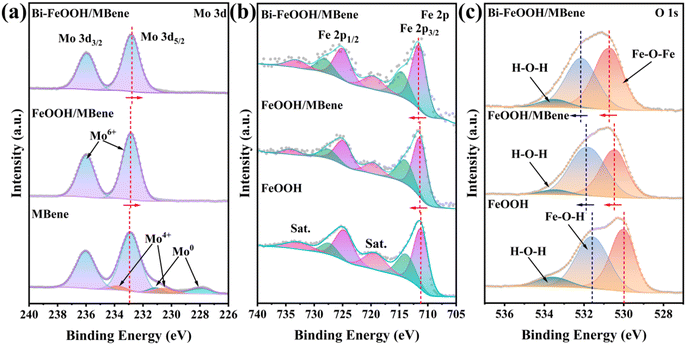 | ||
| Fig. 2 XPS spectra of (a) Mo 3d, (b) Fe 2p, and (c) O 1s for MBene, FeOOH/MBene, and Bi-FeOOH/MBene. | ||
3.2. NRR performances of the catalysts
The NRR performance of the catalyst was tested in an H-type electrolytic cell (Fig. S6). The potential demonstrated was converted to the reversible hydrogen electrode using the mixture of 0.1 M Na2SO4 and 0.01 M LiClO4 aqueous solution as electrolyte, Ag/AgCl as the reference electrode and a carbon rod as the counter electrode. The catalytic materials were dispersed on carbon paper via drop-coating, and the generated NH4+ was detected using the indophenol blue method. The specific preparation procedure is detailed in the SI (Fig. S7). First, Bi-FeOOH/MBene was tested via linear sweep voltammetry (LSV), and the current densities corresponding to different potentials under saturated N2 conditions were discovered to be larger than those under saturated Ar conditions in the range of 0 to −1 V vs. RHE, which was used to preliminarily determine that Bi-FeOOH/MBene possesses electrocatalytic NRR activity (Fig. 3a). To explore the optimal activity potential and best performance of the catalyst, we established a standard curve of NH4+ concentration–absorbance by analyzing the UV-visible absorption spectra of the electrolyte with step-growth NH4+ concentration (Fig. S8), and tested the catalytic materials for the electrocatalytic NRR for 2 h using the time–current method (i–t) in the range of −0.2 to −0.6 V vs. RHE. The current density became progressively higher with increasing applied potential (Fig. S9a). After color development of the tested electrolyte, the UV-visible absorption spectrum obtained showed a peak at 655 nm and exhibited the highest absorbance value in the curve corresponding to −0.4 V vs. RHE, which indicated that the highest ammonia yield was obtained for Bi-FeOOH/MBene at that potential (Fig. S9b). After conversion, it was concluded that the ammonia yield and FE of Bi-FeOOH/MBene increased gradually with the increase of applied potential. The maximum value was reached at −0.4 V vs. RHE, revealing the highest ammonia yield to be 35.96 μg h−1 mg−1, with the highest FE being 38.28% (Fig. 3b and c). When continuing to increase the applied potential, it was found that the ammonia yield and FE began to gradually decrease, which was due to the competing HER becoming stronger at higher applied potentials, severely hindering NRR activity.42 After testing the NRR performance of the precursors of Bi-FeOOH/MBene, it was found that the catalytic performance of Bi-FeOOH/MBene was superior to that of both FeOOH/MBene (optimum ammonia yield and FE of 18.27 μg h−1 mg−1 and 14.26%) and MBene (optimum ammonia yield and FE of 13.95 μg h−1 mg−1 and 8.51%) (Fig. 3d).To determine the source of elemental N in the ammonia produced during the electrocatalytic NRR test as well as to exclude the effect of the experimental environment, we performed the NRR test of Bi-FeOOH/MBene in different environments (Fig. 3e). The ammonia yield of Bi-FeOOH/MBene was calculated to be 36.04 μg h−1 mg−1 after 2 h of NRR testing in the electrolyte saturated with N2, whereas almost zero ammonia yield was obtained under saturated Ar conditions. Concurrently, radioisotope labelling experiments were conducted using 15N2 and 14N2 as nitrogen sources to trace the origin of nitrogen in the generated NH3 (Fig. S10). In the 1H NMR spectrum, the double and triple peaks were attributed to 15NH4+ and 14NH4+, respectively, with coupling constants of 72 Hz and 52 Hz. The results indicate that the elemental N in the ammonia produced in the electrocatalytic NRR experiment is almost entirely derived from the N2 we provided. We then performed the same two-hour test on carbon paper under saturated N2 conditions and found that there was virtually no ammonia production. The potential for carbon paper to demonstrate NRR electrocatalytic activity has been ruled out, thereby affirming that the Bi-FeOOH/MBene catalyst functions as the active component. At the same time, we tested under open-circuit voltage conditions in a saturated N2 electrolyte and detected no ammonia in the post-test electrolyte, suggesting that Bi-FeOOH/MBene requires an applied voltage to convert N2 to ammonia. We also performed i–t tests alternately in electrolytes saturated with N2 and saturated with Ar at −0.4 V vs. RHE. It was found that there was ammonia production under saturated N2 conditions, and the ammonia yield was close to that of the previous test results, while almost no ammonia was found in the electrolyte after the test under saturated Ar conditions, which ruled out the effect of re-release of ammonia on the Nafion membrane with residuals from the previous test. The above test results exclude the influence of environmental factors on the electrocatalytic NRR test and confirm the rigor of the experiment.
To investigate the reasons for the enhanced NRR performance of Bi-FeOOH/MBene, we conducted a series of studies on the electrochemical properties of the catalyst. First, we recorded the cyclic voltammetry (CV) curves of Bi-FeOOH/MBene at different scan rates (Fig. S11a–c). The double layer capacitance (Cdl) values of the three catalytic materials were established through calculation and used to evaluate their corresponding electrochemically active surface area (ECSA) values (Fig. S11d). MBene had the smallest Cdl value of 0.187 mF cm−2, and its complexation with FeOOH QDs yielded a Cdl value for FeOOH/MBene (0.189 mF cm−2) that was not significantly enhanced, due to FeOOH QDs being extremely prone to agglomeration, which is not a significant advantage for the enhancement of the ECSA of catalytic materials. In contrast, the Cdl value of Bi-FeOOH/MBene (0.232 mF cm−2) is much larger than the Cdl values of its two precursors because the doping of Bi improves the self-agglomeration of FeOOH QDs, and the attachment of well-dispersed Bi-FeOOH QDs on the surface of MBene is favourable to enable the increase in the ECSA of the catalyst, which in turn improves its electrochemical NRR activity. The results are consistent with those shown by HRTEM. We also conducted electrochemical impedance spectroscopy (EIS) of the catalytic materials to assess the effect of electron transfer rate on the electrocatalytic NRR performance (Fig. S12). It was found that the electrochemical impedance value of MBene was the smallest, which demonstrated the excellent conductivity of MBene materials. The EIS value of FeOOH/MBene was the largest, which was attributed to the lack of long-range ordering of amorphous FeOOH QDs compared to crystalline materials, resulting in electron migration being impeded. After Bi doping, the impedance of the catalytic materials decreased significantly, indicating enhanced electron transfer due to modulation of their electronic structure by Bi atoms. The HER, the main competing reaction of the NRR, was also evaluated through the HER performance of the catalysts. We tested the catalysts in electrolyte under saturated Ar conditions and plotted LSV curves of the catalytic materials (Fig. S13). It was found that the overpotential of Bi-FeOOH/MBene at a current density of 10 mA cm−2 was as high as −1.83 V, which was much higher than that of FeOOH/MBene (−1.59 V) and MBene (−1.56 V). The Tafel slope of Bi-FeOOH/MBene (541 mV dec−1) is also much higher than that of FeOOH/MBene (468 mV dec−1) and MBene (464 mV dec−1), which suggests that the doping of Bi can inhibit the hydrogen precipitation reaction and slow down the kinetics of the HER to a certain extent, thus promoting the NRR process. Upon investigation, we found that Bi-FeOOH/MBene, with a larger specific surface area, smaller electron transfer resistance and weaker HER activity, exhibited electrochemical properties more inclined to the electrocatalytic NRR, and greater ammonia yield and FE (Fig. 4a).
Meanwhile, we also systematically investigated the stability of the catalysts. First, six consecutive cycling stability tests were performed on the same working electrode at −0.4 V vs. RHE, and the I curve of each test provided similar current densities (Fig. S14). After the NRR reaction, the UV-visible absorption spectra from the six tests almost overlapped with each other (Fig. S15). The ammonia yield and FE obtained from the same electrode sheet after six consecutive tests exhibited minimal fluctuation (Fig. 4b). These results indicate that Bi-FeOOH/MBene has excellent cycling stability. The performance of each catalyst was monitored over a 36 hour period. Bi-FeOOH/MBene maintained a stable current density, exhibiting good long-term stability (Fig. 4c). SEM and XRD analysis revealed no alteration in the catalyst's surface morphology or crystalline structure post-stability testing, indicating that the prepared Bi-FeOOH/MBene exhibits outstanding stability (Fig. S16 and S17). Compared with some recently reported advanced NRR catalysts, Bi-FeOOH/MBene exhibited comparable or superior performance levels (Fig. 4d and Table 1).
| Materials | NH3 yield rate (µg h−1 mg−1) | FE (%) | Ref. |
|---|---|---|---|
| LiFe0.5Mn1.5O4 | 22.45 | 21.52 | 43 |
| Ti-Bi2WO6 | 27.1 | 3.5 | 44 |
| Fe HF | 24.24 | 13.16 | 45 |
| Mo2C–Fe/NC | 28.09 | 24.06 | 46 |
| FeV-WO3 | 17.4 | 32.2 | 47 |
| TiO2−x@COF-Aq | 30.00 | 16.00 | 48 |
| SrCoO3 | 13.86 | 9.36 | 49 |
| B-FeS2 | 26.52 | 12.6 | 50 |
| CuxS–MoS2-5 | 22.37 | 24.22 | 51 |
| Ni/MoO2@NF | 5.865 | 36.14 | 52 |
| TiNb2O7@C | 10.54 | 13.11 | 53 |
| Al2NC | 29.22 | 16.56 | 54 |
| NbNi3 | 25.89 | 33.15 | 55 |
| Cu-doped Nb2O5 | 24.56 | 20.15 | 56 |
| Bi-FeOOH/MBene | 35.96 | 38.28 | This work |
3.3. Catalytic mechanism and DFT calculations
The mechanism of Bi-FeOOH/MBene in the NRR process was investigated by in situ FTIR spectroscopy (Fig. 5a and b). The peak located at 1060 cm−1 corresponds to the N–N stretching vibration.44 The broad band located at about 3400 cm−1 corresponds to the O–H stretching vibration of interfacial water, which serves as a proton source in the NRR process. The peaks located at 1400, 1533, and 3746 cm−1 are attributed to –NH2 wagging, H–N–H bending, and N–H stretching vibrations, respectively, suggesting the generation of NH intermediates at the catalyst surface at −0.4 V vs. RHE.57 Characteristic peaks of symmetric and asymmetric deformation modes (δsNH4+ and δasNH4+) attributed to adsorbed NH4+ appeared at 1700 cm−1 and 1463 cm−1, and the signals were gradually enhanced with increasing time, suggesting that ammonia was produced through the electrocatalytic NRR process of the catalyst.58In situ FTIR confirmed that N2 first interacts with the active site of the catalyst, decomposing H2O to provide protons in the presence of electrical energy, while hydrogenating N atoms and finally forming NH4+. The presence of –NH2 wobbling, H–N–H bending and N–H stretching vibrations confirms that the electrocatalytic NRR process follows the associative pathway on the Bi-FeOOH/MBene surface. We plotted a standard concentration curve of N2H4 following the method of plotting the standard curve of NH4+ concentration to detect the possible by-product N2H4 produced during the NRR process (Fig. S18). The results showed that the UV-visible absorption spectrum of N2H4 in the electrolyte did not display a significant peak at 455 nm after the NRR test (Fig. 5c). This indicates that the catalyst has excellent selectivity, and the conversion of N2 to ammonia by Bi-FeOOH/MBene is consistent with the distal-associative pathway (Fig. 5d), in accordance with the analysis of in situ FTIR and the studies of previous scholars.59The improvement of electrocatalytic (NRR) processes facilitated by Bi-FeOOH/MBene was subsequently examined through DFT calculations. The amorphous Bi-FeOOH was situated on the (220) crystalline surface of MBene constructing a heterogeneous structure and facilitating the building of a computational model (Fig. S19). The adsorption of N2 at the active sites on the surface of the catalyst was investigated, as this represents the first step towards the catalyst's success in the NRR. The results showed that N2 is difficult to adsorb at the Fe site of Bi-FeOOH/MBene, and the corresponding adsorption energy was only −0.571 eV (Fig. 6a). The Mo site at the heterostructure can effectively adsorb N2 with an adsorption energy of −1.157 eV, which indicates that the Mo atom acts as the main NRR active site (Fig. 6b).60 The adsorption energy of the Mo site at the FeOOH/MBene heterostructure is −0.921 eV, which suggests that Bi-doping into the FeOOH QDs effectively enhances the adsorption capacity of the interfacial Mo atom for N2 (Fig. 6c). The differential charge density and Bader charge of Mo sites after adsorption of N2 molecules show that the Mo atoms in Bi-FeOOH/MBene transfer more electrons to *N2, and the electrons lost by the Mo atoms (cyan portion) act on N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N (yellow portion) (Fig. 6d and e). Concurrently, the performance of the HER was evaluated through free energy calculations for H2 generation via proton binding (Fig. S20). The proton adsorption free energy in the FeOOH/MBene material was −0.74 eV. Following bismuth doping, this value decreased to −0.55 eV at the Mo sites, thereby significantly suppressing the HER. This suggests that the doping of Bi changes the electronic structure of Mo atoms at the heterogeneous interface and enhances the electronic interaction between Mo atoms and *N2. Stronger electronic interactions can stabilise the adsorbed N2 and reaction intermediates, providing a firm precondition for the NRR process.61 Increasing electron transfer will provide more energy for the NRR process, accelerating the N2 dissociation process and facilitating the subsequent hydrogenation step. The partial density of states (PDOS) of Mo atoms shows that the introduction of Bi changes the d-band center of interfacial Mo atoms upward towards the Fermi energy level, which suggests that the d-orbitals of the Mo sites are occupied by more electrons (Fig. 6f). The density of states (DOS) curves demonstrate that Bi doping induces orbital hybridization between Fe 3d and Mo 3d at the heterostructure, providing evidence for enhanced d–d orbital coupling. (Fig. 6g and h). This enhanced electronic interaction is more effective in activating the strongly inert N2, in addition to modulating the d-band centre of Mo. Furthermore, the Fe 3d orbitals exhibit spin polarization near the Fermi energy level after Bi introduction. To gain deeper insight into the modulation effect of Bi doping on the d-orbitals, the PDOS of the Fe 3d orbitals was analyzed (Fig. 6i). The PDOS of the Fe 3d orbitals exhibited significant spin polarization following Bi doping. Compared with FeOOH/MBene, Bi-FeOOH/MBene exhibits a larger difference in the integrated area between the spin-up and spin-down state curves near the Fermi level, suggesting that Bi doping induces a partial spin-polarization transition of Fe from a low-spin state to a high-spin state. The spin polarization of Fe enhances the spin density at the Fe sites and transfers spin-polarized electrons to the Mo active site via d–d hybridization. When N2 is adsorbed at the Mo site, these electrons preferentially populate the π* antibonding orbitals of N2, thereby reducing the activation energy barrier.62
N (yellow portion) (Fig. 6d and e). Concurrently, the performance of the HER was evaluated through free energy calculations for H2 generation via proton binding (Fig. S20). The proton adsorption free energy in the FeOOH/MBene material was −0.74 eV. Following bismuth doping, this value decreased to −0.55 eV at the Mo sites, thereby significantly suppressing the HER. This suggests that the doping of Bi changes the electronic structure of Mo atoms at the heterogeneous interface and enhances the electronic interaction between Mo atoms and *N2. Stronger electronic interactions can stabilise the adsorbed N2 and reaction intermediates, providing a firm precondition for the NRR process.61 Increasing electron transfer will provide more energy for the NRR process, accelerating the N2 dissociation process and facilitating the subsequent hydrogenation step. The partial density of states (PDOS) of Mo atoms shows that the introduction of Bi changes the d-band center of interfacial Mo atoms upward towards the Fermi energy level, which suggests that the d-orbitals of the Mo sites are occupied by more electrons (Fig. 6f). The density of states (DOS) curves demonstrate that Bi doping induces orbital hybridization between Fe 3d and Mo 3d at the heterostructure, providing evidence for enhanced d–d orbital coupling. (Fig. 6g and h). This enhanced electronic interaction is more effective in activating the strongly inert N2, in addition to modulating the d-band centre of Mo. Furthermore, the Fe 3d orbitals exhibit spin polarization near the Fermi energy level after Bi introduction. To gain deeper insight into the modulation effect of Bi doping on the d-orbitals, the PDOS of the Fe 3d orbitals was analyzed (Fig. 6i). The PDOS of the Fe 3d orbitals exhibited significant spin polarization following Bi doping. Compared with FeOOH/MBene, Bi-FeOOH/MBene exhibits a larger difference in the integrated area between the spin-up and spin-down state curves near the Fermi level, suggesting that Bi doping induces a partial spin-polarization transition of Fe from a low-spin state to a high-spin state. The spin polarization of Fe enhances the spin density at the Fe sites and transfers spin-polarized electrons to the Mo active site via d–d hybridization. When N2 is adsorbed at the Mo site, these electrons preferentially populate the π* antibonding orbitals of N2, thereby reducing the activation energy barrier.62
4. Conclusions
In summary, we successfully synthesized a novel NRR electrocatalyst, Bi-FeOOH/MBene, by growing amorphous Bi-doped FeOOH QDs on the MBene surface via a simple room-temperature synthesis process using the hydrolysis effect of Fe3+. An ammonia yield of 35.96 μg h−1 mg−1 and a FE of 38.28% with good stability were obtained at a potential of −0.4 V vs. RHE. DFT calculations and experimental tests show that Bi-FeOOH quantum dots can inject electrons into Mo atoms directionally via d–d hybridisation orbitals between heterogeneous interfaces, significantly altering the electronic structure of the Mo active sites on the MBene surface, enhancing the electron transfer and interactions between Mo atoms and N2, and promoting the decomposition and activation of N2. The introduction of Bi can inhibit the competitive HER and promote the kinetics of the NRR to some extent. The doping of Bi atoms can significantly disperse FeOOH QDs and increase the number of heterostructures on the MBene surface, thereby enhancing the number of NRR active sites. In situ FTIR reveals that the electrocatalytic NRR process on the catalyst surface follows the distal-associative pathway in the associative pathway. This work provides new ideas and insights into the electronic regulation of MBene-based catalyst surfaces.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Data availability
Data will be provided upon reasonable request.Supplementary information (SI): experimental section, characterization, electrochemical measurements. See DOI: https://doi.org/10.1039/d5gc04584c.
Acknowledgements
This work was financially supported by the Program for Young Talents of Basic Research in the Universities of Heilongjiang Province (YQJH2024259), the Outstanding Youth Project of the Natural Science Foundation in Heilongjiang Province (YQ2021B011), and the Fundamental Research Fund of Heilongjiang Provincial University (145309201).References
- M. A. Mushtaq, A. Kumar, W. Liu, Q. Ji, Y. Deng, G. Yasin, A. Saad, W. Raza, J. Zhao and S. Ajmal, Adv. Mater., 2024, 36, 2313086 CrossRef CAS.
- A. N. Singh, R. Anand, M. Zafari, M. Ha and K. S. Kim, Adv. Energy Mater., 2024, 14, 2304106 CrossRef CAS.
- Y. Wan, Y. Tang, Y. Zuo, K. Sun, Z. Zhuang, Y. Zheng, W. Yan, J. Zhang and R. Lv, Energy Environ. Sci., 2025, 18, 7460–7469 RSC.
- D. Kim, S. Surendran, G. Janani, Y. Lim, H. Choi, M.-K. Han, S. Yuvaraj, T.-H. Kim, J. K. Kim and U. Sim, Mater. Lett., 2022, 314, 131808 CrossRef CAS.
- H. Yang, G. Ren, Z. Li, Z. Zhang and X. Meng, Appl. Catal., B, 2024, 347, 123795 CrossRef CAS.
- H. He, H. M. Wen, H. K. Li, P. Li, J. Wang, Y. Yang, C. P. Li, Z. Zhang and M. Du, Adv. Sci., 2023, 10, 2206933 CrossRef CAS PubMed.
- W. Liu, N. Yang, Y. Wei, Y. Yu, J. Chen, M. Wei, Y. Huang, X. Li, L. Zhang and F. Saleem, J. Energy Chem., 2024, 93, 471–477 CrossRef CAS.
- J. Zhang, C. Xing, S. An, F. Meng, Z. Liu, R. Ge, M. Ma, J. Ren and J. Tian, Green Chem., 2025, 27, 7129–7136 RSC.
- L. Li, L. Xu, H. Wang, H. Wei, C. Tang, G. Li, Y. Dou, H. K. Liu and S. X. Dou, Energy Environ. Sci., 2024, 17, 9027–9050 RSC.
- F. Chang, W. Gao, J. Guo and P. Chen, Adv. Mater., 2021, 33, 2005721 CrossRef CAS.
- F. Zhang, M. Gao, S. Huang, H. Zhang, X. Wang, L. Liu, M. Han and Q. Wang, Adv. Mater., 2022, 34, 2104562 CrossRef CAS.
- X. Zhao, G. Hu, G. F. Chen, H. Zhang, S. Zhang and H. Wang, Adv. Mater., 2021, 33, 2007650 CrossRef CAS.
- Y. Ren, S. Li, C. Yu, Y. Zheng, C. Wang, B. Qian, L. Wang, W. Fang, Y. Sun and J. Qiu, J. Am. Chem. Soc., 2024, 146, 6409–6421 CrossRef CAS.
- S. Zhang, M. Han, T. Shi, H. Zhang, Y. Lin, X. Zheng, L. R. Zheng, H. Zhou, C. Chen and Y. Zhang, Nat. Sustain., 2023, 6, 169–179 CrossRef.
- H. Zhou, Y. Qu, Y. Fan, Z. Wang, X. Lang, J. Li and Q. Jiang, Appl. Catal., B, 2023, 339, 123133 CrossRef CAS.
- D. Chen, J. Lan, F. Xie, J. Peng, M. Peng, C. Sun, Z. Wei, S. Zhao, M. Wang and P. Liu, Chem. Eng. J., 2023, 475, 146137 CrossRef CAS.
- T. Y. Dai, T. H. Wang, Z. Wen and Q. Jiang, Adv. Funct. Mater., 2024, 34, 2400773 CrossRef CAS.
- S. L. Fereja, A. Mehmood, Q. Ji, W. Raza, A. Hussen, J. Hu, S. Zhai and X. Cai, InfoMat, 2025, 7, e12623 CrossRef.
- J. Lin, X. Wang, Z. Zhao, D. Chen, R. Liu, Z. Ye, B. Lu, Y. Hou and J. Lu, Carbon Energy, 2024, 6, e555 CrossRef CAS.
- S. J. Park, T. H. Nguyen, D. T. Tran, V. A. Dinh, J. H. Lee and N. H. Kim, Energy Environ. Sci., 2023, 16, 4093–4104 RSC.
- Z. Chen, H. Wang, C. Zhang, Y. Gou, Z. Gong, Y. Jiang, H. Zeng, J. Wang, F. Meng and Y. Cui, ACS Catal., 2025, 15, 2885–2895 CrossRef CAS.
- S. Qi, Y. Fan, L. Zhao, W. Li and M. Zhao, Appl. Surf. Sci., 2021, 536, 147742 CrossRef CAS.
- X. Guo, S. Lin, J. Gu, S. Zhang, Z. Chen and S. Huang, Adv. Funct. Mater., 2021, 31, 2008056 CrossRef CAS.
- J. Kang, X. Yang, Q. Hu, Z. Cai, L.-M. Liu and L. Guo, Chem. Rev., 2023, 123, 8859–8941 CrossRef CAS.
- Y. Li, M. S. Hassan, X. Zhao and A. L. Rogach, Adv. Mater., 2025, 37, 2418146 CrossRef CAS.
- Y. Ren, C. Yu, X. Tan, H. Huang, Q. Wei and J. Qiu, Energy Environ. Sci., 2021, 14, 1176–1193 RSC.
- B. Jia, G. Liu, B. Zhang, J. Zheng, K. Yin, J. Lin, C. Han, X. Fan, M. Xu and L. Ye, Adv. Funct. Mater., 2024, 34, 2405867 CrossRef CAS.
- H. Lan, J. Wang, L. Cheng, D. Yu, H. Wang and L. Guo, Chem. Soc. Rev., 2024, 53, 684–713 RSC.
- C. S. Rout, P. V. Shinde, A. Patra and S. M. Jeong, Adv. Sci., 2024, 11, 2308178 CrossRef CAS.
- L. T. Alameda, R. W. Lord, J. A. Barr, P. Moradifar, Z. P. Metzger, B. C. Steimle, C. F. Holder, N. Alem, S. B. Sinnott and R. E. Schaak, J. Am. Chem. Soc., 2019, 141, 10852–10861 CrossRef CAS PubMed.
- Y. Yang, J. Zhou, F. Zhu, Y. Yuan, D. J. Chang, D. S. Kim, M. Pham, A. Rana, X. Tian and Y. Yao, Nature, 2021, 592, 60–64 CrossRef CAS.
- W. Xiong, X. Feng, T. Huang, Z. Huang, X. He, J. Liu, Y. Xiao, X. Wang and Q. Zhang, J. Met. Sci. Technol., 2025, 212, 67–76 CAS.
- W. Xiong, X. Feng, Y. Xiao, T. Huang, X. Li, Z. Huang, S. Ye, Y. Li, X. Ren and X. Wang, Chem. Eng. J., 2022, 446, 137466 CrossRef CAS.
- Z. Ren, Y. Sun, Q. Lei, W. Zhang, Y. Zhao, Z. Yao, J. Si, Z. Li, X. Ren and X. Sun, ACS Nano, 2023, 17, 19144–19154 CrossRef CAS.
- J. He, A. Bhargav and A. Manthiram, Adv. Mater., 2020, 32, 2004741 CrossRef CAS PubMed.
- S. Jin, Z. Shi, R. Wang, Y. Guo, L. Wang, Q. Hu, K. Liu, N. Li and A. Zhou, ACS Nano, 2024, 18, 12524–12536 CrossRef CAS.
- P. Yang, F. Liu, X. Zang, L. Xin, W. Xiao, G. Xu, H. Li, Z. Li, T. Ma and J. Wang, Adv. Energy Mater., 2024, 14, 2303384 CrossRef CAS.
- J. X. Feng, H. Xu, Y. T. Dong, S. H. Ye, Y. X. Tong and G. R. Li, Angew. Chem., Int. Ed., 2016, 55, 3694–3698 CrossRef CAS PubMed.
- M. Cai, Q. Zhu, X. Wang, Z. Shao, L. Yao, H. Zeng, X. Wu, J. Chen, K. Huang and S. Feng, Adv. Mater., 2023, 35, 2209338 CrossRef CAS.
- X. Chen, Q. Wang, Y. Cheng, H. Xing, J. Li, X. Zhu, L. Ma, Y. Li and D. Liu, Adv. Funct. Mater., 2022, 32, 2112674 CrossRef CAS.
- B. Wu, S. Gong, Y. Lin, T. Li, A. Chen, M. Zhao, Q. Zhang and L. Chen, Adv. Mater., 2022, 34, 2108619 CrossRef CAS.
- M. Xie, F. Dai, H. Guo, P. Du, X. Xu, J. Liu, Z. Zhang and X. Lu, Adv. Energy Mater., 2023, 13, 2203032 CrossRef CAS.
- S. Yan, C. Yang, X. Huang, X. Ma, C. Long, L. Chang and Z. Tang, Nano Res., 2025, 18, 94907464 CrossRef.
- H. Wang, C. Zhang, B. Liu, W. Li, C. Jiang, Z. Ke, D. He and X. Xiao, Adv. Mater., 2024, 36, 2401032 CrossRef CAS.
- T. Zhang, Z. Che, Y. Song, R. Yao, J. Li, Y. Sun and G. Liu, Angew. Chem., Int. Ed., 2025, e202514028 CAS.
- S.-Y. Li, Y.-H. Bian, X. Wang, X.-W. Gao, Z. Liu, Z. Zhao, Q. Gu and W.-B. Luo, Chem. Eng. J., 2025, 517, 164457 CrossRef CAS.
- Z. Ji, X. Zhang, W. Gan, Y. Xue, X. Duan, Z. Zhou, Z. Han, S. Zhao, B. Feng and C. Li, Chem. Eng. J., 2025, 515, 163919 CrossRef CAS.
- B. Mishra, S. Biswal, M. Ussama, M. A. Haider and B. P. Tripathi, Appl. Catal., B, 2024, 342, 123395 CrossRef CAS.
- N. Han, W. Zhang, J. Wu, K. Chu, S. Feng, S. Wang, A. R. Puente-Santiago, J. Long, B. Weng and B. L. Su, Angew. Chem., Int. Ed., 2025, 64, e202504601 CrossRef CAS.
- N. Li, J. Zhang, Z. Qiao, D. Gu, D. Ji, Z. Li, D. Yuan, H. Wu and B. Wang, Chem. Eng. J., 2025, 513, 162736 CrossRef CAS.
- L. Li, Y. Li, K. Li, W. Zou, H. Li, Y. Li, L. Li, Q. Zhang, C. Zhang and X. Zhang, ACS Nano, 2024, 19, 1080–1089 CrossRef PubMed.
- T.-Y. An, S. Surendran, J. Lim, D. J. Moon, Y. Yang, S. C. Jesudass, R. P. Sivasankaran, Y. Lim, J. Y. Kim and G. H. Jeong, Adv. Compos. Hybrid Mater., 2024, 7, 201 CrossRef CAS.
- Y. Xue, C. Ma, Q. Yang, X. Wang, S. An, X. Zhang and J. Tian, Chem. Eng. J., 2023, 457, 141146 CrossRef CAS.
- S. Biswas, J. Zhou, X. L. Chen, C. Chi, Y. A. Pan, P. Cui, J. Li, C. Liu and X. H. Xia, Angew. Chem., Int. Ed., 2024, 63, e202405493 CrossRef CAS.
- T. Y. Dai, H. Shi, T. H. Wang, X. Y. Lang and Q. Jiang, Angew. Chem., 2025, e202418035 CAS.
- S. Wang, C. Qian, X. Chen and S. Zhou, ACS Catal., 2024, 14, 7345–7352 CrossRef CAS.
- J. Wang, Q. Zhu, J. Wang, T. Wang, W. Xia, E. Lin, K. Wang, H. Hu, T. Wang and Z. Wang, Angew. Chem., Int. Ed., 2025, 64, e202415208 CrossRef CAS PubMed.
- T.-Y. An, S. Surendran, J. Lim, D. J. Moon, Y. Yang, S. C. Jesudass, R. P. Sivasankaran, Y. Lim, J. Y. Kim and G. H. Jeong, Adv. Compos. Hybrid Mater., 2024, 7, 1–22 CrossRef.
- X. Cui, C. Tang and Q. Zhang, Adv. Energy Mater., 2018, 8, 1800369 CrossRef.
- X. Yan, D. Liu, H. Cao, F. Hou, J. Liang and S. X. Dou, Small Methods, 2019, 3, 1800501 CrossRef.
- X. Wei, C. Chen, X. Z. Fu and S. Wang, Adv. Energy Mater., 2024, 14, 2303027 CrossRef CAS.
- J. Mu, X. W. Gao, T. Yu, L. K. Zhao, W. B. Luo, H. Yang, Z. M. Liu, Z. Sun, Q. F. Gu and F. Li, Adv. Sci., 2024, 11, 2308979 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2026 |





