Tandem depolymerization-electrocatalysis for plastic waste upcycling
Yingxin
Ma†
a,
Mingzhu
Han†
a,
Yufeng
Qian
b,
Dan
Xing
a,
Jizhe
Ma
a,
Yadong
Yu
c,
Bocheng
Qiu
 *a and
Yu
Zhang
*a and
Yu
Zhang
 *bc
*bc
aDepartment of Chemistry, College of Sciences, Nanjing Agricultural University, Nanjing 210095, China. E-mail: bochengqiu@njau.edu.cn
bJiangsu Evalve Co., Ltd, Zhenjiang 212221, China
cCollege of Energy and Power Engineering, Nanjing Institute of Technology, Nanjing 211167, China. E-mail: yu_zhang1225@163.com
First published on 2nd December 2025
Abstract
As persistent environmental pollutants, waste plastics present a valuable carbon resource that can be catalytically converted into high-value commodity chemicals to achieve resource circularity. Advancing the targeted conversion of plastic waste into value-added chemicals is thus a critical strategic pathway. Although various depolymerization techniques yield plastic monomers or platform molecules, these products still suffer from low value, necessitating further catalytic upgrading to obtain high-value chemicals. Electrocatalytic technology, characterized by high reaction selectivity and mild operating conditions, has emerged as an attractive approach for valorizing plastic depolymerization products. The integration of plastic waste depolymerization and electrocatalytic upcycling thus represents a promising pathway for sustainable plastic waste management. In this review, we begin by critically examining plastic depolymerization strategies, with a focus on their compatibility with the following electrocatalytic systems. We subsequently provide a comprehensive analysis of electrocatalytic valorization pathways for the resulting valuable products. Finally, we propose actionable strategies for optimizing current technologies and outline fundamental research directions to unlock greater value from plastic-derived feedstocks.
Green foundation1. This review examines hybrid catalysis strategies for plastic upcycling that integrate depolymerization with electrocatalytic upgrading, emphasizing green chemistry principles, process efficiency, and renewable energy integration.2. Our review highlights recent innovative advances in the depolymerization and electrochemical upcycling of waste plastics, summarizes the production of value-added products with tailored properties, and offers new perspectives for achieving practical upcycling. 3. Future research efforts will focus on integrated recycling platforms that couple depolymerization with electrocatalytic upgrading. These systems are aimed at transforming plastic waste into value-added products to enable efficient resource recovery and advance a circular economy. |
1. Introduction
Plastics have become indispensable to modern life and technological progress due to their advantageous properties, including low weight, high durability, and resistance to degradation, which make them essential materials across diverse industrial sectors.1,2 The relentless increase in plastic production, which is forecast to exceed 500 million metric tons per year by 2050, has directly resulted in a corresponding surge of single-use plastic waste.3 Currently, only 14% of global plastic wastes are recycled, with a large proportion instead ending up in landfills or leaking into the environment, contributing to widespread pollution, including the presence of microplastics even in remote regions such as the Antarctic.4 Moreover, poor management of plastic waste also represents a significant loss of carbon-based resources.5 At present, landfilling and incineration are the primary methods for managing plastic waste, whereas these approaches are economically inefficient and pose significant environmental risks.6,7 These challenges underscore the urgent need for technological innovation and improved recycling strategies to transition toward a truly circular plastics economy. Alternatively, plastic recycling represents a viable solution where discarded plastics are repurposed as raw materials for producing high-value products, supporting sustainable resource utilization and environmental friendliness. The development of advanced plastic waste recycling strategies has become a critical task in building a sustainable and circular materials economy. Mechanical recycling is a common approach for processing plastic waste by reshaping it into new materials; however, variations in chemical, thermal, and mechanical properties among different plastics often result in reduced material quality.8,9 In comparison, another promising approach is chemical processing, where plastic waste is transformed into monomeric components or valuable chemicals, positioning it as a key closed-loop recycling strategy that is garnering increasing attention in both research and industry sectors.8,10,11 This method holds significant potential for enabling a truly circular lifecycle for plastics while mitigating environmental impacts and conserving valuable resources.Catalytic depolymerization is currently the most widely used method for closed-loop plastic recycling. It not only facilitates the recovery of plastic monomers but also generates valuable byproducts through catalytic processes. Within the context of upcycling, the monomers derived from depolymerization can be further valorized to produce value-added chemicals. However, this typically necessitates the implementation of additional processing technologies to upgrade the depolymerized products. Emerging technologies in this field include electrocatalysis, photocatalysis, and photoelectrocatalysis.12–14
Among these, electrocatalysis operates under mild reaction conditions, utilizes electricity sourced from renewable energy, and exhibits high efficiency and selectivity toward the desired reactions. Electrocatalytic technology not only enables the upcycling of plastic depolymerization products in one stage but also supports additional electrocatalytic reactions in a subsequent stage to produce value-added chemical products (Fig. 1). Accordingly, this review summarizes existing plastic depolymerization approaches, with particular emphasis on electrochemical technologies for upgrading depolymerized molecules and their paired electrochemical reactions. The work offers new insights into the integration of plastic depolymerization and electrochemical upcycling processes.
 | ||
| Fig. 1 Typical strategies for plastic waste treatment and the process for the electrochemical upcycling of plastic waste combined with depolymerization. | ||
2. Depolymerization of waste plastics
Electrochemical technologies, driven by renewable energy sources (e.g., wind, hydropower, and solar), represent a mild yet highly efficient strategy for the sustainable upcycling of waste plastics at a relatively low operational cost. However, plastics consist of rigid polymeric structures that demonstrate negligible solubility in conventional electrolyte solutions, rendering direct electrolysis highly challenging.15–17 Recent advances have demonstrated the emerging use of redox mediators in electrochemical plastic recycling, where such mediators enable electron transfer between electrodes and polymers.18,19 This approach represents a significant proof-of-concept step toward the direct conversion of waste plastics. For example, electrochemical techniques enable the depolymerization of polyolefins through targeted cleavage of C–C or C–O bonds. Hourtoule et al. have developed an iron-based electrocatalytic strategy for the direct depolymerization of recalcitrant polystyrene waste into benzoic acid products.20 The reaction proceeds via anodic generation of a high-valent iron-oxo intermediate from an iron–porphyrin catalyst, which facilitates hydrogen abstraction from the benzylic site of polystyrene to generate a carbon radical. Subsequent oxygen capture, cathodic reduction, and β-scission cascade culminate in chain cleavage and oxidation to afford small molecules like benzoic acid, affording a 18.5% yield. Alternatively, Pham et al. achieved highly efficient PET depolymerization under mild conditions by employing a reduced benzimidazolium cation-based electrocatalyst.21 The electrogenerated [N-DMBI]˙ radical serves as a potent electron mediator, driving a synergistic cascade of electron transfer, homolytic cleavage, and oxygen oxidation to facilitate the process. It should be noted that not all electrochemical approaches to plastics are geared toward recycling. For instance, direct mineralization converts plastics into CO2, thereby causing carbon emissions and depleting the material's carbon value.22 Conversely, functionalization primarily serves to tailor the properties of polymers for novel applications, positioning it as a manufacturing technique rather than a recycling solution.23,24 State-of-the-art electrochemical techniques for the direct treatment of plastics are hampered by three principal drawbacks: a prevalent reliance on toxic organic solvents (e.g., dichloromethane, acetonitrile) with associated environmental implications; and critically, low process efficiency. This inefficiency is largely attributed to the inherent poor solubility of polymers and sluggish mass transport, collectively necessitating the development of alternative strategies.Consequently, targeted chemical or biological depolymerization processes must be employed to transform waste plastics into soluble monomeric intermediates suitable for carbon-neutral electrochemical valorization.25,26 Plastics are polymeric materials composed of repeating monomeric units. Their depolymerization pathways exhibit significant variation depending on the specific covalent bonding configurations between these monomers, which differ across various types of plastics. The predominance of C–C, C–O, and C–N covalent bonds in polymeric architectures necessitates targeted bond scission strategies to produce electrochemically processable oligomeric fragments.
For example, C–O linkages appear in polyester plastics containing ester bonds, including PET, polylactic acid (PLA), and polybutylene terephthalate (PBT), which are prone to hydrolysis under alkaline conditions.27 Using PET as a representative case, it exhibits outstanding mechanical properties, including high tensile strength, excellent wear resistance, and low thermal expansion. Owing to these superior characteristics, PET has become one of the most widely used polyester plastics in consumer applications, particularly in beverage bottles, food packaging, and textile fibers.28 Two distinct PET pretreatment pathways exist for electrochemical valorization: (i) complete monomer recovery via alkaline hydrolysis, yielding terephthalic acid (TPA) and ethylene glycol (EG), the latter being amenable to direct electrocatalytic oxidation; or (ii) partial depolymerization creating functionalized intermediates (e.g., carboxylates) as optimized reactants for paired electrolysis systems.29 The first depolymerization approach is typically conducted under alkaline, neutral, or acidic aqueous conditions, with the specific medium selection dictating reaction kinetics and product distribution. Typically, alkaline hydrolysis is typically conducted in KOH/NaOH aqueous solutions, where PET undergoes complete depolymerization to form terephthalate salts (TPA-K/Na) and EG. During alkaline hydrolysis, hydroxide ions (OH−) act as nucleophiles and attack the electrophilic carbonyl carbon of the ester group, leading to the formation of a tetrahedral intermediate (Fig. 2a). Subsequently, this intermediate undergoes cleavage of the ester bond, resulting in the release of the alkoxide group (–OR′). The liberated alkoxide then abstracts a proton from a neighboring hydroxyl group, thereby regenerating a neutral alcohol (–OH).30 This concerted mechanism, formally referred to as carboxylate deprotonation, ultimately yields EG and TPA-K/Na as the primary products under alkaline conditions.31 TPA was obtained by acidifying the hydrolysate with H2SO4/HCl, followed by filtration.
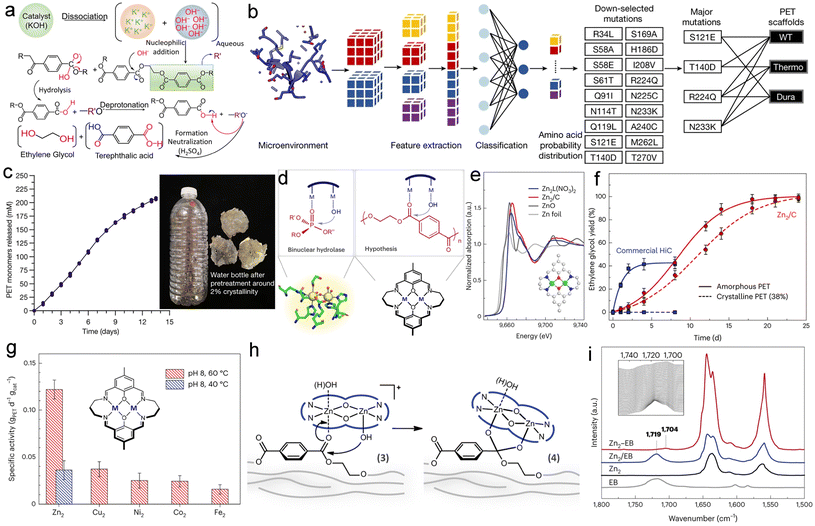 | ||
| Fig. 2 (a) Schematic illustration of the chemical depolymerization of waste PET plastics through alkaline hydrolysis to produce. (b) Machine learning-guided rational engineering of PET hydrolase (c) complete degradation of a pretreated water bottle using FAST-PETase. Reproduced with permission.37 Copyright 2022, Springer Nature. (d) Schematic illustration of the active site and reaction mechanism of the binuclear hydrolase OpdA (left), and of a proposed binuclear catalyst for PET degradation (right). (e) Normalized XANES spectra for Zn2L(NO3)2, Zn2/C, and reference samples are shown; the inset illustrates the molecular structure of Zn2L with atoms color-coded (C: white, N: blue, O: red, Zn: green). (f) Hydrolysis kinetics of am-PET and crystalline PET pellets (38% crystallinity) on Zn2/C and commercial HiC at 60 °C in aqueous NaOH solution (pH 8). (g) Hydrolysis activity of binuclear catalysts with different metal centers against crystalline PET granules (38% conversion) at pH 8 (NaOH aq.) and 60 °C. (h) The proposed mechanism for PET hydrolysis over the binuclear catalyst involves two key steps: co-adsorption of substrates on adjacent zinc sites and stabilization of a six-membered intermediate. (i) DRIFTS spectra comparing the physical mixture (Zn2/EB, blue) and chemisorbed species (Zn2-EB, red) of the PET model compound ethyl benzoate (EB) on the binuclear zinc catalyst (Zn2), with the inset depicting the evolution during removal of free EB. Reproduced with permission.40 Copyright 2023, Springer Nature. | ||
During acidic hydrolysis, the reaction mechanism parallels alkaline processes, wherein H+ ions initially protonate the carbonyl oxygen of PET, facilitating subsequent nucleophilic attack. This stepwise process ultimately yields equimolar quantities of EG and terephthalic acid TPA. During acidic hydrolysis, the reaction mechanism parallels alkaline processes, wherein H+ ions initially protonate the carbonyl oxygen of PET, facilitating subsequent nucleophilic attack. This stepwise process ultimately yields equimolar quantities of EG and terephthalic acid TPA.32 However, acidic hydrolysis typically requires concentrated acid solutions,33 which induce severe equipment corrosion and consequently impede subsequent electrochemical processing.
Neutral hydrolysis of PET in aqueous media typically requires elevated temperatures or pressures to promote the autoionization of water into H+ and OH− ions. The resulting protons react with the carbonyl groups in PET, leading to the formation of hydroxyl species and carbocation intermediates.34 These electrophilic intermediates are readily attacked by nucleophilic water molecules, ultimately resulting in the production of terephthalic acid TPA and ethylene glycol EG. Additionally, Wen et al. demonstrated the continuous and efficient depolymerization of waste PET using ZnO as a catalyst under ambient air pressure without organic solvents, achieving high yields of terephthalic acid (TPA >97%) and ethylene glycol (EG >95%).35 However, this process requires elevated reaction temperatures of 260 °C.
The reliance on concentrated acids, alkalis, and high temperatures in acidic, alkaline, and neutral depolymerization routes significantly elevates carbon emissions in life cycle assessment (LCA) and adversely affects cost-effectiveness in techno-economic analysis (TEA).36 The pursuit of more environmentally benign, energy-efficient, and highly effective depolymerization methods has driven significant advancements in enzymatic catalysis. Lu et al. developed a structure-based machine learning algorithm to engineer FAST-PETase (Fig. 2b), a highly stable and efficient PET hydrolase.37 Remarkably, FAST-PETase achieved complete hydrolysis of intact, non-pretreated PET films derived from water bottles within two weeks at 50 °C (Fig. 2c). Enzymatic catalysis offers a sustainable path for plastic depolymerization beyond PET. Similarly, for PLA, Xie et al. demonstrated through computational design a high-performance esterase mutant that achieved efficient degradation of PLA powder, underscoring the broad potential of this approach.38 Currently, the high production costs and limited availability of synthetic enzymes necessitate further development of efficient, cost-effective enzymatic manufacturing processes.39 To address this challenge, Zhang et al. developed a dinuclear zinc catalyst (Zn2/C) capable of efficiently depolymerizing PET under mild reaction conditions.40 The adjacent metal centers promote substrate proximity (Fig. 2d and e), thereby enhancing reaction rates through enzyme-like intramolecular hydrolysis. Under mild conditions (pH = 8, 40 °C), the Zn2/C catalyst completely depolymerized amorphous PET into monomers within 10 weeks with a specific activity of 36 mgPET d−1gcat−1 (Fig. 2f), whereas conventional catalysts such as zinc acetate and zinc oxide showed no activity. The catalytic activity of the binuclear complexes, which follows the order Zn–Zn > Cu–Cu > Ni–Ni > Co–Co > Fe–Fe, is governed by the Lewis acidity of the metal centers (Fig. 2g). The highly active di-zinc site promotes nucleophilic hydrolysis by exerting pronounced polarization on the PET carbonyl group. The catalytic system, which undergoes an initial induction period for in situ generation of an active hydroxo-bridged binuclear zinc species, demonstrates that the initial compound is essentially a pre-catalyst requiring activation (Fig. 2h). Coordination by the binuclear zinc site activates the PET substrate, as evidenced by a redshift of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretch from 1719 to 1704 cm−1 (Fig. 2i).
O stretch from 1719 to 1704 cm−1 (Fig. 2i).
This shift confirms the weakening of the carbonyl bond and the enhanced electrophilicity of its carbon atom, thereby facilitating nucleophilic attack. This demonstrates the exceptional performance of Zn2/C in breaking down chemically inert PET under environmentally relevant conditions. These methods can depolymerize C–O-containing polyesters but are hampered by low-value monomers and costly separation, as illustrated by EG (boiling point of 197.4 °C). This necessitates the development of approaches like electrochemical upgrading to convert monomers into readily isolable or higher-value molecules.
An emerging approach for recycling polyesters involves tandem reaction systems that integrate depolymerization with in situ valorization of the resulting monomers.41 This integrated methodology enables direct conversion of plastic waste into more separable or higher-value products within a single reactor, significantly enhancing the economic viability of the process. Ma et al. demonstrated the oxidative depolymerization of PET to TPA and oxalic acid by using an Au/HY catalyst, achieving exceptional yields (90%) and selectivity (91%) toward oxalic acid.42 PET was initially depolymerized into terephthalic acid (TPA) and ethylene glycol (EG) under alkaline conditions, followed by the catalytic oxidation of EG to oxalic acid over Au/HY(Fig. 3a). In contrast, the Au/C catalyst exhibited a markedly reduced yield of oxalic acid (26%) under the same conditions, demonstrating a strong support dependency relative to the Au/HY benchmark. Compared to Au/C, the Au/HY system exhibits electron transfer from the HY zeolite support to Au nanoparticles, resulting in an upward shift of the d-band center closer to the Fermi level (Fig. 3b). The introduced electronic modification promotes the adsorption capacity of the catalyst surface toward EG, leading to an enhanced EG conversion rate consequently. The observed profile of glycolic acid, which showed a distinct rise and subsequent fall, confirms its identity as a reaction intermediate in the ethylene glycol (EG) oxidation pathway (Fig. 3c). Density functional theory (DFT) calculations on Au(111) indicate that the rate-determining step for the glycolic acid route (formation of HOO-CHOOH*, ΔG = 0.19 eV) is marginally more favorable than that for the glyoxal route (OHC-CHOOH* formation, ΔG = 0.20 eV) in EG oxidation (Fig. 3d). Combined experimental and computational studies further confirm that the oxidation of EG predominantly proceeds via the glycolic acid pathway, with the active Au sites playing a central role in facilitating this transformation. The separation of oxalic acid from depolymerization media via crystallization with calcium chloride is feasible. Nevertheless, the economic potential of this oxidative depolymerization process is compromised by both the significant alkali requirements in the workup and the low commercial value of the resulting oxalic acid. Therefore, the subsequent transformation of oxalic acid into higher-value products is imperative to establish an economically competitive technology. Notably, electrocatalytic reduction enables the conversion of oxalic acid to valuable products, including glyoxylic acid, glycolic acid, or glycine (by co-reduction with nitrogen oxides), as will be comprehensively discussed in the following chapter.
 | ||
| Fig. 3 (a) Schematic illustration of the oxidative depolymerization of PET over Au/HY. (b) The PET conversion and product concentration as a function of reaction time. (c) The d-band centers of Au/C and Au/HY measured by XPS. (d) Calculated Gibbs free energy changes for each elementary reaction from EG to oxalic acid (OA) via the glyoxal and GA pathways on the Au(111) surface. Reproduced with permission.42 Copyright 2024, Wiley-VCH. (e) Correlation between the pyridine-FTIR spectral characteristics of various supports. (f) Schematic illustration of the passivated Au/P25 catalyst. (g) Profile of the tandem PLA hydrolysis-oxidation process on Au/P25-DPS under the specified conditions: 100 mg PLA, 15 mg catalyst, 3 mL H2O, 140 °C, and 2 bar O2. Reproduced with permission.43 Copyright 2023, Wiley-VCH. | ||
The economic potential of plastic upcycling is undermined by the over-oxidation of monomers to low-value compounds. Therefore, it is imperative to employ precisely controlled reaction parameters and selective catalysts to halt the oxidation at intermediate, high-value stages.
Wang et al. proposed a “hydrolysis-oxidation” strategy that offers an innovative and effective approach to address PLA plastic waste management and recycling challenges.43 The strategy leverages a tunable metal–support interface in Au/TiO2 to achieve highly selective conversion of PLA waste to pyruvic acid under mild aqueous-phase conditions. Control experiments with Ru/TiO2 and Pd/TiO2 showed a pronounced tendency for C–C bond cleavage under identical conditions, resulting in acetic acid as the main product. These findings thereby demonstrate the exceptional capability of gold in suppressing over-oxidation and facilitating selective incomplete oxidation. The Lewis acid strength of various supports, characterized by pyridine-Fourier transform infrared (FTIR) spectroscopy, was investigated to elucidate the metal–support interaction with gold. TiO2 was identified as the support with moderate Lewis acidity. Au/TiO2 provided a markedly superior pyruvic acid yield under the same conditions, with performance displaying a defined correlation to support Lewis acidity. Deviations from the optimal strength, whether too strong (Au/ZrO2) or too weak (Au/SiO2), redirected the reaction pathway toward acetic acid production via side reactions. The selectivity for phthalic anhydride (PA) was significantly enhanced by precisely tailoring the Lewis acid sites on the TiO2 support. Notably, treatment of Au/P25 with diphenylsilane (DPS) effectively passivates excess Lewis acid sites, thereby modulating the catalyst's acidity to an optimal level. Compared to untreated Au/P25, the DPS-modified Au/P25 catalyst achieves 99% pyruvic acid (PA) yield during PLA oxidative depolymerization, which demonstrates exceptional catalytic performance. Kinetic analysis of neutral PLA hydrolysis reveals that the hydrolysis rate to LA significantly exceeds the oxidation rate of LA, demonstrating that PLA oxidative depolymerization to pyruvic acid (PA) proceeds through a sequential hydrolysis-oxidation pathway: initial PLA hydrolysis to LA followed by LA oxidation (Fig. 3g). Unlike conventional PLA depolymerization to lactic acid monomers, this method enables chemical upcycling of waste PLA into valuable products, including pyruvic acid, acetic acid, or an equimolar mixture of acetic acid and formaldehyde. Unlike traditional routes to lactic acid, this method enables acid- and base-free depolymerization of PLA with a markedly reduced carbon footprint, offering a greener alternative. Crucially, pyruvic acid possesses higher economic value than lactic acid and can be further upgraded via electrocatalytic coupling with nitrogen oxides to yield alanine, a higher-value product, as has been experimentally verified.44–46 These aspects shall be explored in depth in the next chapter, which includes a dedicated section on electrochemical methods.
Shifting the focus to C–C bonds, in general, C–C linkages are mainly distributed in polyolefin-based plastics, such as polyethylene (PE), polypropylene (PP), polystyrene (PS), and polyvinyl chloride (PVC).47 Polyolefin plastics are extensively utilized in manufacturing films, packaging materials, containers, and piping systems. With the advancement of the petrochemical industry, polyolefin production has experienced rapid growth, resulting in waste polyolefins now constituting approximately one-third of global plastic output. Polyolefin plastics exhibit inherent chemical inertness and are highly resistant to depolymerization under mild conditions, rendering monomer recovery nearly impossible.48 The stability of these materials arises from their robust C(sp3)–C(sp3) bonds, which are further stabilized by symmetrical electron distribution. This structural feature imposes significant thermodynamic barriers to bond cleavage.49 As a result, the recycling of polyolefins is a fundamentally challenging process.
Current primary methods for polyolefin depolymerization rely on the chemical cleavage of C–C bonds, which typically requires demanding conditions, such as elevated levels of acids and bases, expensive solvents (e.g., ionic liquids), organic catalysts, high temperature, and high-pressure gases (e.g., H2, O2).50–54 For example, hydrogenolysis can effectively convert polyethylene (PE) into low-molecular-weight alkane products, although this process typically requires harsh conditions, including temperatures exceeding 200 °C and hydrogen pressures of 0.5–6 MPa.55,56 The fuel oils and gaseous hydrocarbons from hydrolysis or pyrolysis have low value and poor water solubility, severely hindering their electrochemical upgrading potential. In addition to depolymerizing polyolefins into alkanes, studies on oxidative depolymerization to carboxylic acids are also emerging. The oxygen-containing functional groups in these organic acids render the degradation products both valuable and highly water-soluble, thereby facilitating their subsequent upgrading via electrochemical techniques. Bäckström et al. utilized microwave radiation to treat low-density polyethylene (LDPE) with relatively dilute nitric acid solutions (0.1–0.5 g mL−1), achieving complete conversion of the polymer into organic acids (Fig. 4a).57 Under microwave irradiation, a 0.1 g mL−1 nitric acid solution could be heated to 170 °C, enabling full transformation of waste LDPE plastic bags within 16 hours. The process yielded organic acids with a maximum combined yield of 71% and a carbon process efficiency of 37%. Nevertheless, the process has notable drawbacks, including undesirable CO2 emissions. Additionally, the carbon recovery rate remains suboptimal and requires improvement. Similarly, polystyrene can undergo nitric acid-mediated depolymerization under thermal conditions, yielding organic acids analogous to the degradation pathways of polyethylene. Luo et al. demonstrated the efficient oxidative depolymerization of polystyrene to benzoic acid using nitric acid (20%), achieving a 90% yield with a purity of greater than 95% at 180 °C within 3 hours.58 GPC monitoring revealed significant molecular weight reduction (239 to 196 kDa) and bimodal distribution development within 0.5–1 hour, before benzoic acid formation (Fig. 4b). This profile signifies non-selective backbone cleavage into dispersed fragments. Subsequent breakdown of this bimodal mixture represents a critical preparatory step for ultimate conversion to benzoic acid. Nitric acid is decomposed by heating into NO2 and O2, which subsequently react with carbon-centered radicals generated at the weak linkages of polystyrene chains. Evidence for a radical mechanism in polystyrene degradation comes from radical scavengers and in situ electron paramagnetic resonance (EPR) (Fig. 4c). The inhibited product formation by butylated hydroxytoluene (BHT)/2,2,6,6-tetramethylpiperidine-N-oxyl (TEMPO) and the direct observation of carbon-centered and hydroxyl/superoxide radicals collectively demonstrate a reaction initiated by hydrogen atom transfer from C–H bonds, which produces alkyl radicals and subsequently propagates a radical chain reaction. This process forms peroxyl and hydroxyl radicals through oxidative pathways. The introduction of oxygen-containing functional groups facilitates C–C bond scission, eventually yielding benzoic acid as the predominant product (Fig. 4d). However, the utilization of nitric acid leads to the generation of substantial NOx emissions, which present considerable environmental risks and may contribute to detrimental effects on ecosystems.59,60
 | ||
| Fig. 4 (a) Schematic illustration of the oxidative depolymerization of polyethylene to organic acids via microwave irradiation in nitric acid solution. Reproduced with permission.57 Copyright 2017, American Chemical Society. (b) Evolution of GPC curves for polystyrene plastics under oxidative conditions (180 °C, 20% HNO3). (c) Evolution of the EPR signal for the carbon-centered radical during oxidation (180 °C, 20% HNO3). (d) Reaction mechanism of the oxidative modification of 2,4-diphenylpentane with nitric acid. Reproduced with permission.58 Copyright 2023, Royal Society of Chemistry. (e) Scheme for upgrading waste polyethylene into carboxylic acid chemicals by catalytic oxidation of Co-MCM-41. (f) Product composition as a function of Si/Co ratio and pore size. Here, 200:1-n and 200:1-b denote channels with smaller and larger diameters relative to the standard 200:1 catalyst. (g) Monitoring the degradation products of polyethylene (PE) with varying molecular weights via GC-MS. (h) Schematic degradation mechanism of PE on Co-MCM-41. Reproduced with permission.61 Copyright 2024, Wiley-VCH. (i) The use of TsOMe enables closed-loop recycling of polyarylates (PAs) under mild reaction conditions. (j) Screening of additives for the methanolysis of polyamide-6. (k) Reaction pathway and energy profile for Dimer-1 methanolysis, as determined by DFT calculations. Reproduced with permission.62 Copyright 2025, Springer Nature. | ||
As a result, researchers have been driven to develop innovative oxidative depolymerization technologies aimed at overcoming this environmental challenge. Zhang et al. demonstrated a highly selective oxidative degradation process for converting polyethylene into long-chain diacids by using a Co-MCM-41 catalyst under mild conditions (125 °C, 1 MPa O2) (Fig. 4e).61 The reaction yielded diacids with a total carbon efficiency of 85.9%, and 58.9% selectivity for long-chain diacids, with 46.2% of the product distributed within the C11–C15 range.
The cobalt sites act as the catalytic centers, where both cobalt loading and the pore structure of the molecular sieve critically influence diacid formation and product distribution. Higher cobalt loading, narrower pore channels, and shorter polyethylene chains favor the production of short-chain diacids (Fig. 4f). In contrast, lower cobalt loading, larger pore sizes, and higher polyethylene molecular weights promote the formation of long-chain diacids. To investigate this phenomenon, GC-MS results elucidated distinct oxidation behaviors: n-decane showed minimal reactivity, in contrast to n-triacontane which formed mono- and dicarboxylic acids. This molecular weight-dependent trend continued with PE, with low Mn (3000) giving short-chain diacids and high Mn (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) yielding long-chain diacids. The chain length of diacid products from PE degradation is primarily dictated by the parent polymer's molecular weight, creating a positive correlation whereby higher Mw PE generates longer-chain diacids. Molecular dynamics simulations reveal this relationship is mediated by chain conformation and pore accessibility. For PE with Mw > 8000, the long axis exceeds the pore's upper size limit, preventing passage of extended chains, while the smaller radius of gyration enables entry of coiled chains, thus defining the degradation pathway. Through systematic modulation of these parameters, the oxidative degradation of polyethylene can be precisely tuned to yield a controlled distribution of diacids, ranging from short- to long-chain species. This approach is inherently green, as it requires neither solvents nor precious metal catalysts, generates minimal carbon emissions, and eliminates the production of toxic or hazardous waste.
000) yielding long-chain diacids. The chain length of diacid products from PE degradation is primarily dictated by the parent polymer's molecular weight, creating a positive correlation whereby higher Mw PE generates longer-chain diacids. Molecular dynamics simulations reveal this relationship is mediated by chain conformation and pore accessibility. For PE with Mw > 8000, the long axis exceeds the pore's upper size limit, preventing passage of extended chains, while the smaller radius of gyration enables entry of coiled chains, thus defining the degradation pathway. Through systematic modulation of these parameters, the oxidative degradation of polyethylene can be precisely tuned to yield a controlled distribution of diacids, ranging from short- to long-chain species. This approach is inherently green, as it requires neither solvents nor precious metal catalysts, generates minimal carbon emissions, and eliminates the production of toxic or hazardous waste.
The outlined method efficiently converts polyolefin plastics into water-soluble organic acids like benzoic acid and mixed diacids. The former can be valorized through electrochemical hydrogenation to cyclohexanecarboxylic acid.63,64 For the latter, which poses significant separation challenges, electrocatalytic decarboxylation presents a viable conversion route to high-value, readily separable alkene gases.65–67
C–N linkages are primarily present in polyamide plastics, which have garnered significant attention due to their exceptional performance characteristics. Owing to their outstanding mechanical properties, such as high strength, rigidity, wear resistance, and impact toughness, polyamides are widely used in industries including electronics, automotive manufacturing, construction, and office equipment.68,69 With an annual global production of approximately 7.5 million metric tons, nylon-6 and nylon-66 together account for about 90% of all polyamide products.70,71 Due to their non-biodegradability in the environment, nylon-based plastics persist and accumulate over time.72 Notably, discarded nylon fishing nets constitute approximately 10% of marine plastic waste.73 However, their high chemical stability, manifested by insolubility in most solvents, poses significant challenges for electrochemical reforming and recycling processes. Polyamide depolymerization strategies can be divided into monomer recovery and conversion to other chemical raw materials. From a molecular structural perspective, the C–N linkage in the amide bond exhibits lower polarity than the C–O linkage in the ester bond, resulting in higher bond cleavage reactivity for C–O compared to C–N. This implies that polyamide plastics are generally more resistant to depolymerization than polyester plastics. Nevertheless, the depolymerization experience gained on PET may also be applied to polyamide, including acidolysis and alkaline hydrolysis. Studies on polyamide under acidic conditions revealed that higher acid concentrations significantly accelerate its hydrolysis rate.74 While the mechanism of amide acidolysis remains unclear, the prevailing view favors protonation on the O as the initial step, though protonation on the N has also been proposed.75 To enhance the depolymerization rate of nylon plastics in acidic media, Češarek et al. employed microwave irradiation to rapidly heat the acid solution, thereby achieving efficient polymer hydrolysis.76 In this process, the hydrolysis rate of nylon-66 (PA66) was determined by both the HCl/amide molar ratio and the type of plastic additives (reinforcement additives). Under conditions of 200 °C and an HCl-to-amide molar ratio of 1.25, nylon-66 was completely converted to its constituent monomers (adipic acid and hexamethylenediamine) within 10 minutes. However, the use of acidic catalysts presents several challenges, including difficulties in product separation, generation of acidic waste streams, and corrosion of experimental devices. Consequently, acid-catalyzed depolymerization may not represent the optimal strategy for polymer recycling. Hu et al. demonstrated the hydrolysis of PA6 using subcritical water as a catalyst, achieving near-complete depolymerization under optimal conditions (300 °C, water-to-PA6 mass ratio of 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 60 min).77 This process converted approximately 100% of both virgin PA6 and waste PA6 textiles into ε-caprolactam at yields of 89.9% and 91.6%, respectively. While both acid-catalyzed and subcritical water hydrolysis can effectively depolymerize polyamides, their high-temperature requirements (100–300 °C) and significant energy demands result in limited contributions to carbon neutrality goals. Consequently, there remains a critical need to develop low-temperature and cost-effective depolymerization methods that can recover valuable resources from diverse polyamide-containing plastic wastes. Yang et al. reported a strategy for polyamide depolymerization using methyl p-toluenesulfonate (TsOMe) as an effective activator under mild alcoholysis conditions (Fig. 4i).62 The approach affords near-quantitative yields and high selectivity, precisely converting a range of polyamides into valued monomers encompassing ε-caprolactam, amino acid esters, and diamines or diesters. During the initial research phase, PA6 was used as a model substrate to evaluate alcoholysis additives. Methyl p-toluenesulfonate, which is converted in situ into p-toluenesulfonic acid in methanol, exhibited superior performance in both depolymerization rate and alcoholysis selectivity compared to other Brønsted or Lewis acids (Fig. 4i). The progression of the reaction, monitored by NMR spectroscopy, was marked by a decrease in amide bond signals and the concurrent emergence of ester and protonated amine signals, confirming efficient alcoholysis. This reaction system achieves 83.5% conversion within 140 minutes under the established conditions. Kinetic analysis further revealed that the reaction follows first-order kinetics, with the rate showing a positive correlation with the acid concentration. Theoretical calculations indicate that TsOH initially activates the amide bond via coordination in Int-1 (Fig. 4k), increasing its electrophilicity. Nucleophilic attack by methanol then occurs via the rate-limiting TS-1 to form tetrahedral intermediate Int-2. In the final step, TsOH assists C–N bond cleavage through hydrogen-bonded Int-3 and low-barrier TS-2, leading to carbonyl regeneration and product 1a formation. In summary, TsOH drives the alcoholysis by simultaneously activating the amide group and neutralizing the amino leaving group. This approach proves generally applicable to various polyamides (e.g., PA66, PA11, PA12), enabling their efficient depolymerization and subsequent product isolation through established purification methods like solvent extraction and recrystallization.
1, 60 min).77 This process converted approximately 100% of both virgin PA6 and waste PA6 textiles into ε-caprolactam at yields of 89.9% and 91.6%, respectively. While both acid-catalyzed and subcritical water hydrolysis can effectively depolymerize polyamides, their high-temperature requirements (100–300 °C) and significant energy demands result in limited contributions to carbon neutrality goals. Consequently, there remains a critical need to develop low-temperature and cost-effective depolymerization methods that can recover valuable resources from diverse polyamide-containing plastic wastes. Yang et al. reported a strategy for polyamide depolymerization using methyl p-toluenesulfonate (TsOMe) as an effective activator under mild alcoholysis conditions (Fig. 4i).62 The approach affords near-quantitative yields and high selectivity, precisely converting a range of polyamides into valued monomers encompassing ε-caprolactam, amino acid esters, and diamines or diesters. During the initial research phase, PA6 was used as a model substrate to evaluate alcoholysis additives. Methyl p-toluenesulfonate, which is converted in situ into p-toluenesulfonic acid in methanol, exhibited superior performance in both depolymerization rate and alcoholysis selectivity compared to other Brønsted or Lewis acids (Fig. 4i). The progression of the reaction, monitored by NMR spectroscopy, was marked by a decrease in amide bond signals and the concurrent emergence of ester and protonated amine signals, confirming efficient alcoholysis. This reaction system achieves 83.5% conversion within 140 minutes under the established conditions. Kinetic analysis further revealed that the reaction follows first-order kinetics, with the rate showing a positive correlation with the acid concentration. Theoretical calculations indicate that TsOH initially activates the amide bond via coordination in Int-1 (Fig. 4k), increasing its electrophilicity. Nucleophilic attack by methanol then occurs via the rate-limiting TS-1 to form tetrahedral intermediate Int-2. In the final step, TsOH assists C–N bond cleavage through hydrogen-bonded Int-3 and low-barrier TS-2, leading to carbonyl regeneration and product 1a formation. In summary, TsOH drives the alcoholysis by simultaneously activating the amide group and neutralizing the amino leaving group. This approach proves generally applicable to various polyamides (e.g., PA66, PA11, PA12), enabling their efficient depolymerization and subsequent product isolation through established purification methods like solvent extraction and recrystallization.
The separation of high-boiling-point amines from polyamide hydrolysates is an energy-intensive process. Electrochemical oxidation provides an effective alternative by converting amines into more readily separable nitriles with lower boiling points and solubility. A detailed discussion of this approach will follow in the electrochemistry section.
The chemical composition of different plastic waste streams, including variations in backbone functional groups such as C–O, C–C, and C–N bonds, significantly influences both the selection of viable depolymerization pathways and the determination of optimal processing conditions. The depolymerization strategies for various types of plastics are summarized in Table 1, classified according to the chemical bond types linking the monomeric units. In summary, the depolymerization step is indispensable in plastic waste valorization, which exhibits comparable importance to subsequent electrochemical reforming processes. Currently, many depolymerization methods exhibit limited compatibility with electrochemical reforming, either due to the aqueous insolubility of resulting monomers or the absence of viable pathways for subsequent electrochemical processing. Consequently, future research must focus on developing cost-effective depolymerization strategies that are compatible with electrochemical reforming systems. Such integrated approaches are critical for enabling circular plastic economies and producing renewable carbon-neutral platform chemicals. The molecular platform derived from plastic depolymerization fundamentally dictates the pathways for subsequent electrochemical upgrading, including suitable catalysts, electrolytes, and applied potentials.
| Linkage | Plastics | Conditions | Catalysts | Products | Ref. |
|---|---|---|---|---|---|
| C–O | PET | 1–10 M NaOH/KOH, 40–180 °C | NaOH/KOH | EG, TPA | 78–81 |
| C–O | PET | 0.1 MPa air, H2O, 260 °C | ZnO | EG, TPA | 35 |
| C–O | PET | 2 M PSSA, 2 M H2SO4 | PSSA | EG, TPA | 82 |
| C–O | PET | KH2PO4–NaOH buffer (pH 8) | FAST-PETase | EG, TPA | 37 |
| C–O | PET | NaOH solution (pH 8), 60 °C | Zn2/C | EG, TPA | 40 |
| C–O | PET | 1 MPa O2, 1 M NaOH, 130 °C | Au/NiOv | GA, TPA | 41 |
| C–O | PET | 0.3 MPa O2, 2 M NaOH, 180 °C | Au/HY | OA, TPA | 42 |
| C–O | PLA | 1–10 M NaOH/KOH, 60–180 °C | NaOH/KOH | LA | 81, 83 and 84 |
| C–O | PLA | NH3·H2O, 1 MPa N2, 140 °C | Ru/TiO2 | Alanine | 85 |
| C–O | PLA | H2O, 1 MPa O2, 140 °C | Au/P25 | PA | 43 |
| C–O | PBT | 2 M KOH, 120 °C | KOH | BDO, TPA | 86 |
| C–O | PBS | 2 M KOH, 90 °C | KOH | BDO, succinate | 87 |
| C–C | LDPE | 2 MPa H2, 240 °C | Ru/C-EG120 | Liquid alkanes (C16–C19) | 88 |
| C–C | HDPE, LDPE | 2 MPa He with 5 vol% N2, 280 °C | Ru/HZSM-5(300) | Linear (C1–C6), cyclic (C7–C15) hydrocarbons | 89 |
| C–C | LDPE | 6% HNO3, 180 °C | — | Organic acids | 57 and 65 |
| C–C | HDPE, LDPE | 1 MPa O2, 125–130 °C | Co-MCM-41/LiCoO2 | Dicarboxylic acid | 61 and 90 |
| C–C | HDPE, LDPE | H2O, 1.5 MPa air, 160 °C | Ru/TiO2 | Dicarboxylic acid | 91 |
| C–C | PS | 20% HNO3, 180 °C | — | Benzoic acid | 58 |
| C–N | PA66 | 20% H2SO4, 60 °C | H2SO4 | Hexamethylenediamine, adipic acid | 92 |
| C–N | PA66 | n(HCl/amide) = 1.25, 200 °C | HCl | Hexamethylenediamine, adipic acid | 76 |
| C–N | PA6 |
n(H2O/PA6) = 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 300 °C 1, 300 °C |
— | ε-Caprolactam | 77 |
| C–N | PA6 | 50%(v/v) H2SO4, 100 °C | H2SO4 | 6-Aminohexanoic acid | 93 |
3. Electrochemical upcycling of plastic waste into high-value chemicals
After a systematic evaluation of depolymerization strategies based on the type of bonds linking monomeric units toward plastics, this analysis shifts focus to electrocatalytic approaches that enhance the value of the resulting depolymerized monomers. Electrocatalytic technology not only utilizes renewable electricity for driving chemical transformations but also enables highly selective synthesis of value-added products at both the anode and cathode. For instance, while platform molecules from anodic plastic depolymerization are being oxidized, the cathode can alternatively drive hydrogen evolution reaction (HER), or more valuably, CO2 reduction (CO2RR) or nitrate reduction (NO3−RR) to generate higher-value chemicals than hydrogen.94 This section aims to detail the electrochemical reactions integrated with the preceding chapter's depolymerization strategies, specifically for plastics containing C–O linkages (e.g., polyesters), C–N linkages (e.g., polyamides), and C–C linkages (e.g., polyolefins).3.1 Electrocatalytic upgrading of polyester plastics
Although alkaline hydrolysis is a well-established method for depolymerizing PET and PLA, the low value and high boiling points of the resultant monomers, EG and lactic acid (LA), diminish the economic appeal of purification. To address this, electrochemical conversion emerges as a promising valorization strategy, transforming these monomers into higher-value products to bolster the recycling economics.In the case of PET, the high boiling point (197.4 °C at 1 atm) of ethylene glycol in the alkaline hydrolysate poses a substantial energy burden for its thermal separation. Direct electrochemical upgrading of ethylene glycol in an alkaline medium offers a promising pathway to value-added products, circumventing this issue. Zhou et al. demonstrated an electrocatalytic upgrading strategy employing a CoNiP/NF catalyst and PET hydrolysate electrolyte.95 This system selectively converted EG to formate, which subsequently neutralized the KOH electrolyte by formic acid, followed by producing purified potassium diformate (KDF) through crystallization. With KDF valued at $1590 per ton, a preliminary techno-economic analysis projects net revenues of approximately $350 per ton of recycled PET, achieved under commercially viable current densities exceeding 300 mA cm−2. Furthermore, the cathodic reduction potential can be adequately utilized to generate higher-value products beyond hydrogen. Wang et al. demonstrated a paired electrocatalytic process that simultaneously converts waste PET and CO2 into formic acid with high efficiency.96 The researchers developed cost-effective nickel–cobalt oxide (NiCo2O4/carbon fiber paper (NiCo2O4/CFP)) and tin oxide (SnO2/carbon cloth (SnO2/CC)) catalysts for the electrocatalytic oxidation of PET alkaline hydrolysates and CO2 reduction, respectively. Preliminary techno-economic analysis demonstrates that this integrated strategy enables the co-valorization of PET and CO2 waste into valuable chemicals, generating an estimated net revenue of $557 per ton.
The aforementioned PET depolymerization methods face a critical limitation: their reliance on the use of caustic bases for effective degradation. This approach inevitably complicates subsequent purification of electrocatalytic products and poses significant challenges for wastewater treatment. Enzymatic depolymerization coupled with electrocatalytic conversion has emerged as an environmentally efficient strategy for plastic upcycling.97
With a market price of $4000 per ton, glycolic acid (GA) stands in sharp contrast to formic acid in both value and commercial appeal, attracting greater interest due to its substantial potential in food packaging, cosmetics, and leather processing industries.98,99 Therefore, a selective process for glycolic acid production from PET waste emerges as a more industrially attractive valorization pathway. However, the selective electrosynthesis of GA remains challenging due to uncontrolled C–C bond cleavage.100 This process requires the preferential oxidation of one hydroxyl group at relatively low potentials while preserving the adjacent hydroxyl group, which is difficult to achieve. For GA production, catalyst design strategies primarily focus on suppressing C–C bond cleavage to maximize selectivity toward the desired product. Currently, only noble metal catalysts (e.g., Pt, Pd, Au) demonstrate the capability for selective conversion of EG to GA.101–104 Du and colleagues created an integrated bio-electrochemical cascade for high-yield conversion of waste PET to glycolate under mild conditions.97 This strategy combines enzymatic depolymerization by an engineered LCC enzyme (95.6% efficiency to EG/TPA) with electrocatalytic upgrading using a CO-tolerant Pd/Ni(OH)2 catalyst to produce valuable glycolate. The R158P mutation was incorporated to generate LCCICCG-M3 from LCCICCG-M2, further improving substrate binding. Relative to M2, M3 displayed an 11.3 °C increase in Tm and a 12.1% higher depolymerization efficiency (Fig. 5a). Overall, M3 achieved a 6.3 °C Tm elevation and 78.9% activity increase relative to the parent LCCICCG. This highly effective enzymatic PET depolymerization facilitates downstream electrocatalysis by providing sufficient raw materials. Furthermore, the Pd/Ni(OH)2 catalyst prepared by corrosion synthesis demonstrates both exceptional GA selectivity and strong CO anti-poisoning capability. The observed 0.04 V negative shift in the CO oxidation potential of Pd/Ni(OH)2/NF versus Pd/NF indicates CO can be more readily oxidized and removed from active sites at lower potentials (Fig. 5b). This capability to rapidly clear CO translates to sustained activity and improved poisoning resistance. The consumption of EG reactants is accompanied by substantial glycolate formation and only minor formate generation (Fig. 5c), demonstrating high selectivity. When 5000 C of charge is passed, over 75% of EG is converted, and the glycolate faradaic efficiency remains above 90%. To investigate the glycolate formation pathway on Pd/Ni(OH)2/NF, glycolaldehyde, the initial oxidation intermediate of ethylene glycol, was utilized as the feedstock. In a typical procedure, 20 mM glycolaldehyde was dissolved in 1 M KOH/H218O. After 3 hours of stirring, 18O-labeled geminal diol was produced via keto–enol tautomerism, which subsequently underwent nucleophilic dehydrogenation to yield 18O-labeled glycolate (Fig. 5d). Therefore, glycolate is produced via base-catalyzed nucleophilic addition of the enol to form the geminal diol, and consequently by nucleophilic dehydrogenation of this intermediate. A highly efficient enzymatic-electrochemical sequential process was successfully implemented. It begins with near-quantitative depolymerization of waste PET using LCCICCG-M3 under mild conditions to obtain high-purity ethylene glycol, followed by its selective electrooxidation to glycolic acid (90.6% purity) using a Pd/Ni(OH)2/NF bifunctional electrode at 1.3 V, coupled with green H2 production. This combined approach efficiently transformed 100 g of waste PET into 52.5 g of glycolic acid crystals (92.6% yield) and 21.3 L of H2, showcasing effective resource and energy co-generation from plastic waste and underscoring its promise for circular economy applications (Fig. 5e). Despite higher enzyme costs, the enzymatic-electrochemical system significantly improves operational economics by reducing alkali use. At 200 tons per day capacity, it achieves an $87.7 million annual gross profit, outperforming traditional alkali-electrochemical systems ($21.9–29.9 million) (Fig. 5f). Life cycle analysis confirms a 62% lower carbon footprint (4.81 vs. 12.62 kg CO2 eq. per product unit), mainly due to eliminated alkali-related emissions (Fig. 5g). The technology thus proves both economically viable and environmentally advantageous.
 | ||
| Fig. 5 (a) Comparison of the enzymatic activities of LCCICCG-M3 and LCCICCG in PET hydrolysis at 75 °C and pH 8.0. (b) CO stripping experiments performed on Pd/Ni(OH)2/NF. (c) Concentrations of glycolate and formate, faradaic efficiency (FE) toward glycolate, carbon balance, and EG conversion as functions of charge consumed. (d) Mass spectrum of the glycolate product evolved in 18O-labeled electrolyte. (e) Tandem enzymatic-electrochemical process for integrated upcycling of real-world PET products. (f) Techno-economic analysis compared with previous work. (g) GHG emissions of our cascade approach versus the emerging alkali-catalyzed electrochemical system. Reproduced with permission.97 Copyright 2024, American Chemical Society. (h) Comparative analysis of one-step versus two-step reduction pathways for glycine production. (i) GO FE and productivity assessed via 4 h chronoamperometry across a broad potential window. Reproduced with permission.42 Copyright 2025, Wiley-VCH. | ||
Notably, PET can also be oxidatively depolymerized to oxalic acid and TPA. Although oxalic acid can be readily separated, its low market value and documented nephrotoxicity, which may even lead to renal failure, pose significant economic and safety concerns.15 Consequently, electrochemical upgrading of oxalic acid to higher-value compounds is desirable. In particular, the electrochemical synthesis of glycine (valued at $4200 per ton) from oxalic acid has been demonstrated as a promising approach, further enhancing its economic attractiveness.105–107 Ma et al. developed an innovative thermochemical–electrochemical cascade strategy for the hydrogen-free, efficient, and selective production of glycine from waste PET.42 This integrated approach first employs a highly active and stable Au/HY zeolite catalyst to convert PET into oxalic acid via thermochemical oxidative depolymerization under mild oxygen pressure (0.3 MPa). The resulting oxalic acid is then efficiently transformed into glycine through a two-step electrochemical reduction process utilizing a TiO2 electrocatalyst.
This study successfully established a cascade strategy for electrochemically synthesizing glycine from oxalic acid on a TiO2 nanowire electrode. Addressing the inefficiency in one-step electroreduction arising from the potential mismatch between glyoxylate formation and oxime reduction, a sequential two-stage process was developed (Fig. 5h). The first stage reduces oxalic acid to glyoxylic acid at −0.9 V with 94% faradaic efficiency and a yield of 0.8 mmol cm−2 h−1 (Fig. 5i), while the second stage accomplishes glycine synthesis via hydroxylamine nucleophilic addition and subsequent electroreduction (Fig. 6a). In situ FTIR spectroscopy confirmed the imine pathway in TiO2-catalyzed glycine synthesis (Fig. 6b). The reaction progression was evidenced by the continuous decrease of the oxime C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond vibration at 1650 cm−1, coupled with the concurrent intensification of product signatures at 1485 cm−1 (C–N bond) and 1593 cm−1 (–NH2 group). Crucially, the detection of the characteristic imine intermediate peak at 1668 cm−1 provided direct experimental validation for the imine route, conclusively revealing the complete sequential transformation from oxime to glycine via this key intermediate. DFT calculations provided theoretical insights into the adsorption-driven mechanism of glycine synthesis on TiO2 (Fig. 6c). Horizontal adsorption of oxime on the (001) surface leads to the hydroxylamine pathway with a 0.98 eV rate-limiting barrier, while vertical adsorption activates the imine pathway with a negligible 0.02 eV barrier. The marked energy difference affirms the thermodynamic preference for the imine route and illustrates how intermediate geometry governs pathway selection. The successful demonstration of gram-scale glycine production from waste PET features a continuous process where 50 g of PET is first converted to oxalic acid (85% yield, 97% pure after purification) via Au/HY-catalyzed depolymerization (Fig. 6d). The oxalic acid is then electroreduced to glyoxylic acid (93% yield) in an MEA electrolyzer, and finally transformed to glycine (95% yield) over a TiO2/TM electrode. The overall process delivers 14.2 g of white glycine crystals with 96% purity, representing a 75% total yield from the intermediate GO solution. This cascade upcycling strategy exhibits both economic attractiveness and outstanding environmental performance. Techno-economic analysis for a 300 ton per day PET processing scale shows a net annual profit of $3.5 million with a $68.6 million investment (Fig. 6e), while life cycle assessment confirms a drastically lowered carbon footprint of 72 kg CO2 per kg glycine—84% lower than that of conventional thermochemical approaches (Fig. 6f). It should be noted that the plastic depolymerization process in this study requires significant alkali consumption, which consequently leads to substantial carbon emissions.
N bond vibration at 1650 cm−1, coupled with the concurrent intensification of product signatures at 1485 cm−1 (C–N bond) and 1593 cm−1 (–NH2 group). Crucially, the detection of the characteristic imine intermediate peak at 1668 cm−1 provided direct experimental validation for the imine route, conclusively revealing the complete sequential transformation from oxime to glycine via this key intermediate. DFT calculations provided theoretical insights into the adsorption-driven mechanism of glycine synthesis on TiO2 (Fig. 6c). Horizontal adsorption of oxime on the (001) surface leads to the hydroxylamine pathway with a 0.98 eV rate-limiting barrier, while vertical adsorption activates the imine pathway with a negligible 0.02 eV barrier. The marked energy difference affirms the thermodynamic preference for the imine route and illustrates how intermediate geometry governs pathway selection. The successful demonstration of gram-scale glycine production from waste PET features a continuous process where 50 g of PET is first converted to oxalic acid (85% yield, 97% pure after purification) via Au/HY-catalyzed depolymerization (Fig. 6d). The oxalic acid is then electroreduced to glyoxylic acid (93% yield) in an MEA electrolyzer, and finally transformed to glycine (95% yield) over a TiO2/TM electrode. The overall process delivers 14.2 g of white glycine crystals with 96% purity, representing a 75% total yield from the intermediate GO solution. This cascade upcycling strategy exhibits both economic attractiveness and outstanding environmental performance. Techno-economic analysis for a 300 ton per day PET processing scale shows a net annual profit of $3.5 million with a $68.6 million investment (Fig. 6e), while life cycle assessment confirms a drastically lowered carbon footprint of 72 kg CO2 per kg glycine—84% lower than that of conventional thermochemical approaches (Fig. 6f). It should be noted that the plastic depolymerization process in this study requires significant alkali consumption, which consequently leads to substantial carbon emissions.
 | ||
| Fig. 6 (a) Elucidation of the reaction mechanism for C–N bond formation between GO and NH2OH in glycine synthesis. (b) In situ FTIR study of glyoxylic acid conversion to glycine with NH2OH over TiO2/TM catalyst in 0.5 M H2SO4. (c) Theoretical evaluation of the step-wise Gibbs free energy changes along the hydroxylamine and imine routes for glyoxylate oxime conversion to glycine on TiO2 (001). (d) Schematic diagram of the process for upcycling mixed PET goods into glycine. (e) Techno-economic analysis of the cascade thermocatalysis–electrocatalysis system for 300 ton PET plastic valorization. (f) Analysis of CO2 emission differences between the cascade process and conventional thermochemical synthesis for glycine production. Reproduced with permission.42 Copyright 2025, Wiley-VCH. (g) Catalyst screening for alanine electrosynthesis under conditions of −100 mA cm−2 for 2 h in an electrolyte containing 0.15 M PA, 1.5 M NO3−, and 0.25 M H2SO4. Reproduced with permission.108 Copyright 2025, Wiley-VCH. | ||
To further reduce the carbon footprint, an alternative oxidative depolymerization process has been developed. This approach utilizes water as the solvent and Pt/SiO2 as the catalyst to convert PLA into pyruvic acid, operating without alkali requirements.43,108 Notably, pyruvic acid undergoes spontaneous oximation with hydroxylamine to form pyruvate oxime, which can be further electrochemically reduced to yield high-value alanine (valued at $4539 per ton). Accordingly, researchers designed a lattice-strained CuBi electrocatalyst to enable the co-reduction of pyruvic acid and nitrate to alanine (Fig. 6g). In this system, nitrate is first converted in situ to hydroxylamine, which reacts with pyruvic acid to form pyruvate oxime. This intermediate is then further reduced to yield alanine.108 High-resolution transmission electron microscopy (HRTEM) analysis revealed a contraction in the (012) interplanar spacing from 0.329 nm in pure Bi to 0.323 nm in the CuBi alloy, indicating 1.8% compressive lattice strain induced by Cu doping (Fig. 7a). Geometric phase analysis further identified anisotropic strain distribution, with the most significant lattice distortion occurring along the Exx direction, confirming microstructural changes due to Cu incorporation into the Bi lattice (Fig. 7b). The compressive lattice strain in the CuBi alloy significantly weakens NH2OH adsorption, facilitating its desorption and coupling with pyruvate to form the oxime. Concurrently, alloying optimizes the hydrogen adsorption free energy, markedly promoting oxime hydrogenation kinetics. This synergy between strain effects and dual-site cooperation collectively enhances both the NH2OH desorption and oxime hydrogenation steps, leading to highly efficient alanine synthesis (Fig. 7c). Real-time kinetic analysis under optimized conditions showed a progressive increase in alanine concentration, achieving a 67% yield while maintaining 99% pyruvate conversion (Fig. 7d). These results confirm the exceptional capability of the catalytic system to drive the selective conversion of pyruvate to alanine. In situ differential electrochemical mass spectrometry (DEMS) detected key intermediates (m/z = 46/30/33/103), confirming the three-step alanine synthesis pathway via NH2OH and oxime (Fig. 7e). Imine species detection validated the oxime reduction route (Fig. 7f).
 | ||
| Fig. 7 (a) HRTEM image of CuBi. (b) Structural analysis of the region defined by the green border in (a) using inverse fast Fourier transform, with corresponding strain field maps along the Exx, Eyy, and Exy directions. (c) Catalytic mechanism of alanine synthesis over CuBi catalyst. (d) Time-dependent profiles of PA concentration and alanine production yield. (e) In situ DEMS tracking the evolution of nitrogen-containing intermediates in alanine synthesis from PA and NO3−. (f) In situ DEMS analysis of the pyruvate oxime reduction process. (g) Calculated Gibbs free energy changes for each elementary reaction from pyruvate oxime to alanine via the imine intermediate pathway on CuBi (012) and Bi (012) surfaces. (h) Schematic flowchart of alanine production from mixed PLA plastics, air, and water. (i) TEA comparison for alanine synthesis under specified electricity pricing (0.03 $ per kWh). (j) LCA for alanine production. Reproduced with permission.108 Copyright 2025, Wiley-VCH. | ||
Gibbs calculations identified the rate-determining imine hydrogenation step, with CuBi alloy lowering its barrier to −0.31 eV (−0.04 eV for pure Bi), explaining the enhanced yield and demonstrating alloying kinetics enhancement (Fig. 7g). The successful demonstration of a thermocatalytic–electrocatalytic tandem system enabled the synthesis of alanine from 100 g of waste PLA, air, and water. The process achieved 80% yield to pyruvic acid through Pt/SiO2-catalyzed depolymerization, followed by plasma-assisted nitrate production from air and 166-hour electrolysis, yielding 59 g of alanine crystals with a 66% overall efficiency (Fig. 7h). The integrated thermocatalysis–plasma-electrocatalysis strategy exhibits a strong competitive edge, with techno-economic analysis projecting a $78.6 million annual gross profit at $0.03 per kWh versus $6.82 million for thermocatalysis (Fig. 7i). Its economic resilience is evident as it remains profitable at $0.1 per kWh, where the conventional system fails. Environmentally, the process cuts the carbon footprint by 97% to 3.7 kgCO2 per kg alanine (Fig. 7j), proving its dual competitiveness.
Overall, the depolymerization-electrocatalysis of polyesters enables a diverse range of products. While PET-derived ethylene glycol can be upgraded to formic acid, glycolic acid, or nitrogen-functionalized chemicals, the pathway also extends to sulfur-based products, including hydroxymethanesulfonate (HMS).109 Although chemical compounds such as acetic acid are accessible from polylactic acid, the most viable routes in terms of economic return are confined to its upgrading to pyruvic acid or reductive amination, as other products offer inferior market value.110
3.2 Electrocatalytic upgrading of polyamide plastics
The previous chapter established the waste plastic valorization strategy as an attractive pathway for producing high-value nitrogen-containing chemicals through carbon resource recycling. This approach enables the selective incorporation of additional functional groups (e.g., C–N or C–S bonds) or the targeted transformation of existing functional groups into desired ones during the electrocatalytic process, which is crucial for the efficient synthesis of valuable products.99,109 For example, nylon-66 waste plastics, ranking among the top five global engineering plastics with an annual demand of 1.3 million metric tons, are synthesized via condensation polymerization of two monomers: adipic acid and hexamethylenediamine (HMD).111,112The inherent nitrogen-containing functional groups in HMD-derived hydrolysis products enable direct electrocatalytic functional group modification without requiring additional nitrogen insertion. However, the high boiling point of HMD in polyamide hydrolysates poses a significant energy challenge for its direct extraction from solution. Therefore, electrochemical oxidation of HMD to adiponitrile (ADN) presents an efficient alternative. ADN is recognized as an emerging key industrial feedstock, with its consumption continuously increasing in lithium-ion battery electrolytes.114,115
Xiao et al. developed a multilayered heterostructured electrocatalyst (Ni3S2@Fe2O3) featuring an Ni3S2 outer shell and Fe2O3 inner core.92 This catalyst enables an integrated hydrolysis–electrocatalysis process that converts waste nylon-66 into valuable ADN, adipic acid AA, and H2 (Fig. 8a). The system achieves functional group modification through selective dehydrogenation of C–N bonds in HMD to form C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N bonds, mediated by the Ni3S2@Fe2O3 interface. The distinctive core–shell structure of Ni3S2 and Fe2O3, coupled with their synergistic interfacial interactions, enables these electrocatalysts to achieve selective dehydrogenation of C–N bonds to C
N bonds, mediated by the Ni3S2@Fe2O3 interface. The distinctive core–shell structure of Ni3S2 and Fe2O3, coupled with their synergistic interfacial interactions, enables these electrocatalysts to achieve selective dehydrogenation of C–N bonds to C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N bonds in HMD. At a current density of 100 mA cm−2, HMD was converted to ADN with an FE approaching 100%. Comparative analysis of 1H NMR spectra before and after electrolysis confirmed the complete oxidative transformation of HMD to AND (Fig. 8b). The Ni3S2@Fe2O3 catalyst proves highly efficient and stable for adiponitrile production, delivering near-unity FE over a wide current density range (50–150 mA cm−2) and maintaining 80% efficiency at 250 mA cm−2. Its performance in polymer hydrolysate electrooxidation, characterized by low overpotential and high current density, is superior to reported systems (Fig. 8c). This study demonstrates an integrated strategy for valorizing waste PA-66 plastic into adiponitrile, adipic acid, and hydrogen through sequential steps (Fig. 8d). The process involves acid hydrolysis at 60 °C, followed by cooling and crystallization of adipic acid based on its temperature-dependent solubility. After alkalization to remove potassium sulfate, the remaining HMD-containing filtrate serves as the electrolyte for the selective electrocatalytic oxidation to adiponitrile in an H-cell. The techno-economic analysis highlights the sensitivity of PA-66 electrocatalytic upcycling to electricity cost and current density. Viability is attained at 50 mA cm−2 under $0.10 per kWh electricity, with profits reaching $3131 per ton at 250 mA cm−2 and >80% FE. Conversely, current densities below 10 mA cm−2 lead to high capital costs from extensive electrolyzer needs. With a break-even point of $5535 per ton PA-66, the technology shows clear economic potential (Fig. 8e). This work establishes a promising pathway for the sustainable production of high-value commodity chemicals (AND) and clean hydrogen fuel from waste nylon-66, demonstrating significant potential for circular economy applications.
N bonds in HMD. At a current density of 100 mA cm−2, HMD was converted to ADN with an FE approaching 100%. Comparative analysis of 1H NMR spectra before and after electrolysis confirmed the complete oxidative transformation of HMD to AND (Fig. 8b). The Ni3S2@Fe2O3 catalyst proves highly efficient and stable for adiponitrile production, delivering near-unity FE over a wide current density range (50–150 mA cm−2) and maintaining 80% efficiency at 250 mA cm−2. Its performance in polymer hydrolysate electrooxidation, characterized by low overpotential and high current density, is superior to reported systems (Fig. 8c). This study demonstrates an integrated strategy for valorizing waste PA-66 plastic into adiponitrile, adipic acid, and hydrogen through sequential steps (Fig. 8d). The process involves acid hydrolysis at 60 °C, followed by cooling and crystallization of adipic acid based on its temperature-dependent solubility. After alkalization to remove potassium sulfate, the remaining HMD-containing filtrate serves as the electrolyte for the selective electrocatalytic oxidation to adiponitrile in an H-cell. The techno-economic analysis highlights the sensitivity of PA-66 electrocatalytic upcycling to electricity cost and current density. Viability is attained at 50 mA cm−2 under $0.10 per kWh electricity, with profits reaching $3131 per ton at 250 mA cm−2 and >80% FE. Conversely, current densities below 10 mA cm−2 lead to high capital costs from extensive electrolyzer needs. With a break-even point of $5535 per ton PA-66, the technology shows clear economic potential (Fig. 8e). This work establishes a promising pathway for the sustainable production of high-value commodity chemicals (AND) and clean hydrogen fuel from waste nylon-66, demonstrating significant potential for circular economy applications.
 | ||
| Fig. 8 (a) Sustainable management pathway for recycling end-of-life PA-66. (b) 1H NMR characterization of PA-66 hydrolysate and anolyte samples. (c) Dependence of partial current density and productivity on applied current density for HMD oxidation over Ni3S2@Fe2O3. (d) Conversion pathway of post-consumer PA-66 to adipic acid, adiponitrile (ADN), and hydrogen. (e) TEA of adiponitrile synthesis under varying current densities. Reproduced with permission.92 Copyright 2023, Wiley-VCH. (f) Schematic diagram of Mn-Co3O4 with Mn–O–Co motifs precisely guides the conversion of nylon-66-derived HMD to ADN. (g) Characterization schematic of normalized XANES spectra at Mn K-edge for Mn-Co3O4. (h) Wavelet transform (WT) analysis for the K3-weighted EXAFS data of Mn-Co3O4, and Mn2O3. Reproduced with permission.113 Copyright 2025, Wiley-VCH. | ||
While the electrocatalytic oxidation of HMD had remained largely unexplored, Yang and colleagues developed a Mn-doping approach for Co3O4 to boost its catalytic performance in transforming nylon-66-based HMD (Fig. 8f).113 This strategy enabled deeper investigation into the underlying reaction mechanism through electronic structure modulation. Synchrotron X-ray absorption spectroscopy (XAS) was employed to unambiguously determine the chemical state of Mn dopants in Mn-Co3O4. The Mn K-edge XANES spectrum exhibits close agreement with the Mn2O3 standard, confirming an average Mn oxidation state of +3.0 (Fig. 8g). Wavelet transform analysis not only verifies the atomic dispersion of Mn within the Co3O4 lattice but also provides direct visualization of the local coordination environment dominated by Mn–O–Co bonding motifs (Fig. 8h). Precise structural regulation via Mn doping at octahedral Co3+ sites in Co3O4 tailors its electronic properties, as evidenced by soft X-ray absorption spectra showing suppressed Co3+ signals and enhanced Co–O covalency (Fig. 9a). In situ FTIR and molecular dynamics simulations uniformly demonstrate improved HMD adsorption affinity (Fig. 9b). The dual-site structure mitigates OH−/HMD adsorption competition, thus boosting HMD oxidation performance (Fig. 9c). Consequently, Mn-Co3O4 attains 93% faradaic efficiency for ADN at 200 mA cm−2 and a production rate of 0.87 mmol cm−2 h−1 (Fig. 9d). At a charge of 3000 C, Mn-Co3O4 attained high HMD conversion (>88%) and ADN selectivity (>90% FE), substantially exceeding the performance of Co3O4 (70%, 57% FE). The unimodal concentration profile of AMN validates the reaction pathway as HMD → AMN → AND (Fig. 9e). Kinetic analysis indicated a distinct kinetic enhancement on Mn-Co3O4, with K1 = 0.51 h−1 and K2 = 8.9 h−1, significantly higher than K1 = 0.36 h−1 and K2 = 0.20 h−1 for Co3O4, illustrating synergistic promotion across the sequential steps.
 | ||
| Fig. 9 (a) Electronic structure investigation via Co L-edge XAS for Co3O4 and Mn-Co3O4 catalysts mechanically removed from NF supports. (b) In situ FTIR monitoring of Co3O4/NF and Mn-Co3O4/NF after HMD adsorption and 10 min nitrogen purging. (c) Spatiotemporal characterization of the molecular adsorption process of HMD on Mn-Co3O4 using 3D snapshot imaging. (d) FE and productivity of ADN over Mn-Co3O4/NF under varying current densities. (e) Evolution of reactant and product concentrations for the Mn-Co3O4/NF electrode during electrolysis as a function of charge consumed. (f) Mechanistic investigation of the HOR at the Mn-Co3O4 interface using in situ FTIR spectroscopy. (g) Comparative analysis of HOR free energy profiles and intermediate adsorption geometries on Co3O4 (110) and Mn-Co3O4 (110) surfaces. (h) Process flow for ADN production through valorization of waste nylon-66 cable ties. (i) Economic viability assessment of waste nylon-66 processing: electrochemical upcycling compared with mechanical recycling and incineration. Reproduced with permission.113 Copyright 2025, Wiley-VCH. | ||
In situ FTIR spectroscopy showed a markedly stronger N–H vibration at 3312 cm−1 on Mn-Co3O4 than on Co3O4, reflecting enhanced HMD adsorption and N–H activation. Concurrently, the rising C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N peak at 2251 cm−1 verified efficient ADN formation (Fig. 9f). Gibbs free energy calculations indicated that Mn doping lowers the rate-determining step barrier from 1.76 eV (formation of HNCH2(CH2)4CN* on Co3O4) to 1.31 eV (formation of H2NCH2(CH2)2CN*), significantly improving reaction thermodynamics (Fig. 9g). Experiment and theory concertedly demonstrate that Mn constructs a synergistic dual-site configuration in Co3O4 for cooperative HMD and OH− adsorption, which boosts HMD oxidation by reducing the rate-limiting barrier. A sustainable upcycling strategy converts 130 g of nylon-66 cable ties into 56.7 g of adiponitrile through acid hydrolysis and electrocatalysis in an MEA system (Fig. 9h). With Mn-Co3O4/NF and Pt-NiOOH/NF electrodes operating at 200 mA cm−2 for 329 h, the process attains 95% yield, >99% purity, >98% NMR purity, and >87% FE, while reducing voltage by 410 mV. Its broad applicability is confirmed with nylon-11, polyurethanes, and polyolefin blends, maintaining 91% FE in mixed plastic hydrolysates and proving outstanding generality. Techno-economic analysis confirms the superior profitability of the electrolytic reforming strategy for nylon-66, generating a daily net profit of $127
N peak at 2251 cm−1 verified efficient ADN formation (Fig. 9f). Gibbs free energy calculations indicated that Mn doping lowers the rate-determining step barrier from 1.76 eV (formation of HNCH2(CH2)4CN* on Co3O4) to 1.31 eV (formation of H2NCH2(CH2)2CN*), significantly improving reaction thermodynamics (Fig. 9g). Experiment and theory concertedly demonstrate that Mn constructs a synergistic dual-site configuration in Co3O4 for cooperative HMD and OH− adsorption, which boosts HMD oxidation by reducing the rate-limiting barrier. A sustainable upcycling strategy converts 130 g of nylon-66 cable ties into 56.7 g of adiponitrile through acid hydrolysis and electrocatalysis in an MEA system (Fig. 9h). With Mn-Co3O4/NF and Pt-NiOOH/NF electrodes operating at 200 mA cm−2 for 329 h, the process attains 95% yield, >99% purity, >98% NMR purity, and >87% FE, while reducing voltage by 410 mV. Its broad applicability is confirmed with nylon-11, polyurethanes, and polyolefin blends, maintaining 91% FE in mixed plastic hydrolysates and proving outstanding generality. Techno-economic analysis confirms the superior profitability of the electrolytic reforming strategy for nylon-66, generating a daily net profit of $127![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 when processing 200 tons at $0.1 per kWh electricity, outperforming mechanical recycling and incineration (Fig. 9i). For further enhancement, optimizing key operational parameters is essential, notably developing efficient enzymatic depolymerization under mild conditions to replace the existing acid hydrolysis.
000 when processing 200 tons at $0.1 per kWh electricity, outperforming mechanical recycling and incineration (Fig. 9i). For further enhancement, optimizing key operational parameters is essential, notably developing efficient enzymatic depolymerization under mild conditions to replace the existing acid hydrolysis.
In summary, electrochemical oxidation of polyamide-derived amines to nitriles offers a potentially viable economic route. Nevertheless, the existing approach presents a systemic challenge: the depolymerization requires strongly acidic conditions, while the subsequent electrocatalysis is performed in alkaline media. This discrepancy leads to massive consumption of acids and bases, generating considerable inorganic salt waste and increasing the carbon footprint. Thus, developing mild depolymerization processes or acid-stable electrocatalysts is a pressing need.
3.3 Electrocatalytic upgrading of polyolefin plastics
The chemical inertness of polyolefins, resulting from robust C–C bonds and low polarity, poses a major obstacle to their chemical recycling. Although depolymerization using nitric acid produces organic acid mixtures, their separation is energy-intensive and complex. The complex mixture of these acids presents significant separation challenges, thereby limiting their economic viability as value-added products. Pichler et al. demonstrated the oxidative depolymerization of PE using dilute nitric acid to yield a mixture of organic acids (Fig. 10a).65 The team employed the PE hydrolysate as an electrolyte and successfully converted the contained organic acids into olefins via electrocatalytic upgrading (Fig. 10b). | ||
Fig. 10 (a) Process schematic for producing gaseous hydrocarbons from waste polyethylene via tandem photoelectrochemical integration. (b) Oxidative conversion of polyethylene to dicarboxylic acids, followed by photocatalytic upgrading to alkanes or electrocatalytic decarboxylation to alkenes. (c) Electrolytic decarboxylation of succinic acid solution and real polyethylene depolymerization solution in pH-adjusted aqueous and methanol/water media at 5 V applied voltage. (d) Reaction mechanism of electrocatalytic decarboxylation of succinic acid. Reproduced with permission.65 Copyright 2021, American Chemical Society. (e) TEM image, (f) EDX elemental mapping of Mn2-DAC. (g) Corresponding H2O2 FE of Mn2-DAC at various current densities in a three-electrode flow cell. (h) Schematic illustration of the solid-state electrolyte (SSE) electrolyzer, with inset showing a representative SEM image of the solid-state electrolyte. (i) Schematic illustration of UV-assisted advanced oxidation process (AOP) for functionalizing commercial PE. (j) FTIR spectrum of oxidized polyethylene showing characteristic stretching vibrations of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C–OH groups. (k) High-temperature GPC curves comparing oxidized PE with virgin PE. Reproduced with permission.117 Copyright 2025, Wiley-VCH. O and C–OH groups. (k) High-temperature GPC curves comparing oxidized PE with virgin PE. Reproduced with permission.117 Copyright 2025, Wiley-VCH. | ||
Since succinic acid constitutes the predominant component in the PE depolymerization hydrolysate, it was systematically investigated as a model substrate for electrocatalytic conversion. Voltammetric studies indicated an onset potential of ∼1.5 V (vs. Ag/AgCl) for decarboxylation, yielding ethylene as the main product. Under optimized conditions (2.0–2.5 V, pH 10), a FE of 30% was attained on a carbon paper electrode (Fig. 10c). A practical evaluation of electrocatalytic decarboxylation was conducted using real PE depolymerization solution. In aqueous (pH 10) and methanol/water electrolytes, olefins including ethylene (FE: 4–9%), propylene (0.6–3.9%), and butene were generated. Although methanol enhanced total hydrocarbon FE, the actual yield was limited to 7.6% owing to the low activity of long-chain diacids. With 13.5% CO2 co-produced and less than 4% charge loss from degradation side reactions, the system demonstrated promising stability (Fig. 10c). Mechanistic investigations elucidate a distinct mechanism for the electrocatalytic decarboxylation of succinic acid, which diverges fundamentally from classical Kolbe or Hofer-Moest pathways (Fig. 10d). Rather than involving carbocation intermediates, the process proceeds via sequential or concurrent oxidation of the two carboxyl groups at the anode, leading to the formation of radical intermediates that drive the decarboxylation transformation. In summary, the FEs for hydrocarbon production were significantly higher in methanol than in aqueous solutions under otherwise identical reaction conditions. This two-stage plastic recycling process utilizes renewable electricity to convert polyethylene into valuable gaseous platform chemicals with facile separability, representing a critical first step toward establishing circular chemical recycling pathways for polyethylene waste.
Recent advances in using electrochemically generated reagents present a novel route for plastic valorization. By utilizing in situ produced NaOH, H2, or H2O2 to drive downstream chemical transformations, this electrocatalytic approach combines low energy input, high reaction selectivity, and process efficiency.116–119 Although not a primary electrochemical treatment method, it offers a sustainable strategy for resource recovery from polyolefin waste, including so-called “white pollution”. Huang et al. demonstrated a dual-function catalyst based on high-spin Mn2+ dual-atom centers (Mn2-DAC) that not only mediates efficient H2O2 production in acid but also utilizes the electrogenerated oxidant for direct polyethylene functionalization.117 Structural analysis via X-ray absorption spectroscopy elucidated the coordination environment of the di-Mn sites. The fitting results identified Mn–N (CN = 2.2, R = 1.90 Å) and Mn–O (CN = 2.0, R = 2.02 Å) coordinations, along with a second-shell Mn–O–Mn path at 2.99 Å (Fig. 10e). These findings consistently support a proposed N2-Mn-(μ-O)2-Mn-N2 coordination structure. Mn2-DAC shows remarkably high activity and stability in H2O2 electrosynthesis under flow conditions, sustaining current densities as high as 500 mA cm−2. It delivers 95% FE at 100 mA cm−2 (vs. 38% for Mn-SAC) and retains 82–89% FE beyond 200 mA cm−2 (Fig. 10f). The peak H2O2 production rate reaches 10.8 mol gcat−1 h−1 at 400 mA cm−2, underscoring its excellent ORR activity and efficient mass transport.
Leveraging the superior 2e− ORR capability of Mn2-DAC, we developed an integrated solid-state electrolyte electrolyzer that controls proton flux via anode-driven water oxidation, eliminating proton accumulation and obsolete ion-exchange membranes (Fig. 10g). The in situ generated 1.4 wt% H2O2 enabled efficient PE functionalization: after compounding with iron carboxylates and UV-initiated oxidation (Fig. 10h), ATR-FTIR revealed carbonyl (1712 cm−1) and hydroxyl (3400 cm−1) signals (Fig. 10i), corresponding to 2.0 mol% functionalization. HT-GPC indicated molecular weight reduction, suggesting the material of promise as a compatibilizer with improved adhesion and wettability (Fig. 10j). Hu et al. developed a novel strategy using a RuPt dilute alloy catalyst for efficient PE conversion under low hydrogen pressure, effectively suppressing methane production. The compatibility with electrochemically derived hydrogen paves the way for decentralized and scalable plastic recycling.118 Through rational catalyst design, a Ru9Pt91 dilute alloy was synthesized to direct polyolefin hydrogenolysis (Fig. 11a, steps 1.4–1.7) under low H2 pressure. Pt plays a dual role: enhancing H2 activation for hydrogenation (step 1.5) and diluting Ru to block dehydrogenation (step 1.6) and secondary cracking (step 1.7). This limits methane to <3.2% and enables selective conversion to liquid fuels and lubricants. The use of CeO2 as a support, compared to carbon carriers, leads to notably higher activity and lower hydrogenolysis temperatures, suggesting that the support material is a key determinant of catalytic efficiency (Fig. 11b). A practical demonstration integrating electrolytic H2 production and PE hydrocracking enabled efficient plastic valorization at atmospheric pressure using Ru9Pt91/CeO2 (Fig. 11c). The process completely converted PE powder in 24 h to valuable products (methane ≤3.2%) (Fig. 11d) and was applicable to LDPE, HDPE, and mixed polyolefins, giving C5–C40 yields ≥77.8% (Fig. 11e). From HDPE caps, the product distribution included 10.7% gasoline, 19.4% jet fuel, 38.0% diesel, and 62.5% lubricant base oil (Fig. 11f), confirming the feasibility of decentralized electrolytic H2-assisted plastic upcycling.
 | ||
| Fig. 11 (a) Schematic of methane suppression strategies in n-alkane hydrogenolysis. (b) PE hydrogenolysis performance of Ru9Pt91/CeO2 under varying H2 pressures. (c) Integrated system coupling electrolytic hydrogen production with PE hydrogenolysis. (d) Hydrogenolysis product profiles of various PE substrates: powder, LDPE bags, HDPE caps, and polyolefin mixtures. (e) Carbon distribution profile of HDPE cap hydrogenolysis products. (f) Fuel and lubricant selectivity across carbon ranges: gasoline C5–C12, jet fuel C8–C16, diesel C9–C22, waxes or lubes C20–C40. Reproduced with permission.118 Copyright 2024, Springer Nature. | ||
The electrocatalytic upcycling of polyolefins is hampered by their inherent resistance to dissolution in common electrolytes. Standard approaches involving high-temperature acid treatment decompose polyethylene into organic acid mixtures, but extracting these acids from aqueous solutions proves cost-prohibitive. Although electrochemical decarboxylation facilitates separation by converting acids to gases, the process exhibits systemic limitations, including high carbon intensity and marginal economic viability. An emerging research direction in organic electrocatalysis has demonstrated direct upcycling of polyolefin plastics through selected organic solvents and redox mediators.120,121 While bypassing the need for custom-designed electrocatalysts, this approach establishes electrocatalysis as a viable route for polyolefin waste valorization.
4. Conclusions and outlook
In this review, we summarize the recent advances on the catalytic conversion of waste plastics into high-value products via depolymerization and electrochemical reforming strategies. Electrocatalytic technology holds significant promise for the upcycling of plastic monomers into high-value products, owing to its high reaction selectivity, rapid conversion rates, and the ability to utilize sustainable electricity. However, the chemical diversity and complexity of waste plastics pose significant challenges to the implementation of electrocatalytic technologies, particularly as many conventional systems require the plastic to be dissolved in the electrolyte, which is often impractical. Therefore, special emphasis is placed on discussing depolymerization methods for raw plastics. Despite continuous advances and significant potential opportunities in such tandem catalytic processes, a number of challenges and problems remain to be addressed (Fig. 12).(1) Reaction mechanisms study. To propel the field of plastic conversion toward higher efficiency and selectivity, a synergistic integration of advanced in situ characterization, theoretical calculations, and machine learning is paramount. In situ characterization techniques serve as the “eyes” into the reaction, allowing for real-time monitoring of dynamic processes. For instance, in situ FTIR and Raman spectroscopy can track the evolution of chemical bonds on catalyst surfaces, while in situ X-ray diffraction (XRD) and XAS provide insights into structural phase transitions and electronic changes of active sites. Techniques like in situ EPR are pivotal for detecting transient radical intermediates, thereby elucidating reaction pathways and guiding the rational design of catalysts with tailored surface properties. Complementing these experimental insights, theoretical calculations such as DFT and molecular dynamics simulations (MDS) offer a microscopic understanding of reaction mechanisms. They can calculate adsorption energies, identify rate-determining steps, and reveal the electronic structure requirements for effective catalysis, thus providing a theoretical foundation for catalyst screening. Most importantly, machine learning (ML) acts as a powerful accelerator in this framework. By leveraging vast datasets from both characterization and theoretical studies, ML models can predict novel catalyst compositions with optimal electronic properties, continuously optimize reaction parameters in real-time, and simulate complex reaction networks to forecast product distributions. The convergence of these three pillars, in situ characterization providing real-time experimental data, theoretical calculation deciphering molecular-level details, and machine learning enabling predictive design and optimization, will create a closed-loop research paradigm. This integrated approach promises to unlock deeper mechanistic insights, dramatically accelerate the discovery of high-performance catalysts, and ultimately pave the way for the efficient and selective upcycling of plastic waste into value-added chemicals.
(2) Development of efficient and stable catalysts. The three of catalyst development for plastic conversion lies in the holistic integration of four cornerstone properties: high activity, simple synthesis, and high durability. The primary pursuit of high activity remains paramount, requiring catalysts that not only exhibit exceptional intrinsic activity with low activation barriers but also possess a high density of accessible active sites. This can be achieved through atomic-level engineering strategies, such as constructing single-atom configurations or strain-tuned crystalline facets, to maximize atomic utilization and enhance intrinsic turnover frequency. Furthermore, the brilliance of a catalyst in the lab is meaningless without the capacity for scale-up. The development of simple synthesis methods is critical for industrial translation. The complex must be moved beyond lab-only procedures towards scalable, reproducible, and low-cost manufacturing pathways, such as one-pot synthesis or facile impregnation techniques, to ensure consistent catalyst quality and performance. Finally, all these attributes must be anchored by high durability. The next generation of catalysts must be designed for longevity, with enhanced structural integrity to resist sintering and leaching. This involves engineering self-regenerating functionalities and robust mechanical strength to withstand harsh reaction conditions and numerous regeneration cycles. The practical application of these catalysts demands exceptional impurity resistance to withstand the complex and contaminated real-world plastic waste streams. Advancements in catalytic durability will require interfacial engineering of active sites through protective overlayers or poison-resistant structures that can tolerate impurities while preserving high reactivity.
(3) Large-scale waste plastics conversion. The transition from promising lab-scale catalysis to viable industrial processes represents the most critical frontier in plastic waste valorization. Future efforts must pivot towards bridging this gap through a trinity of interconnected strategies: advancing pilot-scale demonstrations, enabling efficient product separation, and designing fully integrated systems. Scaling up necessitates moving beyond gram-scale reactions to robust pilot-scale operations that replicate industrial challenges. This requires innovative reactor designs that overcome the inherent inefficiencies of solid–liquid interfaces in plastic electrochemistry. As noted, high-temperature electrochemical systems, such as solid oxide fuel cells (SOFCs), offer a transformative pathway by enabling the direct electroreforming or gasification of molten plastics, drastically improving reactant–catalyst contact and charge transfer efficiency. At the pilot scale, the integration of artificial intelligence for real-time process control and optimization of influential parameters (e.g., temperature, pressure, flow rates) will be indispensable for achieving stable, high-throughput operation. Concurrently, the economic viability of the entire process hinges on developing efficient product separation and purification protocols. The complex product streams resulting from plastic conversion demand advanced in situ separation strategies. Future research should explore the integration of selective membranes within reactors, design catalytic systems that facilitate spontaneous product phase separation, or employ solvents for extractive distillation. This focus on streamlining downstream processing is crucial for minimizing energy penalties and maximizing the purity and value of the final products. Ultimately, the highest leverage approach lies in creating intelligent integrated systems. Inspired by the “coupling” concept, future plants could be designed as multi-block facilities. This holistic integration of pretreatment, conversion, separation, and energy management into a single, self-optimizing platform will dramatically reduce costs, enhance overall energy efficiency, and establish a new paradigm for green chemical manufacturing from plastic waste.
(4) Economic analysis and environmental evaluation. The ultimate success of plastic conversion technologies depends on their consistent adherence to green chemistry principles from the initial design phase, verified through TEA and LCA. Future research should shift toward developing processes that are inherently economically advantageous, truly advance sustainable development, and are built on integrated process design. A comprehensive TEA must systematically evaluate costs related to hazardous chemicals (e.g., concentrated acids/bases), precious metal catalysts, energy-intensive operations (e.g., high pressure/temperature), and complex product purification steps. This methodology provides a strategic transition toward earth-abundant catalysts, simplified processes, and embedded energy recovery, identifying routes economically competitive with conventional production. LCAs should encompass the synthesis and regeneration of specialized solvents, energy requirements of high-pressure systems, and potential byproduct toxicity. Furthermore, in alignment with waste prevention principles, processes must be designed to maximize atom economy and minimize hazardous waste generation. This comprehensive LCA approach is essential to prevent burden shifting and ensure environmental credibility through demonstrated reductions in overall impact, rather than focusing narrowly on the conversion step alone. Ultimately, these evaluations should guide an integrated design principle. Future efforts should concentrate on creating systems that intrinsically avoid hazardous materials, incorporate renewable energy sources, and implement in-line separation to minimize purification costs. The systematic incorporation of TEA and LCA into research and development cycles is vital for achieving both economic viability and environmental sustainability, thereby transforming waste plastics from a pollutant into a fundamental component of a circular economy.
Conflicts of interest
There are no conflicts to declare.Data availability
This review did not generate or analyze new data and includes no primary research, software, or code.Acknowledgements
This work was supported by the National Natural Science Foundation of China (22378208, 22302095), the Fundamental Research Funds for the Central Universities (KJYQ2025013), the open research fund of Songshan Lake Materials Laboratory (2023SLABFN17) and the State Key Laboratory of Materials-Oriented Chemical Engineering (SKL-MCE-24B015).References
- V. Tournier, C. M. Topham, A. Gilles, B. David, C. Folgoas, E. Moya-Leclair, E. Kamionka, M. L. Desrousseaux, H. Texier, S. Gavalda, M. Cot, E. Guémard, M. Dalibey, J. Nomme, G. Cioci, S. Barbe, M. Chateau, I. André, S. Duquesne and A. Marty, Nature, 2020, 580, 216–219 CrossRef.
- D. J. Walsh, M. G. Hyatt, S. A. Miller and D. Guironnet, ACS Catal., 2019, 9, 11153–11188 CrossRef.
- H. Sardon and A. P. Dove, Science, 2018, 360, 380–381 CrossRef.
- L. Wimberger, G. Ng and C. Boyer, Nat. Commun., 2024, 15, 2510 CrossRef.
- M. MacLeod, H. P. H. Arp, M. B. Tekman and A. Jahnke, Science, 2021, 373, 61–65 CrossRef PubMed.
- K. L. Law, N. Starr, T. R. Siegler, J. R. Jambeck, N. J. Mallos and G. H. Leonard, Sci. Adv., 2020, 6, eabd0288 CrossRef.
- S. Yang, Y. Li, M. Nie, X. Liu, Q. Wang, N. Chen and C. Zhang, Adv. Mater., 2024, 36, 2404115 CrossRef PubMed.
- J. M. Garcia and M. L. Robertson, Science, 2017, 358, 870–872 CrossRef PubMed.
- K. Cao, S. Zhang, Y. Shi, X. Diao, R. Wei and N. Ji, ACS Nano, 2025, 19, 12734–12761 CrossRef PubMed.
- C. Jehanno, J. W. Alty, M. Roosen, S. De Meester, A. P. Dove, E. Y. X. Chen, F. A. Leibfarth and H. Sardon, Nature, 2022, 603, 803–814 CrossRef PubMed.
- N. A. Rorrer, S. Nicholson, A. Carpenter, M. J. Biddy, N. J. Grundl and G. T. Beckham, Joule, 2019, 3, 1006–1027 CrossRef.
- F. Niu, Z. Wu, D. Chen, Y. Huang, V. V. Ordomsky, A. Y. Khodakov and K. M. Van Geem, Chem. Soc. Rev., 2025, 54, 4948–4972 RSC.
- Z. Ya, S. Zhang, D. Xu, H. Wang and M. Li, ACS Catal., 2025, 15, 5339–5369 CrossRef.
- Y. Ma, H. Tao, X. Guo, P. Yang, D. Xing, V. Nicolosi, Y. Zhang, C. Lian and B. Qiu, J. Mater. Chem. A, 2024, 12, 27220–27228 RSC.
- J. Cho, B. Kim, T. Kwon, K. Lee and S.-I. Choi, Green Chem., 2023, 25, 8444–8458 RSC.
- M. Du, M. Xing, S. Kang, Y. Ma, B. Qiu and Y. Chai, EcoMat, 2022, 4, e12259 CrossRef.
- S. Kang, W. Yuan, X. Guo, Y. Zhang, J. Shang, P. Yang, Y. Ma, V. Nicolosi, L. Cai and B. Qiu, Angew. Chem., Int. Ed., 2025, 64, e202504993 CrossRef PubMed.
- W. Zhang, L. Killian and A. Thevenon, Chem. Sci., 2024, 15, 8606–8624 RSC.
- R. S. Weber and K. K. Ramasamy, ACS Omega, 2020, 5, 27735–27740 CrossRef.
- M. Hourtoule, S. Trienes and L. Ackermann, Angew. Chem., Int. Ed., 2024, 63, e202412689 CrossRef.
- P. H. Pham, S. Barlow, S. R. Marder and O. R. Luca, Chem. Catal., 2023, 3, 100675 Search PubMed.
- T. Esmaeili, J. Hörndl, S. Pokrant, T. Bartschmid, A. Farhadi and G. R. Bourret, ACS Sustainable Chem. Eng., 2025, 13, 8289–8297 CrossRef PubMed.
- G. C. Gilchrist, R. W. Hughes, S. R. Gitter, J. D. Marquez, B. S. Sumerlin and A. M. Evans, J. Am. Chem. Soc., 2025, 147, 8398–8405 CrossRef PubMed.
- Z. Siddiqi and D. Sarlah, J. Am. Chem. Soc., 2021, 143, 21264–21269 CrossRef CAS PubMed.
- Y. Ma, Y. Zhang, W. Yuan, M. Du, S. Kang and B. Qiu, EES Catal., 2023, 1, 892–920 RSC.
- S. Kang, T. Sun, Y. Ma, M. Du, M. Gong, C. Zhou, Y. Chai and B. Qiu, SmartMat, 2023, 4, e1202 CrossRef.
- Y. Li, L. Liu, L. Q. Lee and H. Li, Chem. Commun., 2025, 61, 33–45 RSC.
- S. Yue, Z. Zhao, Y. Liu, M. Yang, T. Zhang, F. Li, K. Liu, P. Wang and S. Zhan, Adv. Funct. Mater., 2025, 35, 09766 CrossRef CAS.
- X. Lou, F. Liu, Q. Li, M. Chu, G. Wang, J. Chen and M. Cao, Chem. Commun., 2024, 60, 2828–2838 RSC.
- H. Abedsoltan, Polym. Eng. Sci., 2023, 63, 2651–2674 CrossRef CAS.
- H. Kopperi, V. Mamidi, G. Suresh and S. Venkata Mohan, Green Chem., 2025, 27, 2359–2373 RSC.
- S. S. Hosseini, S. Taheri, A. Zadhoush and A. Mehrabani-Zeinabad, J. Appl. Polym. Sci., 2007, 103, 2304–2309 CrossRef.
- B. Shojaei, M. Abtahi and M. Najafi, Polym. Adv. Technol., 2020, 31, 2912–2938 CrossRef.
- S. C. Kosloski-Oh, Z. A. Wood, Y. Manjarrez, J. P. de los Rios and M. E. Fieser, Mater. Horiz., 2021, 8, 1084–1129 RSC.
- X. Wen, R. Niu, T. Tang and J. Gong, Appl. Catal., B, 2025, 377, 125485 CrossRef.
- P. Sarda, J. C. Hanan, J. G. Lawrence and M. Allahkarami, J. Polym. Sci., 2022, 60, 7–31 CrossRef.
- H. Lu, D. J. Diaz, N. J. Czarnecki, C. Zhu, W. Kim, R. Shroff, D. J. Acosta, B. R. Alexander, H. O. Cole, Y. Zhang, N. A. Lynd, A. D. Ellington and H. S. Alper, Nature, 2022, 604, 662–667 CrossRef PubMed.
- B. Xie, J. Zhang, H. Sun, R. Bai, D. Lu, Y. Zhu, W. Dong, J. Zhou and M. Jiang, Green Chem., 2024, 26, 7268–7279 RSC.
- C. Guo, L.-Q. Zhang and W. Jiang, Chem, 2023, 9, 363–376 Search PubMed.
- S. Zhang, Q. Hu, Y.-X. Zhang, H. Guo, Y. Wu, M. Sun, X. Zhu, J. Zhang, S. Gong, P. Liu and Z. Niu, Nat. Sustain., 2023, 6, 965–973 CrossRef.
- Q. Chen, H. Yan, K. Zhao, S. Wang, D. Zhang, Y. Li, R. Fan, J. Li, X. Chen, X. Zhou, Y. Liu, X. Feng, D. Chen and C. Yang, Nat. Commun., 2024, 15, 10732 CrossRef PubMed.
- Y. Ma, W. Chen, W. Yuan, Z. Chen, M. Du, L. Cai, W. Wang, M. Xing and B. Qiu, Adv. Energy Mater., 2025, 15, 2404877 CrossRef.
- K. Wang, Z. Sun, W. Guo, M. Chen, C. Zhu, J. Fei, Y. Liu, H. He, Y. Cao and X. Bao, ChemSusChem, 2023, 16, e202301128 CrossRef PubMed.
- G. Wu, Z. Ma, T. Heil, L. Zhang, W. Hu, G. Wu, W. He, L. Dai, Y. Huang and Q. Qin, Adv. Mater., 2025, 37, 2418233 CrossRef PubMed.
- Y. Zhou, R. Duan, Q. e. Huang, C. Ding and C. Li, ACS Catal., 2024, 14, 10164–10171 CrossRef.
- M. Li, Y. Wu, B.-H. Zhao, C. Cheng, J. Zhao, C. Liu and B. Zhang, Nat. Catal., 2023, 6, 906–915 CrossRef.
- S. Zhang, M. Li, Z. Zuo and Z. Niu, Green Chem., 2023, 25, 6949–6970 RSC.
- Y. Ma, X. Jiang, X. Xiang, P. Qu and M. Zhu, Green Chem., 2025, 27, 4040–4093 RSC.
- K. Zheng, Y. Wu, Z. Hu, S. Wang, X. Jiao, J. Zhu, Y. Sun and Y. Xie, Chem. Soc. Rev., 2023, 52, 8–29 RSC.
- W. Han, L. Lin, Z. Cen, Y. Ke, Q. Xu, J. Zhu, X. Mei, Z. Xia, X. Zheng, Y. Wang, Y. Liu, M. He, H. Wu and B. Han, Chem, 2025, 11, 102340 Search PubMed.
- Q. Zhou, W. Gao, D. Wang, Y. Chang, H. Guan, K. H. Lim, X. Yang, P. Liu, W.-J. Wang, B.-G. Li and Q. Wang, Adv. Sci., 2025, 12, 2410574 CrossRef PubMed.
- E. Bäckström, K. Odelius and M. Hakkarainen, ACS Sustainable Chem. Eng., 2019, 7, 11004–11013 CrossRef.
- R. J. Conk, S. Hanna, J. X. Shi, J. Yang, N. R. Ciccia, L. Qi, B. J. Bloomer, S. Heuvel, T. Wills, J. Su, A. T. Bell and J. F. Hartwig, Science, 2022, 377, 1561–1566 CrossRef PubMed.
- G. Zeng, Y. Su, J. Jiang and Z. Huang, J. Am. Chem. Soc., 2025, 147, 2737–2746 CrossRef.
- L. Chen, C. Zhao, X. Yuan, H. Zhang, M. Senanayake, O. Mašek, C. He and Y. S. Ok, Green Chem., 2025, 27, 4867–4897 RSC.
- J. Qin, F. Wu, Y. Dou, D. Zhao, C. Hélix-Nielsen and W. Zhang, Adv. Mater., 2025, 37, 2418138 CrossRef.
- E. Bäckström, K. Odelius and M. Hakkarainen, Ind. Eng. Chem. Res., 2017, 56, 14814–14821 CrossRef.
- X. Luo, J. Zhan, Q. Mei and S. Zhang, Green Chem., 2023, 25, 6717–6727 RSC.
- D. Fowler, M. Coyle, U. Skiba, M. A. Sutton, J. N. Cape, S. Reis, L. J. Sheppard, A. Jenkins, B. Grizzetti, J. N. Galloway, P. Vitousek, A. Leach, A. F. Bouwman, K. Butterbach-Bahl, F. Dentener, D. Stevenson, M. Amann and M. Voss, Philos. Trans. R. Soc., B, 2013, 368, 20130164 CrossRef PubMed.
- A. Ghorani-Azam, B. Riahi-Zanjani and M. Balali-Mood, J. Res. Med. Sci., 2016, 21, 65 Search PubMed.
- Q. Zhang, J. He, X. Wei, C. Shen, P. Ye, W. An, X. Liu, H. Li, S. Xu, Z. Su and Y.-Z. Wang, Angew. Chem., Int. Ed., 2024, 63, e202407510 CrossRef.
- J. Yang, Y. Wang, Y. Fan, B. Xu and T. Tu, Nat. Commun., 2025, 16, 8884 CrossRef CAS PubMed.
- A. Kong, M. Liu, H. Zhang, Z. Cao, J. Zhang, W. Li, Y. Han and Y. Fu, Chem. Eng. J., 2022, 445, 136719 CrossRef CAS.
- Y. Du, X. Chen, W. Shen, H. Liu, M. Fang, J. Liu and C. Liang, Green Chem., 2023, 25, 5489–5500 RSC.
- C. M. Pichler, S. Bhattacharjee, M. Rahaman, T. Uekert and E. Reisner, ACS Catal., 2021, 11, 9159–9167 CrossRef CAS.
- F. J. Holzhäuser, J. B. Mensah and R. Palkovits, Green Chem., 2020, 22, 286–301 RSC.
- Y. Qiu, J. A. Lopez-Ruiz, G. Zhu, M. H. Engelhard, O. Y. Gutiérrez and J. D. Holladay, Appl. Catal., B, 2022, 305, 121060 CrossRef CAS.
- H.-W. He, H. Hu, K.-M. Du, M. Lu, F. Yang, L.-X. Cui, M. Ma, Y.-L. Zhu, Y.-Q. Shi, S. Chen and X. Wang, Green Chem., 2025, 27, 8467–8491 RSC.
- Y. Wu, J. Zhao, C. Wang, T. Li, B.-H. Zhao, Z. Song, C. Liu and B. Zhang, Nat. Commun., 2023, 14, 3057 CrossRef.
- G. Perli, I. Olazabal, L. Breloy, I. Vollmer, F. López-Gallego and H. Sardon, Langmuir, 2025, 41, 6429–6456 CrossRef.
- S. C. H. J. Turk, W. P. Kloosterman, D. K. Ninaber, K. P. A. M. Kolen, J. Knutova, E. Suir, M. Schürmann, P. C. Raemakers-Franken, M. Müller, S. M. A. de Wildeman, L. M. Raamsdonk, R. van der Pol, L. Wu, M. F. Temudo, R. A. M. van der Hoeven, M. Akeroyd, R. E. van der Stoel, H. J. Noorman, R. A. L. Bovenberg and A. C. Trefzer, ACS Synth. Biol., 2016, 5, 65–73 CrossRef PubMed.
- K. Min, J. D. Cuiffi and R. T. Mathers, Nat. Commun., 2020, 11, 727 CrossRef PubMed.
- Y. Tokiwa, B. P. Calabia, C. U. Ugwu and S. Aiba, Int. J. Mol. Sci., 2009, 10, 3722–3742 CrossRef.
- E. Bäckström, K. Odelius and M. Hakkarainen, Glob. Chall., 2021, 5, 2000119 CrossRef PubMed.
- M. N. Khan, Int. J. Chem. Kinet., 2009, 41, 599–611 CrossRef.
- U. Češarek, D. Pahovnik and E. Žagar, ACS Sustainable Chem. Eng., 2020, 8, 16274–16282 CrossRef.
- H. Hu, Q. Xu, L. Sun, R. Zhu, T. Gao, Y. He, B. Ma, J. Yu and X. Wang, ACS Appl. Polym. Mater., 2023, 5, 751–763 CrossRef.
- M. Du, Y. Zhang, S. Kang, X. Guo, Y. Ma, M. Xing, Y. Zhu, Y. Chai and B. Qiu, ACS Catal., 2022, 12, 12823–12832 CrossRef.
- S. Kang, X. Guo, D. Xing, W. Yuan, J. Shang, V. Nicolosi, N. Zhang and B. Qiu, Small, 2024, 20, 2406068 CrossRef PubMed.
- M. Du, T. Sun, X. Guo, M. Han, Y. Zhang, W. Chen, M. Han, J. Ma, W. Yuan, C. Zhou, V. Nicolosi, J. Shang, N. Zhang and B. Qiu, Mater. Horiz., 2025, 12, 3743–3751 RSC.
- T. Uekert, M. F. Kuehnel, D. W. Wakerley and E. Reisner, Energy Environ. Sci., 2018, 11, 2853–2857 RSC.
- H. Abedsoltan, I. S. Omodolor, A. C. Alba-Rubio and M. R. Coleman, Polymer, 2021, 222, 123620 CrossRef.
- Y. Ma, X. Guo, M. Du, S. Kang, W. Dong, V. Nicolosi, Z. Cui, Y. Zhang and B. Qiu, Green Chem., 2024, 26, 3995–4004 RSC.
- Y. Miao, Y. Zhao, J. Gao, J. Wang and T. Zhang, J. Am. Chem. Soc., 2024, 146, 4842–4850 CrossRef.
- S. Tian, Y. Jiao, Z. Gao, Y. Xu, L. Fu, H. Fu, W. Zhou, C. Hu, G. Liu, M. Wang and D. Ma, J. Am. Chem. Soc., 2021, 143, 16358–16363 CrossRef.
- D. Xiong, X. He, X. Liu, K. Zhang, Z. Tu, J. Wang, S.-G. Sun and Z. Chen, ACS Nano, 2024, 18, 20340–20352 CrossRef.
- B. Zhou, K. Shi, X. Teng, Z. Li, L. Chen and J. Shi, Angew. Chem., Int. Ed., 2024, 63, e202411502 CrossRef.
- P. Hu, C. Zhang, M. Chu, X. Wang, L. Wang, Y. Li, T. Yan, L. Zhang, Z. Ding, M. Cao, P. Xu, Y. Li, Y. Cui, Q. Zhang, J. Chen and L. Chi, J. Am. Chem. Soc., 2024, 146, 7076–7087 CrossRef PubMed.
- J. Du, L. Zeng, T. Yan, C. Wang, M. Wang, L. Luo, W. Wu, Z. Peng, H. Li and J. Zeng, Nat. Nanotechnol., 2023, 18, 772–779 CrossRef.
- S. Li, Q. Feng, Q. Li, Y. Xie, P. Xu, Z. Wang, Q. Sun, M. Cao, Q. Zhang and J. Chen, Angew. Chem., Int. Ed., 2025, 64, e202501509 CrossRef PubMed.
- K. Wang, R. Jia, P. Cheng, L. Shi, X. Wang and L. Huang, Angew. Chem., Int. Ed., 2023, 62, e202301340 CrossRef.
- C. Xiao, W. R. Leow, L. Chen, Y. Li and C. Li, Electron, 2023, 1, e14 CrossRef.
- J. de Witt, T. Luthe, J. Wiechert, K. Jensen, T. Polen, A. Wirtz, S. Thies, J. Frunzke, B. Wynands and N. Wierckx, Nat. Microbiol., 2025, 10, 667–680 CrossRef CAS.
- Q. Hu, Z. Zhang, D. He, J. Wu, J. Ding, Q. Chen, X. Jiao and Y. Xie, J. Am. Chem. Soc., 2024, 146, 16950–16962 CrossRef CAS.
- H. Zhou, Y. Ren, Z. Li, M. Xu, Y. Wang, R. Ge, X. Kong, L. Zheng and H. Duan, Nat. Commun., 2021, 12, 4679 CrossRef CAS.
- J. Wang, X. Li, M. Wang, T. Zhang, X. Chai, J. Lu, T. Wang, Y. Zhao and D. Ma, ACS Catal., 2022, 12, 6722–6728 CrossRef CAS.
- M. Du, R. Xue, W. Yuan, Y. Cheng, Z. Cui, W. Dong and B. Qiu, Nano Lett., 2024, 24, 9768–9775 CrossRef CAS.
- P. K. Samantaray, A. Little, D. M. Haddleton, T. McNally, B. Tan, Z. Sun, W. Huang, Y. Ji and C. Wan, Green Chem., 2020, 22, 4055–4081 RSC.
- S. Han, J. Wang, Y. Li, C. Wang, Y. Wu and B. Liu, Adv. Energy Mater., 2025, 15, 2502368 CrossRef CAS.
- J. Hao, T. Wang, J. Cai, G. Gao, Z. Zhuang, R. Yu, J. Wu, G. Wu, S. Lu, X. Wang, M. Du, D. Wang and H. Zhu, Angew. Chem., Int. Ed., 2025, 64, e202419369 CrossRef CAS.
- M. Du, Y. Zhang, S. Kang, C. Xu, Y. Ma, L. Cai, Y. Zhu, Y. Chai and B. Qiu, Small, 2023, 19, 2303693 CrossRef CAS.
- F. Liu, X. Gao, R. Shi, Z. Guo, E. C. M. Tse and Y. Chen, Angew. Chem., Int. Ed., 2023, 62, e202300094 CrossRef CAS PubMed.
- Y. Yan, H. Zhou, S.-M. Xu, J. Yang, P. Hao, X. Cai, Y. Ren, M. Xu, X. Kong, M. Shao, Z. Li and H. Duan, J. Am. Chem. Soc., 2023, 145, 6144–6155 CrossRef CAS PubMed.
- D. Si, B. Xiong, L. Chen and J. Shi, Chem. Catal., 2021, 1, 941–955 CAS.
- X. Kong, C. Liu, Z. Xu, J. Zhao, J. Ni, H. Li, T. Zheng, C. Xia, Z. Geng and J. Zeng, Angew. Chem., Int. Ed., 2024, 63, e202411160 CrossRef CAS PubMed.
- H. Wang, Y. Cheng, Y. Wang, R. Duan, M. Zhou, S. Liu, W. Zhao, H. Qin, J. Yang, Y. Peng, L. Jing, Y. Xu, Q. Zhu, X. Sun, Q. Qian, J. Zhang, X. Kang and B. Han, Nat. Synth., 2025 DOI:10.1038/s44160-025-00892-7.
- M. Xu, M. Wang, H. Wang, P. Yuan, Q. Han and G. Zheng, Adv. Mater., 2025 DOI:10.1002/adma.202507144.
- Y. Ma, X. Guo, M. Han, J. Ma, M. Han, L. Cai, V. Nicolosi, W. Wang, W. Dong, M. Jiang and B. Qiu, Angew. Chem., Int. Ed., 2025, 64, e202511466 CrossRef CAS.
- Q. Xia, S. Gong, J. Wu, Y. Zhai, W. Li, Y. Zhou and X. Zhang, Angew. Chem., Int. Ed., 2025, 64, e202507901 CrossRef CAS PubMed.
- J. Hu, X. Gao, S. Li, Z. Xie, X. Sheng, Z. Yuan, F. Zhang, P. Chen, Y. Zheng and S.-Z. Qiao, Adv. Mater., 2025, 37, 2419578 CrossRef CAS PubMed.
- M. Pelckmans, T. Renders, S. Van de Vyver and B. F. Sels, Green Chem., 2017, 19, 5303–5331 RSC.
- T. Polen, M. Spelberg and M. Bott, J. Biotechnol., 2013, 167, 75–84 CrossRef CAS PubMed.
- P. Yang, Y. Ma, X. Guo, M. Han, D. Xing, S. Kang, J. Ma, J. Shang, V. Nicolosi, N. Zhang and B. Qiu, Angew. Chem., Int. Ed., 2025 DOI:10.1002/anie.202516581.
- S. H. Lee, J.-Y. Hwang, S.-J. Park, G.-T. Park and Y.-K. Sun, Adv. Funct. Mater., 2019, 29, 1902496 CrossRef.
- X. Wang, W. Xue, K. Hu, Y. Li, Y. Li and R. Huang, ACS Appl. Energy Mater., 2018, 1, 5347–5354 CAS.
- T. H. T. Myren, T. A. Stinson, Z. J. Mast, C. G. Huntzinger and O. R. Luca, Molecules, 2020, 25, 2742 CrossRef CAS PubMed.
- H. Huang, M. Sun, K. Chen, Y. Che, X. Tang, Z. Li, K. Nie, S. Qian, J. Fang, H. Wang, Y. Wu, Q. Hu, Y. Wang, X. Sun, J. He, Y.-X. Zhang, Z. Zhuang, L. Zhang and Z. Niu, Angew. Chem., Int. Ed., 2025, 64, e202511844 CrossRef CAS.
- Q. Hu, S. Qian, Y. Wang, J. Zhao, M. Jiang, M. Sun, H. Huang, T. Gan, J. Ma, J. Zhang, Y. Cheng and Z. Niu, Nat. Commun., 2024, 15, 10573 CrossRef CAS.
- Y. Zhang, D. Wu, X. Zeng, B. Qiu, Q. Zhu and J. Zhang, Adv. Sci., 2025, 12, e07913 CrossRef CAS PubMed.
- B. Yan, C. Shi, G. T. Beckham, E. Y.-X. Chen and Y. Román-Leshkov, ChemSusChem, 2022, 15, e202102317 CrossRef CAS PubMed.
- D. E. Fagnani, D. Kim, S. I. Camarero, J. F. Alfaro and A. J. McNeil, Nat. Chem., 2023, 15, 222–229 CrossRef CAS PubMed.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2026 |









