 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Room-temperature chromatographic H2/D2 separation via a solid dihydrogen complex with balanced thermodynamics and kinetics
Tamon
Yamauchi
a,
Taku
Kitayama
a,
Kaiji
Uchida
ab,
Nino
Keuzenkamp
a,
Hiroaki
Iguchi
 c,
Ryojun
Toyoda
c,
Ryojun
Toyoda
 a,
Ryota
Sakamoto
a,
Hao
Xue
a,
Naoki
Kishimoto
a,
Ryota
Sakamoto
a,
Hao
Xue
a,
Naoki
Kishimoto
 a,
Shin-ichiro
Noro
a,
Shin-ichiro
Noro
 d and
Shinya
Takaishi
d and
Shinya
Takaishi
 *ae
*ae
aDepartment of Chemistry, Graduate School of Science, Tohoku University; 6-3 Aramaki-Aza-Aoba, Aoba-Ku, Sendai 980-8578, Japan. E-mail: shinya.takaishi.d8@tohoku.ac.jp
bTokyo Metropolitan Industrial Technology Research Institute, 2-4-10 Aomi, Koto, Tokyo 135-0064, Japan
cDepartment of Materials Chemistry, Graduate School of Engineering, Nagoya University, Furo-cho, Chikusa-ku, Nagoya 464-8603, Japan
dFaculty of Environmental Earth Science, Hokkaido University, Sapporo 060-0810, Japan
ePhysical and Chemical Research Infrastructure Group, RIKEN SPring-8 Center, RIKEN, Sayo, Hyogo, 679–5198, Japan
First published on 23rd October 2025
Abstract
To meet the growing demand for hydrogen isotopes, the development of efficient and practical methods for isotope separation for dihydrogen is essential to replace the current cryogenic distillation method operating at 20 K. One of the most promising alternatives is chemical affinity quantum sieving (CAQS), which exploits differences in adsorption enthalpy (|ΔΔH°|) arising from variations in zero-point vibrational energy (ZPVE) between isotopologues. However, low |ΔΔH°| values of materials have prevented effective separation under ambient conditions. In addition, designing materials with a high |ΔΔH°| value is challenging. Herein, we report the largest |ΔΔH°| value of 5.0 kJ mol−1 observed in the solid-state dihydrogen complex [Mn(PCy3)2(CO)3][BARF], exceeding that of all previously known materials. Quantum chemical calculations and statistical analyses were employed to elucidate the origin of this separation ability. Furthermore, we demonstrated H2/D2 separation at ambient temperature using gas chromatography. This work presents a novel strategy to enhance the efficiency of isotope separation, thereby enabling H2/D2 separation at room temperature.
Green foundation1. Our work advances green chemistry by replacing cryogenic isotope distillation (20 K) with a room-temperature adsorption method. A preliminary estimation indicated that pressure swing adsorption (PSA) using our material could achieve H2/D2 separation with only ∼1/10 of the energy cost of distillation, thereby enabling a sustainable, low-energy pathway.2. Specifically, we demonstrated that [Mn(PCy3)2(CO)3][BARF] exhibited the largest enthalpy difference (ΔΔH° = 5.0 kJ mol−1) reported to date, achieving a separation factor of α = 4.2 at 213 K. Moreover, this compound displayed rapid adsorption–desorption kinetics at room temperature, thereby enabling the first chromatographic isotope separation under ambient conditions. 3. This work could be made greener by designing recyclable column materials and scaling up to industrial PSA processes. Furthermore, by integrating quantum chemical calculations and data science-driven screening, we can propose molecules with α > 5 above 200 K, thereby enabling even lower-energy separations and elevating the sustainability of hydrogen isotope supply chains. |
Introduction
Hydrogen isotopes such as deuterium (D) and tritium (T) play important roles across a wide range of research and industrial applications. D has been used as an NMR solvent, tracer,1 and moderator in nuclear reactors. Recently, D has been used for the termination of silicon surfaces of the transistors,2 polymer optical fibers,3 as well as organic light-emitting diodes (OLEDs).4 In the future, D and T will serve as fuels for nuclear fusion reactors.5 Thus, the demand for the hydrogen isotopes will increase further. T also contaminates water in nuclear waste, and its removal will be important from an environmental point of view. The separation of hydrogen isotopes could be one of the greatest challenges to be addressed.For heavy water (D2O) extraction, the Girdler sulfide (GS) process—which utilizes the equilibrium reaction of H2O + HDS ⇄ HDO + H2S—has been used industrially.6 However, this process contains toxic H2S and gives small separation factors. Recently, new approaches have been proposed, such as penetration into graphene or hexagonal boron nitride,7,8 and metal–organic frameworks (MOFs).9 For the separation of isotopologues of dihydrogen molecules (H2, HD, D2, HT, DT and T2), distillation at 20 K is used in industry, which is also energy-intensive and gives a small separation factor.
In the last decade, “quantum sieving” has attracted much attention as a new method for the isotope separation of dihydrogen molecules. Beenakker et al. proposed the concept of “kinetic quantum sieving” (KQS) in 1995.10 The KQS process utilizes the difference in the diffusion rates within small pores arising from the variation in thermal de Broglie wavelengths of hydrogen isotopes. Its mechanism has been validated by experimental studies using organic cages,11 and MOFs.12 However, KQS requires a low temperature (<77 K) and then high energy consumption for cooling is inevitable. Another mechanism for H2/D2 separation was independently reported in MOFs with open-metal sites by FitzGerald et al. in 201313 and by Oh et al. in 2014.14 This phenomenon was named “chemical affinity quantum sieving (CAQS)”.15 The CAQS process utilizes the difference in the zero-point vibrational energy (ZPVE) and resultant adsorption enthalpy  . The ΔΔH° value of these compounds is so small (<2 kJ mol−1) that the operating temperature is <100 K. In 2017, Weinrauch et al. reported a new CAQS candidate, Cu-MFU-4l, which demonstrated efficient isotope separation at elevated temperatures (≈200 K) through the use of open-metal sites of Cu(I) ions.16 This material exhibited the largest |ΔH°| (32.6 and 35.0 kJ mol−1 for H2 and D2, respectively) among extensive MOF libraries, resulting in a significant enthalpy difference (ΔΔH° = 2.4 kJ mol−1). This pronounced isotope effect arises from orbital interactions (specifically σ-donation and π-back donation) between H2 and Cu(I) open-metal sites, known as the “Kubas interaction”.16,17 The separation factor—defined as the ratio of the equilibrium constant for D2 and H2 adsorption (α ≡ KD2/KH2)—was 2.1 at 203 K for Cu-MFU-4l. Since the discovery of CAQS behaviour in Cu-MFU-4l, materials with a higher separation factor have not been reported,18,19 underscoring the significant challenge of designing MOFs capable of engaging in strong interactions with hydrogen molecules.
. The ΔΔH° value of these compounds is so small (<2 kJ mol−1) that the operating temperature is <100 K. In 2017, Weinrauch et al. reported a new CAQS candidate, Cu-MFU-4l, which demonstrated efficient isotope separation at elevated temperatures (≈200 K) through the use of open-metal sites of Cu(I) ions.16 This material exhibited the largest |ΔH°| (32.6 and 35.0 kJ mol−1 for H2 and D2, respectively) among extensive MOF libraries, resulting in a significant enthalpy difference (ΔΔH° = 2.4 kJ mol−1). This pronounced isotope effect arises from orbital interactions (specifically σ-donation and π-back donation) between H2 and Cu(I) open-metal sites, known as the “Kubas interaction”.16,17 The separation factor—defined as the ratio of the equilibrium constant for D2 and H2 adsorption (α ≡ KD2/KH2)—was 2.1 at 203 K for Cu-MFU-4l. Since the discovery of CAQS behaviour in Cu-MFU-4l, materials with a higher separation factor have not been reported,18,19 underscoring the significant challenge of designing MOFs capable of engaging in strong interactions with hydrogen molecules.
In light of this information, we focused on dihydrogen complexes—the original Kubas-type materials. The first metal dihydrogen complex was reported by Kubas and co-workers in 1984,20 and hundreds of compounds have been reported so far.20–28 Bender et al. reported a difference in adsorption enthalpy between H2 and D2 for the dihydrogen complex W(CO)3(PCy3)2(η2-H2) in THF.24 However, dihydrogen complexes had not been considered as candidates for CAQS until our recent study. Recently, we demonstrated that solid-state [Mn(dppe)2(CO)][BARF] (dppe = 1,2-bis(diphenylphosphino)ethane, BARF = tetrakis(3,5-bis(trifluoromethyl)phenyl)borate; hereafter abbreviated as Mn-dppe) exhibited promising CAQS property at room temperature (α = 2.0 at 313 K).28 This compound showed higher absolute values of adsorption enthalpy (|ΔH°| = 50.2 and 54.4 kJ mol−1 for D2 and H2, respectively) and a larger difference in adsorption enthalpy (ΔΔH° = 4.2 kJ mol−1) than previously reported dihydrogen complexes. A further increase in α would be expected at lower temperatures, but Mn-dppe exhibited very slow adsorption kinetics at <313 K, preventing evaluation of separation performance. We summarized the reported H2/D2 selectivity in CAQS materials in Fig. 1.
 | ||
| Fig. 1 Summary of CAQS materials. Selectivity was estimated by (a) thermal desorption spectroscopy and (b) adsorption isotherms (KD2/KH2). | ||
Herein, we report a new CAQS material candidate, [Mn(PCy3)2(CO)3][BARF] (PCy3 = tricyclohexylphosphine; hereafter abbreviated as Mn-PCy3),29 which improved the kinetics while maintaining a good KD2/KH2. This compound showed a similar |ΔH°| to Cu-MFU-4l (27.0 and 32.0 kJ mol−1 for H2 and D2, respectively) but a much larger difference in the adsorption enthalpy (ΔΔH° = 5.0 kJ mol−1). In addition, Mn-PCy3 showed much faster adsorption kinetics than Mn-dppe, enabling adsorption measurements down to 213 K, giving α = 4.2 at 213 K, thereby combining a higher working temperature with high H2/D2 selectivity. We discussed the origin of these thermodynamic phenomena through quantum chemical calculations, and propose designing guidelines for high-performance CAQS materials. Finally, we demonstrated the gas-chromatography separation of H2/D2 mixture using a column packed with Mn-PCy3, taking advantage of its high separation factor and good kinetics.
Results and discussion
H2 and D2 adsorption isotherms
We measured H2 and D2 adsorption to solid-state Mn-PCy3 between 213 K and 273 K. The adsorption isotherms are shown in Fig. 2(b) and (c). All isotherms were well reproduced by the Langmuir equation, as follows: | (1) |
The thermodynamic parameters were evaluated using the van't Hoff equation, as follows:
 | (2) |
 ,
, 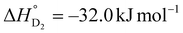 ) was similar to that of Cu-MFU-4l (
) was similar to that of Cu-MFU-4l ( ,
,  ). Conversely, their difference (
). Conversely, their difference ( ) in Mn-PCy3 (5.0 kJ mol−1) was much higher than that of Cu-MFU-4l (2.4 kJ mol−1). This finding suggested that Mn-PCy3 showed a higher separation factor than Cu-MFU-4l at the same temperature. The α values, defined as KD2/KH2, are shown in Fig. 3. Solid lines represent the α value estimated from the fitted ΔH° and ΔS°. Mn-PCy3 showed much larger α than Cu-MFU-4l due to the larger |ΔΔH°| value. It is noteworthy that α of Mn-PCy3 at 273 K was 2.2, which was nearly the same as that of Cu-MFU-4l at 203 K (2.1). This value increased as the temperature decreased, reaching 4.2 at 213 K.
) in Mn-PCy3 (5.0 kJ mol−1) was much higher than that of Cu-MFU-4l (2.4 kJ mol−1). This finding suggested that Mn-PCy3 showed a higher separation factor than Cu-MFU-4l at the same temperature. The α values, defined as KD2/KH2, are shown in Fig. 3. Solid lines represent the α value estimated from the fitted ΔH° and ΔS°. Mn-PCy3 showed much larger α than Cu-MFU-4l due to the larger |ΔΔH°| value. It is noteworthy that α of Mn-PCy3 at 273 K was 2.2, which was nearly the same as that of Cu-MFU-4l at 203 K (2.1). This value increased as the temperature decreased, reaching 4.2 at 213 K.
 | ||
| Fig. 3 Temperature dependence of the H2/D2 separation factor (α ≡ KD2/KH2) in Cu-MFU-4l, Mn-dppe and Mn-PCy3. Solid lines are regression curves using the van't Hoff equation. | ||
Quantum chemical calculations
We performed quantum chemical calculations to reveal the origin of the separation ability of H2 and D2 in Mn-PCy3, Mn-dppe and Cu-MFU-4l. First, we estimated the thermodynamic parameters of H2 and D2 adsorption. For the calculation, we used the cationic fragment ([Mn(PCy3)2(CO)3]+ (Mn-PCy3+) (Fig. S1(a)), [Mn(dppe)2(CO)]+ (Mn-dppe+) (Fig. S1(b)) and Zn3Cu2 cluster as a partial structure of Cu-MFU-4l (Fig. S1(c)). The following equations were used to evaluate and
and  at 298.15 K and 1 bar:
at 298.15 K and 1 bar: | (3) |
 | (4) |
 | (5) |
 | (6) |
 | (7) |
We calculated the thermodynamic parameters of Mn-PCy3, Mn-dppe and Cu-MFU-4l, and then performed structural optimization using various functionals and basis sets. We also estimated the α values at 298.15 K using eqn (7). These have been summarized in Tables S4–S6. At any level of the calculation we examined,  and α were nearly independent and showed good agreement with the experimental trend. This result supported the validity of the calculations. For a more detailed discussion, we used the results of the quantum chemical calculations with wB97X-D functional and def2-TZVP for Mn, aug-cc-pVDZ for H2, and 6-31G for other atoms’ basis sets, which showed good agreement with experimental thermodynamic parameters. We also calculated the α for other pairs of isotopes of hydrogen molecules (HD, HT, DT and T2) (Table S7).
and α were nearly independent and showed good agreement with the experimental trend. This result supported the validity of the calculations. For a more detailed discussion, we used the results of the quantum chemical calculations with wB97X-D functional and def2-TZVP for Mn, aug-cc-pVDZ for H2, and 6-31G for other atoms’ basis sets, which showed good agreement with experimental thermodynamic parameters. We also calculated the α for other pairs of isotopes of hydrogen molecules (HD, HT, DT and T2) (Table S7).
In the CAQS mechanism, differences in the zero-point vibrational energy serve as the driving force for isotope separation. Therefore, it is essential to discuss molecular vibrations derived from adsorbed hydrogen molecules. Fig. 4(a)–(c) show the partial vibrational density of states (p-vDOS) derived from adsorbed hydrogen molecules, estimated through quantum chemical calculations. The motion of the H2 molecule can be described by the six normal modes presented in Fig. 4(d). For all three samples, the vibrational frequencies followed the order: H–H stretching (ν(H–H)) > M–H asymmetric stretching (νasym(M–H)) > symmetric stretching (νsym(M–H)) > in-plane bending ≈ out-of-plane bending > rotation (libration). This order is consistent with the vibrational frequencies reported by Kubas and colleagues, determined from FT-IR spectra of a series of complexes.31
The bending and rotation modes possess relatively low vibrational energies (<800 cm−1) and are highly split due to the coupling with other modes. At room temperature, these modes are likely to be thermally excited in part by considering the Boltzmann distribution, so the contribution from these modes can be less than that from stretching modes. Therefore, we focused on three modes: ν(H–H), νasym(M–H), and νsym(M–H). For ν(H–H), the trend was Cu-MFU-4l > Mn-PCy3 ≈ Mn-dppe, whereas for νasym(M–H) and νsym(M–H), the trend was Cu-MFU-4l < Mn-PCy3 ≈ Mn-dppe. In general, there is a trade-off between the strengths of the M–H bond and the H–H bond.21 These results indicated that the strengths of the M–H bond followed the trend Cu-MFU-4l < Mn-PCy3 ≈ Mn-dppe. Conversely, the trend of |ΔH°| values was Cu-MFU-4l ≈ Mn-PCy3 < Mn-dppe. That is, the |ΔH°| value of Mn-PCy3 was much smaller than that of Mn-dppe despite similar M–H bond strengths. This was probably due to the more rigid structure of Mn-dppe with the five-membered chelate ring structure, resulting in a smaller structural relaxation energy upon H2 desorption.
Statistical analyses of vibrational energy and isotope selectivity
We wished to elucidate the underlying factors contributing to the isotope selectivity observed in hydrogen adsorption systems. Hence, we performed statistical analyses on the vibrational frequencies associated with adsorbed H2 and D2 molecules. To clarify the correlation between these vibrational frequencies and isotope separation performance, we selected the structures of 20 dihydrogen complexes from the Cambridge Crystal Structure Database (CCDC) or modified versions thereof (Fig. S2), and performed quantum chemical calculations to determine ΔΔH° and ΔΔG°. The α at 298.15 K was calculated using eqn (7) and has been summarized in Table S8.The vibrational frequencies of ν(H–H), νasym(M–H), and νsym(M–H) were also evaluated by the quantum chemical calculations. These have been summarized in Fig. S3–S22 and Table S9. The sum of these vibrational frequencies was calculated separately for H2- and D2-derived modes and evaluated as a single descriptor against KD2/KH2.
As shown in Fig. 5a and b, H2 and D2 vibrational frequency sums exhibited strong positive correlations with KD2/KH2. The Pearson correlation coefficient was 0.945 for the H2 vibrational sum and 0.943 for the D2 vibrational sum. These results suggested that the ν(H–H), νasym(M–H), and νsym(M–H) had a dominant role in the thermodynamic differentiation between H2 and D2. Based on regression analyses, we derived a quantitative relationship between isotope selectivity and vibrational energy, KD2/KH2 = (1.98 × 10–3) × ∑ν(H2) − 9.63, where ∑ν(H2) represents the sum of ν(H–H), νasym(M–H), and νsym(M–H) in cm−1. This simple linear expression captured the essence of how vibrational contributions correlated with isotope separation efficiency and could serve as a predictive model for screening new candidate materials. This enables the prediction of separation performance without the need for comparative isotopic experiments, thereby accelerating material discovery.
An additional and important observation from our study was that the top-ranked complexes in terms of α values were cationic first-row transition metal dihydrogen complexes. In general, there is a trade-off between H–H bond strength and M–H bond strength,21 but the principal factor weakening the H–H bond is π-backdonation. The cationic nature and first-row transition metal character contribute to lowering d-orbital energy levels, which, in turn, enhances σ-donation and suppresses the π-backdonation components in the M–H bonding interaction. As a result, the observed complexes exhibited higher overall vibrational frequencies. This insight offers a valuable design principle for the future development of high-performance isotope separation materials.
Adsorption kinetics and chromatographic separation
Adsorption kinetics were examined by monitoring gas pressure during isotherm measurements. Fig. 6 shows the temperature dependence of the adsorption kinetics of H2 and D2 in Mn-PCy3. At 273 K, adsorption kinetics were well represented by single-exponential behaviour, and reached equilibrium within ∼20 s. This was much faster than that of Mn-dppe, which reached equilibrium in ∼1 h. The exact cause was not clear, but in situ PXRD measurements revealed a difference between the two: Mn-dppe gradually became amorphous upon H2 adsorption, whereas Mn-PCy3 showed almost no change in PXRD patterns during the same process (Fig. S2). This finding suggested that, in Mn-dppe, the diffusion of H2 molecules within the crystal impacted the crystalline lattice, suggesting that some factors hindered H2 diffusion, whereas there was little resistance to H2 diffusion in Mn-PCy3. Therefore, the difference in molecular packing in the crystal was considered to influence the difference in kinetics. | ||
| Fig. 6 Adsorption kinetics of (a and b) H2 and (c and d) D2 in Mn-PCy3. (b) and (d) are the magnification of T < 100 s. | ||
With decreasing temperature, the adsorption kinetics gradually became slower and comprised at least two components. The origin of this phenomenon was not revealed. However, it is noteworthy that the adsorption rate was always faster for H2 than for D2, indicating that an isotope effect was also observed in the adsorption kinetics. Such a kinetic isotope effect in a CAQS material has not been reported.
By utilizing the large α value and fast adsorption kinetics at ambient temperature, we conducted an experiment on the separation of H2/D2 by gas chromatography. A 6.35 mm ∅ (4 mm inner diameter) stainless-steel column of ∼90 cm was filled with 8.75 g of Mn-PCy3. Ar was the carrier gas (8 mL min−1) and H2/D2 (8 mL min−1, 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 (v/v)) flowed for 60 s (between 30 s to 90 s). The gases from the column were detected by a mass spectrometer. The results are shown in Fig. 7. At all temperatures, H2 was clearly detected before D2, indicating that D2 was retained more strongly on the column due to its larger KD2 (i.e., longer mean residence time on the complexes at the same temperature). The time difference of each gas exiting the column increased with decreasing temperature. This can be attributed to a higher separation factor at lower temperatures. On the other hand, the lower the temperature, the broader were the respective peak widths. This was probably due to the decrease in adsorption/desorption rate caused by the decrease in temperature (Fig. 6). In the breakthrough experiment (Fig. 7(b)), H2 broke through before D2 at room temperature. This is the first successful chromatographic separation of hydrogen isotopes at ambient temperature. It is also noteworthy that all chromatographic measurements were conducted using the same batch of samples, indicating that these chromatographic separations could be repeatedly performed by purging H2 and D2 gases with Ar flow at room temperature.
50 (v/v)) flowed for 60 s (between 30 s to 90 s). The gases from the column were detected by a mass spectrometer. The results are shown in Fig. 7. At all temperatures, H2 was clearly detected before D2, indicating that D2 was retained more strongly on the column due to its larger KD2 (i.e., longer mean residence time on the complexes at the same temperature). The time difference of each gas exiting the column increased with decreasing temperature. This can be attributed to a higher separation factor at lower temperatures. On the other hand, the lower the temperature, the broader were the respective peak widths. This was probably due to the decrease in adsorption/desorption rate caused by the decrease in temperature (Fig. 6). In the breakthrough experiment (Fig. 7(b)), H2 broke through before D2 at room temperature. This is the first successful chromatographic separation of hydrogen isotopes at ambient temperature. It is also noteworthy that all chromatographic measurements were conducted using the same batch of samples, indicating that these chromatographic separations could be repeatedly performed by purging H2 and D2 gases with Ar flow at room temperature.
 | ||
Fig. 7 Gas chromatograph of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 H2/D2 (v/v) mixture using a packed column of Mn-PCy3. (a) Sixty-second pulse-flow experiment. (b) Continuous flow (breakthrough) experiment. 1 H2/D2 (v/v) mixture using a packed column of Mn-PCy3. (a) Sixty-second pulse-flow experiment. (b) Continuous flow (breakthrough) experiment. | ||
Conclusions
We showed that Mn-PCy3 possessed a higher affinity for D2 than H2. The difference in the adsorption enthalpy between H2 and D2 adsorption was estimated to be 5.0 kJ mol−1, which is the highest among any known material. Our preliminary estimation indicated that pressure swing adsorption using our material could achieve H2/D2 separation with only ∼1/10 of the energy cost of distillation (see page 20 and 21 in the SI). The mechanism of this phenomenon was reasonably explained by the isotopic difference in the ZPVE. We demonstrated the chromatographic H2/D2 separation at ambient temperature. Thus, with its high isotopic selectivity and gas permeability, Mn-PCy3 is a promising material to produce high-purity deuterium and to capture tritium from a low-concentration hydrogen gas mixture by repeating adsorption/desorption at room temperature.Author contributions
T. K. and T. Y. wrote the manuscript together with H. I., R. T, R. S. and S. T. The synthesis and measurements of isotherms were performed by T. K., T. Y., K. U. and S.-i. N. Quantum chemical calculations were performed by N. K., K. U., H. X. and N. K., S. T directed the project.Conflicts of interest
There are no conflicts of interest to declare.Data availability
The data supporting the conclusions reached from our study have been included as part of the supplementary information (SI). Supplementary information: experimental details, experimental and calculated thermodynamic parameters, partial vibrational density of states of a series, thermodynamic parameters, and vibrational frequencies of dihydrogen complexes. See DOI: https://doi.org/10.1039/d5gc04476f.Acknowledgements
This work was supported by JSPS KAKENHI (23H02054, 21H04696, 19H02729), Adaptable and Seamless Technology Transfer Program Through Target-driven R&D from Japan Science and Technology Agency (JPMJTR20T9), Institute for Quantum Chemical Exploration, ENEOS Hydrogen Trust Fund, and KIOXIA Corporation.References
- T. Tani and Y. Ishikawa, Sci. Total Environ., 2023, 903, 166792 CrossRef CAS PubMed.
- J. W. Lyding, K. Hess and I. C. Kizilyalli, Appl. Phys. Lett., 1996, 68, 2526–2528 CrossRef CAS.
- J. Stone and C. A. Burrus, Bell Syst. Tech. J., 1980, 59, 1541–1548 CrossRef CAS.
- H. Tsuji, C. Mitsui and E. Nakamura, Chem. Commun., 2014, 50, 14870–14872 RSC.
- T. S. Pedersen, Nat. Commun., 2016, 7, 13493 CrossRef CAS PubMed.
- H. K. Rae, ACS Symp. Ser., 1978, 68, 1–26 CrossRef CAS.
- M. Lozada-Hidalgo, S. Hu, O. Marshall, A. Mishchenko, A. N. Grigorenko, R. A. W. Dryfe, B. Radha, I. V. Grigorieva and A. K. Geim, Science, 2016, 351, 68–70 CrossRef CAS PubMed.
- S. Yasuda, H. Matsushima, K. Harada, R. Tanii, T. Terasawa, M. Yano, H. Asaoka, J. S. Gueriba, W. A. Diño and K. Fukutani, ACS Nano, 2022, 16, 14362–14369 CrossRef CAS PubMed.
- Y. Su, K.-i. Otake, J. J. Zheng, S. Horike, S. Kitagawa and C. Gu, Nature, 2022, 611, 289–294 CrossRef CAS PubMed.
- J. J. M. Beenakker, V. D. Borman and S. Yu. Krylov, Chem. Phys. Lett., 1995, 232, 379–382 CrossRef CAS.
- M. Liu, L. Zhang, M. A. Little, V. Kapil, M. Ceriotti, S. Yang, L. Ding, D. L. Holden, R. Balderas-Xicohténcatl, D. He, R. Clowes, S. Y. Chong, G. Schütz, L. Chen, M. Hirscher and A. I. Cooper, Science, 2019, 366, 613–620 CrossRef CAS PubMed.
- D. Denysenko, M. Grzywa, M. Tonigold, B. Streppel, I. Krkljus, M. Hirscher, E. Mugnaioli, U. Kolb, J. Hanss and D. Volkmer, Chem. – Eur. J., 2011, 17, 1837–1848 CrossRef CAS PubMed.
- S. A. FitzGerald, C. J. Pierce, J. L. C. Rowsell, E. D. Bloch and J. A. Mason, J. Am. Chem. Soc., 2013, 135, 9458–9464 CrossRef CAS PubMed.
- H. Oh, I. Savchenko, A. Mavrandonakis, T. Heine and M. Hirscher, ACS Nano, 2014, 8, 761–770 CrossRef CAS PubMed.
- I. Savchenko, A. Mavrandonakis, T. Heine, H. Oh, J. Teufel and M. Hirscher, Microporous Mesoporous Mater., 2015, 216, 133–137 CrossRef CAS.
- I. Weinrauch, I. Savchenko, D. Denysenko, S. M. Souliou, H.-H. Kim, M. Le Tacon, L. L. Daemen, Y. Cheng, A. Mavrandonakis, A. J. Ramirez-Cuesta, D. Volkmer, G. Schütz, M. Hirscher and T. Heine, Nat. Commun., 2017, 8, 14496 CrossRef CAS PubMed.
- B. R. Barnett, et al. , J. Am. Chem. Soc., 2021, 143, 14884–14894 CrossRef CAS PubMed.
- Y. Si, X. He, J. Jiang, Z. Duan, W. Wang and D. Yuan, Nano Res., 2021, 14, 518–525 CrossRef CAS.
- L. Li, C. Ji, W. Wang, F. Wu, Y.-X. Tan and D. Yuan, Inorg. Chem. Front., 2022, 9, 1674–1680 RSC.
- G. J. Kubas, R. R. Ryan, B. I. Swanson, P. J. Vergamini and H. J. Wasserman, J. Am. Chem. Soc., 1984, 106, 451–452 CrossRef CAS.
- G. J. Kubas, Chem. Rev., 2007, 107, 4152–4205 CrossRef CAS PubMed.
- R. H. Morris, Coord. Chem. Rev., 2008, 252, 2381–2394 CrossRef CAS.
- G. J. Kubas, in Metal Dihydrogen and σ-Bond Complexes, ed. J. P. Fackler Jr., Kluwer Academic/Plenum Publishers, New York, 2001, pp. 33–51 Search PubMed.
- B. R. Bender, G. J. Kubas, L. H. Jones, B. I. Swanson, J. Eckert, K. B. Capps and C. D. Hoff, J. Am. Chem. Soc., 1997, 119, 9179–9190 CrossRef CAS.
- D. G. Abrecht and B. Fultz, J. Phys. Chem. C, 2012, 116, 22245–22252 CrossRef CAS PubMed.
- K. Uchida, N. Kishimoto, S.-i. Noro, H. Iguchi and S. Takaishi, Dalton Trans., 2021, 50, 12630–12634 RSC.
- K. Uchida, S. Tanaka, S. Adachi, H. Iguchi, R. Sakamoto and S. Takaishi, RSC Adv., 2024, 14, 11452–11455 RSC.
- T. Kitayama, T. Yamauchi, K. Uchida, S. Tanaka, R. Toyoda, H. Iguchi, R. Sakamoto, H. Xue, N. Kishimoto, T. Yoshida, T. Uruga, S.-i. Noro and S. Takaishi, Dalton Trans., 2025, 54, 2621–2627 RSC.
- A. Toupadakis, G. J. Kubas, W. A. King, B. L. Scott and J. Huhmann-Vincent, Organometallics, 1998, 17, 5315–5323 CrossRef CAS.
- A. J. Martínez-Martínez, N. H. Rees and A. S. Weller, Angew. Chem., Int. Ed., 2019, 58, 16873–16877 CrossRef PubMed.
- G. J. Kubas, C. J. Unkefer, B. I. Swanson and E. Fukushima, J. Am. Chem. Soc., 1986, 108, 7000–7009 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2026 |



