Linear paired electrolysis enabled dearomative [3 + 2] cycloadditions of indoles and benzofurans with vinyl azides
Shaoxiong
Yang†
,
Yanren
Zhu†
,
Enfan
Pu
,
Piaopiao
Jiang
,
Xiong
Li
,
Hongbin
Zhang
 * and
Jingbo
Chen
* and
Jingbo
Chen
 *
*
Key Laboratory of Medicinal Chemistry for Natural Resource, Ministry of Education, Yunnan Key Laboratory of Cross-Border Infectious Disease Control and Prevention and Novel Drug Development, Yunnan Provincial Center for Research & Development of Natural Products, School of Chemical Science and Technology, School of Pharmacy, Yunnan University, Kunming, 650500, P. R. China. E-mail: zhanghb@ynu.edu.cn; chenjb@ynu.edu.cn
First published on 4th November 2025
Abstract
Linear paired electrolysis is an ideal yet relatively rare electrochemical approach that offers significant advantages for constructing fused heterocyclic frameworks. Herein, we report an efficient protocol for dearomative [3 + 2] cycloadditions of indoles and benzofurans with vinyl azides via linear paired electrolysis under redox-neutral conditions. This method enables the formation of both rare benzofuro[3,2-b]pyrrolines, indolo[3,2-b]pyrrolines and conventional indolo[2,3-b]pyrrolines with excellent regio- and stereo-selectivity. Notably, this electrochemical approach shows good functional group tolerance and broad substrate scope, making it valuable for synthetic applications and molecular modification in pharmaceutical research.
Green foundation1. A general linear paired electrolysis-driven dearomative [3 + 2] cycloaddition reaction was carried out under metal- and oxidant-free conditions.2. The electrochemical method shows excellent functional group compatibility and a wide substrate applicability range, enabling the construction of various fused compounds with high regio- and stereo-selectivity. 3. The linear paired electrolysis circumvents sacrificial half-reactions on the electrodes, greatly improving energy efficiency and providing a new direction for the synthesis of fused heterocyclic compounds. |
Introduction
2,3-Indoline- and 2,3-dihydrobenzofuran-fused cyclic compounds are widely present in natural products and pharmaceutical molecules,1 and exhibit broad biological activities.2 Among them, indolo[2,3-b]pyrrolidines, as a common class of heterocyclic fused structures, have attracted extensive attention from the organic chemistry community, and numerous synthetic methods have been developed.3 In contrast, indolo- and benzofuro-[3,2-b]pyrrolidines are exceptionally rare in natural products. To date, aristone and phalarine have been reported in the literature as the only natural products containing these two structural frameworks, respectively (Scheme 1a).4 Owing to their distinctive and novel fusion architectures, there have been only a few reports on the synthesis of indolo- and benzofuro-[3,2-b]pyrrolidines over the past decades.5 Notably, the current synthetic approaches for both types of fused-ring compounds often face certain challenges such as lengthy reaction sequences and poor substrate generality. Therefore, establishing a highly efficient protocol for the synthesis of indolo- and benzofuro-[3,2-b]pyrrolidines under mild conditions holds substantial significance for advancing both organic synthesis methodologies and medicinal chemistry research.Electrosynthesis has garnered increasing attention as a powerful tool in organic chemistry due to its ability to utilize sustainable green energy to generate highly reactive radical intermediates via single-electron transfer,6 typically without the need for external oxidants or reductants, thereby offering a green and sustainable synthetic pathway.7 Despite electrosynthesis having made great progress in various research fields like C–H activation,8 cross-coupling reactions,9 and alkene difunctionalization,10 among others,11 these methods often need to be coupled with another sacrificial half-reaction (e.g., proton reduction to form hydrogen) at the counter electrode,12 which greatly reduces energy efficiency. Interestingly, linear paired electrolysis,13 where both electrodes actively participate in the redox transformation, provides an effective and practical strategy to circumvent sacrificial half-reactions. However, its application in the synthesis of fused heterocyclic compounds remains largely underexplored.14 With the rapid development of organic electrochemistry, electrochemical oxidative dearomative [3 + 2] cycloaddition of indoles has emerged as a concise and efficient synthetic method for constructing 2,3-indoline-fused five-membered heterocyclic compounds.15 In 2023, Lei's group reported that electrochemical oxidative coupling between β-ketonitriles and indoles affords furo[3,2-b]indolines via radical cation/nucleophile coupling (Scheme 1b).16 Although this method requires coupling with another sacrificial half-reaction at the counter electrode, it represents the first electrochemical synthesis of furo[3,2-b]indolines. To the best of our knowledge, the electrochemical synthesis of indolo- and benzofuro-[3,2-b]pyrrolines has not been reported yet.
Vinyl azides serve as versatile three-atom synthons, exhibiting both nucleophilic and radical-accepting properties, and have been widely utilized in the synthesis of high-value nitrogen-containing heterocycles.17 However, in the field of electrochemistry, the use of vinyl azides to synthesize nitrogen-containing fused rings remains largely unexplored.18 According to this, we envisioned that the [3 + 2] annulation of indoles and benzofurans with vinyl azides could provide efficient access to indole- and benzofuran-fused pyrrolines under appropriate electrochemical conditions. Herein, we report a linear paired electrolysis strategy for the controlled synthesis of diverse indole- and benzofuran-fused pyrroline scaffolds via a radical cation-mediated dearomative [3 + 2] annulation reaction of indoles and benzofurans with vinyl azides (Scheme 1c). This protocol enables the efficient synthesis of benzofuro[3,2-b]pyrrolines, indolo[3,2-b]pyrrolines and indolo[2,3-b]pyrrolines under mild conditions, with excellent regio- and stereo-selectivity. Importantly, the transformation proceeds in the absence of external oxidants and catalysts, offering a sustainable and practical approach for the construction of structurally complex heterocycles with potential bioactivity.
Results and discussion
To probe the feasibility of this method, we initially selected 3-phenylbenzofuran (1aa) and α-tolyl vinyl azide (2a) as the model substrates to test the reaction conditions (Table 1). Utilizing nBu4NBF4 as the electrolyte and 1,1,1,3,3,3-hexafluoroisopropyl alcohol (HFIP)/MeCN as co-solvents, the dearomative [3 + 2] annulation product 3aa could be obtained in 82% yield under 3.0 mA constant current for 6 h in an undivided cell (entry 1). Poor efficiency for the [3 + 2] annulation reaction was obtained when HFIP or MeCN was used as the sole solvent (entries 2 and 3). The ratio of solvent affects the yield of the reaction to some extent (entry 4). In addition, increasing or decreasing the current reduced the yield of the reaction (entries 5 and 6). As for the electrolyte used, nBu4NPF6 and nBu4NClO4 have a significant detrimental effect (entries 7 and 8). When evaluating different electrode materials, the use of a graphite rod or platinum plate as the anode decreased the yield (entries 9 and 10). Similarly, using a platinum plate as the cathode caused a moderate decrease in the yield (entry 11), possibly due to its lower hydrogen evolution potential. However, replacing the carbon felt cathode with a Ni form cathode led to lower reaction yield (entry 12). Notably, the reaction could be conducted under atmospheric conditions in 70% yield (entry 13). Obviously, no reaction took place without electric current as indicated by the residue of almost all of the starting materials (entry 14).| Entry | Variations | Yieldb/% |
|---|---|---|
| a Reaction conditions: carbon felt electrodes (15 mm × 10 mm × 2.0 mm, distance: 10 mm), 1aa (0.20 mmol), 2a (2.0 equiv.), nBu4NBF4 (1.0 equiv.), solvent (MeCN/HFIP = 2 mL/4 mL), undivided cell, N2, room temperature, constant current = 3.0 mA, 6 h. b Isolated yields are shown. HFIP: hexafluoroisopropanol. n.r.: no reaction. | ||
| 1 | None | 82 |
| 2 | Without MeCN | 35 |
| 3 | Without HFIP | 23 |
| 4 | MeCN/HFIP (4/2) | 65 |
| 5 | 2 mA, 10 h instead of 3 mA, 6 h | 72 |
| 6 | 5 mA, 3.5 h instead of 3 mA, 6 h | 81 |
| 7 | n Bu4NPF6 instead of nBu4NBF4 | 74 |
| 8 | n Bu4NClO4 instead of nBu4NBF4 | 48 |
| 9 | C rod (+) instead of C felt (+) | 45 |
| 10 | Pt (+) instead of C felt (+) | 32 |
| 11 | Pt (−) instead of C felt (−) | 65 |
| 12 | Ni form (−) instead of C felt (−) | 23 |
| 13 | Under air | 70 |
| 14 | No electric current | n.r. |
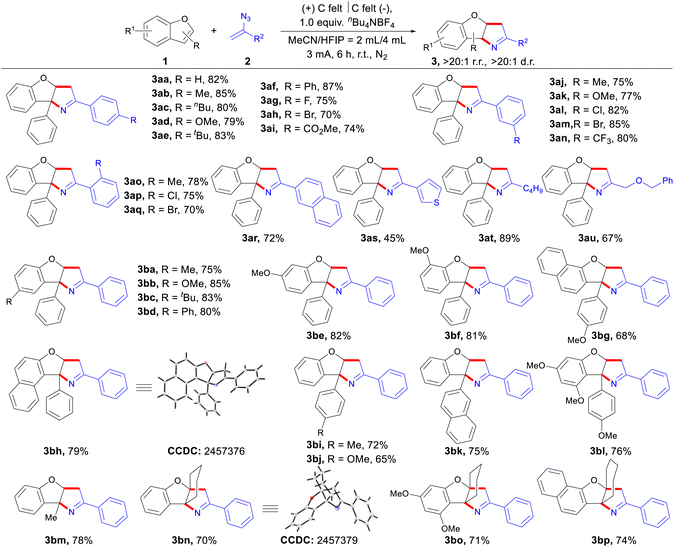
With the optimized reaction conditions in hand, we first turned to explore the substrate scope of α-substituted vinyl azides amenable for the dearomative [3 + 2] annulation with benzofuran derivatives (Scheme 2). The reaction of the electron-neutral parent α-phenyl vinyl azide produced the cycloadduct 3aa in 82% isolated yield. Moreover, aryl vinyl azides bearing electron-donating (e.g., Me, nBu, OMe, tBu, and Ph) or electron-withdrawing (e.g., F, Br, and CO2Me) functional groups at the para-position of the aromatic ring were all well tolerated, giving the corresponding products (3ab–3ai) in generally good yields. As seen in the reactions, regulation of the substitution patterns and steric hindrance at the meta- or ortho-positions of the aromatic ring had no adverse effect on the reaction efficiency, and the expected cycloadducts (3aj–3aq) were isolated in good yields. Naphthyl vinyl azides reacted well to provide 3ar with satisfactory results. Excitedly, thiophenyl and alkyl vinyl azides also participated in the reaction (3as, 3at and 3au).
We then examined the effects of substituents on the benzofuran ring. Introducing various substituents at the C-5 position of the benzofuran ring could provide the corresponding products (3ba–3bd) in moderate to good yields. Additionally, C-6 or C-7 methoxy substituted benzofuran also worked smoothly to provide the relative products (3be and 3bf). Naphthofuran was successfully coupled with the vinyl azide to build larger heterocyclic systems (3bg and 3bh). The structure of 3bh was further confirmed through X-ray crystallography (see the SI for details). Electron-donating substrates at the C-3 phenyl position exhibited higher reactivity (3bi and 3bj). In contrast, phenyl groups of electron-deficient substrates could not react, likely due to their higher oxidation potential. Other aryl groups at the C-3 position of benzofuran, such as naphthyl and methyl, also participated effectively in the reaction (3bk and 3bl). Moreover, a C-3 methyl substituted benzofuran derivative provided the product 3bm. Intriguingly, when benzofurans with C-2/C-3 fused cyclohexane or cycloheptane rings were employed as substrates, three-dimensional bridged products possessing a turbine-blade-like architecture were obtained with excellent regio- and stereo-selectivity (3bn–3bp). The structure of 3bn was also confirmed by X-ray crystallographic analysis (see the SI for details). Our mechanistic studies (Scheme 5c) confirmed that the reaction proceeds via a radical addition mechanism. The C-3 substituted benzofuran radical cation, generated through anodic oxidation, undergoes radical addition preferentially at the C-2 position, which rationalizes the high regioselectivity observed (3aa–3bp). This regioselectivity is further supported by DFT calculations from Lei's work.19
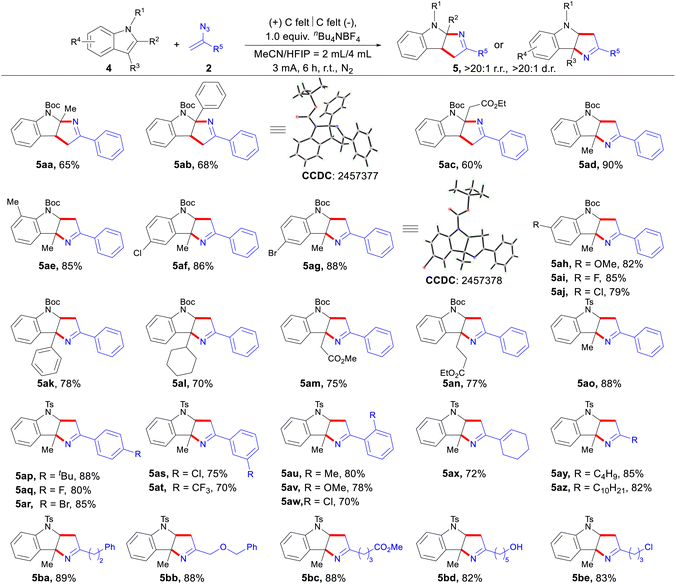
We further applied this method to indoles, enabling their coupling with vinyl azides to form indole-fused pyrrolines (Scheme 3). A series of 2-substituted N-Boc indoles were selectively transformed into indolo[2,3-b]pyrrolines (5aa–5ac), featuring fully substituted carbon centers at the C-2 position in good yields. The structure of 5ab was confirmed by X-ray crystallographic analysis (see the SI for details). Interestingly, when substituents were introduced at the C-3 position of indoles, the reaction proceeded with excellent regioselectivity to afford indolo[3,2-b]pyrrolines bearing a fully substituted carbon center at the C-3 position. Regarding the regioselectivity originating from different substituent positions on the indole ring, our mechanistic studies (Scheme 5b) confirm that the substrate initially forms a radical cation upon anodic oxidation. Subsequently, the conclusions derived from the DFT calculations in Lei's study16 provide an explanation for the high regioselectivity achieved in our work. When the indole nitrogen is substituted with an electron-withdrawing group, the indole radical cation generated through anodic oxidation exhibits similar radical characteristics at both the C-2 and C-3 positions. Consequently, the regioselectivity is primarily governed by the steric effects at the C-2 and C-3 positions, rather than by electronic effects. We initially evaluated a series of N-Boc indoles bearing diverse substituents on the benzene ring. Notably, indole substrates containing either halogen atoms or electron-donating groups (e.g., Me and OMe) at various positions underwent efficient annulation to provide the corresponding products (5ad–5aj) in consistently high yields. Among them, the structure of 5ag was further confirmed through X-ray crystallography (see the SI for details). The [3 + 2] annulation reaction showed moderate efficiency regardless of the substituents at the indole C-3 position, including phenyl, cyclohexyl, and various functionalized alkyl groups (5ak–5an). Additionally, the reaction between diverse functionalized vinyl azides and N-tosylindoles successfully afforded the desired cycloadducts. This encompassed not only various aryl vinyl azides with different substituents (5ao–5aw) but also the more challenging alkyl vinyl azides (5ay–5be). Furthermore, a cyclohexenyl-substituted vinyl azide (5ax) was tolerated.
To further show the use of this protocol for synthesizing polycyclic fused indole and benzofuran compounds, we conducted gram-scale synthesis studies on α-tolyl vinyl azide (2a) with 3-phenylbenzofuran (1be) and N-Boc indole (4ad), respectively. The results confirmed the scalability of the reaction, delivering compounds 3be (75% yield) and 5ad (81% yield) under the optimized conditions (Scheme 4a). The two distinct gram-scale protocols showed acceptable green chemistry metrics: E-factors of 48.46 and 43.67, PMI values of 49.46 and 44.67, atom economies of 92.4% and 92.6%, atom efficiencies of 69.3% and 75.0%, carbon efficiencies reaching 100% and 100%, and reaction mass efficiencies of 49.6% and 54.2%, respectively.20 Furthermore, compounds 3be and 5ad were subjected to further transformations. First, the compound 3be with a cyclic imine group could be further utilized for cyclization reactions, enabling the synthesis of both 2,3-dihydrobenzofuran-fused carbapenem analog 6a and oxaziridine 6b. Then, the NaBH3CN-mediated reduction of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bonds in 3be and 5ad afforded densely functionalized benzofuro[3,2-b]pyrrolidine 6c and indolo[3,2-b]pyrrolidine 7a, respectively, each as a single diastereomer (>20
N bonds in 3be and 5ad afforded densely functionalized benzofuro[3,2-b]pyrrolidine 6c and indolo[3,2-b]pyrrolidine 7a, respectively, each as a single diastereomer (>20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr). Lastly, deprotection of the N-Boc group in 5ad with TFA/DCM at room temperature provided compound 7b in 80% yield (Scheme 4b) (see the SI for the detailed synthesis process).
1 dr). Lastly, deprotection of the N-Boc group in 5ad with TFA/DCM at room temperature provided compound 7b in 80% yield (Scheme 4b) (see the SI for the detailed synthesis process).
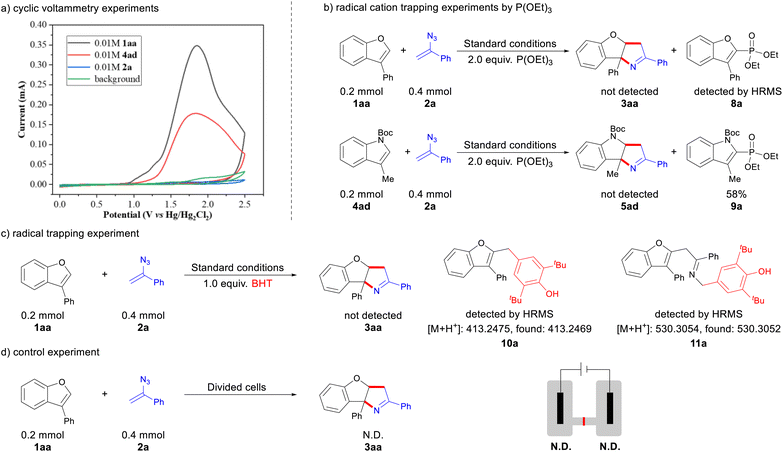
To elucidate the mechanism of the linear paired electrolysis process, we conducted a series of mechanistic studies. Cyclic voltammetry experiments revealed distinct oxidation peaks at 1.91 V (vs. Hg/Hg2Cl2) for 1aa and 1.83 V (vs. Hg/Hg2Cl2) for 4ad. In contrast, no obvious oxidation peak was observed for compound 2a, suggesting that either 1aa or 4ad is preferentially oxidized in the reaction mixture (Scheme 5a). Further evidence was provided by radical cation trapping experiments; when two equivalents of triethyl phosphite were added to the standard reaction, the desired [3 + 2] annulation product was not observed. Instead, the benzofuran phosphorylation product 8a was detected by HRMS, and the indole phosphorylation product 9a was isolated in 58% yield (Scheme 5b). These results confirm the existence of both indole and benzofuran radical cation intermediates. Moreover, the addition of the radical scavenger butylated hydroxytoluene (BHT) completely suppressed the reaction, and HRMS detected the two radical intermediates (10a and 11a) that were trapped by BHT, clearly supporting the involvement of radical species (Scheme 5c). To gain deeper insights into the redox-neutral reaction mechanism, we conducted the experiment in divided cells. The cycloaddition product was not observed, indicating that both anodic oxidation and cathodic reduction are critical to the reaction (Scheme 5d).
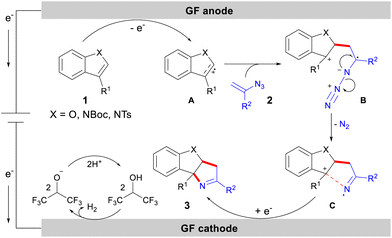
Based on experimental results and literature reports,15,16,19,21 we proposed a possible reaction mechanism (Scheme 6). Under electrochemical conditions, indoles and benzofurans were oxidized to form the radical cation intermediate A. This reactive intermediate then could participate in a regioselective radical addition reaction with vinyl azide 2, affording intermediate B, which then underwent intramolecular denitrogenative cyclization to produce intermediate C. Finally, the cyclization intermediate C could be further reduced at the cathode to afford the target product 3.
Conclusions
In summary, we have developed a linear paired electrolysis method that enables the [3 + 2] cycloaddition of indoles and benzofurans with vinyl azides under mild reaction conditions. This electrochemical approach enables the controlled synthesis of both rare benzofuro[3,2-b]pyrrolines, indolo[3,2-b]pyrrolines and conventional indolo[2,3-b]pyrrolines with excellent regio- and stereo-selectivity. This linear paired electrolysis method provides an efficient platform for synthesizing diverse fused heterocyclic compounds, holding significant importance for advancing both organic synthesis methodologies and medicinal chemistry research.Author contributions
H. Z. and J. C. conceived the project. S. Y. and Y. Z. designed and performed the experimental work. E. P., P. J. and X. L. contributed to the analysis and interpretation of data. J. C., S. Y. and Y. Z. wrote the manuscript. All authors contributed to or approved the final version of the paper.Conflicts of interest
There are no conflicts to declare.Data availability
Data supporting the findings of this study are available within the article and its supplementary information (SI). Supplementary information is available. See DOI: https://doi.org/10.1039/d5gc04148a.CCDC 2457376–2457379 contain the supplementary crystallographic data for this paper.22a–d
Acknowledgements
This work was supported by the National Natural Science Foundation of China (22361051 and U24A20802), the Yunnan Fundamental Research Projects (202301BF070001-022), Yunnan Science and Technology Plan (202449CE340027) and The 2025 Central Government Project for Supporting the Reform and Development of Local Universities – Special Project for the Construction of an Interdisciplinary “Comprehensive Health” Medical-Education Integration Innovation Platform.References
- (a) M. Abou-shoer, K. Suwanborirux, C.-j. Chang and J. M. Cassady, Tetrahedron Lett., 1989, 30, 3385–3388 CrossRef CAS; (b) S. Chakthong, P. Weaaryee, P. Puangphet, W. Mahabusarakam, P. Plodpai, S. P. Voravuthikunchai and A. Kanjana-Opas, Phytochemistry, 2012, 75, 108–113 CrossRef CAS PubMed; (c) N. Ribeiro, F. Thuaud, C. Nebigil and L. Désaubry, Bioorg. Med. Chem., 2012, 20, 1857–1864 CrossRef CAS; (d) J. T. Ndongo, J. N. Mbing, A. Monteillier, M. F. Tala, M. Rütten, D. Mombers, M. Cuendet, D. E. Pegnyemb, B. Dittrich and H. Laatsch, J. Nat. Prod., 2018, 81, 1193–1202 CrossRef CAS PubMed; (e) B. Qin, Y. Wang, X. Wang and Y. Jia, Org. Chem. Front., 2021, 8, 369–383 RSC; (f) Q. Jin, Y.-L. Zhao, Y.-P. Liu, R.-S. Zhang, P.-F. Zhu, L.-Q. Zhao, X.-J. Qin and X.-D. Luo, J. Ethnopharmacol., 2022, 285, 114848 CrossRef CAS PubMed; (g) J. G. M. Eng, M. Shahsavarani, D. P. Smith, J. Hájíček, V. De Luca and Y. Qu, Nat. Commun., 2022, 13, 3335 CrossRef CAS.
- (a) S. Y. Han, J. E. Sweeney, E. S. Bachman, E. J. Schweiger, G. Forloni, J. T. Coyle, B. M. Davis and M. M. Joullié, Eur. J. Med. Chem., 1992, 27, 673–687 CrossRef CAS; (b) K. Soyoung, A. S. Angela, M. S. Steven and A. D. Kinghorn, Anti-Cancer Agents Med. Chem., 2006, 6, 319–345 CrossRef; (c) Z. Xu, Q. Wang and J. Zhu, J. Am. Chem. Soc., 2015, 137, 6712–6724 CrossRef CAS; (d) P. Dhyani, C. Quispe, E. Sharma, A. Bahukhandi, P. Sati, D. C. Attri, A. Szopa, J. Sharifi-Rad, A. O. Docea, I. Mardare, D. Calina and W. C. Cho, Cancer Cell Int., 2022, 22, 206 CrossRef CAS PubMed.
- (a) J. E. Spangler and H. M. L. Davies, J. Am. Chem. Soc., 2013, 135, 6802–6805 CrossRef CAS PubMed; (b) G.-J. Mei, W. L. Koay, C. X. A. Tan and Y. Lu, Chem. Soc. Rev., 2021, 50, 5985–6012 RSC.
- (a) P. A. Cockrum, S. M. Colegate, J. A. Edgar, K. Flower, D. Gardner and R. I. Willing, Phytochemistry, 1999, 51, 153–157 CrossRef; (b) H. R. Arias, M. O. Ortells, D. Feuerbach, V. Burgos and C. Paz, J. Nat. Prod., 2019, 82, 1953–1960 CrossRef CAS.
- (a) I. Kim, H.-K. Na, K. Kim, S. Kim and G. Lee, Synlett, 2008, 2069–2071 CrossRef CAS; (b) V. Sridharan, P. Ribelles, V. Estévez, M. Villacampa, M. T. Ramos, P. T. Perumal and J. C. Menéndez, Chem. – Eur. J., 2012, 18, 5056–5063 CrossRef CAS PubMed; (c) C.-Y. Jin, Y. Wang, Y.-Z. Liu, C. Shen and P.-F. Xu, J. Org. Chem., 2012, 77, 11307–11312 CrossRef CAS PubMed; (d) V. R. Batchu, I. Romero-Estudillo, A. Boto and J. Miguélez, Org. Biomol. Chem., 2014, 12, 9547–9556 RSC; (e) Y. Tokimizu, S. Oishi, N. Fujii and H. Ohno, Angew. Chem., Int. Ed., 2015, 54, 7862–7866 CrossRef CAS; (f) S. A. Morris, T. H. Nguyen and N. Zheng, Adv. Synth. Catal., 2015, 357, 2311–2316 CrossRef CAS PubMed; (g) P. Jia, Q. Zhang, Q. Ou and Y. Huang, Org. Lett., 2017, 19, 4664–4667 CrossRef CAS; (h) E. Y. Schmidt, N. V. Semenova, I. A. Ushakov, A. V. Vashchenko and B. A. Trofimov, Org. Lett., 2021, 23, 4743–4748 CrossRef CAS; (i) A. V. Aksenov, E. V. Aleksandrova, D. A. Aksenov, A. A. Aksenova, N. A. Aksenov, M. A. Nobi and M. Rubin, J. Org. Chem., 2022, 87, 1434–1444 CrossRef CAS.
- (a) K. D. Moeller, Chem. Rev., 2018, 118, 4817–4833 CrossRef CAS; (b) L. F. T. Novaes, J. Liu, Y. Shen, L. Lu, J. M. Meinhardt and S. Lin, Chem. Soc. Rev., 2021, 50, 7941–8002 RSC; (c) W. Zeng, Y. Wang, C. Peng and Y. Qiu, Chem. Soc. Rev., 2025, 54, 4468–4501 RSC.
- R. K. B. Karlsson and A. Cornell, Chem. Rev., 2016, 116, 2982–3028 CrossRef CAS.
- (a) Q.-L. Yang, Y.-Q. Li, C. Ma, P. Fang, X.-J. Zhang and T.-S. Mei, J. Am. Chem. Soc., 2017, 139, 3293–3298 CrossRef CAS PubMed; (b) X. Gao, P. Wang, L. Zeng, S. Tang and A. Lei, J. Am. Chem. Soc., 2018, 140, 4195–4199 CrossRef CAS PubMed; (c) Y. Qiu, M. Stangier, T. H. Meyer, J. C. A. Oliveira and L. Ackermann, Angew. Chem., Int. Ed., 2018, 57, 14179–14183 CrossRef CAS; (d) Y. Liang, F. Lin, Y. Adeli, R. Jin and N. Jiao, Angew. Chem., Int. Ed., 2019, 58, 4566–4570 CrossRef CAS PubMed; (e) Z.-J. Wu, F. Su, W. Lin, J. Song, T.-B. Wen, H.-J. Zhang and H.-C. Xu, Angew. Chem., Int. Ed., 2019, 58, 16770–16774 CrossRef CAS; (f) K.-J. Jiao, Y.-K. Xing, Q.-L. Yang, H. Qiu and T.-S. Mei, Acc. Chem. Res., 2020, 53, 300–310 CrossRef CAS; (g) T. Shen and T. H. Lambert, J. Am. Chem. Soc., 2021, 143, 8597–8602 CrossRef CAS; (h) Y.-K. Xing, X.-R. Chen, Q.-L. Yang, S.-Q. Zhang, H.-M. Guo, X. Hong and T.-S. Mei, Nat. Commun., 2021, 12, 930 CrossRef CAS; (i) Y. Liu, Y. Sun, Y. Deng and Y. Qiu, Angew. Chem., Int. Ed., 2025, 64, e202504459 CrossRef CAS; (j) W. Zeng, C. Peng and Y. Qiu, J. Am. Chem. Soc., 2025, 147, 13461–13470 CrossRef CAS; (k) C. Zhang, H. Tang, X. Zhao, X. Shen and Y. Qiu, J. Am. Chem. Soc., 2025, 147, 23297–23307 CrossRef CAS.
- (a) P. Huang, P. Wang, S. Tang, Z. Fu and A. Lei, Angew. Chem., Int. Ed., 2018, 57, 8115–8119 CrossRef CAS PubMed; (b) Y. Kawamata, J. C. Vantourout, D. P. Hickey, P. Bai, L. Chen, Q. Hou, W. Qiao, K. Barman, M. A. Edwards, A. F. Garrido-Castro, J. N. deGruyter, H. Nakamura, K. Knouse, C. Qin, K. J. Clay, D. Bao, C. Li, J. T. Starr, C. Garcia-Irizarry, N. Sach, H. S. White, M. Neurock, S. D. Minteer and P. S. Baran, J. Am. Chem. Soc., 2019, 141, 6392–6402 CrossRef CAS PubMed; (c) H. Wang, X. Gao, Z. Lv, T. Abdelilah and A. Lei, Chem. Rev., 2019, 119, 6769–6787 CrossRef CAS; (d) K. E. Poremba, S. E. Dibrell and S. E. Reisman, ACS Catal., 2020, 10, 8237–8246 CrossRef CAS; (e) J. L. Röckl, D. Pollok, R. Franke and S. R. Waldvogel, Acc. Chem. Res., 2020, 53, 45–61 CrossRef; (f) J. L. Röckl, D. Schollmeyer, R. Franke and S. R. Waldvogel, Angew. Chem., Int. Ed., 2020, 59, 315–319 CrossRef; (g) Z. Li, W. Sun, X. Wang, L. Li, Y. Zhang and C. Li, J. Am. Chem. Soc., 2021, 143, 3536–3543 CrossRef CAS PubMed; (h) Y. Yuan, J. Yang and A. Lei, Chem. Soc. Rev., 2021, 50, 10058–10086 RSC; (i) H.-J. Zhang, L. Chen, M. S. Oderinde, J. T. Edwards, Y. Kawamata and P. S. Baran, Angew. Chem., Int. Ed., 2021, 60, 20700–20705 CrossRef CAS PubMed; (j) P. Li, Z. Zhu, C. Guo, G. Kou, S. Wang, P. Xie, D. Ma, T. Feng, Y. Wang and Y. Qiu, Nat. Catal., 2024, 7, 412–421 CrossRef CAS; (k) M. C. Lamb, K. A. Steiniger, L. K. Trigoura, J. Wu, G. Kundu, H. Huang and T. H. Lambert, Chem. Rev., 2024, 124, 12264–12304 CrossRef CAS PubMed.
- (a) N. Fu, G. S. Sauer, A. Saha, A. Loo and S. Lin, Science, 2017, 357, 575–579 CrossRef CAS PubMed; (b) K.-Y. Ye, G. Pombar, N. Fu, G. S. Sauer, I. Keresztes and S. Lin, J. Am. Chem. Soc., 2018, 140, 2438–2441 CrossRef CAS PubMed; (c) C.-Y. Cai, X.-M. Shu and H.-C. Xu, Nat. Commun., 2019, 10, 4953 CrossRef CAS; (d) L. Song, N. Fu, B. G. Ernst, W. H. Lee, M. O. Frederick, R. A. DiStasio and S. Lin, Nat. Chem., 2020, 12, 747–754 CrossRef CAS PubMed; (e) H. Huang and T. H. Lambert, J. Am. Chem. Soc., 2021, 143, 7247–7252 CrossRef CAS PubMed; (f) W. Yu, S. Wang, M. He, Z. Jiang, Y. Yu, J. Lan, J. Luo, P. Wang, X. Qi, T. Wang and A. Lei, Angew. Chem., Int. Ed., 2023, 62, e202219166 CrossRef CAS; (g) M. Liu, T. Feng, Y. Wang, G. Kou, Q. Wang, Q. Wang and Y. Qiu, Nat. Commun., 2023, 14, 6467 CrossRef CAS; (h) S. Liu, H. Lin, T. Peng, Z. Yang, P. Wan, J. Li, L. Yang, X. Dai, S. Tu, X. Long, A. Lei, T. Wang and H. Yi, Angew. Chem., Int. Ed., 2025, 64, e202501424 CrossRef CAS; (i) M. Lu, K. Chen, T. Wu and H. Cai, Angew. Chem., Int. Ed., 2025, 64, e202506639 CrossRef CAS.
- (a) J. Xiang, M. Shang, Y. Kawamata, H. Lundberg, S. H. Reisberg, M. Chen, P. Mykhailiuk, G. Beutner, M. R. Collins, A. Davies, M. Del Bel, G. M. Gallego, J. E. Spangler, J. Starr, S. Yang, D. G. Blackmond and P. S. Baran, Nature, 2019, 573, 398–402 CrossRef CAS PubMed; (b) L. Li, Y. Li, N. Fu, L. Zhang and S. Luo, Angew. Chem., Int. Ed., 2020, 59, 14347–14351 CrossRef CAS; (c) T. Shen and T. H. Lambert, Science, 2021, 371, 620–626 CrossRef CAS PubMed; (d) Q. Wang, X. Wang, Y. Liu, J. Zhang, J. Song and C. Guo, J. Am. Chem. Soc., 2025, 147, 8917–8927 CrossRef CAS.
- A. Wiebe, T. Gieshoff, S. Möhle, E. Rodrigo, M. Zirbes and S. R. Waldvogel, Angew. Chem., Int. Ed., 2018, 57, 5594–5619 CrossRef CAS.
- (a) C. Zhu, H. Yue, P. Nikolaienko and M. Rueping, CCS Chem., 2020, 2, 179–190 CrossRef CAS; (b) X. Dong, J. L. Roeckl, S. R. Waldvogel and B. Morandi, Science, 2021, 371, 507–514 CrossRef CAS; (c) J. Strehl, M. L. Abraham and G. Hilt, Angew. Chem., Int. Ed., 2021, 60, 9996–10000 CrossRef CAS PubMed; (d) W. Zhang, N. Hong, L. Song and N. Fu, Chem. Rec., 2021, 21, 2574–2584 CrossRef CAS; (e) N. Sbei, T. Hardwick and N. Ahmed, ACS Sustainable Chem. Eng., 2021, 9, 6148–6169 CrossRef CAS; (f) C. Ma, P. Fang, D. Liu, K.-J. Jiao, P.-S. Gao, H. Qiu and T.-S. Mei, Chem. Sci., 2021, 12, 12866–12873 RSC; (g) S. Zhang, L. Li, J. Li, J. Shi, K. Xu, W. Gao, L. Zong, G. Li and M. Findlater, Angew. Chem., Int. Ed., 2021, 60, 7275–7282 CrossRef CAS; (h) S. Zhang and M. Findlater, Chem. – Eur. J., 2022, 28, e202201152 CrossRef CAS; (i) F. Lian, K. Xu and C. Zeng, Sci. China: Chem., 2023, 66, 540–547 CrossRef CAS; (j) K.-J. Jiao, X.-T. Gao, C. Ma, P. Fang and T.-S. Mei, Isr. J. Chem., 2024, 64, e202300085 CrossRef.
- H.-B. Zhao, P. Xu, J. Song and H.-C. Xu, Angew. Chem., Int. Ed., 2018, 57, 15153–15156 CrossRef CAS PubMed.
- (a) H. Ding, P. L. DeRoy, C. Perreault, A. Larivée, A. Siddiqui, C. G. Caldwell, S. Harran and P. G. Harran, Angew. Chem., Int. Ed., 2015, 54, 4818–4822 CrossRef CAS; (b) K. Liu, S. Tang, P. Huang and A. Lei, Nat. Commun., 2017, 8, 775 CrossRef; (c) K. Liu, W. Song, Y. Deng, H. Yang, C. Song, T. Abdelilah, S. Wang, H. Cong, S. Tang and A. Lei, Nat. Commun., 2020, 11, 3 CrossRef PubMed; (d) Q.-H. Huang, S.-X. Li, J.-C. Kang, R.-X. Liu, Z.-H. Li, F. Xiong, T.-M. Ding and S.-Y. Zhang, Org. Lett., 2024, 26, 5657–5663 CrossRef CAS PubMed.
- X. Liu, D. Yang, Z. Liu, Y. Wang, Y. Liu, S. Wang, P. Wang, H. Cong, Y.-H. Chen, L. Lu, X. Qi, H. Yi and A. Lei, J. Am. Chem. Soc., 2023, 145, 3175–3186 CrossRef CAS PubMed.
- (a) J. Fu, G. Zanoni, E. A. Anderson and X. Bi, Chem. Soc. Rev., 2017, 46, 7208–7228 RSC; (b) E. López and L. A. López, Angew. Chem., Int. Ed., 2017, 56, 5121–5124 CrossRef; (c) X. Huang, X. Li, X. Xie, K. Harms, R. Riedel and E. Meggers, Nat. Commun., 2017, 8, 2245 CrossRef PubMed; (d) V. Kanchupalli and S. Katukojvala, Angew. Chem., Int. Ed., 2018, 57, 5433–5437 CrossRef CAS PubMed; (e) W.-L. Lei, K.-W. Feng, T. Wang, L.-Z. Wu and Q. Liu, Org. Lett., 2018, 20, 7220–7224 CrossRef CAS PubMed; (f) F. Gholami, F. Yousefnejad, B. Larijani and M. Mahdavi, RSC Adv., 2023, 13, 990–1018 RSC; (g) B. Saxena, R. I. Patel, J. Tripathi and A. Sharma, Org. Biomol. Chem., 2023, 21, 4723–4743 RSC; (h) Z. Lin, H. Ren, X. Lin, X. Yu and J. Zheng, J. Am. Chem. Soc., 2024, 146, 18565–18575 CrossRef CAS PubMed; (i) S. Malo, S. Santra, J. Saha, D. Ghosh and I. Das, Chem. Commun., 2024, 60, 12545–12548 RSC; (j) H. Ren, Z. Lin, T. Li, Z. Li, X. Yu and J. Zheng, ACS Catal., 2025, 15, 4634–4643 CrossRef CAS.
- P.-F. Zhong, H.-M. Lin, L.-W. Wang, Z.-Y. Mo, X.-J. Meng, H.-T. Tang and Y.-M. Pan, Green Chem., 2020, 22, 6334–6339 RSC.
- L. Nie, J. Yang, Z. Liu, S. Zhou, S. Chen, X. Qi, A. Lei and H. Yi, J. Am. Chem. Soc., 2024, 146, 31330–31338 CrossRef CAS PubMed.
- (a) A. Beillard, X. Bantreil, T.-X. Métro, J. Martinez and F. Lamaty, Green Chem., 2018, 20, 964–968 RSC; (b) B. Dam, A. K. Sahoo and B. K. Patel, Green Chem., 2022, 24, 7122–7130 RSC; (c) B. Saxena, R. I. Patel, S. Sharma and A. Sharma, Green Chem., 2024, 26, 2721–2729 RSC; (d) Y. Zhang, J. Mao, Z. Wang, L. Tang and Z. Fan, Green Chem., 2024, 26, 9371–9377 RSC; (e) R. I. Patel, B. Saxena and A. Sharma, Green Chem., 2024, 26, 10265–10274 RSC; (f) Z. Ye, Y. Qian, H. Liu, Q. Yin, C. Lv and F. Zhang, Green Chem., 2025, 27, 566–572 RSC.
- (a) J. Wu, Y. Dou, R. Guillot, C. Kouklovsky and G. Vincent, J. Am. Chem. Soc., 2019, 141, 2832–2837 CrossRef CAS PubMed; (b) C. Song, K. Liu, X. Jiang, X. Dong, Y. Weng, C.-W. Chiang and A. Lei, Angew. Chem., Int. Ed., 2020, 59, 7193–7197 CrossRef CAS PubMed; (c) X.-S. Zhou, Z. Zhang, W.-Y. Qu, X.-P. Liu, W.-J. Xiao, M. Jiang and J.-R. Chen, J. Am. Chem. Soc., 2023, 145, 12233–12243 CrossRef CAS PubMed; (d) J. Zi, H. Tang, D. Wang, M. Li, Y. Zhou, S. Lv, D. Liang and L. Shi, Green Chem., 2024, 26, 10397–10403 RSC.
- (a) CCDC 2457376: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2nh36h; (b) CCDC 2457377: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2nh37j; (c) CCDC 2457378: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2nh38k; (d) CCDC 2457379: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2nh39l.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2026 |