Open Access Article
Open Access ArticleOmega-3 PUFAs reduce inflammation by targeting NRF2 and NF-κB activity in an ex vivo model of cardiac mature adipocytes and adipose derived stem cells from atherosclerotic patients
Stefano Quarta a,
Giuseppe Santarpinobcd,
Nadia Calabrisoa,
Maria Annunziata Carluccioa,
Laura Siracusa
a,
Giuseppe Santarpinobcd,
Nadia Calabrisoa,
Maria Annunziata Carluccioa,
Laura Siracusa e,
Tonia Stranoe,
Francesco Cardettac,
Luisa Siculellaf,
Fabrizio Damianof,
Raffaele De Caterina*g and
Marika Massaro
e,
Tonia Stranoe,
Francesco Cardettac,
Luisa Siculellaf,
Fabrizio Damianof,
Raffaele De Caterina*g and
Marika Massaro *a
*a
aInstitute of Clinical Physiology (IFC), National Research Council (CNR), 73100 Lecce, Italy. E-mail: stefanoquarta@cnr.it; mariaannunziata.carluccio@cnr.it; nadia.calabriso@cnr.it; marika.massaro@cnr.it
bDepartment of Clinical and Experimental Medicine, Magna Graecia University of Catanzaro, Italy. E-mail: santarpino@unicz.it
cDepartment of Cardiac Surgery, Città di Lecce Hospital, GVM Care&Research, Lecce, Italy. E-mail: f.cardetta@unicampus.it
dDepartment of Cardiac Surgery, Paracelsus Medical University, Nuremberg, Germany
eInstitute of Biomolecular Chemistry (ICB), National Research Council (CNR), Catania section, Via Paolo Gaifami 18, 95126 Catania, Italy. E-mail: laura.siracusa@icb.cnr.it; tonia.strano@cnr.it
fDepartment of Experimental Medicine (DiMeS), University of Salento, 73100, Lecce, Italy. E-mail: fabrizio.damiano@unisalento.it; luisa.siculella@unisalento.it
gCardiology Division, Pisa University, Hospital, 56126 Pisa, Italy. E-mail: raffaele.decaterina@unipi.it
First published on 7th January 2026
Abstract
Under proatherogenic conditions, epicardial (EAT) and pericardial adipose tissue (PAT) acquire inflammatory/pro-atherogenic phenotypes that contribute to coronary atherosclerosis. Recent data have highlighted a significant inverse relationship between levels of n-3 polyunsaturated fatty acids (PUFAs) eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) in adipose tissue and risk of myocardial infarction. Our study aimed at investigating whether DHA/EPA supplementation of cardiac fat adipocytes attenuates cardiac adipose tissue inflammation. To this aim mature adipocytes and adipose stem cells were isolated from PAT samples collected from coronary artery disease (CAD) patients undergoing coronary artery bypass grafting, exposed to DHA/EPA ex vivo, and evaluated for pro-inflammatory gene expression and activity. PAT adipocytes and stem cell exposure to DHA led to a significant increase in the membrane ratio of omega-3 to omega-6 PUFAs and decreased mRNA expression levels of monocyte chemoattractant protein (MCP)-1, interleukin(IL)-6, matrix metalloproteinase(MMP)-9 and CXC motif chemokine ligand (CXCL)10 (p < 0.05). This downregulation was accompanied by increased expression of uncoupling proteins (UCP)1 and 2 and heme-oxygenase (HO)-1 and of the anti-inflammatory and pro-resolving lipid mediator resolvin D1. Mechanistically, this protective modulation appears to be driven by the upregulation of peroxisome proliferator-activated receptor gamma (PPAR)-γ and nuclear factor erythroid 2-related factor (NRF)2, leading to increased NRF2 activity and suppressed NF-κB signaling. Functionally, supernatants from DHA-conditioned adipocytes exhibited reduced monocyte-attracting activity in chemotaxis assays. While EPA conditioning produced effects similar to DHA, arachidonic acid (AA) showed no significant biological effects. In conclusion, DHA and EPA mitigated the PAT inflammatory profile, highlighting the potential therapeutic role of such PUFAs in reducing cardiac adipose tissue inflammation. These results may have implications for treatment of CAD patients.
Introduction
Adipose tissue is now recognized as a complex endocrine organ that plays a critical role in regulating both local and systemic metabolic homeostasis, and also contributing to metabolic dysfunction.1 This is particularly evident in the cardiac fat depots, epicardial (EAT) and pericardial (PAT) adipose tissues, subtypes of visceral fat that are putatively involved in the pathogenesis of coronary artery disease (CAD) by promoting the development of vulnerable, non-calcified, high-risk plaques.2 This pathogenic connection arises from a substantial reprogramming of gene expression in hypertrophic cardiac adipocytes. Studies using microarray analysis and assessments of mRNA and protein dysregulation have indeed shown that both EAT3 and PAT4 exhibit a pro-atherogenic transcriptional profile in CAD patients. In these individuals, genes encoding various pro-inflammatory cytokines including interleukin(IL)-6, monocyte chemoattractant protein(MCP)-1, and chemokine ligands and receptors, are consistently upregulated in EAT3 and are associated with signs of accelerated transdifferentiation from brown to white fat, as evidenced by a significant decrease in the expression of brown fat-like genes.5 This whitening process has been associated with increased production of reactive oxygen species (ROS)6 and is accompanied by increased activation of the pro-inflammatory transcription factor Nuclear Factor(NF)-κB.7 It has been hypothesized that the whitening of EAT, along with its associated dysfunction, may contribute to the development of a pro-inflammatory microenvironment that disrupts vascular homeostasis. This inflammatory milieu could perpetuate endothelial cell dysfunction, promote the recruitment and activation of monocytes and macrophages, and ultimately increase susceptibility to coronary atherosclerosis.2 The recognition of EAT and PAT as a novel cardiovascular risk factor has thus increased the interest in strategies that may target cardiac adipose tissue expansion and inflammation.Multiple studies have demonstrated that EAT and PAT volumes can be modified through pharmaceutical8 and dietary interventions,9 with potential accompanying changes in underlying adipose gene expression. Midway between a pharmacologic and a dietary therapeutic strategy, supplementation with n-3 polyunsaturated fatty acids (n-3 PUFAs) has been shown to decrease EAT volume in CAD patients,10 in addition to improving various dysmetabolic conditions associated with obesity in both animals and humans.11 Moreover, a higher adipose n3/n6 ratio has recently been shown to predict a reduced occurrence of myocardial infarction.12 Overall, these data suggest that n-3 PUFAs can enhance adipose tissue function and reduce cardiovascular risk, underscoring the need to explore their effects on cardiac fat dysfunction. The aim of this study was to determine whether n-3 PUFAs can ameliorate the pro-inflammatory and dysmetabolic features that characterize hypertrophic cardiac adipocytes and adipose-derived stem cells (ASCs) in patients with coronary atherosclerosis requiring aorto-coronary bypass surgery. Demonstrating whether such protective effects are mediated by beneficial conditioning of EAT and PAT physiology in vivo remains challenging, largely because of the limited availability of reliable experimental models. However, we have recently developed a functional model of human adipocytes derived from PAT collected from coronary patients.13 Using this ex vivo cell model, we investigated whether - and by which mechanisms - the n-3 PUFAs docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) modulate pro-inflammatory gene expression in cardiac adipose tissue. We also examined their influence on functional interactions between adipocytes and monocytes/macrophages, which represent key cellular contributors to atherogenesis.
Material and methods
Chemicals
DHA (22:6 n-3 all cis), EPA (20:5 n-3 all cis) and arachidonic acid (AA) (20:4 n-6, all cis), were obtained as >99% pure sodium salts from Merk Life Science (Milan, Italy). Type 2 collagenase was obtained from Worthington Biochemicals (Lakewood, NJ, USA). Nile red and ActinGreen 488, the latter a selective F-actin probe conjugated with the dye Alexa Fluor 488, are from Invitrogen (distributed by Thermo Fisher Scientific, Waltham, MA, USA). ML385 was obtained from Cayman Chemical Company (Ann Arbor, MI, USA). Unless otherwise indicated, all other reagents were purchased from Merk Life Science.Patient characteristics and PAT sample collection procedures
A total of 12 patients undergoing coronary artery bypass graft surgery (CABG) at Città di Lecce Hospital, Lecce, Italy, were included in the study. The study was conducted in accordance with the ethical guidelines of the Declaration of Helsinki14 and was approved by the Ethics Committee of Lecce (Italy, N. 21, 02072018). All patients gave written informed consent before surgery. The characteristics of the patients are listed in Table 1.| Data are presented as mean ± SD or number of participants (%). BMI, body mass index. | |
|---|---|
| Number of total patients | 12 |
| Age, years | 69.7 ± 5.1 |
| Sex (male %) | 100 |
| Weight, Kg | 82.2 ± 23.8 |
| BMI | 27.6 ± 5.3 |
| Hypertension | 9 (75%) |
| Type 1 diabetes (T1D) | 3 (25%) |
| Type 2 diabetes (T2D) | 3 (25%) |
| Previous stroke or heart attack | 3 (25%) |
Adipocyte cell culture and experimental design
Sodium salt fatty acids were prepared as stock solutions in pyrogen-free distilled water (Merck Life Science) and stored at −80 °C until use in subsequent cell treatment assays. After tissue collection, adipocytes were immediately isolated as previously described13 and incubated for 48 hours with 50 µmol L−1 DHA, EPA or AA in DMEM/F12 supplemented with 10% fetal bovine serum (FBS), 100 mg ml−1 streptomycin and 100 IU ml−1 penicillin. Under these culture conditions, the albumin content of FBS is estimated to be approximately 2.5 g dL−1,15 a concentration sufficient to facilitate PUFA uptake by the cells.16 In selected experiments ML385, at a concentration of 5 µmol L−1, was added for 24 hours before DHA treatment. Following the incubation period, the cells were transferred to sterile tubes and centrifuged at 25 °C for 5 minutes at low speed. The medium below the adipocyte layer was carefully aspirated using a sterile glass Pasteur pipette and used as Conditioned Medium (CM) for the evaluation of chemokine release and chemotaxis assay. The remaining oil cell layers corresponding to PAT adipocytes were then washed with warm PBS before undergoing further analysis. Adipose-derived stem cells (ASCs) were isolated and characterized as previously described13 and expanded until passage 1. Confluent ASCs were then induced to differentiate into mature adipocytes (mAd-ASCs) by exposure to a differentiation medium containing 10% fetal bovine serum (FBS), 100 mg ml−1 streptomycin, 100 IU ml−1 penicillin, 500 µmol L−1 3-isobutyl-1-methylxanthine, 250 nmol L−1 dexamethasone, 1 µmol L−1 rosiglitazone, 5 µg mL−1 insulin for approximately 14 days with medium changes every 3 days.Evaluation of cell viability and cell fluorescent imaging
Cell viability was determined using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltrazolium bromide (MTT) assay, which is based on the ability of viable cells to convert MTT, a soluble tetrazolium salt, into an insoluble formazan precipitate. Briefly, both untreated and treated adipocytes were incubated with MTT solution (at a final concentration of 0.5 mg mL−1) for 2 hours. After incubation sample images were visualized and captured with a stereomicroscope (Nikon, Minato, Tokyo, Japan) equipped with the Nikon NIS-Elements D at 20× magnification. The formazan products were then dissolved with isopropanol, and the absorbance measured at 595 nm using a microplate reader. Total cell protein content was determined using the Lowry method with protein assay reagents from Bio-Rad (Bio-Rad, Laboratories, Segrate, Italy). For the evaluation of cell architecture and lipid content, PAT adipocytes were isolated and made adherent to the bottom of the culture flask by the ceiling technique as previously described.13 After exposing the cells to DHA for 48 hours, lipid distribution was determined by incubating the cells with a 300 nmol L−1 Nile red solution for 30 minutes. The cells were then gently washed and fixed with a 4% formaldehyde solution in PBS for 15 minutes. After washing, the cells were permeabilised with a 0.1% Triton X-100 solution in PBS for 5 minutes and treated with ActinGreen 488 according to the manufacturer's instructions. After additional washing, cell nuclei were stained with DAPI, and images were visualised and captured using an EVOS Floid fluorescence microscope (Thermo Fisher Scientific). Fluorescence from the Nile red staining was quantitatively measured using the Cytation 5 multi-mode microplate reader (BioTek Instruments, Inc., Winooski, VT, USA). For each treatment, 3 photographs of different areas were taken and cell were then analyzed in terms of dimension using NIH ImageJ software.RNA isolation and real-time quantitative polymerase chain reaction
Total RNA was isolated using TRIzol reagent (Thermo Fisher Scientific) according to the manufacturer's protocol and hence retrotranscribed using the High-Capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific) on a GeneAmp PCR System 9700 (Thermo Fisher Scientific) under the following conditions: 10 min at 25 °C, 120 min at 37 °C, and 5 min at 85 °C. Real-time PCR (qPCR) analyses were performed using the CFX96 Touch Real-Time PCR Detection System and software (Bio-Rad, Hercules, CA, USA). All reactions were carried out in a total volume of 25 μL with 50 ng cDNA, 0.3 pmol L−1 of primer pair, and 12.5 μL 2 × SYBR Green PCR master mix (Bio-Rad) under the following conditions: 2 min at 50 °C, 10 min at 95 °C, and 40 cycles of 15 s at 95 °C and 1 min at 60 °C. Reactions were executed in duplicate on three independent sets of RNA. Negative controls (without RNA addition) were processed under the same conditions as the experimental samples. The quantifications were performed using the comparative critical threshold method (ΔΔCT), and β-actin gene was used as internal control for normalization. The primers used for real-time PCR analysis were listed in Table 2.| Gene symbol | Full name | Forward primer sequence | Revers primer sequence | Accession number |
|---|---|---|---|---|
| APN | Adiponectin | AGTCTCACATCTGGTTGGGG | CTCTCTGTGCCTCTGGTTCC | NM_001177800.1 |
| PPAR-γ | Peroxisome proliferator-activated receptor γ | TGCAGGTGATCAAGAAGACG | AGTGCAACTGGAAGAAGGGA | NM_005037.5 |
| MMP-9 | Matrix metalloproteinase-9 | TTGACAGCGACAAGAAGTGG | GCCATTCACGTCGTCCTTAT | NM_004994.2 |
| COX-2 | Cyclooxygenase-2 | TGCTGTGGAGCTGTATCCTG | GAAACCCACTTCTCCACCA | NM_000963.2 |
| MCP-1 | Monocyte chemoattractant protein-1 | CCCCAGTCACCTGCTGTTAT | TCCTGAACCCACTTCTGCTT | NM_002982.3 |
| IL-6 | Interleukin-6 | AGGAGACTTGCCTGGTGAAA | CAGGGGTGGTTATTGCATCT | NM_000600.5 |
| CXCL-10 | C–X–C Motif chemokine ligand-10 | CAAGGATGGACCACACAGAG | GCAGGGTCAGAACATCCACT | NM_001565.2 |
| UCP-1 | Uncoupling protein-1 | TCTCTCAGGATCGGCCTCTA | CCGTGTAGCGAGGTTTGATT | NM_021833.5 |
| UCP-2 | Uncoupling protein-2 | AGCCCACGGATGTGGTAAAG | CTCTCGGGCAATGGTCTTGT | NM_003355.3 |
| NRF2 | Nuclear factor erythroid 2-related factor 2 | GCGACGGAAAGAGTATGAGC | GTTGGCAGATCCACTGGTTT | NM_006164.5 |
| HO-1 | Heme-oxygenase-1 | CTTCTTCACCTTCCCCAACA | CCTGCAACTCCTCAAAGAGC | NM: X14782.2 |
| Rel A | Nuclear factor -κB p65 | CCTGGAGCAGGCTATCAGTC | ATCTTGAGCTCGGCAGTGTT | NM_021975 |
| ATGL | Adipose triglyceride lipase | CTGACCACCCCAACAT | TCACCAGGTACAGATG | NM_020376 |
| SREBP-1 | Sterol regulatory element-binding transcription factor 1 | ACACCATGGGGAAGCACAC | CTTCACTCTCAATGCGCC | NM_004176 |
| DGAT-1 | Diacylglycerol O-acyltransferase 1 | GCTTCAGCAACTACCGTGGCAT | CCTTCAGGAACAGAGAAACCACC | NM_012079 |
| DGAT-2 | Diacylglycerol O-acyltransferase 1 | GCTGACCTGGTTCCCATCTA | CAGGTGTCGGAGGAGAAGAG | NM_032564 |
| CD36 | CD36 molecule | AGATGCAGCCTCATTTCCA | GCCTTGGATGGAAGAACAAA | NM_000072 |
| β-Actin | Beta-actin | GATGAGATTGGCATGGCTTT | CACCTTCACCGTTCCAGTTT | NM_001101.3 |
Measurement of MCP-1, IL-6, MMP-9, CXCL10, APN and lipid mediators by ELISA assay
CM-untreated and DHA/EPA-treated PAT adipocytes were collected, and the levels of secreted cytokines were measured using the corresponding ELISA kits (Boster Bio, Pleasanton, CA, USA). The concentrations of resolvin D1 (RvD1) and prostaglandin E2 (PGE2) were assessed using competitive ELISA kits (Cayman), following the manufacturers’ instructions.NF-κB and NRF2 activation
PAT adipocytes nuclear protein purification was carried out employing the Active Motif Nuclear extract kit (Active Motif, Carlsbad, CA, USA) according to the manufacturer's protocol. The binding activity of NF-κB and NFR2 was assayed using the pertinent “TransAM kit” (Active Motif) following the manufacturer's protocol.PAT adipocytes fatty acid analysis
Control and DHA-treated PAT adipocytes designated for fatty acid analysis were stored at −80 °C until the assay was performed. On the day of the assay, cells were allowed to reach room temperature before being processed as previously described.13 The cell pellet was brought to room temperature and directly methylated to obtain the corresponding fatty acid methyl esters (FAMEs). A more refined procedure for methylation, based on the official method,17 was implemented in our in-house laboratories and applied here. This strategy is particularly suitable for sensitive matrices susceptible to thermal degradation or degradation due to drastic reaction conditions. Briefly, cell pellets (on the order of 100 mg) were accurately weighed and placed in 8 mL amber vials with screw caps and silicone septum (Agilent Technologies, Milan, Italy). Four mL of n-hexane and 1 mL of a 2N KOH solution in methanol (11.2 g of KOH in 100 mL methanol) were then added, the heterogeneous mixture shortly stirred (2 minutes) at room temperature with a magnetic stirrer and then left decanting for at least 2 hours until complete phase separation. The upper clean phase (hexane fraction) was then taken with a glass pasteur pipette, treated with anhydrous sodium sulfate, filtered through Whatman No. 1 paper, placed in 2 mL GC autosampler vials and sent to analytical determinations. Methylations were performed in duplicate for each sample. FAMEs were analyzed in the fast mode on a Shimadzu gas chromatograph Model 17-A equipped with a flame ionization detector (FID) and an operating software Class VP Chromatography Date System version 4.3 (Shimadzu). Analytical conditions were set as follows: DB-5 MS capillary column (15 m × 0.10 mm × 0.10 mm), helium as carrier gas; injection in split mode (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200), injected volume 1 µL, injector and detector temperature 250 and 280 °C, respectively. Linear velocity in column 51 cm sec−1. The oven temperature was held at 80 °C for one minute, then programmed from 80 to 280 °C at 10 °C min−1 constant for 20 minutes. Percentages of compounds were determined from their peak areas in the GC-FID profiles. For FAMEs’ masses determination, gas-chromatography-mass spectrometry (GC-MS) was carried out in the fast mode on a Shimadzu GC-MS mod. GCMS-QP5050A with operating software GCMS solution version 1.02 (Shimadzu). The ionization voltage was 70 eV, the electron multiplier was set at 1000 V and the transfer line temperature was 280 °C. Analytical conditions: SPB-5 Ms (Supelco) capillary column (15 m × 0.10 mm × 0.10 mm), helium as carrier gas. Injection in split mode (1
200), injected volume 1 µL, injector and detector temperature 250 and 280 °C, respectively. Linear velocity in column 51 cm sec−1. The oven temperature was held at 80 °C for one minute, then programmed from 80 to 280 °C at 10 °C min−1 constant for 20 minutes. Percentages of compounds were determined from their peak areas in the GC-FID profiles. For FAMEs’ masses determination, gas-chromatography-mass spectrometry (GC-MS) was carried out in the fast mode on a Shimadzu GC-MS mod. GCMS-QP5050A with operating software GCMS solution version 1.02 (Shimadzu). The ionization voltage was 70 eV, the electron multiplier was set at 1000 V and the transfer line temperature was 280 °C. Analytical conditions: SPB-5 Ms (Supelco) capillary column (15 m × 0.10 mm × 0.10 mm), helium as carrier gas. Injection in split mode (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 96), injected volume 1 µL, injector and detector temperature 250 and 280 °C, respectively. Constant linear velocity in column 50 cm sec−1. The oven temperature was held at 80 °C for one minute, then programmed from 80 to 280 °C at 10 °C min−1 constant for 20 minutes. Analyses were carried out in triplicate. Gas chromatographic peaks were identified based on matching of their mass spectral data whit those compiled in the NIST MS 107, NIST 21, and NIST 14 libraries and comparison of the fragmentation patterns with those reported in literature.
96), injected volume 1 µL, injector and detector temperature 250 and 280 °C, respectively. Constant linear velocity in column 50 cm sec−1. The oven temperature was held at 80 °C for one minute, then programmed from 80 to 280 °C at 10 °C min−1 constant for 20 minutes. Analyses were carried out in triplicate. Gas chromatographic peaks were identified based on matching of their mass spectral data whit those compiled in the NIST MS 107, NIST 21, and NIST 14 libraries and comparison of the fragmentation patterns with those reported in literature.
In vitro THP-1 chemotaxis assay
PAT adipocytes were treated with DHA for 48 hours or left untreated as a control. After exposure, media were collected under sterile conditions, centrifuged to remove cell debris, and frozen at −20 °C until the chemotaxis assay was performed. THP-1 cell migration was analysed using a Boyden chamber (Corning, purchased through Sigma Aldrich) (see Fig. 5B for a schematic representation of the assay), with the upper and lower chambers separated by a polycarbonate membrane with a pore size of 8 µm. THP-1 cells were suspended at a concentration of 2.5 × 106 cells per mL in a chemotaxis buffer consisting of RPMI 1640 with 0.1% BSA and added to the upper chamber, while the adipocyte-conditioned medium was added to the lower chamber. A cell-free culture medium, prepared in the same manner as the experimental samples but without cells, served as the negative control. After 30 minutes of incubation at 37 °C, the upper chambers were removed. The migrated THP-1 cells were determined by adding a solution of 5 mg mL−1 MTT to the lower chamber to reach a final concentration of 0.5 mg mL−1. After 3 hours, the intracellular purple formazan crystals were dissolved and quantified spectrophotometrically. To investigate the role of MCP-1 in the attraction of monocytes, a solution of recombinant MCP-1 was added to the upper chamber, which significantly reduced the migration of monocytes by disrupting the chemotactic gradient. Furthermore, the addition of DHA to PAT adipocyte-conditioned medium derived from untreated cells had no effect, demonstrating that the chemotactic inhibition is dependent on DHA conditioning of the cells themselves.Statistical analysis
The results were expressed as means ± S.D. Student's t-test was used to compare the means between adipocyte culture at different time points post-explantation as well as between the control group and the treated group. A p value of <0.05 was considered as statistically significant.Results
Effect of n-3 PUFAs on cell viability, architecture, and lipid content
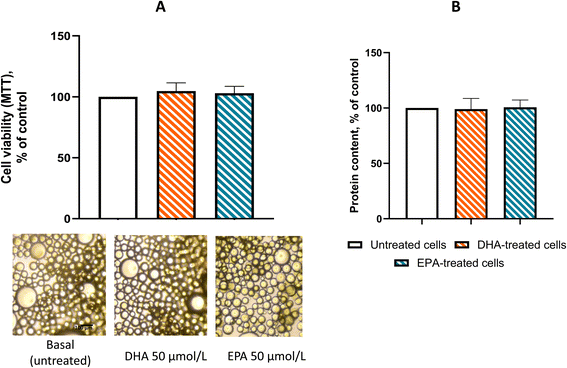
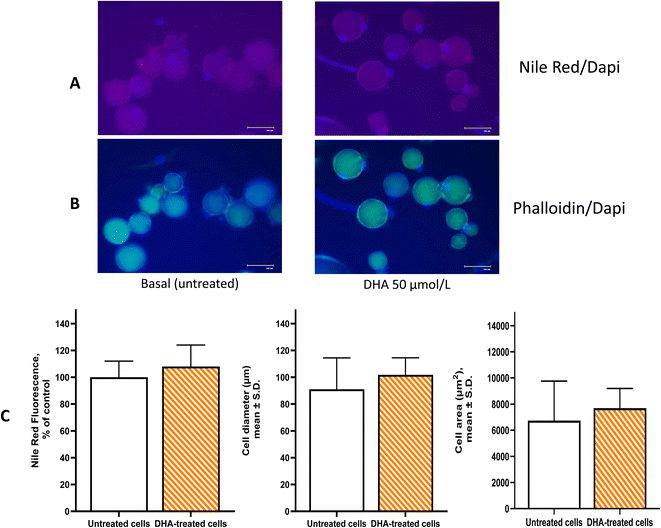
To determine whether DHA and EPA, at concentrations achievable in vivo,18 could cause damage or toxicity to PAT adipocytes, we assessed mitochondrial dehydrogenase activity and total cellular protein content. As shown in Fig. 1A and B, treatment with 50 µmol L−1 DHA or EPA had no impact on cell viability while concentrations of 100 µmol L−1 showed some signs of toxicity (data not shown). At the same concentrations, morphological examination of MTT-loaded cells revealed no significant changes in cell structure (Fig. 1C). At these concentrations, DHA treatment of ASCs and mAd-ASCs had no effect (data not shown).We next investigated whether DHA treatment affects intracellular lipid organization and overall cellular architecture. As shown in Fig. 2A, exposure to 50 µmol L−1 DHA did not alter the pattern of intracellular lipid accumulation, which remained predominantly unilocular in both treated and untreated adipocytes. Structurally, consistent with previous observations in adipocytes from high-fat diet-fed C57BL/6J mice,19 lipid accumulation and the resulting hypertrophy are associated with a redistribution of actin toward the cell cortex. In line with this, phalloidin staining in our samples confirmed the presence of cortical actin enrichment at the cell periphery, a feature that was not modified by DHA treatment (Fig. 2B). Finally, DHA administration did not induce significant changes in total lipid content or cell size (Fig. 2C).
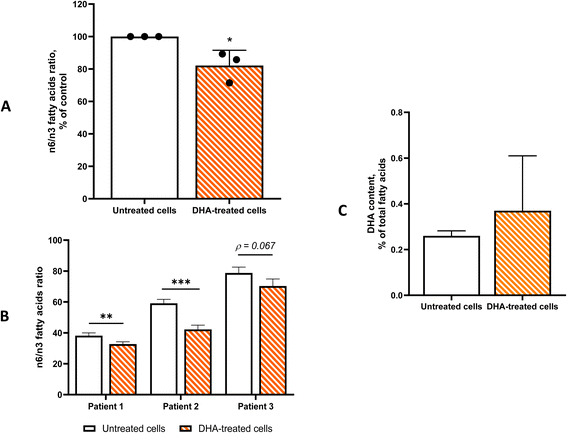
On this basis, all subsequent experiments were conducted using 50 µmol L−1 DHA. Fatty acid profiling revealed no significant alterations in the major fatty acid classes, saturated, monounsaturated, or polyunsaturated fatty acids (data not shown). However, DHA treatment significantly reduced the cellular n-6/n-3 PUFA ratio (Fig. 3A and B). Although an increase in intracellular DHA levels was observed, this change did not reach statistical significance, likely due to variability associated with the high total lipid content of the cells (Fig. 3C).
DHA and EPA modulate inflammatory adipokines and lipid mediators release in cardiac adipocytes from CAD patients
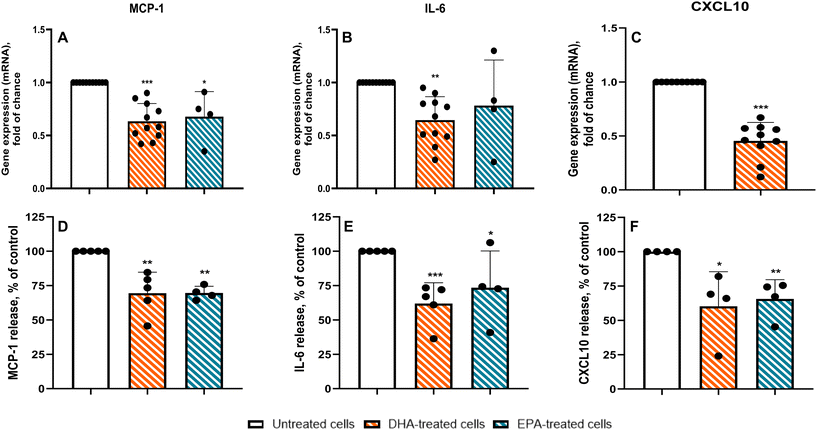
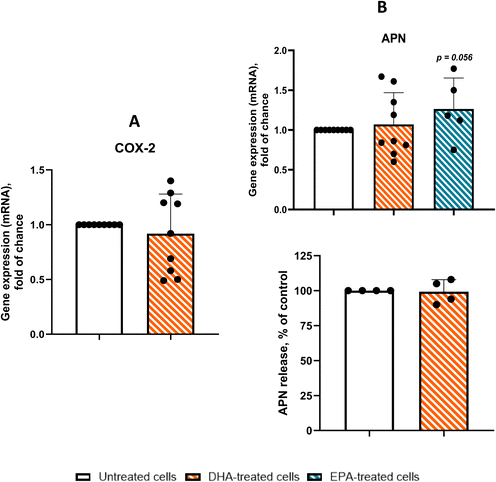
In cardiac adipocytes from CAD patients, we investigated whether n-3 PUFAs modulate inflammatory responses by assessing the expression of MCP-1, IL-6, CXCL10, COX-2, and APN, as well as the release of the lipid mediators PGE2 and RvD1. Fig. 4 summarizes these effects at both the mRNA and protein levels. As shown in Fig. 4A–C (upper panels), DHA and EPA both reduced the expression of the pro-inflammatory genes MCP-1 and IL-6, with DHA consistently showing greater potency. DHA lowered MCP-1 mRNA by approximately 45% and IL-6 mRNA by 40% (p < 0.001 and p < 0.01, respectively), whereas EPA produced reductions of ∼35% and ∼20%. The strongest transcriptional effect was observed for CXCL10, which was reduced by ∼55% following DHA treatment (p < 0.001; Fig. 4C). As expected, exposure to the n-6 PUFA AA did not alter cytokine expression (data not shown). To determine whether these transcriptional effects translated into changes in cytokine secretion, we next measured protein release (Fig. 4A–C, lower panels). DHA again produced robust anti-inflammatory actions, decreasing MCP-1 secretion by ∼30%, IL-6 by ∼40%, and CXCL10 by ∼35%. EPA induced more modest decreases, with reductions of ∼30%, ∼20%, and ∼25% for MCP-1, IL-6, and CXCL10, respectively. DHA treatment did not alter PGE2 release (data not shown), yet it elicited a pronounced and statistically significant increase in RvD1 production relative to controls (15.5 ± 5.2 pg mL−1 vs. 170.4 ± 11.36 pg mL−1, p < 0.001, n = 3), indicating a selective enhancement of pro-resolving mediator synthesis. Together, these data demonstrate that DHA - and, to a lesser extent, EPA - substantially attenuates both the expression and secretion of key inflammatory mediators in cardiac adipocytes from CAD patients.In contrast, as shown in Fig. 5A, COX-2 expression was only slightly reduced following DHA treatment (8%, p = 0.50). However, it is noteworthy that patients clustered into two distinct groups: one group exhibited a significant reduction in COX-2 expression (p < 0.05), while the other showed no appreciable response. Similarly, DHA had no overall effect on APN gene expression or protein secretion (Fig. 5B, upper and lower histograms, respectively). Interestingly, patient responses to DHA treatment were again heterogeneous. One subset of patients showed a significant increase in APN expression (p < 0.05), whereas the remaining patients exhibited no detectable benefit.
These findings indicate that n-3 PUFAs can attenuate the inflammatory response in cardiac adipocytes from CAD patients; however, their effectiveness may vary according to individual responsiveness, leading to differential effects across specific pro-inflammatory molecules.
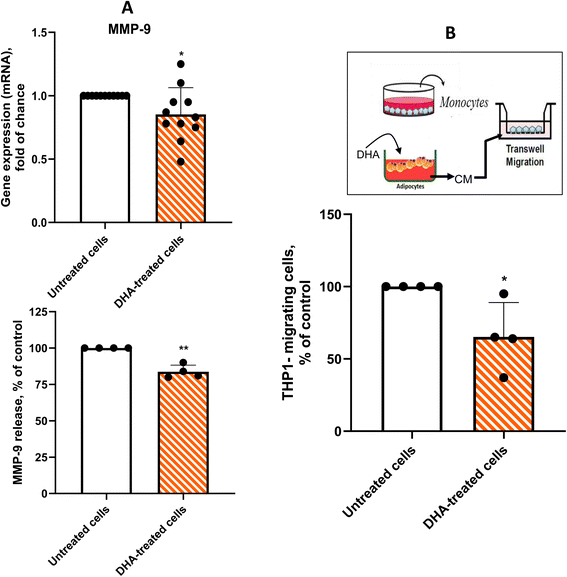
DHA decreases MMP-9 gene expression and monocyte attraction in cardiac adipocytes from CAD patients
MMP-9, which is linked to extracellular matrix remodeling, monocyte chemotaxis, and the rupture of atherosclerotic plaques, is overexpressed in the cardiac fat depots of CAD patients.20 Therefore, we investigated whether DHA could reduce MMP-9 gene expression and protein release in our ex vivo model. We observed a modest but significant reduction of approximately 20% in both MMP-9 gene expression and protein release (p < 0.05) (Fig. 6A).To assess the functional impact of DHA on chemokine overproduction, we evaluated monocyte migration in response to conditioned medium (CM) from PAT adipocyte cultures using a transwell migration assay. CM from untreated adipocytes induced a fourfold increase in monocyte migration compared to cell-free culture medium, which served as the negative control (data not shown). The addition of 10 ng mL−1 human recombinant MCP-1 to the upper chamber reduced monocyte migration by 30% relative to adipocyte CM, highlighting MCP-1's role in monocyte chemotaxis (data not shown). Supporting the anti-inflammatory effect of DHA, treatment of adipocytes with DHA for 48 hours significantly reduced monocyte migration by 35% (p < 0.05), indicating a decreased presence of proinflammatory chemokines following DHA exposure (Fig. 6B). Furthermore, the addition of DHA to PAT adipocyte-conditioned medium derived from untreated cells had no effect, demonstrating that the chemotactic inhibition is dependent on DHA conditioning of the cells themselves.
DHA increases the expression of metabolic genes and key transcription factors in cardiac adipocytes from CAD patients
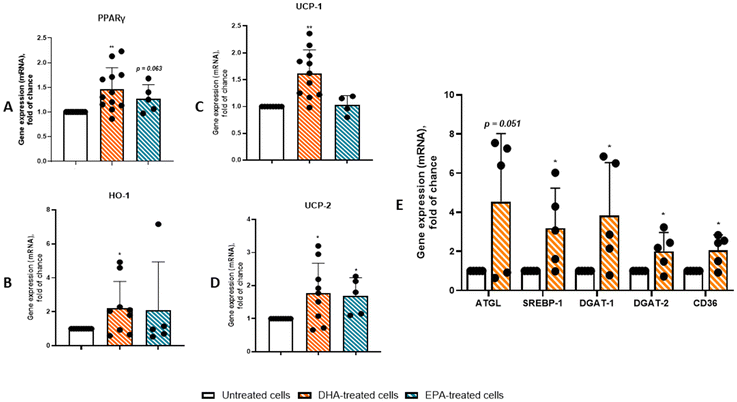
While gene expression profiling analyses of cardiac adipose tissue from CAD patients consistently reveal overexpression of pro-inflammatory genes and pathways, they also indicate potential dysregulation of anti-inflammatory and metabolic genes.4,21,22 As shown in Fig. 7 and 8, DHA treatment significantly increased the expression of PPARγ by 32% (p < 0.05) and NFR2 by 20% (p < 0.05), while the expression of NF-κB p65 (relA) remained unaffected. Conversely, the effects of EPA did not exactly overlap with those of DHA. EPA showed a significant effect in terms of NRF2 induction and only a trend towards upregulation of PPARγ (27%, p = 0.063 and 33%, p < 0.05). Regarding downstream gene expression of PPARγ and NRF2, while DHA induces the UCP-1, UCP-2 and HO-1 gene expression by 42% (p < 0.01), 77% (p < 0.05) and 121% (p < 0.05), respectively (Fig. 7B–D), EPA significantly increased UCP-2 expression (69%, p < 0.05), but had no effect on UCP-1 and HO-1. Additionally, DHA consistently upregulated the expression of a range of genes involved in lipid metabolism, including ATGL, SREBP-1, DGAT-1, DGAT-2, and CD36 by 350%, 220%, 280%, 110%, and 100%, respectively (p < 0.05) (Fig. 7E).DHA suppresses NF-κB and activates NRF2 in PAT adipocytes
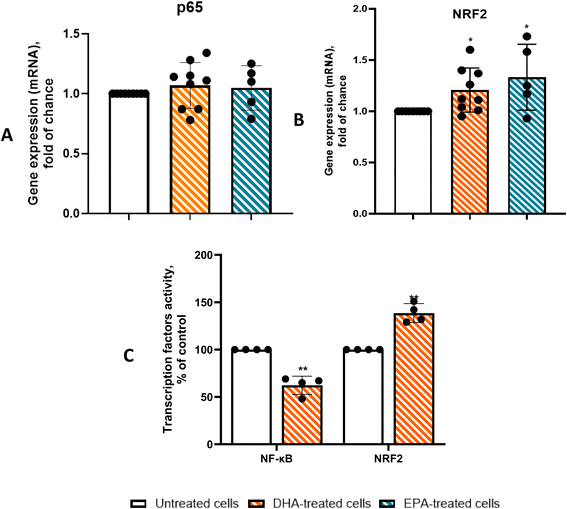
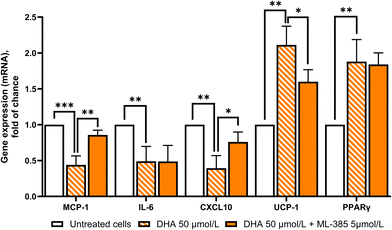
To better understand the mechanism by which DHA reduces inflammation in PAT adipocytes, we examined the expression and activation of NRF2 and NF-κB. In line with the downregulation of MCP-1, IL-6, and CXCL10, genes containing NF-κB binding sequences in their promoters, we observed a significant decrease in NF-κB transactivation activity (Fig. 8C), independent of p65 gene induction (Fig. 8A). At the same time, DHA treatment led to a significant increase in NRF2-binding activity (Fig. 8C), which aligned with both the upregulation of NRF2 gene expression (Fig. 8B) and the increased transcriptional levels of its target gene, HO-1 (Fig. 7D).To investigate the involvement of NRF2 signaling in DHA-induced anti-inflammatory and thermogenic responses, PAT adipocytes from CAD patients were pre-treated with 5 μmol L−1 ML385 (a selective NRF2 inhibitor that blocks NRF2 binding to the antioxidant response element (AREs), preventing the activation of NRF2 target genes) for 24 h prior to DHA exposure. As shown in Fig. 9, DHA markedly reduced the expression of the pro-inflammatory genes MCP-1, IL-6, and CXCL10, lowering their levels by approximately 50–60% relative to untreated cells. Co-treatment with ML385 substantially reverted these effects, restoring gene expression by ∼70–80% toward basal levels (p < 0.01 for MCP-1 and p < 0.05 for CXCL10), indicating that the anti-inflammatory action of DHA is NRF2-dependent for most but not all genes investigated. Conversely, DHA significantly increased UCP-1 expression by roughly 2-fold (p < 0.01), and this induction was almost completely abolished by ML385 co-treatment, indicating strong NRF2 involvement in the thermogenic response. In contrast, the DHA-mediated up-regulation of PPARγ (∼1.7-fold vs. control; P < 0.01) remained unaffected by ML385, suggesting that this transcriptional response occurs independently of NRF2 inhibition.
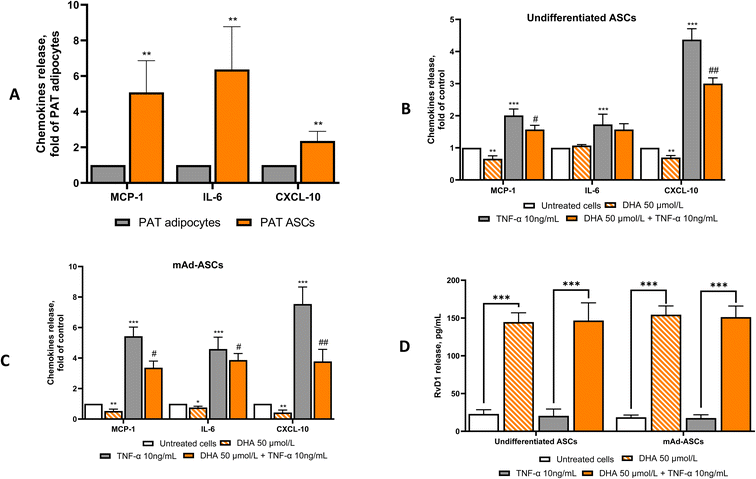
DHA suppresses inflammation in ASCs and mAd-ASCs
ASCs from lean, metabolically healthy individuals display multipotent differentiation capacity, immunosuppressive activity, and directed migration toward sites of injury or inflammation.23,24 In contrast, ASCs from obese individuals develop a metabolically dysfunctional phenotype characterized by increased secretion of pro-inflammatory cytokines such as IL-6, MCP-1, and TNF-α, which activate immune cells and drive macrophage polarization toward a pro-inflammatory state.25–27 To determine whether DHA modulates this inflammatory profile, we treated ASCs in both their undifferentiated state and after adipogenic differentiation. As shown in Fig. 10A, undifferentiated ASCs secreted markedly higher levels of MCP-1 (∼250% of mature adipocytes), IL-6 (∼300%), and CXCL10 (∼150%). DHA treatment significantly reduced the secretion of MCP-1 (55–60%), IL-6 (50–55%), and CXCL10 (40–45%) in both undifferentiated and mAd-ASCs. In parallel, DHA robustly increased the production of RvD1 by approximately 3–4 fold compared with untreated cells (Fig. 10D). These data indicate that DHA exerts consistent anti-inflammatory effects in ASCs regardless of differentiation status, effectively counteracting the pro-inflammatory phenotype associated with metabolically impaired environments. Collectively, these findings indicate that DHA effectively suppresses the pro-inflammatory phenotype of obesity-associated ASCs while promoting the release of anti-inflammatory mediators, highlighting its potential to restore ASC immunomodulatory function.Discussion
Our results highlight the potential of the n-3 PUFAs, DHA and EPA, in mitigating inflammation occurring in the cardiac fat of patients with advanced CAD.This study shows that in mature adipocytes and ASCs isolated from the PAT of patients undergoing CABG, exposure to DHA and EPA modulates the expression of key inflammatory and metabolic genes as well as the expression of transcription factors involved in antioxidant and anti-inflammatory defenses. These changes appear to reduce the attraction of monocytes and potentially limit their infiltration into adipose tissue. To our knowledge, this is the first study to directly examine the protective effects of n-3 PUFAs on cardiac adipocytes and provides mechanistic insights into recent evidence linking higher omega-3/omega-6 lipid ratios to a lower incidence of myocardial infarction.12
In general, PUFAs play essential roles in maintaining cell membrane structure and regulating physiological processes, including signal transduction, cellular metabolism, and tissue homeostasis, thereby contributing to disease prevention.28 They can be obtained directly from the diet or synthesized endogenously through sequential enzymatic reactions involving desaturation, elongation, and peroxisomal β-oxidation.28 The nutritionally essential precursors of n-3 and n-6, respectively α-linolenic acid (C18:3n-3) and linoleic acid (C18:2n-6), are metabolized by Δ6- and Δ5-desaturases and elongases 2 and 5, enzymes whose activity is downregulated by PUFA themselves.29 Although elongation and desaturation enzymes are expressed in many tissues, their contribution to whole-body long-chain PUFA production varies substantially. The liver is considered the primary site of PUFA biosynthesis-including the formation of EPA, DHA, and arachidonic acid (AA)-because it expresses high levels of Δ6- and Δ5-desaturases and elongases and has the capacity to incorporate newly formed fatty acids into lipoproteins for systemic distribution.30,31 Adipose tissue also expresses these enzymes but at lower activity; its main role relates to the storage, remodeling, and local utilization of fatty acids rather than significant de novo long-chain PUFA production.31–33
Experimentally, we observed that exposing pericardial adipocytes to DHA, besides to increase the cell total amount of DHA decreases the omega-6-omega-3 PUFA ratio. This shift is associated with the downregulation of several proinflammatory cytokines and chemokines. Elevated IL-6 mRNA expression in the EAT was found to correlate with increased severity of atherosclerosis, suggesting that high IL-6 levels in the EAT may significantly drive CAD progression.34 Additionally, a review of 40 studies identified IL-6 as a “hub” gene being highly interconnected within gene networks that regulate atherogenesis,21 underscoring IL-6 central role in the pathogenesis of CAD and its possible role as a promising therapeutic target. Our findings indicate that DHA reduces both IL-6 mRNA expression and protein release, aligning with findings from da Cunha de Sá35 and Bargut,36 who reported similar effects in visceral adipocytes and epididymal fat pad isolated from mice on a high-fat diet enriched with fish oil. MCP-1, a chemokine implicated in the pathogenesis of CAD37 and obesity,38 plays a critical role in the recruitment of monocytes to endothelial layers39 and adipose tissue.40 Similarly, CXCL10 has been shown to play a comparable role in the homing and arrest of leukocytes on endothelial cells, particularly in obese individuals.41 Consistent with an anti-inflammatory role of n-3 PUFAs in cardiac adipocytes, our results demonstrate that both DHA and EPA significantly reduce the expression and release of MCP-1, aligning with previous findings in subcutaneous (SAT) and visceral adipose tissue (VAT).35,42,43 Furthermore, DHA also decreased the expression and release of CXCL10, a complementary pro-inflammatory gene that is over-expressed in conjunction with other pro-inflammatory genes in hypertrophic adipose tissue.44 Taken together, these results provide a mechanistic explanation for the observed reduction in the ability of DHA-treated adipocyte-conditioned medium to attract monocytes, highlighting the potential anti-atherogenic role of DHA in modulating the inflammatory environment in coronary plaques.
Cardiac fat in individuals with CAD has also been shown to be a significant source of MMP-9,20 an enzyme involved in tissue remodelling and plaque instability.45 A notable finding of our study was the modulation of MMP-9 gene expression and protein release by DHA. This observation is consistent with previous reports by Kabir,46 who demonstrated similar effects in SAT samples from women with T2D or obesity following fish oil supplementation. However, this is in contrast to the results of Hernandez et al. who found no modulating effect of fish oil in pure adipocytes isolated from adipose tissue of obese subjects.47 This discrepancy could be due to the high doses of fish oil administered (4 g day−1). In contrast, we used more physiological concentrations of DHA and EPA (50 µmol L−1) corresponding to a lower fish oil intake (1–2 g day−1).48
To the best of our knowledge, few studies have investigated the effects of diets enriched in fatty fish on the absolute plasma concentrations of DHA and/or EPA. Most reports, rather, express these changes as the percentage of EPA and/or DHA relative to total fatty acids.49 Among the limited studies that report absolute concentrations, the DHA and EPA levels we applied to inhibit pro-inflammatory gene expression and/or normalize metabolic gene expression fall within the range achievable through in vivo administration of marine lipids. Such interventions typically increase plasma DHA concentrations from the low micromolar range to values exceeding 100 µmol L−1.50 More recently, ingestion of a single 2000 mg dose of DHA has been shown to raise plasma DHA levels to approximately 243 µmol L−1, consistent with single dietary intake of seafood-derived products.51 However, we believe that the physiological relevance of plasma PUFA concentrations is limited in the context of in vitro studies. Cultured cells may take up and metabolize fatty acids more efficiently-or differently-than they do in vivo, owing to the absence of competing processes such as clearance, catabolism, or dilution. Consequently, the same extracellular concentration can lead to substantially greater intracellular accumulation. Moreover, the notion of a “physiological concentration” is inherently complex, as fatty acid levels differ markedly between plasma, tissues, cellular membranes, and subcellular compartments. The primary objective of many in vitro experiments is not to reproduce exact systemic plasma levels, but rather to investigate mechanistic effects under controlled conditions. The concentrations employed often represent plausible upper limits or effect-relevant ranges, rather than steady-state physiological values.52
Recent studies have suggested that EAT and PAT have brown adipose tissue (BAT)-like properties that may decrease with age and under pro-inflammatory conditions associated with obesity and CAD.53 UCP-1 is primarily known as a proton transporter that facilitates the dissipation of mitochondrial membrane potential as heat, thereby enabling non-shivering thermogenesis in BAT.54 Recently, UCP-1 expression has been associated with an active thermogenic phenotype in EAT and PAT.55 This thermogenic activity showed an inverse correlation with markers of inflammation and oxidative stress,55 suggesting a novel role for UCP-1 in attenuating oxidative and proinflammatory conditions in cardiac fat depots.56 The UCP-1 homologue UCP-2 plays a crucial role in regulating the production of ROS,57 insulin secretion,58 mitochondrial fatty acid oxidation and thermogenesis.59
Growing evidence indicates that inflammation plays a central regulatory role in adipose tissue also shaping metabolic function. Among inflammatory mediators, mice lacking of MCP-1 (Ccl2 KO) show increased UCP-1 expression in adipose tissue and increased energy expenditure.60 At the same time, IL-6 modulates adipocyte differentiation by suppressing PPARγ, a key transcription factor required for healthy adipogenesis.61 Furthermore, inflammatory profile also interacts with adipokine metabolic networks: in adipocytes, APN suppresses IL-6, attenuating inflammation and enhancing insulin sensitivity.62 Conversely, anti-inflammatory pathways such as HO-1 induction reduce IL-6 production while increasing APN and PPARγ expression, illustrating a coordinated anti-inflammatory–metabolic axis.63 Exploring how external anti-inflammatory supplements, such as DHA or EPA, shape inflammatory-mediated dysmetabolism may open new therapeutic opportunities to harness metabolic benefits such as improved thermogenesis, adipokine balance, and systemic energy homeostasis. On this background we decided to verify the effects of DHA on UCP-1, UCP-2, and PPRγ, HO-1 and APN. Consistent with the downregulation of the aforementioned inflammatory markers, PAT adipocytes exposed to DHA exhibit increased levels of PPARγ together to increased levels of UCP-1 and UCP-2, ATGL. DGAT-1,-2 and CD36. This is consistent with similar findings observed in mouse/rat models64 and in human peripheral blood mononuclear cell (PBMC).65 Under our experimental conditions, EPA only increased the expression of UCP-2, while the expression of UCP-1 remained unaffected. This lack of modulation of UCP-1 by EPA in PAT adipocytes raises interesting questions for further investigation. In our experimental system, DHA supplementation simultaneously increase the expression of PPARγ, CD36, DGAT1, DGAT2, SREBP-1, and ATGL without producing measurable changes in overall lipid accumulation. As a central transcriptional regulator, PPARγ enhances fatty-acid trafficking and adipokine secretion, partly by up-regulating transporters such as CD36,66 thereby improving the cell's capacity to handle incoming fatty acids. Higher expression of DGAT1 and DGAT2—observed in certain DHA-treated adipose models67 raises the potential for triglyceride synthesis and re-esterification. In parallel, the induction of ATGL, which is positively regulated by PPARγ, enhances lipolytic activity and supports triglyceride mobilization.68 Although SREBP-1 is typically suppressed by n-3 PUFAs,69 context-specific or transient increases have been reported and may reflect adaptive remodeling of lipid metabolism. Together, these coordinated gene-expression changes suggest that DHA promotes a metabolically flexible state characterized by increased lipid flux-greater uptake, synthesis, and mobilization-allowing adipocytes to maintain stable lipid content despite substantial transcriptional activation of lipid-handling pathways.
Overall, our results suggest that both DHA and EPA play a positive role in the regulation of gene networks involved in pro-inflammatory dysmetabolism of cardiac fat. Whether activation of a thermogenic phenotype by fish oils in cardiac adipocytes leads to modulation of EAT and PAT volume remains a matter of debate. While the original papers by Sato10 and Pacifico70 demonstrated a significant reduction in EAT volume in adults with CAD following EPA supplementation and in children with DHA supplementation, more recent research on overweight CAD patients receiving combined EPA + DHA alongside statin therapy found no significant impact.71 This discrepancy may reflect the pronounced effect of statins in reducing EAT volume, potentially hiding the impact of fish oils. However, our results showing an anti-inflammatory effect of DHA and EPA suggest a broader benefit. Future in vivo studies should investigate the effects of DHA not only by looking at changes in EAT volume, but also EAT inflammation using advanced imaging techniques, such as 18F-fluorodeoxyglucose positron emission tomography.72
From a mechanistic perspective, we hypothesize and show that DHA and EPA activate a “protective transcriptional axis” in cardiac fat. This axis involves the upregulation of PPAR-γ and NRF2 expression, both of which are associated with the suppression of pro-inflammatory NF-κB activity, a pathway notably inhibited by omega-3 PUFAs.73
NF-κB activity can be downregulated independently of p65 mRNA expression through mechanisms that affect p65 function or localization. In the resting state, NF-κB dimers, most commonly the p65-p50 dimer, are retained in the cytoplasm in an inactive form by binding to inhibitory proteins known as inhibitors of κB (IκB). A wide range of stimuli can activate NF-κB by inducing the degradation of IκB. This allows the dimers to translocate into the nucleus, where they bind to consensus sequences in the promoters of target genes.74 Drugs and nutraceuticals may affect NF-κB activation through several mechanisms that do not depend on downregulation of p65 mRNA, such as interference with signaling pathways leading to IκB degradation or inhibition of nuclear translocation. These mechanisms prevent the p65 subunit from activating target genes even when the protein is present, providing a layer of regulation separate from transcriptional control of its expression.74 Furthermore, factors that do not directly target NF-κB can also reduce its activity. For example, the Nrf2/HO-1 signaling pathway can shift the cell toward a less inflammatory state, thereby indirectly reducing NF-κB activation. In our data, we clearly observe a reduction in NF-κB activation and we can conclude that this effect is not mediated by regulation of p65 mRNA. Instead, it evidently involves other mechanisms, including the antioxidative and anti-inflammatory actions of the Nrf2–HO-1 axis.75,76
A key feature of inflamed adipocytes is the downregulation of PPARγ gene expression and activity.77 PPARγ is a nuclear receptor that is critical for a positive regulation of adipose metabolism and insulin sensitivity.78 The ability of PPARγ to suppress the synthesis of pro-inflammatory mediators has been widely attributed to the ligand-dependent suppression of key transcription factors, including NF-κB, AP-1 and STAT.79 Consistent with a chronic pro-inflammatory milieu in EAT under pro-atherosclerotic conditions, PPARγ signaling results downregulated.80 Similarly, NRF2 is a well-established master regulator of antioxidant and metabolic signalling pathways.81 It achieves this regulation by binding to a specific DNA sequence known as the antioxidant response element (ARE) located in the promoters of its target genes.81 Through its interaction with ARE, NRF2 orchestrates the cellular defence mechanisms against oxidative stress by enhancing detoxification of ROS, maintaining redox homeostasis, and protecting cells from oxidative damage. Therefore, NRF2, even if not directly targeting NF-κB can reduce its activity shifting the cell toward a less oxidative and inflammatory state, thereby indirectly reducing NF-κB activation.75,76
But NRF2 pathway is critical not only for antioxidant defence but also for supporting metabolic adaptations under stress conditions, further underscoring NRF2's role as a central player in cellular homeostasis and survival.81 Despite conflicting evidence regarding NRF2's role in modulating the dysmetabolic and pro-inflammatory features of obesity82,83 recent studies highlight its tissue-specific protective effects. For example, mice with adipocyte-specific deletion of NRF2 show a deteriorated metabolic profile, underscoring NRF2 role in preserving metabolic homeostasis in the adipose tissue.84 An interplay between PPARγ and NRF2 in the regulation of inflammatory processes is also well established, and there is evidence for their reciprocal regulation. In particular, two AREs have been identified in the PPARγ promoter,82,85 while a putative PPARγ response element (PPRE) is present in the NRF2 promoter region.86 Based on these findings, numerous studies have investigated the potential to improve inflammatory regulation by simultaneously targeting PPARγ and NRF2 signaling pathways with results showing improved therapeutic benefit, further emphasizing the key role of crosstalk between PPARγ and NRF2.87 n-3 PUFAs are well-recognized for their role as activators and gene inducers of both PPARγ and NRF2.88,89 Our results demonstrate that DHA treatment leads to a robust induction of both PPARγ and NRF2 gene expression, accompanied by enhanced NRF2 DNA-binding activity and transcriptional activation of downstream targets. The partial reversal of DHA's effects by the NRF2-specific inhibitor ML385 supports the notion that NRF2 plays a contributory role in mediating DHA's biological activities. However, the incomplete inhibition by ML385 also suggests that other signaling pathways, including PPARγ or additional mechanisms, may be involved in the response to DHA. Collectively, these findings indicate that NRF2 activation is an important mediator of DHA's effects, but it is likely part of a broader network of regulatory pathways. Corroborating these findings previous studies have highlighted the ability of DHA and EPA to induce the expression of NRF2 in inflamed macrophages,90 in PBMC of diabetic patients91 and in endothelial cells,92 and the ability of NRF2 to mediate the anti-inflammatory activities of DHA.90 Similarly, a large body of previous studies have shown the ability for DHA and EPA to activate PPARγ activity and gene expression.93 Under our experimental conditions a boosted expression and activity of PPARγ and NRF2 likely contributes to the upregulation of key downstream targets, including UCP-1 and -2, as well as HO-1 each of which is regulated by either PPARγ or NRF2, or through their synergistic interaction.94 Interestingly, our observations align with those of Walker et al., who reported in the Ossabaw pig model that n-3 PUFA enrichment in EAT was associated with increased PPARγ expression and a shift toward a less inflammatory phenotype.95 To the best of our knowledge, however, our results are the first to demonstrate a direct protective action of DHA in a human cardiac fat model, highlighting its ability to modulate key inflammatory markers such as MCP-1 and IL-6. We clearly observe a reduction in NF-κB activation, and based on our observation, we can conclude that this effect is not mediated by regulation of p65 mRNA. Instead, it evidently involves other mechanisms, including the antioxidative and anti-inflammatory actions of the NRF2-HO-1/PPARγ axis.
We did not pursue in-depth mechanistic analyses of how DHA and EPA exert their effects; however, several complementary pathways are likely involved. These include modifications of membrane fluidity, decreased synthesis of AA-derived pro-inflammatory eicosanoids, and increased formation of specialized pro-resolving mediators such as resolvins and protectins,96 which are known to be reduced in adipose tissue during obesity.97,98 A number of pro-resolving mediators generated from n-3 PUFAs have been shown to limit adipose tissue inflammation, regulate adipokine secretion, and improve insulin sensitivity in animal models and adipocyte cultures,99–101 primarily through attenuation of MAPK and NF-κB signaling. Our observation of increased RvD1 production in mature adipocytes suggests that activation of this pro-resolving pathway contributes to the overall protective effects of DHA.102 Alternatively, this effect may occur through direct interference of PPARγ and NRF2 with NF-κB activation, potentially mediated by the actions of UCP-1, UCP-2, and HO-1 in modulating oxidative stress or upstream redox-sensitive molecular switches and transcription factor activation. Many of these interfering activities remain speculative and deserve further investigation, which will be the subject of future studies.
Adipose tissue is a heterogeneous structure composed of mature adipocytes and a substantial population of adipose-derived stem cells, both of which influence the tissue's inflammatory profile. The ability of DHA to reduce inflammation in both cell types is particularly meaningful, because it suggests a coordinated effect on the entire adipose microenvironment rather than on a single cell population. By dampening pro-inflammatory signaling in mature adipocytes while simultaneously shifting stem cells toward a more anti-inflammatory profile, DHA may help remodel the tissue environment in a way that supports long-term metabolic stability. Over time, this dual action could contribute to sustained reductions in chronic low-grade inflammation, improved adipose function, and a healthier systemic inflammatory balance.
A strength of our research is the use of human mature adipocytes derived from patients as a cell model effective in identifying new prevention and treatment strategies. These adipocytes, which are used immediately after collection, retain the dysmetabolic phenotype of the donors, making them a reliable and physiologically relevant adipocyte model. In addition, they have been shown to be cost-effective, ready-to-use and sensitive to n-3 PUFAs. This approach provides a valuable platform for studying adipose tissue dynamics and responses, providing insights at both patient-specific and population levels.
Limitations of the study
A significant limitation of our study lies in the enrolment of a relatively small cohort of patients, coupled with limited access to comprehensive clinical information and the absence of genetic profiling for the participants. These constraints hinder our ability to fully evaluate potential interindividual variability in the adipose tissue response to the nutraceutical treatment. Such variability could be influenced by genetic factors, metabolic profiles, or other individual-specific determinants,103 which remain unexplored in the current study. Additionally, the collection of a limited amount of original adipose tissue material is another challenge we had to face. The small amount of material from each patient sampling restricted our capacity to isolate sufficient protein for a comprehensive and systematic analysis of cellular protein expression. This limitation, in turn, prevented us from conducting deeper investigations into the molecular mechanisms underlying the observed responses, particularly at the level of specific signalling pathways and protein-level interactions. Future studies addressing these limitations by incorporating larger patient populations, detailed genetic and clinical profiling, and optimized sample collection protocols will be essential for a more robust understanding of the nutraceutical effects of n-3 PUFAs on adipose tissue biology.Conclusion
In conclusion, our results indicate that n-3 PUFAs, particularly DHA and EPA, play a significant role in attenuating pro-inflammatory responses in cardiac adipocytes, potentially reducing tissue remodeling and plaque instability while enhancing metabolic gene expression. These effects appear to be mediated, at least in part, through the upregulation of NRF2- and PPARγ-dependent pathways, leading to suppression of NF-κB signaling and activation of pro-resolving mechanisms, including increased production of resolvin D1. By alleviating inflammation and promoting pro-resolving pathways, n-3 PUFAs may contribute to their cardioprotective properties, targeting both cell- and tissue-specific dysmetabolism. Clinically, these findings suggest that n-3 PUFA supplementation could mitigate cardiac fat–driven inflammation, a key contributor to coronary atherosclerosis and plaque instability, positioning DHA and EPA as potential adjunct therapies for patients with coronary artery disease. In addition, the observed modulation of metabolic and inflammatory pathways implies broader systemic benefits, including improved insulin sensitivity and reduced systemic inflammation. While the precise cardioprotective mechanisms of DHA remain to be fully elucidated, our study underscores the therapeutic potential of n-3 PUFAs and supports further clinical investigations to evaluate their impact on cardiovascular outcomes in high-risk populations.Author contributions
Conceptualization, SQ, MM; methodology, SQ, GS, FC, MM; formal analysis, SQ; investigation, SQ, GS, MAC, NC, LS, TS, LS, FD, MM; data curation, SQ; writing – review & editing, SQ, NC, FD, MAC, LS, RDC; writing – original draft, review & editing, MM, SQ; funding acquisition, GS, MAC, MM; project administration, MM.Conflicts of interest
All authors declare that they have no conflicts of interest.Data availability
The data supporting this article have all been included in the text.Acknowledgements
This work was supported by the Italian Ministry of University and Research to MM (PRIN-2022 Prot. 2022NZNZH8), SQ (PRIN-2022 Prot. 2022NZNZH8) and NC (PRIN 2022 prot. 7 2022CAKEW9). The Authors are grateful to Antonio Danieli (DiMeS) and Gennaro Cagnazzo (CNR-IFC) for their skilled technical support.References
- T. Kawai, M. V. Autieri and R. Scalia, Adipose tissue inflammation and metabolic dysfunction in obesity, Am. J. Physiol.: Cell Physiol., 2021, 320, C375–C391 CrossRef CAS PubMed.
- G. Iacobellis, Epicardial adipose tissue in contemporary cardiology, Nat. Rev. Cardiol., 2022, 19, 593–606 CrossRef CAS PubMed.
- R. Madonna, M. Massaro, E. Scoditti, I. Pescetelli and R. D. Caterina, The epicardial adipose tissue and the coronary arteries: dangerous liaisons, Cardiovasc. Res., 2019, 115, 1013–1025 CrossRef CAS PubMed.
- M. Li, L. Qi, Y. Li, S. Zhang, L. Lin, L. Zhou, W. Han, X. Qu, J. Cai, M. Ye and K. Shi, Association of Pericardiac Adipose Tissue With Coronary Artery Disease, Front. Endocrinol., 2021, 12, 724859 CrossRef PubMed.
- H. S. Sacks, J. N. Fain, S. W. Bahouth, S. Ojha, A. Frontini, H. Budge, S. Cinti and M. E. Symonds, Adult epicardial fat exhibits beige features, J. Clin. Endocrinol. Metab., 2013, 98, E1448–E1455 CrossRef CAS PubMed.
- E. Dozio, E. Vianello, S. Briganti, B. Fink, A. E. Malavazos, E. T. Scognamiglio, G. Dogliotti, A. Sigrüener, G. Schmitz and M. M. Corsi Romanelli, Increased reactive oxygen species production in epicardial adipose tissues from coronary artery disease patients is associated with brown-to-white adipocyte trans-differentiation, Int. J. Cardiol., 2014, 174, 413–414 CrossRef PubMed.
- A. R. Baker, A. L. Harte, N. Howell, D. C. Pritlove, A. M. Ranasinghe, N. F. da Silva, E. M. Youssef, K. Khunti, M. J. Davies, R. S. Bonser, S. Kumar, D. Pagano and P. G. McTernan, Epicardial adipose tissue as a source of nuclear factor-kappaB and c-Jun N-terminal kinase mediated inflammation in patients with coronary artery disease, J. Clin. Endocrinol. Metab., 2009, 94, 261–267 CrossRef CAS PubMed.
- V. Parisi, L. Petraglia, V. D'Esposito, S. Cabaro, G. Rengo, A. Caruso, M. G. Grimaldi, F. Baldascino, A. De Bellis, D. Vitale, R. Formisano, A. Ferro, S. Paolillo, L. Davin, P. Lancellotti, P. Formisano, P. Perrone Filardi, N. Ferrara and D. Leosco, Statin therapy modulates thickness and inflammatory profile of human epicardial adipose tissue, Int. J. Cardiol., 2019, 274, 326–330 CrossRef PubMed.
- L. A. Richardson, A. Basu, L. C. Chien, A. C. Alman and J. K. Snell-Bergeon, Longitudinal Associations of Healthy Dietary Pattern Scores with Coronary Artery Calcification and Pericardial Adiposity in United States Adults with and without Type 1 Diabetes, J. Nutr., 2023, 153, 2085–2093 CrossRef CAS PubMed.
- T. Sato, T. Kameyama, T. Ohori, A. Matsuki and H. Inoue, Effects of eicosapentaenoic acid treatment on epicardial and abdominal visceral adipose tissue volumes in patients with coronary artery disease, J. Atheroscler. Thromb., 2014, 21, 1031–1043 CrossRef CAS PubMed.
- Y. Xiao, Q. Zhang, X. Liao, U. Elbelt and K. H. Weylandt, The effects of omega-3 fatty acids in type 2 diabetes: A systematic review and meta-analysis, Prostaglandins, Leukotrienes Essent. Fatty Acids, 2022, 182, 102456 CrossRef CAS PubMed.
- S. Chiusolo, C. S. Bork, F. Gentile, S. Lundbye-Christensen, W. S. Harris, E. B. Schmidt and R. D. Caterina, Adipose tissue n-3/n-6 fatty acids ratios versus n-3 fatty acids fractions as predictors of myocardial infarction, Am. Heart J., 2023, 262, 38–48 CrossRef CAS PubMed.
- S. Quarta, G. Santarpino, M. A. Carluccio, N. Calabriso, F. Cardetta, L. Siracusa, T. Strano, I. Palamà, G. Leccese, F. Visioli and M. Massaro, Cardiac fat adipocytes: An optimized protocol for isolation of ready-to-use mature adipocytes from human pericardial adipose tissue, J. Mol. Cell. Cardiol., 2024, 196, 12–25 CrossRef CAS PubMed.
- World Medical Association Declaration of Helsinki: Recommendations Guiding Physicians in Biomedical Research Involving Human Subjects, J. Am. Med. Assoc., 1997, 277, 925–926 CrossRef.
- A. F. Oliveira, D. A. Cunha, L. Ladriere, M. Igoillo-Esteve, M. Bugliani, P. Marchetti and M. Cnop, In vitro use of free fatty acids bound to albumin: A comparison of protocols, BioTechniques, 2015, 58, 228–233 CrossRef CAS PubMed.
- B. L. Trigatti and G. E. Gerber, A direct role for serum albumin in the cellular uptake of long-chain fatty acids, Biochem. J., 1995, 308(Pt 1), 155–159 CrossRef CAS PubMed.
- IUPAC, Preparation of Fatty Acid Methyl Ester, in Standard Methods for Analysis of Oils, Fats and Derivatives, Blackwell, Oxford, 1987 Search PubMed.
- S. A. Abdelmagid, S. E. Clarke, D. E. Nielsen, A. Badawi, A. El-Sohemy, D. M. Mutch and D. W. Ma, Comprehensive profiling of plasma fatty acid concentrations in young healthy Canadian adults, PLoS One, 2015, 10, e0116195 CrossRef PubMed.
- B. Hansson, B. Morén, C. Fryklund, L. Vliex, S. Wasserstrom, S. Albinsson, K. Berger and K. G. Stenkula, Adipose cell size changes are associated with a drastic actin remodeling, Sci. Rep., 2019, 9, 12941 CrossRef PubMed.
- V. Miksztowicz, C. Morales, M. Barchuk, G. López, R. Póveda, R. Gelpi, L. Schreier, M. Rubio and G. Berg, Metalloproteinase 2 and 9 Activity Increase in Epicardial Adipose Tissue of Patients with Coronary Artery Disease, Curr. Vasc. Pharmacol., 2017, 15, 135–143 CrossRef CAS PubMed.
- Z. Maghbooli and A. Hossein-Nezhad, Transcriptome and Molecular Endocrinology Aspects of Epicardial Adipose Tissue in Cardiovascular Diseases: A Systematic Review and Meta-Analysis of Observational Studies, BioMed Res. Int., 2015, 2015, 926567 CrossRef PubMed.
- M. Vacca, M. Di Eusanio, M. Cariello, G. Graziano, S. D'Amore, F. D. Petridis, A. D'Orazio, L. Salvatore, A. Tamburro, G. Folesani, D. Rutigliano, F. Pellegrini, C. Sabba, G. Palasciano, R. D. Bartolomeo and A. Moschetta, Integrative miRNA and whole-genome analyses of epicardial adipose tissue in patients with coronary atherosclerosis, Cardiovasc. Res., 2016, 109, 228–239 CrossRef CAS PubMed.
- J. Pestel, F. Blangero and A. Eljaafari, Pathogenic Role of Adipose Tissue-Derived Mesenchymal Stem Cells in Obesity and Obesity-Related Inflammatory Diseases, Cells, 2023, 12, 1–24 CrossRef PubMed.
- A. L. Strong, R. S. Hunter, R. B. Jones, A. C. Bowles, M. F. Dutreil, D. Gaupp, D. J. Hayes, J. M. Gimble, B. Levi, M. A. McNulty and B. A. Bunnell, Obesity inhibits the osteogenic differentiation of human adipose-derived stem cells, J. Transl. Med., 2016, 14, 27 CrossRef PubMed.
- L. Badimon and J. Cubedo, Adipose tissue depots and inflammation: effects on plasticity and resident mesenchymal stem cell function, Cardiovasc. Res., 2017, 113, 1064–1073 CrossRef CAS PubMed.
- M. A. A. Harrison, R. M. Wise, B. P. Benjamin, E. M. Hochreiner, O. A. Mohiuddin and B. A. Bunnell, Adipose-Derived Stem Cells from Obese Donors Polarize Macrophages and Microglia toward a Pro-Inflammatory Phenotype, Cells, 2020, 10, 1–15 CrossRef PubMed.
- C. Serena, N. Keiran, V. Ceperuelo-Mallafre, M. Ejarque, R. Fradera, K. Roche, C. Nunez-Roa, J. Vendrell and S. Fernandez-Veledo, Obesity and Type 2 Diabetes Alters the Immune Properties of Human Adipose Derived Stem Cells, Stem Cells, 2016, 34, 2559–2573 CrossRef CAS PubMed.
- M. Massaro, E. Scoditti, M. A. Carluccio and R. D. Caterina, Basic mechanisms behind the effects of n-3 fatty acids on cardiovascular disease, Prostaglandins, Leukotrienes Essent. Fatty Acids, 2008, 79, 109–115 CrossRef CAS PubMed.
- L. A. Videla, M. C. Hernandez-Rodas, A. H. Metherel and R. Valenzuela, Influence of the nutritional status and oxidative stress in the desaturation and elongation of n-3 and n-6 polyunsaturated fatty acids: Impact on non-alcoholic fatty liver disease, Prostaglandins, Leukotrienes Essent. Fatty Acids, 2022, 181, 102441 CrossRef CAS PubMed.
- D. B. Jump, The biochemistry of n-3 polyunsaturated fatty acids, J. Biol. Chem., 2002, 277, 8755–8758 CrossRef CAS PubMed.
- M. T. Nakamura and T. Y. Nara, Structure, function, and dietary regulation of delta6, delta5, and delta9 desaturases, Annu. Rev. Nutr., 2004, 24, 345–376 CrossRef CAS PubMed.
- P. Sjogren, J. Sierra-Johnson, K. Gertow, M. Rosell, B. Vessby, U. de Faire, A. Hamsten, M. L. Hellenius and R. M. Fisher, Fatty acid desaturases in human adipose tissue: relationships between gene expression, desaturation indexes and insulin resistance, Diabetologia, 2008, 51, 328–335 CrossRef CAS PubMed.
- C. Wang, B. Hucik, O. Sarr, L. H. Brown, K. R. D. Wells, K. R. Brunt, M. T. Nakamura, E. Harasim-Symbor, A. Chabowski and D. M. Mutch, Delta-6 desaturase (Fads2) deficiency alters triacylglycerol/fatty acid cycling in murine white adipose tissue, J. Lipid Res., 2023, 64, 100376 CrossRef CAS PubMed.
- S. Eiras, E. Teijeira-Fernandez, L. G. Shamagian, A. L. Fernandez, A. Vazquez-Boquete and J. R. Gonzalez-Juanatey, Extension of coronary artery disease is associated with increased IL-6 and decreased adiponectin gene expression in epicardial adipose tissue, Cytokine, 2008, 43, 174–180 CrossRef CAS PubMed.
- R. D. C. da Cunha de Sa, M. M. Cruz, T. M. de Farias, V. S. da Silva, J. de Jesus Simao, M. M. Telles and M. I. C. Alonso-Vale, Fish oil reverses metabolic syndrome, adipocyte dysfunction, and altered adipokines secretion triggered by high-fat diet-induced obesity, Physiol. Rep., 2020, 8, e14380 CAS.
- T. C. Bargut, C. A. Mandarim-de-Lacerda and M. B. Aguila, A high-fish-oil diet prevents adiposity and modulates white adipose tissue inflammation pathways in mice, J. Nutr. Biochem., 2015, 26, 960–969 CrossRef CAS PubMed.
- J. Niu and P. E. Kolattukudy, Role of MCP-1 in cardiovascular disease: molecular mechanisms and clinical implications, Clin. Sci., 2009, 117, 95–109 CrossRef CAS PubMed.
- J. Panee, Monocyte Chemoattractant Protein 1 (MCP-1) in obesity and diabetes, Cytokine, 2012, 60, 1–12 CrossRef CAS PubMed.
- L. A. Hardy, T. A. Booth, E. K. Lau, T. M. Handel, S. Ali and J. A. Kirby, Examination of MCP-1 (CCL2) partitioning and presentation during transendothelial leukocyte migration, Lab. Invest., 2004, 84, 81–90 CrossRef CAS PubMed.
- H. Kanda, S. Tateya, Y. Tamori, K. Kotani, K. Hiasa, R. Kitazawa, S. Kitazawa, H. Miyachi, S. Maeda, K. Egashira and M. Kasuga, MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity, J. Clin. Invest., 2006, 116, 1494–1505 CrossRef CAS PubMed.
- B. Moreno, L. Hueso, R. Ortega, E. Benito, S. Martínez-Hervas, M. Peiro, M. Civera, M.-J. Sanz, L. Piqueras and J. T. Real, Association of chemokines IP-10/CXCL10 and I-TAC/CXCL11 with insulin resistance and enhance leukocyte endothelial arrest in obesity, Microvasc. Res., 2022, 139, 104254 CrossRef CAS PubMed.
- J. F. Ferguson, K. Roberts-Lee, C. Borcea, H. M. Smith, Y. Midgette and R. Shah, Omega-3 polyunsaturated fatty acids attenuate inflammatory activation and alter differentiation in human adipocytes, J. Nutr. Biochem., 2019, 64, 45–49 CrossRef CAS PubMed.
- N. Bakker, M. Hickey, R. Shams, C. F. Rivera, J. Vlahos, H. A. Cense, A. Demirkiran, B. Ramkhelawon and A. P. Houdijk, Oral omega-3 PUFA supplementation modulates inflammation in adipose tissue depots in morbidly obese women: A randomized trial, Nutrition, 2023, 111, 112055 CrossRef CAS PubMed.
- S. Kochumon, A. A. Madhoun, F. Al-Rashed, R. Azim, E. Al-Ozairi, F. Al-Mulla and R. Ahmad, Adipose tissue gene expression of CXCL10 and CXCL11 modulates inflammatory markers in obesity: implications for metabolic inflammation and insulin resistance, Ther. Adv. Endocrinol. Metab., 2020, 11, 2042018820930902 CrossRef CAS PubMed.
- T. Li, X. Li, Y. Feng, G. Dong, Y. Wang and J. Yang, The Role of Matrix Metalloproteinase-9 in Atherosclerotic Plaque Instability, Mediators Inflammation, 2020, 2020, 3872367 Search PubMed.
- M. Kabir, G. Skurnik, N. Naour, V. Pechtner, E. Meugnier, S. Rome, A. Quignard-Boulange, H. Vidal, G. Slama, K. Clement, M. Guerre-Millo and S. W. Rizkalla, Treatment for 2 mo with n 3 polyunsaturated fatty acids reduces adiposity and some atherogenic factors but does not improve insulin sensitivity in women with type 2 diabetes: a randomized controlled study, Am. J. Clin. Nutr., 2007, 86, 1670–1679 CrossRef CAS PubMed.
- J. D. Hernandez, T. Li and E. De Filippis, omega-3PUFA supplementation ameliorates adipose tissue inflammation and insulin-stimulated glucose disposal in subjects with obesity: a potential role for apolipoprotein E, Int. J. Obes., 2021, 45, 2286–2287 CrossRef CAS PubMed.
- A. Rusca, A. F. D. Di Stefano, M. V. Doig, C. Scarsi and E. Perucca, Relative bioavailability and pharmacokinetics of two oral formulations of docosahexaenoic acid/eicosapentaenoic acid after multiple-dose administration in healthy volunteers, Eur. J. Clin. Pharmacol., 2009, 65, 503–510 CrossRef CAS PubMed.
- H. R. Superko, S. M. Superko, K. Nasir, A. Agatston and B. C. Garrett, Omega-3 fatty acid blood levels: clinical significance and controversy, Circulation, 2013, 128, 2154–2161 CrossRef PubMed.
- K. H. Bonaa, K. S. Bjerve, B. Straume, I. T. Gram and D. Thelle, Effect of eicosapentaenoic and docosahexaenoic acids on blood pressure in hypertension. A population-based intervention trial from the Tromso study, N. Engl. J. Med., 1990, 322, 795–801 CrossRef CAS PubMed.
- V. A. Mullins, J. M. Snider, B. Michael, L. R. Porter, R. D. Brinton and F. H. Chilton, Impact of fish oil supplementation on plasma levels of highly unsaturated fatty acid-containing lipid classes and molecular species in American football athletes, Nutr. Metab., 2024, 21, 43 CrossRef PubMed.
- N. Alsabeeh, B. Chausse, P. A. Kakimoto, A. J. Kowaltowski and O. Shirihai, Cell culture models of fatty acid overload: Problems and solutions, Biochim. Biophys. Acta, Mol. Cell Biol. Lipids, 2018, 143–151 CrossRef CAS PubMed.
- F. Omran and M. Christian, Inflammatory Signaling and Brown Fat Activity, Front. Endocrinol., 2020, 11, 156 CrossRef PubMed.
- E. Rial, M. M. Gonzalez-Barroso, C. Fleury and F. Bouillaud, The structure and function of the brown fat uncoupling protein UCP1: current status, BioFactors, 1998, 8, 209–219 CrossRef CAS PubMed.
- K. Chechi, P. Voisine, P. Mathieu, M. Laplante, S. Bonnet, F. Picard, P. Joubert and D. Richard, Functional characterization of the Ucp1-associated oxidative phenotype of human epicardial adipose tissue, Sci. Rep., 2017, 7, 15566 CrossRef PubMed.
- K. Chechi, J. Vijay, P. Voisine, P. Mathieu, Y. Bosse, A. Tchernof, E. Grundberg and D. Richard, UCP1 expression-associated gene signatures of human epicardial adipose tissue, JCI Insight, 2019, 4, 1–14 CrossRef PubMed.
- D. Arsenijevic, H. Onuma, C. Pecqueur, S. Raimbault, B. S. Manning, B. Miroux, E. Couplan, M. C. Alves-Guerra, M. Goubern, R. Surwit, F. Bouillaud, D. Richard, S. Collins and D. Ricquier, Disruption of the uncoupling protein-2 gene in mice reveals a role in immunity and reactive oxygen species production, Nat. Genet., 2000, 26, 435–439 CrossRef CAS PubMed.
- C. Y. Zhang, G. Baffy, P. Perret, S. Krauss, O. Peroni, D. Grujic, T. Hagen, A. J. Vidal-Puig, O. Boss, Y. B. Kim, X. X. Zheng, M. B. Wheeler, G. I. Shulman, C. B. Chan and B. B. Lowell, Uncoupling protein-2 negatively regulates insulin secretion and is a major link between obesity, beta cell dysfunction, and type 2 diabetes, Cell, 2001, 105, 745–755 CrossRef CAS PubMed.
- X. Y. Tian, S. Ma, G. Tse, W. T. Wong and Y. Huang, Uncoupling Protein 2 in Cardiovascular Health and Disease, Front. Physiol., 2018, 9, 1060 CrossRef PubMed.
- M. Rajasekaran, O. J. Sul, E. K. Choi, J. E. Kim, J. H. Suh and H. S. Choi, MCP-1 deficiency enhances browning of adipose tissue via increased M2 polarization, J. Endocrinol., 2019, 242, 91–101 CAS.
- S. Almuraikhy, W. Kafienah, M. Bashah, I. Diboun, M. Jaganjac, F. Al-Khelaifi, H. Abdesselem, N. A. Mazloum, M. Alsayrafi, V. Mohamed-Ali and M. A. Elrayess, Interleukin-6 induces impairment in human subcutaneous adipogenesis in obesity-associated insulin resistance, Diabetologia, 2016, 59, 2406–2416 CrossRef CAS PubMed.
- K. M. Ajuwon and M. E. Spurlock, Adiponectin inhibits LPS-induced NF-kappaB activation and IL-6 production and increases PPARgamma2 expression in adipocytes, Am. J. Physiol.: Regul., Integr. Comp. Physiol., 2005, 288, R1220–R1225 CrossRef CAS PubMed.
- A. Burgess, M. Li, L. Vanella, D. H. Kim, R. Rezzani, L. Rodella, K. Sodhi, M. Canestraro, P. Martasek, S. J. Peterson, A. Kappas and N. G. Abraham, Adipocyte heme oxygenase-1 induction attenuates metabolic syndrome in both male and female obese mice, Hypertension, 2010, 56, 1124–1130 CrossRef CAS PubMed.
- J. Lund, L. H. Larsen and L. Lauritzen, Fish oil as a potential activator of brown and beige fat thermogenesis, Adipocyte, 2018, 7, 88–95 Search PubMed.
- S. Moradi, M. Alivand, Y. KhajeBishak, M. AsghariJafarabadi, M. Alipour, P. D. Chilibeck and B. Alipour, The effect of omega3 fatty acid supplementation on PPARgamma and UCP2 expressions, resting energy expenditure, and appetite in athletes, BMC Sports Sci. Med. Rehabil., 2021, 13, 48 CrossRef PubMed.
- J. Wu, J. Luo, Q. He, F. Zhang, C. Shi, J. Zhao, C. Li and W. Deng, CD36 molecule and AMP-activated protein kinase signaling drive docosahexaenoic acid-induced lipid remodeling in goat mammary epithelial cells, Int. J. Biol. Macromol., 2025, 311, 144076 CrossRef CAS PubMed.
- C. W. Huang, Y. J. Chen, J. T. Yang, C. Y. Chen, K. M. Ajuwon, S. E. Chen, N. W. Su, Y. S. Chen, H. J. Mersmann and S. T. Ding, Docosahexaenoic acid increases accumulation of adipocyte triacylglycerol through up-regulation of lipogenic gene expression in pigs, Lipids Health Dis., 2017, 16, 33 CrossRef PubMed.
- E. E. Kershaw, M. Schupp, H. P. Guan, N. P. Gardner, M. A. Lazar and J. S. Flier, PPARgamma regulates adipose triglyceride lipase in adipocytes in vitro and in vivo, Am. J. Physiol. Endocrinol. Metab., 2007, 293, E1736–E1745 CrossRef CAS PubMed.
- X. Deng, Q. Dong, D. Bridges, R. Raghow, E. A. Park and M. B. Elam, Docosahexaenoic acid inhibits proteolytic processing of sterol regulatory element-binding protein-1c (SREBP-1c) via activation of AMP-activated kinase, Biochim. Biophys. Acta, Mol. Cell Biol. Lipids, 2015, 1851, 1521–1529 CrossRef CAS PubMed.
- L. Pacifico, E. Bonci, M. Di Martino, P. Versacci, G. Andreoli, L. M. Silvestri and C. Chiesa, A double-blind, placebo-controlled randomized trial to evaluate the efficacy of docosahexaenoic acid supplementation on hepatic fat and associated cardiovascular risk factors in overweight children with nonalcoholic fatty liver disease, Nutr., Metab. Cardiovasc. Dis., 2015, 25, 734–741 CrossRef CAS PubMed.
- M. Amangurbanova, R. Daher, A. A. Asbeutah, B. Vemuri, H. Mirza, S. Waseem, A. Malik and F. K. Welty, Higher epicardial adipose tissue volume is associated with higher coronary fatty plaque volume and is regulated by waist circumference but not EPA+DHA supplementation, J. Clin. Lipidol., 2024, 18(5), e773–e786 CrossRef PubMed.
- M. Guglielmo, A. Lin, D. Dey, A. Baggiano, L. Fusini, G. Muscogiuri and G. Pontone, Epicardial fat and coronary artery disease: Role of cardiac imaging, Atherosclerosis, 2021, 321, 30–38 CrossRef CAS PubMed.
- M. Massaro, R. Martinelli, V. Gatta, E. Scoditti, M. Pellegrino, M. A. Carluccio, N. Calabriso, T. Buonomo, L. Stuppia, C. Storelli and R. D. Caterina, Transcriptome-based identification of new anti-anti-inflammatory and vasodilating properties of the n-3 fatty acid docosahexaenoic acid in vascular endothelial cell under proinflammatory conditions, PLoS One, 2015, 10, e0129652 CrossRef PubMed.
- A. Hoffmann, G. Cheng and D. Baltimore, NF-κB: master regulator of cellular responses in health and disease, Immun. Inflammation, 2025, 1, 2 CrossRef.
- T. H. Tu, Y. Joe, H. S. Choi, H. T. Chung and R. Yu, Induction of heme oxygenase-1 with hemin reduces obesity-induced adipose tissue inflammation via adipose macrophage phenotype switching, Mediators Inflammation, 2014, 2014, 290708 CrossRef PubMed.
- Z. Wang, Y. Lee, J. S. Eun and E. J. Bae, Inhibition of adipocyte inflammation and macrophage chemotaxis by butein, Eur. J. Pharmacol., 2014, 738, 40–48 CrossRef CAS PubMed.
- R. Yin, L. Fang, Y. Li, P. Xue, Y. Li, Y. Guan, Y. Chang, C. Chen and N. Wang, Pro-inflammatory Macrophages suppress PPARγ activity in Adipocytes via S-nitrosylation, Free Radicals Biol. Med., 2015, 89, 895–905 CrossRef CAS PubMed.
- M. Ahmadian, J. M. Suh, N. Hah, C. Liddle, A. R. Atkins, M. Downes and R. M. Evans, PPARγ signaling and metabolism: the good, the bad and the future, Nat. Med., 2013, 19, 557–566 CrossRef CAS PubMed.
- J. Korbecki, R. Bobinski and M. Dutka, Self-regulation of the inflammatory response by peroxisome proliferator-activated receptors, Inflammation Res., 2019, 68, 443–458 CrossRef CAS PubMed.
- B. Gaborit, N. Venteclef, P. Ancel, V. Pelloux, V. Gariboldi, P. Leprince, J. Amour, S. N. Hatem, E. Jouve, A. Dutour and K. Clément, Human epicardial adipose tissue has a specific transcriptomic signature depending on its anatomical peri-atrial, peri-ventricular, or peri-coronary location, Cardiovasc. Res., 2015, 108, 62–73 CrossRef CAS PubMed.
- A. S. Annie-Mathew, S. Prem-Santhosh, R. Jayasuriya, G. Ganesh, K. M. Ramkumar and D. V. L. Sarada, The pivotal role of Nrf2 activators in adipocyte biology, Pharmacol. Res., 2021, 173, 105853 CrossRef CAS PubMed.
- H. Y. Cho, W. Gladwell, X. Wang, B. Chorley, D. Bell, S. P. Reddy and S. R. Kleeberger, Nrf2-regulated PPAR{gamma} expression is critical to protection against acute lung injury in mice, Am. J. Respir. Crit. Care Med., 2010, 182, 170–182 CrossRef CAS PubMed.
- H. Chen, Q. Guan, S. Gong, Y. Du, Z. Zhang, Y. Liu, L. Zhou, L. Liu, B. Xin, Y. Guo, H. Zhang, Z. Zhou, T. Pei, G. Yu, S. Yin and L. Li, NRF2-REGγ-ACADM/KLF15 Signaling Pathway Regulates the Browning of White Adipose Tissue to Modulate Obesity, Adv. Sci., 2025, e09429 CrossRef CAS PubMed.
- D. V. Chartoumpekis, D. L. Palliyaguru, N. Wakabayashi, M. Fazzari, N. K. H. Khoo, F. J. Schopfer, I. Sipula, Y. Yagishita, G. K. Michalopoulos, R. M. O'Doherty and T. W. Kensler, Nrf2 deletion from adipocytes, but not hepatocytes, potentiates systemic metabolic dysfunction after long-term high-fat diet-induced obesity in mice, Am. J. Physiol. Endocrinol. Metab., 2018, 315, E180–E195 CrossRef CAS PubMed.
- J. Huang, I. Tabbi-Anneni, V. Gunda and L. Wang, Transcription factor Nrf2 regulates SHP and lipogenic gene expression in hepatic lipid metabolism, Am. J. Physiol.: Gastrointest. Liver Physiol., 2010, 299, G1211–G1221 CrossRef CAS PubMed.
- E. Y. Park, I. J. Cho and S. G. Kim, Transactivation of the PPAR-Responsive Enhancer Module in Chemopreventive Glutathione S-Transferase Gene by the Peroxisome Proliferator-Activated Receptor-γ and Retinoid X Receptor Heterodimer, Cancer Res., 2004, 64, 3701–3713 CrossRef CAS PubMed.
- C. Lee, Collaborative Power of Nrf2 and PPARγ Activators against Metabolic and Drug-Induced Oxidative Injury, Oxid. Med. Cell. Longevity, 2017, 2017, 1378175 CrossRef PubMed.
- S. Davinelli, A. Medoro, M. Intrieri, L. Saso, G. Scapagnini and J. X. Kang, Targeting NRF2-KEAP1 axis by Omega-3 fatty acids and their derivatives: Emerging opportunities against aging and diseases, Free Radicals Biol. Med., 2022, 193, 736–750 CrossRef CAS PubMed.
- L. Martinez-Fernandez, L. M. Laiglesia, A. E. Huerta, J. A. Martinez and M. J. Moreno-Aliaga, Omega-3 fatty acids and adipose tissue function in obesity and metabolic syndrome, Prostaglandins Other Lipid Mediators, 2015, 121, 24–41 CrossRef CAS PubMed.
- H. Wang, T. O. Khor, C. L. Saw, W. Lin, T. Wu, Y. Huang and A. N. Kong, Role of Nrf2 in suppressing LPS-induced inflammation in mouse peritoneal macrophages by polyunsaturated fatty acids docosahexaenoic acid and eicosapentaenoic acid, Mol. Pharm., 2010, 7, 2185–2193 CrossRef CAS PubMed.
- P. Golpour, M. Nourbakhsh, M. Mazaherioun, L. Janani, M. Nourbakhsh and P. Yaghmaei, Improvement of NRF2 gene expression and antioxidant status in patients with type 2 diabetes mellitus after supplementation with omega-3 polyunsaturated fatty acids: A double-blind randomised placebo-controlled clinical trial, Diabetes Res. Clin. Pract., 2020, 162, 108120 CrossRef CAS PubMed.
- C. Sakai, M. Ishida, H. Ohba, H. Yamashita, H. Uchida, M. Yoshizumi and T. Ishida, Fish oil omega-3 polyunsaturated fatty acids attenuate oxidative stress-induced DNA damage in vascular endothelial cells, PLoS One, 2017, 12, e0187934 CrossRef PubMed.
- A. R. Ahmadi, F. Shirani, B. Abiri, M. Siavash, S. Haghighi and M. Akbari, Impact of omega-3 fatty acids supplementation on the gene expression of peroxisome proliferator activated receptors-γ, α and fibroblast growth factor-21 serum levels in patients with various presentation of metabolic conditions: a GRADE assessed systematic review and dose–response meta-analysis of clinical trials, Front. Nutr., 2023, 10, 1–10 CrossRef PubMed.
- C. Lee, Collaborative Power of Nrf2 and PPARγ Activators against Metabolic and Drug-Induced Oxidative Injury, Oxid. Med. Cell. Longevity, 2017, 2017, 1378175 CrossRef PubMed.
- M. E. Walker, N. R. Matthan, A. Goldbaum, H. Meng, S. Lamon-Fava, S. Lakshman, S. Jang, A. Molokin, G. Solano-Aguilar, J. F. Urban Jr and A. H. Lichtenstein, Dietary patterns influence epicardial adipose tissue fatty acid composition and inflammatory gene expression in the Ossabaw pig, J. Nutr. Biochem., 2019, 70, 138–146 CrossRef CAS PubMed.
- A. Kar, P. Ghosh, P. Patra, D. S. Chini, A. K. Nath, J. K. Saha and B. Chandra Patra, Omega-3 fatty acids mediated Cellular signaling and its regulation in Human Health, Clin. Nutr. Open Sci., 2023, 52, 72–86 CrossRef.
- A. Neuhofer, M. Zeyda, D. Mascher, B. K. Itariu, I. Murano, L. Leitner, E. E. Hochbrugger, P. Fraisl, S. Cinti, C. N. Serhan and T. M. Stulnig, Impaired local production of proresolving lipid mediators in obesity and 17-HDHA as a potential treatment for obesity-associated inflammation, Diabetes, 2013, 62, 1945–1956 CrossRef CAS PubMed.
- J. Clària, B. T. Nguyen, A. L. Madenci, C. K. Ozaki and C. N. Serhan, Diversity of lipid mediators in human adipose tissue depots, Am. J. Physiol.: Cell Physiol., 2013, 304, C1141–C1149 CrossRef PubMed.
- J. Hellmann, Y. Tang, M. Kosuri, A. Bhatnagar and M. Spite, Resolvin D1 decreases adipose tissue macrophage accumulation and improves insulin sensitivity in obese-diabetic mice, FASEB J., 2011, 25, 2399–2407 CrossRef CAS PubMed.
- A. Gonzalez-Periz, R. Horrillo, N. Ferre, K. Gronert, B. Dong, E. Moran-Salvador, E. Titos, M. Martinez-Clemente, M. Lopez-Parra, V. Arroyo and J. Claria, Obesity-induced insulin resistance and hepatic steatosis are alleviated by omega-3 fatty acids: a role for resolvins and protectins, FASEB J., 2009, 23, 1946–1957 CrossRef CAS PubMed.
- N. Sainz, M. Fernandez-Galilea, A. G. V. Costa, P. L. Prieto-Hontoria, G. M. Barraco and M. J. Moreno-Aliaga, n-3 polyunsaturated fatty acids regulate chemerin in cultured adipocytes: role of GPR120 and derived lipid mediators, Food Funct., 2020, 11, 9057–9066 RSC.
- J. Clària, J. Dalli, S. Yacoubian, F. Gao and C. N. Serhan, Resolvin D1 and Resolvin D2 Govern Local Inflammatory Tone in Obese Fat, J. Immunol., 2012, 189, 2597–2605 CrossRef PubMed.
- M. Francis, C. Li, Y. Sun, J. Zhou, X. Li, J. T. Brenna and K. Ye, Genome-wide association study of fish oil supplementation on lipid traits in 81,246 individuals reveals new gene-diet interaction loci, PLoS Genet., 2021, 17, e1009431 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2026 |