Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A randomised controlled study to investigate the cognitive, mood, metabolic and anti-inflammatory effects of acute oyster mushroom intervention in healthy older adults
Sara
Cha
a,
Lynne
Bell
a,
Derek R.
Fisher
b,
Barbara
Shukitt-Hale
b,
Zicheng
Zhang
c,
Ana
Rodriguez-Mateos
 c and
Claire M.
Williams
c and
Claire M.
Williams
 *a
*a
aUniversity of Reading, School of Psychology & Clinical Language Sciences, Harry Pitt Building, Whiteknights Road, Earley Gate, Reading, RG6 6ES, UK. E-mail: claire.williams@reading.ac.uk
bUSDA-ARS, Human Nutrition Research Center on Aging at Tufts University, Boston, MA, USA
cKing's College London, Department of Nutritional Sciences, School of Life Course and Population Sciences, London, SE1 9NH, UK
First published on 15th December 2025
Abstract
The Pleurotus oyster is a common edible mushroom rich in ergothioneine, a bioactive that has shown benefits to cognition in animals when administered in extract form. The OYSACO study investigated the acute effects of oyster mushroom (OM) on cognition, mood, inflammation and metabolism in healthy older adults. In a cross-over study, 33 participants consumed a noodle soup containing 0.5 (OM0.5), 1 (OM1) and 2 (OM2) servings of OM, and a calorie-matched control soup (OM0), on four separate occasions, each separated by one week. Cognitive function, mood and subjective appetite were assessed at baseline (BL, prior to intervention) and then at 2-, 4- and 6-hours post-consumption. Intervention palatability was recorded after consumption, and a serum sample was taken at 6-hours. Linear Mixed Modelling, with BL as covariate, revealed a significant decline in Positive Affect and concomitant increase in Mental Fatigue over the test day after consuming OM0, while no significant variation in mood was seen throughout the day following OM interventions. Cognitive findings were mixed with no consistent pattern of effects seen following OM interventions compared to OM0. Postprandial inflammatory markers (nitrite, NADPH oxidase 2 and inducible nitric oxide synthase) were significantly lower following OM interventions compared to OM0. Unexpectedly, brain derived neurotrophic factor was found to be dose-dependently lower after consuming OM compared to OM0. Overall, OM interventions helped maintain mood and lower inflammatory markers in healthy older adults, following acute supplementation. Further studies are required to unravel the underlying mechanisms involved.
1. Introduction
Ageing is associated with a deterioration in not just physiological function but also cognitive health.1 Although treatments for age-related conditions remain limited, lifestyle factors, particularly diet, play a crucial role in promoting healthy ageing and reducing the risk of age-related diseases.2 Emerging evidence suggests that diets rich in diverse nutrients and bioactive compounds may benefit both cognitive function and mood during the ageing process.3,4 Among such dietary components, edible mushrooms are gaining attention due to their rich nutrient profile.5 Mushrooms are known to contain a variety of bioactive compounds including ergothioneine, B vitamins, phenolic acids and terpenoids,6 which are thought to be responsible for neurocognitive benefits.7Previous epidemiological research has demonstrated a clear association between mushroom consumption and better cognitive and mental well-being outcomes, when mushrooms were included as part of a vegetable-rich diet.8–11 Whilst these epidemiological studies typically involved Asian populations, findings from our recent epidemiological study involving a UK population suggested that the consumption of more than 1 portion (45 g) of mushrooms per week was associated with better cognitive scores in the domains of episodic memory, executive function and processing speed.12 However, human clinical trials examining mushrooms’ effects on cognition and mood currently remain limited, with most focusing on Lion's Mane mushroom, rather than common edible mushrooms.13 Notably, Lion's Mane supplementation has shown significant cognitive benefits in older adults with cognitive decline, particularly at doses up to 3 g day−1 and for durations over 12-weeks or more.14,15 The effects of other edible mushroom species remain under investigation, and thus definitive conclusions about their cognitive and mood-related benefits, cannot yet be drawn.
The Pleurotus ostreatus oyster species (OM) is a common edible mushroom, ranking as the second-largest cultivated mushroom type worldwide.16 This species is rich in bioactive compounds and nutrients including proteins, vitamins, minerals and dietary fibre.17 Research evidence suggests that OM are also rich in ergothioneine, a sulphur-containing amino acid that has been shown in animal studies to exhibit antidepressant18 and neuroprotective19 effects. Despite the favourable bioactive profile of OM, only a single study to date has investigated the cognitive and mood-related benefits in humans.20 Here the effects of OM supplementation for 12-weeks revealed significant improvement in episodic memory and maintenance of mood compared to placebo; reductions in inflammatory markers compared to baseline levels were also seen for OM-treated participants only. However, no studies have specifically examined the immediate postprandial cognitive and mood effects of OM in a healthy older adult UK population. Therefore, the OYSACO study aimed to investigate the effects of three different doses of dried OM intervention for up to 6-hours (6 h) during the day. The selected doses (40 g, 80 g, and 160 g fresh equivalents) were chosen to reflect realistic intakes of edible mushrooms within a typical diet, while spanning a broad enough range to explore possible dose–response effects. The 80 g portion was chosen as the mid-dose as it aligns with the typical vegetable serving size in the UK. Importantly, whereas many prior mushroom interventions have been employed in encapsulated form, our design focused on OM as a whole food, reflecting its use as a culinary mushroom rather than a medicinal extract. This dosing approach is also consistent with Uffelman and colleagues’ study,21 which employed a similar portion size (84 g) to investigate the effects of OM on cognition and mood.
The focus on the acute postprandial effects in OYSACO is driven by emerging evidence that certain dietary components can influence cognitive function, mood and inflammatory markers within hours of intake. Nutrients such as vitamins, proteins and polyphenols have been shown to exert rapid effects on brain function through mechanisms including modulation of neurotrophic activity, and inflammation. For instance, single-dose interventions with flavonoid-rich foods (e.g. berries) have demonstrated cognitive benefits within an acute timeframe, alongside changes in neurotrophic and inflammatory markers.22 Similarly, acute supplementation with Lion's Mane mushroom has been shown to improve executive function, working memory, and/or mood in adults.23–25 It is noted that oyster mushrooms contain fewer polyphenols than berries, and lower levels of ergothioneine than Lion's Mane. However, in synergy, it is plausible that OM could elicit similar acute cognitive and mood benefits, potentially through modulation of inflammatory and neurotrophic pathways, particularly in an older adult population. Therefore, we hypothesised that the administration of more than 1 portion of dried OM would result in significantly higher cognitive test scores in the domains of episodic and working memory, executive function and psychomotor function, alongside better mood outcomes, up to 6 h post-consumption of OM intervention compared to placebo. Also, we hypothesised that metabolic, and inflammatory markers would be significantly lower, and brain-derived neurotrophic factor (BDNF) would be significantly higher, at 6 h post-consumption of OM intervention compared to placebo. The novel findings from this research will provide a better overview of the optimal dose of OM for acute postprandial cognitive and mood benefits for this age group and examine potential anti-inflammatory and metabolic related mechanisms that may underlie these effects.
2. Materials and methods
This study was given a favourable ethical opinion by the University of Reading Research Ethics Committee (UREC 22/10) and has been registered on ClinicalTrials.gov (NCT05594329).Sample population
At the time we conducted the OYSACO trial, there were no other clinical trials that specifically examined the acute effects of edible mushrooms on cognition and mood, therefore a power calculation using GPower 3.1 was based on research examining the acute benefits of fruit-based interventions on cognitive function in older adults that previously observed a medium effect size (Cohen's d = 0.55).22,26 A sample size of 30 participants was calculated to provide sufficient statistical power. To allow for a 10% attrition rate, 33 healthy adults aged 60–80 years old were recruited from the area local to Reading (UK). Sixty years was selected as the lower age cutoff in line with the WHO definition of “older adults”. Cognitive decline and changes in mood and inflammatory markers can begin to emerge from this age, making it a sensitive period to study dietary interventions.Participants were required to be healthy with normal vision and hearing, non-vegans/vegetarians, non-smokers, and with a body mass index (BMI) less than 30. A complete list of the inclusion and exclusion criteria for our study can be found in Table S1. Antihypertensive or statin medications for controlling blood pressure and cholesterol levels were the only medications permitted during the trial. No other medications or supplements were permitted.
Interventions
A freeze-dried OM powder (Phillips Gourmet, Pennsylvania, USA) was used in this study. Instant noodles sourced from Iceland Groceries (UK) were used as a vehicle for delivering the intervention, as they are nutritionally poor and thus unlikely to confound the effects of the OM intervention. OM was delivered via a savoury instant noodle meal rather than in capsules to better reflect typical culinary consumption of mushrooms and to accommodate the large portion sizes required which would have been impractical with capsules. We acknowledge that mushroom bioavailability may vary depending on food matrix, however mushrooms are typically consumed with other foods rather than in isolation, thereby maintaining real world relevance. An impartial confederate was responsible for blinding the interventions, so participants and researchers remained unaware of the intervention being administered on each occasion.Table 1 summarises the macronutrient, micronutrient, ergothioneine and total phenolic content of the intervention meals. The dried OM portions of 4.70 g (OM0.5), 9.39 g (OM1) and 18.78 g (OM2), were equivalent to 40 g, 80 g and 160 g of fresh OM, respectively. To match the taste, appearance and calorie content of the intervention meals, maltodextrin (Bulk Powders, UK) and cornflour (Lidl, UK) were added. A small pilot trial was conducted prior to carrying out the OYSACO trial, to ensure that the intervention meals were matched for various palatability and satiety measures.
| Nutrient contents | OM0 | OM0.5 | OM1 | OM2 |
|---|---|---|---|---|
| Instant noodles (g) | 30.00 | 30.00 | 30.00 | 30.00 |
| Dried oyster mushroom (g) | — | 4.70 | 9.39 | 18.78 |
| Cornflour (g) | 10.36 | 7.86 | 5.36 | — |
| Maltodextrin (g) | 4.14 | 3.14 | 2.14 | — |
| Energy (kcal) | 188.55 | 189.07 | 189.58 | 188.80 |
| Protein (g) | 3.09 | 4.40 | 5.72 | 8.35 |
| Total fat (g) | 5.21 | 5.37 | 5.54 | 5.86 |
| Saturated fat (g) | 0.40 | 0.42 | 0.45 | 0.50 |
| Carbohydrates (g) | 31.86 | 31.16 | 30.45 | 28.59 |
| Sugars (g) | 0.85 | 1.29 | 1.74 | 2.63 |
| Fibre (g) | 0.85 | 1.75 | 2.64 | 4.43 |
| Total phenolic content (mg) | 2.20 | 3.20 | 4.03 | 4.46 |
| Ergothioneine (mg) | 0.01 | 2.71 | 6.25 | 13.89 |
Procedure
The full study design is summarised in Fig. 1. Any participants who expressed an interest in taking part in the study were sent a link to complete a demographic health questionnaire, and the EPIC-Norfolk Food Frequency Questionnaire (FFQ) to assess their habitual dietary intake. Eligible participants were then asked to attend a familiarisation session at the laboratory during which anthropometric measurements including body mass index (BMI), heart rate (HR), systolic and diastolic blood pressure (SBP & DBP) were checked, along with a finger-prick to check their haemoglobin (Hb) levels (a requirement for blood sampling). Participants also completed the Raven's Progressive Matrices (RPM) as a measure of fluid intelligence (IQ) and were given two full run throughs of the mood and cognitive battery to control for practice effects in subsequent test sessions.One week after the familiarisation session, participants were asked to attend four test visits that took place at 8 am and finished around 4 pm, each separated by a one-week washout. Prior to attending these visits, participants were asked to follow a low polyphenol diet for 48 h and to consume a standardised breakfast of lightly buttered toast with a glass of water at home before coming to the laboratory. The proscribed diet aimed to minimise potential confounds from background dietary intake, as polyphenol intake prior to testing could acutely influence cognitive, mood, metabolic or inflammatory outcomes. During the test visits, participants were asked to complete the battery of cognitive and mood tasks (baseline), and they then received one of the intervention meals (OM0, OM0.5, OM1 or OM2) in counterbalanced order. After consuming the intervention meal, participants were asked to complete the cognitive and mood tests a further three times (at 2 h, 4 h and 6 h). Participants were provided with a standardised lunch, between 12–12:30 pm, containing a chicken sandwich, a packet of crisps and a glass of water. In addition to the cognitive battery, palatability measures were taken immediately after consumption of the intervention meal. At the end of each cognitive test session, ratings of subjective appetite and fullness were recorded using a visual analogue scale. Finally, a 9 ml blood sample was drawn, 6 h post-consumption of the intervention meal. After completing all four test visits, participants were asked to complete a questionnaire relating to their habitual mushroom intake and received a £200 payment.
Primary outcome measures
Secondary outcome measures
Statistical analysis
Data were analysed using IBM SPSS statistics, version 29. Initially, outliers were identified and excluded using boxplots (using 3*IQR rule). For mood and cognitive outcomes, the main analysis was a linear mixed model (LMM) using a maximum likelihood (ML) approach and an unstructured covariance matrix to model repeated testing. Intervention (OM0, OM0.5, OM1, OM2), time (BL, 2 h, 4 h, 6 h), visit (1–4) and time × intervention were entered as fixed factors in the model. Participant number was treated as a random factor in all analyses to further control for non-independence of data. For all measures except for palatability and serum markers, BL performance was included as a covariate. For cognitive, mood and appetite measures, LMM analysis investigated: (a) the main effect of time (within subject factor), (b) the main effect of intervention (between subject factor), (c) the main effect of visit (within subject factor) and (d) the time × intervention interaction. For palatability measures, LMM analysis examined the main effects of visit and intervention, while for serum marker measures, LMM analysis only investigated the main effect of intervention since these measures were only examined at one time point during the test day. For the Switching task, switch trial type was included as an additional fixed factor. Finally, one-way ANOVA was applied in all measures, to examine whether there were any significant differences between the interventions at BL. In all analyses, a Bonferroni correction was applied to post-hoc pairwise comparisons to compare both between- and within-interventions. All significant main effects and post hoc comparisons have been reported (*p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001).3. Results
Cohort characteristics
As shown in Fig. 2, 112 participants expressed an interest in participating in the study, 71 completed the questionnaires, and 38 of these were excluded for health reasons, for not fully completing the questionnaires, or for inability to commit to the study. Thirty-three participants were invited to take part in the trial, of these, one person was excluded at the familiarisation visit due to health reasons. Participants did not report any side effects while participating in the trial.The data from 32 participants (n = 16 females) were included in the analysis with a mean (±SE) age of 67.9 ± 1.1 and with 96.9% being from a British ethnic origin. Regarding the participants’ habitual mushroom intake in the last year, data from the mushroom survey (Table S2) revealed that most participants in the cohort (70%) consumed at least 1 (or more) portion (∼45 g) of mushrooms per week, with the most popular mushroom species being white button, chestnut and portobello mushrooms. Table 2 provides a summary of the cohort sample characteristics.
| 1Cohort sample characteristics (n = 32) | |
|---|---|
| Abbreviations: BMI (body mass index), CRP (C-reactive protein), DBP (diastolic blood pressure), HDL-c (high density lipoprotein-cholesterol), HR (heart rate), LDL-c (low density lipoprotein-cholesterol), SBP (systolic blood pressure), TAG (triglycerides), TC (total cholesterol). 1n (%): Mean (SE), 2n = 30, 3n = 28. | |
| Age | 67.9 (1.1) |
| Gender | |
| Female | 16 (50%) |
| Male | 16 (50%) |
| Nationality | |
| British/Irish | 31 (96.9%) |
| European | 1 (3.1%) |
| 2Fruit & vegetable intake (portions per day) | 6.1 (0.5) |
| Raven's IQ Score (/60) | 50.8 (1.0) |
| BMI (kg m−2) | 25.0 (0.5) |
| HR (beats per minute) | 64.7 (1.4) |
| SBP (mmHg) | 126.7 (2.1) |
| DBP (mmHg) | 80.4 (1.6) |
| Haemoglobin (g L−1) | 144.4 (2.2) |
| 3 Glucose (mmol L−1) | 5.2 (0.2) |
| 3 TC (mmol L−1) | 5.6 (0.2) |
| 3 HDL-c (mmol L−1) | 1.9 (0.1) |
| 3 LDL-c (mmol L−1) | 3.2 (0.2) |
| 3 TAG (mmol L−1) | 1.3 (0.1) |
| 3 Creatinine (mmol L−1) | 84.1 (2.1) |
| 3 CRP (mg L−1) | 1.6 (0.3) |
Mood & cognitive function outcomes
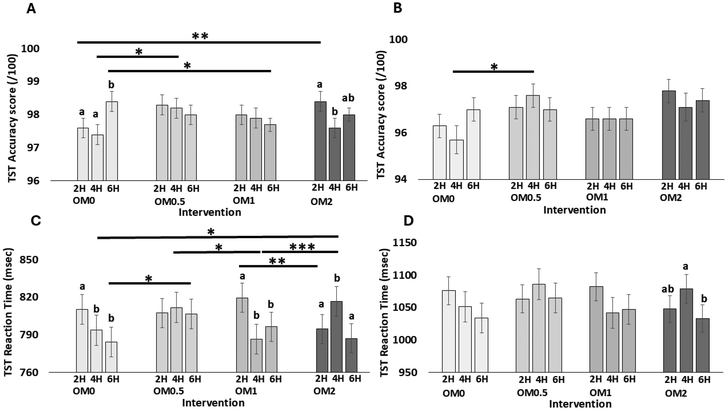
Estimated marginal means and standard errors for all measures and time points are available in Tables S3–10. Only significant main effects of visit, time, intervention, and time × intervention interactions and relevant post hoc comparisons are presented here.For TST reaction time (RT), a significant main effect of time [F(2907) = 11.480, p < 0.001] was shown, with a quicker RT at 6 h compared to 2 h (p < 0.001) and 4 h (p = 0.010). However, intervention-related findings failed to show any clear pattern. A time × intervention interaction [F(6907) = 9.695, p < 0.001] was observed, with between-intervention pairwise comparisons (Fig. 5C), revealing quicker RT for OM0 at 4 h compared to OM2 (p = 0.030), and at 6 h compared to OM0.5 (p = 0.036). Additionally, quicker RT was shown for OM1 at 4 h compared to OM0.5 (p = 0.015) and OM2 (p = 0.001) and slower RT at 2 h compared to OM2 (p = 0.004). Within-intervention pairwise comparisons indicated quicker RT for OM0 at 4 h (p = 0.021) and 6 h (p < 0.001) compared to 2 h, for OM1 at 4 h (p < 0.001) and 6 h (p < 0.001) compared to 2 h and for OM2 at 2 h (p < 0.001) and 6 h (p < 0.001) compared to 4 h. When looking at S1 trial only, data analysis again showed a significant main effect of time [F(2185) = 4.556, p = 0.012], with quicker RT at 6 h compared to 2 h (p = 0.031) and 4 h (p = 0.036). A time × intervention interaction [F(6185) = 2.343, p = 0.033] was also shown, with pairwise comparisons (Fig. 5D) revealing significantly quicker RT for OM2 at 6 h compared to 4 h (p = 0.005). These findings suggest mixed effects of OM interventions on TST performance. Although time-related improvements, particularly faster RT on TST at 6 h were consistently observed, intervention-related effects were inconsistent and lacked a clear pattern. This may be due in part to ceiling effects in accuracy.
No significant main effects or time × intervention interactions were shown for Corsi Blocks Task (CBT), Simple or Complex Finger Tapping Tasks (SFT, CFT).
Palatability & appetite outcomes
Although no significant intervention-related findings were observed across appetite measures, a significant time × intervention interaction [F(9119) = 2.095, p = 0.035] was observed for hunger. Between-intervention pairwise comparisons indicated that participants reported significantly greater hunger for OM1 at 0 h compared to OM0.5 (p = 0.046) and OM2 (p = 0.048), and greater hunger for OM0.5 at 4 h compared to OM2 (p = 0.048). Within-intervention pairwise comparisons showed that OM0.5 was more filling at 0 h compared to 2 h (p = 0.006), 4 h (p = 0.018) and 6 h (p = 0.009), while OM2 was more filling at 0 h compared to 2 h (p < 0.001) and 6 h (p = 0.002) and at 4 h compared to 2 h (p = 0.001) and 6 h (p < 0.001).
Biochemical outcomes
Analysis of BDNF revealed a significant main effect of intervention [F(3.60) = 5.626, p = 0.002], with pairwise comparisons (Fig. 7A) showing significantly lower BDNF levels for OM2 compared to OM0.5 (p = 0.035) and OM0 (p = 0.001) indicating a dose-dependent effect.A significant main effect of intervention was shown for nitrite [F(3.72) = 5.584, p = 0.002], NOX2 [F(3.72) = 5.503, p = 0.002] and iNOS [F(3.72) = 7.377, p < 0.001]. Pairwise comparisons (Fig. 7B–D) revealed significantly lower levels of nitrite (p < 0.001), NOX2 (p = 0.001) and iNOS (p = 0.005) for OM1 compared to OM0. Significantly lower levels of NOX2 were also shown for OM2 compared to OM0 (p = 0.026) and lower levels of iNOS for OM0.5 (p = 0.002) and OM2 (p < 0.001) compared to OM0.
While OM intervention appeared to show an anti-inflammatory effect on some markers, no significant between-intervention differences were observed for glucose, TAG or IL-6.
4. Discussion
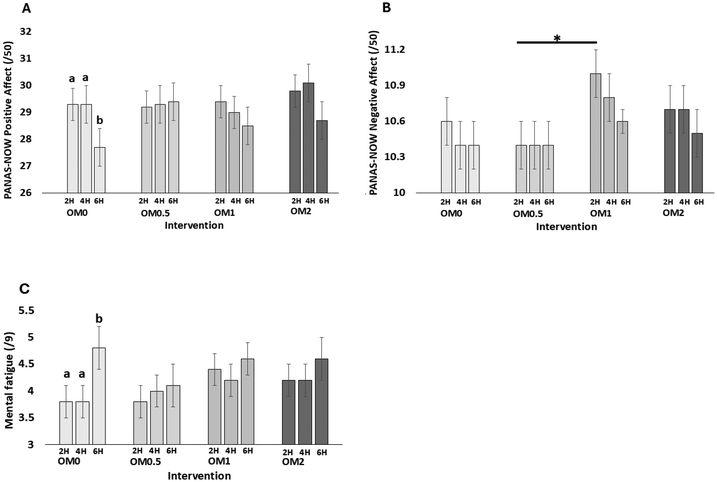
Findings of the OYSACO study have shown that the consumption of 1 portion of dried OM intervention (equivalent to 80 g fresh) demonstrated potential in supporting mood and mental fatigue for up to 6 h in healthy older adults. These mood-stabilising effects were accompanied by significantly lower inflammatory markers, such as nitrite, NOX2 and iNOS, suggesting a possible mechanistic link between inflammation and mood regulation. However, cognitive findings were more variable, with no consistent pattern of episodic memory, executive function, or working memory effects observed following OM intervention. Nevertheless, there was an indication that the effects of OM might be dose dependent, with 1 portion appearing to show an advantage, particularly over half a portion, on measures of both mood and episodic memory, and some inflammatory markers.It is noteworthy that OM intervention appeared to buffer against the typical decline in positive affect and increase in mental fatigue seen over the course of the day, especially under sustained cognitive demand. Compared to the control, which showed expected mood deterioration and increased fatigue during the day, the OM intervention maintained more stable mood and fatigue, suggesting potential acute benefits for supporting mental well-being and resilience during cognitive demanding periods. In terms of cognitive domains, our results are aligned with previous findings observed in polyphenol research. For example, haskap berries, which are rich in anthocyanins, have shown significant improvements in word recall and recognition within 90-minutes of higher-dose intake (200 mg and 400 mg) in healthy older adults, although effects on other cognitive domains such as working memory and executive function have been less consistent.26 This result suggests that acute nutritional supplementation may selectively support certain cognitive domains rather than enhancing cognition more broadly. It is also plausible that shared phytonutrients between OM and other plant-based interventions, such as polyphenols, may underlie these selective effects. It should be noted, though, that OM contains relatively lower polyphenol concentrations than interventions typically used in polyphenol research. Acute mushroom intervention studies have similarly shown benefits to executive function,24 psychomotor function,25 working memory,23 and mood23 in adults, suggesting that mushroom-specific compounds such as ergothioneine may also play a role. However, these studies all investigated Lion's Mane mushroom which is a particularly rich source of ergothioneine compared to OM. Although the OM intervention administered here contained significantly higher levels of ergothioneine compared to the control, serum levels of this metabolite did not significantly increase following consumption, suggesting limited acute bioavailability or uptake within the timeframe assessed. Therefore, observed OM-related effects may be due to a synergy between polyphenols and ergothioneine, or other bioactive macro- or micronutrient compounds present. Interestingly, although the intervention-related effects on executive function – as measured by the TST – were inconsistent and lacked a clear pattern, a noticeable dip in performance for both accuracy and RT was observed predominantly following the OM2 intervention. This decline in performance was not evident in the other cognitive domains or at any other OM dose assessed in this study. This unexpected decline warrants further investigation, especially in relation to potential interactions between OM, mood, mental fatigue, and metabolic factors during the afternoon period.
Findings from the inflammatory measurements add an important layer to the mechanistic understanding of OM intervention. Specifically, our study showed that serum from OM-supplemented older adults can significantly lower the production of inflammatory stress signals, compared to serum obtained following consumption of the placebo (OM0), in LPS-stressed HAPI rat microglial cells, in vitro. This suggests that OM might indirectly benefit cognition through involvement in the nitric oxide (NO) signalling cascade. Prior research has implicated nitric oxide synthase (NOS) and NO dysregulation in vascular dementia and endothelial dysfunction,39 highlighting the need for further exploration of this pathway in relation to mushroom supplementation. Regarding metabolic markers, no significant differences were observed in glucose, TAG and IL-6 measures. This result contrasts with findings from Jayasuriya and colleagues, that showed that when people with type II diabetes consumed an OM intervention daily for 2-weeks – followed by a single OM preload 30-minutes before an oral glucose tolerance test – they exhibited a significant reduction in 2 h glucose and increase in 2 h insulin, measured in serum.40 Differences in health status or the transient nature of these metabolic effects may suggest that alternative mechanisms such as NO signalling may better explain the acute behavioural outcomes observed. Unexpectedly, regarding neurotrophic effects, BDNF levels were significantly lower in a dose-dependent way following OM intervention compared to placebo; however, it remains unclear whether this finding impacted the behavioural outcomes of the study. Previous studies investigating polyphenol-rich interventions have also reported mixed findings. For instance, acute supplementation with blueberries41 or walnuts35 did not result in significant differences in BDNF levels relative to placebo. Although it might seem counterintuitive that BDNF levels at 6 h were dose-dependently lower than placebo, such short-term daily fluctuations in neurotrophic factors are not uncommon and could reflect transient regulatory responses rather than a negative outcome. It is well-documented that BDNF levels naturally decline over the course of the day, which may have contributed to the levels observed in our findings.42
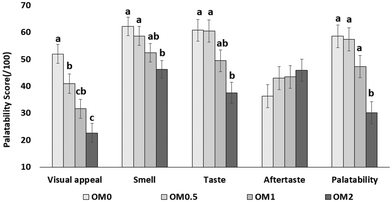
This study is the first to examine the acute effects of ergothioneine-rich OM on cognition and mood in healthy older adults in the UK. While the findings offer promising insights, limitations should be acknowledged. Although the cross-over design enhanced statistical power by allowing participants to serve as their own controls, the lack of consistent intervention effects may reflect overestimation of expected effect sizes during the sample size calculation. Power estimates were based on previous studies using fruit-derived interventions, which may not translate directly to the effects of OM. As such, while the target sample size was met, the study may still have been underpowered if effects associated with OM interventions were smaller than expected. In addition, despite extensive piloting, differences in taste and palatability ratings between interventions emerged, that were not apparent during the pilot phase. While these differences could theoretically influence subjective measures such as mood, the consistency of mood benefits across OM doses suggests that the observed effects are unlikely to be driven by palatability differences. The current findings are limited to the immediate postprandial period, and it remains essential to explore whether these acute effects can be sustained or enhanced though longer-term supplementation, particularly in the context of healthy ageing. An additional limitation is that blood samples were only collected post-dose. Collecting baseline and post-dose samples at each visit would have enabled a clearer assessment of acute biomarker changes and reduced the potential influence of day-to-day variability. However, cannulation/repeated sampling were not permitted under current University of Reading guidelines. A further caveat relates to the cognitive task performance. Participants performed exceptionally well on cognitive tasks at baseline, resulting in ceiling effects, that may have limited the sensitivity of the tasks to detect subtle intervention-related changes. Although a familiarisation session was included to reduce practice effects,43 improvements across subsequent visits for some measures (e.g. TST and CBT) indicated that practice effects persisted. Consequently, in this high-performing healthy cohort, any small protective or enhancing effects of the OM intervention may have been masked or underestimated due to these ongoing practice/visit-related effects.
5. Conclusion
This is the first study to examine the acute effects of OM intervention in healthy older adults. The findings are promising, suggesting maintenance in mood and mental fatigue, alongside lower inflammatory markers, for up to 6 h, post-consumption of ergothioneine-rich freeze-dried OM. However, neurocognitive and metabolic effects were less clear and warrant further investigation to better understand how these physiological effects may contribute to the observed behavioural effects. Longer-term supplementation studies are also needed to determine longer term benefits of OM on cognitive performance and mood during the aging process.Author contributions
C. M. W., B. S.-H. and L. B. designed the study. S. C. conducted the study. B. S.-H., D. F., Z. Z. and A. R.-M. analysed the blood samples. S. C. performed all statistical analyses. S. C., L. B. and C. M. W. drafted the paper. All authors approved the final version of the manuscript.Ethical approval and informed consent
This study has been approved by the University of Reading ethics committee and has, therefore, been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. Informed consent was obtained from each participant prior to attending the study visits.Conflicts of interest
The authors declare that they have no conflict of interest.Data availability
The data supporting this article have been included as part of the supplementary information (SI). Supplementary information is available. See DOI: https://doi.org/10.1039/d5fo03075g.Acknowledgements
This study was conducted as part of a PhD studentship funded by The Mushroom Council®. The funder made no contribution during the design or interpretation of the study, nor in the interpretation of findings to publish. The authors thank all volunteers who participated in the study.References
- T. Mura, N. Coley, H. Amieva, C. Berr, A. Gabelle, P. J. Ousset, B. Vellas, S. Andrieu and G. D. s. group, Cognitive decline as an outcome and marker of progression toward dementia, in early preventive trials, Alzheimers Dement., 2022, 18(4), 676–687, DOI:10.1002/alz.12431.
- F. B. Hu, Diet strategies for promoting healthy aging and longevity: An epidemiological perspective, J. Intern. Med., 2024, 295(4), 508–531, DOI:10.1111/joim.13728 , From 2024 Apr.
- Z. Chrzastek, A. Guligowska, P. Sobczuk and T. Kostka, Dietary factors, risk of developing depression, and severity of its symptoms in older adults—A narrative review of current knowledge, Nutrition, 2023, 106, 111892, DOI:10.1016/j.nut.2022.111892.
- O. van de Rest, A. A. Berendsen, A. Haveman-Nies and L. C. de Groot, Dietary patterns, cognitive decline, and dementia: a systematic review, Adv. Nutr., 2015, 6(2), 154–168, DOI:10.3945/an.114.007617.
- C. W. Phan, P. David and V. Sabaratnam, Edible and Medicinal Mushrooms: Emerging Brain Food for the Mitigation of Neurodegenerative Diseases, J. Med. Food, 2017, 20(1), 1–10, DOI:10.1089/jmf.2016.3740.
- A. Singh, R. K. Saini, A. Kumar, P. Chawla and R. Kaushik, Mushrooms as Nutritional Powerhouses: A Review of Their Bioactive Compounds, Health Benefits, and Value-Added Products, Foods, 2025, 14(5), 741, DOI:10.3390/foods14050741 , From 2025 Feb 22.
- S. N. Rai, D. Mishra, P. Singh, E. Vamanu and M. P. Singh, Therapeutic applications of mushrooms and their biomolecules along with a glimpse of in silico approach in neurodegenerative diseases, Biomed. Pharmacother., 2021, 137, 111377, DOI:10.1016/j.biopha.2021.111377.
- D. M. Ba, X. Gao, L. Al-Shaar, J. Muscat, V. M. Chinchilli, P. Ssentongo, R. B. Beelman and J. Richie, Mushroom intake and cognitive performance among US older adults: the National Health and Nutrition Examination Survey, 2011–2014, Br. J. Nutr., 2022, 128(11), 2241–2248, DOI:10.1017/S0007114521005195.
- L. Feng, I. K. Cheah, M. M. Ng, J. Li, S. M. Chan, S. L. Lim, R. Mahendran, E. H. Kua and B. Halliwell, The Association between Mushroom Consumption and Mild Cognitive Impairment: A Community-Based Cross-Sectional Study in Singapore, J. Alzheimers Dis., 2019, 68(1), 197–203, DOI:10.3233/JAD-180959.
- S. K. Park, C. M. Oh, J. H. Ryoo and J. Y. Jung, The protective effect of mushroom consumption on depressive symptoms in Korean population, Sci. Rep., 2022, 12(1), 21914, DOI:10.1038/s41598-022-26549-5.
- D. M. Ba, X. Gao, L. Al-Shaar, J. E. Muscat, V. M. Chinchilli, R. B. Beelman and J. P. Richie, Mushroom intake and depression: A population-based study using data from the US National Health and Nutrition Examination Survey (NHANES), 2005–2016, J. Affective Disord., 2021, 294, 686–692, DOI:10.1016/j.jad.2021.07.080.
- S. Cha, L. Bell and C. M. Williams, The Relationship between Mushroom Intake and Cognitive Performance: An Epidemiological Study in the European Investigation of Cancer-Norfolk Cohort (EPIC-Norfolk), Nutrients, 2024, 16(3), 353, DOI:10.3390/nu16030353.
- S. Cha, L. Bell, B. Shukitt-Hale and C. M. Williams, A review of the effects of mushrooms on mood and neurocognitive health across the lifespan, Neurosci. Biobehav. Rev., 2024, 158, 105548, DOI:10.1016/j.neubiorev.2024.105548.
- K. Mori, S. Inatomi, K. Ouchi, Y. Azumi and T. Tuchida, Improving effects of the mushroom Yamabushitake (Hericium erinaceus) on mild cognitive impairment: a double-blind placebo-controlled clinical trial, Phytother. Res., 2009, 23(3), 367–372, DOI:10.1002/ptr.2634.
- I.-C. Li, H.-H. Chang, C.-H. Lin, W.-P. Chen, T.-H. Lu, L.-Y. Lee, Y.-W. Chen, Y.-P. Chen, C.-C. Chen and D. P.-C. Lin, Prevention of Early Alzheimer's Disease by Erinacine A-Enriched Hericium erinaceus Mycelia Pilot Double-Blind Placebo-Controlled Study, Front. Aging Neurosci., 2020, 12, 155, DOI:10.3389/fnagi.2020.00155 , Clinical Trial.
- R. S. Jarial, K. Jarial and J. N. Bhatia, Comprehensive review on oyster mushroom species (Agaricomycetes): Morphology, nutrition, cultivation and future aspects, Heliyon, 2024, 10(5), e26539, DOI:10.1016/j.heliyon.2024.e26539 , From NLM.
- M. E. Effiong, C. P. Umeokwochi, I. S. Afolabi and S. N. Chinedu, Assessing the nutritional quality of Pleurotus ostreatus (oyster mushroom), Front. Nutr., 2023, 10, 1279208, DOI:10.3389/fnut.2023.1279208 , From NLM.
- N. Nakamichi, K. Nakayama, T. Ishimoto, Y. Masuo, T. Wakayama, H. Sekiguchi, K. Sutoh, K. Usumi, S. Iseki and Y. Kato, Food-derived hydrophilic antioxidant ergothioneine is distributed to the brain and exerts antidepressant effect in mice, Brain Behav., 2016, 6(6), e00477, DOI:10.1002/brb3.477 , From NLM.
- N. Nakamichi, S. Nakao, M. Nishiyama, Y. Takeda, T. Ishimoto, Y. Masuo, S. Matsumoto, M. Suzuki and Y. Kato, Oral Administration of the Food-Derived Hydrophilic Antioxidant Ergothioneine Enhances Object Recognition Memory in Mice, Curr. Mol. Pharmacol., 2021, 14(2), 220–233, DOI:10.2174/1874467213666200212102710.
- S. Cha, L. Bell, J. Eastwood, D. Fisher, B. Shukitt-Hale, Z. Zhang, A. Rodriguez-Mateos and C. M. Williams, A randomised controlled study to investigate the cognitive, mood, metabolic and anti-inflammatory effects of chronic oyster mushroom intervention in healthy older adults [Unpublished manuscript]. 2025.
- C. N. Uffelman, R. Harold, E. S. Hodson, N. I. Chan, D. Foti and W. W. Campbell, Effects of Consuming White Button and Oyster Mushrooms within a Healthy Mediterranean-Style Dietary Pattern on Changes in Subjective Indexes of Brain Health or Cognitive Function in Healthy Middle-Aged and Older Adults, Foods, 2024, 13(15), 2319, DOI:10.3390/foods13152319.
- L. Bell, D. J. Lamport, L. T. Butler and C. M. Williams, A Review of the Cognitive Effects Observed in Humans Following Acute Supplementation with Flavonoids, and Their Associated Mechanisms of Action, Nutrients, 2015, 7(12), 10290–10306, DOI:10.3390/nu7125538 , From 2015 Dec 9.
- M. B. La Monica, B. Raub, E. J. Ziegenfuss, S. Hartshorn, J. Grdic, A. Gustat, J. Sandrock and T. N. Ziegenfuss, Acute effects of naturally occurring Guayusa tea and Nordic Lion's mane extracts on cognitive performance, Nutrients, 2023, 15(24), 5018 CrossRef CAS PubMed.
- S. Docherty, F. L. Doughty and E. F. Smith, The acute and chronic effects of lion's mane mushroom supplementation on cognitive function, stress and mood in young adults: A double-blind, parallel groups, pilot study, Nutrients, 2023, 15(22), 4842 CrossRef PubMed.
- G. Surendran, J. Saye, S. Binti Mohd Jalil, J. Spreadborough, K. Duong, I. M. Shatwan, D. Lilley, M. Heinrich, G. F. Dodd and S. Surendran, Acute effects of a standardised extract of Hericium erinaceus (Lion's Mane mushroom) on cognition and mood in healthy younger adults: a double-blind randomised placebo-controlled study, Front. Nutr., 2025, 12, 1405796 CrossRef PubMed.
- L. Bell and C. Williams, A pilot dose–response study of the acute effects of haskap berry extract (Lonicera caerulea L.) on cognition, mood, and blood pressure in older adults, Eur. J. Nutr., 2019, 58(8), 3325–3334, DOI:10.1007/s00394-018-1877-9.
- D. Watson, L. A. Clark and A. Tellegen, Development and validation of brief measures of positive and negative affect: the PANAS scales, J. Pers. Soc. Psychol., 1988, 54(6), 1063–1070, DOI:10.1037//0022-3514.54.6.1063.
- K. Kunasegaran, A. M. H. Ismail, S. Ramasamy, J. V. Gnanou, B. A. Caszo and P. L. Chen, Understanding mental fatigue and its detection: a comparative analysis of assessments and tools, PeerJ, 2023, 11, e15744, DOI:10.7717/peerj.15744.
- A. Rey, Rey auditory verbal learning test (RAVLT), L'Examen clinique en psychologie, PUF Paris, 1964 Search PubMed.
- M. G. Miller, D. A. Hamilton, J. A. Joseph and B. Shukitt-Hale, Dietary blueberry improves cognition among older adults in a randomized, double-blind, placebo-controlled trial, Eur. J. Nutr., 2018, 57(3), 1169–1180, DOI:10.1007/s00394-017-1400-8.
- D. Berch, R. Krikorian and E. Huha, The Corsi Block-Tapping Task: Methodological and Theoretical Considerations, Brain Cogn., 1999, 38(3), 317–338, DOI:10.1006/brcg.1998.1039.
- L. Bell, A. R. Whyte, D. J. Lamport, J. P. E. Spencer, L. T. Butler and C. M. Williams, Grape seed polyphenol extract and cognitive function in healthy young adults: a randomised, placebo-controlled, parallel-groups acute-on-chronic trial, Nutr. Neurosci., 2022, 25(1), 54–63, DOI:10.1080/1028415X.2019.1708033.
- N. Cheng, K. L. Barfoot, R. Le Cozannet, P. Fança-Berthon, D. J. Lamport and C. M. Williams, Wild Blueberry Extract Intervention in Healthy Older Adults: A Multi-Study, Randomised, Controlled Investigation of Acute Cognitive and Cardiovascular Effects, Nutrients, 2024, 16(8), 1180, DOI:10.3390/nu16081180.
- A. R. Whyte, N. Cheng, E. Fromentin and C. M. Williams, A Randomized, Double-Blinded, Placebo-Controlled Study to Compare the Safety and Efficacy of Low Dose Enhanced Wild Blueberry Powder and Wild Blueberry Extract (ThinkBlue™) in Maintenance of Episodic and Working Memory in Older Adults, Nutrients, 2018, 10(6), 660, DOI:10.3390/nu10060660 , From NLM.
- L. Bell, G. F. Dodd, M. Jeavons, D. R. Fisher, A. R. Whyte, B. Shukitt-Hale and C. M. Williams, The impact of a walnut-rich breakfast on cognitive performance and brain activity throughout the day in healthy young adults: a crossover intervention trial, Food Funct., 2025, 16(5), 1696–1707, 10.1039/d4fo04832f.
- M. Domínguez-Fernández, Y. Xu, P. Young Tie Yang, W. Alotaibi, R. Gibson, W. L. Hall, L. Barron, I. A. Ludwig, C. Cid and A. Rodriguez-Mateos, Quantitative Assessment of Dietary (Poly)phenol Intake: A High-Throughput Targeted Metabolomics Method for Blood and Urine Samples, J. Agric. Food Chem., 2021, 69(1), 537–554, DOI:10.1021/acs.jafc.0c07055.
- L. Y. Wu, I. K. Cheah, J. R. Chong, Y. L. Chai, J. Y. Tan, S. Hilal, H. Vrooman, C. P. Chen, B. Halliwell and M. K. P. Lai, Low plasma ergothioneine levels are associated with neurodegeneration and cerebrovascular disease in dementia, Free Radicals Biol. Med., 2021, 177, 201–211, DOI:10.1016/j.freeradbiomed.2021.10.019.
- A. A. Mulligan, R. N. Luben, A. Bhaniani, D. J. Parry-Smith, L. O'Connor, A. P. Khawaja, N. G. Forouhi, K. T. Khaw and E.-N. F. Study, A new tool for converting food frequency questionnaire data into nutrient and food group values: FETA research methods and availability, BMJ Open, 2014, 4(3), e004503, DOI:10.1136/bmjopen-2013-004503.
- H. Y. Zhu, F. F. Hong and S. L. Yang, The Roles of Nitric Oxide Synthase/Nitric Oxide Pathway in the Pathology of Vascular Dementia and Related Therapeutic Approaches, Int. J. Mol. Sci., 2021, 22(9), 4540, DOI:10.3390/ijms22094540.
- W. J. Jayasuriya, C. A. Wanigatunge, G. H. Fernando, D. T. Abeytunga and T. S. Suresh, Hypoglycaemic activity of culinary Pleurotus ostreatus and P. cystidiosus mushrooms in healthy volunteers and type 2 diabetic patients on diet control and the possible mechanisms of action, Phytother. Res., 2015, 29(2), 303–309, DOI:10.1002/ptr.5255.
- G. Dodd, C. Williams, L. Butler and J. Spencer, Acute effects of flavonoid-rich blueberry on cognitive and vascular function in healthy older adults, Nutr. Healthy Aging, 2019, 5(2), 119–132, DOI:10.3233/NHA-180056.
- S. W. Choi, S. Bhang and J. H. Ahn, Diurnal variation and gender differences of plasma brain-derived neurotrophic factor in healthy human subjects, Psychiatry Res., 2011, 186(2–3), 427–430, DOI:10.1016/j.psychres.2010.07.028.
- L. Bell, D. J. Lamport, D. T. Field, L. T. Butler and C. M. Williams, Practice effects in nutrition intervention studies with repeated cognitive testing, Nutr. Healthy Aging, 2018, 4(4), 3325–3334, DOI:10.3233/NHA-170038.
| This journal is © The Royal Society of Chemistry 2026 |