Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Effects of incrementally increased plant-based protein intake on gut microbiota and inflammatory–metabolic biomarkers in healthy adults
Samira Prado†
 *a,
Annalena Kamm†
*a,
Annalena Kamm† a,
Katharina Dannenberg
a,
Katharina Dannenberg ab,
Isabel Keidel‡
ab,
Isabel Keidel‡
 a,
Victor Castro-Alves
a,
Victor Castro-Alves c,
Tuulia Hyötyläinenc,
Marleen Lentjes
c,
Tuulia Hyötyläinenc,
Marleen Lentjes ad,
Dirk Repsilbera,
Tatiana M. Marques§
a and
Robert J. Brummer§a
ad,
Dirk Repsilbera,
Tatiana M. Marques§
a and
Robert J. Brummer§a
aNutrition-Gut-Brain Interactions Research Center, School of Medical Sciences, Faculty of Medicine and Health, Örebro University, Södra Grev Rosengatan 42A, SE-701 82, Örebro, Sweden. E-mail: samira.prado@oru.se; annalena.kamm@oru.se; katharina.dannenberg@oru.se; isabel.zurblihn@tum.de; maria.lentjes@oru.se; dirk.repsilber@oru.se; tatiana.marques@oru.se; robert.brummer@oru.se; Tel: +46 19 303470
bClinical Genomics Örebro, Science for Life Laboratory, Örebro University, Örebro, Sweden
cMan-Technology-Environment Research Center, School of Science and Technology, Örebro University, SE-702 81 Örebro, Sweden. E-mail: victor.castro-alves@oru.se; tuulia.hyotylainen@oru.se
dClinical Epidemiology and Biostatistics, School of Medical Sciences, Faculty of Medicine and Health, Örebro University, SE-701 82, Örebro, Sweden
First published on 2nd January 2026
Abstract
Shifting to a plant-based diet naturally alters protein source choices. In many countries, protein from yellow pea is widely used as a main ingredient in meat alternatives. Still, its biological effects, especially regarding gastrointestinal health, remain incompletely understood. The aim of our study was to investigate how a weekly increase in the intake of a well-characterized pea protein isolate affects surrogate markers of health, fecal short-chain fatty acids and gut microbiota composition in healthy individuals. Male and female adults (N = 29) participated in this exploratory intervention study. A 4-week pre-intervention period for questionnaires and fecal samples collection was followed by a 4-week supplementation. Participants consumed isolated pea protein in weekly increasing amounts, starting from 0.25 g per kg body mass per day in week 5 to 1.00 g per kg body mass per day in week 8. Questionnaire data, fecal samples as well as fasting blood and 24 h urine samples were collected weekly. Data from biological samples and questionnaires confirmed a healthy study population and compliance. Fecal calprotectin levels significantly increased only in a subset of participants, which was accompanied by higher fecal water cytotoxicity in vitro. Short-chain fatty acids mainly rose in those subjects with stable calprotectin levels. Relative abundances of Limosilactobacillus frumenti, Odoribacter splanchnicus and Lactobacillus crispatus increased significantly in the total population during the intervention while the relative abundance of Bifidobacterium longum and Bifidobacterium catenulatum decreased. Our results indicate that an increased intake of pea protein isolate affects the growth of certain beneficial bacterial strains and differentially influences markers related to gut inflammation in healthy individuals.
1. Introduction
A societal shift from animal- to plant-based diet is taking place, driven in part by environmental concerns.1 Plant-based proteins from pulses, the edible seeds of legumes, including beans, peas, and lentils, have been shown to support cardiovascular, metabolic health and to exert anti-inflammatory effects.2 Among these, peas have emerged as a prominent source due to their adaptability to grow in moderate climates and their lower allergenic potential compared to soy.3 In addition, peas have favorable technological characteristics such as good emulsifying properties, the ability to form gels,4 and a desirable nutritional quality regarding amino acid profile.5 These attributes may explain the growing investigation of isolated pea protein in Europe, as well as its application in various food products,6–8 for example, commercially available meat alternatives.9,10Although plant-based foods are generally associated with health benefits, their proteins often have a lower digestibility compared to animal proteins. Hence, part of the ingested protein may escape the digestion and absorption in the small intestine, especially when consumed in higher amounts, reaching the colon and undergoing microbial fermentation.11 Colonic fermentation can proceed through two main routes: saccharolytic and proteolytic fermentation. Saccharolytic fermentation utilizes dietary fibers and primarily produces short-chain fatty acids (SCFA), namely butyrate, acetate and propionate. These SCFA are generally considered beneficial for gut health by supporting lipid, glucose, and immune homeostasis.12,13 Proteolytic fermentation uses amino acids and peptides as substrates and is often associated with detrimental effects14 due to the generation of metabolites such as ammonia.15 Ammonia has been linked to intestinal inflammation, intestinal barrier dysfunction, higher intracolonic pH, which affects the metabolic activity of the colonic ecosystem, and increased risk of colorectal cancer.14,16,17 While beneficial SCFA are primarily originated from saccharolytic fermentation, higher protein intake can also alter SCFA patterns by increasing propionate and valerate levels as observed in human dietary intervention studies.18 Moreover, evidence from human in vitro colonic fermentation indicates that dietary protein can also serve as a substrate for butyrate production.19 Such overlaps in protein and saccharolytic SCFA production arise because many metabolic pathways are shared among microbes, and substrate availability determines whether carbohydrates or proteins are preferentially fermented.20
Both ammonia and SCFA are by-products of intestinal microbial fermentation; however, while SCFA generally exert anti-inflammatory and barrier-protective effects,21 ammonia accumulation has been associated with mucosal irritation and inflammation.22 Beyond microbial metabolites, host-derived compounds also serve as indicators of gut inflammatory status, exemplified by fecal calprotectin. Produced by neutrophils, fecal calprotectin acts as a surrogate marker of intestinal inflammation and is routinely measured in clinical and epidemiological settings.23,24 Although the relationship between fecal calprotectin and diet has not been widely explored, animal-based diets have been associated with its increased levels in patients with Crohn's disease and ulcerative colitis.25 In patients with type 2 diabetes, only plant-based but not animal-based protein increased calprotectin in blood.26 Therefore, overall health markers, including blood glucose, insulin, and lipids, along with fecal calprotectin and emerging gut health markers such as SCFA, are useful for assessing whether higher plant-based protein intake affects surrogate indicators of (gut) health.
Although changes in dietary protein sources and amounts do not always alter gut microbial composition, they have been consistently linked to shifts in colonic bacterial metabolism.18,27–29 These metabolic adaptations likely reflect changes in how microbial pathways are engaged depending on substrate availability and local pH. Nevertheless, little is known about how single plant-derived proteins influence these processes in humans. So far, only in vitro fermentation studies have been performed using isolated pea protein30 and data on in vivo effects in humans are scarce. To the best of our knowledge, no human dietary intervention study has yet investigated the association between intake of isolate pea protein and its effects on gut-related health markers in a non-diseased population. Therefore, the aim of this single-arm exploratory dietary intervention study was to evaluate the effect of an increasing intake of isolated pea protein on various surrogate markers of health, fecal SCFA, and gut microbiota composition in healthy individuals. The measurements used to characterize the study population included routine blood markers of health (glucose, insulin, creatinine, urea, high-density lipoprotein (HDL), low-density lipoprotein (LDL), total cholesterol, C-reactive protein (CRP), and triglycerides), urinary markers of protein intake (urea, creatinine, and uric acid), and fecal markers (calprotectin, ammonia, acetate, propionate, butyrate, and valerate) together with gut microbiota composition.
2. Materials and methods
2.1. Study design
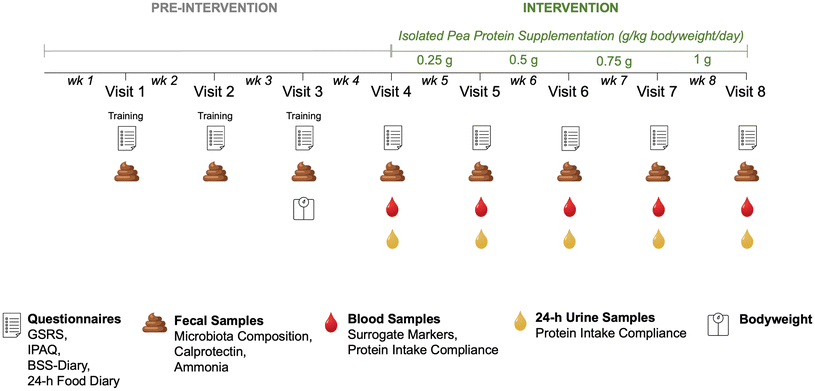
This intervention study was conducted at Örebro University, Sweden in two periods between October–December 2021 and February–April 2022, respectively. The study was approved by the Swedish Ethical Review Authority (Dnr 2021-03256, 08-24-2021), conducted in accordance with the Declaration of Helsinki, and registered at https://www.clinicaltrials.gov (NCT05367804).After assessing eligibility on a first screening visit and after participants have signed an informed consent, participants came to the study center on eight occasions scheduled one week apart (Fig. 1). On the first three study visits, they handed in fecal samples collected at home or at the study site, a completed Gastrointestinal Symptom Rating Scale (GSRS), an International Physical Activity Questionnaire (IPAQ), a Bristol Stool Scale (BSS) diary as well as three 24 h food diaries. Additionally, participants were weighted on visit 3 to calculate the amount of the protein supplement they would receive from visit 4 onwards. Study visits 4–8 were scheduled in the morning after an overnight fast of at least 10 hours and included, in addition to fecal sample collection and completion of questionnaires, the collection of 24 h urine samples as well as fasting blood samples.
2.2. Study subjects
Healthy male and female adults were recruited via social media, the university's webpage as well as posters placed in public areas in Örebro and the University Campus. Applied inclusion criteria were: age 18–45 years; body mass index (BMI) between 18.5–30 kg m−2; stable weight within the previous three months; maintenance of the usual physical activity habits during the study; intake of dietary fiber between 15–25 g day−1 (as evaluated by two food diaries and one 24 h recall); omnivores or vegetarians.Interested individuals were excluded if they fulfilled any of the following exclusion criteria: acute or chronic disease, inflammatory or functional gastrointestinal diseases and any other disease or disorder that could affect the outcome of the study; use of a medication that may interfere the study outcome; eating disorder; high-protein intake (more than 15% of energy or maximum 1.2 g per kg body mass per day as evaluated by two food diaries and one 24 h recall); use of antibiotic medication during the last three months prior the first visit; use of laxative or anti-diarrheal medication within the past three months before the study; regular consumption of probiotic or prebiotic products for the past six weeks before the study; special diet that is considered to affect the study participation and/or study results, for example, high-protein diets; more than five hours of moderate-vigorous exercise/week; pregnancy or breastfeeding; intolerance to dietary supplements that will be used in the study; tobacco and nicotine use; abuse of alcohol or drugs.
2.3. Study product
During the intervention period (visit 4–8), participants consumed protein isolate from yellow pea (Pisane C9, COSUCRA, Belgium), which has been well characterized in the literature,4,5,31,32 and is widely used as food product ingredient.6–8 The protein isolate was manufactured by dehulling and milling the peas, solubilization in water, and decantation to separate the soluble (protein) and insoluble (fiber, starch) fractions, pasteurization of the soluble protein fraction which was followed by purification, concentrating, and spray-drying.8 An isolate was used in the study to minimize the impact of antinutritional factors on the gastrointestinal system and minimize confounders with other food components from the intervention product.5,33 The chemical composition of the provided isolated pea protein was calculated based on a study within our research consortium (SI Table S1).5The amount of protein powder was calculated based on the individual body mass at study visit 3 and increased gradually each week (0.25, 0.50, 0.75 and 1.00 g per kg body mass per day). The protein content of the supplement was provided by the product specification (83.6 g per 100 g of powder). The protein powder was weighed, and the daily amount was packaged in three equal portions which participants were asked to ingest together with breakfast, lunch, and dinner to distribute the protein intake over the day.
2.4. Biological samples
2.4.3.1. Calprotectin. Fecal samples were collected by the participants up to one day before the study visit using an EasySampler Stool Collector Kit (GP Medical Devices, Denmark) and fecal sample collection tubes (Sarstedt, Germany). Participants were instructed to store the tubes at −20 °C and samples were transported to the study center in a frozen transport container (Sarstedt, Germany). The tubes were immediately stored at −20 °C and further transferred to −80 °C until analysis. For fecal calprotectin analysis, samples were thawed at room temperature and extracted using CALEX cap tubes (BÜHLMANN, Switzerland).
All biological samples mentioned above (2.4.1–2.4.3.1) were analyzed at the Laboratory Medicine Clinic (Clinical Chemistry Department, Örebro University Hospital) using clinically automated procedures.
2.4.3.2. Ammonia. Fecal ammonia concentrations were determined using the commercially available Ammonia Kit (Megazyme, Ireland) according to the manufacturer's instructions.
2.4.3.3. Short-chain fatty acids. Fecal samples were also used to measure relative levels of the SCFA butyrate, acetate, propionate, and valerate by ultra-high performance liquid chromatography coupled to time-of-flight high-resolution mass spectrometry (UHPLC-Q-TOF-HRMS) after derivatization of fecal extracts using 3-nitrophenylhydrazine (3-NPH).34 Briefly, 20 mg of fecal sample was mixed with 100 µL cold methanol containing internal standards (10 µg mL−1 each): acetic acid-d4, butyric acid-d8 and propionic acid-d2. After ultrasonication (5 min), the extract was centrifuged (10
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g, 5 min, 4 °C) and a 50 µL-aliquot transferred to a vial. Each sample was further derivatized for 1 h using 50 mM 3-NPH, 50 mM N-ethylcarbodiimide and 7% (v/v) pyridine in aqueous methanol solution. After incubation, 0.2% formic acid was added to stop the derivatization reaction, and the sample was immediately analyzed using a 1290 UHPLC-Q-TOF system (Agilent, USA) equipped using an Acquity BEH C18 column (2.1 × 100 mm, 1.7 µm; Waters Corporation, USA). Mobile phase (A) 0.1% formic acid in water and (B) acetonitrile were eluted at 0.4 mL min−1. The injection volume was 5 µL and autosampler and column temperature were 10 °C and 50 °C, respectively. Data was acquired in negative ion mode. MassHunter Workstation Software (Agilent, USA) was used for data acquisition and processing.
000g, 5 min, 4 °C) and a 50 µL-aliquot transferred to a vial. Each sample was further derivatized for 1 h using 50 mM 3-NPH, 50 mM N-ethylcarbodiimide and 7% (v/v) pyridine in aqueous methanol solution. After incubation, 0.2% formic acid was added to stop the derivatization reaction, and the sample was immediately analyzed using a 1290 UHPLC-Q-TOF system (Agilent, USA) equipped using an Acquity BEH C18 column (2.1 × 100 mm, 1.7 µm; Waters Corporation, USA). Mobile phase (A) 0.1% formic acid in water and (B) acetonitrile were eluted at 0.4 mL min−1. The injection volume was 5 µL and autosampler and column temperature were 10 °C and 50 °C, respectively. Data was acquired in negative ion mode. MassHunter Workstation Software (Agilent, USA) was used for data acquisition and processing.
2.4.3.4. Microbiota analysis. The extraction of DNA and NGS sequencing for the fecal microbiota analysis were performed at Clinical Genomics, Örebro using the QIAsymphony PowerFecal Pro DNA Kit (Qiagen, Germany) on a QIAsymphony SP liquid handler (Qiagen, Germany) following the manufacturer's instructions, with a few minor modifications to the pretreatment step. Briefly, aliquots of approximately the size of a pea were collected from each fecal sample using a sterile 10 µL plastic loop and placed into PowerBead Pro Tubes (Qiagen, Germany) containing 750 µL CD1. Bead beating was performed on a FastPrep 24 bead beater (MP Biomedicals, USA) for 1 min at 6 m s−1. After centrifugation (15
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g, 1 min), the supernatant was transferred to new 2 mL microcentrifuge tubes. Proteinase K digestion was performed by incubation with 30 µL Proteinase K (20 mg mL−1) for 30 min at 56 °C. After digestion, 300 µL CD2 was added, samples were centrifuged (15
000g, 1 min), the supernatant was transferred to new 2 mL microcentrifuge tubes. Proteinase K digestion was performed by incubation with 30 µL Proteinase K (20 mg mL−1) for 30 min at 56 °C. After digestion, 300 µL CD2 was added, samples were centrifuged (15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g, 1 min) and the supernatant was transferred to new 2 mL micro tubes which were loaded on the QIAsymphony. The elution volume was 100 µL. The purified DNA was quantified on a Qubit 2.0 fluorometer (Thermo Fisher, USA) using the Qubit 1X dsDNA HS Assay Kit (Thermo Fisher, USA).
000g, 1 min) and the supernatant was transferred to new 2 mL micro tubes which were loaded on the QIAsymphony. The elution volume was 100 µL. The purified DNA was quantified on a Qubit 2.0 fluorometer (Thermo Fisher, USA) using the Qubit 1X dsDNA HS Assay Kit (Thermo Fisher, USA).Library preparation was performed on NGS STAR (Hamilton, USA) using the Illumina DNA Prep kit and Illumina DNA/RNA UD Indexes (Illumina, USA) according to the manufacturer's instructions. The libraries were quantified on a Qubit 3.0 fluorometer (Thermo Fisher, USA) using the Qubit 1X dsDNA HS Assay Kit (Thermo Fisher, USA) and average fragment lengths determined using the 4200 Tapestation system (Agilent, USA) with the High Sensitivity D5000 screentapes and reagents (Agilent, USA). Sequencing was performed on a NextSeq 2000 sequencer using NextSeq 2000 P3 Reagents (300 Cycles) (Illumina, USA) and sequences from two runs were combined for each sample to gain sufficient read depth. Yielded data was subsequently processed with default settings of the taxprofiler pipeline (version 1.1.2; nextflow version 23.10.0)35 using the MetaPhlAn (4.0.6) taxonomic profiler.
2.5. Questionnaires
During the screening visit, participants were interviewed to verify if they met the study's inclusion and exclusion criteria. Additionally, a 24 h dietary recall was performed together with the participant by trained staff. Participants were also instructed and trained on how to properly fill in two 24 h food diaries on two separate days after the screening visit. As support, participants received a portion guide containing pictures of different portion sizes for all main food groups and an information leaflet with additional instructions. The portion guide and the food diaries were provided by the Swedish Food Agency.36 The 24 h recall and the two 24 h food diaries were used to evaluate if dietary fiber and protein intake were matching with the inclusion criteria. During the continuation of the study, three 24 h food diaries were collected each week on two weekdays and one weekend day of choice with at least one day in-between.The GSRS was filled in by the participants on the evening before each visit to monitor gastrointestinal symptoms. The questionnaire includes 15 questions on symptoms during the past seven days based on a seven-point graded Likert-type scale.37 Specifically, a score of 1 represents no discomfort at all, 2 minor discomfort, 3 mild discomfort, 4 moderate discomfort, 5 moderately severe discomfort, 6 severe discomfort, and 7 very severe discomfort. Additionally, participants were asked to document every bowel movement in a BSS diary regarding stool consistency and frequency. The BSS classifies stool consistency on a scale from 1 (hard stool) to 7 (liquid stool) as an estimation of gastrointestinal transit time.38 Furthermore, an IPAQ questionnaire was completed on the evening before each visit. The questionnaire covers different physical activity levels during the past seven days and was applied as a proxy of lifestyle maintenance.39
2.6. Cell culture
Human colonic epithelial cell caco-2 were cultured in Dulbecco's Modified Eagle Medium (DMEM) containing penicillin and streptomycin with 10% Fetal Bovine Serum (FBS) at 37 °C in a humidified atmosphere of 5% CO2. Caco-2 cells were kindly provided by Dr Ignacio Rangel (Örebro University, Örebro, Sweden). Cells were passed to new culture plates by using trypsin/EDTA when they reached 80–90% of confluence. Subsequently, cells were used to evaluate cytotoxicity of fecal water.2.7. Resazurin cell viability and Lactate Dehydrogenase (LDH) assays
Cells (1 × 104 cells per well) were plated in a 96-well plate overnight, then 10% (v/v) of the culture medium was replaced with filtered (0.22 µm) fecal water, which was resuspended in sterile phosphate buffered saline (PBS) at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 (w/v) ratio. A cell control had 10% of DMEM medium replaced by sterile PBS, and a cell death control was treated with 0.02% Triton X-100 (Sigma-Aldrich, Germany). After 24 h of incubation, 20 µL of cell supernatant was transferred to measure extracellular LDH, while cells were added with 20 µL of resazurin dye solution (Sigma-Aldrich, Germany) and incubated for 3 h.
10 (w/v) ratio. A cell control had 10% of DMEM medium replaced by sterile PBS, and a cell death control was treated with 0.02% Triton X-100 (Sigma-Aldrich, Germany). After 24 h of incubation, 20 µL of cell supernatant was transferred to measure extracellular LDH, while cells were added with 20 µL of resazurin dye solution (Sigma-Aldrich, Germany) and incubated for 3 h.
For LDH determination, the commercially available LDH Activity Assay Kit was used (Sigma-Aldrich, Germany) according to the manufacturer's instructions. Briefly, nicotinamide adenine dinucleotide hydrogen standards were used, and 50 µL of the master reaction mix was added to each well. The plate was mixed using a horizontal shaker protected from the light for 2 min and absorbance was measured at 450 nm in a microplate reader at 37 °C (Bio-Rad, USA), taking measurements every 5 min until the most active sample is greater than the highest standard. The final measurement was the one at which the most active sample was near to exceeding the highest standard, but which fell within the linear range of the standard curve. For the resazurin assay, fluorescence was monitored at a wavelength of 590 nm using an excitation wavelength of 560 nm (Bio-Rad, USA).
2.8. Power calculation
The power calculation was based on the investigation of SCFA associated with protein fermentation in the gut. Prior research showed that dietary plant-based protein supplementation induced 8.42% of increase in butyrate and a 7.54% of increase in isovalerate (both expressed as relative proportions of total SCFA) over one week.40 The standard deviation of these differences was 7.54. For our study, we anticipated detecting at least 50% of that effect size in a population with normal weight, using a Wilcox signed-rank test, 80% power, and a 2-tailed significance level of 0.05. Our calculations indicated that a sample size of 32 subjects would detect a 0.53 standard deviation change in fecal SCFA.2.9. Statistical analyses
Week 1–3 was considered a training period for diary and questionnaire completion to ensure reliable data during week 4–8. Therefore, the first three weeks were not included in the final analysis. As collected questionnaire data included information of multiple days over a course of one week (24 h food diary), or whole weeks in general (GSRS, BSS and IPAQ) we refer to the timepoints as week rather than visit to highlight the different dimensions in the collected data.In general, a repeated measures one-way analysis of variance (rmANOVA) for within group analyses and two-way rmANOVA for between group analyses was performed for normally distributed data after log-transformation. In case of missing values, a mixed-effects-analysis was used instead. Post-hoc analyses were conducted using the Dunnett's and Šídák's multiple comparisons test, respectively. In contrast, cell proliferation was analyzed using an ordinary one-way analysis of variance with Tukey's multiple comparisons and differences in cytotoxicity were assessed with paired t-tests.
Non-normally distributed data was analyzed using the Friedman test for within group analyses, followed by the Dunn's multiple comparison test. Between-group analyses were performed using the multiple Mann–Whitney test with Holm–Šídák multiple comparisons.
Preprocessing for gut microbiota composition analysis included agglomeration to species level, filtering Chordata sequences and transforming counts to relative counts per sample (per cent). Repeated measures correlation analyses were performed on log-transformed, scaled and sparsity-reduced data using Spearman's rank correlation. The sparsity reduction removed operational taxonomic units with reads in the lower 30% percentile and that appeared in less than two samples. Multiple comparisons were accounted for by using the Benjamini–Hochberg correction. Additionally, a random forest model (package randomForest; version 4.7-1.1) using package caret (version 6.0-94) for the optimization of the hyperparameter mtry was trained to classify before (visit 1–4) and under intervention samples (visit 5–8).
Statistical analyses were performed using GraphPad Prism Version 10.4.1 (GraphPad Software, USA) and R (version 4.3.2) using the package phyloseq (version 1.44.0). For all described analyses, results with a p-value of p < 0.05 were considered statistically significant. Results are presented as mean ± SD for parametric data and median (IQR) for non-parametric data.
3. Results
A total of 311 people expressed interest in participating in the study, of whom 79 were screened, and 37 met eligible criteria. Following screening, six individuals were no longer interested in participating, and during the study, two participants withdrew due to personal reasons or a desire to discontinue the protein supplementation. Consequently, the study was completed with 29 participants (SI Fig. S1). Baseline characteristics of the study population are summarized in Table 1.| Median (IQR) | n | |
|---|---|---|
| Age (years) | ||
| Total | 26 (22–33) | 29 |
| Female | 25 (21–29) | 20 |
| Male | 29 (24–37) | 9 |
| Body mass index (kg m−2) | ||
| Total | 23.00 (21.90–26.28) | 28 |
| Female | 22.50 (21.90–26.40) | 19 |
| Male | 24.30 (21.95–26.30) | 9 |
3.1. Protein intake compliance, gastrointestinal symptoms, dietary and lifestyle habits
Neither energy nor dietary fiber intake changed significantly during the intervention. However, the intake of carbohydrates was significantly lower from week 7 onwards compared to week 4 (week 7: p = 0.0002, week 8: p = 0.0329), and fat intake was significantly reduced in week 8 (p = 0.0013). As expected, protein intake increased significantly each week both when expressed as energy percentage (week 5: p = 0.0004, week 6: p < 0.0001, week 7: p < 0.0001, week 8: p < 0.0001) as well as per g per kg body mass per day (week 5: p = 0.0026, week 6: p < 0.0001, week 7: p < 0.0001, week 8: p < 0.0001). Protein intake measured as g per kg body mass per day, calculated from 24 hour urinary nitrogen also showed a significant increase from visit 7 onwards (visit 7: p = 0.0056, visit 8: p = 0.0114). Original data of all analyzed parameters are summarized in Table 2. As a proxy of compliance, 95.1% of the empty protein supplements bags were returned by the participants.| Week 4 | Week 5 | Week 6 | Week 7 | Week 8 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Median (IQR) | n | Median (IQR) | n | Median (IQR) | n | Median (IQR) | n | Median (IQR) | n | |
| Dietary characteristics | ||||||||||
| Energy (kcal day−1) | 2104 (1735–2654) | 25 | 1974 (1757–2413) | 26 | 2160 (1945–2468) | 25 | 2017 (1933–2320) | 26 | 2195 (1941–2493) | 26 |
| Fiber (g day−1) | 20.6 (16.5–25.1) | 25 | 22.5 (15.7–25.6) | 26 | 19.2 (16.4–22.4) | 25 | 19.0 (15.8–23.8) | 26 | 21.6 (17.2–24.0) | 26 |
| Carbohydrates (E %) | 45.4 (41.6–48.6) | 25 | 42.6 (38.5–48.5) | 26 | 41.5 (38.4–44.7) | 25 | 39.2 (37.5–42.2)*** | 26 | 40.1 (37.3–44.5)* | 26 |
| Fat (E %) | 38.5 (35.8–41.8) | 25 | 38.9 (35.5–42.4) | 26 | 37.1 (34.3–41.5) | 25 | 35.9 (31.0–39.7) | 26 | 34.3 (29.7–36.7)*** | 26 |
| Protein (E %) | 15.2 (13.6–17.8) | 25 | 17.3 (15.3–23.1)*** | 26 | 21.0 (18.0–24.2) **** | 25 | 23.2 (21.0–26.0)**** | 26 | 24.5 (22.4–27.0)**** | 26 |
| Protein (g per kg body mass per day) | 1.1 (0.9–1.2) | 25 | 1.3 (1.1–1.5)** | 26 | 1.6 (1.5–1.9)**** | 25 | 1.7 (1.6–1.9)**** | 26 | 2.0 (1.8–2.2)**** | 26 |
| 24 h urea nitrogen | Visit 4 | Visit 5 | Visit 6 | Visit 7 | Visit 8 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Data is shown as median (IQR) and includes results from a mixed-effects analysis including Dunnett's multiple comparisons of each intervention visit/week vs. the baseline (visit/week 4); statistical results are based on log-transformed data; *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001; E %: energy percentage. | ||||||||||
| Protein (g per kg body mass per day) | 1.2 (0.8–1.6) | 16 | 1.6 (1.1–2.0) | 18 | 1.9 (1.3–2.2) | 13 | 1.6 (0.8–1.8)** | 12 | 2.0 (1.6–2.4)* | 17 |
The median of the weekly reported BSS during the intervention period ranged from 3.8–4.3 which lies within the normal range of 3–5. The daily stool frequency during the intervention ranged from 0.9–1.1. There were no statistically significant differences in stool type and frequency during the intervention indicating no alterations due to the increased protein intake (SI Table S2). Since the study population is healthy, the average scores for the GSRS were low, as expected, with a median ranging from 1–2. Statistical analyses revealed no significant changes of the total GSRS score during the intervention (SI Table S2). Physical activity levels measured in MET-min/week were statistically significantly different (p = 0.0031), however post-hoc multiple comparisons failed to detect significances. Physical activity, measured in physical activity levels (PAL) did not change significantly (SI Table S2).
3.2. Surrogate markers of health
From visits 4–8, fasting blood, urine, and fecal samples were collected, and their correspondent biochemical measurements are summarized in Table 3. Plasma triglycerides were significantly decreased comparing visit 6 and 8 to visit 4 (p = 0.0361), while plasma urea was significantly increased at visit 8 (p = 0.0092). Plasma creatinine levels were significantly lower on all intervention visits (visit 5: p = 0.0022, visit 6: p = 0.0198, visit 7: p = 0.0344, visit 8: p = 0.0453). Despite limitations due to incomplete sample collection (53% of completed samples), 24 h urine urea levels showed a significant increase by visit 8 (p = 0.0494). In fecal samples, calprotectin levels increased significantly (visit 5: p = 0.0194, visit 6: p = 0.0.0236, visit 7: p = 0.0.0236, visit 8: p = 0.0021), whereas fecal ammonia levels decreased significantly (visit 5: p = 0.0343, visit 7: p < 0.0001, visit 8: p = 0.0005). For SCFA, valerate significantly increased (visit 8: p = 0.0315). Pearson correlation between ammonia in feces and urea in blood resulted in R2 = 0.8443, r = −0.9189 (p = 0.0274).| Visit 4 | Visit 5 | Visit 6 | Visit 7 | Visit 8 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Median (IQR) | n | Median (IQR) | n | Median (IQR) | n | Median (IQR) | n | Median (IQR) | n | |
| Data is shown as median (IQR) and includes results from a mixed-effects analysis including Dunnett's multiple comparisons of each intervention visit vs. the baseline (visit 4); fold change was calculated in relation to the baseline average values; statistical results are based on log-transformed data; *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001; CRP: C-reactive protein, HDL: high-density lipoprotein, LDL: low-density lipoprotein. | ||||||||||
| Blood samples | ||||||||||
| Glucose (mmol L−1) | 5.2 (5.1–5.6) | 25 | 5.4 (5.1–5.6) | 23 | 5.3 (5.0–5.6) | 23 | 5.4 (5.1–5.5) | 25 | 5.3 (5.2–5.6) | 26 |
| Serum samples | ||||||||||
| Insulin (mIU L−1) | 6.8 (5.2–12.6) | 25 | 6.9 (6. 0–9.1) | 24 | 6.9 (6.0–9.3) | 26 | 7.5 (5.4–10.6) | 20 | 7.7 (5.7–10.1) | 26 |
| Plasma samples | ||||||||||
| CRP (mg L−1) | 0.4 (0.2–0.7) | 25 | 0.5 (0.3–0.7) | 24 | 0.4 (0.3–0.5) | 26 | 0.4 (0.3–0.7) | 25 | 0.5 (0.3–0.9) | 26 |
| HDL cholesterol (mmol L−1) | 1.5 (1.3–1.8) | 25 | 1.3 (1.2–1.8) | 24 | 1.5 (1.1–1.8) | 26 | 1.4 (1.2–1.8) | 25 | 1.5 (1.2–1.7) | 26 |
| LDL cholesterol (mmol L−1) | 2.7 (2.2–3.2) | 25 | 2.4 (2.0–2.9) | 24 | 2.5 (2.1–3.0) | 26 | 2.8 (2.2–3.2) | 25 | 2.6 (2.2–3.0) | 26 |
| Cholesterol (mmol L−1) | 4.6 (3.9–5.0) | 25 | 4.1 (3.5–4.7) | 24 | 4.3 (3.4–4.8) | 26 | 4.5 (3.7–4.8) | 25 | 4.2 (3.7–4.7) | 26 |
| Triglycerides (mmol L−1) | 0.8 (0.7–1.0) | 25 | 0.7 (0.6–0.9) | 24 | 0.6 (0.6–0.7)* | 26 | 0.7 (0.6–1.0) | 25 | 0.7 (0.6–0.9) | 26 |
| Urea (mmol L−1) | 4.6 (3.6–5.4) | 25 | 4.5 (3.5–5.6) | 24 | 4.8 (3.6–6.5) | 26 | 5.1 (4.4–6.1) | 25 | 5.9 (4.4–6.6)* | 26 |
| Creatinine (µmol L−1) | 60.0 (52.0–73.0) | 25 | 53.0 (47.3–64.0)** | 24 | 56.5 (46.5–69.5)* | 26 | 54.0 (46.5–67.5)* | 25 | 60.0 (50.0–69.0)* | 26 |
| 24 h urine samples | ||||||||||
| Urea (mmol day−1) | 354.0 (286.4–505.0) | 16 | 481.0 (350.7–659.5) | 18 | 511.5 (365.8–700.6) | 13 | 435.2 (357.8–714.0) | 12 | 575.7 (410.8–714.0)* | 17 |
| Uric acid (mmol day−1) | 3.2 (2.6–3.4) | 16 | 4.0 (3.0–5.0) | 18 | 3.8 (3.3–6.1) | 13 | 3.3 (2.3–5.4) | 12 | 4.5 (3.5–6.1) | 17 |
| Creatinine (mmol day−1) | 11.2 (8.4–14.7) | 16 | 12.3 (10.1–18.3) | 18 | 11.7 (9.2–17.3) | 13 | 9.7 (7.6–13.2) | 12 | 15.1 (7.9–17.8) | 16 |
| Fecal samples | ||||||||||
| Calprotectin (mg kg−1) | 10.0 (10.0–30.3) | 28 | 26.5 (10.0–46.5)* | 28 | 27.0 (10.0–52.0)* | 29 | 26.0 (10.0–65.5)* | 29 | 27.0 (10.0–70.0)** | 29 |
| Ammonia (mg L−1) | 21.0 (13.0–26.5) | 29 | 14.0 (11.0–19.0)* | 29 | 17.0 (12.0–24.0) | 29 | 4.0 (2.0–8.0)**** | 29 | 2.0 (0.0–9.0)*** | 29 |
| Butyrate (fold change) | 0.9 (0.4–1.3) | 29 | 1.1 (0.6–1.5) | 29 | 1.0 (0.6–1.5) | 29 | 0.9 (0.6–1.5) | 29 | 1.3 (0.8–1.6) | 29 |
| Propionate (fold change) | 0.7 (0.4–1.4) | 29 | 1.2 (0.6–1.5) | 29 | 0.8 (0.5–1.6) | 29 | 1.0 (0.6–1.5) | 29 | 1.1 (0.7–1.6) | 29 |
| Acetate (fold change) | 0.8 (0.5–1.6) | 29 | 1.0 (0.6–1.5) | 29 | 1.0 (0.5–1.6) | 29 | 1.1 (0.4–1.6) | 29 | 1.0 (0.6–1.8) | 29 |
| Valerate (fold change) | 0.8 (0.4–1.3) | 29 | 1.1 (0.7–1.8) | 29 | 1.2 (0.6–2.2) | 29 | 1.1 (0.6–1.5) | 29 | 1.2 (0.9–2.3)* | 29 |
3.3. Subgrouping by fecal calprotectin
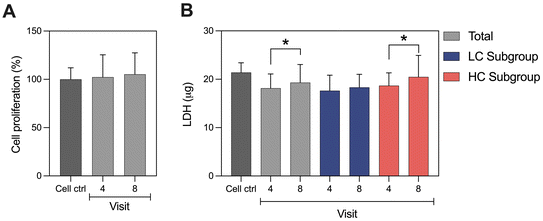
Interestingly, together with a significant increase in fecal calprotectin over time during the intervention, the variability in calprotectin levels among participants also increased. To investigate whether this variability was driven by a subset of participants, we divided the group based on fecal calprotectin levels at visit 8, splitting participants at the median into a high-calprotectin (HC) and a low-calprotectin (LC) subgroup for further analysis. These groups were not significantly different regarding age, sex, BMI and their dietary fiber intake (results not shown). Fig. 2A illustrates that calprotectin increased only in the HC subgroup significantly compared with visit 4 (visit 6: p = 0.0367, visit 7: p = 0.0320, visit 8: p < 0.0001). No significant changes were observed in the LC subgroup. The subgroups differed significantly starting from visit 6 onwards (visit 6: p = 0.0075, visit 7: p = 0.0051, visit 8: p < 0.0001). The baseline comparison at visit 4 showed a trend for significance (p = 0.0706). Further analysis revealed a general trend of increasing SCFA levels over time for LC subgroup (Fig. 2B), and levels of valerate and butyrate were significantly higher at visit 8 (p = 0.0365 and p = 0.0427, respectively). No significant changes were observed in the HC subgroup nor between LC and HC subgroups.3.4. Colonic cell viability and cytotoxicity
Cell proliferation was not differently affected by the fecal water from baseline (visit 4) and from the intervention's end (visit 8). Additionally, no differences were observed comparing fecal water samples to the control cells only treated with cell culture medium (Fig. 3A).To further assess cytotoxicity, we measured LDH, a cytosolic enzyme released from damaged cells. Extracellular LDH levels increased significantly at visit 8 compared to visit 4 (p = 0.0100). However, no differences were observed comparing fecal water to the control cells. Since calprotectin can promote cytotoxicity in epithelial cells,41 we investigated whether fecal water would exert distinct cytotoxic effects in LC and HC subgroups. The HC subgroup exhibited higher LDH release after the intervention (p = 0.0077), while no change was observed for the LC subgroup between visits 4 and 8 (Fig. 3B).
3.5. Microbiota composition
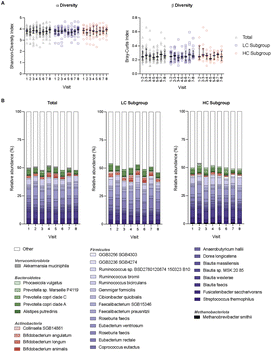
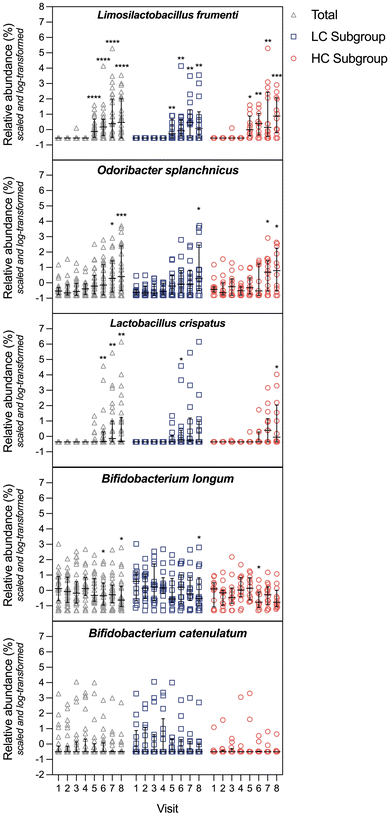
The average read length and depth per sample was 144 bp and 13.9 Mio, respectively. There was no significant difference in α and β diversity as measured by the Shannon-Diversity Index and the Bray–Curtis Index during the intervention and within LC and HC subgroups (Fig. 4A). The most abundant phylum among the study population was Firmicutes, followed by Bacteroidetes, Actinobacteria and Verrucomicrobiota (Fig. 4B).To investigate if the intervention has led to changes in species abundances, we analyzed if relative abundances correlate with visits 4–8. We found a significant and positive correlation for the species L. frumenti (correlation coefficient: 0.55, p < 0.0001), O. splanchnicus (correlation coefficient: 0.53, p < 0.001), and L. crispatus (correlation coefficient: 0.43, p = 0.002). In contrast, relative abundances of B. longum and B. catenulatum were significantly negatively correlated with the intervention visits (correlation coefficient: −0.39, p = 0.010 and correlation coefficient: −0.37, p = 0.019, respectively). If participants were grouped into LC and HC, a significant positive correlation remained for L. frumenti (correlation coefficient: 0.59, p = 0.003), O. splanchnicus (correlation coefficient: 0.59, p = 0.003) and L. crispatus (correlation coefficient: 0.57, p = 0.004) in the HC subgroup. Subsequently, we analyzed relative abundances of the correlating species to further explore potential differences (Fig. 5). In the total population, significantly higher abundances of L. frumenti were present at visit 5–8 compared to visit 4 (visit 5: p < 0.0001, visit 6: p < 0.0001, visit 7: p < 0.0001, visit 8: p < 0.0001). This was also observed in the LC subgroup (visit 5: p = 0.0064, visit 6: p = 0.0014, visit 7: p = 0.0012, visit 8: p = 0.0095) and in the HC subgroup (visit 5: p = 0.0208, visit 6: p = 0.0020, visit 7: p = 0.0042, visit 8: p = 0.0001). Relative abundances of O. splanchnicus increased significantly at visit 7 and 8 (visit 7: p = 0.0144, visit 8: p = 0.0002) in the total population. Whereas the observed differences at visit 7 and 8 were maintained for HC (visit 7: p = 0.0383, visit 8: p = 0.0141), only differences at visit 8 remained significant within the LC subgroup (p = 0.0288). For L. crispatus, results showed a significantly increased abundance at visits 6–8 (visit 6: p = 0.0056, visit 7: p = 0.0038, visit 8: p = 0.0012) in the total population. Within the subgroups we observed significantly higher abundances at visit 6 for LC (p = 0.0363) and visit 8 for HC (p = 0.0162). In contrast to the previously mentioned species, relative abundances of B. longum were significantly lower at visit 6 and visit 8 in the total population (visit 6: p = 0.0131, visit 8: p = 0.0006). Subgroup analyses revealed lower abundances at visit 8 in the LC subgroup (p = 0.0157) and lower abundances at visit 6 in the HC subgroup (p = 0.0027). For B. catenulatum, the Friedman tests was significant for the total population (p = 0.0004) and the LC subgroup (p = 0.0063), however, multiple comparisons failed to detect significances. Within the HC subgroup, none of the conducted tests were significant.
There were no significant differences in relative abundances of the correlating species between the subgroups.
The random forest model used to identify important variables confirmed results from correlation analysis, with L. frumenti as the by far most important variable (permutation p-value of 0 in 200 repetitions). Subsequently, we investigated if the six relevant species also correlate with any other biological markers in the intervention study. In the total population, relative abundances of L. frumenti significantly correlated with fecal ammonia (correlation coefficient: −0.41, p = 0.001), plasma urea (correlation coefficient: 0.37, p = 0.009) and fecal calprotectin (correlation coefficient: 0.33, p = 0.009). O. splanchnicus significantly correlated with fecal ammonia (correlation coefficient: −0.34, p = 0.009) and L. crispatus significantly correlated with fecal ammonia (correlation coefficient: −0.33, p = 0.009) as well as plasma urea (correlation coefficient: 0.31, p = 0.025). Furthermore, we found a significant correlation between relative abundances of B. longum and plasma cholesterol (correlation coefficient: −0.32, p = 0.025). In the HC subgroup, L. frumenti and L. crispatus significantly correlated with fecal calprotectin (correlation coefficient: 0.57, p = 0.0007 and correlation coefficient: 0.45, p = 0.013, respectively) as well as fecal ammonia (correlation coefficient: −0.45, p = 0.013 and correlation coefficient: −0.46, p = 0.013, respectively).
4. Discussion
This is the first dietary intervention to demonstrate a significant increase in fecal calprotectin levels in a subset of healthy participants in response to increasing amounts of isolated pea protein supplementation. Additionally, fecal butyrate and valerate levels were significantly elevated in participants with stable fecal calprotectin. These findings highlight the importance of assessing plant-based protein intake in healthy individuals to establish potential links with disease risk in the future. Furthermore, this study extends the existing literature on generalized dietary effects by providing insights into which biomarkers exhibit interindividual variability under comparable dietary conditions.4.1. Effects of protein supplementation on biomarkers of gut health and microbiota composition
Fecal calprotectin is a non-specific but sensitive marker of intestinal inflammation, released by neutrophils in the intestinal mucosa during inflammatory processes and commonly used to monitor inflammatory bowel disease.42,43 In our study, the divergence between individuals with increasing versus stable fecal calprotectin levels suggested the presence of responders and non-responders in terms of their tolerance to plant-based protein supplementation. The differences between subgroup baseline values of calprotectin showed a trend towards significance, indicating that an increased intake of plant-based proteins may promote inflammation in predisposed individuals.44 In these individuals, the progressive rise in fecal calprotectin may reflect a low-grade inflammatory or mucosal stress response to the escalating protein load, even though most calprotectin values remained below the clinical cut-off for intestinal inflammation. Given that fecal calprotectin primarily reflects neutrophil activity, its increase may not necessarily signify overt inflammation but could also represent subtle shifts in immune or epithelial homeostasis.23Nevertheless, we observed higher LDH cytotoxicity of fecal water only in the HC subgroup in line with previous research,41 highlighting that LDH cytotoxicity may be linked to increased inflammation.45 In contrast, participants who maintained stable calprotectin levels simultaneously displayed higher amount of SCFA, consistent with a metabolically favorable fermentation profile, potentially supporting both energy supply to colonocytes and microbial nitrogen recycling. Although there is limited evidence in the literature on the association between SCFA and fecal calprotectin, fecal calprotectin levels are strongly correlated with intestinal mucosal inflammation where SCFA concentrations play a key role in colonocyte energy metabolism and immune regulation.23,46,47 Taken together, these findings point towards a potential link between SCFA-mediated modulation of gut inflammation and lower calprotectin levels. Further studies including complementary inflammatory and permeability markers are warranted to clarify the underlying mechanisms.
Valerate is strongly linked to proteolytic fermentation,48 and its increase was observed in overweight adults receiving soy protein supplementation compared to control or casein groups,40 aligning with our findings. Additionally, valerate formation is associated with branched-chain amino acid metabolism and other proteolytic processes,49 whereas butyrate is typically linked to carbohydrate fermentation.13 However, bacteria can also produce butyrate from protein, as shown in vitro, where the protein-to-fiber ratio was a key determinant of butyrate production.19 Despite similar protein-to-fiber ratios, the LC and HC subgroups exhibited different SCFA trends. Only LC subgroup showed increased butyrate, with a tendency for higher propionate and acetate. Therefore, our observations indicate that the shifts in SCFA levels were most likely not driven by changes in the fermentation of indigestible carbohydrates since both valerate and butyrate can be produced through overlapping microbial routes depending on substrate availability.20 These collective results highlight the presence of responder and non-responder phenotypes, emphasizing the importance of considering individual gut status and host-microbiota interactions when evaluating the effects of a high plant-based protein intake.
Interestingly, the typically detrimental metabolite ammonia, resulting from proteolytic fermentation, decreased in feces with an increase in protein intake. This finding aligns with another study showing that labeled nitrogen excretion in feces tended to be lower in a high-protein diet compared with low-protein diet.50 Ammonia can be used by the gut bacteria or be absorbed by the colonic mucosa and converted in the liver to urea, being excreted in the urine later.51 Such metabolism goes hand in hand with the negative correlation between fecal ammonia and plasma urea, indicating an increased availability of ammonia in the gut to being taken up by the host. Another explanation for the decrease of fecal ammonia is its utilization by certain microbial taxa in the gut as a nitrogen source for generating microbial biomass.52 The observed increase of relative abundance of L. frumenti, L. crispatus, and O. splanchnicus and their negative correlations with fecal ammonia may strengthen this hypothesis. While literature on underlying metabolic pathways of the specific strains is scarce, research on lactic acid bacteria has described their proteolytic properties and their growth dependence on nitrogen, even though the extent of utilization can differ between strains and the nitrogen source.53,54 Species within the genus Lactobacillus have been linked to beneficial effects in the past, especially for their probiotic characteristics.55,56 Probiotics are “live microorganisms which when administered in adequate amounts, confer a health benefit on the host”,57 for instance by promoting intestinal barrier integrity and function, improving insulin sensitivity and exerting immunomodulatory effects.58 However, research on the role specific species mentioned above in human health is still sparse. In addition, the presence of other phytochemicals in the protein isolate, such as flavonoids,59 may have influenced the observed changes in relative abundance of the gut microbiota composition.60,61
This exploratory study suggests the presence of both species-dependent and species-independent microbiota-driven mechanisms triggered by dietary components. While the observed changes in species abundance, SCFA, and calprotectin indicate that unabsorbed pea protein isolate reached the colon, further studies combining targeted metabolite profiling with microbial functional analyses could elucidate the respective contributions of saccharolytic and proteolytic metabolism.
4.2. Strengths and limitations
The combination of self-reported and several objective measurements of protein intake compliance (24 h food diaries, returned protein bags, as well as blood and 24 h urine urea levels) suggests high adherence to the intervention. Additionally, participants maintained an isocaloric diet by slightly decreasing their carbohydrate and fat intake, which remained close to or within the Nordic Nutrition Recommendations for a normal diet (45–60 E % and 25–40 E %, respectively).62 Still, this could limit the ability to attribute all observed effects solely to pea protein isolate. To ensure a homogeneous study population, we included only participants within predefined dietary fiber and protein intake ranges, further strengthening the observed results.We concluded the study with 29 participants, three less than anticipated. However, we decided not to proceed with further recruitment, a decision influenced by the ongoing COVID-19 pandemic and logistical constraints. Additionally, our subgroup analyses based on fecal calprotectin were of exploratory nature, wherefore they may lack sufficient sample size. In addition, the predominance of young female participants limits the generalizability of our findings to other populations and the assessment of sex-related differences.
The weekly increment in protein intake was designed to assess the feasibility of protein supplementation and to determine the threshold for metabolic changes. However, this approach limits our ability to distinguish whether the observed effects were driven by intervention duration or by the increasing protein dose. Nevertheless, the gradual increase facilitated compliance and adaptation while allowing participants to reach a high-protein intake without drastic dietary shifts. The absence of a control group also makes it challenging to rule out potential reactivity effects due to sample and questionnaire collection. However, the inclusion of a 4 week baseline period before the intervention likely mitigated such biases, as participants had time to adapt to study procedures before the intervention began.
5. Conclusion
As societal shifts in protein intake influence both its quantity and source, it is essential to investigate the resulting impact on health biomarkers and gut microbiota. Our findings suggest that increasing doses of isolated pea protein can modulate the growth of beneficial bacteria and affect fecal calprotectin and SCFA levels in a subset of healthy participants. Lower calprotectin levels were associated with a tendency towards increased fecal SCFA, while participants with higher calprotectin showed only minimal SCFA changes and increased fecal water cytotoxicity in vitro. These results highlight the importance of assessing health markers related to plant-based protein intake in healthy populations, as well as the identification for biological differential responses. Future research should include larger cohorts and control groups to further explore subgroup differences in gut inflammation.Author contributions
S. P.: conceptualization; data curation; formal analysis; funding acquisition; investigation; methodology; project administration; resources; supervision; validation; visualization; writing – original draft; writing – review & editing. A. K.: data curation; formal analysis; investigation; methodology; supervision; validation; visualization; writing – original draft; writing – review & editing. K. D.: formal analysis; methodology; visualization; writing – review & editing. I. K. data curation; methodology; writing – review & editing. V. C. A.: formal analysis; methodology; visualization; writing – review & editing. T. H.: methodology; writing – review & editing. M. L.: conceptualization; methodology; writing – review & editing. D. R.: formal analysis; methodology; visualization; writing – review & editing. T. M. M.: conceptualization; project administration; supervision; validation. R. J. B.: conceptualization; funding acquisition; investigation; project administration; resources; supervision; validation; writing – review & editing. All authors had final approval of the submitted manuscript and published versions.Conflicts of interest
The authors declare no conflict of interest.Data availability
The data are pseudonymised, and the key variable may not be destroyed. According to Swedish ethics regulations, the raw data cannot be shared without an approved ethics application from the Swedish Ethical Review Authority. An ethical permit can only be obtained for research conducted within Sweden. Data can be requested as a public document via forskningsdata@oru.se and will undergo a confidentiality assessment before any potential release.Supplementary information (SI) is available. See DOI: https://doi.org/10.1039/d5fo02653a.
Acknowledgements
We thank all participants, the interns Sofie Raemisch and Giulia Bevilacqua, the study nurses who assisted in this dietary intervention and Lantmännen for providing the isolated pea protein used in the study. Furthermore, we would also like to acknowledge Clinical Genomics Örebro, Science for Life Laboratory, for providing expertise and service with sequencing. This work was supported by Örebro University Food and Health (ORU 1.4.1-00125/2021) and FORMAS (2020-02843 and 2021-02037). The DAAD and ERASMUS programs supported interns. This study was conducted within the PAN Sweden Research Centre.References
- V. Abe-Inge, R. Aidoo, M. Moncada de la Fuente and E. M. Kwofie, Plant-based dietary shift: Current trends, barriers, and carriers, Trends Food Sci. Technol., 2024, 143, 104292, DOI:10.1016/j.tifs.2023.104292.
- I. Luzardo-Ocampo and E. Gonzalez de Mejia, Plant proteins and peptides as key contributors to good health: A focus on pulses, Food Res. Int., 2025, 211, 116346, DOI:10.1016/j.foodres.2025.116346.
- M. Benković, A. Jurinjak Tušek, T. Sokač Cvetnić, T. Jurina, D. Valinger and J. Gajdoš Kljusurić, An Overview of Ingredients Used for Plant-Based Meat Analogue Production and Their Influence on Structural and Textural Properties of the Final Product, Gels, 2023, 9(12), 921, DOI:10.3390/gels9120921.
- K. Broucke, B. Duquenne, B. De Witte, E. De Paepe, S. De Smet and G. Van Royen, Effect of salt, phosphate and protein content on the techno-functionality of plant-based proteins for hybrid meat product formulation, Eur. Food Res. Technol., 2025, 251, 1117–1126, DOI:10.1007/s00217-025-04692-3.
- J. Auer, M. Alminger, M. Marinea, M. Johansson, G. Zamaratskaia, A. Högberg and M. Langton, Assessing the digestibility and estimated bioavailability/bioaccessibility of plant-based proteins and minerals from soy, pea, and faba bean ingredients, LWT, 2024, 197, 115893, DOI:10.1016/j.lwt.2024.115893.
- T. P. Czaja, S. N. Beldring, C. Renaud and S. B. Engelsen, Mimicking the properties of commercial chocolate mousses using plant proteins as foaming stabilisers. Texture, rheology, color and proton mobility, Food Res. Int., 2025, 212, 116450, DOI:10.1016/j.foodres.2025.116450.
- S. Ebert, M.-C. Baune, K. Broucke, G. Van Royen, N. Terjung, M. Gibis and J. Weiss, Buffering capacity of wet texturized plant proteins in comparison to pork meat, Food Res. Int., 2021, 150, 110803, DOI:10.1016/j.foodres.2021.110803.
- P. Moll, H. Salminen, O. Seitz, C. Schmitt and J. Weiss, Characterization of soluble and insoluble fractions obtained from a commercial pea protein isolate, J. Dispersion Sci. Technol., 2023, 44(13), 2417–2428, DOI:10.1080/01932691.2022.2093214.
- B. J. Plattner, S. Hong, Y. Li, M. J. Talavera, H. Dogan, B. S. Plattner and S. Alavi, Use of Pea Proteins in High-Moisture Meat Analogs: Physicochemical Properties of Raw Formulations and Their Texturization Using Extrusion, Foods, 2024, 13(8), 1195, DOI:10.3390/foods13081195.
- H.-G. Zhu, H.-Q. Tang, Y.-Q. Cheng, Z.-G. Li and L.-T. Tong, Potential of preparing meat analogue by functional dry and wet pea (Pisum sativum) protein isolate, LWT, 2021, 148, 111702, DOI:10.1016/j.lwt.2021.111702.
- L. Boven, R. Akkerman and P. de Vos, Sustainable diets with plant-based proteins require considerations for prevention of proteolytic fermentation, Crit. Rev. Food Sci. Nutr., 2024, 65(14), 2829–2839, DOI:10.1080/10408398.2024.2352523.
- W. Campos-Perez and E. Martinez-Lopez, Effects of short chain fatty acids on metabolic and inflammatory processes in human health, Biochim. Biophys. Acta, Mol. Cell Biol. Lipids, 2021, 1866(5), 158900, DOI:10.1016/J.BBALIP.2021.158900.
- Y. Yao, X. Cai, W. Fei, Y. Ye, M. Zhao and C. Zheng, The role of short-chain fatty acids in immunity, inflammation and metabolism, Crit. Rev. Food Sci. Nutr., 2022, 62(1), 1–12, DOI:10.1080/10408398.2020.1854675.
- K. Windey, V. De Preter and K. Verbeke, Relevance of protein fermentation to gut health, Mol. Nutr. Food Res., 2012, 56(1), 184–196, DOI:10.1002/mnfr.201100542.
- D. Levitt and M. Levitt, A model of blood-ammonia homeostasis based on a quantitative analysis of nitrogen metabolism in the multiple organs involved in the production, catabolism, and excretion of ammonia in humans, Clin. Exp. Gastroenterol., 2018, 11, 193–215, DOI:10.2147/CEG.S160921.
- F. Blachier, F. Mariotti, J. F. Huneau and D. Tomé, Effects of amino acid-derived luminal metabolites on the colonic epithelium and physiopathological consequences, Amino Acids, 2007, 33(4), 547–562, DOI:10.1007/s00726-006-0477-9.
- K. Fung, C. Ooi, M. Zucker, T. Lockett, D. Williams, L. Cosgrove and D. Topping, Colorectal Carcinogenesis: A Cellular Response to Sustained Risk Environment, Int. J. Mol. Sci., 2013, 14(7), 13525–13541, DOI:10.3390/ijms140713525.
- I. E. K. Mak, Y. Yao, M. T. T. Ng and J. E. Kim, Influence of dietary protein and fiber intake interactions on the human gut microbiota composition and function: a systematic review and network meta-analysis of randomized controlled trials, Crit. Rev. Food Sci. Nutr., 2025, 65(30), 6861–6879, DOI:10.1080/10408398.2025.2452362.
- R. Jackson, T. Yao, N. Bulut, T. M. Cantu-Jungles and B. R. Hamaker, Protein combined with certain dietary fibers increases butyrate production in gut microbiota fermentation, Food Funct., 2024, 15(6), 3186–3198, 10.1039/D3FO04187E.
- M. Yuan, K. Gao, K. Peng, S. Bi, X. Cui and Y. Liu, A review of nutritional regulation of intestinal butyrate synthesis: Interactions between dietary polysaccharides and proteins, Foods, 2025, 14(21), 3649, DOI:10.3390/foods14213649.
- M. A. R. Vinolo, H. G. Rodrigues, R. T. Nachbar and R. Curi, Short-chain fatty acids as key regulators of inflammation and immunity, Nutrients, 2011, 3(10), 858–876, DOI:10.3390/nu3100858.
- Y. Li, L. Pan, X. Zeng, R. Zhang, X. Li, J. Li, H. Xing and J. Bao, Ammonia exposure causes the imbalance of the gut-brain axis by altering gene networks associated with oxidative metabolism, inflammation and apoptosis, Ecotoxicol. Environ. Saf., 2021, 224, 112668, DOI:10.1016/j.ecoenv.2021.112668.
- A. Jukic, L. Bakiri, E. F. Wagner, H. Tilg and T. E. Adolph, Calprotectin: from biomarker to biological function, Gut, 2021, 70(10), 1978–1988, DOI:10.1136/gutjnl-2021-324855.
- I. Stríz and I. Trebichavský, Calprotectin - a pleiotropic molecule in acute and chronic inflammation, Physiol. Res., 2004, 53(3), 245–253 Search PubMed , PMID: 15209531.
- L. A. Bolte, A. Vich Vila, F. Imhann, V. Collij, R. Gacesa, V. Peters, C. Wijmenga, A. Kurilshikov, M. J. E. Campmans-Kuijpers, J. Fu, G. Dijkstra, A. Zhernakova and R. K. Weersma, Long-term dietary patterns are associated with pro-inflammatory and anti-inflammatory features of the gut microbiome, Gut, 2021, 70(7), 1287–1298, DOI:10.1136/gutjnl-2020-322670.
- M. Markova, L. Koelman, S. Hornemann, O. Pivovarova, S. Sucher, J. Machann, N. Rudovich, R. Thomann, R. Schneeweiss, S. Rohn, A. F. H. Pfeiffer and K. Aleksandrova, Effects of plant and animal high protein diets on immune-inflammatory biomarkers: A 6-week intervention trial, Clin. Nutr., 2020, 39(3), 862–869, DOI:10.1016/j.clnu.2019.03.019.
- L. Alvarenga, J. A. Kemp, B. G. Baptista, M. Ribeiro, L. S. Lima and D. Mafra, Production of Toxins by the Gut Microbiota: The Role of Dietary Protein, Curr. Nutr. Rep., 2024, 13(2), 340–350, DOI:10.1007/s13668-024-00535-x.
- L. A. David, C. F. Maurice, R. N. Carmody, D. B. Gootenberg, J. E. Button, B. E. Wolfe, A. V. Ling, A. S. Devlin, Y. Varma, M. A. Fischbach, S. B. Biddinger, R. J. Dutton and P. J. Turnbaugh, Diet rapidly and reproducibly alters the human gut microbiome, Nature, 2014, 505(7484), 559–563, DOI:10.1038/nature12820.
- S. Wu, Z. Bhat, R. Gounder, I. Mohamed Ahmed, F. Al-Juhaimi, Y. Ding and A. Bekhit, Effect of Dietary Protein and Processing on Gut Microbiota—A Systematic Review, Nutrients, 2022, 14(3), 453, DOI:10.3390/nu14030453.
- J. Karlsson, P. Lopez-Sanchez, T. M. Marques, T. Hyötyläinen, V. Castro-Alves, A. Krona and A. Ström, Physico-chemical properties of pea fibre and pea protein blends and the implications for in vitro batch fermentation using human inoculum, Food Hydrocolloids, 2024, 150, 109732, DOI:10.1016/j.foodhyd.2024.109732.
- T. Schumacher, T. Steinmacher, E. Köster, A. Wagemans, J. Weiss and M. Gibis, Physico-chemical characterization of ten commercial pea protein isolates, Food Hydrocolloids, 2025, 162, 110996, DOI:10.1016/j.foodhyd.2024.110996.
- A. Y. J. Tiong, S. Crawford, N. C. Jones, G. H. McKinley, W. Batchelor and L. van ‘t Hag, Pea and soy protein isolate fractal gels: The role of protein composition, structure and solubility on their gelation behaviour, Food Struct., 2024, 40, 100374, DOI:10.1016/j.foostr.2024.100374.
- F. Ashkar and J. Wu, Effects of Food Factors and Processing on Protein Digestibility and Gut Microbiota, J. Agric. Food Chem., 2023, 71(23), 8685–8698, DOI:10.1021/acs.jafc.3c00442.
- P. Seeburger, H. Forsman, G. Bevilacqua, T. M. Marques, L. O. Morales, S. B. R. Prado, Å. Strid, T. Hyötyläinen and V. Castro-Alves, From farm to fork… and beyond! UV enhances Aryl hydrocarbon receptor-mediated activity of cruciferous vegetables in human intestinal cells upon colonic fermentation, Food Chem., 2023, 426, 136588, DOI:10.1016/j.foodchem.2023.136588.
- S. Stamouli, M. E. Beber, T. Normark, T. A. Christensen, L. Andersson-Li, M. Borry, M. Jamy and J. A. Fellows Yates, nf-core/taxprofiler: highly parallelised and flexible pipeline for metagenomic taxonomic classification and profiling, bioRxiv, 2023, preprint, DOI:10.1101/2023.10.20.563221.
- Livsmedelsverket, Portionsguiden, https://www.livsmedelsverket.se/matvanor-halsa-miljo/matvanor--undersokningar/portionsguiden (accessed December 2024).
- K. R. Kulich, A. Madisch, F. Pacini, J. M. Piqué, J. Regula, C. J. Van Rensburg, L. Újszászy, J. Carlsson, K. Halling and I. K. Wiklund, Reliability and validity of the Gastrointestinal Symptom Rating Scale (GSRS) and Quality of Life in Reflux and Dyspepsia (QOLRAD) questionnaire in dyspepsia: A six-country study, Health Qual. Life Outcomes, 2008, 6(1), 12, DOI:10.1186/1477-7525-6-12.
- S. J. Lewis and K. W. Heaton, Stool Form Scale as a Useful Guide to Intestinal Transit Time, Scand. J. Gastroenterol., 1997, 32(9), 920–924, DOI:10.3109/00365529709011203.
- C. L. Craig, A. L. Marshall, M. Sjöström, A. E. Bauman, M. L. Booth, B. E. Ainsworth, M. Pratt, U. Ekelund, A. Yngve, J. F. Sallis and P. Oja, International Physical Activity Questionnaire: 12-Country Reliability and Validity, Med. Sci. Sports Exercise, 2003, 35(8), 1381–1395, DOI:10.1249/01.MSS.0000078924.61453.FB.
- M. Beaumont, K. J. Portune, N. Steuer, A. Lan, V. Cerrudo, M. Audebert, F. Dumont, G. Mancano, N. Khodorova, M. Andriamihaja, G. Airinei, D. Tomé, R. Benamouzig, A.-M. Davila, S. P. Claus, Y. Sanz and F. Blachier, Quantity and source of dietary protein influence metabolite production by gut microbiota and rectal mucosa gene expression: a randomized, parallel, double-blind trial in overweight humans, Am. J. Clin. Nutr., 2017, 106(4), 1005–1019, DOI:10.3945/ajcn.117.158816.
- F. Shabani, M. Mahdavi, M. Imani, M. Hosseinpour-Feizi and N. Gheibi, Calprotectin (S100A8/S100A9)-induced cytotoxicity and apoptosis in human gastric cancer AGS cells: Alteration in expression levels of Bax, Bcl-2, and ERK2, Hum. Exp. Toxicol., 2020, 39(8), 1031–1045, DOI:10.1177/0960327120909530.
- B. Bressler, R. Panaccione, R. N. Fedorak and E. G. Seidman, Clinicians’ guide to the use of fecal calprotectin to identify and monitor disease activity in inflammatory bowel disease, Can. J. Gastroenterol. Hepatol., 2015, 29(7), 369–372, DOI:10.1155/2015/852723.
- N. Kapel, H. Ouni, N. A. Benahmed and L. Barbot-Trystram, Fecal Calprotectin for the Diagnosis and Management of Inflammatory Bowel Diseases, Clin. Transl. Gastroenterol., 2023, 14(9), e00617, DOI:10.14309/ctg.0000000000000617.
- M. A. Mendall, D. Chan, R. Patel and D. Kumar, Faecal calprotectin: factors affecting levels and its potential role as a surrogate marker for risk of development of Crohn's Disease, BMC Gastroenterol., 2016, 16(1), 126, DOI:10.1186/s12876-016-0535-z.
- A. A. M. Kämpfer, P. Urbán, S. Gioria, N. Kanase, V. Stone and A. Kinsner-Ovaskainen, Development of an in vitro co-culture model to mimic the human intestine in healthy and diseased state, Toxicol. in Vitro, 2017, 45, 31–43, DOI:10.1016/j.tiv.2017.08.011.
- X. Liu, J. Shao, Y.-T. Liao, L.-N. Wang, Y. Jia, P. Dong, Z. Liu, D. He, C. Li and X. Zhang, Regulation of short-chain fatty acids in the immune system, Front. Immunol., 2023, 14, 1186892, DOI:10.3389/fimmu.2023.1186892.
- D. Parada Venegas, M. K. De la Fuente, G. Landskron, M. J. González, R. Quera, G. Dijkstra, H. J. M. Harmsen, K. N. Faber and M. A. Hermoso, Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases, Front. Immunol., 2019, 10, 277, DOI:10.3389/fimmu.2019.00277.
- E. Neis, C. Dejong and S. Rensen, The Role of Microbial Amino Acid Metabolism in Host Metabolism, Nutrients, 2015, 7(4), 2930–2946, DOI:10.3390/nu7042930.
- P. Van den Abbeele, J. Ghyselinck, M. Marzorati, A.-M. Koch, W. Lambert, J. Michiels and T. Chalvon-Demersay, The effect of amino acids on production of SCFA and bCFA by members of the porcine colonic microbiota, Microorganisms, 2022, 10(4), 762, DOI:10.3390/microorganisms10040762.
- K. Windey, V. De Preter, T. Louat, F. Schuit, J. Herman, G. Vansant and K. Verbeke, Modulation of Protein Fermentation Does Not Affect Fecal Water Toxicity: A Randomized Cross-Over Study in Healthy Subjects, PLoS One, 2012, 7(12), e52387, DOI:10.1371/journal.pone.0052387.
- O. M. Wrong and A. Vince, Urea and ammonia metabolism in the human large intestine, Proc. Nutr. Soc., 1984, 43(1), 77–86, DOI:10.1079/PNS19840030.
- W. G. Bergen and G. Wu, Intestinal Nitrogen Recycling and Utilization in Health and Disease, J. Nutr., 2009, 139(5), 821–825, DOI:10.3945/jn.109.104497.
- M. Hu, D. Wang, X. Tang, Q. Zhang, J. Zhao, B. Mao, H. Zhang and S. Cui, Improving the utilization efficiency of nitrogen source through co-culture of Lactobacillus strains with different nitrogen source metabolisms, LWT, 2024, 191, 115701, DOI:10.1016/j.lwt.2023.115701.
- M. Liu, J. R. Bayjanov, B. Renckens, A. Nauta and R. J. Siezen, The proteolytic system of lactic acid bacteria revisited: a genomic comparison, BMC Genomics, 2010, 11(1), 36, DOI:10.1186/1471-2164-11-36.
- E. Dempsey and S. C. Corr, Lactobacillus spp. for Gastrointestinal Health: Current and Future Perspectives, Front. Immunol., 2022, 13, 840245, DOI:10.3389/fimmu.2022.840245.
- A. B. Shah, A. Baiseitova, M. Zahoor, I. Ahmad, M. Ikram, A. Bakhsh, M. A. Shah, I. Ali, M. Idress, R. Ullah, F. A. Nasr and M. Al-Zharani, Probiotic significance of Lactobacillus strains: a comprehensive review on health impacts, research gaps, and future prospects, Gut Microbes, 2024, 16(1), 2431643, DOI:10.1080/19490976.2024.2431643.
- C. Hill, F. Guarner, G. Reid, G. R. Gibson, D. J. Merenstein, B. Pot, L. Morelli, R. B. Canani, H. J. Flint, S. Salminen, P. C. Calder and M. E. Sanders, The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic, Nat. Rev. Gastroenterol. Hepatol., 2014, 11(8), 506–514, DOI:10.1038/nrgastro.2014.66.
- S. Gul and E. Durante-Mangoni, Unraveling the Puzzle: Health Benefits of Probiotics—A Comprehensive Review, J. Clin. Med., 2024, 13(5), 1436, DOI:10.3390/jcm13051436.
- A. Cosson, E. Meudec, C. Ginies, A. Danel, P. Lieben, N. Descamps, V. Cheynier, A. Saint-Eve and I. Souchon, Identification and quantification of key phytochemicals in peas – Linking compounds with sensory attributes, Food Chem., 2022, 385, 132615, DOI:10.1016/j.foodchem.2022.132615.
- S. Mithul Aravind, S. Wichienchot, R. Tsao, S. Ramakrishnan and S. Chakkaravarthi, Role of dietary polyphenols on gut microbiota, their metabolites and health benefits, Food Res. Int., 2021, 142, 110189, DOI:10.1016/j.foodres.2021.110189.
- M. Zhou, J. Ma, M. Kang, W. Tang, S. Xia, J. Yin and Y. Yin, Flavonoids, gut microbiota, and host lipid metabolism, Eng. Life Sci., 2024, 24(5), 2300065, DOI:10.1002/elsc.202300065.
- R. Blomhoff, R. Andersen, E. K. Arnesen, J. J. Christensen, H. Eneroth, M. Erkkola, I. Gudanaviciene, Þ. I Halldórsson, A. Høyer-Lund, E. W. Lemming, H. M. Meltzer, T. Pitsi, I. Siksna, I. Þórsdóttir and E. Trolle, Nordic Nutrition Recommendations, 2023. DOI:10.6027/nord2023-003.
Footnotes |
| † These authors contributed equally as co-first authors. |
| ‡ Present address: Isabel Keidel, Chair of Nutrition and Immunology, Technical University Munich, Germany, DE-85354, Freising, Germany. |
| § These authors contributed equally as last authors. |
| This journal is © The Royal Society of Chemistry 2026 |