Open Access Article
Open Access ArticleCoffee silverskin extract-functionalised pectin: a sustainable substrate to obtain chicken meatballs with antioxidant and improved sensory properties
U. Gianfranco Spizzirri†
a,
Eva Scarcelli† b,
Matteo Carletta
b,
Matteo Carletta c,
Rosa Nicolettid,
Cinzia Benincasad,
Donatella Restucciae,
Domizia Vescovoe,
Maria Stefania Sinicropib,
Annalisa Seriof,
Rosa Di Capuaa,
Francesca Aiello
c,
Rosa Nicolettid,
Cinzia Benincasad,
Donatella Restucciae,
Domizia Vescovoe,
Maria Stefania Sinicropib,
Annalisa Seriof,
Rosa Di Capuaa,
Francesca Aiello *b and
Maria Martuscellif
*b and
Maria Martuscellif
aIonian Department of Law, Economics and Environment, University of Bari Aldo Moro, 74123 Taranto, Italy
bDepartment of Pharmacy, Health and Nutritional Sciences, University of Calabria, 87036 Rende, Italy. E-mail: francesca.aiello@unical.it
cDoctoral School, PhD course in Economic and Social Science, University of Teramo, Via R. Balzarini, 1, 64100 Teramo, Italy
dCouncil for Agricultural Research and Economics (CREA), Research Centre for Olive, Fruit and Citrus Crops, Via Settimio Severo 83, 87036 Rende, Italy
eDepartment of Management, Sapienza University of Rome, 00161 Rome, Italy
fDepartment of Bioscience and Technology for Food, Agriculture and Environment, University of Teramo, Via R. Balzarini, 1, 64100 Teramo, Italy
First published on 7th January 2026
Abstract
High-methoxyl pectin, functionalised with phenolic compounds extracted from coffee silverskin, was used in chicken meatballs formulation for improving antioxidant and sensory properties of the final product. An enzymatic conjugation process was applied to obtain the functional pectin then added at the level of 2.5%. At the same time, non-spiked chicken meatballs were prepared as control. At day 0, functionalised meatballs (PLF0) exhibited a total polyphenol content of 10.44 mg GAE per g, significantly higher than controls (PB, 2.74 mg GAE per g). Antioxidant capacity, expressed as IC30 against the ABTS˙+ radical, was 0.0127 mg mL−1 in PLF0 compared to 0.8982 mg mL−1 in PB0. After 5 and 10 days of storage at +4 °C, functionalised samples retained markedly higher antioxidant potential, with IC30 values nearly 9-fold lower than PB5 and 14-fold lower than PB10, respectively. Cooking preserved these benefits: PLFC maintained a TPC of 9.33 mg GAE per g and an IC30 of 0.1129 mg mL−1, while controls showed lower TPC (5.54 mg GAE per g) and weaker activity (1.4920 mg mL−1). Sensory evaluation revealed that the incorporation of functionalised pectin did not adversely affect the visual appearance, odor, or texture of chicken meatballs. Both raw and cooked products maintained comparable acceptability to controls, with no significant differences in overall liking. Notably, the functionalised meatballs retained their characteristic sensory profile after 10 days of refrigerated storage, confirming that the enrichment with coffee silverskin-derived conjugates did not impair consumer-relevant attributes. Overall, these results demonstrated that functionalisation of chicken meatballs with coffee silverskin extract enhanced the polyphenol content and the antioxidant stability while preserving desirable sensory qualities, making this approach a promising strategy for developing antioxidant-enriched meat products.
Sustainability spotlightThis study highlights a sustainable approach to functional food development by valorising coffee silverskin, an abundant by-product of the coffee roasting industry. Through enzymatic functionalization, bioactive phenolic compounds were successfully incorporated into high-methoxyl pectin, generating a natural antioxidant conjugate that was applied as an ingredient in chicken meatballs. This strategy not only reduced waste by converting a low-value residue into a functional additive but also improved the nutritional and technological quality of a widely consumed meat product. The functionalized meatballs demonstrated enhanced polyphenol content, remarkable antioxidant activity during storage and after cooking, and maintained favourable sensory attributes, supporting their potential for consumer acceptance. By integrating food industry by-products into novel formulations, this work provides a model for circular economy practices, contributing to waste reduction, resource efficiency, and the development of healthier protein-rich foods. |
1 Introduction
Coffee, initially classified as an anti-fatigue stimulant,1 has gradually evolved into one of the most consumed beverages worldwide. In 2022/23, global coffee consumption reached approximately 173.1 million 60 kg bags,2 with a slight increase to 177 million bags during the 2023/24 coffee year.3 When the roasting process takes place,4 the coffee silverskin (CSS) is obtained in large quantities (i.e. around 1 ton of CSS for every 120 tons of roasted coffee).5 This is one of the primary wastes derived from coffee processing, made by a thin layer that covers green coffee beans. Considering a coffee consumption of 10.386 million kg, approximately 2.077 million kg of CSS are generated every year.6 Given that total global coffee intake continues to rise, annual CSS production is estimated to increase accordingly,7 soliciting several attempts to explore new strategies for its valorisation and not only for its recycling. This approach directly supports the achievement of SDG Target 12.3 of the 2030 Agenda.8 In this context, the adoption of circular models for waste management is emphasised.9Numerous ways have been investigated to reuse CSS, including packaging realised by incorporating CSS into biodegradable polylactic acid (PLA polymer) resulting in reinforced bio-nanocomposite films.10,11 In addition it was used in the production of biobutanol.12 More recent valorisation routes, consider its exploitation as a low-cost fertilizer (∼€20–30 per ton) due to its moderate nitrogen and potassium content13 or as a substrate in bioethanol production, with theoretical yields between 0.2–0.3 g ethanol per g dry CSS.14 However, low economic return high pretreatment costs and low conversion efficiency were underlined as severe limitations. CSS has also been applied in the food sector: examples of beverages with antioxidant properties,15 biscuits as a natural source of fibre,16 bread formulated to reduce the risk of chronic diseases17 and with prebiotic and antioxidant effects,18 as well as chicken meat burgers19 can be found in literature. Recently, to facilitate the transition towards more sustainable production and consumption patterns, the reuse of CSS has been proposed as a functional ingredient in bakery products. Compared to composting methods, this alternative scenario yielded beneficial results, leading to a 50% reduction in disposal costs and a 96% reduction in environmental impact.20 Moreover, this strategy ranked higher within the waste hierarchy proposed by the European Commission.21 In this sense, traditional disposal methods, such as landfilling or incineration, are the least desirable in the waste treatments22 as they not only present environmental burdens but also neglect the material's potential.
The cosmetic industry has also explored CSS extracts as exfoliating agents or antioxidants, although these attempts remain niche applications and largely experimental.23 In contrast, the reuse of CSS for the extraction of polyphenols to formulate functional food ingredients is a high-value and sustainable alternative. CSS contains notable amounts of chlorogenic acids and other phenolic compounds (up to 25–30 mg GAE per g dry weight)4 which exhibit strong antioxidant capacity. Additionally, the presence of high-value biological compounds like caffeic acid5 enhances CSS as a raw material with notable health and nutritional properties.24 In particular, the high polyphenols content of CSS has prompted investigations to valorise their recognised antioxidant activity.25 Antioxidant molecules, whether naturally origin or synthetically derived, have the ability to neutralise free radicals and reactive oxygen species (ROS). The growing interest in this field derives from the possibility to include these bioactive molecules into food and beverage products, therefore defined as “functional”. This property is crucial to offer protection against free radical-induced damage, which is involved in the onset and development of chronic diseases, such as cardiovascular diseases, ageing, heart disease, anaemia, cancer, inflammation and neurodegenerative disorders.26 Caffeic acid, a phenolic secondary metabolite also found in CSS, exhibits high antioxidant power, attributed to the presence of a phenolic second hydroxyl group, which enhance scavenging activity through resonance stabilisation and o-quinones formation.27 This may explain its greater antioxidant power compared to other phenolic acids, such as ferulic acid.28 Moreover, caffeic acid is not only able to scavenge radicals directly via hydrogen atom transfer mechanisms but also enables the formation of new metabolites via oxidative degradation.29 Therefore, an important objective is the development of a novel functional food ingredient, characterized by a high content of phenolic acids, such as caffeic acid, with potent antioxidant activity, specifically intended for incorporation into meat products. In particular, chicken meatballs represent an ideal application due to their sensory qualities,30 but also for being a primary source of high-quality protein, vitamins and minerals, with low fat and cholesterol concentrations.31 Moreover, chicken meat contains a higher proportion of unsaturated fatty acids and is more easily digestible than red meat.32 Nevertheless, quality determinants of the final product are influenced by the ingredients used throughout the production chain, which not always involve the use of unhealthy ingredients.33 In this sense, there is a clear consumer demand for healthier formulations, enriched with bioactive compounds to enhance the overall nutritional profile of the product.
Such CSS valorisation strategy, aligns with the European Bioeconomy Strategy,34 reduces reliance on synthetic antioxidants, and offers a scalable solution with added health benefits. This approach not only prevents waste remediation costs but converts an environmental burden into a functional ingredient, in compliance to the green chemistry principles and the sustainable food innovation.
In light of these considerations, the aim of this study was to develop a sustainable strategy for valorising coffee silverskin by extracting its phenolic fraction and using it to produce an enzymatically functionalised pectin with enhanced antioxidant properties. This upcycled biopolymer was then incorporated into chicken meatballs with three specific objectives: (i) enrich the formulations with polyphenols, (ii) enhance their antioxidant capacity during refrigerated storage, and (iii) verify that these improvements could be achieved without compromising the sensory quality of raw or cooked products. In this way, the study explores how a coffee-processing by-product can be repurposed into a functional ingredient that fits within circular bioeconomy principles and contributes to more sustainable food formulations.
2 Materials and methods
2.1. Chemicals and reagents
All reagents, analytical standards and LC/MS-grade solvents were obtained from Sigma-Aldrich (Riedel-de Haën, Laborchemikalien, Seelze, Germany), Extrasynthése (Genay, France), Merck (Darmstadt, Germany) and VWR International (Milan, Italy). Ultrapure water was produced using a Milli-Q plus system (Millipore, Bedford, MA, USA).2.2. Coffee silverskin
The CSS were provided by the roasting company Torrefazione Adriatica s.p.a. (Giulianova, Italy) and stored, protected from light and heat sources, until processing. Samples were classified according to coffee variety and mixing into two matrices: 100% Robusta (Coffee canephora var. Uganda), designated as “ROB”; a blend (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) of C. canephora var. Uganda, C. canephora var. India Parchment and C. arabica var. India Arabica, designated as “MIX”.
1) of C. canephora var. Uganda, C. canephora var. India Parchment and C. arabica var. India Arabica, designated as “MIX”.
2.3. Extraction of polyphenols from coffee silverskin
| CS | Sieve (µm) | ||
|---|---|---|---|
| 355 | 75 | Bottom | |
| a CS = coffee silverskins; ROB = CS originated from the roasting of Coffea canephora (Robusta); MIX = CS originated from the roasting of a blend of Coffea canephora (Robusta) and Coffea arabica (Arabica). Mean ± SD values with different letters are significantly different (Tukey HSD, p < 0.05). | |||
| ROB (%) | 52.04 ± 2.41b | 47.42 ± 2.11a | 0.54 ± 0.02b |
| MIX (%) | 63.88 ± 3.01a | 33.08 ± 1.48b | 3.04 ± 0.12a |
Samples (20 g) were extracted by ultrasound system (AU-32, ARGOLAB, Italy) at 40 °C, 40 kHz and 120 W for 30 min, using two different solvent mixes: (A) 105 mL of absolute ethanol with 45 mL of distilled water or (B) 105 mL of absolute ethanol with 45 mL of acidified water (pH = 2.0, adjusted with 37% HCl (w/w)). The supernatant was filtered using Whatman no. 3 paper and 0.21 µm filters. The extracts obtained were concentrated using a rotary evaporator under reduced pressure and stored at +4 °C until analysis. All extraction yields (%) are reported in Table 2.
| Sample | Plant matrix mass (g) | Extraction solvent | Volume (ml) | Temperature (°C) | Time (min) | Yield (%) | Yield (%) |
|---|---|---|---|---|---|---|---|
| a ROB1A = Robusta, with particle size greater than 355 µm, in water/ethanol; ROB2A = Robusta, with particle size greater than 75 µm, in water/ethanol; MIX1A = mixture, with particle size greater than 355 µm, in water/ethanol; MIX2A = mixture, with particle size greater than 75 µm, in water/ethanol ROB1B = Robusta, with particle size greater than 355 µm, in acid water/ethanol; MIX1B = mixture, with particle size greater than 355 µm, in acid water/ethanol; ROB2B = Robusta, with particle size between 75 µm and 355 µm, in acid water/ethanol; MIX2B = mixture, with particle size between 75 µm and 355 µm, in acid water/ethanol. Values are expressed as mean ± SD (n = 3). Different letters indicate significant differences according to Tukey's HSD test at p < 0.0. | |||||||
| ROB1A | 20 | Ethanol/water (70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 v/v) 30 v/v) |
150 | 40 | 30 | 0.746 ± 0.03d | 3.73 ± 0.11e |
| ROB2A | 20 | Ethanol/water (70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 v/v) 30 v/v) |
150 | 40 | 30 | 1.188 ± 0.04a | 5.94 ± 0.22a |
| MIX1A | 20 | Ethanol/water (70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 v/v) 30 v/v) |
150 | 40 | 30 | 1.011 ± 0.04b | 5.05 ± 0.21b |
| MIX2A | 20 | Ethanol/water (70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 v/v) 30 v/v) |
150 | 40 | 30 | 0.497 ± 0.02f | 2.49 ± 0.10g |
| ROB1B | 20 | Ethanol/acid water (pH = 2, 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 v/v) 30 v/v) |
150 | 40 | 30 | 0.835 ± 0.03d | 4.18 ± 0.19d |
| MIX1B | 20 | Ethanol/acid water (pH = 2, 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 v/v) 30 v/v) |
150 | 40 | 30 | 0.909 ± 0.04c | 4.55 ± 0.18c |
| ROB2B | 20 | Ethanol/acid water (pH = 2, 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 v/v) 30 v/v) |
150 | 40 | 30 | 0.741 ± 0.03d | 3.71 ± 0.16e |
| MIX2B | 20 | Ethanol/acid water (pH = 2, 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 v/v) 30 v/v) |
150 | 40 | 30 | 0.608 ± 0.02e | 3.04 ± 0.12f |
2.4. Synthesis of antioxidant pectin conjugates
In order to obtain the pectin–polyphenol conjugate, high methoxylated (HM) pectin was functionalised with the chosen extract (ROB1B) employing two methods: the polymerisation technique via grafting (g), performed according to the literature35 and the polymerisation technique via enzymatic reaction (e), catalysed by porcine pancreatic lipase adapted from Zhang et al. (2020).36 The resulting polymers (eROB1B and gROB1B, details in SI) were subjected to comparative chemical analysis.2.5. 1H-NMR spectroscopy of CS extracts
1H-NMR spectra were acquired using a Brucker 300 MHz spectrometer. Samples were dissolved in DMSO-d6 with tetramethylsilane (TMS) as the internal standard, at 25 °C. Chemical shifts (δ) were expressed in parts per million (ppm). The signals assignment was performed comparing the data already present in published papers available in literature, and using Human Metabolome Database, Chem Spectrum, and nmrdb.org (https://www.nmrdb.org/).2.6. LC-MS/MS analysis of CS extracts and pectin conjugates
Samples were analysed by electrospray ionization tandem mass spectrometry (ESI-MS/MS) using an API 4000 Q-Trap mass spectrometer (Applied Biosystems, Sciex) interfaced with an HPLC 1200 series instrument (Agilent Technologies, Santa Clara, California). The first diagnostic evaluation was carried out by direct infusion analysis,37 operating in negative-ion mode in full scan product ion scan, and precursor ion scan. Afterwords, quantitative analysis were obtained by multiple reaction monitoring (MRM).The mass spectrometer parameters were set as follows: ion spray voltage, 4500 V; curtain gas, 20 psi; source temperature, 400 °C; ion source gases 1 and 2 set at 40 and 35 psi, respectively. Compound-specific parameters such as declustering potential (DP), entrance potential (EP), collision energy (CE), and collision cell exit potential (CXP) were optimized individually for each MRM transition. Chromatographic separation of analytes was performed using an Eclipse XDB-C8-A HPLC column (5 µm particle size, 150 mm length, 4.6 mm i.d.; Agilent Technologies, Santa Clara, CA, USA) at a flow rate of 300 µL min−1 with an injection volume of 10 µL. The binary mobile phase consisted of 0.1% formic acid in water (solvent A) and methanol (solvent B). The gradient elution was as follows: 10% to 100% B over 10 min, held at 100% B for 2 min, and returned to the initial conditions (90% A, 10% B) over the next 8 min. The total run time was 20 min per injection. Quantitative analyses were performed using external calibration curves built through least-squares linear regression analysis. To this end, standard stock solutions were prepared by dissolving in methanol the standard compound of interest. The correlation coefficients (R2) of the calibration curves ranged between 0.9994 and 0.9997. The standards used for phenols characterization and detection were: caffeic acid (CA), ferulic acid (FA), catechol (CAT), tyrosol (TYR), vanillin (VAN), vanillic acid (VA), Luteolin (LUT), Verbascoside (VER), Rutin (RUT). To assess analyte losses during the analytical procedure and to determine the accuracy, recovery tests on spiked solutions were conducted. Additionally, the limit of detection (LOD) and limit of quantification (LOQ) were calculated by analyzing procedural blanks.
2.7. Chicken meatballs preparation and analysis
Chicken meat was from retail (Amadori Group, Cesena, Italy). Chicken meatballs were prepared using fresh boneless chicken breast, ground through a 3 mm plate. Formulation included ground chicken breast 83%, water 9.6%, maize starch 3.5%, high methoxyl pectin (PLB) or functionalised HM pectin (PLF) 2.5% and salt 1.4%. All ingredients were mixed uniformly to ensure homogeneous distribution. The meat batter was then portioned into 12 g spheres and rolled manually to ensure consistent shape and size. Raw samples were analysed for colour and antioxidant features, immediately after preparation (T0) and after 5 (T5) and 10 (T10) days of refrigerated storage (4 °C) under commercial packaging film. Samples were cooked by a preheated air fryer (ENKHO, Eurospin Italia S.p.a., San Martino Buon Albergo, VR) at 180 °C for 20 min. After cooking, products were tested for sensory attributes (cooled at 55 ± 5 °C) and underwent instrumental analysis for colour and texture evaluation (at room temperature).
 | (1) |
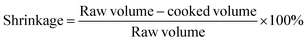 | (2) |
The coordinates a* and b* were used to calculate hue angle value according to the following eqn (3):
 | (3) |
The colour difference (ΔE) between the sample colour 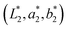 and the reference colour
and the reference colour 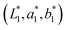 was determined according to the following eqn (4):38,39
was determined according to the following eqn (4):38,39
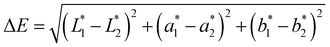 | (4) |
2.8. Antioxidant evaluation of CSS extracts, pectin conjugates and chicken meatballs
2.9. Microbial analysis
Meatballs, prepared with pectin (PLB) and functionalised pectin (PLF), were subjected to the determination of total mesophilic and psychrophlic aerobic counts during 10 days of storage at 4 °C in aerobic conditions. In detail, 10 grams of meatballs, representative of the whole sample, were aseptically cut and diluted 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 in sterile saline solution. They were homogenized by a Stomacher (200 rpm for 90 s) and serially diluted. Three different samples were analysed for each sampling time (0, 5 and 10 days). Plate count agar was prepared and inoculated with the appropriate dilutions, then the Petri dished were incubated at 25 °C × 48 h and 4 °C × 7 days, respectively for Total Mesohilic and Total Psycrophilic counts. The analyses were performed in triplicate, and the results obtained were expressed as log CFU per g.
10 in sterile saline solution. They were homogenized by a Stomacher (200 rpm for 90 s) and serially diluted. Three different samples were analysed for each sampling time (0, 5 and 10 days). Plate count agar was prepared and inoculated with the appropriate dilutions, then the Petri dished were incubated at 25 °C × 48 h and 4 °C × 7 days, respectively for Total Mesohilic and Total Psycrophilic counts. The analyses were performed in triplicate, and the results obtained were expressed as log CFU per g.
2.10. Statistical analysis
All experiments were performed in triplicate, and results were presented as mean ± standard deviation (SD). Differences among groups were analysed using one-way analysis of variance (ANOVA). When the ANOVA indicated significant differences (p < 0.05), Tukey's Honestly Significant Difference (HSD) post-hoc test was applied to identify which means differed significantly. Statistical analyses were performed using GraphPad Prism 8.3.0 (GraphPad Software, Inc., San Diego, CA, USA).3 Results and discussion
3.1. Ultrasound-assisted extraction of phenolic compounds from CSS
In this study, phenolic compounds were isolated using ultrasound-assisted extraction (UAE). Compared with conventional extraction approaches, UAE is recognized for its efficiency, reproducibility, and reduced processing times, and is therefore considered a reliable and sustainable method.48Prior to extraction, CSS samples were subjected to a sieving procedure using stainless-steel sieves of 355 µm and 75 µm mesh size. This allowed the separation of the raw material into three granulometric fractions: particles larger than 355 µm, particles between 75 and 355 µm, and particles smaller than 75 µm. The extraction yields obtained from each fraction are reported in Table 2, providing insight into the effect of particle size on extraction efficiency.
Additionally, two solvent mixtures were employed: (i) ethanol/water (70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 v/v) and (ii) ethanol/water acidified to pH 2.0 with HCl (37% w/w), in the same volumetric ratio. The use of hydroalcoholic and acidified aqueous-organic mixtures is consistent with the principles of green chemistry and supported by the evidence that such mixtures outperform pure organic solvents in extracting phenolic constituents.49
30 v/v) and (ii) ethanol/water acidified to pH 2.0 with HCl (37% w/w), in the same volumetric ratio. The use of hydroalcoholic and acidified aqueous-organic mixtures is consistent with the principles of green chemistry and supported by the evidence that such mixtures outperform pure organic solvents in extracting phenolic constituents.49
For clarity, the different extraction conditions were labelled using a coding system based on coffee type, granulometry, and solvent mixture. The CSS samples originated from the roasting of either Coffea canephora (Robusta, ROB) or a blend of Coffea canephora and Coffea arabica (MIX). The granulometric fractions were classified as particles larger than 355 µm (coded as 1) or between 75 and 355 µm (coded as 2). Solvent systems were distinguished as hydroalcoholic (coded as A) or acidified hydroalcoholic (coded as B). In this way, a total of eight extracts were obtained, and the extraction yields under different conditions are reported in Table 2.
Overall, yields expressed as percentages, were comparable across different granulometric fractions of the plant matrix and no direct correlation was observed between particle size reduction and extraction efficiency. In general, smaller particle sizes provide a larger surface-to-volume ratio, facilitating solvent access and enhancing the diffusion of intracellular compounds into the medium. However, excessively fine powders may lead to particle agglomeration, reduced solvent circulation, and difficulties in solid–liquid separation, which can counteract these advantages.50 Particle size is known to influence extraction performance due to its effect on surface area, solvent penetration, and mass transfer kinetics. For this reason, extraction efficiency does not always increase linearly with particle size reduction, and in some cases coarser fractions may yield comparable or even superior results. In some instances, coarser powders (MIX1A, ROB1B, MIX1B) even resulted in higher yields than finer ones (MIX2A, ROB2B, MIX2B), despite the latter exhibited a larger surface area. The highest yields were recorded for samples ROB2A (5.94%) and MIX1A (5.05%). The extraction yields reported here are consistent with previous studies on UAE of plant phenolics, where values typically ranged between 4–7% depending on solvent system and plant species.48,51 Notably, the absence of a clear dependence on particle size was also observed in other studies, suggesting that cavitation phenomena compensated for surface area limitations by efficiently disrupting cellular structures. Moreover, the performance of hydroalcoholic and acidified mixtures aligns with literature findings, emphasizing the role of solvent polarity and pH in enhancing phenolic recovery.52 Taken together, these findings reinforce the robustness of UAE as a green and versatile extraction technique for phenolic compounds, also highlighting the importance of solvent composition over granulometric factors in determining extraction efficiency.
3.2. Characterisation of CSS extracts
| Sample | TPC (mg GAE per g) | FC (mg CT per g) | Scavenger activity IC50 (mg mL−1) | |
|---|---|---|---|---|
| DPPH | ABTS | |||
| a ROB1A = Robusta, with particle size greater than 355 µm, in water/ethanol; ROB2A = Robusta, with particle size greater than 75 µm, in water/ethanol; MIX1A = mixture, with particle size greater than 355 µm, in water/ethanol; MIX2A = mixture, with particle size greater than 75 µm, in water/ethanol ROB1B = Robusta, with particle size greater than 355 µm, in acid water/ethanol; MIX1B = mixture, with particle size greater than 355 µm, in acid water/ethanol; ROB2B = Robusta, with particle size between 75 µm and 355 µm, in acid water/ethanol; MIX2B = mixture, with particle size between 75 µm and 355 µm, in acid water/ethanol. TPC = total polyphenol content; FC = flavonoid content; DPPH = 2,2-diphenyl-1-picrylhydrazyl; ABTS = 2,2′-azino-bis(3-ethylbenzothiazolin-6-sulphonic acid). Values are expressed as mean ± SD (n = 3). Different letters indicate significant differences according to Tukey's HSD test at p < 0.05. | ||||
| ROB1A | 370 ± 13b | 758 ± 28b | 0.0448 ± 0.0018e | 0.0869 ± 0.0031e |
| ROB2A | 186±6d | 720 ± 28b | 0.0598 ± 0.0021d | 0.1334 ± 0.0032c |
| MIX1A | 140±5e | 770 ± 29b | 0.0646 ± 0.0027c | 0.1480 ± 0.0040b |
| MIX2A | 89±3f | 266 ± 11e | 0.1665 ± 0.0074a | 0.2125 ± 0.0082a |
| ROB1B | 422 ± 17a | 760 ± 32b | 0.0678 ± 0.0027c | 0.1162 ± 0.0042d |
| MIX1B | 287 ± 12c | 838 ± 36a | 0.0736 ± 0.0029c | 0.1207 ± 0.0043d |
| ROB2B | 180±7d | 439 ± 17d | 0.1269 ± 0.0058b | 0.1438 ± 0.0063 |
| MIX2B | 41±2g | 281 ± 12e | 0.1706 ± 0.0077a | 0.1445 ± 0.0063b |
ROB1B showed an increase in TPC of ∼14% compared to its non-acidified counterpart ROB1A (422 vs. 370 mg GAE per g), demonstrating that mild acidic treatment effectively cleaves glycosidic bonds and releases bound phenolic compounds, as reported in previous studies.53 FC followed a similar trend, ranging from 266 ± 11 mg of catechin (CT) per gram of extract in MIX2A to 838 ± 36 mg CT per g in MIX1B. The highest flavonoid concentrations were observed in ROB1B (760 ± 32 mg CT per g) and MIX1B (838 ± 36 mg CT per g), indicating that acidified hydroalcoholic extraction and intermediate particle sizes (75–355 µm) can favor flavonoid solubilization. Interestingly, larger particle fractions (>355 µm) generally returned higher TPC and flavonoid values, suggesting that these particles may retain polyphenols better during processing and facilitate their release upon extraction.
Antioxidant activity, expressed as IC50, correlated strongly with TPC and flavonoid content. ROB1A and ROB1B displayed the lowest IC50 values for DPPH (0.0448 ± 0.0018 and 0.0678 ± 0.0027 mg mL−1, respectively) and ABTS (0.0869 ± 0.0031 and 0.1162 ± 0.0042 mg mL−1), indicating superior radical scavenging capacity compared to mixed extracts. In contrast, MIX2B, the extract with the lowest TPC and flavonoid content, showed markedly higher IC50 values for both radicals (DPPH: 0.1706 ± 0.0077 mg mL−1; ABTS: 0.1445 ± 0.0063 mg mL−1). These results confirmed the direct relationship between polyphenol content and antioxidant performance. Notably, ABTS-based IC50 values were generally higher than DPPH values, likely reflecting differences in solubility and reaction kinetics of hydrophilic versus lipophilic antioxidants. Acid hydrolysis emerged as a key factor in increasing the bioavailability of phenolic compounds, whereas larger particle fractions retained higher levels of bioactive compounds. Robusta extracts obtained with acidified hydroalcoholic solvent and coarser particle size demonstrated the most promising combination of high TPC and FC values, and low IC50 values, confirming their suitability for innovative applications including nutraceutical formulations or polymer functionalisation.
| Extract | Compound | 1H NMR (DMSO) splitting δ (ppm) and multiplicitya |
|---|---|---|
| a s: Singlet; d: doublet; m: multiplet. ROB1B = Robusta, with particle size greater than 355 µm, in acid water/ethanol. | ||
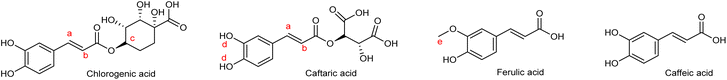
| ROB1B | Chlorogenic acid | 4.37 CH–O (m, 1H) |
6.26 CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–C CH–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (d, 1H, J = 7.0Hz) O (d, 1H, J = 7.0Hz) |
||
| 6.78 CH-6, (d, 1H, J = 9.2Hz) | ||
| 7.08 CH-2, (s, 1H, J=) | ||
7.47 CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH (d, 1H, J = 15.6Hz) CH (d, 1H, J = 15.6Hz) |
||
| Caftaric acid | 9.22 OH (s, 2H) | |
6.46 CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–C CH–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (d, 1H, J = 15.9Hz) O (d, 1H, J = 15.9Hz) |
||
| Ferulic acid | Ethylenic signals overlapped with chlorogenic ones | |
| 3.89 O–CH3 (s, 3H) | ||
| Caffeic acid | Ethylenic signals and many of the aromatic protons, overlapped with chlorogenic ones | |
7.00 CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH partially overlapped (d, 1H, J = 13.8 Hz) CH partially overlapped (d, 1H, J = 13.8 Hz) |
||
3.3. Synthesis and characterisation of pectin conjugates
Hydrophilic natural polymers exhibit three key characteristics: biocompatibility, biodegradability, and non-toxicity. These properties make them highly attractive for applications in food preservation, biomedical devices, and environmental remediation.54 Moreover, their functional versatility allows the introduction of additional bioactive properties through chemical modification.In this study, HM pectin was functionalised via grafting, following a modified procedure based on Restuccia et al. (2018).55 Hydrophilic natural polymers, such as pectin, provide an ideal scaffold for the covalent attachment of antioxidant molecules due to their abundant hydroxyl groups and inherent safety profile. The grafting reaction was initiated using the ROB1B extract (equivalent to 75 mg of gallic acid) in combination with a redox pair consisting of L-ascorbic acid and hydrogen peroxide. This system operates at relatively low temperatures, reducing the risk of forming toxic by-products. The ascorbyl and hydroxyl radicals generated in situ abstract hydrogen atoms from the pectin hydroxyl groups, activating the polymer chain and facilitating the formation of covalent bonds with the phenolic acids present in the extract.56 The resulting grafted polymer, designated gROB1B, was subsequently characterized using spectrophotometric assays to determine its antioxidant capacity and polyphenol content.
In parallel, enzymatic conjugation has been reported as an effective alternative for grafting phenolic compounds onto polysaccharides skeleton. This strategy engages enzymes, such as laccases and peroxidases, which promote selective esterification under mild conditions.57,58 Following this approach, ROB1B extract was conjugated to HM pectin using porcine pancreatic lipase (PPL), which catalyses both the hydrolysis of HM pectin methyl esters in aqueous solution and the subsequent esterification between the hydroxyl groups of phenolic acids and the carboxyl groups of pectin.36,59,60
The resulting polymer, denoted eROB1B, along with non-functionalised pectin (PB), was evaluated for total polyphenol content and radical scavenging activity against ABTS, allowing direct comparison of the antioxidant efficacy between the chemically and enzymatically functionalised conjugates (Table 5).
| Sample | TPC (mg GAE per g) | IC30 (mg mL−1) radical ABTS |
|---|---|---|
| a ROB1B = Robusta, with particle size greater than 355 µm, in acid water/ethanol; eROB1B = pectin conjugate with ROB1B, by enzymatic reaction; gROB1B = pectin conjugate, with ROB1B, by grafting; values expressed as averages (n = 3). TPC = total polyphenol content; ABTS = 2,2′-azino-bis(3-ethylbenzothiazolin-6-sulphonic acid). Values are expressed as mean ± SD (n = 3). Different letters indicate significant differences according to Tukey's HSD test at p < 0.05.10 = Not detectable or below the limit of quantification of the assays. | ||
| eROB1B | 10.96 ± 0.48a | 0.091 ± 0.003a |
| gROB1B | 2.02 ± 0.08b | 17.881 ± 0.741b |
| PB | — | — |
The results indicate that HM pectin functionalised via porcine pancreatic lipase (eROB1B) exhibited a significantly higher content of phenolic groups compared to the same polymer functionalised through the grafting approach (gROB1B). These findings were supported by the eROB1B's enhanced antioxidant performance, as demonstrated by the IC30 values, confirming the superior radical-scavenging ability of the enzymatically conjugated pectin against the ABTS˙+ cation radical. The lower IC30 values observed for PL indicate that a smaller concentration of the polymer is required to achieve 30% inhibition, highlighting the greater efficacy of the enzymatic functionalisation method in introducing bioactive phenolic moieties onto the polysaccharide backbone. Furthermore, the hypothesis that the phenolic groups in the conjugates, and consequently their observed antioxidant activity, originated exclusively from the ROB1B extract was supported by the negative results obtained from identical assays conducted on the non-functionalised pectin (PB), which served as the experimental control. The absence of detectable radical-scavenging activity in PB confirms that the intrinsic HM pectin matrix does not significantly contribute to the measured antioxidant effect. These results emphasize the critical role of chemical or enzymatic modification in enhancing the functional properties of natural polysaccharides, demonstrating that the method of conjugation not only determines the quantity of incorporated phenolic compounds, but also directly influences the resulting bioactivity. Overall, these data confirmed literature findings, suggesting that enzymatic functionalisation with ROB1B represents a more efficient strategy for the incorporation of antioxidant phenolic compounds into pectin backbone, providing a polymer conjugate with both higher phenolic content and stronger radical-scavenging capability compared to grafting-based modification.61 This enhanced performance may be attributed to the mild reaction conditions and the dual catalytic role of ROB1B, which facilitates both hydrolysis and esterification reactions, leading to more effective covalent attachment of phenolic groups to the polysaccharide chain.
3.4. LC-MS/MS analysis of polyphenols in CSS extracts and functionalised pectin
As a preliminary exploratory step prior to the quantitative evaluation, a qualitative analysis on both extracts, ROB1A and ROB1B, was conducted. These experiments led to the identification of predominant metabolites that facilitated a deeper understanding of the phenolic profile of the samples. Based on metabolic annotation, few compounds stood up, primarily derived from caffeic acid, quinic acid, and ferulic acid. Among the identified compounds, chlorogenic acid (also known as 3-caffeoylquinic acid) was detected and its presence was confirmed based on its characteristic fragmentation pattern. The molecular ion was observed at m/z 352.8, and its identification was supported by the presence of key product ions: m/z 191.3, corresponding to quinic acid, m/z 172.8, attributed to a dehydrated form of quinic acid, and m/z 178.9, indicative of the release of caffeic acid. Similarly, feruloylquinic acid was identified through its molecular ion at m/z 193.05, with a fragmentation pattern showing product ions at m/z 191.3 (quinic acid), m/z 172.8 (dehydrated quinic acid), and m/z 193.1, consistent with ferulic acid, thereby supporting the structural assignment. Furthermore, the presence of the molecular ion at m/z 515.5, and fragments at m/z 352.9 (chlorogenic acid), 191.3 (quinic acid) and 178.9 (caffeic acid) strongly suggested the presence of two caffeoyl moieties esterified to a quinic acid core, identified as dicaffeoylquinic acid. All these compounds are well known in literature for their health-promoting properties. Chlorogenic acid, the most abundant caffeoylquinic acid in foods like coffee itself, exhibited an interesting potential role in the prevention of type 2 diabetes and cardiovascular disease through the modulation of lipid and glucose metabolism. It is also extensively metabolized, mainly in the colon, into active derivatives that may bind to serum proteins or accumulate in tissues, potentially mediating effects in vivo.62 Feruloylquinic acid is a potent free radical scavengers and xanthine oxidase (XO) inhibitor.63 Dicaffeoylquinic acid and its derivatives showed strong antioxidant activity, effectively scavenging free radicals and inhibiting Cu2+-mediated LDL oxidation in a dose-dependent manner. These effects were proved to be due mainly to their caffeoyl moieties, which also contribute to metal ion chelation.64Following the qualitative evaluation, quantitative analyses of the extracts and polymers were performed. The results revealed a significant presence of the phenolic compounds under study in ROB1B. Indeed, the concentration of catechol and tyrosol was found to be four to six times higher than that observed in ROB1A (Table 6). Among phenolic acids, caffeic and ferulic acids exhibited an impressive increase in ROB1B, suggesting an enhanced extraction or stabilization under the acidified conditions used. Likewise, significant differences were noted in the flavonoid profile: compounds such as rutin and luteolin were considerably more abundant in ROB1B, with rutin reaching levels nearly ten times higher than in ROB1A. Notably, vanillin was detected exclusively in the ROB1B extract, indicating that acidic conditions may either promote its release from bound forms or enhance its chemical stability during extraction.
| Compound | ROB1A | ROB1B | eROB1B | gROB1B |
|---|---|---|---|---|
| a ROB1B = Robusta, with particle size greater than 355 µm, in acid water/ethanol; eROB1B = pectin conjugate with ROB1B, by enzymatic reaction; gROB1B = pectin conjugate, with ROB1B, by grafting <LOD = below limit of detection; <LOQ = below limit of quantification. Different letters indicate significant differences according to Tukey's HSD test at p < 0.05. | ||||
| Catechol | 5.89 ± 0.24b | 42.79 ± 2.51a | <LOD | <LOD |
| Tyrosol | 131.03 ± 9.35b | 238.54 ± 11.74a | <LOQ | <LOQ |
| Vanillin | <LOD | 11.94 ± 1.01 | <LOD | <LOD |
| Vanillic acid | 4.28 ± 0.19b | 7.24 ± 0.50a | <LOD | <LOD |
| Caffeic acid | 61.23 ± 3.19b | 390.91 ± 22.43a | 3.21 ± 0.14c | 1.92 ± 0.02c |
| Ferulic acid | 58.84 ± 2.47b | 337.39 ± 17.33a | <LOQ | <LOQ |
| Luteolin | 7.18 ± 0.47b | 30.84 ± 2.04a | 6.00 ± 0.36b | 5.10 ± 0.21b |
| Rutin | 1.04 ± 0.08b | 10.80 ± 0.65a | 1.00 ± 0.02b | 0.84 ± 0.01b |
| Verbascoside | 16.67 ± 1.12b | 51.72 ± 2.65a | <LOD | <LOD |
The enhanced phenolic profile observed in the acidified extract (ROB1B) strongly suggested that solvent acidification with HCl played a key role in improving the extraction efficiency of phenolic compounds from coffee silverskin. Several factors are likely to contribute to this effect. First, acidic conditions seemed to promote the hydrolysis of ester and glycosidic bonds, which commonly link phenolic compounds to structural components of the plant matrix, including lignin, hemicellulose, and pectin. Coffee silverskin showed no exception, as its lignin content has been confirmed by the detection of guaiacyl (G) and syringyl (S) units, characteristic of lignin polymers.65 This hydrolytic action increased the availability of free phenolics that would otherwise have remained bound within the insoluble polymeric fraction. Further support for the role of acidification in enhancing phenolic extraction came from a comparative study undertaken by Petreska Stanoeva et al. (2020), who found that HCl-acidified solvent systems consistently yielded higher concentrations of total phenolic compounds, especially anthocyanins, from Aronia melanocarpa, compared to systems acidified with acetic or formic acid. This improvement is attributed to the strongly acidic environment (pH ≈ 0.7), which facilitated the stabilization and solubilization of phenolic structures.66 The acidified environment played a key role in enhancing both the solubility and extractability of specific phenolic acids, particularly caffeic and ferulic acid. These compounds possess ionizable functional groups that, under low pH conditions, remain predominantly in their protonated form. This protonation improves their solubility in ethanol-rich solvents while reducing interactions with matrix components such as proteins, polysaccharides, and lignin. Flavonoids such as rutin and luteolin, which are often unstable under neutral or alkaline conditions, also appeared to benefit from acidification, which likely mitigated degradation and oxidation during extraction. Among these phenolics, caffeic acid was notably abundant in CSS, where it is frequently bound to lignin or polysaccharide structures. Acidic conditions (pH < 3.7) have been shown to significantly enhance the extraction efficiency of hydroxycinnamic acids by limiting their ionic interactions within the matrix and increasing their solubility in organic solvents. This dual effect of reduced binding and improved solubility under acidic conditions supported the observed increase in extractable phenolic content.67 The analysis of the two polymers, gROB1B and eROB1B, revealed a significant reduction in free phenolic content compared to the original extract ROB1B. Most phenolic compounds were below the limit of detection (LOD) or quantification (LOQ), indicating limited retention or accessibility.
However, a few phenolics, such as caffeic acid, luteolin, and rutin, remained detectable, suggesting partial incorporation or stability during polymer formation. Notably, eROB1B showed slightly higher levels of these phenolics, indicating a marginally better phenolic retention. However, while both enzymatic and chemical strategies offered viable approaches to functionalised biopolymers, enzymatic strategy was more effective in preserving and integrating phenolic structures into the pectin backbone, leading to significantly higher antioxidant performance. In contrast, the radical grafting approach (gROB1B) showed much lower phenolic incorporation according to the TPC values and poorer scavenging capacity recorded.
This superior performance of enzymatic modification is also consistent with literature reports: for example, Zhang et al. (2021) demonstrated that lipase-catalyzed grafting of gallic acid onto pectin significantly enhanced antioxidant activity compared to native pectin. Enzymatic methods thus appear to provide a more gentle and selective modification route, avoiding harsh conditions or radical side-reactions, and enabling a higher retention of bioactive phenolics.36,68
3.5. Evaluations on raw and cooked chicken meatballs
To evaluate the phenolic content and antioxidant activity of the prepared meatballs, extractions were performed by a literature protocol with some modification.41,42 Chicken meatballs were prepared using 83% minced chicken, 9.6% water, 3.5% corn starch, 2.5% functionalised pectin, and 1.4% salt.
The pectin concentration (2.5%, w/w) was chosen following preliminary trials (1–5% range), where 2.5% offered optimal textural and binding properties. Similar inclusion levels have been reported in the literature for comminuted meat products employing pectin as a functional binder.37,69
The mixture was homogenized and divided into four portions (10 g each), with three stored at +4 °C for shelf-life analyses and one cooked by an air fryer at 180 °C for 20 min. Control meatballs containing 2.5% non-functionalised pectin were prepared in parallel for comparison. Spectrophotometric assays were conducted on freshly prepared meatballs (t = 0 day), refrigerated samples (t = 5 and 10 days) and cooked meatballs (Table 7). These experiments aimed to assess whether the antioxidant activity was maintained during storage under typical household refrigeration conditions with PVC film covering. The results clearly demonstrated that meatballs containing enzymatically functionalised pectin retained significant antioxidant activity, consistent with the performance observed in the ROB1B extract and polymer conjugates. In contrast, non-functionalised meatballs (controls) exhibited minimal radical-scavenging activity, likely due to interference from other ingredients or the protein matrix of chicken meat. Despite these factors, functionalised meatballs showed a markedly higher TPC and enhanced antioxidant capacity, which was preserved even after cooking. The TPC and ABTS radical-scavenging activity (IC30) of the meatballs are summarized in Table 7. Freshly prepared functionalised meatballs (PLF0) exhibited a TPC of 10.44 ± 0.47 mg GAE per g, which was nearly four times higher than the control (PLB0, 2.74 ± 0.11 mg GAE per g), demonstrating the effective incorporation of antioxidant phenolic compounds through enzymatic functionalisation. Correspondingly, the IC30 for ABTS radical scavenging was 0.0127 ± 0.0004 mg mL−1 for PLF0, compared to 0.8982 ± 0.0320 mg mL−1 for PLB0, highlighting a substantially higher radical-scavenging capacity in functionalised samples. Quantitative comparisons further highlighted the impact of functionalisation: the concentration of PLF5 (functionalised meatball stored for 5 days) required to achieve a 30% reduction in initial ABTS absorbance was nearly nine times lower than that of the control. After 10 days of storage, the difference between PLF10 and PLB10 increased to approximately fourteen-fold, unequivocally demonstrating superior antioxidant performance of the functionalised samples relative to controls. After 5 days (PLF5), TPC slightly decreased to 9.93 ± 0.41 mg GAE per g, with an IC30 of 0.4574 ± 0.0153 mg mL−1. After 10 days (PLF10), TPC further decreased to 6.51 ± 0.27 mg GAE per g and IC30 to 0.3146 ± 0.0111 mg mL−1. Despite this reduction, functionalised meatballs maintained significantly higher phenolic content and antioxidant activity than their non-functionalised counterparts, confirming the protective effect of the conjugated pectin. Notably, the IC30 values indicate that a much lower concentration of functionalised meatball extract was required to achieve 30% ABTS inhibition, with reductions ranging from approximately ninefold at 5 days to fourteenfold at 10 days compared to controls. Once again, these findings underscored the superior antioxidant efficiency of the functionalised samples. The antioxidant activity of raw meatballs remained stable during refrigeration, showing excellent efficacy in aqueous environments. No significant differences were observed in ABTS radical inhibition between PLF5 and PLF10, suggesting that the antioxidant capacity was preserved after 5 days of storage, despite a reduction relative to freshly prepared samples (PLF0). Cooked functionalised meatballs (PLFC) also exhibited strong antioxidant properties. At a concentration of 0.1129 mg mL−1, PLFC achieved a 30% reduction in ABTS absorbance, whereas the corresponding control (PLBC) required 1.4920 mg mL−1 to achieve the same effect. The TPC was consistently higher in PLFC than in PLBC, aligning with observations by Devatkal et al. (2010), who reported increased TPC in pomegranate peel-enriched meatballs compared to controls.42 Specifically, Folin–Ciocâlteu assays revealed a 4.89% decrease after 5 days and a 37.64% decrease after 10 days, with TPC remaining substantially higher than in control samples. These results are comparable to those reported by Al-Juhaimi et al. (2018), where a 3.57% reduction in TPC was observed in chicken meatballs containing Argel leaf extract after 5 days of refrigeration.41 Overall, these findings confirmed that pectin functionalised with CSS extract imparted substantial antioxidant capacity, which is preserved in meatball preparations under both refrigerated storage and cooking conditions. This highlights the potential of enzymatically functionalised pectin as a valuable ingredient for developing functional foods with improved bioactive properties.
| Sample | Refrigeration time (days) | TPC (mg GAE per g) | IC30 (mg mL−1) radical ABTS |
|---|---|---|---|
| a TPC = total polyphenol content; PLF0 = raw functionalised chicken patty, 0 days; PLB0 = raw chicken patty (control), 0 days; PLF5 = raw functionalised chicken patty, 5 days; PLB5 = raw chicken patty (control), 5 days; PLF10 = raw functionalised chicken meatball, 10 days; PLB10 = raw chicken meatball (control), 10 days; PLFC = cooked functionalised chicken meatball; PLBC = cooked chicken meatball (control). Values are expressed as mean ± SD (n = 3). Different letters indicate significant differences according to Tukey's HSD test at p < 0.05.10 = Not detectable or below the limit of quantification of the assays. | |||
| PLF0 | 0 | 10.44 ± 0.47a | 0.0127 ± 0.0004h |
| PLB0 | 0 | 2.74 ± 0.11c | 0.8982 ± 0.0320d |
| PLF5 | 5 | 9.93 ± 0.41a | 0.4574 ± 0.0153e |
| PLB5 | 5 | 2.63 ± 0.12c | 4.0226 ± 0.1401b |
| PLF10 | 10 | 6.51 ± 0.27b | 0.3146 ± 0.0111f |
| PLB10 | 10 | 2.60 ± 0.09c | 4.3885 ± 0.1623a |
| PLFC | — | 9.33 ± 0.41a | 0.1129 ± 0.0042g |
| PLBC | — | 2.54 ± 0.21c | 1.4920 ± 0.0430c |
| Time (days) | ||||
|---|---|---|---|---|
| 0 | 5 | 10 | ||
| a PLF = raw functionalised chicken patty; PLB = raw chicken patty (control). Different lowercase letters indicate statistically significant differences between time points based on Tukey's HSD post-hoc test (p < 0.05); different uppercase letters indicate significant differences between formulations within the time point assessed by Student's t-test (p < 0.05). | ||||
| PLB | L* | 56.7 ± 1.63Aab | 58.84 ± 0.96Aa | 52.48 ± 3.96b |
| a* | 0.70 ± 0.44 | 0.34 ± 0.33A | 0.23 ± 1.14A | |
| b* | 11.17 ± 0.79a | 11.66 ± 1.58Bab | 11.89 ± 0.55Ba | |
| ΔE | 2.47 ± 0.98 | 4.45 ± 2.38 | ||
| PLF | L* | 46.84 ± 1.83Bab | 46.26 ± 0.73Bb | 48.60 ± 1.04a |
| a* | 0.49 ± 0.17a | −1.45 ± 0.24Bb | −1.88 ± 0.47Bb | |
| b* | 9.70 ± 1.22b | 9.56 ± 1.08Aab | 9.02 ± 1.17Ab | |
| ΔE | 2.34 ± 0.69 | 3.24 ± 0.49 | ||
| PLF vs. PLB | ΔE | 9.99 ± 1.13b | 12.89 ± 0.52a | 5.79 ± 1.66c |
Indeed, the functionalised pectin was obtained after a binding reaction with a CSS polyphenolic extract. Despite the selectivity of the extraction procedure, it could be rational that typical CSS pigments were also co-extracted.
In PLF (treated sample), a* value correlated with total polyphenols content (r, 0.75) and with antioxidant activity (IC30) (r, −0.85), as expected; moreover, the hue angle value (h°) correlated with total polyphenols content (r, −0.71) and with antioxidant activity (IC30) (r, 0.86). No significant correlation was observed in the control (PLB).
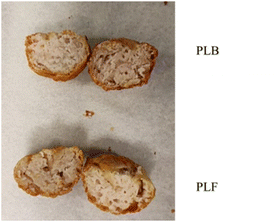
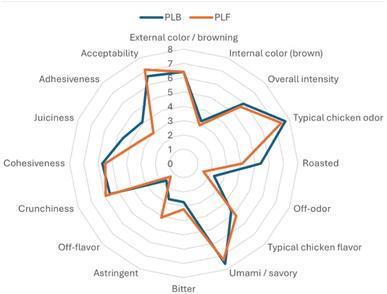
The total colour difference parameter (ΔE) revealed differences in colour between two formulations (ΔE > 5), meanwhile no differences were observed for each investigated sample among time of the storage. Colour is a crucial for driving consumers to purchase decision;70 the effect of plant extracts on the colour of meat formulated could be partially due to raw matter used:71 chicken meat has a naturally low myoglobin level, so the effect of pigments in newly formulated samples may appear more evident. Furthermore, the occurrence of antioxidants could prevent the change in colour during the refrigerated storage, making the myoglobin more stable in the raw functionalised chicken patty (PLF).19 Representative images of the cooked meatballs are shown in Fig. 1. Colorimetric values for the cooked samples (T0), both for the meatballs' interior or crusts, are reported in Fig. 2. Control (PLB) and functionalised (PLF) samples showed similar results for the different colorimetric variables, except for the redness value a* for which differences are statistically significant, although minimal. Table 9 summarizes results obtained for texture profiles analysis and cooking properties. Cooked samples didn't show statistically significant differences in any of the explored parameters. These results were further supported by sensory Quantitative Descriptive Analysis (QDA) (Fig. 4), as, on average, panellists evaluated in a similar way the different sensory descriptors. All data suggested that the functionalised HM pectin did not alter the sensory quality of the final product.
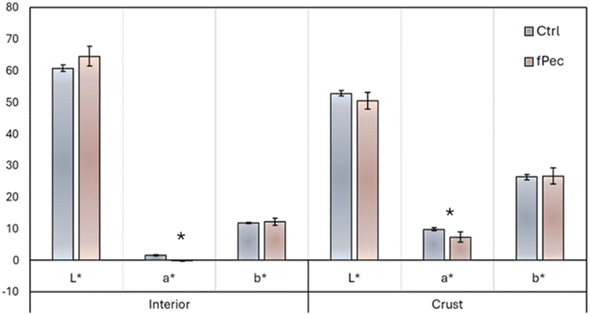 | ||
| Fig. 2 Interior and crust colorimetric values for control (PFB) and functionalised (PLF) cooked samples (T0). Significant differences computed by Student's t-test (p < 0.05) are indicated by *. | ||
| PLB | PLF | Sign. | ||
|---|---|---|---|---|
| a TPA = texture profile analysis; PLF = raw functionalised chicken patty; PLB = raw chicken patty. No significant differences are computed by Student's t-test (p < 0.05). | ||||
| TPA parameters | Hardness | 11.97 ± 1.15 | 11.55 ± 1.89 | n.s. |
| Fracturability | 6.06 ± 1.02 | 6.28 ± 0.91 | n.s. | |
| Cohesiveness | 0.430 ± 0.029 | 0.417 ± 0.029 | n.s. | |
| Springiness | 0.690 ± 0.038 | 0.678 ± 0.03 | n.s. | |
| Gumminess | 5.14 ± 0.64 | 4.81 ± 0.87 | n.s. | |
| Chewiness | 3.57 ± 0.63 | 3.25 ± 0.48 | n.s. | |
| Resilience | 0.143 ± 0.025 | 0.152 ± 0.025 | n.s. | |
| Cooking properties | Cooking loss % | 8.01 ± 1.04 | 8.88 ± 0.94 | n.s. |
| Shrinkage % | 46.9 ± 1.08 | 48.7 ± 1.37 | n.s. | |
Our findings support the use of HM pectin functionalised with CSS extracts as natural preservatives in clean-label meat products, in alternative to synthetic additives such as butylated hydroxytoluene (BHT) and butylated hydroxyanisole (BHA) the use of which worries consumers for their potential toxicity (especially hepatotoxicity, nephrotoxicity, and neurotoxicity).72
Total mesophilic (M) and psychrophilic (P) counts were determined for the control (PLB) and modified (PLF) raw chicken meatballs; these microbial parameters allowed to obtain a frame of the microbial contamination and growth dynamics during time of refrigerated storage of the samples in aerobic conditions (under the same conditions as other tests carried out). Results are reported in Fig. 3. The counts at T0 were quite high, nevertheless, they were in accordance with data observed by other researchers on chicken meatballs prepared in laboratory conditions.73 Other authors recently demonstrated that psycrotrophic counts can exceed 5.80 log CFU per g in chicken breast.74 Both the microbiological parameters investigated showed higher counts for the treated meatballs, starting from T0 until T10 days of refrigeraton, with statistically significant differences (p < 0.05) with respect to the control samples, in all the sampling points with the exception of mesophilic count at 10 days. The data reflected the hand-made preparation of the meatballs, particularly for PLF samples. It could be inferred that the different steps of processing (e.g. extraction, liophilization, etc.) could determine a higher contamination, thus suggesting to devote special attention to handling, in order to reduce microbial contamination as much as possible. Moreover, starting from day 5, the high counts revealed for both kind of samples underlined the need to use Modified Atmosphere Packaging and/or other hurdles to contain microbial growth and to guarantee the product stability over time. This would allow to further take advantage of the valuable results obtained for the oxidative stability of the meatballs.
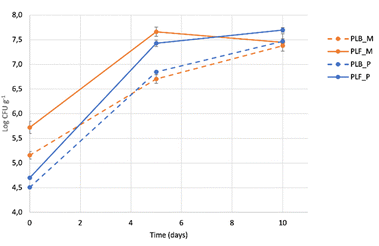 | ||
| Fig. 3 Total mesophylic (M) and psychrophilic (P) dynamics of control (PLB) and modified (PLB) meatballs during 10 days at 4 °C. | ||
4 Conclusions
The incorporation of high-methoxyl pectin functionalised with coffee silverskin extract into chicken meatballs significantly enhanced total polyphenol content and ABTS˙+ radical scavenging activity, both in raw and cooked products. IC30 values for functionalised patties were up to 14 times lower than controls, demonstrating effective antioxidant activity. The stability of these compounds was maintained during storage at 4 °C for 5 and 10 days and after cooking, suggesting that enzymatic pectin functionalisation not only preserved but may have enhanced the polyphenol stability under thermal processing conditions. From a technological perspective, the addition of functionalised pectin slightly altered the colorimetric properties of raw meatballs, mainly due to the pigments in the coffee silverskin extract. However, no significant differences were observed in texture profile analysis, cooking loss, or sensory attributes of the cooked product at T0, indicating that the overall organoleptic and technological quality was preserved.In terms of sustainability, the proposed approach offers a promising strategy to valorise an abundant coffee industry by-product, converting it into a functional food ingredient. This contributes to waste reduction and resource efficiency, aligning with circular economy principles, while potentially reducing the need for synthetic additives and responding to consumer demand for healthier and natural products.
Regarding shelf-life, although the functionalised meatballs maintained antioxidant activity, further optimization of formulation and storage conditions is warranted to ensure microbiological stability and extend product longevity.
Strategies such as active or modified atmosphere packaging, refrigeration optimization, or the addition of natural preservatives could further enhance safety and quality during storage. Overall, the coffee silverskin extract-functionalised pectin represents a promising strategy for developing healthier, antioxidant-rich, and environmentally sustainable meat products, offering both functional and technological benefits without compromising sensory quality.
Author contributions
U. Gianfranco Spizzirri; conceptualization, investigation, methodology, data curation, validation, visualization, writing – original draft, writing review & editing. Eva Scarcelli; formal analysis, investigation, data curation, methodology, validation, visualization, writing – original draft, writing review & editing. Matteo Carletta; formal analysis, data curation, writing – original draft. Rosa Nicoletti; formal analysis, data curation, writing – original draft. Cinzia Benincasa; conceptualization, validation, funding acquisition, data curation, writing – original draft. Donatella Restuccia; conceptualization, funding acquisition, writing – original draft. Domizia Vescovo; formal analysis, data curation. Annalisa Serio; writing – original draft. Maria Stefania Sinicropi; funding; supervision. Rosa Di Capua; formal analysis, data curation. Francesca Aiello; funding acquisition, conceptualization, writing – original draft, writing review & editing, project administration. Maria Martuscelli; funding acquisition, conceptualization, writing – original draft, writing review & editing, project administration.Conflicts of interest
The authors declare no conflict of interest.Data availability
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.Supplementary information (SI) is available. See DOI: https://doi.org/10.1039/d5ay01895a.
Acknowledgements
The authors wish to thank Torrefazione Adriatica S. P. A. (Giulianova, TE, Italy) for supplying the coffee silverskin (CSS) used in this research. Master course “Nutraceuticals 4.0 and Clinical Nutrition”, granted by “Patti Territoriali per l’Alta Formazione delle Imprese” project, CUP: H22C24000120001 (M. S. Sinicropi). The manuscript reports about a sensory analysis carried out thanks to the participation of voluntaries, trained and expert panelists belonging to the Department of Bioscience and Technology for Food, Agriculture and Environment of the University of Teramo, matching requisites for sensory tests as described in (ISO) 8589:2007.References
- H. Zhou, J. Li, C. Zhang, Y. Huang, H. Jiang and L. Chen, Nutr. Metabol. Cardiovasc. Dis., 2025, 35, 103855 CrossRef PubMed.
- I. C. O. ICO, Coffee Report and Outlook, 2023, accessed sep-2025, https://www.ico.org/ Search PubMed.
- M. Ridder, Global coffee consumption 2012/13–2023/24, https://www.statista.com/statistics/292595/global-coffee-consumption/, accessed sep, 2025.
- A. Santanatoglia, L. Alessandroni, C. Mannozzi, R. Marconi, D. Piatti, G. Sagratini, S. Vittori and G. Caprioli, Future Postharvest and Food, 2024, 1, 61–81 CrossRef.
- M. Martuscelli, L. Esposito, C. Di Mattia, A. Ricci and D. Mastrocola, Foods, 2021, 10, 1367 CrossRef PubMed.
- N. Miler, P. Wojewódzki, A. Woźny, D. Rymarz and A. Gołębiewska, Agronomy, 2025, 15, 1633 CrossRef.
- H. Salim, C. S. Ahmed, N. Mokhtari-Soulimane, B. S. Fadia, S. Mimoun and H. Merzouk, Biomass Convers. Biorefinery, 2023, 15, 1069–1082 CrossRef.
- U. Nations, #Envision 2030 Goal 12: Responsible Consumption and Production, https://www.un.org/development/desa/disabilities/envision2030-goal12.html, sep-2025.
- F. Ciccullo, M. Fabbri, N. Abdelkafi and M. Pero, J. Clean. Prod., 2022, 340, 130673 CrossRef.
- F. Sarasini, F. Luzi, F. Dominici, G. Maffei, A. Iannone, A. Zuorro, R. Lavecchia, L. Torre, A. Carbonell-Verdu, R. Balart and D. Puglia, Polymers, 2018, 10, 1256 CrossRef PubMed.
- S. H. Sung, Y. Chang and J. Han, Carbohydr. Polym., 2017, 169, 495–503 CrossRef PubMed.
- M. Hijosa-Valsero, J. Garita-Cambronero, A. I. Paniagua-García and R. Díez-Antolínez, Microb. Cell Factories, 2018, 17, 154 CrossRef PubMed.
- A. K. Singh and R. Sharma, J. Crit. Rev., 2020, 7, 686–691 Search PubMed.
- L. M. F. Pardo, N. V. Castillo, Y. M. V. Durán, J. A. J. Rosero and J. A. Lozano Moreno, Chem. Eng. Process. Process Intensif., 2022, 182, 109183 CrossRef.
- N. Martinez-Saez, M. Ullate, M. A. Martin-Cabrejas, P. Martorell, S. Genovés, D. Ramon and M. D. del Castillo, Food Chem., 2014, 150, 227–234 CrossRef PubMed.
- E. Garcia-Serna, N. Martinez-Saez, M. Mesias, F. Morales and M. Castillo, Pol. J. Food Nutr. Sci., 2014, 64, 243–251 CrossRef.
- A. Guglielmetti, B. Fernandez-Gomez, G. Zeppa and M. D. Del Castillo, Pol. J. Food Nutr. Sci., 2019, 69, 157–166 CrossRef.
- A. Pourfarzad, H. Mahdavian-Mehr and N. Sedaghat, LWT–Food Sci. Technol., 2013, 50, 599–606 CrossRef.
- M. Martuscelli, L. Esposito and D. Mastrocola, Foods, 2021, 10, 1833 Search PubMed.
- G. Ansanelli, G. Fiorentino, R. Chifari, K. Meisterl, E. Leccisi and A. Zucaro, Sustainability, 2023, 15, 16281 CrossRef.
- E. Commission, Directive 2008/98/EC of the European Parliament and of the Council of 19 November 2008 on waste, sep-2025, 2008, https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32008L0098.
- J. Cristóbal, V. Castellani, S. Manfredi and S. Sala, Waste Manage., 2018, 72, 3–16 Search PubMed.
- É. M. d. Santos, L. M. d. Macedo, L. L. Tundisi, J. A. Ataide, G. A. Camargo, R. C. Alves, M. B. P. P. Oliveira and P. G. Mazzola, Trends Food Sci. Technol., 2021, 111, 280–291 Search PubMed.
- Y. Narita and K. Inouye, Food Res. Int., 2014, 61, 16–22 Search PubMed.
- D. Stagos, Antioxidants, 2019, 9, 19 Search PubMed.
- C. Zehiroglu and S. B. Ozturk Sarikaya, J. Food Sci. Technol., 2019, 56, 4757–4774 CrossRef.
- J. Terao, H. Karasawa, H. Arai, A. Nagao, T. Suzuki and K. Takama, Biosci., Biotechnol., Biochem., 2014, 57, 1204–1205 CrossRef.
- C. M. Spagnol, R. P. Assis, I. L. Brunetti, V. L. B. Isaac, H. R. N. Salgado and M. A. Corrêa, Spectrochim. Acta, Part A, 2019, 219, 358–366 CrossRef PubMed.
- A. Purushothaman, S. S. Babu, S. Naroth and D. Janardanan, Free Radical Res., 2022, 56, 617–630 CrossRef PubMed.
- M. A. Hashem, M. Y. Ali, M. J. Ferdwsi, M. A. Haque and M. A. Islam, SAARC J. Agric., 2018, 16, 205–213 CrossRef.
- M. Bonoli, M. Caboni, M. Rodriguezestrada and G. Lercker, Food Chem., 2007, 101, 1327–1337 CrossRef.
- D. Alexandrakis, G. Downey and A. G. M. Scannell, Food Bioprocess Technol., 2009, 5, 338–347 CrossRef.
- A. K. Verma, V. Pathak, V. P. Singh and P. Umaraw, J. Appl. Anim. Res., 2015, 44, 409–414 CrossRef.
- E. Commission, A sustainable bioeconomy for Europe: strengthening the connection between economy, society and the environment – updated bioeconomy strategy, accessed sep-2025, 2018, DOI:10.2777/792130.
- U. G. Spizzirri, O. I. Parisi, F. Iemma, G. Cirillo, F. Puoci, M. Curcio and N. Picci, Carbohydr. Polym., 2010, 79, 333–340 CrossRef.
- G. Zhang, B. Huang, C. Zheng, Q. Chen and P. Fei, J. Agric. Food Chem., 2020, 69, 1234–1241 CrossRef PubMed.
- E. Sharefiabadi and M. Serdaroğlu, Food Health, 2021, 7, 64–74 CrossRef.
- J. Delgado-Ospina, R. Lucas-González, M. Viuda-Martos, J. Fernández-López, J. Á. Pérez-Álvarez, M. Martuscelli and C. Chaves-López, J. Food Process. Preserv., 2022, 46, e16752 CAS.
- S. Rout and P. P. Srivastav, J. Food Sci. Technol., 2025, 1–11 Search PubMed.
- M. Tura, M. A. Gagliano, E. Valli, M. Petracci and T. Gallina Toschi, Poult. Sci., 2024, 103, 104083 CrossRef.
- F. Y. Al-Juhaimi, S. A. Shahzad, A. S. Ahmed, O. Q. Adiamo, I. A. Mohamed Ahmed, O. N. Alsawmahi, K. Ghafoor and E. E. Babiker, J. Food Sci. Technol., 2018, 55, 1797–1805 CrossRef CAS.
- S. K. Devatkal, K. Narsaiah and A. Borah, Meat Sci., 2010, 85, 155–159 CrossRef CAS.
- U. G. Spizzirri, F. Iemma, F. Puoci, G. Cirillo, M. Curcio, O. I. Parisi and N. Picci, Biomacromolecules, 2009, 10, 1923–1930 CrossRef CAS PubMed.
- S. Rout and P. P. Srivastav, Food Chem., 2024, 447, 138914 CrossRef CAS.
- F. Aiello, R. Malivindi, M. F. Motta, P. Crupi, R. Nicoletti, C. Benincasa, M. L. Clodoveo, V. Rago, U. G. Spizzirri and D. Restuccia, Int. J. Mol. Sci., 2023, 24, 15075 CrossRef CAS.
- D. Restuccia, V. Sicari, T. M. Pellicanò, U. G. Spizzirri and M. R. Loizzo, Food Res. Int., 2017, 102, 303–312 CrossRef CAS PubMed.
- G. Carullo, U. G. Spizzirri, M. Montopoli, V. Cocetta, B. Armentano, M. Tinazzi, F. Sciubba, G. Giorgi, M. Enrica Di Cocco, T. Bohn, F. Aiello and D. Restuccia, Int. J. Food Sci. Technol., 2022, 57, 4086–4095 CrossRef CAS.
- F. Chemat, N. Rombaut, A.-G. Sicaire, A. Meullemiestre, A.-S. Fabiano-Tixier and M. Abert-Vian, Ultrason. Sonochem., 2017, 34, 540–560 CrossRef CAS PubMed.
- H. Metrouh-Amir, C. M. M. Duarte and F. Maiza, Ind. Crops Prod., 2015, 67, 249–256 CrossRef CAS.
- L. Wang and C. L. Weller, Trends Food Sci. Technol., 2006, 17, 300–312 CrossRef CAS.
- F. Dahmoune, L. Boulekbache, K. Moussi, O. Aoun, G. Spigno and K. Madani, Ind. Crops Prod., 2013, 50, 77–87 CrossRef CAS.
- C. Rodríguez-Pérez, R. Quirantes-Piné, A. Fernández-Gutiérrez and A. Segura-Carretero, Ind. Crops Prod., 2015, 66, 246–254 CrossRef.
- K. Pyrzynska, Separations, 2024, 11, 334 CrossRef CAS.
- K. Halake, H. J. Kim, M. Birajdar, B. S. Kim, H. Bae, C. Lee, Y. J. Kim, S. Kim, S. Ahn, S. Y. An, S. H. Jung and J. Lee, J. Ind. Eng. Chem., 2016, 40, 16–22 CrossRef CAS.
- D. Restuccia, G. Giorgi, U. Gianfranco Spizzirri, F. Sciubba, G. Capuani, V. Rago, G. Carullo and F. Aiello, Int. J. Food Sci. Technol., 2018, 54, 1313–1320 CrossRef.
- U. G. Spizzirri, P. Caputo, C. Oliviero Rossi, P. Crupi, M. Muraglia, V. Rago, R. Malivindi, M. L. Clodoveo, D. Restuccia and F. Aiello, Foods, 2022, 11, 158 CrossRef CAS PubMed.
- N. Karaki, A. Aljawish, C. Humeau, L. Muniglia and J. Jasniewski, Enzyme Microb. Technol., 2016, 90, 1–18 CrossRef CAS.
- S. Slagman, H. Zuilhof and M. C. R. Franssen, ChemBioChem, 2017, 19, 288–311 CrossRef PubMed.
- D. M. Scheibel, I. P. I. Gitsov and I. Gitsov, Molecules, 2024, 29, 989 CrossRef CAS PubMed.
- A. A. Mendes, P. C. Oliveira and H. F. de Castro, J. Mol. Catal. B: Enzym., 2012, 78, 119–134 Search PubMed.
- P. Wang, P. Fei, C. Zhou and P. Hong, LWT, 2021, 147, 111615 CrossRef CAS.
- M. N. Clifford, A. Kerimi and G. Williamson, Compr. Rev. Food Sci. Food Saf., 2020, 19, 1299–1352 CrossRef CAS PubMed.
- H. Boulebd, M. Carmena-Bargueño and H. Pérez-Sánchez, Antioxidants, 2023, 12, 1669 Search PubMed.
- T. M. Hung, M. Na, P. T. Thuong, N. D. Su, D. Sok, K. S. Song, Y. H. Seong and K. Bae, J. Ethnopharmacol., 2006, 108, 188–192 Search PubMed.
- V. Gottstein, M. Bernhardt, E. Dilger, J. Keller, C. M. Breitling-Utzmann, S. Schwarz, T. Kuballa, D. W. Lachenmeier and M. Bunzel, Foods, 2021, 10, 1705 CrossRef CAS.
- J. Petreska Stanoeva, E. Balshikevska, M. Stefova, O. Tusevski and S. G. Simic, Nat. Prod. Commun., 2020, 15(7) DOI:10.1177/1934578X20934675.
- Y. Yan-Ying, Z. Wei and C. Shu-Wen, Chin. J. Anal. Chem., 2007, 35, 1726–1730 Search PubMed.
- B. Huang, Z. Zhang, N. Ding, B. Wang, G. Zhang and P. Fei, Int. J. Biol. Macromol., 2021, 190, 343–350 CrossRef CAS PubMed.
- M. Zhang, Z. Wang, J. Wu, J. Lu, D. Liu, Y. Huang and G. Lv, Lwt, 2023, 176, 114486 CrossRef CAS.
- M. Al-Hijazeen, E. Lee, A. Mendonca and D. Ahn, Antioxidants, 2016, 5, 18 Search PubMed.
- J. Fernández-López, N. Zhi, L. Aleson-Carbonell, J. A. Pérez-Alvarez and V. Kuri, Meat Sci., 2005, 69, 371–380 CrossRef.
- J. Ren, Z. Li, X. Li, L. Yang, Z. Bu, Y. Wu, Y. Li, S. Zhang and X. Meng, Foods, 2025, 14, 1095 CrossRef CAS PubMed.
- M. Biplob, M. I. Hossen, H. Khatun and M. M. Rahman, Meat Res., 2024, 4, 106 Search PubMed.
- L. Necidová, A. Zouharová, D. Haruštiaková, Š. Bursová, K. Bartáková and J. Golian, Poult. Sci., 2024, 103, 104290 CrossRef.
Footnote |
| † Equally contributed. |
| This journal is © The Royal Society of Chemistry 2026 |