Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Sustainable valorization of tomato waste through life cycle assessment of lycopene extraction methods
Natalia A. Di Clemente ,
Andrea Gomez-Zavaglia
,
Andrea Gomez-Zavaglia * and
Esteban Gerbino
* and
Esteban Gerbino
Center for Research and Development in Food Science and Technology (CIDCA, CCT-CONICET La Plata) RA1900, La Plata, Buenos Aires, Argentina. E-mail: angoza@qui.uc.pt; Fax: +54(221)4249287; Tel: +54(221)4890741
First published on 12th December 2025
Abstract
Tomato processing produces substantial quantities of residues that constitute both an environmental challenge and a potential resource for recovery. This study assessed the environmental performance of four lycopene extraction strategies from tomato discards through a life cycle assessment approach. Conventional and ultrasound-assisted extractions were compared with either a hexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acetone
acetone![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethanol (50
ethanol (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25
25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25, v/v) mixture or ethyl acetate as solvent, defining four scenarios: Scenario 1 (C-HAE, conventional extraction with hexane
25, v/v) mixture or ethyl acetate as solvent, defining four scenarios: Scenario 1 (C-HAE, conventional extraction with hexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acetone
acetone![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethanol); Scenario 2 (C-EA, conventional extraction with ethyl acetate); Scenario 3 (U-HAE, ultrasound-assisted extraction with hexane
ethanol); Scenario 2 (C-EA, conventional extraction with ethyl acetate); Scenario 3 (U-HAE, ultrasound-assisted extraction with hexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acetone
acetone![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethanol); Scenario 4 (U-EA, ultrasound-assisted extraction with ethyl acetate). The functional unit was defined as 1 kg of lycopene-equivalent extract. Environmental impacts were calculated with the ReCiPe 2016 Midpoint (H) method, considering eighteen impact categories. Scenario 4 exhibited the lowest environmental impact (117.3 ± 27.6 mg/100 g of lycopene yield; 1187.9 ± 103.5 µg Txeq per gww) whereas Scenario 2 the highest, with the lowest lycopene yield (18.6 ± 18.5 mg/100 g) but the highest antioxidant capacity per unit of lycopene. A sensitivity analysis based on the antioxidant activity-to-lycopene ratio confirmed Scenario 4 as the most favorable option. Beyond energy efficiency and solvent selection, the extraction process also affected lycopene isomerization: ultrasound-assisted methods promoted Z-isomers formation over E-ones, with increases of 41% for the hexane
ethanol); Scenario 4 (U-EA, ultrasound-assisted extraction with ethyl acetate). The functional unit was defined as 1 kg of lycopene-equivalent extract. Environmental impacts were calculated with the ReCiPe 2016 Midpoint (H) method, considering eighteen impact categories. Scenario 4 exhibited the lowest environmental impact (117.3 ± 27.6 mg/100 g of lycopene yield; 1187.9 ± 103.5 µg Txeq per gww) whereas Scenario 2 the highest, with the lowest lycopene yield (18.6 ± 18.5 mg/100 g) but the highest antioxidant capacity per unit of lycopene. A sensitivity analysis based on the antioxidant activity-to-lycopene ratio confirmed Scenario 4 as the most favorable option. Beyond energy efficiency and solvent selection, the extraction process also affected lycopene isomerization: ultrasound-assisted methods promoted Z-isomers formation over E-ones, with increases of 41% for the hexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acetone
acetone![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethanol solvent mixture and 140% for ethyl acetate. Since Z-isomers are more bioaccessible and display comparable or higher antioxidant activity than E-isomers, this shift may further enhance the functional value of the recovered pigment. Overall, combining green solvents with ultrasound technologies can reduce the environmental burdens and support sustainable valorization of tomato waste.
ethanol solvent mixture and 140% for ethyl acetate. Since Z-isomers are more bioaccessible and display comparable or higher antioxidant activity than E-isomers, this shift may further enhance the functional value of the recovered pigment. Overall, combining green solvents with ultrasound technologies can reduce the environmental burdens and support sustainable valorization of tomato waste.
Sustainability spotlightThis study advances sustainable food systems by demonstrating that lycopene extraction from tomato residues can be significantly optimized through the combined use of green solvents and ultrasound-assisted technologies. By integrating Life Cycle Assessment (LCA) with functional efficiency metrics, the work highlights extraction routes that substantially reduce energy demand, solvent use, and environmental burdens while increasing the added value of agro-industrial by-products. These findings directly support SDG 12 (Responsible Consumption and Production) by promoting circular resource use, SDG 9 (Industry, Innovation and Infrastructure) through the development of cleaner and more efficient processing technologies, and SDG 13 (Climate Action) by identifying lower-impact production pathways. These results support the transition toward low-impact, circular processing strategies that promote waste valorization, resource efficiency, and more sustainable food production chains. |
1 Introduction
Tomatoes represent one of the most important agricultural commodities worldwide and, in 2023, ranked as the leading vegetable crop, with a global production of approximately 192 million tons.1 They are particularly valued for their rich composition of antioxidants, including ascorbic acid (vitamin C), tocopherol (vitamin E), carotenoids (lycopene and β-carotene), and various phenolic compounds, including quercetin, kaempferol, naringenin, lutein, and chlorogenic acids.2 Beyond their health-promoting properties, these compounds also contribute to the sensory attributes of tomatoes, influencing aroma, taste, and texture.3Despite their remarkable nutritional and sensory qualities, postharvest losses in tomatoes remain a major global concern, estimated between 25% and 42% annually.4 Food loss and waste not only generate significant socio-economic costs but also account for about 8% of global greenhouse gas emissions and lead to the depletion of natural resources (e.g., water, soil, energy) used to produce food that is never consumed, which ultimately occupies 21% of landfill capacity.5 To mitigate these challenges, international organizations have established initiatives aimed at reducing food loss and waste. The 2030 Agenda for Sustainable Development Goals (SDGs) includes Target 12.3, which seeks to halve per capita global food waste at the retail and consumer levels by 2030 while also reducing food losses along production and supply chains, including postharvest stages.6 The European Commission has aligned its policies with this objective through the “Farm to Fork Strategy”, a central component of the European Green Deal.7
Accordingly, the valorization of tomato discards (losses, wastes) represents a promising approach. Recovering bioactive compounds from agro-industrial residues not only adds value through the extraction of nutrients and functional ingredients but also reduces the environmental impacts associated with waste disposal.8 Likewise, the valorization strategy can be extended to surplus tomatoes, which are often discarded during periods of high production due to their seasonal nature and high perishability. The reuse of these derivatives can simultaneously provide additional income sources, reduce disposal costs, and enhance the nutritional value of functional foods.9
The use of synthetic dyes has raised increasing health and regulatory concerns. Some artificial colorants, such as Erythrosine (Red No. 3), have been associated with potential adverse effects, including genotoxicity10 thyroid dysfunction, and hyperactivity in children.11,12 Due to such evidence, the Food and Drug Administration (FDA) banned the use of Red No. 3 in cosmetics and topical drugs and is moving towards restricting its use in food products.13,14 Several studies have further linked synthetic dyes to allergic reactions and behavioral effects, prompting stricter regulations and a growing consumer preference for safer, natural alternatives.15 Within this framework, natural pigments offer innovative opportunities for replacement. Their use has expanded across multiple industries, including food, pharmaceutical, textile, dairy, and seafood sectors. In this context, lycopene, a key carotenoid predominantly found in tomatoes, is increasingly valued in the food industry as a natural and safe coloring agent, owing to both its vivid red hue and its strong antioxidant capacity.16 Because tomatoes account for 80-90% of dietary lycopene intake, with levels ranging from 0.72 to 4.2 mg per 100 g depending on ripeness, temperature, and soil conditions, they constitute a major and reliable source for natural pigment-based applications.17
The growing consumer preference for natural, health-promoting, and sustainable products further drives the demand for functional foods enriched with natural antioxidants such as lycopene.16,18–21 The global carotenoids market reflects this trend, being valued at USD 2 billion in 2023 and projected to reach USD 2.9 billion by 2029, at a compound annual growth rate (CAGR) of 6.7%.17
Despite increasing interest in lycopene extraction from tomato residues, the environmental implications of these processes remain largely underexplored. The available research typically focuses on extraction yields, antioxidant properties, or process optimization,22 but few works integrate these metrics within a comprehensive environmental quantification.23,24 Moreover, no study to date has jointly evaluated the combined effect of extraction conditions and lycopene isomerization on both environmental performance and functional efficiency.
Given this context, the aim of this study is to compare the environmental impact of different extraction techniques used for the recovery of bioactive compounds from tomato discards. Specifically, the study evaluates conventional extraction methods employing petrochemical solvents against sustainable alternatives such as ultrasound-assisted extraction (UAE) and the use of low-toxicity, biodegradable solvents like ethyl acetate. A life cycle assessment (LCA) approach was applied to quantify the environmental performance of each extraction process in terms of energy consumption, greenhouse gas emissions, and solvent use. The outcomes aimed to identify the most sustainable strategies for the valorization of tomato residues, contributing to the development of greener extraction technologies aligned with sustainable food processing.
2 Materials and methods
2.1. Materials
Analytical-grade hexane, acetone, ethanol, and ethyl acetate, together with HPLC-grade hexane, tetrahydrofuran, acetonitrile, and methanol, were obtained from ANEDRA (Research, ARG) and CICCARELLI (Reagents S.A., ARG). The HPLC calibration curve was prepared using a lycopene standard (HPLC grade, 98% purity, from tomato; Sigma-Aldrich Chemie GmbH, Germany).2.2. Methods
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acetone
acetone![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethanol (50
ethanol (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25
25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25) and ethyl acetate] and two methods (conventional and ultrasound-assisted extraction). For each extraction, 1 g of concentrated tomato waste was used in triplicate, maintaining a sample-to-solvent ratio of 1
25) and ethyl acetate] and two methods (conventional and ultrasound-assisted extraction). For each extraction, 1 g of concentrated tomato waste was used in triplicate, maintaining a sample-to-solvent ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16 in 15 mL Falcon tubes. The conventional method, modified according to,25 consisted of mixing 1 g of the homogenized sample with the solvent mixture [hexane
16 in 15 mL Falcon tubes. The conventional method, modified according to,25 consisted of mixing 1 g of the homogenized sample with the solvent mixture [hexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acetone
acetone![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethanol (50
ethanol (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25
25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25) or ethyl acetate] for 1 minute in a vortex, and then incubating it at 40 °C for 1 minute. Ultrasound-assisted extraction was carried out using a Branson 2510 ultrasonic cleaner (tank capacity: 1/2 gallon) operating at a maximum power of 300 W and a frequency of 40 kHz for 29 minutes at 60 °C, according to26 with modifications. Ten milliliters of distilled water were added to the lycopene extracts to remove any interfering water-soluble compounds.27 The mixture was then centrifuged at 4000 rpm for one minute in a centrifuge 5430 (Eppendorf AG, Hamburg, Germany). Finally, the organic phase was collected and filtered through 0.45 µm nylon filters prior to lycopene analysis. All extraction procedures were performed in the dark to minimize carotenoid degradation.
25) or ethyl acetate] for 1 minute in a vortex, and then incubating it at 40 °C for 1 minute. Ultrasound-assisted extraction was carried out using a Branson 2510 ultrasonic cleaner (tank capacity: 1/2 gallon) operating at a maximum power of 300 W and a frequency of 40 kHz for 29 minutes at 60 °C, according to26 with modifications. Ten milliliters of distilled water were added to the lycopene extracts to remove any interfering water-soluble compounds.27 The mixture was then centrifuged at 4000 rpm for one minute in a centrifuge 5430 (Eppendorf AG, Hamburg, Germany). Finally, the organic phase was collected and filtered through 0.45 µm nylon filters prior to lycopene analysis. All extraction procedures were performed in the dark to minimize carotenoid degradation.For the HPLC analysis, the samples were evaporated and resuspended in a mixture of tetrahydrofuran, acetonitrile and methanol (15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30
30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 55 v/v/v) via ultrasonication for 15 minutes to ensure the solubilization of the extracts. Chromatographic analyses were performed using a Waters Spherisorb C18 column (250 mm × 4.6 mm, 5 µm particle size) maintained at 35 °C under isocratic conditions. The mobile phase consisted of acetonitrile and water (90
55 v/v/v) via ultrasonication for 15 minutes to ensure the solubilization of the extracts. Chromatographic analyses were performed using a Waters Spherisorb C18 column (250 mm × 4.6 mm, 5 µm particle size) maintained at 35 °C under isocratic conditions. The mobile phase consisted of acetonitrile and water (90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, v/v) containing 9 µM triethylamine (TEA), with a flow rate of 1 mL min−1. The system was equipped with a Waters 1525 binary HPLC pump and a Waters 2998 photodiode array detector, set at λmax = 472 nm. The mobile phase was freshly prepared prior to each run.
10, v/v) containing 9 µM triethylamine (TEA), with a flow rate of 1 mL min−1. The system was equipped with a Waters 1525 binary HPLC pump and a Waters 2998 photodiode array detector, set at λmax = 472 nm. The mobile phase was freshly prepared prior to each run.
2.2.4.1 Study design and goal definition. The goal of this study was to compare the environmental performance of conventional and ultrasound-assisted extraction techniques for the recovery of bioactive compounds -particularly carotenoids, predominantly lycopene-from tomato discards. The assessment focused on four extraction strategies, referred to as scenarios (Table 1), which describe the different scenarios based on the solvents and extraction methodologies employed in this study.
| Scenario | Method | Solvent |
|---|---|---|
| 1 | Convectional | Hexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Acetone Acetone![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ethanol Ethanol |
| 2 | Convectional | Ethyl acetate |
| 3 | Ultrasonic | Hexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Acetone Acetone![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ethanol Ethanol |
| 4 | Ultrasonic | Ethyl acetate |
Scenario 1 (C-HAE): conventional solvent extraction using a mixture of hexane, acetone, and ethanol (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25
25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25, v/v) with a solid-to-solvent ratio of 1
25, v/v) with a solid-to-solvent ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16 (w/v). The mixture was vortexed for 1 min and maintained in a water bath at 40 °C for 1 min.
16 (w/v). The mixture was vortexed for 1 min and maintained in a water bath at 40 °C for 1 min.
Scenario 2 (C-EA): conventional solvent extraction with ethyl acetate and a solid-to-solvent ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16 (w/v). The mixture was vortexed for 1 min and maintained in a water bath at 40 °C for 1 min.
16 (w/v). The mixture was vortexed for 1 min and maintained in a water bath at 40 °C for 1 min.
Scenario 3 (U-HAE): Ultrasound-assisted extraction using a mixture of hexane, acetone, and ethanol (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25
25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25, v/v) with a solid-to-solvent ratio of 1
25, v/v) with a solid-to-solvent ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16 (w/v). The extraction was performed in an ultrasonic bath for 29 min at 60 °C, 40 kHz, 300 W.
16 (w/v). The extraction was performed in an ultrasonic bath for 29 min at 60 °C, 40 kHz, 300 W.
Scenario 4 (U-EA): Ultrasound-assisted extraction with ethyl acetate at a solid-to-solvent ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16 (w/v). The extraction was carried out in an ultrasonic bath for 29 min at 60 °C, 40 kHz, 300 W.
16 (w/v). The extraction was carried out in an ultrasonic bath for 29 min at 60 °C, 40 kHz, 300 W.
A LCA framework was applied in accordance with ISO 14040 and ISO 14044 standards (International Organization for Standardization [ISO].28
2.2.4.2 Functional unit and system boundaries. The functional unit was defined as 1 kg of lycopene-equivalent extract obtained from tomato discards. This unit allows for the comparison of extraction methods based on the same quantity of target compound produced, independent of yield variations.
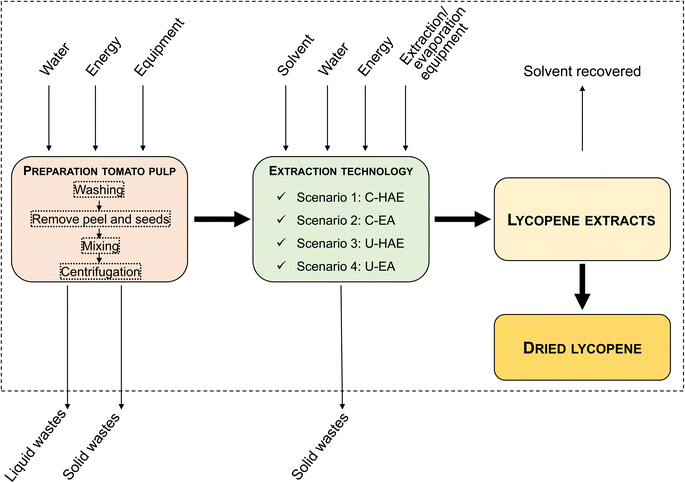
The system boundaries considered a gate-to-gate approach, encompassing all processes from tomato pulp preparation (tomato discards collection, washing, removal of peel and seeds, mixing and centrifugation) through lycopene extraction (Scenarios 1 to 4) and solvent recovery (Fig. 1). Upstream agricultural production, downstream product formulation (e.g., encapsulation or food incorporation) and waste management were excluded to isolate the environmental impact of the extraction stage.
2.2.4.3 Life cycle inventory (LCI): Inventory data collection. The LCI involved the systematic collection of input and output data related to each stage of the process for the four scenarios. The inventory considered the consumption of electricity, tomatoes, solvents (hexane, acetone, ethanol, and ethyl acetate), and water (tap and deionized), as well as the generation of liquid, organic, and chemical wastes, solvent losses, and the production of dried lycopene. Detailed data corresponding to the four evaluated scenarios are presented in Table 2.
| Unit | Scenario 1 | Scenario 2 | Scenario 3 | Scenario 4 | |
|---|---|---|---|---|---|
| Inputs | |||||
| Tomatoes pulp preparation | |||||
| Tomatoes | Kg | 5214 | 58![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 841 841 |
5196 | 5889 |
| Electricity | MJ | 6100 | 68![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 844 844 |
6079 | 6890 |
| Tap water | L | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 428 428 |
117![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 682 682 |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 391 391 |
11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 779 779 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Lycopene extraction | |||||
| Solvent | L | ||||
| Hexane | 4406 | 4390 | |||
| Acetone | 2203 | 2195 | |||
| Ethanol | 2203 | 2195 | |||
| Ethyl acetate | 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 441 441 |
9953 | |||
| Deionized water | L | 5507 | 62![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 151 151 |
5488 | 6221 |
| Electricity | MJ | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 658 658 |
154![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 133 133 |
37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 455 455 |
42![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 455 455 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Solvent recovery | |||||
| Lycopene in extract | Kg | 2533 | 78![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 933 933 |
2495 | 8350 |
| Electricity | MJ | 242 | 8200 | 241 | 821 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Outputs | |||||
| Tomatoes pulp preparation | |||||
| Liquid waste | L | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 158 158 |
137![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 209 209 |
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 116 116 |
13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 733 733 |
| Organic waste | Kg | 2933 | 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 098 098 |
2923 | 3313 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Lycopene extraction | |||||
| Organic waste | Kg | 550 | 6214 | 548 | 621 |
| Chemical waste | Kg | 8972 | 62![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 151 151 |
8941 | 6221 |
| Lost solvent | Kg | ||||
| Hexane | 378 | 406 | |||
| Acetone | |||||
| Ethanol | |||||
| Ethyl acetate | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 763 763 |
628 | |||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Solvent recovery | |||||
| Solvent | Kg | 2532 | 78![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 932 932 |
2494 | 8349 |
| Dried lycopene | Kg | 1 | 1 | 1 | 1 |
Energy data were obtained from direct laboratory measurements, and emission factors for electricity and solvents were sourced from the ELCD 3.2 database (Greendelta v2.18 correction20220908).
Environmental impacts were evaluated using the ReCiPe 2016 Midpoint method, adopting the hierarchist perspective and World (2010) (H) normalization approach.29,30 The impact categories selected for this analysis (Table 3) included global warming (GW, kg CO2-eq), stratospheric ozone depletion (SOD, kg CFC-11 eq), ionizing radiation (IR, kBq Co-60 eq), ozone formation (human health) (OHh, kg NOx eq), ozone formation (terrestrial ecosystems) (OTe, kg NOx eq), fine particulate matter formation (FPMF, kg PM2.5 eq), terrestrial acidification (TA, kg SO2 eq), freshwater eutrophication (FE, kg P eq), marine eutrophication (ME, kg N eq), terrestrial ecotoxicity (TE, kg 1,4-DCB eq), freshwater ecotoxicity (FEco, kg 1,4-DCB eq), marine ecotoxicity (MEco, kg 1,4-DCB eq), human carcinogenic toxicity (HCT, kg 1,4-DCB eq), human non-carcinogenic toxicity (HNCT, kg 1,4-DCB eq), land use (L, m2 years crop eq), mineral resource scarcity (MRS, kg Cu eq), fossil resource scarcity (FRS, kg oil eq), and water consumption (WC, m3).
| Impact categories | Abbreviation | Unit |
|---|---|---|
| Fine particulate matter formation | FPMF | kg PM2.5 eq |
| Fossil resource scarcity | FRS | kg oil eq |
| Freshwater ecotoxicity | FEco | kg 1,4-DCB |
| Freshwater eutrophication | FE | kg P eq |
| Global warming | GW | kg CO2 eq |
| Human carcinogenic toxicity | HCT | kg 1,4-DCB |
| Human non-carcinogenic toxicity | HNCT | kg 1,4-DCB |
| Ionizing radiation | IR | kBq Co-60 eq |
| Land use | L | m2a crop eq |
| Marine ecotoxicity | MEco | kg 1,4-DCB |
| Marine eutrophication | ME | kg N eq |
| Mineral resource scarcity | MRS | kg Cu eq |
| Ozone formation, human health | OHh | kg NOx eq |
| Ozone formation, terrestrial ecosystems | OTe | kg NOx eq |
| Stratospheric ozone depletion | SOD | kg CFC11 eq |
| Terrestrial acidification | TA | kg SO2 eq |
| Terrestrial ecotoxicity | TE | kg 1,4-DCB |
| Water consumption | WC | m3 |
Electrical consumption (MJ) was calculated according to eqn (1),31 where P is the power of the equipment (kW) (bath, mixer, ultrasonic bath, rotavapor system) and t is the duration of use (hour).
| Electrical consumption = P × t | (1) |
2.2.4.4 Life cycle assessment. Calculations were performed using Open LCA software. Impact categories were normalized and weighted to facilitate comparison among the four scenarios (Section 2.2.4.1). Sensitivity analyses were conducted to evaluate the impact of solvent recovery on antioxidant efficiency and the total environmental impact. To achieve this, the inputs and outputs were adjusted according to the ratio of extracted lycopene to antioxidant activity, as well as to the functional unit.
2.2.4.5 Data quality and assumptions. Primary data were collected from controlled laboratory-scale experiments, complemented by literature data where necessary. Assumptions included a 90% solvent recovery rate for ethyl acetate, and 80% for hexane, consistent with prior studies.32,33
| y = 22.069x − 14.311 | (2) |
3 Results and discussion
3.1. Lycopene content and antioxidant activity
The lycopene content of the tomato concentrate showed significant differences in the four extraction strategies (Table 4). Unexpectedly, Scenario 2 (conventional extraction with ethyl acetate) yielded the lowest lycopene concentration, as determined by both UV-vis and HPLC analyses, with values of 18.6 ± 0.3 and 18.6 ± 18.5 mg/100 g ww (wet weight), respectively. Scenario 4 followed, showing 186.3 ± 3.0 mg/100 g ww by UV-vis and 117.3 ± 27.6 mg/100 g ww by HPLC. Scenarios 1 and 3 exhibited the highest lycopene contents, with 207.6 ± 4.5 and 168.4 ± 18.5 mg/100 g ww, and 208.2 ± 6.0 and 147.0 ± 39.3 mg/100 g ww, respectively. These values are consistent with the higher extraction efficiencies generally reported for ultrasound-assisted and mixed-solvent systems, as shown in Table 5.35–37| Scenario | Method | Solventa | Concentration (UV-vis, 472 nm) (mg/100gww)b | Concentration (HPLC) (mg/100gww)b | Antioxidant activity (µg Tx eq per gww) | Lycopene (µg per gww) | Antioxidant activity: Lycopene | % E-isomers | % Z-isomers | E/Z |
|---|---|---|---|---|---|---|---|---|---|---|
a H![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A A![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) E: Hexane E: Hexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Acetone Acetone![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ethanol; EA: Ethyl acetate.b ww: wet weight of concentrated tomato; different letters in columns indicate significant differences (p < 0.05). Ethanol; EA: Ethyl acetate.b ww: wet weight of concentrated tomato; different letters in columns indicate significant differences (p < 0.05). |
||||||||||
| 1 | Convectional | H![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A A![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) E E |
208.2 ± 6.0A | 147.0 ± 39.3A | 734.7 ± 97.0C | 1470.4 ± 392.7A | 0.5 | 82.4 | 17.6 | 5.6 |
| 2 | Convectional | EA | 18.6 ± 0.3C | 18.6 ± 18.5B | 630.1 ± 49.1C | 186.3 ± 23.3B | 3.4 | 93.6 | 6.4 | 15.4 |
| 3 | Ultrasonic | H![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A A![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) E E |
207.6 ± 4.5A | 168.4 ± 18.5A | 934.8 ± 26.9B | 1683.6 ± 185.5A | 0.6 | 75.2 | 24.8 | 3.1 |
| 4 | Ultrasonic | EA | 186.3 ± 3.0B | 117.3 ± 27.6A | 1187.9 ± 103.5A | 1173.3 ± 275.5A | 1.0 | 84.6 | 15.4 | 5.5 |
| Extraction method | Sample | Solvent | Yield | Parameters | Reference |
|---|---|---|---|---|---|
a UAE: ultrasound-assisted extraction; v/w: volume-to-weight ratio; S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) S solvent-to-sample ratio. S solvent-to-sample ratio. |
|||||
| UAE | Freeze-dried tomato pomace | Ethyl lactate/Ethyl acetate (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) 3) |
133 mg/100 g dw | 100 mL g−1 S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) S 63.4 °C; 20 min S 63.4 °C; 20 min |
35 |
| UAE | Freeze-dried tomato pulp | Hexane/acetone/ethanol (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 v/v/v) 25 v/v/v) |
511 mg/100 g dw | 74.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mL g−1 S 1 mL g−1 S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) S 47.6 °C; 45.6 min S 47.6 °C; 45.6 min |
36 |
| Non-UAE (shaking) | Tomato puree | Hexane/ethanol/acetone (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 v/v/v) 25 v/v/v) |
15.8 mg/100 g w/w | 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 S 1 S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) S S |
37 |
Interestingly, while the UV-vis results revealed statistically significant differences for Scenario 4 compared with Scenarios 1 and 3, the HPLC data did not confirm these differences (Table 4). This discrepancy may be attributed to the presence of co-extracted compounds (β-carotenes, lutein) with overlapping absorbance in the visible range, which could affect the UV-vis quantification but are more effectively resolved by HPLC analysis.38
Despite these differences, Scenario 4 exhibited the highest antioxidant activity (1187.9 µg Tx eq per g ww), indicating that antioxidant compounds other than lycopene may contribute to the overall activity.38 The ratio between antioxidant activity and lycopene content was markedly higher in Scenario 2 (approximately 3.4-fold greater) than in the other scenarios, suggesting that the extraction solvent and processing conditions may differentially influence both lycopene yield and antioxidant potential. In fact, the extraction conditions in Scenario 2 could favor the co-extraction of other highly active compounds, despite its poor lycopene recovery. In contrast, the lower ratios observed for Scenarios 1 and 3 reflect a weaker functional contribution per unit of lycopene extracted. Scenario 4 showed an intermediate behavior, supporting the idea that ultrasound combined with ethyl acetate enhances the extraction of a more functionally efficient antioxidant profile than in the other scenarios. These results underscore the importance of considering functional efficiency -not only compound yield-when evaluating extraction performance.
3.2. Environmental profile
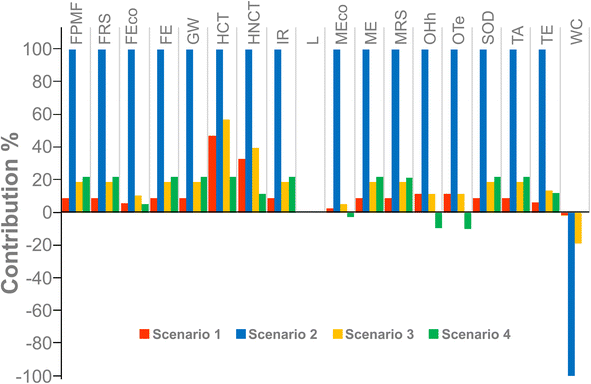
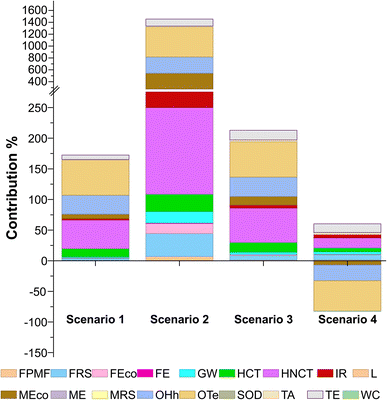
Fig. 2 and 3 show the relative contribution of each environmental impact category across the different scenarios for obtaining 1 kg of lycopene-equivalent extract from tomato discards. Scenario 2 exhibited the highest impact in all categories, followed by Scenarios 3 and 1, whereas Scenario 4 was the most favorable overall (Fig. 2). The lycopene extraction stage represented the main hotspot in all scenarios (data not shown), exceeding the impacts associated with tomato pulp preparation. This predominance is particularly evident in Scenario 2, where the lower lycopene yield led to a greater amount of tomato and solvent required to achieve the functional unit of 1 kg of lycopene, thereby amplifying the overall environmental burden.Fig. 3 shows that the categories Ozone formation-Terrestrial ecosystems (OTe), Ozone formation-Human health (OHh), Marine ecotoxicity (MEco), Terrestrial ecotoxicity (TE), Fossil resource scarcity (FRS), and Human non-carcinogenic toxicity (HNCT) were the main contributors to the overall environmental impact. Regarding OTe and OHh, ozone is not directly emitted into the atmosphere but is formed through photochemical reactions involving nitrogen oxides (NOx) and non-methane volatile organic compounds (NMVOCs). In the atmosphere, NOx and NMVOCs are transformed into ozone, which can subsequently be inhaled by humans or absorbed by plants.29 The energy consumption associated with lycopene extraction processes, together with solvent use, had a significant impact on both ecosystems and human health, with Scenario 2 contributing up to 643.1 kg NOx eq (Table 6).
| Impact categoriesb | Unit | Scenario 1 | Scenario 2 | Scenario 3 | Scenario 4 |
|---|---|---|---|---|---|
| a Bold rows indicate the most impactful categories.b FPMF: fine particulate matter formation; FRS: fossil resource scarcity; FEco: freshwater ecotoxicity; FE: freshwater eutrophication; GW: global warming; HCT: human carcinogenic toxicity; HNCT: human non-carcinogenic toxicity; IR: ionizing radiation; L: land use; MEco: marine ecotoxicity; ME: marine; eutrophication; MRS: mineral resource scarcity; OHh: ozone formation, human health; OTe: ozone formation, terrestrial ecosystems; SOD: stratospheric ozone depletion; TA: terrestrial acidification; TE: terrestrial ecotoxicity; WC: water consumption. | |||||
| FPMF | kg PM2.5 eq | 15.2 | 176.1 | 33.3 | 38.2 |
| FRS | kg oil eq | 3.2 × 103 | 3.7 × 104 | 6.9 × 103 | 7.9 × 103 |
| FEco | kg 1.4-DCB | 1.1 | 20.2 | 2.1 | 1.0 |
| FE | kg P eq | 0 | 1 | 0 | 0 |
| GW | kg CO2eq | 1.3 × 104 | 1.5 × 105 | 2.8 × 104 | 3.3 × 104 |
| HCT | kg 1.4-DCB | 36.4 | 77.9 | 44.1 | 16.9 |
| HNCT | kg 1.4-DCB | 7.0 × 103 | 2.1 × 104 | 8.4 × 103 | 2.4 × 103 |
| IR | kBq Co−60eq | 1.1 × 103 | 1.2 × 104 | 2.3 × 103 | 2.7 × 103 |
| L | m2a crop eq | 0 | 0 | 0 | 0 |
| MEco | kg 1.4-DCB | 7.6 | 274.5 | 14.5 | −7.2 |
| ME | kg N eq | 0.2 | 2.8 | 0.5 | 0.6 |
| MRS | kg Cu eq | 2.2 | 25.1 | 4.8 | 5.4 |
| OHh | kg NOxeq | 634.9 | 5643.1 | 649.6 | −530.9 |
| OTe | kg NOxeq | 1.0 × 103 | 9.0 × 103 | 1.0 × 103 | −8.8 × 102 |
| SOD | kg CFC11 eq | 2.5 × 10−3 | 2.9 × 10−2 | 5.5 × 10−3 | 6.4 × 10−3 |
| TA | kg SO2 eq | 52.5 | 606.4 | 114.8 | 131.5 |
| TE | kg 1.4-DCB | 7.6 × 103 | 1.2 × 105 | 1.6 × 104 | 1.5 × 104 |
| WC | m3 | −2.3 × 100 | −1.5 × 102 | −2.8 × 101 | −3.2 × 101 |
In turn, the results for MEco and TE categories were expressed in kg 1,4-dichlorobenzene equivalents (1,4-DCB eq) to characterize pollutant emissions. Scenario 2 contributed 274.5 and 1.2 × 105 kg 1,4-DCB eq per FU (functional unit), respectively, whereas Scenarios 1 and 3 showed corresponding values of 7.6 and 7.6 × 103, and 14.5 and 1.6 × 104 kg 1,4-DCB eq per FU, respectively.
For the FRS category, as mentioned previously, the lycopene extraction process was identified as the main environmental hotspot, where most of the energy consumption is concentrated. In La Plata (Buenos Aires, Argentina), natural gas and oil are the primary fuels used for thermal electricity generation, thereby contributing to fossil resource scarcity. Scenario 2 consumed 3.7 × 104 kg oil eq per FU, whereas Scenarios 3 and 4 showed values of 6.9 × 103 and 7.9 × 103 kg oil eq per FU, respectively (Table 6).
For the category HNCT, expressed in kg 1,4-dichlorobenzene equivalents (kg 1,4-DCB eq), Scenarios 1 and 3 showed comparable values of 7.0 × 103 and 8.4 × 103 kg 1,4-DCB eq, respectively, while Scenario 2 contributed 2.1 × 104 kg 1,4-DCB eq. The use of toxic solvents and the fuel consumption associated with electricity production generated industrial process emissions that have been linked to adverse effects on human health.39,40
Finally, Freshwater ecotoxicity (FEco), Human carcinogenic toxicity (HCT), Ionizing radiation (IR) and Terrestrial acidification (TA) contributed to the environmental impact to a minor degree. However, Scenario 2 showed the highest values compared to the other scenarios. FEco and HCT contributed 20.2 and 77.9 kg 1,4-DCB eq, respectively, Ionizing radiation (IR), with 1.2 × 104 kBq Co-60 eq, and Terrestrial acidification (TA), with 606.4 kg SO2 eq. All of these are related to the emission of gases generated during industrial processes that contribute to the toxicity and acidification of the terrestrial ecosystem, with direct impacts on human health and environmental toxicity.
3.3. Sensitivity analysis
Lycopene in fresh tomatoes occurs predominantly in the all-E form (more than 90%).41 However, in the human body, the Z-isomers of lycopene are more soluble than E-ones, and form micelles, thereby facilitating intestinal absorption.42 Furthermore, studies indicate that the intestinal absorption and antioxidant activity of Z-isomers, particularly 5-Z-lycopene, are superior. Isomerization occurs not only during digestion, but also as a result of temperature, for example, during extraction processes.41 Nevertheless, many studies have reported that the Z-isomers of carotenoids exhibit equal or even higher antioxidant capacity compared with their all-E counterparts,43 and display similar functionality, such as anti-obesity activity.44 The increased formation of Z-isomers may enhance lycopene bioaccessibility and antioxidant potential, as these isomeric forms are generally more stable than E-isomers against heat and light and more readily incorporated into biological membranes.In this work, the isomerization analysis demonstrated that, within each solvent system (Scenarios 1 vs. 3 and Scenarios 2 vs. 4), the introduction of ultrasound shifted the carotenoid profile toward a greater proportion of Z-isomers, whereas the conventional extractions consistently favored the formation of E-isomers (Table 4). In other words, Scenarios 3 and 4 showed higher proportions of Z-isomers compared with their counterparts obtained without ultrasound-assisted extraction (Scenarios 1 and 2, respectively). This shift in lycopene isomerization can be attributed to the temperature increase during the ultrasound-assisted extraction process.42
When comparing the effect of the solvents on the functionality of the extracts, it is worth noting that the use of ethyl acetate led to a higher proportion of E-isomers than the mixture of hexane, acetone, and ethanol (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25
25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25, v/v), because E-isomers are less soluble than Z-isomers in non-polar solvents such as hexane.43 This finding is consistent with the higher antioxidant activity-to-lycopene ratio observed under these conditions (Scenario 2 vs. 1 and Scenario 4 vs. 3) (Table 4).
25, v/v), because E-isomers are less soluble than Z-isomers in non-polar solvents such as hexane.43 This finding is consistent with the higher antioxidant activity-to-lycopene ratio observed under these conditions (Scenario 2 vs. 1 and Scenario 4 vs. 3) (Table 4).
As shown in Section 3.1, Scenario 2 resulted in the lowest lycopene yield, considerably lower than that obtained in the other three scenarios (Table 4). However, it exhibited the highest antioxidant capacity relative to the amount of lycopene extracted. Therefore, the functional unit of the baseline LCA was adjusted to account for this relationship, and a sensitivity analysis was performed by adjusting the inputs and outputs according to the ratio between extracted lycopene content and antioxidant activity (Table 7).
| Unit | Scenario 1 | Scenario 2 | Scenario 3 | Scenario 4 | |
|---|---|---|---|---|---|
| Inputs | |||||
| Tomatoes pulp preparation | |||||
| Tomatoes | Kg | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 428 428 |
17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 652 652 |
9352 | 5889 |
| Electricity | MJ | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 653 653 |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 942 942 |
6890 |
| Tap water | L | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 855 855 |
35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 304 304 |
18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 704 704 |
11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 779 779 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Lycopene extraction | |||||
| Solvent | L | ||||
| Hexane | 8811 | 7903 | |||
| Acetone | 4406 | 3951 | |||
| Ethanol | 4406 | 3951 | |||
| Ethyl acetate | 29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 832 832 |
9953 | |||
| Deionized water | L | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 014 014 |
18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 645 645 |
9878 | 6221 |
| Electricity | MJ | 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 315 315 |
46![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 240 240 |
67![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 419 419 |
42![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 455 455 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Solvent recovery | |||||
| Lycopene in extract | Kg | 5066 | 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 680 680 |
4491 | 8350 |
| Electricity | MJ | 484 | 2460 | 434 | 821 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Outputs | |||||
| Tomatoes pulp preparation | |||||
| Liquid waste | L | 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 316 316 |
41![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 163 163 |
21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 808 808 |
13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 733 733 |
| Organic waste | Kg | 5866 | 9929 | 5261 | 3313 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Lycopene extraction | |||||
| Organic waste | Kg | 1099 | 1864 | 986 | 621 |
| Chemical waste | Kg | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 944 944 |
18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 645 645 |
16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 094 094 |
6221 |
| Lost solvent | Kg | ||||
| Hexane | 757 | 731 | |||
| Acetone | |||||
| Ethanol | |||||
| Ethyl acetate | 3229 | 628 | |||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Solvent recovery | |||||
| Solvent | Kg | 5064 | 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 680 680 |
4490 | 8349 |
| Dried lycopene | Kg | 2 | 0.3 | 1.8 | 1 |
After this adjustment, Scenario 4 represented the most favorable situation, and Scenario 2 shifted from the least sustainable state to an intermediate level of sustainability (Fig. 4). Scenarios 1 and 3, however, exerted negative effects on most environmental impact categories. In Scenario 4, ozone formation in terrestrial ecosystems (OTe), ozone formation in human health (OHh) and marine ecotoxicity (MEco) presented negative values, which could reflect environmental benefits mainly resulting from ‘avoided’ emissions, either by directly reducing NOx and VOC concentrations or by indirectly avoiding electricity consumption.45,46 The main contributing categories remained consistent with the baseline LCA (OHh, OTe, MEco, TE, FRS, HNCT). Nevertheless, Human carcinogenic toxicity (HCT) appeared in Scenarios 1 and 3 (Fig. 5), likely associated with secondary emissions from industrial processes, such as those arising from energy production, metals, or combustion by-products, rather than from the use of solvents (e.g., hexane or ethanol).47,48 Additionally, Scenario 4 required the smallest amount of tomato discards to achieve the same level of antioxidant activity relative to the amount of lycopene extracted (Table 7).49 Therefore, its potential application at the pilot scale appears highly promising, given the proven scalability of ultrasound-assisted extraction, its efficiency, and its well-documented and widespread implementation at the industrial level.50–52
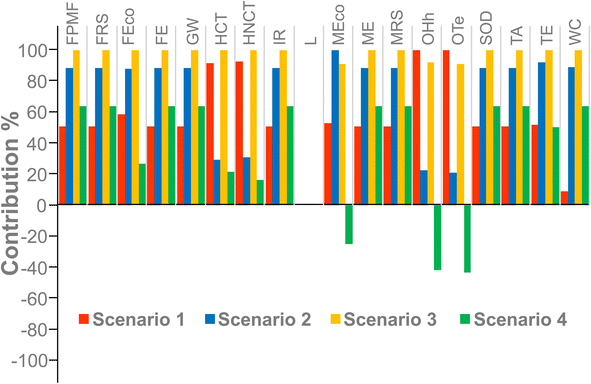 | ||
| Fig. 4 Sensitivity analysis illustrating the relative environmental performance of the four extraction scenarios after adjusting the functional unit based on the antioxidant activity. | ||
4 Conclusions
This study demonstrates that the valorization of tomato discards through lycopene recovery can contribute to waste reduction and resource efficiency within sustainable food systems. The comparative LCA revealed that ultrasound-assisted extraction with ethyl acetate (Scenario 4) achieved the best environmental performance, primarily due to its lower energy requirements and reduced fossil resource consumption. Conversely, conventional extraction with ethyl acetate (Scenario 2) initially appeared as the least sustainable; however, when lycopene yield and antioxidant capacity were jointly considered, its relative performance improved substantially.The sensitivity analysis, performed by adjusting the inputs and outputs of the four scenarios according to the antioxidant activity-to-lycopene ratio, revealed markedly different results, shifting the least favorable scenario (Scenario 2) to an intermediate position. This analysis proved essential for refining the interpretation of results and highlighted the importance of considering LCA as a dynamic process that incorporates feedback among different stages of analysis to improve accuracy and avoid misinterpretation. Integrating functional efficiency metrics, such as antioxidant activity, into environmental assessments enables a comprehensive evaluation of sustainability outcomes. In addition, since energy consumption represents the primary hotspot in the lycopene extraction stage, its influence was particularly evident in impact categories such as ozone formation, human non-carcinogenic toxicity, terrestrial ecotoxicity, and fossil resource scarcity, which are directly associated with electricity use and energy origin. Therefore, future improvements should prioritize substituting conventional energy sources with cleaner alternatives, such as wind power, in order to further enhance the environmental performance and overall sustainability of the lycopene recovery process.
Finally, the results of this work indicate that solvent selection and energy optimization are key levers for reducing environmental impacts in bioactive compound extraction, and that adopting green solvents together with ultrasound-assisted technologies provides a viable route toward low-impact, circular processes that increase the added value of tomato residues while supporting sustainable food production and waste valorization.
Author contributions
NDC conducted the experimental work, methodology design, data interpretation and curation, and formal analysis. E. G. contributed to the conceptualization, methodology design, supervision of the experimental procedures, and data interpretation. A. G.-Z. conceived the study, coordinated the research, contributed to the writing, review, and editing of the manuscript, and funding acquisition. All authors read and approved the final version of the manuscript.Conflicts of interest
The authors declare that they have no conflict of interest.Data availability
The data supporting the findings of this study are included within the article. Additional datasets related to the analytical determinations and LCA calculations are available from the corresponding author upon reasonable request.Acknowledgements
This work was supported by the Argentinean Agency for Scientific and Technological Promotion (ANPCyT) (Project PICT(2020)/0482 and PICT(2019)/2428). A. G-Z. and E. G. are members of the research career of the Argentinean Research Council (CONICET). N. D. C. is a postdoctoral fellow of CONICET. Prof. C. Franca is acknowledged for generously providing the tomato waste from Quinta Flores.References
- Z. Guan, T. Biswas and F. Wu, The US Tomato Industry: an Overview of Production and Trade, University of Florida IFAS Extension, Gainesville, FL, USA, 2025, EDIS document FE1027, DOI:10.32473/edis-fe1027-2017.
- E. J. Collins, C. Bowyer, A. Tsouza and M. Chopra, Biology, 2022, 11(2), 239, DOI:10.3390/biology11020239.
- E. M. Rizk, A. T. El-Kady and A. R. El-Bialy, Ann. Agric. Sci., 2014, 59, 53–61, DOI:10.1016/j.aoas.2014.06.008.
- FAO, Agricultural production statistics 2010–2023, FAOSTAT Analytical Briefs, Rome, 2024, https://openknowledge.fao.org/handle/20.500.14283/cd3755en accessed 8 November 2025 Search PubMed.
- A. Arias, G. Feijoo and M. T. Moreira, Food Bioprod. Process., 2022, 135, 178–189, DOI:10.1016/j.fbp.2022.08.003.
- United Nations, Sustainable Development Goal 12: Ensure Sustainable Consumption and Production Patterns, United Nations Department of Economic and Social Affairs, 2015, https://sdgs.un.org/goals/goal12 Search PubMed.
- European Commission, The European Green Deal: Communication from the Commission to the European Parliament, the European Council, the Council, the European Economic and Social Committee, the Committee of the Regions and the European Investment Bank, EUR-Lex, Brussels, 2019, https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A52019DC0640 accessed 8 November 2025 Search PubMed.
- B. Socas-Rodríguez, J. Álvarez-Rodríguez, M. Herrera-Hernández, M. Rodríguez-Delgado and M. Á. Rodríguez-Delgado, Trends Food Sci. Technol., 2021, 108, 98–115, DOI:10.1016/j.tifs.2021.01.002.
- K. Szabo, L. Mitrea, L. F. Călinoiu, B. E. Teleky, G. A. Martău, D. Plamada, M. S. Pascuta, S. A. Nemeş, R. A. Varvara and D. C. Vodnar, Molecules, 2022, 27(22), 7977, DOI:10.3390/molecules27227977.
- J. Gingrich, Memorandum: Color Additive Petition from Center for Science in the Public Interest, Request to Revoke Color Additive Listing for Use of FD&C Red No. 3 in Food and Ingested Drugs, Department of Health and Human Services, Food and Drug Administration, Division of Food Ingredients, Regulatory Branch, 2024, Docket No. FDA-2023-N-0437, 21 CFR Part 74 Search PubMed.
- S. Kobylewski and M. F. Jacobson, Int. J. Occup. Environ. Health, 2012, 18(3), 220–246, DOI:10.1179/1077352512Z.00000000034.
- D. McCann, A. Barrett, A. Cooper, D. Crumpler, L. Dalen and K. Grimshaw, et al., Lancet, 2007, 370(9598), 1560–1567, DOI:10.1016/S0140-6736(07)61306-3.
- Food and Agriculture Organization (FAO), Color Additive Petition from Center for Science in the Public Interest, 2025, https://www.federalregister.gov/documents/2025/01/16/2025-00830/color-additive-petition-from-center-for-science-in-the-public, accessed 8 November 2025.
- Commission Regulation (EU) 2022/63, Official Journal of the European Union, 2022, http://data.europa.eu/eli/reg/2022/63/oj Search PubMed.
- A. Negi, Sustain. Chem., 2025, 6, 23, DOI:10.3390/suschem603002.
- M. Baker, Z. Xu and C. Jones, Food Qual. Prefer., 2022, 97, 104475, DOI:10.1016/j.foodqual.2021.104475.
- BBC Research, The Global Market for Carotenoids, https://www.bccresearch.com/market-research/food-and-beverage/the-global-market-for-carotenoids.html, accessed 8 November 2025.
- J. Bogue and L. Repar, Trends Food Sci. Technol., 2023, 132, 45–57, DOI:10.1016/j.tifs.2022.12.015.
- K. Topolska, A. Florkiewicz and A. Filipiak-Florkiewicz, Food Qual. Prefer., 2021, 91, 104197, DOI:10.1016/j.foodqual.2021.104197.
- A. Rashidinejad, Future Postharvest Food, 2024, 1(2), 266–273, DOI:10.1002/fpf2.12022.
- D. Boone-Villa, S. López and A. Martínez, J. Funct. Foods, 2019, 57, 75–88, DOI:10.1016/j.jff.2019.03.015.
- J. Pinheiro, R. Ganhão, E. M. Gonçalves and C. L. M. Silva, Foods, 2019, 8(12), 649, DOI:10.3390/foods8120649.
- S. Ghnimi, A. Nikkhah, J. Dewulf and S. van Haute, Sci. Rep., 2021, 11, 1–12, DOI:10.1038/s41598-021-92211-1.
- R. D. Yadav and P. B. Dhamole, Biomass Convers. Bioref., 2024, 14(20), 25495–25511, DOI:10.1007/s13399-023-04676-x.
- E. Nemli, S. Ozakdogan and M. Tomas, et al., Food Biophys, 2021, 16, 355–364, DOI:10.1007/s11483-021-09674-y.
- Z. Lianfu and L. Zelong, Ultrason. Sonochem., 2008, 15(5), 731–737, DOI:10.1016/j.ultsonch.2007.12.001.
- F. Periago, M. D. Rincón, G. Aguera and G. Ros, J. Agric. Food Chem., 2004, 52, 5796–5802, DOI:10.1021/jf049345h.
- International Organization for Standardization, ISO 14044:2006, Environmental Management – Life Cycle Assessment – Requirements and Guidelines, ISO, Geneva, 2006 Search PubMed.
- M. A. J. Huijbregts, Z. J. N. Steinmann and P. M. F. Elshout, et al., Int. J. Life Cycle Assess., 2017, 22, 138–147, DOI:10.1007/s11367-016-1246-y.
- A. Agraso-Otero, A. Tehseen, R. Rebolledo-Leiva and S. González-García, Ind. Crops Prod., 2025, 232, 121290, DOI:10.1016/j.indcrop.2025.121290.
- E. Gerbino, C. Quentier and C. Pénicaud, Data Brief, 2022, 45, 108478, DOI:10.1016/j.dib.2022.108478.
- F. Chemat, M. Abert-Vian, A. S. Fabiano-Tixier, J. Strube, L. Uhlenbrock, V. Gunjevic and G. Cravotto, TrAC, Trends Anal. Chem., 2019, 118, 248–263, DOI:10.1016/j.trac.2019.05.037.
- C. M. Galanakis, Trends Food Sci. Technol., 2012, 26, 68–87, DOI:10.1016/j.tifs.2012.03.003.
- N. J. Miller, C. Castelluccio, L. Tijburg and C. Rice-Evans, FEBS Lett., 1996, 392, 40–44 CrossRef PubMed.
- Y. P. A. Silva, T. A. P. C. Ferreira, G. B. Celli and M. S. Brooks, Waste Biomass Valor, 2019, 10(10), 2851–2861, DOI:10.1007/s12649-018-0317-7.
- A. L. Eh and S. Teoh, Ultrason. Sonochem., 2012, 19, 151–159, DOI:10.1016/j.ultsonch.2011.05.019.
- G. D. Sadler and D. J. Davis, J. Food Sci., 1990, 55(5), 1460–1461, DOI:10.1111/j.1365-2621.1990.tb03958.x.
- A. I. Olives Barba, M. Cámara Hurtado, M. C. Sánchez Mata, V. Fernández Ruiz and M. López Sáenz De Tejada, Food Chem., 2006, 95(2), 328–336, DOI:10.1016/j.foodchem.2005.02.028.
- R. Metcheva, P. Ostoich and M. Beltcheva, BioRisk – Biodivers. Ecosyst. Risk Assess., 2022, 17, 7–18, DOI:10.3897/biorisk.17.77438.
- Y. Zhang, A. Han, S. Deng, X. Wang, H. Zhang, S. Hajat, J. S. Ji, W. Liang and C. Huang, Glob. Transit., 2023, 5, 117–124, DOI:10.1016/j.glt.2023.07.001.
- Y. Li, Y. Zhang and J. Chen, Food Sci., 2023, 44(19), 330–349, DOI:10.7506/spkx1002-6630-20221010-090.
- J. Arballo, J. Amengual and J. W. Erdman Jr, Antioxidants, 2021, 10(3), 342, DOI:10.3390/antiox10030342.
- M. Honda, H. Kageyama, T. Hibino, Y. Zhang, W. Diono, H. Kanda, R. Yamaguchi, R. Takemura, T. Fukaya and M. Goto, Molecules, 2019, 24(11), 2149, DOI:10.3390/molecules24112149.
- S. Fenni, J. Astier, L. Bonnet, E. Karkeni, E. Gouranton, L. Mounien, C. Couturier, F. Tourniaire, V. Böhm and H. Hammou, et al., Mol. Nutr. Food Res., 2019, 63(5), e1800788, DOI:10.1002/mnfr.201800788.
- N. Elginoz, K. Khatami, I. Owusu-Agyeman and Z. Cetecioglu, Front. Sustain. Food Syst., 2020, 4(23) DOI:10.3389/fsufs.2020.00023.
- P. Szulc, J. Kasprzak, Z. Dymaczewski and P. Kurczewski, Energies, 2021, 14(356) DOI:10.3390/en14020356.
- M. Z. Hauschild, R. K. Rosenbaum and S. I. Olsen, Life Cycle Assessment: Theory and Practice, Springer International Publishing, Cham, 2018, DOI:10.1007/978-3-319-56475-3.
- European Chemicals Agency (ECHA), Substance Information: n-Hexane; Ethanol, 2023, https://echa.europa.eu/substance-information, accessed 8 November 2025.
- M. Honda, Y. Watanabe, K. Murakami, R. Takemura, T. Fukaya, Wahyudiono, H. Kanda and M. Goto, LWT, 2017, 86, 69–75, DOI:10.1016/j.lwt.2017.07.046.
- F. Chemat, N. Rombaut, A. G. Sicaire, A. Meullemiestre, A.-S. Fabiano-Tixier and M. Abert-Vian, Ultrason. Sonochem., 2017, 34, 540–560, DOI:10.1016/j.ultsonch.2016.06.035.
- J. Tamminen, J. Holappa, D. V. Gradov and T. Koiranen, Ultrason. Sonochem., 2022, 90, 106171, DOI:10.1016/j.ultsonch.2022.106171.
- I. Quaratesi, I. Calinescu, V. Lavric, V. Ferrara, E. Badea, P. Chipurici, E. G. Dumbravă, R. R. Constantinescu, N. D. Ignat and I. Popa, Agronomy, 2024, 14, 1452, DOI:10.3390/agronomy14071452.
| This journal is © The Royal Society of Chemistry 2026 |