Open Access Article
Open Access ArticleEnhanced recovery of antioxidant compounds from avocado pomace using ultrasound-assisted extraction systems
Dayvison Mendes Moreiraa,
Jailton Ribeiro dos Santos Juniora,
Luiz Carlos Corrêa-Filho b,
Lourdes Maria Correa Cabralc and
Renata Valeriano Tonon
b,
Lourdes Maria Correa Cabralc and
Renata Valeriano Tonon *c
*c
aGraduate Program in Food Science (PPGCAL), Institute of Chemistry (IQ), Federal University of Rio de Janeiro (UFRJ), Cidade Universitária, Rio de Janeiro, RJ 21941-909, Brazil. E-mail: dayvison.engpesca@gmail.com
bGraduate Program in Food Science and Technology (PPGCTA), Federal Rural University of Rio de Janeiro (UFRRJ), Seropédica, Rio de Janeiro, RJ 23890-000, Brazil
cEmbrapa Food Technology, Guaratiba, Rio de Janeiro, RJ 23020-470, Brazil
First published on 2nd January 2026
Abstract
Avocado oil production generates large amounts of residues, including peels, seeds, and exhausted pulp, which are rich sources of phenolic compounds with antioxidant activity. Ultrasound-assisted extraction (UAE) is a promising green technology for recovering bioactive compounds from agro-industrial wastes. This study compared the hydroethanolic UAE of phenolic compounds from avocado pomace using two system configurations: with and without recirculation. Ethanol concentration (0–100%) and extraction time (0–24 min for the non-recirculating system, and 0–180 min for the recirculating system) were evaluated. UAE was effective in enhancing phenolic recovery, providing higher phenolic yields in reduced processing time (1056 mg GAE/100 g after 15 min of extraction), when compared to the conventional extraction method (656 mg GAE/100 g after 180 min of extraction). For both systems, 15 min of extraction with 50% ethanol was identified as the best condition for antioxidant recovery. However, the recirculating system provided better temperature control. This configuration is also more suitable for processing larger sample volumes, showing greater potential for process scale-up.
Sustainability spotlightThis work contributes to sustainable food processing by valorizing avocado pomace, a major by-product of oil production, through ultrasound-assisted extraction of natural antioxidants. The process employs hydroethanolic solvents and ultrasonic technology, known for reducing both extraction time and environmental impact when compared with conventional methods. The recirculating system evaluated enhances temperature control and scalability, facilitating industrial implementation. This study supports the United Nations Sustainable Development Goals, particularly SDG 12 (Responsible Consumption and Production) and SDG 9 (Industry, Innovation and Infrastructure), by promoting circular bioeconomy practices and the development of cleaner, resource-efficient extraction technologies. |
1. Introduction
Avocado oil, extracted from the fruit pulp, is rich in monounsaturated fatty acids, particularly oleic acid, which supports cardiovascular health, and contains vitamin E with antioxidant properties.1,2 During oil extraction, large amounts of residues are generated, including peels, seeds and pulp, which are rich sources of phenolic compounds exhibiting high antioxidant activity.3 Thus, they are considered potential natural antioxidants for food systems.4The extraction step is essential for recovering bioactive compounds, and traditional solid–liquid extraction methods such as mechanical agitation are increasingly being replaced by more efficient and environmentally sustainable technologies.5
Ultrasound-assisted extraction (UAE) is an efficient and sustainable alternative to conventional extraction techniques, offering higher compound recovery and in significantly shorter processing times. Recognized as a green, simple, and cost-effective method, UAE enhances the release and diffusion of bioactive compounds from diverse matrices.6 Its mechanism is based on acoustic cavitation, which promotes the formation, expansion, and implosion of microbubbles, which disrupts cell structures, increases matrix porosity, and facilitates solute release, thereby improving mass transfer and extraction yield.7
Ultrasound-assisted extraction is typically performed in open systems, such as ultrasonic baths or probe setups. However, these systems pose notable challenges regarding temperature control during extraction. The propagation of ultrasonic waves generates heat due to cavitation and particle agitation, which can lead to the degradation of thermolabile compounds such as phenolics and antioxidants.6
In this context, the use of a continuous-circulation ultrasound-assisted extraction system represents a promising alternative. In this configuration, the solid matrix and solvent do not remain in constant contact with the ultrasonic probe but instead circulate continuously through the system. This design minimizes heat accumulation, especially when coupled with a cooling bath, allowing for longer extraction periods under controlled thermal conditions. In addition, it is better suited for processing larger sample volumes and for potential scale-up applications.
Most studies on the extraction of phenolic compounds from avocado waste have focused on isolated residues, such as seeds, peels, or leaves, rather than the actual by-products generated by oil-processing industries, which include a mixture of seeds, peels, and pulp.8,9 Regarding ultrasonic systems, existing research has primarily employed bath or open-probe configurations, revealing a lack of studies involving continuous recirculation systems.
The present study aimed to compare ultrasound-assisted hydroethanolic extraction processes conducted with and without recirculation systems, focusing on their effects on the recovery of phenolic compounds from avocado pomace. Additionally, the influence of ethanol concentration and extraction time on the yield of phenolic compounds and the antioxidant capacity of the resulting extracts was also evaluated. Finally, UAE was compared to a conventional mechanical agitation extraction.
2. Material and methods
2.1 Material
The Hass avocado (Persea americana) pomace, consisting of a mixture of pulp, peel, and seeds, was supplied by Fazenda Irarema (São Sebastião da Grama, Brazil). The material was dried in a tray dryer at 45 °C for 24 h, ground in a knife mill, and sieved through a 1 mm mesh. The obtained material was vacuum-packed in metallized bags and stored at 10 °C until processing.2.2 Ultrasound-assisted extraction
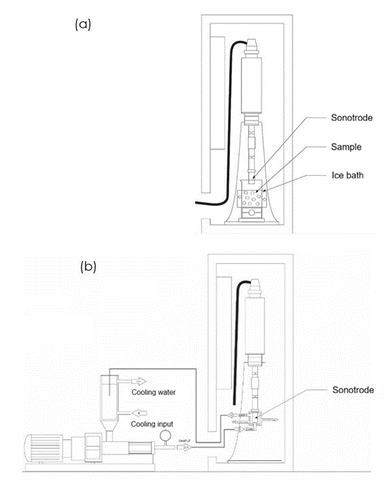
The extraction of antioxidant compounds from avocado pomace was carried out using an ultrasonic system UIP1000hdT (Hielscher, Germany) equipped with an ultrasonic probe (34 mm diameter) operating with nominal (input) power of 200 W. In this system, the vibration amplitude is not fixed, as the device automatically adjusts the amplitude to reach and maintain the selected nominal power during operation. Two extraction modes were evaluated: (i) a batch system without recirculation, and (ii) a continuous circulation system, in which both the pomace and the extraction solvent were recirculated through the ultrasonic chamber, as illustrated in Fig. 1.The recirculating system comprises an ultrasonic transducer fitted with a sonotrode and integrated with a peristaltic pump and a thermostatic bath, allowing continuous circulation of the sample mixture (pomace + solvent) through an external loop. This configuration enhances medium homogenization and provides improved thermal regulation during processing. During the extraction process, flow rate was 3409 mL min−1, and each portion of the sample mixture resulted in an average of 3.4 complete cycles per minute through the cavitation zone, resulting in a cumulative local residence time of approximately 9.6 s min−1 within the active ultrasonic region.
Initially, to evaluate the effect of ethanol concentration on the extraction of antioxidant compounds, hydroethanolic solutions containing 0, 10, 30, 50, 70, 90, and 100% (v/v) ethanol were employed under both extraction modes. Each extraction was performed for 15 min. Based on the ethanol concentration that provided the highest extraction efficiency, the kinetics of antioxidant compound extraction were subsequently investigated under both operating conditions: without circulation (up to 24 min) and with circulation (up to 180 min).
For comparison, a conventional extraction by mechanical agitation was also conducted using an orbital shaker at 30 rpm, for 180 min at 40 °C, employing the same solvent composition selected for the ultrasound-assisted process.
A solid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) liquid ratio of 1
liquid ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 was used in both extraction systems based on preliminary tests, which showed that lower proportions in the non-recirculating system left the extract too viscous and difficult to filter. The extracts were initially centrifuged at 5000 rpm for 15 min to remove suspended particles. Then, they were filtered using qualitative round filter paper (0.45 µm pore size, 90 mm diameter) and stored in amber bottles at −18 °C until analysis.
20 was used in both extraction systems based on preliminary tests, which showed that lower proportions in the non-recirculating system left the extract too viscous and difficult to filter. The extracts were initially centrifuged at 5000 rpm for 15 min to remove suspended particles. Then, they were filtered using qualitative round filter paper (0.45 µm pore size, 90 mm diameter) and stored in amber bottles at −18 °C until analysis.
2.3 Total phenolic compounds
The Folin-Ciocalteu method was employed to quantify the total phenolic content, following the procedure described by Singleton, Joseph, and Rossi (1965),10 with some modifications. In a test tube, 250 µL of the sample was mixed with 1.25 mL of 10% Folin-Ciocalteu reagent, vortexed, and allowed to react in the dark for 2 min. Then, 1 mL of 7.5% sodium carbonate solution was added, the mixture was vortexed and incubated in a water bath at 50 °C for 15 min, and absorbance was measured at 760 nm. The total phenolic content was quantified based on a gallic acid calibration curve and expressed as mg gallic acid equivalents (GAE) per 100 g of dried avocado pomace.2.4 Antioxidant capacity
2.5 Phenolic profile of the extract
The analysis of free phenolic acids and flavonoids was performed according to Nascimento et al. (2017).13 The hydroethanolic extract was filtered and analyzed using a high-performance liquid chromatograph (HPLC) Alliance e2695, equipped with a 2998 UV-visible PDA detector, quaternary pump, vacuum degasser, and autosampler (Waters). Chromatographic separation was carried out on a reversed-phase C18 column (Kromasil, 150 mm × 4.6 mm, 5 µm particle size), maintained at 40 °C. The mobile phase consisted of (A) an aqueous solution of 0.15% phosphoric acid and (B) acetonitrile, using a gradient elution program. The flow rate was set at 1.0 mL min−1, and the injection volume was 5 µL. The PDA detector scanned from 200 to 600 nm, with quantification performed at selected wavelengths (270, 290, 310, 325, and 370 nm). Results were expressed in mg/100 g of dried pomace.2.6 Statistical analyses
All analyses were performed in triplicate, and the results were subjected to analysis of variance (ANOVA). Significant differences between mean values were assessed using Tukey's test at a 5% significance level (p < 0.05). Statistical analyses were conducted using STATISTICA® software, version 10.0 (Statsoft, Tulsa, USA).3. Results and discussion
3.1 Effect of ethanol concentration
Extraction technology and solvent selection are critical factors in the extraction processes, particularly for food and pharmaceutical applications. To ensure safety and quality, solvents classified as “Generally Recognized As Safe” (GRAS) are presumed suitable for use in foods, additives, and industrial application, provided they are used within established regulatory limits. Ethanol is one such solvent and is widely accepted for use in foods and beverages due to its recognized safety.14Fig. 2 presents the total phenolic content and antioxidant capacity (ABTS+ and DPPH) obtained using hydroethanolic solutions with different ethanol concentrations in the recirculating and non-recirculating ultrasound-assisted extraction systems.
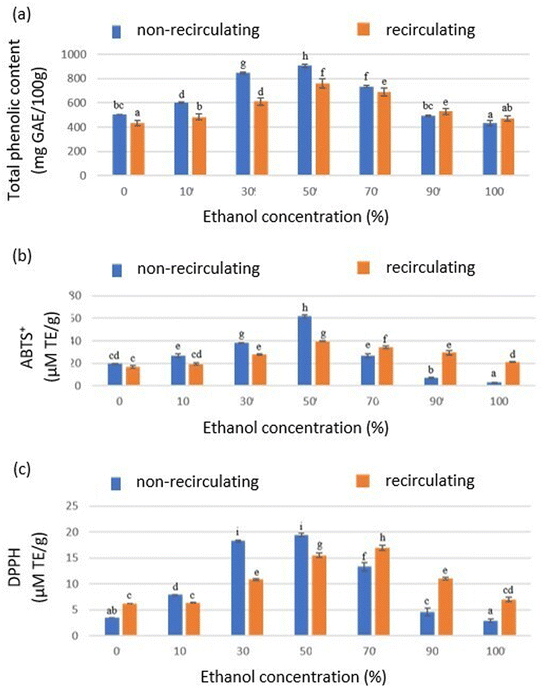 | ||
| Fig. 2 (a) Total phenolic content, (b) antioxidant capacity determined by the ABTS+ method, and (c) antioxidant capacity determined by the DPPH method in avocado pomace. | ||
Factors such as polarity, solubilization capacity, surface tension, and viscosity are known to affect the extraction performance.15–17 Extraction with 50% ethanol produced the highest yields for total phenolic content and antioxidant capacity in both ultrasound-assisted systems. This enhanced extraction efficiency can be attributed to the synergistic effect between ethanol and water. Water hydrates the plant matrix, swelling it and weakening cell wall structures, which, combined with ultrasonic cavitation, enhances porosity and facilitates the release of compounds. Additionally, the complementary solvent properties of ethanol and water improve the solubility of phenolic compounds and other antioxidants, making solvent mixtures more effective than individual solvents alone.
A 50% hydroethanolic solution exhibits intermediate polarity, as it combines highly polar water with less polar ethanol. This polarity profile favors the extraction of moderately polar compounds, including most phenolic molecules, which contain hydroxyl groups (–OH) attached to aromatic rings.19 Moreover, using a water–ethanol mixture as a solvent reduces the use of pure organic solvents, rendering the process safer, more sustainable, and environmentally friendly.
Previous studies support the benefits of using solvent mixtures in the extraction of phenolic compounds. Vizzotto and Pereira (2011)20 reported that combinations of solvents improved phenolic recovery from blackberry (Rubus sp.) extracts. While ultrapure water alone was less efficient than methanol, ethanol, or acetone, its combination with organic solvents created a moderately polar medium that facilitated polyphenol extraction. Similarly, the use of hydroalcoholic mixtures, such as ethanol–water combinations, has been shown to be highly effective for extracting bioactive phenolic compounds from food industry by-products. These mixtures enhanced the recovery of flavonoids, anthocyanins, and phenolic acids from residues such as grape skins, apple peels, and peach by-products.16 Compared to single solvents, hydroalcoholic mixtures provided higher extraction yields due to the complementary properties of ethanol and water. Moreover, the extracts exhibited significant antioxidant activity, as measured by DPPH and FRAP assays, highlighting the functional potential of the recovered compounds.
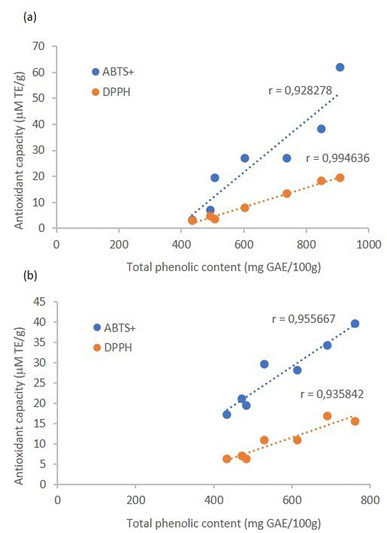
The antioxidant capacity measured by both methods followed the same trend observed for total phenolics, indicating that they are strongly correlated, as confirmed by the Pearson correlation coefficients (r), which were higher than 0.9 (Fig. 3). This indicates that the antioxidant activity of the extracts can be greatly attributed to their phenolic compounds. The ABTS+ antiradical activity after 15 minutes of extraction was higher than that reported by Gonzales (2024)21 for the extraction of bioactive compounds from avocado pomace using magnetic stirring with water as solvent. In that study, extraction was conducted for 15 min at a maximum temperature of 60 °C, resulting in an average of 12.90 µM Trolox g−1. In comparison, the ultrasound-assisted system achieved 19.53 µM Trolox g−1 without circulation and 17.16 µM Trolox g−1 with circulation under the same duration and solvent conditions. These results demonstrate that the application of ultrasound significantly enhances the efficiency of bioactive compound extraction from avocado pomace, highlighting the feasibility of this technique to obtain extracts with higher antioxidant capacity.
3.2 Effect of extraction time
Based on the 50% hydroethanolic solution, identified as the most suitable solvent for the extraction process, kinetics assays were performed to evaluate the influence of extraction time on the recovery of antioxidant compounds in both ultrasound-assisted systems. The total phenolic content and antioxidant capacity of the hydroethanolic extracts obtained at different extraction times, as well as the final extract temperature and energy consumption (provided by the ultrasound device) of each process, are presented in Table 1. The extraction time of 0 min corresponds to the initial control condition of the sample, in which the mixture was prepared and immediately filtered without any exposure to ultrasound.| Extraction time (min) | Total phenolic content (mg GAE/100 g) | ABTS (µM TE g−1) | DPPH (µM TE g−1) | T (°C) | Energy spent (kWh) |
|---|---|---|---|---|---|
| a Different letters within a column indicate significant differences according to Tukey's test (p < 0.05). | |||||
| Non-recirculating system | |||||
| 0 | 461.61 ± 19.86a | 29.91 ± 0.19 ab | 11.98 ± 0.10a | 15 | — |
| 3 | 550.47 ± 12.46b | 32.92 ± 1.32abc | 11.48 ± 0.11a | 21 | 7.5 |
| 6 | 688.25 ± 7.45c | 41.03 ± 3.39c | 14.15 ± 0.48b | 26 | 14.9 |
| 9 | 873.55 ± 18.75d | 55.10 ± 1.70d | 15.48 ± 0.92b | 30 | 22.4 |
| 12 | 912.08 ± 11.93d | 63.27 ± 1.51efg | 17.37 ± 0.33c | 33 | 29.9 |
| 15 | 1019.59 ± 30.11e | 70.43 ± 0.38gh | 18.93 ± 0.51 cd | 39 | 37.2 |
| 18 | 1049.90 ± 23.83e | 74.20 ± 0.75hi | 19.32 ± 0.25de | 40 | 44.7 |
| 21 | 1168.38 ± 47.14f | 79.22 ± 2.30i | 20.21 ± 0.88def | 48 | 52.2 |
| 24 | 1211.08 ± 25.14f | 91.16 ± 0.65j | 21.70 ± 0.17 fg | 55 | 59.6 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Recirculating system | |||||
| 0 | 480.83 ± 8.36 ab | 26.61 ± 1.21a | 12.00 ± 0.10a | 14 | — |
| 15 | 1056.09 ± 12.47e | 37.31 ± 2.64bc | 19.90 ± 0.44de | 30 | 49.9 |
| 45 | 1070.57 ± 31.34e | 57.31 ± 5.66de | 20.68 ± 0.86 fg | 40 | 130.6 |
| 90 | 1046.43 ± 22.70e | 57.44 ± 4.66ef | 22.51 ± 0.92gh | 41 | 223.1 |
| 120 | 1057.46 ± 06.21e | 64.67 ± 5.71 fg | 22.57 ± 0.83gh | 43 | 297.6 |
| 180 | 1082.99 ± 34.89e | 65.61 ± 2.83fgh | 23.40 ± 0.10h | 45 | 449.5 |
In both systems, a near-equilibrium concentration for total phenolics was achieved after 15 min of extraction, with little or no increase at more prolonged times. There was not significant difference in total phenolic content of the extracts obtained in both systems for this extraction time (Table 1). When considering the feasibility of industrial-scale implementation, prolonged extraction times can increase solvent volatilization, energy consumption, equipment use, and operational costs.22 In this work, extraction longer than 15 minutes resulted in higher energy demands and increased final extract temperatures, mainly in the system without recirculation (Table 1). The use of a circulating ultrasound-assisted extraction system allows for extended extraction periods with better temperature control, minimizing the possible degradation of thermosensitive compounds and preserving their bioactivity.
In the absence of solvent circulation, local saturation near the matrix can limit extraction rates, while prolonged extraction allows compounds that are more difficult to release to gradually diffuse into the solvent. Additionally, ultrasound-induced cavitation acts progressively, disrupting cells over time and facilitating compound availability, although extended periods may risk the degradation of sensitive antioxidants.
The extraction of antioxidant compounds is generally limited by the solubility of the target compounds in the solvent, and an equilibrium is expected once this limit is reached, beyond which no further increase occurs despite longer extraction times or higher process intensity.
In the non-circulating ultrasound system, phenolic content remained constant after 15 min. This suggests that the system may have approached a partial equilibrium, where local solvent saturation and limited diffusion slow the release of additional compounds. Additionally, prolonged extraction times may contribute to the degradation of thermosensitive antioxidants, further limiting the net increase in extracted compounds.
The energy efficiency (EE), expressed in mg GAE kWh−1, was calculated based on the total phenolic content obtained at the selected extraction time (15 min) and the corresponding energy consumption. The non-recirculating system showed an EE of approximately 0.27 mg GAE kWh−1, whereas the recirculating system presented 0.21 mg GAE kWh−1. Although recirculation enhances solution homogenization and tends to improve thermal control by reducing local temperature gradients, the overall energy demand of the process increases, primarily due to the continuous operation of the pump responsible for fluid recirculation.
Several authors reported the degradation of antioxidants due to high temperature and prolonged exposure. De Lima Marsiglia et al., (2023),23 when evaluating the thermal stability of bioactive compounds in jaboticaba peel using 50% ethanol and ultrasound, observed that at 90 °C, approximately 55% of the phenolic content was reduced, indicating that elevated temperatures accelerate the degradation of bioactive compounds. Wu et al., (2024),24 in their literature review on thermal processing of fruits and vegetables, corroborated how high temperatures impact food processing and emphasized the application of emerging technologies, such as ultrasound, for bioactive compound extraction. In the present study, the maximum temperature reached was 55 °C at 24 minutes in the non-circulating system, and 45 °C at 180 minutes in the circulating system, indicating that thermal degradation of antioxidant compounds was unlikely.
Comparable findings were reported by Bezerra et al. (2022)25 in the ultrasound-assisted extraction of phenolic compounds and antioxidants from avocado peel (Persea americana Mill) using a 50% hydroalcoholic solution, where extraction times of 20 and 30 minutes did not result in statistically significant differences in antioxidant activity measured by ABTS+ and DPPH assays, with differences observed only for total phenolic content. The Folin-Ciocalteu assay measures total reducing capacity, reacting with any reducing substance that may also have been extracted which may overestimate the antioxidant activity due to interference of non-phenolic reducing substances.
The DPPH method primarily detects antioxidants capable of donating hydrogen atoms to the radical, being more effective for lipophilic or moderately lipophilic compounds.18 Given the use of a hydrophilic extracting solution and the low lipid content of avocado residue, low variation was observed between different ultrasound systems. In contrast, ABTS+ values in the present study under non-circulating conditions at 15 minutes were significantly higher (70.43 µM TE g−1) than those in the circulating system (37.31 µM TE g−1), suggesting that a smaller sample circulation area, influenced by the beaker circumference and sonotrode position, enhanced cavitation forces within a reduced space. This likely facilitated the extraction of bioactive compounds—including phenolics, flavonoids, carotenoids, and tocopherols—that were more susceptible to release and measurable by the ABTS+ assay.26
The antioxidant capacity determined by the ABTS+ method in this study was higher than that reported by Gonzales (2024),21 who found that avocado pomace extracts obtained at different extraction temperatures by orbital agitation over 15 min exhibited values as mentioned, in both systems, a near-equilibrium total phenolic content was achieved after 15 min of extraction. Regarding the type of system employed, the circulating ultrasound system demonstrated advantages in antioxidant compound extraction, primarily due to better temperature control of the sample. In addition, for potential industrial-scale applications, the circulating system would allow processing of larger sample volumes with improved parameter control.
3.3 Comparison between conventional extraction and ultrasound-assisted extraction
The results of total phenolic content and antioxidant capacity of 50% hydroethanolic extracts obtained at different extraction times under orbital agitation over 180 min, representing the conventional extraction process, are presented in Table 2.| Extraction time (min) | Total phenolic content (mg GAE/100 g) | ABTS (µM TE g−1) | DPPH (µM TE g−1) | T (°C) |
|---|---|---|---|---|
| a Different letters within a column indicate significant differences according to Tukey's test (p < 0.05). | ||||
| Orbital shaking extraction | ||||
| 15 | 538.41 ± 08.35b | 33.46 ± 0.38a | 13.52 ± 0.14b | 40 |
| 45 | 544.88 ± 05.18b | 37.36 ± 0.57 ab | 13.92 ± 0.17bc | 40 |
| 90 | 587.32 ± 04.28bc | 38.86 ± 0.57 ab | 14.64 ± 0.19bc | 40 |
| 120 | 600.93 ± 05.14c | 39.66 ± 0.43 ab | 15.13 ± 0.03c | 40 |
| 180 | 656.32 ± 03.15d | 40.89 ± 1.35b | 17.48 ± 0.05f | 40 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Ultrasound with circulation system | ||||
| 15 | 1056.09 ± 12.47a | 37.31 ± 2.64 ab | 19.90 ± 0.44d | 30 |
| 45 | 1070.57 ± 31.34a | 57.31 ± 5.66c | 20.68 ± 0.86de | 40 |
| 90 | 1046.43 ± 22.70a | 57.44 ± 4.66c | 22.51 ± 0.92ae | 41 |
| 120 | 1057.46 ± 06.21a | 64.67 ± 5.71c | 22.57 ± 0.83a | 43 |
| 180 | 1082.99 ± 34.89a | 65.61 ± 2.83d | 23.40 ± 0.10a | 45 |
Extraction under mechanical agitation showed lower results compared to ultrasound-assisted extraction with circulation, both in total phenolic content and antioxidant capacity. Total phenolic content increased by more than 95% after 15 min of ultrasound-assisted extraction in the circulating system compared to conventional extraction, and by approximately 65% after 180 min.
The cavitation process, hydration, fragmentation, and pore formation in the plant tissue matrix increase solute exposure through cell wall rupture, facilitating its release into the solvent. In the ABTS+ assay, ultrasound with circulation enhanced extraction by over 23% after 15 minutes, while in the DPPH assay, the increase exceeded 47% over the same period. Overall, the conventional approach tends to be less effective compared to ultrasound-assisted extraction, which offers higher efficiency, faster processing, and better preservation of bioactive compounds.
This mechanism reduces extraction time and, when coupled with a recirculating system equipped with a thermal bath, enables improved temperature control, thereby helping to preserve the integrity of heat-sensitive antioxidants and minimizing degradation of phenolic compounds, flavonoids, and vitamins.
3.4 Phenolic profile of the extract
The phenolic acid and flavonoid contents of the avocado pomace extract obtained in the circulation system after 15 minutes of extraction are presented in Table 3.| Compound | (mg/100 g dried pomace) |
|---|---|
| Protocatechuic acid | 0.66 |
| 4-Hydroxybenzoic acid | 0.34 |
| Epicatechin | 10.94 |
| p-Coumaric acid | 0.16 |
| Chlorogenic acid | 5.58 |
| Quercetin | 3.74 |
Epicatechin was the predominant compound in the obtained extract among the standards analyzed. Belonging to the phenolic class, flavonoids possess antioxidant properties, which contribute to the neutralization of free radicals and the reduction of oxidative stress in the body.27 Epicatechin has a chemical structure containing hydroxyl groups, conferring polarity to the molecule and favoring its solubility in polar solvents such as water and ethanol.
Restrepo-Serna; Cardona-Alzate, (2024),28 when evaluating Hass avocado peel using different extraction technologies, reported higher values for epicatechin, which may be related to the longer extraction time (60 minutes) conducted at 60 °C in an ultrasonic bath with 750 W power, in addition to the different type of raw material.
Chlorogenic acid was the second most abundant compound identified, followed by quercetin. Both compounds exhibit antioxidant and anti-inflammatory properties. Their polarity suggests greater solubility in the hydroethanolic solvent used during extraction, facilitating the recovery of these compounds from the avocado plant matrices.
Research on the anti-inflammatory mechanisms of chlorogenic acid has intensified, as it acts through a dual molecular strategy to release bioactive molecules, neutralizing the propagation of inflammation by inhibiting nitric oxide synthesis.29 Vollmannová et al., (2024),30 highlighted in their study the effects of quercetin in protecting against chronic diseases, attributed to its multiple biological activities. In addition to its anti-inflammatory properties, quercetin modulates the immune response and reduces chronic inflammation, factors that contribute to the development of various long-term pathologies.
Phenolic acids such as protocatechuic, 4-hydroxybenzoic, and p-coumaric acids, present in lower amounts in avocado extract, also exhibit antioxidant, antimicrobial, and anti-inflammatory properties.31 They act in neutralizing free radicals, helping to protect cells from oxidative stress. This combination of activities indicates a high potential for their incorporation into foods to improve preservation, enhance nutritional value, and potentially provide health benefits.
These compounds function as natural antioxidants, helping to delay the oxidation of lipids and other components, thereby prolonging the shelf life of food products. Furthermore, their presence may contribute to the functional properties of foods. However, deliberate incorporation of these acids into foods must comply with specific regulations from health surveillance agencies, ensuring the safety and acceptability of the final product.
4. Conclusions
Based on the results obtained in this study, ultrasound-assisted extraction can be considered an efficient technique for obtaining phenolic and antioxidant compounds from avocado pomace, offering advantages over traditional methods, such as increased extraction yield in a reduced processing time.Regarding the ultrasound system, with or without circulation, a better temperature control was observed in the circulating system, and no statistically significant differences were observed between treatments at the 15-min extraction time, suggesting that the choice of system should consider the specific objectives of the extraction. For industrial-scale applications, the circulating system presents advantages by allowing the processing of larger volumes and easier temperature control; on the other hand, the non-circulating system requires less operational effort but demands greater attention to thermal control and limits the volume of sample that can be processed.
Author contributions
Dayvison Moreira: conceptualization, methodology, formal analysis, writing – original draft. Jailton Junior: formal analysis, methodology. Luiz Corrêa-Filho: formal analysis, writing – review and editing. Lourdes Cabral: conceptualization and supervision. Renata Tonon: conceptualization, funding acquisition, project administration, writing – reviewing and editing and supervision.Conflicts of interest
The authors declare no conflicts of interest.Data availability
All data supporting this article are presented within the paper, and all information sourced from the literature has been properly cited.Acknowledgements
The authors are grateful for the financial support provided by CAPES (Finance code 001), FAPERJ (processes number E-26/200.370/2023 and E-26/202.520/2019) and CNPq (processes number 311529/2021-6 and 311496/2018-0).References
- P. F. Duarte, M. A. Chaves, C. D. Borges and C. R. B. Mendonça, Cienc. Rural, 2016, 46(4), 747–754 CrossRef CAS.
- M. Flores, C. Saravia, C. E. Vergara, F. Ávila, H. Valdés and J. Ortiz-Viedma, Molecules, 2019, 24, 2172 CrossRef CAS.
- R. G. Araújo, R. M. Rodriguez-Jasso, H. A. Ruiz, M. M. E. Pintado and C. N. Aguilar, Trends Food Sci. Technol., 2018, 80, 51–60 CrossRef.
- B. Rodríguez-Martínez, B. Gullón and R. Yáñez, Antioxidants, 2021, 10(10), 1630 CrossRef PubMed.
- Y. Yao, Y. Pan and S. Liu, Ultrason. Sonochem., 2020, 62, 104722 CrossRef CAS.
- F. Chemat, N. Rombaut, A. Sicaire, A. Meullemiestre, A. Fabiano-Tixier and M. Abert-Vian, Ultrason. Sonochem., 2017, 34, 540–560 CrossRef CAS PubMed.
- K. Kumar, S. Srivastav and V. S. Sharanagat, Ultrason. Sonochem., 2021, 70, 105325 CrossRef CAS.
- B. Melgar, M. Inês, A. Ciric and M. Soković, Ind. Crops Prod., 2018, 111, 212–218 CrossRef CAS.
- W. Wang, T. R. Bostic and L. Gu, Food Chem., 2010, 122(4), 1193–1198 CrossRef CAS.
- V. L. Singleton and J. A. Rossi, Am. J. Enol. Vitic., 1965, 16(3), 144–158 CrossRef CAS.
- A.-B. Maestre-Hernández, J.-J. Vicente-López, F. Pérez-Llamas, M.-E. Candela-Castillo, M.-T. García-Conesa, M.-J. Frutos, A. Cano, J. Hernández-Ruiz and M. B. Arnao, Processes, 2023, 11, 1400 CrossRef.
- W. Brand-Williams, M. E. Cuvelier and C. Berset, Food Sci. Technol., 1995, 25–30 CAS.
- L. S. M. Nascimento, M. C. P. A. Santiago, E. M. M. Oliveira, R. G. Borguini, E. C. O. Braga, V. C. Martins, S. Pacheco, M. C. Souza and R. L. O. Gogoy, Nutr. Food Technol., 2017, 3(3), 1–7 Search PubMed.
- M. A. Tremocoldi, P. L. Rosalen, M. Franchin, A. P. Massarioli, C. Denny, É. R. Daiuto, J. A. R. Paschoal, P. S. Melo and S. M. Alencar, PLoS One, 2018, 13(2), e0192577 CrossRef.
- U. Roobab, R. M. Aadil, S. S. Kurup and S. Maqsood, Ultrason. Sonochem., 2025, 114, 107252 CrossRef CAS PubMed.
- E. Gil-Martín, T. Forbes-Hernández, A. Romero, D. Cianciosi, F. Giampieri and M. Battino, Food Chem., 2022, 378, 131918 CrossRef.
- M. Kaveh, F. Sharifian, E. Khalife, S. Keramat, B. Ghaysari, M. Dolatkhoh and F. Jadidi, Sci. Rep., 2025, 15, 31327 CrossRef CAS PubMed.
- Y. Lang, N. Gao, Z. Zang, X. Meng, Y. Lin, S. Yang, Y. Yang, Z. Jin and B. Li, J. Future Foods, 2024, 5, 255–272 Search PubMed.
- S. Ma, C. Cai, Q. Lu and Z. Tan, Food Chem., 2025, 448, 137950 Search PubMed.
- M. Vizzotto and M. C. Pereira, Rev. Bras. Frutic., 2011, 33, 1223–1230 Search PubMed.
- C. M. Gonzales, Piracicaba, 2024, 34–45 Search PubMed.
- R. Foujdar, M. B. Bera and H. K. Chopra, Biomed. Food Appl., 2020, 751–780 CAS.
- W. I. M. de Lima Marsiglia, L. de, S. C. Oliveira, R. L. J. Almeida, N. C. Santos, J. M. da S. Neto, Â. M. Santiago, B. C. A. de Melo and F. L. H. da Silva, J. Indian Chem. Soc., 2023, 100, 100995 CrossRef CAS.
- Y. Wu, Y. Liu, Y. Jia, C. Feng, H. Zhang, F. Ren and G. Zhao, Food Res. Int., 2024, 180, 113784 Search PubMed.
- R. A. D. Bezerra, N. M. Silva, B. F. Tuzzi, F. E. De Marchi, A. C. Feihrmann and G. T. dos Santos, Res. Soc. Dev., 2022, 11, 4–6 Search PubMed.
- K. V. L. Bastos and F. M. Souza, Biomed. J. Sci. Tech. Res., 2025, 61(1), 4–9 Search PubMed.
- I. J. S. German, K. T. Pomini, J. C. Andreo, J. V. T. C. Shindo, M. V. M. de Castro, C. R. P. Detregiachi, A. C. Araújo, E. L. Guiguer, L. F. Laurindo, P. C. de S. Bueno, M. de S. S. de Souza, M. Gabaldi, S. M. Barbalho and A. L. Shinohara, Nutrients, 2024, 16, 326 CrossRef CAS.
- D. L. Restrepo-Serna and C. A. Cardona-Alzate, Sustain. Chem. Pharm., 2024, 39, 101562 CrossRef.
- J. Huang, M. Xie, L. He, X. Song and T. Cao, Front. Pharmacol, 2023, 14, 1218015 CrossRef CAS PubMed.
- A. Vollmannová, T. Bojňanská, J. Musilová, J. Lidiková and M. Cifrová, Heliyon, 2024, 10, e30612 CrossRef.
- D. J. Bhuyan, M. A. Alsherbiny, S. Perera, M. Low, A. Basu, O. A. Devi, M. S. Barooah, C. G. Li and K. Papoutsis, Antioxidants, 2019, 8, 42 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2026 |