Open Access Article
Open Access ArticleAntioxidant, antimicrobial, and cytotoxic properties of chitosan–PVA films functionalised with chitooligosaccharide and gallic acid for shelf-life extension of perishable foods
Shuva Bhowmik abc,
Dominic Agyei
abc,
Dominic Agyei d and
Azam Ali
d and
Azam Ali *ab
*ab
aCentre for Bioengineering and Nanomedicine, Faculty of Dentistry, Division of Health Sciences, University of Otago, 310 Great King Street North, Dunedin 9016, New Zealand. E-mail: azam.ali@otago.ac.nz
bSir John Walsh Research Institute (SJWRI), Faculty of Dentistry, Division of Health Sciences, University of Otago, Dunedin 9016, New Zealand
cDepartment of Fisheries and Marine Science, Noakhali Science and Technology University, Noakhali, 3814, Bangladesh
dSchool of Chemistry, Faculty of Science, Monash University, Clayton, VIC-3800, Australia
First published on 20th December 2025
Abstract
Environment friendly, non-toxic, and functional packaging films are gaining interest worldwide for extending the shelf-life of perishable food items. Hence, this study explored the antioxidant, antimicrobial, and cytotoxicity patterns of earlier fabricated chitosan films containing polyvinyl alcohol (PVA), chitooligosaccharide (COS), and gallic acid (GA). In addition, the active properties of chitosan films were reconfirmed using tomatoes, green grapes, and pearl fish fillets as model perishable food products. Chitosan films containing COS and GA demonstrated strong DPPH (99.48 ± 0.24%) and ABTS (98.06 ± 0.91%) radical scavenging capacity and robust potentiality in ferric reducing activity (230.93 ± 1.42, equivalent µM Fe2+ per g sample). Additionally, the inhibitory rates of the fabricated films towards Escherichia coli, Listeria innocua, and Saccharomyces cerevisiae were 59.49 ± 9.17%, 79.29 ± 0.94%, and 79.55 ± 8.45%, respectively. Furthermore, MTT assay exhibited that chitosan films were biocompatible with viability greater than 90% on HaCaT cells and 75% on Caco-2 cells. The fabricated films demonstrated non-cytotoxicity in vitro and show potential suitability for packaging applications. Moreover, the application of chitosan films on tomatoes and green grapes showed the lowest weight loss compared to the control film at room temperature (23 °C) for up to 7 and 9 days, respectively. Additionally, these films also led to a reduction in total volatile basic nitrogen (TVBN) levels in the preservation of pearl fish fillets, indicating the potential for shelf-life extension.
Sustainability spotlightThis research focuses on the development of functional, non-toxic, and sustainable active packaging films made from chitosan blended with polyvinyl alcohol, chitooligosaccharides, and gallic acid. The resulting active films demonstrated strong antioxidant properties against DPPH and ABTS radicals. Additionally, they displayed significant antimicrobial activity against E. coli, L. innocua, and S. cerevisiae, along with strong biocompatibility with HaCaT and Caco-2 cells. These films effectively reduced weight loss in tomatoes and grapes, as well as minimised total volatile basic nitrogen production during the preservation of pearl fish fillets, highlighting their potential as functional packaging solutions for extending the shelf life of perishable foods. |
1 Introduction
Microbial contamination is a common phenomenon during the storage of perishable foods, leading to degraded food quality and a threat to consumers' health.1 It is estimated that over 200 million tons of food could be wasted by 2050 due to improper packaging and the short shelf-life of perishable food items.2 Additionally, the use of petroleum-based materials in food packaging can lead to significant environmental pollution, posing a threat to both terrestrial and aquatic life.3 Moreover, the use of plastic in food packaging generates microplastics, which accumulate in living terrestrial and aquatic environments through the food chain and enter humans through biomagnification.4 It is reported that microplastics have been detected in human blood, which can cause severe health risks.5 In this regard, using biodegradable and functional food packaging materials in the food industry may protect food by preventing contamination, reducing food waste, ensuring food safety, and contributing to the United Nations' Sustainable Development Goals (SDGs).6,7Several polysaccharides, including chitosan, pectin, starch, and alginate, are considered suitable for developing active food packaging materials.8 Among them, chitosan is gaining interest in functional packaging materials due to its film-forming capacity, antioxidant and antimicrobial activity, non-toxicity, biodegradability, and biocompatibility.9,10 Although chitosan has functional properties in the development of packaging films, it still has some drawbacks, including insufficient antioxidant and antimicrobial abilities, which greatly limit the application of neat chitosan in active food packaging.11,12 Additionally, the incorporation of polyvinyl alcohol (PVA) is increasing in the development of active chitosan films due to its excellent film-forming ability, biocompatibility, and improved flexibility, although it offers fewer functional properties. Therefore, chitosan derivatives and phenolic compounds are incorporated to fabricate functional chitosan films.13 Chitooligosaccharide (COS) and gallic acid are highly water-soluble and obtained from the enzymatic depolymerisation of chitosan and secondary metabolites in plant materials (e.g., grapes, apples, blueberries, tea, etc.), respectively.14 Studies have reported that COS and gallic acid possess several functional activities, such as antioxidant, antimicrobial, and antiviral properties.15–17 The incorporation of COS in the development of active chitosan films, through conjugation with caffeic acid, showed potential antioxidant activity, particularly in scavenging DPPH radicals.18 Additionally, the combination of gallic acid with corn starch and pullulan in the production of functional films demonstrated both DPPH radical scavenging properties and antimicrobial activity against Escherichia coli, Staphylococcus aureus, and Pseudomonas aeruginosa.19 Hence, there has been growing interest in including COS and gallic acid in developing active food packaging materials.
In general, active food packaging materials come into direct contact with food and release bioactive compounds to extend product shelf life by reducing lipid oxidation and microbial growth.20,21 Even though using biomaterials in the development of packaging films is safe, the interaction of raw materials during the film's synthesis process could alter the packaging film's biocompatibility patterns.22 Therefore, the cytotoxicity patterns of packaging films need to be tested before practical application for extending food shelf-life. In addition, the packaging materials used in food applications should not release any toxic compounds that could harm human health, as stated by the Food Safety Act, 1991, in EC 1935/2004.23 Thus, the main objective of the current study is to explore the antioxidant, antimicrobial, and cytotoxicity patterns of developed chitosan films containing polyvinyl alcohol, COS, and gallic acid.
2 Materials and methods
2.1 Materials
Chitosan (91% degree of deacetylation and 503 kDa molecular weight, Mw) was acquired from Weseta International in Shanghai, China. Chitooligosaccharide (COS) was synthesised following the method of Rajabi et al.24 Polyvinyl alcohol (≥99% hydrolysed and 89![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–98
000–98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1 Mw) and gallic acid (97.5% and 170 g mol−1 Mw) were provided by Sigma-Aldrich, New Zealand. TPTZ (2,4,6-Tris(2-pyridyl)-s-triazine), Mw: 312.33, and ≥98%), ABTS (2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid, Mw: 548.68 g mol−1, and ≥98%), and DPPH (2,2-diphenyl-1-picrylhydrazyl and molecular weight: 394.32) were obtained from Sigma-Aldrich, Switzerland, Canada, and Germany respectively. Potassium persulfate (Mw: 270.32 and ≥99%) was procured from Sigma-Aldrich. Methanol (CAS-No.: 67-56-1, >99.8%, and Mw: 32.04) was supplied by Fisher Scientific, UK, Ltd, India. Glacial acetic acid (CAS No.: 64-19-7 and >99.7%), sodium acetate trihydrate (Mw: 136.08, and 99% from Biolab (Aust) Ltd), ferrous sulphate (CAS No.: 7782-63-0 and 99%), iron(III) chloride hexahydrate (CAS No.: 10025-77-1, Mw: 270.30 g mol−1, and 97%) were also obtained from Sigma-Aldrich, USA, for this study. Escherichia coli (ESR 916), Saccharomyces cerevisiae (ICMP 10067), and Listeria innocua (ESR 3024) were acquired from the Microbiological Lab culture collection of the Department of Food Science, University of Otago. Luria broth (LB), nutrient yeast peptone dextrose broth (YPD), and tryptic soy broth (TSB) were supplied by Becton, Dickinson, and Company, Le Pont de Claix, France. The Caco-2 cells (P6, human colon cells) and HaCaT cells (P38, human keratinocyte skin cells) were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA). DMEM (Dulbecco's modified Eagle's medium), FBS (fetal bovine serum), anti–anti (antibiotic–antimycotic), 0.25% trypsin-EDTA, DPBS (Dulbecco's phosphate buffered saline), calcein AM, and propidium iodide were obtained from Gibco (Life Technologies Corporation, Grand Island, USA). Additionally, ethylene tetrazolium bromide (3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H, MTT) was purchased from Thermo Fisher Scientific (Life Technologies Corporation, Eugene, USA), and dimethyl sulfoxide (CAS No.: 67-68-5, molecular weight: 78.13 g mol−1, and ≥99.7%, DMSO) was procured from Sigma-Aldrich, USA. Potassium carbonate anhydrous (K2CO3, Mw: 138.21, 99% assay) was purchased from M & B Laboratory Chemicals (May & Baker Ltd, Dagenham, England). Methyl red (C15H15N3O2), bromocresol green (C21H14Br4O5S), and phenolphthalein (C20H14O4) were acquired from Hopkin & Williams Ltd, Chadwell Heath, Essex, England. Boric acid (H3BO3, Mw: 61.83, 99.5% assay) was supplied by Ajax Chemicals, Auburn, Australia. Analytical grade ethanol (C2H5OH, 99.5%) was procured from the Department of Chemistry, University of Otago. Analytical reagent grade hydrochloric acid (HCl, Mw: 36.46, CAS-No.: 7647-01-0, and 37%) was supplied by Fisher Scientific, UK. Sodium hydroxide (NaOH, CAS-No.: 1310-73-2, Mw: 40, and >98% assay) was provided by Sigma-Aldrich, New Zealand. Fresh tomatoes, green grapes, and pearl fish fillets were purchased from the local supermarkets in Dunedin, New Zealand.
000 g mol−1 Mw) and gallic acid (97.5% and 170 g mol−1 Mw) were provided by Sigma-Aldrich, New Zealand. TPTZ (2,4,6-Tris(2-pyridyl)-s-triazine), Mw: 312.33, and ≥98%), ABTS (2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid, Mw: 548.68 g mol−1, and ≥98%), and DPPH (2,2-diphenyl-1-picrylhydrazyl and molecular weight: 394.32) were obtained from Sigma-Aldrich, Switzerland, Canada, and Germany respectively. Potassium persulfate (Mw: 270.32 and ≥99%) was procured from Sigma-Aldrich. Methanol (CAS-No.: 67-56-1, >99.8%, and Mw: 32.04) was supplied by Fisher Scientific, UK, Ltd, India. Glacial acetic acid (CAS No.: 64-19-7 and >99.7%), sodium acetate trihydrate (Mw: 136.08, and 99% from Biolab (Aust) Ltd), ferrous sulphate (CAS No.: 7782-63-0 and 99%), iron(III) chloride hexahydrate (CAS No.: 10025-77-1, Mw: 270.30 g mol−1, and 97%) were also obtained from Sigma-Aldrich, USA, for this study. Escherichia coli (ESR 916), Saccharomyces cerevisiae (ICMP 10067), and Listeria innocua (ESR 3024) were acquired from the Microbiological Lab culture collection of the Department of Food Science, University of Otago. Luria broth (LB), nutrient yeast peptone dextrose broth (YPD), and tryptic soy broth (TSB) were supplied by Becton, Dickinson, and Company, Le Pont de Claix, France. The Caco-2 cells (P6, human colon cells) and HaCaT cells (P38, human keratinocyte skin cells) were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA). DMEM (Dulbecco's modified Eagle's medium), FBS (fetal bovine serum), anti–anti (antibiotic–antimycotic), 0.25% trypsin-EDTA, DPBS (Dulbecco's phosphate buffered saline), calcein AM, and propidium iodide were obtained from Gibco (Life Technologies Corporation, Grand Island, USA). Additionally, ethylene tetrazolium bromide (3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H, MTT) was purchased from Thermo Fisher Scientific (Life Technologies Corporation, Eugene, USA), and dimethyl sulfoxide (CAS No.: 67-68-5, molecular weight: 78.13 g mol−1, and ≥99.7%, DMSO) was procured from Sigma-Aldrich, USA. Potassium carbonate anhydrous (K2CO3, Mw: 138.21, 99% assay) was purchased from M & B Laboratory Chemicals (May & Baker Ltd, Dagenham, England). Methyl red (C15H15N3O2), bromocresol green (C21H14Br4O5S), and phenolphthalein (C20H14O4) were acquired from Hopkin & Williams Ltd, Chadwell Heath, Essex, England. Boric acid (H3BO3, Mw: 61.83, 99.5% assay) was supplied by Ajax Chemicals, Auburn, Australia. Analytical grade ethanol (C2H5OH, 99.5%) was procured from the Department of Chemistry, University of Otago. Analytical reagent grade hydrochloric acid (HCl, Mw: 36.46, CAS-No.: 7647-01-0, and 37%) was supplied by Fisher Scientific, UK. Sodium hydroxide (NaOH, CAS-No.: 1310-73-2, Mw: 40, and >98% assay) was provided by Sigma-Aldrich, New Zealand. Fresh tomatoes, green grapes, and pearl fish fillets were purchased from the local supermarkets in Dunedin, New Zealand.
2.2 Chitosan film fabrication
The chitosan films were fabricated using the solvent casting method, following the process established by Bhowmik et al.25 Briefly, 2% chitosan (CH) solution was obtained by mixing 0.4 g of CH in 20 mL of 2% acetic acid solution. After that, 0.2 g (50% based on CH) of polyvinyl alcohol (PVA), 0.02–0.04 g (5–10% based on CH) of chitooligosaccharide (COS), and 0.02–0.04 g (5–10% based on CH) of gallic acid (GA) were added and stirred for 30 min at room temperature (23 °C). Then, the film-forming solution was poured into square Petri dishes (10 cm × 10 cm) and kept for 72 h at 23 °C. The fabricated films were manually separated from the Petri dishes. The film containing only chitosan was regarded as the CH film, followed by CP2 (CH + 0.2 g PVA), CP5 (CH + 0.2 g PVA + 0.02 g COS), CP6 (CH + 0.2 g PVA + 0.04 g COS), CP7 (CH + 0.2 g PVA + 0.02 g GA), CP8 (CH + 0.2 g PVA + 0.04 g GA), CP9 (CH + 0.2 g PVA + 0.02 g COS + 0.02 g GA), CP10 (CH + 0.2 g PVA + 0.04 g COS + 0.04 g GA), CP11 (CH + 0.2 g PVA + 0.04 g COS + 0.02 g GA) and CP12 (CH + 0.2 g PVA + 0.02 g COS + 0.02 g GA) films based on previous work (Table S1), where the physical, mechanical, structural, thermal, and biodegradation properties of the films were included.252.3 Antioxidant activity of developed chitosan films
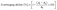 | (1) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 with a 50% methanol solution to create a workable solution. The absorbance of the working solution at 734 nm using a UV/Visible Spectrophotometer (Ultrospec 3300 Pro, Biochrom Ltd, Cambridge, England) was measured and found to be 0.96 (<1). After that, 1.5 mL of working solution and 5 mg of film sample were mixed, and the mixture was left in the dark. At various times (6 min–4 h), the absorbance at 734 nm was measured. Antioxidant ability was calculated in triplicate using eqn (2).
10 with a 50% methanol solution to create a workable solution. The absorbance of the working solution at 734 nm using a UV/Visible Spectrophotometer (Ultrospec 3300 Pro, Biochrom Ltd, Cambridge, England) was measured and found to be 0.96 (<1). After that, 1.5 mL of working solution and 5 mg of film sample were mixed, and the mixture was left in the dark. At various times (6 min–4 h), the absorbance at 734 nm was measured. Antioxidant ability was calculated in triplicate using eqn (2).
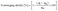 | (2) |
2.4 Antimicrobial properties of films
The antimicrobial activities and the integrity of microbial cell membranes of chitosan films were assessed towards Gram-positive bacteria (Listeria innocua), Gram-negative bacteria (Escherichia coli), and yeast (Saccharomyces cerevisiae) following the method of Bi et al.29 and Sadiq et al.30 with some modifications. E. coli and L. innocua were cultured in liquid Luria broth and tryptic soy broth media at 37 °C to the logarithmic phase, and S. cerevisiae was cultured in yeast peptone dextrose medium at 30 °C. After that, 0.2 mL microbial suspensions (with an initial absorbance of 1 at OD600) of E. coli, S. cerevisiae, and L. innocua were diluted 50 times using 10 mL of LB, YPD, and TSB medium, respectively, and incubated with the films (6 mm × 6 mm) with 0.2 mL liquid medium for 24 h. Then, the optical density at 600 nm was measured using a plate reader (CLARIOstarPlus, BMG LABTECH, Germany) at different time intervals (0–24 h), and the antimicrobial rate was analysed in triplicate using eqn (3).| Antimicrobial rate (%) = (OD1 − OD2)/OD1 × 100 | (3) |
To assess the integrity of microbial cell membranes, 0.15 mL of microbial suspension was obtained after 24 hours, and it was centrifuged (1–16 K, SIGMA, Germany) at 11000×g for five minutes. Utilising 0.1 mL of the supernatant, the quantities of cellular constituents were assessed as proposed by Bi et al.29 by determining the absorbance at 260 nm with a plate reader (CLARIOstarPlus, BMG LABTECH, Germany). The control group consisted of the microbial suspension supernatant that had not been film-treated.
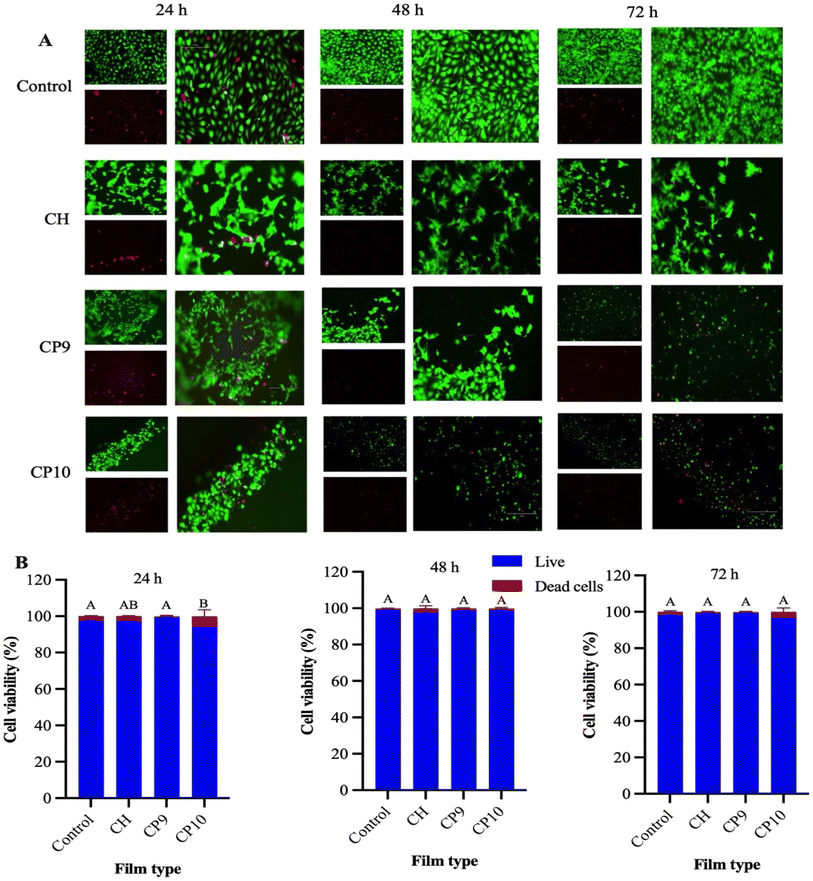
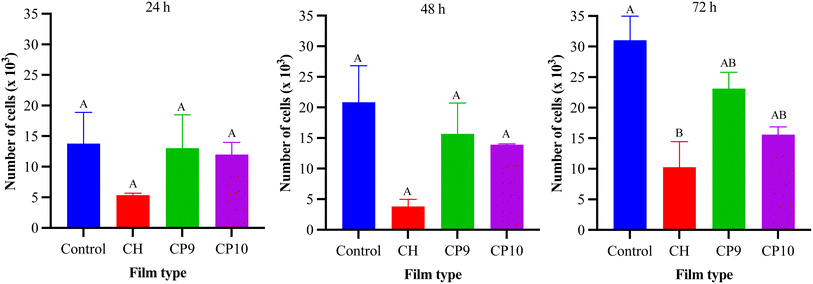
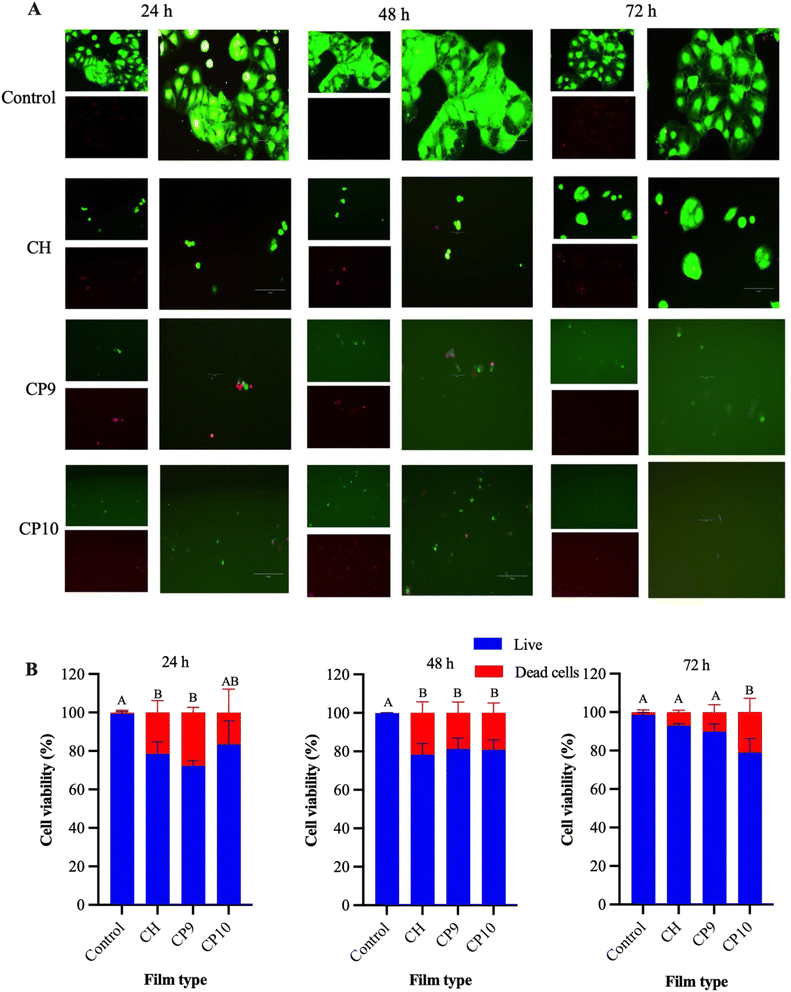
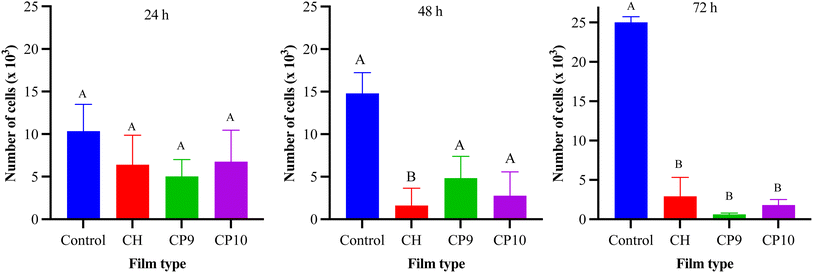
2.5 Cell assay, viability, and proliferation assays
| Cell viability (%) = Number of live cells/Total cell number × 100 | (4) |
2.6 Evaluation of storage quality of tomato and green grape
The storage qualities of tomatoes and green grapes were assessed as model food products to investigate the active properties of the developed films (CH, CP9, and CP10). The prepared tomatoes (3.2–3.5 g) and green grapes (3.1–3.3 g) were kept in a Petri dish (3.5 cm × 3.5 cm) covered with a lid. The films (1.3 cm × 1.3 cm) were inserted inside the Petri dish, and the setups containing tomatoes and grapes were stored for 7 and 9 days, respectively, at room temperature (23 °C, relative humidity 46%). Tomatoes and green grapes in the Petri dish without a film were regarded as the control, and those without a lid and film were designated as non-packaging (NP). The Petri dish was weighed every day, and the weight was recorded. The weight loss of tomatoes and grapes was measured in triplicate using eqn (5).36| Weight loss (%) = (W1 − W2)/W1 × 100 | (5) |
2.7 Evaluation of storage quality of pearl fish fillet
The purchased pearl fish fillets were cut (20–25 g) and kept in a Petri dish with films (1.3 cm × 1.3 cm) separately at room temperature (23 °C, relative humidity 46%). The pH, drip loss, and total volatile basic nitrogen (TVBN) were measured daily in triplicate to detect the storage quality of pearl fish fillets. The pearl fish fillet without a film was considered the control, and the one without a film and lid was regarded as the NP group.The pH of pearl fish fillet was detected following the method of Pang et al.37 with some modifications. Five (5) grams of pearl fish fillet were vigorously homogenised in 45 mL of distilled water. After that, the pH of the pearl fish fillet filtrate was measured in triplicate using a pH meter (HI 5222, Hanna Instruments, USA). Additionally, the drip loss of packaged pearl fish fillets with films in a Petri dish was assessed using the weighing method.36 The pearl fish fillets in a Petri dish were weighed daily using an analytical balance, and eqn (6) was used to calculate the drip loss.
| Drip loss (%) = (W1 − W2)/W1 × 100 | (6) |
The TVBN content of pearl fish fillets was measured following the method of Lee et al.38 with some modifications. Briefly, 5 g of ground pearl fish fillets were inserted in a 50 mL Falcon tube containing 45 mL distilled water and kept at room temperature (23 °C) for approximately 35 min. After that, Whatman No. 1 filter paper was used to filter the samples, and the supernatant was analysed. Before sample analysis, the Conway indicator solution was prepared by dissolving 66 mg of bromocresol green and 66 mg of methyl red in 100 mL of ethanol (99.5%). Additionally, a 0.01 N boric acid solution was prepared by dissolving 0.3 g boric acid in 150 mL of ethanol, and the volume was increased to 500 mL using 350 mL of distilled water. Also, a 50% potassium carbonate solution was made by dissolving 50 g of K2CO3 in 100 mL of distilled water. In the analysis of TVBN in pearl fish fillets, 1 mL of H3BO3 and 100 µL of indicator solution were included in the inner part of the Conway unit, and 1 mL of supernatant and 1 mL of K2CO3 solution were added in the outer chamber of the Conway unit. Then, the Conway unit was kept at 37 °C for 2 h in an oven (THERMOTEC 2000, Lower Hutt, New Zealand). After that, 0.01 N HCl was incorporated into the inner part of the Conway unit until the formation of the pink colour. The TVBN content was calculated in triplicate using eqn (7).
| TVBN (mg/100 g) = 0.14 × (b − a) × f × d/W × 100 | (7) |
2.8 Statistical analysis
The statistical analysis used GraphPad Prism version 9.4.1 (GraphPad Software, Boston, USA). The obtained data from at least three replicates are presented as mean ± standard deviation (SD). To look for significant differences (P ≤ 0.05) in the generated films, cell viability, and total cell number, Tukey's test and ordinary one-way analysis of variance (ANOVA) were employed.3 Results and discussion
3.1 Antioxidant activity of developed chitosan films
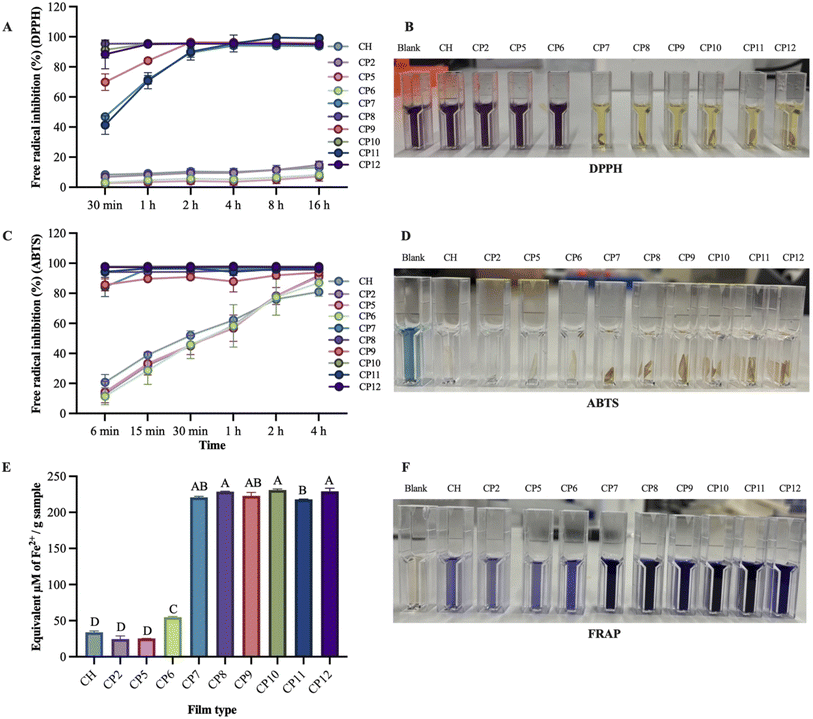
The antioxidant activity of fabricated chitosan films is presented in Fig. 1. In the DPPH scavenging assay, the fabricated films showed different activities during 0.5–16 h. Films CP8 (95.37 ± 0.57–95.51 ± 0.56%), CP10 (91.37 ± 5.52–95.38 ± 0.08%), and CP12 (88.30 ± 9.51–95.44 ± 0.25%) exhibited the highest DPPH scavenging activities with greater consistency during the time periods, whereas CP7 (46.80 ± 1.44–94.12 ± 1.85%), CP9 (69.82 ± 5.48–96.30 ± 0.04%), and CP11 (41.31 ± 6.14–99.48 ± 0.24%) demonstrated linearity in different time intervals. CH (8.31 ± 0.43–13.13 ± 4.26%), CP2 (6.88 ± 0.17–14.80 ± 2.45%), CP5 (2.76 ± 1.79–7.16 ± 3.13%), and CP6 (3.13 ± 0.21–8.24 ± 2%) films presented the lowest percentage of DPPH scavenging activities. These research findings are consistent with Zhang et al.,39 who reported that a neat chitosan film (15%) demonstrated the lowest DPPH scavenging capacity compared to a gallic acid-loaded film (50–83%), indicating that the addition of gallic acid in the chitosan film enhanced antioxidant activity due to the interaction between the native antioxidant properties of gallic acid moieties and the chitosan backbone. The addition of PVA and COS in the chitosan film did not improve the DPPH radical scavenging activity in the chitosan films. However, the inclusion of gallic acid and COS significantly enhanced the DPPH radical scavenging capacity of the chitosan–PVA films labelled CP9–CP12. These findings align with the research conducted by Yuan et al.,18 who elucidated that COS and caffeic acid-loaded chitosan films significantly increased scavenging ability (93.43%) compared to the control film (10%). Additionally, similar studies reported by Lee et al.40 indicated that including gallic acid in the preparation of chitosan active films exhibited strong scavenging properties (95.7%) due to the presence of a phenolic hydroxyl group.Similarly, in the ABTS scavenging assay, CP10 (97.85 ± 1.01–98.06 ± 0.91%), CP12 (97.41 ± 0.69–97.57 ± 0.53%), CP8 (94.12 ± 5.05–95.79 ± 2.63%), and CP11 (94.34 ± 2.87–96.68 ± 0.38%) showed the highest percentage of ABTS scavenging properties, whereas CP7 (84.23 ± 9.09–96.50 ± 0.38%) and CP9 (85.60 ± 5.59–93.87 ± 0.67%) demonstrated different activity with linearity in the experimental time, followed by CH (20.76 ± 7.30–80.99 ± 1.28%), CP2 (12.80 ± 0.07–91.90 ± 4.81%), CP5 (14.23 ± 10.02–90.94 ± 4.81%), and CP6 (11.48 ± 8–87.06 ± 12.72%). Similar findings were stated by Zhao et al.,11 who described chitosan films (22%) as having the lowest ABTS scavenging activity, whereas adding gallic acid to chitosan films enhanced hydrogen supply capacity, improving ABTS radical scavenging capacity (85%).
Likewise, the highest FRAP (equivalent µM Fe2+ per g sample) was obtained from the CP10 (230.93 ± 1.42) film, followed by CP12 (229.13 ± 4.20), CP8 (228.69 ± 0.49), CP9 (222.88 ± 4.76), CP7 (220.73 ± 1.36) and CP11 (218.11 ± 0.24), whereas the lowest FRAP was exhibited by CP6 (54.66 ± 1.11), CH (33.88 ± 1.79), CP5 (25.04 ± 0.06), and CP2 (24.43 ± 4.39) films. Adding COS and gallic acid to the chitosan–PVA film enhanced its ability to reduce Fe3+ to Fe2+, potentially indicating synergistic effects. These findings align with those of Gulzar et al.,28 who reported that adding COS and tannic acid to chitosan films demonstrated the highest FRAP activity, which depends on the amounts of COS and tannic acids in film development. A film with the capacity to convert ferric to ferrous ions and delay the production of free radicals might delay the lipid oxidation of foods and avoid oxidative stress.41 The highest FRAP activities by incorporating COS and gallic acid reconfirmed their active role in developing chitosan films with effective antioxidant properties.
3.2 Antimicrobial properties of fabricated films
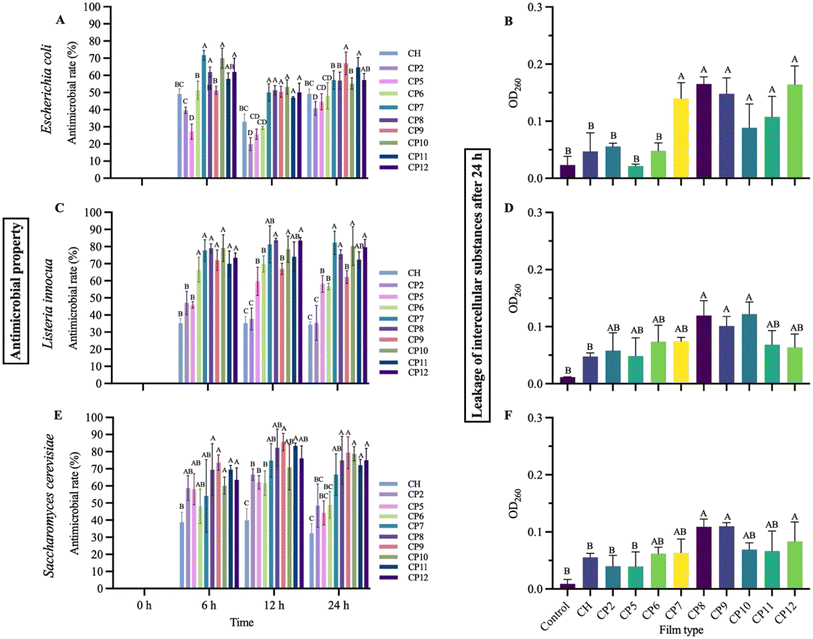
The antimicrobial activity of the developed chitosan film towards Escherichia coli (Gram-negative bacteria), Listeria innocua (Gram-positive bacteria), and Saccharomyces cerevisiae (yeast) is presented in Fig. 2. Overall, the COS and gallic acid loaded chitosan films showed higher antimicrobial activity and leakage of greater intracellular substances compared to the neat chitosan film. These findings suggested that adding COS and gallic acid effectively improved the antimicrobial properties of the chitosan film. The inclusion of COS and gallic acid in the chitosan film resulted in antimicrobial capacities of 59.49 ± 9.17% (CP10), 79.29 ± 0.94% (CP10), and 79.55 ± 8.45% (CP9). The findings of the study are in line with Zhao et al.,11 who demonstrated that the addition of gallic acid improved antimicrobial activity towards Gram-positive (Staphylococcus aureus) and Gram-negative (Escherichia coli) bacteria due to the synergistic effect of the positively charged amino group of chitosan and gallic acid, which disrupts the bacterial cell wall and results in bacteria death. Adding PVA and COS alone in the chitosan film did not significantly improve the antimicrobial properties of the chitosan film. These findings are consistent with those of Gulzar et al.28 and Kanatt et al.,42 who reported that elevated concentrations of COS in the film failed to inhibit the growth of microbes (Listeria monocytogenes and Escherichia coli) effectively, and PVA in the chitosan film did not show any significant changes in microbial growth (Staphylococcus aureus and Bacillus cereus), respectively. However, combining COS and gallic acid in the chitosan film improved antimicrobial activity in the current study due to the synergistic interaction with chitosan. Additionally, the release of cellular components is higher in COS and gallic acid-loaded films (CP7-CP12 (Escherichia coli), CP8-CP10 (Listeria innocua), and CP8, CP9, and CP12 (Saccharomyces cerevisiae)) than in the chitosan film. This could have happened due to the presence of an abundance of free amino groups, contributed by COS, in the film matrix, leading to electrostatic interactions with the phosphate groups of the microbial cell membrane to damage membrane integrity, resulting in the release of intercellular substances.29,43 Additionally, the potential interaction of GA with the chitosan film matrix caused membrane pores of microbes to destroy cell membranes and enhance their cell permeability, resulting in leakage of cell constituents.44 Also, we hypothesise that the inclusion of COS and gallic acid in the chitosan film acts synergistically to improve membrane permeability and physical disruption of microbial cells, leading to death. These findings are consistent with those of Hou et al.,17 who reported that incorporating gallic acid and tannic acid into a cellulose/chitosan film matrix created an active film with enhanced antimicrobial activity against Escherichia coli and Staphylococcus aureus due to their synergistic interactions. Therefore, the OD260 values of COS and gallic acid-loaded chitosan films were higher than those of the neat chitosan film, reconfirming the enhancement of antimicrobial activity in the fabricated films of the current study.3.3 Cytotoxicity patterns of chitosan films
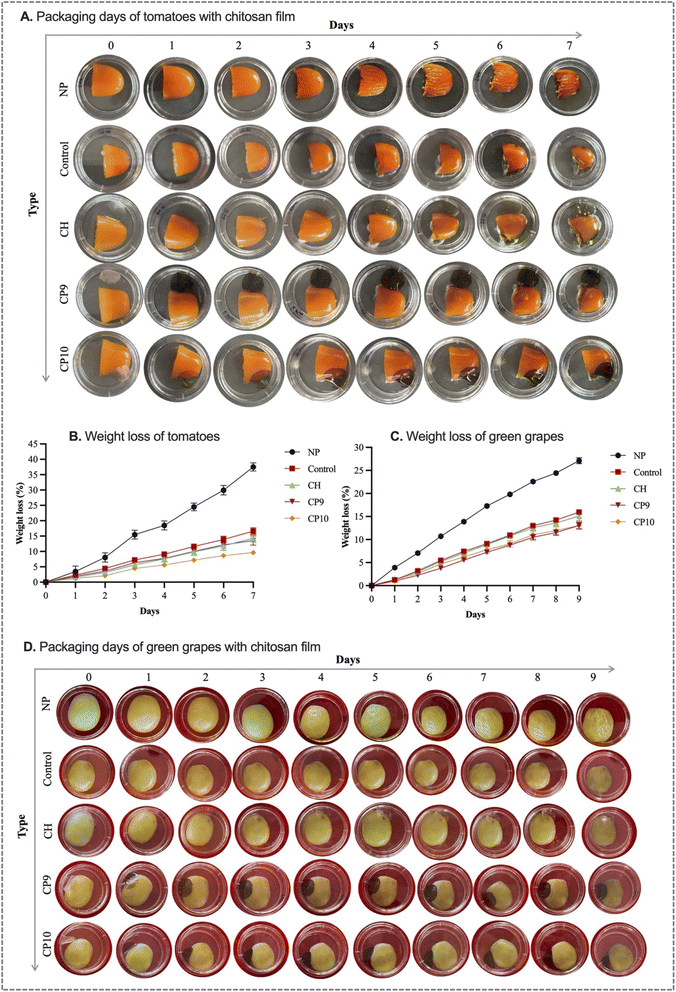
3.4 Application of films in tomato and green grape packaging
The appearance and weight loss of tomatoes under different pack-film conditions are presented in Fig. 7A and B. It was observed that the NP tomatoes gradually lost their appearance, and the surface became wrinkly due to the loss of turgidity in the cells. However, no microbial growth was seen in NP tomatoes compared to other film-packed groups. This observation could be explained by the fact that the NP samples, being without a lid and film, did not accumulate moisture droplets from the respiration of the tomatoes. The development of humidity inside the film-packed samples (control-CP10) created a suitable atmosphere for the growth of microbes. In the case of the control group, microbial growth was observed on day 2. In contrast, the development of microbes in the film-packed tomatoes with CH, CP9, and CP10 films was detected on days 3, 4, and 5, respectively. In other words, films such as CP10 delayed the onset of microbial growth. The current research findings confirm the point that the fabricated films had antimicrobial ability that extended the shelf life of the tomatoes. On day 1, all the tomatoes (NK: 3.48 ± 1.76%, control: 2.30 ± 0.38%, CH: 1.64 ± 0.38%, CP9: 1.94 ± 0.04%, CP10: 1.25 ± 0.07%) showed more or less similar weight loss. However, by day 7, the NK group demonstrated the highest weight loss (37.5 ± 1.32%), followed by the control (16.57 ± 1.11%), CH (14.29 ± 1.25%), CP9 (13.56 ± 1.5%), and CP10 (9.63 ± 0.55%). These findings are similar to those of Gasti et al.,54 who reported unpacked green chillies demonstrating the highest weight loss compared to chitosan and gallic acid-loaded chitosan film-packed green chillies.A similar observation was made for the grape samples. Non-packaged green grapes demonstrated the highest weight loss (27.11 ± 0.62%) and shrivelling during the storage periods compared to film-packed green grapes (control: 15.95 ± 0.25%, CH: 14.77 ± 1.2%, CP9: 13.1 ± 0.94%, and CP10: 13.54 ± 0.47%) (Fig. 7C and D). The current research findings align with those of Zhao et al.,11 who observed the highest percentage of fresh-cut apple weight loss in an unpacked group compared to apple storage with the chitosan film and PVA-gallic acid incorporated chitosan film. Another study reported that non-packaged kiwifruit demonstrated shrinking and the highest weight loss compared to packing kiwifruit with the polylactic acid film containing chitosan and alizarin.36 The growth of microbes was not observed with the naked eye in green grapes during the entire storage. This could be possible due to the presence of phenolic compounds, including tannins, anthocyanins, and flavonols, in grapes, which have preventive ability towards microbial growth.55 Sun et al.56 reported that unpacked grapes (Vitis vinifera L. Kyoho) demonstrated microbial growth and spoilage signs after 14 days of storage periods. In contrast, grapes packaged with chitosan enriched with montmorillonite and lauroyl arginate ethyl showed no microbial growth up to 20 days of storage periods.
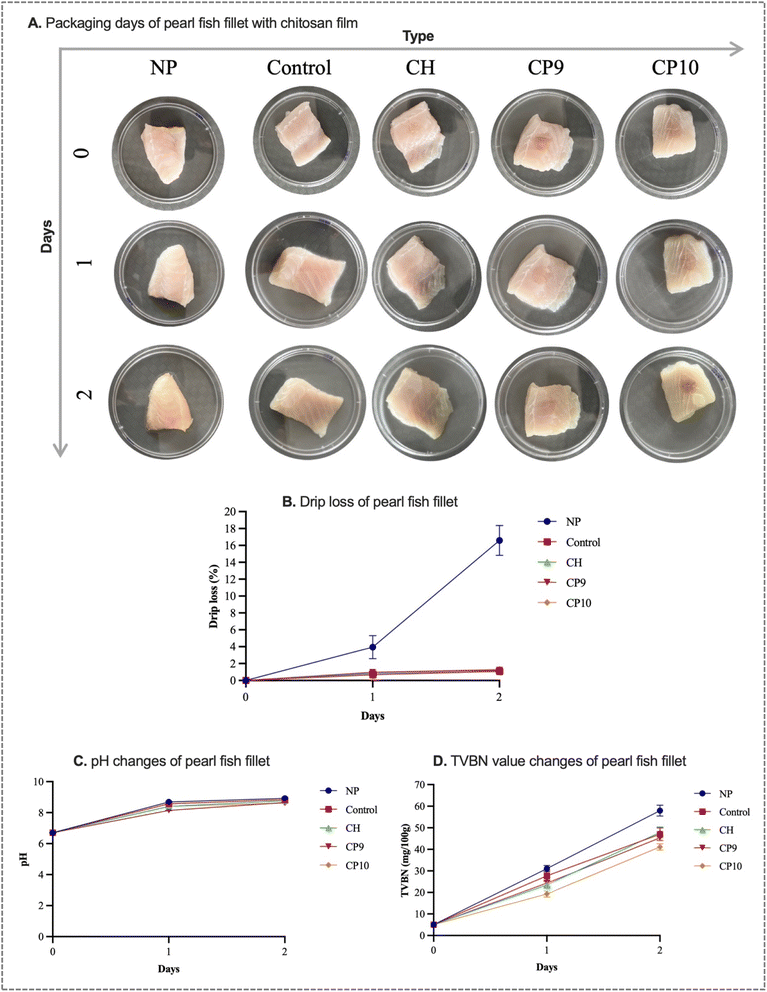
3.5 Application of films in pearl fish fillet packaging
The appearance, drip loss, pH, and TVBN value of pearl fish fillets under different film-packaging conditions are presented in Fig. 8. It can be seen that the pearl fish fillets look shiny and show a ‘fresh’ colour on day 0 compared to day 2. Also, the shrinkage of pearl fish fillets was observed from day 2, especially in the NP group (Fig. 8A). There were significant differences in the drip loss of pearl fish fillets between the NP group and the film-packed group (Fig. 8B). The non-packaging and control pearl fish fillets demonstrated 16.27% and 3.33% drip loss on day 2, respectively, whereas film-packed group showed below 3.22% drip loss. However, there are no significant differences in the drip loss of pearl fish fillets between the control and film-packed groups. This observation could be attributed to the fact that, in the current study, a small portion of the films was used, as compared to other studies where whole fish fillets were wrapped in the packaging film.36,57The pH of fresh pearl fish fillets was 6.7 ± 0.02, and it can be seen from Fig. 8C that the pH of both the film-packed and NP groups of pearl fish fillets increased during storage. The pH of the non-packaging group pearl fish was increased to 8.92 ± 0.03 on day 2, followed by the control (8.82 ± 0.01), CH (8.76 ± 0.03), CP9 (8.64 ± 0.01), and CP10 (8.61 ± 0.01) films. The pH value of pearl fish fillets was significantly lower in the CP9 and CP10 film-packed fish compared to the NP, control, and CH packaging groups. The findings indicate that CP9 and CP10 films in packaging pearl fish could minimise the microorganism action and inhibit chemical deteriorative action, resulting in lower pH compared to other groups. The findings are consistent with the work of Yan et al.,58 Amaregouda et al.,59 and Wu et al.36
The TVBN (mg/100 g) value of fresh pearl fish fillets was 5.04 ± 0.01. The TVBN value of NP and film-packed pearl fish fillets was increased during storage (Fig. 8D). The TVBN value of NP (31.08 ± 1.45) and control (27.72 ± 2.52) pearl fish fillets was significantly higher than that of CH (23.51 ± 1.44), CP9 (24.36 ± 1.44), and CP10 (19.32 ± 1.46) film-packed fish fillets on day 1. However, the TVBN value increased to 57.96, 47.04, and 47.88 on day 2 in NP, control, and CH-packed pearl fish fillets, respectively, which is significantly higher than the changes observed in CP9 (45.36) and CP10 (41.16) films. The findings imply that fabricated films could minimise the decomposition of pearl fish fillets by diminishing antimicrobial action and lipid oxidation.60 The findings in this study align with those of Chen et al.61 and Dong et al.,62 who developed active packaging films for hairtail fish and shrimp for shelf life extension and identified lowered TVBN compared to the control, respectively. Also, several research articles stated that fish is considered spoiled when the TVBN value exceeds 30 mg/100 g.63,64 In the current study, the TVBN value of pearl fish fillets was much lower in CP9 and CP10 film-packed fish on day 1; however, it exceeded the 30 mg/100 g cutoff value on day 2. These findings indicate that the films can potentially extend the shelf life of pearl fish fillets.
4 Conclusion
In the present research work, the functional properties of chitosan films containing PVA, COS, and gallic acid were investigated and evaluated. The COS and gallic acid-loaded chitosan–PVA films showed the highest scavenging capacity towards DPPH and ABTS radicals, and the highest FRAP was obtained from the film containing 10% COS and 10% gallic acid (CP10). In addition, the CP10 film demonstrated the highest antimicrobial efficacy towards Escherichia coli and Listeria innocua, and CP9 exhibited the highest leakage of cellular components towards Saccharomyces cerevisiae. Also, the highest cell viability of HaCaT and Caco-2 cells was observed on CH and CP9 films compared to CP10. Moreover, the application of CP9 and CP10 films on tomatoes and green grapes showed the lowest weight loss, and pearl fish fillets demonstrated the lowest pH and TVBN compared to CH, control, and non-packaging groups. Based on current research findings, chitosan films containing PVA, COS, and gallic acid could be considered functional and suitable packaging materials to extend the shelf life of perishable foods. Further studies may be necessary to examine the release profile of COS and gallic acid, focusing on their effects on cellular responses, microbial load data, and the quality indices of tomatoes and green grapes. Furthermore, a shelf ageing study of the packaging film could be conducted to evaluate the long-term stability of its functional properties during storage. This research could improve the understanding of the film's functional performance and the synergistic interactions between COS and gallic acid in practical food packaging applications.Author contributions
Shuva Bhowmik: conceptualization, investigation, methodology, data curation, visualization, writing – original draft. Dominic Agyei: supervision, writing – review & editing. Azam Ali: supervision, conceptualization, project administration, writing – review & editing.Conflicts of interest
The authors declare that they have no conflicts of interest.Data availability
The authors affirm that the data supporting this study's findings are available within the research article and will also be shared upon request.Supplementary information (SI) is available. See DOI: https://doi.org/10.1039/d5fb00775e.
Acknowledgements
Shuva Bhowmik would like to acknowledge the University of Otago, Dunedin, New Zealand, for supporting the PhD research and studies through the Otago Doctoral Scholarship.References
- F. Ghaderi, A. Shakerian, Z. Mashak, E. Rahimi and S. M. Jafari, J. Food Meas. Char., 2024, 1–18 Search PubMed.
- V. Guillard, S. Gaucel, C. Fornaciari, H. Angellier-Coussy, P. Buche and N. Gontard, Front. Nutr., 2018, 5, 121 CrossRef PubMed.
- Y. Yin and M. W. Woo, Sustainable Food Technol., 2024, 2(3), 548–566 RSC.
- K. Ma, F. Li, T. Zhe, X. Sun, X. Zhang, P. Wan, H. Na, J. Zhao and L. Wang, Food Chem., 2024, 435, 137552 CrossRef CAS PubMed.
- H. A. Leslie, M. J. Van Velzen, S. H. Brandsma, A. D. Vethaak, J. J. Garcia-Vallejo and M. H. Lamoree, Environ. Int., 2022, 163, 107199 CrossRef CAS PubMed.
- I. Hamed, A. N. Jakobsen and J. Lerfall, Compr. Rev. Food Sci. Food Saf., 2022, 21, 198–226 CrossRef PubMed.
- J. Paul, J. Jacob, M. Mahmud, M. Vaka, S. G. Krishnan, A. Arifutzzaman, D. Thesiya, T. Xiong, K. Kadirgama and J. Selvaraj, Int. J. Biol. Macromol., 2024, 130850 CrossRef CAS PubMed.
- Q.-B. Yao, F. Huang, Y.-H. Lu, J.-M. Huang, M. Ali, X.-Z. Jia, X.-A. Zeng and Y.-Y. Huang, Trends Food Sci. Technol., 2024, 104390 CrossRef CAS.
- S. Bhowmik, D. Agyei and A. Ali, Food Packag. Shelf Life, 2022, 34, 100962 CrossRef CAS.
- A. A. Hunashyal, S. P. Masti, L. K. Kurabetta, M. N. Gunaki, S. Madihalli, J. P. Pinto, M. B. Megalamani, B. Thokchom, R. B. Yarajarla and R. B. Chougale, Sustainable Food Technol., 2025, 3, 2088–2107 RSC.
- Y. Zhao, L. Yang, M. Xu, H. Wang, X. Gao, B. Niu and W. Li, Int. J. Biol. Macromol., 2022, 222, 2987–3000 CrossRef CAS PubMed.
- J. P. Pinto, M. H. Anandalli, A. A. Hunashyal, Priyadarshini, S. P. Masti, R. B. Chougale, V. Gudihal and R. F. Bhajantri, J. Mater. Sci.: Mater. Electron., 2025, 36, 1035 CrossRef CAS.
- Q. Zhou, W. Lan and J. Xie, Int. J. Biol. Macromol., 2024, 254, 127917 CrossRef CAS PubMed.
- A. Singh, A. Mittal and S. Benjakul, Food Rev. Int., 2023, 39, 2297–2319 CrossRef CAS.
- Y. Sun, J. Cui, L. Tian, Y. Mi and Z. Guo, Mar. Drugs, 2023, 21, 535 CrossRef CAS PubMed.
- W. Weian, Y. Yunxin, W. Ziyan, J. Qianzhou and G. Lvhua, Biomater. Sci., 2024, 12(6), 1405–1424 RSC.
- T. Hou, R. Venkatesan, T. Dhilipkumar, V. Mayakrishnan, C. J. Raorane, S. S. Sana, M. A. Ansari and S.-C. Kim, Int. J. Biol. Macromol., 2025, 285, 138276 CrossRef CAS PubMed.
- Y. Yuan, W. Tan, C. Lin, J. Zhang, Q. Li and Z. Guo, Food Hydrocolloids, 2023, 138, 108431 CrossRef CAS.
- M. Zhang, B. Yang, Z. Yuan, Q. Sheng, C. Jin, J. Qi, M. Yu, Y. Liu and G. Xiong, Food Chem.: X, 2023, 100782 CAS.
- E. Messinese, O. Pitirollo, M. Grimaldi, D. Milanese, C. Sciancalepore and A. Cavazza, Food Bioprocess Technol., 2024, 17, 606–627 CrossRef.
- M. N. Gunaki, S. P. Masti, L. K. Kurabetta, S. Madihalli, A. A. Hunashyal, R. B. Chougale, V. Holeyannavar and S. kumar Vootla, J. Environ. Chem. Eng., 2025, 118397 CrossRef CAS.
- B. Tian, J. Liu, W. Yang and J.-B. Wan, J. Agric. Food Chem., 2023, 71, 1325–1347 CrossRef CAS PubMed.
- N. Kumar, Pratibha, J. Prasad, A. Yadav, A. Upadhyay, Neeraj, S. Shukla, A. T. Petkoska, Heena and S. Suri, Food Eng. Rev., 2023, 15, 718–747 CrossRef CAS.
- M. Rajabi, J. Cabral, S. Saunderson and M. A. Ali, Carbohydr. Polym., 2022, 295, 119884 CrossRef CAS PubMed.
- S. Bhowmik, D. Agyei and A. Ali, Cellulose, 2024, 1–17 CAS.
- A. Riaz, S. Lei, H. M. S. Akhtar, P. Wan, D. Chen, S. Jabbar, M. Abid, M. M. Hashim and X. Zeng, Int. J. Biol. Macromol., 2018, 114, 547–555 CrossRef CAS PubMed.
- M. Božič, S. Gorgieva and V. Kokol, Carbohydr. Polym., 2012, 87, 2388–2398 CrossRef.
- S. Gulzar, M. Tagrida, K. Nilsuwan, T. Prodpran and S. Benjakul, Food Hydrocolloids, 2022, 133, 107916 CrossRef CAS.
- J. Bi, C. Tian, G.-L. Zhang, H. Hao and H.-M. Hou, Food Chem., 2021, 365, 130534 CrossRef CAS PubMed.
- M. B. Sadiq, J. Tarning, T. Z. Aye Cho and A. K. Anal, Molecules, 2017, 22, 47 CrossRef PubMed.
- S. S. Sali, M. L. Gould, M. Qasim and M. A. Ali, J. Mater. Chem. B, 2021, 9, 1557–1567 RSC.
- D. S. B. Anugrah, G. Delarosa, P. Wangker, R. Pramitasari and D. Subali, Packag. Technol. Sci., 2023, 36(8), 681–697 CrossRef CAS.
- M. L. Gould, J. T. Ratnayake, N. Ramesh, T. J. Powlay, O. J. Curnow, M. P. Staiger and G. J. Dias, J. Polym. Environ., 2023, 31, 1335–1350 CrossRef CAS.
- B. Mahaling, N. Pandala, H.-C. Wang and E. B. Lavik, ACS Bio Med Chem Au, 2022, 2, 499–508 CrossRef CAS PubMed.
- X. Deng, M. Gould, R. Katare and M. Ali, Biomed. Mater., 2024, 19, 055007 Search PubMed.
- Y. Wu, Y. Ma, Y. Gao, Y. Liu and C. Gao, Int. J. Biol. Macromol., 2022, 214, 348–359 CrossRef CAS PubMed.
- G. Pang, C. Zhou, X. Zhu, L. Chen, X. Guo and T. Kang, J. Food Saf., 2023, 43, e13045 CrossRef CAS.
- H. Lee, M. S. Kim, W.-H. Lee and B.-K. Cho, Sens. Actuators, B, 2018, 259, 532–539 CrossRef CAS.
- X. Zhang, J. Liu, C. Qian, J. Kan and C. Jin, Food Hydrocoll., 2019, 89, 1–10 CrossRef CAS.
- C. R. Lee, S. J. Lee, T. I. Kim, K. Chathuranga, J. S. Lee, S. Kim, M. H. Kim and W. H. Park, Food Chem., 2025, 463, 141322 CrossRef CAS PubMed.
- A. Mittal, A. Singh, S. Benjakul, T. Prodpran, K. Nilsuwan, N. Huda and K. de la Caba, Food Hydrocoll., 2021, 111, 106384 CrossRef CAS.
- S. R. Kanatt, M. Rao, S. Chawla and A. Sharma, Food Hydrocoll., 2012, 29, 290–297 CrossRef CAS.
- Z. Fang, W. Cong, H. Zhou, J. Zhang and M. Wang, J. Funct. Foods, 2024, 116, 106219 CrossRef CAS.
- S. Keyvani-Ghamsari, M. Rahimi and K. Khorsandi, Nutr. Food Sci., 2023, 11, 5856–5872 CrossRef CAS PubMed.
- X. Yang, B. Wang and D. Sha, ACS Appl. Polym. Mater., 2021, 3(8), 3867–3877 CrossRef CAS.
- Z. Riaz, S. Baddi, F. Gao and C.-L. Feng, Eur. Polym. J., 2024, 206, 112778 CrossRef CAS.
- Y. Liu, R. Wang, D. Wang, Z. Sun, F. Liu, D. Zhang and D. Wang, Food Hydrocolloids, 2022, 127, 107546 CrossRef CAS.
- S. C. Forester and A. L. Waterhouse, J. Agric. Food Chem., 2010, 58, 5320–5327 Search PubMed.
- I. Salcedo, C. Aguzzi, G. Sandri, M. C. Bonferoni, M. Mori, P. Cerezo, R. Sánchez, C. Viseras and C. Caramella, Appl. Clay Sci., 2012, 55, 131–137 CrossRef CAS.
- M. C. Di Santo, A. Alaimo, A. P. D. Rubio, R. De Matteo and O. E. Pérez, Biochem. Biophys. Rep., 2020, 24, 100842 Search PubMed.
- I. Y. Ho, A. Abdul Aziz and S. Mat Junit, Sci. Rep., 2020, 10, 9987 CrossRef CAS PubMed.
- K. Mu and D. D. Kitts, J. Agric. Food Chem., 2023, 71, 3022–3032 Search PubMed.
- E.-B. Ko, Y.-G. Jang, C.-W. Kim, R.-E. Go, H. K. Lee and K.-C. Choi, Biomol. Ther., 2021, 30, 151 Search PubMed.
- T. Gasti, S. Dixit, R. B. Chougale and S. P. Masti, Sustainable Food Technol., 2023, 1, 390–403 Search PubMed.
- S. Augustine, V. Kudachikar, V. Vanajakshi and R. Ravi, J. Food Sci. Technol., 2013, 50, 332–338 Search PubMed.
- Z. Sun, J. Hao, H. Yang and H. Chen, Food Bioprocess Technol., 2018, 11, 1853–1862 Search PubMed.
- L. Zhang, D. Yu, Y. Xu, Q. Jiang, W. Xia and D. Yu, Food Biosci., 2023, 54, 102941 Search PubMed.
- J. Yan, R. Cui, Y. Qin, L. Li and M. Yuan, Int. J. Biol. Macromol., 2021, 177, 328–336 Search PubMed.
- Y. Amaregouda, K. Kamanna and T. Gasti, Int. J. Biol. Macromol., 2022, 218, 799–815 Search PubMed.
- W.-Y. J. Kam, H. Mirhosseini, F. Abas, N. Hussain, S. Hedayatnia and H.-L. F. Chong, Food Control, 2018, 90, 66–72 Search PubMed.
- M. Chen, T. Yan, J. Huang, Y. Zhou and Y. Hu, Int. J. Biol. Macromol., 2021, 179, 90–100 CrossRef CAS PubMed.
- S. Dong, Y. Zhang, D. Lu, W. Gao, Q. Zhao and X. Shi, Food Packag. Shelf Life, 2023, 35, 101022 Search PubMed.
- F. Wang, C. Xie, H. Tang, H. Li, J. Hou, R. Zhang, Y. Liu and L. Jiang, Int. J. Biol. Macromol., 2023, 252, 126423 Search PubMed.
- Y. Liu, J. Chen, H. Li and Y. Wang, Int. J. Biol. Macromol., 2024, 259, 128934 Search PubMed.
| This journal is © The Royal Society of Chemistry 2026 |