Open Access Article
Open Access ArticleAn antibacterial γ-CD-MOF complex functionalized film for fish meat safety and freshness
Meimei
Guo
a,
Tahirou
Sogore
a,
Jin
Huang
a,
Xinyu
liao
c,
Mofei
Shen
*b and
Tian
Ding
 *ac
*ac
aCollege of Biosystems Engineering and Food Science, Zhejiang University, Hangzhou, 310058, China. E-mail: tding@zju.edu.cn
bDepartment of Food Science and Engineering, Zhejiang University of Technology, Hangzhou, Zhejiang 310014, China. E-mail: mfshen@zjut.edu.cn
cInnovation Center of Yangtze River Delta, Zhejiang University, Jiaxing, 314102, China
First published on 4th December 2025
Abstract
In this study, we developed a functionalized film for packaging refrigerated fish fillets. The functionalized film was produced using a γ-cyclodextrin metal–organic framework (γ-CD-MOF) loaded with gold nanoparticles (γ-CD-MOF complex), with polydimethylsiloxane serving as the substrate. The obtained functionalized film significantly enhanced antioxidant activity, demonstrating a 44% 2,2-diphenyl-1-picrylhydrazyl radical (DPPH˙+) scavenging rate and a 58% 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) radical (ABTS˙+) scavenging rate, much higher than those of the pure film. Furthermore, the functionalized film reduced the bacterial counts of Escherichia coli O157:H7 (E. coli O157:H7) by approximately 99.4% and Staphylococcus aureus (S. aureus) by approximately 80% within 24 hours. Moreover, the functionalized film maintained the pH within 6.70 ± 0.02 and the volatile basic nitrogen (TVB-N) value within 8.71 mg/100 g on day 4. It also exhibited a lower total viable count (TVC) value compared to both the control and pure film groups during the first four days, significantly improving the storage safety of refrigerated fish meat. Additionally, the embedded γ-CD-MOF complex affected the tensile strength, water contact angle, and water vapor transmission rate (WVTR) of the functionalized film. In conclusion, this functionalized film increased the shelf life of refrigerated fish fillet samples by inhibiting microbial growth and reducing the oxidation rate, compared to the control sample.
Sustainability spotlightThis study drives sustainable food packaging innovation by tackling fish spoilage and eco-harmful nanoparticle synthesis. We develop a functionalized film using a γ-CD-MOF (a biocompatible, “edible” material from γ-cyclodextrin and alkali metals) for in situ gold nanoparticle synthesis, requiring no toxic surfactants/stabilizers needed and thereby avoiding hazards of traditional methods and limitations of biological synthesis. With polydimethylsiloxane (low-cost, stable, inert) as the substrate, the film delivers dual value: its strong antioxidant/antimicrobial properties reduce fish TVB-N, pH, and TVC, cutting spoilage-related food waste (meat products often lose up to 40% of production). Its biocompatible components also minimize post-use environmental impact, outperforming conventional packaging. By integrating green synthesis, seafood waste reduction, and safe packaging, this research aligns with SDG 12 (Responsible Consumption and Production) and supports aquatic product sustainability, offering a practical path for eco-friendly food packaging. |
1 Introduction
Ensuring the safety and quality of food products during storage is a critical issue for both industries and governments. Meat products, in particular, are highly susceptible to spoilage, with up to 40% of production potentially lost.1,2 Fish is a rich source of high-quality protein, providing essential amino acids, minerals, and highly unsaturated fatty acids.3 These nutrients are crucial for human nutrition and digestive processes. However, during the postmortem stage of fish, the various nutrients within the tissue undergo enzymatic and microbial degradation, resulting in the formation of new chemical compounds, such as reactive aldehydes and other degradation by-products. These compounds are primarily responsible for the spoilage process of fish.4 As such, fish with an extended shelf life is essential for both producers and consumers.Fish processing and preservation techniques have been developed to inhibit or slow microbial growth. Recently, nanocomposite films incorporating antimicrobial agents such as essential oils, bacteriocins, antimicrobial enzymes, or metal nanoparticles have been developed. Metal nanoparticles are widely recognized for their promising antimicrobial properties, owing to their small size and high surface area-to-volume ratio, which provide an extensive reactive surface capable of interacting effectively with microorganisms.5 They can be readily integrated with other biomaterials to enhance antibacterial activity.6 For example, CuO nanoparticle complexes have been incorporated into polylactic acid/poly(butylene adipate-co-terephthalate) composite films and used for antimicrobial food packaging materials.7 A silver–carrageenan (Ag/Carr) nanocomposite film for food packaging was reported. The antimicrobial properties of the synthesized films were explored, and these films were employed for the storage of cottage cheese (dairy product) and strawberries (fruit).8 Additionally, antimicrobial composite films for fruits containing ZnGlu MOF particles have been reported, with jasmine essential oil as the active component incorporated into the MOF channels.9 Furthermore, gold, silver, copper, cobalt, indium, tungsten, tin, aluminum, chromium, zinc, manganese, tantalum, and titanium-based nanoparticle thin films are reported to have good antimicrobial activity.10–12 Among these metal nanoparticles, gold nanoparticles have garnered significant interest from researchers due to their low toxicity and excellent biocompatibility.13
Gold nanoparticles are generally synthesized via physicochemical and biological methods, but both have limitations: the former uses hazardous/toxic substances, harming health and the environment;14 the latter is restricted by limited biomaterials, long synthesis, poor organism regulation, and large particle size variability.15 Therefore, in this study, gold nanoparticles were green-synthesized in situ using a γ-CD-MOF, which is composed of γ-cyclodextrin and alkali metal ions (e.g., K+). As a biocompatible multifunctional material with permanent porosity, the γ-CD-MOF not only contains abundant hydroxyl groups but also features –OCCO– binding groups on its primary and secondary faces, enabling complexation with metal ions. Additionally, its porous structure allows absorption of guest molecules (e.g., nanoparticles), collectively supporting the in situ green synthesis without the need for additional reagents.16,17 In contrast, in the production of plant fiber-based food packaging materials, chemical compounds such as monomers and additives are necessary. Furthermore, modified or bleached plant fibers may pose safety risks due to unclear toxicity mechanisms and toxicological effects.18 Additionally, the release of microplastics from plastic packaging has raised concerns about human health and safety. Microplastics with small particle sizes can accumulate in various organs and tissues, and may have adverse effects on human health.19 Therefore, the “edible” nature of the γ-CD-MOF offers a safety advantage, making it a promising material for applications in food packaging.20,21 Furthermore, polydimethylsiloxane has been used in the packaging field due to its exceptional properties, including excellent thermal stability, biocompatibility, resistance to corrosion, flexibility, low cost, ease of fabrication, and chemical inertness, among others.22–24
In this study, a functionalized film was successfully synthesized and characterized in terms of its appearance, thermal stability, and mechanical properties. Subsequently, this film was applied to the preservation of fish meat. The results indicated that the functionalized film exhibited excellent antioxidant and antimicrobial properties. Moreover, compared with a pure film, the functionalized film could reduce the total volatile basic nitrogen (TVB-N), pH, and total viable count (TVC) values of fish meat to a certain extent. As a result, this functionalized film shows great potential for use in fish meat packaging.
2 Materials and methods
2.1 Materials
2,2′-Azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) (98%) and 2,2-diphenyl-1-picrylhydrazyl (98.5%) were purchased from Shanghai Macklin Biochemical Co. Ltd (Shanghai, China). γ-CD (98%) and potassium hydroxide (KOH, 90%) were purchased from Shanghai Macklin Biochemical Co. Ltd (Shanghai, China). Polyethylene glycol 8000 (PEG 8000) was purchased from Shanghai Aladdin Bio-Chem Technology Co. Ltd (Shanghai, China). Methanol (MeOH, 99.5%), acetonitrile (99.5%), chloroauric acid (47.8%) and dichloromethane (99.5%) were obtained from Sinopharm Chemical Reagents Co. Ltd (Shanghai, China). Experimental ultra-pure water was processed by a Smart-N system (Heal Force Biotech, Hk). Staphylococcus aureus (ATCC 25923), Escherichia coli O157:H7 (ATCC 35150), LB Broth, and LB Nutrient Agar were purchased from Qingdao Hope Bio-technology Co., Ltd (Qingdao, China). Phosphate Buffer Saline (PBS) was purchased from Beijing Solarbio Science & Technology Co., Ltd (Beijing, China). The large yellow croakers used in the experiment were all purchased from a supermarket.2.2 Preparation of films
The γ-CD-MOF and its complex were synthesized according to the methods outlined in our previous studies.13 The preparation of the pure film and functionalized films followed the procedure described in our earlier work.13 More detailed information is listed in SI.2.3 Characterization of films
A scanning electron microscope (SEM, Gemini SEM 360, ZEISS, Germany) fitted with an energy-dispersive X-ray spectroscopy (EDS) detector was used to investigate the morphological features of the functionalized film. To obtain a smooth, uncontaminated surface for cross-sectional observation, the functionalized film was first frozen and then fractured using liquid nitrogen. Before the SEM measurement, the fractured film samples were attached to conductive adhesive tape and sputter-coated with a thin layer of platinum to improve electrical conductivity.Powder X-ray diffraction (PXRD) (D8 Advance, Bruker, Germany) was performed on the samples. The experimental conditions were 40 kV tube voltage and 40 mA tube current. All the samples were analyzed over a 2θ angle at a stepwise scan increment of 0.02° with a scanning speed of 0.1 s per step. A Fourier transform infrared spectrometer (FTIR) (Nicolet IS50, Thermo Fisher, USA) was used to collect the spectra of the samples. The samples were thoroughly ground and mixed with potassium bromide before being pressed into a translucent sheet for testing. The wave number range was 500–4000 cm−1 with a resolution of 4 cm−1. Additionally, an X-ray photoelectron spectrometer (XPS) (AXIS SUPRA+, Shimadzu, Japan) was employed to characterize the surface elemental composition and chemical environments of the γ-CD-MOF complex.
Thermogravimetric analysis (TGA) was carried out on the functionalized film with a STARe System TGA2 instrument (Mettler Toledo, Switzerland). Approximately 5 mg of the functionalized film sample was used for each TGA test, which was placed in a Tzero aluminum crucible. The sample was heated from 25 °C up to 700 °C at a constant heating rate of 10 °C per minute, and a nitrogen flow of 50 mL min−1 was kept throughout the process to protect the sample from oxidation. An ultraviolet spectrophotometer (UV-2600, Shimadzu, Japan) was employed to confirm whether γ-CD-MOF complex crystals were present in the functionalized film. The verification process involved two main steps: first, both the γ-CD-MOF complex and the functionalized film were soaked in pure water to prepare solutions with a concentration of 1 mg mL−1. Then, ultraviolet-visible (UV-Vis) absorbance spectra of these two solutions were measured, covering the wavelength range from 300 nm to 700 nm.
2.4 Mechanical properties and water contact angle analysis
A universal testing machine (model Z020, ZwickRoell, Germany) was utilized to assess the mechanical properties of the functionalized film. During the tests, the stretching speed was adjusted to 50 mm per minute, and all measurements were carried out under room temperature conditions. To ensure data reliability, each mechanical test was conducted in triplicate. For the determination of water contact angles, sample discs with a diameter of 1 cm were cut from both the pure film and the functionalized film. To obtain accurate contact angle values, five separate measurements were taken for each film sample, and the results were averaged for subsequent analysis.2.5 Antioxidant activity
Antioxidant activities of the functionalized films were assessed using DPPH˙+ and ABTS˙+ scavenging methods.25,26 For DPPH analysis, a fresh methanolic solution of DPPH (0.2 mM) was prepared, and 50 mg of the film sample was added to a 10 mL DPPH solution. The mixture was incubated for 30 minutes at room temperature, and then the absorbance was measured at 517 nm. For ABTS analysis, potassium sulfate (2.45 mM) was mixed with ABTS solution (7 mM) in equal volumes and incubated for 12–16 hours in the dark to prepare the ABTS assay solution. Then, 500 mg of film sample was added to 10 mL of the ABTS assay solution, and the mixture was incubated at room temperature for 30 minutes in the dark. The absorbance was then measured at 734 nm. The antioxidant activity of all the films was determined as follows:where Ac and As represent the absorbance of DPPH and ABTS for the control and film samples, respectively. The test was repeated in triplicate.
2.6 Antibacterial activity
To assess the films' antibacterial activity, two model bacterial strains were selected: S. aureus and E. coli O157:H7. First, these bacterial strains were grown in LB medium. The culture was maintained at 37 °C with constant shaking at 180 rpm for a 17-hour period. Using the plate count technique, the bacterial suspension was diluted with sterile PBS to a final concentration of 1 × 106 CFU mL−1. Next, 100 µL of this standardized bacterial solution was pipetted onto the surface of two types of films: pure films and functionalized films. Each film had a diameter of 18 mm. To ensure thorough contact between the bacterial solution and the film surface, a second film was placed on top of the bacterial suspension, creating a sandwich-like structure. These film–bacteria assemblies were then incubated at 37 °C for 24 hours. Post-incubation, 4900 µL of PBS was added to the system to rinse the films and dilute any remaining bacterial solution. The number of viable bacterial colonies was subsequently quantified using the plate count method once more. Additionally, a transmission electron microscope (TEM) (JEM-1230 TEM, HITACHI, Japan) was used to examine the morphological changes in the bacterial cells.2.7 Antibacterial mechanism
Cell membrane integrity was evaluated by quantifying the leakage of nucleic acids and proteins into the bacterial suspension. This quantification was performed using an ultraviolet-visible spectrophotometer (Model: NanoDrop One/One C, Manufacturer: Thermo Scientific, U.S.A.). Specifically, nucleic acid concentration was determined by measuring the optical density (OD) at a wavelength of 260 nm, while protein concentration was assessed via OD measurement at 280 nm, in accordance with previously established protocols.27–29 For sample preparation, bacterial solutions exposed to functionalized films for different time intervals were subjected to centrifugation. The centrifugation process was conducted at 8000 rpm for 10 minutes at a temperature of 25 °C. Following centrifugation, the supernatant was collected to determine the absorbance values at 260 nm and 280 nm. Separately, the mitochondrial membrane potential of bacteria was evaluated using the Rhodamine 123 (Rh 123) fluorescent staining assay.30,31 Bacterial solutions after treatment were first centrifuged at 25 °C, 8000 rpm for 10 minutes. The resulting bacterial pellet was then washed three times with PBS. After washing, the bacteria were resuspended in 1 mL of Rh 123 solution (with a final concentration of 2 µg mL−1) and incubated in the dark at room temperature for 30 minutes. Post-incubation, the bacterial samples were washed twice with PBS to eliminate unbound Rh 123. The fluorescence spectra of the samples were subsequently measured using a multimode microplate reader. The detection parameters were set as follows: excitation wavelength at 480 nm, emission wavelength at 530 nm, and a gain value of 80.2.8 Freshness of large yellow croakers during storage
2.9 Statistical analysis
All data were analyzed using analysis of variance (ANOVA) in IBM SPSS Statistics 25.0. When the overall P value for the experiment was below the significance threshold (P < 0.05), Tukey's test was used to compare the means. Graphs were generated using Origin 2018 and GraphPad Prism 9, based on data from three independent replicate experiments.3 Results and discussion
3.1 Characterization of films
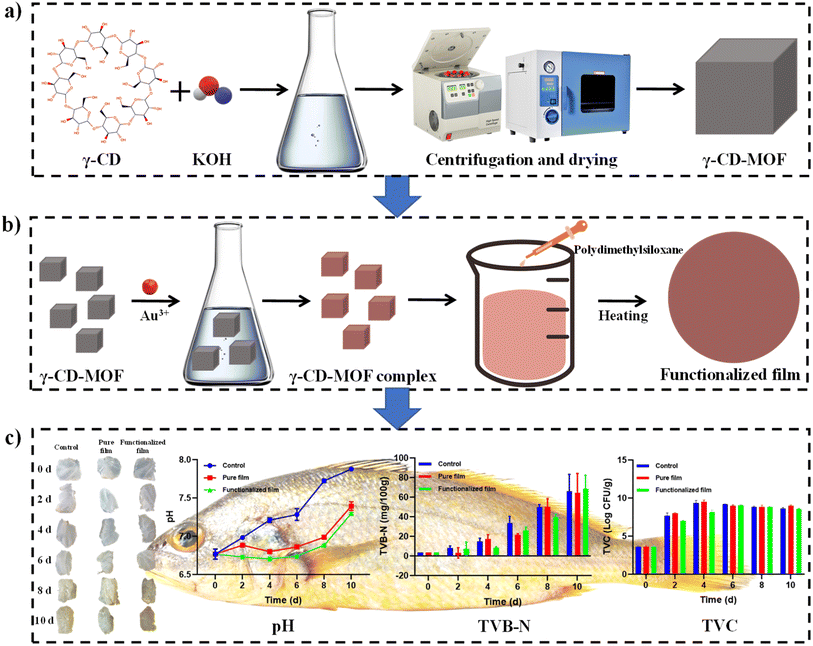
Scheme 1 presents the preparation process and application of the functionalized film. As exhibited in Fig. 1a, the SEM image of the functionalized film surface displays a large number of cubic crystals corresponding to the γ-CD-MOF complex. Fig. 1b demonstrates partial integration of the γ-CD-MOF complex crystals into the matrix of the functionalized film. In contrast, Fig. 1c and d provide the surface and cross-sectional SEM images of the pure film, respectively, with no embedded crystals observed in either case.Fig. 1e presents the PXRD patterns of different samples. As shown in the figure, the γ-CD-MOF complex exhibits distinct and intense characteristic diffraction peaks, whereas the pure film and functionalized film display extremely weak characteristic peaks without the emergence of new diffraction peaks. This indicates that the incorporation of the γ-CD-MOF complex into the film is likely mediated by physical interactions. To further verify the interaction between the γ-CD-MOF complex and the functionalized film, FTIR was performed on the samples (Fig. 1f). The results reveal that the FTIR spectrum of the functionalized film is almost identical to that of the pure film, with no new absorption bands formed. This confirms that the interaction between the γ-CD-MOF complex and the functionalized film is of a physical nature.
The effect of partially embedded γ-CD-MOF complex crystals on the thermal stability of the functionalized film is illustrated in Fig. 1g and h. A prominent weight loss of the γ-CD-MOF complex is observed in the temperature range of 250–380 °C, which is attributed to the thermal degradation of the cyclodextrin component and the full decomposition of the γ-CD-MOF structure.34,35 For the pure film, a 50% weight loss is achieved at 595 °C, which aligns with findings reported in previous literature.36 By comparison, the functionalized film exhibits a 50% weight loss at 520 °C, a temperature notably lower than that of the pure film. These results indicate that the incorporation of γ-CD-MOF complex crystals impairs the thermal stability of the functionalized film. While the incorporation of the γ-CD-MOF complex moderately compromises the thermal stability of the functionalized film, the film still retains satisfactory thermal stability. As illustrated in Fig. 1g, the functionalized film exhibits negligible weight loss until the heating temperature exceeds 300 °C. Notably, the temperature employed in traditional thermal processing of foodstuffs generally does not exceed 250 °C.37 Furthermore, to pursue healthier and more nutritious food products, non-thermal processing technologies have been increasingly adopted in recent years.38
Fig. 2a shows the UV-Vis absorption peaks of the γ-CD-MOF complex and the functionalized film after immersion in water, with these peaks being ascribed to gold nanoparticles.39 This observation confirms the successful incorporation of γ-CD-MOF complex crystals into the functionalized film. To further verify the formation of gold nanoparticles, XPS analysis was performed on the γ-CD-MOF complex. The results revealed characteristic peaks at 87.8 eV and 84.1 eV, which are attributed to Au 4f5/2 and Au 4f7/2, respectively (Fig. S1). These findings confirm the successful formation of gold nanoparticles. Additionally, the gold content in the γ-CD-MOF complex was determined, with a loading efficiency of approximately 2.2% (Fig. S2). In Fig. 2b, the potassium signal verifies the presence of the γ-CD-MOF, while the gold signal confirms the existence of gold nanoparticles again. Furthermore, Fig. 2b reveals that γ-CD-MOF complex crystals are uniformly distributed on the surface of the functionalized film.40 Finally, the cytotoxicity of the γ-CD-MOF complex was investigated, and the results demonstrated that it exhibits no cytotoxicity within the concentration range of 62.5–1000 µg mL−1 (Fig. S3).
 | ||
| Fig. 2 (a) UV-Vis absorbance spectra of the γ-CD-MOF complex and functionalized film immersed in water; (b) EDS elemental mapping of the functionalized film (K signal: blue; Au signal: yellow). | ||
3.2 Mechanical properties and water contact analysis
Mechanical properties are of critical importance to food packaging films during the packaging process, thereby highlighting the necessity of guaranteeing their superior performance.41,42 As presented in Fig. 3a, the stress–strain curve of the functionalized film is analogous to that of the pure film, which implies that the two films possess comparable elastic behaviors. Fig. 3b demonstrates that the tensile strength of the functionalized film is on par with that of the pure film. Furthermore, the incorporation of γ-CD-MOF complex crystals remarkably improves the elastic modulus of the functionalized film, while simultaneously reducing its elongation at break, as evidenced in Fig. 3c and d.Hydrophilic food packaging materials are prone to being affected by humid environments, which severely restricts their application in the preservation and transportation of food products. Consequently, improving the water resistance of these packaging materials is of great significance.43 To assess the hydrophobic properties of the films, water contact angle analysis was conducted. As shown in Fig. 3e, the functionalized film exhibits hydrophobic characteristics, though its water contact angle is notably lower than that of the pure film. This phenomenon can be ascribed to the high hydrophilicity of the γ-CD-MOF, whose structure contains a large number of hydrophilic hydroxyl groups.20 Despite the inherent hydrophobicity of polydimethylsiloxane, the introduction of γ-CD-MOF complex crystals leads to a reduction in its water contact angle. Additionally, a comparison of the WVTR between the pure film and the functionalized film was conducted (Fig. S4). The results demonstrate that under identical conditions, the WVTR values of the pure film and the functionalized film are 150.68 and 173.37 (g (m2 24 h)−1), respectively. This observation indicates that the incorporation of the γ-CD-MOF complex moderately enhances the WVTR of the functionalized film.
3.3 Antioxidant properties
The antioxidant activity and free radical scavenging ability of food packaging films are critical, as free radicals can cause food discoloration, rancidity, and off-flavors.44 The antioxidant activity of the films was evaluated based on ABTS and DPPH free radical scavenging assays, with the results presented in Fig. 3f. The functionalized film showed 44% DPPH scavenging activity and 58% ABTS scavenging activity. Although the DPPH and ABTS free radical scavenging capacities of the functionalized film were lower than those of organoselenocyanate-tethered methyl anthranilate hybrids and organodiselenide-tethered methyl anthranilates,45,46 they were significantly higher than those of the pure film, as the latter exhibited only about 8% DPPH scavenging activity and 5% ABTS scavenging activity. The antioxidant activity of the functionalized film is attributed to the released gold nanoparticles, which exhibit significant antioxidant properties due to the unique characteristics of their nanoparticle surfaces.47,48 Furthermore, the free radical scavenging activity of the functionalized film, as measured by the ABTS assay, was slightly higher than that observed with the DPPH assay. This can be explained by the greater solubility of the γ-CD-MOF in pure water, which facilitates enhanced release of gold nanoparticles.443.4 Antibacterial properties
To evaluate the antibacterial effectiveness of the films, both E. coli O157:H7 and S. aureus were used as test microorganisms. The antibacterial performance against E. coli O157:H7 is shown in Fig. 4a. In comparison to the control, the bacterial population on the pure film increased after 24 hours, indicating that E. coli O157:H7 adhered to the pure film surface, promoting bacterial growth.49 This suggests that the pure film does not effectively inactivate E. coli O157:H7. In contrast, after 24 hours of exposure to the functionalized film, a significant reduction in bacterial count was observed, highlighting the functionalized film's strong antibacterial properties against E. coli O157:H7. Similarly, Fig. 4d further demonstrates that the functionalized film also significantly reduced the bacterial counts of S. aureus. Taken together, these findings indicate that the functionalized film exhibits potent antibacterial activity, likely due to the release of gold nanoparticles, which are known to have intrinsic antibacterial properties.13TEM images in Fig. 4b and c illustrate the morphology of untreated and treated E. coli O157:H7, respectively. As shown in Fig. 4b, the control E. coli O157:H7 retains an intact cell membrane and characteristic structure. In contrast, after exposure to the functionalized film, E. coli O157:H7 exhibits severe damage, including cell lysis, membrane disruption, and abnormal cellular swelling (Fig. 4c).50 Analogously, Fig. 4e and f demonstrate that control S. aureus has intact membranes, while treated S. aureus shows extensive damage. A large number of gold nanoparticles are observed adhering to S. aureus cell surfaces, resulting in membrane rupture and leakage of intracellular contents.50,51 Additionally, the gold nanoparticles may interact with other organelles such as the cell membrane and may also inhibit specific proteins involved in cellular respiration, thus causing cell death.52–54
3.5 Antibacterial mechanism
In bacteria, the cell membrane functions as a protective barrier. Disruption of the cell membrane by antimicrobial agents leads to the leakage of intracellular ions (e.g., K+ and Na+) and other macromolecules.28 Additionally, macromolecules such as nucleic acids and proteins exhibit characteristic absorbance peaks at 260 nm and 280 nm, respectively.55Fig. 5a and b depict the leakage of nucleic acids and proteins from E. coli O157:H7, while Fig. 5d and e show the same for S. aureus. The results reveal that the absorbance values of all experimental groups are significantly higher than those of the control groups. This confirms that treatment with the functionalized film caused severe disruption, thereby impairing the integrity and permeability of the bacterial cell membrane.28,56Rh 123, a positively charged probe that enters cells through diffusion, was employed to assess the mitochondrial membrane potential.57 Under normal circumstances, cells sustain stable intracellular membrane potentials, which are crucial for maintaining normal cellular function.58 The results of the membrane potential assays for E. coli O157:H7 and S. aureus are presented in Fig. 5c and f, respectively. These results show that the mitochondrial membrane potential in the control groups was higher than that in the experimental groups. Furthermore, the fluorescence intensities of both control and experimental groups decreased steadily over time. This implies that an increasing proportion of bacteria might have entered an inactive state with prolonged incubation.59,60 Taken together, these findings confirm that treatment with the functionalized film induced a reduction in the mitochondrial membrane potential of the tested bacteria.
3.6 Freshness of large yellow croakers during storage
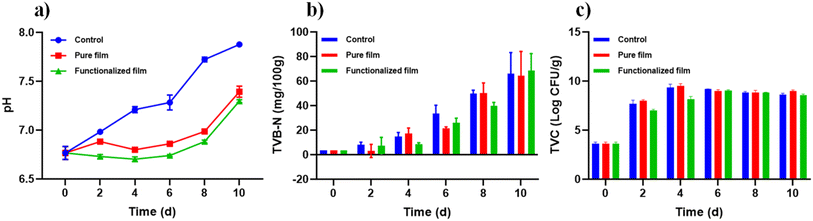 | ||
| Fig. 6 Effects of the pure film and functionalized film on various parameters, including (a) pH, (b) TVB-N, and (c) TVC of fish fillets stored under controlled conditions at 4 °C. | ||
The primary cause of the initial pH decline is the hydrolysis of ATP to phosphoric acid following the death of the fish, along with the anaerobic degradation of glycogen to lactic acid. This process leads to the accumulation of acidic metabolites, resulting in a decrease in pH.62 In the later stages, as the fish enters the autolytic spoilage phase, proteolytic enzymes and alkaline microorganisms break down proteins into volatile compounds such as ammonia, dimethylamine, and trimethylamine. This process leads to an increase in pH due to the accumulation of these alkaline substances.63
4 Conclusion
In this study, a functionalized film was successfully fabricated by immobilizing a γ-CD-MOF complex onto a polydimethylsiloxane substrate. Characterization revealed that the γ-CD-MOF complex modulated the film's physicochemical properties, including the tensile strength and water contact angle, which are key parameters for packaging applicability. The film exhibited remarkable antioxidant activity with DPPH˙+ and ABTS˙+ scavenging rates of 44% and 58%, respectively, significantly higher than those of the pure PDMS film, as well as potent antibacterial efficacy that reduced E. coli O157:H7 by 99.4% and S. aureus by 80% within 24 hours. During 4-day refrigerated storage of fish fillets, it effectively maintained pH at 6.70 ± 0.02 and TVB-N at 8.71 mg/100 g while suppressing TVC to levels substantially lower than those in the control and pure film groups. Collectively, the functionalized film prolongs the shelf life of refrigerated fish fillets by inhibiting microbial proliferation and retarding lipid oxidation, ensuring storage safety and freshness. Future work will focus on expanding applicability to more aquatic products, and investigating biodegradability to align with green packaging trends, thereby promoting its practical use in food preservation and packaging.Author contributions
Meimei Guo: writing – review & editing, writing – original draft, software, methodology, investigation, formal analysis, data curation. Tahirou Sogore: writing – review & editing, formal analysis. Jin Huang: writing – review & editing, formal analysis. Xinyu liao: writing – review & editing, formal analysis. Mofei Shen: writing – review & editing, formal analysis. Tian Ding: writing – review & editing, validation, supervision, conceptualization, funding acquisition.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Data availability
The data supporting the findings of this study and supplementary information are available from the corresponding author upon reasonable request.Supplementary information (SI): Fig. S1 shows the high-resolution Au 4f XPS spectra obtained from the γ-CD-MOF complex; Fig. S2 shows the gold ion loading rate of the γ-CD-MOF complex; Fig. S3 shows the in vitro cell viability of Caco-2 cells exposed to the γ-CD-MOF complex; Fig. S4 shows the water vapor transmittance rate of pure films and functionalized films; Fig. S5 shows the UV-Vis absorbance spectra of the γ-CD-MOF complex stored at different temperatures for 7 days. See DOI: https://doi.org/10.1039/d5fb00743g.
Acknowledgements
This work was supported by the National Key Research and Development Program of China (2022YFF1102700), and the Starry Night Science Fund of Zhejiang University Shanghai Institute for Advanced Study (Grant No. SN-ZJU-SIAS-004).References
- J. M. Lorenzo, R. Batlle and M. Gómez, Extension of the shelf-life of foal meat with two antioxidant active packaging systems, LWT–Food Sci. Technol., 2014, 59, 181–188 CrossRef CAS.
- R. Eshaghi, M. Mohsenzadeh and J. F. Ayala-Zavala, Bio-nanocomposite active packaging films based on carboxymethyl cellulose, myrrh gum, TiO2 nanoparticles and dill essential oil for preserving fresh-fish (Cyprinus carpio) meat quality, Int. J. Biol. Macromol., 2024, 263, 129991 CrossRef CAS PubMed.
- J. Tavares, A. Martins, L. G. Fidalgo, V. Lima, R. A. Amaral, C. A. Pinto, A. M. Silva and J. A. Saraiva, Fresh Fish Degradation and Advances in Preservation Using Physical Emerging Technologies, Foods, 2021, 10, 10040780 Search PubMed.
- M. N. Madhubhashini, C. P. Liyanage, A. U. Alahakoon and R. P. Liyanage, Current applications and future trends of artificial senses in fish freshness determination: A review, J. Food Sci., 2024, 89, 33–50 CrossRef CAS PubMed.
- E. Sánchez-López, D. Gomes, G. Esteruelas, L. Bonilla, A. L. Lopez-Machado, R. Galindo, A. Cano, M. Espina, M. Ettcheto, A. Camins, M. S. Amélia, D. Alessandra, S. Antonello, L. G. Maria and B. S. Eliana, Metal-Based Nanoparticles as Antimicrobial Agents: An Overview, Nanomaterials, 2020, 10(2), 292 CrossRef PubMed.
- A. Evans and A. K. Kevin, Evaluation of metal-based antimicrobial compounds for the treatment of bacterial pathogens, J. Med. Microbiol., 2021, 70(5), 001363 CrossRef CAS PubMed.
- X. Xie, J. Tian, J. Wu, R. Guo, J. Zhang, D. Fu, H. Wang, B. Niu and H. Yan, Polylactic Acid/Poly(Butylene Adipate-Co-Terephthalate) Packing Films Doped with Novel Antimicrobial Agent CuO@γ-CD-MOFs, ChemistrySelect, 2025, 10, e02743 CrossRef CAS.
- S. Kumari, A. Kumari and R. Sharma, Safe and sustainable food packaging: Argemone albiflora mediated green synthesized silver-carrageenan nanocomposite films, Int. J. Biol. Macromol., 2024, 264, 130626 CrossRef CAS PubMed.
- A. M. Pak, E. N. Zakharchenko, E. A. Maiorova and V. V. Novikov, Bicompatible Metal-Organic Framework for Functional Packing of Food Products, Russ. J. Coord. Chem., 2023, 49, 97–103 CrossRef CAS.
- M. Sriubas, K. Bockute, P. Palevicius, M. Kaminskas, Z. Rinkevicius, M. Ragulskis, S. Simonyte, M. Ruzauskas and G. Laukaitis, Antibacterial Activity of Silver and Gold Particles Formed on Titania Thin Films, Nanomaterials, 2022, 12(7), 1190 CrossRef CAS PubMed.
- D. Mitra, E. T. Kang and K. G. Neoh, Antimicrobial Copper-Based Materials and Coatings: Potential Multifaceted Biomedical Applications, ACS Appl. Mater. Interfaces, 2020, 12(19), 21159–21182 CrossRef CAS PubMed.
- E. M. Cazalini, W. Miyakawa, G. R. Teodoro, A. S. S. Sobrinho, J. E. Matieli, M. Massi and C. Y. Koga-Ito, Antimicrobial and anti-biofilm properties of polypropylene meshes coated with metal-containing DLC thin films, J. Mater. Sci. Mater. Med., 2017, 28(6), 97 CrossRef PubMed.
- M. Guo, M. Shen, Y. Zhu, T. Sogore and T. Ding, Ultra-small gold nanoparticles embedded cyclodextrin metal-organic framework composite membrane to achieve antibacterial and humidity-responsive functions, Carbohydr. Polym., 2024, 340, 122200 CrossRef CAS PubMed.
- J. Jeevanandam, S. F. Kiew, S. Boakye-Ansah, S. Y. Lau, A. Barhoum, M. K. Danquah and J. Rodrigues, Green approaches for the synthesis of metal and metal oxide nanoparticles using microbial and plant extracts, Nanoscale, 2022, 14, 2534–2571 RSC.
- B. A. Suliasih, S. Budi and H. Katas, Synthesis and application of gold nanoparticles as antioxidants, Pharmacia, 2024, 71, 1–19 CrossRef CAS.
- I. Roy and J. F. Stoddart, Cyclodextrin Metal-Organic Frameworks and Their Applications, Acc. Chem. Res., 2021, 54(6), 1440–1453 CrossRef CAS PubMed.
- A. Hamedi, A. Anceschi, A. Patrucco and M. Hasanzadeh, A γ-cyclodextrin-based metal-organic framework (γ-CD-MOF): a review of recent advances for drug delivery application, J. Drug Targeting, 2021, 30(4), 381–393 CrossRef PubMed.
- M. Tang, C. Chen, J. Song, Y. Ni, B. Xiang, J. Zou and D. Xu, Safety of plant fiber-based food contact materials: Overview of the discovery, identification, detection and risk assessment of unknown risk substances, Food Packag. Shelf Life, 2024, 43, 101281 CrossRef CAS.
- J. Čurlej, P. Zajác, J. Čapla and L. Hleba, Safety issues of microplastics released from food contact materials, J. Microbiol. Biotechnol. Food Sci., 2023, 13(2), e10317 CrossRef.
- R. A. Smaldone, R. S. Forgan, H. Furukawa, J. J. Gassensmith, A. M. Z. Slawin, O. M. Yaghi and J. . F. Stoddart, Metal-Organic Frameworks from Edible Natural Products, Angew. Chem., Int. Ed., 2010, 49, 8630–8634 CrossRef CAS PubMed.
- S. Li, X. Hu, S. Chen, X. Wang, H. Shang, Y. Zhou, J. Dai, L. Xiao, W. Qin and Y. Liu, Synthesis of γ-cyclodextrin metal-organic framework as ethylene absorber for improving postharvest quality of kiwi fruit, Food Hydrocoll., 2023, 136, 108294 CrossRef CAS.
- R. Ariati, F. Sales, A. Souza, R. A. Lima and J. Ribeiro, Polydimethylsiloxane Composites Characterization and Its Applications: A Review, Polymers, 2020, 13(23), 4258 CrossRef PubMed.
- Y. Song, S. Sun, Q. Hao, S. Gao, W. Wang and H. Hou, Effect of polydimethylsiloxane on the structure and barrier properties of starch/PBAT composite films, Carbohydr. Polym., 2024, 336, 122119 CrossRef CAS PubMed.
- F. Wang, L. Qiu and Y. Tian, Super Anti-Wetting Colorimetric Starch-Based Film Modified with Poly(dimethylsiloxane) and Micro-/Nano-Starch for Aquatic-Product Freshness Monitoring, Biomacromolecules, 2021, 22, 3769–3779 CrossRef CAS PubMed.
- S. Roy, R. Priyadarshi and J. Rhim, Gelatin/agar-based multifunctional film integrated with copper-doped zinc oxide nanoparticles and clove essential oil Pickering emulsion for enhancing the shelf life of pork meat, Food Res. Int., 2022, 160, 111690 CrossRef CAS PubMed.
- S. Roy and J. Rhim, Gelatin/agar-based functional film integrated with Pickering emulsion of clove essential oil stabilized with nanocellulose for active packaging applications, Colloids Surf., A, 2021, 627, 127220 CrossRef CAS.
- B. Zhang, Y. Zang, Q. Mo, L. Sun, M. Tu, D. Shu, Y. Li, F. Xue, G. Wu and X. Zhao, Antibacterial activity and mechanism of slightly acidic electrolyzed water (SAEW) combined with ultraviolet light against Staphylococcus aureus, LWT, 2023, 182, 114746 CrossRef CAS.
- H. Gao, M. Sun, Y. Duan, Y. Cai, H. Dai and T. Xu, Controllable synthesis of lignin nanoparticles with antibacterial activity and analysis of its antibacterial mechanism, Int. J. Biol. Macromol., 2023, 246, 125596 CrossRef CAS PubMed.
- L. Dou, R. Xue, W. Deng, J. Wang, X. Liao, R. Yu, X. Duan and Y. Xiong, Design, synthesis and evaluation of phenyl sulfonyl fluoride substituted ruthenium polypyridine complex as antibacterial agent targeting cell membrane, J. Mol. Struct., 2025, 1319, 139591 CrossRef CAS.
- J. Comas and J. Vives-Rego, Assessment of the effects of gramicidin, formaldehyde, and surfactants on Escherichia coli by flow cytometry using nucleic acid and film potential dyes, Cytometry, 1997, 29, 58–64 CrossRef CAS PubMed.
- Y. Zhang, X. Liu, Y. Wang, P. Jiang and S. Quek, Antibacterial activity and mechanism of cinnamon essential oil against Escherichia coli and Staphylococcus aureus, Food Control, 2015, 59, 282–289 CrossRef.
- X. Fan, L. Yin, J. Zhu, P. Sun, Y. Zhu, Q. Chen, B. Kong, Q. Liu and H. Wang, The improvement of storage quality of Harbin red sausage by coating with oregano essential oil loaded zein-pectin-chitosan nanoparticles, Food Packag. Shelf Life, 2024, 43, 101274 CrossRef CAS.
- A. Lv, G. Fan, Z. Yang, Z. Zhang, M. M. Khan and X. Fu, Polydopamine-immobilized lysozyme for the fabrication of antibacterial films and its application in the preservation of fresh pork, Food Packag. Shelf Life, 2024, 43, 101288 CrossRef CAS.
- Z. Hu, S. Li, S. Wang, B. Zhang and Q. Huang, Encapsulation of menthol into cyclodextrin metal-organic frameworks: Preparation, structure characterization and evaluation of complexing capacity, Food Chem., 2021, 338, 127839 CrossRef CAS PubMed.
- Z. Qin, Q. Jiang, Y. Zou, M. Chen, J. Li, Y. Li and H. Zhang, Synthesis of Nanosized γ-Cyclodextrin Metal-Organic Frameworks as Carriers of Limonene for Fresh-Cut Fruit Preservation Based on Polycaprolactone Nanofibers, Small, 2024, 20(29), 2400399 CrossRef CAS PubMed.
- S. Chen, J. Cao and J. Zheng, Reprocessable Silyl Ether-Based Dynamic Covalent Poly(dimethylsiloxane) Networks with Superb Thermal Stability and Creep Resistance, ACS Appl. Polym. Mater., 2024, 6, 4215–4225 CrossRef CAS.
- M. Zhao, Z. Liu, W. Zhang, G. Xia, C. Li, K. Rakariyatham and D. Zhou, Advance in aldehydes derived from lipid oxidation: A review of the formation mechanism, attributable food thermal processing technology, analytical method and toxicological effect, Food Res. Int., 2025, 203, 115811 CrossRef CAS PubMed.
- S. White, A. Jackson-Davis, K. Gordon, K. Morris, A. Dudley, A. Abdallah-Ruiz, K. Allgaier, K. Sharpe, A. K. Yenduri, K. Green and F. Santos, A Review of Non-thermal Interventions in Food Processing Technologies, J. Food Prot., 2025, 88(6), 100508 CrossRef CAS PubMed.
- K. Seku, G. Bhagavanth Reddy, A. I. Osman, S. S. Hussaini, N. S. Kumar, M. Al-Abri, B. Pejjai, S. B. Alreshaidan, A. S. Al-Fatesh and K. K. Kadimpati, Modified frankincense resin stabilized gold nanoparticles for enhanced antioxidant and synergetic activity in in-vitro anticancer studies, Int. J. Biol. Macromol., 2024, 278, 134935 CrossRef CAS PubMed.
- Y. He, X. Wang, C. Zhang, J. Sun, J. Xu and D. Li, Near-Infrared Light-Mediated Cyclodextrin Metal-Organic Frameworks for Synergistic Antibacterial and Anti-Biofilm Therapies, Small, 2023, 19(35), 2300199 CrossRef CAS PubMed.
- H. Zhou, T. Li, E. Zhu, S. Wang, Q. Zhang, X. Li, L. Zhang, Y. Fan, J. Ma and Z. Wang, Dissolving-co-catalytic strategy for the preparation of flexible and wet-stable cellulose film towards biodegradable packaging, Int. J. Biol. Macromol., 2024, 275, 133454 CrossRef CAS PubMed.
- Y. He, T. Zhong, Y. Liu, M. Wan, L. Sun, Y. Zhao and Z. Wang, Development of a multifunctional active food packaging film based on electrospun polyvinyl alcohol/chitosan for preservation of fruits, Int. J. Biol. Macromol., 2024, 277, 134636 CrossRef CAS PubMed.
- X. Lan, T. Luo, Z. Zhong, D. Huang, C. Liang, Y. Liu, H. Wang and Y. Tang, Green cross-linking of gelatin/tea polyphenol/ε-poly (L-lysine) electrospun nanofibrous film for edible and bioactive food packaging, Food Packag. Shelf Life, 2022, 34, 100970 CrossRef CAS.
- C. Gao, P. Chen, Y. Ma, L. Sun, Y. Yan, Y. Ding and L. Sun, Multifunctional polylactic acid biocomposite film for active food packaging with UV resistance, antioxidant and antibacterial properties, Int. J. Biol. Macromol., 2023, 253, 126494 CrossRef CAS PubMed.
- B. Al-Abdallah, Y. S. Al-Faiyz and S. Shaaban, Anticancer, Antimicrobial, and Antioxidant Activities of Organodiselenide-Tethered Methyl Anthranilates, Biomolecules, 2022, 12(12), 1765 CrossRef CAS PubMed.
- B. Al-Abdallah, Y. S. Al-Faiyz and S. Shaaban, Organoselenocyanates Tethered Methyl Anthranilate Hybrids with Promising Anticancer, Antimicrobial, and Antioxidant Activities, Inorganics, 2022, 10(12), 246 CrossRef CAS.
- J. Beurton, I. Clarot, J. Stein, B. Creusot, C. Marcic, E. Marchioni and A. Boudier, Long-lasting and controlled antioxidant property of immobilized gold nanoparticles for intelligent packaging, Colloids Surf., B, 2019, 176, 439–448 CrossRef CAS PubMed.
- B. Zhu, N. Xie, L. Yue, K. Wang, M. Z. Bani-Fwaz, H. Hussein Osman, A. F. El-kott and X. Bai, Formulation and characterization of a novel anti-human endometrial cancer supplement by gold nanoparticles green-synthesized using Spinacia oleracea L. Leaf aqueous extract, Arab. J. Chem., 2022, 15, 103576 CrossRef CAS.
- F. Pan, M. Liu, S. Altenried, M. Lei, J. Yang, H. Straub, W. W. Schmahl, K. Maniura-Weber, O. Guillaume-Gentil and Q. Ren, Uncoupling bacterial attachment on and detachment from polydimethylsiloxane surfaces through empirical and simulation studies, J. Colloid Interface Sci., 2022, 622, 419–430 CrossRef CAS PubMed.
- Y. Wen, W. Chen, R. Wu, J. Guo, X. Liu, B. Shi, C. Zhang, L. Wu, Y. Zheng, A. Liu and L. Lin, Chitosan-Stabilized PtAu Nanoparticles with Multienzyme-Like Activity for Mixed Bacteria Infection Wound Healing and Insights into Its Antibacterial Mechanism, Small Struct., 2024, 5(6), 2300553 CrossRef CAS.
- J. Xin, Q. Pu, R. Wang, Y. Gu, L. He, X. Du, G. Tang and D. Han, Antibacterial activity and mechanism of chelerythrine against Streptococcus agalactiae, Front. Vet. Sci., 2024, 11, 1408376 CrossRef PubMed.
- S. Shaaban, A. M. Abu-Dief, M. Alaasar, A. S. Al-Janabi, N. S. Alsadun, O. K. Al Duaij and T. A. Yousef, Novel Fe (III), Cu (II), and Zn (II) Chelates of Organoselenium-Based Schiff Base: Design, Synthesis, Characterization, DFT, Anticancer, Antimicrobial, and Antioxidant Investigations, Appl. Organomet. Chem., 2024, 39(1), e7776 CrossRef.
- S. Shaaban, K. T. Abdullah, K. Shalabi, T. A. Yousef, O. K. Al Duaij, G. M. Alsulaim, H. A. Althikrallah, M. M. Alaasar, A. S. Al-Janabi and A. M. Abu-Dief, Synthesis, Structural Characterization, Anticancer, Antimicrobial, Antioxidant, and Computational Assessments of Zinc(II), Iron(II), and Copper(II) Chelates Derived From Selenated Schiff Base, Appl. Organomet. Chem., 2024, 38(12), e7712 CrossRef CAS.
- W. S. Hamama, G. G. El-Bana, S. Shaaban and H. H. Zoorob, Synthetic Approach to Some New Annulated 1,2,4-Triazine Skeletons with Antimicrobial and Cytotoxic Activities, J. Heterocycl. Chem., 2018, 55(4), 971–982 CrossRef CAS.
- R. Qin, S. Yang, B. Fu, Y. Chen, M. Zhou, Y. Qi, N. Xu, Q. Wu, Q. Hua, Y. Wu and Z. Liu, Antibacterial activity and mechanism of the sesquiterpene δ-cadinene against Listeria monocytogenes, LWT, 2024, 203, 116388 CrossRef CAS.
- C. Song, S. Luo, M. He, X. Zhao and M. Sun, Antibacterial mechanism of water-soluble CeO2 nanoflowers and its product design in antibacterial application, Appl. Surf. Sci., 2025, 679, 161249 CrossRef CAS.
- A. Baracca, G. Sgarbi, G. Solaini and G. Lenaz, Rhodamine 123 as a probe of mitochondrial film potential: Evaluation of proton flux through F0 during ATP synthesis, Biochim. Biophys. Acta, Bioenerg., 2003, 1606, 137–146 CrossRef CAS PubMed.
- L. D. Zorova, V. A. Popkov, E. Y. Plotnikov, D. N. Silachev, I. B. Pevzner, S. S. Jankauskas, V. A. Babenko, S. D. Zorov, A. V. Balakireva, M. Juhaszova, S. J. Sollott and D. B. Zorov, Mitochondrial film potential, Anal. Biochem., 2018, 552, 50–59 CrossRef CAS PubMed.
- A. M. Abdula, G. L. Mohsen, B. H. Jasim, M. S. Jabir, A. I. Rushdi and Y. Baqi, Synthesis, pharmacological evaluation, and in silico study of new 3-furan-1-thiophene-based chalcones as antibacterial and anticancer agents, Heliyon, 2024, 10, e32257 CrossRef CAS PubMed.
- X. Wang, F. Cheng, X. Wang, T. Feng, S. Xia and X. Zhang, Chitosan decoration improves the rapid and long-term antibacterial activities of cinnamaldehyde-loaded liposomes, Int. J. Biol. Macromol., 2021, 168, 59–66 CrossRef CAS PubMed.
- R. Zheng, G. Liao, J. Kang, S. Xiong and Y. Liu, An intelligent myofibrillar protein film for monitoring fish freshness by recognizing differences in anthocyanin (Lycium ruthenicum)-induced color change, Food Res. Int., 2024, 192, 114777 CrossRef CAS PubMed.
- S. Zhuang, H. Hong, L. Zhang and Y. Luo, Spoilage-related microbiota in fish and crustaceans during storage: Research progress and future trends, Compr. Rev. Food Sci. Food Saf., 2020, 20, 252–288 CrossRef PubMed.
- Y. Yang, X. Yu, Y. Zhu, Y. Zeng, C. Fang, Y. Liu, S. Hu, Y. Ge and W. Jiang, Preparation and application of a colorimetric film based on sodium alginate/sodium carboxymethyl cellulose incorporated with rose anthocyanins, Food Chem., 2022, 393, 133342 CrossRef CAS PubMed.
- O. Romruen, T. Karbowiak, R. Auras and S. Rawdkuen, Smart bilayer film: Quality monitoring for freshness of fish and minced pork delights, Food Packag. Shelf Life, 2024, 44, 101299 CrossRef CAS.
- S. You, X. Zhang, Y. Wang, Y. Jin, M. Wei and X. Wang, Development of highly stable color indicator films based on κ-carrageenan, silver nanoparticle and red grape skin anthocyanin for marine fish freshness assessment, Int. J. Biol. Macromol., 2022, 216, 655–669 CrossRef CAS PubMed.
- N. Wells, D. Yusufu and A. Mills, Colourimetric plastic film indicator for the detection of the volatile basic nitrogen compounds associated with fish spoilage, Talanta, 2019, 194, 830–836 CrossRef CAS PubMed.
- M. Saed, R. D. Ayivi, J. Wei and S. O. Obare, Gold nanoparticles antibacterial activity: Does the surface matter?, Colloid Interface Sci. Commun., 2024, 62, 100804 CrossRef CAS.
- A. Khan, P. Ezati and J. Rhim, Chitosan/gelatin-based multifunctional film integrated with green tea carbon dots to extend the shelf life of pork, Food Packag. Shelf Life, 2023, 37, 101075 CrossRef CAS.
- R. Sasikumar, S. Saranya, L. Lourdu Lincy, L. Thamanna and P. Chellapandi, Genomic insights into fish pathogenic bacteria: A systems biology perspective for sustainable aquaculture, Fish Shellfish Immunol., 2024, 154, 109978 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2026 |