 Open Access Article
Open Access ArticleUltrasound assisted extraction of betacyanin from dragon fruit (Hylocereus polyrhizus) for sustainable natural colorant production
Ruri Aditya Sari a,
Yudi Pranoto
a,
Yudi Pranoto *a,
Arima Diah Setiowati
*a,
Arima Diah Setiowati a and
Indriana Kartini
a and
Indriana Kartini b
b
aDepartment of Food and Agricultural Product Technology, Faculty of Agricultural Technology, Universitas Gadjah Mada, Jl. Flora No. 1, Bulaksumur, Yogyakarta, 55281, Indonesia. E-mail: pranoto@ugm.ac.id
bDepartment of Chemistry, Faculty of Mathematics and Natural Sciences, Universitas Gadjah Mada, Sekip Utara, Bulaksumur, 55281 Yogyakarta, Indonesia
First published on 9th January 2026
Abstract
Betacyanin, a natural pigment with potential antioxidant properties, is widely used in the food, cosmetic and pharmaceutical applications. However, it is sensitive to heat and light, presenting challenges in extraction and processing. This study comparatively evaluated conventional extraction and ultrasound-assisted extraction (UAE) for recovering betacyanin from Hylocereus polyrhizus flesh and peel. The novelty of this work lies in its integrated assessment of extraction efficiency, betacyanin yield, thermal degradation kinetics and bioactive compound profiles across two distinct plant matrices. Both methods were analyzed at varying solvent ratios and temperatures, and their effects on betacyanin yield were analyzed using three-way ANOVA, which revealed significant main and interaction effects. Spectrophotometric quantification, antioxidant assays, total phenolic content (TPC), total flavonoid content (TFC) and HPLC profiling were used to characterize extract quality. The highest betacyanin content was obtained using UAE with water as solvent at 45 °C for 10 minutes and 2.5 W g−1 for flesh (430.25 mg/100 g), and 0.5 W g−1 for peel (117.989 mg/100 g) respectively. UAE shortened the extraction time from 60 min to 10 min, an 83% reduction, indicating substantial energy savings while eliminating organic solvents and supporting green processing principles. Betacyanin showed marked degradation above 60 °C, especially under light exposure, confirming its thermolabile, photosensitive behavior. First-order-kinetics predominated under all thermal and photochemical conditions. Peel extracts exhibited slightly greater thermal stability, whereas flesh extracts demonstrated stronger antioxidant activity (66.61%). HPLC profiling confirmed betanin as the dominant pigment in the extract. Overall, UAE effectively enhances betacyanin recovery while retaining pigment integrity, offering a rapid, energy efficient and sustainable approach suitable for food grade natural colorant production.
Sustainability spotlightThis study advances sustainable natural colorant production by optimizing ultrasound assisted extraction (UAE) of betacyanins from dragon fruit flesh and peel, valorizing an agricultural by-product. Using food grade solvents shortens processing time and improves recovery while limiting thermal and photodegradation, thereby reducing energy input and solvent burden relative to prolonged extractions. The approach enables food manufacturers to substitute stable, bioactive, plant derived pigments for synthetic colorants. |
1. Introduction
Betacyanins are water-soluble nitrogenous pigments mostly found in plants of the Cactaceae family, notably dragon fruit (Hylocereus polyrhizus). They have drawn interest because of their vibrant color and potential health advantages. These pigments are well-known for their antioxidant properties, which are connected to several health advantages, including neuroprotective, anti-inflammatory, and anticancer activities. Notable sources of betacyanins include dragon fruit, especially Hylocereus polyrhizus. Often called pitaya, dragon fruit is a tropical fruit high in betanin and isobetanin, which help explain its unique color and health-promoting qualities.Dragon fruit flesh can be directly consumed to obtain its nutritional benefits. However, betacyanin extraction has several advantages, including broader applications. Consumers can obtain similar functional benefits from concentrated extracts at a far lower consumption than from the raw fruit. In addition, betacyanin extracts can be utilized more widely across food, nutraceutical, and cosmetic industries, where standardized pigment levels and functional properties are required. Betacyanins in fresh fruit are highly perishable and prone to enzymatic degradation, oxidation, and color loss. Extraction enables betacyanin stabilization, thereby improving shelf life.
Nowadays, consumers prefer natural ingredients because of their alleged improved quality, health benefits, and safety over synthetic components.1 Several natural food colorants have demonstrated comparable effectiveness to synthetic dyes.2 As a natural colorant and bioactive compound, betacyanin has been gradually used in the food, cosmetics, and pharmaceutical industries.3,4 Due to the sensitivity of dragon fruit against environmental factors, such as temperature, pH, and light, extracting betacyanin becomes challenging.5,6 The extraction method and conditions greatly affect the efficiency of betacyanin extraction and the recovery of related bioactive components in dragon fruit. Conventional extraction techniques may compromise thermolabile compounds by requiring large amounts of solvent use and long processing times. Often used solvents include water and ethanol because of their safety, economy, and sustainability for food-grade uses.7
Due to its complex matrix and the divergent solubilities of target compounds, extracting betacyanin from dragon fruit flesh and peel is technically challenging. There were differences in the extraction methods used, which included the composition of flesh or peel and the solvent. Priatni & Pradita8 and Khoo et al.9 used the composition of flesh/peel to solvent ratios ranging from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 to 1
4 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 under different extraction conditions. Thus, it is necessary to conduct related research to obtain a higher betacyanin content from both flesh and peel. Advanced techniques such as ultrasonic-assisted extraction (UAE) have been investigated to minimize solvent consumption and shorten processing times using acoustic cavitation, providing improved extraction of bioactive compounds, including betacyanin, while mitigating these constraints.10
10 under different extraction conditions. Thus, it is necessary to conduct related research to obtain a higher betacyanin content from both flesh and peel. Advanced techniques such as ultrasonic-assisted extraction (UAE) have been investigated to minimize solvent consumption and shorten processing times using acoustic cavitation, providing improved extraction of bioactive compounds, including betacyanin, while mitigating these constraints.10
Recent studies further highlight the significance of UAE as an energy-efficient and green technology for extracting bioactive compounds. Nabi et al.11 demonstrated that UAE markedly enhanced extraction efficiency while retaining pigment stability through the synergy of cavitation and eco-friendly solvent systems. Similarly, Ghiasvand et al.12 reported that UAE significantly increased mass transfer rates and reduced solvent usage by over 40% compared with conventional maceration. These findings confirm the capability of UAE to intensify extraction kinetics, improve compound recovery, and align with sustainability goals in natural product processing. Accordingly, integrating UAE for betacyanin extraction from Hylocereus polyrhizus may provide a comparable advantage by combining mechanical disruption and controlled energy input to release pigments efficiently without extensive solvent or temperature stress. In addition, Anwar13 highlighted the valorisation of agro-waste via green extraction, especially using UAE, pulsed electric fields, and other non-thermal methods for recovering high-value bioactive compounds in alignment with circular bioeconomy goals. These previous studies underline the importance of integrating sustainability metrics, solvent minimisation, non-thermal process intensification, and matrix valorisation, all elements that are inherent in this current study of UAE on the flesh and peel of dragon fruit.
UAE uses ultrasonic vibrations to destroy cell walls, release intracellular molecules, and reduce heat deterioration.11,12 Improved efficiency, lower energy usage, and shorter extraction times are among the advantages of UAE over traditional extraction techniques.13,14 Several factors determine the effectiveness of UAE, such as the type of solvent, the material-to-solvent ratio, the extraction time, the temperature, and the ultrasonic power.15 Altering parameters of the cavitation process, such as the temperature and intensity of ultrasonic waves, influences the extraction rate, thus improving the solubility of betacyanin and enabling mass transfer from the raw material to the solvent.15–18
Previous studies show that altering these parameters can significantly enhance the extraction of betacyanins and other bioactive compounds, including phenolics and flavonoids, which are closely associated with antioxidant activity.19 Although various studies have optimized UAE for plant matrices rich in betacyanin, such as beetroot and prickly pear, most focused on a single matrix type (flesh or peel). The process intensification of UAE affects factors such as betacyanin content under rapid energy input, the selective recovery of coexisting bioactive compounds, and extraction kinetics from different plant matrices (flesh and peel) with distinct cellular architectures. Kaur et al.20 optimized UAE conditions for beetroot with a primary focus on maximizing pigment yield without explicitly modelling the combined effects of solvent ratio, ultrasonic power, and temperature. Similarly, Linares and Rojas10 and Thuy et al.21 investigated UAE in Hylocereus polyrhizus, but neither used a full factorial design nor simultaneously compared flesh and peel matrices. Therefore, limited factor interactions, such as matrix type, extraction conditions, solvent type, and other critical scientific questions, remain unresolved. The current study fills this research gap by performing three-way ANOVA-based analyses that integrate solvent ratio, temperature, and ultrasonic power to evaluate their main and interactive effects on betacyanin content in both flesh and peel. This dual-matrix approach is unprecedented among prior UAE optimization studies of dragon fruit, beetroot, and prickly pear. Apart from assessing betacyanin levels, evaluating antioxidant capacity, total phenolic content, and total flavonoid content, and profiling specific betacyanin components are necessary to provide a whole picture of the bioactive capacity of the obtained molecules.22,23
The study aimed to analyse the effect of solvent type, material-to-solvent ratio, and extraction time on the betacyanin content. It also evaluates ultrasonic-assisted extraction parameters, including solvent ratio, extraction temperature, and ultrasonic power, to improve the extraction of betacyanin from dragon fruit (Hylocereus polyrhizus) from both the flesh and peel. Betacyanin integrity and stability (pigment retention and thermal kinetic degradation), bioactive qualities (antioxidant, phenolic, and flavonoid analysis), and betacyanin profile were investigated on the sample with the highest betacyanin content to ascertain how the extraction technique affected the integrity, stability, bioactive compounds, and betacyanin profile. The results of this work should improve the development of sustainable and efficient techniques for synthesizing bioactive compounds and natural pigments from dragon fruit.
2. Materials and methods
2.1 Materials
Dragon fruit (Hylocereus polyrhizus) was cultivated in Sambirejo Village, Banyuwangi Regency, East Java, Indonesia (8°30′09.9″ Latitude, 114°09′26.8″ Longitude) and was purchased from the local market of Sayur Pawon (Yogyakarta, Indonesia) with a maturity index of harvest index IV (maturity degree about 11–13.5°Brix with a fruit weight ranging from 400–650 grams). The plantation is located in a low-elevation tropical coastal area (average temperature 28–33 °C; relative humidity 70–85%) with volcanic loam soil and drip irrigation. Seventy percent ethanol (Merck, analytical grade) and distilled water served as solvents. Folin–Ciocalteu reagent (Supelco), sodium carbonate (Supelco), citric acid (Merck), sodium phosphate (Merck), gallic acid (Sigma-Aldrich), 2,2-diphenyl-1-picrylhydrazyl (DPPH; Sigma-Aldrich), sodium nitrate (Merck), sodium hydroxide (Merck), sodium acetate (Supelco), betanin standard (Sigma-Aldrich), aluminium chloride (Merck), acetonitrile (Supelco), and formic acid (Merck) were used in this study. All the reagents used were of analytical grade.As pre-treatment, the dragon fruit was well cleaned with water, then sliced and separated into peel and flesh. The peel was diced into small pieces and dehydrated at 40 °C for 24 hours. The dried peel was then ground and sieved through a 100 mesh sieve. The peel powder was preserved in a hermetically sealed aluminium foil bag at 30 °C until further examination. The flesh was homogenized for 5 minutes using a Waring blender and thereafter kept at 5 °C for future examination.
2.2 Conventional extraction
Conventional betacyanin extraction of dragon fruit flesh and peel was performed using distilled water and 70% ethanol at various material to solvent ratios (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, and 1
3, and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 v/w for flesh and 1
5 v/w for flesh and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, 1
10, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30, and 1
30, and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 v/w for peel). The mixtures were then swirled in a water bath set at 50 °C for 25, 60, 120, and 180 minutes following previously reported static maceration methods for betacyanin matrices.8,20 Afterwards, the mixtures were centrifuged for 5 minutes at 2500 rpm, and the supernatant was collected. The supernatant was further filtered using a muslin cloth, resulting in filtrate and residue. The filtrate was concentrated using a rotary evaporator at 45 °C for 20 minutes to separate the extract from the solvent.
50 v/w for peel). The mixtures were then swirled in a water bath set at 50 °C for 25, 60, 120, and 180 minutes following previously reported static maceration methods for betacyanin matrices.8,20 Afterwards, the mixtures were centrifuged for 5 minutes at 2500 rpm, and the supernatant was collected. The supernatant was further filtered using a muslin cloth, resulting in filtrate and residue. The filtrate was concentrated using a rotary evaporator at 45 °C for 20 minutes to separate the extract from the solvent.
Water and 70% ethanol were selected as extraction solvents based on their polarity, safety, and ability to solubilize betacyanin. The material-to-solvent ratios and extraction times were selected based on preliminary trials (data not shown). Lower ratios ensure sufficient solvent availability to dissolve betacyanins from soft matrices such as flesh, whereas higher ratios are required for the peel due to its thicker cell walls, higher fiber content, and reduced solvent diffusivity. Extraction times were chosen to capture both the rapid pigment release under UAE and the slower diffusion-driven release occurring during conventional extraction.20 This conventional extraction process served as a baseline without ultrasonic assistance to quantify the enhancement by UAE in terms of betacyanin content, time reduction, and pigment retention under comparable solvent conditions.
To maintain pigment stability, extraction was performed in a dark environment, and the pH of both aqueous and ethanolic solvents was maintained at pH 5. This slightly acidic range was selected because it provides the most stable pH conditions for betacyanin, minimizing color degradation and deprotonation of chromophores.5,24,25
2.3 Ultrasound assisted extraction (UAE)
An ultrasound homogenizer with a probe (UP200ST Ultrasound Processor, Hielscher, Germany) was used to perform the betacyanin extraction from the flesh and peel of dragon fruit. The material was mixed with water at various material-to-solvent ratios. The mixture was then exposed to different ultrasound powers (0.5 W g−1, 2.5 W g−1, and 4.5 W g−1) and varying temperatures of 30 °C, 45 °C, and 60 °C for 10 minutes. The selected range of 30–60 °C was chosen to account for betacyanin's thermal tolerance, since degradation typically accelerates above 60 °C.26,27 Temperatures below 30 °C reduce solubility and mass transfer efficiency.28 Similarly, the ultrasonic power range of 0.5–4.5 W g−1 represented the optimal cavitation intensity spectrum for pigment extraction while avoiding localized overheating, as reported by previous studies.14,20 Lower power levels (<0.5 W g−1) cause incomplete cell disruption, whereas higher power levels (>4.5 W g−1) may induce pigment oxidation through excessive cavitation collapse. In addition, ultrasonic power in this study was expressed as W g−1 rather than absolute power (W) to accurately represent the acoustic energy delivered per unit mass of the sample.2.4 Betacyanin content
Following Morales,24 the betacyanin content in all extracts was measured at 535 nm using a UV/Vis spectrophotometer (Thermofisher, USA). The following equation calculates the betacyanin concentration. Here, A is the absorbance; DF is the dilution factor; V is the volume; W is the sample weight; L is the cuvette path length. In this study, the molar extinction coefficients (ε) and molecular weight (MW) were applied to calculate concentration and absorbance values. The parameters are defined as follows: MW = 550 g mol−1; ε in H2O = 65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 L mol−1 cm−1. Results were expressed as mg per 100 g of material. The betacyanin standard was dissolved in a McIlvaine buffer solution at pH 6.5 using 4.0 mL of McIlvaine buffer as the blank.
000 L mol−1 cm−1. Results were expressed as mg per 100 g of material. The betacyanin standard was dissolved in a McIlvaine buffer solution at pH 6.5 using 4.0 mL of McIlvaine buffer as the blank.
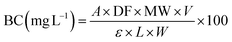 | (1) |
2.5 Betacyanin retention
The betacyanin retention was calculated using the equation proposed by Otálora et al.29 by considering the initial content of betacyanin in the sample and the final content after a determined amount of time (at 20–120 minutes) at 40 °C, 50 °C, 60 °C, 70 °C, and 80 °C under two different conditions (light exposure and dark conditions). The selected temperature range was based on the known thermolability of betacyanin and reflects the typical thermal conditions encountered in industrial food processing. In addition, the selected exposure times were chosen to capture both early-stage degradation kinetics and prolonged thermal effects.The light exposure treatment was performed using a white fluorescent light source with an average intensity of 5000 ± 200 lux (approximately 9.1 W m−2). Furthermore, the dark conditions were achieved by wrapping the samples in aluminum foil to eliminate external light interference. Betacyanin retention can be calculated using eqn (2).
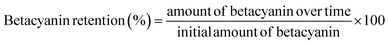 | (2) |
2.6 Thermal kinetic degradation
The selection of the kinetic model used in this study followed both mechanistic considerations and statistical goodness of fit criteria. The best-fitting kinetic model was selected based on regression performance, with the model having the highest coefficient of determination (R2) and the lowest residual error.The kinetic rate constant (k) of thermal degradation was calculated by regression of the experimental data of the initial concentration with time, as described by Rosa et al.:30
CB = CB0 − kt![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) zero-order kinetic zero-order kinetic
| (3) |
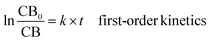 | (4) |
The half-life time (t1/2), defined as the time needed for the response of interest to be reduced by 50%, was estimated as follows:
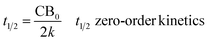 | (5) |
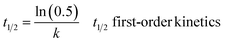 | (6) |
2.7 Bioactive compound analysis
The antioxidant activity (DPPH assay), total phenolic content (TPC), and total flavonoid content (TFC) of the extract were determined on the sample with the highest betacyanin content to ascertain how the extraction technique affected the bioactive substance. All analyses were performed in triplicate (n = 3), and the results were expressed on a dry weight basis (mg GAE per 100 g DW for TPC; mg QE per 100 g DW for TFC).
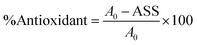 | (7) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 v/v), 2 mL of 20% w/v sodium carbonate solution, and 2 mL of distilled water. The sample was kept in the dark for 1 hour, and then its absorbance was measured at 700 nm using a UV-Vis spectrophotometer. A standard gallic acid curve spanning 50 to 550 mg L−1 was developed, yielding R2 = 0.998, to measure concentration. The results were expressed as mg gallic acid equivalents per 100 g dry weight (mg GAE per 100 g DW).
3 v/v), 2 mL of 20% w/v sodium carbonate solution, and 2 mL of distilled water. The sample was kept in the dark for 1 hour, and then its absorbance was measured at 700 nm using a UV-Vis spectrophotometer. A standard gallic acid curve spanning 50 to 550 mg L−1 was developed, yielding R2 = 0.998, to measure concentration. The results were expressed as mg gallic acid equivalents per 100 g dry weight (mg GAE per 100 g DW).2.8 Betacyanin profile
The betacyanin profile was investigated on the sample with the highest betacyanin content to ascertain how the extraction technique affected the betacyanin profile. HPLC analysis was performed using a Shimadzu Prominence system. An HPLC system equipped with an SPD M20A detector was run at 30 °C following the method by Faridah et al.32 Data were processed using LabSolution software. The HPLC system was equipped with a Shim-pack Gist column (5 µm, 150 × 4.6 mm) coupled with a guard column (5 µm, 10 × 4.0 mm). Beginning at 20% solvent B (80% acetonitrile in water) in solvent A (formic acid, pH 3) and working to 40% B in A + B at a flow rate of 1 mL min−1, a linear gradient was applied over 40 minutes. An injection volume of 20 µL was used and the compounds were detected at 530 nm for the flesh betacyanin extract.2.9 Statistical analysis
Every study was performed in triplicate at two levels of replication (biological and analytical replicates, n = 3); data were presented as mean values ± standard deviation. Post-hoc comparisons were made using Tukey's HSD test at a 95% confidence level (p < 0.05) and were examined using one-way ANOVA to evaluate variations among the outcomes. Differences between treatments were evaluated using three-way Analysis of Variance (ANOVA) with factors (solvent ratio × time × solvent type) for conventional extraction and (solvent ratio × temperature × ultrasonic power) for UAE. These factors exerted statistically significant impacts on betacyanin content (p < 0.001). To facilitate direct comparison, the same solvent, ratios, and sample masses were used in both extraction methods, and data were normalized per 100 g dry weight. The analysis computed partial Eta2 and adjusted R2 to determine the effect size and model fit for each parameter combination. All analyses were performed using SPSS v21.0.3. Results and discussion
3.1 Effect of material to solvent ratio, solvent type and extraction time on betacyanin content
In general, increasing the solvent ratio under water extraction exhibited a positive trend (p < 0.05) on betacyanin content for both flesh and peel matrices. This suggests that a larger solvent volume enhances pigment solubilization, increases the concentration gradient, and promotes better mass transfer during extraction.33For flesh, the trend showed that the extraction time influenced betacyanin content, where betacyanin content initially increased, reached a peak, and then slightly declined at prolonged durations, which indicates a potential onset of thermal degradation. In the flesh extracted with water at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio, betacyanin content increased from 253.83 mg/100 g at 25 minutes to a maximum of 268.86 mg/100 g at 60 minutes and slightly decreased at 120 and 180 minutes (Table 1). At a 1
1 ratio, betacyanin content increased from 253.83 mg/100 g at 25 minutes to a maximum of 268.86 mg/100 g at 60 minutes and slightly decreased at 120 and 180 minutes (Table 1). At a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 ratio, the betacyanin content increased from 322.86 mg/100 g to 339.83 mg/100 g before declining. A similar trend was observed at a 1
3 ratio, the betacyanin content increased from 322.86 mg/100 g to 339.83 mg/100 g before declining. A similar trend was observed at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 ratio, with the optimum at 60 minutes (465.63 mg/100 g), followed by a slight decrease. This indicates that the optimal water extraction for flesh occurs at 60 minutes, after which degradation may reduce pigment content.34 For the peel samples, the trend was consistent, higher solvent ratios led to higher betacyanin content across all times (p < 0.05). However, in the peel, the effect of time was less pronounced, with a more stable betacyanin content observed over 60 minutes. At a 1
5 ratio, with the optimum at 60 minutes (465.63 mg/100 g), followed by a slight decrease. This indicates that the optimal water extraction for flesh occurs at 60 minutes, after which degradation may reduce pigment content.34 For the peel samples, the trend was consistent, higher solvent ratios led to higher betacyanin content across all times (p < 0.05). However, in the peel, the effect of time was less pronounced, with a more stable betacyanin content observed over 60 minutes. At a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 ratio, the yield increases from 26.28 mg per 100 g (25 min) to 36.06 mg per 100 g (120 min) before slightly decreasing (Table 2). At a 1
10 ratio, the yield increases from 26.28 mg per 100 g (25 min) to 36.06 mg per 100 g (120 min) before slightly decreasing (Table 2). At a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 ratio, the values increased from 40.62 mg/100 g to 45.31 mg/100 g, then declined to 35.00 mg/100 g at 180 minutes. At the 1
30 ratio, the values increased from 40.62 mg/100 g to 45.31 mg/100 g, then declined to 35.00 mg/100 g at 180 minutes. At the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 ratio, the maximum reached 56.46 mg/100 g at 60 minutes, supporting that water is an effective solvent, especially at higher ratios and intermediate times.
50 ratio, the maximum reached 56.46 mg/100 g at 60 minutes, supporting that water is an effective solvent, especially at higher ratios and intermediate times.
| Sample | Solvent | Extraction time (minutes) | Betacyanin (mg per 100 g) | ||
|---|---|---|---|---|---|
Material to solvent ratio (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
Material to solvent ratio (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) 3) |
Material to solvent ratio (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) 5) |
|||
| a Values expressed as mean ± S. D. (n = 3). Values with different lowercase letters within the same column indicate significant differences (p < 0.05) from each other; values with different uppercase letters within the same row indicate significant differences (p < 0.05) from each other. | |||||
| Flesh | Water | 25 | 253.83 ± 0.86eA | 322.86 ± 0.70gB | 333.40 ± 0.60eC |
| 60 | 268.86 ± 0.71gA | 339.83 ± 0.02hB | 465.63 ± 0.75 hC | ||
| 120 | 260.62 ± 0.01fA | 320.65 ± 0.62fA | 447.14 ± 0.74gC | ||
| 180 | 243.05 ± 0.02cA | 295.68 ± 0.60eB | 377.95 ± 0.80fC | ||
| Ethanol | 25 | 211.05 ± 0.80aA | 239.49 ± 0.82bB | 278.66 ± 0.33bC | |
| 60 | 248.96 ± 0.71dA | 262.29 ± 0.82dB | 292.74 ± 0.83dC | ||
| 120 | 230.67 ± 0.71bA | 245.70 ± 0.85cB | 283.69 ± 0.78cC | ||
| 180 | 210.39 ± 0.82aA | 220.39 ± 0.82aB | 263.03 ± 0.70aC | ||
| Sample | Solvent | Extraction time (minutes) | Betacyanin (mg per 100 g) | ||
|---|---|---|---|---|---|
Material to solvent ratio (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) 10) |
Material to solvent ratio (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30) 30) |
Material to solvent ratio (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) 50) |
|||
| a Values expressed as mean ± S. D. (n = 3). Values with different lowercase letters within the same column indicate significant differences (p < 0.05) from each other; values with different uppercase letters within the same row indicate significant differences (p < 0.05) from each other. | |||||
| Peel | Water | 25 | 26.28 ± 0.05eA | 40.62 ± 0.26dB | 43.73 ± 0.25bC |
| 60 | 36.64 ± 0.14gA | 50.59 ± 0.30gB | 56.46 ± 0.82fC | ||
| 120 | 36.06 ± 0.23gA | 45.31 ± 0.32fB | 49.90 ± 0.43dC | ||
| 180 | 30.69 ± 0.05fA | 35.00 ± 0.80cB | 44.30 ± 0.66bC | ||
| Ethanol | 25 | 16.63 ± 0.14bA | 29.24 ± 0.10aB | 39.27 ± 0.43aC | |
| 60 | 22.59 ± 0.33dA | 46.17 ± 0.30fB | 58.72 ± 0.40gC | ||
| 120 | 18.87 ± 0.60cA | 42.45 ± 0.15eB | 52.94 ± 0.66eC | ||
| 180 | 12.13 ± 0.05aA | 33.09 ± 0.30bB | 48.19 ± 0.25cC | ||
A similar positive effect (p < 0.05) of increasing the solvent ratio was observed with ethanol as a solvent, although the overall yields were lower than those observed with water. The ratio effect was consistent across all times in both flesh and peel, but time-related trends varied between matrices. For flesh extracted with ethanol, it increased by around 60 minutes and then declined. For instance, at lower solvent volume (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), betacyanin levels initially increased with extraction time but later declined as prolonged heating promoted pigment degradation. In contrast, at a higher solvent ratio (1
1), betacyanin levels initially increased with extraction time but later declined as prolonged heating promoted pigment degradation. In contrast, at a higher solvent ratio (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5), the pigment continued to accumulate throughout the extraction period, reaching a maximum and then decreasing at extended durations, indicating the typical balance between enhanced diffusion and time-dependent thermal degradation. This indicated that flesh pigments were more prone to degradation under extended ethanol extraction, possibly as a result of ethanol's lower polarity, which reduced pigment stability.28 In contrast, peel extracted with ethanol displayed higher stability and a clear ratio-dependent trend. At a 1
5), the pigment continued to accumulate throughout the extraction period, reaching a maximum and then decreasing at extended durations, indicating the typical balance between enhanced diffusion and time-dependent thermal degradation. This indicated that flesh pigments were more prone to degradation under extended ethanol extraction, possibly as a result of ethanol's lower polarity, which reduced pigment stability.28 In contrast, peel extracted with ethanol displayed higher stability and a clear ratio-dependent trend. At a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 ratio, betacyanin showed a modest increase over time, while at 1
10 ratio, betacyanin showed a modest increase over time, while at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 and 1
30 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 ratios, the extraction reached progressively higher maxima before plateauing, reflecting the enhanced diffusion capacity afforded by greater solvent availability, surpassing ethanol extracted flesh at the same ratio and time. These values indicated that the peel structure offers protection, possibly due to fiber and pectin components that reduce oxidation and facilitate controlled release.34
50 ratios, the extraction reached progressively higher maxima before plateauing, reflecting the enhanced diffusion capacity afforded by greater solvent availability, surpassing ethanol extracted flesh at the same ratio and time. These values indicated that the peel structure offers protection, possibly due to fiber and pectin components that reduce oxidation and facilitate controlled release.34
Moreover, flesh generally contained higher betacyanin concentrations, reflecting its native pigment abundance. However, peel exhibited higher stability during extraction, particularly in ethanol, where yields were better maintained over time. This stability was due to the structural matrix of the peel, which protects pigments from rapid degradation.34 Importantly, the positive effect of increasing the material-to-solvent ratio was observed universally across all matrices, times, and solvents, highlighting it as the dominant factor for optimizing extraction. In contrast, extraction time played a matrix-specific role: the flesh was time-sensitive, while the peel remained stable, especially during ethanol extraction.
The higher the solvent-to-material ratio, the greater the concentration difference between the material and the solvent. This results in a more optimal release of betacyanin into both solvents (water and ethanol).34 The use of excess solvent could facilitate mass transfer efficiency due to the imbalance between the solvent volume and the material's surface area.35 At higher solvent ratios (above 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5), the concentration gradient between the material and the solvent becomes lower. A low concentration gradient reduces the diffusion rate of betacyanin from the material to the solvent, thereby slowing down the extraction process.28 These findings align with previous research that underscores the need to adjust solvent to material ratios to get greater pigment content.30 This also aligns with the findings by Esquivel et al.,36 who reported that excessive solvent volume can facilitate mass transfer efficiency. At a ratio of 1
5), the concentration gradient between the material and the solvent becomes lower. A low concentration gradient reduces the diffusion rate of betacyanin from the material to the solvent, thereby slowing down the extraction process.28 These findings align with previous research that underscores the need to adjust solvent to material ratios to get greater pigment content.30 This also aligns with the findings by Esquivel et al.,36 who reported that excessive solvent volume can facilitate mass transfer efficiency. At a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, the balance between the solvent volume and material surface area maximizes pigment extraction efficiency. Peel extraction required a higher ratio of 1
5, the balance between the solvent volume and material surface area maximizes pigment extraction efficiency. Peel extraction required a higher ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 for optimal results due to the peel's more complex cellular structure, requiring greater solvent penetration.30 The betacyanin content improved from a 1
50 for optimal results due to the peel's more complex cellular structure, requiring greater solvent penetration.30 The betacyanin content improved from a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 to a 1
10 to a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 ratio, by approximately 35% for water extraction (Table 2). Water-based extraction consistently produced higher betacyanin content than ethanol-based extraction at all material-to-solvent ratios for fruit flesh.
50 ratio, by approximately 35% for water extraction (Table 2). Water-based extraction consistently produced higher betacyanin content than ethanol-based extraction at all material-to-solvent ratios for fruit flesh.
The observed increase in betacyanin content with higher solvent ratios was consistent with Fick's law of diffusion, which predicts that a greater concentration gradient promotes faster solute migration.37 However, excessive solvent volume reduced the driving force once the solute concentration approached equilibrium, explaining the plateau at high ratios.37 The difference in results could be attributed to water's higher polarity and dielectric constant, which enhanced cavitation efficiency and betacyanin solubility and diffusion phenomenon.32,33,37,38 Betacyanins are highly polar molecules containing multiple hydroxyl and imine groups, thus exhibiting strong solubility in aqueous environments.39 A few hydroxyl groups (–OH) existing in betacyanin compounds, which lead to charge polarization and hydrogen bonding, are responsible for the hydrophilic properties of betacyanins. Water, with its high dielectric constant, facilitates hydrogen bonding and increases mass transfer coefficients at the solid–liquid interface, thereby accelerating pigment diffusion from intracellular vacuoles into the solvent phase. Conversely, ethanol's lower polarity index and weaker hydrogen-bonding ability result in reduced pigment solubility and a lower dielectric constant that limit charge stabilization within the chromophore system.
The results were similar to the findings of Das et al.,40 who reported a similar trend in dragon fruit flesh when extracting anthocyanin using water and ethanol. Moreover, the results demonstrated that extraction time significantly (p < 0.05) affected the yield of betacyanin, irrespective of whether water or ethanol was used as the solvent across all ratios.
Extraction time affected betacyanin content following a saturation trend initially governed by fast surface diffusion, then transitioning to internal diffusion control as walls become depleted.32 Mechanistically, the positive influence of temperature (up to 60 °C) and extraction time (≤60 min) reflected improved cell wall permeability and diffusion kinetics. The highest betacyanin content was observed for both fruit flesh and peel at 60 minutes of extraction time. The plateau beyond 60 minutes suggested attainment of extraction equilibrium, where additional exposure only promoted degradation of the thermolabile betacyanin chromophore.
The results revealed that extending the extraction time to 120 and 180 minutes did not increase the betacyanin content, indicating that the extraction process reaches a saturation point after a specific duration. This observation is consistent with the findings of da Silva et al.4 and Sokolova et al.,25 who reported that prolonged extraction times can lead to betacyanin oxidation and the degradation of thermolabile compounds, thereby reducing pigment stability and yield. Beyond this optimal period, the decrease in betacyanin content is primarily attributed to pigment degradation caused by extended exposure to light and oxygen, or thermal effects.6
As for the peel, the maximum betacyanin content for both water and ethanol extraction was achieved at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 solvent ratio after 60 minutes of extraction. The slightly superior performance of ethanol over water for peel was due to its ability to penetrate the peel's fibrous structure and extract non-polar compounds.39 These results are consistent with a study by Khoo et al.,9 who observed that the peel of fruits generally required the highest solvent ratios for optimal extraction, possibly due to its denser composition. The peel of dragon fruit, being more fibrous and less pigmented than the flesh, requires more solvent to facilitate efficient extraction. However, it is worth noting that water application resulted in only a slight decrease in betacyanin content (approximately 1 mg/100 g). To conclude this step, the optimal ratio was 1
50 solvent ratio after 60 minutes of extraction. The slightly superior performance of ethanol over water for peel was due to its ability to penetrate the peel's fibrous structure and extract non-polar compounds.39 These results are consistent with a study by Khoo et al.,9 who observed that the peel of fruits generally required the highest solvent ratios for optimal extraction, possibly due to its denser composition. The peel of dragon fruit, being more fibrous and less pigmented than the flesh, requires more solvent to facilitate efficient extraction. However, it is worth noting that water application resulted in only a slight decrease in betacyanin content (approximately 1 mg/100 g). To conclude this step, the optimal ratio was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 for flesh and 1
5 for flesh and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 for peel for both solvents, with an extraction time of 60 minutes and water as the solvent. The three-way ANOVA revealed that all main effects (solvent type, extraction time, and solvent ratio) had statistically significant impacts on betacyanin content (p < 0.001). These effects were substantiated by large F-values and very high effect sizes, confirming that the model robustly explains nearly all observed variations in betacyanin content. Partial Eta2 represents the effect size and reflects the magnitude or strength of the effect. The adjusted R2 value of 0.999 further supports the model's explanatory power, aligning with previous findings on solid–liquid extraction dynamics.35 The interaction analysis demonstrated different levels of influence in flesh and peel matrices (Table 3).
50 for peel for both solvents, with an extraction time of 60 minutes and water as the solvent. The three-way ANOVA revealed that all main effects (solvent type, extraction time, and solvent ratio) had statistically significant impacts on betacyanin content (p < 0.001). These effects were substantiated by large F-values and very high effect sizes, confirming that the model robustly explains nearly all observed variations in betacyanin content. Partial Eta2 represents the effect size and reflects the magnitude or strength of the effect. The adjusted R2 value of 0.999 further supports the model's explanatory power, aligning with previous findings on solid–liquid extraction dynamics.35 The interaction analysis demonstrated different levels of influence in flesh and peel matrices (Table 3).
| Sample | Interaction | F value | Sig. | p-Value | Partial Eta2 |
|---|---|---|---|---|---|
| a R2 = 0.996 (adjusted R2 = 0.994). | |||||
| Flesh | Solvent × time | 32.396 | 0 | <0.001 | 0.669 |
| Solvent × ratio | 567.586 | 0 | <0.001 | 0.959 | |
| Time × ratio | 44.512 | 0 | <0.001 | 0.848 | |
| Solvent × time × ratio | 92.95 | 0 | <0.001 | 0.921 | |
| Peel | Solvent × time | 61.149 | 0 | <0.001 | 0.793 |
| Solvent × ratio | 2412.904 | 0 | <0.001 | 0.990 | |
| Time × ratio | 151.699 | 0 | <0.001 | 0.950 | |
| Solvent × time × ratio | 193.809 | 0 | <0.001 | 0.960 | |
In the flesh, the interaction between the solvent and ratio yielded the highest F value (F = 567.586; partial Eta2 = 0.959), indicating a strong dependency of pigment recovery on the volume of solvent used in relation to the solid material.
This supports the principle that optimal solvent availability enhances diffusion gradients, which are central to mass transfer efficiency.41 Excessive solvent beyond this point may dilute the concentration gradient and reduce mass transfer efficiency due to the imbalance between the solvent volume and material surface area.21 This phenomenon is also supported by diffusion theory, where a lower concentration gradient reduces the diffusion rate of solutes into the solvent, ultimately slowing the extraction process.24 However, excessive solvent may dilute the system and reduce the driving force for diffusion, as also noted by Cacace and Mazza.33
The interaction between time and ratio showed significant effects (F = 44.512, p < 0.001), as well as the interaction between solvent and time (F = 32.396, p < 0.001), suggesting that these parameters jointly determine the efficiency of pigment liberation from the matrix. Overexposure or insufficient time can both impair recovery due to thermal degradation or incomplete diffusion.36 The three-way interaction (interaction of solvent, time, and ratio) in the flesh was also significant (F = 92.950, partial Eta2 = 0.921, p < 0.001), indicating the synergistic influence of all extraction variables. This suggests that optimal betacyanin content is not the result of one parameter alone but of their combined configuration. These findings align with the principles of mass transfer during solid–liquid extraction, where solute migration depends heavily on the solvent polarity, concentration gradients, and contact time.21,26 The solvent plays a pivotal role in pigment solubilization: polar solvents such as water enhance the release of hydrophilic pigments like betacyanin, whereas less polar solvents (i.e., ethanol) may show lower affinity.4 A significant interaction between solvent and extraction time (F = 61.149, p < 0.001) indicates that the optimal extraction time varies by the solvent. Water-based extractions demonstrated higher stability and pigment retention between 60 and 120 minutes, in line with Delgado-Vargas et al.42
In contrast, the peel matrix exhibited even more pronounced effects; the solvent and ratio interaction had an exceptionally high F value (F = 2412.904, partial Eta2 = 0.990), underscoring the criticality of solvent accessibility in penetrating the more fibrous and pectin-rich structure of the peel. Interaction between time and ratio (F = 151.699) and between solvent and time (F = 61.149) further demonstrated substantial influences, affirming the necessity for well timed, matrix specific extraction strategies. In addition, the three-way interaction in peel (F = 193.809, partial Eta2 = 0.960) confirms that maximum pigment extraction from tougher matrices like peel demands optimized tuning across all variables.
3.2 Ultrasound-assisted extraction on betacyanin content
Ultrasound-assisted extraction (UAE) was done to extract betacyanin from both flesh and peel using water as a solvent with different ultrasound powers (0.5 W g−1, 2.5 W g−1, and 4.5 W g−1) and temperatures (30 °C, 45 °C, and 60 °C) for 10 minutes. A clear trend was observed in the influence of ultrasonic power, temperature, and solvent ratio on the betacyanin content from flesh and peel (Tables 4 and 5). The enhancement in extraction efficiency under UAE conditions can be explained by acoustic cavitation. Ultrasonic waves induce alternating compression and rarefaction cycles in the solvent, generating microbubbles that collapse violently upon reaching the critical pressure. This implosion produces localized microjets and shockwaves, disrupting plant cell walls and increasing the permeability of cytoplasmic membranes.42 The cavitation-induced shear stresses disintegrate the middle lamella and facilitate solvent penetration into the cellular matrix, enhancing pigment leaching and intracellular diffusion.42| Power | Material to solvent ratio | Betacyanin content (mg per 100 g) | ||
|---|---|---|---|---|
| 30 °C | 45 °C | 60 °C | ||
| a Values expressed as mean ± S. D. (n = 3). Values with different lowercase letters within the same column indicate significant differences (p < 0.05) from each other; values with different uppercase letters within the same row indicate significant differences (p < 0.05) from each other. | ||||
| 0.5 W g−1 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
234.346 ± 0.40aB | 262.449 ± 0.40bD | 198.579 ± 0.69bA |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
242.657 ± 1.20bC | 281.240 ± 0.60aA | 215.695 ± 1.21cB | |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
256.613 ± 0.90dC | 303.330 ± 0.83aA | 234.996 ± 0.60dB | |
| 2.5 W g−1 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
291.199 ± 0.40fE | 416.929 ± 0.69eH | 305.144 ± 0.40gG |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
302.624 ± 0.32gE | 423.023 ± 0.81fH | 324.472 ± 1.60hG | |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
321.199 ± 0.80hF | 430.245 ± 1.21gH | 337.502 ± 1.40iG | |
| 4.5 W g−1 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
247.446 ± 0.80cC | 297.679 ± 0.81cF | 264.191 ± 0.81eD |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
287.565 ± 1.07eD | 317.035 ± 1.60dF | 273.930 ± 0.54aA | |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
289.126 ± 1.61eD | 319.372 ± 1.41dF | 293.571 ± 1.21fE | |
| Power | Material to solvent ratio | Betacyanin content (mg per 100 g) | ||
|---|---|---|---|---|
| 30 °C | 45 °C | 60 °C | ||
| a Values expressed as mean ± S. D. (n = 3). Values with different lowercase letters within the same column indicate significant differences (p < 0.05) from each other; values with different uppercase letters within the same row indicate significant differences (p < 0.05) from each other. | ||||
| 0.5 W g−1 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
74.462 ± 0.93eA | 90.388 ± 0.47cD | 89.664 ± 0.62dB |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 30 |
42.333 ± 0.51bB | 73.863 ± 1.34aF | 67.440 ± 0.88aA | |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 50 |
102.641 ± 0.83iC | 117.989 ± 0.01gF | 97.845 ± 0.01fB | |
| 2.5 W g−1 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
85.734 ± 0.89gC | 102.902 ± 0.18dG | 94.732 ± 0.99eE |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 30 |
51.383 ± 0.51dB | 83.205 ± 0.01bG | 56.346 ± 0.51bE | |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 50 |
104.080 ± 0.83aA | 112.713 ± 0.83fE | 109.356 ± 1.44hD | |
| 4.5 W g−1 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
79.425 ± 0.82fB | 108.176 ± 1.29eH | 98.868 ± 0.47fF |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 30 |
45.252 ± 0.51cC | 107.437 ± 0.01eH | 84.373 ± 0.51cG | |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 50 |
97.844 ± 0.01hB | 112.234 ± 1.44fE | 103.121 ± 0.83gC | |
At 2.5 W g−1 power levels in the UAE, mechanical effects dominated over thermal effects, promoting pigment release without excessive degradation. In contrast, excessive power (4.5 W g−1) increased thermal hotspots (>70 °C) that accelerated oxidative degradation of the chromophore, leading to color fading. It was confirmed that UAE efficiency was governed not merely by time and temperature but also by controlled microscale energy transfer at the solvent–matrix interface.43 At a fixed ultrasonic power, increasing the extraction temperature generally enhanced the betacyanin content. This was particularly prominent at 2.5 W g−1, where a temperature rise from 30 °C to 45 °C improved betacyanin content considerably. However, further increases at 60 °C did not always result in proportional gains. This suggests the thermal degradation of betacyanin at higher temperatures.36 Similarly, higher solvent ratios consistently yielded more betacyanin content, particularly up to a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 ratio in flesh and 1
5 ratio in flesh and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 in peel. This enhancement was attributed to greater concentration gradients that favor solute diffusion.44
50 in peel. This enhancement was attributed to greater concentration gradients that favor solute diffusion.44
Nevertheless, at high ultrasonic power (4.5 W g−1), betacyanin content decreased, indicating possible degradation due to cavitation induced thermal hotspots.34,45 A moderate ultrasonic power (2.5 W g−1) was consistently more effective than lower or higher settings. This was evident in the flesh at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 ratio and 45 °C, where power at 2.5 W g−1 yielded 423.02 mg/100 g compared to 281.24 mg/100 g (0.5 W g−1) and 317.03 mg/100 g (4.5 W g−1). Peel data mirrored this pattern. Excessive power may cause local overheating and pigment degradation, while insufficient power leads to limited cell disruption.40 When comparing temperatures at a fixed solvent ratio, increasing temperature from 30 °C to 45 °C generally increased betacyanin content before significantly decreasing at 60 °C for both flesh and peel. Increased temperature enhances solubility and mass transfer, while excessive heat may cause oxidative deterioration.45
3 ratio and 45 °C, where power at 2.5 W g−1 yielded 423.02 mg/100 g compared to 281.24 mg/100 g (0.5 W g−1) and 317.03 mg/100 g (4.5 W g−1). Peel data mirrored this pattern. Excessive power may cause local overheating and pigment degradation, while insufficient power leads to limited cell disruption.40 When comparing temperatures at a fixed solvent ratio, increasing temperature from 30 °C to 45 °C generally increased betacyanin content before significantly decreasing at 60 °C for both flesh and peel. Increased temperature enhances solubility and mass transfer, while excessive heat may cause oxidative deterioration.45
At each constant temperature, increasing the solvent ratio resulted in higher betacyanin contents. In the flesh at 45 °C and power at 2.5 W g−1, the betacyanin content improved from 416.93 mg/100 g (at a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to 430.25 mg/100 g (at a ratio of 1
1) to 430.25 mg/100 g (at a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5). In the peel, the same trend was observed at 45 °C where the 1
5). In the peel, the same trend was observed at 45 °C where the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 ratio yielded 112.71 mg/100 g compared to 73.86 mg/100 g at a ratio of 1
50 ratio yielded 112.71 mg/100 g compared to 73.86 mg/100 g at a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30. Higher ratios enhanced solvent availability and diffusivity, although beyond a certain threshold, the gain plateaus due to dilution effects.37 The observed ratio of 1
30. Higher ratios enhanced solvent availability and diffusivity, although beyond a certain threshold, the gain plateaus due to dilution effects.37 The observed ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 for the flesh and 1
5 for the flesh and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 for the peel, showed a balance between solvent availability and the solute concentration gradient. A higher solvent-to-material ratio generally enhances diffusion by maintaining a steep concentration gradient between the plant matrix and the bulk solvent, which accelerates mass transfer and pigment solubilization.46 However, beyond a certain point, further increases in the solvent volume no longer enhance diffusion efficiency due to the system becoming overly diluted, thereby diminishing the effective concentration gradient per unit of solute released.36 This phenomenon has been reported in other pigment extraction studies, where an optimal ratio exists that maximizes diffusivity before solvent dilution leads to a plateau in extraction yield.36,46
50 for the peel, showed a balance between solvent availability and the solute concentration gradient. A higher solvent-to-material ratio generally enhances diffusion by maintaining a steep concentration gradient between the plant matrix and the bulk solvent, which accelerates mass transfer and pigment solubilization.46 However, beyond a certain point, further increases in the solvent volume no longer enhance diffusion efficiency due to the system becoming overly diluted, thereby diminishing the effective concentration gradient per unit of solute released.36 This phenomenon has been reported in other pigment extraction studies, where an optimal ratio exists that maximizes diffusivity before solvent dilution leads to a plateau in extraction yield.36,46
The flesh consistently produced higher betacyanin content than the peel under all tested conditions. At 2.5 W g−1 (45 °C) and a maximum ratio, the flesh yielded 430.25 ± 1.21 mg/100 g compared to peel's content of 112.71 ± 0.83 mg/100 g. This disparity is due to differences in matrix composition, with the peel having higher fiber and pectin content, limiting pigment diffusion.37 The greater betacyanin content in the extraction with UAE was attributed to ultrasound's cavitation effects, which disrupt cell walls, facilitate the release of pigments, and enhance mass transfer.37,45 The peel's fibrous structure appears particularly responsive to ultrasound-assisted disruption. These results align with Bitwell et al.,17 who demonstrated that UAE increases the extraction efficiency of bioactive compounds by enhancing solvent penetration into plant cells through ultrasonic cavitation. A similar observation was reported by Shen et al.14
Ultrasound extraction produces ultrasonic waves that attack the integrity of plant cellular walls. This results in increased permeability of the cytoplasmic membranes, allowing more solvent to enter the plant cells and releasing more compounds into the solvent.43 The application of UAE in this study is consistent with previous reports that ultrasound enhances the extraction of betacyanin and other bioactive compounds by Thuy et al.,21 who found that the main source of betacyanin is the flesh of dragon fruit, explaining the variation in betacyanin content between the flesh and peel; the flesh constantly yields a greater concentration of betacyanin. Kaur et al.20 demonstrated that UAE improved the extraction of betalains from beetroot and anthocyanins from red cabbage, respectively. Compared to conventional extraction, UAE achieved better yields in significantly shorter times.
Prior studies showed that conventional flesh extraction produced 465.63 mg/100 g at 60 minutes, while UAE yielded betacyanin content of about 430.25 mg/100 g from the flesh in 10 minutes, which was slightly lower than the best conventional extraction. Higher betacyanin content was achieved with an ultrasonic power of 0.5 W g−1 at 45 °C, extraction time of 10 min, and at a material to solvent ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50. UAE produced 117.99 mg/100 g from the peel compared to that obtained with the best conventional extraction (56.46 mg/100 g) in only 10 minutes, instead of 60. This corresponded to a 2.1-fold improvement in pigment recovery using UAE and represented an 83% reduction in extraction time and an estimated energy savings of around 65%, assuming proportional scaling between acoustic and thermal energy input. The comparison explicitly demonstrated that ultrasound cavitation significantly accelerated pigment diffusion and release, achieving a higher yield within the extraction time under identical solvent and ratio conditions. This enhancement was attributed to mechanical disruption of peel cell walls by cavitation microjets, which increased solvent accessibility and facilitated rapid mass transfer without excessive solvent consumption. Furthermore, excessive power led to the formation of radicals (·OH and ·H) and microjets that degraded betacyanin to betalamic acid, a well-documented phenomenon.34 This result indicated that the ultrasound-assisted process markedly enhanced extraction kinetics through cavitation-induced microchannel formation and accelerated solvent penetration, enabling pigment release comparable to or even higher than the conventional method but within a significantly shorter timeframe. Hence, UAE can be regarded as a time-efficient technique that exhibits high extraction efficiency with minimal pigment degradation, offering a practical advantage for industrial-scale processing where rapid throughput is crucial.37 This further supports that ultrasonic cavitation enhances and accelerates mass transfer and solute release by disrupting cellular structures.40
50. UAE produced 117.99 mg/100 g from the peel compared to that obtained with the best conventional extraction (56.46 mg/100 g) in only 10 minutes, instead of 60. This corresponded to a 2.1-fold improvement in pigment recovery using UAE and represented an 83% reduction in extraction time and an estimated energy savings of around 65%, assuming proportional scaling between acoustic and thermal energy input. The comparison explicitly demonstrated that ultrasound cavitation significantly accelerated pigment diffusion and release, achieving a higher yield within the extraction time under identical solvent and ratio conditions. This enhancement was attributed to mechanical disruption of peel cell walls by cavitation microjets, which increased solvent accessibility and facilitated rapid mass transfer without excessive solvent consumption. Furthermore, excessive power led to the formation of radicals (·OH and ·H) and microjets that degraded betacyanin to betalamic acid, a well-documented phenomenon.34 This result indicated that the ultrasound-assisted process markedly enhanced extraction kinetics through cavitation-induced microchannel formation and accelerated solvent penetration, enabling pigment release comparable to or even higher than the conventional method but within a significantly shorter timeframe. Hence, UAE can be regarded as a time-efficient technique that exhibits high extraction efficiency with minimal pigment degradation, offering a practical advantage for industrial-scale processing where rapid throughput is crucial.37 This further supports that ultrasonic cavitation enhances and accelerates mass transfer and solute release by disrupting cellular structures.40
The results in Table 6 reveal that all interactions among ultrasonic power, extraction temperature, and solvent ratio significantly influenced betacyanin content in both flesh and peel samples (p < 0.001).
| Sample | Interaction | F value | Sig. | p-Value | Partial Eta2 |
|---|---|---|---|---|---|
| a R2 = 1.000 (adjusted R2 = 1.000). | |||||
| Flesh | Power × temperature | 5853.936 | 0 | <0.001 | 0.998 |
| Power × ratio | 116.849 | 0 | <0.001 | 0.896 | |
| Temperature × ratio | 56.615 | 0 | <0.001 | 0.807 | |
| Power × temperature × ratio | 167.831 | 0 | <0.001 | 0.961 | |
| Peel | Power × temperature | 338.817 | 0 | <0.001 | 0.962 |
| Power × ratio | 533.289 | 0 | <0.001 | 0.975 | |
| Temperature × ratio | 927.756 | 0 | <0.001 | 0.986 | |
| Power × temperature × ratio | 213.606 | 0 | <0.001 | 0.969 | |
The F values and partial Eta2 values indicate that these interactions were statistically significant (p < 0.001) and carried substantial effect sizes. Among the interactions in the flesh sample, the interaction between power and temperature showed the highest F value (F = 5853.936, partial Eta2 = 0.998), indicating that temperature variations had the most dominant influence depending on the power level used.
This suggests a strong synergistic relationship where moderate ultrasonic power (2.5 W g−1) combined with an optimal temperature (45 °C) maximizes pigment release. Similar trends were observed in the peel samples.
However, the magnitude of F values was generally lower, reflecting the tougher matrix of the peel with more fiber and pectin, which inhibits solvent penetration and pigment diffusion. In peel, the interaction between power and temperature showed the highest F value (F = 927.756, partial Eta2 = 0.986). The interaction between power and ratio (F = 116.849, partial Eta2 = 0.896) in flesh and peel (F = 533.289, partial Eta2 = 0.975) also had a significant effect (p < 0.001), suggesting that increasing the solvent volume alone was insufficient unless accompanied by substantial energy input.47
Excessive power input (4.5 W g−1) may damage the solute, whereas insufficient power leads to suboptimal diffusion efficiency. In addition, the interaction between temperature and ratio for flesh (F = 56.615, partial Eta2 = 0.807) and peel (F = 927.756, partial Eta2 = 0.986) supports the idea that solvent diffusion is temperature dependent. As the temperature increases, solvent viscosity decreases and solubility increases, enhancing the diffusion gradient.44 However, due to thermal oxidation, overly high temperatures (e.g., 60 °C) may disrupt pigment stability, especially at low solvent volume.36
The three-way interaction (interaction between power, temperature, and ratio) was also highly significant (F = 167.831, partial Eta2 = 0.961, p < 0.001) in the flesh and peel (F = 213.606, partial Eta2 = 0.969, p < 0.001), demonstrating that betacyanin content is not a product of individual variables but a combined function of all three. The highest yields were observed when all variables were tuned synergistically, i.e., at 2.5 W g−1 at 45 °C and a high solvent ratio (e.g.,1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 ratio).
5 ratio).
Van et al.48 reported that the maximum betacyanin content from dragon fruit peel obtained under the optimal UAE conditions (material![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water ratio of 1
water ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, at 3.5 W g−1 and 7.5 min extraction time) was 34.02 mg/100 g. While in this study, the best conditions resulted in 117.989 mg/100 g for peel (material: water ratio of 1
10, at 3.5 W g−1 and 7.5 min extraction time) was 34.02 mg/100 g. While in this study, the best conditions resulted in 117.989 mg/100 g for peel (material: water ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 ratio at 0.5 W g−1 and 10 min extraction time), demonstrating that UAE under the right solvent ratio and matrix conditions can yield more than three times the pigment recovery with only a modest energy input. This comparison underscores the potential of UAE for process intensification, while further confirming that water remains the better solvent due to its molecular compatibility with betacyanin's polar structure and its green chemistry attributes.
50 ratio at 0.5 W g−1 and 10 min extraction time), demonstrating that UAE under the right solvent ratio and matrix conditions can yield more than three times the pigment recovery with only a modest energy input. This comparison underscores the potential of UAE for process intensification, while further confirming that water remains the better solvent due to its molecular compatibility with betacyanin's polar structure and its green chemistry attributes.
3.3 Betacyanin retention
This part of the study investigated betacyanin retention in both dragon fruit flesh and peel extracts under two conditions (light exposure and dark conditions). Betacyanin is known for its red violet color, water solubility, and vulnerability to environmental factors such as light and temperature. Its stability is crucial for application in food colorants and nutraceuticals.42 Betacyanin degradation during heat and light exposure can be explained by well-established chromophoric degradation pathways. Thermal energy initiates decarboxylation and dehydrogenation of betacyanin molecules, disrupting the conjugated double-bond systems responsible for the red-violet color.27 Under oxidative conditions, photooxidation and hydrolysis of the imine (–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) HN–) linkage between betalamic acid and cyclo-DOPA lead to pigment fragmentation into colorless derivatives such as betalamic acid and dopaxanthin.26
HN–) linkage between betalamic acid and cyclo-DOPA lead to pigment fragmentation into colorless derivatives such as betalamic acid and dopaxanthin.26
At 40 °C, betacyanin retention in the flesh extract remained above 88% up to 80 minutes and decreased moderately to 86% after 100 minutes. These findings demonstrated that heat exerted a stronger influence on pigment degradation than light during prolonged exposure. Similar to Esatbeyoglu et al.,49 thermal treatment led to a gradual transformation of betacyanin into colorless or brown compounds, lowering both color intensity and antioxidant potential. Retention levels after 120 minutes (55–60%) confirmed partial degradation under moderate heat, which may still be acceptable for processing conditions requiring mild heating. Consequently, optimizing temperature–time parameters remains crucial for maximizing pigment stability during storage.
The results showed that higher temperatures led to faster degradation of betacyanin pigments in flesh and peel. Across all matrices, 40 °C yielded the highest pigment retention, while 80 °C resulted in the most substantial losses. The data reveal that peel exhibits better thermal stability than flesh but still follows a similar trend at higher temperatures (80 °C), accelerating pigment degradation.
Based on the results, in the flesh, retention dropped to 7.39% ± 0.16 (light exposure) and 14.42% ± 0.16 (dark conditions) at 120 minutes at 80 °C, compared to 85.44% ± 0.16 (light exposure) and 48.50% ± 0.28 (dark conditions) retention at 40 °C over the same period (Fig. 1). This degradation pattern is attributed to the thermal sensitivity of betacyanins, which are prone to oxidative and structural breakdown at elevated temperatures. The peel showed better pigment stability at lower temperatures (40 °C). At 40 °C under light exposure, the peel retained 78.08% ± 0.08 after 120 minutes, while only 15.8% ± 0.31 was retained at 80 °C (Fig. 2). This pattern supports the understanding that betacyanin degrades via hydrolysis, decarboxylation, and dehydrogenation reactions, which are strongly temperature dependent.46,50
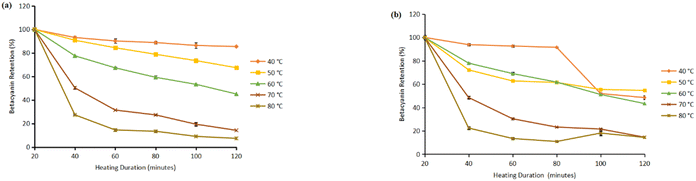 | ||
| Fig. 1 Retention of betacyanin obtained from the flesh: under (a) light exposure and (b) dark conditions. | ||
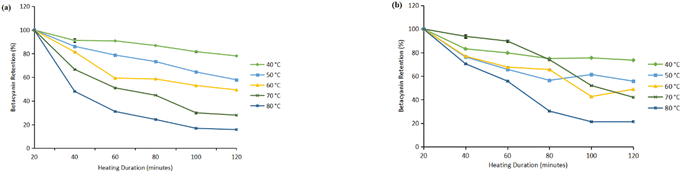 | ||
| Fig. 2 Retention of betacyanin obtained from the peel: under (a) light exposure and (b) dark conditions. | ||
The peel showed slightly higher initial stability due to its pectin rich cell wall matrix, which slows heat and oxygen diffusion. However, this protection is lost above 60 °C as the matrix breaks down.27,42,51 Thus, the observed thermal sensitivity and pigment retention patterns are mechanistically consistent with coupled diffusion reaction kinetics, validating that betacyanin degradation involves both transport (the diffusion of heat, oxygen and reactive species toward the pigment molecules) and chemical transformation processes.
This degradation can be attributed to photo-oxidative stress. When exposed to light, betacyanin undergoes structural cleavage in the aldimine linkage, decreasing color intensity and stability.52 Over time, retention declined sharply, particularly after prolonged exposure. The concentration of betacyanin decreased significantly as a function of time, particularly at higher temperatures. At 70 °C and 80 °C, the retention drops drastically within the first 20 minutes, falling below 30%. These results indicate that betacyanin is highly thermolabile, and exposure to high temperatures accelerates pigment degradation. At 80 °C, retention was reduced to around 20% by 20 minutes and remained low throughout the 120-minute period. This demonstrated that exposure to lower temperatures better preserves the molecular integrity of the pigment. Herbach et al.53 and Azeredo34 reported that betacyanin is susceptible to thermal breakdown through decarboxylation and isomerization reactions.
Betacyanin degradation occurred more rapidly under light exposure than in dark environments at 40 °C, particularly during prolonged heating. In contrast, pigment degradation proceeded more slowly in the dark, especially at 50 °C. After 120 minutes of heating at 50 °C, pigment retention in the flesh was 67.5% under light exposure and 54.6% in the dark. Although darkness initially provided a slight protective effect, this advantage diminished at higher temperatures (80 °C). These observations indicate that, beyond a certain thermal threshold, pigment loss is primarily driven by thermal degradation rather than the photooxidative process. Similar findings were reported by Sokolova et al.,25 who reported that betacyanin degradation involves the breakdown of aldimine bonds and decarboxylation reactions under heat stress. Light accelerates betacyanin degradation via photooxidation, producing free radicals that compromise pigment stability. This suggests that light is a critical stressor in the degradation process. Without light, thermal degradation remains the primary factor, and its effect is less pronounced, especially at 60 °C. The findings are in agreement with previous studies emphasizing the photosensitivity of betacyanin pigments and the protective role of dark environments in pigment stability.27,42 Light exposure increases the generation of reactive oxygen species (ROS), which react with the chromophore structure of betacyanin, leading to pigment bleaching and structural instability.54 In addition, light exposure had a more substantial effect on the peel samples. At 80 °C for 120 minutes, retention in light was 15.8%, whereas it was slightly higher (21.3%) under dark conditions.
Conversely, betacyanin was more stable at 40 °C, as indicated by the highest pigment retention observed in the flesh. Betacyanin retention in flesh extract at 40 °C remained above 88% up to 80 minutes and only showed a substantial drop to 86% after 100 minutes. These results reinforce that betacyanin is more sensitive to heat than light over extended exposure times. This confirms the findings from Esatbeyoglu et al.,55 who demonstrated that thermal induced degradation of betacyanin produces colorless or brown compounds, reducing the color intensity and antioxidant capacity. At the 120-minute mark, retention remained at about 55–60%, indicating moderate thermal degradation. These conditions may present a compromise in pigment preservation, which is suitable for industrial processes that require some degree of heating. Longer exposure, even at lower temperatures, eventually leads to pigment loss. However, the rate and extent of degradation are significantly magnified under high thermal stress. Thus, optimizing temperature and time is essential for maximizing betacyanin retention during storage.
3.4 Thermal kinetic degradation
The thermal degradation of betacyanin in both dragon fruit flesh and peel was analyzed at different temperatures (40–80 °C) and under light exposure conditions. As summarized in Table 7, first-order kinetics consistently provided the best fit (R2) across all conditions, indicating that the rate of pigment loss depended on pigment concentration.| Source | Temperature | Light exposure | Order | R2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a a |
ka | t1/2 (minute)a | Unit |
|---|---|---|---|---|---|---|---|
| a R2 = regression correlation, k = degradation rate constant; t1/2 = half life. | |||||||
| Flesh | 40 °C | Yes | First | 0.7727 | −0.0077 | 89.87 | minutes−1 |
| No | First | 0.9070 | −0.0015 | 462.00 | minutes−1 | ||
| 50 °C | Yes | First | 0.8255 | −0.0055 | 126.00 | minutes−1 | |
| No | First | 0.9970 | −0.0038 | 182.40 | minutes−1 | ||
| 60 °C | Yes | First | 0.9878 | −0.0079 | 87.70 | minutes−1 | |
| No | First | 0.9797 | −0.0075 | 92.40 | minutes−1 | ||
| 70 °C | Yes | First | 0.9239 | −0.0176 | 39.60 | minutes−1 | |
| No | First | 0.9539 | −0.0182 | 38.10 | minutes−1 | ||
| 80 °C | Yes | First | 0.4908 | −0.0151 | 12.90 | minutes−1 | |
| No | First | 0.8536 | −0.0234 | 29.60 | minutes−1 | ||
| Peel | 40 °C | Yes | First | 0.9612 | −0.0023 | 495.00 | minutes−1 |
| No | First | 0.7765 | −0.0027 | 256.70 | minutes−1 | ||
| 50 °C | Yes | First | 0.9918 | −0.0052 | 133.30 | minutes−1 | |
| No | First | 0.8014 | −0.0053 | 130.80 | minutes−1 | ||
| 60 °C | Yes | First | 0.8935 | −0.0069 | 100.40 | minutes−1 | |
| No | First | 0.8729 | −0.0077 | 90.00 | minutes−1 | ||
| 70 °C | Yes | First | 0.9681 | −0.0127 | 54.60 | minutes−1 | |
| No | First | 0.9095 | −0.0090 | 77.00 | minutes−1 | ||
| 80 °C | Yes | First | 0.9292 | −0.018 | 38.50 | minutes−1 | |
| No | First | 0.9544 | −0.017 | 40.80 | minutes−1 | ||
The half-life values (t1/2) range broadly, from under 10 minutes at elevated temperatures (flesh at 80 °C, light exposure) to several hundred minutes under milder conditions (peel at 40 °C, dark). The markedly shorter t1/2 at higher temperatures compared to lower temperatures reflects the strong temperature dependence of the degradation constant k. According to Arrhenius' behavior, higher temperatures accelerate molecular motion, increase collision frequency and energy, and thereby enhance pigment breakdown.56
This pattern is consistent with previous studies suggesting that betacyanin degradation is more accurately modeled by first-order kinetics under oxidative or thermal stress, especially when pigments are freely soluble and not bound in cellular structures.27 Temperature had a significant influence on degradation rates. As the temperature increased from 40 °C to 80 °C, the degradation rate constant (k) increased in magnitude (more negative), indicating faster degradation. In flesh, at 40 °C, betacyanin was found to slightly degrade, representing the most stable condition among those temperatures.
This result suggests that, under lower thermal stress, pigment breakdown is primarily controlled by external factors such as heat and light rather than substrate concentration. As heating time extended, the degradation gradually shifted toward a first-order kinetic model, demonstrating that prolonged exposure increased the dependency of degradation on pigment concentration. This behavior is often observed when pigments are present in high concentrations or are stabilized in a matrix that limits diffusion.51
In contrast, under dark conditions, the degradation at 40 °C was better described by a first-order kinetic model (R2 = 0.9070). This implies that, in the absence of light, betacyanin molecules degraded in a concentration dependent manner, even at lower temperatures. Notably, under both conditions, the degradation profile revealed a sharp decline in betacyanin retention after 80 minutes of heating, especially at elevated temperatures (≥70–80 °C). This pronounced reduction highlights the cumulative effect of thermal stress on pigment stability, suggesting that beyond a certain heating threshold, betacyanin molecules undergo accelerated decomposition. Similar findings have been reported in earlier studies on betalain stability, where extended heating at high temperatures significantly increased the rate of pigment loss due to structural breakdown of the chromophore system.49,57 A similar trend was observed in the peel, betacyanin degradation at 40 °C followed a first-order kinetic model, with a higher correlation under light exposure (R2 = 0.9612) compared to dark conditions (R2 = 0.7765). As the temperature increased (50–80 °C), degradation rates consistently increased, especially at 70 °C and 80 °C, where retention profiles showed a rapid decrease between 60 and 80 minutes of heating. These results confirm that extended exposure to higher temperatures markedly accelerates betacyanin degradation in the peel. This trend aligns with the Arrhenius principle, where elevated temperature increases molecular motion and accelerates the breakdown of thermolabile compounds such as betacyanin.58
Light exposure generally exhibited higher degradation rates and a better fit to first-order kinetics. Light exposure promotes photooxidation, especially in aqueous environments, leading to pigment instability.49,59
These findings support that UV visible light causes excitation of betalain electrons, thereby increasing their reactivity and accelerating color degradation, with pigment loss reaching up to 15–16% due to light and oxygen exposure.60 In addition, rapid degradation at high temperature and/or light exposure can be mechanistically linked to structural changes in the betacyanin molecule. Betacyanin consists of a betalamic acid core conjugated via a imine (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) linkage to a cyclo-DOPA skeleton; exposure to heat and photons can induce cleavage of the imino bond, decarboxylation, and formation of betalamic acid and other breakdown products, which lose the vivid red violet chromophore and shift to yellowish pigments.61,62 These kinetic insights were critical for food product development. Based on the kinetic degradation results, for industrial extraction or product formulation using betacyanin from Hylocereus polyrhizus, it is advisable to limit thermal exposure to temperatures <60 °C and avoid intense light exposure during storage or processing. Low-temperature processes at 40–50 °C and under dark conditions are ideal for beverages, colorants, or supplements intended to preserve betacyanin's antioxidant value. On the other hand, applications requiring thermal processing, such as baked goods or thermally treated jellies, may tolerate partial degradation, provided optimized temperature exposure is used to retain color integrity. More importantly, the results highlight the need for betacyanin stabilization methods to improve its stability and broaden its applications.
N) linkage to a cyclo-DOPA skeleton; exposure to heat and photons can induce cleavage of the imino bond, decarboxylation, and formation of betalamic acid and other breakdown products, which lose the vivid red violet chromophore and shift to yellowish pigments.61,62 These kinetic insights were critical for food product development. Based on the kinetic degradation results, for industrial extraction or product formulation using betacyanin from Hylocereus polyrhizus, it is advisable to limit thermal exposure to temperatures <60 °C and avoid intense light exposure during storage or processing. Low-temperature processes at 40–50 °C and under dark conditions are ideal for beverages, colorants, or supplements intended to preserve betacyanin's antioxidant value. On the other hand, applications requiring thermal processing, such as baked goods or thermally treated jellies, may tolerate partial degradation, provided optimized temperature exposure is used to retain color integrity. More importantly, the results highlight the need for betacyanin stabilization methods to improve its stability and broaden its applications.
3.5 Bioactive compounds
Appreciable variations in antioxidant activity, total phenolic content (TPC), and total flavonoid content (TFC) were observed across different portions of the fruit (non-extracted, flesh and peel) and flesh betacyanin extract with water as a solvent. The analysis of bioactive compounds in the betacyanin extract was conducted on the sample with the highest betacyanin content, which was obtained from dragon fruit flesh using a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 (w/v) material to solvent ratio with water as the solvent. The extraction was performed using ultrasound-assisted extraction (UAE) at 2.5 W g−1 and 45 °C, which yielded the highest antioxidant capacity, consistent with the maximum betacyanin content reported under the same extraction parameters. This strong correlation suggested that the enhanced radical-scavenging ability of the extract was mechanistically linked to increased pigment concentration and to the efficient release of polyphenolic cofactors through cavitation-induced disruption of the cellular matrix.
5 (w/v) material to solvent ratio with water as the solvent. The extraction was performed using ultrasound-assisted extraction (UAE) at 2.5 W g−1 and 45 °C, which yielded the highest antioxidant capacity, consistent with the maximum betacyanin content reported under the same extraction parameters. This strong correlation suggested that the enhanced radical-scavenging ability of the extract was mechanistically linked to increased pigment concentration and to the efficient release of polyphenolic cofactors through cavitation-induced disruption of the cellular matrix.
Table 8 shows the antioxidant activity of the extracted and non-extracted sample. The non-extracted sample was measured using the raw flesh and peel as the sample. According to Table 8, extraction significantly boosts the antioxidant activity of betacyanin. The extract exhibited the maximum antioxidant activity of 66.61 ± 0.82%, followed by the flesh at 47.49 ± 0.27% and the peel at 26.35 ± 0.09%. Jamilah et al.63 recorded 45–55% antioxidant activity in dragon fruit. The increased quantity of betacyanin in the pigmented flesh explained the stronger antioxidant activity in the flesh as compared to the peel.55,64
| Sample | Antioxidant (%) | Total phenolic content (mg GAE per 100 g) | Total flavonoid content (mg QE per 100 g) |
|---|---|---|---|
| a Values expressed as mean ± S. D. (n = 3) values with different lowercase letters within the same column indicate significant differences (p < 0.05) from each other. | |||
| Flesh | 47.49 ± 0.27b | 18.00 ± 0.01c | 7.06 ± 0.03b |
| Peel | 26.35 ± 0.09a | 12.00 ± 0.00b | 4.61 ± 0.05a |
| Flesh betacyanin extract | 66.61 ± 0.82c | 8.48 ± 0.64a | 47.48 ± 1.02c |
Fathordoobady et al.45 observed increased antioxidant activity (70–80%) in betacyanin extract. These findings align with studies by Choo et al.65 showing that betacyanin extract has more antioxidant capacity. This suggests that betacyanin is a potentially efficient radical scavenger.60 Betacyanin is the component responsible for the antioxidant capacity of dragon fruit.
The total phenolic content (TPC) was highest in the flesh of dragon fruit (18.00 ± 0.01 mg GAE per 100 g) and lowest in the peel (12.00 ± 0.00 mg GAE per 100 g). This result is similar to those findings by Wu et al.,66 who reported TPC values of 15–20 mg GAE per 100 g in dragon fruit flesh. Extraction parameters such as the choice of the solvent and the application of ultrasound tend to be more selective in extracting free flavonoid compounds compared to other phenolic compounds, particularly those bound within the cell wall matrix.53
The antioxidant and phenolic content of Hylocereus polyrhizus extracts revealed a distinct pattern between the flesh and peel matrices. Although both parts exhibited strong radical scavenging capacities, the flesh consistently demonstrated higher antioxidant activity than the peel. This enhanced activity, however, cannot be attributed solely to betacyanin content. Several studies have shown that dragon fruit flesh also contains other phenolic compounds such as hydroxybenzoic acids, ferulic acid, and flavonol derivatives, which contribute to its total antioxidant potential.57 Hence, the higher antioxidant response in flesh may arise from a composite effect of these phenolics rather than from betacyanin alone.
Correlation analysis between total betacyanin content and antioxidant activity revealed a moderate positive relationship (R2 = 0.71), indicating that while betacyanin significantly contributes to free radical scavenging, other co-extracted phenolic constituents also play an additional role.36,67
At 45 °C, solvent viscosity decreased, improving mass transfer coefficients and promoting the diffusion of hydrophilic antioxidants into the solvent. Simultaneously, an ultrasonic power of 2.5 W g−1 provided sufficient acoustic energy to rupture vacuolar membranes without causing pigment oxidation. These conditions enhanced both betacyanin and co-extraction of phenolics and flavonoids, as confirmed by the elevated antioxidant activity in the UAE extract compared to conventional methods.36
The solvent effect was another determinant. Water, as a highly polar solvent, favors the dissolution of betacyanin and phenolic hydroxyl groups through hydrogen bonding.68,69 The increase in total flavonoid content after UAE treatment suggested that ultrasound enhanced the release of bound flavonoids from cell wall polysaccharides, improving their accessibility to the solvent phase.67
The lower phenolic content observed in the betacyanin extract obtained through UAE compared to the flesh and peel could be attributed to the selective physicochemical nature of the extraction process.45 UAE primarily promotes the release of free, water-soluble compounds such as betacyanins and flavonoids through acoustic cavitation, while bound phenolics, typically ester- or ether-linked to lignin, pectin, and hemicellulose, remain largely unreleased under the extraction conditions.45 The use of highly polar water as a solvent enhanced pigment solubilization but limits the dissolution of moderately polar phenolics, resulting in selective enrichment of betacyanin rather than total phenolic recovery. Moreover, cavitation induced microbubble collapse generates hydroxyl radicals that can oxidize or depolymerize phenolic compounds, reducing measurable total phenolic content despite the overall increase in antioxidant activity. These findings align with previous studies reporting that a short-duration, high-energy environment during UAE enhances pigment yield and antioxidant potential but can lead to selective extraction and partial oxidative degradation of phenolics.40,45
3.6 Betacyanin profile
The betacyanin profile determined by HPLC was obtained from the flesh extract with the highest betacyanin content, which was extracted using UAE at 2.5 W g−1 and 45 °C, with a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, and compared with a commercial betanin standard. The HPLC chromatogram of the betacyanin extract (Fig. 3) from flesh with the UAE-treated sample exhibited nine peaks with retention times between 1.423 and 5.36 minutes, consistent with those reported by Herbach et al.53 However, the identification of compounds in this study relied exclusively on retention time comparison with reference standards, without further confirmation using spectrophotometric or mass spectrophotometric techniques. While retention time alignment provides a preliminary indication of compound identity, it cannot distinguish between structural isomers or co-eluting derivatives that may exhibit chromatographic behavior. This limitation is particularly relevant for betalain-rich matrices such as Hylocereus polyrhizus, where isobetanin and betanidin derivatives can exhibit overlapping retention times under reverse-phase conditions. Moreover, variations in solvent composition, gradient program, and stationary-phase selectivity can slightly shift retention behavior, potentially leading to the underestimation of minor pigments. Without complementary evidence from UV-Vis absorption spectra or mass-to-charge ratio data obtained from HPLC-DAD or LC-MS/MS, the structural confirmation of individual betacyanin species remains tentative. Nevertheless, the retention time patterns observed here closely match literature data for betanin-dominant extracts, and the results remain valuable for comparative profiling and process evaluation across different extraction treatments. Future studies should employ HPLC-DAD-MS coupling to improve compound discrimination, quantify degradation intermediates, and verify pigment stability under ultrasonic processing.
5, and compared with a commercial betanin standard. The HPLC chromatogram of the betacyanin extract (Fig. 3) from flesh with the UAE-treated sample exhibited nine peaks with retention times between 1.423 and 5.36 minutes, consistent with those reported by Herbach et al.53 However, the identification of compounds in this study relied exclusively on retention time comparison with reference standards, without further confirmation using spectrophotometric or mass spectrophotometric techniques. While retention time alignment provides a preliminary indication of compound identity, it cannot distinguish between structural isomers or co-eluting derivatives that may exhibit chromatographic behavior. This limitation is particularly relevant for betalain-rich matrices such as Hylocereus polyrhizus, where isobetanin and betanidin derivatives can exhibit overlapping retention times under reverse-phase conditions. Moreover, variations in solvent composition, gradient program, and stationary-phase selectivity can slightly shift retention behavior, potentially leading to the underestimation of minor pigments. Without complementary evidence from UV-Vis absorption spectra or mass-to-charge ratio data obtained from HPLC-DAD or LC-MS/MS, the structural confirmation of individual betacyanin species remains tentative. Nevertheless, the retention time patterns observed here closely match literature data for betanin-dominant extracts, and the results remain valuable for comparative profiling and process evaluation across different extraction treatments. Future studies should employ HPLC-DAD-MS coupling to improve compound discrimination, quantify degradation intermediates, and verify pigment stability under ultrasonic processing.
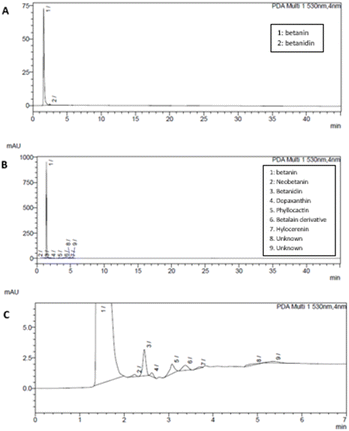 | ||
| Fig. 3 Betacyanin profile monitored at 530 nm. (A) Betanin standard; (B) betacyanin from the flesh extract; (C) enlarged view of betacyanin from the flesh extract showing minor peaks. | ||
Peak 1 is designated by the standard as betanin (betanidin-5-O-β-glucoside) at 1.43 minutes, as determined by the retention durations noted in the chromatographic profile of the commercial betanin standard above 2. The phyllocactin and hylocerenin peaks were detected at 3.08 minutes and 3.68 minutes, respectively (Table 9). Based on the area, betanin constituted about 99.4% of the total peak area. This result was close to the betanin standard. Stintzing et al.70 identified betanin as the primary betacyanin in the Hylocereus species at a 1.3–1.5 minute retention time. According to Stintzing et al.,71 there were six major betacyanin pigments, i.e., betanin, isobetanin, phyllocactin, isophyllocactin, hylocerenin, and isohylocerenin in the fruit flesh extract of Hylocereus polyrhizus species as detected by HPLC, electrospray MS/MS, and 1H NMR techniques. Due to the lower molecular weight, phyllocactin eluted earlier than hylocerenin. The six pigments of red pitaya constituted three pairs of compounds exhibiting decreasing overall polarity, beginning with the most polar betanin, followed by phyllocactin and hylocerenin, as indicated by HPLC analysis.71
| Peak | Compound | Retention time (min) | Area (%) |
|---|---|---|---|
| 1 | Betanin | 1.43 | 99.39 |
| 2 | Neobetanin | 2.23 | 0.03 |
| 3 | Betanidin | 2.45 | 0.23 |
| 4 | Dopaxanthin | 2.62 | 0.03 |
| 5 | Phyllocactin | 3.08 | 0.13 |
| 6 | Betalain derivative | 3.37 | 0.08 |
| 7 | Hylocerenin | 3.68 | 0.02 |
| 8 | Unknown | 4.92 | 0.04 |
| 9 | Unknown | 5.36 | 0.05 |
Betanin is the strongest red violet chromophore and one of the most potent antioxidant species among betacyanins due to its conjugated imine aromatic structure and electron donating hydroxyl groups.64–66,72,73 Therefore, extracts enriched in betanin are expected to exhibit greater color intensity, improved thermal resilience and stronger radical scavenging efficiency than those dominated by phyllocactin or hylocerenin.53,70
4. Conclusions
These results clearly suggested the efficiency of UAE in enhancing pigment diffusion and recovery through cavitation-induced microchannel formation within the peel's fibrous matrix, while simultaneously reducing extraction time and thermal degradation risk. UAE achieved high betacyanin yields within 10 minutes through cavitation enhanced pigment diffusion, producing 430.25 mg/100 g (flesh) and 117.99 mg/100 g (peel), the latter representing a 2.1 fold increase over conventional extraction. Conventional extraction yielded slightly higher pigment from flesh but required 60 minutes and greater thermal exposure.Betacyanin stability decreased sharply above 60 °C and under light, following first-order kinetics under oxidative conditions. Betanin was identified as the dominant pigment, and UAE extracts retained strong antioxidant activity despite reduced phenolics, due to cavitation induced oxidation and enhanced flavonoid release. Overall, UAE offers a rapid energy-efficient, and thermally protective method for producing stable natural colorants.
These results position UAE as a promising method for the scalable production of natural pigments and functional ingredients suitable for food and beverage applications requiring color stability and nutritional integrity. UAE presents a sustainable and scalable strategy for producing natural colorants, offering significant reductions in processing time, energy requirements, and solvent consumption. However, it should be noted that this study did not compare betacyanin profiles between flesh and peel, nor between the conventional and UAE methods; thus, the detailed pigment composition could not be fully evaluated. Future studies are therefore recommended to include comprehensive profiling to address this gap.
Author contributions
Ruri Aditya Sari, Yudi Pranoto, Arima Diah Setiowati, and Indriana Kartini conceived and designed the experimentation. Ruri Aditya Sari performed the experiments and data curation and wrote the original manuscript. Arima Diah Setiowati and Indriana Kartini validated the method and reviewed the manuscript. Yudi Pranoto validated and reviewed the final manuscript. All authors have read and approved the final version of the manuscript.Conflicts of interest
There are no conflicts to declare.Data availability
All data generated or analyzed during this study are included in this published article and comply with research standards.Acknowledgements
We would like to express our gratitude to the Indonesian Education Scholarship (BPI), the Centre for Higher Education Funding and Assessment (PPAPT) Ministry of Higher Education, Science, and Technology of Republic Indonesia and the Indonesia Endowment Fund for Education (LPDP) for a Doctoral scholarship awarded to one of us (Ruri Aditya Sari), under contract no. 011047/PPAT.1.2/BPI.06/02/2025.References
- N. Martins, C. Roriz, P. Morales, L. Barros and I. C. F. Ferreira, Food colorants: Challenges, opportunities and current desires of agro-industries to ensure consumer expectations and regulatory practices, Trends Food Sci. Technol., 2016, 52, 1–15 CrossRef CAS.
- D. B. Rodriguez-Amaya, Natural Food Pigments and Colorants, Curr. Opin. Food Sci., 2016, 7, 20–26 Search PubMed.
- A. Kumorkiewicz-Jamro, T. Świergosz, K. Sutor, A. Spórna-Kucab and S. Wybraniec, Multi-colored shades of betalains: Recent advances in betacyanin chemistry, Nat. Prod. Rep., 2021, 38(12), 2315–2346 RSC.
- D. V. T. da Silva, D. dos Santos Baião, F. de Oliveira Silva, G. Alves, D. Perrone and E. M. Del Aguila, et al., Betanin, a natural food additive: Stability, bioavailability, antioxidant and preservative ability assessments, Molecules, 2019, 24(3), 458 Search PubMed.
- G. B. Celli and M. S. L. Brooks, Impact of extraction and processing conditions on betalains and comparison of properties with anthocyanins—A current review, Food Res. Int., 2016, 1, 1–9 Search PubMed.
- N. M. Iffah, N. Jin Samsudin, R. Nordin, B. M. R. Zahirrah and A. Z. Mohsin, Thermal and photostability of betacyanin from dragon fruit (Hylocereus polyrhizus), J. Agrobiotechnol., 2024, 15(S1), 88–97 CrossRef.
- J. Azmir, I. S. M. Zaidul, M. M. Rahman, K. M. Sharif, A. Mohamed and F. Sahena, et al., Techniques for extraction of bioactive compounds from plant materials: A review, J. Food Eng., 2013, 117(4), 426–436, DOI:10.1016/j.jfoodeng.2013.01.014.
- S. Priatni and A. Pradita, Stability Study of Betacyanin Extract from Red Dragon Fruit (Hylocereus Polyrhizus) Peels, Procedia Chem., 2015, 16, 438–444 Search PubMed.
- H. E. Khoo, X. He, Y. Tang, Z. Li, C. Li and Y. Zeng, et al., Betacyanins and Anthocyanins in Pulp and Peel of Red Pitaya (Hylocereus polyrhizus cv. Jindu), Inhibition of Oxidative Stress, Lipid Reducing, and Cytotoxic Effects, Front. Nutr., 2022, 9, 1–11 Search PubMed.
- G. Linares and M. L. Rojas, Ultrasound-Assisted Extraction of Natural Pigments From Food Processing By-Products: A Review, Front. Nutr., 2022, 9, 1–17 Search PubMed.
- P. Nabi, A. Heydarinasab, S. Shahriari and A. Vaziri, Ultrasound - Assisted Extraction of Crocin from Saffron Using Natural Deep Eutectic Solvents : Cryogenic Grinding Technology , Yield Optimization , and Kinetic Analysis, Food Bioprocess Technol., 2025, 7160–7178, DOI:10.1007/s11947-025-03875-w.
- M. K. H. Ghiasvand, A. Heydarinasab and S. Shahriari, Ultrasound-assisted extraction of Trans-Anethole from fennel seeds using deep eutectic solvents : Insights into optimization, yield, and kinetic modeling, J. Mol. Liq., 2025, 426, 127477, DOI:10.1016/j.molliq.2025.127477.
- M. M. J. Anwar, Advances in Green Technologies for Bioactive Extraction and Valorization of Agro-Waste in Food and Nutraceutical Industries, Haya, 2025, 6221, 184–195 CrossRef.
- L. Shen, S. Pang, M. Zhong, Y. Sun, A. Qayum and Y. Liu, et al., A comprehensive review of ultrasonic assisted extraction (UAE) for bioactive components: Principles, advantages, equipment, and combined technologies, Ultrason. Sonochem., 2023, 101, 106646, DOI:10.1016/j.ultsonch.2023.106646.
- A. G. Demesa, S. Saavala, M. Pöysä and T. Koiranen, Overview and Toxicity Assessment of Ultrasound-Assisted Extraction of Natural Ingredients from Plants, Foods, 2024, 13(19), 1–13 Search PubMed.
- S. T. Mgoma, M. Basitere, V. V. Mshayisa and D. De Jager, A Systematic Review on Sustainable Extraction, Preservation, and Enhancement in Food Processing: The Advancement from Conventional to Green Technology Through Ultrasound, Processes, 2025, 13(4), 1–16 Search PubMed.
- C. Bitwell, S. S. Indra, C. Luke and M. K. Kakoma, A review of modern and conventional extraction techniques and their applications for extracting phytochemicals from plants, Sci. Afr., 2023, 19, e01585, DOI:10.1016/j.sciaf.2023.e01585.
- A. Carreira-Casais, M. Carpena, A. G. Pereira, F. Chamorro, A. Soria-Lopez and P. G. Perez, Critical Variables Influencing the Ultrasound-Assisted Extraction of Bioactive Compounds—A Review, Chem. Proc, 2021, 5, 50 Search PubMed.
- R. Vardanega, D. T. Santos and M. A. De Almeida, Intensification of bioactive compounds extraction from medicinal plants using ultrasonic irradiation, Pharmacogn. Rev., 2014, 8(16), 88–95 Search PubMed.
- G. Kaur, S. Mohanty and R. Sharma, Enhanced extraction of betalains using ultrasound-assisted techniques, Food Bioprocess Technol., 2021, 14, 352–360 Search PubMed.
- N. M. Thuy, P. T. B. Ngoc and N. V. Tai, Effect of conventional and ultrasonic-assisted extracts on betacyanin content of red dragon fruit (Hylocereus polyrhizus), Food Res., 2022, 6(3), 389–395 Search PubMed.
- Y. Yao, Enhancement of mass transfer by ultrasound: Application to adsorbent regeneration and food drying/dehydration, Ultrason. Sonochem., 2016, 31, 512–531, DOI:10.1016/j.ultsonch.2016.01.039.
- D. Panda and S. Manickam, Cavitation technology-the future of greener extraction method: A review on the extraction of natural products and process intensification mechanism and perspectives, Appl. Sci., 2019, 9, 766 CrossRef CAS.
- N. X. Morales, Y. V. Katherine, M. Ralf and T. D. Grethel, Stabilisation of betalains and phenolic compounds extracted from red cactus pear (Opuntia ficus-indica) by spray and freeze-drying using OCA (Oxalis tuberosa) starch as drying aid, Food Sci. Technol. Int., 2020, 27(5), 456–469 Search PubMed.
- D. V. Sokolova, N. A. Shvachko, A. S. Mikhailova, V. S. Popov, A. E. Solovyeva and E. K. Khlestkina, Characterization of Betalain Content and Antioxidant Activity Variation Dynamics in Table Beets (Beta vulgaris L.) with Differently Colored Roots, Agronomy, 2024, 14(5), 999 CrossRef CAS.
- J. Kim, J. Ryu, S. H. Lee, J. H. Kim, D. G. Kim and T. H. Ha, Evaluation of Anthocyanin Profiling, Total Phenolic and Flavonoid Content , and Antioxidant Activity of Korean Rubus Accessions for Functional Food Applications and Breeding, Antioxidants, 2025, 14(8), 1012 CrossRef CAS PubMed.
- I. Sadowska-Bartosz and G. Bartosz, Biological properties and applications of betalains, Molecules, 2021, 26(9), 1–36 CrossRef PubMed.
- K. M. Herbach, F. C. Stintzing and R. Carle, Betalain stability and degradation—structural and environmental aspects, Phytochem. Rev., 2006, 5(2), 445–459 Search PubMed.
- M. Otálora, J. Carriazo, L. Iturriaga, C. Osorio and M. Nazareno, Encapsulating Betalains from Opuntia ficus-indica fruits by ionic gelation: pigment chemical stability during storage of beads, Food Chem., 2016, 202, 373–382 CrossRef PubMed.
- C. H. Rosa, F. Antelo and G. R. Rosa, Kinetics of Thermal-Degradation of Betanins: A Teaching Mini-Project for Undergraduates Employing the Red Beet, J. Food Sci. Educ., 2018, 17(4), 104–110 CrossRef.
- M. Arivalagan, G. Karunakaran, T. Roy, M. Dnsh, S. Sindhu and V. Shilpashree, et al., Biochemical and nutritional characterization of dragon fruit (Hylocereus species), Food Chem., 2021, 353, 129426 CrossRef CAS PubMed.
- A. Faridah, R. Holinesti and D. Syukri, Identifikasi Pigmen Betasianin Dari Kulit Buah Naga Merah (Hylocereus Polyrhizus), Journal of Education and Family (Jurnal Pendidikan dan Keluarga), 2015, 7(18), 147–154 Search PubMed.
- J. E. Cacace and G. Mazza, Mass transfer process during extraction of phenolic compounds from milled berries, J. Food Eng., 2003, 59(4), 379–389 CrossRef.
- H. M. Azeredo, etalains: Properties, sources, applications, and stability - A review, Int. J. Food Sci. Technol., 2009, 44(12), 2365–2376 CrossRef CAS.
- C. Galanakis, Recovery of high added-value components from food wastes: Conventional, emerging technologies and commercialized applications, Trends Food Sci. Technol., 2012, 26(2), 68–87 CrossRef CAS.
- P. Esquivel, F. C. Stintzing and R. Carle, Phenolic compound profiles and their corresponding antioxidant capacity of purple pitaya (Hylocereus sp.) genotypes, Food Chem., 2019, 275, 390–398 CrossRef PubMed.
- L. Wang and C. L. Weller, Recent advances in extraction of nutraceuticals from plants, Trends Food Sci. Technol., 2006, 17(6), 300–312 CrossRef CAS.
- S. Vilkhu, R. Mawson, L. Simons and D. Bates, Applications and opportunities for ultrasound assisted extraction in the food industry—A review, Innovative Food Sci. Emerging Technol., 2008, 9(2), 161–169 CrossRef.
- D. Butera, L. Tesoriere, F. D. Gaudio, A. Bongiorno, M. Allegra and A. M. Pinatudi, et al., Stability of betalains in prickly pear extract under thermal and solvent stress, J. Agric. Food Chem., 2002, 50, 6895–6901 CrossRef CAS PubMed.
- M. Das, A. Saeid, M. F. Hossain, G. H. Jiang, J. B. Eun and M. Ahmed, Influence of extraction parameters and stability of betacyanins extracted from red amaranth during storage, J. Food Sci. Technol., 2019, 56(2), 643–653, DOI:10.1007/s13197-018-3519-x.
- R. Castellar, J. Obón, M. Alacid and J. Fernández-López, Color properties and stability of betacyanins from Opuntia fruits, J. Agric. Food Chem., 2003, 51(9), 2772–2776 CrossRef CAS PubMed.
- F. Delgado-Vargas, A. R. Jiménez and O. Paredes-López, Natural pigments: carotenoids, anthocyanins, and betalains—characteristics, biosynthesis, processing, and stability, Crit. Rev. Food Sci. Nutr., 2000, 40(3), 173–289 CrossRef CAS PubMed.
- F. Chemat, N. Rombaut, A. G. Sicaire, A. Meullemiestre, A. S. Fabiano-Tixier and M. Abert-Vian, Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review, Ultrason. Sonochem., 2017, 34, 540–560 CrossRef CAS PubMed.
- S. J. Calva-Estrada, M. Jiménez-Fernández and E. Lugo-Cervantes, Betalains and their applications in food: The current state of processing, stability and future opportunities in the industry, Food Chem.: Mol. Sci., 2022, 4, 100089 CAS.
- F. Fathordoobady, H. Mirhosseini, J. Selamat and M. Manap, Effect of solvent type and ratio on betacyanins and antioxidant activity of extracts from Hylocereus polyrhizus flesh and peel, Food Chem., 2016, 202, 70–80 CrossRef CAS PubMed.
- H. Naczk and F. Shahidi, Extraction and Analysis of Phenolics in Food, J. Chromatogr. A, 2004, 1054(1–2), 95–111 CrossRef PubMed.
- Q. W. Zhang, L. G. Lin and W. C. Ye, Techniques for extraction and isolation of natural products: A comprehensive review, Chin. Med., 2018, 13(1), 1–26, DOI:10.1186/s13020-018-0177-x.
- M. P. Van, D. T. Duc, H. Dam, T. Thanh and H. T. Chi, Comparison of ultrasound assisted extraction and enzyme assisted extraction of betacyanin from red dragon fruit peel, in E3S Web of Conferences, 2020, Available from: https://www.e3s-conferences.org/articles/e3sconf/pdf/2020/47/e3sconf_tsae2020_04004.pdf Search PubMed.
- T. Esatbeyoglu, A. E. Wagner, V. B. Schini-Kerth and G. Rimbach, Betanin—a food colorant with biological activity, Mol. Nutr. Food Res., 2015, 59(1), 36–47 CrossRef CAS PubMed.
- D. Pingret, A. Fabiano-Tixier and F. Chemat, Degradation During Application of Ultrasound in Food Processing: A Review, Food Control, 2013, 31(2), 593–606 CrossRef.
- V. T. Pham, D. D. L. Nguyen, T. N. T. Nguyen, T. T. V. Dao, T. T. Tran and L. K. V. Tran, et al., Study on the production process of betacyanin pigment from dragon fruit (Hylocereus polyrhizus) peel by microwave and ultrasonic methods, Food Res., 2025, 9(4), 202–208 Search PubMed.
- N. S. Ramli, P. Ismail and A. Rahmat, Influence of conventional and ultrasonic-assisted extraction on phenolic contents, betacyanin contents, and antioxidant capacity of red dragon fruit (Hylocereus polyrhizus), Sci. World J., 2014, 2014, 964731 Search PubMed.
- K. M. Herbach, F. C. Stintzing and R. Carle, Stability and color changes of thermally treated betanin, phyllocactin, and hylocerenin solutions, J. Agric. Food Chem., 2006, 54(2), 390–398 Search PubMed.
- J. Dai and G. M. Russell, Plant phenolics: extraction, analysis and their antioxidant and anticancer properties, Molecules, 2010, 15(10), 7313–7352 CrossRef CAS PubMed.
- A. Esatbeyoglu, P. Wagner and G. Winterhalter, Stability and antioxidant capacity of betacyanin pigments in vitro, Food Res. Int., 2014, 64, 42–49 Search PubMed.
- S. M. Duyar, F. Sarı and H. A. Karaoglan, Degradation kinetics of betalains in red beetroot juices throughout fermentation process and storage, Ital. J. Food Sci., 2024, 36(4), 229–239 CAS.
- F. C. Stintzing and R. Carle, Functional properties of anthocyanins and betalains in plants, food and in human nutrition, Trends Food Sci. Technol., 2004, 15(1), 19–38 Search PubMed.
- J. Bassama, A. Tamba, M. Ndong, K. Dieu, D. Sarr and M. Cissé, Degradation kinetics of betacyanins during the pasteurization and storage of cactus pear (Opuntia dillenii Haw.) juice using the arrhenius, eyring, and ball models, Beverages, 2020, 7(2), 1–12, DOI:10.3390/beverages7010002.
- M. I. Khan, Stabilization of betalains: A review, Food Chem., 2016, 197, 1280–1285 Search PubMed.
- K. Ravichandran, N. M. M. T. Saw, A. A. A. Mohdaly, A. M. M. Gabr, A. Kastell and H. Riedel, et al., Impact of processing of red beet on betalain content and antioxidant activity, Food Res. Int., 2013, 50(2), 670–675, DOI:10.1016/j.foodres.2011.07.002.
- C. M. Otálora, E. L. Bonifazi, E. N. Fissore, M. F. Basanta and L. N. Gerschenson, Thermal Stability of Betalains in By-Products of the Blanching and Cutting of Beta vulgaris L. Var conditiva, Pol. J. Food Nutr. Sci., 2020, 70(1), 15–24 CrossRef.
- L. Aztatzi-Rugerio, S. Y. Granados-Balbuena, Y. Zainos-Cuapio, E. Ocaranza-Sanches and M. Rojas-Lopez, Analysis of the degradation of betanin obtained from beetroot using Fourier transform infrared spectroscopy, J. Food Sci. Technol., 2019, 56, 3677–3686 CrossRef CAS PubMed.
- B. Jamilah, C. Shu, M. Kharidah, M. Dzulkifly and A. Noranizan, Physico-chemical characteristics of red pitaya (Hylocereus polyrhizus) peel, Int. Food Res. J., 2011, 18(1), 279–286 CAS.
- G. Tenore, E. Novellino and A. Basile, Nutraceutical potential and antioxidant benefits of red pitaya (Hylocereus polyrhizus) consumption, J. Funct. Foods, 2012, 4(1), 129–136 CrossRef CAS.
- K. Y. Choo, Y. Y. Ong, R. L. H. Lim, C. P. Tan and C. W. Ho, Study on bioaccessibility of betacyanins from red dragon fruit (Hylocereus polyrhizus), Food Sci. Biotechnol., 2019, 28(4), 1163–1169, DOI:10.1007/s10068-018-00550-z.
- L. Wu, H. Hsu, Y. Chen, C. Chiu, Y. Lin and J. Ho, Antioxidant and antiproliferative activities of red pitaya, Food Chem., 2016, 95(2), 319–327 CrossRef.
- P. Esquivel, F. C. Stintzing and R. Carle, Pigment pattern and expression of colour in fruits from different Hylocereus sp genotypes, Innovative Food Sci. Emerging Technol., 2007, 8, 451–453 CrossRef CAS.
- T. S. Kujala, M. S. Vienola, K. D. Klika, J. M. Loponen and K. Pihlaja, Betalain and phenolic compositions of four beetroot (Beta vulgaris) cultivars, Eur. Food Res. Technol., 2002, 214(6), 505–510 Search PubMed.
- Q. D. Do, A. E. Angkawijaya, P. L. Tran-Nguyen, L. H. Huynh, F. E. Soetaredjo and S. Ismadji, et al., Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatica, J. Food Drug Anal., 2014, 22(3), 296–302, DOI:10.1016/j.jfda.2013.11.001.
- F. C. Stintzing, J. Conrad, I. Klaiber, U. Beifuss and R. Carle, Structural investigations on betacyanin pigments by LC NMR and 2D NMR spectroscopy, Phytochemistry, 2004, 65(4), 415–422 CrossRef CAS PubMed.
- F. C. Stintzing and R Carle, Betalains – emerging prospects for food scientists, Trends Food Sci. Technol., 2007, 18(10), 514–525 Search PubMed.
- K. Woo, F. Ngou, L. Ngo, W. Soong and P. Tang, Stability of betacyanin from red dragon fruit (Hylocereus polyrhizus) and its potential application as a natural colourant in beverages, Int. J. Food Sci. Technol., 2011, 46(5), 954–960 Search PubMed.
- I. M. Yusoff, Z. Mat Taher, Z. Rahmat and L. S. Chua, A review of ultrasound-assisted extraction for plant bioactive compounds: Phenolics, flavonoids, thymols, saponins and proteins, Food Res. Int., 2022, 157, 111268, DOI:10.1016/j.foodres.2022.111268.
| This journal is © The Royal Society of Chemistry 2026 |
