 Open Access Article
Open Access ArticleOptimization and nanoencapsulation of dandelion leaf extract for herbal tea: a comparative study of spray and freeze drying
Qudsiya Ayaz,
Nadira Anjum,
Sehrish Mustafa,
Abdul Rouf,
Imtiyaz Ahmad Zargar and
Sajad Mohd Wani *
*
Division of Food Science and Technology, Sher-e-Kashmir University of Agricultural Sciences and Technology (SKUAST), Kashmir 190025, Shalimar, India. E-mail: wanisajad82@gmail.com; Tel: +91 9858445878
First published on 19th January 2026
Abstract
This study focuses on optimizing the brewing conditions and nanoencapsulation of dandelion tea extract (DTE) to enhance its stability and bioavailability. The most suitable brewing conditions were determined by using Response Surface Methodology (RSM) with a Box-Behnken design resulting in an extraction time of 110 min, temperature of 85.28 °C and water-to-tea ratio of 150 mL/5 g. The extracted polyphenols were nanoencapsulated using maltodextrin via spray and freeze-drying. The spray-dried powder exhibited higher encapsulation efficiency (88.5%) and controlled release (89.92%) compared to freeze-dried powder (83.51% and 75.76%, respectively), whereas freeze-dried powder showed higher antioxidant activity (85.87%) compared to spray-dried powder (81.35%) indicating better preservation of bioactive compounds. FTIR confirmed successful encapsulation via peak shifts, while SEM showed dented spray-dried nanoparticles and flaky, irregular freeze-dried nanoparticles. The findings suggest that nanoencapsulation of DTE via spray drying enhances stability and bioavailability compared to freeze drying, making it an ideal ingredient for herbal tea, and functional beverages.
Sustainability spotlightThis study promotes sustainable food innovation by valorising dandelion (Taraxacum officinale) leaves -an underutilized wild resource from the Himalayan region into a functional herbal tea via nanoencapsulation. Using maltodextrin, a biodegradable plant-derived carrier, the process enhances the stability and bioavailability of natural antioxidants and polyphenols while minimizing dependence on synthetic additives. Following optimization of the brewing conditions, the extract was nanoencapsulated using spray- and freeze-drying techniques promoting the utilization of locally available plant based resources supporting the development of sustainable, nutritionally enriched functional beverages with improved environmental value. |
1. Introduction
The herbal tea market has recently witnessed significant growth due to shifting consumer preferences toward health and wellness.1,2 Herbal teas, often called tisanes, have been appreciated in different cultures for hundreds of years due to their therapeutic benefits and stimulating flavour. Historically, herbal teas were mainly consumed for medicinal and healing properties, with each herb providing distinct therapeutic benefits. However, nowadays they have become more popular not just for health benefits but also due to the variety of flavors and enriching aromas.3Taraxacum officinale commonly known as dandelion is a highly perishable and underutilized plant, despite its potential health benefits. Dandelion (Taraxacum officinale) belongs to the Asteraceae (sunflower) family, and grows in temperate climates. Dandelion tea is a well known herbal beverage, known for its powerful antioxidants and anti-inflammatory and anti-cancer effects.4 It is rich in phytochemicals, including phenolic acids, triterpenes, coumarins, and flavonoids, all of which are thought to impart antioxidant, immune-boosting, anti-atherosclerotic, antitumor, antimicrobial and other beneficial effects. With innovation in preservation and processing methods, dandelion could become a valuable, sustainable ingredient in the beverage sector.5 However, to fully realize its potential, it is crucial to optimize the extraction and processing conditions to maximize the retention of bioactive compounds, particularly phenolics and flavonoids. Response Surface Methodology (RSM) is an analytical technique that uses a quadratic model to examine relationships between response variables and independent factors aiming to evaluate and optimize experimental conditions.
Instantization of tea converts traditional brewed tea into a soluble powder that dissolves quickly in hot water. Instant tea is commonly produced through extraction followed by drying, most frequently by spray drying or freeze drying, because these methods preserve key bioactive compounds while enabling convenient preparation.6
Spray and freeze-drying are widely utilized techniques for developing instant tea. Spray-drying is cost-effective for commercial use, while freeze-dried tea retains more aromatic compounds.7 Advances in encapsulation technologies such as nanoencapsulation incorporates active compounds into microparticle matrices, regulating their controlled release and shielding them from external factors.8 Among the various drying methods for instant tea production, spray drying and freeze drying are the most prevalent techniques. Freeze drying is particularly effective for preserving heat-sensitive and volatile compounds due to its low-temperature, although it is associated with higher operational costs. In contrast, spray drying is more economical and easily scalable, which has led to its widespread application in nanoencapsulation.9 However, a systematic comparison of these techniques in terms of encapsulation efficiency, antioxidant retention, and controlled release is therefore necessary forming the basis for the present study.
Instant tea production often requires carrier agents to transform liquid extracts into stable powders. Maltodextrin is a popular choice because it is affordable, highly soluble, low in viscosity, and capable of forming films,8,10 which help protect sensitive phytochemicals during drying. Its ability to create a protective matrix makes it an effective wall material for maintaining the stability, dispersibility, and controlled release of polyphenols in both spray- and freeze-dried systems.11,12
To our knowledge, no studies have explored nano-encapsulation technology using both spray-drying and freeze-drying for instant herbal tea production from dandelion leaves. The study aimed to optimize the brewing conditions for tea extraction using RSM with a Box-Behnken design, and to nanoencapsulate the extracted tea polyphenols with maltodextrin through spray and freeze-drying methods.
2 Materials and methods
2.1 Materials
Dandelion leaves were collected at higher altitudes in the Northern Himalayas in Kreeri baramulla with latitude: 34.17°N and longitude: 74.47°E. The samples were promptly cleaned to ensure their safety, followed by sanitization using a 100 ppm sodium hypochlorite solution for 10 minutes to reduce microbial load. After rinsing with distilled water, leaves were dehydrated for three hours in a cabinet dryer at 65 °C. An electric mill was used to grind the dry sample, which was then sieved through a 20-mesh screen, packed, and kept in an airtight container for later use.2.2. Methods
| Process variables | Code | Variables level codes | ||
|---|---|---|---|---|
| −1 | 0 | +1 | ||
| Time (min) | A | 50 | 80 | 110 |
| Temperature (°C) | B | 70 | 85 | 100 |
| Water/Tea (ml) | C | 30 | 90 | 150 |
Dandelion tea infusions (DTIs) were brewed under different conditions as outlined in Table 2. Using a reflux condenser equipped with a magnetic stirrer and a temperature-sensor, heat-reflux extraction (HRE) was carried out. Five grams of dandelion tea leaves boiled in varying percentages of water were added to each DTI. Following the completion of the brewing process, the tea infusion was rapidly chilled and strained using Whatman filter No. 1. After that, the dandelion tea extracts were centrifuged for ten minutes at 5000 rpm using a Weswox WT-24BL. A rotating vacuum evaporator (Buchi Rotavapor R-80, Switzerland) was then used to concentrate the tea extracts in their best optimal state under lower pressure (mmHg) and at a temperature of no more than 35 °C. It was then stored in sealed plastic vials at −18 °C for additional examination after being dried in an oven at a temperature of no more than 27 °C.
| Run | Time (min) | Temp (°C) | Water/tea (ml) | TPC (mg GAE/g) | TFC (mg QE/100 g) | DPPH (%) | Total yield (%) |
|---|---|---|---|---|---|---|---|
| 1 | 80 | 85 | 90 | 74.02 | 63.03 | 63.02 | 23.24 |
| 2 | 50 | 70 | 90 | 64.48 | 56.89 | 51.02 | 20.74 |
| 3 | 80 | 85 | 90 | 70.98 | 65.12 | 59.89 | 22.95 |
| 4 | 50 | 100 | 90 | 75.38 | 65.23 | 68.56 | 19.94 |
| 5 | 50 | 85 | 30 | 65.59 | 57.58 | 58.98 | 18.57 |
| 6 | 80 | 85 | 90 | 72.25 | 61.89 | 62.36 | 24.36 |
| 7 | 110 | 100 | 90 | 75.27 | 65.87 | 66.15 | 29.01 |
| 8 | 80 | 70 | 30 | 63.18 | 58.42 | 59 | 23.78 |
| 9 | 110 | 70 | 90 | 77.67 | 67.12 | 67.98 | 27.25 |
| 10 | 80 | 85 | 90 | 74.12 | 64.56 | 63.21 | 26.6 |
| 11 | 80 | 85 | 90 | 76.36 | 63.11 | 60.01 | 24.02 |
| 12 | 110 | 85 | 30 | 71.58 | 61.55 | 62.23 | 28.69 |
| 13 | 80 | 70 | 150 | 73.57 | 64.63 | 60.12 | 23.11 |
| 14 | 50 | 85 | 150 | 72.48 | 63.98 | 64.69 | 17.89 |
| 15 | 110 | 85 | 150 | 77.78 | 68.01 | 70.25 | 30.25 |
| 16 | 80 | 100 | 150 | 72.59 | 66.45 | 71.25 | 25.69 |
| 17 | 80 | 100 | 30 | 71.12 | 62.23 | 61.09 | 24.31 |
2.3 Quantitative analysis
The following formula was used to determine the extraction yield:
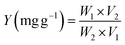 | (1) |
2.4 Preparation of dandelion leaf extract (DLE) for determination of antioxidant properties
DLE was dissolved in 10 mL distilled water, mixed via vortexing, heated at 30 °C for 30 minutes, and centrifuged at 4000 rpm for 20 minutes, and the supernatant was collected for further analysis.15| Inhibition Percentage (IP) % = [(Acontrol − Asample)/Acontrol] × 100 | (2) |
2.5 Preparation of nanoencapsulated powders
Maltodextrin DE 20 (10 g) was dissolved in 100 mL of purified water and left overnight. After adding 1% w/w of Tween 80 as a surfactant, the mixture was agitated for an hour. The wall material and dehydrated dandelion tea extract were mixed in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (w/v) ratios. A sonicator (QSONICA; model Q700) was used to homogenize the solution at 20 KHz for 15 minutes in order to increase the effectiveness of encapsulation and reduce the size of the particles to the nanoscale. The solution was then dehydrated using lyophilisation and spray drying methods.10
1 (w/v) ratios. A sonicator (QSONICA; model Q700) was used to homogenize the solution at 20 KHz for 15 minutes in order to increase the effectiveness of encapsulation and reduce the size of the particles to the nanoscale. The solution was then dehydrated using lyophilisation and spray drying methods.10
A Mini Spray Dryer (BUCHI, B-290), Switzerland, was used to dry the nanoemulsion. The inlet temperature of the spray dryer was set at 120 °C, with a hot air flow velocity of 2.5 m3 min−1 and a peristaltic pump flow rate of 5 cm3 min−1 at 0.06 MPa. The outlet temperature stabilized at approximately 68 °C. The resultant encapsulation products were gathered and kept in an airtight container for further analysis.
Freeze drying involved freezing the nanoemulsion at −20 °C and then drying it using a lyophilization system (BUCHI, Lyovapor L-200, Switzerland). For 48 hours, the ice condenser's temperature was kept at −56 °C, and the vacuum was adjusted to 0.074 Mbar. After 48 hours of drying, the powder was placed in sealed bags for further examination.
2.6. Physical properties of nanoencapsulated powders
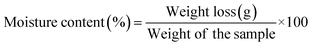 | (3) |
A water activity measurement device from the Aqualab (Model 3 TE) series was used to measure the water's activity.
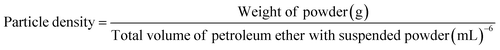 | (4) |
The powder samples' porosity was calculated using the relationship between tapped and particle densities, as shown in eqn 5
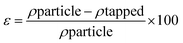 | (5) |
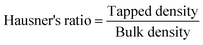 | (6) |
 | (7) |
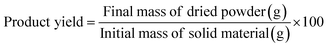 | (8) |
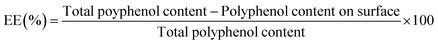 | (9) |
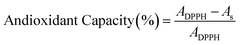 | (10) |
For IC50 determination, antioxidant capacity was evaluated at multiple sample concentrations, and the IC50 value was calculated as the concentration required for reaching 50% antioxidant capacity.
2.7 Characterization of nanoencapsulated powders
3. Results and discussion
3.1. Fitting of the model
The analysis of variance (ANOVA) presented in Table 3 indicates that independent factors have a substantial (p < 0.05) effect on the models developed for every response. All the selected parameters had extremely significant coefficients of determination (R2), which ranged from 0.9052 to 0.9590, suggesting the adequate fit of mathematical models with real data. Predicted R2 and adjusted R2 were found to be reasonably in agreement for all significant models. Additionally, for every model, the precision and coefficient of variation fell between 12.339 and 17.859 and 1.77 and 5.04% respectively, confirming the accuracy, dependability, and reproducibility of the results, as shown in Table 3. Lack of fit for all the models was found to be non-significant, validating their close fit with the observed data. Additionally, the adequate precision was more than 4 for all the models, which guarantees high reliability and is appropriate for validation.| Responses | R2 | Predicted R2 | Adjusted R2 | Regression adequate precision | C.V. | p-value | Lack of fit |
|---|---|---|---|---|---|---|---|
| Total phenolic content | 0.9402 | 0.8631 | 0.8633 | 12.3395 | 2.20 | <0.0017 | 0.9727 |
| Total flavonoid content | 0.9480 | 0.7440 | 0.8812 | 12.4092 | 1.77 | <0.0010 | 0.7584 |
| DPPH | 0.9590 | 0.7304 | 0.9064 | 16.9682 | 2.42 | <0.0005 | 0.5944 |
| Total yield | 0.9052 | 0.8412 | 0.8833 | 17.8592 | 5.04 | <0.0001 | 0.7687 |
3.2 Effect of independent variables (extraction parameters) on dependent variables of dandelion tea
| TPC = 73.55 + 3.05A + 1.93B + 3.12C − 2.39C2 | (11) |
| TFC = 63.54 + 23.6A + 1.59B + 2.91C − 2.40AB | (12) |
From eqn (12), it was observed that the independent variables i.e. extraction time, (A) extraction temperature (B) and water: tea ratio (C), had a positive significant (p < 0.05) effect on TFC. The total flavonoid content increases with increasing extraction time and temperature. This could be because tea leaves are easier to penetrate, which increases their surface area for interaction with water and facilitates a higher level of flavonoid extraction throughout. Additionally, a longer extraction period allows for the dissolution of more flavonoids.30 Wang et al.31 noted similar findings on the optimization of brewing conditions for vine tea. Additionally, it was shown that a larger water to solid ratio enhanced the TFC. This behaviour is most likely caused by a larger volume of solvent, which speeds up compound diffusion by effectively dissolving flavonoids and extracting more solid materials from cells, improving the extraction process and overall enhancing the TFC.32 Insang et al.33 noted similar findings during the mulberry leaf extraction process. Moreover, the interactive effect between extraction time and temperature (AB) revealed a significant negative impact (p < 0.05) on TFC.
| DPPH = 61.70 + 2.92A + 3.62B + 3.13C − 4.84AB | (13) |
The fitted model shows that all three independent variables have a substantial (p < 0.05) positive linear impact on DPPH. DPPH increased when A, B, and C increased, based on the positive values of linear components of extraction time (A), extraction temperature (B), and solid liquid ratio (C). The reason for this could be due to the higher temperatures which speed up the extraction process and make antioxidants more soluble and easier to release from the tea leaves; increasing both time and temperature boosts the overall antioxidant activity. In contrast to less stable, less soluble antioxidants, heat-stable antioxidants in particular are less likely to degrade at higher temperatures, enabling a faster extraction rate.34 Similar outcomes were noted by Lee et al.35 Furthermore, the antioxidant activity increased when the water-to-solid ratio increased as shown inTable 4 This might be because of the larger volume of the solvent, which increases the possibility that antioxidants will come into contact with the extraction solvent and help move bioactive chemicals into the liquid phase.29 Wati et al.36 also noted a similar pattern. Moreover, tea's antioxidant activity was significantly (p < 0.05) impacted negatively by the interaction of extraction time (A) and temperature (B).
| Values | Total phenolic content (mg GAE/g) | Total flavonoid content (mg QE/100 g) | DPPH (%) | Total yield (%) |
|---|---|---|---|---|
| Predicted values | 77.78 | 68.04 | 70.68 | 29.10 |
| Actual values | 76.58 | 67.12 | 69.88 | 28.28 |
| Variation (%) | 1.54 | 1.35 | 1.13 | 2.81 |
| TY = 24.14 + 4.76A | (14) |
From the regression eqn (14), TY increased significantly (p < 0.05) as extraction time (A) increased. This could be explained by the fact that longer extraction times improve the extraction of more antioxidant substances as well as tea polyphenols, allowing more bioactive substances to be discharged into the liquid medium and enhancing the proportion of total yield.37 Similar findings regarding the Hirsute leaf extraction technique were noted by Pham et al.38
3.3 Validation of the model and optimization
The optimal conditions were determined using the desirability function approach, yielding a desirability score of 0.969 (Fig. 1). The best conditions for making dandelion tea were determined to be 110 minutes extraction time, 85.28 °C extraction temperature, and 150.00 mL/5 g water-to-tea ratio. The predicted and experimentally obtained values for responses were 77.78 and 76.68 mg GAE/g for TPC, 68.04 and 67.12 mg QE/g for TFC, 70.68 and 69.88% for DPPH antioxidant activity, and 29.10 and 28.28% for total yield respectively. A strong connection between the predicted and experimental results confirmed the validity of the current RSM model. The experimental results closely matched the predicted response values, with a difference of less than 4%.3.4 Physical characteristics of nanoencapsulated powders
| Parameters | Freeze dried | Spray dried |
|---|---|---|
| a All values are reported as mean ± standard deviation from three replicates. Statistically significant differences (p ≤ 0.05) between the freeze-dried and spray-dried encapsulates within rows are represented by different lowercase letters (a and b). | ||
| Moisture content (%) | 3.49 ± 0.31a | 2.69 ± 0.42b |
| Water activity (aw) | 0.29 ± 0.02a | 0.2 ± 0.02b |
| Bulk density (g cm−3) | 0.42 ± 0.02b | 0.59 ± 0.03a |
| Tapped density (g cm−3) | 0.46 ± 0.03b | 0.71 ± 0.01a |
| Particle density (g cm−3) | 1.48 ± 0.04b | 1.65 ± 0.02a |
| Hygroscopicity (%) | 12.25 ± 0.07b | 14.59 ± 0.09a |
| Porosity (%) | 68.91 ± 1.63a | 57.57 ± 1.63b |
| Carr's index (flowability) | 8.69 ± 0.04b | 17.14 ± 0.04a |
| Hausner ratio (cohesiveness) | 1.09 ± 0.01b | 1.20 ± 0.01a |
Particle density is a significant variable for estimating the true density of particulates, eliminating the void regions among them. The particle densities of both powders differed significantly (p < 0.05) as shown in Table 5. The spray-dried sample had a higher particle density of 1.65 g cm−3, while the freeze-dried sample showed a lower particle density of 1.48 g cm−3. The difference in powdered density between the spray-dried and freeze-dried samples may be the reason for the higher particle density in the former. Less air space between the particles makes higher bulk density powders ideal for storing. During spray drying, compact, spherical particles are formed as a result of rapid evaporation of water due to high temperature which leads to smaller sizes and greater uniformity; these particles pack more tightly, hence increasing the particle density. In addition, a higher bulk density can result in superior flow characteristics, lower settling down, and less vulnerability to moisture absorption, all of which enhance performance and extend shelf life in a variety of applications.45 Our findings are consistent with the outcomes of Bashir et al.46
Porosity is a crucial component of powder reconstitution and represents an additional important feature in food processing. Since porosity quantifies the percentage of total volume occupied by void spaces, it is related to bulk density.45 A statistically significant difference (p ≤ 0.05) was observed in the porosity of both powders with spray dried tea powder having a porosity of 57.57% and freeze-dried tea powder showing a porosity of 68.91% as given in Table 5. Lower porosity was exhibited by spray dried powder while higher porosity was shown by freeze dried powder. The reason for lower porosity might be due to the higher bulk density and particle arrangement of the spray-dried nano-encapsulated samples. Because of their smaller size, the particles are packed closer together, creating fewer spaces between them and reducing the material's porosity compared to the freeze-dried powder. Additionally, the lower bulk density values of the freeze-dried powder samples can be explained by their higher porosity, which resulted from phase transition of ice that creates voids, thereby increasing the product's porosity.48 Previous studies by Karam et al.,49 revealed the same outcomes with freeze-dried and spray-dried mango powders.
The powder's bulk and tapped densities are used to calculate the CI and HR values. Greater cohesion and poor flowability are indicated by higher values of the Hausner Ratio (HR) and Carrs Index (CI). Table 5 indicates that the CI and HR values for freeze-dried tea powder varied between 8.69 and 1.09, while the values for spray-dried tea powder were 17.14 and 1.20, respectively. In contrast to the freeze-dried powder, which showed greater flowability and lower cohesiveness, the spray-dried powder had higher HR and CI values, suggesting that the substance is more cohesive and has poor flowability. This might be because of the low moisture content, which has been demonstrated to improve powder particle cohesiveness and decrease flowability.51 Furthermore, the homogeneity and fine particle size in the freeze-dried powder sample decreased interstitial spaces, maximizing surface availability in contact with the surrounding air. This consequently results in lower values for the Hausner Ratio and Carr Index in freeze-dried powder,.52 and our findings correlate with those of Caliskan and Dirim.53
| Parameters | Freeze dried | Spray dried |
|---|---|---|
| a All values are expressed as mean ± standard deviation from three replicates. Statistically significant differences (p ≤ 0.05) between the freeze-dried and spray-dried encapsulates within each row are denoted by different lowercase letters (a and b). | ||
| Product yield (%) | 85.29 ± 1.51a | 75.96 ± 1.31b |
| Encapsulation efficiency (%) | 83.51 ± 0.77b | 88.5 ± 0.94a |
| Antioxidant capacity (%) | 85.87 ± 1.53 a | 81.35 ± 0.67 b |
| IC50 (mg mL−1) | 1.71 ± 0.03 a | 1.94 ± 0.06 b |
| Particle size (nm) | 290.26 ± 2.36a | 180.45 ± 1.54b |
| Polydispersity index | 0.29 ± 0.02b | 0.34 ± 0.02a |
| Zeta potential (mv) | −24.4 ± 0.63b | −20.5 ± 1.37a |
The IC50 values derived from antioxidant capacity measurements indicate that the freeze-dried powder exhibited slightly higher antioxidant potency, as reflected by its lower IC50 value (1.71 mg mL−1) compared with the spray-dried powder (1.94 mg mL−1).This outcome reflects the advantage of low-temperature processing in preserving phenolic antioxidants. Similar trends were reported by Liang et al.61 for Spiranthes sinensis, where minimally heated extracts showed lower IC50 values. Turkiewicz et al.62 also observed higher IC50 values in spray dried medicinal-plant extracts.
3.5 Characterization of encapsulated powders
3.5.1.1 Particle size. Particle size plays a key role in determining stability, encapsulation efficiency, and the overall accessibility of the core substance. It's crucial for enhancing targeted nutrition and medicinal applications, ensuring the effectiveness of the product. Table 6 depicts the particle size of the tea powder nanoparticles that were encapsulated using freeze and spray drying. The freeze-dried tea samples exhibited a larger particle size of 290.26 nm, while the spray dried powder samples had a particle size of 180.45 nm. The reduction in the particle size of the spray-dried sample may be due to the high inlet temperature and atomization.63 Moreover, the larger size of the particles in freeze drying can be attributed to the agglomeration of particles during the formation of ice crystals due to the lower temperature used in the process and the inability of forces to break the frozen medium into tiny droplets. Our results are in line with Chen et al.64 for spray and freeze drying of water pea.
3.5.1.2 Polydispersity index (PDI). The polydispersity index (PDI) is essential for evaluating uniformity and stability of the particle size dispersion. The distribution of particle size is measured by the PDI value, which is greater for samples exhibiting greater size diversity than for samples with uniformly sized particles.65 Furthermore, a polydispersity index below 0.4 suggests a narrow particle size distribution.66 Table 6 presents the PDI values for the freeze dried and spray dried powders and were recorded as 0.29 and 0.34 respectively. The freeze-dried samples exhibited a lower PDI, whereas the spray dried samples showed a higher PDI. This is because in freeze drying, each sample is frozen separately and then dried at low temperatures and pressures, which might cause cell rupture and enhance the capsule's internal environment that promotes the production of smaller particles. However, spray drying can result in particle aggregation and produces larger particles by spraying the liquid sample and drying it at high temperatures.67 Consequently, the polydispersity index (PDI) will probably be more uniform in freeze drying. Our results for the polydispersity index (PDI) are consistent with those of Ma et al.,52 who compared freeze-drying (FD) and spray-drying (SD) in stable silybin nanosuspensions.
3.5.1.3 Zeta potential. Zeta-potential plays a vital role in evaluating how stable a colloidal system is. It is an essential component in estimating the degree of stability of microparticles and serves as an indicator of net surface charge. The stability of nanoparticles is generally determined by their charge. A zeta potential exceeding +30 mV or falling below −30 mV indicates an ideal condition for maintaining long-term colloidal stability. In general, the z-potential can be used to calculate the extent of both stability and instability of suspensions. The probability of particle aggregation due to electric repulsion decreases with increasing z-potential.68 From Table 6, the zeta potentials of the freeze-dried and spray-dried tea powders were −24.4 mV and −20.5 mV, respectively. The lower zeta potential in freeze-dried samples is primarily due to an increase in particle size which decreases the surface area available for charge dispersion and electrostatic repulsion.69 Comparable observations were reported by Ledari et al.,70 when they assessed two drying methods for chlorophyll encapsulation.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) stretching region characteristic of carbohydrates showed distinct movement, shifting from 1358.9 cm−1 in dandelion extract and 1344.3 cm−1 in maltodextrin to 1365.4 cm−1 in the freeze-dried powder and 1372.7 cm−1 in the spray-dried powder. The consistent fingerprint band at around 989–990 cm−1 in all samples is attributed to C–H stretching of methylene and methyl groups confirming that the aromatic ring structure of polyphenols remains intact after encapsulation aligning with previous research by Kang et al.73 Similar FT-IR shifts for O–H and C–H stretching bands have been reported in earlier microencapsulation studies involving dandelion leaf extract by the spray drying process by Martinic et al.74 These findings demonstrate that the encapsulation process, along with the drying methods, has a significant effect on the molecular interactions between the tea extract and maltodextrin, with slight differences observed between freeze-dried and spray-dried powders. The variations in vibrational peaks between spray-dried and freeze-dried powder samples could stem from the higher thermal conditions employed during spray drying that causes structural modifications that ultimately affect the oscillatory behaviour of the functional groups. While investigating the sea buckthorn fava bean composite instant powder, similar observations were made by Li et al.75 for spray and freeze-drying effects on physicochemical properties, bio actives and sensory attributes.
O) stretching region characteristic of carbohydrates showed distinct movement, shifting from 1358.9 cm−1 in dandelion extract and 1344.3 cm−1 in maltodextrin to 1365.4 cm−1 in the freeze-dried powder and 1372.7 cm−1 in the spray-dried powder. The consistent fingerprint band at around 989–990 cm−1 in all samples is attributed to C–H stretching of methylene and methyl groups confirming that the aromatic ring structure of polyphenols remains intact after encapsulation aligning with previous research by Kang et al.73 Similar FT-IR shifts for O–H and C–H stretching bands have been reported in earlier microencapsulation studies involving dandelion leaf extract by the spray drying process by Martinic et al.74 These findings demonstrate that the encapsulation process, along with the drying methods, has a significant effect on the molecular interactions between the tea extract and maltodextrin, with slight differences observed between freeze-dried and spray-dried powders. The variations in vibrational peaks between spray-dried and freeze-dried powder samples could stem from the higher thermal conditions employed during spray drying that causes structural modifications that ultimately affect the oscillatory behaviour of the functional groups. While investigating the sea buckthorn fava bean composite instant powder, similar observations were made by Li et al.75 for spray and freeze-drying effects on physicochemical properties, bio actives and sensory attributes.
 | ||
| Fig. 4 In vitro bioactive release behaviour of freeze dried and spray dried tea nanoparticles in simulated gastric digestion (0–2 h) and simulated intestinal digestion (2–6 h). | ||
| Sample | Zero order | First order | Higuchi | Hixon–Crowell | Korsmeyer–Peppas | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| K0 | R2 | K1 | R2 | KH | R2 | KHC | R2 | KKP | R2 | n | |
| Freeze dried | 0.265 | 0.8570 | 0.005 | 0.9428 | 4.288 | 0.9105 | 0.001 | 0.9459 | 1.712 | 0.93210 | 0.667 |
| Spray dried | 0.263 | 0.9852 | 0.004 | 0.9562 | 4.206 | 0.9157 | 0.001 | 0.9784 | 0.560 | 0.9927 | 0.866 |
The delivery of the active component is influenced by variations in particle size and surface area, according to the Hixson-Crowell model, while the Higuchi model characterizes the release mechanism as being predominantly regulated by diffusion. The Korsmeyer-Peppas model provided the optimal fit for the present data. The regression coefficient R2 values obtained by the Korsmeyer-Peppas model for dandelion tea nanoparticles was recorded to be 0.9321 0 for freeze-dried nanoparticles and 0.9927 for spray-dried nanoparticles. This model also aids in characterizing the release mechanism through the diffusion exponent (n). If n ≤ 0.5, the release follows Fickian diffusion. For values of n ≥ 0.89, the release mechanism indicates super case II transport through polymeric chain erosion. When n is between 0.45 and 0.89, an anomalous (non-Fickian) release mechanism occurs, involving both diffusion and swelling. The n values for nanoparticles obtained through freeze-dried and spray-dried nanoparticles (0.667–0.866) suggest a non-Fickian diffusion transport mechanism. Similar findings were reported by Ledari et al.70 during encapsulation of chlorophyll using freeze drying and spray drying techniques.
4. Conclusion
Instant herbal dandelion tea powder was developed by nanoencapsulation of dandelion leaf extracts by optimizing the brewing conditions using Box-Behnken design. Comparative evaluation of spray- and freeze-dried nanoencapsulated powders demonstrated that spray-dried particulates exhibited superior encapsulation efficiency, surface morphology, flowability, and reconstitution behaviour parameters that are critical for practical application in herbal tea formulations. The study revealed that spray-dried nanoparticulates with polyphenolic compounds exhibited superior encapsulation efficiency, surface characteristics, and release behaviour. The release profile closely followed the Korsmeyer–Peppas model, which exhibited the highest R2 values across the samples and was therefore identified as the best-fit model for sustained-release formulations. However, the zero-order model also provided an excellent fit for the spray-dried nanoparticulates, further supporting their sustained-release behaviour. These findings indicate that nano-encapsulation of dandelion tea extract with maltodextrin is a promising approach for enhancing its stability under simulated gastrointestinal conditions. In addition to lower energy requirements for industrial-scale processing, enhanced solubility, dispersibility, and stability further support spray drying as the preferred method for producing functional nanoencapsulated dandelion tea powders. The developed product offers an innovative nutraceutical beverage which can be scaled up to the industrial set up. Future studies should explore the incorporation of these encapsulated nanoparticles into herbal tea formulations to evaluate their stability, controlled release properties, and potential health benefits.Author contributions
Qudsiya Ayaz: writing – original draft, software, formal analysis, data curation, Nadira Anjum: validation, software, Sehrish Mustafa: writing review & editing, Abdul Rouf: supervision, project administration, Imtiyaz Ahmad Zargar: supervision and project administration, and Sajad Mohd Wani: supervision, resources, project administration, investigation.Conflicts of interest
There are no conflicts to declare.Data availability
Data will be made available on request.Acknowledgements
This work has been financially supported by the Department of Biotechnology (DBT), New Delhi. BT/PR45204/NER/95/1931/2022.References
- G. E. Okpiaifo, B. Dormoy-Smith, B. Kassas and Z. Gao, Perception and demand for healthy snacks/beverages among US consumers vary by product, health benefit, and color, PLoS One, 2023, 18(6), e0287232 CrossRef CAS PubMed.
- V. Puri, M. Nagpal, I. Singh, M. Singh, G. A. Dhingra, K. Huanbutta, D. Dheer, A. Sharma and T. Sangnim, A comprehensive review on nutraceuticals: therapy support and formulation challenges, Nutrients, 2022, 14(21), 4637 CrossRef CAS PubMed.
- X. Long, S. Ranjitkar, A. Waldstein, H. Wu, Q. Li and Y. Geng, Preliminary exploration of herbal tea products based on traditional knowledge and hypotheses concerning herbal tea selection: a case study in Southwest Guizhou, China, J. Ethnobiol. Ethnomed., 2024, 20(1), 1 CrossRef PubMed.
- M. S. Hosen and B. Madhu, Health benefits of herbal tea: a review, Daffodil International University, 2023, vol. 10, pp. 1–10 Search PubMed.
- Y. Zhu, W. Gu, R. Tian, C. Li, Y. Ji, T. Li, C. Wei and Z. Chen, Morphological, physiological, and secondary metabolic responses of Taraxacum officinale to salt stress, Plant Physiol. Biochem., 2022, 189, 71–82 CrossRef CAS PubMed.
- C. Someswararao and P. P. Srivastav, A novel technology for production of instant tea powder from the existing black tea manufacturing process, Innovative Food Sci. Emerging Technol., 2012, 16, 143–147 CrossRef CAS.
- V. Kraujalytė, E. Pelvan and C. Alasalvar, Volatile compounds and sensory characteristics of various instant teas produced from black tea, Food Chem., 2016, 194, 864–872 CrossRef.
- L. M. Sagis, Microencapsulation and Microspheres for Food Applications, Academic Press, 2015, 1–550 Search PubMed.
- A. Gharsallaoui, G. Roudaut, O. Chambin, A. Voilley and R. Saurel, Applications of spray-drying in microencapsulation of food ingredients: an overview, Food Res. Int., 2007, 40(9), 1107–1121 CrossRef CAS.
- F. Sansone, T. Mencherini, P. Picerno, M. d'Amore, R. P. Aquino and M. R. Lauro, Maltodextrin/pectin microparticles by spray drying as carrier for nutraceutical extracts, J. Food Eng., 2011, 105(3), 468–476 CrossRef CAS.
- A. Tolun, Z. Altintas and N. Artik, Microencapsulation of grape polyphenols using maltodextrin and gum arabic as two alternative coating materials: development and characterization, J. Biotechnol., 2016, 239, 23–33 CrossRef CAS PubMed.
- S. Y. Hundre, P. Karthik and C. Anandharamakrishnan, Effect of whey protein isolate and β-cyclodextrin wall systems on stability of microencapsulated vanillin by spray–freeze drying method, Food Chem., 2015, 174, 16–24 CrossRef CAS PubMed.
- L. Yao, X. Liu, Y. Jiang, N. Caffin, B. D'Arcy, R. Singanusong and Y. Xu, Compositional analysis of teas from Australian supermarkets, Food Chem., 2006, 94(1), 115–122 CrossRef CAS.
- A. Kumaran and R. J. Karunakaran, In vitro antioxidant activities of methanol extracts of five Phyllanthus species from India, LWT–Food Sci. Technol., 2007, 40(2), 344–352 CrossRef CAS.
- I. O. Pfingstgraf, M. Taulescu, R. M. Pop, R. Orăsan, L. Vlase, A. Uifalean and A. E. Pârvu, Protective effects of Taraxacum officinale L. (dandelion) root extract in experimental acute on chronic liver failure, Antioxidants, 2021, 10(4), 504 CrossRef CAS PubMed.
- L. G. L. Gerolis, F. S. Lameiras, K. Krambrock and M. J. Neves, Effect of gamma radiation on antioxidant capacity of green tea, yerba mate, and chamomile tea as evaluated by different methods, Radiat. Phys. Chem., 2017, 130, 177–185 CrossRef CAS.
- G. W. Shuen, L. Y. Yi, T. S. Ying, G. C. Y. Von, Y. A. B. Yusof and P. L. Phing, Effects of drying methods on the physicochemical properties and antioxidant capacity of Kuini powder, Braz. J. Food Technol., 2021, 24, e2020086 CrossRef CAS.
- N. Jinapong, M. Suphantharika and P. Jamnong, Production of instant soymilk powders by ultrafiltration, spray drying and fluidized bed agglomeration, J. Food Eng., 2008, 84(2), 194–205 CrossRef.
- J. A. Zokti, B. Sham Baharin, A. S. Mohammed and F. Abas, Green tea leaves extract: microencapsulation, physicochemical and storage stability study, mol., 2016, 21(8), 940 CrossRef PubMed.
- J. Mujica-Álvarez, O. Gil-Castell, P. A. Barra, A. Ribes-Greus, R. Bustos, M. Faccini and S. Matiacevich, Encapsulation of vitamins A and E as spray-dried additives for the feed industry, mol., 2020, 25(6), 1357 CrossRef.
- S. Saikia, N. K. Mahnot and C. L. Mahanta, Optimisation of phenolic extraction from Averrhoa carambola pomace by response surface methodology and its microencapsulation by spray and freeze drying, Food Chem., 2015, 171, 144–152 CrossRef CAS PubMed.
- P. Filippou, S. T. Mitrouli and P. Vareltzis, Sequential membrane filtration to recover polyphenols and organic acids from red wine lees: the antioxidant properties of the spray-dried concentrate, Membr., 2022, 12, 353 CrossRef CAS PubMed.
- V. Sanna, G. Caria and A. Mariani, Effect of lipid nanoparticles containing fatty alcohols having different chain length on the ex vivo skin permeability of econazole nitrate, Powder Technol., 2010, 201(1), 32–36 CrossRef CAS.
- R. Rashid, S. M. Wani, S. Manzoor, F. A. Masoodi and A. Altaf, Nanoencapsulation of pomegranate peel extract using maltodextrin and whey protein isolate: characterisation, release behaviour and antioxidant potential during simulated in vitro digestion, Food Biosci., 2022, 50, 102135 CrossRef CAS.
- R. Pulicharla, C. Marques, R. K. Das, T. Rouissi and S. K. Brar, Encapsulation and release studies of strawberry polyphenols in biodegradable chitosan nanoformulation, Int. J. Biol. Macromol., 2016, 88, 171–178 CrossRef CAS PubMed.
- J. Li and Z. Guo, Concurrent extraction and transformation of bioactive phenolic compounds from rapeseed meal using pressurized solvent extraction system, Ind. Crops Prod., 2016, 94, 152–159 CrossRef CAS.
- S. Parvez, I. A. Wani and F. A. Masoodi, Extraction optimization of green tea beverage (Noon Chai) for yield, polyphenols and caffeine using response surface methodology, Arab. J. Sci. Eng., 2021, 46, 1–13 Search PubMed.
- A. Mokrani and K. Madani, Effect of solvent, time and temperature on the extraction of phenolic compounds and antioxidant capacity of peach (Prunus persica L.) fruit, Sep. Purif. Technol., 2016, 162, 68–76 CrossRef CAS.
- K. N. Prasad, F. A. Hassan, B. Yang, K. W. Kong, R. N. Ramanan, A. Azlan and A. Ismail, Response surface optimisation for the extraction of phenolic compounds and antioxidant capacities of underutilised Mangifera pajang Kosterm. peels, Food Chem., 2011, 128(4), 1121–1127 CrossRef CAS.
- J. Liu, C. Li, G. Ding and W. Quan, Artificial intelligence assisted ultrasonic extraction of total flavonoids from Rosa sterilis, Molecules, 2021, 26(13), 3835 CrossRef CAS PubMed.
- L. Wang, H. Zhang, M. Xie, J. Wan, J. Li, S. Yang and Y. Xiong, Optimization of brewing process vine tea and flavor analysis of different brewing processes, J. Food Biochem., 2024, 48(1), 8858457 Search PubMed.
- A. D. Sousa, A. I. V. Maia, T. H. S. Rodrigues, K. M. Canuto, P. R. V. Ribeiro, R. D. C. A. Pereira and E. S. de Brito, Ultrasound-assisted and pressurized liquid extraction of phenolic compounds from Phyllanthus amarus and its composition evaluation by UPLC-QTOF, Ind. Crops Prod., 2016, 79, 91–103 CrossRef CAS.
- S. Insang, I. Kijpatanasilp, S. Jafari and K. Assatarakul, Ultrasound-assisted extraction of functional compound from mulberry (Morus alba L.) leaf using response surface methodology and effect of microencapsulation by spray drying on quality of optimized extract, Ultrason. Sonochem., 2022, 82, 105806 CrossRef CAS PubMed.
- Y. Liu, L. Luo, C. Liao, L. Chen, J. Wang and L. Zeng, Effects of brewing conditions on the phytochemical composition, sensory qualities and antioxidant activity of green tea infusion: a study using response surface methodology, Food Chem., 2018, 269, 24–34 CrossRef CAS.
- L. S. Lee, N. Lee, Y. H. Kim, C. H. Lee, S. P. Hong, Y. W. Jeon and Y. E. Kim, Optimization of ultrasonic extraction of phenolic antioxidants from green tea using response surface methodology, Molecules, 2013, 18(11), 13530–13545 CrossRef CAS PubMed.
- R. Wati, S. Sampanvejsobha and N. Punbusayakul, Effect of extraction conditions on Assam green tea, Agric. Sci. J., 2009, 40, 47–50 Search PubMed.
- A. P. Tambunan, A. Bahtiar and R. R. Tjandrawinata, Influence of extraction parameters on the yield, phytochemical, TLC-densitometric quantification of quercetin, and LC–MS profile, and how to standardize different batches for long term from Ageratum conyzoides L. leaves, Pharmacogn. J., 2017, 9(6), 767–774 CrossRef CAS.
- H. N. T. Pham, V. T. Nguyen, Q. V. Vuong, M. C. Bowyer and C. J. Scarlett, Effect of extraction solvents and drying methods on the physicochemical and antioxidant properties of Helicteres hirsuta Lour. leaves, Technol., 2015, 3(4), 285–301 Search PubMed.
- S. A. Mahdavi, S. M. Jafari, E. Assadpoor and D. Dehnad, Microencapsulation optimization of natural anthocyanins with maltodextrin, gum Arabic and gelatin, Int, J. Biol. Macromol., 2016, 85, 379–385 CrossRef PubMed.
- Z. Abdullah, F. S. Taip, S. M. M. Kamal and R. Z. A. Rahman, Nonlinear model-based inferential control of moisture content of spray dried coconut milk, Foods, 2020, 9(9), 1177 CrossRef PubMed.
- L. S. Kuck and C. P. Z. Norena, Microencapsulation of grape (Vitis labrusca var. Bordo) skin phenolic extract using gum Arabic, polydextrose, and partially hydrolyzed guar gum as encapsulating agents, Food Chem., 2016, 194, 569–576 CrossRef CAS PubMed.
- R. V. D. B. Fernandes, S. V. Borges and D. A. Botrel, Influence of spray drying operating conditions on microencapsulated rosemary essential oil properties, Food Sci. Technol., 2013, 33, 171–178 CrossRef.
- D. W. Dadi, S. A. Emire, A. D. Hagos and J. B. Eun, Physical and functional properties, digestibility, and storage stability of spray- and freeze-dried microencapsulated bioactive products from Moringa stenopetala leaves extract, Ind. Crops Prod., 2020, 156, 112891 CrossRef CAS.
- H. Mitra, H. A. Pushpadass, M. E. E. Franklin, R. K. Ambrose, C. Ghoroi and S. N. Battula, Influence of moisture content on the flow properties of basundi mix, Powder Technol., 2017, 312, 133–143 CrossRef CAS.
- S. Parvez, I. A. Wani and F. A. Masoodi, Extraction optimization of green tea beverage (Noon Chai) for yield, polyphenols and caffeine using response surface methodology, Arab. J. Sci. Eng., 2022, 1–13 Search PubMed.
- I. Bashir, S. M. Wani, A. A. Bhat, A. A. Khan, S. Z. Hussain, S. A. Ganai and N. Anjum, Effect of freeze drying and spray drying on physical properties, morphology and in vitro release kinetics of vitamin D3 nanoparticles, Powder Technol., 2024, 432, 119164 CrossRef CAS.
- R. V. Tonon, C. Brabet and M. D. Hubinger, Influence of process conditions on the physicochemical properties of açaí (Euterpe oleraceae Mart.) powder produced by spray drying, J. Food Eng., 2008, 88(3), 411–418 CrossRef.
- L. A. Egas-Astudillo, N. Martínez-Navarrete and M. D. M. Camacho, Quality of a powdered grapefruit product formulated with biopolymers obtained by freeze-drying and spray-drying, J. Food Sci., 2021, 86(6), 2255–2263 CrossRef CAS PubMed.
- M. C. Karam, J. Petit, D. Zimmer, E. B. Djantou and J. Scher, Effects of drying and grinding in production of fruit and vegetable powders: A review, J. Food Eng., 2016, 188, 32–49 CrossRef.
- K. Muzaffar, B. N. Dar and P. Kumar, Assessment of nutritional, physicochemical, antioxidant, structural and rheological properties of spray dried tamarind pulp powder, J. Food Meas. Charact., 2017, 11, 746–757 CrossRef.
- J. J. Fitzpatrick, K. Barry, P. S. M. Cerqueira, T. Iqbal, J. O'Neill and Y. H. Roos, Effect of composition and storage conditions on the flowability of dairy powders, Int. Dairy J., 2007, 17(4), 383–392 CrossRef CAS.
- Y. Ma, J. Gao, W. Jia, Y. Liu, L. Zhang, Q. Yang and Y. Wang, A comparison of spray-drying and freeze-drying for the production of stable silybin nanosuspensions, J. Nanosci. Nanotechnol., 2020, 20(6), 3598–3603 CrossRef CAS PubMed.
- G. Caliskan and S. N. Dirim, The effect of different drying processes and the amounts of maltodextrin addition on the powder properties of sumac extract powders, Powder Technol., 2016, 287, 308–314 CrossRef CAS.
- H. Aryaee, P. Ariaii, D. Zare, S. Mirdamadi and S. N. Raeisi, Evaluation of the physicochemical characteristics of a blend fruit juice powder mixed with Lactiplantibacillus plantarum: A comparison of spray drying and freeze drying, J. Food Process. Preserv., 2023, 1, 5597647 Search PubMed.
- Q. Chen, F. Zhong, J. Wen, D. McGillivray and S. Y. Quek, Properties and stability of spray-dried and freeze-dried microcapsules co-encapsulated with fish oil, phytosterol esters, and limonene, Dry. Technol., 2013, 31(6), 707–716 CrossRef CAS.
- X. Xin, S. Essien, K. Dell, M. W. Woo and S. Baroutian, Effects of spray-drying and freeze-drying on bioactive and volatile compounds of smoke powder food flavouring, Food Bioprocess Technol., 2022, 15, 785–794 CrossRef CAS.
- M. E. da Silva Júnior, M. V. R. L. Araújo, A. C. S. Martins, M. dos Santos Lima, F. L. H. Da Silva, A. Converti and M. I. S. Maciel, Microencapsulation by spray-drying and freeze-drying of extract of phenolic compounds obtained from ciriguela peel, Sci. Rep., 2023, 13(1), 15222 CrossRef PubMed.
- P. Vareltzis, D. Fotiou, V. Papatheologou, S. Kyroglou, E. Tsachouridou and A. M. Goula, Optimized solid–liquid separation of phenolics from lavender waste and properties of the dried extracts, Sep., 2024, 11(3), 67 CAS.
- P. Vareltzis, A. Stergiou, K. Kalinderi and M. Chamilaki, Antioxidant potential of spray- and freeze-dried extract from oregano processing wastes, using an optimized ultrasound-assisted method, Foods, 2023, 12(13), 2628 CrossRef CAS PubMed.
- W. F. Gomes, F. R. França, M. Denadai, J. K. Andrade, E. M. da Silva Oliveira, E. S. de Brito, S. Rodrigues and N. Narain, Effect of freeze- and spray-drying on physico-chemical characteristics, phenolic compounds and antioxidant activity of papaya pulp, J. Food Sci. Technol., 2018, 55(6), 2095–2102 CrossRef CAS PubMed.
- C. P. Liang, C. H. Chang, C. C. Liang, K. Y. Hung and C. W. Hsieh, In vitro antioxidant activities, free radical scavenging capacity, and tyrosinase inhibitory of flavonoid compounds and ferulic acid from Spiranthes sinensis (Pers.) Ames, Molecules, 2014,(19), 4681–4694 CrossRef CAS PubMed.
- I. P. Turkiewicz, A. Wojdyło, K. Tkacz, K. Lech, A. Michalska-Ciechanowska and P. Nowicka, The influence of different carrier agents and drying techniques on physical and chemical characterization of Japanese quince (Chaenomeles japonica) microencapsulation powder, Food Chem., 2020,(323), 126830 CrossRef CAS PubMed.
- F. H. Brishti, S. Y. Chay, K. Muhammad, M. R. Ismail-Fitry, M. Zarei, S. Karthikeyan and N. Saari, Effects of drying techniques on the physicochemical, functional, thermal, structural and rheological properties of mung bean (Vigna radiata) protein isolate powder, Food Res. Int., 2020, 138, 109783 CrossRef CAS PubMed.
- W. Chen, H. T. Chiu, Z. Feng, E. Maes and L. Serventi, Effect of spray-drying and freeze-drying on the composition, physical properties, and sensory quality of pea processing water (Liluva), Foods, 2021, 10(6), 1401 CrossRef CAS PubMed.
- S. Ahmadian, R. E. Kenari, Z. R. Amiri, F. Sohbatzadeh and M. H. H. Khodaparast, Fabrication of double nano-emulsions loaded with hyssop (Hyssopus officinalis L.) extract stabilized with soy protein isolate alone and combined with chia seed gum in controlling the oxidative stability of canola oil, Food Chem., 2024, 430, 137093 CrossRef CAS PubMed.
- Y. Ma, S. Chen, W. Liao, L. Zhang, J. Liu and Y. Gao, Formation, physicochemical stability, and redispersibility of curcumin-loaded rhamnolipid nanoparticles using the pH-driven method, J. Agric. Food Chem., 2020, 68(27), 7103–7111 CrossRef CAS PubMed.
- D. E. Walton, The morphology of spray-dried particles: A qualitative view, Dry. Technol., 2000, 18(9), 1943–1986 CrossRef CAS.
- A. Seyfoddin and R. Al-Kassas, Development of solid lipid nanoparticles and nanostructured lipid carriers for improving ocular delivery of acyclovir, Drug Dev. Ind. Pharm., 2013, 39(4), 508–519 CrossRef CAS PubMed.
- I. E. Agarry, Z. Wang, T. Cai, J. Kan and K. Chen, Chlorophyll encapsulation by complex coacervation and vibration nozzle technology: Characterization and stability study, Innov. Food Sci. Emerg. Technol., 2022, 78, 103017 CrossRef CAS.
- S. A. Ledari, J. M. Milani, S. A. Shahidi and A. Golkar, Comparative analysis of freeze drying and spray drying methods for encapsulation of chlorophyll with maltodextrin and whey protein isolate, Food Chem. X, 2024, 21, 101156 CrossRef PubMed.
- H. Tiernan, B. Byrne and S. G. Kazarian, ATR-FTIR spectroscopy and spectroscopic imaging for the analysis of biopharmaceuticals, Spectrochim. Acta A Mol. Biomol. Spectrosc., 2020, 241, 118636 CrossRef CAS PubMed.
- T. Arumugham, R. Krishnamoorthy, J. AlYammahi, S. W. Hasan and F. Banat, Spray-dried date fruit extract with a maltodextrin/gum arabic binary blend carrier agent system: Process optimization and product quality, Int. J. Biol. Macromol., 2023, 238, 124340 CrossRef CAS PubMed.
- Y. R. Kang, Y. K. Lee, Y. J. Kim and Y. H. Chang, Characterization and storage stability of chlorophylls microencapsulated in different combinations of gum arabic and maltodextrin, Food Chem., 2019, 272, 337–346 CrossRef CAS PubMed.
- A. Martinić, A. Kalušević, S. Lević, V. Nedović, A. Vojvodić Cebin, S. Karlović, I. Špoljarić, G. Mršić, K. Žižek and D. Komes, Microencapsulation of dandelion (Taraxacum officinale L.) leaf extract by spray drying, Food Technol. Biotechnol., 2022, 60(2), 237–252 Search PubMed.
- S. Li, X. Fu, J. Wen, L. Jiang, L. Shao, Y. Du and C. Shan, Characterization of physicochemical properties, bioactivities, and sensory attributes of sea buckthorn–fava bean composite instant powder: Spray-drying versus freeze-drying coupled with carriers, Foods, 2024, 13(23), 3944 CrossRef CAS PubMed.
- R. A. Kurniasih, E. N. Dewi and L. Purnamayati, Effect of different coating materials on the characteristics of chlorophyll microcapsules from Caulerpa racemosa, IOP Conf. Ser. Earth Environ. Sci., 2018, 116(1), 012030 CrossRef.
- K. Yadav, R. K. Bajaj, S. Mandal and B. Mann, Encapsulation of grape seed extract phenolics using whey protein concentrate, maltodextrin and gum arabic blends, J. Food Sci. Technol., 2020, 57, 426–434 CrossRef CAS PubMed.
- J. R. da Rosa, G. C. C. Weis, K. I. B. Moro, S. S. Robalo, C. E. Assmann, L. P. P. da Silva, E. I. Muller, C. D. B. da Silva, C. R. de Menezes and C. S. da Rosa, Effect of wall materials and storage temperature on anthocyanin stability of microencapsulated blueberry extract, LWT, 2021, 142, 111027 CrossRef.
- A. M. Ribeiro, M. Shahgol, B. N. Estevinho and F. Rocha, Microencapsulation of vitamin A by spray-drying, using binary and ternary blends of gum arabic, starch, and maltodextrin, Food Hydrocoll., 2020, 108, 106029 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2026 |



