Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Antimicrobial active packaging with biopolymers and natural extracts: sustainable solutions and technological challenges
Shubhajit Sarkhel a,
Samandeep Kaur
a,
Samandeep Kaur b,
Rahul Dasc,
Aditi Sharmad,
Ankan Kheto
b,
Rahul Dasc,
Aditi Sharmad,
Ankan Kheto e,
Debapam Sahaf and
Yogesh Kumar
e,
Debapam Sahaf and
Yogesh Kumar *g
*g
aCollege of Food Technology, Indira Gandhi Krishi Vishwavidyalaya, Raipur, Chhattisgarh 492012, India
bSchool of Engineering and Technology, National Forensic Sciences University, Gandhinagar, Gujarat 382007, India
cICAR-Central Institute of Post-Harvest Engineering and Technology, Ludhiana, Punjab 141004, India
dDepartment of Food Engineering and Technology, Sant Longowal Institute of Engineering and Technology, Longowal, Sangrur, Punjab 148106, India
eDepartment of Food Process Engineering, National Institute of Technology, Rourkela, Odisha 769008, India
fDepartment of General Science, Calcutta Institute of Technology, Howrah, West Bengal 711316, India
gDepartment of Food Science and Technology, Graphic Era University, Dehradun, Uttarakhand 248002, India. E-mail: kyogesh2u@gmail.com
First published on 16th December 2025
Abstract
The growing demand for sustainable food packaging solutions has increased research into antimicrobial active packaging systems based on biodegradable biopolymers and natural extracts. This review provides a comprehensive overview of recent progress in their development, functionality, and existing challenges, with particular emphasis on plant-derived compounds, animal-derived antimicrobials, and microbial metabolites. These bioactive agents exhibit various antimicrobial mechanisms, including membrane disruption, enzyme inhibition, and oxidative stress induction, and are often incorporated into biopolymer matrices through solvent casting, extrusion, or encapsulation techniques. However, the critical technological challenge lies in achieving the stability, compatibility, and controlled release of these agents in complex food matrices, which directly impacts their real-world applicability. Regulatory restrictions, economic feasibility, and consumer safety concerns further complicate commercialization. By synthesizing current findings, this review emphasizes that emerging encapsulation strategies and smart delivery systems not only enhance antimicrobial efficacy but also represent a pivotal step toward scalable, eco-friendly, and truly sustainable packaging solutions. Therefore, this review highlights how emerging technologies and policy approaches can integrate material innovation with sustainability goals to create safer, more effective, and eco-friendly antimicrobial packaging.
Sustainability spotlightThis review highlights the potential of biopolymer- and natural extract-based antimicrobial active packaging to reduce food spoilage, extend shelf-life, and minimize reliance on petroleum-based plastics. The advancements in their fabrication and application directly support global sustainability goals, including SDG 12 (Responsible Consumption and Production) and SDG 14 (Life Below Water), by addressing both food waste and plastic pollution. This article also emphasizes the importance of encapsulation and controlled-release technologies to increase the stability and efficacy of natural antimicrobial agents, paving the way for scalable, safe, and multifunctional packaging solutions. Through a comprehensive synthesis of recent advances, regulatory frameworks, and engineering strategies, this work contributes to the development of sustainable food preservation technologies that align with consumer expectations, environmental mandates, and circular economy principles. |
1. Introduction
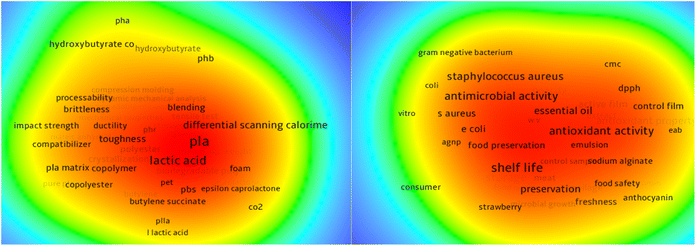
Research on food packaging is evolving, from containment to now being active, intelligent, and smart. Petroleum-based packaging continues to dominate owing to its superior protective properties, but its persistence in the environment has resulted in serious ecological and health concerns, including the spread of microplastics that now affect both humans and other living organisms. According to the World Health Organization,1 health risks occur throughout the plastic lifecycle, from production to disposal, whereas global initiatives such as the Sustainable Development Goals (SDGs), particularly SDG 12 (Responsible Consumption and Production) and SDG 14 (Life Below Water), emphasize the need for sustainable packaging and reduced plastic pollution.2 In this context, biopolymers are emerging as promising alternatives because of their nontoxicity, biodegradability, film-forming ability, and wide availability. Keyword network analysis (Fig. 1) focusing on “biodegradable AND packaging” shows that lactic acid and its derivatives currently dominate research in this area, with recent studies doubling in number over the past five years compared with the previous fifteen. Despite this, other underexplored biopolymers, such as starch, proteins, and fibers, hold substantial potential. Importantly, these materials have demonstrated versatility in active packaging applications, particularly when combined with plant-based extracts,3,4 natural antioxidants,5 colorimetric indicators,6 and other functional additives to increase protection. One especially promising direction is the development of antimicrobial biopolymer films, which can be safely functionalized with natural extracts that typically possess low or no human toxicity, making them suitable for multifunctional food packaging.The relevance of antimicrobial active packaging has grown as the food industry faces significant annual losses, with large volumes of food becoming unfit for consumption owing to microbial activity. Microorganisms such as bacteria, yeasts, and molds are the leading causes of spoilage, as their metabolic processes lead to undesirable sensory changes, including off-flavors, color alterations, and textural defects.7 While physical and chemical processes such as moisture loss or lipid oxidation also contribute to quality decline, microbial spoilage remains the most complex and persistent challenge. This issue is amplified by long supply chains and extended storage periods, which increase the possibility of contamination and reduce the effectiveness of conventional preservation strategies.8,9 In addition to the economic and nutritional costs of food loss, spoilage also contributes to environmental pressures, particularly greenhouse gas emissions associated with waste disposal. In this context, despite their environmental drawbacks, conventional plastics play a role in limiting spoilage and thereby reducing emissions from food waste.10 This highlights a critical sustainability trade-off. While plastics mitigate food waste, they simultaneously worsen ecological harm through persistence and microplastic pollution. Therefore, the challenge is not only to replace plastics with biodegradable alternatives but also to develop systems that balance environmental responsibility with food preservation efficacy.
In addition to these technical challenges, the shift toward antimicrobial active packaging is accelerated by strong market and policy drivers. For example, the global antimicrobial packaging market size was valued at USD 11.11 billion in 2023 and is projected to grow at a CAGR of 7.6% from 2024 to 2030.11 Within this market, the biopolymer-based antimicrobial packaging segment is expected to experience the fastest growth during the forecast period. In addition, consumers are increasingly demanding clean-label products with natural preservation methods while also expecting packaging solutions that align with broader sustainability goals. At the same time, corporations are under pressure to meet ambitious environmental commitments, such as reducing single-use plastics and transitioning to recyclable or compostable alternatives. However, life cycle assessments suggest that simply replacing plastics with other materials can, in some cases, result in higher greenhouse gas emissions, underscoring the need for solutions that also minimize food waste.12 Regulatory bodies add another layer of pressure, enforcing stricter rules on food-contact materials and encouraging the adoption of safer, more sustainable technologies. Against this backdrop, antimicrobial active packaging based on biodegradable polymers must be critically assessed not only for its environmental credentials but also for its technological robustness. Advanced engineering strategies such as nanocarriers, nanostructured composites, and smart encapsulation systems are increasingly recognized as pivotal solutions to overcome the limitations of stability, compatibility, and controlled release.
Therefore, this review aims to explore the recent progress in antimicrobial active packaging based on biopolymers and natural extracts, with a focus on studies published after 2015. It critically examines how plant-derived (essential oils, flavonoids, polyphenols, tannins), animal-derived (lysozyme, lactoferrin, chitosan derivatives), and microbial (nisin, bacteriocins, peptides) antimicrobial components interact with biopolymer matrices to extend shelf-life and enhance food safety. Particular attention is given to the mechanisms governing antimicrobial action, release behavior, and material performance, as well as to emerging engineering strategies such as encapsulation, nanostructuring, and advanced coatings that improve stability and efficacy. Moreover, the review highlights associated challenges, such as compound instability and matrix compatibility, as well as safety and regulatory challenges. By highlighting these sustainability trade-offs and integrating advanced engineering strategies, the present review establishes antimicrobial biopolymer packaging as an important step toward more sustainable ways to preserve food.
2. Natural antimicrobial extract
The active ingredients within the food packaging system play a significant role in food quality. It enhances the shelf-life of food by releasing desirable components that inactivate microbes. In addition, bioactive compounds from natural extracts are strongly compatible with biopolymers and often produce synergistic effects, enhancing antimicrobial activity through the controlled release of active compounds. The mechanisms involved in reducing a wide range of microbes using natural extracts are discussed in further sections. Moreover, Table 1 presents a list of some common antimicrobial components in natural extracts, their sources and their mechanism of action.| Antimicrobial components | Sources | Mechanism of action | Optimal working factors | References |
|---|---|---|---|---|
| Cinnamaldehyde | Cinnamon essential oil | PMK-1 mediated p38 signaling pathway against P. aeruginosa | MIC of 10 mg L−1 and 100 mg L−1 | Shu et al.14 |
| Eugenol | Clove essential oil | Alters membrane permeability, resulting in leakage of intracellular contents against E. coli | MIC of 0.0312 to 8 µg mL−1 within 4 h | Jeyakumar & Lawrence15 |
| 1,8-Cineole and α-pinene | Rosemary essential oil | Cell wall stress, destruction of organelle membranes, vacuolar segregation, mitochondrial depolarization, and cell cycle arrest at G1/S phase against Candida albicans | MIC of 4500 µg mL−1 for 1,8-cineole and 3125 µg mL−1 for α-pinene | Shahina et al.16 |
| Carvacrol | Lamiaceae essential oil | B. subtilis 168 elongation and S. aureus BAA-41 enlargement and inhibition of cell division | MIC of 4–16 µg mL−1 | Zhi et al.17 |
| Citral | Citrus essential oils | Decreased intracellular ATP concentration, and cell membrane hyperpolarization against Cronobacter sakazakii | MIC of 0.27 to 0.54 mg mL−1 | Shi et al.18 |
| Chrysoeriol-7-O-β-D-xyloside, luteolin-7-O-β-D-apiofuranosyl-1→2-β-D-xylopyranoside, chrysoeriol-7-O-β-D-apiofuranosyl-1→2-β-D-xylopyranoside, chrysoeriol-7-O-α-L-rhamnopyranosyl-1→6-β-D-4″-hydrogeno sulfate glucopyranoside, and isorhamnetin-3-O-α-L-rhamnopyranosyl-1→6-β-D-glucopyranoside | G. glandulosum | Nucleic acids lost through a damaged cytoplasmic membrane against V. cholerae | MIC value of 64 µg mL−1 for was recorded with compound chrysoeriol-7-O-α-L-rhamnopyranosyl-1→6-β-D-4″-hydrogeno sulfate glucopyranoside | Tagousop et al.19 |
| Flavonoids glycosides | ||||
| Pentagalloyl-O-β-D-glucose | Cytinus tannin extracts | Destruction of membranes and chelation of metal ions against Staphylococcus aureus, Staphylococcus epidermidis, Enterococcus faecium | MIC of 125 to 500 µg mL−1 | Maisetta et al.20 |
| 1,5-Di-O-caffeoyl quinic acid | L. crithmoides phenolic extracts | Destruction of cell wall against Staphylococcus aureus ATCC 25923, and Pseudomonas aeruginosa ATCC 27853, Escherichia coli ATCC 3521, Enterococcus faecalis ATCC 29212, and Salmonellatyphimurium LT2 DT104 | ZI > 1–16 mm | Jallali et al.21 |
| Oleiferasaponin D3 | C. oleifera cake saponin extract | Destruction of cell membrane structure, leakage of cell contents and inhibited the growth of mycelium, reduced cell adhesion and aggregation, and effectively inhibited the formation of biofilm against C. albicans, S. cerevisiae, and Penicillium | MIC of 0.078, 0.156, and 0.156 mg mL−1 | Yu et al.22 |
| Amyloids fibrils, peptide residues, and worms | Hen egg white lysozyme | Electrostatic binding to the cell walls and membranes of the microorganisms, followed by membrane disruption against S. aureus and C. albicans | MIC of 0.01 mg mL−1 | Kummer et al.23 |
| Lactoferricin Lfcin and lactoferrampin Lfampin | Red deer Cervus elaphus milk lactoferrin | Form amphipathic structures with net hydrophobic and positively charged surfaces against E. coli ATCC 25922 and L. acidophilus ATCC 4356 | MIC of 240–480 µg mL−1 | Wang et al.24 |
| Lactoperoxidase, glucose oxidase, α-D-glucose, potassium thiocyanate, and hydrogen peroxide | Lactoperoxidase system | Oxidization of essential sulphydryl groups in bacterial enzymes and proteins against psychrotrophic bacteria and Pseudomonas spp. | Concentration of 5–7.5% | Farshidi et al.25 |
| Thiazolium moieties, quaternized 4-2-4-methylthiazol-5-yl ethoxy-4-oxobutanoic acid | Chitosan from shrimp shells | Permeabilize the cell wall against L. innocua, C. albicans, S. epidermidis, S. aureus, E. coli | MIC of 8–32 µg mL−1 | Muñoz-Nuñez et al.26 |
| Nisin | Lactobacillus sp. | Forming pores and inhibiting cell wall synthesis against E. faecalis strains ATCC 29212, OG1RF, and strain E | MICs of 1 mg mL−1 | Tong et al.27 |
| Reuterin | Lactobacillus reuteri | Targets lipid and amino acid metabolism, leading to cell membrane damage, subsequently results in energy metabolism disorder against Staphylococcus aureus | MIC of 18.25 mM | Sun et al.28 |
2.1 Plant derivative extract
Plant-derived extracts such as essential oils, polyphenols, and phytochemicals are widely incorporated into packaging material matrices because of their effective bioactive properties. For example, essential oils are volatile aromatic secondary metabolites that are extracted from various parts of angiospermic plant tissues and serve ecological functions in pollination and defense against microbial pathogens.13 Similarly, essential oils are compatible with biopolymer-based packaging in the form of films and coatings. When mixed with a biopolymer solution, they form a stable emulsion that, upon drying, becomes embedded in the polymer matrix, providing antimicrobial activity through the migration of active compounds from the matrix to the food. Scanning electron microscopy (SEM) images confirm that the essential oils cause structural alterations in the film during drying, which results from the complex interactions that take place between the lipid, the protein and the solvent. These phenomena increase the stability of the essential oils in the matrix. The bioavailability of these active compounds is directly correlated with the concentration of the essential oils being incorporated and the temperature of the system.29 Furthermore, its molecular weight is less than 300 Da, which results in high vapor pressure at room temperature, allowing it to exist in a gaseous state.30 The antimicrobial activity of essential oils is directly associated with the presence of bioactive volatile chemical compounds, primarily monoterpenes, sesquiterpene hydrocarbons and their oxygenated derivatives.31 The mechanism of action of essential oils against microbes involves disruption of bacterial cellular membranes, which leads to increased permeability and leakage of intracellular contents. This is due to the accumulation of bioactive components of the essential oils, which are adsorbed onto the cell surface and subsequently penetrate the phospholipid bilayer of the membrane. Alterations in membrane permeability result in the leakage of essential intercellular components, including proteins, reducing sugars, ATP, and DNA. It simultaneously disrupts ATP generation and associated enzymatic activities, ultimately leading to cellular destruction and electrolyte loss.32 Moreover, it also affects bacterial dissipation of the proton motive force and adenosine triphosphate depletion. Even prolonged exposure to essential oils triggers apoptosis and necrosis in bacterial cells, disrupts ion transport via interactions with membrane proteins and intercellular compounds, impairs enzyme activity, and causes electrolyte loss (K+, Na+, Ca2+).30 These effects arise through multiple pathways and are influenced by their hydrophobic and hydrophilic nature, bacterial type, and cell wall structure. For example, Gram-positive bacteria are particularly susceptible to essential oils, as their peptidoglycan cell wall allows hydrophobic compounds (mostly present in essential oils) to penetrate and interact with the cytoplasm. However, in a few cases, Gram-negative bacteria resist infection via an additional outer membrane consisting of proteins and lipopolysaccharides around the cell wall.33On the other hand, polyphenols such as flavonoids, flavan-3-ols, flavonols, flavanones, phenolic acids, stilbenoids, and tannins are nonvolatile plant secondary metabolites with multiple phenol rings and are incorporated in active packing material matrices because of their antimicrobial properties.34 Sachets, absorbent pads, biopolymer coatings, etc., are commonly used as vehicles for polyphenol-based antimicrobial packaging materials.34 The incorporation and stability are accomplished using intermolecular bonding via hydrophobic forces and electrostatic interactions between the functional groups of the packaging material matrix and polyphenols. Furthermore, antimicrobial action occurs by contact diffusion of active compounds of polyphenols from films or coating into food.35 The bioavailability of these active compounds is directly correlated with the amount of fat and moisture present in the food matrix.29 These polyphenols inhibit bacterial biofilm formation, a protective layer against adverse factors, through mechanisms such as reducing motility and surface adhesion, blocking detection, and inhibiting the expression of virulence factors associated with pathogenic behavior.36,37 The inhibition of biofilm formation can be achieved by modifying the activity of curli genes (csgA, csgB) in E. coli O157:H7, increasing the level of protein IsaA (immunodominant staphylococcal antigen A) for S. aureus and the formation of hydrogen bonds via hydroxyl groups.34 Additionally, polyphenols disrupt the cell membranes of Gram-positive and Gram-negative bacteria by altering their phospholipid or lipid bilayers, resulting in reduced fluidity, increased permeability, and impaired ion transportation.38 It binds with the peptidoglycan of the cell wall, thereby lowering osmotic pressure and increasing ionic strength, which reduces cell tolerance.39 Moreover, it leaks small molecules from the liposome inner space to agglomerate the liposome, causing membrane lipid bilayer damage and apoptosis.40 The B rings of flavonoids inhibit DNA and RNA synthesis by intercalating or hydrogen bonding with nucleic acid bases, while they also block nucleic acid synthesis by inhibiting DNA gyrase.41 In addition, polyphenols bind proteins both noncovalently and covalently, exerting antibacterial activities by sequestration and denaturation of proteins into soluble or insoluble complexes.34
In addition to polyphenols and essential oils, several other compounds in plant-derived extracts exhibit strong antimicrobial activity. Alkaloids, terpenoids, lectins, quinone, coumarin, polypeptides, tannins (nonphenolic), and sulfide-containing compounds interfere with microbial DNA replication, RNA transcription, and protein synthesis; inhibit biofilms, cell division, and enzyme activity; and disrupt the cell wall.42–45 In conclusion, plant-derived extracts exhibit strong antimicrobial potential through various mechanisms. However, their application can be limited by factors such as high volatility, which affects the organoleptic properties of food due to their strong aroma, poor water solubility, heat and light sensitivity, and regulatory and safety considerations.29 In addition, the incorporation of these extracts reduced the film strength and increased the water vapor permeability.46 The possible accumulation and uneven distribution of these extracts on the surface of the film and deterioration of its properties (e.g., oxidation, thermal degradation) were also observed.47 Therefore, advanced stabilization and controlled-release strategies such as encapsulation or nanodelivery systems are commonly employed, as discussed later.
2.2 Animal derivative extract
Numerous animal-derived biopolymers, enzymes and proteins, such as lysozyme, lactoferrin, chitosan derivatives, and lactoperoxidase, have been effectively leveraged in active packaging materials to inhibit microbial growth. Lysozyme (N-acetylmuramic hydrolase) is a cysteine-rich disulfide-linked hydrolytic enzyme of animal origin (found in egg and milk) that is generally recognized as safe (GRAS) and is widely recognized for its antimicrobial activity.43 Lysozymes exhibit excellent stability and solubility in water, making them blend effectively with a film-forming solution. Therefore, it can be used as an edible film, coating, or applied directly to the surface of foods. To apply a coating, the food product is immersed in the film-forming solution and subsequently dried, resulting in the formation of a microfilm on the food surface. The bioavailability of lysozyme was highest with chitosan. It is evenly distributed in chitosan-based biopolymer films through hydrogen bonds (N–H in lysozyme and O–H in chitosan) and shows high antimicrobial activity via pH-dependent controlled release.48 During storage, lysozyme hydrolyzes chitosan to smaller molecular weights with higher antimicrobial activity while retaining its potential as an antimicrobial agent.49 Several factors, such as pH, temperature, ionic strength, and salt type, significantly influence lysozyme bioavailability. It exhibits optimum activity at pH 5, while its stability decreases under highly acidic and alkaline conditions. Low concentrations of salts normally increase the catalytic efficiency of lysozyme, but high ionic strengths inhibit its activity. Lysozyme is a thermally stable enzyme. It remains active even at high temperatures, and its activity can be further protected by sugars and polyols.50,51 Lysozyme cleaves the β-(1,4)-glycosidic linkage between N-acetylmuramic acid and N-acetylglucosamine in peptidoglycan chains, a key structural element of the cell wall of Gram-positive bacteria, and this hydrolytic activity weakens wall integrity, ultimately leading to bacterial lysis. However, they are ineffective against Gram-negative bacteria because their outer membrane is rich in phospholipids, lipopolysaccharides, and lipoproteins that block access to peptidoglycan. However, in some cases, as a cationic protein, lysozyme can still form pores in bacterial cells via electrostatic interactions with membrane phospholipids, causing lysis independent of peptidoglycan hydrolysis.52 The cationic, hydrophilic, and lipophilic properties of lysozyme increase its affinity for the negatively charged outer membrane of bacteria, subsequently leading to disruption of the integrity of the plasma membrane.53 Therefore, lysozyme can be incorporated into packing matrices to develop effective antimicrobial packaging systems.On the other hand, lactoferrin is an iron-binding glycoprotein present in milk that has antimicrobial effects on Gram-positive and Gram-negative bacteria, viruses, and fungi through iron deprivation. It interacts with bacterial membranes through its lactoferricin peptide.54 Its function depends on its ability to bind iron, which results in iron deficiency, restricting bacterial growth.43 Lactoferrins are compatible with biopolymer films or coatings such as gelatin, chitosan, cellulose, and nanocellulose via electrostatic interactions. They also exhibit antimicrobial activity through direct contact migration and controlled release.55 Furthermore, the lactoperoxidase system (LPOS), which is naturally present in milk and human saliva, comprises three essential components, namely, lactoperoxidase (LPO), hydrogen peroxide (H2O2), and thiocyanate (SCN−), and only displays antimicrobial effects when they coexist. They are mostly bioavailable with chitosan-, whey protein- and alginate-based biopolymer films and are stable via hydrogen bonding or charge–charge interactions.48 These films show antimicrobial activity through the controlled diffusion of active agents to food surfaces.56 Within this system, LPO catalyzes the oxidation of SCN− to generate hypothiocytosis (OSCN−) and hypothiocytosis acid (HOSCN), which are reactive species that oxidize sulfhydryl (–SH) groups in microbial proteins and enzymes, thereby disrupting cellular metabolism.56 The oxidation of the SH group damaged the cell wall structure. This damage makes the wall more permeable, allowing important nutrients and cell components, such as ions, amino acids, and peptides, to leak out. This can hinder the growth of microorganisms and may lead to cell death. By this mechanism, LPOS exhibits broad-spectrum antimicrobial activity against both Gram-positive and Gram-negative bacteria, along with antifungal effects, making it effective for antimicrobial packing applications.
In addition to these proteins and enzymes, chitosan and its derivatives, polysaccharides obtained from crustacean shells, have also gained prominence as animal-derived biopolymer films in antimicrobial packaging. Chitosan-based polymeric materials can be formed into fibers, films, gels, sponges, beads or nanoparticles. It exerts its activity despite its cationic amino groups, which interact electrostatically with negatively charged microbial cell membranes, leading to increased permeability, leakage of intercellular components, and eventual cell death. The bioavailability of chitosan in terms of its antimicrobial effect on a packaging material significantly depends upon several intrinsic and extrinsic factors, such as concentration, pH, molecular weight, and solubility. Chitosan has a concentration-dependent antimicrobial effect. Similarly, at low concentrations, its protonated amino groups bind to negatively charged bacterial surfaces, disrupt membrane integrity, and promote leakage of intercellular components, whereas at higher concentrations, chitosan forms a uniform coating on the cell surface, which suppresses further leakage. The optimal condition is 1–5% chitosan (w/w) with LDPE for packaging applications.57,58 Low-molecular-weight (<4.6 kDa) chitosan can also enter the cell's nucleus and block the transcription of RNA from DNA through adsorption to DNA molecules,59 exerting antimicrobial effects on bacteria, yeasts, and fungi.60 Furthermore, at a relatively high charge density of 83.7%, strong electrostatic interactions occur, leading to an outstanding antimicrobial effect.61 The hydrophilic–hydrophobic balance of chitosan is another important factor determining its antimicrobial activity. The poor water solubility of chitosan creates major restrictions. However, hydrophobic modifications, including N-acylation, improve the affinity of this polymer for microbial membranes. Chitosan becomes highly soluble and polycationic in acids. Therefore, its antimicrobial activity is far stronger at low pH and decreases close to neutral. Moreover, it has a high chelating ability toward metal ions such as Zn2+, Fe2+, and Mg2+ at low pH, showing antimicrobial effects by destabilizing microbial cell walls through the sequestration of essential ions.57,60,62
Furthermore, saturated fatty acids (SAFAs), monounsaturated fatty acids (MUFAs) and polyunsaturated fatty acids (PUFAs) extracted from Salmo salar fish waste oil showed antimicrobial activity against Gram-positive and Gram-negative bacteria. The extracted fatty acids alter bacterial cell membrane hydrophobicity, charge and integrity, which results in electron leakage and subsequent cell death.63 Similarly, α-helical pardaxins isolated from the skin glands of Red Sea Moses sole (Pardachirus marmoratus) show antimicrobial activity against Gram-positive and Gram-negative bacteria via pore-forming activities. Additionally, α-helical peptides (pleurocidins and piscidins) from the skin mucus of winter flounder (Pleuronectes americanus) inhibit bacterial DNA, RNA, and protein synthesis.64 Overall, animal-derived extracts show strong antimicrobial potential, highlighting their value in active packaging. However, their sensitivity to processing and low compatibility limit their commercial use. For example, lactoferrin is highly unstable under certain conditions, such as high temperature and low pH, which cause conformational changes and loss of activity.55 On the other hand, the compatibility of LPOS with biopolymers is limited, as it is effectively restricted to alginate, chitosan, gelatin, and whey protein. Moreover, it depends on a continuous or sequential cofactor supply, making its direct incorporation into packaging films difficult.56 Overall, compared with the plant-derived extract, neither group was universally superior. Each performs best under specific food and packaging conditions, and their optimal choice depends on the desired release behavior, target microorganisms, and product formulation. Moreover, combining them with plant-based extracts may offer synergistic, sustainable, and multifunctional food packaging solutions. Therefore, more research should focus on immobilization, encapsulation, and compatibility with a wide range of biopolymers, as well as synergistic and comparative studies with other natural extracts, to improve their use in real-world food packaging applications.
2.3 Microbial derivative extract
Antimicrobial compounds extracted from bacterial cells, known as bacteriocins, are gaining potential for use in packaging material matrices because of their ability to resist microbes and tolerate high temperatures and acidic environments. These are metabolic byproducts that bacteria produce as part of their defense mechanism. The bacteriocins produced by lactic acid bacteria, known as nisin, are acceptable because of their traditional role in the food fermentation process. Nisin belongs to the lantibiotic class of bacteriocins and is composed of 34 amino acids. It interacts with the bacterial cell wall, forming pores that disrupt the pH equilibrium and proton motive force, causing cellular fluid leakage and cell death. Nisin is more effective against spores than it is against vegetative microorganisms because of its sporostatic potential. It interacts with biopolymers through a polymer network during film preparation, becoming stable and exhibiting antimicrobial activity through pH-dependent diffusion from the biopolymer matrix to the microbial surface. The compatibility and bioavailability of nisin with the packaging material matrix are excellent, with a polyvinyl dichloride lacquer coating (800 ± 7 IU dm−2), chitosan films (1.234–1.347 × 10–13 m2 s−1), and composite films (0.6–2.0 wt% of total released). During nisin release from food packaging, water first diffuses into the material matrix, causing structural relaxation and swelling, which enables the antimicrobial agent to migrate through the matrix and ultimately reach and act upon the microbial surface.65–67On the other hand, pediocin forms a random coil in aqueous solution, which becomes partially helical with distinct hydrophobicity in nonaqueous media. Its N-terminal cationic and C-terminal hydrophobic regions enable pore formation in bacterial membranes, causing leakage of cellular fluids, ATP loss, proton motive force collapse, and cell death.68 The polylactic acid–sawdust particle biocomposite film surface was impregnated with pediocin by diffusion coating, which resulted in an antimicrobial effect. The sawdust particles increased the surface hydrophilicity, which facilitated the adsorption and controlled release of hydrophilic pediocin to the surface of the bacteria.69 Similarly, reuterin (β hydroxypropionaldehyde), produced from glycerol by certain strains of Lactobacillus reuteri, is another antimicrobial agent attracting interest because of its high water solubility, resistance to heat, proteolytic and lipolytic enzymes, and stability over a wide range of pH values. Alginate–konjac gum film containing L. reuteri cells can produce reuterin through anaerobic glycerol bioconversion, resulting in antimicrobial activity.70 The aldehyde functional group in reuterin acts as a bioactive component, which triggers oxidative stress in microbes by altering thiol groups in proteins and small molecules.71 An alternative mechanism suggests that the dimeric form of reuterin interferes with DNA synthesis, thereby suppressing microbial growth and causing cell death.72 In addition to microbe-derived biochemical extracts, bacteriophages may serve as effective antimicrobial agents within active packaging materials composed of acetate cellulose. The incorporation of bacteriophage into the cellulose film demonstrated observable diffusion into the bacterial medium through contact with the inoculated environment.73 Bacteriophages encode enzymes that effectively hydrolyze the cell wall peptidoglycan core and lyse bacterial host cells during the terminal phase of their lytic cycle.74 All these microbiological derivatives highlight their potential for use as antimicrobial packing agents. While their natural origin, stability and broad antimicrobial mechanism make them attractive, challenges are present. For example, direct incorporation of pediocin in the packaging material matrix faces notable challenges, as it can be rapidly degraded by proteolytic enzymes and adsorbed by the food matrix, leading to diminished activity during long-term storage or under inadequate temperature conditions.68 Similarly, when unencapsulated nisin is incorporated into a packaging material matrix, it quickly loses its activity through degradation due to environmental factors such as temperature, pH, oxygen, etc.66 However, in comparison with plant and animal extracts, microbial metabolites demonstrate greater reliability in controlled-release packaging systems.
Moreover, active packaging systems with natural extracts often lack the ability to distinguish between harmful pathogens and beneficial or neutral microbiota. Therefore, future research should prioritize the development of smart-responsive packaging, genetic engineering of microbes, phage–biofilm synergy, and hybrid nanostructures for embedding these compounds. Future research should also be conducted in the design and optimization of engineered antimicrobial packaging to target pathogens while preserving or leaving beneficial or neutral microbiota. Variations in membrane charge, peptidoglycan architecture, and lipid composition in many pathogens make them inherently more susceptible to the action of mechanisms that disrupt membranes. Moreover, few bioactive agents, such as lysozyme or nisin, act upon specific targets, which leads to stronger effects on Gram-positive or spore-forming organisms. Moreover, the MICs and release rates further guide the formulations to maintain concentrations that impede pathogens while nontarget microbes are unaffected.
2.4 Encapsulation methods for natural extracts
Natural extracts from plants, animals, and microorganisms face challenges such as environmental sensitivity and instability. Several encapsulation techniques, such as solvent evaporation, phase inversion, spray drying, freeze drying, cross-linking, and ionic gelation, might be beneficial, as they preserve the effectiveness of these natural extracts and improve target-specific delivery, oxidation stability, and solubility. The solvent evaporation method is a widely used low-energy encapsulation method that involves dissolving the natural extract in a polymer–organic solvent, emulsifying it in an aqueous phase, and solidifying it as the solvent evaporates, forming polymeric microspheres. Natural extracts from plants are primarily encapsulated using this method.75 Another low-energy encapsulation method is phase inversion. In this method, changes in temperature or composition alter the surfactant affinity, which leads to the formation of fine oil-in-water emulsions. This material is effectively used to encapsulate bioactive compounds such as quercetin, epigallocatechin-3-gallate, flavonoids, and vitamin E.76In contrast, encapsulation using the spray-drying method results in the formation of a protective shell around the active core ingredients. Natural or synthetic polymers are used for this purpose, with protein- and polysaccharide-based biopolymers being the most common owing to their biocompatibility and biodegradability. This technique has been effectively applied to encapsulate a wide range of bioactive compounds, including plant-derived substances such as essential oils and polyphenols;77,78 animal-derived components such as lysozyme,79 lactoferrin,80 and lactoperoxidase;81 and bacterial derivatives such as bacteriocins.82 Natural extracts such as polyphenols, lysozyme, and bacteriocins can also be encapsulated using cross-linking methods. In this method, biopolymers, e.g., proteins and polysaccharides, are chemically cross-linked using various agents, resulting in a three-dimensional network that entraps these bioactive compounds.83–85
Freeze drying, on the other hand, is suitable for heat-sensitive plant bioactive compounds; bacterial extract encapsulated as the feed emulsion is frozen at subzero temperatures, and the ice crystals are sublimated under low pressure.75 Additionally, for heat-sensitive hydrophobic compounds, the encapsulation method is ionic gelation, where a polymeric solution with an active compound is atomized into an ionic solution, forming spherical gel structures. This simple and cost-effective method does not require special equipment.86
Nanotechnology-enabled encapsulation enhances the stability, bioavailability, and controlled release of natural extracts by incorporating them into nanoscale carriers such as liposomes, nano emulsions, and nanoparticles. Liposomes are spherical vesicles that range in size from 30 nanometers to the micrometer scale. They are composed of phospholipids that spontaneously arrange themselves into lipid bilayers. Liposomes are used to encapsulate and deliver essential oils such as Zanthoxylum tingoassuiba and Laurel,87 as well as polyphenols such as curcumin, resveratrol, quercetin, honokiol, and anthocyanins.88 Additionally, liposomes can be loaded with β-carotene through co-modification with chitosan and lactoferrin.89 On the other hand, nano emulsions are conventional emulsions with droplet sizes smaller than 200 nm. While they are thermodynamically unstable, their small size offers long-term stability, enhanced bioavailability, and improved transparency. They are widely used to encapsulate essential oils, polyphenols, lysozyme, and bacteriocins.90–93 Studies have shown that nisin can be loaded with mesoporous silica nanoparticles, poly-γ-glutamic acid/poly-L-lysine nanoparticles, and silver nanoparticles.66
However, the shelf-life and stability of these encapsulated natural extracts may change under different storage conditions. For example, temperature has a role in the oxidative stability of encapsulated essential oils during storage. Oxidative degradation was lower in Citrus reticulata essential oil stored at 5 °C than in that stored at 50 °C for 30 days.94 Similarly, the oxidative stability of encapsulated P. jaubertii extract was greater at 4 °C than at 25 °C.95 The activity of chitosan–thyme oil nanocapsules stored at 4 °C was greater than that of those stored at 25 °C for 5 weeks.96 On the other hand, moisture is a crucial factor in terms of the storage stability of lysozyme. The accelerated stability at 40 °C and 75% RH showed that crystallized lysozyme retained 95% activity after 20 weeks, whereas the activity of the spray-dried samples decreased to 87%.97 Additionally, storage studies of freeze-dried encapsulated bovine lactoferrin powder at 4 °C and 40% RH for nine years revealed that it has antimicrobial activity against Salmonella enteritidis.98
Among the encapsulation methods, spray drying offers the highest scalability and moderate cost with the balance of good bioactivity; hence, it is commercially suitable. Freeze-drying results in greater preservation of sensitive compounds but is expensive and less scalable, whereas nano emulsion and nanoparticle systems ensure good retention of bioactivity but require complex and expensive processing. In general, all methods have a trade-off between cost and industrial feasibility with respect to bioactive stability; selection depends on the target compound and intended packaging application. Moreover, the major drawbacks of these encapsulation methods include low efficacy, loss of bioactive compounds, instability during storage, and decreased industrial viability. Ionic gelation can lead to uneven gelation, resulting in soft cores. Therefore, future studies should focus on hybrid methods and scalable low-energy processes to enhance industrial applicability.
2.5 Limitations and safety considerations
One of the primary challenges associated with the application of these natural antimicrobial extracts in food packaging materials is the inadequate compatibility between the polymer-based matrix and the bioactive compounds present in the extract. In most cases, these compounds are not properly incorporated into the polymer matrix. Additionally, the irregular migration of these active compounds toward target microorganisms presents further difficulties. Moreover, growing concerns regarding innovative food packaging continue to impede advancements in the development of effective antimicrobial packaging solutions. Regulatory compliance is essential to ensure consumer safety when packaging comes into direct contact with food. In the European Union, natural antimicrobials such as plant extracts and essential oils are listed as flavoring substances (Regulation EC 872/2012), with some approved as food additives (Regulation EC 1333/2008). In accordance with regulation EC 450/2009, any antimicrobial agents utilized in active packaging that are intended to be released into food products must adhere to regulation EC 1333/2008. This stipulation mandates that such agents be included on the list of approved food additives.99 In the United States, the Food and Drug Administration (FDA) regulates active packing under the Federal Food, Drug, and Cosmetic Act (FFDCA), which classifies incorporated antimicrobials as food additives or GRASs and is also governed by Regulation 1282/2011 on active and intelligent materials, amending Regulations 1935/590/EC and 1989/109/EC.433. Biopolymer–natural extract synergy
The synergy between biopolymers and natural extracts influences both the structural integrity and functional performance of antimicrobial packaging systems. To provide a better understanding of these dual contributions, material synergy and functional synergy can be distinguished. Material synergy refers to how polymer–extract interactions affect the mechanical strength, barrier behavior, compatibility, and overall film morphology. In contrast, functional synergy focuses on the controlled release and migration behavior of bioactive compounds, which ultimately governs antimicrobial efficacy.3.1 Types of biopolymers for antimicrobial packaging
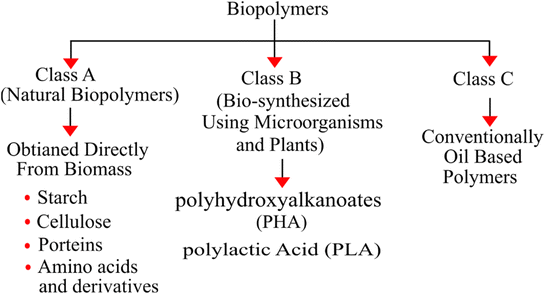
Biopolymers used for antimicrobial packaging can be broadly classified into two different categories: natural biopolymers and synthetic biopolymers. Natural biopolymers can be further divided into three major groups: polysaccharides (chitosan, cellulose, and starch), proteins, and polynucleotides. Polysaccharides, including chitosan, cellulose, and starch, are composed of a number of sugar molecules connected by glycoside linkages. Proteins, such as gelatin and casein, consist of amino acid monomers connected by peptide bonds. Polynucleotides, such as DNA and RNA, are formed from nucleotide monomers. In contrast, synthetic biopolymers include materials such as polylactic acid (PLA), polyesters such as polyhydroxyalkanoates (PHA), and polybutylene succinate (PBS). Biopolymers are eco-friendly and cutting-edge packaging materials derived from renewable sources, including plants, animals, and microbes, and they are increasingly being explored as greener substitutes for traditional petroleum-derived plastics. Polymers can be utilized in their pure form or can be used in the form of biocomposites with antimicrobial agents such as essential oils or different types of nanoparticles. For a better understanding, the typical classification of biopolymers on the basis of their method of production is illustrated in Fig. 2.3.2 Material synergy
The compatibility between bioactive compounds and biopolymer matrices is primarily determined by three major groups of factors: (i) the physicochemical properties of the components, (ii) the polar matrix characteristics, and (iii) the environmental and processing conditions.113,114 The physicochemical properties of the bioactive compounds and biopolymer matrices include polarity/hydrophilicity/hydrophobicity matching, hydrophobic–hydrophilic balance, solubility, volatility, affinity, chemical and structural interactions (stereochemistry, conformational flexibility, and molecular weight, size, and volume), and their ability to engage in specific intermolecular interactions. These interactions involve steric effects, electrostatic attractions or repulsion, and hydrogen bonding, all of which strongly influence the miscibility of lipophilic compounds with hydrophilic biopolymeric materials and their dispersion within the matrix. Polymer matrix characteristics such as crystallinity, free-volume distribution, glass transition temperature (Tg), and overall molecular mobility also strongly influence the degree of miscibility and prevent phase separation during film formation.113 The environmental and processing conditions, including pH, water activity, temperature, ionic strength, solvent type, component concentration and mixing ratio, also affect polymer–extract interactions by altering molecular dynamics, solubility behavior, and dispersion efficiency.115
These compatibility factors are commonly evaluated using a range of advanced analytical techniques such as spectroscopic analyses (chemical interactions), thermal analyses (miscibility and molecular mobility), structural and morphological analyses (dispersion and microstructure), crystallinity and molecular organization, surface and interfacial analyses, and functional migration and release tests. Spectroscopic methods include Fourier transform infrared spectroscopy (FTIR), nuclear magnetic resonance (NMR), and mass spectrometry (MS).116–118 FTIR is employed to detect new hydrogen bonds or shifts in characteristic peaks that indicate chemical interactions. NMR reveals structural changes, molecular mobility, and chemical environment modifications, whereas MS confirms molecular identity and detects chemical changes after the incorporation of bioactive compounds. However, thermal analyses, including differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA), assess how the addition of bioactive compounds affects the thermal behavior of a polymer.119 DSC is used to monitor changes in the Tg or melting behavior, reflecting miscibility or plasticization effects, whereas TGA is used to monitor the thermal stability and verify whether thermal degradation occurs due to incompatibility. In addition, structural and morphological analyses (scanning electron microscopy (SEM), atomic force microscopy (AFM), and transmission electron microscopy (TEM)) are used to examine how uniformly the bioactive compound is distributed within the polymer matrix.119,120 SEM and AFM reveal surface and cross-sectional homogeneity and nanoscale surface topology and roughness, whereas TFM confirms particle encapsulation and dispersion at the nanoscale. Crystallinity and molecular organization determined via X-ray diffraction (XRD) analyses reveal whether bioactive incorporation disrupts or enhances polymer crystallinity.118 Additional tools, such as surface and interfacial analyses by contact angle measurements and surface energy analysis, are used to assess the interaction strength (hydrophilicity/hydrophobicity changes and interfacial compatibility) and wetting behavior (adhesion and affinity) between the polymer and the active compound, whereas migration assays and release kinetic studies provide functional evidence of how the compatibility of the polymer extract influences the stability, diffusivity, retention, and release of active compounds.
Another study by Akhter et al.124 developed innovative functional packaging films by blending chitosan, pectin, and starch (0.75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5
1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.75 w/w) and incorporating 0.5% rosemary essential oil, mint essential oil, nisin, and lactic acid. The results revealed that the incorporated rosemary and mint essential oils substantially improved the moisture barrier properties; mechanical properties, including tensile strength, Young's modulus, and percentage elongation at break; and thermal stability of the developed films compared with those of the control sample. The reduced tensile strength (7.72 MPa) observed in the control biocomposite film may be attributed to the presence of glycerol, whose hygroscopic nature introduces additional moisture into the polymer matrix, thereby weakening the tensile properties of the film.125 The experimental results revealed that the tensile strength and elongation at break increased by 236.14% and 246.01%, respectively. The improvement in film strength following the incorporation of rosemary and mint essential oils can be attributed to their rich polyphenolic contents, such as carnosic acid, carnosol, rosmarinic acid, α-pinene, citronellol, and methyl eugenol, which are thereby capable of forming hydrogen bonds with the abundant polar hydroxyl groups in the chitosan–pectin matrix. Therefore, stronger bonding between the essential oils and the chitosan–pectin matrix may have tightened the polymer network, resulting in the films being more resistant to stress. This observation was consistent with previously reported studies in which clove oil was incorporated into citrus–pectin edible films.126 Interactions between the carbohydrate polymer and essential oil created a cross-linked structure that restricted molecular mobility and improved tensile strength. Moreover, the mechanical strengths of the nisin extract- and lactic acid-containing protein-based and polysaccharide-based films did not differ significantly. Similar results were obtained for tea extract-incorporated biocomposite films.127 However, Akhter et al.124 demonstrated that a biocomposite film without the addition of any extracts was clearer and more transparent. The incorporation of essential oils influenced the color parameters (L*, a*, b*) and opacity of the biocomposite films. Specifically, the L* and a* values significantly decreased (p ≤ 0.05) from 64.85 to 61.73 and −2.05 to −1.29, respectively, whereas the b* values did not significantly change, suggesting a shift toward a darker and slightly redder appearance. This alteration in optical properties may help protect packaged foods from visible and ultraviolet light, thereby reducing nutrient degradation, discoloration, and off-flavors.128
0.75 w/w) and incorporating 0.5% rosemary essential oil, mint essential oil, nisin, and lactic acid. The results revealed that the incorporated rosemary and mint essential oils substantially improved the moisture barrier properties; mechanical properties, including tensile strength, Young's modulus, and percentage elongation at break; and thermal stability of the developed films compared with those of the control sample. The reduced tensile strength (7.72 MPa) observed in the control biocomposite film may be attributed to the presence of glycerol, whose hygroscopic nature introduces additional moisture into the polymer matrix, thereby weakening the tensile properties of the film.125 The experimental results revealed that the tensile strength and elongation at break increased by 236.14% and 246.01%, respectively. The improvement in film strength following the incorporation of rosemary and mint essential oils can be attributed to their rich polyphenolic contents, such as carnosic acid, carnosol, rosmarinic acid, α-pinene, citronellol, and methyl eugenol, which are thereby capable of forming hydrogen bonds with the abundant polar hydroxyl groups in the chitosan–pectin matrix. Therefore, stronger bonding between the essential oils and the chitosan–pectin matrix may have tightened the polymer network, resulting in the films being more resistant to stress. This observation was consistent with previously reported studies in which clove oil was incorporated into citrus–pectin edible films.126 Interactions between the carbohydrate polymer and essential oil created a cross-linked structure that restricted molecular mobility and improved tensile strength. Moreover, the mechanical strengths of the nisin extract- and lactic acid-containing protein-based and polysaccharide-based films did not differ significantly. Similar results were obtained for tea extract-incorporated biocomposite films.127 However, Akhter et al.124 demonstrated that a biocomposite film without the addition of any extracts was clearer and more transparent. The incorporation of essential oils influenced the color parameters (L*, a*, b*) and opacity of the biocomposite films. Specifically, the L* and a* values significantly decreased (p ≤ 0.05) from 64.85 to 61.73 and −2.05 to −1.29, respectively, whereas the b* values did not significantly change, suggesting a shift toward a darker and slightly redder appearance. This alteration in optical properties may help protect packaged foods from visible and ultraviolet light, thereby reducing nutrient degradation, discoloration, and off-flavors.128
3.3 Functional synergy
Biodegradable polymers, such as starch, PLA, pectin, chitosan, gelatin, and cellulose derivatives, provide excellent carriers for such active agents.129 Their physicochemical properties, such as hydrophilicity, crystallinity, and molecular integrity, significantly influence the migration rate and release kinetics of the active compounds. Compared with conventional nonbiodegradable plastic polymers, these biopolymers not only provide environmentally friendly alternatives but also enable adjustable release behavior through the modification of complex polymer compositions or incorporation of natural plant-derived extracts, essential oils, plasticizers, etc. Numerous studies have explored the use of these biopolymers in designing active packaging systems with controlled release profiles tailored to specific food preservation needs (Table 2). These controlled release phenomena represent the core functional advantages of biopolymer-extract systems, distinguishing them from simple material enhancements and making release kinetics the central mechanism that determines antimicrobial effectiveness.
| Source of biopolymer | Natural extract/active agent | Type of active compound | Release behavior/migration profile | Major findings | References |
|---|---|---|---|---|---|
| Chitosan | Essential oils (ginger, tea tree, rosemary, sage, thyme) | Phenolic compounds, terpenoids | Exponential growth to a maximum; equilibrium within 48 h | • Release proportional to phenolic content of EO; ginger EO showed highest diffusion (release ratio 0.92); behavior followed Fickian diffusion | Souza et al.134 |
| Hydroalcoholic extracts | Polyphenols | Negligible diffusion | • Strong interaction between polymer and extract; no detectable migration to fatty food simulant | ||
| Thyme essential oil | Thymol | Release rate varied with solvent polarity: slowest in 95% ethanol (up to 300 min), fastest in 50% ethanol | • The addition of polysaccharides (xanthan gum, pullulan, gum tragacanth, Arabic gum) altered essential oil release | Lian et al.135 | |
| • Arabic gum and pullulan delayed release in 95% ethanol due to stronger polymer–oil interactions | |||||
| Chitosan–Arabic gum film (applied to nectarine) | Thyme essential oil (thymol) | Antifungal agent | Release of thymol vapor from film surface provided antifungal protection | • Composite film effectively inhibited fungal growth on nectarine due to controlled release of thymol | |
| Chitosan (CS) and whey-based laminate | Cinnamon extract (encapsulated in CS nanoparticles) | Polyphenols/antioxidant and antimicrobial compounds | Similar desorption profile to ROS; ≈10–15% release after 24 h (within EU OML limit) | • The coatings displayed high antioxidant activity (>90% in liquid; ∼40% when coated); release dependent on chitosan–polyphenol interactions | Potrč et al.136 |
| • Better antifungal activity observed | |||||
| • Extracts are key drivers of microbial inhibition | |||||
| Polysaccharides film | EOs from various plant sources | Terpenes, terpenoids, phenols, aldehydes, alcohols, ketones, amines, and amides | Diffusion-controlled; dependent on EO miscibility with food matrix | • EOs migrated from the matrix phase to the film surface and further contact with foods | Anis et al.137 |
| • EOs levels significantly decreased within the films with the time | |||||
| Cellulose acetate | Pink pepper EO | Volatile terpenes and phenolic compounds | Migration observed at 4 °C over 12 days | • The lipid components of cheese facilitated EO migration from the polymer matrix into the food | Dannenberg et al.;138 Tian et al.139 |
| Cellulose nanofibers (CNF) | Purple sweet potato anthocyanins | Natural dye/pH indicator | Responds to pH changes from 2–12; color changes from red to yellow | • pH-sensitive release observed via color change | Chen et al.140 |
| Oregano essential oil (OEO) | Antimicrobial agent | Incorporated into film; likely gradual antimicrobial effect | • Antimicrobial effect likely due to gradual release of OEO | ||
| Anthocyanins + oregano essential oil | pH indicator + antimicrobial | Not explicitly stated; pH-sensitive and antimicrobial properties combined | • Combined functionality observed; pH-sensitive color change and antimicrobial activity indicate active agents are effectively incorporated, but specific migration behavior not detailed |
The controlled release behavior of biopolymer extracts varies significantly across different food matrices.130 This is due mainly to the physicochemical nature of the targeted food matrix, which influences both diffusion and the polymer network as well as solvent–polymer interactions and the molecular weight of the bioactive ingredient.131 High-fat foods generally promote faster release of hydrophobic/lipophilic bioactive compounds owing to enhanced partitioning into lipid phases, whereas low-fat or aqueous food matrices retain these molecules longer, resulting in slower diffusion. Moisture-rich foods (water acts as a plasticizer) facilitate polymer swelling and increase the free volume within hydrophilic matrices, accelerating the mobility of active compounds, whereas dry or low-moisture foods restrict swelling and limit release.130 In general, nonbiodegradable polymers exhibit diffusion as a dominant mechanism for molecular transport, whereas biodegradable polymers, swelling, and degradation or erosion are also involved.132 The pH and ionic strength of food also modulate release kinetics by altering the polymer charge, electrostatic interactions, and network structure.133 For example, pH-sensitive polymers such as carboxymethyl chitosan, alginate, and chitosan exhibit markedly different release patterns: deprotonation under alkaline conditions increases electrostatic repulsion and swelling (thus faster release), whereas protonation at low pH can tighten the network and retard diffusion. Additionally, viscosity and matrix consistency influence molecular transport, with viscous or semisolid foods reducing migration compared with liquid foods. To describe and predict these release behaviors, several kinetic and mathematical models have been applied, including Fickian diffusion models based on concentration gradients, the Hixson–Crowell model for the dissolution rate, the Higuchi model for diffusion-controlled systems, the Baker–Lonsdale or modified Higuchi model for bioactive release from spherical matrices, the Korsmeyer–Peppas model for distinguishing between Fickian and non-Fickian mechanisms for bioactive release from polymeric matrices, Poiseuille's law of laminar flow for bioactive release from membrane matrices and the empirical Weibull and Peleg models to capture complex release patterns.130,141 Partition coefficient based models are also used to evaluate the distribution of active compounds between the film and food, especially in lipid-rich products. Together, these models help characterize release mechanisms and forecast performance across diverse food environments. For a better understanding of the mathematical models, detailed empirical and semiempirical release kinetics models are illustrated in Table 3.
| Model | Equation | Model parameters | Application | References |
|---|---|---|---|---|
| Zero order | Mt = M0 − k0t | M0: initial concentration of encapsulated substance | • Describing release kinetics of matrix systems slabs, low solubility, or coated material, osmotic systems | Malekjani & Jafari132 |
| Mt: concentration at any time t | • Not common mechanism due to rapid dissolution of most food materials | |||
| k0: zero-order equation rate constant (concentration per time) | ||||
| First order | Mt = M0e−k1t | k1: first-order equation rate constant (time−1) | • Best describes the release kinetics of water-soluble material in porous matrices and ionizable oil or water-soluble materials from W/O/W emulsions | Malekjani & Jafari132 |
| Higuchi model | f1 = Mt = KHt0.5 | KH: dissolution constant (concentration/time0.5) | • Best suited for modeling the release of water-soluble or low solubility encapsulated compounds from solid or semisolid matrices | Higuchi144 |
| Hixson–Crowell model | M01/3 − Mt1/3 = KHCt | KHC: model constant incorporating the surface volume relation (concentration/time1/3) | • Applicable for planar systems where dissolution occurs uniformly across surfaces parallel to the active compound | Hixson & Crowell145 |
| Ritger–Peppas and Korsmeyer–Peppas model (power law) | 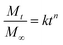 |
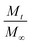 : released fraction at any time t : released fraction at any time t |
• Applied when release mechanism is not known or when more than one release mechanism is involved | Korsmeyer et al.;146 Ritger & Peppas147 |
| k: rate constant influenced by system structure and geometry (release velocity constant) | ||||
| Baker–Lonsdale or modified Higuchi model | 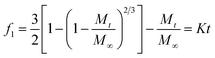 |
K: model constant | • Suitable for microspheres or microcapsules | Baker & Lonsdale148 |
| • Bioactive release from spherical matrices | ||||
| Weibull model | 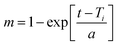 |
a: scale parameter (determines the process time scale) | • Suitable for wide range of release situations and is useful for comparing release profiles in matrix systems | Weibull149 |
| Ti: location parameter indicating lag time before release begins (often zero) | ||||
| Hopfenberg model | 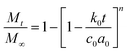 |
c0: initial concentration | • Applicable for modeling optimized oil spheres that show site specific, biphasic release behavior | Katzhendler et al.151 |
| a0: radius (sphere or cylinder radius, or half-thickness for slab) | ||||
| k0: erosion rate constant | ||||
| n: for slab, cylinder, and the sphere is 1, 2, and 3, respectively | ||||
| Poiseuille's law of laminar flow | 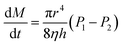 |
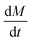 : drug/bioactive release rate : drug/bioactive release rate |
— | Bayer141 |
| c: solute concentration inside the matrix | ||||
| r: radius of the cylindrical pore/orifice | ||||
| η: viscosity of the fluid (matrix or carrier) | ||||
| h: membrane thickness (length of channel) | ||||
| P1 − P2 = pressure difference across the membrane | ||||
| Peppas–Sahlin model | 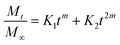 |
K1 and K2, are constants | • Useful to calculate the approximate two contribution mechanisms such as diffusional and relaxational in an anomalous release process | Peppas & Sahlin150 |
| m: exponent representing the Fickian diffusion contribution based on the system's geometry | ||||
| K1tm term represents the Fickian diffusion contribution and K2t2m represents the polymer relaxation contribution | ||||
| Brazel and Peppas model | 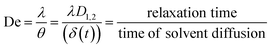 |
De: Deborah (diffusing number) | • Useful for analyzing how water uptake and polymer swelling influence drug release in swellable or erodible matrices | Bruschi152 |
| λ: relaxation time of the polymer | • Helps compare the relative rates of water diffusion and polymer relaxation to optimize controlled-release formulations | |||
| θ: characteristic water-diffusion time in a swellable matrix | ||||
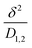 : ratio of diffusing distance to diffusing coefficient of water in the polymer : ratio of diffusing distance to diffusing coefficient of water in the polymer |
A study by Souza et al.134 developed chitosan films incorporated with essential oil and hydroalcoholic extracts and reported that the migration of these films into fatty food simulant media occurred after 10 days of storage. The results of the migration assay indicated negligible diffusion toward a fatty food simulant, indicating strong retention within the polymer and limited release. These outcomes suggested that the active compounds within the extracts remained tightly bound to the complex polymeric matrix, indicating a greater affinity for the packaging material than for the fatty food simulant. In contrast, chitosan films with essential oils (EOs) exhibited an “exponential growth to a maximum” release profile, reaching equilibrium within 48 h of storage. The total phenolic content (TPC) released was proportional to the TPC in the crude EOs, with ginger and tea EOs resulting in greater release and rosemary, sage, and thyme EOs resulting in lower release. Most of the films, with the exception of the chitosan–ginger EO (0.92), had a release ratio of 0.34–0.52, reflecting differences in polymer–active compound interactions. These behaviors were satisfactorily associated with Fickian diffusion models, which present their interactions and concentration gradients in controlling release kinetics from active packaging. However, the migration behavior of active compounds is influenced by various factors, including (a) polymer characteristics such as morphology, crystallinity, molecular weight, and microcavity distribution, which affect diffusion pathways; (b) the physicochemical characteristics of active agents, including polarity, solubility, and molecular structure; and (c) the interactions between the polymer and the incorporated compounds, such as plasticizing or anti-plasticizing effects.142 Environmental factors also exert strong control over release rates, with moisture being particularly influential. Water acts as a plasticizer in hydrophilic biopolymers, disrupting intermolecular hydrogen bonding and increasing the free volume within the matrix. This expansion of chain spacing within the polymer matrix accelerates the diffusion of entrapped active compounds, increases mobility and can dramatically shift films from glassy to rubbery states. For example, the diffusion coefficient of carvacrol (a natural monoterpenoid phenol) in soy protein films increased from 0.11 × 10−16 m2 s−1 at 60% RH to 7.5 × 10−16 m2 s−1 at 100% RH.143
However, pH-responsive polymers, such as carboxymethyl chitosan and alginate, also exhibit adjustable release behavior. Under alkaline conditions, deprotonation of carboxyl groups increases electrostatic repulsion and matrix swelling, enlarging the pore size and promoting faster release of active compounds. A similar pattern of release kinetics was observed in hydrophilic pectin-based films153 and chitosan-based films.154 However, the concentration of EOs released in a controlled manner from food is important and governs the shelf stability of foods. At any point during storage, the concentration of EOs at the food surface should remain above the minimum inhibitory concentration (MIC) required to suppress microbial growth. A previous study by Anis et al.137 suggested that the rapid diffusion rate may cause the EO concentration to drop below the MIC too quickly, resulting in detrimental effects on the antimicrobial properties and shelf-life of the EO. Additionally, if the diffusion rate is too slow, the EO concentration at the food surface may not reach an effective antimicrobial level. In this case, biopolymers incorporated with EO films may not be capable of protecting food. An earlier study by Souza et al.134 demonstrated that the quantities of glycerol and EOs were constant and that variations in their migration profiles are attributed mainly to differences in the chemical composition and polarity of the EOs. The greater release observed for ginger EO may result from its greater solubility in ethanol (95%) and the lipophilic nature of its active constituents, as also noted by Reis et al.155
Importantly, controlled release systems are closely coupled with migration safety considerations, as bioactive compounds can be transferred from packaging materials into food systems. Previously published literature emphasized that migration must remain within regulatory limits and must not generate toxicologically relevant concentrations.156 Safety evaluation basically involves quantifying the amount of migrated compound via validated analytical methods such as chromatographic methods (HPLC or GC-MS), FTIR spectroscopy, NMR spectroscopy, thermal analyses such as DSC and TGA, electrochemical methods, and release cell systems (e.g., Franz diffusion cells), followed by comparisons with specific migration limits (SMLs) set by regulatory agencies.156 On the other hand, the biological activity of the released compounds is typically evaluated through antimicrobial assays, including MIC determination and agar diffusion tests, ensuring that the released concentration remains microbiologically effective throughout storage.157 These assessments also examine whether the release results in the formation of any degradation or reaction byproducts and confirm that all the incorporated natural extracts or essential oils are food-grade or GRAS substances. Migration testing is often carried out using standardized food simulants under controlled conditions of time, temperature, humidity, and pH to mimic realistic storage environments, ensuring that the packaging maintains both functional performance and consumer safety.158
4. Advances in the fabrication of biopolymer-based antimicrobial films
The fabrication method plays a key role in determining the scalability, processing stability, and release behavior of biopolymer-based antimicrobial films. Rather than focusing only on formulations or lab steps, an engineering-oriented approach considers how factors such as temperature, shear, drying rate, solvent removal, and layer formation influence film uniformity, mechanical strength, diffusion pathways, and industrial applicability. The following subsections examine major fabrication techniques from this engineering perspective, highlighting how each method affects production efficiency, large-scale manufacturing potential, and the controlled release of antimicrobial agents.4.1 Film-forming techniques
Different film-forming methods influence the processing stability, scalability, and release behavior of antimicrobial agents in biopolymer films. Each technique produces a distinct microstructure, which affects the mechanical strength, barrier properties, and diffusion pathways. Solvent casting is widely used in laboratory studies because it allows easy mixing of polymers, plasticizers, and active compounds. However, it is difficult to scale due to long drying times, high energy use, and sensitivity to temperature and humidity. Uneven solvent evaporation can create variable film thicknesses and internal micro voids, which lead to inconsistent mechanical properties and unpredictable release rates of antimicrobial agents.159 For example, chitosan films prepared by solvent casting with a thyme-oil nanoemulsion (TH-NE) exhibited enhanced antimicrobial activity and increased flexibility, attributed to their greater elongation at break. However, achieving thicker, uniform films requires prolonged drying at elevated temperatures (50–55 °C for 24 hours), conditions that limit industrial feasibility.160 In another study on a solvent-cast system, chickpea–whey protein films enriched with quercetin demonstrated how formulation changes can influence film structure and release behavior. Increasing the whey protein content strengthened intermolecular interactions and improved the barrier and mechanical performance up to 40%, whereas increasing the whey protein content caused structural defects. The films also showed medium-dependent quercetin release, highlighting how casting-based formulations allow tuning of the microstructure and controlled release through adjustment of the protein composition.161Extrusion provides a more stable and continuous process and is currently the most industry-ready technique. Controlled heat and mechanical shear during extrusion allow precise structuring of the polymer matrix, resulting in uniform films with consistent performance.162 Co-extrusion enables multilayer films where barrier layers can be engineered to regulate moisture transport and slow the release of active agents. Studies on alginate-based films have shown that plasticizer content strongly affects melt viscosity and thermal stability and that higher glycerol levels help maintain mechanical stability even at elevated processing temperatures.163 Nanocomposite films produced by melt-mixing extrusion also demonstrate how extrusion can improve processing stability and film performance. For example, mLLDPE films reinforced with graphene nanoplatelets (GNPs) showed excellent filler dispersion and more stable processing, with certain GNP levels reducing torque and pressure during extrusion. The GNPs acted as nucleating agents, increasing the crystallinity and enhancing the oxygen and water-vapor barrier properties while also increasing the Young's modulus. These results show that extrusion can effectively incorporate nanofillers and tailor the film structure and barrier behavior in a scalable manner.164 Twin-screw (multiscrew) extrusion is often preferred over single-screw systems because the intense shear in single-screw extrusion can generate excess heat, which may degrade thermally sensitive antimicrobial compounds. In contrast, the more controlled, positive-displacement flow in multiscrew extrusion helps reduce thermal hotspots and better preserves the activity of thermolabile bioactive agents.165 In addition to food packaging, advances in additive manufacturing, particularly material extrusion, have shown that similar extrusion-based processes can be adapted for high-precision applications such as printable electronics and bioengineered scaffolds. These systems rely on the formulation of printable inks from colloidal suspensions, polymer melts, and biopolymer-based composites with tunable mechanical and functional properties. Insights from this field highlight the broader versatility of extrusion, including its compatibility with a wide range of composite formulations and active materials (e.g., nanomaterials, sensors, or bioactives), which may inform the next generation of functional and responsive packaging films.166 Extrusion not only supports higher throughput but also avoids solvent-related variability and maintains structural integrity during long-term storage, whereas solvent-cast films are more sensitive to humidity-induced recrystallization and mechanical weakening.167
Electrospinning is a versatile technique used to fabricate nanofiber-based films for active food packaging applications. A typical electrospinning setup consists of four main components: a high-voltage power supply, an injection pump, a capillary tube (or spinneret), and a grounded metal collector. In this process, a polymer solution is loaded into the capillary tube, and a high-voltage electric field is applied between the needle tip and the collector. The electric field induces the formation of a Taylor cone, from which a charged jet of the polymer solution is ejected. As the jet travels toward the collector, the solvent rapidly evaporates, resulting in the deposition of solid nanofibers on the surface.168 Electrospinning produces nanofibrous films with very high surface areas and porosities, which can enhance antimicrobial effectiveness because the active agents can diffuse more easily. It is also useful for encapsulating sensitive bioactive compounds within nanofibers.169 For example, electrospun nanofiber mats made from cold-water fish gelatin supplemented with up to 20% bovine lactoferrin showed significant antimicrobial activity against several foodborne pathogens, including Pseudomonas fluorescens and E. coli.170 Although high bioactive loading reduced the fiber diameter and tensile strength, the system demonstrated strong potential for active packaging applications. Despite its advantages, electrospinning faces several technical limitations. The method is highly sensitive to the solution viscosity, voltage, humidity, and temperature. These variations make continuous, large-scale production difficult. Traditional single-needle electrospinning has low throughput, and although newer multi-nozzle or free-surface systems improve productivity, industrial adoption is still limited. Thus, electrospinning offers excellent functional performance but currently lacks the scalability required for mass production.171
4.2 Layer-by-layer and coating technologies
Compared with single-layer films, multilayer and coating-based fabrication techniques provide much better control over the film structure and release behavior. From a process-engineering perspective, these methods allow the precise adjustment of layer thickness, interfacial interactions, and drying dynamics, which directly influence processing stability, scalability, and antimicrobial release profiles. Techniques such as layer-by-layer (LbL) assembly, dip coating, spray coating, and roll coating enable the formation of films with tailored barrier properties and controlled diffusion pathways, supporting the development of high-performance and scalable biopolymer films.168In industrial and research applications, spraying can be achieved through air spray atomization, air-assisted airless atomization, or pressure atomization, each of which differ in how compressed air and pressure generate droplet formation. The nozzle configuration—pneumatic or hydraulic, with designs such as a solid stream, hollow cone, flat spray, or full cone—plays a critical role in determining droplet size, deposition pattern, and coverage.181 This method facilitates the formation of thin, uniform, and continuous coatings without excessive material waste, offering a distinct advantage over immersion-based techniques.
Several studies have demonstrated the potential of spraying in enhancing food preservation. For example, Ribeiro et al.182 reported that carrageenan-based coatings containing calcium chloride, when sprayed on strawberries, effectively reduced microbial growth and maintained fruit firmness during refrigerated storage. Similarly, Saberi et al.183 reported that a pea starch–guar gum–lipid composite coating containing shellac and oleic acid delayed firmness loss and minimized ethylene production in oranges, highlighting the ability of spray-coated films to increase postharvest quality. Furthermore, spray coating has been successfully employed for chitosan-, gelatin-, and starch-based coatings on fruits, vegetables, and meat products, where it enhances their mechanical stability, barrier properties, and shelf life.176
From a process engineering standpoint, the atomization pressure and droplet size are critical parameters influencing coating homogeneity and film integrity. Excessive pressure (above approximately 3.5 bar) can disrupt the film-forming network, resulting in nonuniform surface coverage or droplet rebound. The technique also supports multilayer and composite applications, such as alternating sprays of alginate and calcium chloride to produce cross-linked gel layers, which provide improved water resistance and mechanical strength.179 Compared with dipping, the spraying method offers superior controllability, reduced drying time, and lower solution waste, making it highly suitable for continuous and automated coating lines. Overall, spray coating represents an efficient, scalable, and adaptable approach for fabricating biopolymer-based edible films with enhanced barrier, antimicrobial, and mechanical properties that meet the requirements of modern sustainable packaging systems.
4.3 Nanotechnology-enabled approaches
The integration of nanotechnology into biopolymer-based packaging has revolutionized the development of antimicrobial films by enhancing their physicochemical, mechanical, and functional properties. Nanomaterials, typically in the 1–100 nm range, impart unique characteristics such as a high surface area, tunable reactivity, and enhanced barrier strength that are not achievable with conventional fillers. The incorporation of nanosized reinforcements, including metallic nanoparticles (AgNPs, ZnO NPs, TiO2 NPs), nanoclays, and biogenic nanocellulose, into biodegradable polymer matrices such as chitosan, starch, and polyvinyl alcohol significantly improves the tensile strength, gas impermeability, and antimicrobial efficiency. These improvements arise from the creation of tortuous diffusion paths and dense interfacial bonding networks within the polymer matrix, which effectively restrict oxygen and moisture permeation while allowing the controlled release of antimicrobial agents.184 Among metallic nanofillers, silver nanoparticles are the most extensively used owing to their strong antibacterial activity against both Gram-positive and Gram-negative bacteria, which is achieved through membrane disruption and reactive oxygen species (ROS) generation.185 Moreover, compared with metallic silver, zinc oxide and titanium dioxide nanoparticles exhibit photocatalytic antimicrobial action, offering lower toxicity and improved stability.184Recent studies have highlighted the use of hybrid nanostructures that combine nanoparticles with natural bioactive compounds to achieve synergistic antimicrobial and antioxidant effects. For example, cellulose-based nanocomposite films incorporating curcumin and modified with organosilane exhibited a Janus architecture with asymmetric hydrophilic–hydrophobic surfaces, enhancing UV resistance, moisture barrier, and antioxidant activity while enabling visual freshness monitoring and maintaining full biodegradability.186 A study on PVA–chitin nanofibril nanocomposites revealed that incorporating 10% fungal-derived nanofibrils reduced water vapor permeability by nearly 70% and significantly increased film stiffness, demonstrating the strong reinforcing potential of biobased nanofillers. Because fungal chitin provides a renewable feedstock, this approach also supports environmentally sustainable packaging, as confirmed by life cycle assessment. These results highlight how nanoscale fillers can simultaneously enhance the mechanical, barrier, and sustainability performance of biopolymer films. Another study demonstrated that a multilayer film incorporating electrospun zein nanofibers loaded with cumin essential oil (CEO) revealed how nanoscale structures can enhance film performance and control release behavior. Increasing the nanofiber layer thickness improved the mechanical strength, barrier properties, crystallinity, and thermal stability, while the FESEM images confirmed a more compact structure. The films also exhibited stronger antimicrobial activity and sustained CEO release in aqueous medium, demonstrating the effectiveness of nanofiber-based architectures in tuning both functionality and controlled diffusion.187
In addition to inorganic nanostructures, organic nanomaterials such as cellulose nanocrystals (CNCs) and chitosan nanoparticles (CSNPs) have gained substantial attention because of their compatibility and biodegradability. Their high crystallinity, abundant hydroxyl groups, and ability to form strong hydrogen bonds facilitate uniform dispersion within polymer matrices, resulting in enhanced mechanical integrity and moisture barrier properties. For example, CNC-reinforced chitosan films demonstrated an improved tensile modulus and reduced water vapor permeability due to the formation of dense interfacial hydrogen-bonding networks between cellulose nanocrystals and the chitosan matrix.188 In a previous study, chitosan–ZnO nanocomposite coatings on LDPE films effectively reduced bacterial (by 63%) and fungal (twofold) growth on okra during 12 days of storage at 25 °C without affecting quality parameters. This demonstrates their strong antimicrobial efficiency and suitability for active food packaging applications.189 Moreover, nanocomposite films fabricated via solution casting or electrospinning have shown superior antimicrobial performance when integrated with natural bioactive agents such as essential oils, polyphenols, or curcumin nanoparticles. These bioactives can be encapsulated within nanoemulsions or nanoliposomes to prevent volatility and degradation, allowing for controlled release at the food–film interface and extended antimicrobial efficacy.184
Recent studies have emphasized hybrid nanostructures that couple nanoparticles with natural bioactive compounds for synergistic antimicrobial and antioxidant effects. For example, cellulose-based nanocomposite films incorporating curcumin and modified with organosilane exhibited a Janus architecture with asymmetric hydrophilic–hydrophobic surfaces, enhancing UV resistance, moisture barrier, and antioxidant activity while enabling visual freshness monitoring and maintaining full biodegradability.186
Despite these advantages, safety and regulatory concerns regarding nanoparticle migration into food remain significant. The potential leaching of metallic nanoparticles such as AgNPs and ZnONPs into food matrices may pose toxicity risks, necessitating detailed migration studies and risk assessments.186 Consequently, biogenic or green synthesis approaches using plant extracts or microbial routes are increasingly favored to minimize toxicity and ensure compliance with safety standards.190 The future of nanotechnology-enabled food packaging lies in the design of multifunctional nanocomposite films that combine mechanical strength, biodegradability, and intelligent sensing capabilities, enabling real-time freshness monitoring while ensuring environmental sustainability. These innovations are paving the path toward the next generation of smart, bioresponsive, and fully compostable food packaging systems, which are aligned with circular economy principles and global sustainability goals.
5. The “intelligent” and “integrated” antimicrobial package
Smart packaging can be broadly classified into two main types: active packaging and intelligent packaging. By releasing antimicrobial agents, moisture regulators, and gas scavengers, active packaging can interact dynamically with a food product or its surrounding environment to improve preservation.191 Conversely, intelligent packaging incorporates sensors and indicators such as biosensors, time–temperature indicators (TTIs), and gas detectors to continuously monitor the freshness and quality of food in real time.192 Recent developments increasingly combine these two functionalities, creating integrated, multifunctional systems in which a single packaging matrix is capable of both preserving food and reporting its status, often through pH-, enzyme-, or volatile-responsive mechanisms. These systems provide critical data on spoilage, contamination, and storage conditions, enabling timely interventions to increase food safety and reduce waste.193 Additionally, known as smart packaging, intelligent packaging specifically detects certain attributes of a food or its environment and conveys this information to manufacturers, distributors, and consumers.194 These pH- and volatile-responsive indicator systems have recently been enhanced by combining indicators with antimicrobial agents within the same matrix. Recent examples include chitosan/gelatin/ZnO composite films loaded with pomegranate flower anthocyanin nanocapsules for fish freshness monitoring195 and active chitosan films containing single and double nanoemulsions of Oliveria decumbens essential oil with eggplant anthocyanins for chicken preservation,196 highlighting the emerging trend of combining antimicrobial efficacy with real-time, colorimetric freshness indications.5.1 Microbial contamination and active packaging
Microbial contamination, which potentially occurs at any stage of the supply chain, remains a significant challenge for food safety and shelf-life. Such contamination can degrade sensory qualities or pose serious health hazards to consumers.197 Antimicrobial active packaging involves the embedding of controlled-release compounds that inhibit microbial growth, regulate humidity, and prevent spoilage.198 Biodegradable films serve as sustainable carriers for antimicrobial agents and are often enhanced with bioactive compounds such as essential oils and phenolics to increase their antimicrobial and antioxidant capacity. Biopolymer-based biodegradable packaging, including protein-derived films, is increasingly favored as an eco-friendly alternative to conventional plastics. The incorporation of antimicrobial substances such as essential oils or chitosan composites into these materials has proven effective in prolonging product freshness and shelf life.194 Chitosan, which has inherent antimicrobial properties, inhibits microbial proliferation and helps stabilize the microenvironment within packaging. Combining chitosan with essential oils, metallic nanoparticles, or natural phenolic compounds results in synergistically improved pathogen resistance and food preservation.194 For example, multifunctional biocomposites fabricated by integrating biosynthesized silver nanoparticles (AgNPs) and grapefruit peel extract into polyvinyl alcohol (PVA) matrices exhibit robust antimicrobial effects against foodborne pathogens, coupled with antioxidant activity, suggesting a promising, cost-effective, and environmentally sustainable packaging platform.1995.2 Metal-based antimicrobial packaging
Metals such as silver (Ag), copper (Cu), and zinc (Zn) are the most frequently used elements for antimicrobial meat packaging because their effective microbial inhibition is balanced with considerations of cost and safety. Antimicrobial action primarily involves the release of metal ions. For example, Ag+ ions interact with bacterial proteins by forming bonds with thiol groups, disrupting electron transport, and inhibiting DNA replication.200 However, single-metal systems pose limitations; silver ions are susceptible to oxidation, diminishing their efficacy, and copper ions may alter the visual appearance of packaging materials. To overcome these challenges, recent research has focused on combining or doping multiple metals to enhance antimicrobial activity through synergistic effects and improved physicochemical properties. Nonetheless, issues surrounding metal ion migration and potential toxicity require rigorous assessment to guarantee safe application in direct food contact.201 Currently, comprehensive toxicity evaluation frameworks for metal antimicrobial agents in meat packaging remain underdeveloped.5.3 Bacteriocins and regenerated cellulose-based packaging
Historically, regenerated cellulose-based materials, such as cellophane, have been valued for their mechanical integrity, transparency, and antimicrobial capabilities, especially when combined with agents such as nisin.202 Bacteriocins, bacterially produced ribosomal peptides, are increasingly recognized as natural antimicrobial agents because of their resistance to heat and acidic environments.73 Nisin, for example, acts through electrostatic interactions with the negatively charged bacterial membrane, leading to cellular leakage and death. It is notably effective against Gram-positive bacteria such as Micrococcus luteus, Staphylococcus aureus, and Bacillus cereus and is often incorporated into polymer-based packaging to increase meat safety.203,2045.4 Biopolymer-based antimicrobial packaging films
Biopolymer-based antimicrobial packaging films (BFPFs) have demonstrated efficacy in inhibiting spoilage organisms through controlled release strategies, thus improving shelf-life and safety. However, industrial adoption faces challenges, including higher production costs than synthetic plastics, the migration of components exceeding permissible limits, diminished effectiveness under refrigeration, and potential microbial resistance arising from biofilms or horizontal gene transfer.205 Emerging antimicrobial technologies such as photodynamic inactivation (PDI) harness a photosensitizer activated by light and oxygen to generate reactive oxygen species (ROS), which selectively kill microbes. PDI offers broad-spectrum antimicrobial action, rapid targeting of microbial cells, efficient inactivation under mild conditions, and a low propensity for inducing resistance, regardless of the microbial antibiotic profile.205,206 For example, curcumin (CUR), a widely investigated photosensitizer in PDI, also serves as an antioxidant and cross-linking agent in biopolymeric films. CUR-incorporated films exhibit strong antibacterial effects against various pathogens and extend the shelf-life of foods such as grapes and pork by generating ROS that suppress microbial growth.205,207 Composites containing both CUR and ε-polylysine, which are natural antimicrobial polymers recognized as safe by the FDA, show improved water resistance and prevent the spoilage of refrigerated seafood by bacteria.208 Encapsulation of CUR within polymer matrices enhances its photostability and antibacterial efficacy under illumination.209 Riboflavin (RB) has emerged as a cost-effective alternative to CUR, exhibiting superior acid and thermal stability. RB-incorporated chitosan films show improved mechanical strength, antimicrobial activity, and ultraviolet absorption, making them promising candidates for active food packaging applications.2055.5 Photothermal and nanomaterial-enhanced packaging
Complementary photothermal films may use nanomaterials such as gold nanoparticles or MXenes to absorb light and convert it into localized heat, disrupting microbial membranes and enhancing antimicrobial efficacy.210 Wang et al.211 developed an antibacterial composite by incorporating black phosphorus quantum dots (BPQDs) into a tannic acid-functionalized zeolitic imidazolate framework (ZIF-8-TA). This system showed synergistic antimicrobial effects via photothermal ablation (808 nm NIR, 1.5 W cm−2), photodynamic ROS generation, and polyphenol-mediated membrane disruption. When added to chitosan films, it preserved grapes by retaining 92.3% ascorbic acid and limiting weight loss to under 5% over 14 days at 4 °C. Compared with conventional packaging, beef has a 3.8 log CFU g−1 lower microbial count and extends shelf-life to 18 days. It inactivated 89.7% of the Escherichia coli and 93.2% of the Staphylococcus aureus within 2 hours of NIR exposure.2115.6 Commercial biobased packaging
While numerous startups and larger enterprises are advancing biobased plastics to compete with traditional petroleum-derived materials, many commercial biopackaging products currently lack active antimicrobial components. Examples of commercial antimicrobial films include Zeomic™ and Microgarde™.212 Companies such as TIPA® manufacture compostable films containing between 20% and 80% biobased content that are fully compostable. These films perform well under freezing conditions but degrade when exposed to heat or high-liquid-content foods, limiting their applicability.213 Polyhydroxybutyrate (PHB), a bacterial biopolymer from the polyhydroxyalkanoate family, represents a promising biodegradable, biocompatible, and nontoxic alternative. Its microbial synthesis avoids competition with food crops, supporting sustainable packaging material production.214 Cellulose derivatives such as carboxymethylcellulose (CMC) are incorporated into composite films with curcumin and zinc oxide nanoparticles to generate packaging materials with enhanced antimicrobial and water vapor barrier properties; however, the intrinsic hydrophilicity of cellulose sometimes limits its practical application.215 Edible packaging films blending alginate, aloe vera, and garlic oil have been reported to provide strong barriers and antimicrobial functionalities, highlighting the potential for active packaging applications.216 The addition of garlic extract and titanium dioxide nanoparticles to CMC/Arabic gum/gelatin composite films improved their mechanical properties, water vapor and oxygen permeability, and antimicrobial efficacy.2175.7 Intelligent packaging and spoilage detection
In intelligent packaging, nanomaterials such as quantum dots have been exploited to detect bacterial growth through fluorescence signals. Cadmium selenide/zinc sulfide quantum dots exhibit blue fluorescence upon microbial binding, indicating rapid contamination detection. Upconversion fluorescence systems detect important biomolecules, including proteins, enzymes, and bacteria, enabling the development of test kits for pathogens such as Escherichia coli.218 Other microbial spoilage indicators include pH-sensitive chitosan-based films that change color upon contamination: chitosan films containing dyes from Bauhinia blakeana turn from brown to green upon pork spoilage,219 whereas anthocyanins from red cabbage incorporated into chitosan exhibit color variation, indicating milk spoilage.220 Bacterial cellulose–polypyrrole–zinc oxide nanoparticle films can be used to assess both pH and spoilage in chicken meat, and polyvinyl alcohol–gelatin films loaded with Amaranthus leaf extract exhibit colorimetric changes due to the use of betalain pigments for fish and poultry freshness detection.221 An ammonia-sensitive film composed of polyvinyl alcohol, quaternary ammonium chitosan, and cactus pear extract has demonstrated real-time shrimp freshness detection through distinct color shifts from pink to yellow, concurrently providing antioxidant and antimicrobial effects.222Recent advances include pectin-based antimicrobial hydrogels infused with cinnamon essential oil, which absorb moisture within bread packaging and release the oil to suppress mold growth.223 Additionally, enzyme-responsive nanofiber membranes that release thymol in response to the presence of pectinase have been developed to inhibit Aspergillus niger in citrus fruits.224 Colorimetric analysis and fluorescence spectroscopy are commonly used for evaluating film responsiveness. Crosslinking caseinate films with glyoxal produces insoluble networks capable of gradual enzymatic release and sustained antimicrobial activity against pathogens such as E. coli and S. aureus. Gallic acid coatings enhance these effects, demonstrating potential for food packaging applications requiring prolonged microbial inhibition.225
6. Applications across food products
Biopolymers and natural extract-based antimicrobial packaging films have been explored for various types of food products (Table 4).| Antimicrobial extract | Biopolymers | Application | Major findings | References |
|---|---|---|---|---|
| Zein–magnolol | Sodium carboxymethyl cellulose | Fresh-cut jackfruit | Minimizes weight loss, retains flavor, and delays the ripening process for 8 days at 25 °C | Wei et al.226 |
| Genipin | Chitosan, and gelatin | Strawberries | Better firmness and soluble solids content up to 5 days | Li et al.227 |
| Chlorogenic acid | Agar | Cherry | Spoilage rate reduces to 12.5% after 9 days at 25 °C with 75% relative humidity | Han et al.228 |
| Phenolic-rich substance from white and red varieties | Polylactic acid | Sunflower oil | After 30 days, peroxide index was lesser | de Freitas et al.229 |
| Lemongrass oil | Polyvinyl alcohol | Gimbap | Microbial growth was very less after 12 h at 25 °C with 90% of relative humidity | Ahn et al.230 |
| Apple polyphenols | Gelatin, carboxymethyl cellulose, and chitosan | Beef | Prevent inhibition of E. coli and S. aureus, up to 3 days at 4 °C | Ahmad et al.233 |
| Diacetyl | Sodium stearate and cyclodextrin nanosponges | Beef | More than 90% of inhibition rate against Pseudomonas spp. and lactic acid bacteria after 11 days at 4 °C | Rupérez et al.234 |
| Berberine | Gelatin, dialdehyde nanocellulose, and ε-polylysine | Pork | Delaying spoilage by reducing TVBN and TBARS for 10 days at 4 °C | Xu et al.235 |
| Microalgal | Pectin and gelatin | Chicken meat | E. coli and S. aureus inhibition rate were 80 and 20%, respectively, after 7 days | Karabulut & Goksen236 |
| Cinnamaldehyde | Chitosan, PVA and gelatin | Trout fillets | Under 4 °C, no qualitative changes till 12 days | Hosseini et al.237 |
| Betalain | Starch and gum tragacanth | Shrimp | Film color change (pink to pale yellow) with increased TVBN after 48 h at 20 °C | Thakur et al.110 |
| Curcumin | Nanocellulose and PVA | Chinese grass carp | It prevents the generation of TVBN by hindering the microbial and enzymatic growth for 9 days at 4 °C | Wang et al.238 |
| Citric acid | Chitosan | Tilapia fillets | Under 4 °C, TVBN, water loss, and oxidative degradation became lowest for 10 days | Shi et al.239 |
| Thyme essential oil and shikonin | Soy lipophilic protein | Salmon | Overall appearance and color did not show any significant changes at 4 °C for 6 days. Additionally, it able to retains freshness and Pseudomonas growth rate up to 9 days | Sun et al.240 |
| Geraniol | Starch, pullulan, and glycerol | Salmon | After 5 days at 4 °C, overall appearance was higher than control. Meanwhile, weight loss, and pH values were lowest | Zou et al.241 |
| Lemon peel oil | Gelatin and acetic acid | Kashar cheese | Under 4 °C, it hinders the growth of gram-positive, yeast, mold, and aerobic mesophilic bacteria for 21 days | Doğan et al.242 |
| αs2-casein182–207 and αs2-casein151–181 peptide | Whey protein | Cheese | No observable changes in yeasts and molds were identified after 28 days in packed at 4 °C | Zhang et al.243 |
| Rosemary oil | Chitosan | Karish cheese | Color and flavor attributes were almost similar to control till 5 days (4 °C) | Gadallah et al.244 |
| Carvacrol and thymol | Thermoplastic biopolymers | Brie cheese | Under 5 °C, it prevents the growth of Salmonella typhimurium and Listeria monocytogenes for 7 days | Di Matteo et al.245 |
| Anethum graveolens oil | Gellan gum and pineapple nanocellulose | Bread | No visual changes and fungal growth at room temperature for 14 days | Sripahco et al.246 |
| Carvacrol | Aluminosilicates | Crisps | Lipid oxidation rate was very less up to 30 days at 60 °C | Wrona et al.247 |
6.1 Fresh produce
To preserve fresh-cut jackfruit, Wei et al.226 incorporated a zein–magnolol mixture (0.5 v/v) into a sodium carboxymethyl cellulose film, which can hinder color change and visible mold growth until 8 days at 25 °C. The hydrophobic nature of zein provides a moisture barrier, while magnolol's phenolic chemistry contributes to its antioxidant and antimicrobial activities. This dual functionality minimized weight loss (22.65% vs. 32.51% in unpacked samples), delayed lipid peroxidation (lowest malondialdehyde content), and preserved sensory attributes such as flavor and texture. Li et al.227 developed an egg tray-shaped aerogel using gelatin and genipin-crosslinked chitosan, which preserved strawberry quality for five days without visible mycelia. The protein–polysaccharide matrix enhanced firmness and soluble solids retention by reducing water vapor permeability, while cytotoxicity assays confirmed its biocompatibility (93% cell viability at 625 µg mL−1). This demonstrates how food matrix chemistry (high water activity in strawberries) requires packaging with strong moisture control and antimicrobial release. Han et al.228 added chlorogenic acid (0.3 mg mL−1) to agar films, reporting superior antimicrobial ability. The phenolic hydroxyl groups of chlorogenic acid disrupted the microbial membranes, extending the cherry shelf time to 9 days at 25 °C with 75% RH and reducing spoilage to 12.5%. Sensory evaluation confirmed an acceptable taste and texture, linking chemical stabilization directly to consumer acceptability. de Freitas et al.229 incorporated grape stalk phenolics (6% wt.) into polylactic acid films, lowering the peroxide index of sunflower oil after 30 days. Here, the antioxidant-rich phenolic chemistry reduced oxidative rancidity, revealing how packaging can be tailored to lipid-rich matrices. Ahn et al.230 introduced lemongrass oil into polyvinyl alcohol films for gimbap storage at 25 °C and 90% RH. Compared with that in the packed samples, the microbial growth in the unpacked samples increased to 6.8 log CFU mL−1 after 12 h, whereas it was 4.2 log CFU mL−1 in the packed samples. However, rapid release (∼60% within 24 h) led to diminished antimicrobial efficacy, highlighting the need for controlled release systems to match the high-moisture, high-pH food matrix of gimbap. Xu et al.231 added chitosan/ZIF-67-Cu powders (0.5–2%) into carboxymethyl cellulose films for cherry tomatoes. The hybrid system provided antimicrobial Cu ion release while maintaining low migration (86.4% cell viability at 0–400 µg mL−1), reducing weight loss to ∼4% over 12 days. This finding illustrates how balancing antimicrobial efficacy with toxicological safety is critical in fresh produce applications. Zhu et al.232 reported that compared with unpacked controls (7.01%), oxidized corn starch/polybutylene adipate terephthalate films reduced weight loss in cherry tomatoes (3.69%) and grapes (3.65%). The low water vapor permeability of the starch–polyester blend was directly correlated with reduced dehydration and better sensory firmness. Dang et al.248 reported that gallic acid in chitosan/N,N-methylenebisacrylamide films minimized grape weight loss (12.13% vs. 18.73% control) and improved hardness (19.55 N vs. 12.55 N). The dense polymer structure restricted oxygen permeation, linking matrix chemistry to oxidative stability and texture preservation. Ashraf et al.249 used zein–nisin nanofillers (15%), pullulan, deep eutectic solvent plasticizers, and carboxymethyl chitosan to coat mangoes stored at 25 °C and 70% RH. The antimicrobial peptide nisin inhibited bacterial growth, whereas zein improved barrier properties, resulting in the highest firmness, polyphenol retention, and lowest weight loss. These findings demonstrate how multifunctional coatings can address microbial spoilage, oxidative degradation, and sensory quality simultaneously.6.2 Meat and poultry products
In modern-day industrial processing, extending the shelf-life of poultry products and their derivatives with conventional packaging poses severe challenges to preserving their poultry-fresh quality because of their increased susceptibility to microbial spoilage. As a result, several innovative approaches that integrate both biobased and functional materials have been developed. Ahmad et al.233 fabricated tripolymeric films integrating gelatin, carboxymethyl cellulose, and chitosan with apple polyphenols (APs) for packaging beef. According to the XRD and FTIR results, AP incorporation improved H-bonding, crystallinity, and film structural stability. Additionally, the antioxidant and antimicrobial capacities substantially increased, as evidenced by the effective inhibition of E. coli and S. aureus, with a shelf-life extension of beef of up to three days at 4 °C. The films resulted in less water loss and lipid/protein oxidation of the product with higher UV barrier properties (60% reduction in transmittance). Natural extracts and biobased coatings rich in antimicrobial and antioxidant properties have also been investigated to extend the shelf-life of meat. Khoshdouni Farahani et al.250 reported that clove extract effectively inhibited lipid oxidation and microbial spoilage in chicken fillets stored at 4 °C. Compared with the control, the 3% clove extract significantly reduced the pH, peroxide value, thiobarbituric acid content, TVN, and microbial count. Moreover, the 3% treatment resulted in the highest sensory score, confirming its potential to prolong the shelf-life of chicken fillets. In contrast, Rupérez et al.234 proposed active sachets containing diacetyl, a lactic acid bacterial metabolite with proven antimicrobial action, entrapped in sodium stearate gels and cyclodextrin nanosponges (CDNS). Here, the volatilization mechanism of diacetyl and CDNS was considered within beef packaging environments (at 4 °C) to control pathogens such as Salmonella and spoilage microbiota without direct contact. Interestingly, a 77% inhibition rate against Salmonella was reported after 11 days. Moreover, Pseudomonas spp. and lactic acid bacteria were more than 90% effective for 11 days. Furthermore, some green approaches, such as citrus peel-derived silver nanoparticle films251 and pea protein–corn starch films with green tea polyphenols,252 were found to be effective against major pathogens and lipid oxidation in beef. Another example of active packaging involves a bilayer packaging system consisting of an outer layer of gelatin and LAPONITE® (a synthetic clay) with an inner layer composed of electrospun zein fibers incorporating eugenol.253 The bilayer packaging proved to be beneficial for pork meat, as the LAPONITE® content improved the mechanical and barrier performance, whereas the zein-eugenol layer provided concentration-dependent antimicrobial and antioxidant activities via Fickian diffusion, as inferred from previous studies. A study conducted by Xu et al.235 evaluated the dual antimicrobial action of photodynamic food packaging using gelatin and a dialdehyde nanocellulose matrix loaded with berberine-based nanoparticles and ε-polylysine. Eventually, the composite film exhibited robust mechanical strength, fair hydrophobicity, minimal ultraviolet transmittance, and high transparency (86.57%). The antimicrobial mechanism involved synergistic reactive oxygen species generation and membrane disruption under visible light to increase the bacterial count by more than 6 log CFU in 15 min. This mechanism also maintained the shelf-life of pork with lower TVB-N and TBARS values, delaying spoilage for 10 days at 4 °C. For broad-spectrum antimicrobial activity, pectin–gelatin films with microalgal extracts in deep eutectic solvents were proposed by Karabulut & Goksen236 for chicken meat. They reported that the inhibition of E. coli and S. aureus increased by 80 and 20%, respectively, with the total mesophilic bacteria reducing to 5.59 log CFU g−1 after 7 days. Thus, active packaging renders multifunctional solutions to these concerning constraints involved in poultry processing and preservation.6.3 Seafood and fishery products
The seafood and fishery processing industries are currently centered around green production lines without any exception to their packaging. The packaging line has thus gradually shifted toward the utilization of natural extracts and biopolymer-based active packaging for these products. As fish and other seafoods are among the most easily spoiled food products, researchers are investigating the antibacterial characteristics of natural resources to replace synthetic chemicals. Several investigations have shown that natural extracts have immense potential to hinder microbial growth and lipid oxidation, the primary constraints to the industrial storage of seafood products. For example, Shi et al.239 developed chitosan films plasticized with citric acid-based deep eutectic agents (CA-DEAs) and optimized the film composition at 0.5% CA-DEA. The film exhibited a tensile strength of 47.98 MPa and improved antibacterial zones for E. coli and S. aureus (up to 23.6 mm), with strong DPPH and ABTS radical scavenging properties. Particularly for tilapia, it notably reduced water and protein loss, maintained TVB-N below 20 mg/100 g, and extended shelf-life by more than 10 days at 4 °C. Similarly, Babaei et al.254 reported that gelatin–sodium alginate composite coatings enriched with phycocyanin effectively delayed the spoilage of whiteleg shrimp during refrigerated storage. Compared with the control conditions, the 10% and 20% phycocyanin treatments significantly reduced bacterial growth, oxidative spoilage, and chemical deterioration. Moreover, the sensory attributes remained stable, highlighting phycocyanin as a promising natural antioxidant additive for extending shrimp shelf-life. In another study, Jeon et al.255 incorporated malic acid (MA) into whey protein isolate coatings for steamed fish paste. They then applied an in-package cold plasma treatment to enhance the coating efficacy and observed an increase in the MA diffusion rate of up to 1.5 times. The treatment was effective enough for microbial inhibition of Salmonella (∼2.5 log CFU g−1 reduction) and Listeria monocytogenes (∼1.8 log CFU g−1) for over 28 days at 4 °C, with stable product color and pH. Wu et al.256 also developed multifunctional chitosan–quercetin-based films for green packaging with superior mechanical properties, >90% antimicrobial efficacy, and rapid free radical scavenging (80% in 5 minutes). Additionally, the film provides real-time spoilage monitoring with a 70-day self-degradation limit and has been found to significantly slow TVB-N increases in stored fish. Wang et al.238 designed a self-healing cellulose nanofibril–PVA hydrogel system with curcumin-loaded ZIF-8 to preserve grass carp fillets. It exhibited 193.5% greater tensile strength and DPPH scavenging rates up to 95%. They could track the extension of shelf life up to 9 days with an in-package ammonia-induced color shift, indicating freshness.Several works have also demonstrated advanced nanotech-based antimicrobial packaging with natural inputs, such as engineered biopolymer films embedded with cinnamaldehyde-loaded chitosan nanoparticles in a chitosan–PVA–fish gelatin matrix237 or nanofiber films with nanochitin–nicin complexes fabricated via coaxial electrospinning.257 The homogeneously dispersed nanoparticles improved the film tensile strength, thermal stability, and UV-barrier properties, along with an inhibition zone of up to 6.6 mm for foodborne bacteria such as S. aureus, L. monocytogenes, E. coli, and S. enteritidis. The sustained release of antimicrobials promoted the extension of the shelf-life of rainbow trout fillets to 12 days at 4 °C. Nanotech-based smart packaging is also promising when designed with lyophilic proteins from legumes such as soy coupled with nanoemulsions and hydroxypropyl methylcellulose taken as a matrix.240 The bilayer design with pH-responsive indicators (shikonin) enhanced the water-barrier performance, with a shelf-life extension of up to 9 days for salmon. For salmon preservation, edible starch–pullulan films with geraniol are also effective and can achieve hydrophobicity (contact angle of 68.1°), reduced water solubility (37.6%), and high DPPH/ABTS scavenging (up to 56–66%) with product freshness for up to 7 days (maintaining bacterial counts under 6 log CFU g−1).241 As the packaging industry has been encouraged to use environmentally benign natural polymers such as proteins, polysaccharides, and their derivatives, shrimp packaging is also notably reshaped from conventional packaging via synthetic plastics. For example, Feng et al.258 developed sodium alginate films embedded with cobalt-based metal–organic framework nanoparticles, which achieved a tensile strength of 96.4 MPa, high antibacterial rates of up to 99.9% for S. aureus and rapid color changes with ammonia sensitivity, indicating shrimp spoilage. Furthermore, Thakur et al.110 demonstrated the antibacterial efficacy of kodo millet starch–gum tragacanth films with beetroot peel extract, which markedly improved the water vapor barrier properties and visual pH sensitivity for shrimp freshness, which was correlated with TVB-N levels.
6.4 Dairy and soy products
To delay the deterioration of tofu, Cui et al.259 added nisin (0.312 mg g−1), which effectively inhibited bacillus growth. Nisin is a cationic peptide that interacts with bacterial cell membranes to form pores that disrupt viability. Its incorporation maintained its textural firmness and color stability for 10 days at both 4 °C and 37 °C, demonstrating how peptide chemistry can be modified in high-moisture, protein-rich matrices. In another study, Gao et al.260 used dialdehyde cellophane for tofu packaging, preventing color changes for 3 days at room temperature. The aldehyde groups crosslinked with protein structures, stabilizing the surface appearance, although microbial counts and sensory attributes indicated limited long-term efficacy. This underscores the need for controlled release systems in high-pH soy matrices.Zhang et al.243 extracted peptides (αs2-casein182–207 and αs2-casein151–181) from dairy products and incorporated them into whey protein films. These peptides restricted E. coli growth to <1 log CFU g−1 for 21 days and prevented yeast/mold growth for 28 days at 4 °C. The peptide–protein synergy illustrates how food matrix chemistry (casein–whey interactions) can be exploited for targeted antimicrobial release while maintaining sensory neutrality. Essential oils are often unsuitable for dairy due to pungency and volatility. Gadallah et al.244 addressed this by encapsulating rosemary oil in chitosan nanoparticles for Karish cheese. This nanocarrier system masked strong odors, stabilized the oil against light degradation, and preserved flavor and color attributes for 28 days. Quantitatively, microbial reductions were dramatic: A. flavus (4.97 log), C. albicans (6.82 log), and P. aeruginosa (7.72 log) within 3 days, with complete elimination by day 15. This highlights how encapsulation connects sensory acceptability with antimicrobial potency. However, Doğan et al.242 introduced lemon peel oil into gelatin–acetic acid films to preserve Kashar cheese at 4 °C. The hydrophobic terpenes in lemon oil provided broad-spectrum antimicrobial activity against Gram-positive bacteria, yeasts, and molds, extending the shelf-life to 21 days. However, sensory evaluation highlighted the challenge of balancing antimicrobial efficacy with flavor acceptability, a critical performance metric in dairy systems. Similarly, Di Matteo et al.245 introduced carvacrol and thymol into thermoplastic biopolymers, which inhibited Salmonella typhimurium and Listeria monocytogenes in brie cheese at 5 °C. Carvacrol at an 8% concentration strongly inhibited Listeria after 10 days, although Lactobacillus remained unaffected, indicating that the selective antimicrobial action preserved the beneficial microflora. However, authors such as Anari et al.261 modified LDPE films with acrylic acid and natamycin for doogh packaging. This ensured that the yeast counts remained below the Iranian standard limit (87 CFU mL−1 vs. 100 CFU mL−1) after 23 days at 25 °C, confirming regulatory compliance and microbial stability in acidic dairy beverages. Similarly, Bruni et al.262 applied PHBV films treated with lauroyl arginate ethyl to spreadable cheese at 4 °C. The antimicrobial agent diffused into cheese at 21.5 mg, within the safety threshold (28.9 mg per body weight), effectively inhibiting Penicillium roqueforti and Micrococcus luteus. Importantly, migration studies confirmed compliance with intake limits (0.5 mg per kg body weight), linking toxicological safety with functional antimicrobial performance.
6.5 Bakery- and cereal-based products
To extend the shelf-life of bread, Sripahco et al.246 incorporated Anethum graveolens oil into gellan gum–pineapple nanocellulose films and incubated them for 14 days at room temperature. The hydrophilic nanocellulose provided structural integrity and moisture regulation, while dill oil contributed to antifungal activity. Bread samples showed no visual changes for up to 21 days, confirming that the volatile compounds of the oil effectively suppressed fungal growth without altering sensory attributes such as color or texture. Kim et al.263 evaluated insect protection in instant noodles by mixing rice chaff, vulgare, and Tanacetum cinerariifolium with LLDPE films. At the 0.5% concentration, the repellent efficacy was markedly greater against P. interpunctella (8.12-fold reduction) than against T. castaneum (2.17-fold reduction). This demonstrates how food matrix chemistry (low-moisture, starch-rich noodles) requires packaging with insect-repellent properties rather than microbial control, broadening the functional scope of antimicrobial packaging.Wrona et al.247 added encapsulated carvacrol to aluminosilicate–LDPE films to protect crisps. The phenolic chemistry of carvacrol provided strong antioxidant activity, reducing lipid oxidation rates at a 30% concentration after 30 days at 60 °C. The malondialdehyde (MDA) content was 45% lower than that of the controls, suggesting that chemical stabilization directly affects the sensory preservation of crisp flavors and aromas. Peighambardoust et al.264 loaded polypropylene films with sorbic acid for yogurt dough storage under refrigeration. The moisture content remained stable for 20 days, whereas sorbic acid migration (1.4–3.3 mg dm−2) was well below the permitted limit (10 mg dm−2). At a concentration of 6%, sorbic acid effectively hindered mold growth and preserved sensory quality, demonstrating regulatory compliance and functional efficacy in high-moisture dough matrices. Settier-Ramírez et al.265 incorporated casein hydrolysates into polyvinyl alcohol films for homemade paste creams. The peptides exhibited strong antimicrobial activity, extending the shelf-life to 13 days at 4–24 °C. Their protein-derived chemistry provided controlled release, maintaining microbial stability without altering sensory properties, highlighting the compatibility of dairy-derived antimicrobials with starch-rich bakery matrices.
7. Challenges and limitations
Despite the promising potential of antimicrobial active packaging using biopolymers and natural extracts, several challenges restrict their adoption and commercialization. When incorporated into complex biopolymer matrices, natural antimicrobial extracts often exhibit reduced efficacy. Additionally, factors such as pH, moisture content, temperature and food composition can affect the release kinetics and antimicrobial activity of active compounds. For example, essential oils and polyphenols may degrade or volatilize during processing (especially at high temperatures) and storage. Moreover, the interaction between food components and active agents can lead to compound neutralization, which can reduce their effectiveness. Therefore, attaining uniform dispersion and stabilization of incorporated natural extracts remains important. In addition, the phase separation and poor miscibility between hydrophobic compounds such as essential oils and hydrophilic polymers such as starch could result in poor film integrity. Likewise, the mechanical, barrier, and optical properties of the films may be affected by the incorporation of extract/bioactives. Therefore, an optimization of the formulation and processing conditions is needed.In addition to technical challenges, the regulatory aspects of antimicrobial packaging are complex and depend on the region. In the EU, active substances must comply with EC regulations 1333/2008 and 450/2009, whereas in the US, the FDA classifies them as food additives or GRAS substances under the FFDCA. However, most natural extracts also lack standardized safety evaluations or approved usage limits. This further complicates existing issues and commercial adoption. Moreover, the lack of knowledge of any extract or compound may increase consumer concerns regarding the safety, allergenicity, and sensory impact of natural extracts. Some natural extracts, particularly those derived from animal or microbial sources, may pose allergenic risks. Essential oils, for example, may impart strong odors or flavors that alter food acceptability. Animal-derived components such as lysozyme or lactoferrin may trigger allergic reactions or raise ethical concerns. Additionally, the long-term effects of chronic exposure to bioactive compounds released from packaging materials remain underexplored. Therefore, research and development priorities must decisively address regulatory compliance through standardized toxicological testing, migration studies, and harmonized global guidelines; cost-effective manufacturing by developing scalable encapsulation, extrusion, and hybrid processing technologies that ensure reproducibility and reduce production costs; and robust performance stability under realistic supply chain conditions, including variable temperature, humidity, and mechanical stress. By focusing on these priorities, future research can bridge the gap between laboratory innovation and industrial commercialization, ensuring that antimicrobial biopolymer packaging evolves into a safe, effective, and market-ready solution.
8. Future perspectives
Several strategies are emerging to overcome the limitations of current antimicrobial active packaging systems. Compared with traditional extraction methods, sustainable extraction methods, including supercritical fluid extraction, ultrasound-assisted extraction, and enzyme-assisted techniques, are gaining significant research attention for isolating bioactive compounds with minimal environmental impact. These methods not only improve yield but also reduce solvent use and preserve compound integrity. Additionally, green solvents and biodegradable carriers are being explored to increase formulation compatibility. Future research must prioritize scalable, eco-efficient extraction methods coupled with stabilization strategies such as encapsulation and nanocarrier systems. These approaches ensure consistent bioactive compound quality, industrial feasibility, and a reduced environmental footprint. Advanced release systems such as nanocarriers, liposomes, and smart-responsive systems (pH- or temperature-triggered release) offer controlled and targeted delivery of antimicrobial agents. The next decade should focus on quantifying release kinetics in real food matrices, optimizing dosage efficiency, and minimizing sensory interference to bridge the gap between laboratory success and commercial adoption. New hybrid encapsulation methods, such as combining spray drying with gel or cross-linking techniques, help make large-scale production easier by improving the stability of active ingredients, reducing material loss, and allowing continuous, cost-effective manufacturing. Future work must decisively address scalability by integrating continuous processing, cost modeling, and industrial pilot trials to validate the commercial readiness of biopolymer/extract/nanocarrier hybrid systems. Biopolymer-based antimicrobial packaging supports the circular economy by reducing food waste, improving compostability, and reducing dependence on fossil-based plastics. Further advances in recycling, biodegradation, and life cycle assessment must be coupled with clear regulatory frameworks and consumer awareness strategies to ensure market acceptance and alignment with SDG 12 and SDG 14. Overall, the strategic outlook for the next decade lies in engineering and designing smart, multifunctional packaging systems that combine antimicrobial, antioxidant, and sensing features while simultaneously addressing scalability, regulatory clarity, and consumer trust.9. Conclusions
Antimicrobial active packaging using biopolymers and natural extracts is a promising alternative for sustainable food preservation. Exploiting and optimizing the inherent antimicrobial properties of plant, animal, and microbial derivatives for incorporation in biopolymer-based food packaging systems can address spoilage-related losses. The current state of the field demonstrates strong laboratory-scale evidence of efficacy, but translation to industrial-scale applications remains limited. Challenges such as compound stability, compatibility with the polymer matrix, regulatory barriers, and scalability must be explored and addressed to realize the full potential of biopolymer/natural extract films. Therefore, advances in the fields of encapsulation and controlled release systems are offering better stabilization as well as improving the efficacy of bioactive compounds. Various studies and reports confirm that integrating antimicrobial packaging into circular economy frameworks and food systems can significantly contribute to sustainable goals (SDG 12 and SDG 14). Strategic priorities for the next decade must focus on quantifying antimicrobial performance under real-world supply chain conditions, developing scalable manufacturing technologies, and establishing standardized testing protocols to ensure reproducibility and safety. Clear regulatory guidelines and efforts to increase consumer awareness could play crucial roles in ensuring the market acceptance of these biopolymer/natural extract-based antimicrobial films. Ultimately, sustained interdisciplinary strategies, engineering-driven innovation, and investment in industrial partnerships are crucial for developing safe, effective, and scalable antimicrobial packaging systems that align with environmental sustainability and public health goals.Author contributions
Shubhajit Sarkhel, Samandeep Kaur, Rahul Das, Aditi Sharma, Ankan Kheto, Debapam Saha: writing – original draft, investigation; formal analysis; Yogesh Kumar: conceptualization, writing – original draft, writing – review & editing, validation, supervision.Conflicts of interest
The authors declare that there are no conflicts of interest.Data availability
All findings and supporting data are available through the respective published sources cited in this work.References
- World Health Organization, Plastics and health initiative [Internet], World Health Organization, Geneva, [cited 2025 Sep 13], available from: https://www.who.int/initiatives/plastics-and-health-initiative Search PubMed.
- United Nations, Sustainable consumption and production [Internet], United Nations Sustainable Development, [cited 2025 Sep 13], available from: https://www.un.org/sustainabledevelopment/sustainable-consumption-production/ Search PubMed.
- R. F. Luz, R. D. Ferreira, C. N. Silva, B. M. Miranda, R. H. Piccoli, M. S. Silva and K. A. Batista, Development of a halochromic, antimicrobial, and antioxidant starch-based film containing phenolic extract from jaboticaba peel, Foods, 2023, 12(3), 653 CrossRef PubMed.
- P. A. Freitas, C. González-Martínez and A. Chiralt, Antioxidant starch composite films containing rice straw extract and cellulose fibres, Food Chem., 2023, 400, 134073 CrossRef.
- N. Revutskaya, E. Polishchuk, I. Kozyrev, L. Fedulova, V. Krylova, V. Pchelkina and E. Kotenkova, Application of natural functional additives for improving bioactivity and structure of biopolymer-based films for food packaging: a review, Polymers, 2024, 16(14), 1976 CrossRef PubMed.
- Y. Kumar, Y. Bist, D. Thakur, M. Nagar and D. C. Saxena, A review on the role of pH-sensitive natural pigments in biopolymers based intelligent food packaging films, Int. J. Biol. Macromol., 2024, 276, 133869 CrossRef.
- Y. Madhumala and V. S. Soraganvi, Microbiology of food spoilage, in Frontiers in Food Biotechnology, Springer Nature Singapore, Singapore, 2024, pp. 67–80 Search PubMed.
- J. Huang, M. Zhang, A. S. Mujumdar and Y. Ma, Technological innovations enhance postharvest fresh food resilience from a supply chain perspective, Crit. Rev. Food Sci. Nutr., 2024, 64(30), 11044–11066 CrossRef.
- S. A. Siddiqui, S. Singh, N. A. Bahmid and A. Sasidharan, Applying innovative technological interventions in the preservation and packaging of fresh seafood products to minimize spoilage: a systematic review and meta-analysis, Heliyon, 2024, 10(8), e Search PubMed.
- F. Meng, M. Brandão and J. M. Cullen, Replacing plastics with alternatives is worse for greenhouse gas emissions in most cases, Environ. Sci. Technol., 2024, 58(6), 2716–2727 CrossRef.
- Grand View Research, Antimicrobial packaging market size, share & trends analysis report by material, by technology, by application, by region, and segment forecasts, 2024–2030 [Internet], 2025, [cited 2025 Sep 15], available from: https://www.grandviewresearch.com/industry-analysis/antimicrobial-packaging-market.
- F. Bauer, T. D. Nielsen, L. J. Nilsson, E. Palm, K. Ericsson, A. Fråne and J. Cullen, Plastics and climate change—Breaking carbon lock-ins through three mitigation pathways, One Earth, 2022, 5(4), 361–376 CrossRef.
- R. Ribeiro-Santos, M. Andrade, N. R. Melo and A. Sanches-Silva, Use of essential oils in active food packaging: recent advances and future trends, Trends Food Sci. Technol., 2017, 61, 132–140, DOI:10.1016/j.tifs.2016.11.021.
- C. Shu, L. Ge, Z. Li, B. Chen, S. Liao, L. Lu and M. Qu, Antibacterial activity of cinnamon essential oil and its main component cinnamaldehyde and the underlying mechanism, Front. Pharmacol, 2024, 15, 1378434, DOI:10.3389/fphar.2024.1378434.
- G. E. Jeyakumar and R. Lawrence, Mechanisms of bactericidal action of eugenol against Escherichia coli, J. Herb. Med., 2021, 26, 100406, DOI:10.1016/j.hermed.2020.100406.
- Z. Shahina, H. R. Al, J. D. W. Price, M. Whiteway, T. Sultana and T. E. S. Dahms, Rosemary essential oil and its components 1,8-cineole and α-pinene induce ROS-dependent lethality and ROS-independent virulence inhibition in Candida albicans, PLoS One, 2022, 17(11), e0277097, DOI:10.1371/journal.pone.0277097.
- Z. Zhi, P. Zhou, T. He, S. Chen, X. Qian, Y. Ye and W. Yuan, Study of the antimicrobial activity of carvacrol and its mechanism of action against drug-resistant bacteria, Biochem. Biophys. Res. Commun., 2025, 757, 151643, DOI:10.1016/j.bbrc.2025.151643.
- C. Shi, K. Song, X. Zhang, Y. Sun, Y. Sui, Y. Chen and X. Xia, Antimicrobial activity and possible mechanism of action of citral against Cronobacter sakazakii, PLoS One, 2016, 11(7), e0159006, DOI:10.1371/journal.pone.0159006.
- C. N. Tagousop, J. D. Tamokou, S. E. Ekom, D. Ngnokam and L. Voutquenne-Nazabadioko, Antimicrobial activities of flavonoid glycosides from Graptophyllum grandulosum and their mechanism of antibacterial action, BMC Compl. Alternative Med., 2018, 18(1), 252, DOI:10.1186/s12906-018-2321-7.
- G. Maisetta, G. Batoni, P. Caboni, S. Esin, A. C. Rinaldi and P. Zucca, Tannin profile, antioxidant properties, and antimicrobial activity of extracts from two Mediterranean species of parasitic plant Cytinus, BMC Compl. Alternative Med., 2019, 19(1), 82, DOI:10.1186/s12906-019-2487-7.
- I. Jallali, M. B. Jemaa, F. Medini, P. Waffo-Téguo, J. M. Mérillon, H. Ben Ahmed and R. Ksouri, Isolation of the major phenolic of Limbarda crithmoïdes L. and evaluation of its contribution in the phytochemical variability and the biological activities among localities and organs, Biochem. Syst. Ecol., 2025, 123, 105109, DOI:10.1016/j.bse.2025.105109.
- Z. Yu, X. Wu and J. He, Study on the antifungal activity and mechanism of tea saponin from Camellia oleifera cake, Eur. Food Res. Technol., 2022, 248(3), 783–795, DOI:10.1007/s00217-021-03929-1.
- N. Kummer, T. Wu, K. J. De France, F. Zuber, Q. Ren, P. Fischer and G. Nyström, Self-assembly pathways and antimicrobial properties of lysozyme in different aggregation states, Biomacromolecules, 2021, 22(10), 4327–4336, DOI:10.1021/acs.biomac.1c00870.
- Y. Wang, J. D. Morton, A. E.-D. A. Bekhit, A. Carne and S. L. Mason, Amino acid sequences of lactoferrin from red deer (Cervus elaphus) milk and antimicrobial activity of its derived peptides lactoferricin and lactoferrampin, Foods, 2021, 10(6), 1305, DOI:10.3390/foods10061305.
- M. Farshidi, M. Yousefi and A. Ehsani, The combined effects of lactoperoxidase system and whey protein coating on microbial, chemical, textural, and sensory quality of shrimp (Penaeus merguiensis) during cold storage (4 ± 1 °C), Food Sci. Nutr., 2018, 6(6), 1378–1386, DOI:10.1002/fsn3.669.
- C. Muñoz-Nuñez, R. Cuervo-Rodríguez, C. Echeverría, M. Fernández-García and A. Muñoz-Bonilla, Synthesis and characterization of thiazolium chitosan derivative with enhanced antimicrobial properties and its use as component of chitosan based films, Carbohydr. Polym., 2023, 302, 120438, DOI:10.1016/j.carbpol.2022.120438.
- Z. Tong, Y. Zhang, J. Ling, J. Ma, L. Huang and L. Zhang, An in vitro study on the effects of nisin on the antibacterial activities of 18 antibiotics against Enterococcus faecalis, PLoS One, 2014, 9(2), e89209, DOI:10.1371/journal.pone.0089209.
- M. C. Sun, D. D. Li, Y. X. Chen, X. J. Fan, Y. Gao, H. Ye and C. Zhao, Insights into the mechanisms of reuterin against Staphylococcus aureus based on membrane damage and untargeted metabolomics, Foods, 2023, 12(23), 4208, DOI:10.3390/foods12234208.
- S. Sharma, S. Barkauskaite, A. K. Jaiswal and S. Jaiswal, Essential oils as additives in active food packaging, Food Chem., 2021, 343, 128403, DOI:10.1016/j.foodchem.2020.128403.
- R. Borah, M. D. Purkayastha, M. Malakar, A. Mukherjee, A. Roy and S. Kumar, Progress in essential oil extraction and their applications in food packaging and preservation, Food Biomacromolecules, 2024, 1(2), 140–154, DOI:10.1002/fob2.12014.
- M. Hyldgaard, T. Mygind and R. L. Meyer, Essential oils in food preservation: mode of action, synergies, and interactions with food matrix components, Front. Microbiol., 2012, 3, 12, DOI:10.3389/fmicb.2012.00012.
- S. Chouhan, K. Sharma and S. Guleria, Antimicrobial activity of some essential oils—present status and future perspectives, Medicines, 2017, 4(3), 58, DOI:10.3390/medicines4030058.
- N. Khorshidian, M. Yousefi, E. Khanniri and A. M. Mortazavian, Potential application of essential oils as antimicrobial preservatives in cheese, Innovative Food Sci. Emerging Technol., 2018, 45, 62–72, DOI:10.1016/j.ifset.2017.09.020.
- J. Y. Bae, Y. H. Seo and S. W. Oh, Antibacterial activities of polyphenols against foodborne pathogens and their application as antibacterial agents, Food Sci. Biotechnol., 2022, 31(8), 985–997, DOI:10.1007/s10068-022-01058-3.
- H. Yong and J. Liu, Active packaging films and edible coatings based on polyphenol-rich propolis extract: a review, Compr. Rev. Food Sci. Food Saf., 2021, 20(2), 2106–2145, DOI:10.1111/1541-4337.12697.
- S. Galié, C. García-Gutiérrez, E. M. Miguélez, C. J. Villar and F. Lombó, Biofilms in the food industry: health aspects and control methods, Front. Microbiol., 2018, 9, 898, DOI:10.3389/fmicb.2018.00898.
- D. Khokhani, C. Zhang, Y. Li, Q. Wang, Q. Zeng, A. Yamazaki and C. H. Yang, Discovery of plant phenolic compounds that act as type III secretion system inhibitors or inducers of the fire blight pathogen Erwinia amylovora, Appl. Environ. Microbiol., 2013, 79(18), 5424–5436, DOI:10.1128/AEM.00845-13.
- F. Nazzaro, F. Fratianni, L. De Martino, R. Coppola and V. De Feo, Effect of essential oils on pathogenic bacteria, Pharmaceuticals, 2013, 6(12), 1451–1474, DOI:10.3390/ph6121451.
- W. H. Zhao, Z. Q. Hu, Y. Hara and T. Shimamura, Inhibition of penicillinase by epigallocatechin gallate resulting in restoration of antibacterial activity of penicillin against penicillinase-producing Staphylococcus aureus, Antimicrob. Agents Chemother., 2002, 46(7), 2266–2268, DOI:10.1128/aac.46.7.2266-2268.2002.
- P. Buzzini, B. Turchetti, F. Ieri, M. Goretti, E. Branda, N. Mulinacci and A. Romani, Catechins and proanthocyanidins: naturally occurring O-heterocycles with antimicrobial activity, in Bioactive Heterocycles IV, ed. M. T. H. Khan, Springer, Berlin, 2007, pp. 239–263, DOI: DOI:10.1007/7081_2007_065.
- M. Oblak, M. Kotnik and T. Solmajer, Discovery and development of ATPase inhibitors of DNA gyrase as antibacterial agents, Curr. Med. Chem., 2007, 14(19), 2033–2047, DOI:10.2174/092986707781368414.
- S. B. Bhatwalkar, R. Mondal, S. B. N. Krishna, J. K. Adam, P. Govender and R. Anupam, Antibacterial properties of organosulfur compounds of garlic (Allium sativum), Front. Microbiol., 2021, 12, 613077, DOI:10.3389/fmicb.2021.613077.
- R. K. Deshmukh and K. K. Gaikwad, Natural antimicrobial and antioxidant compounds for active food packaging applications, Biomass Convers. Biorefin., 2024, 14(4), 4419–4440, DOI:10.1007/s13399-022-02623-w.
- Q. Huang, X. Liu, G. Zhao, T. Hu and Y. Wang, Potential and challenges of tannins as an alternative to in-feed antibiotics for farm animal production, Anim. Nutr., 2018, 4(2), 137–150, DOI:10.1016/j.aninu.2017.09.004.
- Y. Yan, X. Li, C. Zhang, L. Lv, B. Gao and M. Li, Research progress on antibacterial activities and mechanisms of natural alkaloids: a review, Antibiotics, 2021, 10(3), 418, DOI:10.3390/antibiotics10030318.
- S. A. Varghese, S. Siengchin and J. Parameswaranpillai, Essential oils as antimicrobial agents in biopolymer-based food packaging: a comprehensive review, Food Biosci., 2020, 38, 100785, DOI:10.1016/j.fbio.2020.100785.
- A. Tomić, O. Šovljanski and T. Erceg, Insight on incorporation of essential oils as antimicrobial substances in biopolymer-based active packaging, Antibiotics, 2023, 12(9), 1473, DOI:10.3390/antibiotics12091473.
- W. Zhang and J. W. Rhim, Functional edible films/coatings integrated with lactoperoxidase and lysozyme and their application in food preservation, Food Control, 2022, 133, 108670, DOI:10.1016/j.foodcont.2021.108670.
- J. Duan, K. Kim, M. A. Daeschel and Y. Zhao, Storability of antimicrobial chitosan-lysozyme composite coating and film-forming solutions, J. Food Sci., 2008, 73(6), M321–M329, DOI:10.1111/j.1750-3841.2008.00850.x.
- B. Masschalck and C. W. Michiels, Antimicrobial properties of lysozyme in relation to foodborne vegetative bacteria, Crit. Rev. Microbiol., 2003, 29(3), 191–214, DOI:10.1080/713610448.
- T. Silvetti, S. Morandi, M. Hintersteiner and M. Brasca, Use of hen egg white lysozyme in the food industry, in Egg Innovations and Strategies for Improvements, ed. Hester P. Y., Academic Press, Cambridge (MA), 2017, pp. 233–242, DOI:10.1016/B978-0-12-800879-9.00022-6.
- N. Khorshidian, E. Khanniri, M. R. Koushki, S. Sohrabvandi and M. Yousefi, An overview of antimicrobial activity of lysozyme and its functionality in cheese, Front. Nutr., 2022, 9, 833618, DOI:10.3389/fnut.2022.833618.
- M. Derde, V. Lechevalier, C. Guérin-Dubiard, M. F. Cochet, S. Jan, F. Baron, M. Gautier, V. Vié and F. Nau, Hen egg white lysozyme permeabilizes Escherichia coli outer and inner membranes, J. Agric. Food Chem., 2013, 61(41), 9922–9929, DOI:10.1021/jf4029199.
- M. Tavassoli, B. Bahramian, R. Abedi-Firoozjah, A. Ehsani, Y. Phimolsiripol and S. P. Bangar, Application of lactoferrin in food packaging: a comprehensive review on opportunities, advances, and horizons, Int. J. Biol. Macromol., 2024, 273, 132969, DOI:10.1016/j.ijbiomac.2024.132969.
- I. Abad, C. Conesa and L. Sánchez, Development of encapsulation strategies and composite edible films to maintain lactoferrin bioactivity: a review, Materials, 2021, 14(23), 7358, DOI:10.3390/ma14237358.
- M. Yousefi, A. Nematollahi, M. Shadnoush, A. M. Mortazavian and N. Khorshidian, Antimicrobial activity of films and coatings containing lactoperoxidase system: a review, Front. Nutr., 2022, 9, 828065, DOI:10.3389/fnut.2022.828065.
- S. H. Lim and S. M. Hudson, Synthesis and antimicrobial activity of a water-soluble chitosan derivative with a fiber-reactive group, Carbohydr. Res., 2004, 339(2), 313–319, DOI:10.1016/j.carres.2003.10.024.
- K. V. Reesha, S. K. Panda, J. Bindu and T. O. Varghese, Development and characterization of an LDPE/chitosan composite antimicrobial film for chilled fish storage, Int. J. Biol. Macromol., 2015, 79, 934–942, DOI:10.1016/j.ijbiomac.2015.06.016.
- L. A. M. van den Broek, R. J. I. Knoop, F. H. J. Kappen and C. G. Boeriu, Chitosan films and blends for packaging material, Carbohydr. Polym., 2015, 116, 237–242, DOI:10.1016/j.carbpol.2014.07.039.
- M. Kong, X. G. Chen, K. Xing and H. J. Park, Antimicrobial properties of chitosan and mode of action: a state of the art review, Int. J. Food Microbiol., 2010, 144(1), 51–63, DOI:10.1016/j.ijfoodmicro.2010.09.012.
- M. Kong, X. Chen, Y. Xue, C. Liu, L. Yu, Q. Ji, D. S. Cha and H. J. Park, Preparation and antibacterial activity of chitosan microspheres in a solid dispersing system, Front. Mater. Sci. China, 2008, 2(2), 214–220, DOI:10.1007/s11706-008-0036-2.
- Y. Hu, Y. Du, J. Yang, J. F. Kennedy, X. Wang and L. Wang, Synthesis, characterization and antibacterial activity of guanidinylated chitosan, Carbohydr. Polym., 2007, 67(1), 66–72, DOI:10.1016/j.carbpol.2006.04.015.
- L. Inguglia, M. Chiaramonte, V. Di Stefano, D. Schillaci, G. Cammilleri, L. Pantano and V. Arizza, Salmo salar fish waste oil: fatty acids composition and antibacterial activity, PeerJ, 2020, 8, e9299, DOI:10.7717/peerj.9299.
- G. Caruso, R. Floris, C. Serangeli and L. Di Paola, Fishery wastes as a yet undiscovered treasure from the sea: biomolecules sources, extraction methods and valorization, Mar. Drugs, 2020, 18(12), 622, DOI:10.3390/md18120622.
- H. Mathur, D. Field, M. C. Rea, P. D. Cotter, C. Hill and R. P. Ross, Bacteriocin-antimicrobial synergy: a medical and food perspective, Front. Microbiol., 2017, 8, 1205, DOI:10.3389/fmicb.2017.01205.
- E. E. Popa, A. C. Miteluţ, M. Râpă, P. A. Popescu, M. C. Drăghici, M. Geicu-Cristea and M. E. Popa, Antimicrobial active packaging containing nisin for preservation of products of animal origin: an overview, Foods, 2022, 11(23), 3820, DOI:10.3390/foods11233820.
- H. Wang, R. Zhang, H. Zhang, S. Jiang, H. Liu, M. Sun and S. Jiang, Kinetics and functional effectiveness of nisin loaded antimicrobial packaging film based on chitosan/poly(vinyl alcohol), Carbohydr. Polym., 2015, 127, 64–71, DOI:10.1016/j.carbpol.2015.03.058.
- N. Khorshidian, E. Khanniri, M. Mohammadi, A. M. Mortazavian and M. Yousefi, Antibacterial activity of pediocin and pediocin-producing bacteria against Listeria monocytogenes in meat products, Front. Microbiol., 2021, 12, 709959, DOI:10.3389/fmicb.2021.709959.
- W. Woraprayote, Y. Kingcha, P. Amonphanpokin, J. Kruenate, T. Zendo, K. Sonomoto and W. Visessanguan, Anti-listeria activity of poly(lactic acid)/sawdust particle biocomposite film impregnated with pediocin PA-1/AcH and its use in raw sliced pork, Int. J. Food Microbiol., 2013, 167(2), 229–235, DOI:10.1016/j.ijfoodmicro.2013.09.009.
- F. J. Rodrigues, M. F. Cedran, J. L. Bicas and H. H. Sato, Inhibitory effect of reuterin-producing Limosilactobacillus reuteri and edible alginate-konjac gum film against foodborne pathogens and spoilage microorganisms, Food Biosci., 2023, 52, 102443, DOI:10.1016/j.fbio.2023.102443.
- S. Langa, J. M. Landete, I. Martín-Cabrejas, E. Rodríguez, J. L. Arqués and M. Medina, In situ reuterin production by Lactobacillus reuteri in dairy products, Food Control, 2013, 33(1), 200–206, DOI:10.1016/j.foodcont.2013.02.035.
- S. Vollenweider and C. Lacroix, 3-Hydroxypropionaldehyde: applications and perspectives of biotechnological production, Appl. Microbiol. Biotechnol., 2004, 64(1), 16–27, DOI:10.1007/s00253-003-1497-y.
- D. M. Gouvêa, R. C. S. Mendonça, M. L. Soto and R. S. Cruz, Acetate cellulose film with bacteriophages for potential antimicrobial use in food packaging, LWT–Food Sci. Technol., 2015, 63(1), 85–91 CrossRef.
- Ł. Grabowski, K. Łepek, M. Stasiłojć, K. Kosznik-Kwaśnicka, K. Zdrojewska, M. Maciąg-Dorszyńska and A. Węgrzyn, Bacteriophage-encoded enzymes destroying bacterial cell membranes and walls, and their potential use as antimicrobial agents, Microbiol. Res., 2021, 248, 126746, DOI:10.1016/j.micres.2021.126746.
- A. Rezvankhah, Z. Emam-Djomeh and G. Askari, Encapsulation and delivery of bioactive compounds using spray and freeze-drying techniques: a review, Dry. Technol., 2020, 38(1–2), 235–258, DOI:10.1080/07373937.2019.1653906.
- N. Anton and T. F. Vandamme, The universality of low-energy nano-emulsification, Int. J. Pharm., 2009, 377(1), 142–147, DOI:10.1016/j.ijpharm.2009.05.014.
- M. Beyki, S. Zhaveh, S. T. Khalili, T. Rahmani-Cherati, A. Abollahi, M. Bayat and A. Mohsenifar, Encapsulation of Mentha piperita essential oils in chitosan–cinnamic acid nanogel with enhanced antimicrobial activity against Aspergillus flavus, Ind. Crops Prod., 2014, 54, 310–319, DOI:10.1016/j.indcrop.2014.01.033.
- S. Sarkhel, D. Manvi, C. T. Ramachandra, M. Manjunath and U. K. Nidoni, Studies on supercritical fluid extraction and spray drying effect on the quality of instant tea of Morus alba L, Meas. Food, 2022, 7, 100052, DOI:10.1016/j.meafoo.2022.100052.
- C. B. Amara, N. Eghbal, P. Degraeve and A. Gharsallaoui, Using complex coacervation for lysozyme encapsulation by spray-drying, J. Food Eng., 2016, 183, 50–57, DOI:10.1016/j.jfoodeng.2016.03.016.
- B. Wang, Y. P. Timilsena, E. Blanch and B. Adhikari, Characteristics of bovine lactoferrin powders produced through spray and freeze drying processes, Int. J. Biol. Macromol., 2017, 95, 985–994, DOI:10.1016/j.ijbiomac.2016.10.087.
- M. Jacquot, P. De Donato, O. Barres, M. N. Pons, J. Scher, A. Miclo and D. Poncelet, Physicochemical characterisation of the lactoperoxidase system powders: comparison of two drying techniques, Powder Technol., 2002, 128(2), 205–212, DOI:10.1016/S0032-5910(02)00198-5.
- G. Mauriello, M. Aponte, R. Andolfi, G. Moschetti and F. Villani, Spray-drying of bacteriocin-producing lactic acid bacteria, J. Food Prot., 1999, 62(7), 773–777, DOI:10.4315/0362-028X-62.7.773.
- Q. Bu, Y. Chen, Y. Ding, K. Zhang, Y. C. Li, X. You and G. Zhao, Preparation and characterization of tea polyphenol composite microspheres encapsulated using sodium alginate and crosslinked starch, LWT–Food Sci. Technol., 2023, 184, 114888, DOI:10.1016/j.lwt.2023.114888.
- P. Hiwale, S. Lampis, G. Conti, C. Caddeo, S. Murgia, A. M. Fadda and M. Monduzzi, In vitro release of lysozyme from gelatin microspheres: effect of cross-linking agents and thermoreversible gel as suspending medium, Biomacromolecules, 2011, 12(9), 3186–3193, DOI:10.1021/bm200679w.
- L. Wei, D. Wong, T. Jeoh and M. L. Marco, Intestinal delivery of encapsulated bacteriocin peptides in cross-linked alginate microcapsules, Food Res. Int., 2024, 188, 114473, DOI:10.1016/j.foodres.2024.114473.
- L. E. Kurozawa and M. D. Hubinger, Hydrophilic food compounds encapsulation by ionic gelation, Curr. Opin. Food Sci., 2017, 15, 50–55, DOI:10.1016/j.cofs.2017.06.004.
- M. Guidotti-Takeuchi, L. N. de Morais Ribeiro, F. A. L. dos Santos, D. A. Rossi, F. Della Lucia and R. T. de Melo, Essential oil-based nanoparticles as antimicrobial agents in the food industry, Microorganisms, 2022, 10(8), 1504, DOI:10.3390/microorganisms10081504.
- B. Enaru, S. Socaci, A. Farcas, C. Socaciu, C. Danciu, A. Stanila and Z. Diaconeasa, Novel delivery systems of polyphenols and their potential health benefits, Pharmaceuticals, 2021, 14(10), 946, DOI:10.3390/ph14100946.
- S. Gao, X. Yi, X. Gao, Z. Long, J. Guo, G. Xia and X. Shen, Stabilization of β-carotene liposomes with chitosan–lactoferrin coating system: vesicle properties and anti-inflammatory in vitro studies, Foods, 2025, 14(6), 968, DOI:10.3390/foods14060968.
- R. Becerril, C. Nerín and F. Silva, Encapsulation systems for antimicrobial food packaging components: an update, Molecules, 2020, 25(5), 1134, DOI:10.3390/molecules25051134.
- A. Maurya, V. K. Singh, S. Das, J. Prasad, A. Kedia, N. Upadhyay, N. K. Dubey and A. K. Dwivedy, Essential oil nanoemulsion as eco-friendly and safe preservative: bioefficacy against microbial food deterioration and toxin secretion, mode of action, and future opportunities, Front. Microbiol., 2021, 12, 751062, DOI:10.3389/fmicb.2021.751062.
- C. Qi, G. Liu, Y. Ping, K. Yang, Q. Tan, Y. Zhang, G. Chen, X. Huang and D. Xu, A comprehensive review of nano-delivery system for tea polyphenols: construction, applications, and challenges, Food Chem.:X, 2023, 17, 100571, DOI:10.1016/j.fochx.2023.100571.
- Z. Wang, W. Tang, Z. Sun, F. Liu and D. Wang, An innovative Pickering W/O/W nanoemulsion co-encapsulating hydrophilic lysozyme and hydrophobic perilla leaf oil for extending shelf life of fish products, Food Chem., 2024, 439, 138074, DOI:10.1016/j.foodchem.2023.138074.
- A. A. Mahdi, Q. A. Al-Maqtari, J. K. Mohammed, W. Al-Ansi, S. M. Aqeel, H. Cui and L. Lin, Nanoencapsulation of mandarin essential oil: fabrication, characterization, and storage stability, Foods, 2022, 11(1), 54, DOI:10.3390/foods11010054.
- Q. A. Al-Maqtari, J. K. Mohammed, A. A. Mahdi, W. Al-Ansi, M. Zhang, A. Al-Adeeb, M. Wei, H. M. Phyo and W. Yao, Physicochemical properties, microstructure, and storage stability of Pulicaria jaubertii extract microencapsulated with different protein biopolymers and gum arabic as wall materials, Int. J. Biol. Macromol., 2021, 187, 939–954, DOI:10.1016/j.ijbiomac.2021.07.180.
- R. M. González-Reza, H. Hernández-Sánchez, D. Quintanar-Guerrero, L. Alamilla-Beltrán, Y. Cruz-Narváez and M. L. Zambrano-Zaragoza, Synthesis, controlled release, and stability on storage of chitosan-thyme essential oil nanocapsules for food applications, Gels, 2021, 7(4), 212, DOI:10.3390/gels7040212.
- A. A. Elkordy, R. T. Forbes and B. W. Barry, Stability of crystallised and spray-dried lysozyme, Int. J. Pharm., 2004, 278(2), 209–219, DOI:10.1016/j.ijpharm.2004.02.027.
- Y. Hu, S. Luo, Y. Jiang, J. Lin, B. Xu, Z. H. Zhang, B. Adhikari, T. Xu and B. Wang, Stability and functionality of bovine lactoferrin powder after 9 years of storage, Curr. Res. Food Sci., 2025, 10, 101036, DOI:10.1016/j.crfs.2025.101036.
- A. Papadochristopoulos, J. P. Kerry, N. Fegan, C. M. Burgess and G. Duffy, Natural anti-microbials for enhanced microbial safety and shelf-life of processed packaged meat, Foods, 2021, 10(7), 1598, DOI:10.3390/foods10071598.
- S. Chen, M. Wu, P. Lu, L. Gao, S. Yan and S. Wang, Development of pH indicator and antimicrobial cellulose nanofibre packaging film based on purple sweet potato anthocyanin and oregano essential oil, Int. J. Biol. Macromol., 2020, 149, 271–280, DOI:10.1016/j.ijbiomac.2020.01.231.
- V. M. Rangaraj, K. Rambabu, F. Banat and V. Mittal, Natural antioxidants-based edible active food packaging: an overview of current advancements, Food Biosci., 2021, 43, 101251 CrossRef.
- P. Umaraw and A. K. Verma, Comprehensive review on application of edible film on meat and meat products: an eco-friendly approach, Crit. Rev. Food Sci. Nutr., 2017, 57(6), 1270–1279 CrossRef PubMed.
- Q. Xie, G. Liu, Y. Zhang, J. Yu, Y. Wang and X. Ma, Active edible films with plant extracts: an updated review of their types, preparations, reinforcing properties, and applications in muscle foods packaging and preservation, Crit. Rev. Food Sci. Nutr., 2023, 63(32), 11425–11447, DOI:10.1080/10408398.2022.2092058.
- L. A. Vasconcelos, L. C. B. Reis, Ê. R. Dias, G. P. Camilloto and A. Branco, Characterization of a flavonol-rich antioxidant fraction from Spondias purpurea L. pulp and the effect of its incorporation on cellulose acetate-based film, J. Sci. Food Agric., 2021, 101(8), 3270–3279 CrossRef PubMed.
- H. Yong, X. Wang, R. Bai, Z. Miao, X. Zhang and J. Liu, Development of antioxidant and intelligent pH-sensing packaging films by incorporating purple-fleshed sweet potato extract into chitosan matrix, Food Hydrocoll., 2019, 90, 216–224 CrossRef.
- S. Zhang, Z. He, F. Xu, Y. Cheng, G. I. N. Waterhouse, D. Sun-Waterhouse and P. Wu, Enhancing the performance of konjac glucomannan films through incorporating zein–pectin nanoparticle-stabilized oregano essential oil Pickering emulsions, Food Hydrocoll., 2022, 124, 107222, DOI:10.1016/j.foodhyd.2021.107222.
- V. Kola and I. S. Carvalho, Plant extracts as additives in biodegradable films and coatings in active food packaging, Food Biosci., 2023, 54, 102860 CrossRef.
- J. Ma, G. Ye, S. Jia, H. Ma, D. Jia, J. He, J. Lv, X. Chen, F. Liu, K. Gou and R. Zeng, Preparation of chitosan/peony (Paeonia suffruticosa Andr.) leaf extract composite film and its application in sustainable active food packaging, Int. J. Biol. Macromol., 2022, 222, 2200–2211, DOI:10.1016/j.ijbiomac.2022.10.012.
- M. Hasan, R. Rusman, I. Khaldun, L. Ardana, M. Mudatsir and H. Fansuri, Active edible sugar palm starch-chitosan films carrying extra virgin olive oil: barrier, thermo-mechanical, antioxidant, and antimicrobial properties, Int. J. Biol. Macromol., 2020, 163, 766–775 CrossRef PubMed.
- R. Thakur, H. Singhi, V. R. Suryavanshi, R. Santhosh, S. Roy, K. Gul and P. Sarkar, pH-sensitive intelligent packaging films from kodo millet (Paspalum scrobiculatum) starch and gum tragacanth incorporated with beetroot peel extract for monitoring shrimp freshness, Food Packag. Shelf Life, 2024, 46, 101405, DOI:10.1016/j.fpsl.2024.101405.
- T. Li, J. Zhang, A. Hu, F. Guo, H. Zhou and Q. Wang, Effect of transglutaminase and laccase on pea protein gel properties compared to that of soybean, Food Hydrocoll., 2024, 156, 110314 CrossRef CAS.
- G. G. de Lima, I. C. B. Zakaluk, M. A. Artner, A. C. Pedro, P. H. Gonzalez de Cademartori, G. I. B. D. Muniz and W. L. E. Magalhães, Enhancing barrier and antioxidant properties of nanocellulose films for coatings and active packaging: a review, ACS Appl. Nano Mater., 2025, 8(9), 4397–4421 CrossRef CAS.
- G. F. Nogueira, R. A. Oliveira, J. I. Velasco and F. M. Fakhouri, Methods of incorporating plant-derived bioactive compounds into films made with agro-based polymers for application as food packaging: a brief review, Polymers, 2020, 12(11), 2518, DOI:10.3390/polym12112518.
- N. Benbettaïeb, F. Debeaufort and T. Karbowiak, Bioactive edible films for food applications: mechanisms of antimicrobial and antioxidant activity, Crit. Rev. Food Sci. Nutr., 2019, 59(21), 3431–3455, DOI:10.1080/10408398.2018.1494132.
- A. M. Kusova, A. E. Sitnitsky and Y. F. Zuev, The role of pH and ionic strength in the attraction–repulsion balance of fibrinogen interactions, Langmuir, 2021, 37(34), 10394–10401 CrossRef CAS PubMed.
- T. G. Abedelmaksoud, M. I. Younis, A. B. Altemimi, R. H. Tlay and N. A. Hassan, Bioactive compounds of plant-based food: extraction, isolation, identification, characteristics, and emerging applications, Food Sci. Nutr., 2025, 13(6), e70351 CrossRef CAS PubMed.
- P. C. Tsaousis, M. Sarafidou, A. Soto Beobide, G. N. Mathioudakis, K. Filippi, D. Bartzialis, K. S. Andrikopoulos, K. D. Giannoulis, N. G. Danalatos, A. A. Koutinas and G. A. Voyiatzis, Quantification of plant biomass composition via a single FTIR absorption spectrum supported by reference component extraction/isolation protocols, Biomass Convers. Biorefin., 2025, 15(18), 25273–25288, DOI:10.1007/s13399-025-06858-1.
- N. P. Sunesh, I. Suyambulingam, D. Divakaran, H. Pulikkalparambil, M. R. Sanjay and S. Siengchin, Pedalium murex plant-based bioplasticizer reinforced polylactic acid films: a promising approach for biodegradable fruit packaging applications, Int. J. Biol. Macromol., 2024, 270, 132392, DOI:10.1016/j.ijbiomac.2024.132392.
- S. Shivangi, D. Dorairaj, P. S. Negi and N. P. Shetty, Development and characterisation of a pectin-based edible film that contains mulberry leaf extract and its bio-active components, Food Hydrocoll., 2021, 121, 107046, DOI:10.1016/j.foodhyd.2021.107046.
- X. Tian, Y. Li, Z. Xu, X. Feng, Q. Kong and X. Ren, Efficient binding paradigm of protein and polysaccharide: preparation of isolated soy protein-chitosan quaternary ammonium salt complex system and exploration of its emulsification potential, Food Chem., 2023, 407, 135111, DOI:10.1016/j.foodchem.2022.135111.
- F. Rol, M. Billot, M. Bolloli, D. Beneventi and J. Bras, Production of 100% cellulose nanofibril objects using the molded cellulose process: a feasibility study, Ind. Eng. Chem. Res., 2020, 59(16), 7670–7679 CrossRef.
- Y. Wang, Q. Wang, S. Liu, X. Ji, G. Yang and J. Chen, Lipase induced highly hydrophobic nanofibrillated cellulose film for strain sensor application, Carbohydr. Polym., 2022, 284, 119193 CrossRef PubMed.
- T. Periyasamy, S. P. Asrafali and J. Lee, Recent advances in functional biopolymer films with antimicrobial and antioxidant properties for enhanced food packaging, Polymers, 2025, 17(9), 1257, DOI:10.3390/polym17091257.
- R. Akhter, F. A. Masoodi, T. A. Wani and S. A. Rather, Functional characterization of biopolymer based composite film: incorporation of natural essential oils and antimicrobial agents, Int. J. Biol. Macromol., 2019, 137, 1245–1255, DOI:10.1016/j.ijbiomac.2019.06.214.
- P. Farshi, S. N. Mirmohammadali, B. Rajpurohit, J. S. Smith and Y. Li, Pea protein and starch: functional properties and applications in edible films, J. Agric. Food Res., 2024, 15, 100927, DOI:10.1016/j.jafr.2023.100927.
- T. Nisar, Z. C. Wang, X. Yang, Y. Tian, M. Iqbal and Y. Guo, Characterization of citrus pectin films integrated with clove bud essential oil: physical, thermal, barrier, antioxidant and antibacterial properties, Int. J. Biol. Macromol., 2018, 106, 670–680 CrossRef.
- K. K. N. C. Perazzo, A. C. V. Conceição, J. C. P. Santos, D. J. Assis, C. O. Souza and J. I. Druzian, Properties and antioxidant action of cassava starch films incorporated with green tea and palm oil extracts, PLoS One, 2014, 9(9), e105199 CrossRef.
- W. Lou, Z. Huang, Q. Shao, Y. Shan, D. Shi, Z. Chen, J. Zhang, W. Yu, J. Wang, H. Yang and M. Cai, Recent advances in active packaging: insights into novel functional elements, response strategies and applications for food preservation, Food Packag. Shelf Life, 2025, 49, 101489, DOI:10.1016/j.fpsl.2025.101489.
- S. G. G. Aziz and H. Almasi, Physical characteristics, release properties, and antioxidant and antimicrobial activities of whey protein isolate films incorporated with thyme (Thymus vulgaris L.) extract-loaded nanoliposomes, Food Bioprocess Technol., 2018, 11(8), 1552–1565, DOI:10.1007/s11947-018-2121-6.
- M. Sabaghi, S. Tavasoli, S. Z. Hoseyni, M. R. Mozafari, P. Degraeve and I. Katouzian, A critical review on approaches to regulate the release rate of bioactive compounds from biopolymeric matrices, Food Chem., 2022, 382, 132411, DOI:10.1016/j.foodchem.2022.132411.
- S. Boostani and S. M. Jafari, A comprehensive review on the controlled release of encapsulated food ingredients; fundamental concepts to design and applications, Trends Food Sci. Technol., 2021, 109, 303–321 CrossRef.
- N. Malekjani and S. M. Jafari, Modeling the release of food bioactive ingredients from carriers/nanocarriers by the empirical, semiempirical, and mechanistic models, Compr. Rev. Food Sci. Food Saf., 2021, 20(1), 3–47 CrossRef PubMed.
- H. Rajabi, S. M. Jafari, G. Rajabzadeh, M. Sarfarazi and S. Sedaghati, Chitosan-gum Arabic complex nanocarriers for encapsulation of saffron bioactive components, Colloids Surf., A, 2019, 578, 123644, DOI:10.1016/j.colsurfa.2019.123644.
- V. G. L. Souza, P. F. Rodrigues, M. P. Duarte and A. L. Fernando, Antioxidant migration studies in chitosan films incorporated with plant extracts, J. Renewable Mater., 2018, 6(5), 548–558 Search PubMed.
- H. Lian, J. Shi, X. Zhang and Y. Peng, Effect of the added polysaccharide on the release of thyme essential oil and structure properties of chitosan based film, Food Packag. Shelf Life, 2020, 23, 100467, DOI:10.1016/j.fpsl.2020.100467.
- S. Potrč, L. Fras Zemljič, M. Sterniša, S. Smole Možina and O. Plohl, Development of biodegradable whey-based laminate functionalised by chitosan–natural extract formulations, Int. J. Mol. Sci., 2020, 21(10), 3668, DOI:10.3390/ijms21103668.
- A. Anis, K. Pal and S. M. Al-Zahrani, Essential oil-containing polysaccharide-based edible films and coatings for food security applications, Polymers, 2021, 13(4), 575, DOI:10.3390/polym13040575.
- G. S. Dannenberg, G. D. Funck, C. E. S. Cruxen, J. L. Marques, W. P. Silva and Â. M. Fiorentini, Essential oil from pink pepper as an antimicrobial component in cellulose acetate film: potential for application as active packaging for sliced cheese, LWT–Food Sci. Technol., 2017, 81, 314–318, DOI:10.1016/j.lwt.2017.04.002.
- B. Tian, J. Liu, W. Yang and J. B. Wan, Biopolymer food packaging films incorporated with essential oils, J. Agric. Food Chem., 2023, 71(3), 1325–1347 CrossRef PubMed.
- H. Chen, J. Wang, G. Jiang, H. Guo, X. Liu and L. Wang, Active packaging films based on chitosan and Herba lophatheri extract for the shelf life extension of fried bighead carp fillets, Int. Food Res. J., 2020, 27(4), 720–726 Search PubMed.
- I. S. Bayer, Controlled drug release from nanoengineered polysaccharides, Pharmaceutics, 2023, 15(5), 1364 CrossRef.
- M. S. Brewer, Natural antioxidants: sources, compounds, mechanisms of action, and potential applications, Compr. Rev. Food Sci. Food Saf., 2011, 10(4), 221–247 CrossRef CAS.
- T. Hou, S. Ma, F. Wang and L. Wang, A comprehensive review of intelligent controlled release antimicrobial packaging in food preservation, Food Sci. Biotechnol., 2023, 32(11), 1459–1478, DOI:10.1007/s10068-023-01344-8.
- T. Higuchi, Rate of release of medicaments from ointment bases containing drugs in suspension, J. Pharm. Sci., 1961, 50(10), 874–875, DOI:10.1002/jps.2600501018.
- A. W. Hixson and J. H. Crowell, Dependence of reaction velocity upon surface and agitation, Ind. Eng. Chem., 1931, 23(8), 923–931 CrossRef CAS.
- R. W. Korsmeyer, R. Gurny, E. Doelker, P. Buri and N. A. Peppas, Mechanisms of solute release from porous hydrophilic polymers, Int. J. Pharm., 1983, 15(1), 25–35, DOI:10.1016/0378-5173(83)90064-9.
- P. L. Ritger and N. A. Peppas, A simple equation for description of solute release I. Fickian and non-fickian release from non-swellable devices in the form of slabs, spheres, cylinders or discs, J. Contr. Release, 1987, 5(1), 23–36, DOI:10.1016/0168-3659(87)90034-4.
- R. W. Baker and H. K. Lonsdale, Controlled release: mechanisms and rates, Adv. Exp. Med. Biol., 1974, 15, 15–71 CrossRef.
- W. Weibull, A statistical distribution function of wide applicability, J. Appl. Mech., 1951, 18, 293–297 CrossRef.
- N. A. Peppas and J. J. Sahlin, A simple equation for the description of solute release. III. Coupling of diffusion and relaxation, Int. J. Pharm., 1989, 57(2), 169–172, DOI:10.1016/0378-5173(89)90306-2.
- I. Katzhendler, A. Hoffman, A. Goldberger and M. Friedman, Modeling of drug release from erodible tablets, J. Pharm. Sci., 1997, 86(1), 110–115, DOI:10.1021/js9600538.
- M. L. Bruschi, Mathematical models of drug release, in Strategies to Modify the Drug Release from Pharmaceutical Systems, ed. Bruschi M. L., Woodhead Publishing, Cambridge, 2015, pp. 63–86, DOI:10.1016/B978-0-08-100092-2.00005-9.
- J. D. Wicochea-Rodríguez, P. Chalier, T. Ruiz and E. Gastaldi, Active food packaging based on biopolymers and aroma compounds: how to design and control the release, Front. Chem., 2019, 7, 398 CrossRef.
- J. Liu, S. Liu, Q. Wu, Y. Gu, J. Kan and C. Jin, Effect of protocatechuic acid incorporation on the physical, mechanical, structural and antioxidant properties of chitosan film, Food Hydrocoll., 2017, 73, 90–100, DOI:10.1016/j.foodhyd.2017.06.035.
- C. A. Reis, A. Gomes and P. J. do Amaral Sobral, Films based on biopolymers incorporated with active compounds encapsulated in emulsions: properties and potential applications—a review, Foods, 2023, 12(19), 3602, DOI:10.3390/foods12193602.
- N. Szczepańska, B. Kudłak and J. Namieśnik, Recent advances in assessing xenobiotics migrating from packaging material—a review, Anal. Chim. Acta, 2018, 1023, 1–21 CrossRef.
- S. H. Yakobi, S. Mkhize and O. J. Pooe, Screening of antimicrobial properties and bioactive compounds of Pleurotus ostreatus extracts against Staphylococcus aureus, Escherichia coli, and Neisseria gonorrhoeae, Biochem. Res. Int., 2023, 2023(1), 1777039 Search PubMed.
- R. K. Rout, A. Kumar and D. P. S. Rao, Encapsulation of oregano (Origanum vulgare) leaf polyphenols: development, characterization and in-vitro release study, Food Hydrocolloids Health, 2021, 1, 100028, DOI:10.1016/j.fhfh.2021.100028.
- L. B. Avila, C. Schnorr, L. F. Silva, M. M. Morais, C. C. Moraes, G. S. da Rosa and M. Naushad, Trends in bioactive multilayer films: perspectives in the use of polysaccharides, proteins, and carbohydrates with natural additives for application in food packaging, Foods, 2023, 12(8), 1692 CrossRef CAS.
- S. Elshamy, K. Khadizatul, K. Uemura, M. Nakajima and M. A. Neves, Chitosan-based film incorporated with essential oil nanoemulsion foreseeing enhanced antimicrobial effect, J. Food Sci. Technol., 2021, 58(9), 3314–3327 CrossRef CAS PubMed.
- M. Ş. Karakuş, Structural properties, mechanical behavior, and food protecting ability of chickpea protein-derived biopolymer films, Polymers, 2025, 17(14), 1938 CrossRef.
- S. Mangaraj, A. Yadav, L. M. Bal, S. K. Dash and N. K. Mahanti, Application of biodegradable polymers in food packaging industry: a comprehensive review, J. Packag. Technol. Res., 2019, 3(1), 77–96 CrossRef.
- Z. Eslami, S. Elkoun, M. Mirzapour and M. Robert, Development of a continuous extrusion process for alginate biopolymer films for sustainable applications, Polymers, 2025, 17(13), 1818 CrossRef CAS PubMed.
- M. L. Dias, B. A. Ribeiro, A. L. Toledo and V. J. Pita, Enhanced thermal, mechanical, and barrier properties of packaging grade linear low-density polyethylene (LLDPE) nanocomposites with commercial graphene nanoplatelets, J. Appl. Polym. Sci., 2025, 142(44), e57689 CrossRef CAS.
- H. Patil, R. V. Tiwari and M. A. Repka, Hot-melt extrusion: from theory to application in pharmaceutical formulation, AAPS PharmSciTech, 2016, 17(1), 20–42 CrossRef CAS.
- D. Li, Y. Yang, A. L. Elias, N. Yan and F. Guo, Biopolymer composites material extrusion and their applications: a review, Adv. Eng. Mater., 2023, 25(21), 2301048 CrossRef CAS.
- M. Reuther, N. Rollet, F. Debeaufort and O. Chambin, Orodispersible films prepared by hot-melt extrusion versus solvent casting, Int. J. Pharm., 2025, 675, 125536 CrossRef CAS.
- Q. Wang, W. Chen, W. Zhu, D. J. McClements, X. Liu and F. Liu, A review of multilayer and composite films and coatings for active biodegradable packaging, npj Sci. Food, 2022, 6(1), 18 CrossRef.
- M. Zhang, A. Ahmed and L. Xu, Electrospun nanofibers for functional food packaging application, Materials, 2023, 16(17), 5937 CrossRef CAS.
- E. Alp-Erbay, A. F. Yeşilsu and M. Türe, Fish gelatin antimicrobial electrospun nanofibers for active food-packaging applications, J. Nano Res., 2019, 56, 80–97 CAS.
- A. M. Mohammadi, S. M. Hosseini and M. Yousefi, Application of electrospinning technique in development of intelligent food packaging: a short review of recent trends, Food Sci. Nutr., 2020, 8, 4656–4665 CrossRef.
- E. Poverenov, S. Danino, B. Horev, R. Granit, Y. Vinokur and V. Rodov, Layer-by-layer electrostatic deposition of edible coating on fresh-cut melon model: anticipated and unexpected effects of alginate–chitosan combination, Food Bioprocess Technol., 2014, 7, 1424–1432 CrossRef CAS.
- I. M. Brasil, C. Gomes, A. Puerta-Gomez, M. E. Castell-Perez and R. G. Moreira, Polysaccharide-based multilayered antimicrobial edible coating enhances quality of fresh-cut papaya, LWT–Food Sci. Technol., 2012, 47, 39–45 CrossRef CAS.
- H. Arnon and E. Poverenov, Improving food products' quality and storability by using layer-by-layer edible coatings, Trends Food Sci. Technol., 2018, 75, 81–92 CrossRef.
- K. T. Chaudhary, Thin film deposition: solution based approach, in Thin Films, IntechOpen, London, 2021 Search PubMed.
- R. Suhag, N. Kumar, A. T. Petkoska and A. Upadhyay, Film formation and deposition methods of edible coating on food products: a review, Food Res. Int., 2020, 136, 109582 CrossRef CAS PubMed.
- L. P. Wigati, A. A. Wardana, F. N. Nkede, M. Fanze, M. H. Wardak, M. A. Reshaka Kavindi and F. Tanaka, Influence of cellulose nanocrystal formulations on the properties of pregelatinized cornstarch and cornmint essential oil films, Sci. Rep., 2025, 15(1), 18997 CrossRef CAS PubMed.
- L. Atieno, W. Owino, E. M. Ateka and J. Ambuko, Influence of coating application methods on the postharvest quality of cassava, Int. J. Food Sci., 2019, 2019(1), 2148914 Search PubMed.
- K. Priya, N. Thirunavookarasu and D. V. Chidanand, J. Agric. Food Res., 2023, 12, 100623 CAS.
- L. Doveri, Y. A. Diaz Fernandez, G. Dacarro, P. Grisoli, C. Milanese, M. Urena and P. Pallavicini, Active pectin/carboxymethylcellulose composite films for bread packaging, Molecules, 2025, 30(11), 2257 CrossRef CAS PubMed.
- R. D. Andrade, O. Skurtys and F. A. Osorio, Atomizing spray systems for application of edible coatings, Compr. Rev. Food Sci. Food Saf., 2012, 11(3), 323–337 CrossRef CAS.
- C. Ribeiro, A. A. Vicente, J. A. Teixeira and C. Miranda, Optimization of edible coating composition to retard strawberry fruit senescence, Postharvest Biol. Technol., 2007, 44(1), 63–70 CrossRef CAS.
- B. Saberi, J. B. Golding, J. R. Marques, P. Pristijono, S. Chockchaisawasdee, C. J. Scarlett and C. E. Stathopoulos, Application of biocomposite edible coatings based on pea starch and guar gum on quality, storability and shelf life of Valencia oranges, Postharvest Biol. Technol., 2018, 137, 9–20 CrossRef CAS.
- J. Mahmud, E. Sarmast, S. Shankar and M. Lacroix, Advantages of nanotechnology developments in active food packaging, Food Res. Int., 2022, 154, 111023 CrossRef CAS PubMed.
- M. Carbone, D. T. Donia, G. Sabbatella and R. Antiochia, Silver nanoparticles in polymeric matrices for fresh food packaging, J. King Saud Univ. Sci., 2016, 28(4), 273–279 CrossRef.
- W. Wang, X. Liu, F. Guo, Y. Yu, J. Lu, Y. Li and G. Yu, Biodegradable cellulose/curcumin films with Janus structure for food packaging and freshness monitoring, Carbohydr. Polym., 2024, 324, 121516 CrossRef CAS PubMed.
- D. Ayazi, M. Zandi, A. Ganjloo and N. Dardmeh, Electrospun zein nanofiber-reinforced multilayer biodegradable films with cumin essential oil, J. Food Meas. Charact., 2025, 19(10), 7339–7353 CrossRef.
- M. Yadav, K. Behera, Y. H. Chang and F. C. Chiu, Cellulose nanocrystal reinforced chitosan based UV barrier composite films for sustainable packaging, Polymers, 2020, 12(1), 202 CrossRef.
- L. Al-Naamani, J. Dutta and S. Dobretsov, Nanocomposite zinc oxide-chitosan coatings on polyethylene films for extending storage life of okra (Abelmoschus esculentus), Nanomaterials, 2018, 8(7), 479 CrossRef PubMed.
- F. K. Alsammarraie, W. Wang, P. Zhou, A. Mustapha and M. Lin, Green synthesis of silver nanoparticles using turmeric extracts and investigation of their antibacterial activities, Colloids Surf., B, 2018, 171, 398–405 CrossRef PubMed.
- A. Siddique and Y. W. Park, Effect of iron fortification on microstructural, textural, and sensory characteristics of caprine milk Cheddar cheeses under different storage treatments, J. Dairy Sci., 2019, 102(4), 2890–2902 CrossRef PubMed.
- S. Neethirajan, V. Ragavan, X. Weng and R. Chand, Biosensors for sustainable food engineering: challenges and perspectives, Biosensors, 2018, 8(1), 23 CrossRef PubMed.
- L. Du, X. Huang, Z. Li, Z. Qin, N. Zhang, X. Zhai and Y. Wang, Application of smart packaging in fruit and vegetable preservation: a review, Foods, 2025, 14(3), 447 CrossRef PubMed.
- S. S. Rajan and K. M. Wani, A review of smart food and packaging technologies: revolutionizing nutrition and sustainability, Food Human., 2025, 4, 100593 CrossRef.
- S. Choubaki, H. Baghaei and A. Mohammadi Nafchi, Smart indicator for fish freshness monitoring: a chitosan/gelatin/zinc oxide nanoparticle composite incorporating pomegranate flower anthocyanin extract nanocapsules, Food Sci. Nutr., 2025, 13(9), e70944 CrossRef PubMed.
- S. Shiryanpour, L. Nouri, M. Azizi, A. Najafi and A. M. Nafchi, Active chitosan film containing single and double nanoemulsions of Oliveira decumbens Vent essential oil/anthocyanin of eggplant for chicken preservation, J. Food Meas. Charact., 2025, 19(10), 7381–7403 CrossRef.
- I. Ahmed, H. Lin, L. Zou, Z. Li, A. L. Brody, I. M. Qazi and L. Sun, An overview of smart packaging technologies for monitoring safety and quality of meat and meat products, Packag. Technol. Sci., 2018, 31(7), 449–471 CrossRef.
- C. Vasile and M. Baican, Progresses in food packaging, food quality, and safety—controlled-release antioxidant and/or antimicrobial packaging, Molecules, 2021, 26(5), 1263 CrossRef.
- N. Yaqoob, A. Zahira, S. Kamal, M. Almas and S. Rehman, Development of multifunctional bioactive food packaging based on silver nanoparticles/grapefruit peel extract reinforced PVA composites, Mater. Today Commun., 2023, 37, 107529 CrossRef.
- E. Smiechowicz, B. Niekraszewicz, M. Strzelinska and M. Zielecka, Antibacterial fibers containing nanosilica with immobilized silver nanoparticles, Autex Res. J., 2020, 20(4), 441–448 CrossRef.
- G. Subramani and R. Manian, Bioactive chitosan films: integrating antibacterial, antioxidant, and antifungal properties in food packaging, Int. J. Biol. Macromol., 2024, 278, 134596 CrossRef PubMed.
- Y. Liu, S. Ahmed, D. E. Sameen, Y. Wang, R. Lu, J. Dai, S. Li and W. Qin, A review of cellulose and its derivatives in biopolymer-based food packaging application, Trends Food Sci. Technol., 2021, 112, 532–546, DOI:10.1016/j.tifs.2021.04.016.
- D. Maresca and G. Mauriello, Development of antimicrobial cellulose nanofiber-based films activated with nisin for food packaging applications, Foods, 2022, 11(19), 3051 CrossRef PubMed.
- R. H. B. Setiarto, L. Anshory and A. A. Wardana, Biosynthesis of nisin, antimicrobial mechanism and its applications as a food preservation: a review, IOP Conf. Ser. Earth Environ. Sci., 2023, 1169(1), 012105 CrossRef.
- J. Guo, S. Rawdkuen, W. Zhang, P. Kingwascharapong and G. Xia, Research progress of biopolymer-based food packaging films/coatings functionalized with edible photosensitizers, Trends Food Sci. Technol., 2025, 105078 CrossRef.
- Y. Zhang, Y. Huang, Y. Chen and Z. Qin, Multifunctional and pH-responsive dialdehyde cellulose/polyvinyl alcohol composite film reinforced with curcumin, J. Appl. Polym. Sci., 2024, 141(9), e55035 CrossRef.
- Y. Lv, P. Li, L. Cen, F. Wen, R. Su, J. Cai and W. Su, Gelatin/carboxymethylcellulose composite film combined with photodynamic antibacterial: new prospect for fruit preservation, Int. J. Biol. Macromol., 2024, 257, 128643 CrossRef PubMed.
- X. Yang, M. Zhang, B. Zhao, X. Zou and R. Song, Development, characteristics and biological activities of bilayer composite film based on gelatin/κ-carrageenan loading curcumin and ε-polylysine, Food Hydrocoll., 2024, 150, 109646 Search PubMed.
- T. Ma, Y. Chen, X. Zhi and B. Du, Cellulose laurate films containing curcumin as photoinduced antibacterial agent for meat preservation, Int. J. Biol. Macromol., 2021, 193, 1986–1995 CrossRef PubMed.
- Q. Zhang, Y. Li and F. Huang, Gold nanorod-chitosan photothermal films: rapid Salmonella inactivation via localized thermal shock, ACS Appl. Mater. Interfaces, 2023, 15(18), 22345–22357 Search PubMed.
- J. Wang, S. Zhou, F. Lu, S. Wang and Q. Deng, Polyphenols functionalized MOF encapsulated BPQDs for synergistic photothermal/photodynamic antibacterial properties and multifunctional food preservation, Food Chem., 2024, 451, 139451 CrossRef PubMed.
- M. S. Firouz, K. Mohi-Alden and M. Omid, A critical review on intelligent and active packaging in the food industry: research and development, Food Res. Int., 2021, 141, 110113 Search PubMed.
- J. R. Westlake, M. W. Tran, Y. Jiang, X. Zhang, A. D. Burrows and M. Xie, Biodegradable biopolymers for active packaging: demand, development and directions, Sustainable Food Technol., 2023, 1(1), 50–72 RSC.
- B. McAdam, M. Brennan Fournet, P. McDonald and M. Mojicevic, Production of polyhydroxybutyrate (PHB) and factors impacting its chemical and mechanical characteristics, Polymers, 2020, 12(12), 2908 CrossRef PubMed.
- Z. Miao, Y. Zhang and P. Lu, Novel active starch films incorporating tea polyphenols-loaded porous starch as food packaging materials, Int. J. Biol. Macromol., 2021, 192, 1123–1133 Search PubMed.
- M. S. A. Aziz and H. E. Salama, Developing multifunctional edible coatings based on alginate for active food packaging, Int. J. Biol. Macromol., 2021, 190, 837–844 CrossRef PubMed.
- A. M. Youssef, H. S. El-Sayed, E. N. Islam and S. M. El-Sayed, Preparation and characterization of novel bionanocomposites based on garlic extract for preserving fresh Nile tilapia fish fillets, RSC Adv., 2021, 11(37), 22571–22584 RSC.
- Y. Palanisamy, V. Kadirvel and N. D. Ganesan, Recent technological advances in food packaging: sensors, automation, and application, Sustainable Food Technol., 2025, 3, 161–180 RSC.
- X. Zhang, S. Lu and X. Chen, A visual pH sensing film using natural dyes from Bauhinia blakeana Dunn, Sens. Actuators, B, 2014, 198, 268–273 CrossRef.
- P. Shao, L. Liu, J. Yu, Y. Lin, H. Gao, H. Chen and P. Sun, An overview of intelligent freshness indicator packaging for food quality and safety monitoring, Trends Food Sci. Technol., 2021, 118, 285–296 CrossRef.
- P. Ezati and J. W. Rhim, pH-responsive chitosan-based film incorporated with alizarin for intelligent packaging applications, Food Hydrocoll., 2020, 102, 105629 Search PubMed.
- X. Yao, H. Hu, Y. Qin and J. Liu, Development of antioxidant, antimicrobial and ammonia-sensitive films based on quaternary ammonium chitosan, polyvinyl alcohol and betalains-rich cactus pears (Opuntia ficus-indica) extract, Food Hydrocoll., 2020, 106, 105896 CrossRef.
- Y. Wang, C. Yuan, Y. Liu and B. Cui, Fabrication of kappa–carrageenan hydrogels with cinnamon essential oil/hydroxypropyl–β–cyclodextrin composite: evaluation of physicochemical properties, release kinetics and antimicrobial activity, Int. J. Biol. Macromol., 2021, 170, 593–601 CrossRef.
- T. Min, L. Zhou, X. Sun, H. Du, X. Bian, Z. Zhu and Y. Wen, Enzyme-responsive food packaging system based on pectin-coated poly(lactic acid) nanofiber films for controlled release of thymol, Food Res. Int., 2022, 157, 111256 CrossRef.
- L. W. Wong, C. Y. Hou, C. C. Hsieh, C. K. Chang, Y. S. Wu and C. W. Hsieh, Preparation of antimicrobial active packaging film by capacitively coupled plasma treatment, LWT–Food Sci. Technol., 2020, 117, 108612 CrossRef.
- F. Wei, C. Shi, H. Wang, W. Li, X. Zhang and X. Zhang, Zein-magnolol complexes assisted hydrogel film: physical, antioxidant, antimicrobial properties and application on food packaging, Food Res. Int., 2025, 116672, DOI:10.1016/j.foodres.2025.116672.
- Y. Li, H. Du, X. Liu, L. Liang, X. Yun, Z. Wang and Z. Zhu, A novel bio-based aerogel for fragile fruit packaging with cushioning and antimicrobial properties, Food Hydrocoll., 2025, 167, 111438, DOI:10.1016/j.foodhyd.2025.111438.
- X. Han, J. Chen, Q. Wang, J. Zhang, J. Mi, J. Feng and W. Zhang, Photodynamically activated chlorogenic acid-based antimicrobial packaging films for cherry preservation, Food Chem., 2025, 479, 143857, DOI:10.1016/j.foodchem.2025.143857.
- P. A. V. de Freitas, S. Meyer, E. Hernández-García, D. Rebaque, F. Vilaplana and A. Chiralt, Antioxidant and antimicrobial extracts from grape stalks obtained with subcritical water: potential use in active food packaging development, Food Chem., 2024, 451, 139526, DOI:10.1016/j.foodchem.2024.139526.
- S. Ahn, U. Park, H. Kim, C. Ban and S. Lim, Controlled release system inducing change in stability of volatile essential oil emulsion incorporated in a hydrogel using a responsive polymer for practical active food packaging, Food Hydrocoll., 2025, 112015, DOI:10.1016/j.foodhyd.2025.112015.
- X. Xu, W. Zhang, S. Jia, Y. Chen, X. Zhou and Y. Ding, Antimicrobial packaging films based on MOF nanozymes engineered via biomineralization and bimetallic hybridization for fruit preservation, Food Chem., 2025, 484, 144324, DOI:10.1016/j.foodchem.2025.144324.
- X. Zhu, P. Yang, Y. Zeng, L. Chen, X. Guo and T. Kang, An antimicrobial packaging film based on a copolymer of lipoic acid-quaternary ammonium salt derivative and chitosan for its utilization in the preservation of grapes, Food Res. Int., 2025, 117162, DOI:10.1016/j.foodres.2025.117162.
- H. N. Ahmad, Y. Yong, N. Munawar, R. Li, Y. Wen and J. Zhu, Biomimetic antimicrobial packaging: Malus sylvestris polyphenols derived tripolymeric active films for extended shelf life and quality maintenance of beef, Food Chem., 2025, 490, 145033, DOI:10.1016/j.foodchem.2025.145033.
- D. Rupérez, C. Nerín and F. Silva, Controlling beef microbial spoilage with diacetyl-based active packaging sachet, Food Res. Int., 2025, 209, 116176, DOI:10.1016/j.foodres.2025.116176.
- L. Xu, R. Chen and T. Ren, Fabrication of gelatin-based photodynamic food packaging with dual-antimicrobial activity for enhanced pork preservation, Food Hydrocoll., 2025, 166, 111361, DOI:10.1016/j.foodhyd.2025.111361.
- G. Karabulut and G. Goksen, Development of pectin-gelatin biopolymer films with DES-based Chlorella vulgaris extracts as plasticizers for enhanced UV-blocking, antioxidant, and antimicrobial food packaging application, Food Packag. Shelf Life, 2025, 49, 101534, DOI:10.1016/j.fpsl.2025.101534.
- S. F. Hosseini, J. Ghaderi and M. C. Gómez-Guillén, Tailoring physico-mechanical and antimicrobial/antioxidant properties of biopolymeric films by cinnamaldehyde-loaded chitosan nanoparticles and their application in packaging of fresh rainbow trout fillets, Food Hydrocoll., 2022, 124, 107249, DOI:10.1016/j.foodhyd.2021.107249.
- S. Wang, Y. Ma, F. Wang, C. Lu, Y. Liu, S. Zhang and L. Wang, Development of cellulose-based self-healing hydrogel smart packaging for fish preservation and freshness indication, Carbohydr. Polym., 2025, 348, 122806, DOI:10.1016/j.carbpol.2024.122806.
- P. Shi, X. Wang, S. Ali, L. Y. Zeng, X. Xian, J. Huang and H. U. Javed, Development of eco-friendly chitosan films enhanced with bioactive citric acid-based deep eutectic agents for sustainable food packaging and fish preservation, Food Hydrocoll., 2025, 168, 111571, DOI:10.1016/j.foodhyd.2025.111571.
- Y. Sun, Y. Ma, Y. Hou, S. Jia, S. Cheng, W. Su and H. Wang, Preparation and characterization of a pH-responsive smart film based on soy lipophilic protein/hydroxypropyl methylcellulose for salmon freshness monitoring and packaging, Food Packag. Shelf Life, 2025, 47, 101436, DOI:10.1016/j.fpsl.2025.101436.
- Y. Zou, J. Ren, Y. Fan, Y. Cao, Y. Xie, X. Xiao and F. Liu, Enhanced hydrophobicity, antioxidant activity, and antimicrobial efficacy of edible starch-based films incorporating pullulan and geraniol for fresh salmon preservation, Food Packag. Shelf Life, 2025, 49, 101530, DOI:10.1016/j.fpsl.2025.101530.
- N. Doğan, C. Doğan, A. K. Eticha, M. Gungor and Y. Akgul, Centrifugally spun micro-nanofibers based on lemon peel oil/gelatin as novel edible active food packaging: fabrication, characterization, and application to prevent foodborne pathogens E. coli and S. aureus in cheese, Food Control, 2022, 139, 109081, DOI:10.1016/j.foodcont.2022.109081.
- R. Zhang, B. Wang, F. Zhang, K. Zheng and Y. Liu, Milk-derived antimicrobial peptides incorporated whey protein film as active coating to improve microbial stability of refrigerated soft cheese, Int. J. Food Microbiol., 2024, 419, 110751, DOI:10.1016/j.ijfoodmicro.2024.110751.
- A. H. Gadallah, R. S. Hafez, K. M. Fahim and L. I. Ahmed, Application of rosemary oil nano-emulsion as antimicrobial and antioxidant natural alternative in pasteurized cream and Karish cheese, Int. J. Food Microbiol., 2024, 422, 110823, DOI:10.1016/j.ijfoodmicro.2024.110823.
- A. Di Matteo, M. Stanzione, E. Orlo, C. Russo, M. Lavorgna, G. G. Buonocore and M. Isidori, Biodegradable films enriched with thymol and carvacrol with antioxidant and antibacterial properties to extend cheese shelf-life, Food Control, 2026, 179, 111541, DOI:10.1016/j.foodcont.2025.111541.
- T. Sripahco, S. Khruengsai and P. Pripdeevech, Biodegradable antifungal films from nanocellulose-gellan gum incorporated with Anethum graveolens essential oil for bread packaging, Int. J. Biol. Macromol., 2023, 243, 125244, DOI:10.1016/j.ijbiomac.2023.125244.
- M. Wrona, S. Manso, F. Silva, L. Cardoso, J. Salafranca, C. Nerín and M. Á. Caballero, New active packaging based on encapsulated carvacrol, with emphasis on its odour masking strategies, Food Packag. Shelf Life, 2023, 40, 101177, DOI:10.1016/j.fpsl.2023.101177.
- X. Dang, S. Han, Y. Du, Y. Fei, B. Guo and X. Wang, Engineered environment-friendly multifunctional food packaging with superior nonleachability, polymer miscibility and antimicrobial activity, Food Chem., 2025, 466, 142192, DOI:10.1016/j.foodchem.2024.142192.
- J. Ashraf, J. Zhang, T. Tufail, M. Awais, Y. Qi, Z. Ahmed and B. Xu, Deep eutectic solvent: a promising way to enhance the physicomechanical and antimicrobial properties of pullulan/carboxymethyl chitosan films impregnated with zein–nisin nanofillers for sustainable food packaging, Food Hydrocoll., 2025, 111866, DOI:10.1016/j.foodhyd.2025.111866.
- Z. Khoshdouni Farahani, P. Mahasti Shotorbani, A. Akhondzadeh Basti, H. Hamedi, B. Hassani and A. Mohammadi Nafchi, Assessing coating with clove extract on improving the shelf life of chicken fillet at refrigeration storage temperature, Food Sci. Nutr., 2025, 13(8), e70786 CrossRef CAS PubMed.
- D. Trak, A. Soyuçok, B. Kabak, E. Kendüzler and Y. Arslan, Citrus-derived silver nanoparticles for food safety: biodegradable packaging with antimicrobial and oxidative stability, Food Chem., 2025, 145113, DOI:10.1016/j.foodchem.2025.145113.
- F. M. Alshabrmi and E. A. Alatawi, Multifunctional pea protein-corn starch films with green tea polyphenols: enhanced antimicrobial activity in beef packaging, Food Chem., 2025, 146466, DOI:10.1016/j.foodchem.2025.146466.
- M. Chen, L. Li, W. Ji, W. Lu, X. Tang, S. Jiang and H. Wang, Zein/eugenol electrospun mats on gelatin/laponite substrate for developing bilayer functional food packaging, Food Res. Int., 2025, 117463, DOI:10.1016/j.foodres.2025.117463.
- S. Babaei, S. Z. Juneghani, M. Mojarrad, T. Roshanzamir, A. Gholamhosseini and A. M. Nafchi, Do phycocyanin-enriched gelatin/alginate coatings decrease bacterial spoilage and improve the physicochemical and sensory properties of Whiteleg shrimp during storage?, J. Food Meas. Charact., 2025, 1–11 Search PubMed.
- Y. J. Jeon, G. E. Myung and S. C. Min, In-package cold plasma treatment enhances the antimicrobial efficacy of malic acid-incorporated whey protein edible coating against Salmonella and Listeria monocytogenes in steamed fish paste, Food Packag. Shelf Life, 2022, 33, 100905, DOI:10.1016/j.fpsl.2022.100905.
- S. Wu, S. Wu, T. Feng, X. Shi, Y. Yan, Y. Wang and F. Ding, Development of multifunctional green food packaging based on chitosan and quercetin for visual monitoring of fish spoilage, Food Packag. Shelf Life, 2025, 52, 101605, DOI:10.1016/j.fpsl.2025.101605.
- Y. Yuan, H. Jiang, W. Liu, M. Duan, J. Pang and C. Wu, Novel core-shell pullulan-gelatin-zein nanofiber films loaded with nanochitin-nisin complexes for antibacterial food packaging: custom development and physicochemical properties, Carbohydr. Polym., 2025, 124295, DOI:10.1016/j.carbpol.2025.124295.
- S. Feng, Q. Tang, Z. Xu, K. Huang, H. Li and Z. Zou, Development of novel Co-MOF loaded sodium alginate-based packaging films with antimicrobial and ammonia-sensitive functions for shrimp freshness monitoring, Food Hydrocoll., 2023, 135, 108193, DOI:10.1016/j.foodhyd.2022.108193.
- B. Cui, K. Wang, N. Hu, Y. Sun, C. Mao, P. Ye and Y. Wang, Combination of radio frequency heating and antibacterial agents for microorganism control in the production of packaged tofu, Food Control, 2023, 154, 110015, DOI:10.1016/j.foodcont.2023.110015.
- P. Gao, R. Cha, H. Luo, Y. Xu, P. Zhang, L. Han and X. Jiang, Development of antimicrobial oxidized cellulose film for active food packaging, Carbohydr. Polym., 2022, 278, 118922, DOI:10.1016/j.carbpol.2021.118922.
- H. N. B. Anari, M. Majdinasab, S. Shaghaghian and M. Khalesi, Development of a natamycin-based non-migratory antimicrobial active packaging for extending shelf-life of yogurt drink (Doogh), Food Chem., 2022, 366, 130606, DOI:10.1016/j.foodchem.2021.130606.
- J. Bruni, F. Licciardello, F. Gaubiac, V. Guillard and F. Coffigniez, Tailored antimicrobial PHBV-based packaging for extended shelf life of processed cheese, Food Packag. Shelf Life, 2024, 44, 101319, DOI:10.1016/j.fpsl.2024.101319.
- S. H. Kim, S. E. Kang, Y. D. Kim and M. K. Park, Industrial-scale blown active packaging film with essential oils: properties, dual-functional performance, and box packaging application of instant noodles, Food Packag. Shelf Life, 2024, 43, 101276, DOI:10.1016/j.fpsl.2024.101276.
- S. H. Peighambardoust, A. K. Davarani and S. H. Fasihnia, Effect of active antimicrobial films on quality parameters and shelf-life of fresh yufka dough, Heliyon, 2024, 10(4), e25972, DOI:10.1016/j.heliyon.2024.e25972.
- L. Settier-Ramírez, G. López-Carballo, R. Gavara and P. Hernández-Muñoz, Broadening the antimicrobial spectrum of nisin-producing Lactococcus lactis subsp. Lactis to Gram-negative bacteria by means of active packaging, Int. J. Food Microbiol., 2021, 339, 109007, DOI:10.1016/j.ijfoodmicro.2020.109007.
| This journal is © The Royal Society of Chemistry 2026 |