Open Access Article
Open Access ArticlePulsed electric field-assisted copigmentation via endogenous phenolic acids enhances the chemical stability of anthocyanins in pomegranate juice
Iuri Procopio Castro Brito and
Eric Keven Silva *
*
Universidade Estadual de Campinas (UNICAMP), Faculdade de Engenharia de Alimentos (FEA), Rua Monteiro Lobato, 80, Campinas-SP, CEP:13083-862, Brazil. E-mail: ekeven@unicamp.br
First published on 1st January 2026
Abstract
Pomegranate juice is valued for its high content of anthocyanins and phenolic acids, which contribute to its vivid color and antioxidant properties but are sensitive to thermal degradation. This study investigated the impact of pulsed electric field (PEF) treatments at 5, 10, 15, and 20 kV cm−1 in continuous flow on anthocyanin stability, phenolic profile, physicochemical properties, and color of pomegranate juice. High-intensity ultrasound (HIUS) and thermal treatments at 90 °C/1 min and 120 °C/1 min were applied for comparison. PEF treatments preserved most anthocyanins and maintained juice quality, notably inducing electric field-driven copigmentation between anthocyanins and the juice's own phenolic acids, without adding external copigments. This intrinsic copigmentation significantly enhanced color stability and intensity, with the strongest effect observed at 15 kV cm−1. Higher intensity (20 kV cm−1) led to some anthocyanin degradation. Thermal treatment at 90 °C/1 min caused moderate anthocyanin loss (11%), while severe heating at 120 °C/1 min resulted in substantial degradation (up to 52%) and color loss. Despite some losses, thermal processing increased punicalagin content by 16–23%. Overall, PEF emerges as a promising non-thermal alternative for preserving phenolic compounds and stabilizing anthocyanins in pomegranate juice through a novel mechanism of copigmentation that relies on the natural phenolic constituents of the juice itself, without the need for added copigments. This approach opens new opportunities for developing clean-label products with enhanced color stability and functional properties.
Sustainability spotlightThis study demonstrates a sustainable, non-thermal, and clean-label strategy for enhancing the stability and functional quality of pomegranate juice. By applying continuous-flow pulsed electric field (PEF) processing, the research minimizes energy consumption and eliminates the need for additives or external copigments. The electric field-induced copigmentation between naturally occurring phenolic acids and anthocyanins improves color intensity and chemical stability without compromising nutritional value. This approach reduces processing waste, preserves bioactives, and aligns with circular and green processing principles, contributing to the development of eco-efficient food technologies for the functional beverage sector. |
1. Introduction
Pomegranate juice is widely recognized for its high nutritional value and rich content of bioactive compounds, especially phenolic compounds such as anthocyanins, ellagitannins, ellagic acid derivatives, flavonoids, phenolic acids, which are responsible for its intense reddish-purple color and antioxidant properties. Among these, anthocyanins are notably heat-sensitive and prone to degradation when exposed to conventional thermal processing, resulting in significant losses of color intensity and bioactivity. While thermal treatments are traditionally employed to ensure microbial safety and extend shelf life, they frequently result in the breakdown of these sensitive compounds and compromise the sensory and nutritional quality of the juice.1,2In recent years, non-thermal technologies have gained increasing attention as potential alternatives to conventional processing methods. These emerging techniques aim to preserve the nutritional, functional, and sensory properties of foods while ensuring safety and self-life. Among the most studied non-thermal approaches are pulsed electric fields (PEF), high-intensity ultrasound (HIUS), cold plasma, and high-pressure processing. Unlike thermal treatments, which frequently degrade heat-labile compounds, non-thermal methods operate under milder conditions and reduce damage to vitamins, pigments, and phenolic compounds, while aligning with sustainability and clean-label trends in food processing.3–8
Among these, PEF stand out due to their ability to inactivate microorganisms and enzymes while minimizing nutritional and sensory losses. PEF involves the application of high-voltage pulses (typically 1–80 kV cm−1) over very short durations, promoting charge redistribution across cell membranes and macromolecules and leading to microbial and enzymatic inactivation. The efficiency of PEF treatments is influenced by several process parameters, including field strength, pulse width, frequency, treatment time, membrane polarity, electrode configuration, sample conductivity, and the nature of the food matrix. Beyond preservation, PEF shows promise for enhancing bioactive compound extraction, modifying biopolymers, and assisting processes, such as freezing/thawing and drying. Additionally, PEF has demonstrated the ability to preserve the integrity of heat-sensitive compounds, such as anthocyanins, while also inducing beneficial structural and molecular changes within food systems.9–12
Recent studies indicate that PEF may not only preserve heat-sensitive phenolic compounds but also influence molecular interactions within complex food matrices, including potential copigmentation effects among anthocyanins, ellagitannins, and other phenolics naturally present in pomegranate juice. These interactions play a key role in color expression, stability, and antioxidant behavior. However, despite increasing interest in PEF, research has primarily focused on the retention of individual compounds or microbial inactivation, while the impact of PEF on intermolecular interactions, chemical environment, and copigmentation phenomena in phenolic-rich systems remains largely unexplored. To date, no studies have investigated how different PEF intensities modulate the phenolic profile or the copigmentation dynamics of pomegranate juice.
Although recent studies hint that PEF may influence molecular interactions, these observations are incidental rather than the result of targeted investigation. In most cases, analytical approaches were not designed to monitor intermolecular phenomena, and interpretations were restricted to changes in concentration rather than interaction mechanisms. Thus, the role of PEF in promoting or disrupting copigmentation remains unresolved.13,14 In pomegranate juice, a complex matrix rich in phenolic compounds, including both heat-sensitive compounds, such as anthocyanins and more stable molecules like ellagitannins and ellagic acid, PEF may influence the organization, association, or reactivity of these compounds. Such interactions can affect important quality parameters, including chemical stability, functionality, and appearance of the final product. Despite the growing interest in PEF, previous studies have focused almost exclusively on microbial inactivation and the retention of individual phenolic compounds. As a result, the chemical environment and intermolecular interactions, including copigmentation, have been largely overlooked. This is mainly because most studies rely on global analytical markers (e.g., total phenolics, antioxidant assays) that are insufficient to capture subtle molecular reorganizations. Although several studies report changes in phenolic content after PEF, they do not resolve individual compounds or their interaction patterns. This limitation reflects the common use of low-resolution methods and the prevailing assumption that PEF acts primarily by preserving compounds rather than modulating their interactions. Consequently, the potential effects of PEF on molecular associations, such as copigmentation, have not been investigated. This gap highlights the need for further research to fully understand the broader effects of PEF on the molecular architecture and quality attributes of functional beverages.
A growing body of evidence suggests that PEF can modulate non-covalent interactions among phenolic compounds. In the context of pomegranate juice, we hypothesize that the electric field promotes dipole alignment and closer spatial approximation between anthocyanins and endogenous phenolic acids, facilitating supramolecular arrangements that may enhance color intensity and stability. Despite the promising nature of these effects, there is still limited understanding of how PEF conditions modulate molecular interactions and how its performance compares to other techniques such as HIUS or thermal processing. In this context, particular emphasis is placed on the effects of PEF on the bioactive compound profile of pomegranate juice, given the comprehensive monitoring carried out in this study. Therefore, the present work aims to evaluate the effects of PEF in continuous mode at intensities of 5, 10, 15, and 20 kV cm−1 on the profile and chemical stability of phenolic compounds, in addition to physicochemical properties, antioxidant activity, and visual appearance of pomegranate juice. A detailed and comprehensive monitoring of individual phenolic compounds was carried out, allowing for an in-depth evaluation of how different PEF intensities influence the chemical composition of the juice. HIUS and thermal treatments were also conducted to contextualize the specific impact of each technology on juice quality and chemical stability of bioactive compounds.
2. Material and methods
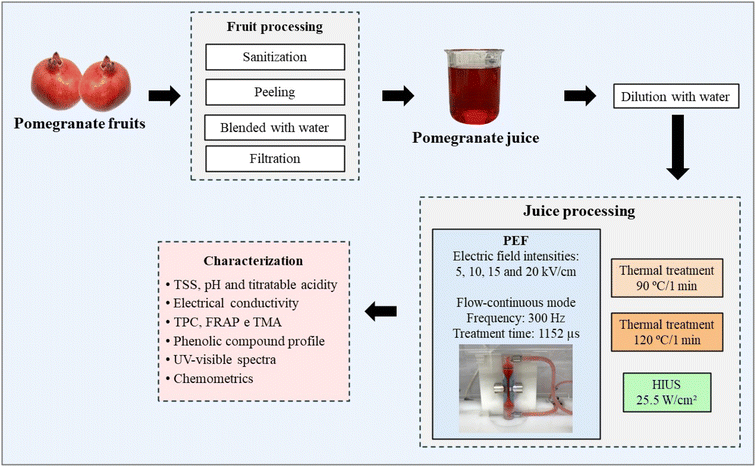
Fig. 1 illustrates the juice preparation process, the experimental design for continuous-flow PEF treatment, and the subsequent chemical characterization of the treated samples. Following the pre-processing steps, which included sanitization, extraction, and filtration, this study evaluated the impact of PEF at varying field intensities (5–20 kV cm−1) and HIUS, in comparison with reference thermal treatments conducted at 90 °C and 120 °C. The samples were subsequently subjected to multiparametric characterization, including physicochemical, spectroscopic, and chemometric analyses, to determine how each technology affects the retention of bioactive compounds, including phenolics and antioxidants, as well as the overall quality of the final product.2.1 Raw materials and chemicals
Pomegranate (Punica granatum L.) fruits of the Wonderful variety, imported from Peru, were purchased at a local retail market in Campinas, São Paulo (Brazil). Sodium carbonate, Folin–Ciocalteu reagent, and ferric chloride were obtained from Dinâmica (Indaiatuba, Brazil). Sodium hydroxide was obtained from Synth (Diadema, Brazil). Gallic acid, 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox), and TPTZ (2,4,6-tris(2-pyridyl)-s-triazine) were obtained from Sigma-Aldrich (St. Louis, USA).2.2 Pomegranate juice preparation
Pomegranate fruits were sanitized with 200 ppm sodium hypochlorite, peeled, and blended with water at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio (fruit
2 ratio (fruit![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water) using a blender (MX1300XTX, Waring Commercial Inc., McConnellsburg, USA) operating at 1.3 kW for 90 seconds. The mixture was then filtered through a stainless-steel sieve. The resulting concentrated juice was stored in plastic containers, protected from light, at −20 °C until further use. Subsequently, the juice was filtered through a 100-mesh sieve (150 µm opening) and centrifuged at 10
water) using a blender (MX1300XTX, Waring Commercial Inc., McConnellsburg, USA) operating at 1.3 kW for 90 seconds. The mixture was then filtered through a stainless-steel sieve. The resulting concentrated juice was stored in plastic containers, protected from light, at −20 °C until further use. Subsequently, the juice was filtered through a 100-mesh sieve (150 µm opening) and centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 minutes. Finally, the concentrated juice was diluted with distilled water at a 1
000 rpm for 10 minutes. Finally, the concentrated juice was diluted with distilled water at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 ratio (juice:water, v/v). This dilution step was performed to adjust the electrical conductivity of the sample (<2000 µS cm−1), preventing dielectric breakdown and excessive Joule heating during PEF processing. It is important to note that the dilution step applied in this study was specifically designed to standardize the physicochemical properties of the juice and to ensure compatibility with the operational limits of high electric field strength application, particularly by controlling electrical conductivity and minimizing excessive Joule heating. As such, the PEF conditions investigated here are directly applicable to standardized pomegranate beverages or diluted juice formulations. The direct application of similar high-field PEF conditions to industrial pomegranate juice obtained by mechanical pressing, which typically exhibits higher electrical conductivity, would require further process optimization, including adjustments in field strength, pulse parameters, flow configuration, or pre-conditioning strategies. Therefore, future studies are necessary to adapt and optimize PEF processing parameters for undiluted industrial pomegranate juices while maintaining non-thermal conditions and product quality.
3 ratio (juice:water, v/v). This dilution step was performed to adjust the electrical conductivity of the sample (<2000 µS cm−1), preventing dielectric breakdown and excessive Joule heating during PEF processing. It is important to note that the dilution step applied in this study was specifically designed to standardize the physicochemical properties of the juice and to ensure compatibility with the operational limits of high electric field strength application, particularly by controlling electrical conductivity and minimizing excessive Joule heating. As such, the PEF conditions investigated here are directly applicable to standardized pomegranate beverages or diluted juice formulations. The direct application of similar high-field PEF conditions to industrial pomegranate juice obtained by mechanical pressing, which typically exhibits higher electrical conductivity, would require further process optimization, including adjustments in field strength, pulse parameters, flow configuration, or pre-conditioning strategies. Therefore, future studies are necessary to adapt and optimize PEF processing parameters for undiluted industrial pomegranate juices while maintaining non-thermal conditions and product quality.
2.3 Pomegranate juice processing
The acoustic power, ultrasound intensity, and specific energy of the treatment were 33.8 ± 0.1 W, 25.5 ± 0.1 W cm−2, and 338 kJ kg−1, respectively.
The pomegranate juice was pumped through the treatment chamber at a flow rate of 0.3 L min−1, corresponding to a residence time of 0.192 s, which was calculated according to eqn (1). Pulses were applied at a frequency of 300 Hz, with a pulse duration of 20 µs and an inter-pulse interval of 20 µs. The juice conductivity was measured at 1483 µS cm−1 prior to treatment. All treatments were performed in a single-pass mode without recirculation.
 | (1) |
Eqn (2) and (3) were used to calculate the energy density of the PEF treatments. Electric field intensities of 5, 10, 15, and 20 kV cm−1 were applied, corresponding to treatment times of 1152 µs and specific energy inputs of 43, 171, 384, and 683 kJ kg−1, respectively. The selection of these intensities was based on ranges commonly used in previous studies applying PEF to fruit and vegetable juices, which typically employ electric fields between 5 and 25 kV cm−1 to evaluate physicochemical modifications and bioactive compound stability.9 The treatments at intensities of 5, 10, 15, and 20 kV cm−1 were designated as PEF05, PEF10, PEF15, and PEF20, respectively. The experiments were performed in triplicate.
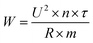 | (2) |
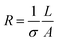 | (3) |
2.4 Pomegranate juice characterization
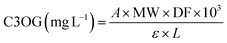 | (4) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 L mol−1 cm−1), L is the path length (considered as 0.87784 cm due to the use of microplates), and 103 is the conversion factor from grams to milligrams.
900 L mol−1 cm−1), L is the path length (considered as 0.87784 cm due to the use of microplates), and 103 is the conversion factor from grams to milligrams.Copigmentation parameters, such as hyperchromic and bathochromic effects, and colorimetric parameters were determined for the pomegranate juices as described by Wang et al. (2024).20 These effects indicate changes in the color and its intensity in the sample. The maximum absorption wavelength (λmax) was determined, and the hyperchromic shift (%ΔAmax) and bathochromic shift (%Δλmax) at this wavelength were calculated using eqn (5) and (6), respectively. Color intensity (CI) was determined by summing the absorbance values measured at wavelengths of 420, 520, and 620 nm.
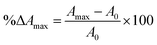 | (5) |
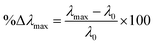 | (6) |
To rule out the formation of melanin-like pigments in pomegranate juice treated with PEF, the samples were centrifuged, as melanin-type pigments are insoluble in water. Melanin-type pigments are soluble in basic media and typically exhibit a characteristic absorbance peak near 250 nm. The supernatant was separated, and the pellet formed at the bottom was resuspended in 0.1 M NaOH at a concentration of 0.5 mg mL−1. A UV-visible spectral scan was then performed from 200 to 780 nm using a spectrophotometer.
2.5 Chemometrics
Principal Component Analysis (PCA) was performed to explore the variables most representative of pomegranate juice quality and bioactivity. The dataset included a wide range of physicochemical and bioactive parameters: pH, TSS, titratable acidity, electrical conductivity, TPC, FRAP, TAC, hyperchromic shift (%ΔAmax), and bathochromic shift (%Δλmax). In addition, the concentrations of individual anthocyanins (cyanidin-3-O-glucoside, cyanidin-3,5-O-diglucoside, delphinidin-3-O-glucoside, delphinidin-3,5-O-diglucoside), total anthocyanins, individual phenolic compounds (galloyl-glucoside, gallic acid, digalloyl-HHDP-glucoside, punicalagin A and B, ellagic acid-glucoside, ellagic acid-arabinoside, ellagic acid, p-coumaric acid, ferulic acid, caffeic acid, sinapic acid, trans-cinnamic acid), as well as total phenolic acids and total phenolic compounds were included in the analysis.Experimental replicates were treated as separate observations in the PCA. Prior to analysis, data were autoscaled by centering each variable to its mean and scaling to unit variance. The PCA was conducted in R (https://www.r-project.org/) using the FactoMineR and factoextra packages.
Sample clustering was performed using Hierarchical Cluster Analysis (HCA). The data were autoscaled, the distance between samples was calculated using Euclidean distance, and clustering was conducted using Ward's method. The analyses were carried out using the factoextra packages in R (https://www.r-project.org/).
2.6 Statistical analysis
All experiments were conducted in at least in duplicate and results are presented as mean ± standard deviation. Statistical comparisons among treatments were performed using one-way analysis of variance (ANOVA) at a 95% confidence level, followed by Tukey's honest significant difference (HSD) post hoc test to identify significant differences. Data analysis was carried out using Minitab 21® software (Minitab Inc., State College, PA, USA).3. Results and discussion
3.1 pH, total soluble solids, electrical conductivity, and titratable acidity
The stability of pH, total soluble solids (TSS), and titratable acidity are key parameters in maintaining the sensory and physicochemical quality of fruit juices. Table 1 presents the effects of electric field intensity on the pH, total soluble solids (TSS), and titratable acidity values of pomegranate juice, and compares these effects with thermal treatments and HIUS processing. The results indicate that, regardless of the processing method, there were no significant differences among the samples for TSS and titratable acidity (p-value = 0.109 and p-value = 0.435, respectively). However, pH showed a statistically significant overall difference according to ANOVA (p-value = 0.031). Nevertheless, Tukey's test did not reveal significant pairwise differences to justify the formation of distinct groups. These findings suggest that PEF represent a promising technology for fruit juice processing, as they do not alter critical quality parameters of the product. These results are also consistent with previous studies indicating that PEF, HIUS and moderate thermal processing tend to preserve the fundamental physicochemical characteristics of fruit juices.4,9 These results can be interpreted considering the underlying mechanisms of PEF action. The primary effect of PEF in liquid matrices is the electroporation of cellular membranes, which may occur in either a reversible or irreversible manner depending on the electric field intensity, treatment duration, and matrix structure. In previously extracted juices, such as pomegranate juice, most plant cells have already been disrupted before processing, which substantially diminishes the potential impact of electroporation on bulk chemical parameters. Consequently, even if PEF induces an additional, residual degree of membrane permeabilization, this effect is insufficient to release meaningful quantities of intracellular constituents capable of modifying pH, total soluble solids, or titratable acidity.| Treatment | pH | TSS (°Brix) | Titratable acidity (mg citric acid/100 mL) | Electrical conductivity (µS cm−1) |
|---|---|---|---|---|
| a Different letters in the same column indicate that there is a significant difference for the variable values at a 95% significance level according to the Tukey test. Columns without letters indicate that there is no significant difference between the treatments. | ||||
| Untreated | 3.28 ± 0.01 | 2.60 ± 0.01 | 823 ± 7 | 1483 ± 2 b |
| TT1 | 3.23 ± 0.01 | 2.55 ± 0.01 | 823 ± 13 | 1521 ± 1 ab |
| TT2 | 3.23 ± 0.01 | 2.55 ± 0.01 | 844 ± 3 | 1582 ± 8 a |
| HIUS | 3.25 ± 0.01 | 2.55 ± 0.01 | 814 ± 7 | 1493 ± 10 ab |
| PEF05 | 3.24 ± 0.01 | 2.60 ± 0.01 | 804 ± 7 | 1454 ± 55 b |
| PEF10 | 3.26 ± 0.01 | 2.60 ± 0.01 | 823 ± 7 | 1482 ± 9 b |
| PEF15 | 3.29 ± 0.03 | 2.68 ± 0.11 | 809 ± 53 | 1451 ± 27 b |
| PEF20 | 3.29 ± 0.03 | 2.60 ± 0.01 | 797 ± 3 | 1470 ± 21 b |
Furthermore, pH, TSS, and titratable acidity are relatively stable parameters that depend predominantly on the intrinsic chemical composition of the juice, particularly sugars, organic acids, and ionic species. Because PEF is a non-thermal technology and does not promote degradation or significant chemical transformation of these compounds, it is theoretically expected that such variables remain unaltered. This trend was also observed for moderate thermal treatments and HIUS, which, under the conditions applied, did not reach intensities capable of triggering chemical reactions or oxidative degradations that could influence these parameters.
The electrical conductivity of pomegranate juice exhibited significant alterations (p-value = 0.008) following thermal and HIUS treatments. This phenomenon can be attributed to the degradation of certain compounds, such as chlorophyll, which contains a magnesium metallic atom within its structure. The release of this metallic ion into the medium increases electrical conductivity. Another contributing factor to the elevated electrical conductivity observed in these treatments is the disruption of cellular membranes in residual tissues present in the juice. Despite centrifugation, complete removal of cellular material from the juice is not achievable, thus, residual cells persist. This disruption releases previously compartmentalized ions into the surrounding medium, thereby increasing electrical conductivity. In contrast, PEF treatments did not significantly affect this parameter when compared to untreated samples.
3.2 Total phenolic content, total anthocyanins and antioxidant activity
Fig. 2 shows the effects of electric field intensity on TPC (A) and FRAP (B) compared to thermal and ultrasound treatments. Based on the results presented, thermal processing, HIUS, and PEF techniques at different intensities can preserve the phenolic compounds and antioxidant activity of pomegranate juice (p-value = 0.283 for TPC and p-value = 0.234 for FRAP). The approximate content of phenolic compounds was 575 µg of GAE per milliliter, while the antioxidant activity was 10 µg of TE per milliliter. Pomegranate juice is a rich source of bioactive compounds. Therefore, retaining these compounds after processing is essential to preserve its bioactive properties, including antioxidant, antimicrobial, and anti-inflammatory activities, among others.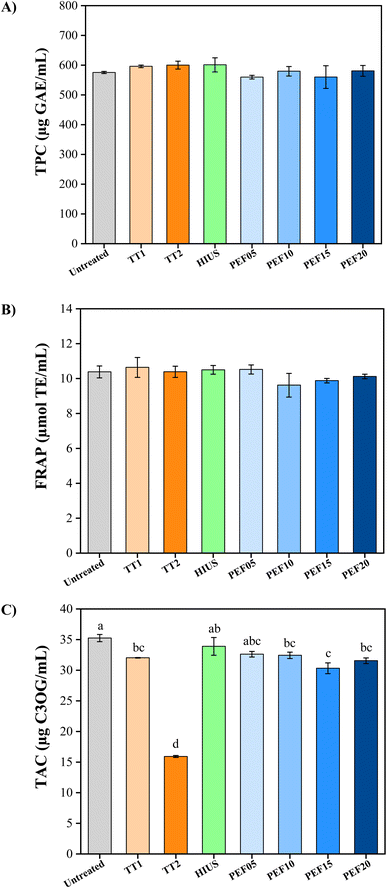 | ||
| Fig. 2 Effect of thermal, HIUS and PEF treatments on: (A) TPC; (B) FRAP; and (C) TAC. Graphs without letters indicate that there is no significant difference between the treatments. | ||
Anthocyanins are highly sensitive to heat, and exposure to high temperatures can cause their degradation into colorless or structurally modified molecules, resulting in a significant reduction of their levels in the juice. Fig. 2C shows the effects of electric field intensity on TAC compared to thermal and ultrasound treatments. The anthocyanins present in pomegranate juice were affected differently depending on the processing method. All treatments led to a significant reduction in the content of these pigments, except for HIUS and PEF05 (p-value < 0.001). The untreated sample contained 35.25 µg mL−1 of C3OG. PEF15 resulted in a 14% reduction in TAC, whereas TT2 led to a 55% decrease. This reduction may lead to changes in the color of the pomegranate juice and potentially diminish its bioactive properties. Heat treatments conducted at temperatures exceeding 60 °C can lead to a substantial decline in anthocyanin levels, as these compounds are prone to degradation under elevated thermal conditions.21 Ozkan et al.22 applied PEF to a fruit juice blend with high levels of anthocyanins and flavonoids, using energy densities of 100 and 120 kJ L−1. Their results showed a significant increase in flavonoid and anthocyanin contents in the PEF-treated samples compared to the control. This finding contrasts with the results of the present study, indicating that process- and matrix-related factors may still influence whether these bioactive compounds increase or decrease after treatment. On the other hand, antioxidant activity remained unchanged; thus, although the levels of these bioactive compounds increased, the observed bioactive property did not differ. The reduction in anthocyanin content associated with PEF at intensities above 5 kV cm−1 may be related to chemical reactions, such as oxidative reactions, and intermolecular rearrangements occurring in the pomegranate juice samples. Consequently, although higher electric field strengths induced some pigment degradation, PEF processing remains a promising alternative to severe thermal treatments (such as TT2), as it demonstrates a superior capacity to retain anthocyanins and maintain the bioactive profile of the product.
3.3 Phenolic compound profile
Fig. 3 shows the effects of electric field intensity on the anthocyanins detected in pomegranate juice: cyanidin-3-O-glucoside, cyanidin-3,5-O-diglucoside, delphinidin-3-O-glucoside, and delphinidin-3,5-O-diglucoside. Among these, cyanidin-3-O-glucoside and cyanidin-3,5-O-diglucoside were the most abundant, with cyanidin-3-O-glucoside being the predominant anthocyanin, initially present at 18.42 µg mL−1. The different processing methods significantly affected the content of this pigment (p-value < 0.001). TT1, HIUS, PEF05, PEF15, and PEF20 reduced cyanidin-3-O-glucoside by approximately 11%. In contrast, the more intense thermal treatment (TT2) led to a 50% reduction, potentially compromising visual attributes and bioactivity. Severe heat treatments, such as heating at 120 °C, promote anthocyanin degradation through thermal hydrolysis and oxidative reactions.23 For the other anthocyanins, PEF treatments had no significant effect, indicating the preservation of both delphinidin derivatives and diglucosylated cyanidins. The TT1 and HIUS selectively reduced cyanidin forms, while delphinidins remained stable under these milder conditions.When analyzing total anthocyanins, PEF treatments at all intensities preserved their content. In contrast, HIUS and TT1 caused slight reductions (approximately 11%), and the high-intensity thermal treatment (TT2) led to a 52% decrease. These results demonstrate that PEF is a promising non-thermal technology capable of preserving heat-sensitive compounds, such as anthocyanins. Truong et al.24 applied PEF to raspberry juice and evaluated both individual and total anthocyanins. The results indicated that PEF preserved the total anthocyanin content; however, cyanidin-3-2G-glucosyl rutinoside showed a slight decrease, which was similar to our findings, in which the content of cyanidin-3-O-glucoside was reduced.
Table 2 presents the impact of electric field intensity on the phenolic compound profile of pomegranate juice compared to thermal and HIUS treatments. Quantitative and statistical analyses revealed significant changes in the levels of phenolic compounds, reflecting a dynamic balance between their release and degradation during processing. Galloyl-glucoside and digalloyl-HHDP-glucoside remained stable across most treatments, except at 120 °C, indicating their general resistance to various processing conditions.
| Phenolic compound and derivatives | Untreated | TT1 | TT2 | HIUS | PEF05 | PEF10 | PEF15 | PEF20 |
|---|---|---|---|---|---|---|---|---|
| a n. d.: not detected. Values are expressed as the mean ± standard deviation. Means followed by the same letter within rows do not differ significantly according to Tukey's test. | ||||||||
| Galloyl-glucoside | 3.2 ± 0.1 a | 3.11 ± 0.03 a | 1.1 ± 0.1 b | 3.1 ± 0.1 a | 3.22 ± 0.04 a | 3.28 ± 0.01 a | 3.3 ± 0.1 a | 3.2 ± 0.1 a |
| Gallic acid | n. d. | 0.93 ± 0.01 | 5.3 ± 0.1 | n. d. | n. d. | n. d. | n. d. | n. d. |
| Digalloyl-HHDP-glucoside | 4.0 ± 0.1 ab | 4.20 ± 0.13 a | 3.9 ± 0.1 ab | 4.1 ± 0.1 ab | 3.9 ± 0.2 ab | 3.8 ± 0.1 ab | 3.82 ± 0.03 ab | 3.7 ± 0.1 b |
| Punicalagin A | 56.2 ± 0.7 b | 65.18 ± 0.71 a | 64.32 ± 0.83 a | 66.02 ± 0.52 a | 49.30 ± 1.74 c | 48.7 ± 0.2 c | 46.00 ± 2.12 c | 48.96 ± 0.01 c |
| Punicalagin B | 129.4 ± 0.2 c | 159.98 ± 1.30 a | 151.8 ± 1.9 ab | 146.71 ± 1.84 b | 122.28 ± 6.43 c | 121.94 ± 4.84 c | 117 ± 1 c | 120 ± 1 c |
| Ellagic acid-glucoside | 14.04 ± 0.03 a | 14.0 ± 0.4 a | 14.87 ± 0.01 a | 13.84 ± 0.22 a | 11.97 ± 0.27 b | 11.87 ± 0.40 b | 11.07 ± 0.50 b | 12.2 ± 0.1 b |
| Ellagic acid-arabinoside | 3.32 ± 0.01 a | 3.3 ± 0.1 a | 2.50 ± 0.04 b | 3.3 ± 0.1 a | 2.7 ± 0.1 b | 2.70 ± 0.12 b | 2.50 ± 0.13 b | 2.77 ± 0.02 b |
| Ellagic acid | 4.92 ± 0.02 b | 5.27 ± 0.01 b | 8.58 ± 0.14 a | 4.9 ± 0.1 b | 3.70 ± 0.1 c | 3.60 ± 0.23 c | 3.3 ± 0.3 c | 3.8 ± 0.1 c |
| p-Coumaric acid | n. d. | n. d. | 0.38 ± 0.01 | n. d. | n. d. | n. d. | n. d. | n. d. |
| Ferulic acid | n. d. | 0.01 ± 0.001 | 0.26 ± 0.01 | n. d. | n. d. | n. d. | n. d. | n. d. |
| Caffeic acid | n. d. | n. d. | 0.50 ± 0.01 | n. d. | n. d. | n. d. | n. d. | n. d. |
| Sinapic acid | n. d. | n. d. | 0.11 ± 0.01 | n. d. | n. d. | n. d. | n. d. | n. d. |
| Trans-cinnamic acid | n. d. | n. d. | 0.08 ± 0.01 | n. d. | n. d. | n. d. | n. d. | n. d. |
| Total phenolic acids | 215.2 ± 0.6 b | 255.9 ± 0.2 a | 253.6 ± 3.1 a | 241.9 ± 1.8 a | 197.1 ± 8.8 c | 195.9 ± 5.8 c | 187 ± 4 c | 194 ± 1 c |
| Total phenolic compounds (total phenolic acids + total anthocyanins) | 253.4 ± 1.4 c | 290 ± 1 a | 272.0 ± 3.4 b | 276.8 ± 2.1 ab | 234.4 ± 7.8 d | 234.2 ± 5.3 d | 223.5 ± 3.8 d | 230.9 ± 0.2 d |
Gallic acid and ferulic acid were detected only in thermally treated samples. Additionally, p-coumaric, caffeic, sinapic, and trans-cinnamic acids appeared exclusively in the TT2, suggesting that high thermal energy may convert precursors into these phenolic acids.
The main phenolic compounds identified were punicalagin A, punicalagin B, ellagic acid-glucoside, ellagic acid, and ellagic acid-arabinoside, consistent with previous literature. These findings indicate that ellagic acid is present both in its native form and glycosylated with monosaccharides. Both thermal treatments increased punicalagin levels, with rises of approximately 16% and 23% for the A and B forms, respectively. Furthermore, the TT2 increased ellagic acid content by 74%, while ellagic acid-arabinoside decreased by 25% compared to the untreated sample. This increase may result from ellagitannin breakdown and the conversion of glycosylated derivatives, facilitated by the rupture of cell walls, which promotes the release of intracellular compounds.25–27
HIUS processing preserved nearly all phenolic compounds and even increased punicalagin A and B levels by 17% and 13%, respectively, highlighting ultrasound as a method that not only retains bioactives but may also enhance their release.
Conversely, PEF treatments led to reductions in certain phenolics: approximately 13% (punicalagin A), 6% (punicalagin B), 16% (ellagic acid-glucoside), 19% (ellagic acid-arabinoside), and 27% (ellagic acid). These reductions may result from structural modifications that rendered these compounds undetectable by standard analytical methods, or from interactions with other juice constituents leading to the formation of new compounds. In the case of anthocyanins, although PEF is generally considered a non-thermal preservation method, there is growing evidence that high electric field intensities can trigger electrochemical reactions at the electrode-medium interface, resulting in the formation of reactive oxygen species (ROS), such as hydrogen peroxide and hydroxyl radicals. These ROS can attack the anthocyanin structure, particularly the C-ring, promoting ring cleavage and formation of degradation products such as protocatechuic acid and quinonoid derivatives.23 Therefore, part of the observed reductions may also be attributed to anthocyanin degradation via oxidative pathways. Such losses may negatively impact the functional properties of the juice, reinforcing the importance of optimizing PEF parameters to minimize the generation of ROS and preserve phenolic integrity.
Regarding total phenolic acids and total phenolic content, both thermal treatments (TT1 and TT2) and HIUS led to significant increases compared to the untreated sample (p-value < 0.001). In contrast, PEF treatments caused reductions of approximately 10% in both parameters. Among all treatments, the TT1 process was the most effective, preserving about 90% of anthocyanins while enhancing phenolic acid release. Consequently, this treatment resulted in improved bioactive properties of the pomegranate juice.
3.4 Color and visual appearance
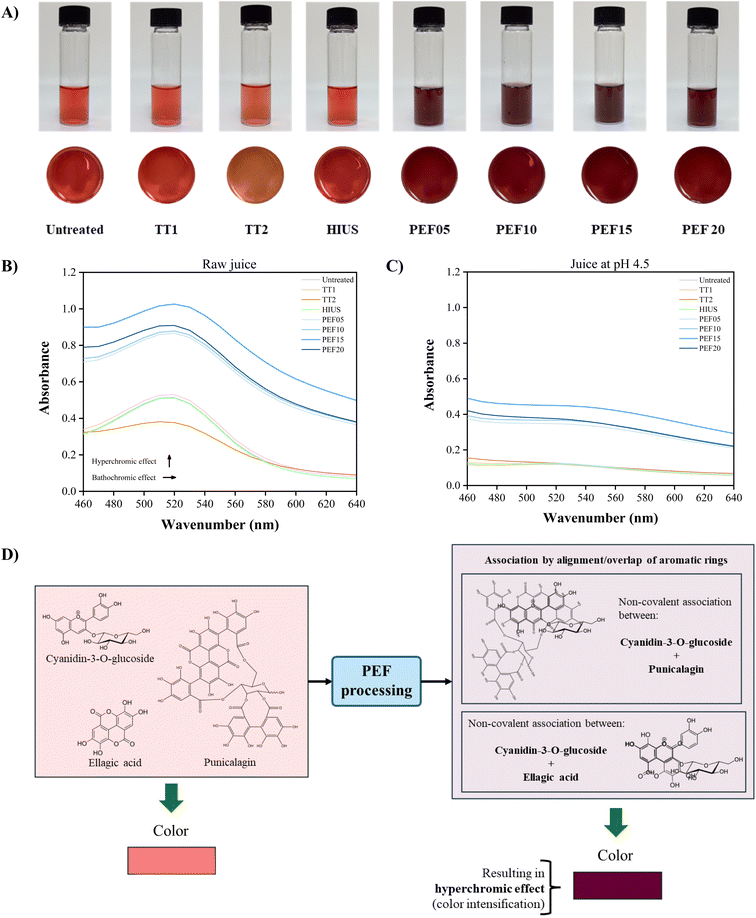
Fig. 4A illustrates the effects of electric field intensity on the visual appearance of pomegranate juice compared to thermal and HIUS treatments. The untreated sample exhibited a vivid red color. Similarly, the samples processed at TT1 and by HIUS showed a visual appearance very close to the control. In contrast, the sample treated at TT2 showed a marked color change to yellow-brown. This indicates that the 120 °C treatment was severe enough to degrade anthocyanins, resulting in noticeable color alteration, as previously discussed in Sections 3.2 and 3.3. PEF treatments, on the other hand, intensified the red color of the juice, yielding a deep red-purple appearance.Fig. 4B presents the UV-visible spectra of pomegranate juice samples processed with different PEF intensities, and compares them with untreated, thermal, and HIUS-treated samples. The analyzed region ranged from 460 to 640 nm, where pomegranate juice displays its most intense color, with red tones typically observed around 520 nm. In line with the macroscopic appearance, the samples processed at TT1 and by HIUS exhibited spectra similar to the untreated juice sample. However, the sample treated at TT2 showed a reduction in absorbance at 520 nm, indicating a decrease in red color intensity, which matched its visual appearance. PEF treatments led to an increase in absorbance at 520 nm, confirming an intensification of the red color. This enhancement is known as the hyperchromic effect, typically resulting from copigmentation processes. In pomegranate juice, such copigmentation may occur between anthocyanins and other phenolic acids, flavonoids, and aromatic compounds.
Table 3 presents the copigmentation parameters of pomegranate juice as influenced by different processing methods, with particular emphasis on the effects of PEF treatments at varying intensities, in comparison with thermal and ultrasound processing. All PEF treatments resulted in a notable increase in maximum absorbance (%ΔAmax), confirming the hyperchromic effect of PEF on the juice. Additionally, a significant difference was observed between PEF intensities (p-value < 0.001), with the PEF15 showing the greatest color enhancement among all processing conditions. This suggests a novel function of PEF as a technology capable of inducing anthocyanin copigmentation in beverages. However, processing parameters must be carefully evaluated, as PEF20 showed a decrease in the hyperchromic effect, indicating that this condition may be too severe (overprocessing). This observation is supported by the visual appearance in Fig. 4A, where the PEF20 exhibited a slightly different shade, an intense red, but not as deep or purple-toned as seen in other PEF-treated samples. Moreover, the %ΔAmax also confirmed the loss of red intensity in the TT2, supporting the visual loss of vivid red color in that condition.
| Treatment | %ΔAmax | %Δλmax | Color intensity |
|---|---|---|---|
| a Means followed by the same letter within column do not differ significantly according to Tukey's test. | |||
| Untreated | 0 c | 0 | 1.0 ± 0.1 c |
| TT1 | 2.8 ± 2.6 c | 0 | 1.07 ± 0.01 c |
| TT2 | −26 ± 2 d | −1.92 | 0.95 ± 0.02 c |
| HIUS | −1 ± 3 c | 0 | 1.00 ± 0.02 c |
| PEF05 | 68 ± 8 b | 0 | 2.42 ± 0.13 b |
| PEF10 | 71 ± 3 b | 0 | 2.48 ± 0.02 b |
| PEF15 | 99.3 ± 6.4 a | 0 | 3.1 ± 0.2 a |
| PEF20 | 77 ± 9 b | −0.96 | 2.58 ± 0.13 b |
When a shift in the maximum absorption wavelength occurs, this is referred to as the bathochromic effect, which alters the hue of the color. This wavelength shift was observed in the samples TT2 and with PEF20, indicating that these processing techniques not only affect color intensity but also its tone. Copigmentation is a particularly interesting phenomenon, as it can lead to enhanced color stability by protecting anthocyanins from degradation, making them more resistant to pH changes, heat, and other environmental factors.
Table 3 shows the effects of different electric field intensities on the color intensity of pomegranate juice compared to thermal and ultrasound treatments. Samples processed using both thermal treatments and HIUS preserved the juice color. However, PEF treatments promoted an increase in color intensity (p-value < 0.001). Among the PEF intensities, the PEF15 demonstrated the highest ability to enhance color. In contrast, the PEF20 intensity resulted in a lower color intensity compared to PEF15, suggesting that this electric field strength may represent an overly intense treatment. Therefore, PEF has the potential to intensify the color of pomegranate juice, possibly through an anthocyanin copigmentation process.
Fig. 4C displays the UV-visible spectra of pomegranate juice processed by PEF at varying intensities, compared to samples treated by other technologies, all measured at pH 4.5. At this pH, anthocyanins are colorless. Therefore, their absence in the spectrum allows for inferences about other contributors to absorbance. In this condition, the untreated sample, thermal treatments, and HIUS showed extremely low absorbance. In contrast, PEF-treated samples maintained relatively higher absorbance values at pH 4.5, indicating that pigments, once copigmented through PEF, may retain some color even under normally colorless conditions. Notably, PEF15 yielded the highest absorbance. A slight increase was observed in PEF05 and PEF10, with 10 kV cm−1 showing higher absorbance between the two. A significant absorbance increase occurred in PEF15, while a decrease PEF20 suggests pigment degradation may occur under excessive processing intensity, even after copigmentation.
Therefore, the modification of pigment properties by PEF represents a promising strategy for color modulation in anthocyanin- and copigment-containing products. The enhancement of color intensity may allow for the reduction of added colorants or other inputs in food formulation, potentially lowering production costs without requiring additional ingredients or higher concentrations of dyes. Accordingly, further studies are needed to optimize PEF as an assisting technology for copigmentation, aiming to better understand the phenomenon, improve process control, and expand its application potential.
3.5 Potential mechanism of copigmentation by PEF on pomegranate juice
Copigmentation reactions, driven by molecular interactions between anthocyanins and colorless phenolic compounds, such as ferulic, sinapic, syringic, gallic, caffeic, ellagic, and rosmarinic acids, as well as catechin, have been shown to significantly enhance the chemical stability of anthocyanins. These interactions protect anthocyanins from degradation, helping preserve color intensity and prolong pigment longevity in food systems.Anthocyanin copigmentation occurs through intermolecular interactions, including hydrogen bonding, van der Waals forces, and hydrophobic interactions with other molecules. Additionally, copigmentation involving metal ions is also possible.20,28,29 However, the electrical conductivity values discussed in Section 3.1 indicate that PEF treatment did not alter the conductivity of the juice. Therefore, interactions between metal ions and anthocyanins were unlikely, and the hypothesis of metal-induced copigmentation in pomegranate juice was ruled out.
Given the observed color intensification in pomegranate juice following PEF treatment, the potential formation of melanin-like pigments was considered. To investigate this, the juice was centrifuged, and the pellet was resuspended in 0.1 M NaOH. The UV-visible spectrum of the resuspended solution (SI) showed a peak at 205 nm, which corresponds to peptide bonds. This indicates that the pellet was composed of proteins and peptides that became insoluble following PEF application. Moreover, the juice retained the same color tone and intensity even after centrifugation, further supporting the exclusion of melanin-like pigment formation due to the absence of the characteristic absorbance near 250 nm.
In this context, the most plausible explanation for the copigmentation effect observed after PEF treatment involves interactions between anthocyanins and phenolic acids. Although HPLC analysis (Fig. 3) showed that cyanidin-3-O-glucoside levels were largely preserved compared to thermal treatments, a slight numerical reduction was observed in PEF samples. This behavior, when analyzed alongside the reduction of specific phenolic acids (Table 2), supports the occurrence of copigmentation. The decrease in free phenolic co-factors (such as ellagic acid derivatives) suggests their involvement in intermolecular interactions with anthocyanins. These copigmentation complexes likely mitigated further degradation of the pigments, resulting in the high retention rates observed, despite the slight initial loss due to processing stress. Compared to thermal and HIUS treatments, which either preserved or increased the concentration of these compounds, the reduction following PEF treatment suggests that non-covalent molecular associations formed between anthocyanins and phenolic acids may have rendered these compounds undetectable by the analytical method used. This provides a strong indication of copigmentation, as the newly formed complexes may no longer be measurable by conventional techniques, even at mild PEF intensities such as 5 kV cm−1.
Fig. 4D proposes a possible mechanism for PEF-induced copigmentation. Pomegranate juice contains a variety of phenolic compounds, particularly anthocyanins and phenolic acids. Among the most abundant anthocyanins is cyanidin-3-O-glucoside, while punicalagins and ellagic acid (including glycosylated forms) are among the predominant phenolic acids. Anthocyanins contribute to the red color of the juice, whereas phenolic acids are colorless. Instead of random collisions, the high-intensity electric field promotes the polarization and alignment of the molecular dipoles of these aromatic compounds along the electric field lines. This directional alignment facilitates the close approach of the planar aromatic rings, effectively promoting intermolecular π–π stacking interactions between the anthocyanins and the copigments. This phenomenon enhances color intensity (a hyperchromic effect) and increases anthocyanin stability, allowing pigmentation even under pH conditions where anthocyanins are typically colorless, as discussed in Section 3.4.
Furthermore, these anthocyanin-phenolic acid complexes may no longer be detected by standard quantification methods. Thus, PEF-mediated copigmentation represents a promising strategy for innovating the development and stabilization of natural pigments, particularly anthocyanins, which are inherently sensitive to environmental and food processing conditions.
3.6 Principal component analysis (PCA)
PCA was applied to evaluate how different processing techniques influenced the chemical composition and bioactive properties of pomegranate juice. The first two principal components accounted for 62.54% and 21.88% of the total variance, respectively, together explaining 84.42% of the dataset's overall variability. This high cumulative variance indicates that PCA effectively captured the chemical diversity among samples, allowing for a comprehensive assessment of processing-induced changes.Fig. 5A displays the PCA score plot, where the distribution of treatment groups is shown based on the first two components (PC1 and PC2). PC1 clearly distinguishes the TT2 from all other treatments. It also separates the HIUS and TT1 samples from the PEF-treated and untreated samples. Interestingly, PC1 reveals a strong similarity between untreated samples and those processed with PEF at various intensities, indicating shared chemical characteristics. Conversely, PC2 clusters the PEF-treated samples and TT2 into a separate group, distinguishing them from the untreated, HIUS, and TT1 samples.
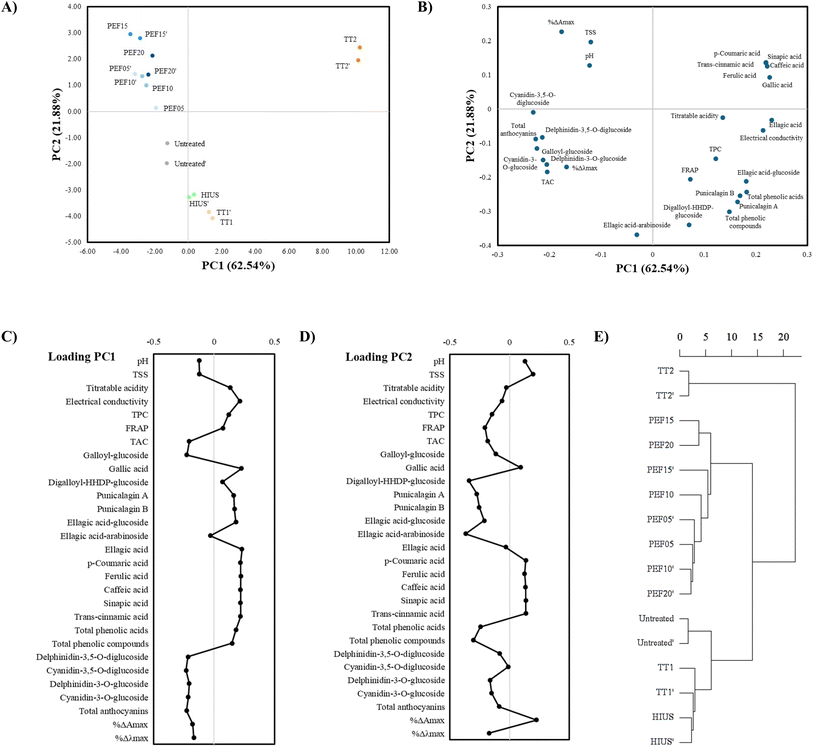 | ||
| Fig. 5 Principal component analysis (PCA): (A) scores plot; (B) loadings plot; (C) loadings of PC1; (D) loadings of PC2; and (E) Hierarchical Cluster Analysis (HCA). | ||
Fig. 5B presents the PCA loading plot, showing a well-distributed set of variables across the two principal components. This indicates that each sample group is defined by distinct chemical and functional traits contributing variably to the total variance. All treatments showed high reproducibility, as both replicates of each process clustered closely together, confirming the consistency of the results.
Regarding PC1, the TT2 are primarily defined by the presence of several phenolic acids, including gallic, ferulic, caffeic, sinapic, p-coumaric, and trans-cinnamic acids. These compounds were not detected in other treatments, suggesting that intense thermal processing led to their formation. Additionally, these samples are associated with increased electrical conductivity, higher levels of ellagic acid and its glucoside, total phenolic acids, punicalagin A and B, and overall total phenolic content, indicators of enhanced bioactive compound concentration and physicochemical changes.
PEF-treated samples across different intensities are mainly characterized by higher values of the hyperchromic effect (%ΔAmax), total soluble solids (TSS), and pH. These variables point to the occurrence of copigmentation phenomena promoted by PEF, which stands out as a distinct effect among the treatments. Copigmentation represents a promising innovation avenue in food technology, contributing to improved color stability and sensory attributes of products.
The HIUS and TT1 samples are associated with elevated levels of ellagic acid-arabinoside, digalloyl-HHDP-glucoside, and FRAP activity, suggesting a preservation or enhancement of antioxidant capacity and specific phenolic compounds in these treatments.
Fig. 5C illustrates the contribution of each variable to the formation of PC1. Variables that positively correlated with PC1 included electrical conductivity, gallic acid, ellagic acid, p-coumaric, ferulic, caffeic, sinapic, and trans-cinnamic acids, as well as total phenolic content, most of which defined the TT2 group. In contrast, variables negatively correlated with PC1 were TMA, galloyl-glucoside, cyanidin-3-O-glucoside, cyanidin-3,5-O-diglucoside, delphinidin-3-O-glucoside, delphinidin-3,5-O-diglucoside, and total anthocyanin content. This indicates that the anthocyanin response was consistent across treatments, regardless of processing method.
Fig. 5D shows the contribution of variables to PC2. Positively correlated variables included TSS, p-coumaric, ferulic, caffeic, sinapic, and trans-cinnamic acids, as well as the hyperchromic effect (%ΔAmax), reinforcing the unique chemical modifications induced by PEF. On the other hand, negatively correlated variables were FRAP, TMA, digalloyl-HHDP-glucoside, punicalagin A and B, ellagic acid-arabinoside, and total phenolic compounds, highlighting their association with untreated and mildly processed samples.
Fig. 5E shows the Hierarchical Cluster Analysis (HCA) performed to elucidate the similarity between samples based on their multivariate profiles. The dendrogram revealed three distinct clusters defined by linkage distance: a divergent group isolating the intense thermal treatment (TT2), a cohesive cluster containing all PEF-treated samples, which exhibited internal sub-clustering consistent with field intensity, and a third group comprising the untreated, TT1, and HIUS samples. Notably, the short linkage distance between TT1 and HIUS indicates a high degree of similarity in their effects on the matrix, suggesting that high-intensity ultrasound induced modifications analogous to mild thermal processing. These hierarchical patterns fully corroborate the spatial distribution observed in the PCA score plot, confirming that while TT2 induces the most drastic changes, PEF creates a unique treatment signature distinct from both the control and other processing methods.
PCA successfully distinguished the effects of the various processing methods applied to pomegranate juice by reducing the complexity of the multivariate data while preserving essential chemical insights. These findings were corroborated by HCA, which validated the multivariate distinctiveness of the PEF treatment and the high similarity between HIUS and mild thermal processing. This combined approach enabled a deeper understanding of how each technique, including PEF, influenced the quality attributes, appearance, and bioactive profile of the juice, clearly highlighting the distinct impact of each treatment.
4. Conclusion
This study demonstrated that continuous-flow PEF processing is a viable non-thermal alternative for pomegranate juice preservation, effectively maintaining total anthocyanin content and visual appearance compared to conventional thermal treatments. While thermal processing (TT2) resulted in significant anthocyanin degradation, PEF treatments preserved the bioactive profile, although optimization is required to minimize the loss of specific phenolic acids.A major finding of this work was the observation of electric field-induced copigmentation between anthocyanins and phenolic acids (such as punicalagins and ellagic acid) naturally present in the matrix. This phenomenon suggests that PEF can enhance color stability without external additives. However, future studies are needed to fully elucidate this mechanism; specifically, techniques such as Nuclear Magnetic Resonance (NMR) are needed to structurally confirm the non-covalent associations generated between these compounds.
Furthermore, while physicochemical parameters showed promising results, the industrial adoption of this technology requires holistic validation. Future research must evaluate the long-term stability of the copigmented complexes, the microbiological safety of the processed juice, and the impact of PEF on sensory characteristics through consumer panels. Ultimately, PEF stands out as an energy-efficient technology that aligns with sustainable processing goals. PEF provides a promising pathway for producing high-quality, functional pomegranate juice with extended shelf life by balancing process intensity to maximize copigmentation while ensuring microbial safety.
Conflicts of interest
The authors declare that part of the results reported in this manuscript were used to support a patent application.Data availability
All data supporting the findings of this study are included within the article. Additional datasets generated or analyzed during the current work are available from the corresponding author upon reasonable request.Supplementary information (SI) is available. See DOI: https://doi.org/10.1039/d5fb00657k.
Acknowledgements
This study was financed, in part, by the São Paulo Research Foundation (FAPESP), Brasil. Process Numbers #2020/11255-3, #2023/01876-9, and #2024/04051-3. Eric Keven Silva thanks the Brazilian National Council for Scientific and Technological Development (CNPq, Process No. 312695/2025-0) for the Research Productivity Fellowship.References
- L. El Hosry, C. Bou-Mitri, M. Bou Dargham, M. Abou Jaoudeh, A. Farhat, J. El Hayek, J. M. Bou Mosleh and E. Bou-Maroun, Food Biosci., 2023, 55, 103034 CrossRef CAS.
- S. Shahkoomahally, D. Shin, F. Habibi, J. Kim and A. Sarkhosh, Food Chem. Adv., 2023, 2, 100225 CrossRef.
- R. A. Juliato, I. P. C. Brito and E. K. Silva, Food Chem., 2025, 481, 144037 CrossRef CAS PubMed.
- M. M. Strieder, H. S. Arruda, G. M. Pastore and E. K. Silva, Food Hydrocolloids, 2023, 139, 108489 CrossRef CAS.
- E. K. Silva, H. S. Arruda, G. M. Pastore, M. A. A. Meireles and M. D. A. Saldaña, Ultrason. Sonochem., 2020, 63, 104942 CrossRef CAS PubMed.
- I. P. C. Brito and E. K. Silva, Food Hydrocolloids, 2025, 166, 111342 CrossRef CAS.
- S. S. Arya, N. Nachiappan, R. Waghmare and M. S. Bhat, Food Prod., Process. Nutr., 2023, 5, 36 CrossRef.
- F. V. Silva and A. Sulaiman, Foods, 2022, 11(13), 1942 CrossRef CAS PubMed.
- I. P. C. Brito and E. K. Silva, Food Res. Int., 2024, 184, 114207 CrossRef CAS PubMed.
- P. Achayuthakan, R. Wongsagonsup, J. Sriprablom, M. Suphantharika and P. Intra, Polymers, 2023, 15(20), 4087 CrossRef CAS PubMed.
- B. Llavata, G. A. Collazos-Escobar, J. V. García-Pérez and J. A. Cárcel, Innovative Food Sci. Emerging Technol., 2024, 92, 103591 CrossRef CAS.
- I. C. Nyoto and F. Gómez Galindo, Biochem. Biophys. Rep., 2023, 35, 101515 CAS.
- N. El Darra, M. F. Turk, M.-A. Ducasse, N. Grimi, R. G. Maroun, N. Louka and E. Vorobiev, Food Chem., 2016, 194, 944–950 CrossRef CAS PubMed.
- N. El Darra, H. N. Rajha, M.-A. Ducasse, M. F. Turk, N. Grimi, R. G. Maroun, N. Louka and E. Vorobiev, Food Chem., 2016, 213, 352–360 CrossRef CAS PubMed.
- T. J. Mason, J. P. Lorimer, D. M. Bates and Y. Zhao, Ultrason. Sonochem., 1994, 1, S91–S95 CrossRef CAS.
- H. S. Arruda, G. A. Pereira, D. R. de Morais, M. N. Eberlin and G. M. Pastore, Food Chem., 2018, 245, 738–749 CrossRef CAS PubMed.
- W. Chen, C. Xie, Q. He, J. Sun and W. Bai, Food Chem.:X, 2023, 17, 100535 CAS.
- M. M. Giusti and R. E. Wrolstad, Curr. Protoc. Food Anal. Chem., 2001, F1.2.1 Search PubMed.
- F. T. Borsoi, H. S. Arruda, L. M. Reguengo, I. A. Neri Numa and G. M. Pastore, Food Chem., 2025, 483, 144254 CrossRef CAS PubMed.
- X. Wang, J. Cheng, Y. Zhu, T. Li, Y. Wang and X. Gao, Food Res. Int., 2024, 190, 114632 CrossRef CAS PubMed.
- M. J. Almeida, M. A. Ribeiro-Sanches, B. Guimarães, E. S. Lago-Vanzela and J. Telis-Romero, J. Food Process Eng., 2025, 48, e70049 CrossRef.
- G. Ozkan, M. E. Oner, A. Fischer, A. Juadjur, K. Aganovic, G. Dräger, E. Capanoglu and T. Esatbeyoglu, Food Chem.:X, 2025, 31, 103000 CAS.
- H. S. Arruda, E. K. Silva, N. M. Peixoto Araujo, G. A. Pereira, G. M. Pastore and M. R. Marostica Junior, Molecules, 2021, 26(9), 2632 CrossRef CAS PubMed.
- N. Q. A. Truong, A. Puzovic, D. Pavon-Vargas, K. Simkova, I. Rabeeah, H. Murray, M. C. Grohar, M. Mikulič-Petkovšek, K. Mandl, M. Gössinger, S. Rainieri, H. Halbwirth, L. Cattani and M. Rinaldi, Innovative Food Sci. Emerging Technol., 2025, 104, 104101 CrossRef CAS.
- J. Piecko, M. Mieszczakowska-Frąc, K. Celejewska and J. Szwejda-Grzybowska, Foods, 2024, 13(8), 1231 CrossRef CAS PubMed.
- G. Yildiz and R. M. Aadil, J. Food Sci. Technol., 2020, 57, 1462–1468 CrossRef CAS PubMed.
- F. Van de Velde, M. E. Pirovani and S. R. Drago, J. Food Compos. Anal., 2018, 72, 22–31 CrossRef CAS.
- B. Bay Yılmaz and N. Türker, J. Food Meas. Charact., 2024, 18, 1499–1516 CrossRef.
- M. Türkyılmaz, F. Hamzaoğlu, M. Akpınar Uzun and M. Özkan, Food Chem., 2025, 486, 144611 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2026 |