Open Access Article
Open Access ArticleProtein extraction from apple seeds for waste valorization for sustainable food systems
Sehrish
Mustafa
a,
Iqra
Bashir
a,
Sajad Mohd
Wani
 *a,
Sajad Ahmad
Sofi
b,
Tawheed
Amin
a,
Abdul Rouf
Malik
c,
Faheem-ullah
Khan
d,
Imtiyaz
Murtaza
e,
Imran
Khan
f,
Qudsiya
Ayaz
a and
Khansa
Rasool
a
*a,
Sajad Ahmad
Sofi
b,
Tawheed
Amin
a,
Abdul Rouf
Malik
c,
Faheem-ullah
Khan
d,
Imtiyaz
Murtaza
e,
Imran
Khan
f,
Qudsiya
Ayaz
a and
Khansa
Rasool
a
aDivision of Food Science and Technology, Sher-e-Kashmir University of Agricultural Sciences and Technology-Kashmir, Jammu and Kashmir 190025, India. E-mail: wanisajad82@gmail.com
bDepartment of Food Science, University of Arkansas, Fayetteville, Arkansas 72704, USA
cDivision of Fruit Science, Sher-e-Kashmir University of Agricultural Sciences and Technology-Kashmir, Jammu and Kashmir 190025, India
dDivision of Floriculture and Landscape Architecture, Sher-e-Kashmir University of Agricultural Sciences and Technology-Kashmir, Jammu and Kashmir 190025, India
eDivision of Basic Sciences and Humanities, Sher-e-Kashmir University of Agricultural Sciences and Technology-Kashmir, Jammu and Kashmir 190025, India
fDivision of Agri-Statistics, Sher-e-Kashmir University of Agricultural Sciences and Technology-Kashmir, Jammu and Kashmir 190025, India
First published on 30th October 2025
Abstract
Proteins are essential to the food industry because they provide both functional and nutritional advantages. This study explores apple seeds, a largely overlooked agro-industrial by-product, as a sustainable source of plant proteins. Utilizing apple seed proteins can add value to agro-industrial waste, promote sustainability, and support circular economy. Apple seed hydrolysate (ASPH) and protein concentrate (ASPC) were prepared through extraction and enzymatic hydrolysis and assessed for their composition, functional properties, and antioxidant activity. ASPC exhibited a protein content of 59.21%, while ASPH achieved a higher protein content of 77.48% due to enzymatic hydrolysis. ASPH demonstrated enhanced solubility (72.5%) at neutral pH, and superior water absorption capacity (2.45 g g−1), and emulsifying properties compared to ASPC. Additionally, ASPH showed stronger antioxidant activity, with DPPH and ABTS scavenging rates increasing by 28% and 35%, respectively. These results highlight the potential of apple seed proteins for use in nutraceuticals, bakery products, and plant-based food formulations.
Sustainability spotlightThis research highlights the sustainable utilization of apple seeds, a largely discarded agro-industrial by-product, as a novel plant protein source. By developing protein concentrate and hydrolysate from apple seeds, the study promotes waste valorization, circular economy principles, and resource use efficiency in the food industry. The enhanced functional properties and bioactivity of these proteins provide new opportunities for their application in nutraceuticals and plant-based food formulations, offering a sustainable alternative to conventional protein sources. This innovation not only reduces food waste but also supports environmental conservation by utilizing an abundant yet underutilized resource. Additionally, integrating apple seed proteins into food systems contributes to addressing global challenges related to food security and protein availability reinforcing the need for eco-friendly and waste-reducing solutions in agricultural and food processing industries. |
1 Introduction
Proteins are a crucial component of the food industry, since they offer both desirable nutritional benefits and functional properties. Animal-derived proteins have traditionally been chosen over plant-based proteins because they exhibit superior amino acid compositions and higher absorption.1 The limited supply of animal-based proteins, however, is unable to meet increasing consumer demands. In order to partially replace animal proteins, research into finding new plant sources of protein has garnered attention recently. In comparison to animal proteins, plant proteins show a competitive advantage in terms of financial appeal and long-term availability. Plant proteins are also inexpensive and widespread. Plant proteins used as food or food additives are significantly influenced by their functional properties, which include solubility, hydration, foaming, emulsifying, hydrophobicity, water and oil binding, gelling, and viscosity behaviors.2 Non-thermal processing techniques are applied to modify the functional, structural and physicochemical properties of proteins, enhancing their potential for diverse applications in food formulations.3 Having proteins with suitable functionalities is essential for industrial production, and as a result, novel plant proteins have garnered growing interest.4 Protein concentrates, containing 70–90% protein, are functional food ingredients obtained by removing non-protein components like fats and carbohydrates. They offer enhanced nutritional value and key functional properties such as solubility, emulsification, and gelling, making them versatile for use in bakery products, dairy alternatives, and plant-based meat substitutes. Proteolysis can improve nutritional properties by removing anti-nutritional compounds and production of peptides that have therapeutic potential against various chronic diseases, and generation of such kind of peptides depends upon protease enzymes employed.5–7To ensure a steady supply of protein for the diet, protein valorization from agro-industrial waste streams has become a viable and sustainable approach. This strategy tackles the problem of global food security in addition to reducing food waste. Agro-industrial waste streams represent a cost-effective and environmentally sustainable protein source. Utilization of these by-products which are available at no additional costs can help to create a protein adjunct with added value and reduce solid waste, both of which can lead to environmental sustainability.8 Apple seeds which constitute over 5% of the fruit, are an agro-industrial waste product of the apple processing industry and a sustainable, beneficial source of proteins and nutrient-dense components.9 As per FAO/WHO guidelines, apple seeds are underutilized for the extraction of proteins with a range of nutritious compositions, such as adequate amounts of essential amino acids, lipid content exceeding 20%, a fiber content of 4%, and protein content ranging from 30% to 50%.10 With possible biological properties like antioxidant, anti-obesity, anti-diabetic, and antimicrobial properties, apple seed proteins promote the sustainable economy.11 Moreover, apple seed proteins are hypoallergenic and helpful in demonstrating structural, functional, and nutraceutical qualities for plant-based meat alternatives, baked goods, and drinks.12,13 Recent literature revealed the use of apple seeds for preparation of protein isolates and evaluated their techno-functional properties to emphasize their use in the development of functional ingredients in food product formulations.14–17
Growing interest in plant protein, the use of apple seed protein showed significant potential and underutilized sources of proteins to support nutritional functional aspects. Recent literature related to apple seed protein focused on structural and functional aspects of apple seed protein. However, there is limited study on protein concentrates and hydrolysate preparation and their comparison based on structural, functional and antioxidant activity. Therefore, standardizing the process for utilization of apple seed for protein extraction for development of functional and nutraceutical ingredients in the form of hydrolysates and peptides can find numerous applications in different food industries. Therefore, the objective of the present study was to valorize apple seeds to prepare protein concentrates and protein hydrolysates from apple seeds and their characterization and comparison for various functional and bioactive properties.
2 Materials and methods
2.1 Materials
Apple seeds were purchased from a nearby apple processing industry. Prior to extraction of proteins, amygdalin, a cyanogenic glycoside, naturally present in the kernels and seeds of several fruits, like apples, apricots, almonds, cherries, plums, and peaches is removed.18 This compound plays a defensive role in plants, protecting them from herbivores; however, its consumption poses serious health risks to humans.19 The method described by Bolarinwa et al. was used to extract amygdalin from the apple seeds.14 All standard chemicals and reagents used were of analytical grade and procured from HiMedia Laboratories Pvt. Ltd unless otherwise mentioned.2.2 Preparation of apple seed protein concentrate (ASPC)
Following the removal of amygdalin, seeds of apple were ground in a lab mixer/grinder and dried until they had an 8.5% moisture content. ASPC was prepared by following the method given by Ghorad et al.15 The dry seed flour was allowed to soak in hexane (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) at 25 °C for six hours, stirring periodically, in order to extract the fat. Following solvent separation, extractions were carried out thrice using fresh solvent. A decantation of the solvent was performed following each extraction. The free fat meal was then ground to fit through a 240 μm mesh sieve and allowed to air dry at room temperature. In order to preserve it in the refrigerator for future research, the finished product was packaged in polyethylene pouches.
10) at 25 °C for six hours, stirring periodically, in order to extract the fat. Following solvent separation, extractions were carried out thrice using fresh solvent. A decantation of the solvent was performed following each extraction. The free fat meal was then ground to fit through a 240 μm mesh sieve and allowed to air dry at room temperature. In order to preserve it in the refrigerator for future research, the finished product was packaged in polyethylene pouches.
2.3 Preparation of apple seed protein hydrolysate (ASPH)
100 grams of ASPC was mixed with one liter of tap water and brought to a pH of 9.00 using NaOH (1.0 N). The slurry was centrifuged (Weswox WT-24BL) at 5000×g for 15 minutes at 5 °C after being agitated for 1 hour at 35 °C. Whatman no. 1 filter paper was used to filter the supernatant.16 Proteins were coagulated once the filtered supernatant was brought to the standard pH of 4.5 using 1 M HCl. For 15 minutes, the precipitate and supernatant were centrifuged at 5000×g at 5 °C. After neutralizing, curd protein with 0.1 M NaOH (0.1 M), was adjusted to pH 7 after being re-dispersed in distilled water. After that, it was frozen, ground in a lab mill, and sieved through a 60 mesh screen. Additionally, distilled water was used to suspend 1% (w/v) Apple Seed Protein Isolate (ASPI), which was subsequently hydrolyzed using the pepsin enzyme. The hydrolysis pH was 2 and hydrolysis time was 60 minutes, and the enzyme to substrate ratio (Es) was 2.5% (w/w). The pH was kept constant during hydrolysis, and 37 °C was the reaction's operating temperature. At 100 °C, the hydrolysates were immersed in a water bath for ten minutes in order to deactivate the enzymes. The supernatant was centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 20 minutes. Additionally, a dried protein hydrolysate was produced by freeze-drying the supernatant, and was subjected to further study.
000 rpm for 20 minutes. Additionally, a dried protein hydrolysate was produced by freeze-drying the supernatant, and was subjected to further study.
 | (1) |
2.4 Characterization of ASPC and ASPH
The obtained ASPC and ASPH were characterized for their proximate composition and functional properties. Antioxidant attributes were analyzed using assays such as DPPH (1,1-diphenyl-2-picrylhydrazyl), ABTS (2,2′-azino-bis 3-ethylbenzothiazoline-6-sulfonic acid) radical scavenging activities, and FRAP (Ferric Reducing Antioxidant Power). Additionally, SDS-PAGE was performed to assess the molecular weight distribution of the proteins.| CHO(%) = 100 − (% moisture + % protien + % fat + % ash + % fibre) | (2) |
2.4.2.1 Water absorption capacity (WAC) and oil absorption capacity (OAC). The method provided by Kakar et al.20 was used to measure the WAC and OAC of samples. The sample (1 g) was dispersed in 10 mL distilled water or refined sunflower oil and the sample was vortexed for 30 minutes followed by centrifugation at 3000 g for 25 minutes. The supernatant or unabsorbed oil was then decanted, and the residue was weighed. eqn (3) was used to determine the WAC and OAC:
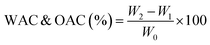 | (3) |
2.4.2.2 Foam measurements. The foaming stability (FS) and foaming capacity (FC) were calculated by the method given by Chaudhary and Kumar.21 In a graduated cylinder, the sample (1.0 g) was dispersed with 50 mL of distilled water at 30 ± 2 °C. The suspension was mixed and shaken for five min to produce foam. The volume of foam at 30 seconds after whipping was converted to foaming capacity using eqn (4).
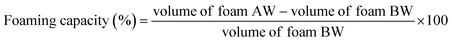 | (4) |
The FS was calculated using a similar procedure, except that the samples were allowed to stand at room temperature for 30 minutes, and the volume of residual foam was quantified.
Eqn (5) was used to calculate FS:
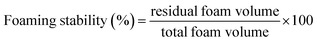 | (5) |
2.4.2.3 Protein solubility. The method described by Sofi et al.22 was used to determine solubility and was calculated according to eqn (6):
 | (6) |
2.4.2.4 Protein precipitability. Protein precipitability (PP) was obtained according to the method of Rao et al.23 and was computed by using eqn (7);
 | (7) |
P 1 and P2 are mg of protein in 1 mL of V1 and V2, respectively.
2.4.2.5 Buffer capacity. The buffer capacity of the samples was measured using the method outlined by Rao et al.23 A known volume of NaOH (0.1 M) solution was added in small increments after 1 g sample had been dispersed in 50 mL of distilled water. The pH variations that resulted were then noted. A separate section was treated with HCl, and the corresponding changes in pH were recorded. The amount of NaOH or HCl added was plotted against pH, and buffer capacity was expressed in terms of mmol of HCl or NaOH required to change pH by 1 unit per g of ASPC or ASPH.
2.4.3.1 ABTS radical scavenging activity. The Xu et al.24 method was used to measure the antioxidant activity of ASPC and ASPH. To create the ABTS stock solution, equal parts of 2.45 mmol L−1 potassium persulfate and 7 mmol L−1 ABTS were dissolved in distilled water. In order to form the ABTS radical cation, the mixture was left in the dark for sixteen hours. Methanol was then added to the stock solution until its absorbance at 734 nm was 0.70 ± 0.02. Next, 2.0 mL of ABTS radical cation solution was combined with 50 μL of each sample extract (25 μL mL−1, 50 μL mL−1, and 100 μL mL−1) and left to react for six minutes. A UV-vis double beam spectrophotometer (IG-28DS) was used to measure the absorbance at 734 nm in relation to a blank (methanol). As a positive control, ascorbic acid was utilized. Each experiment was carried out in triplicate, and the scavenging activity was computed using eqn (8):
 | (8) |
2.4.3.2 DPPH radical scavenging activity. The extracts' ability to scavenge DPPH radicals was assessed using a slightly modified version of the method of Xu et al.24 Two milliliters of sample extracts at different concentrations (25, 50, and 100 μg mL−1) were mixed with two milliliters of DPPH solution (0.1 mmol L−1 in methanol), and the mixture was allowed to stand in the dark for 30 minutes. A UV-vis double beam spectrophotometer (IG-28DS) was used to measure the absorbance of sample extracts at 517 nm in relation to a blank (methanol). Each experiment was carried out in triplicate and ascorbic acid was used as the positive control. To calculate the scavenging effect, eqn (9) was utilized.
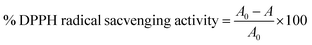 | (9) |
2.4.3.3 Ferric reducing antioxidant power (FRAP). FRAP assay was carried out by following the procedure given by Sonawane and Arya.16 The mixture used to prepare the FRAP reagents included onefold 2,4,6-tripyridyl-s-triazine (TPTZ), onefold ferric chloride (FeCl3), and tenfold acetate buffers. After 15 minutes of incubation at 37 °C, the reaction mixture was supplemented with 20 μL of the sample and 200 μL of FRAP reagent. Next, the absorbance was measured at 593 nm.
3.4.3.4 Protein pattern analysis (SDS-PAGE). SDS-PAGE was used to visualize the protein profile using the method of Gani et al.25 To make the protein sample, 2.7 mL of a 5% (w/v) SDS solution was combined with 0.3 g of the protein sample. After two minutes of vortexing the mixture, the homogenate was incubated for an hour at 85 °C. A centrifuge (eppendrof, centrifuge) was used to centrifuge the samples at 17,000 g for 20 minutes at room temperature (26–28 °C). The Biuret method was used to determine protein concentration in the supernatant. The mixture was heated for three minutes after the dissolved samples and sample buffer were combined in a 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v) ratio. The samples (15 μg protein) were electrophoresed using a Mini Protein III unit at a constant current of 15 mA per gel on polyacrylamide gels comprising 15% running gel and 4% stacking gel. Following electrophoresis, the gel was stained with 0.02% (w/v) Coomassie Blue R-250 in 50% (v/v) methanol and 7.5% (v/v) acetic acid, and then destained with 50% (v/v) methanol and 7.5% (v/v) acetic acid. The molecular weight of the proteins was estimated using protein standards with protein markers that ranged in size from 10 kDa to 180 kDa.
1 (v/v) ratio. The samples (15 μg protein) were electrophoresed using a Mini Protein III unit at a constant current of 15 mA per gel on polyacrylamide gels comprising 15% running gel and 4% stacking gel. Following electrophoresis, the gel was stained with 0.02% (w/v) Coomassie Blue R-250 in 50% (v/v) methanol and 7.5% (v/v) acetic acid, and then destained with 50% (v/v) methanol and 7.5% (v/v) acetic acid. The molecular weight of the proteins was estimated using protein standards with protein markers that ranged in size from 10 kDa to 180 kDa.
3.4.3.5 Statistical analysis. With three distinct lots of samples, triplicate experiments were conducted, and the results were reported as mean ± standard deviation. SPSS statistics software (v.250 16, Inc., Chicago, IL) was utilized to do a one-way analysis of variance (ANOVA) at a 5% level of significance. Duncan's Multiple Range Test (DMRT) was then employed to determine the difference between means.
3 Results and discussion
3.1 Degree of hydrolysis
Protein hydrolysate serves as the major source of bioactive peptides following hydrolysis.26 The degree of hydrolysis (DH) is considered as a criterion to determine the proteolysis and hydrolysis yield.27 It is an important consideration in assessing the protein's ability to hydrolyze, which may indicate the functionality and biological activities of proteins.28 The pepsin enzyme was used to hydrolyze the ASPH powder. The optimal hydrolysis conditions for ASPH were found to be pH 2 at 60 °C and an enzyme: substrate ratio of 2.5% (w/w). For ASPH, the degree of hydrolysis was 37.45 ± 0.57%. Similar results were reported in tamarind seed protein hydrolysate using papain enzyme.293.2 Proximate analysis
The data presented in Table 1 revealed that proximate composition of ASPC and ASPH varied significantly (p ≤ 0.05). Apple seed protein hydrolysate presented higher moisture content (10.63 ± 0.38%) than apple seed protein concentrate (7.85 ± 0.23%) due to the differences in processing methods. Proteins are hydrolyzed in the presence of water to produce smaller peptides and amino acids, which frequently leaves the finished product with some moisture. Protein concentrates, on the other hand, are usually treated to exclude water and other non-protein ingredients to boost protein stability and purity. Because of this, protein concentrates possess lower moisture content than hydrolysates.| Parameter (%) | ASPC | ASPH |
|---|---|---|
| a Values are expressed as mean ± standard deviation. | ||
| Moisture content | 7.85 ± 0.23a | 10.63 ± 0.38b |
| Protein content | 59.21 ± 1.10a | 77.48 ± 1.24b |
| Fat | 0.57 ± 0.04a | 0.34 ± 0.02b |
| Total ash | 4.68 ± 0.18a | 2.79 ± 0.10b |
| Fiber | 9.24 ± 0.26a | 5.33 ± 0.17b |
| Carbohydrate content | 18.45 ± 0.31a | 3.43 ± 0.13b |
The protein content of ASPH was recorded to be 77.48 ± 1.24% while the protein content of ASPC was found to be 59.21 ± 1.10% (Table 1). The higher protein content of ASPH compared to ASPC may be attributed to the increased availability of peptide bonds in ASPC for hydrolysis by pepsin during protein digestion, leading to the release of more free amino acids.30 Despite using the same raw material, the protein hydrolysates may contain varying amounts of protein, demonstrating the impact of the enzymes' substrate specificities. The hydrolysates' protein content was similar to that of pumpkin seed protein hydrolysates, ranging from 81.07 to 92.22%.31
Fat contents of ASPC and ASPH were statistically significant (p ≤ 0.05). ASPC recorded higher crude fat content than ASPH. The difference between them can be explained by variations in their processing methodologies. ASPC is produced by concentrating the protein from apple seeds, which involves partial defatting but does not fully remove all non-protein components. As a result, ASPC retains a small amount of residual fat, as the primary aim of this process is protein concentration rather than exhaustive fat removal. In contrast, ASPH is obtained by subjecting ASPC to enzymatic hydrolysis, which breaks down the protein structure into smaller peptides and amino acids. This enzymatic process not only enhances protein digestibility but also facilitates a reduction in non-protein residues, including fats. The enzymatic breakdown in ASPH production may aid in further releasing or separating residual fats from the protein matrix, resulting in a lower fat content in ASPH compared to ASPC. Therefore, the additional hydrolysis step in ASPH processing accounts for its lower crude fat content relative to ASPC. This difference in crude fat content highlights how enzymatic treatment in protein hydrolysate production can more effectively reduce unwanted components, thus impacting the composition and potential applications of the final product.
Table 1 depicts the values of crude fiber for ASPC and ASPH, and the results varied significantly (p ≤ 0.05). The crude fiber content in ASPC and ASPH was observed to be 9.24 ± 0.26% and 5.33 ± 0.17%, respectively. The reason for higher fiber content in ASPC is that it is produced by concentrating the protein from apple seeds, which retains a certain amount of non-protein components, including fiber. In contrast, ASPH, which is derived from ASPC through enzymatic hydrolysis, undergoes further breakdown of the protein into smaller peptides and amino acids. This additional processing reduces the non-protein components, including fiber, leading to lower crude fiber content in ASPH.
From Table 1, the ash content of ASPC was found to be 4.68 ± 0.18% while for ASPH the ash content was recorded to be 2.79 ± 0.10%. ASPC is produced by concentrating the protein, which still contains a significant amount of inorganic minerals, salts, and other non-protein components. These minerals are not completely removed during the concentration process. In contrast, ASPH, which is obtained by enzymatically hydrolyzing ASPC exhibited a lower total ash content. This reduction in ash content is a result of the hydrolysis process, which further breaks down the proteins into smaller peptides and amino acids, leading to the removal or reduction of some of the inorganic components.
3.3 Functional properties
Complex interactions between the conformation, structure, composition, and physicochemical characteristics of proteins under the influence of the environment and other food ingredients are represented by their functional properties.32 These properties fall into three main categories: (a) moisturizing properties, influenced by protein–water interactions (such as water retention and solubility); (b) interfacial qualities, like foaming and emulsification; and (c) gelling properties, determined by protein–protein and protein–water interactions.33Fig. 1 presents some of the observed functional properties of ASPC and ASPH. The ability of protein molecules to absorb water shows how they interact with one another, which influences how the protein behaves in various intricate food systems.25 ASPH had a higher WAC of 143.21 ± 3.14%, whereas ASPC had a WAC of 115.25 ± 2.43%. The higher WAC of ASPH can be attributed to its high protein content which may have altered the composition and structural conformation of protein molecules, thereby increasing their ability to bind water through their hydrophilic sites.34 Another explanation for this might be that proteins separate into subunits, each of which has a greater number of water binding sites.35 | ||
| Fig. 1 Functional properties of apple seed protein concentrate (ASPC) and apple seed protein hydrolysate (ASPH). | ||
Oil absorption capacity (OAC) of proteins is a crucial functional characteristic of food. It affects mouthfeel, flavor, texture, and yield. Physical oil entrapment in proteins and noncovalent forces such as hydrogen bonding, hydrophobicity, and electrostatics that are involved in lipid–protein interactions have been blamed for OAC.36 OAC of ASPC and ASPH varied significantly (p ≤ 0.05), and was found to be 173.67 ± 2.21% and 126.84 ± 2.10%, respectively. The oil absorption capacities, in contrast, were observed to reduce enzyme hydrolysis because of the presence of polar amino acids and oil absorption capacity is associated with their ability to reduce the interfacial tension between the hydrophobic and hydrophilic elements of the protein sample.23 Foaming characteristics like foam volume and foam stability are significant for processing the foods. The movement, penetration, and the reorganization of molecules at the air/water interface affect foam formation, which is mostly determined by the size, hydrophobicity, and flexibility of the structure of protein molecules.37 The amount of interfacial area that can be produced by a protein is known as its foaming capacity whereas foam stability is defined as the ability of a protein to stabilize against gravitational and mechanical stresses.38 ASPH presented a foaming capacity and stability of 67.19 ± 1.26% and 22.15 ± 0.48%, respectively, while ASPC presented a foaming capacity and foaming stability of 43.18 ± 1.18% and 13.56 ± 0.26%, respectively (Fig. 1). It was found that ASPC had a lower foam capacity and significantly reduced foam stability due to hydrolysis. This is most likely because more air can be incorporated when the polypeptide content first increases. Nevertheless, the polypeptides lack the strength necessary to produce stable foam. Further hydrolysis is likely to result in peptides, which lack the ability to stabilize the air cells of the foam.34
Solubility is one of the most crucial functional properties of proteins. For a protein-based product to be suitable for use in manufactured foods, it must exhibit high solubility, as this is essential for desirable functional and rheological properties such as foaming capacity. 39 The solubility of ASPH was measured to be higher than that of ASPC and was found to be 85.12 ± 0.72%. This improved solubility in ASPH was the outcome of hydrolysis, which can liberate low molecular weight peptides and increase the amount of ionizable groups, which increases the solubility and encourages interactions between the hydrolysate and water.40
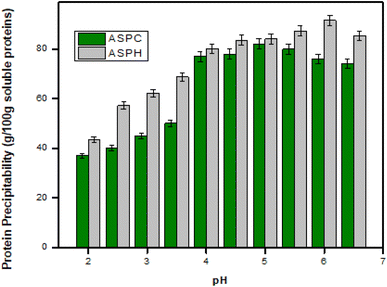
As shown in Fig. 2, the highest protein content extractable in ASPC and ASPH was found at pH 5 (82.10 ± 2.13 g/100 g) and 6 (91.53 ± 2.18 g/100 g), respectively. The remaining protein, 17.9 g/100 g and 8.47 g/100 g was in solution. The soluble protein's perceptibility decreased on both sides of pH 5 and pH 6, indicating that these values were optimal for precipitating the extractable protein in ASPC and ASPH, respectively.
To change the pH by one unit at acidic pH (2–6), an average of 0.412 ± 0.013 mmol and 0.204 ± 0.010 mmol of hydrochloric acid were needed per gram of ASPC and ASPH, respectively. Similarly, to bring about a change of a single pH unit in the alkaline pH range (6–10) 0.082 ± 0.07 mmol and 0.063 ± 0.04 mmol of sodium hydroxide was required per gram of ASPC and ASPH, respectively. Increased buffer capacity of both ASPC and ASPH was seen in an acidic medium as revealed in Fig. 3.
3.4 Antioxidant activities
Proteins are unique antioxidants as they can prevent lipid oxidation in a number of ways, for instance by scavenging free radicals, chelating prooxidative transition metals, reducing hydroperoxides, inactivating reactive oxygen species, and changing the physical characteristics of food systems.41 The antioxidant activities of ASPC and ASPH through different methods are presented in Fig. 4. It is usually recommended to utilize more than two assays to estimate antioxidant activity as the DPPH, FRAP, and ABTS assays measure different radicals, which may be irregular. The FRAP assay was utilized to reflect the ferric-reducing activity, while the DPPH and ABTS assays assess the scavenging capacity.A marked difference was observed in the protein hydrolysate (Lane B), which exhibited the near-complete disappearance of intact protein bands seen in the concentrate. Instead, only a faint smear corresponding to low-molecular-weight peptides below 20 kDa was visible, reflecting extensive enzymatic cleavage of the parent proteins. This confirms that hydrolysis by proteolytic enzymes effectively disrupted the quaternary and tertiary structures, generating a wide distribution of short peptide fragments. The absence of well-defined bands in the hydrolysate highlights the efficiency of hydrolysis and the high susceptibility of apple seed proteins to enzymatic degradation. Such degradation is desirable as it reduces the size of proteins into bioactive peptides with improved solubility, digestibility, and functional properties.
The results also align with earlier findings in oilseed proteins such as soybean, flaxseed, and rapeseed, where enzymatic hydrolysis led to the breakdown of major storage proteins into smaller peptides with enhanced bioactivity. Several studies have demonstrated that peptides in the range of <20 kDa are associated with antioxidant, antihypertensive, and anti-inflammatory effects, suggesting that the apple seed protein hydrolysates obtained here may hold potential nutraceutical value. Moreover, the generation of low-molecular-weight peptides is advantageous for applications in functional foods, as these fragments are more readily absorbed in the gastrointestinal tract compared to intact proteins. The clear contrast between the intact protein profile of the concentrate and the fragmented peptide distribution in the hydrolysate validates the success of the enzymatic treatment employed in this study. From a technological standpoint, such structural modifications in proteins can also contribute to improved techno-functional properties such as emulsification, foaming, and solubility, which are critical in food formulations. Thus, the SDS-PAGE results not only demonstrate the effective conversion of apple seed proteins into hydrolysates but also support their potential application in the development of value-added functional food products and nutraceutical formulations.
4 Conclusion
Apple seeds are a byproduct of the apple processing industry and serve as a sustainable protein source with desirable nutritional and functional properties. The production of ASPC and ASPH highlights a novel approach for protein valorization from agro-industrial waste. Enzymatic hydrolysis of apple seed protein improves techno-functional properties and antioxidant properties, making it suitable for diverse food formulations. Increased solubility and bioactive potential of apple seed protein hydrolysates enhance its potential as a nutraceutical ingredient for novel product formulations. Utilizing apple seeds for extraction of protein isolates directly supports environmental sustainability and advances the circular economy by transformation into a valuable functional food ingredient in the form of protein hydrolysates. Future studies should focus on optimizing extraction methods and scaling up production to enable industrial applications of apple seed proteins.Author contributions
Sehrish Mustafa: methodology, writing – original draft, investigation. Iqra Bashir: methodology, writing – original draft. Sajad Mohd Wani: conceptualization, supervision, project administration. Sajad Ahmad Sofi: writing review and editing. Tawheed Amin: data curation, resources. Ab. Raouf Malik: data curation, resources. Faheem-ullah Khan: validation, formal analysis. Imtiyaz Murtaza: data curation, resources. Imran Khan: data curation, resources. Qudsiya Ayaz: writing review and editing. Khansa Rasool: writing review and editing.Conflicts of interest
The authors declare that there is no conflict of interest.Data availability
The data used are provided in the manuscript.References
- L. Day, J. A. Cakebread and S. M. Loveday, Trends Food Sci. Technol., 2022, 119, 428–442 CrossRef CAS
.
- K. K. Ma, M. Greis, J. Lu, A. A. Nolden, D. J. McClements and A. J. Kinchla, Foods, 2022, 11(4), 594 CrossRef CAS PubMed
.
- J. Zhang, L. Liu, H. Liu, A. Yoon, S. S. Rizvi and Q. Wang, Crit. Rev. Food Sci. Nutr., 2019, 59(20), 3267–3280 CrossRef CAS
.
- C. G. Rizzello, D. Tagliazucchi, E. Babini, G. S. Rutella, D. L. T. Saa and A. Gianotti, J. Funct. Foods, 2016, 27, 549–569 CrossRef CAS
.
- J. Vioque, M. Alaiz and J. Girón-Calle, Food Chem., 2012, 132(1), 67–72 CrossRef CAS PubMed
.
- B. Hernández-Ledesma, M. Ramos and J. Á. Gómez-Ruiz, Small Rumin. Res., 2011, 101, 196–204 CrossRef
.
- A. G. P. Samaranayaka and E. C. Y. Li-Chan, J. Funct. Foods, 2011, 3(4), 229–254 CrossRef CAS
.
- M. Sakthivel and P. Palani, Int. J. Biol. Macromol., 2016, 86, 390–401 CrossRef CAS PubMed
.
- V. I. Candrawinata, J. B. Golding, P. D. Roach and C. E. Stathopoulos, CyTA--J. Food, 2015, 13(2), 293–299 CrossRef CAS
.
- M. Kumar, M. D. Barbhai, T. Esatbeyoglu, B. Zhang, V. Sheri, S. Dhumal and J. M. Lorenzo, Food Biosci., 2022, 50, 102155 CrossRef
.
- C. Ji and Y. Luo, J. Agric. Food Res., 2023, 12, 100604 CAS
.
- M. N. Nasrabadi, A. S. Doost and R. Mezzenga, Food Hydrocoll., 2021, 118, 106789 CrossRef
.
- S. A. Sofi, S. Rafiq, D. Majid, M. A. Binobead, M. A. Ali, M. S. Elshikh and B. N. Dar, Radiat. Phys. Chem., 2024, 225, 112139 CrossRef CAS
.
- I. F. Bolarinwa, C. Orfila and M. R. Morgan, Food Chem., 2014, 152, 133–139 CrossRef CAS PubMed
.
- A. S. Ghorad, D. T. Bornare, M. M. Humane and A. H. Munde, BIOINFOLET-A Q. J. Life Sci., 2018, 15(2), 240–241 Search PubMed
.
- S. K. Sonawane and S. S. Arya, 3 Biotech, 2017, 7(3), 1–11 CrossRef PubMed
.
- AOAC., AOAC Official Methods of Analysis, Association of Official Analytical Chemists, Arlington, 18th edn, 2005 Search PubMed.
- A. Kakar, T. F. Miano, A. H. Soomro, A. Yar, S. A. Memon and B. Khan, Int. J. Ecosyst. Ecol. Sci., 2022, 12(2), 585–594 CrossRef
.
-
G. B. Donald, Medical Toxicology of Natural Substances, 2009, vol. 55, pp. 336–352 Search PubMed
.
- D. Ganjewala, S. Kumar, D. S. Asha and K. Ambika, Acta Biol. Szeged., 2010, 54, 1–14 Search PubMed
.
- V. Chaudhary and V. Kumar, Chem. Sci. Rev. Lett., 2020, 9(33), 98–108 CAS
.
- S. A. Sofi, J. Singh, K. Muzaffar and B. N. Dar, ACS Food Sci. Technol., 2021, 1(5), 802–812 CrossRef CAS
.
- N. Rao, P. G. Prabhakara Rao and G. Rao, Int. Food Res. J., 2011, 18(3), 949–955 CAS
.
- Y. Xu, M. Fan, J. Ran, T. Zhang, H. Sun, M. Dong and H. Zheng, Saudi J. Biol. Sci., 2016, 23(3), 379–388 CrossRef CAS
.
- A. Gani, Z. ul Ashraf, N. Noor and I. A. Wani, Ultrason. Sonochem., 2022, 86, 106010 CrossRef CAS PubMed
.
- I. Gill, R. López-Fandiño, X. Jorba and E. N. Vulfson, Enzyme Microb. Technol., 1996, 18(3), 162–183 CrossRef CAS PubMed
.
-
F. Guerard, R. Ravakkec-Ple, D. De La Broise, A. Binet and L. Dufosse, in Engineering and Manufacturing for Biotechnology, Springer, Dordrecht, 2002, pp. 39–50 Search PubMed
.
- M. Manzoor, J. Singh, Z. F. Bhat and S. Jaglan, Int. J. Biol. Macromol., 2024, 257, 128553 CrossRef CAS PubMed
.
-
H. R. Horton, L. A. Moran, R. S. Ochs, J. D. Rawn and K. G. Scrimgeour, Principles of Biochemistry, Prentice Education, 3rd edn, 2002 Search PubMed
.
- M. B. Bagul, S. K. Sonawane and S. S. Arya, 3 Biotech, 2018, 8, 1–8 CrossRef PubMed
.
- V. Muhamyankaka, C. F. Shoemaker, M. Nalwoga and X. M. Zhang, Int. Food Res. J., 2013, 20(5), 2227 Search PubMed
.
- L. Mirmoghtadaie, S. S. Aliabadi and S. M. Hosseini, Food Chem., 2016, 199, 619–627 Search PubMed
.
- O. A. Higuera-Barraza, C. L. Del Toro-Sanchez, S. Ruiz-Cruz and E. Márquez-Ríos, Ultrason. Sonochem., 2016, 31, 558–562 CAS
.
- A. A. Nnamezie, A. A. Famuwagun and S. O. Gbadamosi, Food Prod., Process. Nutr., 2021, 3, 1–14 Search PubMed
.
- R. Sinha, C. Radha, J. Prakash and P. Kaul, Food Chem., 2007, 101(4), 1484–1491 CAS
.
- N. Wang, L. Maximiuk, D. Fenn, M. T. Nickerson and A. Hou, Cereal Chem., 2020, 97(6), 1111–1117 CrossRef CAS
.
- M. A. Malik, H. K. Sharma and C. S. Saini, Ultrason. Sonochem., 2017, 39, 511–519 CrossRef CAS PubMed
.
- L. Banupriya and T. P. Vijayakumar, Int. J. Adv. Res., 2016, 4(1), 1288–1296 CAS
.
- S. P. Samaei, M. Ghorbani, D. Tagliazucchi, S. Martini, R. Gotti, T. Themelis, F. Tesini, A. Gianotti, T. G. Toschi and E. Babini, Food Chem., 2020, 330, 127120 CrossRef CAS PubMed
.
- R. K. C. Jeewanthi, N. K. Lee and H. D. Paik, Food Sci. Anim. Resour., 2015, 35(3), 350 CrossRef PubMed
.
- R. J. Elias, S. S. Kellerby and E. A. Decker, Crit. Rev. Food Sci. Nutr., 2008, 48(5), 430–441 CrossRef CAS PubMed
.
- Z. Xie, J. Huang, X. Xu and Z. Jin, Food Chem., 2008, 111(2), 370–376 CrossRef CAS PubMed
.
- C. F. Ajibola, J. B. Fashakin, T. N. Fagbemi and R. E. Aluko, Int. J. Mol. Sci., 2011, 12(10), 6685–6702 CAS
.
- A. A. Famuwagun, A. M. Alashi, S. O. Gbadamosi, K. A. Taiwo, J. D. Oyedele, O. C. Adebooye and R. E. Aluko, Pol. J. Food Nutr. Sci., 2020, 70(4), 429–443 CAS
.
| This journal is © The Royal Society of Chemistry 2026 |