Open Access Article
Open Access ArticleOxidative stability of baked products incorporated with Phyllanthus emblica seed extract: a functional alternative to synthetic antioxidants
Ahinsa Lankanayaka ab,
K. G. L. R. Jayathungea,
Pasan C. Bandaraa,
Danushika C. Manatunga
ab,
K. G. L. R. Jayathungea,
Pasan C. Bandaraa,
Danushika C. Manatunga a,
Sameera Samarakoonc,
Nimal Punyasiric and
Chathuri M. Senanayake
a,
Sameera Samarakoonc,
Nimal Punyasiric and
Chathuri M. Senanayake *a
*a
aDepartment of Biosystems Technology, Faculty of Technology, University of Sri Jayewardenepura, Homagama, 10200, Sri Lanka. E-mail: chathurisnnk@sjp.ac.lk
bFaculty of Graduate Studies, University of Sri Jayewardenepura, Gangodawila, Nugegoda, 10250, Sri Lanka
cInstitute of Biochemistry, Molecular Biology and Biotechnology, University of Colombo, Colombo 00300, Sri Lanka
First published on 19th December 2025
Abstract
Lipid oxidation limits shelf-life of bakery products, typically controlled by synthetic antioxidants (BHT, BHA, and TBHQ) with safety concerns. This study aimed to evaluate ethyl acetate extract of Phyllanthus emblica seed (PSE) as a natural antioxidant to extend the shelf-life of sponge cakes and biscuits. The extract was assessed for antioxidant activity, thermal stability, antimicrobial activity, and toxicity prior to application. HPLC analysis identified seven major phenolic compounds: gallic, vanillic, sinapic, p-coumaric, and ellagic acids, myricetin, and quercetin. PSE showed significantly (p < 0.05) higher antioxidant activity than synthetic antioxidants, with lower IC50 in DPPH (41.23 µg mL−1) and ABTS˙+ (29.31 µg mL−1) and higher FRAP (180.34 µg mL−1). PSE retained over 90% antioxidant activity at 180 °C for 2 h, exceeding BHT (62%), BHA (63%), and TBHQ (87%). Toxicity assessment indicated low toxicity, with an IC50 of 1025.37 µg mL−1 (Caco-2 cells) and an LC50 of 1832.71 µg mL−1 (zebrafish embryos), and no effects on survival, hatching, or heartbeat were observed at 200 µg mL−1, the bakery application concentration. Sponge cakes and biscuits were treated with PSE (200 µg mL−1) and compared with BHT, BHA, TBHQ, and control (no antioxidant). Sponge cakes with PSE retained peroxide value (PV) and TBARS below thresholds (0.5 meq per kg and 0.3 mg kg−1) for 14 days, while controls exceeded limits within 7 days. In biscuits, PV remained below 1.0 meq per kg for 56 days with PSE, BHT, and TBHQ. PSE extended microbial shelf-life to 12 days in cakes and 84 days in biscuits without affecting sensory quality. Findings highlight PSE as a thermally stable, non-toxic, and effective natural alternative to synthetic antioxidants in bakery products.
Sustainability spotlightThis research aligns strongly with global sustainability efforts by addressing multiple Sustainable Development Goals (SDGs). Specifically, it supports SDG 3: good health and well-being, by developing a natural antioxidant alternative that reduces reliance on synthetic antioxidants with potential health risks. At the same time, it advances SDG 12: responsible consumption and production, by valorizing Phyllanthus emblica seeds, an underutilized by-product that is often discarded as waste, thereby enhancing resource efficiency and minimizing environmental impact. By extending the oxidative stability of baked products with natural antioxidants, this study not only enhances food safety and shelf-life but also promotes more sustainable, eco-friendly food systems that contribute to long-term food security. |
1 Introduction
The challenge of food preservation has become increasingly complex in recent years as lipid-rich foods demand effective protection against oxidative deterioration to ensure extended shelf life and quality retention.1 Lipid oxidation remains one of the most significant causes of quality loss in baked products, which contain high fat levels and are subjected to high processing temperatures (100–200 °C).2 During oxidation, unsaturated fatty acids react with oxygen to form hydroperoxides (primary products) and aldehydes and ketones (secondary products), leading to undesirable changes in flavor, aroma, texture, and color.3 Consequently, these reactions limit the storage stability of baked products by accelerating rancidity, nutritional degradation, and reducing consumer acceptability. Thus, controlling lipid oxidation is essential for maintaining product freshness and safety since these products are commonly stored under ambient conditions for long period.To mitigate oxidative deterioration, antioxidants are commonly incorporated into bakery formulations. These compounds interrupt free radical chain reactions, decompose peroxides, and chelate pro-oxidant metals, thereby enhancing the oxidative stability and sensory quality of food products.4,5 The Sri Lankan Food (Antioxidants) Regulation of 2009 limits antioxidant usage in baked products to 200 mg kg−1,6 ensuring product safety while maintaining quality. Widely used synthetic antioxidants include butylated hydroxytoluene (BHT), butylated hydroxyanisole (BHA), tert-butylhydroquinone (TBHQ), and propylgallate (PG).7 BHA is commonly used in low-fat foods, including cereal products such as breakfast cereals and cake mixtures. BHT is typically used in bakery products at levels of 0.01–0.04%, which is more effective than BHA.8 TBHQ is considered the best synthetic antioxidant for frying oils, but not for baked products because of high volatility at baking temperatures.9 Nevertheless, synthetic antioxidants are found to be efficient and inexpensive, but their long-term usage is questionable. Recent research findings revealed that the prolonged usage of synthetic antioxidants adversely affects living beings, such as rodents and monkeys, and has a potential carcinogenic effect.10 For example, BHA and BHT have been associated with tumor development in humans and rats, particularly affecting the forestomach and liver.11 Studies have also demonstrated that high doses of BHA and BHT can enhance carcinogenesis in monkeys.12 Moreover, the long-term usage of TBHQ with high dosage has been found to increase the risk of development of carcinogenicity, cytotoxicity, and potential adverse effects on human health.13,14 Baran et al.15 observed that exposure to BHA and/or TBHQ can increase the production of reactive oxygen species (ROS), leading to apoptosis and oxidative DNA damage in zebrafish models. Moreover, some synthetic antioxidants such as BHA and BHT are highly volatile at high baking temperatures, making them less suitable for heat-processed foods. These drawbacks have encouraged researchers and consumers alike to seek natural, safe, and thermally stable alternatives from plant sources.
Natural plant extracts have emerged as promising alternatives to synthetic antioxidants due to their abundance of bioactive compounds such as polyphenols, flavonoids, tannins, and carotenoids, which effectively scavenge free radicals and inhibit lipid oxidation.16 Several studies have demonstrated that natural antioxidants retain higher stability at baking temperature than synthetic ones. For instance, rosemary extract remained active at 200 °C, successfully preventing rancidity in multigrain bread for up to 10 days, whereas BHT and BHA lost over 70% of their activity at 150 °C.17 Masoud et al.18 reported that incorporating 10% muskmelon seed flour into biscuits significantly enhanced their antioxidant potential, increasing total phenolic content (85.52 to 210.54 mg GAE per g), total flavonoid content (54.84 to 142.28 mg CE per g), and DPPH inhibition (48.63 to 65.54%), while yielding the most acceptable sensory results, confirming its value as a natural antioxidant source. Cakir et al.19 reported that incorporating Prunus spinosa powder into gluten-free rice cakes significantly enhanced total phenolic content (35.91 ± 0.25 to 73.39 ± 0.58 mg GAE per g) while reducing the estimated glycemic index. Similarly, Psidium guineense Sw. leaf extract incorporated into cakes reduced peroxide and hexanal formation more effectively than BHT, while improving sensory properties.20 Such findings confirm the potential of plant-based antioxidants to enhance both oxidative stability and product acceptability in bakery systems.
In addition to functional advantages, the use of fruit-processing by-products such as seeds, peels, pomace, and skins offers an environmentally sustainable solution to reduce food waste while recovering valuable bioactive compounds.21 Studies have shown that substituting refined flour with tomato pomace in bread and muffins extended shelf life by 4–7 days.22 In another study, Reddy et al.23 investigated that the ethanolic extracts from amla (Phyllanthus emblica), drumstick leaves (Moringa oleifera), and raisins (Vitis vinifera) provided greater antioxidant protection in biscuits than BHA. These examples highlight the dual benefits of natural extracts in food preservation and waste valorization.
Phyllanthus emblica (Indian gooseberry or amla) is a medicinal plant widely recognized for its rich composition of phenolics, flavonoids, and hydrolyzable tannins such as emblicanin A and B, phyllantine, and phyllantidine, which exhibit strong antioxidant and antimicrobial activities.24,25 While the fruit pulp is extensively used in functional foods and nutraceuticals, the seeds are typically discarded as waste despite their high antioxidant potential. The Phyllanthus emblica seed extract (PSE) contains potent phenolic compounds such as resveratrol, gallocatechin, and quercetin, which contribute to its radical scavenging capacity and antibacterial effects.26 Previous studies have shown that incorporating Phyllanthus emblica extracts into meat products effectively reduced lipid peroxidation and maintained desirable sensory qualities during storage. For instance, Bariya et al.27 reported that goat meat patties enriched with Phyllanthus emblica fruit and seed coat extracts showed significantly lower TBARS values (below 1 mg malonaldehyde per kg after 21 days) and reduced free fatty acid formation compared to controls, while maintaining superior color, flavor, and overall acceptability during storage. Nevertheless, there is currently no research investigating the application of Phyllanthus emblica seed in bakery products, where both thermal stability and oxidative protection are essential.
However, this study is the first to systematically evaluate the effectiveness of the ethyl acetate extract of Phyllanthus emblica seed with major synthetic antioxidants (BHT, BHA, and TBHQ) in controlling both oxidative and microbial spoilage in baked products, supported by a comprehensive toxicological profile. The objectives of this research are to determine the antioxidant and antimicrobial activities of Phyllanthus emblica seed extract, evaluate its thermal stability at baking temperatures, and assess its toxicological safety through both in vitro and in vivo assays. Furthermore, the study aims to examine the oxidative and microbial stability of baked products incorporated with PSE during storage, with the ultimate goal of exploring its potential for food application and performance evaluation as a natural antioxidant.
2 Materials and methods
2.1 Materials
Freshly harvested Phyllanthus emblica fruits (mature green stage) were obtained from a home garden in Matale, Sri Lanka, and authenticated (Specimen No. 2024/322) by the National Herbarium, Peradeniya. Bacterial cultures (Staphylococcus aureus ATCC 25923, Bacillus cereus ATCC 11778, Escherichia coli ATCC 25922, Salmonella typhi ATCC 6539) were taken from the Microbial Storage Bank, Department of Biosystems Technology, Faculty of Technology, University of Sri Jayewardenepura. The Caco-2 cell line was obtained from the Institute of Biochemistry, Molecular Biology, and Biotechnology, University of Colombo. Zebrafish (Danio rerio) embryos were sourced from the Medical Research Institute, Colombo. Reagents and solvents (HPLC/analytical/food grade) were from Sigma Aldrich (St. Louis, MO) and Merck (Darmstadt, Germany). Chemicals included BHA, BHT, TBHQ, Folin–Ciocalteu reagent, gallic acid, quercetin, methanol, acetonitrile, acetic acid, DPPH, ABTS, fetal bovine serum (sterile-filtered, Capricorn, South America), Dulbecco's Modified Eagle Medium (high glucose 4.5 g L−1, with L-glutamine, sodium pyruvate and phenol red), SRB dye, anti-chlorine water, thiobarbituric acid, Mueller–Hinton agar (pH 7.3 ± 0.1 at 25 °C), ampicillin, total plate count agar (pH 7.0 ± 0.2 at 25 °C), and potato dextrose agar (pH 5.6 ± 0.2 at 25 °C). Ingredients for baked products (wheat flour, sugar, margarine, eggs, salt, and baking powder) were sourced locally.2.2 Preparation of phenolic extracts
Phyllanthus emblica seeds were separated manually and dried at 40 °C for 12 h in an oven (LDO-150F, Labtech Co., Ltd, Korea). The dried seeds were milled into fine powder (<180 µm) using a grinder (SL-T2-550, SILIL, Sri Lanka) and stored at 4 °C until use. Phyllanthus emblica seeds extract (PSE) was prepared using ultrasound-assisted extraction (UAE) according to the method reported by Irakli et al.28 An aliquot (1.0 g) of dried powder was mixed with 3 mL of 100% ethyl acetate and introduced to an ultrasonicator (LD-LUHS-A16, Labtron, USA) and extracted at 40 kHz for 10 min (<40 °C). Ethyl acetate was chosen as the extraction solvent owing to its moderate polarity, which effectively solubilizes a broad spectrum of phenolic acids and flavonoids of mid-to-low polarity, contributing to extracts with enhanced antioxidant activity.29 After ultrasonic-assisted extraction, the crude extract of Phyllanthus emblica seed was centrifuged at 1500×g for 10 min using a centrifuge (Sorvall ST 8R, Thermo Fisher Scientific, Germany, 2021) and the supernatant was filtered through Whatman No.1 paper. Then, the filtrate was evaporated under vacuum at 40 °C on a rotary evaporator (RE-301, Yamato Scientific Co., Ltd, USA), and stored in dark glass vials at −20 °C until further use.2.3 Total phenolic content
The total phenolic content of PSE was determined using the Folin–Ciocalteu method.30 An aliquot (0.1 mL) of phenolic extract was mixed with 0.5 mL Folin–Ciocalteu reagent (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 diluted) and neutralized with 0.4 mL sodium carbonate (7.5%, w/v). After 1 h incubation in the dark at room temperature, absorbance was measured at 765 nm using a spectrophotometer (G10S UV-vis, Thermo Fisher Scientific, USA). The total phenolic content was calculated from a gallic acid standard curve (y = 0.0121x + 0.0112, r2 = 0.999) and expressed as g GAE per kg DW.
10 diluted) and neutralized with 0.4 mL sodium carbonate (7.5%, w/v). After 1 h incubation in the dark at room temperature, absorbance was measured at 765 nm using a spectrophotometer (G10S UV-vis, Thermo Fisher Scientific, USA). The total phenolic content was calculated from a gallic acid standard curve (y = 0.0121x + 0.0112, r2 = 0.999) and expressed as g GAE per kg DW.
2.4 Total flavonoid content
The total flavonoid content of PSE was determined using the aluminum chloride colorimetric method.31 An aliquot (0.5 mL) of phenolic extract was mixed with 1.5 mL of solvent, 0.1 mL of 10% aluminum chloride, and 0.1 mL of 1 M sodium acetate, and the volume was adjusted to 3 mL with solvent. After 30 min incubation in the dark at room temperature, absorbance was measured at 415 nm using a spectrophotometer (G10S UV-vis, Thermo Fisher Scientific, USA). The total flavonoid content was calculated from a quercetin standard curve (y = 0.0066x + 0.0078, r2 = 0.997) and expressed as g QE per kg DW.2.5 HPLC profile
Identification of phenolic compounds and flavonoids was performed by HPLC (high-performance liquid chromatography) according to Aguilar-Hernandez et al.32 The extracts (10 µL) were injected into an HPLC system (Agilent 1260 Infinity, Agilent Technologies, SA) equipped with a photodiode array detector fitted with a C18 reverse-phase column (5 µm particle size, 4.6 mm diameter, 150 mm long; Agilent Eclipse XDB, Agilent Technologies). The isocratic mobile phase consisted of 53% methanol and 2% acetic acid at a flow rate of 0.4 mL min−1. The peak area was detected at 280–365 nm. Compounds were quantified by calibration curves of standards (gallic acid, vanillic acid, sinapic acid, p-coumaric acid, ellagic acid, myricetin, and quercetin) at 25–200 µg mL−1. The results were expressed in mg per g DW.2.6 Antioxidant activity
| Scavenging activity (%) = [(A0 − A1)/A0] × 100 | (1) |
| Reducing power (%) = [(A1/A0) − 1] × 100 | (2) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio and incubated overnight at 4 °C in the dark to form a dark green stock solution. Before use, it was diluted with methanol to get an absorbance of 0.70 ± 0.02 at 734 nm. A 0.2 mL of different sample concentrations (10, 15, 20, 25, 30, 35 µg mL−1) was mixed with 5.8 mL ABTS working solution, and absorbance was recorded after 6 min using a spectrophotometer (G10S UV-vis, Thermo Fisher Scientific, USA). Scavenging activity was calculated using eqn (3), where A0 and A1 are the absorbance of the control and sample, respectively, and expressed as IC50.
1 ratio and incubated overnight at 4 °C in the dark to form a dark green stock solution. Before use, it was diluted with methanol to get an absorbance of 0.70 ± 0.02 at 734 nm. A 0.2 mL of different sample concentrations (10, 15, 20, 25, 30, 35 µg mL−1) was mixed with 5.8 mL ABTS working solution, and absorbance was recorded after 6 min using a spectrophotometer (G10S UV-vis, Thermo Fisher Scientific, USA). Scavenging activity was calculated using eqn (3), where A0 and A1 are the absorbance of the control and sample, respectively, and expressed as IC50.| Scavenging activity (%) = [(A0 − A1)/A0] × 100 | (3) |
2.7 Thermal stability
The thermal stability of PSE at different concentrations (50, 100, 150, 200, and 250 µg mL−1) and of the synthetic antioxidants was assessed by measuring the retention of antioxidant activity after heating at 180 °C for 2 h in an oven (LDO-150F, Labtech Co., Ltd, Korea) according to the method reported by Seneviratne et al.36 The stability was expressed as a percentage of the initial (non-heated) FRAP values (Section 2.5.2) and compared with the retained percentages of BHT, BHA, and TBHQ.2.8 Antimicrobial activity
The antimicrobial activity of PSE was evaluated using the disc diffusion method37 and compared with BHT, BHA, and TBHQ. Four food-borne pathogenic bacteria were tested: Staphylococcus aureus (ATCC 25923) and Bacillus cereus (ATCC 11778) as Gram-positive, and Escherichia coli (ATCC 25922) and Salmonella typhi (ATCC 6539) as Gram-negative. Subcultures were prepared by inoculating 100 µL of each strain into 10 mL of peptone water and standardizing bacterial suspensions to 0.5 McFarland. Mueller–Hinton agar plates were inoculated with the bacterial cultures, and filter paper discs soaked in 100 µL of the sample were placed on the surface. Ampicillin served as the positive control, and 100% ethyl acetate was the negative control. Plates were incubated at 37 °C for 18–24 h, and inhibition zones were measured using calipers. All tests were performed in triplicate.2.9 In vitro cytotoxicity
The cytotoxicity of PSE was assessed using the method reported by Mandim et al.38 and compared with BHT, BHA, and TBHQ. Caco-2 cells were maintained in DMEM supplemented with 10% fetal bovine serum, 2 mM glutamine, and antibiotics (penicillin 100 U mL−1 and streptomycin 100 mg mL−1) at 37 °C with 5% CO2. Cells with 70–80% confluence were treated with antioxidants at different concentrations (50, 100, 200, 400, and 800 µg mL−1), while untreated cells served as controls. After adherence, cells (0.5 × 104 per well) were incubated for 24, 48, and 72 h. The reaction was stopped with 20 µL of 10% (w/v) TCA, incubated at 4 °C for 1 h, washed, dried, and stained with 50 µL of 0.4% (w/v) SRB for 15 min. Excess SRB was removed with 1% (v/v) acetic acid, and adhered SRB was solubilized with 100 µL of 10 mM Tris base. Absorbance was recorded at 540 nm (Synergy HT, Biotek, USA). The cell viability (%) and IC50 values were calculated.2.10 In vivo toxicity
Zebrafish (Danio rerio) embryos were exposed to PSE, BHA, BHT, and TBHQ following OECD guideline.39 At 6 hours post-fertilization (hpf), healthy embryos were selected and 10 fertilized eggs per concentration were transferred to individual wells of 6-well plates. Embryos were exposed to five concentrations (50, 100, 200, 400, and 800 µg mL−1) of extracts in 2 mL of distilled water, while the control group was kept with diluted anti-chlorine water. All the treatments were carried out in triplicate at approximately 28 °C. Embryos were observed under a 1600x USB Digital Microscope (China) at 24, 48, 72, and 96 hpf to record survival rate (%), hatching rate (%), mortality rate (%), heartbeat (bpm), and developmental anomalies (pericardial edema, yolk sac edema, spinal curvature, and non-structural deformities). Toxicity was assessed by calculating the lethal concentration (LC50).40,412.11 Effect of antioxidants on lipid oxidation, microbial shelf-life and sensory quality of baked products
Once the baked products were prepared, their proximate composition, color, and textural properties were analyzed. Throughout the storage period, formation of peroxides and TBARS, microbial activity, and sensory properties were determined to assess the effectiveness of the natural antioxidants in preserving product stability and consumer appeal.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (w/v) ratios. The mixture was then shaken in an orbital shaker for 20 minutes. The lipid extract was decanted, and the extraction process was repeated twice with fresh portions of hexane. All extracts were combined, dried over anhydrous sodium sulfate, and filtered. The hexane was evaporated using a rotary evaporator (RE-301, Yamato Scientific Co., Ltd, USA). The lipid extract was then stored at −20 °C until further analysis.
3 (w/v) ratios. The mixture was then shaken in an orbital shaker for 20 minutes. The lipid extract was decanted, and the extraction process was repeated twice with fresh portions of hexane. All extracts were combined, dried over anhydrous sodium sulfate, and filtered. The hexane was evaporated using a rotary evaporator (RE-301, Yamato Scientific Co., Ltd, USA). The lipid extract was then stored at −20 °C until further analysis.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol (7
methanol (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 v/v) and vortexed for 2 s. Ammonium thiocyanate solution (50 µL) was added and vortexed again for 2 s. Then, 50 µL of iron(II) chloride solution was added and mixed well. Incubation was carried out for 5 min in the dark, and the absorbance was measured using a spectrophotometer (G10S UV-vis, Thermo Fisher Scientific, USA) at 500 nm against the blank, which contains all reagents except the lipid sample. To construct the calibration curve, a stock solution of iron(III) with a concentration of 10 µg mL−1 was prepared, and the procedure was followed for different concentrations of the working solution (1–10 µg mL−1).
3 v/v) and vortexed for 2 s. Ammonium thiocyanate solution (50 µL) was added and vortexed again for 2 s. Then, 50 µL of iron(II) chloride solution was added and mixed well. Incubation was carried out for 5 min in the dark, and the absorbance was measured using a spectrophotometer (G10S UV-vis, Thermo Fisher Scientific, USA) at 500 nm against the blank, which contains all reagents except the lipid sample. To construct the calibration curve, a stock solution of iron(III) with a concentration of 10 µg mL−1 was prepared, and the procedure was followed for different concentrations of the working solution (1–10 µg mL−1).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol (7
methanol (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 v/v), mixed with 5 mL of TBA–TCA solution and vortexed. The resulting solution was boiled at 95 °C for 15 min in a water bath (SWB-C, Biobase Co., Ltd, China), cooled immediately, and the absorbance was measured using a spectrophotometer (G10S UV-vis, Thermo Fisher Scientific, USA). A calibration curve was prepared using 1,1,3,3-tetraethoxy propane as the standard solution. The results were expressed using eqn (4), where A is the absorbance of oil at 532 nm, 72.3 is the molecular weight of malondialdehyde, C is the slope of the calibration curve and W is the sample weight (g).
3 v/v), mixed with 5 mL of TBA–TCA solution and vortexed. The resulting solution was boiled at 95 °C for 15 min in a water bath (SWB-C, Biobase Co., Ltd, China), cooled immediately, and the absorbance was measured using a spectrophotometer (G10S UV-vis, Thermo Fisher Scientific, USA). A calibration curve was prepared using 1,1,3,3-tetraethoxy propane as the standard solution. The results were expressed using eqn (4), where A is the absorbance of oil at 532 nm, 72.3 is the molecular weight of malondialdehyde, C is the slope of the calibration curve and W is the sample weight (g).| TBARS (mg malondialdehyde equivalent/g of oil) = (A × 72.3)/(C × W) | (4) |
2.12 Statistical analysis
All samples were analyzed in triplicate unless otherwise specified. All the data were subjected to analysis of variance (ANOVA) using Minitab version 17 statistical package (Minitab, LCC), while the mean separation was performed by Tukey's pairwise test with a significance level of p < 0.05. The study results were graphically represented using the OriginPro® 2025 (OriginLab Corporation, Northampton, MA, USA) software.3 Results and discussion
3.1 Total phenolic and flavonoid content
In the ethyl acetate extract of Phyllanthus emblica seed, TPC was 22.72 ± 0.10 g GAE per kg and TFC was 15.62 ± 0.05 g QE per kg seed. The selection of a suitable extraction method and the solvent are very important when considering extraction efficiency. Kaur et al.55 reported that ultrasound-assisted PSE provided the highest TPC (5.82 ± 0.05) compared to maceration (1.71 ± 0.05) and shaking (3.08 ± 0.05) methods due to structural disruption in plant cell walls, resulting in direct contact with the solvent. In a similar study, Mishra et al.56 found that the extraction yield (%) of total phenolics from Phyllanthus emblica seed was significantly higher in ethyl acetate extracts (57.2 ± 2.01) compared to butanol (24.31 ± 1.20) and aqueous extracts (14.88 ± 1.16).3.2 HPLC profile
HPLC analysis of the PSE identified seven major phenolic acids and flavonoids (Fig. 1). They were detected at different retention times ranging from 3.9 to 12.8 min (Table S1). The identified compounds included gallic acid, vanillic acid, sinapic acid, p-coumaric acid, ellagic acid, myricetin, and quercetin. As denoted in Fig. 1, quercetin (30.03 ± 0.24 mg per g DW) was present in the highest concentration, followed by ellagic acid (11.35 ± 0.08 mg per g DW), p-coumaric acid (9.99 ± 0.04 mg per g DW), gallic acid (7.79 ± 0.08 mg per g DW), sinapic acid (6.53 ± 0.06 mg per g DW), and myricetin (4.81 ± 0.03 mg per g DW), whereas vanillic acid (3.22 ± 0.14 mg per g DW) was present in the lowest concentration. The high abundance of quercetin and ellagic acid indicates strong potential antioxidant and antimicrobial properties, as both compounds are well recognized for their radical scavenging activity, metal chelating ability, and bioactive effects.57,58 Gallic acid further contributes to the antioxidant capacity of the extract due to its high redox potential and capacity to neutralize free radicals. Although present in lower concentrations, vanillic acid, sinapic acid, p-coumaric acid, and myricetin may provide complementary or synergistic effects, thereby enhancing the overall bioactivity of the extract.59 Importantly, several of these phenolic acids, such as gallic acid, ellagic acid, and quercetin, have been reported to exhibit relatively high thermal stability. For instance, gallic acid shows limited degradation at moderate to high temperatures (at 180 °C),60 while ellagic acid retains stability under high temperature conditions (at 200 °C),61 enabling it to retain bioactivity during thermal treatment. Quercetin, though somewhat sensitive to prolonged heating, can remain functionally active under controlled processing conditions, particularly when present in complex food matrices.62A similar study by Nambiar et al.63 identified the phenolic profile of the methanolic extract of P. emblica seeds (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 w/v). They reported the presence of phenolic compounds such as caffeic acid (0.022 µg mg−1), syringic acid (0.003 µg mg−1), p-coumaric acid (0.013 µg mg−1), myricetin (71.280 µg mg−1), and tannic acid (6.461 µg mg−1), which contribute to the total antioxidant activity depending on both their relative concentrations and synergistic interactions. The phenolic profile of P. emblica seed extract obtained in this study differed markedly from that reported by Nambiar et al.63 While their methanolic extract was dominated by myricetin (71.28 mg g−1), our ethyl acetate extract contained comparatively low levels of myricetin (4.81 mg per g DW) but a high abundance of quercetin (30.03 ± 0.24 mg per g DW), which was not detected in their study. These variations can be primarily attributed to the differences in solvent polarity and extraction techniques. Methanol, being highly polar, facilitates efficient extraction of polar flavonols such as myricetin, whereas ethyl acetate, with moderate polarity, is more selective for less polar flavonoids and free phenolic acids, thereby increasing quercetin elution. In addition, the UAE applied in the present study may have induced partial hydrolysis of glycosidic conjugates, liberating free quercetin that would otherwise remain undetected in conventional maceration.
20 w/v). They reported the presence of phenolic compounds such as caffeic acid (0.022 µg mg−1), syringic acid (0.003 µg mg−1), p-coumaric acid (0.013 µg mg−1), myricetin (71.280 µg mg−1), and tannic acid (6.461 µg mg−1), which contribute to the total antioxidant activity depending on both their relative concentrations and synergistic interactions. The phenolic profile of P. emblica seed extract obtained in this study differed markedly from that reported by Nambiar et al.63 While their methanolic extract was dominated by myricetin (71.28 mg g−1), our ethyl acetate extract contained comparatively low levels of myricetin (4.81 mg per g DW) but a high abundance of quercetin (30.03 ± 0.24 mg per g DW), which was not detected in their study. These variations can be primarily attributed to the differences in solvent polarity and extraction techniques. Methanol, being highly polar, facilitates efficient extraction of polar flavonols such as myricetin, whereas ethyl acetate, with moderate polarity, is more selective for less polar flavonoids and free phenolic acids, thereby increasing quercetin elution. In addition, the UAE applied in the present study may have induced partial hydrolysis of glycosidic conjugates, liberating free quercetin that would otherwise remain undetected in conventional maceration.
3.3 Antioxidant activity
The antioxidant activity was evaluated using three different assays: DPPH radical scavenging activity, ferric reducing antioxidant power (FRAP), and ABTS˙+ scavenging activity (Fig. S1). A lower IC50 value corresponds to higher antioxidant activity, as it reflects the concentration required to inhibit 50% of the free radicals. In the DPPH assay (Table 1), PSE demonstrated the strongest antioxidant activity because its IC50 was significantly (p < 0.05) lower (41.23 ± 0.29 µg mL−1) compared to BHT (213.76 ± 0.47 µg mL−1), BHA (295.43 ± 1.40 µg mL−1) and TBHQ (44.35 ± 2.15). Similarly, in the ABTS assay (Table 1), PSE exhibited significantly (p < 0.05) higher antioxidant activity (29.31 ± 0.27 µg mL−1) than BHT (65.62 ± 0.55 µg mL−1), BHA (54.21 ± 0.16 µg mL−1), and TBHQ (30.67 ± 0.38 µg mL−1). The FRAP assay (Table 1) further highlighted the highest reducing power percentage of PSE (180.34 ± 1.03%) compared to BHT (45.36 ± 1.21%) and BHA (36.76 ± 1.24%), with TBHQ (153.31 ± 4.06%) again showing the highest antioxidant activity. Jaiboonma et al.29 reported that the antioxidant activity of PSE depends on the polarity of the extraction solvent. Ethyl acetate, with moderate polarity, exhibited the highest activity by efficiently extracting a balanced mix of phenolic compounds and flavonoids, whereas non-polar hexane mainly extracted lipophilic compounds with low antioxidant potential and highly polar butanol primarily extracted non-antioxidant sugars. Thus, the optimal solvent selectivity makes it more effective in enhancing antioxidant activity across DPPH, FRAP, and ABTS˙+ assays. Comparatively, as reported by Jaiboonma et al.,29 ethyl acetate extracts showed the highest FRAP activity (751.92 ± 5.22 mg AAE per g extract) and the strongest ABTS˙+ scavenging activity (IC50 = 31.53 ± 0.36 µg mL−1). A similar study was conducted by Mishra et al.,56 in which PSE was extracted using water, diethyl ether, butanol and ethyl acetate, and the maximum DPPH free radical scavenging activity (61.07 ± 1.63%) was achieved by the ethyl acetate fraction. The DPPH radical scavenging activity of the aqueous fraction (0.098 ± 0.98%) and butanol fraction (12.99 ± 2.25%) remained lower compared to the ethyl acetate fraction. The reason for the poor DPPH free radical scavenging activity of the aqueous extract of the seed may be that the major portion of phenolic acids and flavonoids present in the seed has poor solubility in water and hence could not be partitioned into water. According to our study, the antioxidant activity of the tested compounds followed the ranking order: PSE > TBHQ > BHT > BHA, which showed that PSE exhibited stronger antioxidant potential than the commonly used synthetic antioxidants BHT, BHA and TBHQ. The antioxidant activity of PSE is likely due to its content of flavonoids and phenolic compounds including myricetin, tannic acid, syringic acid, and coumaric acid.64 However, TBHQ exhibited significantly (p < 0.05) higher antioxidant activity among other synthetic antioxidants. The highest activity of TBHQ can be attributed to its highly reactive structure, which enhances hydrogen donation and radical scavenging efficiency.65 Phenolic compounds and flavanoids present in PSE are well known for their strong antioxidant potential, primarily due to their ability to neutralize free radicals and chelate metal ions.66 These findings demonstrate that PSE is a highly effective natural antioxidant, with activity comparable to and in some cases exceeding that of synthetic antioxidants such as BHT and BHA.| DPPH˙ IC50 (µg mL−1) | ABTS˙+ IC50 (µg mL−1) | FRAP reduced percentage (%) | |
|---|---|---|---|
| a Each data point represented mean ± SD (n = 3). Different lowercase letters showed a significant difference in the same column (p < 0.05). IC50: half maximal inhibitory concentration, PSE: Phyllanthus emblica seed extract, BHT: butylated hydroxytoluene, BHA: butylated hydroxyanisole, and TBHQ: tert-butylhydroquinone. | |||
| PSE | 41.23 ± 0.29c | 29.31 ± 0.27c | 180.34 ± 1.03a |
| BHT | 213.76 ± 0.47b | 65.62 ± 0.55a | 45.36 ± 1.21c |
| BHA | 295.43 ± 1.40a | 54.21 ± 0.16b | 36.76 ± 1.24d |
| TBHQ | 44.35 ± 2.15c | 30.67 ± 0.38c | 153.31 ± 4.06b |
The strong antioxidant activity of PSE is likely due to the combined and complementary effects of its phenolic compounds, including gallic, vanillic, sinapic, p-coumaric, and ellagic acids, as well as myricetin and quercetin. The hydroxybenzoic and hydroxycinnamic acids (such as gallic, vanillic, p-coumaric, and sinapic acids) act as fast hydrogen donors, neutralizing free radicals at both water-based and lipid interfaces.67,68 Flavonols such as quercetin and myricetin, on the other hand, have strong chain-breaking abilities and can stabilize free radicals through resonance, thereby helping to protect lipid membranes from oxidative damage.69 Ellagic acid enhances this protection by effectively binding metal ions, thereby reducing the formation of highly reactive radicals through Fenton-type reactions.70 Moreover, the smaller phenolic acids can regenerate flavonols through redox interactions and extending the overall antioxidant activity of the mixture.68 These combined mechanisms of phenolic acids and flavonoids create a synergistic effect that provides greater antioxidant protection than any single compound alone.71 This synergy explains why the crude extract shows superior performance compared to individual synthetic antioxidants under real food storage conditions.
Extraction is a critical step in unlocking the potential of phenolic antioxidants from plant matrices, and recent advances in extraction technologies have enabled greater efficiency, reduced solvent consumption, lower energy usage, and shorter processing times. Among these, ultrasound-assisted extraction (UAE) has emerged as a “green” technique that employs acoustic cavitation to disrupt plant cell walls, enhance mass transfer, and improve the yield of target bioactive compounds. Studies have consistently shown that UAE significantly increases total phenolic content (TPC), total flavonoid content (TFC), and overall antioxidant activity compared to conventional solvent extraction. For instance, Yi et al.72 demonstrated that sonication is more efficient than reflux or Soxhlet extraction, and that 80% methanol yields higher recovery of phenolic compounds than ethanol or other aqueous solvent mixtures, highlighting the importance of both solvent selection and extraction parameters. Similarly, for the leaves of Phyllanthus emblica, UAE achieved a phenolic yield of 56.82% compared to only 16.78% using conventional vortex mixing, illustrating the superior efficiency of ultrasound-assisted techniques. The high yield, reduced processing time, and lower solvent requirement make UAE highly suitable for further research studies.73 The scalability and compatibility with green extraction principles further enhance its applicability for sustainable utilization of plant by-products and the development of natural antioxidant-enriched food systems.
3.4 Thermal stability
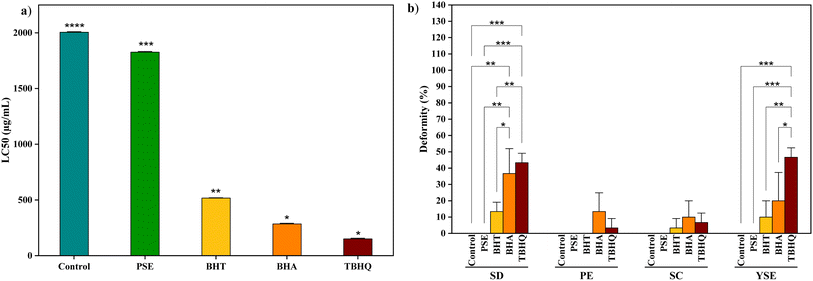
The thermal stability of antioxidants is crucial in determining their suitability for incorporation into baked products at baking temperatures, as antioxidants lose their stability due to their volatility at high temperatures. In this study, the thermal stability of the antioxidants at 200 µg mL−1 concentration was evaluated because it aligns with the recommended concentration for baked products.74 The results revealed that the stability of all tested antioxidants was decreased after exposure to the high-temperature treatment. However, the retained thermal stability varied significantly among the samples. Notably, PSE exhibited the highest retained stability of 91% compared to all the synthetic antioxidants, including TBHQ (87%), BHT (62%), and BHA (63%). Mehta et al.25 identified that PSE consists of tannins such as ellagic acid and punicalagin, which have the ability to form stable intermolecular bonds to withstand high temperatures. Therefore, PSE may exhibit higher thermal stability. TBHQ demonstrates higher thermal stability than BHA and BHT due to its hydroquinone structure and low volatility, which provide stronger electron-donating properties and greater resistance to thermal degradation.75 In contrast, BHA and BHT are more prone to oxidative cleavage at high temperatures due to their reactive hydroxyl groups, which break down under oxidative conditions.75,76 They are also volatile compounds, making them prone to evaporation when exposed to heat and leading to a loss of antioxidant effectiveness.76 This makes them less stable compared to natural antioxidants such as PSE. The thermal stability of the antioxidants can be ordered as PSE > TBHQ > BHA > BHT. This thermal resilience of PSE enhances its applicability as a natural alternative to synthetic antioxidants in baked products.3.5 Antimicrobial activity
The investigated antioxidants at 200 µg mL−1 exhibited antibacterial activity against four foodborne pathogenic bacterial strains including two Gram-positive bacterial strains (Staphylococcus aureus and Bacillus cereus) and two Gram-negative bacterial strains (Escherichia coli and Salmonella typhi). The results of the antibacterial potential are presented in Table 2. As shown in Table 2, TBHQ showed significantly higher (p < 0.05) antibacterial inhibition among the tested antioxidants. In the present study, PSE showed zone inhibition against pathogenic bacteria as follows: S. aureus (8.83 ± 0.62 mm), B. cereus (14.17 ± 1.03 mm), S. typhi (7.50 ± 0.41 mm), and E. coli (10.83 ± 0.62 mm). The results revealed that the tested antioxidants have a more inhibitory effect on the growth of S. aureus due to their high sensitivity. Previous research by Anbuselvi and Jha77 reported that the ethyl acetate fractions of crude PSE showed a 7.2 mm zone of inhibition against S. aureus, because bioactive compounds, including quercetin, phyllantine, phyllantidine, gallic acid, ascorbic acid, emblicanin A and emblicanin B in PSE, are responsible for targeting pathogenic bacteria that impede their growth.26 Several studies have reported that Gram-positive bacterial strains are more sensitive to bioactive compounds present in extracts relative to Gram-negative bacterial strains,37 owing to the cell walls of Gram-negative bacteria being constructed with lipopolysaccharides, which restrict the access of bioactive compounds in extracts.78 Martin et al.79 reported that ethanolic extracts of yerba mate showed antimicrobial activity against S. aureus and L. monocytogenes but not against E. coli. Among the tested strains, Gram-positive bacteria were much more sensitive to almost all the antioxidants, while BHA and BHT showed the lowest inhibition against Gram-negative bacteria strains (E. coli and S. typhi) (Table 2). An observational study by Walter et al.80 presented that BHT was the least effective against Gram-negative bacteria such as Salmonella sp. and E. coli compared to the Gram-positive ones. Agada et al.81 reported that BHA at 200 µg mL−1 was bactericidal to S. aureus, while S. typhi and E. coli were inhibited by 400 µg mL−1 of BHA. According to Shelef and Liang,82 the effect of BHA was bacteriostatic at a concentration less than 200 µg mL−1. By comparing the values, it can be stated that synthetic antioxidants BHT and BHA have significantly lower antibacterial activity than other tested antioxidants. Although the standard antibiotic (ampicillin) and TBHQ exhibited stronger inhibitory effects, PSE still demonstrated notable and broad antimicrobial activity in addition to its primary antioxidant function. This dual antioxidant and antibacterial activity highlights PSE as a promising multi-functional natural preservative for baked products.| Diameter of the inhibition zone (mm) | ||||
|---|---|---|---|---|
| S. aureus | B. cereus | E. coli | S. typhi | |
| a Each data point represented mean ± SD (n = 3). Different lowercase letters show significant differences in the same column (p < 0.05). PSE: Phyllanthus emblica seed extract, BHT: butylated hydroxytoluene, BHA: butylated hydroxyanisole, and TBHQ: tert-butylhydroquinone. | ||||
| PSE | 8.83 ± 0.62b | 14.17 ± 1.03a | 10.83 ± 0.62b | 7.50 ± 0.41c |
| BHT | 6.77 ± 0.21c | 6.00 ± 0.00d | 6.00 ± 0.00d | 6.00 ± 0.00d |
| BHA | 6.50 ± 0.00c | 6.00 ± 0.00d | 6.00 ± 0.11d | 6.00 ± 0.00d |
| TBHQ | 8.57 ± 0.33b | 6.57 ± 0.33c | 9.50 ± 0.41c | 8.00 ± 0.10b |
| Ampicillin | 11.50 ± 0.41a | 9.50 ± 0.40b | 19.50 ± 0.41a | 15.83 ± 0.24a |
3.6 In vitro cytotoxicity
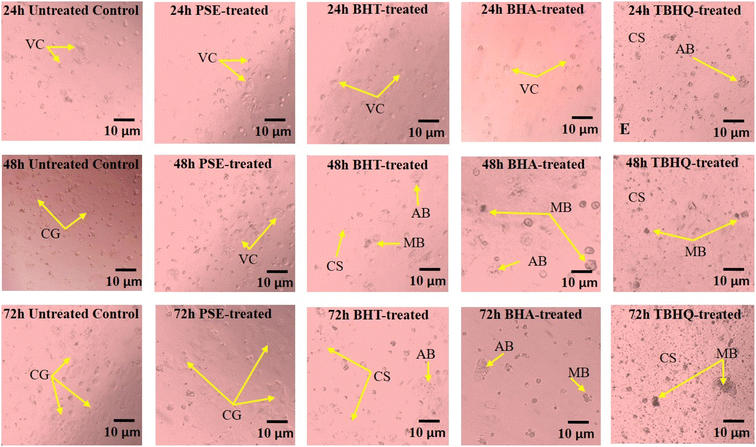
The Caco-2 cell line is known for its ability to develop into cells resembling intestinal epithelium, which makes it a valuable model for testing the safety of antioxidants. It also helps in understanding how these compounds are absorbed and metabolized, and how they impact intestinal cells.83 The higher IC50 suggest a higher cyto-compatibility effect over Caco-2 cells from the extracts, while lower IC50 represent higher cytotoxic activity. In the present study, cytotoxicity evaluation was focused on the concentration of 200 µg mL−1, aligning with the recommended antioxidant concentration for incorporation into food products. After 72 h of incubation, TBHQ exhibited the lowest IC50 (53.48 ± 5.36 µg mL−1) among the synthetic antioxidants (Table 3), followed by BHA (375.34 ± 0.14) and BHT (596.10 ± 2.35). A study by Esazadeh et al.84 reported TBHQ-induced oxidative stress and apoptosis in intestinal epithelial cells with an IC50 value below 100 µg mL−1, confirming high toxicity. Saito et al.85 also confirmed that BHA and BHT induce ROS generation and disrupt cellular membranes, though with milder effects than TBHQ. In the present study, PSE exhibited the highest IC50 value (1025.37 ± 4.14), as displayed in Table 3. Martínez et al.17 studied Phyllanthus emblica extract and reported an IC50 > 1000 µg mL−1 in Caco-2 cells, similar to this study. Chowdhury et al.86 found that Phyllanthus niruri extract showed mild cytotoxicity (IC50 – 900 µg mL−1) in HepG2 cells, further confirming the low toxicity of Phyllanthus species. Harikumar and Kuttan87 demonstrated that Phyllanthus amarus extract protects intestinal cells from oxidative stress, contrasting with the pro-oxidant nature of synthetic antioxidants. The results revealed that the PSE, BHT, and BHA cell viability increased up to 48 h and then gradually decreased after 72 h. However, the cell viability of TBHQ was decreased with time because TBHQ can get metabolized into tert-butyl-p-benzoquinone, which is more toxic and highly volatile resulting in the induction of apoptosis in affected cells.88 Furthermore, Fig. 2 illustrates the effect of PSE and synthetic antioxidants on the morphology of Caco-2 cells over 24 h, 48 h, and 72 h of incubation at 200 µg mL−1. The micrographs indicate that the untreated control cells maintained viable cells and consistent growth throughout the observation period, confirming the absence of any external cytotoxic influence. Similarly, PSE-treated cells exhibited active cell growth with no significant signs of cytotoxicity over time, suggesting high cell viability and low toxicity. In contrast, cells treated with synthetic antioxidants displayed varying degrees of morphological alterations indicative of cytotoxic effects. BHT-treated cells showed mild cytotoxic effects with slightly reduced cell density over time. BHA treatment resulted in noticeable cell damage at 72 h compared to 24 h and 48 h, suggesting that BHA induces cytotoxicity upon prolonged exposure. Importantly, TBHQ-treated Caco-2 cells exhibited increased cell shrinkage, apoptotic bodies and membrane blebbing alongside a yellowish-red discoloration of the media. Thus, TBHQ may cause severe oxidative stress and cytotoxicity. These effects are consistent with previous findings by Khezerlou et al.,89 who reported similar cytotoxic and oxidative stress-related changes in cells exposed to TBHQ. The observed cytotoxicity trend was TBHQ > BHA > BHT > PSE. TBHQ and BHA are widely used synthetic antioxidants that have been previously reported to induce oxidative stress and trigger apoptosis in cells in the gastrointestinal tract.84 Similarly, BHA and BHT are known to exert cytotoxic effects due to their ability to disrupt cellular membranes and induce ROS production.85 In contrast, PSE is derived from a natural source that displays significantly lower cytotoxicity. The reduced cytotoxicity of PSE can be attributed to the presence of polyphenols such as tannins and flavonoids, which not only provide antioxidant activity but also exhibit protective effects against oxidative stress-induced cell damage.90| Extract | IC50 value (µg mL−1) | ||
|---|---|---|---|
| 24 h | 48 h | 72 h | |
| a Each data point represented mean ± SD (n = 3). Different lowercase letters show significant difference in the same column and different uppercase letters show significant difference in the same row (p < 0.05). PSE: Phyllanthus emblica seed extract, BHT: butylated hydroxytoluene, BHA: butylated hydroxyanisole, and TBHQ: tert-butylhydroquinone. | |||
| PSE | 740.98 ± 1.25 aC | 1167.11 ± 2.30 aA | 1025.37 ± 4.14 aB |
| BHT | 574.93 ± 2.41bC | 681.62 ± 4.21bA | 596.10 ± 2.35bB |
| BHA | 428.52 ± 3.64 cB | 494.61 ± 5.27 cA | 375.34 ± 0.14 cC |
| TBHQ | 210.21 ± 1.21 dA | 109.32 ± 0.92 dB | 53.48 ± 5.36 dC |
3.7 In vivo toxicity
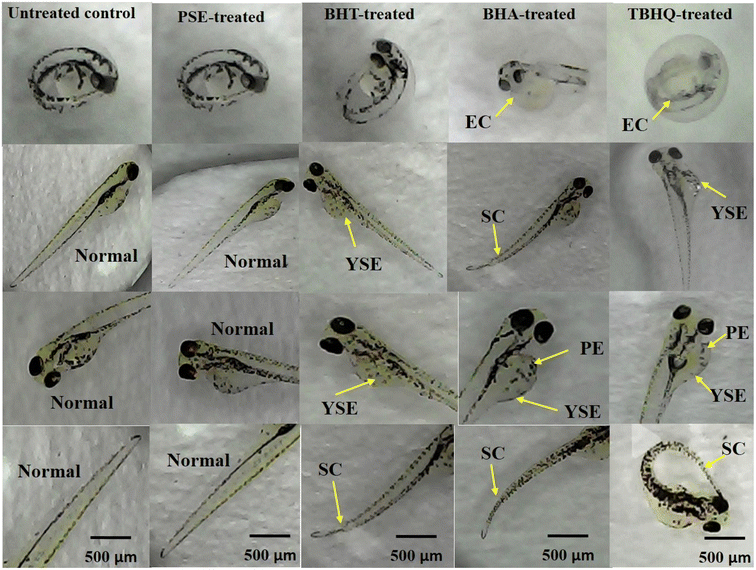
Several studies have reported that high doses of polyphenolic-rich foods can potentially cause adverse effects through pro-oxidative effects.91 The toxicological assessment of Danio rerio embryos exposed to natural and synthetic antioxidants at 24, 48, 72, and 96 hpf revealed distinct differences in their development. As shown in Fig. 2, embryos treated with PSE demonstrated consistently high survival rates (>90%), low mortality (<10%) and hatching rates exceeding 85% by 72 hpf. In contrast, embryos exposed to TBHQ exhibited significant developmental abnormalities. By 96 hpf, survival dropped below 50%, mortality exceeded 40% and hatching remained under 30%. Embryos treated with BHA and BHT showed intermediate levels of toxicity. BHT-exposed embryos had a survival rate of 70%, hatching rate of 60–65% and mortality of around 25%. In comparison, BHA exposure led to a slightly higher toxicity, with survival falling to 60%, hatching to 50%, and mortality nearing 35%. The normal heartbeat rate of zebrafish embryos ranges from 120 to 180 beats per minute (bpm).92 The heartbeat rate of embryos exposed to antioxidants at 200 µg mL−1 was evaluated. The heartbeat evaluation started at 48 hpf because their heart begins to beat normally by 48 hpf.93 As shown in Fig. 3, there was no significant (p > 0.05) difference in the mean heartbeat rate (bpm) of PSE-treated embryos (150.00 ± 2.05) compared to the control group (154.00 ± 0.47). In contrast, BHT, BHA, and TBHQ exhibited dose-dependent cardiotoxic effects, with TBHQ (128.00 ± 1.25) showing the most significant reduction in heart rate, followed by BHA (132.00 ± 1.25) and BHT (145.00 ± 2.49). These results suggest that PSE exhibits a high level of biocompatibility and minimal toxicity. This effect may be attributed to its richness in phenolic compounds, particularly gallic acid, tannic acid, myricetin, and syringic acid, which possess strong free radical scavenging properties and contribute to the enhancement of cellular defense mechanisms during early embryonic development.LC50 refers to the concentration of a substance required to cause mortality in 50% of a test population, typically within a specified exposure period. A lower LC50 value indicates higher toxicity, while a higher LC50 value reflects lower toxicity. Fig. 4 represents a comparison of the LC50 (µg mL−1) of PSE with synthetic antioxidants: BHT, BHA, and TBHQ over post-fertilization. PSE consistently shows the highest LC50 at 96 hpf, indicating the lowest toxicity among the tested antioxidants. Maintaining an LC50 threshold above 200 µg mL−1 is essential for our study as this level indicates low toxicity and aligns with the recommended concentration limits for safe food application. Synthetic antioxidants show significantly (p < 0.05) lower LC50 due to higher toxicity over time compared to PSE. Among the synthetic antioxidants tested, TBHQ was found to be the most toxic with an LC50 of 189.25 ± 1.27 µg mL−1 at 96 hpf, which falls below the safety threshold of 200 µg mL−1. BHT showed the lowest toxicity (400.21 ± 0.45 µg mL−1), while BHA (204.28 ± 0.28 µg mL−1) was more harmful than BHT but still less toxic than TBHQ. These results emphasize the importance of identifying safer alternatives with LC50 values above 200 µg mL−1. Promisingly, the current study found that PSE had an LC50 of 1832.71 ± 1.22 µg mL−1 with a significantly safer profile compared to synthetic antioxidants. Furthermore, Yang et al.94 conducted research by exposing zebrafish embryos to four synthetic phenolic antioxidants: BHA, BHT, TBHQ, and 2,2′-methylenebis (6-tert-butyl-4-methylphenol) (AO2246). The 96 hpf LC50 values indicated varying degrees of acute toxicity, with the toxicity ranking as AO2246 > TBHQ > BHA > BHT. Notably, exposure to BHA, TBHQ, and AO2246 resulted in decreased hatching rates because non-lethal concentrations of BHA (≤20 µM), TBHQ (≤20 µM), BHT (≤200 µM), and AO2246 (≤2 µM) resulted in reduced heart rates and body lengths in a dose-dependent manner.
Fig. 5 reveals distinct differences in developmental toxicity among the tested antioxidants. Embryos exposed to PSE exhibited predominantly normal morphology, similar to the untreated control, resulting in minimal toxic effects. In contrast, embryos exposed to synthetic antioxidants such as BHT, BHA, and TBHQ showed visible signs of developmental toxicity. Malformations such as spinal curvature, yolk sac edema, pericardial edema, and even egg coagulation were commonly observed, especially in the BHA- and TBHQ-treated groups (Fig. 5). These findings are in agreement with those of Leonard et al.95 and Yang et al.,94 who observed that TBHQ disrupts redox homeostasis, induces apoptosis, and causes morphological deformities in zebrafish embryos such as pericardial edema and spinal curvature. Also, Baran et al.15 and Chen et al.96 reported that both BHA and BHT can interfere with antioxidant enzyme systems, delay embryonic development, and increase the incidence of malformations such as yolk sac edema and axial deformities. These deformities suggest that synthetic antioxidants may interfere with normal embryonic development and raising concerns about their safety. In contrast, the natural extract appeared to be much safer for reinforcing its potential as a more biocompatible and less toxic alternative for food applications.
3.8 Effect of antioxidants on the shelf-life of baked products
| Moisture | Ash | Fat | Protein | Fiber | Carbohydrate | |
|---|---|---|---|---|---|---|
| a Each data point represented mean ± SD (n = 3). | ||||||
| Sponge cake | 14.47 ± 0.12 | 1.03 ± 0.03 | 29.69 ± 0.11 | 10.19 ± 0.46 | 1.38 ± 0.07 | 43.23 ± 0.23 |
| Biscuits | 8.53 ± 0.94 | 0.92 ± 0.23 | 22.55 ± 0.20 | 0.92 ± 0.47 | 2.97 ± 0.33 | 60.49 ± 0.18 |
Table 5 shows that both sponge cakes and biscuits are not significantly (p > 0.05) different in resilience, cohesiveness, springiness, and color except hardness and chewiness. When considering the hardness, there is a significant (p < 0.05) difference across all sponge cakes and biscuits. In both sponge cakes and biscuits containing PSE, the hardness was lower than that of products containing synthetic antioxidants. The reduced hardness in PSE-added samples could be attributed to the inherent properties of the natural phenolic compounds, which may interact with the product matrix to retain moisture content and prevent excessive stiffness during storage.100 For example, research by Sulaiman et al.101 found that sponge cakes containing phenolic extracts from mango peel exhibited lower hardness than those containing synthetic antioxidants, likely due to the moisture-retaining capacity of phenolic compounds. Similarly, biscuits formulated with moringa leaf extract showed reduced hardness compared to BHT-containing biscuits.102
| Control | BHT-added | BHA-added | TBHQ-added | PSE-added | |
|---|---|---|---|---|---|
| a Each data point represented mean ± SD (n = 3). Different lowercase letters show significant differences in the same row (p < 0.05), L*, lightness; a*, redness/greenness; b*, yellowness/blueness. PSE: Phyllanthus emblica seed extract, BHT: butylated hydroxytoluene, BHA: butylated hydroxyanisole, and TBHQ: tert-butylhydroquinone. | |||||
| Sponge cake | |||||
| Hardness (g) | 2564.50 ± 0.15b | 2648.00 ± 0.24a | 2640 21 ± 0.27a | 2456.33 ± 0.11c | 2325.33 ± 1.35d |
| Resilience | 0.29 ± 0.45a | 0.30 ± 0.70a | 0.25 ± 0.20a | 0.27 ± 1.25a | 0.28 ± 0.64a |
| Cohesiveness | 0.82 ± 0.05a | 0.79 ± 0.15a | 0.80 ± 0.00a | 0.81 ± 0.07a | 0.79 ± 1.62a |
| Springiness (mm) | 0.72 ± 0.64a | 0.69 ± 0.06a | 0.65 ± 1.32a | 0.70 ± 0.08a | 0.73 ± 0.64a |
| Chewiness (mJ) | 356.22 ± 0.17b | 400.33 ± 0.71a | 435.66 ± 0.10a | 236.33 ± 0.91d | 296.35 ± 0.01c |
| Color L* | 76.21 ± 0.78a | 71.56 ± 0.61a | 75.50 ± 0.27a | 74.47 ± 0.42a | 75.28 ± 0.15a |
| a* | 3.93 ± 0.06a | 3.90 ± 0.31a | 3.90 ± 0.03a | 4.03 ± 0.15a | 3.93 ± 0.06a |
| b* | 24.37 ± 0.50a | 25.60 ± 0.58a | 23.85 ± 0.29a | 24.04 ± 0.25a | 24.37 ± 0.50a |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Biscuits | |||||
| Hardness (g) | 5075.00 ± 1.11c | 5167.00 ± 0.16b | 5644.00 ± 0.60a | 4967.0 ± 0.10d | 4658.26 ± 1.15e |
| Adhesiveness (mJ) | 92.00 ± 0.33a | 88.00 ± 0.27a | 85.67 ± 0.19a | 89.37 ± 0.11a | 90.84 ± 0.61a |
| Resilience | 0.04 ± 0.10a | 0.03 ± 0.09a | 0.04 ± 0.34a | 0.02 ± 0.05a | 0.02 ± 0.55a |
| Cohesiveness | 1.11 ± 0.13a | 1.24 ± 0.87a | 1.04 ± 0.88a | 1.13 ± 0.45a | 1.05 ± 0.05a |
| Springiness (mm) | 29.22 ± 0.55a | 30.45 ± 0.50a | 28.64 ± 0.15a | 32.88 ± 0.41a | 31.68 ± 0.44a |
| Chewiness (mJ) | 1618.80 ± 0.05b | 1645.99 ± 0.14a | 1538.36 ± 0.60d | 1534.72 ± 0.10d | 1572.20 ± 1.22c |
| Color L* | 67.40 ± 1.05a | 65.12 ± 0.45a | 65.64 ± 0.22a | 66.28 ± 0.16a | 67.34 ± 0.15a |
| a* | 1.64 ± 0.04a | 0.98 ± 0.44a | 1.22 ± 0.08a | 0.88 ± 0.17a | 0.97 ± 0.34a |
| b* | 25.23 ± 0.47a | 24.00 ± 0.50a | 25.33 ± 1.25a | 24.28 ± 0.33a | 24.89 ± 0.12a |
As depicted in Table 5, the cohesiveness and springiness values of both sponge cakes and biscuits were not significantly (p > 0.05) different from each other in samples containing antioxidants as well as the controls. A study by de la Rosa et al.103 examined the addition of phenolic compounds to bread and found that these natural antioxidants did not significantly affect the cohesiveness and springiness of the bread, indicating that the internal structure remained stable. Similar results were reported by Zhang et al.,10 where the addition of 1%, 2%, 5% of grape seed extract in wheat-based cakes did not significantly affect cohesiveness and springiness compared to control samples. The chewiness of PSE-added products was moderate. The results were lower than synthetic antioxidant-added samples but higher than the TBHQ-added sponge cakes and biscuits (Table 5). A similar study by de la Rosa et al.103 also found that the inclusion of phenolic compounds in bread formulations resulted in moderate chewiness due to interactions between phenolic compounds and gluten proteins, which altered the dough's rheological properties.
Importantly, the addition of antioxidants did not impact the color parameters (L*, a*, b*) of sponge cakes and biscuits. This indicates that it doesn't cause significant discoloration or browning. This finding is crucial for consumer acceptability because color serves as a primary indicator of freshness and quality.104 De la Rosa et al.103 also reported that the addition of phenolic compounds did not significantly alter the color parameters of the bread, indicating no noticeable discoloration or browning.
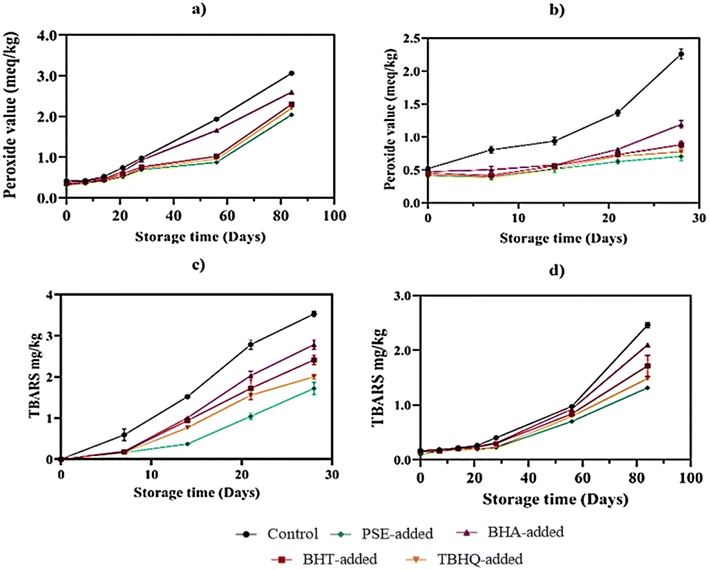
The findings of this study align with those of Kozłowska et al.,105 who reported that bakery fats with peroxide values (PVs) exceeding 3 meq per kg can still maintain acceptable quality, as moderate PV levels do not necessarily correlate with sensory deterioration. In their study, the addition of 1.0% green tea extract (GTE) significantly reduced lipid oxidation, resulting in a PV of 3.2 meq per kg after 28 days, compared to 4.2 meq per kg in cakes containing 0.02% BHT and 6.0 meq per kg in control cakes. Similarly, Zaky et al.106 observed a significant (p < 0.05) increase in PV in biscuits during storage, with control samples showing the highest PV of 21.84 meq per kg by the end of the storage period. Biscuits containing 0.5%, 1%, 2%, and 3% grape pomace extract (GPE) showed PVs ranging from 1.38 ± 0.03 to 12.11 ± 0.04 meq per kg, whereas those with BHA ranged from 1.45 ± 0.03 to 15.13 ± 0.05 meq per kg. After six months, PV values for the GPE-added biscuits were 12.11 ± 0.07, 11.08 ± 0.08, 7.54 ± 0.02, and 6.31 ± 0.06 meq per kg, respectively, compared to 15.13 ± 0.05 meq per kg for the BHA-added biscuits. In the same study, Zaky et al.106 also compared the effects of BHA and GPE on TBARS levels in biscuits over six months. Initially, GPE caused a slight reduction in TBARS values (0.381–0.377 mg kg−1) compared to the control (0.385 mg kg−1) and BHA (0.383 mg kg−1). Over time, GPE demonstrated the greatest protective effect, retaining TBARS values below 0.563 mg kg−1, while the control and BHA samples reached 0.805 mg kg−1 and 0.669 mg kg−1, respectively.
According to the current study, PSE-added baked products were more effective than those containing synthetic antioxidants such as BHT and BHA in reducing lipid oxidation. The lower PV and TBARS values indicate that the phenolic acids and flavonoids present in PSE can readily donate hydrogen atoms to free radicals, thereby interrupting the oxidative chain reaction and enhancing oxidative stability.107
A study by Senanayake et al.53 evaluated the effects of coconut oil meal (CME) and sesame oil meal (SME) phenolic extracts (20 mg/100 g of margarine) on the shelf life of vanilla cake. The findings revealed that both CME and SME extended the microbial shelf life of the cakes up to 13 days. In contrast, cakes with no added antioxidants (control) and those with the synthetic antioxidant butylated hydroxytoluene (BHT) exceeded the maximum allowed microbial counts by day 7 and day 11, respectively. This suggests that CME and SME are effective natural alternatives to synthetic antioxidants in enhancing both microbiological and chemical stability of cakes during storage.
Current research findings suggest that, baked products enriched with antioxidants demonstrated notable antimicrobial activity compared to control products without added antioxidants. Among the tested samples, those containing PSE and TBHQ exhibited significantly (p < 0.05) higher antimicrobial effects suggesting their potential in enhancing the microbial safety of baked goods during storage and reducing the risk of spoilage caused by microbial contamination.
In the sensory evaluation of biscuits, as shown in Fig. 8, the sensory properties of control biscuits were significantly (p < 0.05) decreased by day 28. This degradation of color, odor, taste, texture, and overall acceptability is due to the microbial contamination, lipid oxidation and extreme storage conditions. Among all the synthetic antioxidants, the sensory attributes of the BHA-added biscuits significantly (p < 0.05) decreased within the storage. However, the overall acceptability remained above acceptable levels until day 42 (Fig. 8). TBHQ-added biscuits demonstrated better sensory stability compared to BHA and BHT-added biscuits. Color, odor, and taste showed minor changes up to day 42, with significant reductions becoming apparent after day 56. In contrast, PSE-added biscuits maintained higher acceptability throughout storage with minimal reductions even after 84 days. The overall acceptability remained high with only a slight decline from day 0 to day 84 (Fig. 8).
These results are consistent with the findings of Hefnawy and El-Shourbagy,110 who evaluated the sensory properties of biscuits prepared with water extract of grape leaf (GLE), ethanolic (70%) extract of carrot (CE), and water extract of turmeric (TPE) over a two-month storage period at 25 ± 2 °C. The biscuits were formulated with either 200 ppm of synthetic antioxidants (BHA and TBHQ) or 1% (w/w) of the plant extracts. The study found that biscuits containing phenolic extracts were well accepted in terms of color, crumb color, texture, and mouthfeel, showing sensory characteristics comparable to both the control samples and those containing BHA and TBHQ. This research study indicates that addition of natural antioxidants does not affect the sensory parameters of baked products. For instance, a study by Kozłowska et al.105 on sponge cakes fortified with 1.0% green tea extract (GTE) showed that while some consumers detected a slight difference in flavor, the overall acceptability remained high. Moreover, Zaky et al.106 evaluated the effect of 0.5%, 1%, 2%, and 3% grape pomace extract (GPE) on the sensory characteristics of biscuits and found no significant differences in sensory scores between GPE-enriched and control biscuits. These findings suggest that the addition of natural antioxidants do not adversely affect sensory quality. The moderate reduction in hardness and chewiness in PSE-added sponge cakes and biscuits may be perceived as a positive attribute, indicating a softer and more pleasant mouthfeel. This contributes to higher consumer acceptance, as evidenced by the high sensory scores.
4 Conclusion
Phyllanthus emblica seed extract (PSE) emerges as a powerful natural antioxidant with strong potential to replace synthetic preservatives in food systems. Beyond its antioxidant potential, PSE exhibits notable antimicrobial properties. Compared to synthetic antioxidants such as BHT, BHA, and TBHQ, PSE demonstrates superior thermal stability making it highly suitable for baked products. Toxicological assessments, including cytotoxicity assays and the zebrafish (Danio rerio) embryonic toxicity test, indicate that PSE is significantly safer than conventional synthetic antioxidants, presenting minimal toxic effects. These findings support its potential application as a safer ingredient for food preservation. The incorporation of PSE into baked products enhances their oxidative stability by mitigating the formation of primary and secondary oxidation products, thus preventing rancidity and off-flavors. Furthermore, the antimicrobial properties of PSE contribute to the extended microbial shelf life due to inhibiting microbial growth, maintaining product freshness. Sensory evaluations further confirm that PSE maintains the sensory attributes (color, taste, aroma, texture, and overall acceptability) of these products compared to those enriched with synthetic antioxidants.While the present study demonstrates the strong potential of PSE as a natural antioxidant and antimicrobial agent in baked products, several areas want further investigation. Future research should explore its application across diverse food matrices, including meat, dairy, and oil/fat-based products, to assess its broad-spectrum effectiveness. Long-term in vivo safety evaluations and chronic exposure studies are needed to confirm its safety for regular consumption. Additionally, the development of encapsulation or other delivery strategies could enhance the stability, bioavailability, and controlled release of PSE in complex food systems. Mechanistic studies to elucidate the interactions and synergistic effects of its phenolic compounds would provide deeper insights into its antioxidant and antimicrobial actions. Finally, examining the performance of PSE under varying storage conditions and processing methods, along with evaluating its commercial feasibility and consumer acceptability, will facilitate its practical application as a safe and effective alternative to synthetic preservatives in the food industry.
Ethical approval
The animal study was approved by the Ethics Review Committee (ERC), Faculty of Graduate Studies, USJ, SL (FGS/ERCAS/2024/11/03), and the sensory evaluation procedure was approved by the ERC, Faculty of Medical Sciences, USJ, Sri Lanka (ERC 11/24).Author contributions
Ahinsa Lankanayaka (0009-0009-7356-5938): conceptualization, investigation, visualization, and writing—original draft. K. G. L. R. Jayathunge: supervision, review, and editing. Pasan C. Bandara: supervision, review and editing. Danushika C. Manatunga: supervision, review, and editing. Sameera Samarakoon: supervision, review, and editing. Nimal Punyasiri: supervision, review, and editing. Chathuri M. Senanayake (0000-0002-7116-6396): conceptualization, funding acquisition, supervision, review and editing. All authors read and approved the final version of the manuscript.Conflicts of interest
The authors declare no conflict of interest.Data availability
The datasets used and/or analyzed during the present study are included in this article and its supplementary information (SI). Supplementary information: Fig. S1: antioxidant activities of phenolic extract and synthetic antioxidants: (a) DPPH radical scavenging activity, (b) reducing power, and (c) ABTS radical scavenging activity; Table S1: phenolic compounds and flavonoids identified in ethyl acetate extract of Phyllanthus emblica seed; Table S2: effect of phenolic and synthetic antioxidants on aerobic plate count and yeast and mold count of sponge cakes; Table S3: effect of phenolic and synthetic antioxidants on aerobic plate count and yeast and mould count of biscuits. See DOI: https://doi.org/10.1039/d5fb00609k.Acknowledgements
This work was funded by the Research Council of the University of Sri Jayewardenepura, Sri Lanka, under the research grant number ASP/01/RE/TEC/2022/76. The authors sincerely acknowledge the Institute of Biochemistry, Molecular Biology and Biotechnology (IBMBB), University of Colombo, Sri Lanka, for providing the necessary research facilities and technical support essential for the successful completion of this study. Financial assistance from the Research Council of the University of Sri Jayewardenepura, Sri Lanka, under the research grant ASP/01/RE/TEC/2022/76 is gratefully recognized, and the continuous institutional support extended by the Department of Biosystems Technology, Faculty of Technology, University of Sri Jayewardenepura, Sri Lanka, is also appreciated.References
- D. Wang, H. Xiao, X. Lyu, H. Chen and F. Wei, Lipid oxidation in food science and nutritional health: A comprehensive review, Oil Crop Sci., 2023, 8(1), 35–44, DOI:10.1016/j.ocsci.2023.02.002.
- Z. Sabouri, M. Barzegar, M. A. Sahari and H. B. Naghdi, Antioxidant and antimicrobial potential of Echinacea purpurea extract and its effect on extension of cake shelf life, J. Med. Plants Res., 2012, 11, 28–40 Search PubMed.
- F. Shahidi and A. Hossain, Role of lipids in food flavor generation, Molecules, 2022, 27(15), 5014, DOI:10.3390/molecules27155014.
- F. Shahidi and Y. Zhong, Lipid oxidation and improving the oxidative stability, Chem. Soc. Rev., 2010, 39(11), 4067–4079, 10.1039/b922183m.
- A. Zahid, J. K. Seo, Y. J. Park, J. Y. Jeong, S. K. Jin and T. S. Park, et al., The effects of natural antioxidants on protein oxidation, lipid oxidation, color, and sensory attributes of beef patties during cold storage at 4 °C, Korean J. Food Sci. Anim. Resour., 2018, 38(5), 1029–1042, DOI:10.5851/kosfa.2018.e36.
- Food (Antioxidants) Regulations, 2009, Sri Lanka, Gazette Extraordinary No. 1617/16, Entered into force March 1, 2010 Search PubMed.
- K. N. Seneviratne, W. C. Prasadani and B. Jayawardena, Phenolic extracts of coconut oil cake: A potential alternative for synthetic antioxidants, Food Sci. Technol., 2016, 36(4), 591–597, DOI:10.1590/1678-457X.07316.
- B. Nanditha and P. Prabhasankar, Antioxidants in Bakery Products: A Review, Crit. Rev. Food Sci. Nutr., 2008, 49(1), 1–27, DOI:10.1080/10408390701764104.
- V. C. Ramalho and N. Jorge, Antioxidants used in oils, fats and fatty foods, Quim. Nova, 2006, 29(4), 755–760 CrossRef CAS.
- Y. Zhang, X. Li, S. Wang, Y. Chen and H. Zhao, Effects of natural antioxidants on texture and shelf-life of bakery products, Food Chem., 2021, 345, 128756, DOI:10.1016/j.foodchem.2020.128756.
- Y. Murakami, A. Kawata, T. Katayama and S. Fujisawa, Anti-inflammatory activity of the artificial antioxidants 2-tert-butyl-4-methoxyphenol (BHA), 2,6-di-tert-butyl-4-methylphenol (BHT), and 2,4,6-tri-tert-butylphenol (TBP), and their various combinations, In Vivo, 2015, 29, 197–206 CAS.
- O. Ousji and L. Sleno, Identification of in vitro metabolites of synthetic phenolic antioxidants BHT, BHA, and TBHQ by LC-HRMS/MS, Int. J. Mol. Sci., 2020, 21(24), 9525, DOI:10.3390/ijms21249525.
- N. Bensid, A. El Abed, J. M. Houicher, J. M. Regenstein and F. Ozogul, Antioxidant and antimicrobial preservatives: Properties, mechanism of action, and applications in food – A review, Crit. Rev. Food Sci. Nutr., 2022, 62(11), 2985–3001, DOI:10.1080/10408398.2020.1862046.
- S. C. Lourenço, M. Moldao-Martins and V. D. Alves, Antioxidants of natural plant origins: From sources to food industry applications, Molecules, 2019, 24(22), 4132, DOI:10.3390/molecules24224132.
- A. Baran, S. Yildirim, A. Ghosigharehaghaji, I. Bolat, E. Sulukan and S. B. Ceyhun, An approach to evaluating the potential teratogenic and neurotoxic mechanism of BHA based on apoptosis induced by oxidative stress in zebrafish embryo (Danio rerio), Hum. Exp. Toxicol., 2020, 39(12), 1647–1662, DOI:10.1177/0960327120952140.
- A. Lankanayaka, N. D. Lakshan, L. Jayathunge, P. Bandara, D. C. Manatunga and C. M. Senanayake, A review of sustainable strategies for encapsulating antioxidant-rich plant polyphenolic extracts using nanoemulsification to enhance the oxidative stability of edible oils, Discover Food, 2025, 5, 65, DOI:10.1007/s44187-025-00331-8.
- M. Martínez, G. Ros and G. Nieto, Hydroxytyrosol: Health benefits and use as functional ingredient in meat, Med. Chem., 2021, 21(1), 1–15 Search PubMed.
- N. Al Masoud, S. A. Hassan, T. S. Alomar, W. Mujahid and R. M. Aadil, Enhancing biscuits with muskmelon seed flour: A study of physicochemical, textural, and nutritional characteristics, Qual. Assur. Saf. Crops Foods, 2024, 16(3), 139–151, DOI:10.15586/qas.v16i3.1503.
- E. Cakir, G. Ozulku, H. Bekiroglu, M. Arici and O. Sagdic, Technological quality, bioactive features, and glycemic index of gluten-free cakes formulated with lyophilized wild Prunus spinosa fruit, Qual. Assur. Saf. Crops Foods, 2024, 16(2), 1–11, DOI:10.15586/qas.v16i2.1398.
- C. M. Senanayake, H. Hapugaswatta, N. Jayathilaka and K. N. Seneviratne, Phenolic extracts of the leaves of Psidium guineense Sw. improve the shelf life of sunflower oil and baked cake and antioxidant status of Wistar rats, J. Food Biochem., 2018, e12632, DOI:10.1111/jfbc.12632.
- A. A. K. Lankanayaka, D. S. Matharage, C. M. Senanayake, K. G. L. R. Jayathunge, P. C. Bandara and D. C. Manatunga, Extraction of bioactive compounds from food waste and their potential applications in the food industry: a review, Braz. J. Dev., 2024, 10(4), e69187, DOI:10.34117/bjdv10n4-06.
- D. Mehta, P. Prasad, R. S. Sangwan and S. K. Yadav, Tomato processing byproduct valorization in bread and muffin: Improvement in physicochemical properties and shelf life stability, J. Food Sci. Technol., 2018, 55, 2560–2568, DOI:10.1007/s13197-018-3176-0.
- V. Reddy, A. Urooj and A. Kumar, Evaluation of antioxidant activity of some plant extracts and their application in biscuits, Food Chem., 2005, 90(1–2), 317–321, DOI:10.1016/j.foodchem.2004.05.038.
- A. Chowdhury, S. Santra and S. Mandal, Phytochemical screening and evaluation of cytotoxic activity of Phyllanthus niruri L. against human hepatocellular carcinoma HepG2 cells, J. Pharmacogn. Phytochem., 2020, 9(4), 1794–1798 Search PubMed.
- S. Mehta, P. K. Rai, D. K. Rai, N. K. Rai, A. K. Rai and D. Bicanic, et al., LIBS-based detection of antioxidant elements in seeds of Emblica officinalis, J. Elem. Anal., 2010, 5(3), 186–192, DOI:10.1007/s11483-010-9158-z.
- G. P. Singh, A. S. Jain, P. B. Sridhara Setty, S. Bv, S. S. Patil and K. P. Suresh, et al., Antimicrobial, antioxidant, and anti-inflammatory activities of seeds from Emblica officinalis (Gaertn.), Bioinformation, 2022, 18(8), 683–691, DOI:10.6026/97320630018683.
- A. Bariya, D. Patel, V. Gamit and R. B. Parmar, Assessment of antioxidant and sensory properties of amla (Emblica officinalis) fruit and seed coat powder incorporated cooked goat meat patties, Int. J. Curr. Microbiol. Appl. Sci., 2018, 7(7), 3306–3318, DOI:10.20546/ijcmas.2018.707.385.
- M. Irakli, P. Chatzopoulou and L. Ekateriniadou, Optimization of ultrasound-assisted extraction of phenolic compounds: Oleuropein, phenolic acids, phenolic alcohols, and flavonoids from olive leaves and evaluation of its antioxidant activities, Ind. Crops Prod., 2018, 124, 382–388, DOI:10.1016/j.indcrop.2018.07.070.
- A. Jaiboonma, W. Janvikul and S. Poeaim, The bioactivities of seed coat and embryo extracts from Indian gooseberry (Phyllanthus emblica), Int. J. Agric. Technol., 2018, 14(2), 183–191 CAS.
- V. L. Singleton and J. A. Rossi, Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents, Am. J. Enol. Vitic., 1965, 16, 144–158 CrossRef CAS.
- M. A. Hossain, M. Khatun, M. Islam and R. Huque, Enhancement of antioxidant quality of green leafy vegetables upon different cooking methods, Prev. Nutr. Food Sci., 2017, 22(3), 216–222 Search PubMed.
- G. Aguilar-Hernandez, M. L. Garcia-Magana, M. L. A. Vivar-Vera, S. G. Ssyago-Ayerdi, J. A. Sanchez-Burgos and J. Morales-Castro, Optimization of ultrasound-assisted extraction of phenolic compounds from Annona muricata by-products and pulp, Molecules, 2019, 24(5), 904, DOI:10.3390/molecules24050904.
- T. Hatano, H. Kagawa, T. Yasuhara and T. Okuda, Two new flavonoids and other constituents in licorice root: Their relative astringency and radical scavenging effects, Chem. Pharm. Bull., 1988, 36(6), 2090–2097 CrossRef CAS PubMed.
- M. Oyaizu, Studies on products of browning reaction: Antioxidative activities of products of browning reaction prepared from glucosamine, Jpn. J. Nutr. Diet., 1986, 44(17), 307–315 CrossRef CAS.
- R. Re, N. Pellegrini, A. Proteggente, A. Pannala, M. Yang and C. Rice-Evans, Antioxidant activity applying an improved ABTS radical cation decolorization assay, Free Radicals Biol. Med., 1999, 26(9–10), 1231–1237 CrossRef CAS PubMed.
- K. P. Seneviratne, N. V. P. Anjali, C. M. Senanayake, N. Jayathilaka and K. N. Seneviratne, Ethanolic extract of rice bran: a thermally stable preservative for edible oils and cake, Food Prod., Process. Nutr., 2022, 4, 14, DOI:10.1186/s43014-022-00094-0.
- J. Bonilla and P. J. do A. Sobral, Antioxidant and antimicrobial properties of ethanolic extracts of guarana, boldo, rosemary, and cinnamon, Braz. J. Food Technol., 2017, 20, e2016024, DOI:10.1590/1981-6723.2416.
- F. Mandim, M. I. Dias, J. Pinela, P. Barracosa, M. Ivanov and D. Stojkovic, Chemical composition and in vitro biological activities of cardoon (Cynara cardunculus L. var. altilis DC.) seeds as influenced by viability, Food Chem., 2020, 323, 126838, DOI:10.1016/j.foodchem.2020.126838.
- OECD, Test No. 236: Fish embryo acute toxicity (FET) test, OECD Guidelines for the Testing of Chemicals, Section 2, OECD Publishing, 2013, DOI:10.1787/9789264203709-en.
- M. D. Ramli, N. Chong, N. Hussin, M. Mohd Uzid and J. Sanusi, An evaluation of toxicity assessment in zebrafish (Danio rerio) embryo induced by Mitragyna speciosa, Int. J. Med. Toxicol. Legal Med., 2020, 23, 31, DOI:10.5958/0974-4614.2020.00007.8.
- W. Thitinarongwate, R. Mektrirat, W. Nimlamool, P. Khonsung, S. Pikulkaew, S. Okonogi and P. Kunanusorn, Phytochemical and safety evaluations of Zingiber ottensii Valeton essential oil in zebrafish embryos and rats, Toxics, 2021, 9(5), 102, DOI:10.3390/toxics9050102.
- T. Janjarasskul, K. Tananuwong, V. Kongpensook, S. Tantratian and S. Kokpol, Shelf life extension of sponge cake by active packaging as an alternative to direct addition of chemical preservatives, LWT–Food Sci. Technol., 2016, 166–174, DOI:10.1016/j.lwt.2016.04.049.
- W. Klunklin and G. Savage, Effect of substituting purple rice flour for wheat flour on physicochemical characteristics, in vitro digestibility, and sensory evaluation of biscuits, J. Food Qual., 2018, 8, 8052847, DOI:10.1155/2018/8052847.
- AOAC International, Official Methods of Analysis of the Association of Official Analytical Chemists, AOAC International, 20th edn, 2012 Search PubMed.
- A. Y. Guadarrama-Lezama, H. Carrillo-Navas, C. Perez-Alonso, E. J. Vernon Carter and J. Alvarez-Ramirez, Thermal and rheological properties of sponge cake batters and texture and microstructural characteristics of sponge cake made with native corn starch in partial or total replacement of wheat flour, LWT–Food Sci. Technol., 2016, 46–54, DOI:10.1016/j.lwt.2016.02.031.
- S. M. Man, L. Stan, A. Paucean, M. S. Chiş, V. Mureşan and S. A. Socaci, et al., Nutritional, sensory, texture properties, and volatile compounds profile of biscuits with roasted flaxseed flour partially substituting for wheat flour, Appl. Sci., 2021, 11, 4791, DOI:10.3390/app11114791.
- B. R. Nanditha, B. S. Jena and P. Prabhasankar, Influence of natural antioxidants and their carry-through property in biscuit processing, J. Sci. Food Agric., 2008, 89(2), 288–298, DOI:10.1002/jsfa.3440.
- N. C. Shantha and E. A. Decker, Rapid, sensitive, iron-based spectrophotometric methods for determination of peroxide values of food lipids, J. AOAC Int., 1994, 77(2), 421–424, DOI:10.1093/jaoac/77.2.421.
- F. S. Gómez and M. P. Almajano, Pineapple waste extract for preventing oxidation in model food systems, J. Food Sci., 2016, 81(7), 1692–1699, DOI:10.1111/1750-3841.13327.
- J. J. H. Wang, N. J. Park, S. D. Choi, D. H. Ha and D. H. Oh, Microbiological analysis of rice cake processing in Korea, J. Food Prot., 2016, 79(1), 157–162 CrossRef PubMed.
- BAM, Bacteriological Analytical Manual: Department of Health and Human Services. U.S. Food and Drug Administration. Bacteriological Analytical Manual: 2011, Food Sampling and Preparation of Sample Homogenate, BAM, Bam, Iran, 2001 Search PubMed.
- International Organization for Standards, ISO 7218-1 Microbiology of the Food and Animal Feeding Stuffs-General Requirements and Guidance for Microbiological Examinations, ISO, Geneva, Switzerland, 2014 Search PubMed.
- C. M. Senanayake, C. H. Algama, R. L. Wimalasekara, W. N. M. T. D. N. Weerakoon, N. Jayathilaka and K. N. Seneviratne, Improvement of oxidative stability and microbial shelf life of vanilla cake by coconut oil meal and sesame oil meal phenolic extracts, J. Food Qual., 2019, 1–8, DOI:10.1155/2019/1263629.
- S. Bajaj, A. Urooj and P. Prabhasankar, Effect of incorporation of mint on texture, colour, and sensory parameters of biscuits, Int. J. Food Prop., 2006, 9(4), 691–700, DOI:10.1080/10942910600547632.
- M. Kaur, A. Sharma, P. Bhardwaj, H. Kaur and S. K. Uppal, Evaluation of physicochemical properties, nutraceuticals composition, antioxidant, antibacterial and antifungal potential of waste amla seed coat (Phyllanthus emblica, variety Neelam), Journal of Food Measurement and Characterization, 2021, 15, 1201–1212, DOI:10.1007/s11694-020-00721-9.
- P. Mishra, N. Dutta and C. L. Mahanta, Partial extraction and identification of phenolics in Amla (Emblica officinalis) seed coat powder, J. Food Sci. Technol., 2015, 52(11), 6990–7001, DOI:10.1007/s13197-015-1835-y.
- F. Aghababaei and M. Hadidi, Recent advances in potential health benefits of quercetin, Pharmaceuticals, 2023, 16(7), 1020, DOI:10.3390/ph16071020.
- J. Sharifi-Rad, C. Quispe, C. M. S. Castillo, R. Caroca, M. A. Lazo-Vélez and H. Antonyak, et al., Ellagic acid: A review on its natural sources, chemical stability, and therapeutic potential, Oxid. Med. Cell. Longevity, 2022, 3848084, DOI:10.1155/2022/3848084.
- W. Sun and M. H. Shahrajabian, Therapeutic potential of phenolic compounds in medicinal plants—Natural health products for human health, Molecules, 2023, 28(5), 1845, DOI:10.3390/molecules28051845.
- M. Y. Jung and D. S. Choi, Protective effect of gallic acid on the thermal oxidation of corn and soybean oils during high temperature heating, Food Sci. Biotechnol., 2016, 25(6), 1577–1582, DOI:10.1007/s10068-016-0243-z.
- N. Gama, D. D. Evtyugin, A. Lourenço, C. Lopes and D. V. Evtuguin, Ellagic acid as stabilizer in the thermo-oxidative degradation of thermoplastic polyurethane, Polym. Degrad. Stab., 2023, 215, 110456, DOI:10.1016/j.polymdegradstab.2023.110456.
- H. Cao, O. Saroglu, A. Karadag, Z. Diaconeasa, G. Zoccatelli and C. A. Conte-Junior, Available technologies on improving the stability of polyphenols in food processing, Food Front., 2021, 1–31, DOI:10.1002/fft2.65.
- S. S. Nambiar, M. Paramesha and N. P. Shetty, Comparative analysis of phytochemical profile, antioxidant activities, and foam prevention abilities of whole fruit, pulp, and seeds of Emblica officinalis, J. Food Sci. Technol., 2015, 52(11), 7254–7262, DOI:10.1007/s13197-015-1844-x.
- S. H. Hassanpour and A. Doroudi, Review of the antioxidant potential of flavonoids as a subgroup of polyphenols and partial substitute for synthetic antioxidants, Avicenna J. Phytomed., 2023, 13(4), 354–376, DOI:10.22038/AJP.2023.21774.
- A. Zeb, Concept, mechanism, and applications of phenolic antioxidants in foods, J. Food Biochem., 2020, e13394, DOI:10.1111/jfbc.13394.
- M. C. Christodoulou, J. C. Orellana Palacios, G. Hesami, S. Jafarzadeh, J. M. Lorenzo and R. Domínguez, Spectrophotometric methods for measurement of antioxidant activity in food and pharmaceuticals, Antioxidants, 2022, 11(11), 2213, DOI:10.3390/antiox11112213.
- I. Bayram and E. A. Decker, Underlying mechanisms of synergistic antioxidant interactions during lipid oxidation, Trends Food Sci. Technol., 2023, 133, 219–230, DOI:10.1016/j.tifs.2023.02.003.
- B. Huseyin, p-Coumaric acid in cereals: presence, antioxidant and antimicrobial effects, Int. J. Food Sci. Technol., 2015, 50(11), 2323–2328, DOI:10.1111/ijfs.12898.
- M. Leopoldini, N. Russo, S. Chiodo and M. Toscano, Iron chelation by the powerful antioxidant flavonoid quercetin, J. Agric. Food Chem., 2006, 54(17), 6343–6351, DOI:10.1021/jf060986h.
- A. Scarano, B. Laddomada, F. Blando, S. De Santis, G. Verna, M. Chieppa and A. Santino, The chelating ability of plant polyphenols can affect iron homeostasis and gut microbiota, Antioxidants, 2023, 12(3), 630, DOI:10.3390/antiox12030630.
- D. Skroza D, V. Simat V, L. Vrdoljak, N. Jolic, A. Skelin, M. Cagalj, R. Frleta and I. Generalić Mekinic, Investigation of Antioxidant Synergisms and Antagonisms among Phenolic Acids in the Model Matrices Using FRAP and ORAC Methods, Antioxidants, 2022, 11(9), 1784, DOI:10.3390/antiox11091784.
- T. Yi, Q. Chen, X. He, S. So, Y. Lo, L. Fan, J. Xu, Y. Tang, J. Zhang, Z. Zhao and H. Chen, Chemical quantification and antioxidant assay of four active components in Ficus hirta root using UPLC-PAD-MS fingerprinting combined with cluster analysis, Chem. Cent. J., 2013, 7(1), 115, DOI:10.1186/1752-153X-7-115.
- C. C. Tsai, C. H. Chou, Y. C. Liu and C. W. Hsieh, Ultrasound-assisted extraction of phenolic compounds from Phyllanthus emblica L. and evaluation of antioxidant activities, Int. J. Cosmet. Sci., 2014, 36(5), 471–476, DOI:10.1111/ics.12143.
- D. Ahmed, M. Khan and R. Saeed, Comparative analysis of phenolics, flavonoids, and antioxidant and antibacterial potential of methanolic, hexanic, and aqueous extracts from Adiantum caudatum leaves, Antioxidants, 2015, 4(2), 394–409, DOI:10.3390/antiox4020394.
- S. Dai, M. Liang, H. Cheng, C. Yu, W. Li and F. Lai, et al., Thermal decomposition characteristics of BHT and its peroxide (BHTOOH), BMC Chem., 2024, 18(1), 11, DOI:10.1186/s13065-024-01190-7.
- J. Tsaknis, S. Lalas and E. Protopapa, Effectiveness of the antioxidants BHA and BHT in selected vegetable oils during intermittent heating, Grasas Aceites, 2002, 53(2), 199–205, DOI:10.3989/gya.2002.v53.i2.309.
- S. Anbuselvi and M. Jha, Phytochemical and antimicrobial activity of Emblica officinals seed extract, World J. Pharm. Res., 2015, 4(8), 1336–1341 CAS.
- N. De C. C. Pinto, L. M. Campos, A. C. S. Evangelista, A. S. O. Lemos, T. P. Silva and R. C. N. Melo, et al., Antimicrobial Annona muricata L. (soursop) extract targets the cell membranes of Gram-positive and Gram-negative bacteria, Ind. Crops Prod., 2017, 107, 332–340, DOI:10.1016/j.indcrop.2017.05.054.
- J. G. P. Martin, E. Porto, S. M. de Alencar, E. M. da Glória, C. B. Corrêa and I. S. R. Cabral, Antimicrobial activity of yerba mate (Ilex paraguariensis St. Hil.) against food pathogens, Rev. Argent. Microbiol., 2013, 45(2), 93–98, DOI:10.1016/s0325-7541(13)70006-3.
- A. Walter, W. Samuel, A. Peter and O. Joseph, Antibacterial activity of Moringa oleifera and Moringa stenopetala methanol and n-hexane seed extracts on bacteria implicated in water borne diseases, Afr. J. Microbiol. Res., 2011, 5(2), 153–157 Search PubMed.
- D. E. Agada, T. T. Sar, J. A. Ujoh and L. O. Ameh, Antibacterial susceptibility of Staphylococcus aureus, Salmonella typhi, Bacillus subtilis and Escherichia coli to snail slime, Afr. Health Sci., 2023, 23(4), 177–182, DOI:10.4314/ahs.v23i4.20.
- L. A. Shelef and P. Liang, Antibacterial effects of butylated hydroxyanisole (BHA) against Bacillus species, J. Food Sci., 1982, 47(7982), 799 Search PubMed.
- A. S. M. C. Saliba, A. G. O. Sartori, P. S. Batista, J. E. P. G. Amaral, N. O. Silva and M. Ikegaki, et al., Simulated gastrointestinal digestion/Caco-2 cell transport: Effects on biological activities and toxicity of a Brazilian propolis, Food Chem., 2023, 403, 134330, DOI:10.1016/j.foodchem.2022.134330.
- K. Esazadeh, J. E. N. Dolatabadi, H. Andishmand, H. Mohammadzadeh-Aghdash, M. Mahmoudpour and M. N. Kermanshahi, et al., Cytotoxic and genotoxic effects of tert-butylhydroquinone, butylated hydroxyanisole, and propyl gallate as synthetic food antioxidants, Food Sci. Nutr., 2024, 12(11), 7004–7016, DOI:10.1002/fsn3.4373.
- M. Saito, H. Sakagami and S. Fujisawa, Cytotoxicity and apoptosis induction by butylated hydroxyanisole (BHA) and butylated hydroxytoluene (BHT), Anticancer Res., 2003, 23(6C), 4693–4701 CAS.
- N. Chaudhary, L. Sabikhi, S. A. Hussain, R. Kumar and U. Choudhary, Emblicanin-rich Emblica officinalis encapsulated double emulsion and its antioxidant stability during storage, Eur. J. Lipid Sci. Technol., 2020, 122(10), 1900316 CrossRef CAS.
- K. B. Harikumar and R. Kuttan, An extract of Phyllanthus amarus protects mouse chromosomes and intestine from radiation induced damages, J. Radiat. Res., 2007, 48(6), 469–476, DOI:10.1269/jrr.07039.
- M. Eskandani, E. Hamishehkar and J. E. N. Dolatabadi, Cytotoxicity and DNA damage properties of tert-butylhydroquinone (TBHQ) food additive, Food Chem., 2014, 153, 315–320, DOI:10.1016/j.foodchem.2013.12.087.
- A. Khezerlou, A. P. Akhlaghi, A. M. Alizadeh, P. Dehghan and P. Maleki, Alarming impact of the excessive use of tert-butylhydroquinone in food products: A narrative review, Toxicol Rep, 2022, 9, 1066–1075, DOI:10.1016/j.toxrep.2022.04.027.
- S. Sriwatcharakul, Evaluation of bioactivities of Phyllanthus emblica seed, Energy Rep., 2020, 6, 442–447, DOI:10.1016/j.egyr.2019.08.088.
- K. R. Martin, Polyphenols as dietary supplements: A double-edged sword, Nutr. Diet. Suppl., 2009, 1, 1–12, DOI:10.2147/NDS.S6422.
- W. Thitinarongwate, R. Mektrirat, W. Nimlamool, P. Khonsung, S. Pikulkaew and S. Okonogi, et al., Phytochemical and safety evaluations of Zingiber ottensii Valeton essential oil in zebrafish embryos and rats, Toxics, 2021, 9(5), 102, DOI:10.3390/toxics9050102.
- C. L. Kemmler, F. W. Riemslagh, H. R. Moran and C. Mosimann, From stripes to a beating heart: Early cardiac development in zebrafish, J. Cardiovasc. Dev. Dis., 2021, 8(2), 17, DOI:10.3390/jcdd8020017.
- X. Yang, Z. Sun, W. Wang, Q. Zhou, G. Shi and F. Wei, et al., Developmental toxicity of synthetic phenolic antioxidants to the early life stage of zebrafish, Sci. Total Environ., 2018, 643, 559–568, DOI:10.1016/j.scitotenv.2018.06.213.
- E. R. Leonard, E. S. Marques, M. A. Roy, S. M. Conlin, R. Ranjan and A. R. Timme-Laragy, Dietary exposure to the food preservative tert-butylhydroquinone (tBHQ) impairs zebrafish (Danio rerio) survival, growth, organ development, and gene expression in Nrf2a-dependent and independent ways, Food Chem. Toxicol., 2023, 176, 113788, DOI:10.1016/j.fct.2023.113788.
- H. Cheng, Y. Li, F. Liu, S. Zhu, L. Chen and H. Lu, et al., Butylated hydroxytoluene (BHT) induces zebrafish spinal cord defects and scoliosis by inhibiting the hedgehog pathway, Sci. Rep., 2025, 15(1), 28858, DOI:10.1038/s41598-025-14524-9.
- C. Caleja, L. Barros, A. L. Antonio, M. B. P. P. Oliveira and I. C. F. R. Ferreira, A comparative study between natural and synthetic antioxidants: Evaluation of their performance after incorporation into biscuits, Food Chem., 2017, 216, 342–346, DOI:10.1016/j.foodchem.2016.08.075.
- A. Gill, N. John, N. Iqbal, T. A. Faridi and S. Noor, Assessment of biochemical profile among patients of microbiological quality assessment of bakery products available in Lahore, Pakistan, Diet Factor, 2020, 1(1), 19–23, DOI:10.54393/df.v1i01.1.
- M. C. Caruso, F. Galgano, M. A. Colangelo, N. Condelli, T. Scarpa and R. Tolve, et al., Evaluation of the oxidative stability of bakery products by OXITEST method and sensory analysis, Eur. Food Res. Technol., 2017, 243(7), 1183–1191, DOI:10.1007/s00217-016-2831-9.
- I. Arcan and A. Yemenicioğlu, Antioxidant activity of pomegranate peel extracts in different food models, Food Chem., 2011, 129(4), 1118–1128, DOI:10.1016/j.foodchem.2011.05.093.
- A. Sulaiman, M. Y. Soo, M. M. Yusoff and M. M. Sahri, Effect of mango peel phenolics on texture and oxidative stability of sponge cake, LWT–Food Sci. Technol., 2019, 110, 15–21, DOI:10.1016/j.lwt.2019.04.073.
- R. Kumar, S. Sharma, A. Gupta and R. Bhardwaj, Influence of Moringa oleifera leaf extract on physicochemical properties of functional biscuits, J. Food Process. Preserv., 2020, 44(7), e14480, DOI:10.1111/jfpp.14480.
- L. A. de la Rosa, J. O. Moreno-Escamilla, J. Rodrigo-García and E. Alvarez-Parrilla, Addition of phenolic compounds to bread: antioxidant benefits and implications for dough rheology and sensory properties, Food Prod., Process. Nutr., 2021, 3, 25, DOI:10.1186/s43014-021-00068-8.
- R. R. Indiarto, R. Reni, G. L. Utama, E. Subroto, A. D. Pangawikan and M. Djali, The physicochemical, antioxidant, and sensory properties of chocolate biscuits incorporated with encapsulated mangosteen (Garcinia mangostana L.) peel extract, Int. J. Food Prop., 2023, 26(1), 122–138, DOI:10.1080/10942912.2022.2159429.
- M. Kozłowska, A. Zbikowska, A. Szpicer and A. Połtorak, Oxidative stability of lipid fractions of sponge-fat cakes after green tea extracts application, J. Food Sci. Technol., 2019, 56(5), 2628–2638, DOI:10.1007/s13197-019-03750-5.
- A. A. Zaky, E. Asiama, S. Y. El-Faham, M. M. S. Ashour and A. M. Sharaf, Utilization of grape pomace extract as a source of natural antioxidant in biscuits, Eur. Acad. Res., 2020, 8(1) Search PubMed.
- J. Gómez-Estaca, C. López-de-Dicastillo, P. Hernández-Muñoz, R. Catalá and R. Gavara, Advances in antioxidant active food packaging, Trends Food Sci. Technol., 2014, 35(1), 42–51, DOI:10.1016/j.tifs.2013.10.008.
- H. F. Ismail, Z. Hashim, W. T. Soon, N. S. Ab Rahman, A. N. Zainudin and A. Majid, Comparative study of herbal plants on the phenolic and flavonoid content, antioxidant activities, and toxicity on cells and zebrafish embryo, J. Tradit. Complementary Med., 2017, 7, 452–465 CrossRef PubMed.
- U.S. Food and Drug Administration, Revised Guidelines for the Assessment of Microbiological Quality of Processed Foods (Circular No. 2013(010)), U.S. Food and Drug Administration, 2013, https://www.fda.gov Search PubMed.
- T. H. Hefnawy and G. A. El-Shourbagy, Evaluation of antioxidant activity of some plant extracts and their effects on the stability of lipids in biscuits, J. Food Dairy Sci., 2014, 5(9), 667–678 CrossRef.
| This journal is © The Royal Society of Chemistry 2026 |