Open Access Article
Open Access ArticleIntelligent & edible biopolymer-based packaging technologies for postharvest shelf-life enhancement of litchi (Litchi chinensis) fruit: a comprehensive review
Sonu Kumar
ab,
Shikha Sharma
ab and
Vimal Katiyar
 *ab
*ab
aDepartment of Chemical Engineering, Indian Institute of Technology (IIT) Guwahati, Guwahati, Assam 781039, India. E-mail: vkatiyar@iitg.ac.in
bCentre for Sustainable Polymer, Indian Institute of Technology (IIT) Guwahati, Guwahati, Assam 781039, India
First published on 5th January 2026
Abstract
Litchi (Litchi chinensis) is a sub-tropical fruit of high nutrition and commercial value, but its quality and safety deteriorate during storage due to physical, chemical, enzymatic, and microbial damage. The shelf-life can be improved by various methods such as sulfur (SO2) fumigation, cold storage, gamma irradiation, high-pressure processing, edible packaging, etc. SO2 fumigation is one of the most explored methods for preserving litchi fruit but due to its negative environmental and human health effects, its application is limited. Edible and intelligent coatings and films comprised of various biopolymers can be a promising solution to prolong the shelf-life of litchi and they help to achieve SDGs 12.3 (halve per capita global food waste at the retail and consumer levels), 13.2 (integrate climate change measures into national policies, strategies, and planning) and 14.1 (prevent and significantly reduce marine pollution of all kinds). This article reviews the recent advances in using different biopolymers to develop intelligent & edible films and coatings to improve the storage life of litchi fruit. Edible packaging based on polysaccharides, proteins, lipids, additives, nanoparticles, etc. has been examined. These protective layers reduce the respiration and transpiration rates, protect against mechanical stress, microbial contamination, enzymatic browning and UV light, and enhance the quality by adding bioactive agents, nanomaterials, probiotics, etc. The opportunities to use bioactive compounds, essential oils and plant extract in various biopolymeric edible coatings are explored. Finally, regulatory aspects associated with edible intelligent packaging have been discussed. Overall, the application of edible and intelligent packaging can be a potential alternative for delaying the degradative changes, extending the shelf-life, and reducing the economic losses caused by the spoilage of litchi.
Sustainability spotlightThis review highlights the role of intelligent and edible packaging technologies in reducing postharvest losses of litchi, a highly perishable fruit. By compiling and critically analyzing recent advances in biopolymer-based films and coatings, natural antioxidant incorporations, and intelligent freshness indicators, the paper provides a sustainable framework for replacing synthetic packaging with eco-friendly alternatives. Such insights promote waste reduction, extend fruit shelf-life, and lower environmental impacts associated with plastic use. The review aligns with the UN Sustainable Development Goals, particularly SDG 12.3, SDG 13.2 and SDG 14.1, by encouraging sustainable material choices and resource-efficient food preservation practices that can guide both researchers and industry toward greener innovations. |
1. Introduction
Litchi (Litchi chinensis) is a non-climacteric fruit that is an extremely popular horticulture produce among its consumers due to its unique taste, distinctive flavor, and attractive pink appearance. This fruit has high commercial value in the international and domestic market due to its numerous health benefits and high consumer demand.1 Litchi is a source of several nutrients including vitamins, phenolics, flavonoids, sugar, and minerals. Litchi is a rich source of antioxidants due to the presence of a broad range of phenolics and flavonoids which are highly effective against human health problems like heart diseases, dyspepsia, diabetes and smallpox.2,3 Litchi is particularly recommended to be consumed during pregnancy in order to meet the nutritional requirements for maternal health. This fruit is extremely susceptible to spoilage and can only be stored for a maximum period of 2–3 days under normal atmospheric conditions and 3–5 days under cold storage conditions.4 Historical accounts state that litchi seed, pulp, and whole fruit were utilised in herbal remedies in China.5 The market value of this fruit is reported as USD 7.10 billion in 2024 and is expected to reach USD 9.27 billion by 2029 which is equivalent to a compound annual growth rate (CAGR) of 5.5% during the time scale of 2024–29.6 However, the short shelf life of this fruit limits its full potential both in terms of economics as well as nutritional value. Pericarp browning, fungal infestation, textural loss, weight decay, and microcracking development are major problems associated with the shelf life and storage of fruit. The fast pericarp browning of litchi fruit is mainly reported due to the high action of enzymes found namely polyphenol oxidase (PPO) and peroxidase (POD).7,8 Therefore, one of the major challenges associated with the producer is maintaining the appealing red-pink color of this fruit, especially during importing this internationally. A wide group of fungi have been reported for major post-harvest diseases associated with fruit deterioration by various research communities actively working with this fruit.9–11To date, different conventional practices have been implemented to remove these post-harvest problems including SO2 fumigation,12 refrigeration,13 irradiation process14 and controlled and modified atmosphere packaging.15 Sulfur fumigation is a commercial technique to address the issues of fungal growth and browning of litchi fruit. However, consumer preference, government regulation regarding sulfur use and environmental problems have recently raised a lot of questions about its application.16 Petroleum-based plastic packaging has been utilized for processed food and farm produce for decades because of its cost effectiveness, production simplicity, convenience for users, and favourable chemical characteristics. Nevertheless, consumer demands have shifted toward eco-friendly, renewable, biodegradable, and sustainable packaging materials because of growing public awareness of plastics' use of non-renewable resources, safety concerns, and huge accumulations and contaminations in the environment. Adopting edible films and coatings is one way to meet the current demand which could apply to a wide range of horticulture produce including litchi for enhancing their shelf life (reducing post-harvest loss).
In the last 2 decades, edible films and coatings for fruits and vegetables have attracted the attention of a large scientific community due to its various properties including biodegradability, biocompatibility, sustainable nature, and increase in the awareness of people toward human health and environment. Along with these unique and important properties, edible films and coatings maintain the organoleptic quality of coated components for longer periods. The efficacy of these novel edible composite films incorporating supplemental functional substances hinges upon the inherent physical and chemical properties, as well as the biological activity, of the additives themselves and the edible film matrix, in addition to their encapsulation. The edible coating is a thin extra layer applied on the surface of horticulture produce which can be consumed along with fruits and vegetables,17–19 whereas the use of edible films is applicable as an inner layer that directly interacts with the foods in packages that consist of non-edible films.20 Their main mechanism includes the regulation of respiration and transpiration phenomena associated with fruits and vegetables along with microbes and browning control. Edible coating formulation has been reported for various fruit commodities as a sustainable approach for enhanced shelf-life including apples, avocados, strawberries, cherries, and oranges.21–23 This type of packaging is based on polysaccharides (e.g., cellulose, starch, chitosan, and gums), protein (whey and milk protein), and lipids (waxes and oils) and may contain active components for active edible coating. In a manner analogous to conventional packaging films, active packaging possesses the capability to offer both barrier and mechanical safeguards for food products. Furthermore, active packaging has been engineered to extend the shelf life and enhance the quality of food items through the release of antioxidants and antimicrobials or the absorption of O2, CO2, ethylene, and various odors. Active packaging (active edible films and coatings) introduces antioxidant, antimicrobial, and UV-resistant properties which could be the potential solution for browning and fungal attack problems on litchi fruit.
A new category of food packaging materials known as intelligent packaging combines the protective function of traditional edible films with the capacity to detect and convey variations in food quality. Intelligent packaging is made to interact with its surroundings and provide visible or quantifiable cues regarding the freshness, safety, or spoilage of food, in contrast to conventional packaging, which merely serves as a passive barrier.24,25 These systems are especially useful for perishable goods like fruits, vegetables, dairy products, and seafood, where quality degradation happens quickly and often goes undetected until it is too late.
The application of various packaging materials on various fruits and vegetables is also extensively discussed in numerous research articles.26–28 For researchers, identifying a desirable film and coating component for a specific horticulture product can be a challenging task. These components and materials for a particular fruit must be identified based on data scattered across a wide range of literature. There has been no detailed review of edible films and coatings suitable for litchi fruit recently, that is why this review was planned. The use of various intelligent & edible films and coatings on litchi fruit is summarized and analyzed in this paper. This review aims to cover various major post-harvest challenges associated with the litchi fruit which limits the shelf life of this fruit significantly along with their preservation technique. This review discusses about the emerging sustainable intelligent and edible biopolymer-based packaging technologies to replace conventional treatments including sulfur fumigation, application of fungicides, and irradiation treatments for enhancing postharvest storability of litchi fruit which is in accordance with Sustainable Development Goals 12, 13 and 14. Also, the required properties of edible films and coatings for maintaining the desired nutritional and quality parameters of litchi fruit for shelf-life enhancement will be discussed. This paper will cover applications of different edible coating formulations which were developed by researchers for prolonging the storage life of litchi fruit and reducing its postharvest loss. This is an essential step towards achieving SDG 12.3 which is with regard to reducing global food losses and waste. Additionally, biodegradable polymeric food packaging is an environment-friendly technology that can lead to low carbon footprint, greenhouse gas emission and microplastic generation, and brings us a step closer towards SDG 13.2 and 14.1. The overall discussion will help in choosing better components for intelligent and edible coating formulation and standardization.
2. Brief overview of the litchi fruit
Litchi fruit is a member of the Sapindaceae family which includes fruits like logans and mangosteen which are believed to originate from the southern part of current China.29,30 The litchi fruit is characterized by a thin and sturdy peel that easily peels away, revealing a pearl-white, jelly-like pulp with a delightful taste resulting from the harmonious combination of acids and sugars.31 With a high fiber content and low protein and lipid amount, litchi is an excellent source of carbohydrates (∼16.5 g/100 g). The fruit possesses a higher level of various micronutrients including phenolics (∼178 mg GAE/100 g), flavonoids (∼54 mg QE/100 g), tannins, carotenoids, minerals (iron, phosphorous, and calcium), and vitamins (mainly C and trace amounts of A and K).32 It has been shown in vitro and in vivo that litchi has valuable bioactivities such as antioxidant, antimicrobial, antidiabetic, nontoxic, anticancer and antidiabetic characteristics.22,33,34 Besides being consumed as fresh produce it is utilized in various processed forms including canned litchi, litchi juice & wine, litchi honey, and pickles. Litchi pericarp (15% of fruit weight) which is discarded as waste also contains a significant amount of bioactive compounds (phenolics, flavonoids, and tannins).35It is widely produced in tropical and subtropical regions, with major producers located in Asia, Africa, and the Americas. The two largest producers are China (∼70% of total production) and India followed by countries like Thailand, Vietnam, Bangladesh, and South Africa.3,36 India produces 737![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 metric tons of litchi and produces 7.43 metric tons per hectare at present on an area of about 99
200 metric tons of litchi and produces 7.43 metric tons per hectare at present on an area of about 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 170 hectares.37 It is interesting to note that despite the smaller output, Vietnam has become the world's second-largest exporter of litchis in the year 2018. The demand for Vietnamese litchi is increasing due to better fruit quality than China and India.38 According to FAOSTAT 2020, the United States imports a majority of litchi, driven by the market demand for exotic fruits.39 The total size of the European litchi import sector is estimated to fluctuate between ∼20
170 hectares.37 It is interesting to note that despite the smaller output, Vietnam has become the world's second-largest exporter of litchis in the year 2018. The demand for Vietnamese litchi is increasing due to better fruit quality than China and India.38 According to FAOSTAT 2020, the United States imports a majority of litchi, driven by the market demand for exotic fruits.39 The total size of the European litchi import sector is estimated to fluctuate between ∼20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and 25
000 and 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tonnes per year. Madagascar is recognized as the primary supplier of litchi to the European region, introducing a significant amount of exotic fruits, predominantly litchi, totalling ∼15.5 thousand tonnes in 2017.40
000 tonnes per year. Madagascar is recognized as the primary supplier of litchi to the European region, introducing a significant amount of exotic fruits, predominantly litchi, totalling ∼15.5 thousand tonnes in 2017.40
As a non-climacteric fruit after harvesting, litchi stops ripening and becomes insensitive to exogenous ethylene. Once this fruit reaches full maturity on the tree, it must be harvested.41 One crucial factor in determining when to harvest the fruit is its color (red pericarp color and flattened tubercle).42 Another factor that decides when to harvest the litchi fruit (optimum condition) is the maturity index parameter (ratio of TSS and TA). The optimum value of TSS and TA for the harvesting is reported as 19° brix and ∼0.3–0.4%, respectively.43 However, these criteria will vary from cultivar to cultivar, country to country, and climate area to climate area. In tropical countries like India, post-harvest problems of fruits are of utmost importance. Litchi fruit is highly perishable, with a shelf life of two to three days at ambient temperature. The main factors preventing the growth of litchi-based farming and industries have been identified as pericarp browning, desiccation problems, chilling injury, fungal growth, and post-harvest decay. The pericarp browning of fruit is a major concern because consumers base their acceptance of it on its appearance. The quantitative and qualitative losses in litchi fruits from harvest to consumption have a significant impact, diminishing fruit availability and elevating transportation and marketing costs per unit. The average cumulative loss experienced within the supply chain of litchi varies between 35.3% and 43.8%, indicating significant inefficiencies and challenges in the preservation and distribution processes of this particular fruit commodity.44 In this context, edible films and coatings can be a viable and sustainable solution to resolve this issue.
3. Postharvest losses related to litchi
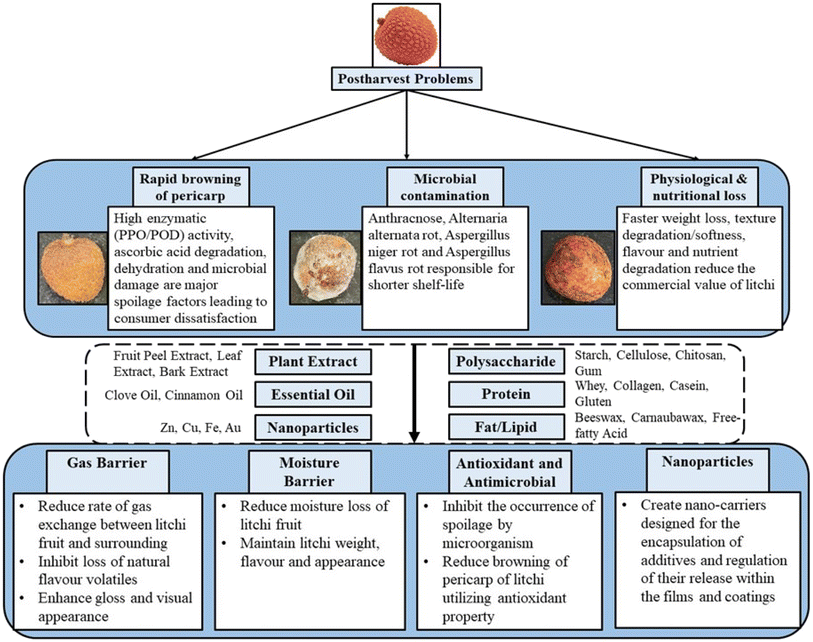
In this section, we have provided a concise summary of key mechanisms that contribute to litchi fruit deterioration as depicted in Fig. 1. These mechanisms must be considered when developing effective edible coatings to enhance the shelf life, quality, and safety of litchi.3.1 Pericarp browning
In litchi fruit, browning is a postharvest physiological disorder that has a significant impact on the fruit's quality and marketability. This change happens quickly, with about half of the fruit's skin turning brown within 12 hours of harvesting when kept at room temperature.45 This is an accelerated process that completely browns the skin after the 2nd or 3rd day after storage at room temperature (>20 °C).3 Associated phenomena for pericarp browning are desiccation, mechanical damage,46 fungal attack,47 high activity of PPO and POD enzyme48 and unsuitable low temperature (injury caused by chilling).49 High activities of polyphenol oxidase and peroxidase enzymes have been reported as a major cause of browning of the pericarp due to oxidation degradation of the red color pigment (anthocyanins). The activity of the PPO enzyme is reduced by the application of several types of antioxidants and is enhanced with the divalent cations of calcium and magnesium. Numerous studies have reported the PPO activity/amount variation with time for establishing the direct relationship between the PPO and browning effect on the pericarp of fruits. The activity of PPO (polyphenol oxidase) in litchi fruit storage appears to be unpredictable based on numerous studies. These findings highlight the inconsistent nature of PPO activity in litchi fruit storage, with some studies showing an increase,4 others indicating no significant change, and others reporting a gradual reduction. A study performed by Qinqin et al.50 reported that POD is involved in the enzymatic browning of litchi fruit due to its ability to oxidize 4-methyl catechol in the presence of H2O2, thereby forming brown polymeric pigments. The author's research findings support the hypothesis that both PPO and POD play a role in litchi pericarp browning. Recent findings have reported that when the activities of PPO and POD were inhibited, it resulted in a delay in the browning process of litchi pericarp.51,52Ascorbic acid degradation is reported as another factor that is responsible for the browning of the fruit pericarp. The overall mechanism is still not well understood, but it has been concluded that hydrogen peroxide is produced from the degradation of ascorbic acid which accelerates the oxidative degradation of anthocyanins. An increase in ascorbic acid oxidation has been reported with an increase in pericarp browning. However, a smaller amount of ascorbic acid in mature fruit pericarp makes it a non-significant factor for browning problems.53
Water loss and pH change are also correlated with the browning development of the pericarp.54 Underhillab et al.55 have studied the effect of water loss and pH change on pericarp browning and concluded that both these events increase the browning significantly. The pH of the pericarp tends to rise with moisture loss; after 48 hours at 25 °C and 60% relative humidity, it reaches a range of ∼4.15–4.52. The presence of anthocyanins, which give litchi their red colour, is impacted by this pH shift. Recently, a study was conducted by Liu et al.56 to establish a relationship between water loss from the pericarp and browning development of the pericarp of packed and unpacked litchi. In the end the researchers concluded that both these phenomena are highly correlated (R ∼ 0.92 & 0.65). Since water loss was significantly low (p < 0.05) for unpacked litchi as compared to packed ones, it was concluded in the end that packaging affects the browning significantly. However, whether this water loss is of biological significance is still not very clear.
3.2 Fungal infestation
Fungal attack is one of the most visible factors in the litchi fruit industry causing major post-harvest loss which lowers fruit value resulting in consumer rejection and economical loss. Generally, litchi is ready for harvest during the hot and humid season. However, this time of the year also makes them highly perishable and potentially susceptible to fungi infection. The coarse consistency of the litchi pericarp offers an optimal surface for the attachment of conidia and the proliferation of fungi. The litchi aril possesses a diminished pH level, elevated sugar concentration, and abundant nutrients, all of which are conducive to the flourishing of fungal entities, particularly Penicillium spp. This results in the fruit quickly decaying, losing its value for sale, and not surviving long.57 Various species of fungi have been reported to cause different diseases to the fruit including Alternaria, Botryodiplodia, Penicillium species and Colletotrichum gloeosporioides.16The primarily reported disease affecting the litchi fruit is anthracnose among several other fruit rots (caused by various species of fungus). Anthracnose is a postharvest lychee disease that is prevalent globally and is primarily caused by the fungal pathogen Colletotrichum gloeosporioides, with occasional contributions from C. acutatum.58 Lychee anthracnose is characterized by browning of the pericarp, where the infection is usually localized. The fleshy part of the lychee, or aril, is usually unaffected and collapses only sometimes. While infections may arise during cultivation in the field, they typically remain latent until post-harvest stages. In the year 2015 pathogens responsible for this disease were separated and identified in Oaxaca, Mexico as Colletotrichum gloeosporioides Penz.59 They reported anthracnose as the brown lesion on the fruit's outer surface. Zhao et al.60 reported the first case of anthracnose disease in China and explained that a brown spot developed on the fruit or the leaves of the litchi. This experiment concluded that the main pathogen responsible for this disease is Colletotrichum karstii (a newly identified pathogen) along with C. gloeosporioides.
Additionally, there are various other fruit rots which are responsible for the postharvest deterioration of the litchi fruit. Kumar et al.61 studied the effect of Alternaria alternata pathogen on litchi fruit in India (Bihar) and concluded that this pathogen causes fast and complete drying of the outer pericarp. According to the authors it is estimated that around 40% of the fruits get damaged by Alternaria alternata. Aspergillus niger rot and Aspergillus flavus rot are also reported in various literature studies which cause a severe damage during postharvest storage of the fruit. Aspergillus niger rot first manifests as a light brown lesion encircling the fruit's stalks, which subsequently turns deep brown. Four to five days after infections, black conidial heads can be distinguished. In the affected areas some white marks are often observed.
3.3 Additional factors responsible for postharvest changes
Accelerated water and firmness loss are two physiological parameters which limit the shelf life of litchi fruit. Water loss from the fruit increases weight loss, whereas firmness loss results in the textural loss of litchi fruits. Because of these losses (sensorial loss) the overall acceptability by the consumer is highly diminished.2 In various experimental studies it is well concluded that when litchi fruit was used as the control and was compared with other preservation techniques such as edible coating, MAP packaging and refrigerated condition storage, a significantly high weight and firmness loss was observed.62,63 The phenomenon of water reduction in litchi fruit is a manifestation of the respiration rates and the intricate interplay with the surrounding environment. The decrease in litchi firmness as the fruit ripens is predominantly attributed to a biochemical mechanism characterized by the breakdown of pectin and cellular elements through the action of hydrolytic enzymes, known as hydrolases. Additionally, this process is impacted by variables like loss of weight and moisture, which play a role in the reduction of tissue volume and damage to the fruit's skin.64 There is also a correlation given by the researchers in which excess water loss in litchi and other logan fruits contributes to the browning of the pericarp.65,66 Additionally, litchi fruit manifests a discernible physiological detriment (postharvest damage) when subjected to suboptimal temperatures below 10 °C (above the freezing temperature) referred to as the chilling injury. The fruit tissues lose strength, because they are unable to perform normal metabolic functions required at this reduced temperature and result in dysfunction.494. Intelligent & edible film and coating preservation mechanism for litchi fruit postharvest storability
This section provides an overview of the key parameters, working mechanisms, and essential functional attributes (as illustrated in Fig. 2) of the developed edible films and coatings intended for litchi preservation. The discussion focuses on crucial properties such as physical barrier and mechanical strength, which maintain the structural integrity of the film and protect the fruit from external stress; controlled moisture and gas exchange, which help to regulate respiration and prevent dehydration; antioxidant and antimicrobial activity, which inhibits oxidative degradation and microbial spoilage; and UV-resistant properties, which safeguards bioactive components from photodegradation.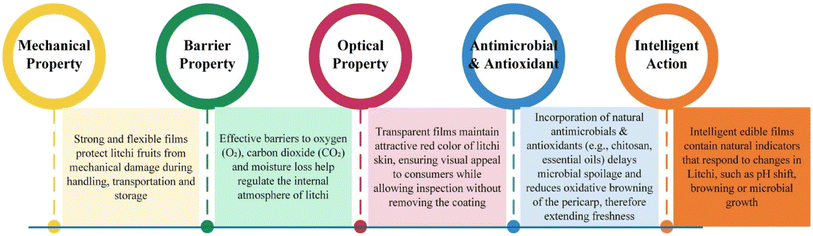 | ||
| Fig. 2 Functional properties offered by edible films and coatings for prolonging the storability of litchi fruit. | ||
4.1 Physical barrier and mechanical strength
Edible films and coatings create a protective external layer (like the existing natural cuticle layer) for fruits and vegetables after complete drying. This created barrier separates the external layer of produce and open environment or prevents direct contact.67,68 As a result, the fruit is less susceptible to direct exposure to detrimental elements in the external surroundings, microbes, pollution (dust) and other impurities. Thus, optimized edible films and coatings on litchi fruit will reduce fungal decay resulting in enhanced shelf life. When a chitosan based edible coating with pomelo extract and citric acid was applied on litchi fruit, fungal growth was reduced significantly.69 The film formed on the surface of the produce possesses some mechanical strength, which is a desirable requirement during the transportation of the fruits. Several factors such as tensile strength, elongation, plasticizer concentration and type, molecular weight of the film, solvent classification, and thickness of the coating influence the mechanical strength of a film.70 A study conducted on transportation of litchi from Muzaffarpur (Bihar) to Delhi concluded that 15.8% of fruits get damaged (compressed, bruised and attacked by fungi) in transit.61 In this context, mechanical strength provided by the applied film and coating can significantly overcome this loss due to the stress developed in fruits during transportation.A carrageenan based film utilizing grapefruit essential oil was formulated for active edible packaging. The amount of carrageenan was kept constant at 2% and grapefruit essential oil was varied from 0.1 to 0.3%. The tensile strength increases from 65 to 98 MPa as the amount of oil increases.71 This enhancement in tensile strength was due to the presence of the large range of ketones and phenolics in grapefruit essential oil. Similarly, sericin, chitosan and carbon dot based edible film exhibited maximum tensile strength and elongation at break of 11.63 MPa and 54.71% respectively, which further was applied as an edible coating on litchi fruit and has significantly enhanced its shelf life up to 6 days at open storage (25 °C and 85% RH).72
4.2 Controlled moisture and gas exchange
Desiccation is a vital problem associated with the limited shelf life of litchi fruits, which is also one of the factors responsible for the browning action of the pericarp and rapid weight loss of the litchi fruits.73 Edible films and coatings in this context are particularly important to regulate the rate of water and gaseous losses. The efficacy of a high-quality coating and film is determined by its ability to regulate the transfer of mass between the internal composition of the fruit and the external surroundings. This migration phenomena involves the passage of water vapor via transpiration, the flow of gases through respiration, the dispersion of gases, and specific volatile organic compounds.70 The water vapor permeability (WVP) of the edible film is significantly influenced by the hydrophobic characteristics of the polymer matrix used in its preparation, whereas the gas permeability of the edible film is significantly influenced by the cohesive energy, free volume, and crystallinity of the polymer matrix.74 The gas permeation in biopolymeric films is governed by the interplay of sorption and diffusion mechanisms, which are intricately influenced by the molecular dimensions and structural characteristics of the diffusing species.75 Mathematically, gaseous transfer can be formulated by Fick's law of gas diffusion and Henry's law of solubility as shown in eqn (1) and (2) respectively.
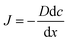 | (1) |
| P = C/S | (2) |
In eqn (1), J is the gas diffusion, D is the diffusivity constant and the other term is the concentration gradient. In eqn (2), P is the permeability, C is the permeate concentration and S is the solubility.
Thereby, matrix selection is a very technical component for any produce while developing edible films and coatings for an optimum level of gaseous and water vapor transfer rate. A proficiently formulated coating that exhibits effective water vapor and oxygen permeability is of greater importance in inhibiting textural deterioration, desiccation of fruits, reduction in size, as well as chemical or enzymatic processes, thereby ensuring the longevity of litchi fruit freshness. Increase in film thickness and lipid content cause WVP to reduce. When carnauba wax was incorporated into arrowroot starch and an edible film was developed, it was observed that WVP decreases from 6.7 to 3.6 (×10−7) g H2O per m h per Pa when the fraction of carnauba wax increases in the film.76 Recently, shellac (SH) and oleic acid (OA) were plasticized and a cast film was developed for efficacy in packaging application. Here, shellac was kept fixed and oleic acid was varied from 0 to 50%. SH–OA 10% shows 2.55 g per m2 per day WVTR as compared to 8.83 g per m2 per day of only the neat SH film. The improved barrier was probably due to the absence of any crack and hole on the surface and the amphiphilic nature of oleic acid. However, when the concentration of oleic acid was increased from 10% to 50%, then again there was enhancement probably related to an increase in free volume.77 A film of chitosan (CH) and cinnamon (CN) nano emulsion was prepared and its functionalisation by cold plasma treatment was performed. The CH and CH–CN in the developed film showed OTR less than 0.05 cm3 per m2 per day probably due to the well-arranged hydrogen bond structure.78
However, the developed film and coating should not completely block the passage of O2 and CO2 to maintain optimum level of oxygen to reduce potential fermentation development. Therefore, an ideal edible film or coating substance must possess semi-permeability to respiratory gases and inhibit respiration while avoiding unwanted fermentation.
4.3 Antioxidant and antimicrobial properties
The antioxidant and antibacterial characteristics play a crucial role as functional attributes of edible films and coatings. These properties are essential in inhibiting the oxidation of food and safeguarding it against microbial spoilage respectively, thereby preserving food quality and ultimately prolonging its shelf life. In the context of litchi fruit, these two active properties play an important role in shelf-life preservation, as antioxidants and antimicrobials can be highly effective against browning (reduce oxidation/activity of PPO/POD) and the fungal infestation problem of the litchi fruit, respectively. The active component is obtained through both synthetic (butylated hydroxy anisole (BHA), butylated hydroxytoluene (BHT), butyl hydroquinone (TBHQ) etc.) and natural sources (plant extracts and essential oils).29 These phytochemical actions in plant extracts and essential oils are due to the presence of phenolics, flavonoids and tannins.79,80 In recent times, there has been a growing interest among producers and consumers in the biodegradable packaging system that utilizes natural antioxidant and antimicrobial agents. Resources for active components include natural additives and extracts made from plants, herbs, spices, animals, and microorganisms.The ability of films and coatings to exhibit antioxidant and antimicrobial activities is related to their capacity to release the active components, and typically, this ability is directly correlated with the concentration of the active compound present in the packaging material.81,82 Antimicrobial action proceeds through two mechanisms in general: (I) via interfering with the chemical or biological process, (II) circumventing the natural defence mechanism/antibacterial resistance. The major action includes (I) interfering with the cell wall synthesis, (II) cell membrane degradation, (III) inhibiting DNA replication, RNA and protein synthesis, (IV) disturbing the drug delivery and metabolic pathway.
Bhatia et al.83 developed a chia seed mucilage based edible film incorporating cinnamon oil nanoemulsion as the active ingredient for antioxidant and antimicrobial characteristics. Incorporation of the cinnamon oil emulsion into the matrix significantly enhances the antioxidant activity (87.03 ± 0.30% DPPH radical scavenging capacity) and effectiveness against E. coli and S. aureus. The authors reported that increase in the antioxidant and antimicrobial capacity of the edible film could be due to the large range of bioactive compounds present in cinnamon oil. Therefore, this active edible film could significantly improve the shelf life of litchi fruit. Sajimon et al.84 have formulated an edible film of whey protein concentrate incorporating different concentrations of a nanoemulsion of oregano essential oil (1, 2, 3, and 4% (w/w)). The formulated film shows positive inhibition against E. coli and S. aureus (25.36 ± 0.52–28.0 ± 0.5 mm) and high antioxidant properties in terms of DPPH (86.89 ± 0.087%) and FRAP (51.24 ± 0.031%) respectively. The high activity could be easily correlated with the presence of various phytochemicals present in oregano essential oil.
4.4 UV resistance properties
The assessment of transmittance plays a crucial role in the evaluation of the optical barrier characteristics of films, indicating their efficacy in blocking UV and visible light.85,86 The potential of ultraviolet (UV) radiation to shorten the shelf life of agricultural commodities is evident. Long exposure to light, especially ultraviolet light, degrades food quality through photolysis and photooxidation reactions. As a result, active oxygen and free radicals are generated, forming unpleasant odors and flavours, reducing nutritional value, and discoloring food, thereby promoting food degradation. Thus, in this context UV resistant edible films and coatings could be a potential solution for minimizing the phytochemical losses in litchi fruit and other horticulture produce.87Abdel Aziz et al.88 have developed an alginate based edible film containing aloe vera gel as the active component for the enhancement of antioxidant, antimicrobial and UV shielding. The study concluded that neat alginate based edible film shows poor UV shielding properties showing UV transmission up to 90%. Thereafter, when aloe vera gel was incorporated in 50% and 66.7% volume, significantly reduced UV transmission up to 15.3 and 6.01% was obtained. This reduction was attributed to the presence of different UV absorbing substances in aloe vera gel. Chen et al.89 have formulated a nano lignocellulosic biomass and gluten based composite film targeting the high UV barrier properties for packaging litchi fruit. Nearly 100% UV barrier was displayed by the nano lignocellulosic and gluten based composite film attributed to the presence of aromatic rings. This study concluded that fruits packed with the developed film show optimum firmness and low weight loss as compared to open and conventionally packed litchi.
4.5 Intelligent action
According to our conceptual framework, a package is deemed “intelligent” if it possesses the capability to monitor the product, perceive the environmental conditions both internally and externally, and engage in communication with humans.24 For instance, an intelligent package is characterized by its ability to assess the quality and safety parameters of a food product while offering timely alerts to the consumer or food producer. Intelligent edible coatings utilizing natural biopolymers, including chitosan, pullulan, and gum arabic, and integrated with pH-sensitive indicators, may be applied as a protective layer on litchi fruit. These coatings mitigate respiration, inhibit enzymatic browning, and prevent microbial proliferation, thus preserving fruit quality and enhancing shelf life. Concurrently, the intelligent components, such as anthocyanins or curcumin, react to pericarp pH fluctuations and metabolite release during spoilage, exhibiting a colorimetric change that indicates fruit freshness.25 According to a research, a bilayer antibacterial chromogenic material was developed using chitosan and hydroxyethyl cellulose as the base, mulberry anthocyanins as freshness indicators, and nano-TiO2 as an antimicrobial layer. When applied to litchi, it inhibited microbial growth, delayed browning, and maintained quality. The intelligent role of mulberry anthocyanins was to respond to pH changes during deterioration, showing visible color shifts that signaled the fruit's freshness. Thus, the material functioned both as a preservative and a real-time quality indicator as represented in Fig. 3.90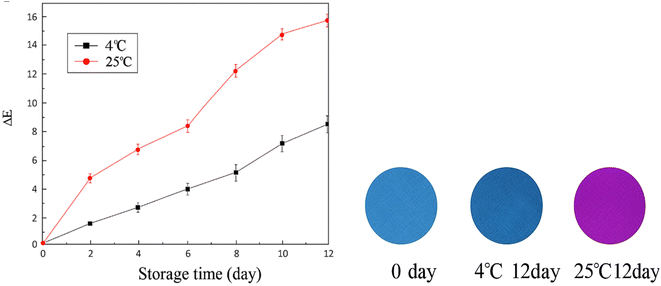 | ||
| Fig. 3 Changes in chromatic aberration of a two-layer antibacterial chromogenic material in litchi fruit during storage at 4 and 25 °C for 12 days (left), and pre- & post-intelligent color modulation of this double-layered substance at 4 and 25 °C on the 12th day as compared to the 0th day (right); reproduced with permission from Luo et al. (2022),90 open access. | ||
5. Intelligent & edible films and coatings on litchi fruit
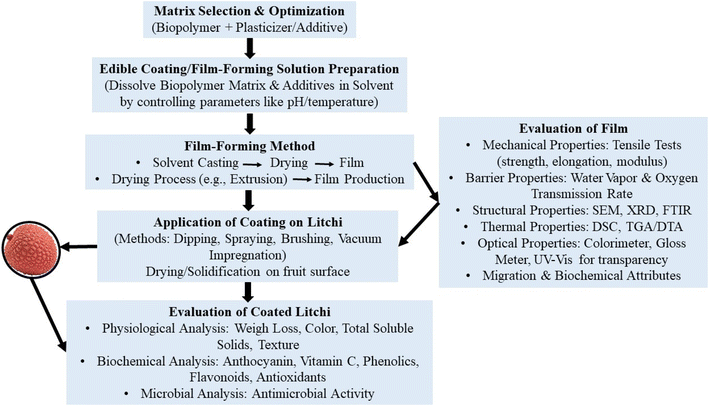
Edible films and coatings are used to enhance the storage life and quality of litchi fruit by controlling the diffusion of gases and moisture, protecting against physical, chemical and microbial damage, slowing down the deteriorative changes and improving the sensorial attributes. There are several bio-based polymers, i.e., polysaccharides, proteins and lipids, plasticizers, fillers, additives and nanomaterials which are used as intelligent & edible packaging ingredients and they are mentioned below with the suitable cases of applications to litchi fruit. In Table 1, different matrices have been tabulated with their advantages and disadvantages which are important before selecting the matrix for formulation of edible films and coatings for different products. More specifically, polysaccharides form transparent, biodegradable films and coatings with good gas barrier properties but exhibit limited water resistance due to their hydrophilic nature. Lipid-based coatings provide excellent water vapor barriers and reduce moisture loss but have low mechanical strength and limited flexibility. Wax-based primary packaging effectively reduces water loss and enhances surface gloss, yet it offers weak gas barrier properties and may alter the sensory attributes of food when applied in excess. Hence, combining these materials can yield composite films with improved functional and protective properties. After developing the film solution, it is either cast or extruded to obtain the dried film that is characterised for its mechanical, WVTR, OTR, thermal, structural, optical, migration and antimicrobial properties. The coating solution is applied to litchi through dipping, spraying, brushing, etc.. The coated litchi is then evaluated for its physiochemical, biochemical, microbial and sensory properties. The complete workflow is illustrated in Fig. 4. Furthermore, in Table 2, research conducted over the past five years has been summarized which discusses in detail about the application of various developed edible and intelligent packaging formulations on litchi fruit under both room and refrigerated storage conditions.| Matrix | Advantages | Limitations | References |
|---|---|---|---|
| Cellulose and its derivatives (e.g., hydroxypropyl methylcellulose – HPMC) | • Better film-forming properties | • Poor WVTR properties | 91–93 |
| • High transparency and mechanical strength | • Requires plasticizers to improve flexibility | ||
| • Biodegradable, non-toxic and inexpensive | |||
| Starch | • Abundant and cost-effective | • Poor water resistance | 94–96 |
| • Film forming properties and good barrier to O2 and CO2 | • Poor mechanical properties thus requires modification or blending with other materials | ||
| Chitosan | • Antimicrobial/antioxidant properties | • Solubility issues in water | 97 and 98 |
| • Excellent film-forming ability, mechanical strength and gaseous barrier properties | • High permeability to water molecules | ||
| • At higher concentrations sensory properties get affected | |||
| Pectin | • Good film-forming properties | • Less mechanical strength | 99–101 |
| • Excellent gelling agent/emulsifier and thickener | • Poor barrier to water vapour | ||
| • Good barrier to O2 and CO2 | |||
| Alginate | • Good gel-forming ability, used as a thickening and emulsifying agent | • Weak mechanical properties | 102 and 103 |
| • Biodegradable and non-toxic | • Requires calcium ions for gel formation or crosslinking process | ||
| Pullulan | • Excellent film-forming ability and barrier to O2 and CO2 | • Weak water vapor barrier properties | 104–106 |
| • High transparency and flexibility | • High price, low solubility | ||
| Carrageenan | • Good gel-forming and film-forming properties, low cost | • Sensitive to moisture | 107 and 108 |
| • Enhances biodegradability and non-toxic | • Requires plasticizers for improved flexibility | ||
| • Better mechanical and UV protection properties | |||
| Xanthan gum | • Good film-forming ability and inexpensive | • Poor resistance to water | 109 |
| • High viscosity and stability | • Requires blending with other materials to improve properties | ||
| • Biodegradable and non-toxic | |||
| Guar gum | • Good film-forming properties | • Poor mechanical properties | 110 and 111 |
| • Biodegradable and non-toxic | • Highly hydrophilic | ||
| • Good viscosity and stability | |||
| Agar | • Excellent gel-forming and film-forming properties | • Highly brittle | 112–114 |
| • Biodegradable and non-toxic | • Poor mechanical and barrier properties | ||
| • Transparent and flexible | |||
| Dextran | • Good film-forming properties | • Poor mechanical strength | 115 and 116 |
| • Biodegradable and non-toxic | • Requires blending with other materials to improve properties | ||
| • High water solubility | |||
| Beeswax | • Superhydrophobic exhibiting good water barrier properties | • Poor mechanical strength | 117–119 |
| • Biodegradable and derived from renewable sources | • Low melting temperature and gaseous barrier | ||
| • Suitable for use in vegan and vegetarian products (GRASS approved) | |||
| Carnauba wax | • High gloss and excellent moisture barrier (highly hydrophobic) | • Brittle at room temperature | 120–122 |
| • Biodegradable and food-grade (GRASS approved) | • Difficult to process without emulsifiers | ||
| Shellac wax | • Excellent gloss and moisture barrier | • Brittle and requires plasticizers for flexibility | 123 and 124 |
| • Derived from natural sources | • Limited heat resistance | ||
| • Biodegradable (GRASS approved) | |||
| Rice bran wax | • Good barrier properties | • Moderate mechanical strength | 125 and 126 |
| • Biodegradable and derived from renewable sources | • Limited availability and higher cost compared to some other waxes | ||
| • Suitable for use in vegan and vegetarian products (GRASS approved) | |||
| Gelatin | • Good mechanical strength and transparency | • Highly hygroscopic | 127–129 |
| • Low cost | • Animal derived, possible concern for vegetarians/vegans | ||
| Whey protein | • Good film-forming properties | • Moderate barrier properties to gases and moisture | 130–132 |
| • Flexible film, resistance to oil | • Possible allergenicity | ||
| Soy protein | • Good mechanical properties | • Poor moisture barrier properties | 130 and 131 |
| • Renewable and biodegradable | • Potential allergenicity | ||
| Casein | • Excellent film-forming properties | • Sensitive to moisture | 133–135 |
| • Good mechanical strength and transparency | • Derived from animal sources | ||
| Collagen | • Good film-forming ability | • Poor mechanical strength | 136 and 137 |
| • Biocompatible and biodegradable | • Requires crosslinking agents | ||
| Corn zein | • Excellent water barrier properties | • Brittle nature | 138–140 |
| • Medium gas barrier, resistance to oil | • Insoluble in water |
| Edible packaging formulation | Method of application | Storage conditions | Outcome | Reference |
|---|---|---|---|---|
| Trehalose | Dipping | 25 °C with 85 ± 5% RH for 8 days | Trehalose treatment effectively delayed browning in litchi, as evidenced by a ∼15.1% higher chromaticity value on the 8th day compared to the control. It also promoted anthocyanin biosynthesis and inhibited the activities of PPO and POD | 164 |
| Glutamic acid (Glu), methionine (Met) | Dipping | 25 ± 1 °C | Glu, Met and Mix (the combination of Glu and Met notably delayed pericarp browning and pulp degradation). Mix treatment increased sucrose degradation, slowed sugar oximation & cell wall breakdown | 165 |
| Cellulose, Fe-doped ZIF-8, epigallocatechin gallate | Wrapped in film | 25 °C & 60–70% RH | Storage life was prolonged to 9 days with concurrent inhibition of browning reactions and mildew formation. Mass loss was curtailed by ∼40%, while bioactive compounds including anthocyanins, ascorbic acid, phenolic components, and flavonoids showed enhanced stability | 166 |
| Chitosan, vanillin-based deep eutectic solvents | Dipping | Ambient conditions (25 ± 2 °C; RH 80–90%) | Shelf life was extended to 5 days. Browning and weight loss were minimized. Vitamin C & anthocyanin contents were maintained over the storage period. Antibacterial activities of the developed formulation effectively inhibited E. coli and S. aureus activity | 167 |
| Peach gum | Dipping | 25 ± 1 °C with 80–85% RH for 8 days | The optimized solution reduces the browning rate, retards anthocyanin degradation, maintains weight loss, TSS and other organoleptic properties | 168 |
| Liposome, chitosan | Dipping | 20 °C, 75% RH up to 9 days | Coating enhanced appearance quality (browning index ↓ ∼40%), nutritional retention, moisture retention, membrane integrity, antioxidant activity, enzymatic control, disease resistance | 169 |
| Chitosan, aloe vera gel and combination with UV radiation | Dipping | Refrigerated condition storage (2 °C for 20 days) | Reduced browning, preserving phenolic compounds, flavonoids, anthocyanins, antioxidant activity, lowering physiological and biochemical degradation during refrigerated storage | 170 |
| Starch, gum, citrus peel extract | Room temperature for a 6 day storage study | Effectively reduced weight loss, microbial load, and colour degradation in litchi during 6 days of storage. The optimized formulation (starch-to-gum ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 0.4% peel extract, particle size 300 µ) preserved quality attributes more effectively than the control 1, 0.4% peel extract, particle size 300 µ) preserved quality attributes more effectively than the control |
171 | |
| Sodium alginate, calcium chloride, cellulose nanocrystals, nisin | Dipping | 25.1 ± 1.2 °C, 30.0 ± 2.1 RH% for 11 days | Shelf life extended by 2.6 times; browning index reduced by 38.1% and decay rate by 62.5%; moisture loss minimized; firmness, pH, and quality attributes maintained | 172 |
| Chitosan, oxidized fucoidan, cinnamaldehyde | Dipping | Room temperature for 8 days | Shelf life increased to 8 days. Total phenolics content was ∼72.5% higher than control; vitamin C was ∼59% higher. TA, TSS, weight loss & hardness were maintained | 173 |
| Starch, selenium nanoparticles | Wrapped in edible film | 25 °C, 50% RH for 15 days | The developed film showed enhanced properties in terms of UV barrier, mechanical strength, antioxidant and antimicrobial properties. Improved biodegradability (∼11 days in soil) and biocompatibility was reported. The developed formulation reduces the moisture loss and decay incidence & enhances the shelf life till 15 days | 174 |
| Cellulose, methylcellulose, acacia gum | 27 ± 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C, 82 ± 5% RH for 7 days °C, 82 ± 5% RH for 7 days |
Optimized formulation retained TSS (∼65%), TA (∼42%), ascorbic acid (∼24%) & phenolic acids (∼40%) in treated litchi juice as compared to uncoated ones. Physiological weight loss and decay of fruits were also significantly maintained | 175 | |
| Alginate oligosaccharides | Spraying | 5 °C with 85% RH for 21 days | Reduced weight loss (∼12%), better texture & color attributes and lower rate of decay incidence (∼15%) in fruit were observed as compared to the control group | 176 |
| Gluten, starch nanocrystal, montmorillonite | Edible film packaging | 4 °C for 20 days | The developed biocomposite demonstrated higher tensile strength (∼57 MPa) & lower WVTR characteristics. Treatment with the optimized formulation reduced the action of browning causing phenol PPO & POD. Similarly, coating treatment maintained weight loss (∼7.36%), surface hardness (∼19.86 N) & retained a higher level of anthocyanin and ascorbic acid | 64 |
| Egg albumin, chitosan, carbon dots | Dipping | ∼25 °C and ∼85 RH for 6 days | The film displayed super antimicrobial, antioxidant and light hindrance quality. The developed formulation displayed constant values of vitamin C, color chromaticity & other organoleptic characteristics | 156 |
| Fisetin silver nanoparticles (AgNPs) | Dipping | ∼25 °C and 36% RH | The film showed greater tensile strength (∼61 MPa) and lower WVP (∼20 kg per m2 per day). The developed coating displayed reduce rate of fruit decay & enhanced overall organoleptic properties | 163 |
| Zein, chitosan curcumin, zeolitic imidazolate framework-8 | Edible film packaging | ∼25 °C and ∼70% RH for 8 days | The developed film demonstrated enhanced tensile strength and OTR (∼9.2 and 1.5-fold fruit). Freshness and overall appearance were maintained till day 8 as compared to control litchi | 177 |
| Vanillin–taurine antioxidant compound | Dipping | ∼25 °C and ∼90% RH | Reduced level of PPO and POD enzyme & enhanced level of SOD during storage was reported. The coated fruit displayed lower weight & textural loss compared to the control. Similarly, browning was significantly reduced in the coated fruit | 178 |
| Silver nanoparticles | Dipping | Room temperature for 5 days and at 4 °C for 25 days | Minimum browning index, least decay, and least weight & firmness loss were observed | 179 |
| Chitosan, hydroxyethyl cellulose, mulberry anthocyanins, titanium dioxide nanoparticles | Edible film as sealing material | ∼25 °C and 4 °C | Reduced weight loss, texture degradation and browning index in coated litchi were observed. Coating retained physiological and organoleptic attributes for longer storage length | 180 |
| Silk sericin (SS), chitosan, carbon dots | Soaking | 25 °C and 85% RH for 6 days | The developed biocomposite demonstrated enhanced properties such as antioxidant activity, overall flexibility and antimicrobial action. Treated fruits showed reduced water loss, firmness, TSS, TA and anthocyanins during the storage period | 72 |
| Zinc oxide nanoparticles (ZnO-NPs) | Spraying | 25 ± 2 °C. After 7 days | Maximum mycelial growth inhibition (∼70%) was observed for litchi fruit coated with 1 mg per ml ZnO-NPs | 181 |
| Aloe vera chitosan, corn starch, calcium chloride, salicylic acid | Dipping | Room temperature (non-refrigerated storage) | Optimized formulation reduced the weight loss & fruit hardness till day 8. The fruit cracking, skin browning, and fruit decay were reduced. Overall organoleptic attributes were maintained during the storage period | 182 |
| Calcium chloride, oxalic acid, ice treatment | Dipping | Ambient temperature | Delayed disease incidence, extended shelf life, reduced physiological weight loss, maintained firmness, lowered pericarp browning index, regulated TSS and titratable acidity, preserved vitamin C content, and stabilized pH in postharvest litchi fruit | 183 |
| Chitosan, pullulan, pomegranate peel extract | Dipping | ∼25 °C and RH ∼ 45% and cold storage (∼4 °C and RH ∼ 95%) | Lower weight loss after 18 days, regulated TSS and TA, maintained pH, higher phenolics and flavonoids, and antioxidant activity, improved sensory qualities (notably on day 9 under both storage conditions) | 2 |
5.1 Polysaccharide-based edible coatings
Polysaccharides are long chains of monosaccharides and disaccharides as repeating units bonded by the glycosidic linkage. There are various polysaccharides, for example, chitosan, cellulose, pullulan, starch, sodium alginate, pectin, gums, etc., which can be used to coat the litchi fruit and are discussed below. These biopolymers have excellent film forming properties and are abundantly available at affordable rates. They possess superior mechanical and gas barrier properties but inferior water vapor barrier properties.141–143 Therefore, they are used in combination as a composite edible packaging material to obtain the desired properties for specific applications.Recently, Liang et al.147 have developed a composite film of chitosan (CS), fucoidan (FU) and encapsulated cinnamaldehyde (CA). First, chitosan and fucoidan derivatives were developed and then cinnamaldehyde was added. The crude emulsion was degassed in an ultrasonic cleaning bath, then spread on a Petri dish and dried at 45 ± 1 °C for 12 h. The dried coating films were then removed and stored in a dryer at 25 °C and 50% relative humidity for future use. The coating solution was then used for coating litchi fruit and various parameters (weight, texture, colour, vitamin, phenolic content) were determined at room temperature. CA/CS–FU-0.1 coated fruit showed the least microbial growth, least weight loss and maintained the highest firmness (1.36 ± 0.05 N), researchers concluded that the high antimicrobial activity and barrier properties shown by the CA/CS–FU-0.1 film were responsible for these characteristics. Similarly, significantly higher values of vitamin C (29.14 ± 0.50 mg 100 g−1) and phenolics (8.38 ± 0.30 g kg−1) were reported for CA/CS–FU-0.1 coated litchi till the 8th day, again due to the high antimicrobial and barrier properties of the solution. Therefore, the authors concluded that CA/CS–FU-0.1 coating solution is sufficient to improve the shelf-life of litchi as compared to uncoated references (Fig. 5).
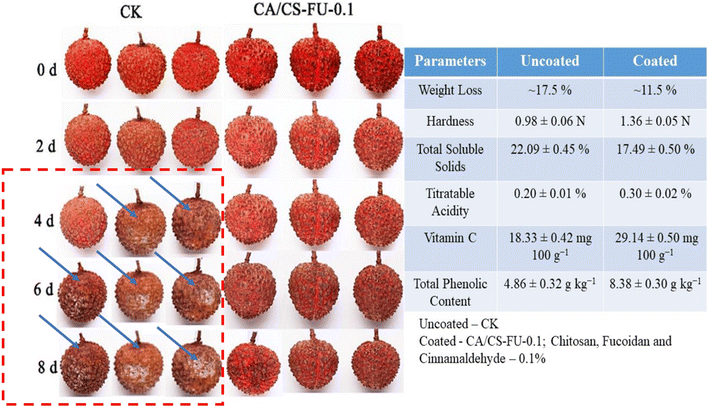 | ||
| Fig. 5 Chitosan–fucoidan encapsulated cinnamaldehyde composite coating on litchi fruits and its quality parameter comparison with control ones; reproduced with permission from Liang et al. (2024),147 Elsevier. | ||
A gluten-based film with montmorillonite (MMT) and starch nanocrystals (SNCs) reinforced into chitosan was formulated by Sharma et al.148 Thereafter, its efficacy analysis was done and finally the same was used as an edible film for shelf-life preservation of litchi up to 20 days. MMT and SNC increase the tensile strength (up to 57 MP) and reduce the water vapour transmission rate (0.68 × 10−3 g m−2 h−1). However, SNC/chitosan was superior to the MMT/chitosan-based film due to the excellent barrier properties. Coated litchis show superior organoleptic properties as compared to uncoated litchis: (i) lower enzyme activity (PPO/POD), (ii) higher bioactive components (anthocyanins, phenolics and flavonoids), (iii) less alteration in firmness (8.47% and 7.36%) and physiological weight loss (24.04% and 19.86%). In conclusion, the developed packaging can be used as a potential preservation technique for litchi fruit.
Litchi fruit was immersed in carboxymethyl cellulose (CMC) biopolymeric solution and stored under ambient conditions, i.e., 27 °C and 82% RH for 7 days in order to extend the shelf life.151 CMC coating had a significant effect on coated litchi. CMC retained total soluble solids (∼65%), and had higher titratable acidity (∼42%), ascorbic acid content (∼24%), and total phenolic content (∼40%) than the control fruit samples. Furthermore, enzymatic activity such as polyphenol oxidase (∼25%) and peroxidise (∼28%) activities were reduced. Weight loss, pericarp browning and decay incidence were lowered down in the case of treated litchi fruits as compared to uncoated ones.
Liu et al.152 have made a novel film having a regulated release of chlorine dioxide gas on the litchi fruit surface. The idea behind the work was that a regulated amount of chlorine dioxide can control the respiration and transpiration rate as well as destroy the bacteria and fungi on the surface. Here NaClO2 was encapsulated in EC (ethyl cellulose) as the shell and was finally placed in PVA (poly vinyl alcohol). Thereafter, a small amount of citric acid was introduced, which results in a regulated release of ClO2 gas.
| 5ClO2− + 4H+ → 4ClO2↑ + Cl− + 2H2O |
The mechanical strength (58.66 MPa) and gas barrier (36.1% H2O & 32.3% O2 migration rate reduction) characteristics of the films were highly appropriate for the manufacturing of litchi storage pouches at a microcapsule content of 6.0% (w/w). Additionally, the storage bags exhibited the highest ClO2 concentration of 263.22 ± 12.34 mg m−3, effectively eradicating pathogenic bacteria (E. coli andL. monocytogenes) and impeding the development of B. cinerea. The authors reported that this optimized film enhanced the shelf life of litchi fruit, thus the proposed PVA/EC–NaClO2 active package films have the potential to enhance the shelf life of litchi fruit.
A chitosan and pullulan edible coating enriched with pomegranate peel extract were dip-coated on litchi fruit.2 The treated fruits were stored for 18 days at room temperature, i.e., 23 °C and 40–45% RH and refrigerated temperature, i.e., 4 °C and 90–95% RH. The results suggested that 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 chitosan:pullulan added with 5% extract of pomegranate peel decreased physiological weight loss, total soluble solids, acidity and browning. The weight loss was reduced to 44.5% and 55.7% at room temperature whereas 10.4% and 12.6% under refrigerated conditions for treated and control litchi on the 18th day, respectively. Edible coated litchi showed lesser increase in total soluble solids and titratable acidity. Additionally, the pH, phenolic and flavonoid contents, and antioxidant activity were reduced to a lower extent in the coated fruits.
50 chitosan:pullulan added with 5% extract of pomegranate peel decreased physiological weight loss, total soluble solids, acidity and browning. The weight loss was reduced to 44.5% and 55.7% at room temperature whereas 10.4% and 12.6% under refrigerated conditions for treated and control litchi on the 18th day, respectively. Edible coated litchi showed lesser increase in total soluble solids and titratable acidity. Additionally, the pH, phenolic and flavonoid contents, and antioxidant activity were reduced to a lower extent in the coated fruits.
Bham et al.154 applied guar gum, xanthan gum and methyl cellulose (low and high viscosity) at various concentrations on litchi as an edible coating for enhancing the quality and shelf life. The treated fruits were studied for 12 days under refrigerated conditions (4 °C and 90% RH). 1.5% guar gum coated fruits which were stored in a perforated brown paper bag showed minimum weight loss and fruit shrinkage%, pericarp browning and highest overall sensory attributes.
5.2 Protein-based edible coatings
Proteins are made up of 20 amino acids linked by peptide bonds. Proteins such as collagen, keratin, gelatin, zein, casein, gluten, soy protein, egg protein and whey protein are employed on litchi for its storage and preservation. Proteins have good mechanical, optical and gas barrier properties, however they have poor water vapor permeability.155 In the context of litchi, proteins can be used with other biopolymers including polysaccharides and lipids to compensate their weaknesses and obtain the film of required properties.An edible coating formulated with silk-sericin, chitosan and carbon dots was applied on litchi by the immersion method and thereafter fruits were stored at 25 °C and 85% RH for 6 days.72 The study reported that edible coatings decreased the browning of litchi peel, weight loss, change in total soluble solids and titratable acidity, and malondialdehyde content while maintaining a higher value of antioxidant and vitamin C content as compared to the control litchi fruit.
Su et al.156 investigated the effect of an egg albumin, chitosan, and carbon dot based edible coating in the volume ratio of 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 on litchi fruits which were stored at 25 °C and 85–90% RH for 6 days. Coated litchi showed lower pericarp browning, weight loss% and reducing sugar whereas higher chromaticity a*, b*, and L*, vitamin C, total sugar, and sucrose content were retained (Fig. 6).
1 on litchi fruits which were stored at 25 °C and 85–90% RH for 6 days. Coated litchi showed lower pericarp browning, weight loss% and reducing sugar whereas higher chromaticity a*, b*, and L*, vitamin C, total sugar, and sucrose content were retained (Fig. 6).
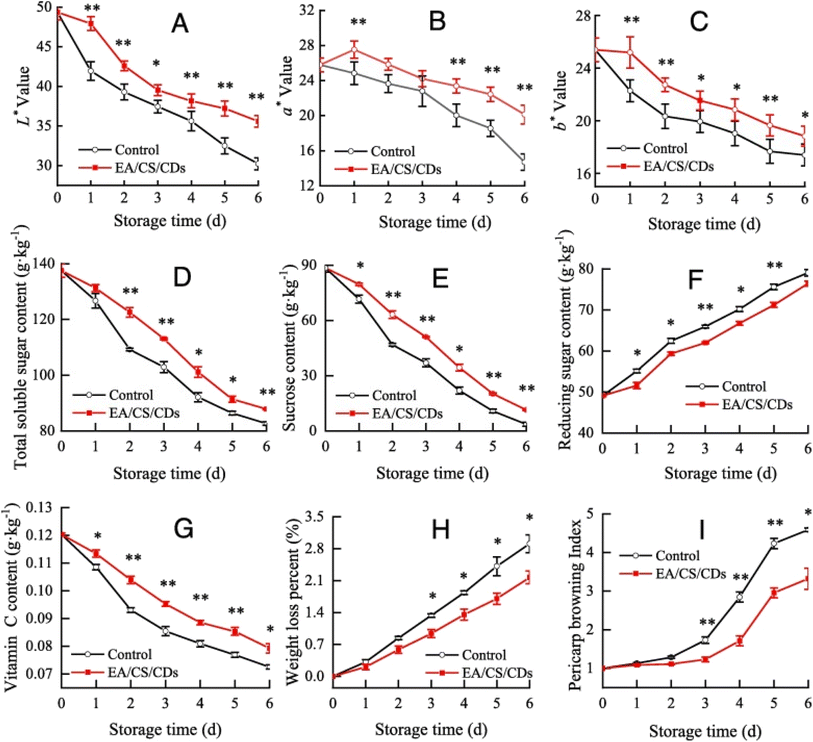 | ||
| Fig. 6 Quality parameters of litchi fruit coated with the egg albumin, chitosan, and carbon dots and its comparison with the control including pericarp color attributes: L* value (A), a* value (B), and b* value (C); pulp total soluble sugar content (D); sucrose content (E); reducing sugar content (F); vitamin C content (G); fruit weight loss percentage (H); and pericarp browning index (I); reproduced with permission from Su et al. (2023),156 Elsevier. | ||
5.3 Lipid-based edible coatings
Lipids include phospholipids, mono-, di- and tri-glycerides, phosphatide, terpenes, cerebrosides, natural wax, fatty acids, fatty alcohol, and surfactants.155 They are used in edible packaging because they provide surface gloss and water vapor barrier properties. However, they offer poor mechanical and optical properties. They are fragile, non-cohesive, and greasy, with a waxy taste and texture. These can be balanced by using other biomaterials in combination with lipids for film formation on litchi and other agro-commodities.A research study depicted the effect of a 9% carnauba wax nanoemulsion (CWN) edible coating on post harvested lychee fruits which were stored at 16 °C and 70% RH.157 This edible coating significantly reduced the weight loss by 4% and pericarp browning and maintained total soluble solids and pH compared to control fruits after 96 h of storage. When 9% CWN was supplemented with 1% Mentha piperita essential oil, the weight loss was 10.6% after 168 h while it was 18.2% for the control. The coating effectively maintained the pH, TSS, TA, firmness and color of coated litchi fruits. The incidence of disease was 20% and 60% for treated and untreated fruits.
Another study conducted by Nanglia et al.52 analysed the effect of hydrochloric acid and shellac wax edible coating on litchi under low temperature storage (2–3 °C and 90–95% RH) for a duration of 14 days. The edible coated fruits showed lesser weight loss, browning index value and decay incidences while maintaining higher firmness and bioactive value. The coated fruits showed retarded activities of enzymes such as polyphenol oxidases, peroxidase, and phenylalanine ammonia lyase as compared to uncoated fruits.
5.4 Additive-based edible films and coatings
Edible packaging is fortified with additives such as antioxidants, antimicrobials, essential oils, preservatives, and browning inhibitors to maintain food quality. Plasticizers, emulsifiers, and cross-linking agents enhance structural and barrier properties. Bioactive ingredients like probiotics, plant extracts, nutrients, and nutraceuticals further improve functionality and health benefits.155 These are added in permissible limits to enhance physical, chemical and functional properties of coatings or films and further, slow down deteriorative changes in food. For instance, plant oils enhance food quality and safety by providing water vapor, oxygen and UV barrier, antioxidant and antimicrobial properties in packaging.158One such research showed that when litchi fruit was given an edible coating with 50% aloe vera gel, an active additive, and stored at 20 °C and 90% RH for 8 days, the browning was significantly delayed.159 The weight loss, superoxide anion, relative electrolyte leakage, hydrogen peroxide and malondialdehyde content were also reduced as compared to untreated fruits. The enzyme activities such as peroxidase and polyphenol oxidase were decreased. Treated samples showed a higher total anthocyanin content, ascorbic acid content, total phenolic concentration, total soluble solids, titratable acidity and enzyme activities such as catalase, superoxide dismutase and ascorbate peroxidase activities.
Li et al.160 developed an edible coating solution utilizing Lysimachia foenum-graecum Hance based oil, eggplant pill anthocyanin, and Ginkgo biloba leaf extract and utilized them for coating litchi fruit. After coating the fruit, they were stored for a period of 12 days at 25 °C. The superior preservation effect on litchi is evident with the utilization of 0.5% essential oil extract treatment, ranking above both 0.5% Ginkgo biloba extract and 0.05% potassium sorbate treatment. The latter treatments exhibit comparable effects that surpass the efficacy of 0.5% anthocyanin extract. The findings illustrated that the three types of botanical extracts exhibited a deceleration in water evaporation, suppression of enzymatic browning, mitigation of decay progression in litchi fruit to a certain degree, and exerted a specific influence on the retention of quality and vitality.
5.5 Nanoparticles in edible coatings
Nanomaterials are nanoscale structures that have at least one dimension in the range of 1 to 100 nm. They can be categorised as organic and inorganic. Micelles, dendrimers, liposomes, nanogels, polymeric nanoparticles, and layered biopolymers are some examples of organic nanomaterials. Inorganic nanomaterials do not contain carbon and can be divided into metal nanoparticles (gold, silver, iron, copper, aluminium, etc.), metal-oxide nanoparticles (zinc oxide, titanium dioxide, iron oxide, etc.), ceramic nanoparticles (hydroxyapatite, alumina, silica, etc.), bionanomaterials (exosomes, magnetosomes, lipoproteins, viruses and ferritin) and carbon-based nanomaterials (quantum dots, graphene, carbon black, carbon nanotubes and nanofibers).161 Due to their morphology and high surface area to volume distribution ratio, they provide improved physicochemical attributes. They exhibit antibacterial and antioxidant properties and are therefore employed for food packaging applications in permitted doses and pretested conditions.A research study was conducted in which quaternary ammonium salted chitin, epigallocatechin gallate, selenium nanoparticles, carboxymethyl cellulose and corn starch were used for formulating an edible film in order to preserve litchi stored at 25 °C and 50% RH for 15 days.162 The weight loss%, decay rate%, and texture deterioration were minimum whereas soluble solid content, pH, and titratable acids were better maintained in the edible film wrapped litchi over a period of 15 days as compared to control fruits.
Using a similar approach fisetin chelated silver nanoparticles (FT-AgNPs) with a chitosan/pullulan (CS/PUL) matrix was formulated.163 It was observed that the formulated novel film displayed great tensile strength (61.2 MPa) and lower WVP (below 20 kg per m2 per day). The decay of litchi fruit was effectively inhibited by the 0.6% FT-AgNPs/CP treatment during storage at 25 °C, resulting in 15 days storage compared to 9 days for the control group. Furthermore, the presence of 0.024 µg kg−1 of residual Ag+ in litchi pulp confirmed the safety of utilizing the 0.6% FT-AgNPs/CP treatment.
6. Formation of edible and intelligent films and coatings
Different techniques exist for applying films and coating materials to litchi fruits and different horticulture produce on a small scale in the laboratory. These include dipping, brushing, extrusion, spraying, and solvent casting. However, transitioning to large-scale industrial production may require new processing equipment that can integrate well with existing packaging machinery. Common methods for forming films typically involve either casting solutions (wet method) or processing melted materials (dry method). The dry method includes processes like extrusion, injection molding, and thermoforming. This section briefly discusses potential edible film and coating development technology for litchi fruits with their advantages and limitations.6.1 Edible coating development technology
6.2 Edible film developing technology
7. Safety and regulatory aspects of intelligent and edible films and coatings for litchi fruits
Edible films and coatings for litchi fruit are developed with different components like polysaccharides, lipids, fats independently or in combination with a plasticizer, surfactant and different bioactive components. Since edible films and coatings are applied directly onto the fruit surface (edible portion) of the produce, developing edible films and coatings for litchi involves meeting rigorous safety and regulatory standards to ensure they are safe for consumption and do not negatively impact the fruit's quality. The safety aspect mainly includes toxicological safety, microbial safety and chemical safety.1967.1 Toxicological safety
Components of edible films and coatings must be examined and tested for toxicity. Ingredients should have a “Generally Recognized as Safe” (GRAS) status from the U.S. Food and Drug Administration (FDA) or an equivalent designation from other regulatory authorities. This involves thorough testing for acute and chronic toxicity, potential carcinogenic effects, and allergenicity.7.2 Microbial and chemical safety
The edible films and coatings must not promote the growth of pathogenic microorganisms and should be free from toxic chemical residue. Therefore, compliance with microbial limits set by food safety authorities and residue limits for pesticides, heavy metals, and other contaminants as per Codex Alimentarius or regional regulations is essential. In this context, use of natural and food-grade materials, natural antioxidants and antimicrobial agents could be effective.197One of the major components of the regulatory aspect is labelling and consumer information. Different types of coating materials applied in the development of edible coatings have the potential to induce allergic responses in certain consumers. The utilization of corn zein, soybean protein, fish components, and collagen in the formulation of edible coatings is associated with the occurrence of allergic reactions.198 Similarly, natural bioactive compounds are safe to use for active properties and are approved by FDA and EU but may act as allergens in higher doses.199
Therefore, labels should include information about the coating materials, potential allergens, and any additives used. In this regard the manufacturer must follow labelling regulations such as those outlined in the FDA's Food Labeling200 or EU Regulation No 1169/2011.201 Another critical parameter is quality control and assurance, which requires implementing systems such as good manufacturing practices (GMP) and Hazard Analysis and Critical Control Points (HACCP). Regular monitoring and testing are required following the GMP guidelines and HACCP principles from controlling bodies like FDA, EFSA, and Codex Alimentarius.
8. Conclusions and future perspectives
Litchi is a highly perishable food commodity which becomes more susceptible to contamination after harvesting. Quantitatively, up to ≈45% postharvest loss is reported. The major quality deterioration includes rapid pericarp browning, fungal attack and textural loss. The major postharvest treatment used conventionally is sulfur fumigation mainly targeting the browning problem. However, the associated health related concerns limit its applicability. Therefore, in this context edible films and coatings as novel technology and its recent applications have been discussed along with the postharvest problem of litchi, conventional treatment for its preservation, properties of edible films and coatings and their manufacturing technology. At the end of the review, safety and regulatory aspects of films and coatings are discussed. Edible films and coatings having good antioxidant, antimicrobial and barrier properties are particularly appropriate for litchi fruit controlling the browning and microbial growth. Various developed formulations have enhanced the shelf life of litchi fruit significantly under room temperature storage. Therefore, it could be a sustainable method for litchi preservation specifically targeting the farmers of this fruit. The overall discussion will be beneficial to researchers for the formulation, optimization and application of novel films and coatings for litchi fruit.A wide volume of research has been performed on the development of intelligent and edible film and coating formulation and shelf life study of litchi fruit. But, potentially none of them have been commercialized yet. Therefore, more targeted research is required in this area for further optimization and application.
(I) Cost–benefit analysis study of the developed films and coatings is very limited; thus, more research is required in this area. In particular, machinery, material cost, manpower, infrastructure cost, etc., should be considered.
(II) Integration of advance processing technology should be done during the formulation of edible films and coatings. In this context, nanotechnology in edible films and coatings is a novel and effective strategy to enhance the active properties but very few studies are done for litchi fruit. Thus, investigation in this direction needs to be done more.
(III) Scalability studies under real environment conditions for commercial viability are required to be explored for food processors to meet the market standard and consumer acceptability.
(IV) The toxicology, biocompatibility, digestibility and possible allergenic reactions need to be investigated in detail for meeting food regulatory standards delivering safer and high-quality products to consumers.
In conclusion, optimized intelligent & edible films and coatings could be a promising technology for preservation of postharvest litchi fruits which is a safer, low cost, durable, and environment-friendly alternative.
Author contributions
Sonu Kumar: writing – review & editing, writing – original draft, visualization, investigation, formal analysis, conceptualization. Shikha Sharma: writing – review & editing, writing – original draft, visualization, investigation, formal analysis, conceptualization. Vimal Katiyar: validation, supervision, conceptualization, project administration.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Data availability
All data and information discussed in this review are drawn from the published literature and are fully presented within the manuscript with suitable references. No additional datasets were generated in the preparation of this work.Acknowledgements
The authors thank the Department of Chemical Engineering, Centre for Sustainable Polymer (CoE-SUSPOL) and Lakshminath Bezbaroa Central Library, Indian Institute of Technology (IIT) Guwahati, India for providing support and facilities for literature access and completion of this review article. Vikrant Bodana and Mandavi Goswami (Research Scholar) of CoE-SUSPOL are also highly acknowledged for their discussion whenever required during the completion of the work.References
- J. Liu, Y. Bao, S. Liu, L. Zhu, X. Xu, G. Jiang and Z. Zhang, Physiological and transcriptomic analyses reveal mechanisms of exogenous strigolactones to regulate cold tolerance in litchi fruit, Postharvest Biol. Technol., 2024, 210(09255214), 112764, DOI:10.1016/j.postharvbio.2024.112764.
- N. Kumar, Neeraj, Pratibha and M. Singla, Enhancement of Storage Life and Quality Maintenance of Litchi (Litchi Chinensis Sonn.) Fruit Using Chitosan:pullulan Blend Antimicrobial Edible Coating, Int. J. Fruit Sci., 2020, 20, S1662–S1680, DOI:10.1080/15538362.2020.1828224.
- D. Sivakumar, L. A. Terry and L. Korsten, An overview on litchi fruit quality and alternative postharvest treatments to replace sulfur dioxide fumigation, Food Rev. Int., 2010, 26, 162–188, DOI:10.1080/87559121003590516.
- V. Deshi, F. Homa, V. Y. Tokala, H. Mir, M. A. Aftab and M. W. Siddiqui, Regulation of pericarp browning in cold-stored litchi fruit using methyl jasmonate, J. King Saud Univ. Sci., 2021, 33(5), 101445, DOI:10.1016/j.jksus.2021.101445.
- P. Yao, Y. Gao, J. Simal-Gandara, M. A. Farag, W. Chen, D. Yao, D. Delmas, Z. Chen, K. Liu, H. Hu, J. Xiao, X. Rong, S. Wang, Y. Hu and Y. Wang, Litchi (Litchi chinensis Sonn.): a comprehensive review of phytochemistry, medicinal properties, and product development, Food Funct., 2021, 12, 9527–9548, 10.1039/d1fo01148k.
- Mordor Intelligence, Lychee Market Size & Share Analysis - Growth Trends & Forecasts (2025-2030), 2025, accessed August 14, 2025, https://www.mordorintelligence.com/industry-reports/lychee-market Search PubMed.
- Z. Zhang, X. Pang, D. Xuewu, Z. Ji and Y. Jiang, Role of peroxidase in anthocyanin degradation in litchi fruit pericarp, Food Chem., 2005, 90, 47–52, DOI:10.1016/j.foodchem.2004.03.023.
- Y. Jiang, X. Duan, D. Joyce, Z. Zhang and J. Li, Advances in understanding of enzymatic browning in harvested litchi fruit, Food Chem., 2004, 88, 443–446, DOI:10.1016/j.foodchem.2004.02.004.
- M. Xing, J. Zhao, J. Zhang, Y. Wu, R. A. A. Khan, X. Li, R. Wang, T. Li and T. Liu, 6-Pentyl-2H-pyran-2-one from Trichoderma erinaceum is Fungicidal against Litchi Downy Blight Pathogen Peronophythora litchii and Preservation of Litchi, J. Agric. Food Chem., 2023, 71, 19488–19500, DOI:10.1021/acs.jafc.3c03872.
- X. Yu, J. Chen, J. Zhong, W. Deng, Z. Zhang, Y. Wu and Q. Zheng, Antifungal efficacy of LEDs against major postharvest pathogens of litchi fruit in vitro and in vivo, Food Control, 2023, 154(09567135), 110019, DOI:10.1016/j.foodcont.2023.110019.
- Y. Jiang, L. Yao, A. Lichter and J. L., Postharvest biology and technology of litchi fruit, J. Food Agric. Environ., 2003, 1, 76–81 Search PubMed , accessed August 14, 2025, https://dtisartec.senasica.gob.mx:8080/biblioteca/libros/articulos/Jiang,Y._etal_2003_2.pdf.
- P. Laborda, D. D. Herrera-Balandrano, F. Q. Liu and S. Y. Wang, Melatonin interplay with reactive oxygen, nitrogen, and sulfur species during ripening and postharvest storage of agricultural produce, Oxygen, Nitrogen and Sulfur Species in Post-harvest Physiology of Horticultural Crops, 2024, pp. 273–301, DOI:10.1016/B978-0-323-91798-8.00002-3.
- R. Kumar, A. Dongariyal, R. Rai, M. K. Arya and N. Mishra, Effect of edible coating and packaging on postharvest life and quality of litchi (Litchi chinensis Sonn.) fruits during storage, J. Pharmacogn. Phytochem., 2020, 9, 3018 Search PubMed , accessed August 14, 2025., https://www.phytojournal.com.
- H. H. Fang, W. L. Lee, K. T. Chiu, H. Y. Ma, S. H. Yang, C. Y. Hung, H. L. Chen, C. W. Tung and Y. C. Tsai, Irradiation with green light at night has great effects on the management of Conopomorpha sinensis and maintains favorable litchi fruit quality, Sci. Hortic., 2023, 312, 111830, DOI:10.1016/j.scienta.2023.111830.
- R. Passafiume, P. Roppolo, I. Tinebra, A. Pirrone, R. Gaglio, E. Palazzolo and V. Farina, Reduction of Pericarp Browning and Microbial Spoilage on Litchi Fruits in Modified Atmosphere Packaging, Horticulturae, 2023, 9(6), 651, DOI:10.3390/horticulturae9060651.
- V. Kumar and A. K. D. Anal, Postharvest Diseases of Litchi and Their Management, Postharvest Handling and Diseases of Horticultural Produce Book, 2021, ch. 19, DOI:10.1201/9781003045502.
- M. A. Khalid, B. Niaz, F. Saeed, M. Afzaal, F. Islam, M. Hussain, Mahwish, H. M. S. Khalid, A. Siddeeg and A. Al-Farga, Edible coatings for enhancing safety and quality attributes of fresh produce: a comprehensive review, Int. J. Food Prop., 2022, 25, 1(10942912), 1817–1847, DOI:10.1080/10942912.2022.2107005.
- B. Maringgal, N. Hashim, I. S. M. A. Tawakkal and M. T. M. Mohamed, Recent advance in edible coating and its effect on fresh/fresh-cut fruits quality, Trends Food Sci. Technol., 2020, 96, 253–267, DOI:10.1016/j.tifs.2019.12.024.
- B. Yousuf, O. S. Qadri and A. K. Srivastava, Recent developments in shelf-life extension of fresh-cut fruits and vegetables by application of different edible coatings: a review, LWT--Food Sci. Technol., 2018, 89, 198–209, DOI:10.1016/j.lwt.2017.10.051.
- K. Priya, N. Thirunavookarasu and D. V. Chidanand, Recent advances in edible coating of food products and its legislations: a review, J. Agric. Food Res., 2023, 12, 100623, DOI:10.1016/j.jafr.2023.100623.
- Z. A. Belay, T. G. Mashele, W. J. Botes and O. J. Caleb, Effects of zein-nisin edible coating on physicochemical and microbial load of ‘Granny Smith’ apple after long term storage, CyTA - J. Food, 2023, 21, 334–343, DOI:10.1080/19476337.2023.2199833.
- A. De Bruno, A. Gattuso, D. Ritorto, A. Piscopo and M. Poiana, Effect of Edible Coating Enriched with Natural Antioxidant Extract and Bergamot Essential Oil on the Shelf Life of Strawberries, Foods, 2023, 12(3), 488, DOI:10.3390/foods12030488.
- E. A. Luna-Zapién, J. A. Zegbe and J. A. Meza-Velázquez, Applying an alginate and mucilage-based edible coating to avocado halves favors some physical attributes and consumer acceptance, J. Prof. Assoc. Cactus Dev., 2023, 25, 244–256, DOI:10.56890/jpacd.v25i.530.
- S. Kalpana, S. R. Priyadarshini, M. M. Leena, J. A. Moses and C. Anandharamakrishnan, Intelligent packaging: trends and applications in food systems, Trends Food Sci. Technol., 2019, 93, 145–157, DOI:10.1016/J.TIFS.2019.09.008.
- C. Wu, H. Jiang, J. Zhao, M. Humayun, S. Wu, C. Wang, Z. Zhi and J. Pang, A novel strategy to formulate edible active-intelligent packaging films for achieving dynamic visualization of product freshness, Food Hydrocoll., 2022, 133, 107998, DOI:10.1016/J.FOODHYD.2022.107998.
- K. H. Le, M. D. B. Nguyen, L. D. Tran, H. P. N. Thi, C. Van Tran, K. Van Tran, H. P. N. Thi, N. D. Thi, Y. S. Yoon, D. D. Nguyen and D. D. La, A novel antimicrobial ZnO nanoparticles-added polysaccharide edible coating for the preservation of postharvest avocado under ambient conditions, Prog. Org. Coating, 2021, 158, 106339, DOI:10.1016/j.porgcoat.2021.106339.
- M. C. Strano, C. Restuccia, R. De Leo, S. Mangiameli, E. Bedin, M. Allegra, A. Quartieri, G. Cirvilleri and A. Pulvirenti, Efficacy of an antifungal edible coating for the quality maintenance of Tarocco orange fruit during cold storage, Crop Prot., 2021, 148, 105719, DOI:10.1016/j.cropro.2021.105719.
- J. Yan, Z. Luo, Z. Ban, H. Lu, D. Li, D. Yang, M. S. Aghdam and L. Li, The effect of the layer-by-layer (LBL) edible coating on strawberry quality and metabolites during storage, Postharvest Biol. Technol., 2019, 147, 29–38, DOI:10.1016/j.postharvbio.2018.09.002.
- S. P. Bangar, M. Kumar, W. S. Whiteside, M. Tomar and J. F. Kennedy, Litchi (Litchi chinensis) seed starch: structure, properties, and applications - a review, Carbohydr. Polym. Technol. Appl., 2021, 2, 100080, DOI:10.1016/j.carpta.2021.100080.
- C. Menzel, The physiology of growth and cropping in lychee, Acta Hortic., 2001, 175–184, DOI:10.17660/ActaHortic.2001.558.24.
- S. Emanuele, M. Lauricella, G. Calvaruso, A. D'Anneo and M. Giuliano, Litchi chinensis as a Functional Food and a Source of Antitumor Compounds: An Overview and a Description of Biochemical Pathways, Nutrients, 2017, 9, 992, DOI:10.3390/NU9090992.
- K. A. Marak, H. Mir, P. Singh, M. W. Siddiqui, T. Ranjan, D. R. Singh, M. H. Siddiqui and M. Irfan, Exogenous melatonin delays oxidative browning and improves postharvest quality of litchi fruits, Sci. Hortic., 2023, 322, 112408, DOI:10.1016/J.SCIENTA.2023.112408.
- S. Ali, A. S. Khan, A. U. Malik, M. A. Anjum, A. Nawaz and H. M. S. Shah, Modified atmosphere packaging delays enzymatic browning and maintains quality of harvested litchi fruit during low temperature storage, Sci. Hortic., 2019, 254, 14–20, DOI:10.1016/J.SCIENTA.2019.04.065.
- F. Garcia and G. Davidov-Pardo, Recent advances in the use of edible coatings for preservation of avocados: a review, J. Food Sci., 2021, 86, 6–15, DOI:10.1111/1750-3841.15540.
- S. Emanuele, M. Lauricella, G. Calvaruso, A. D’Anneo and M. Giuliano, Litchi chinensis as a functional food and a source of antitumor compounds: an overview and a description of biochemical pathways, Nutrients, 2017, 9(9), 992, DOI:10.3390/nu9090992.
- Shilpa, B. V. C. Mahajan, N. P. Singh, K. S. Bhullar and S. Kaur, Forced air cooling delays pericarp browning and maintains post-harvest quality of litchi fruit during cold storage, Acta Physiol. Plant., 2022, 44(6), 66, DOI:10.1007/S11738-022-03405-7.
- H. Pathak, M. R. Chandrakar, V. K. Choudhary, S. Singh, S. K. Thakur and G. Langangmeilu, Growth Rate of Area Production and Productivity of Litchi Fruit in Jashpur District of Chhattisgarh, India, Int. J. Environ. Clim. Chang., 2023, 13, 1173–1182, DOI:10.9734/IJECC/2023/v13i92343.
- A. K. Das, V. Rajkumar, P. K. Nanda, P. Chauhan, S. R. Pradhan and S. Biswas, Antioxidant Efficacy of Litchi (Litchi chinensis Sonn.) Pericarp Extract in Sheep Meat Nuggets, Antioxidants, 2016, 5(2), 16, DOI:10.3390/antiox5020016.
- Lychee Production in India, https://www.fao.org/4/ac684e/ac684e08.htm, accessed August 8, 2025 Search PubMed.
- S. K. Singh, E. S. Marboh and V. Nath, Litchi, Fruit and Nut Crops, 2023, pp. 1–28, DOI:10.1007/978-981-99-1586-6_12-1.
- L. Li, A. Lichter, D. Chalupowicz, D. Gamrasni, T. Goldberg, O. Nerya, R. Ben-Arie and R. Porat, Effects of the ethylene-action inhibitor 1-methylcyclopropene on postharvest quality of non-climacteric fruit crops, Postharvest Biol. Technol., 2016, 111, 322–329, DOI:10.1016/j.postharvbio.2015.09.031.
- V. Deshi, M. W. Siddiqui, F. Homa and J. P. Singh, Postharvest hydrogen sulfide infiltration modulates antioxidative metabolism and increases shelf life of litchi, Acta Physiol. Plant., 2020, 42(5), 67, DOI:10.1007/s11738-020-03056-6.
- B. Bhushan, A. Pal, R. Narwal, V. S. Meena, P. C. Sharma and J. Singh, Combinatorial approaches for controlling pericarp browning in Litchi (Litchi chinensis) fruit, J. Food Sci. Technol., 2015, 52, 5418–5426, DOI:10.1007/s13197-015-1712-8.
- V. Kumar and A. K. D. Anal, Postharvest Diseases of Litchi and Their Management, Postharvest Handling and Diseases of Horticultural Produce, CRC Press, 2021, ch 19, pp. 231–238, DOI:10.1201/9781003045502-19.
- S. J. R. Underhill and D. H. Simons, Lychee (Litchi chinensis Sonn.) pericarp desiccation and the importance of postharvest micro-cracking, Sci. Hortic., 1993, 54, 287–294, DOI:10.1016/0304-4238(93)90107-2.
- M. Kumar, P. Teotia, R. Prasad, A. Varma and V. Kumar, The lychee fruit: post harvest handling techniques, in The Lychee Biotechnology, Springer, Singapore, 2017, pp. 193–211, DOI:10.1007/978-981-10-3644-6_6.
- S. Huang, X. Lv, L. Zheng and D. Guo, Exiguobacterium acetylicum Strain SI17: A Potential Biocontrol Agent against Peronophythora litchii Causing Post-Harvest Litchi Downy Blight, Horticulturae, 2024, 10, 888, DOI:10.3390/HORTICULTURAE10080888/S1.
- R. Tang, Y. Zhou, Z. Chen, L. Wang, Y. Lai, S. K. Chang, Y. Wang, H. Qu, Y. Jiang and H. Huang, Regulation of browning and senescence of litchi fruit mediated by phenolics and energy status: a postharvest comparison on three different cultivars, Postharvest Biol. Technol., 2020, 168, 111280, DOI:10.1016/J.POSTHARVBIO.2020.111280.
- D. Ye, X. Wang, J. Guo, J. Ren, B. Li, Q. Li, Y. Chen, X. Wang, M. Ntakatsane and P. Chen, The underpinning mechanisms and applications of glycine betaine on chilling injury in fruit and vegetables, Microchem. J., 2025, 212, 113290, DOI:10.1016/J.MICROC.2025.113290.
- G. Qinqin, T. Shiping, Partial characterization of soluble peroxidase in pericarp of litchi fruitProg. Biochem. Biophys., 2002, 29, 6891–896, https://www.pibb.ac.cn/pibben/article/abstract/20020615?st=article_issue Search PubMed.
- X. y. Bai, Z. m. Yang, W. j. Shen, Y. z. Shao, J. k. Zeng and W. Li, Polyphenol treatment delays the browning of litchi pericarps and promotes the total antioxidant capacity of litchi fruit, Sci. Hortic., 2022, 291, 110563, DOI:10.1016/j.scienta.2021.110563.
- S. Nanglia, B. V. C. Mahajan, N. Singh, S. Kapoor, K. S. Bhullar, S. Kaur and V. Kumar, Combined effect of acids and shellac coating on pericarp browning, enzymatic activities, and biochemical attributes of litchi fruit during storage, J. Food Process. Preserv., 2022, 46(5), e16535, DOI:10.1111/JFPP.16535.
- D. M. Holcroft and E. J. Mitcham, Postharvest physiology and handling of litchi (Litchi chinensis Sonn.), Postharvest Biol. Technol., 1996, 9(3), 265–281 CrossRef.
- M. Reichel, J. Wellhöfer, R. Triani, P. Sruamsiri, R. Carle and S. Neidhart, Postharvest control of litchi (Litchi chinensis Sonn.) pericarp browning by cold storage at high relative humidity after enzyme-inhibiting treatments, Postharvest Biol. Technol., 2017, 125, 77–90, DOI:10.1016/J.POSTHARVBIO.2016.10.002.
- S. Underhillab and C. Critchleyb, Anthocyanin decolorisation and its role in lychee pericarp browning, Aust. J. Exp. Agric., 1994, 34(1), 115–122 CrossRef.
- B. Liu, W. w. Xue, Z. l. Guo, S. y. Liu, Q. n Zhu, X. q. Pang, Z. q. Zhang and F. Fang, Water loss and pericarp browning of litchi (Litchi chinensis) and longan (Dimocarpus longan) fruit maintain seed vigor, Sci. Hortic., 2021, 290, 110519, DOI:10.1016/j.scienta.2021.110519.
- X. Yu, J. Chen, J. Zhong, W. Deng, Z. Zhang, Y. Wu and Q. Zheng, Antifungal efficacy of LEDs against major postharvest pathogens of litchi fruit in vitro and in vivo, Food Control, 2023, 154, 110019, DOI:10.1016/J.FOODCONT.2023.110019.
- J. M. Anderson, E. A. B. Aitken, E. K. Dann and L. M. Coates, Morphological and molecular diversity of Colletotrichum spp. causing pepper spot and anthracnose of lychee (Litchi chinensis) in Australia, Plant Pathol., 2013, 62(2), 279–288, DOI:10.1111/j.1365-3059.2012.02632.x.
- M. M. Bolaños, D. T. Ortiz, A. M. Aguilera, G. V. Ponce, D. N. Ángel, E. G. Pérez, V. S. López, Anthracnose (Colletotrichum gloeosporioides Penz.) of litchi fruit (Litchi chinensis Soon.) in Oaxaca, México, Revista Mexicana de Fitopatología, 2015, 332, https://www.scielo.org.mx/scielo.php?pid=S0185-33092015000200140&script=sci_arttext&tlng=en Search PubMed.
- J. Zhao, Z. Yu, Y. Wang, Q. Li, L. Tang, T. Guo, S. Huang, J. Mo and T. Hsiang, Litchi Anthracnose Caused by Colletotrichum karstii in Guangxi, China, Plant Dis., 2021, 105(10), 3295, DOI:10.1094/PDIS-01-21-0196-PDN.
- V. Kumar, S. K. Purbey and A. K. D. Anal, Losses in litchi at various stages of supply chain and changes in fruit quality parameters, Crop Prot., 2016, 79, 97–104, DOI:10.1016/J.CROPRO.2015.10.014.
- M. V. Fernandez, A. Z. Colombo, C. Lires, C. Cova, M. V. Agüero and R. J. Jagus, Improving the Overall Quality and Safety of Fruit and Vegetable Smoothies During Refrigerated Storage Through Gamma Irradiation and Natural Antimicrobials, J. Food Saf., 2025, 45, e70023, DOI:10.1111/JFS.70023.
- F. Shahzad, M. Doron, S. A. Sargent and J. K. Brecht, Development and feasibility of an approach utilizing modified atmosphere packaging (MAP) and ethylene scrubbing to allow extended international transport and marketing of tree-ripe mangos, Postharvest Biol. Technol., 2025, 225, 113488, DOI:10.1016/J.POSTHARVBIO.2025.113488.
- T. Sharma, G. Kaur, A. Singh, V. Kumar and B. N. Dar, Enhancing litchi shelf life with gluten-based nanocomposite films: a comparative study of montmorillonite and starch nanocrystals reinforced with chitosan, Sci. Hortic., 2024, 332, 113239, DOI:10.1016/j.scienta.2024.113239.
- Y. Chen, J. Li, D. Zhu, D. Han, C. Zhou, Y. Li, Y. Liu, Y. Ma, T. Luo, B. Wen and Z. Wu, Water migration and browning mechanisms in postharvest litchi pericarp: insights from LF NMR, Postharvest Biol. Technol., 2025, 230, 113768, DOI:10.1016/J.POSTHARVBIO.2025.113768.
- H. Guan, S. Tao, Y. Pan, W. Zhang, Z. Han, H. Yang and Y. Tan, Water transport, vascular bundle integrity, and enzymatic activity in rambutan pericarp and spine browning, Postharvest Biol. Technol., 2025, 228, 113685, DOI:10.1016/J.POSTHARVBIO.2025.113685.
- D. Shi, B. Zhao, P. Zhang, P. Li, X. Wei and K. Song, Edible composite films: enhancing the postharvest preservation of blueberry, Hortic Environ Biotechnol, 2024, 65(3), 355–373, DOI:10.1007/s13580-023-00581-4.
- M. Sultan, O. M. Hafez, M. A. Saleh and A. M. Youssef, Smart edible coating films based on chitosan and beeswax-pollen grains for the postharvest preservation of Le Conte pear, RSC Adv., 2021, 11, 9572–9585, 10.1039/d0ra10671b.
- C. Yang, F. W. Lee, Y. J. Cheng, Y. Y. Chu, C. N. Chen and Y. C. Kuan, Chitosan coating formulated with citric acid and pomelo extract retards pericarp browning and fungal decay to extend shelf life of cold-stored lychee, Sci. Hortic., 2023, 310, 111735, DOI:10.1016/J.SCIENTA.2022.111735.
- S. O. Olunusi, N. H. Ramli, A. Fatmawati, A. F. Ismail and C. C. Okwuwa, Revolutionizing tropical fruits preservation: emerging edible coating technologies, Int. J. Biol. Macromol., 2024, 130682, DOI:10.1016/j.ijbiomac.2024.130682.
- S. Bhatia, Y. A. Shah, A. Al-Harrasi, M. Jawad, E. Koca and L. Y. Aydemir, Enhancing Tensile Strength, Thermal Stability, and Antioxidant Characteristics of Transparent Kappa Carrageenan Films Using Grapefruit Essential Oil for Food Packaging Applications, ACS Omega, 2024, 9, 9003–9012, DOI:10.1021/acsomega.3c07366.
- S. Mei, B. Fu, X. Su, H. Chen, H. Lin, Z. Zheng, C. Dai and D. P. Yang, Developing silk sericin-based and carbon dots reinforced bio-nanocomposite films and potential application to litchi fruit, LWT--Food Sci. Technol., 2022, 164, 113630, DOI:10.1016/j.lwt.2022.113630.
- H. Huang, H. Liu, L. Wang and X. Xiang, Cuticular wax metabolism responses to atmospheric water stress on the exocarp surface of litchi fruit after harvest, Food Chem., 2023, 414, 135704, DOI:10.1016/j.foodchem.2023.135704.
- D. Das, P. S. Panesar, C. S. Saini and J. F. Kennedy, Improvement in properties of edible film through non-thermal treatments and nanocomposite materials: a review, Food Packag. Shelf Life, 2022, 32, 100843, DOI:10.1016/J.FPSL.2022.100843.
- Y. A. Shah, S. Bhatia, A. Al-Harrasi, M. Tarahi, H. Almasi, R. Chawla and A. M. M. Ali, Insights into recent innovations in barrier resistance of edible films for food packaging applications, Int. J. Biol. Macromol., 2024, 132354, DOI:10.1016/j.ijbiomac.2024.132354.
- J. G. de Oliveira Filho, C. C. de, O. N. Bezerra, B. R. Albiero, F. C. A. Oldoni, M. Miranda, M. B. Egea, H. M. C. de Azeredo and M. D. Ferreira, New approach in the development of edible films: the use of carnauba wax micro- or nanoemulsions in arrowroot starch-based films, Food Packag. Shelf Life, 2020, 26, 100589, DOI:10.1016/J.FPSL.2020.100589.
- A. Ahuja and V. K. Rastogi, Physicochemical and thermal characterization of the edible shellac films incorporated with oleic acid to enhance flexibility, water barrier and retard aging, Int. J. Biol. Macromol., 2024, 269, 132136, DOI:10.1016/j.ijbiomac.2024.132136.
- M. Kongboonkird, P. Chuesiang, V. Ryu and U. Siripatrawan, Enhancing functional properties of active chitosan film incorporated with cinnamon oil nanoemulsion using argon dielectric barrier discharge cold plasma treatment, Food Hydrocoll., 2024, 156, 110171, DOI:10.1016/J.FOODHYD.2024.110171.
- N. Benbettaïeb, F. Debeaufort and T. Karbowiak, Bioactive edible films for food applications: mechanisms of antimicrobial and antioxidant activity, Crit. Rev. Food Sci. Nutr., 2019, 59, 3431–3455, DOI:10.1080/10408398.2018.1494132.
- A. M. Ribeiro, B. N. Estevinho and F. Rocha, Preparation and Incorporation of Functional Ingredients in Edible Films and Coatings, Food Bioprocess Technol., 2021, 14, 209–231, DOI:10.1007/S11947-020-02528-4/TABLES/2.
- E. Abdollahzadeh, A. Nematollahi and H. Hosseini, Composition of antimicrobial edible films and methods for assessing their antimicrobial activity: a review, Trends Food Sci. Technol., 2021, 110, 291–303, DOI:10.1016/j.tifs.2021.01.084.
- E. Díaz-Montes and R. Castro-Muñoz, Edible films and coatings as food-quality preservers: an overview, Foods, 2021, 10(2), 249, DOI:10.3390/foods10020249.
- S. Bhatia, A. Al-Harrasi, S. Ullah, Y. A. Shah, M. S. Al-Azri, M. Jawad, M. K. Anwer, M. F. Aldawsari, M. S. Al-Jassasi, E. Koca and L. Y. Aydemir, Fabrication, characterization and antioxidant activities of pectin and gelatin based edible film loaded with Citrus reticulata L. essential oil, J. Food Process Eng., 2024, 47(4), e14583, DOI:10.1111/JFPE.14583.
- A. Sajimon, A. S. Edakkadan, A. J. Subhash and M. Ramya, Incorporating oregano (Origanum vulgare L.) Essential oil onto whey protein concentrate based edible film towards sustainable active packaging, J. Food Sci. Technol., 2023, 60, 2408–2422, DOI:10.1007/s13197-023-05763-7.
- P. Ezati, A. Khan, R. Priyadarshi, T. Bhattacharya, S. K. Tammina and J. W. Rhim, Biopolymer-based UV protection functional films for food packaging, Food Hydrocoll., 2023, 142, 108771, DOI:10.1016/j.foodhyd.2023.108771.
- A. Botalo, T. Inprasit, S. Ummartyotin, K. Chainok, S. Vatthanakul and P. Pisitsak, Smart and UV-Resistant Edible Coating and Films Based on Alginate, Whey Protein, and Curcumin, Polymers, 2024, 16, 447, DOI:10.3390/POLYM16040447.
- D. Zhou, Q. Liu, T. Zhu, T. Li, G. Fan, X. Li and C. Wu, Effects of ultraviolet C on the quality and aroma volatile in peach fruit during postharvest storage, Food Chem., 2024, 139906, DOI:10.1016/j.foodchem.2024.139906.
- M. S. A. Aziz and H. E. Salama, Developing multifunctional edible coatings based on alginate for active food packaging, Int. J. Biol. Macromol., 2021, 190, 837–844, DOI:10.1016/J.IJBIOMAC.2021.09.031.
- Y. Chen, Y. Li, S. Qin, S. Han and H. Qi, Antimicrobial, UV blocking, water-resistant and degradable coatings and packaging films based on wheat gluten and lignocellulose for food preservation, Composites, Part B, 2022, 238, 109868, DOI:10.1016/J.COMPOSITESB.2022.109868.
- J. Luo, G. Xia, L. Liu, A. Ji and Q. Luo, Fabrication of Chitosan/Hydroxyethyl Cellulose/TiO2 Incorporated Mulberry Anthocyanin 3D-Printed Bilayer Films for Quality of Litchis, Foods, 2022, 11, 3286, DOI:10.3390/FOODS11203286.
- S. Mukherjee, A. Sengupta, S. Preetam, T. Das, T. Bhattacharya and N. Thorat, Effects of fatty acid esters on mechanical, thermal, microbial, and moisture barrier properties of carboxymethyl cellulose-based edible films, Carbohydr. Polym. Technol. Appl., 2024, 7, 100505, DOI:10.1016/J.CARPTA.2024.100505.
- O. M. Atta, S. Manan, M. Ul-Islam, A. A. Q. Ahmed, M. W. Ullah and G. Yang, Development and characterization of plant oil-incorporated carboxymethyl cellulose/bacterial cellulose/glycerol-based antimicrobial edible films for food packaging applications, Adv. Compos. Hybrid Mater., 2022, 5, 973–990, DOI:10.1007/S42114-021-00408-9/TABLES/4.
- P. Singh, S. Magalhães, L. Alves, F. Antunes, M. Miguel, B. Lindman and B. Medronho, Cellulose-based edible films for probiotic entrapment, Food Hydrocoll., 2019, 88, 68–74, DOI:10.1016/J.FOODHYD.2018.08.057.
- K. D. Tafa, N. Satheesh and W. Abera, Mechanical properties of tef starch based edible films: development and process optimization, Heliyon, 2023, 9, 13160, DOI:10.1016/j.heliyon.2023.e13160.
- Y. Wang, J. Ju, Y. Diao, F. Zhao and Q. Yang, The application of starch-based edible film in food preservation: a comprehensive review, Crit. Rev. Food Sci. Nutr., 2024, 65(14), 2731–2764, DOI:10.1080/10408398.2024.2349735.
- F. M. Pelissari, D. C. Ferreira, L. B. Louzada, F. Dos Santos, A. C. Corrêa, F. K. V. Moreira and L. H. Mattoso, Starch-Based Edible Films and Coatings: An Eco-friendly Alternative for Food Packaging, Starches for Food Application: Chemical, Technological and Health Properties, 2019, pp. 359–420, DOI:10.1016/B978-0-12-809440-2.00010-1.
- A. I. Bourbon, A. C. Pinheiro, M. A. Cerqueira, C. M. R. Rocha, M. C. Avides, M. A. C. Quintas and A. A. Vicente, Physico-chemical characterization of chitosan-based edible films incorporating bioactive compounds of different molecular weight, J. Food Eng., 2011, 106, 111–118, DOI:10.1016/J.JFOODENG.2011.03.024.
- A. Pavinatto, A. V. de Almeida Mattos, A. C. G. Malpass, M. H. Okura, D. T. Balogh and R. C. Sanfelice, Coating with chitosan-based edible films for mechanical/biological protection of strawberries, Int. J. Biol. Macromol., 2020, 151, 1004–1011, DOI:10.1016/J.IJBIOMAC.2019.11.076.
- N. S. Said, I. F. Olawuyi, H. S. Cho and W. Y. Lee, Novel edible films fabricated with HG-type pectin extracted from different types of hybrid citrus peels: effects of pectin composition on film properties, Int. J. Biol. Macromol., 2023, 253, 127238, DOI:10.1016/J.IJBIOMAC.2023.127238.
- M. Z. Mulla, J. Ahmed, A. Vahora and S. Pathania, Effect of pectin incorporation on characteristics of chitosan based edible films, J. Food Meas. Char., 2023, 17, 5569–5581, DOI:10.1007/S11694-023-02047-8/METRICS.
- W. A. Asfaw, K. D. Tafa and N. Satheesh, Optimization of citron peel pectin and glycerol concentration in the production of edible film using response surface methodology, Heliyon, 2023, 9(3), e13724, DOI:10.1016/j.heliyon.2023.e13724.
- M. S. Nair, M. Tomar, S. Punia, W. Kukula-Koch and M. Kumar, Enhancing the functionality of chitosan- and alginate-based active edible coatings/films for the preservation of fruits and vegetables: a review, Int. J. Biol. Macromol., 2020, 164, 304–320, DOI:10.1016/J.IJBIOMAC.2020.07.083.
- E. C. Martínez-Molina, Y. Freile-Pelegrín, S. L. Ovando-Chacón, F. A. Gutiérrez-Miceli, M. Á. Ruiz-Cabrera, A. Grajales-Lagunes, M. C. Luján-Hidalgo and M. Abud-Archila, Development and characterization of alginate-based edible film from Sargassum fluitans incorporated with silver nanoparticles obtained by green synthesis, J. Food Meas. Char., 2022, 16, 126–136, DOI:10.1007/S11694-021-01156-6/METRICS.
- T. Ghosh, R. Priyadarshi, C. Krebs de Souza, B. L. Angioletti and J. W. Rhim, Advances in pullulan utilization for sustainable applications in food packaging and preservation: a mini-review, Trends Food Sci. Technol., 2022, 125, 43–53, DOI:10.1016/J.TIFS.2022.05.001.
- L. Gan, G. Jiang, Y. Yang, B. Zheng, S. Zhang, X. Li, Y. Tian and B. Peng, Development and characterization of levan/pullulan/chitosan edible films enriched with ε-polylysine for active food packaging, Food Chem., 2022, 388, 132989, DOI:10.1016/J.FOODCHEM.2022.132989.
- M. Gniewosz, K. Pobiega, K. Kraśniewska, A. Synowiec, M. Chaberek and S. Galus, Characterization and Antifungal Activity of Pullulan Edible Films Enriched with Propolis Extract for Active Packaging, Foods, 2022, 11, 2319, DOI:10.3390/FOODS11152319.
- T. R. Martiny, B. S. Pacheco, C. M. P. Pereira, A. Mansilla, M. S. Astorga-España, G. L. Dotto, C. C. Moraes and G. S. Rosa, A novel biodegradable film based on κ-carrageenan activated with olive leaves extract, Food Sci. Nutr., 2020, 8, 3147–3156, DOI:10.1002/FSN3.1554.
- C. Cheng, S. Chen, J. Su, M. Zhu, M. Zhou, T. Chen and Y. Han, Recent advances in carrageenan-based films for food packaging applications, Front. Nutr., 2022, 9, 1004588, DOI:10.3389/FNUT.2022.1004588/BIBTEX.
- S. Bhatia, A. Al-Harrasi, Y. A. Shah, A. N. S. Alrasbi, M. Jawad, E. Koca, L. Y. Aydemir, J. A. Alamoudi, Y. Almoshari and S. Mohan, Structural, mechanical, barrier and antioxidant properties of pectin and xanthan gum edible films loaded with grapefruit essential oil, Heliyon, 2024, 10(3), e25501, DOI:10.1016/j.heliyon.2024.e25501.
- R. Tanwar, L. Kumar, P. Kumar and K. K. Gaikwad, Characterization of Guar Gum Based Edible Film Containing Microwave-Assisted Mint Leaves Extract and Citric Acid for Ber (Ziziphus Mauritiana) Fruit Packaging, SSRN Electron. J., 2021, 1–23, DOI:10.2139/SSRN.3978979.
- R. K. Dhaka, N. Kumar, Pratibha and A. Upadhyay, Optimization, Characterization, and Influence of Microfluidization on Almond Gum-based Composite Edible Film, Starch, 2021, 73(5/6), 2000101, DOI:10.1002/STAR.202000101.
- P. Wongphan and N. Harnkarnsujarit, Characterization of starch, agar and maltodextrin blends for controlled dissolution of edible films, Int. J. Biol. Macromol., 2020, 156, 80–93, DOI:10.1016/J.IJBIOMAC.2020.04.056.
- S. Roy, R. Chawla, R. Santhosh, R. Thakur, P. Sarkar and W. Zhang, Agar-based edible films and food packaging application: a comprehensive review, Trends Food Sci. Technol., 2023, 141, 104198, DOI:10.1016/J.TIFS.2023.104198.
- F. S. Mostafavi and D. Zaeim, Agar-based edible films for food packaging applications - a review, Int. J. Biol. Macromol., 2020, 159, 1165–1176, DOI:10.1016/J.IJBIOMAC.2020.05.123.
- E. Díaz-Montes, P. Lukova and G. Pierre, Dextran: Sources, Structures, and Properties, Polysaccharides, 2021, 2, 554–565, DOI:10.3390/POLYSACCHARIDES2030033.
- L. Wu, B. Cui, D. Dong, Z. Wu, J. Li, L. Lu, N. Wang, K. Nishinari and M. Zhao, Effect of mixture microstructure/compatibility on the properties of type-A gelatin-dextran edible films, Carbohydr. Polym., 2024, 329, 121733, DOI:10.1016/J.CARBPOL.2023.121733.
- V. A. Kumar, M. Pravitha, A. Yadav, R. Pandiselvam and P. P. Srivastav, Influence of ultrasonic application on soybean aqueous extract based composite edible film: characterization and their food application, Food Hydrocoll., 2023, 135, 108210, DOI:10.1016/J.FOODHYD.2022.108210.
- S. F. Hosseini, Z. Mousavi and D. J. McClements, Beeswax: a review on the recent progress in the development of superhydrophobic films/coatings and their applications in fruits preservation, Food Chem., 2023, 424, 136404, DOI:10.1016/J.FOODCHEM.2023.136404.
- L. D. Pérez-Vergara, M. T. Cifuentes, A. P. Franco, C. E. Pérez-Cervera and R. D. Andrade-Pizarro, Development and characterization of edible films based on native cassava starch, beeswax, and propolis, NFS J., 2020, 21, 39–49, DOI:10.1016/J.NFS.2020.09.002.
- S. Galus, M. Gaouditz, H. Kowalska and F. Debeaufort, Effects of Candelilla and Carnauba Wax Incorporation on the Functional Properties of Edible Sodium Caseinate Films, Int. J. Mol. Sci., 2020, 21, 9349, DOI:10.3390/IJMS21249349.
- L. S. Devi, S. Kalita, A. Mukherjee and S. Kumar, Carnauba wax-based composite films and coatings: recent advancement in prolonging postharvest shelf-life of fruits and vegetables, Trends Food Sci. Technol., 2022, 129, 296–305, DOI:10.1016/J.TIFS.2022.09.019.
- M. S. Butt, M. Akhtar, A. A. Maan and M. Asghar, Fabrication and characterization of carnauba wax-based films incorporated with sodium alginate/whey protein, J. Food Meas. Char., 2023, 17, 694–705, DOI:10.1007/S11694-022-01636-3/METRICS.
- T. Han, W. Chen, Q. Zhong, W. Chen, Y. Xu, J. Wu and H. Chen, Development and Characterization of an Edible Zein/Shellac Composite Film Loaded with Curcumin, Foods, 2023, 12, 1577, DOI:10.3390/FOODS12081577/S1.
- Y. Yuan, N. He, Q. Xue, Q. Guo, L. Dong, M. H. Haruna, X. Zhang, B. Li and L. Li, Shellac: a promising natural polymer in the food industry, Trends Food Sci. Technol., 2021, 109, 139–153, DOI:10.1016/J.TIFS.2021.01.031.
- P. Abhirami, N. Modupalli and V. Natarajan, Novel postharvest intervention using rice bran wax edible coating for shelf-life enhancement of Solanum lycopersicum fruit, J. Food Process. Preserv., 2020, 44, e14989, DOI:10.1111/JFPP.14989.
- B. L. Tan, M. E. Norhaizan and L. C. Chan, Rice Bran: From Waste to Nutritious Food Ingredients, Nutrients, 2023, 15, 2503, DOI:10.3390/NU15112503.
- Y. Shen, Z. J. Ni, K. Thakur, J. G. Zhang, F. Hu and Z. J. Wei, Preparation and characterization of clove essential oil loaded nanoemulsion and pickering emulsion activated pullulan-gelatin based edible film, Int. J. Biol. Macromol., 2021, 181, 528–539, DOI:10.1016/J.IJBIOMAC.2021.03.133.
- A. Sancakli, B. Basaran, F. Arican and O. Polat, Effects of bovine gelatin viscosity on gelatin-based edible film mechanical, physical and morphological properties, SN Appl. Sci., 2021, 3, 1–11, DOI:10.1007/S42452-020-04076-0/FIGURES/8.
- J. T. Sánchez, A. V. García, A. Martínez-Abad, F. Vilaplana, A. Jiménez and M. C. Garrigós, Physicochemical and Functional Properties of Active Fish Gelatin-Based Edible Films Added with Aloe Vera Gel, Foods, 2020, 9, 1248, DOI:10.3390/FOODS9091248.
- L. Tavares, H. K. S. Souza, M. P. Gonçalves and C. M. R. Rocha, Physicochemical and microstructural properties of composite edible film obtained by complex coacervation between chitosan and whey protein isolate, Food Hydrocoll., 2021, 113, 106471, DOI:10.1016/j.foodhyd.2020.106471.
- M. I. Socaciu, M. Fogarasi, C. A. Semeniuc, S. A. Socaci, M. A. Rotar, V. Mureşan, O. L. Pop and D. C. Vodnar, Formulation and characterization of antimicrobial edible films based on whey protein isolate and tarragon essential oil, Polymers, 2020, 12, 8(20734360), 1748, DOI:10.3390/POLYM12081748.
- L. M. Fernandes, J. T. Guimarães, R. Silva, R. S. Rocha, N. M. Coutinho, C. F. Balthazar, R. N. Calvalcanti, C. W. Piler, T. C. Pimentel, R. P. C. Neto, M. I. B. Tavares, E. A. Esmerino, M. Q. Freitas, M. C. Silva and A. G. Cruz, Whey protein films added with galactooligosaccharide and xylooligosaccharide, Food Hydrocoll., 2020, 104(0268005X), 105755, DOI:10.1016/j.foodhyd.2020.105755.
- S. Deng, J. Liao, H. Wu, M. Cao, M. Fan, H. Essawy, G. Du and X. Zhou, Multifunctional modification of biodegradable casein-microcrystalline cellulose composite film with UV-absorbing property using wood bark extract, Ind. Crops Prod., 2023, 192(09266690), 116080, DOI:10.1016/j.indcrop.2022.116080.
- E. De Gregorio, F. M. Miliani, F. M. Vivaldi, N. Calisi, N. Poma, A. Tavanti, C. Duce, F. Nardella, S. Legnaioli, A. G. Carota, L. Strambini, D. Biagini, T. Lomonaco, F. Di Francesco and P. Salvo, A casein-based biodegradable and sustainable capacitive sensor, Mater. Chem. Phys., 2024, 314(02540584), 128888, DOI:10.1016/j.matchemphys.2024.128888.
- M. R. Khan, S. Volpe, M. Valentino, N. A. Miele, S. Cavella and E. Torrieri, Active casein coatings and films for perishable foods: structural properties and shelf-life extension, Coatings, 2021, 11, 8(20796412), 899, DOI:10.3390/coatings11080899.
- L. Li, X. Luo, Y. Liu, M. Teng, X. Liu, X. Zhang and X. Liu, Edible packaging revolution: enhanced functionality with natural collagen aggregates, Food Hydrocoll., 2024, 156, 110331, DOI:10.1016/J.FOODHYD.2024.110331.
- L. M. Júnior, P. R. Rodrigues, R. G. da Silva, R. P. Vieira and R. M. V. Alves, Sustainable Packaging Films Composed of Sodium Alginate and Hydrolyzed Collagen: Preparation and Characterization, Food Bioprocess Technol., 2021, 14, 2336–2346, DOI:10.1007/S11947-021-02727-7/FIGURES/5.
- H. Motalebinejad, B. Bazargani-Gilani and M. Pajohi-Alamoti, Corn Zein edible film containing Sumac fruit extract and encapsulated Thymus daenensis Celak essential oil to improving the shelf life of chicken fillet, J. Food Meas. Char., 2023, 17, 5989–6002, DOI:10.1007/S11694-023-02099-W/METRICS.
- X. Chen, F. Cui, H. Zi, Y. Zhou, H. Liu and J. Xiao, Development and characterization of a hydroxypropyl starch/zein bilayer edible film, Int. J. Biol. Macromol., 2019, 141, 1175–1182, DOI:10.1016/J.IJBIOMAC.2019.08.240.
- C. K. Mouzakitis, V. Sereti, A. Matsakidou, K. Kotsiou, C. G. Biliaderis and A. Lazaridou, Physicochemical properties of zein-based edible films and coatings for extending wheat bread shelf life, Food Hydrocoll., 2022, 132, 107856, DOI:10.1016/J.FOODHYD.2022.107856.
- S. Sharma, K. Nakano, S. Kumar and V. Katiyar, Edible packaging to prolong postharvest shelf-life of fruits and vegetables: a review, Food Chem. Adv., 2024, 4, 100711, DOI:10.1016/J.FOCHA.2024.100711.
- K. Das, S. Sharma, S. Kumar, S. Mahajan, S. K. Banerjee and V. Katiyar, Chitosan nanoparticles and neem essential oil functionalized pullulan/gum arabic active edible biocomposites for fresh-cut guava preservation, Int. J. Biol. Macromol., 2024, 283, 136936, DOI:10.1016/J.IJBIOMAC.2024.136936.
- S. Kumar, P. Shukla, K. Das and V. Katiyar, Chitosan/water caltrop pericarp extract reinforced active edible film and its efficacy as strawberry coating for prolonging shelf life, Int. J. Biol. Macromol., 2025, 307, 142115, DOI:10.1016/J.IJBIOMAC.2025.142115.
- K. Das, S. Sharma, S. Kumar, S. Mahajan, S. K. Banerjee and V. Katiyar, Chitosan nanoparticles and neem essential oil functionalized pullulan/gum arabic active edible biocomposites for fresh-cut guava preservation, Int. J. Biol. Macromol., 2024, 283, 136936, DOI:10.1016/J.IJBIOMAC.2024.136936.
- M. Heras, C. C. Huang, C. W. Chang and K. H. Lu, Trends in chitosan-based films and coatings: a systematic review of the incorporated biopreservatives, biological properties, and nanotechnology applications in meat preservation, Food Packag. Shelf Life, 2024, 42, 101259–22142894, DOI:10.1016/j.fpsl.2024.101259.
- C. Yang, F. Lee, Y. Cheng, Y. Chu, C. Chen and Y. Kuan, Chitosan coating formulated with citric acid and pomelo extract retards pericarp browning and fungal decay to extend shelf life of cold-stored lychee, Scientia Horticulturae, 2023, 310, 111735, DOI:10.1016/j.scienta.2022.111735.
- F. Liang, C. Liu, J. Geng, N. Chen, W. Lai, H. Mo and K. Liu, Chitosan–fucoidan encapsulating cinnamaldehyde composite coating films: preparation, pH-responsive release, antibacterial activity and preservation for litchi, Carbohydr. Polym., 2024, 333, 121968, DOI:10.1016/J.CARBPOL.2024.121968.
- T. Sharma, G. Kaur, A. Singh, V. Kumar and B. N. Dar, Enhancing litchi shelf life with gluten-based nanocomposite films: a comparative study of montmorillonite and starch nanocrystals reinforced with chitosan, Sci. Hortic., 2024, 332, 113239, DOI:10.1016/J.SCIENTA.2024.113239.
- G. Zhao, J. Du, W. Chen, M. Pan and D. Chen, Preparation and thermostability of cellulose nanocrystals and nanofibrils from two sources of biomass: rice straw and poplar wood, Cellulose, 2019, 26, 8625–8643, DOI:10.1007/s10570-019-02683-8.
- Y. Liu, S. Ahmed, D. E. Sameen, Y. Wang, R. Lu, J. Dai, S. Li and W. Qin, A review of cellulose and its derivatives in biopolymer-based for food packaging application, Trends Food Sci. Technol., 2021, 112, 532–546, DOI:10.1016/j.tifs.2021.04.016.
- P. Kumar, S. Sethi and S. L. Nayak, Impact of Edible Hydrocolloids on Quality and Shelf Life of Litchi (Litchi chinensis Sonn.) Under Ambient Conditions, Appl. Fruit Sci., 2024, 66, 1119–1127, DOI:10.1007/S10341-024-01081-0/METRICS.
- R. Liu, X. Zhu, J. Wang and C. Huang, Activated release of chlorine dioxide gas from polyvinyl alcohol microcapsule (ethylcellulose/sodium-chlorite) hybrid films for active packaging of litchi during postharvest storage, Postharvest Biol. Technol., 2023, 196, 112173, DOI:10.1016/J.POSTHARVBIO.2022.112173.
- J. Ahmed, R. Santhosh, R. Thakur, M. Mulla and P. Sarkar, Thermo-mechanical, rheological, microstructural, and barrier properties of gum-based edible packaging: a review, Food Packag. Shelf Life, 2023, 38(22142894), 101117, DOI:10.1016/j.fpsl.2023.101117.
- S. Bham, N. K. Mishra, R. Rai, A. Dongariyal, R. Kumar and T. Pratap, Effect of Edible Coating and Packaging on Physiological and Sensory Attributes of Litchi (Litchi chinensis Sonn.) Fruits, Int. J. Curr. Microbiol. Appl. Sci., 2020, 9, 517–526, DOI:10.20546/ijcmas.2020.911.063.
- S. Sharma, K. Nakano, S. Kumar and V. Katiyar, Edible packaging to prolong postharvest shelf-life of fruits and vegetables: a review, Food Chem. Adv., 2024, 4, 100711, DOI:10.1016/J.FOCHA.2024.100711.
- X. Su, H. Lin, B. Fu, S. Mei, M. Lin, H. Chen, Z. Zheng, H. Bo, D. P. Yang and Y. Lin, Egg-yolk-derived carbon dots@albumin bio-nanocomposite as multifunctional coating and its application in quality maintenance of fresh litchi fruit during storage, Food Chem., 2023, 405, 134813, DOI:10.1016/J.FOODCHEM.2022.134813.
- C. W. T. Fukuyama, L. G. R. Duarte, I. C. Pedrino, M. C. Mitsuyuki, S. B. Junior and M. D. Ferreira, Effect of carnauba wax nanoemulsion associated with Syzygium aromaticum and Mentha piperita essential oils as an alternative to extend lychee post-harvest shelf, Sustain. Food Technol., 2024, 2, 426–436, 10.1039/d3fb00251a.
- M. Andrade, A. T. Sanches-Silva, H. C. Moghadas, R. Chauhan and J. S. Smith, Application of Plant Oils as Functional Additives in Edible Films and Coatings for Food Packaging: A Review, Foods, 2024, 13, 997, DOI:10.3390/FOODS13070997.
- S. Ali, A. S. Khan, A. Nawaz, M. A. Anjum, S. Naz, S. Ejaz and S. Hussain, Aloe vera gel coating delays postharvest browning and maintains quality of harvested litchi fruit, Postharvest Biol. Technol., 2019, 157(09255214), 110960, DOI:10.1016/j.postharvbio.2019.110960.
- L. Li, X. Yan and J. Li, The Prolonging Effect of Natural Plant Extracts on the Storage Period of Postharvest Litchi, Hortic. Sci. Technol., 2021, 39, 254–262, DOI:10.7235/HORT.20210023.
- B. H. Alshammari, M. M. A. Lashin, M. A. Mahmood, F. S. Al-Mubaddel, N. Ilyas, N. Rahman, M. Sohail, A. Khan, S. S. Abdullaev and R. Khan, Organic and inorganic nanomaterials: fabrication, properties and applications, RSC Adv., 2023, 13, 13735–13785, 10.1039/d3ra01421e.
- Y. Yu, J. Zhou, Q. Chen, F. Xie, D. Zhang, Z. He, S. Cheng and J. Cai, Self-reinforced multifunctional starch nanocomposite film for litchi fruit postharvest preservation, Chem. Eng. J., 2024, 486, 150262, DOI:10.1016/J.CEJ.2024.150262.
- Z. Qi, P. Xie, C. Yang, X. Xue, H. Chen, H. Zhou, H. Yuan, G. Yang and C. Wang, Developing fisetin-AgNPs incorporated in reinforced chitosan/pullulan composite-film and its application of postharvest storage in litchi fruit, Food Chem., 2023, 407, 135122, DOI:10.1016/J.FOODCHEM.2022.135122.
- G. Liu, S. Liu, J. Liu, Y. Xiang, L. Zhu, X. Xu and Z. Zhang, Trehalose delays postharvest browning of litchi fruit by regulating antioxidant capacity, anthocyanin synthesis and energy status, Postharvest Biol. Technol., 2025, 219, 113249, DOI:10.1016/J.POSTHARVBIO.2024.113249.
- L. Wei, Z. You, P. Liu, H. Zhu, X. Duan, Y. Jiang and Z. Yun, The role of amino acids in delaying the pulp breakdown of litchi fruit during postharvest storage, Food Chem., 2025, 489, 144977, DOI:10.1016/J.FOODCHEM.2025.144977.
- W. Zhao, Y. Liang, Y. Zhang, Y. Deng, H. Fu, X. Yuan, Z. Wang, X. Lu and B. Lin, Active cellulose-based film with synergistic enhanced photothermal antibacterial effects by Fe-doped ZIF-8 and EGCG for efficient litchi preservation, Chem. Eng. J., 2025, 521, 166462, DOI:10.1016/J.CEJ.2025.166462.
- H. U. Javed, J. Lai, J. Du, S. Zhong, H. Wu, W. Jiang, M. He, J. Shang, M. Zhang, S. Ali, Y. Liu, W. Xin, H. Yao, D. Lu, X. Shu and L. Y. Zeng, Development of chitosan-based films enhanced with vanillin-based deep eutectic solvents (V-DEA) for prolonging litchi shelf life and postharvest quality, Int. J. Biol. Macromol., 2025, 319, 145144, DOI:10.1016/J.IJBIOMAC.2025.145144.
- F. Yin, F. Wang, N. Xu, L. Shuai, Y. Liang, M. Song, M. He, W. Cai and Y. Liu, Peach gum edible coating film delays the browning of postharvest litchi and maintains its quality, J. Food Sci. Technol., 2025, 62, 562–571, DOI:10.1007/S13197-024-06046-5/FIGURES/5.
- B. Luo, S. Xuan, X. Wang, K. Ding, P. Jin, Y. Zheng and Z. Wu, Liposome/chitosan coating film bioplastic packaging for Litchi fruit preservation, Food Chem., 2025, 464, 141850, DOI:10.1016/J.FOODCHEM.2024.141850.
- N. Chandra, S. Chand, O. Singh, R. K. Srivastava, A. Singh, B. Mamgain, S. Kumar and R. Sharma, Storage Stability Assessment of the Postharvest Quality of the Litchi Cultivar ‘Rose Scented’ Via Gamma Radiation and Surface Edible Coating, Appl. Fruit Sci., 2025, 67, 1–11, DOI:10.1007/S10341-025-01435-2/FIGURES/2.
- S. Patidar, P. Kumari, A. Singh, N. C. Shahi, K. Chand, A. Hussain, W. Ahmad, U. C. Lohani and S. Kumar, Formulation and optimization of plant based organic coating with citrus peel extract for enhancing shelf life and quality of postharvest litchi fruit, J. Food Meas. Char., 2025, 19, 2350–2365, DOI:10.1007/S11694-025-03115-X/FIGURES/1.
- X. Yin, Y. Zhou, Y. Tang, D. Kong, W. Xiao, L. Gan, J. Huang and Y. Zhang, A crosslinked and percolation network alginate coating for litchi prevention, Int. J. Biol. Macromol., 2024, 275, 133252, DOI:10.1016/J.IJBIOMAC.2024.133252.
- F. Liang, C. Liu, J. Geng, N. Chen, W. Lai, H. MO and K. Liu, Chitosan–fucoidan encapsulating cinnamaldehyde composite coating films: Preparation, pH-responsive release, antibacterial activity and preservation for litchi, Carbohydr. Polym., 2024, 333, 121968, DOI:10.1016/j.carbpol.2024.121968.
- Y. Yu, J. Zhou, Q. Chen, F. Xie, D. Zhang, Z. He, S. Cheng and J. Cai, Self-reinforced multifunctional starch nanocomposite film for litchi fruit postharvest preservation, Chem. Eng. J., 2024, 486, 150262, DOI:10.1016/J.CEJ.2024.150262.
- P. Kumar, S. Sethi and S. L. Nayak, Impact of Edible Hydrocolloids on Quality and Shelf Life of Litchi (Litchi chinensis Sonn.) Under Ambient Conditions, Appl. Fruit Sci., 2024, 66, 1119–1127, DOI:10.1007/S10341-024-01081-0/FIGURES/3.
- J. Shen, S. Wan, H. Tan, Postharvest litchi (Litchi chinensis Sonn.) quality preservation by alginate oligosaccharides, arXiv, 2024, arXiv:2403.01383, DOI:10.48550/arXiv.2403.01383.
- C. Geng, X. Liu, J. Ma, H. Ban, H. Bian and G. Huang, High strength, controlled release of curcumin-loaded ZIF-8/chitosan/zein film with excellence gas barrier and antibacterial activity for litchi preservation, Carbohydr. Polym., 2023, 306, 120612, DOI:10.1016/J.CARBPOL.2023.120612.
- H. U. Javed, R. Liu, C. Li, S. Zhong, J. Lai, M. Hasan, X. Shu and L. Y. Zeng, Preparation of Vanillin-Taurine Antioxidant Compound, Characterization, and Evaluation for Improving the Post-Harvest Quality of Litchi, Antioxidants, 2023, 12, 618, DOI:10.3390/ANTIOX12030618.
- X. Lin, Y. Lin, Z. Liao, X. Niu, Y. Wu, D. Shao, B. Shen, T. Shen, F. Wang, H. Ding, B. Ye and Y. Li, Preservation of Litchi Fruit with Nanosilver Composite Particles (Ag-NP) and Resistance against Peronophythora litchi, Foods, 2022, 11(19), 2934, DOI:10.3390/FOODS11192934.
- J. Luo, G. Xia, L. Liu, A. Ji and Q. Luo, Fabrication of Chitosan/Hydroxyethyl Cellulose/TiO2 Incorporated Mulberry Anthocyanin 3D-Printed Bilayer Films for Quality of Litchis, Foods, 2022, 11, 3286, DOI:10.3390/FOODS11203286.
- J. Ahmed, M. Ali, H. M. Sheikh, M. O. Al-Kattan, Farhana, U. Haroon, M. Safaeishakib, M. Akbar, A. Kamal, M. S. Zubair and M. F. H. Munis, Biocontrol of Fruit Rot of Litchi chinensis Using Zinc Oxide Nanoparticles Synthesized in Azadirachta indica, Micromachines, 2022, 13(9), 1461, DOI:10.3390/MI13091461.
- A. Mani, A. B. Sharangi and P. K. Sahu, Aloe vera Based Dipping Treatments on Shelf Life and Physico-Chemical Properties of Litchi During Ambient Storage, Proc. Natl. Acad. Sci., India, Sect. B, 2021, 91, 521–532, DOI:10.1007/s40011-021-01248-9.
- M. S. Akter, M. R. Sarker, S. Choudhury, M. R. Sarkar, N. Islam and J. Uddain, Effect of postharvest treatments on shelf life and quality of litchi stored at ambient temperature, Int. J. Postharvest Technol. Innovation, 2020, 7, 319–334, DOI:10.1504/IJPTI.2020.110883.
- S. M. Jogdand, O. Singh, R. L. Lal, K. K. Mishra and N. C. Shahi, Effect of α-tocopherol based coating formulation on pericarp browning of litchi, Pharm. Innov., 2022, 11, 370–376 Search PubMed , https://www.thepharmajournal.com.
- A. Valdés, M. Ramos, A. Beltrán, A. Jiménez, M. C. Garrigós and S. Farris, State of the art of antimicrobial edible coatings for food packaging applications, Coatings, 2017, 17, 18(20734360), 2472, DOI:10.3390/coatings7040056.
- R. Suhag, N. Kumar, A. T. Petkoska and A. Upadhyay, Film formation and deposition methods of edible coating on food products: a review, Food Research International, n.d., 2020, 136, 109582, DOI:10.1016/j.foodres.2020.109582.
- A. Matloob, H. Ayub, M. Mohsin, S. Ambreen, F. A. Khan, S. Oranab, M. A. Rahim, W. Khalid, G. A. Nayik, S. Ramniwas and S. Ercisli, A Review on Edible Coatings and Films: Advances, Composition, Production Methods, and Safety Concerns, ACS Omega, 2023, 8, 28932–28944, DOI:10.1021/ACSOMEGA.3C03459.
- Q. Fan, J. Ma, Q. Xu, W. An and R. Qiu, Multifunctional coatings crafted via layer-by-layer spraying method, Prog. Org. Coating, 2018, 125, 215–221, DOI:10.1016/j.porgcoat.2018.09.015.
- S. Jesmin, T. Debnath, J. M. M. Islam, M. S. Rahaman, A. M. Kamal, M. S. Rahaman, S. E. Kabir and M. A. khan, Edible Transparent Coating of Irradiated Oligo-Chitosan to Preserve Aesthetic View and Taste of Litchi Fruit, J. Food Microbiol., 2018, 2(1), 6–11 Search PubMed , https://www.alliedacademies.org/journal-food-microbiology/.
- M. K. I. Khan, A. Nazir and A. A. Maan, Electrospraying: a Novel Technique for Efficient Coating of Foods, Food Eng. Rev., 2017, 9, 112–119, DOI:10.1007/s12393-016-9150-6.
- A. Sohel, R. Rani, D. Mehta, M. K. Nayak and M. K. Patel, Quality evaluation of strawberries coated with water-in-oil based emulsion using an advanced electrostatic spray coating system, J. Food Sci. Technol., 2023, 61(8), 1492–1502, DOI:10.1007/s13197-023-05915-9.
- A. A. Lipin and A. G. Lipin, Prediction of coating uniformity in batch fluidized-bed coating process, Particuology, 2022, 61, 41–46, DOI:10.1016/j.partic.2021.03.010.
- E. Tavassoli-Kafrani, M. V. Gamage, L. F. Dumée, L. Kong and S. Zhao, Edible films and coatings for shelf life extension of mango: a review, Crit. Rev. Food Sci. Nutr., 2022, 62, 2432–2459, DOI:10.1080/10408398.2020.1853038.
- Y. Yu, J. Zhou, Q. Chen, F. Xie, D. Zhang, Z. He, S. Cheng and J. Cai, Self-reinforced multifunctional starch nanocomposite film for litchi fruit postharvest preservation, Chem. Eng. J., 2024, 486, 150262, DOI:10.1016/j.cej.2024.150262.
- Y. Wu, H. Wu and L. Hu, Recent Advances of Proteins, Polysaccharides and Lipids-Based Edible Films/Coatings for Food Packaging Applications: a Review, Food Biophys., 2024, 19, 29–45, DOI:10.1007/S11483-023-09794-7.
- S. Moreno and B. Giménez, Digestibility and Toxicology of Edible Films and Coatings, Edible Films and Coatings, 2016, accessed June 11, 2024, https://www.taylorfrancis.com/chapters/edit/10.1201/9781315373713-40/digestibility-toxicology-edible-films-coatings-silvia-moreno-bego%C3%B1a-gim%C3%A9nez Search PubMed.
- J. P. Gh, B. J. Sinclair, G. K. Perinbarajan, R. Dutta, R. Shekhawat, N. Saikia, R. Chidambaram and A. T. Mossa, An overview on smart and active edible coatings: safety and regulations, Eur. Food Res. Technol., 2023, 249, 1935–1952, DOI:10.1007/s00217-023-04273-2.
- A. Yadav, N. Kumar, A. Upadhyay, S. Sethi and A. Singh, Edible coating as postharvest management strategy for shelf-life extension of fresh tomato (Solanum lycopersicum L.): an overview, J. Food Sci., 2022, 87, 2256–2290, DOI:10.1111/1750-3841.16145.
- E. P. R. Pereira, J. Silva da Graça, B. M. Ferreira, C. F. Balthazar, D. Xavier-Santos, F. F. Bezerril, M. Magnani and A. S. Sant’Ana, What are the main obstacles to turning foods healthier through probiotics incorporation? a review of functionalization of foods by probiotics and bioactive metabolites, Food Research International, 2024, 176, 113785, DOI:10.1016/j.foodres.2023.113785.
- L. D. Díaz, V. Fernández-Ruiz and M. Cámara, An international regulatory review of food health-related claims in functional food products labeling, J. Funct.Foods, 2020, 68, 103896, DOI:10.1016/J.JFF.2020.103896.
- V. Paganizza, A European overview on Regulation (EU) No 1169/2011 after the entry into force, R. di diritto alimentare, R. di diritto alimentare, 2020, pp. 20-35 Search PubMed.
| This journal is © The Royal Society of Chemistry 2026 |