Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Formulation of casein–curcumin nanodispersions using microfluidization and nano-precipitation methods: cytotoxicity and in vitro release in a mango drink
Maanya
Mehrotra†
a,
Shashank
Singh†
a,
Nikita
Kansal†
a,
Kiran
Verma
 b,
Anit
Kumar
*c and
Narashans Alok
Sagar
b,
Anit
Kumar
*c and
Narashans Alok
Sagar
 *de
*de
aDepartment of Food Technology, Harcourt Butler Technical University, Kanpur, 208002, India
bDepartment of Food Science and Technology, National Institute of Food Technology Entrepreneurship and Management, Kundli, Sonepat, Haryana 131028, India
cDepartment of Food Science and Postharvest Technology, Bihar Agricultural College, Bihar Agricultural University, Sabour, Bhagalpur, Bihar 813210, India. E-mail: anitkumarsingh09@gmail.com
dDepartment of Biotechnology, University Institute of Biotechnology, Chandigarh University, Mohali, Punjab 140413, India. E-mail: narashans.alok@gmail.com
eUniversity Centre for Research and Development (UCRD), Chandigarh University, Mohali, Punjab 140413, India
First published on 17th November 2025
Abstract
Curcumin is a bioactive compound obtained from turmeric (Curcuma longa). It has several biological properties, such as antioxidant, anti-inflammatory, antimicrobial and anticancer activities. The therapeutic efficacy and bioavailability of curcumin are low due to its poor water solubility and instability. Nanoencapsulation has emerged as a promising approach for curcumin delivery in food systems that can address the aforementioned challenges. In this study, casein–curcumin nanodispersions were developed by two different technologies, microfluidization and nano-precipitation, to enhance the stability, cytocompatibility and the controlled release of curcumin in a mango-based beverage. The results showed that the nanoparticles made from nanoprecipitation were bigger than those made from microfluidization. The PDI values showed that the microfluidized particles were more uniform in size, while the nano-precipitated ones were almost uniform. The zeta potential of the nanoprecipitated particles (−23.63 mV) showed that they were more stable than the microfluidized particles, which had lower values (−13.5 mV and −8 mV). Cytotoxicity was assessed using the MDCK (normal) and HepG2 (cancer) cell lines, indicating that the curcumin nanoparticles reduced cancer cell viability, particularly at higher concentrations, while maintaining a high biocompatibility with normal cells. From the in vitro release analysis, the nanoprecipitated curcumin dispersions exhibited a more controlled and sustained release in the mango drink than the microfluidized samples, which exhibited a faster initial release. Overall, our results show that the casein–curcumin nanodispersions produced by both methods (the nanoprecipitation and microfluidization methods) hold potential as effective functional food ingredients for targeted and sustained curcumin delivery.
Sustainability spotlightThis study promotes sustainable food innovation using casein (a natural milk protein) to encase curcumin from turmeric. This process improves its solubility, stability, and therapeutic effectiveness in a mango-based drink. By applying eco-friendly nano-encapsulation techniques like microfluidization and nano-precipitation, the research presents a safe, biocompatible, and functional delivery system for curcumin that supports health and cuts down on the need for synthetic additives. These methods not only enhance the value of natural bioactives but also support sustainable food system goals by encouraging plant- and protein-based functional ingredients with potential uses in nutraceuticals and health-focused beverages. |
1 Introduction
Curcumin is a bioactive phytopolyphenol extracted from the rhizomes of the plant Curcuma longa, generally known as turmeric. It is a yellow-coloured pigment that has been commonly used as a household spice and natural food coloring agent in South Asian (Indian, Pakistani, Bangladeshi, and Chinese) food preparations since ancient times.1 The use of turmeric in these countries dates back more than 2000 years. Curcumin is known to have a broad spectrum of biological activities, including antioxidant, antimicrobial, anti-inflammatory and anticancer activities.2 Because inflammation has been shown to be a causative factor behind the proliferation of tumour cells, curcumin, with its potent anti-inflammatory properties, can inhibit carcinogenesis.1 As an antioxidant, curcumin is known to hinder the formation of various reactive oxygen species. However, the poor pharmacokinetic and pharmacodynamic properties of curcumin, which are mainly related to its low water solubility, short half-life, photodegradation, chemical instability, and rapid metabolism in the gastrointestinal tract, result in its low bioavailability, vastly reducing its remedial potential. To overcome these problems, researchers have considered numerous approaches, including the preparation of nanocurcumin in the form of nanoemulsions, liposomes, suspensions and dispersions.2–4 Furthermore, the loading of curcumin into micelles can provide a promising approach for stabilizing the compound and developing formulations suitable for further pharmaceutical and food studies. The nanoencapsulation of curcumin helps with its easy incorporation into different beverages and can improve its poor solubility. Furthermore, casein–curcumin-based nano-particles are relatively stable under the processing conditions, and are easy to prepare, and their size distribution can be easily monitored. Various research and review studies have been published on the nanoencapsulation of bioactive compounds using protein-based emulsions.5–7 Because casein has distinct hydrophilic and hydrophobic domains, various mechanical approaches have been generally used to reduce the particle/droplet size of systems that contain curcumin. These mechanical approaches include homogenization, sonication and high-pressure homogenization (microfluidization) utilizing high energy.8Microfluidization is a novel technique in the field of food processing, although it has been widely used for pharmaceutical applications. Microfluidization is a type of advanced high-pressure homogenization process that creates finely dispersed suspensions and emulsions on a nanoscale. A fluid passes through microchannels that divide the fluid stream into two fine jets in the interaction chamber. The two fine jets are then directed at each other at right angles at high pressures of up to 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 psi. As the two microstreams collide, there is a sudden pressure drop, and as a result of turbulence, cavitation and shear effects, the impact leads to the production of a fluid with much smaller droplets than the initial fluid. Microfluidization reduces the mean particle size depending on the number of passes and applied pressure. As the number of passes and pressure increase, the droplet size decreases up to a certain size depending upon the fluid. According to Bucci et al.,9 there is little reduction in particle size beyond the third pass. Microfluidization can be applied to the improvement of the bioavailability and fast release of curcumin.10
000 psi. As the two microstreams collide, there is a sudden pressure drop, and as a result of turbulence, cavitation and shear effects, the impact leads to the production of a fluid with much smaller droplets than the initial fluid. Microfluidization reduces the mean particle size depending on the number of passes and applied pressure. As the number of passes and pressure increase, the droplet size decreases up to a certain size depending upon the fluid. According to Bucci et al.,9 there is little reduction in particle size beyond the third pass. Microfluidization can be applied to the improvement of the bioavailability and fast release of curcumin.10
On the other hand, nanoprecipitation is a conventional approach for producing nanoparticles. This approach involves a partially water-miscible solvent (saturated in water) and an organic phase, which are then emulsified in a solution containing a stabilizer, leading to the formation of nanoparticles.11 Any delivery system used to deliver curcumin should be able to release it within the gastrointestinal tract after ingestion. The slow release of curcumin from a nanoparticulate formulation increased the bioavailability of the delivered curcumin.2
There is very limited information available on the formulation and cytotoxicity of casein–curcumin nanodispersions prepared using microfluidization and nanoprecipitation, as well as their application in beverages.12–14 Hence, this study is necessary for advancing the understanding and exploration of the potential applications of casein–curcumin nanodispersions. The present study investigates microfluidization and nanoprecipitation as methods for utilizing casein as a delivery vehicle for curcumin, providing an alternative approach for food applications. Additionally, microfluidization and nanoprecipitation are both are eco-friendly methods due to their reduced energy and solvent consumption, avoidance of toxic chemicals and minimal waste creation. This study is divided into three sections: the first section focuses on the development of curcumin nanoparticles using microfluidization and nanoprecipitation, the second section presets analyses of the particle size, zeta potential and cytotoxicity of curcumin, and the third section presents the application of curcumin nanoparticles in a mango drink and a discussion of their release behavior.
2 Materials and methods
2.1. Chemicals and reagents
Casein and curcumin were received from Sigma-Aldrich Bangalore, India. All the reagents, polyvinyl alcohol (PVA), Dimethyl Sulfoxide (DMSO), ethanol, chloroform, phosphate-buffered saline (PBS), Tween-80, DMEM and trypsin, used for the experiments were purchased from Sigma-Aldrich, Bangalore, India. The MDCK and HepG2 cell lines were procured from NCCS, Pune, India. PLGA 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 (inherent viscosity 0.16–0.24 dl g−1, acid terminated), which was kindly provided by BSBE, Indian Institutes of Technology, Kanpur, India, was used to prepare the nanoparticles. Ripe mangoes (Mangifera indica cv. Alphonso) were procured from a local market in Kanpur, India.
50 (inherent viscosity 0.16–0.24 dl g−1, acid terminated), which was kindly provided by BSBE, Indian Institutes of Technology, Kanpur, India, was used to prepare the nanoparticles. Ripe mangoes (Mangifera indica cv. Alphonso) were procured from a local market in Kanpur, India.
2.2. Preparation of nanoparticles using microfluidization
The microfluidized casein–curcumin dispersions were made following the method described by Pinheiro et al.15 Briefly, 100 mg of curcumin, with and without 30 mg casein, was dissolved in 500 ml of ethanol. The resultant mixture was then homogenized (Ultra Turrax, T18 digital, Germany) at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 2 minutes. The homogenized solution was then passed through a microfluidizer (Model M110P, Microfluidics Corporation, USA) at 20
000 rpm for 2 minutes. The homogenized solution was then passed through a microfluidizer (Model M110P, Microfluidics Corporation, USA) at 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 psi pressure and the sample was collected after different passes (1st, 3rd and 5th). However, for the standard curcumin solution, 100 mg curcumin was dissolved in 500 ml of ethanol and a similar procedure to that used for the casein–curcumin dispersions was followed. The sample codes of the different samples are presented in Table 1.
000 psi pressure and the sample was collected after different passes (1st, 3rd and 5th). However, for the standard curcumin solution, 100 mg curcumin was dissolved in 500 ml of ethanol and a similar procedure to that used for the casein–curcumin dispersions was followed. The sample codes of the different samples are presented in Table 1.
| S. no. | Treatment | Codes |
|---|---|---|
| 1 | 100 mg curcumin in 500 ml ethanol without microfluidization | A0 |
| 2 | 100 mg curcumin in 500 ml ethanol after 1st pass | A1 |
| 3 | 100 mg curcumin in 500 ml ethanol after 3rd pass | A2 |
| 4 | 100 mg curcumin in 500 ml ethanol after 5th pass | A3 |
| 5 | 100 mg curcumin and 30 mg casein in 500 ml ethanol without microfluidization | B0 |
| 6 | 100 mg curcumin and 30 mg casein in 500 ml ethanol after 1st pass | B1 |
| 7 | 100 mg curcumin and 30 mg casein in 500 ml ethanol after 3rd pass | B2 |
| 8 | 100 mg curcumin and 30 mg casein in 500 ml ethanol after 5th pass | B3 |
| 9 | Casein–curcumin nanoparticles prepared by nanoprecipitation method | C0 |
2.3. Preparation of nanoparticles using nanoprecipitation
The casein–curcumin nanoparticles (C0) were prepared using a nanoprecipitation method adapted with slight modifications from Joshi and Thakur.16 Casein (15 mg) and curcumin (1 mg) were dissolved in 1 ml of DMSO and vortexed for 10 minutes, yielding a uniformly dispersed solution of particles. This mixture was then ultrasonicated for 8 min in an ice-bath surrounding the Eppendorf tube to prevent excessive heat and avoid casein denaturation. The resulting solution appeared completely transparent with a reddish-brown hue. Using a syringe infusion pump, this solution was then added into 10 ml of 3% aqueous PVA at a consistent flowrate of 120 ml h−1 under moderate stirring (700 rpm) with a magnetic stirrer. A syringe needle was submerged in the PVA solution to ensure controlled mixing. Stirring was maintained for 30 minutes, which facilitated the thorough blending of DMSO and the aqueous PVA solution. Following this step, DMSO was evaporated from the mixture using a rotary vacuum evaporator at 40 °C for 2 hours.To prepare the casein–curcumin nanoparticles, a stock solution was created by dissolving the casein–curcumin dispersion in DMSO after applying a disaggregation protocol. From this stock solution, a volume equivalent to 75 µg of casein–curcumin solution and 15 mg of PLGA was added, reaching a final volume of 1 ml in DMSO. The nanoparticles were then collected by ultracentrifugation at 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g for 50 minutes (Sorvall Micro-ultracentrifuge, Thermo Scientific Ltd, Rotor S-55-S). After the first centrifugation, the supernatant was removed, and the nanoparticles at the bottom of the tube were collected and washed with 1 mL of distilled water. Ultracentrifugation was repeated at 60
000 g for 50 minutes (Sorvall Micro-ultracentrifuge, Thermo Scientific Ltd, Rotor S-55-S). After the first centrifugation, the supernatant was removed, and the nanoparticles at the bottom of the tube were collected and washed with 1 mL of distilled water. Ultracentrifugation was repeated at 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g for 10 minutes, followed by an additional wash with 1 mL of distilled water.
000 g for 10 minutes, followed by an additional wash with 1 mL of distilled water.
2.4. Size, polydispersity index and zeta potential analysis
The nanoparticles were characterized by their size, polydispersity index (PDI), and zeta potential using dynamic light scattering (DLS, Malvern Zetasizer Nano ZS90, UK) equipped with a 633-nm laser source and a detector positioned at a 90° angle. The particle size and distribution were represented as the intensity-weighted hydrodynamic diameter and the number of particles by percentage (%), respectively; these parameters are mathematically well correlated. The zeta potential measurements were calculated using the Smoluchowski approximation, following the method described by Joshi and Thakur.152.5. Cell line assay
A cell line assay was carried out as detailed by Fan et al.17 Briefly, the MDCK and HepG2 cell lines were cultured prior to the experiment. The cell lines were then placed in trypsin and incubated for 10 min at 37 °C and a CO2 concentration of 10%. After incubation, DMEM (2 mL) was added to deactivate trypsin and the cell lines were allowed to grow for 24 h. Different concentrations of the curcumin nanoparticles (25, 50 and 75 µL) and a control (without nanoparticles) were then added for determining the effect of the nanoparticles on the cells. DMEM (2 mL) and 10% fetal bovine serum (FBS) were added to the cells, which were kept for 24 hours. At the end of this period, readings were taken on a plate reader. Using the control reading for 100 cells, the readings of the various concentrations were calculated relative to the control of 100 cells; the readings were taken in triplicate.2.6. In vitro release
A mango was peeled and the pulp was extracted using a grinder. The fibers were then removed from the pulp and the juice was stored in sterilized glass bottles. The initial total soluble solids (TSS) and pH of the mango juice were 12° Brix and 3.62, respectively. To prepare the mango drink, the TSS was adjusted to 14° Brix by adding a sugar syrup solution. The mixture was then placed on a shaker for 20 minutes, which ensured uniform blending. The prepared drink was then pasteurized at 80 °C for 5 minutes and stored in sterilized glass bottles at a refrigeration temperature of 4 °C ± 2 °C.The in vitro release was assessed in the mango-drink matrix. A volume of 600 µL of the mango drink was dispensed into each well of a 24-well plate and mixed with 300 µL of the microfluidized and nanoprecipitated casein–curcumin nanoparticles. The mixture was placed on a shaker, and 100 µL aliquots were collected at various time intervals (0, 5, 10, 20, 30, 45, and 90 minutes). Each 100 µL sample was then mixed with 200 µL of chloroform to dissolve the casein nanoparticles. The absorbance readings were taken using a SpectraMax M3 Multi-Mode Microplate Reader (Molecular Devices).
2.7. Statistical analysis
All the experiments were performed in triplicate (n = 3) and the obtained data were analyzed using one-way ANOVA with the application of the sigmaPlot software (version 12.0, Systat Software Inc., San Jose, California, USA). Duncan's multiple range test was utilized to separate means and the results were presented as the mean ± S.D.3 Results and discussion
3.1 Size, polydispersity index and zeta potential analysis
The size, polydispersity index and zeta potential of the nanoparticles prepared with different methods are presented in Table 2. These results show that microfluidization reduced the particle size up to 80 nm. However, this reduction of the particle size of curcumin in ethanol was achieved after the 2nd pass and in the presence of casein a similar reduction in particle size was achieved after the 3rd pass, which can be attributed to the hindrance provided by casein. Indeed, it has been previously reported that there is a hydrophobic interaction between curcumin and casein that forms a thick interfacial layer around lipophilic compounds.18 The decrease in particle size was likely due to the increase in the number of passes, which further degraded the particles, and their decreased stability was likely due to the formation of the aggregated nanoparticles. The particle size of the nanoparticles obtained by nanoprecipitation was larger than those in the microfluidized dispersions.| Sample | Particle size (d, nm) | PDI | Zeta potential (mV) |
|---|---|---|---|
| a Values in mean ± SD. | |||
| A0 | 160.100 ± 6.151 | 0.152 ± 0.030 | −21.867 ± 2.155 |
| A1 | 151.800 ± 6.954 | 0.101 ± 0.009 | −16.233 ± 0.737 |
| A2 | 81.090 ± 1.369 | 0.215 ± 0.010 | −12.180 ± 2.238 |
| A3 | 80.177 ± 1.793 | 0.209 ± 0.018 | −13.500 ± 3.132 |
| B0 | 189.567 ± 15.683 | 0.206 ± 0.015 | −15.467 ± 0.404 |
| B1 | 190.133 ± 12.716 | 0.332 ± 0.036 | −11.167 ± 2.686 |
| B2 | 103.893 ± 17.803 | 0.337 ± 0.108 | −8.607 ± 3.248 |
| B3 | 88.113 ± 0.989 | 0.167 ± 0.017 | −8.810 ± 0.053 |
| C0 | 195.944 ± 30.790 | 0.401 ± 0.088 | −23.633 ± 3.732 |
The polydispersibility index (PDI) provides information about the distribution of particles. Sadeghi et al.19 reported that suspensions with a PDI value lower than 0.3 with single-size distribution peaks are close to being classified as a monodispersed suspension. The PDI value for the microfluidized nanoparticles was lower than 0.3, while it was greater than 0.3 for the nanoprecipitated nanoparticles. However, an increase in the PDI value of the microfluidized nanoparticles was likely due to the encapsulation of curcumin by casein, which could have caused the particles to accumulate. The range of the PDI value of the dispersions indicated that microfluidization produced a dispersion that was monodispersed, while the nanoprecipitated nanoparticles were close to possessing a monodispersed nature. The results also show a narrow and uniform distribution of particles within the desired limit of 200 nm.20
The zeta potential of a dispersion, which is based on the superficial charge present on the surface of the particles, determines the particle stability in such a system. The zeta potential of the nanoparticles should be either less than 30 mV or higher than +30 mV; at these ranges, they are considered to have highly charged surface particles, which contributes to the stability of their suspensions.21 The zeta potential of the nanoprecipitated nanoparticles was observed to be −23.63 mV, which indicated a higher stability than the microfluidized nanoparticles with and without casein (−13.5 and −8 mV, respectively). Raviadaran et al.22 also reported the optimized nanoemulsion of curcumin using 1–10 cycles of microfluidizer at a pressure of 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 305.3 psi (140 MPa). They used Tween 80 as a surfactant for stabilizing the nanoemulsion, which gave an average zeta potential of −56, making the nanoemulsion stable.
305.3 psi (140 MPa). They used Tween 80 as a surfactant for stabilizing the nanoemulsion, which gave an average zeta potential of −56, making the nanoemulsion stable.
3.2 Cell line analysis
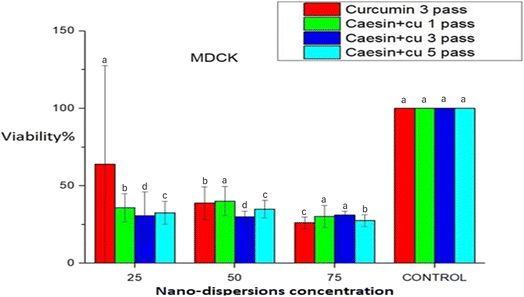
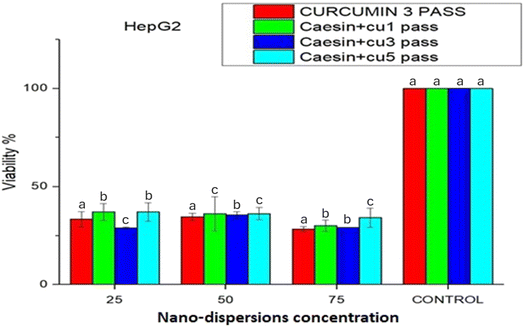
The cell viability of the MDCK and HepG 2 cells was determined by a plate reader machine. Cell line analysis showed that the MDCK cells had almost 100% viability at all concentrations of the nanoprecipitated curcumin nanoparticles indicating that the curcumin dispersions did not have adverse effects on the MDCK cells. However, the viability of the cancer cells (HepG 2) decreased drastically. On the other hand, the viable cell count of the MDCK cells exposed to the nanoprecipitated nanoparticles increased as the concentration decreased from 75 to 25 µL. When the concentration of the curcumin nanoparticles was 25 µL, the viable cells increased to a maximum. This observation can be attributed to the fact that when the cells were given low concentrations of the foreign curcumin nanoparticles for the first time, they adapted and replicated more than at other higher concentrations. The viability of the cancer cells (HepG2) decreased as the concentration of the nanoparticles increased. There was a low number of viable cancer cells at a 75-µL concentration of curcumin nanoparticles. This observation was likely due to the anti-carcinogenic property of curcumin, which kills more cancer cells at high concentrations than at low concentrations (Fig. 1). The inhibition of FAS (fatty acid synthase) in cancer cells has been observed to induce apoptosis, which suggests that inhibiting the activity of FAS should be a reasonable approach for the treatment of cancer. Moreover, it was reported that curcumin is a natural FAS inhibitor.23 Curcumin was able to induce HepG2 cell apoptosis by inhibiting intracellular FAS activity, downregulating FAS expression and the mRNA level. This novel finding supported the important role of FAS in cancer cells. These results suggest that curcumin can be considered to have potential applications in the treatment of cancer, providing some useful ideas and new clues in developing target-directed anticancer drugs for further in vivo studies.17In the case of the microfluidized nanoparticles, the viability of both the MDCK and HepG2 cells decreased regardless of the number of microfluidizer passes. For the MDCK cells, the curcumin control sample (3 passes) showed a decline in cell viability as the nanoparticle concentration increased from 25 to 75 µL. The lowest viability was observed in the first-pass sample at a 75 µL concentration, compared to the 50 µL and 25 µL samples. This reduction in cell viability may be attributed to the increased concentration of the free, non-encapsulated curcumin nanoparticles, which likely overwhelmed the cells due to their smaller size and cytotoxic nature, leading to increased cell death. Among all the microfluidized samples, the curcumin nanoparticles subjected to three passes demonstrated the most effective results (Fig. 2). For the HepG2 cancer cells, a similar trend was observed, where higher curcumin nanoparticle concentrations (particularly at 75 µL) led to a greater reduction in cell viability compared to 25 µL and 50 µL. However, for the third-pass sample, the lowest cell viability was unexpectedly recorded at 25 µL, although a difference across concentrations was not found. In the fifth-pass sample, cell viability exhibited a minimal variation across concentrations, with the lowest viability at 75 µL; additionally, the standard deviations were the smallest observed, indicating consistent results.
These results suggest that microfluidization created fine and more uniform nanoparticles due to high shear force, which not only improved their stability, but could have caused a partial breakdown of the casein matrix, releasing more free curcumin and increasing cytotoxicity at higher concentrations. In contrast to microfluidization, nanoprecipitation occurred under milder conditions, which likely led to a better encapsulation of curcumin within the casein matrix. This results suggest that larger, but more biocompatible nanoparticles release curcumin gradually, maintaining high cell viability in normal cells and retaining their anticancer effects. Thus, although microfluidization enhanced particle uniformity, it likely increased cytotoxicity at higher concentrations, while nanoprecipitation improved biocompatibility through a controlled curcumin release; however, the third-pass sample showed an anomalous behavior in the HepG2 cell line, which could not be clearly explained (Fig. 3).
3.3 Release study of the mango drink
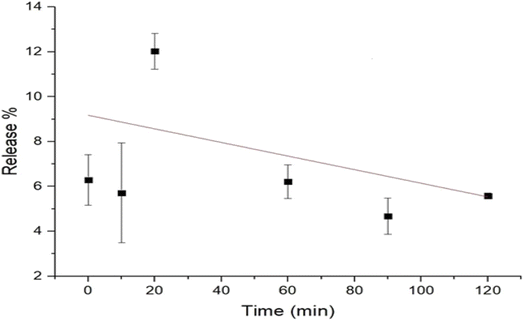
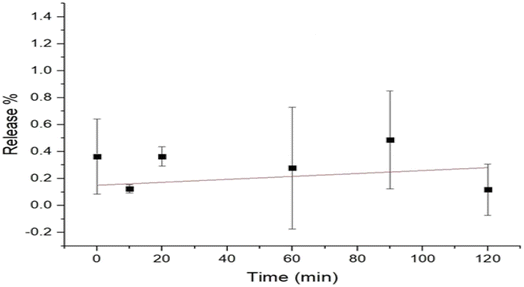
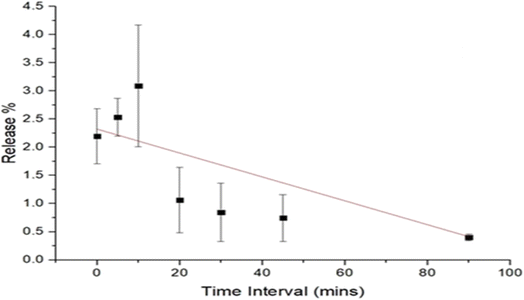
The results of the release study of the curcumin (microfluidized and nanoprecipitated) nanoparticles are presented in Fig. 4. The release of the curcumin nanoparticles was determined using the optical density method, which shows the degradation of the curcumin nanoparticles. The results obtained for the microfluidized curcumin nanoparticles showed a gradual release of curcumin, which decreased with time (Fig. 4). Initially, the release of curcumin was 9.1%, which continuously decreased at a rate of 0.03% per min, reaching 5.5% at 120 min. However, the release of curcumin from the nanoprecipitated curcumin nanoparticles increased with time. Initially, the release was very low (0.15%) compared to the microfluidized curcumin nanoparticles (9.1%); however, it increased at a rate of 0.001% per min and finally reached 0.27% after 120 min (Fig. 5). The higher initial burst release of the microfluidized curcumin nanoparticles was due to the partial exposure of the curcumin molecules on the surface of the nanoparticles, which allowed them to diffuse quickly into the surrounding medium. Over time, as the surface-associated curcumin was depleted, the release rate decreased, which led to a gradual decline in the curcumin concentration.The nanoprecipitated curcumin nanoparticles had a lower curcumin release than the microfluidized curcumin nanoparticles, showing that the effective encapsulation of curcumin is crucial for the slow release of curcumin. The release study of the nanoprecipitated sample in the mango drink showed that the release of curcumin from the mango drink exhibited a gradual decrease (Fig. 6). Initially, the curcumin release was 2.3%, which decreased at the rate of 0.02% per min and reached 0.4% after 90 min. Obeid et al.24 reported a similar trend in the release study of niosomal nanoparticles from the encapsulated curcumin. They also used microfluidization for sample preparation, concluding that microfluidization allowed for the small and controlled distribution of particles, which led to a sudden release of curcumin that reached a maximum within two days. In another study, Li et al.25 also reported an initial burst release of curcumin. However, this trend was not observed in this study for the microfluidized sample of curcumin nanoparticles loaded into the mango drink, but it was similar for the nanoparticles prepared from nanoprecipitation. Taha et al.26 recently studied curcumin-loaded milk proteins and observed that among the curcumin-loaded caseins, the highest release of curcumin was from the curcumin-loaded casein nanoparticles, α-lactalbumin and β-lactoglobulin nanoparticles. The release of curcumin from the food matrix can be dependent upon the hydrophobic interaction of curcumin with food constituents. The stronger the interaction of curcumin with other compounds, the slower the release of curcumin from the matrix.27
Conclusions
This study concluded that the curcumin nanodispersions with relatively small particle size, low polydispersity index and controlled zeta potential were developed by using microfluidization. A slight delay in the reduction of the particle size of curcumin resulted from the hydrophobic interaction of casein with curcumin. The microfluidized nanodispersions exhibited better monodispersity than the nano-precipitated ones, supported by the obtained PDI values below 0.3, indicating a uniform particle distribution for the microfluidized nanodispersions. However, the zeta potential values indicated that the nanoprecipitated nanodispersions were relatively more electrostatically stable compared to the microfluidized nanodispersions. Based on the physicofunctional properties, microfluidization was effective in producing uniform nanoparticles (<200 nm); however, the surface charge and stability can be further improved by using appropriate encapsulating agents or stabilizers. The biofunctional properties of the curcumin nanodispersions prepared by the nanoprecipitation method showed no cytotoxic effects on the MDCK cells and demonstrated dose-dependent anticancer activity against the HepG2 cells. Comparatively, a reduced cell viability in both cell types was found for the microfluidized nanodispersions. The release studies of the mango drink revealed a faster initial release of curcumin followed by a gradual decline for the microfluidized nanodispersions, while the nanoprecipitated particles showed a much slower and sustained release. These findings suggest that the casein–curcumin nanodispersions developed using the nanoprecipitation and microfluidization techniques are promising as functional food ingredients for the targeted and sustained delivery of curcumin. In future studies, blank nanocarriers (casein nanoparticles without curcumin) could be used as controls to better understand any carrier-specific effects on cell lines.Author contributions
Maanya Mehrotra: methodology, formal analysis, extraction data, and writing – original and draft; Shashank Singh: methodology, formal analysis, extraction data, and writing – original draft; Nikita Kansal: formal analysis, extraction data, and writing – original draft; Kiran Verma: formal analysis and data curation; Anit Kumar: administration, supervision, and conceptualization; Narashans Alok Sagar: editing and reviewing.Conflicts of interest
The authors declare no conflict of interest.Data availability
Data are provided within the manuscript.Acknowledgements
The author(s) received no financial support for the research, authorship, and/or publication of this article. The authors are obliged to the Vice-Chancellor of Bihar Agricultural University, Sabour, Bihar and HBTU, Kanpur, as well as Nabodita Sinha (PhD Research Scholar) and Ashwani Kumar Thakur (Professor, BSBE Department) from the Indian Institute of Technology, Kanpur, India for providing the necessary facilities to carry out the work for this study.References
- W. H. Lee, C. Y. Loo, M. Bebawy, F. Luk, R. S. Mason and R. Rohanizadeh, Curcumin and its Derivatives: Their Application in Neuropharmacology and Neuroscience in the 21st Century, Curr. Neuropharmacol., 2013, 11(4), 338–378, DOI:10.2174/1570159X11311040002.
- T. P. Sari, B. Mann, R. Kumar, R. R. B. Singh, R. Sharma, M. Bhardwaj and S. Athira, Preparation and Characterization of Nanoemulsion Encapsulating Curcumin, Food Hydrocoll., 2015, 43, 540–546, DOI:10.1016/j.foodhyd.2014.07.011.
- K. Verma, A. Tarafdar and P. C. Badgujar, Microfluidics Assisted Tragacanth Gum Based Sub-Micron Curcumin Suspension and its Characterization, LWT–Food Sci. Technol., 2021, 135, 110269, DOI:10.1016/j.lwt.2020.110269.
- A. Araiza-Calahorra, M. Akhta and A. Sarkar, Rrecent Advances in Emulsion-Based Delivery Approaches for Curcumin: From Encapsulation to Bioaccessibility, Trends Food Sci. Technol., 2018, 71, 155–169, DOI:10.1016/j.tifs.2017.11.009.
- T. Dissanayake and N. Bandara, Protein-Based Encapsulation Systems for Codelivery of Bioactive Compounds: Recent Studies and Potential Application, Curr. Opin. Food Sci., 2024, 57, 101181, DOI:10.1016/j.cofs.2024.101181.
- H. Cheng, W. Chen, J. Jiang, M. A. Khan and L. L. Wusigale, A Comprehensive Review of Protein-Based Carriers with Simple Structures for the Co-Encapsulation of Bioactive Agents, Compr. Rev. Food Sci. Food Saf., 2023, 22, 2017–2042, DOI:10.1111/1541-4337.13139.
- M. Pateiro, B. Gómez, P. E. S. Munekata, F. J. Barba, P. Putnik, D. B. Kovačević and J. M. Lorenzo, Nanoencapsulation of Promising Bioactive Compounds to Improve their Absorption, Stability, Functionality and the Appearance of the Final Food Products, Molecules, 2021, 26(6), 1547, DOI:10.3390/molecules26061547.
- D. J. McClements, Nanoemulsions Versus Microemulsions: Terminology, Differences, and Similarities, Soft Matter, 2012, 8(6), 1719–1729, 10.1039/C2SM06903B.
- A. J. Bucci, D. L. Van. Hekken, M. H. Tunick, J. A. Renye and P. M. Tomasula, The Effects of Microfluidization on the Physical, Chemical and Coagulation Properties of Milk, J. Dairy Sci., 2018, 101(8), 6990–7001, DOI:10.3168/jds.2017-13907.
- P. Ganesan, G. Karthivashan, S. Park, J. Kim and D. K. Choi, Microfluidization Trends in the Development of Nanodelivery Systems and Applications in Chronic Disease Treatments, Int. J. Nanomed., 2018, 13, 6109–6121, DOI:10.2147/IJN.S178077.
- S. Salatin, J. Barar, M. Barzegar-Jalali, K. Adibkia, F. Kiafar and M. Jelvehgari, Development of a Nanoprecipitation Method for the Entrapment of a Very Water Soluble Drug into Eudragit RL Nanoparticles, Res. Pharm. Sci., 2017, 12(1), 1–14, DOI:10.4103/1735-5362.199041.
- K. Verma, A. Tarafdar, D. Kumar, T. P. Sari, P. C. Badgujar, S. Pareek, E. Assadpour and S. M. Jafari, Microfluidization Based Nano/Sub-Micron Curcumin Formulations for Food and Nutraceuticals: Physico-Functional Characteristics and Safety Aspects, Food Chem., 2025, 485, 144402, DOI:10.1016/j.foodchem.2025.144402.
- K. Verma, A. Tarafdar, R. Maurya, D. Kumar, P. C. Badgujar, K. K. Kondepudi and N. Dilbagh, Characterization, Cytotoxicity, and Stability Evaluation of Novel Nanocurcumin Functionalized Cream Powder Under Accelerated Storage Conditions, Powder Technol., 2023, 428, 118809, DOI:10.1016/j.powtec.2023.118809.
- K. Verma, A. Tarafdar, D. Kumar, Y. Kumar, J. S. Rana and P. C. Badgujar, Formulation and Characterization of Nano-Curcumin Fortified Milk Cream Powder Through Microfluidization and Spray Drying, Food Res. Int., 2022, 160, 111705, DOI:10.1016/j.foodres.2022.111705.
- A. C. Pinheiro, M. Lad, H. D. Silva, M. A. Coimbra, M. Boland and A. A. Vicente, Unravelling the Behaviour of Curcumin Nanoemulsions During in Vitro Digestion: Effect of the Surface Charge, Soft Matter, 2013, 9(11), 3147–3154, 10.1039/C3SM27527B.
- A. S. Joshi and A. K. Thakur, Biodegradable Delivery System Containing a Peptide Inhibitor of Polyglutamine Aggregation: A Step Toward Therapeutic Development in Huntington's Disease, J. Pept. Sci., 2014, 20, 630–639, DOI:10.1002/psc.2640.
- H. Fan, W. Tian and X. Ma, Curcumin Induces Apoptosis of HepG2 Cells Via Inhibiting Fatty Acid Synthase, Targeted Oncol., 2014, 9(3), 279–286, DOI:10.1007/s11523-013-0286-5.
- D. D. Kumar, B. Mann, R. Pothuraju, R. Sharma and R. Bajaj and Minaxi, Formulation and Characterization of Nanoencapsulated Curcumin Using Sodium Caseinate and its Incorporation in Ice Cream, Food Funct., 2016, 7(1), 417–424, 10.1039/c5fo00924c.
- R. Sadeghi, S. Gh. Etemad, E. Keshavarzi and M. Haghshenasfard, Investigation of Alumina Nanofluid Stability by UV–Vis Spectrum, Microfluid. Nanofluidics, 2015, 18(5–6), 1023–1030, DOI:10.1007/s10404-014-1491-y.
- S. Wohlfart, S. Gelperina and J. Kreuter, Transport of Drugs Across the Blood–Brain Barrier by Nanoparticles, J. Contr. Release, 2012, 161, 264–273, DOI:10.1016/j.jconrel.2011.08.017.
- H. D. Silva, M. A. Cerqueira and A. A. Vicente, Nanoemulsions for Food Applications: Development and Characterization, Food Bioprocess Technol., 2012, 5(3), 854–867, DOI:10.1007/s11947-011-0683-7.
- R. Raviadaran, D. Chandran, L. H. Shin and S. Manickam, Optimization of Palm Oil in Water Nano-Emulsion with Curcumin Using Microfluidizer and Response Surface Methodology, LWT–Food Sci. Technol., 2018, 96, 58–65, DOI:10.1016/j.lwt.2018.05.022.
- J. Zhao, X. B. Sun, F. Ye and W. X. Tian, Suppression of Fatty Acid Synthase, Differentiation and Lipid Accumulation in Adipocytes by Curcumin, Mol. Cell. Biochem., 2011, 351, 19–28, DOI:10.1007/s11010-010-0707-z.
- M. A. Obeid, I. Khadra, A. Albaloushi, M. Mullin, H. Alyamani and V. A. Ferro, Microfluidic Manufacturing of Different Niosomes Nanoparticles for Curcumin Encapsulation: Physical Characteristics, Encapsulation Efficacy, and Drug Release, Beilstein J. Nanotechnol., 2019, 10(1), 1826–1832, DOI:10.3762/bjnano.10.177.
- Z. L. Li, S. F. Peng, X. Chen, Y. Q. Zhu, L. Q. Zou, W. Liu and C. M. Liu, Pluronics Modified Liposomes for Curcumin Encapsulation: Sustained Release, Stability and Bioaccessibility, Food Res. Int., 2018, 108, 246–253, DOI:10.1016/j.foodres.2018.03.048.
- S. Taha, I. El-Sherbiny, T. Enomoto, A. Salem, E. Nagai, A. Askar, G. Abady and M. Abdel-Hamid, Improving the Functional Activities of Curcumin Using Milk Proteins as Nanocarriers, Foods, 2020, 9(8), 986 CrossRef CAS PubMed.
- J. Gao, J. Ming, B. He, Y. Fan, Z. Gu and X. Zhang, Preparation and Characterization of Novel Polymeric Micelles for 9-Nitro-20(S)-Camptothecin Delivery, Eur. J. Pharm. Sci., 2008, 34(2–3), 85–93, DOI:10.1016/j.ejps.2008.01.016.
Footnote |
| † Authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2026 |