Open Access Article
Open Access ArticlePolysaccharide-based layer-by-layer edible coatings for shelf-life extension of fresh tomatoes
Aulal Munaab,
Rizky Aflaha c,
Syahla Salsabilaab,
David Ruslimanab,
Kuwat Triyana
c,
Syahla Salsabilaab,
David Ruslimanab,
Kuwat Triyana c,
Aditya Rianjanu
c,
Aditya Rianjanu de,
Condro Wibowo
de,
Condro Wibowo *a and
Hutomo Suryo Wasisto
*a and
Hutomo Suryo Wasisto *bf
*bf
aDepartment of Food Technology, Faculty of Agriculture, Universitas Jenderal Soedirman, Jl. Dr Soeparno No. 63, Purwokerto 53122, Indonesia. E-mail: condro.wibowo@unsoed.ac.id
bPT Foodfuture Icon Nusantara, Purwokerto 53146, Indonesia
cDepartment of Physics, Faculty of Mathematics and Natural Sciences, Universitas Gadjah Mada, Sekip Utara, BLS 21, Yogyakarta 55281, Indonesia
dDepartment of Materials Engineering, Faculty of Industrial Technology, Institut Teknologi Sumatera, Terusan Ryacudu, Way Hui, Jati Agung, Lampung Selatan 35365, Indonesia
eCenter for Green and Sustainable Materials, Institut Teknologi Sumatera, Terusan Ryacudu, Way Hui, Jati Agung, Lampung 35365, Indonesia
fPT Biostark Analitika Inovasi, Bandung 40375, Indonesia. E-mail: h.wasisto@biostark-ai.com
First published on 10th December 2025
Abstract
High yield loss in tomatoes due to suboptimal post-harvest handling and storage demands innovation in preservation technology that is effective and environmentally friendly. Here, we develop polysaccharide-based layer-by-layer edible coatings made of chitosan and hydroxypropyl methylcellulose (chitosan/HPMC) and evaluate their effectiveness for extending the shelf-life of fresh tomatoes using three different application methods (i.e., dipping, air-gun spraying, and manual air-pressure spraying). The coating films are formulated and characterized based on physical, mechanical, and permeability properties. The coated tomatoes have a significantly improved storability of 12 days longer than their uncoated counterpart. Among the investigated application methods, air-gun spraying proves to be the most effective, consistent, and practical method for implementing chitosan/HPMC layer-by-layer on fresh tomatoes. It can limit final weight loss to 7.27%, maintain firmness at 5.44 MPa, and retain ∼68% of the total soluble solids after 24 days of storage. These findings provide an important foundation to design efficient edible coating strategies for sustainable post-harvest preservation.
Sustainability spotlightThis study contributes to sustainable food technology by advancing eco-friendly post-harvest preservation methods. The polysaccharide-based edible coatings developed here are biodegradable, non-toxic, and derived from renewable resources, offering an alternative to synthetic packaging. By effectively extending the shelf life of fresh tomatoes while reducing reliance on plastic-based storage solutions, this approach supports waste reduction, resource efficiency, and improved food system resilience. |
1 Introduction
Improper handling, inadequate storage, and post-harvest limitations in tomato farming lead to yield reductions of 20% to more than 40%.1 This loss not only reduces food availability but also disrupts market stocks and economic stability and causes a waste of resources and an increase in carbon footprint.2–4 Therefore, innovations are needed to overcome these problems, especially for the development of post-harvest preservation technology that is able to extend the storage period and reduce fruit spoilage.5–10 One solution that has been offered and developed by many researchers is the utilization of edible coatings because they can be an ideal, environmentally friendly alternative to reduce the use of conventional plastics.11–13Edible coating is a widely used strategy to protect perishable fresh horticultural products,14–17 such as citrus,18–20 mango,21 pomegranate,22 and mushroom.23 The utilization of edible coatings can prevent weight loss, delay physiological aging, and maintain product quality during storage.15,16 Edible coatings applied to the surface of the fruit can regulate respiration and transpiration and act as a barrier to pathogenic microorganisms.24–27 During their development, edible coatings have been made using natural biopolymers, including proteins, lipids, and polysaccharides, because of their abundance in nature and good biocompatibility.6,9,28–35 Among the available polysaccharides, hydroxypropyl methylcellulose (HPMC) is one of the most widely used due to its ability to form thin films and repel moisture.36–38 Unfortunately, the use of a single biopolymer is often found to result in low coating performance. Thus, an edible coating composed of more than one material has emerged as a promising solution to unlock the potential of the coating film having excellent gas permeability control, mechanical strength, and antimicrobial properties.39
From previous studies, a combined coating film comprising chitosan and HPMC (chitosan/HPMC) has shown synergistic performance, where chitosan acts as an antimicrobial agent10,40–43 and HPMC serves as a film-forming matrix.44,45 To realize this film, the layer-by-layer deposition method is considered promising because it can deploy oppositely charged biopolymers sequentially to form uniform layers. Although chitosan/HPMC layer-by-layer edible coating offers various benefits (e.g., chitosan not only improves the mechanical resistance of the film structure but also regulates the gradual release of active compounds),22,40,42,46–48 it still faces various challenges, such as optimization of mechanical strength, applicability, and production on an industrial scale.45,49,50 Previous studies have used dipping as a typical method to apply edible coatings to fruits.45,46,51–55 Despite its simple process, the dipping method still has several limitations (e.g., low solution efficiency, inhomogeneous coating distribution, possible cross-contamination, poor scalability, and low reproducibility).
Here, we introduce a comparative evaluation of new spraying-based techniques (e.g., air-gun spraying and manual air-pressure spraying) alongside conventional dipping to demonstrate how application methods influence coating performance, film homogeneity, and shelf-life extension on fresh tomatoes. Furthermore, by monitoring various properties of the coated tomatoes during long-term storage (i.e., weight loss, firmness, total soluble solids, vitamin C, and color), a more comprehensive understanding of the effects of different coating application methods on the fruits can be obtained. As a result, this study can be used as a basis for designing efficient coating strategies for sustainable post-harvest preservation.
2 Materials and methods
2.1 Materials
For the main materials, hydroxypropyl methylcellulose (HPMC) powder (purity ≥99%, viscosity grade ∼3000 mPa S) and chitosan flakes were purchased from Prima Chemical Store (Purwokerto, Indonesia) and Chitafood (Yogyakarta, Indonesia), respectively. Since the molecular weight and degree of deacetylation were not specified in the product documentation, we used viscosity grade (for HPMC) and commercial grade (for chitosan) as the primary characterization parameters in this study. Glacial acetic acid purchased from Prima Chemical & Packaging was used as chitosan solvent. Tomato (Solanum lycopersicum ‘Servo’) samples were hand-picked at uniform maturity (light red to red) and with consistent dimensions (diameter of 4–5 cm and weight of 40–70 g) from local farmers in Sumbang, Banyumas, Central Java, Indonesia. Sodium chloride (NaCl) from Merck, Darmstadt, Germany, was used to control the environment in the permeability test. For vitamin C assessment, two reagents—amylum (Merck, Germany) and iodine (Sigma-Aldrich, Singapore)—were employed. All materials and samples were used as received without any further purification or pre-treatment.2.2 Edible film production
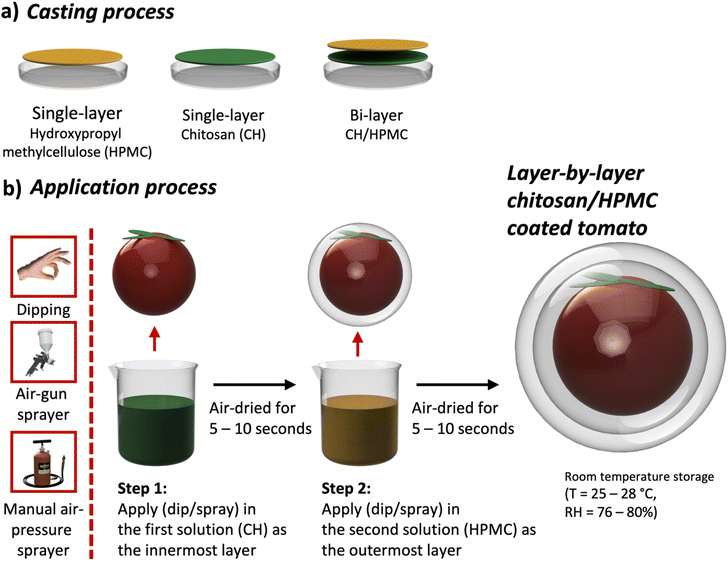
HPMC powder was dissolved in distilled water that had been heated at 45–50 °C for ∼10–15 minutes with three different concentrations of 0.4%, 0.8%, and 1.2% (w/v). After adding HPMC, the mixed solutions were stirred using a hot plate stirrer for ∼15 minutes until 60–70 °C and then cooled to 30 °C to produce the HPMC solution. Separately, chitosan solutions were prepared by dissolving chitosan powder with three different concentrations of 0.5%, 1.0%, and 1.5% (w/v) in 1% (v/v) acetic acid. The chitosan solutions were then stirred using a hot plate stirrer (T = ∼50 °C for ∼15–20 minutes) and subsequently cooled to 30 °C until the solutions became clear. Both HPMC and chitosan solutions were then poured into separate plastic molds with a thickness of ∼2–3 cm and then dried in a food dehydrator at ∼30 °C for 12–14 hours to obtain HPMC and chitosan single-layer films. In addition to those single-layer films, bi-layer chitosan-combined HPMC (chitosan/HPMC) films were prepared using a layer-by-layer technique by depositing the chitosan and HPMC solutions sequentially. All fabricated films are shown in Table 1 and illustrated in Fig. 1a. All concentrations used in this study were determined from preliminary trials based on previous studies.45,56 These concentrations were selected to achieve proper polymer dispersion and coating uniformity without causing excessive viscosity that may hinder spraying performance.| Material base | Layer type |
|---|---|
| HPMC | Single-layer |
| Chitosan | Single-layer |
| Chitosan/HPMC | Bi-layer |
2.3 Edible film characterization
Each single-layer film was placed on a white paper surface for color analysis based on the ASTM E1347 standard using a color reader (Chnspec CS-10) at 10 different points. The results were represented as L, a*, and b* values and subtracted from the white plate standard to obtain ΔL, Δa, and Δb, which were then calculated to obtain the ΔE value using eqn (1):
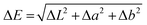 | (1) |
After being cut to a size of 4 cm × 1 cm, the film was measured in terms of its thickness (d) and subsequently inserted into a UV-Vis spectrophotometer to read the absorbance at a wavelength of 600 nm (A600) for obtaining its opacity using eqn (2):48
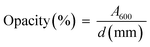 | (2) |
Water vapor permeation (WVP) values were generated using the ASTM E96 standard with a circular sample with a diameter of 4.5 cm. A film sheet was used for the cup containing silica gel and placed in a modified chamber using saturated sodium chloride (RH = 60–70%, ΔP = 4245 Pa). The cup was weighed (Δw) regularly at certain intervals (Δt) and calculated using eqn (3):
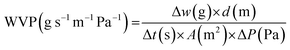 | (3) |
To evaluate the mechanical properties of the film, tensile strength (TS) and elongation at break (EAB) values were determined using ASTM D882 standards. The mechanical strength testing was carried out using a rectangular membrane cut to dimensions of 10 cm × 2 cm and performed on a texture analyzer (Stable Microsystems TA-XT Plus) under conditions of 50 mm initial grip separation (L), 50 mPa force, and 50 mm s−1 constant velocity. Force (F) and final length before break (L′) data were recorded as test result data. The TS and EAB values were then calculated using eqn (4) and (5), respectively:
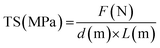 | (4) |
 | (5) |
Surface morphology, chemical composition, and wettability were studied on the film to obtain its optimum coating order. The surface morphology of the edible coating film was observed using scanning electron microscopy (SEM, JEOL JSM 6510) by cutting the sample into a 2 cm × 2 cm size. Morphological observations were made in the form of surface structure and uniformity. The chemical composition of the film was analyzed using Fourier-transform infrared (FTIR) spectroscopy (Thermo Scientific Nicolet iS10) in the range of 400–4000 cm−1. The obtained spectra were used to identify the functional groups of the films and observe the molecular interactions between HPMC and chitosan, as well as evaluate the coating integrity. The wettability of the film was evaluated in terms of its water contact angle (WCA) using the sessile drop method (Fumalife). The 6 µL of deionized water was gently placed on the film surface using a micro-syringe, and images were captured at 0–30 s intervals using an optical camera. The resulting images from the WCA observation were then analyzed using ImageJ software. The WCA was measured in three repetitions (n = 3) for each sample, and the standard deviation (SD) of the repetition calculation was used as the measurement error.
2.4 Tomato applications
Tomatoes were harvested from local farmers (Sumbang, Banyumas, Central Java, Indonesia) and strictly selected based on color (light red to red), appearance (no physical damage), a weight of 40–70 g, and a diameter of 4–5 cm. To ensure that microbial effects did not interfere with the evaluation of coating performance, all tomatoes were carefully pre-treated by washing and sanitizing prior to coating application, following standard post-harvest handling practices.57 The selected tomatoes were then divided into nine groups. The different groups represented treatment differences in terms of the sequence (i.e., single-layer HPMC, single-layer chitosan, and bi-layer chitosan/HPMC) and application technique (i.e., dipping, air-gun spray, and manual air-pressure spray). Tomatoes were washed and air-dried prior to coating, as illustrated in Fig. 1b. All tomato samples were then put into plastic trays and covered with plastic nets to prevent pest infestation after receiving the treatments. Those samples were stored at room temperature (T = 25–28 °C; RH = 76–80%; with no light exposure). Observations were carried out for 24 days, and evaluations were made every 6 days.2.5 Tomato assessment
Tomatoes were evaluated based on physical (i.e., color, weight loss, and firmness) and chemical (i.e., total soluble solids and vitamin C) properties every 6 days. First, the surface color was observed using a color reader (Chnspec CS-10). Observations were made at 5 random places on the surface of the tomato. The data obtained in the form of L, a*, and b* were calculated as Minolta color values using eqn (6):
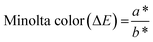 | (6) |
These results were then compared with the United States Department of Agriculture (USDA) classification of tomato fruit maturity stages in Table 2. Tomatoes were weighed using an analytical balance to determine the final weight on the day of observation (b) and compared with the initial weight from the first day of storage (a). Based on these values, the amount of weight loss (WL) can be determined based on the calculation using eqn (7):
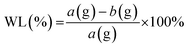 | (7) |
| Minolta color values (a*/b*) | USDA tomato color stages |
|---|---|
| −0.59 to −0.47 | Green |
| −0.47 to −0.27 | Breaker |
| −0.27 to 0.08 | Turning |
| 0.08 to 0.60 | Pink |
| 0.60 to 0.95 | Light red |
| 0.96 to 1.21 | Red |
To determine the firmness of the tomatoes, a texture analyzer (Stable microsystem TA.XT plus) with a 5 mm diameter cylindrical probe was used with defined settings (i.e., pre-test speed of 3.0 mm s−1, test speed of 1.0 mm s−1, post-test speed of 30 mm s−1, and penetration distance of 3 mm). For chemical analysis, tomatoes were cut and crushed into pulp. A total of 1–2 mL of tomato pulp was then added to a digital hand refractometer (Alla France 950.0100 PPT-ATC) to obtain the total soluble solids (TSS). TSS data were collected in units of °Brix. To determine the vitamin C value, tomato pulp was diluted in distilled water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 v/v) and filtered using filter paper to produce tomato extract. A total of 20–30 mL of tomato extract was mixed with 1% amylum indicator and titrated using iodine 0.01 N until the color turned blue. The volume of iodine (Viodine) was used to calculate vitamin C using eqn (8):45
10 v/v) and filtered using filter paper to produce tomato extract. A total of 20–30 mL of tomato extract was mixed with 1% amylum indicator and titrated using iodine 0.01 N until the color turned blue. The volume of iodine (Viodine) was used to calculate vitamin C using eqn (8):45
| Vitamin C (%) = Viodine × 0.88 × dilution ratio | (8) |
2.6. Statistical analysis
Data obtained from observation were analyzed using one-way analysis of variance (ANOVA) with a p ≤ 0.05 significance level and a post hoc test using Duncan's multiple range test (DMRT) for parametric data. Non-parametric data were analyzed using the Kruskal–Wallis test with a significance level of p ≤ 0.05 and the Mann–Whitney U-test as a post hoc test method. All statistical analyses were conducted using Statistical Product and Service Solutions (SPSS) software.3 Results and discussion
3.1 Edible film characteristics
Fig. 2a shows the water vapor permeability (WVP) test results of HPMC films with various concentrations of 0.4%, 0.8%, and 1.2%. The WVP values obtained are 6.98 × 10−11, 5.80 × 10−11, and 3.85 × 10−11 g s−1 m−1 Pa−1 for HPMC concentrations of 0.4%, 0.8%, and 1.2%, respectively. The WVP value of the HPMC films decreased as the concentration increased. This phenomenon may occur when the concentration of the matrix solution is increased and the film structure becomes more compact, limiting its interaction with water.58 The water vapor permeability (WVP) test is known as a parameter to observe material and water interaction.43,59 On the other hand, the oxygen transmission rate (OTR) is typically used as one of the important parameters for evaluating the gas barrier functionality of edible films.60–63 However, in this study, we focused on WVP as the primary barrier property, since moisture loss is the dominant factor influencing tomato deterioration. Additionally, the polysaccharide coatings applied here are inherently more effective against water vapor transfer compared to oxygen diffusion. Fig. 2b and c show the color and opacity measurement results of the HPMC films. These two parameters (i.e., color and opacity) are crucial to evaluate, as they are influential in the product appearance and good consumer acceptance. As the concentration of HPMC increases, the resulting ΔE value becomes higher. The increase in the ΔE value indicates a decrease in the clarity of the film. These results are then supported by the opacity value, which continues to increase with values of 4.24%, 5.08%, and 6.52% for HPMC concentrations of 0.4%, 0.8%, and 1.2%, respectively. These results show that both parameters increase with increasing concentration of HPMC used in film fabrication, which is believed to be due to an increase in the solid content of the HPMC film.64In addition to the appearance of the edible film, mechanical strength is also an important parameter to be considered, as it affects the film resistance to physical contact in its application as an edible coating. The mechanical strength of the HPMC-based edible film was evaluated based on the tensile strength and elongation at break values, as shown in Fig. 2d. The tensile strength values are (0.26 ± 0.11) MPa, (0.50 ± 0.11) MPa, and (0.59 ± 0.24) MPa, while the elongation at break values are (3.74 ± 3.25)%, (3.82 ± 1.70)%, and (5.06 ± 2.20)%, for HPMC films with concentrations of 0.4%, 0.8%, and 1.2%, respectively. The increase in both values indicates that the mechanical strength of the film rises as the concentration of HPMC used increases. This phenomenon is very likely to occur because, with an increase in the concentration used, the density of the resulting film increases, making the resulting film stronger. The appearance of the fabricated HPMC-based edible film for each concentration can be observed based on the results of the photographs shown in Fig. 2e. The detailed data of HPMC film characteristics can be found in Table S1 in the SI.
Fig. 3a shows the WVP measurement results of chitosan-based edible films with various concentrations, with values of 1.26 × 10−11, 1.22 × 10−11, and 1.06 × 10−11 g s−1 m−1 Pa−1 for chitosan concentrations of 0.5%, 1.0%, and 1.5%, respectively. Similar to the WVP results of HPMC-based films, the WVP values of chitosan-based films decreased with increasing concentration, which is believed to be due to the same reason as that of HPMC-based films.58 However, the WVP value of chitosan-based films for each concentration is much lower than that of HPMC-based films, indicating that the chitosan film has a tighter structure. This phenomenon is supported by chitosan's ability to create a vapor barrier by forming a zigzag path to resist water diffusion.65 Fig. 3b and c show the color and opacity measurement results of chitosan-based films. Both values are almost equal to those of HPMC-based film and increase with increasing concentration, as seen in HPMC-based films. In contrast to HPMC-based films, the different color pigments of chitosan are believed to be the main factor determining the color and opacity of the fabricated films.66 Chitosan contains the crustacean-derived pigment astaxanthin. This pigment causes an increase in the ΔE value of the fabricated film, which then results in a decrease in the opacity value when compared to that of the HPMC-based film.
The tensile strength and elongation at break values of the chitosan-based film can be seen in Fig. 3d. The tensile strength values of the chitosan-based film are (0.23 ± 0.07) MPa, (11.20 ± 2.51) MPa, and (0.29 ± 0.05) MPa, with elongation at break values of (13.34 ± 1.92)%, (0.32 ± 0.08)%, and (13.79 ± 4.87)% for concentrations of 0.5%, 1.0%, and 1.2%, respectively. It is clearly seen that the tensile strength values of chitosan-based films with various concentrations are lower than those of HPMC-based films. In contrast, the elongation at break value of chitosan is much higher when compared to that of the HPMC-based film. These results indicate that chitosan has less strength but much higher elasticity. The mechanical properties are necessary to evaluate because in the practical applications, the polymer may face different stresses.67,68 The appearance of the chitosan-based film can be seen in Fig. 3e. The detailed values of chitosan film characteristics are presented in Table S2 in the SI.
3.2 Layer-by-layer characteristics
Fig. 4 shows the Fourier-transform infrared (FTIR) spectra of the fabricated edible films. The HPMC monolayer has characteristic peaks at 3415 cm−1, 2902 cm−1, and 1644 cm−1 associated with O–H, C–H, and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibrations, respectively, which indicate the presence of hydroxyl, methyl, and carbonyl groups. The peaks in the fingerprint region (500–1500 cm−1) are associated with C–H (1454 cm−1 and 956 cm−1) and C–O–C (1327 cm−1 and 1052 cm−1) vibrational bands, which are recognized as methyl and ether bonds in the matrix. These results are similar to previous studies.69 For the chitosan monolayer, the peaks at 3167 cm−1, 1605 cm−1, and 1529 cm−1 define O–H and N–H stretching, C
O stretching vibrations, respectively, which indicate the presence of hydroxyl, methyl, and carbonyl groups. The peaks in the fingerprint region (500–1500 cm−1) are associated with C–H (1454 cm−1 and 956 cm−1) and C–O–C (1327 cm−1 and 1052 cm−1) vibrational bands, which are recognized as methyl and ether bonds in the matrix. These results are similar to previous studies.69 For the chitosan monolayer, the peaks at 3167 cm−1, 1605 cm−1, and 1529 cm−1 define O–H and N–H stretching, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching, and N–H bending vibrations associated with hydroxyl, amide, and amine groups. These findings are in agreement with previously reported investigation results.45 With the combination of HPMC as the outer surface on chitosan, the same functional groups as the monolayer were found in the spectra of the chitosan/HPMC bi-layer film, namely hydroxyl, amine, methyl, and ether. However, broader peaks near 3000–3500 cm−1 were found in the chitosan/HPMC bi-layer, which are responsible for O–H and N–H stretching vibrations. This broadening, accompanied by a slight shift to lower wavenumbers, suggests the strengthening of hydrogen bonding between the hydroxyl group of HPMC and the amino/hydroxyl groups of chitosan. Additionally, noticeable shifts in the amide I (≈1600–1650 cm−1) and amide II (≈1500–1580 cm−1) bands indicate electrostatic interactions between the protonated –NH3+ groups of chitosan and the hydroxyl-rich HPMC matrix. These spectra confirm that the layer-by-layer assembly enhances intermolecular bonding and contributes to the formation of a more compact and stable bi-layer structure.65
O stretching, and N–H bending vibrations associated with hydroxyl, amide, and amine groups. These findings are in agreement with previously reported investigation results.45 With the combination of HPMC as the outer surface on chitosan, the same functional groups as the monolayer were found in the spectra of the chitosan/HPMC bi-layer film, namely hydroxyl, amine, methyl, and ether. However, broader peaks near 3000–3500 cm−1 were found in the chitosan/HPMC bi-layer, which are responsible for O–H and N–H stretching vibrations. This broadening, accompanied by a slight shift to lower wavenumbers, suggests the strengthening of hydrogen bonding between the hydroxyl group of HPMC and the amino/hydroxyl groups of chitosan. Additionally, noticeable shifts in the amide I (≈1600–1650 cm−1) and amide II (≈1500–1580 cm−1) bands indicate electrostatic interactions between the protonated –NH3+ groups of chitosan and the hydroxyl-rich HPMC matrix. These spectra confirm that the layer-by-layer assembly enhances intermolecular bonding and contributes to the formation of a more compact and stable bi-layer structure.65
The surface morphology of the fabricated films is shown in Fig. 5a according to SEM images. Slight cracks are identified on the single-layer HPMC and rough surfaces are found on the single-layer chitosan and bi-layer chitosan/HPMC. Among them, the HPMC film depicts a more uniform, dense, and smooth surface. Such characteristics can provide a stable and better barrier as a coating layer. While the chitosan film has almost similar results, it is slightly rougher, and some irregularities, such as the presence of small porous structures, are detected. On the other hand, the bi-layer chitosan/HPMC shows a rough and structured surface morphology, potentially indicating a multilayered structure due to electrostatic interactions between layers. In particular, chitosan as the base layer provides a more compact, dense, and less rough foundation, while HPMC as the outer layer results in a smoother and less lumpy surface. Therefore, chitosan/HPMC with HPMC as the outer layer appears to have a better structure. Although morphological properties are not the only crucial factors to determine coating suitability, they still need to be well controlled to accomplish better surface characteristics (i.e., homogeneous, smooth, compact, and uniform). Earlier studies have found that adding plasticizers (e.g., glycerol, sorbitol, and gelatin) in certain amounts can prevent cracks and produce a smoother surface on the films.57,70 Thus, it can enhance other properties such as better adhesion, less permeation, and separation.
Fig. 5b shows the water contact angle (WCA) test results of all three films (i.e., HPMC, chitosan, and chitosan/HPMC films) to evaluate their wettability. HPMC and chitosan films exhibit different wettability behavior. The HPMC film has a low WCA value of (69 ± 5)°, indicating its hydrophilic properties. This result is consistent with its smooth and continuous surface morphology, which can be one factor that influences surface wettability. On the other hand, chitosan has a higher WCA value of (88 ± 5)°, indicating its more hydrophobic properties. Apart from the rougher surface morphology, the high WCA of the chitosan film is also caused by its chemical structure. Here, chitosan only has amino bonds, making it less likely to interact with water.71,72
Meanwhile, the chitosan/HPMC film shows a moderate WCA value (located between those from HPMC and chitosan films), which amounts to (81 ± 5)°. This WCA value indicates that the deposition of chitosan followed by HPMC results in enhanced interfacial interaction, leading to a more uniform film with optimal wettability. Thus, the chitosan/HPMC film could potentially offer a controlled barrier effect, limiting dehydration while allowing gas exchange. These findings suggest that chitosan/HPMC coatings provide optimal conditions between surface wettability and film integrity, making them the most suitable treatment for preserving tomato freshness. Although the mechanistic characterizations (i.e., SEM, FTIR, and wettability) were carried out on standalone bi-layer films rather than tomato surfaces, the results provide a strong indication of the coating behavior when applied to target fruits (tomatoes).
3.3 Application to tomatoes
The different coating application methods (dipping, air-gun spraying, and manual air-pressure spraying) showed different weight loss patterns. On day 24, the manual spraying method resulted in the highest weight loss of 11.78%, while the dipping and air-gun spraying methods recorded mass losses of 7.42% and 7.27%, respectively (p > 0.05). Although the difference was not statistically significant, this phenomenon of different mass losses can be attributed to the variation in the nozzle diameter. Manual air-pressure spraying uses a large diameter nozzle, which tends to produce an uneven and excessive coating, which can accelerate water loss. In contrast, an air-gun with a small nozzle (0.6 mm) produces more homogeneous droplets. This finding is consistent with a previous study,56 which emphasized the importance of controlling the nozzle size, spraying distance, pressure, and flow rate for optimal coating formation.
The firmness observation in Fig. 6b shows that for each coating method, the firmness value decreases. On day 6, the firmness values were (8.6 ± 0.7) N, (9.6 ± 0.0) N, and (10.3 ± 0.0) N, and on day 24, they became (6.3 ± 0.0) N, (5.4 ± 0.0) N, and (5.2 ± 0.0) N for the dipping, air-gun spraying, and manual air-pressure spraying coating methods, respectively. Although the dipping coating showed the smallest firmness value on day 6 compared to the other methods, it did not decrease significantly on day 24, making it the highest firmness value. Meanwhile, for air-gun spraying, its highest firmness value was found on day 6 and started to decrease significantly on day 24. This phenomenon occurs because the coating film produced by the dipping method possesses a higher thickness than that processed by the air-gun spraying method. Although air-gun spraying can provide a more even coating, it cannot be as thick as the dipping method. This makes tomatoes coated by the dipping method maintain firmness better. The increase in firmness durability observed after layer-by-layer coating is in line with the results of a previous study conducted on Japanese pears (Kosui).50
Meanwhile, the vitamin C content of each method also gives striking results, where the trend shows that the air-gun spraying method has the advantage in maintaining nutrient stability. The trend of the highest vitamin C content from the air-gun spraying method persists until the observation on day 24, which is believed because this method can produce a very thin and even layer that can provide optimal protection to tomatoes, aligning with the weight loss observation. On the other hand, the highest decrease in vitamin C occurs in the dipping method, which is assumed to produce a layer that is too thick and can prevent optimal gas exchange. This excess coating thickness also has the potential to create an internal environment that accelerates vitamin degradation due to oxidative stress or atmospheric imbalance.57,75
The highest Minolta color value was achieved by the air-gun spraying method (1.84), followed by manual air-pressure spraying (1.62) and dipping (1.07). While all variants are still within the “ripe red” range according to the USDA, the coating produced by the air-gun is more homogeneous and thinner, allowing for reasonable ripening without introducing off-flavor effects or immaturity. The photographs of the coated tomatoes using different application methods are displayed in Fig. 6f. In contrast, thick layers from the dipping end delay ripening due to limited gas exchange and light penetration.76
These differences are linked to coating thickness uniformity and gas-moisture regulation capability. Dipping produced thicker coating accumulation in certain areas, leading to anaerobic zones and tissue softening. Manual air-pressure spraying provided thinner but less uniform coatings, resulting in variability across samples. In contrast, air-gun spraying provided finer and more consistent atomization, a continuous and smoother layer, better automation, reduced material waste, and improved process control. Its coating uniformity also contributed to enhanced barrier properties. Therefore, air-gun spraying has been identified as a robust method to realize layer-by-layer applications on an industrial scale to extend the shelf-life of horticultural products. To contextualize these findings, a comparative summary with recent tomato-coating studies is provided in Table S4 in the SI. This comparison emphasizes the key advances of the current work, particularly in demonstrating that the application method plays a decisive role in coating uniformity, barrier functionality, and shelf-life enhancement. Overall, while most previous studies relied solely on static immersion, this study introduces a novel comparison of three application methods and demonstrates that spraying, particularly air-gun spraying, provides similar physical and quality improvement and achieves a longer shelf-life duration compared to some bioactive-enriched formulations. This highlights the practical applicability and process efficiency of the present work.
Despite the successful demonstration of the effective chitosan/HPMC coating in extending the shelf-life of tomatoes, the effects of particle size modification in the mixed solutions on the coating characteristics are of interest for feasibly improving the application coating performance. Here, detailed particle analysis is therefore needed, where different material characterization methods (e.g., dynamic light scattering (DLS)) can be conducted to determine the particle size distributions.18,20,55,65,77,78 Additionally, for further study, microbial analysis is considered an important action for post-harvest tomato preservation to provide more quantitative data on possibly detected microorganisms, besides the physicochemical and quality attributes of coated tomatoes. Future optimization regarding mechanical conveyance and continuous spraying integration will further enhance cost-efficiency, enabling scalable adoption in fresh-produce supply chains.
4 Conclusions
The development and application of polysaccharide-based layer-by-layer edible coatings have been successfully conducted using a combination of chitosan and hydroxypropyl methylcellulose (HPMC), which can significantly improve the quality and shelf-life of fresh tomatoes up to 12 days longer than their uncoated counterpart. Among three different application methods (i.e., dipping, air-gun spraying, and manual air-pressure spraying), air-gun spraying has been proven to be the most effective, consistent, and practical method for implementing chitosan/HPMC layer-by-layer on fresh tomatoes. It can limit final weight loss to 7.27%, maintain firmness at 5.44 MPa, and retain ∼68% of the total soluble solids after 24 days of storage. This method also has strong potential to be applied to other climacteric fruits with similar deterioration behavior such as papaya, mango, and eggplant due to their high respiration rate, sensitivity to water loss, and perishability under ambient storage.Author contributions
Aulal Muna: conceptualization, investigation, formal analysis, visualization, writing – original draft, and writing – review & editing. Rizky Aflaha: investigation, visualization, writing – original draft, and writing – review & editing. Syahla Salsabila and David Rusliman: investigation. Kuwat Triyana: supervision, resources. Aditya Rianjanu: validation and writing – review & editing. Hutomo Suryo Wasisto and Condro Wibowo: conceptualization, supervision, validation, investigation, funding acquisition, resources, and writing – review & editing.Conflicts of interest
All authors declare that they have no conflicts of interest.Data availability
All data to support the findings are available within the article, supplementary information (SI) and cited references. Supplementary information: comprises information on physicochemical properties of hydroxypropyl methylcellulose (HPMC) and chitosan edible films with various concentrations, physicochemical properties of the selected chitosan/HPMC edible coating treatments for fresh tomatoes during 24 days of storage, visualization of edible coating effects on fresh tomatoes during 24 days of storage, and comparative summary between this work and the previous studies. Additional information can be provided by corresponding authors upon reasonable request. See DOI: https://doi.org/10.1039/d5fb00555h.Acknowledgements
This research was funded by the Directorate of Higher Education, Research, and Technology, Ministry of Research, Technology, and Higher Education, for the funding project of “Penelitian dan Pengabdian Kepada Masyarakat” year 2023. The authors thank the Taste and Odor Research Center (TOR-C), Universitas Gadjah Mada, which provided several instrumental setups for testing the edible film.References
- A. Beaulah, K. R. Rajadurai, T. Anitha, J. Rajangam and S. Maanchi, Postharvest handling and value added products of tomato to enhance the profitability of farmers, Plant Sci. Today, 2025, 12, 1–13, DOI:10.14719/pst.5794.
- A. Mohan, R. Krishnan, K. Arshinder, J. Vandore and U. Ramanathan, Management of postharvest losses and wastages in the Indian tomato supply chain—A temperature-controlled storage perspective, Sustainability, 2023, 15, 1331, DOI:10.3390/su15021331.
- G. Abera, A. M. Ibrahim, S. F. Forsido and C. G. Kuyu, Assessment on post-harvest losses of tomato (Lycopersicon esculentem Mill.) in selected districts of East Shewa Zone of Ethiopia using a commodity system analysis methodology, Heliyon, 2020, 6, e03749, DOI:10.1016/j.heliyon.2020.e03749.
- H. M. P. C. Kumarihami, N. Kumar, P. Pratibha, A. T. Petkoska and N. Neeraj, Effect of natural materials on thermal properties of food packaging film: An overview, in Natural Materials for Food Packaging, ed. J. Parameswaranpillai, A. Jayakumar, E. K. Radhakrishnan, S. Siengchin and S. Radoor, Wiley-VSH Verlag GmbH, Germany, 2023, vol. 12, pp. 255–274, DOI:10.1002/9783527837304.ch12.
- J. Prasad, N. Kumar, P. Pratibha, R. Jaiswal, A. Yadav, S. P. Sharma, O. A. Fawole and N. Kaushik, Biopolymer based composite packaging: A sustainable approach for fruits and vegetables preservation, Appl. Food Res., 2025, 5, 101211, DOI:10.1016/j.afres.2025.101211.
- N. Kumar and N. Neeraj, Polysaccharide-based component and their relevance in edible film/coating: a review, Nutr. Food Sci., 2019, 49, 793–823, DOI:10.1108/NFS-10-2018-0294.
- A. T. Petkoska, D. Daniloski, N. Kumar, P. Pratibha and A. T. Broach, Biobased materials as a sustainable potential for edible packaging, in Sustainable Packaging: Environmental Footprints and Eco-Design of Product and Processes, ed. S. S. Muthu, Springer, Singapore, 2021, vol. 1, pp. 111–135, DOI:10.1007/978-981-16-4609-6_5.
- A. T. Petkoska, D. Daniloski, N. Kumar, P. Pratibha and A. T. Broach, Active edible packaging: A sustainable way to deliver functional bioactive compounds and nutraceuticals, in Sustainable Packaging: Environmental Footprints and Eco-Design of Products and Processes, ed. S. S. Muthu, Springer, Singapore, 2021, vol. 1, pp. 225–264, DOI:10.1007/978-981-16-4609-6_9.
- N. Feng, J. Zhang, J. Tian, Y. Zhang, M. Li, X. Guo, Q. Han, Y. Wang, A. Gao, Y. Wang, L. Yan, J. Kong and P. Yang, Preserving fruit freshness with amyloid-like protein coatings, Nat. Commun., 2025, 16, 5060, DOI:10.1038/s41467-025-60382-4.
- N. Kumar, P. Pratibha, A. T. Petkoska, E. Khojah, R. Sami and A. Al-Mushhin, Chitosan edible films enhanced with pomegranate peel extract: Study in physical, biological, and thermal properties, Materials, 2021, 14, 3305, DOI:10.3390/ma14123305.
- R. R. Krishnan, A. D. Olasupo, K. H. Patel, I. F. Bolarinwa, M. O. Oke, C. Okafor, C. Nkwonta and I. Ifie, Edible biopolymers with functional additives coatings for postharvest quality preservation in horticultural crops: A review, Front. Sustain. Food Syst., 2025, 9, 1569458, DOI:10.3389/fsufs.2025.1569458.
- X. Yu, J. Zhu, J. Wu, Y. Cheng, Y. Gao, Y. Liu and F. Jiang, Edible coating based on Konjac glucomannan loading Ocimum gratissimum essential oil for postharvest preservation of orange, Polymers, 2025, 17, 1217, DOI:10.3390/polym17091217.
- P. Chavan, K. Lata, T. Kaur, A. R. Jambrak, S. Sharma, S. Roy, A. Sinhmar, R. Thory, G. P. Singh, K. Aayush and A. Rout, Recent advances in the preservation of postharvest fruits using edible films and coatings: A comprehensive review, Food Chem., 2023, 418, 135916, DOI:10.1016/j.foodchem.2023.135916.
- G. Romanazzi and M. Moumni, Chitosan and other edible coatings to extend shelf life, manage postharvest decay, and reduce loss and waste of fresh fruits and vegetables, Curr. Opin. Biotechnol., 2022, 78, 102834, DOI:10.1016/j.copbio.2022.102834.
- B. Maringgal, N. Hashim, I. S. M. A. Tawakkal and M. T. M. Mohamed, Recent advance in edible coating and its effect on fresh/fresh-cut fruits quality, Trends Food Sci. Technol., 2020, 96, 253–267, DOI:10.1016/j.tifs.2019.12.024.
- J. G. de Oliveira Filho, M. Miranda, M. D. Ferreira and A. Plotto, Nanoemulsions as edible coatings: A potential strategy for fresh fruits and vegetables preservation, Foods, 2021, 10, 2438, DOI:10.3390/foods10102438.
- S. K. Paul, Edible films and coatings for fruits and vegetables, in Reference Module in Materials Science and Materials Engineering, ed. S. Hashimi and I. A. Choudhury, Elsevier, Netherlands, 2020, Vol. 5, pp. 363–376, DOI:10.1016/B978-0-12-803581-8.11509-7.
- N. Kumar, A. Upadhyay and S. Shukla, Effects of essential oils and ultrasonic treatments on properties of edible coatings and their application on citrus fruits, Starch, 2023, 76, 2300104, DOI:10.1002/star.202300104.
- N. Kumar, A. Upadhyay, S. Shukla, V. K. Bajpai, M. Kieliszek, A. Yadav and V. Kumaravel, Next generation edible nanoformulations for improving post-harvest shelf-life of citrus fruit, J. Food Meas. Char., 2024, 18, 1825–1856, DOI:10.1007/s11694-023-02287-8.
- N. Kumar, A. A. Khan, D. Pyngrope, A. M. Alanazi, A. Upadhyay and S. Shukla, Development and characterization of novel starch (mango kernel and litchi seed) based active edible coatings and films using ultrasonication: Effect on postharvest shelf life of Khasi mandarins, Sustain. Chem. Pharm., 2024, 39, 101610, DOI:10.1016/j.scp.2024.101610.
- N. Kumar, P. Pratibha, A. Upadhyay, A. T. Petkoska, M. Gniewosz and M. Kieliszek, Extending the shelf life of mango (Mangifera indica L.) fruits by using edible coating based on xanthan gum and pomegranate peel extract, J. Food Meas. Char., 2023, 17, 1300–1308, DOI:10.1007/s11694-022-01706-6.
- N. Makhathini, N. Kumar and O. A. Fawole, Enhancing circular bioeconomy: Alginate-cellulose nanofibre films/coatings functionalized with encapsulated pomegranate peel extract for postharvest preservation of pomegranate arils, Int. J. Biol. Macromol., 2025, 309, 142848, DOI:10.1016/j.ijbiomac.2025.142848.
- N. Kumar, P. Pratibha, N. Neeraj and A. T. Petkoska, Application of edible packaging for the preservation of mushrooms, in Food Packaging; Safety, Management, and Quality, ed. W. F. Lai, Nova Publisher, USA, 2022, DOI:10.52305/SEPV4757.
- S. O. Olunusi, N. H. Ramli, A. Fatmawati, A. F. Ismail and C. C. Okwuwa, Revolutionizing tropical fruits preservation: Emerging edible coating technologies, Int. J. Biol. Macromol., 2024, 264, 130682, DOI:10.1016/j.ijbiomac.2024.130682.
- T. T. Pham, L. L. Nguyen, M. S. Dam and L. Baranyai, Application of edible coating in extension of fruit shelf life: Review, AgriEngineering, 2023, 5, 520–536, DOI:10.3390/agriengineering5010034.
- F. J. Blancas-Benitez, B. Montaño-Leyva, L. Aguirre-Güitrón, C. L. Moreno-Hernández, Á. Fonseca-Cantabran, L. del Carmen Romero-Islas and R. R. González-Estrada, Impact of edible coatings on quality of fruits: A review, Food Control, 2022, 139, 109063, DOI:10.1016/j.foodcont.2022.109063.
- N. Kumar, P. Pratibha and N. Neeraj, Functional properties of pomegranate peel in edible coating/film: a review, Int. J. Postharvest Technol. Innovation, 2020, 7, 205–216, DOI:10.1504/IJPTI.2020.110422.
- C. Cannata, C. A. C. Rutigliano, C. Restuccia, G. Muratore, L. Sabatino, E. Geoffriau, C. Leonardi and R. P. Mauro, Effects of polysaccharide-based edible coatings on the shelf life of fresh-cut carrots with different pigmentations, J. Agric. Food Res., 2025, 20, 101765, DOI:10.1016/j.jafr.2025.101765.
- D. M. Lieu, T. T. K. Dang and H. T. Nguyen, Protein and polysaccharide edible coatings: A promising for fruits preservation - recent advances, Food Chem.:X, 2025, 27, 102388, DOI:10.1016/j.fochx.2025.102388.
- Y. Fan, J. Ren, X. Xiao, Y. Cao, Y. Zou, B. Qi, X. Luo and F. Liu, Recent advances in polysaccharide-based edible films/coatings for food preservation: fabrication, characterization, and applications in packaging, Carbohydr. Polym., 2025, 364, 123779, DOI:10.1016/j.carbpol.2025.123779.
- P. S. U. Putra, D. R. Adhika, G. Genecya, M. S. Al Madanie and L. A. T. W. Asri, Evaluation of chitosan-encapsulated lemongrass (Cymbopogon citratus) essential oil nanoemulsion for fruit edible coating, OpenNano, 2025, 24, 100246, DOI:10.1016/j.onano.2025.100246.
- A. Salaria, S. S. Patel, K. Sridhar, P. Chawla and M. Sharma, Extraction and characterization of biopolymers from rice bran oil cake: Development of antimicrobial edible coating for extending shelf-life of strawberry fruit, Food Biosci., 2025, 65, 106053, DOI:10.1016/j.fbio.2025.106053.
- N. Kumar, S. Tripathi, P. Pratibha, M. Mehra, H. Heena and A. T. Petkoska, Whey protein based edible coatings: Recent trends, in Whey Valorirization, ed. A. Poonia and A. T. Petkoska, Springer, Singapore, 2023, vol. 1, pp. 187–209, DOI:10.1007/978-981-99-5459-9_10.
- A. K. A. N. W. M. R. K. Thamarsha, N. Kumar, P. Pratibha, K. Mor, A. Upadhyay, H. P. K. Sudhani and V. S. Pamu, A review of functional properties and applications of legume-based edible coatings, Legume Sci., 2024, 6, e70004, DOI:10.1002/leg3.70004.
- N. Begum, S. K. Paul, P. Kumar, J. K. Sahu and S. A. Husain, Development of tulsi impregnated starch-based edible coating to extend the shelf life of tomatoes, Pharma Innov., 2017, 6, 249–255 Search PubMed.
- M. Liu, P. Zhang, Y. Wu and J. Ouyang, Chitosan-hydroxypropyl methylcellulose and sodium alginate bilayer edible films for chestnut preservation, Food Chem., 2025, 466, 142254, DOI:10.1016/j.foodchem.2024.142254.
- S. Z. Iqbal, M. Hussain, H. Ali, A. Haider, S. Ali, A. Hussain, M. A. Javed and M. Jawaid, Preparation and application of hydroxypropyl methylcellulose blended with beeswax and essential oil edible coating to enhance the shelf life of sweet cherries, Int. J. Biol. Macromol., 2024, 272, 132532, DOI:10.1016/j.ijbiomac.2024.132532.
- Y. Zhang, J. Bonilla, I. J. Joye and L. T. Lim, Physicochemical properties of edible films formed by phase separation of zein and hydroxypropyl methylcellulose, LWT, 2025, 218, 117504, DOI:10.1016/j.lwt.2025.117504.
- L. Wang, Z. Luo, J. Yan, Z. Ban, M. Yang, M. Qi, Y. Xu, F. Wang and L. Li, Ultrasonic nebulization-assisted layer-by-layer assembly based on carboxymethyl chitosan: An emerging alternative for promoting phenylpropanoid metabolism, Ultrason. Sonochem., 2020, 68, 105184, DOI:10.1016/j.ultsonch.2020.105184.
- J. Prasad, N. Kumar and S. P. Sharma, Shelf-life extension of guava and eggplant fruits using chitosan-sodium alginate based antimicrobial composite coating functionalized with clove-soy lecithin nanoemulsion, Plant Sci. Today, 2025, 12, 1–11, DOI:10.14719/pst.7980.
- N. Kumar, N. Neeraj, P. Pratibha and M. Singla, Enhancement of storage life and quality maintenance of litchi (Litchi Chinensis Sonn) fruit using chitosan:pullulan blend antimicrobial edible coating, Int. J. Fruit Sci., 2020, 20, S1662–S1680, DOI:10.1080/15538362.2020.1828224.
- S. K. Paul, H. Dutta, S. Chakraborty, G. Deka, S. Sarkar, L. N. Sethi and S. K. Gosh, Development and characterization of bael (Aegle marmelos) leaf extract incorporated chitosan-based functional edible coating and its application on stored tomatoes, Sustainable Food Technol., 2024, 2, 1709–1723, 10.1039/D4FB00160E.
- S. K. Paul, S. Sarkar, L. N. Sethi and S. Ghosh, Effect of variation in composition on water vapor resistance and solubility properties of chitosan-based edible film, Pharma Innov., 2018, 7, 530–535 Search PubMed.
- H. Arnon, R. Granit, R. Porat and E. Poverenov, Development of polysaccharides-based edible coatings for citrus fruits: A layer-by-layer approach, Food Chem., 2015, 166, 465–472, DOI:10.1016/j.foodchem.2014.06.061.
- S. Jurić, M. S. Bureš, K. Vlahoviček-Kahlina, K. S. Stracenski, G. Fruk, N. Jalšenjak and L. M. Bandić, Chitosan-based layer-by-layer edible coatings application for the preservation of mandarin fruit bioactive compounds and organic acids, Food Chem. X., 2023, 17, 100575, DOI:10.1016/j.fochx.2023.100575.
- M. Meena, S. Pilania, A. Pal, S. Mandhania, B. Bhushan, S. Kumar, G. Gohari and V. Saharan, Cu-chitosan nano-net improves keeping quality of tomato by modulating physio-biochemical responses, Sci. Rep., 2020, 10, 21914, DOI:10.1038/s41598-020-78924-9.
- N. Kumar, P. Pratibha, N. Neeraj, A. T. Petkoska, S. A. AL-Hilifi and O. A. Fawole, Effect of chitosan–pullulan composite edible coating functionalized with pomegranate peel extract on the shelf life of mango (Mangifera indica), Coatings, 2021, 11, 764, DOI:10.3390/coatings11070764.
- N. Kumar, P. Pratibha, A. T. Petkoska, E. Khojah, R. Sami and A. A. M. Al-Mushhin, Chitosan edible films enhanced with pomegranate peel extract: Study on physical, biological, thermal, and barrier properties, Materials, 2021, 14, 3305, DOI:10.3390/ma14123305.
- C. Niu, L. Liu, A. Farouk, C. Chen and Z. Ban, Coating of layer-by-layer assembly based on chitosan and CMC: Emerging alternative for quality maintenance of citrus fruit, Horticulturae, 2023, 9, 715, DOI:10.3390/horticulturae9060715.
- N. Hira, O. W. Mitalo, R. Okada, M. Sangawa, K. Masuda, N. Fujita, K. Ushijima, T. Akagi and Y. Kubo, The effect of layer-by-layer edible coating on the shelf life and transcriptome of ‘Kosui’ Japanese pear fruit, Postharvest Biol. Technol., 2022, 185, 111787, DOI:10.1016/j.postharvbio.2021.111787.
- C. Wibowo, S. Salsabila, A. Muna, D. Rusliman and H. S. Wasisto, Advanced biopolymer-based edible coating technologies for food preservation and packaging, Compr. Rev. Food Sci. Food Saf., 2024, 23, e13275, DOI:10.1111/1541-4337.13275.
- K. Y. Perera, S. Sharma, B. Duffy, S. Pathania, A. K. Jaiswal and S. Jaiswal, An active biodegradable layer-by-layer film based on chitosan-alginate-TiO2 for the enhanced shelf life of tomatoes, Food Packag. Shelf Life, 2022, 34, 100971, DOI:10.1016/j.fpsl.2022.100971.
- D. A. Carillo-Lomelí, M. A. Cerqueira, V. Moo-Huchin, A. I. Bourbon, V. G. L. Souza, A. Lestido-Cardama, L. M. Pastrana, Y. M. Ochoa-Fuentes, F. D. Hernádez-Castillo, J. A. Villarreal-Quintanilla and D. J. de Rodríguez, Influence of edible multilayer coatings with Opuntia stenopetala polysaccharides and Flourensia microphylla extract on the shelf-life of cherry tomato (Solanum lycopersicum L.), Sci. Hortic., 2024, 332, 113224, DOI:10.1016/j.scienta.2024.113224.
- M. Younis, R. M. Kamel, M. Abdin, E. El Fadly, M. Saleh, S. M. S. Shawir, D. O. Abdelkarim and M. A. Salama, Prediction of tomato postharvest quality using artificial neural networks: a case study on edible coating treatments, Int. J. Food Sci. Technol., 2025, 60, vvaf165, DOI:10.1093/ijfood/vvaf165.
- M. L. Flores-López, J. M. Vieira, C. M. R. Rocha, J. M. Lagarón, M. A. Cerqueira, D. J. de Rodríguez and A. A. Vicente, Postharvest quality improvement of Tomato (Solanum lycopersicum L.) fruit using nanomultilayer coating containing aloe vera, Foods, 2024, 13, 83, DOI:10.3390/foods13010083.
- W. Silva-Vera, M. Zamorano-Riquelme, C. Rocco-Orellana, R. Vega-Viveros, B. Gimenez-Castillo, A. Silva-Weiss and F. Osorio-Lira, Study of spray system applications of edible coating suspensions based on hydrocolloids containing cellulose nanofibers on grape surface (Vitis vinifera L.), Food Bioprocess Technol., 2018, 11, 1575–1585, DOI:10.1007/s11947-018-2126-1.
- S. K. Paul, S. Sarkar, L. N. Sethi and S. K. Ghosh, Development of chitosan based optimized edible coating for tomato (Solanum lycopersicum) and its characterization, J. Food Sci. Technol., 2018, 55, 2446–2456, DOI:10.1007/s13197-018-3162-6.
- H. R. Arifin, M. Djali, B. Nurhadi, S. A. Hasim, A. Hilmi and A. V. Puspitasari, Improved properties of corn starch-based bio-nanocomposite film with different types of plasticizers reinforced by nanocrystalline cellulose, Int. J. Food Prop., 2022, 25, 509–521, DOI:10.1080/10942912.2022.2052085.
- N. Kumar, D. Daniloski, P. Pratibha, N. Neeraj, N. D’cunha, N. Naumovski and A. T. Petkoska, Pomegranate peel extract – A natural bioactive addition to novel active edible packaging, Food Res. Int., 2022, 156, 111378, DOI:10.1016/j.foodres.2022.111378.
- V. T. T. Thuy, L. T. Hao, H. Jeon, J. M. Koo, J. Park, E. S. Lee, S. Y. Hwang, S. Choi, J. Park and D. X. Oh, Sustainable, self-cleaning, transparent, and moisture/oxygen-barrier coating films for food packaging, Green Chem., 2021, 23, 2658–2667, 10.1039/D0GC03647A.
- M. Sultan, O. M. Hafez, M. A. Saleh and A. M. Youssef, Smart edible coating films based on chitosan and beeswax-pollen grains for the postharvest preservation of Le Conte pear, RSC Adv., 2021, 11, 9572–9585, 10.1039/D0RA10671B.
- Y. Du, F. Yang, H. Yu, W. Yao and Y. Xie, Controllable fabrication of edible coatings to improve the match between barrier and fruits respiration through layer-by-layer assembly, Food Bioprocess Technol., 2022, 15, 1778–1793, DOI:10.1007/s11947-022-02848-7.
- H. Nguyen, T. H. Tran, L. T. Hao, H. Jeon, J. M. Koo, G. Shin, D. S. Hwang, S. Y. Hwang, J. Park and D. X. Oh, Biorenewable, transparent, and oxygen/moisture barrier nanocellulose/nanochitin-based coating on polypropylene for food packaging applications, Carbohydr. Polym., 2021, 271, 118421, DOI:10.1016/j.carbpol.2021.118421.
- F. Zhao, L. Jiang, C. Wang, S. Li, D. Sun, Q. Ma, Z. Yu, Y. Liu and W. Jiang, Flexibility, dissolvability, heat-sealability, and applicability improvement of pullulan-based composite edible films by blending with soluble soybean polysaccharide, Ind. Crops Prod., 2024, 215, 118693, DOI:10.1016/j.indcrop.2024.118693.
- X. Yu, Q. Liu, Z. Jin and A. Jiao, Preparation and characterization of hydroxypropyl methylcellulose/hydroxypropyl starch composite films reinforced by chitosan nanoparticles of different sizes, Mater. Today Commun., 2023, 35, 105714, DOI:10.1016/j.mtcomm.2023.105714.
- J. G. de Oliveira Filho, M. R. V. Bertolo, S. S. Fernandes, A. C. Lemes, G. da Cruz Silva, S. B. Junior, H. M. C. de Azeredo, L. H. C. Matosso and M. B. Egea, Intelligent and active biodegradable biopolymeric films containing carotenoids, Food Chem., 2024, 434, 137454, DOI:10.1016/j.foodchem.2023.137454.
- R. K. Dhaka, N. Kumar, P. Pratibha and A. Upadhyay, Optimization, characterization, and influence of microfluidization on almond gum-based composite edible film, Starch, 2021, 73, 2000101, DOI:10.1002/star.202000101.
- N. Kumar, P. Pratibha, A. T. Petkoska and M. Singla, Natural gums for fruits and vegetables preservation: A Review, in Gums, Resins and Latexes of Plant Origin: Chemistry, Biological Activites and Uses, ed. H. M. Murthy, Springer Nature, Switzerland, 2022, vol. 1, pp. 81–116, DOI:10.1007/978-3-030-91378-6_4.
- N. M. da Silva, J. de Matos Fonseca, H. K. Feldhaus, L. S. Soares, G. A. Valencia, C. E. Maduro de Campos, M. Di Luccio and A. R. Monteiro, Physical and morphological properties of hydroxypropyl methylcellulose films with curcumin polymorphs, Food Hydrocolloids, 2019, 97, 105217, DOI:10.1016/j.foodhyd.2019.105217.
- T. R. Putri, A. Adhitasari, V. Paramita, M. E. Yulianto and H. D. Ariyanto, Effect of different starch on the characteristics of edible film as functional packaging in fresh meat or meat products: A review, Mater. Today Proc., 2023, 87, 192–199, DOI:10.1016/j.matpr.2023.02.396.
- S. Takeshita, S. Zhao, W. J. Malfait and M. M. Koebel, Chemistry of chitosan aerogels: Three-dimensional Pore control for tailored applications, Angew. Chem., Int. Ed., 2021, 60, 9828–9851, DOI:10.1002/anie.202003053.
- L. Katriani, R. Aflaha, A. H. As’ari, P. Nurwantoro, R. Roto and K. Triyana, Nanofiber-coated quartz crystal microbalance with chitosan overlay for highly sensitive room temperature ammonia gas sensor, Microchem. J., 2024, 206, 111532, DOI:10.1016/j.microc.2024.111532.
- H. T. Duguma, Potential applications and limitations of edible coatings for maintaining tomato quality and shelf life, Int. J. Food Sci. Technol., 2022, 57, 1353–1366, DOI:10.1111/ijfs.15407.
- S. Ali, M. A. Ullah, A. Nawaz, S. Naz, A. A. Shah, G. Gohari, F. Razavi, G. Khaliq and K. Razzaq, Carboxymethyl cellulose coating regulates cell wall polysaccharides disassembly and delays ripening of harvested banana fruit, Postharvest Biol. Technol., 2022, 191, 111978, DOI:10.1016/j.postharvbio.2022.111978.
- N. D. Lakshan, C. M. Senanayake, T. Liyanage and A. Lankanayaka, Clove essential oil emulsions-loaded arrowroot starch-beeswax-based edible coating extends the shelf life and preserves the postharvest quality of fresh tomatoes (Solanum lycopersicum L.) stored at room temperature, Sustainable Food Technol., 2024, 2, 1052–1068, 10.1039/D4FB00033A.
- Y. A. Shah, S. Bhatia, A. Al-Harrasi, M. Tarahi, H. Almasi, R. Chawla and A. M. M. Ali, Insights into recent innovations in barrier resistance of edible films for food packaging applications, Int. J. Biol. Macromol., 2024, 271, 132354, DOI:10.1016/j.ijbiomac.2024.132354.
- M. L. Zambrano-Zaragoza, E. Mercado-Silva, P. Ramirez-Zamorano, M. A. Cornejo-Villegas, E. Gutiérrez-Cortez and D. Quintanar-Guerrero, Use of solid lipid nanoparticles (SLNs) in edible coatings to increase guaca (Psidium guajava L.) shelf life, Food Res. Int., 2013, 51, 946–953, DOI:10.1016/j.foodres.2013.02.012.
- M. L. Zambrano-Zaragoza, E. Mercado-Silva, A. Del Real L., E. Gutiérrez-Cortez, M. A. Cornejo-Villegas and D. Quintanar-Guerrero, The effect of nano-coatings with α-tocopherol and xanthan gum on shelf-life and browning index of fresh-cut “Red Delicious” apples, Innov. Food Sci. Emerg. Technol., 2014, 22, 188–196, DOI:10.1016/j.ifset.2013.09.008.
| This journal is © The Royal Society of Chemistry 2026 |