Open Access Article
Open Access ArticleRecent advances in microalgal pigments as a source of natural colors and their application in next-generation functional foods
Sajesh Chettri†
a,
Ammar B. Altemimib,
Puja Das†c,
Rattan Singhd,
Prashant Pandharinath Saide,
Pinku Chandra Nath *h,
Vinay Kumar Pandey
*h,
Vinay Kumar Pandey *fi and
Sarvesh Rustagi
*fi and
Sarvesh Rustagi gj
gj
aDepartment of Processing and Food Engineering, College of Agricultural Engineering and Post-Harvest Technology, Central Agricultural University, Ranipool 737135, Sikkim, India
bFood Science Department, College of Agriculture, University of Basrah, Basrah 61004, Iraq
cUttaranchal Institute of Technology, SALS, Uttaranchal University, Dehradun 248007, Uttarakhand, India
dDepartment of Food Technology, School of Applied and Life Sciences, Uttaranchal University, Dehradun 248007, Uttarakhand, India
eCollege of Food Technology, Central Agricultural University, Imphal 795004, Manipur, India
fResearch and Development Cell, Manav Rachna International Institute of Research and Studies (Deemed to be University), Faridabad 121004, Haryana, India. E-mail: v.k.pandey30@gmail.com; nathpinku005@gmail.com
gDepartment of Food Technology, School of Agricultural Sciences, Shri Guru Ram Rai University, Dehradun, Uttarakhand, India
hDepartment of Bioengineering, School of Engineering and Architecture, Techno India University, Agartala 799004, Tripura, India
iDepartment of Microbiology, Graphic Era (Deemed to be University), Dehradun, Uttarakhand, India
jDepartment of Food Technology, Dev Bhoomi Uttarakhand University, Dehradun 248007, Uttarakhand, India
First published on 25th November 2025
Abstract
Microalgae-derived pigments represent promising natural alternatives to synthetic food colouring agents, meeting the growing demand for sustainable, clean-label ingredients. As the demand for clean-label, plant-based, and functional food products grows, microalgal pigments are being explored as promising natural alternatives to synthetic dyes. These pigments, including phycobiliproteins (PBPs), carotenoids, and chlorophylls, offer multifunctional applications without synthetic dye-related risks. Sustainably sourced from microalgae, they support eco-friendly production aligned with circular economy principles. Pigment-rich microalgal biomass is being incorporated into next-generation functional foods such as protein bars, dairy alternatives, and beverages, contributing both color and nutritional value. The bioactive potential of microalgal pigments includes antioxidant, anti-inflammatory, and anticancer properties. Integrating microalgae pigments into food manufacturing presents both challenges and opportunities, requiring the optimization of extraction techniques. Extraction technologies such as microwave-assisted and pressurized liquid extraction are advancing, but require further improvement to enhance their stability, yield, and cost-efficiency. Techno-economic analyses emphasize production costs, scalability, and regulatory compliance as important for commercial viability. Techno-economic assessments highlight key factors such as production costs, scalability, and profitability. Current extraction methods need refinement to improve their yield and cost-efficiency. Microalgae pigments hold great promise as sustainable colorants. However, challenges still remain in terms of pigment stability during processing and sensory acceptance in food applications. Nevertheless, current biotechnological and downstream processing advancements are increasing the practicality of microalgal pigment functional foods in smart food systems. This review explores their potential for smart food applications, analysing their production costs, market prospects, and regulatory aspects.
Sustainability spotlightMicroalgal pigments offer a renewable, eco-friendly alternative to synthetic food colorants, reducing the environmental impact while supporting circular economy principles. Cultivation requires minimal land and water, promotes carbon sequestration, and ensures year-round production. Their integration into functional foods advances sustainable nutrition, aligning health benefits with global goals for greener, resilient food systems. |
1 Introduction
Microalgal pigments have a wide range of applications across various industries, including food,1 nutraceuticals,2 pharmaceutical,3 aquaculture,4 and cosmetics.5 Because of their fluorescent qualities, they are also utilized in clinical and research laboratories to mark antibody receptors. Phycobiliproteins (PBPs) show anti-inflammatory, antioxidant, hepatoprotective, neuroprotective effects.6 Also, the cultivation of microalgae is eco-friendly, and thus their use is expanding in aquaculture for aviculture feed, feed premixes, and water quality improvement through bioremediation. They are also used to enhance animal coloration, such as through astaxanthin, which improves the appearance of species.7 Microalgae are the simplest plant-like entities, which can be single-celled or filamentous. They efficiently capture solar energy, leading to significant biomass accumulation through photosynthesis. They are categorized based on their coloration including Chlorophyceae (green), Cyanophyceae (blue–green), Rhodophyceae (red), and Phaeophyceae (brown).8 Microalgae produce pigments such as chlorophylls, carotenoids, and PBPs, which give rise to various colors, ranging from green and yellow to red and brown. Natural pigments such as β-carotene (yellow, from Dunaliella), phycocyanin (deep blue, from Spirulina), and astaxanthin (yellow to red, from Haematococcus) have gained popularity due to their non-toxic and non-cancer-causing characteristics, categorizing them as a safer alternative to synthetic colorants.9 Recently, consumer acceptance has increased for natural color food ingredients to prioritize healthy diets and food safety; there is an increasing preference for nutritious, natural, and clean-label food products. Algae will drive the food, nutraceutical, pharmaceutical, and cosmetic industries to explore the generation of high-value industrial natural substances, especially pigments for food usage. Many commercially available pigments are currently produced using chemical synthesis, which has raised health issues regarding their safety because of possible toxicity resulting from their processing techniques or molecular structures. Alternatively, natural pigments are considered safe, with no harmful side effects, and often enhance nutrition, while providing various biological benefits.10 The widespread availability of pigments generated from plants has improved health positive aspects.11 The use of natural microalgal pigments is growing in popularity among producers and customers. The market for these pigments is expected to reach 452.4 million by 2025, achieving a high-value compound annual growth rate (CAGR) of 4%.11 Microalgae are important sources of lipids, proteins, vitamins, polysaccharides, and polyunsaturated fatty acids (PUFAs), all with significant commercial and health benefits. Pigments from microalgae, such as carotenoids, chlorophylls, and PBPs, are among the fast-growing and eco-friendly cultivations. This makes them highly competitive as natural colorants in the food sector.12 The quality and quantity of pigments are constantly being improved by sustainable farming practices and extraction process developments, increasing the potential of these pigments for a broad range of industrial uses. This review presents an in-depth analysis of microalgae-derived pigments as natural food colorants, focusing on their techno-economic feasibility, potential for integration within the smart food industry, and the challenges they face. Additionally, it seeks to highlight recent advancements in sustainable cultivation and extraction techniques that improve the quality and yield of pigments, thereby expanding their applicability across various sectors.A thorough search of the literature was carried out to find relevant papers on microalgal pigments and their use in functional meals. This review evaluates databases from 2014–2025 published in Scopus, Web of Science (Elsevier and Springer), and PubMed. “Microalgae” and “pigment” or “phycocyanin” or “astaxanthin” or “chlorophyll” or “carotenoid” and “functional food application” or “extraction” were the keywords and Boolean combinations used to find the papers. Original research and review publications published in English with an emphasis on the extraction techniques, characterisation, functional properties, market trends, and health applications of microalgae-derived pigments in food and associated industries were the inclusion criteria. This review excluded non-peer-reviewed publications that lacked significant experimental or review data for pigment use as well as studies restricted to non-food industrial purposes. Duplicate articles were identified and eliminated using reference management software. To ensure reproducibility, the search approaches, keywords, and screening procedures conformed to standard reporting criteria.
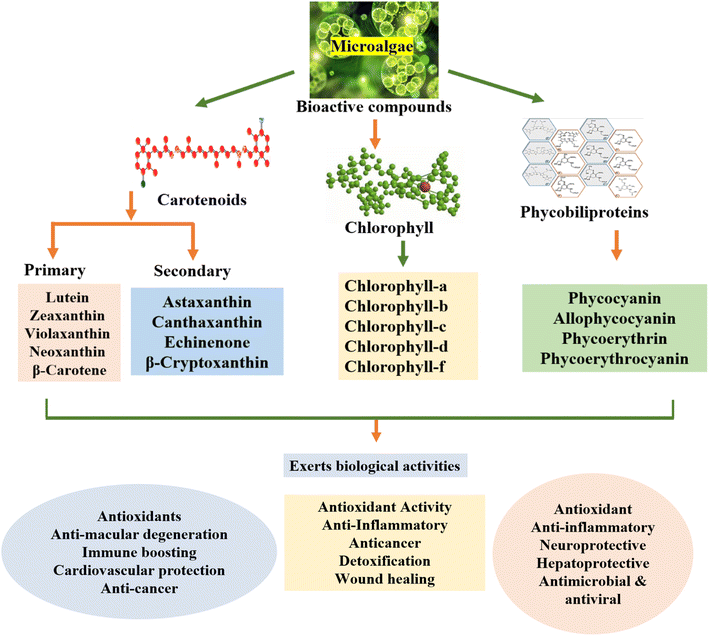
2 Microalgae and pigments
Microalgae are a diverse group of cryptogamic plants. They take on diverse shapes, including filamentous, siphonaceous, colonial, and unicellular organisms.13 The oxygenic photosynthetic prokaryotes known as cyanobacteria are among these species, which show a great deal of diversity in terms of their morphology, physiology, ecology, and other characteristics.14 The Chlorophyta phylum includes unicellular, multicellular, filamentous, and siphonous algae, predominantly found in freshwater environments. In contrast, cryptophytes are primarily unicellular and widely distributed across freshwater and marine ecosystems.15 Dinophytes, also unicellular, are characterized by two distinct flagella and mainly found in aquatic habitats. Each microalgae species is associated with a unique set of pigments, contributing to their varying colors and ecological functions.Microalgae are significant sources of high-value compounds that are economically and nutritionally important. These microscopic organisms produce a diverse range of beneficial substances, viz., proteins, lipids, complex carbohydrates, vital minerals, vitamins, coloured compounds, and beneficial fatty acids. These products have substantial commercial potential and offer considerable health benefits.16 The word “microalgae” comprises eukaryotic photosynthetic microorganisms as well as prokaryotic cyanobacteria, which transform light energy into chemical energy through photosynthesis. These organisms contain three primary pigment classes, carotenoids (typically ranging from 0.1–0.2% of their dry weight, and possibly up to 14% in certain species), chlorophylls (0.5–1.0% of their dry weight), and PBPs (up to 8% of their dry weight). These pigments are crucial for photosynthetic processes and cellular development. Unlike pigment sources from other natural origins such as fruits, plants, and animals, microalgal pigments demonstrate exceptional physiological properties, including powerful antioxidant and antibacterial capabilities, with extensive potential health applications. Moreover, these pigments offer remarkable versatility in food coloration, presenting a diverse palette of shades and natural tones. This characteristic gives microalgal pigments a significant competitive advantage as natural food colorants, enabling them to effectively replicate and enhance the visual appeal of various food products.17
The harvesting of microalgae and production of biomass are illustrated in Fig. 1. The most compelling benefit of microalgae stems from their unique cultivation characteristics. These organisms represent a consistent and sustainable natural pigment source that can be produced industrially with remarkable environmental adaptability. Unlike traditional agricultural products, microalgae are not constrained by seasonal variations, climate limitations, or specific environmental conditions. Their exceptional qualities include rapid reproduction, high pigment concentration, and remarkable resilience to challenging growth environments. Additionally, microalgae do not compete with conventional agriculture for arable land, which significantly enhances their economic and ecological value. These distinctive attributes position microalgae as increasingly attractive and competitive alternatives for generating natural pigments, offering significant advantages over conventional biological pigment sources.18
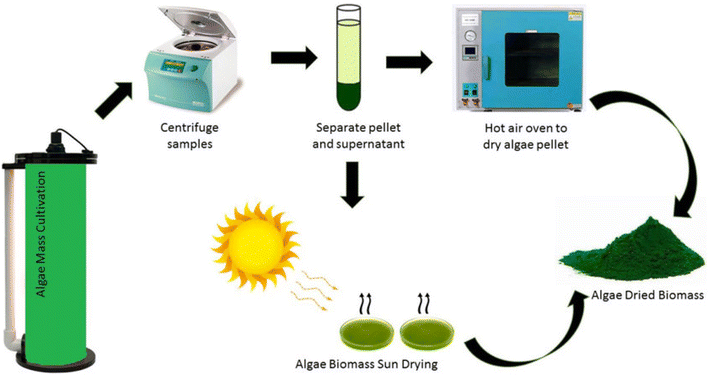 | ||
| Fig. 1 Microalgae harvesting and biomass production (Yaakob et al., 2021).19 | ||
Researchers are actively investigating various cultivation techniques to maximize pigment production across different microalgal species. Several prominent pigments have already achieved industrial-scale production and widespread commercial application, including astaxanthin (pigment sourced from Haematococcus, which is yellow-to-red), PBPs (pigment sourced from Spirulina and Galdieria sulphuraria, which is blue), and β-carotene (pigment sourced from Dunaliella, which is yellow). These pigments have found widespread use across multiple industries, including food, pharmaceuticals, fishery, nutraceuticals and cosmetics. Within microalgae, pigment synthesis occurs during both normal vegetative growth and under stress conditions. The final product quality is significantly influenced by various biotic and abiotic factors throughout the production process. Emerging trophic strategies and innovative techniques are proving increasingly effective in improving the production efficiency of pigment, offering promising advancements in microalgal biotechnology.20 Through the phases of extraction, purification, and food processing, pigment structures can be vulnerable to degradation or complete destruction, which can significantly compromise their colouring capabilities and nutritional properties in foodstuffs. Consequently, it is critically important to develop and implement refined extraction and processing techniques that effectively preserve the structural integrity, stability, and functional activity of these valuable pigment compounds.
3 Characterization of microalgae pigments
Microalgae produce three main types of pigments for photosynthetic processes: phycobiliproteins, chlorophylls, and carotenoids, which include carotenes and xanthophylls. Phycobilins are water soluble, whereas carotenoids and chlorophylls are usually fat soluble.21 The absorption spectra and molecular structures of these chlorophylls vary slightly (Fig. 2). The colors of chlorophylls a and b are blue–green and bright green, whereas the colors of chlorophylls c, d, and f are yellow–green, forest green, and emerald green, respectively. The extraction of chlorophylls from microalgae is simple and affordable. The majority of vascular plants contain green pigments, i.e. chlorophylls a and b. The most prevalent pigment needed for photosynthesis, chlorophyll-a, is found in all photosynthetic organisms. All chlorophytes, their progeny, and green plants contain chlorophyll-b, the second most prevalent form, but only dinoflagellates, haptophytes, heterokonts, and cryptophytes contain chlorophyll-c. Certain cyanobacteria have been found to contain chlorophyll-f, while certain red algae (rhodophytes) are unique in having chlorophyll-d.9 The tetrapyrrole ring structure of the porphyrin macrocycle that makes up the chlorophyll molecule has 4 carbon atoms and 1 nitrogen atom in each of the pyrrole ring. These nitrogen atoms form a central cavity that readily binds an Mg2+ ion.7 In chlorophyll a, the methyl group in ring II is substituted by a formyl group found in chlorophyll-b. Consequently, chlorophyll has a blue–green hue, absorbing light most effectively at wavelengths in the range of 660–665 nm. In contrast, chlorophyll-b is green–yellow, with its maximum absorbance ranging from 642 to 652 nm. Exposure to oxygen, weak acids or light can lead to various chlorophyll degradation products, owing to its accelerated oxidation.22Carotenoids are terpenoid pigments extracted from a forty-carbon polyene chain, giving them unique molecular structures and light-absorption characteristics, which are crucial for photosynthesis. Carotenoids can possess cyclic and oxygen-containing functional groups, distinguishing hydrocarbon carotenoids, known as carotenes, from their oxygenated derivatives, called xanthophylls.23 Carotenoids are classed as primary (e.g., xanthophylls) and secondary carotenoids; the former play essential roles in the photosynthetic apparatus, while the latter are produced in higher quantities in response to specific environmental stimuli. Xanthophylls are generally hydrophobic, and frequently connected with membranes or noncovalently coupled to certain proteins; they are typically found in the thylakoid membrane, whereas secondary carotenoids are found within lipid vesicles. Carotenoids are usually extracted using organic solvents including methanol, acetone, and dimethyl sulfoxide (DMSO).23
PBPs, found predominantly in Cyanophyceae and Cryptophyceae, are light-harvesting pigments that are categorized into 3 main types based on their amino acid sequences and spectroscopic properties including phycoerythrin (PE, red in color), phycocyanin (PC, blue in color), and allophycocyanin (APC).24 These oligomeric proteins are made up of polypeptides that bear chromophores and come from two families, potentially sharing one ancestor. PBPs help in photosynthesis by assembling into phycobilisomes and adhering to the thylakoid surface. The main PBPs in cyanobacteria include C-PC, which typically consists of two noncovalently associated subunits with molecular weights of 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–20
000–20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 daltons. These proteins exhibit distinct spectral properties due to their individual absorption spectra. The absorbance maximum for the different types of PBPs include C-PC at 615–640 nm, C-PE at 565–575 nm, phycoerythrocyanin at 577 nm, and C-APC at 650–655 nm.25 PC, a PBP produced by Galdieria sulphuraria, is a thermostable, acid-resistant pigment with strong antioxidant properties,26 making it useful in food, pharmaceutical, and cosmetics applications.27 Heterotrophic and mixotrophic cultivation considerably increase the yield of phycocyanin by adjusting the carbon/nitrogen ratios and organic carbon sources.28 G. sulphuraria beats traditional sources such as Spirulina in terms of biomass production, pigment stability, and the ability to endure difficult growing conditions and generate high-quality phycocyanin on a large-scale. Research also shows that it converts carbon efficiently in oxygen-balanced mixotrophic cultivation, allowing sustainable production with low contamination hazards.27
000 daltons. These proteins exhibit distinct spectral properties due to their individual absorption spectra. The absorbance maximum for the different types of PBPs include C-PC at 615–640 nm, C-PE at 565–575 nm, phycoerythrocyanin at 577 nm, and C-APC at 650–655 nm.25 PC, a PBP produced by Galdieria sulphuraria, is a thermostable, acid-resistant pigment with strong antioxidant properties,26 making it useful in food, pharmaceutical, and cosmetics applications.27 Heterotrophic and mixotrophic cultivation considerably increase the yield of phycocyanin by adjusting the carbon/nitrogen ratios and organic carbon sources.28 G. sulphuraria beats traditional sources such as Spirulina in terms of biomass production, pigment stability, and the ability to endure difficult growing conditions and generate high-quality phycocyanin on a large-scale. Research also shows that it converts carbon efficiently in oxygen-balanced mixotrophic cultivation, allowing sustainable production with low contamination hazards.27
4 Microalgae-derived edible pigments
4.1 Carotenoids
Among the more than 400 carotenoids present in nature, β-carotene is one of the most important. The chemical structure of carotenoid is illustrated in Fig. 3. Numerous carotenoids, such as β-carotene, are provitamin A and have a number of biological roles, which are very beneficial for human health.29 Their yellow, orange, and red hues are caused by carotenoid pigments, which are essential for photosynthesis and cellular defence. These pigments, including β-carotene, lutein, astaxanthin, and zeaxanthin, absorb light in regions that chlorophyll cannot, thus enhancing light capture for photosynthesis. Carotenoids have several strong antioxidants, protecting cells from oxidative damage produced by reactive oxygen species and excessive light.30 They also provide significant health advantages, including improved immunity, less inflammation, and support for eye health. The nutritional and functional qualities of microalgal carotenoids make them popular in animal feed, cosmetics, and nutraceuticals.31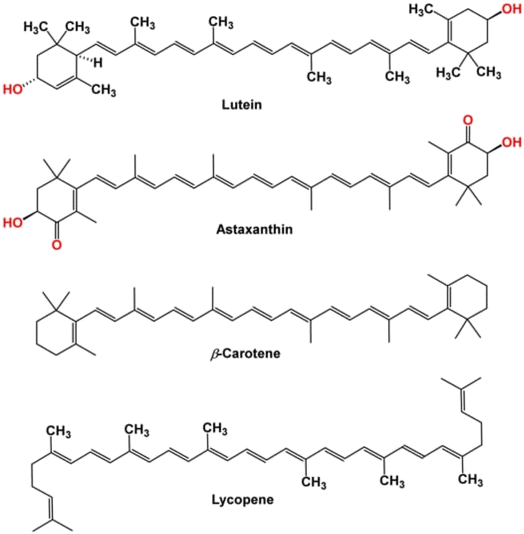 | ||
| Fig. 3 Common structure of carotenoids (Metibemu & Ogungbe, 2022).32 | ||
4.2 Chlorophyll
Chlorophyll, a green pigment, is abundant in microalgae and enables them to transform light energy into chemical energy. Microalgae get their distinctive colour from chlorophyll, which reflects green light and absorbs it mostly in the blue and red wavelengths. Chlorophyll-a, which is necessary for oxygenic photosynthesis, and chlorophyll-b, aiding in absorbing extra light energy, are the two primary forms present in microalgae.33 In addition to photosynthesis, chlorophyll possesses anti-inflammatory and antioxidant qualities, which make it useful in cosmetics and nutraceuticals. Because of their high chlorophyll content, microalgae are becoming more and more popular in the biofuel, health supplement, and sustainable food industries. The anti-inflammatory, detoxifying, and antioxidant qualities of microalgal chlorophyll provide several health benefits.31 By neutralizing free radicals, it lowers oxidative stress and the likelihood of developing chronic illnesses such as cancer and heart disease. Chlorophyll promotes liver detoxification through the removal of toxins from the body. Furthermore, it enhances metabolic processes, which can help in weight management, and improves gut health, facilitating better digestion. Furthermore, detoxification and general immune support are supported by the ability of chlorophyll to bind with heavy metals. Fig. 4 shows the molecular structures of various chlorophyll molecules.34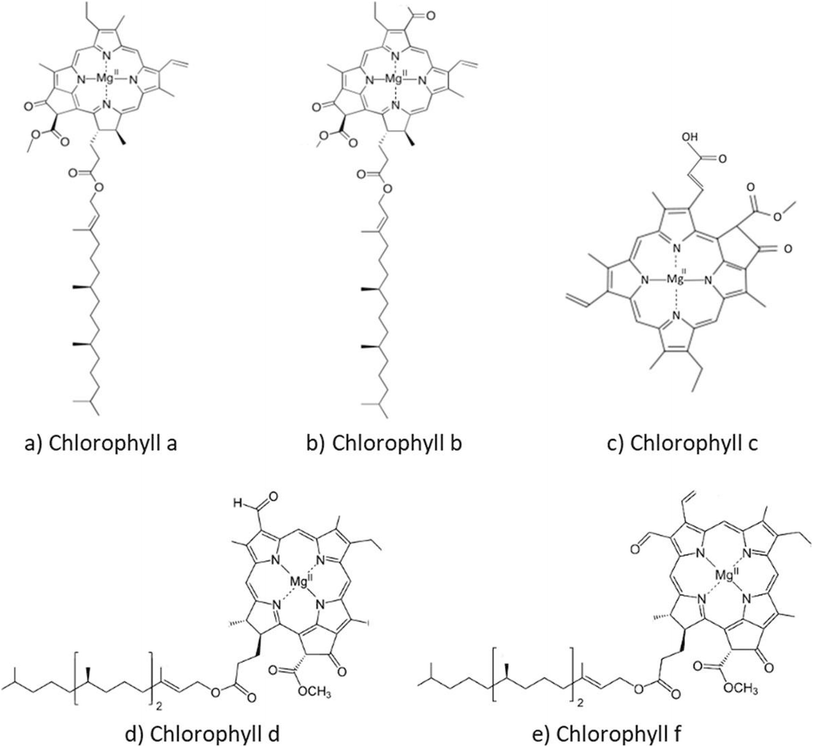 | ||
| Fig. 4 Molecular structures of various chlorophyll molecules. (a) chlorophyll-a, (b) chlorophyll-b, (c) chlorophyll-c, (d) chlorophyll-d, (e) chlorophyll-f (Yip et al., 2024).35 | ||
4.3 PBPs
PBPs are extremely important pigments found in microalgae, especially in red algae and cyanobacteria (such as Spirulina). C-APC, C-PC, and C-PE are examples of these water-soluble pigments.36 By efficiently absorbing light in the orange, red, and yellow spectra that chlorophyll is unable to collect, these pigments are essential for photosynthesis. Chlorophyll receives this energy, which improves its photosynthetic activity, particularly under low-light conditions.37 The potent anti-inflammatory, antioxidant, and immune-stimulating qualities of PBPs have also attracted attention for their health advantages. Because of its bioactive qualities and use as a natural colourant, C-PC, in particular, is extensively employed in the food, cosmetics, and pharmaceutical industries.38 Because of their special fluorescence, these pigments are also used as fluorescent markers in biomedical research. PBPs provide an environmentally friendly and adaptable remedy for a number of applications, making them increasingly popular in health supplements, functional foods, and biotechnological advancements.5 Factors affecting the production of microalgal pigments
5.1 Light
Light is an essential component in algal cultivation and growth, given that its duration and intensity directly impact photosynthesis and the biochemical makeup of microalgal materials.6 It was observed that to develop the photobioreactors or open pond systems, a greater surface area is required because the light intensity in culture systems diminishes with depth.39 Alternatively, although light is necessary for photosynthesis, the photosynthetic system can be harmed by excessive light levels that exceed its tolerance limits. Various wavelengths of light have been studied to determine their effects on algal growth and pigment yield. Water molecules and particles absorb shorter wavelengths, allowing blue and green light to penetrate deeper into the water. Green algae possess chlorophyll a and b as their primary pigments, which enhance their growth rates with an increase in light intensity. Light needs vary during different growth phases, such as β-carotene production in Dunaliella salina, which requires 135.3 and 245.6 µmoL m2 s−1 for growth and high β-carotene yields, respectively. Precise photoperiod and light intensity control are critical for maximizing microalgae growth.40 For example, in Chlorella pyrenoidosa, their chlorophyll-a levels decreased under blue light. However, they increased under a combination of blue and red light, while their chlorophyll-b levels were significantly low in red light.41 PBPs help absorb green light and stabilize the photosynthetic apparatus by transferring energy to essential pigments.42 Chlorophyll-a, which absorbs blue and red light, is critical in converting light into chemical energy, while carotenoids help dissipate excess energy. In Gracilaria birdiae, green and blue light with 8 and 14 h photoperiods increased their C-PE and C-PC concentrations, respectively. Similarly, Meristotheca papulosa produced the maximum C-PE and C-PC levels under green and blue light and low amounts in red and white light, respectively.425.2 Temperature
In phototrophic microalgae, temperature has a significant impact on biomass production. It affects not just their development but also the molecular architecture and metabolic processes in their cells. Studies show that elevated temperatures can enhance pigment synthesis in microalgae.6 For example, blue–green microalgae can produce higher amounts of carotenoids, especially β-carotene, in response to increased temperatures. The optimal growth temperature for microalgae varies depending on the strain and its temperature tolerance.43 It has been reported that 22 °C was the optimal temperature for Dunaliella salina to produce β-carotene.6 This implies that temperature significantly influences β-carotene accumulation. Another research group observed that compared to Chlorella vulgaris KNUA104, Chlorella sorokiniana strains KNUA114 and KNUA122 generated more fatty acids at higher temperatures, suggesting that they could be used as bioavailable resources. Chlorella vulgaris grew the best at 30 °C and decreased at higher degrees, but C. closterium flourished at 30 °C and D. tertiolecta and T. suecica developed more quickly at 18 °C.44 Temperature also affects the physiological activity and structural organization of the cells in microalgae, influencing their pigment yield. The response of microalgae to temperature also depends on the characteristics of their cell envelope. Both low and high temperatures affect their cell envelope, causing it to become stiffer or more pliable.6 Sudden temperature changes can stress microalgae, and higher temperatures can disrupt their cell hydrophobicity. Dunaliella triolet, algae without cell walls, are particularly sensitive to temperature fluctuations.445.3 pH
Algal pigment synthesis is significantly influenced by pH. pH levels below 5.0 or above 8.5 can inhibit microalgae growth, where lower pH values promote the synthesis of yellow pigments, while higher pH values influence red pigment production.40 The regulation of pH affects solubility and nutrient uptake in algal cells. For example, Tetraselmis sp. and Nannochloropsis sp. demonstrated a high cell density and elevated lipid, protein, and carbohydrate levels when cultivated at pH 7.5 and 8.5. Similarly, Spirulina platensis (known as Arthrospira platensis) achieved the highest levels of carotenoids (2.4 mg g−1 DW), chlorophyll-a (10.6 mg g−1 DW), C-phycocyanin (91 mg g−1 DW), and PBPs (159 mg g−1) at pH 8.5 and 9.0. These findings underscore the importance of pH in enhancing the production of antioxidants in S. platensis, with the potential for regulating their production through pH adjustment.6However, drastic pH changes can negatively impact the synthesis of carotenoids and chlorophylls, eventually reducing the growth of microalgae and biomass production.45 Pigment extraction yields from frozen and wet Chlorella vulgaris biomass varied with pH. For example, at pH 7, the molecular weight of the protein fraction was higher than that at pH 9, which can affect the characteristics of PBPs. A number of variables, such as species, media, and cultivation conditions, affect the optimum pH level for algal growth and pigment production. Variations in pH exhibited little impact on the protein fraction nutritional value, whereas alkaline environments improved the antioxidant activity. Nannochloropsis biomass had higher protein concentrations at pH 8.5, 11, and 12, yielding 6, 12.2, and 20.5 mg g−1, respectively, with the highest concentrations observed at pH 11 during aqueous extraction.6
5.4 Salinity
Cellular osmosis is an important process in pigment generation in marine microalgae cultures. When exposed to high concentrations of NaCl in the surrounding environment, cells form a hypertonic solution, causing them to shrink due to cellular dehydration and internal damage.46 According to,47 Chaetoceros muelleri exhibited the highest fucoxanthin content at salinities below 55%, achieving 2.92 mg g−1 and 0.072 mg per L per day. In contrast, halotolerant Amphora sp. showed optimal productivity at salinities above 55%, with 1.2 mg g−1 and 0.053 mg per L per day. The tolerance of microalgae to varying salt content levels is also influenced by their cultivation mode, such as semi-continuous cultivation, and a gradual increase in salinity levels over time. Increased salinity tolerance in microalgae is linked to the congregation of lower molecular weight organic solutes, including mannitol, glycerol, proline and sucrose. For example, Chlorella vulgaris exhibited a substantial reduction in total chlorophyll and carotenoid content as the salt levels increased, and thus the NaCl levels were kept between 0.0 and 0.1 M for the best pigment and biomass yields.48 Tetraselmis sp. and Nannochloropsis sp. achieved a high cell density, lipid and carbohydrate contents and protein at a salinity of 28 ppt. Tetraselmis suecica had a total carotenoid concentration of 0.35% to 1.1% and a chlorophyll content of 2.8% to 5.5%, respectively, at NaCl concentrations ranging from 15 to 60 g L−1. Another study observed that C. clostridium grown in media at different salt contents (30% and 60%) exhibited slower growth in a higher salinity environment. However, both cultures entered exponential growth after 6 days, reaching 0.65 and 0.60 × 106 cells per mL, respectively. Tolerance to salinity in microalgae also has practical applications, such as reducing salinity from seawater and lowering the cost of reverse osmosis in potable water production.6Pigment development by microalgae is also influenced by other variables, including nutritional content, herbicides, and heavy metals. The effects of inhibition of pesticides on PBP production have been reported by numerous psychobiologists.49 According to,50 pesticide exposure damages the intracellular thylakoid membrane of PBPs, resulting in their detachment. Nitrogen is a key nutrient for growth in microalgal cultures. The absence of nitrogen or nitrogen starvation can stress these organisms, halting cell division given that nitrogen is essential for metabolic activities.51
6 Technologies for the extraction of pigments from microalgae
6.1 Solvent-assisted extraction
Traditionally, solvent extraction has been used to extract microalgal pigments.52 During this process, the solvent acts as an extractant for intracellular compounds by penetrating the cell wall, leading to perforation or dissolution of the membrane. The efficiency of the solvent is determined by its chemical affinity for the target pigments, as well as the nature of the microalgal membrane or wall. Non-polar solvents are commonly used for the extraction of carotenoids because of their high hydrophobicity. Fig. 5 illustrates the extraction of bioactive pigments using various emerging technologies.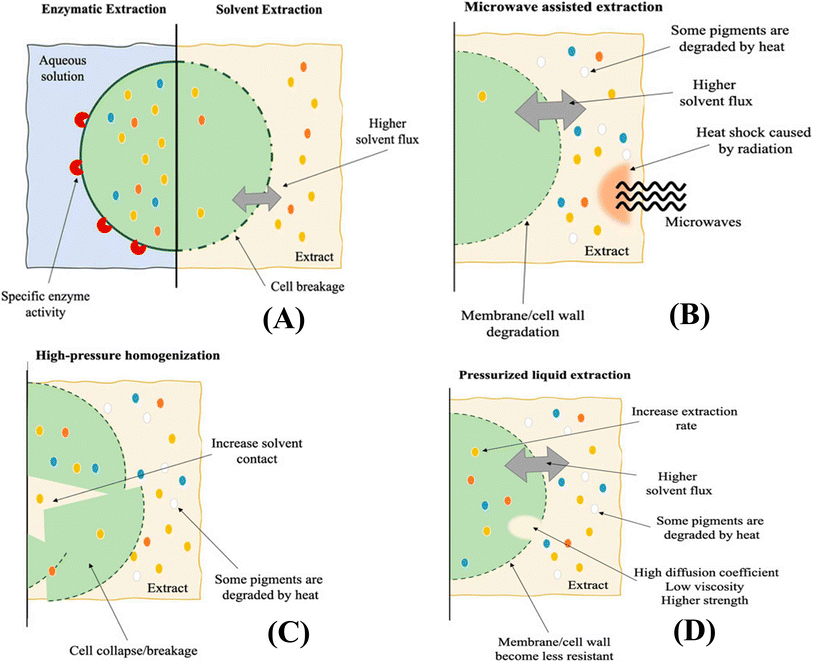 | ||
| Fig. 5 Extraction of bioactive pigments using (A) enzymatic and solvent assisted (B) microwave assisted (C) high-pressure homogenization (D) pressurized liquid extraction (Pagels, Pereira, et al., 2021).53 | ||
For example, the best solvent for the extraction of lutein from wet Chlorella vulgaris was hexane/ethanol (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v). Ethanol can be used to extract fucoxanthin from Isochrysis galbana and leave it undamaged.54 Alternatively, the traditional methods for extracting astaxanthin from Haematococcus pluvialis employ acetone/ethyl acetate/ethanol or chloroform/methanol. Also, the appropriate temperatures should be used for solvent extraction because natural pigments are heat-sensitive. Although applying heat can increase the extraction rates, prolonged exposure at high temperatures (usually above 65 °C) can cause pigment degradation, particularly in carotenoids and chlorophylls.55 In cases where the solvent lacks sufficient cell-disruptive effects, they may be combined with other agents such as chelating agents, hypochlorite, antibiotics, detergents, chaotropic, bases, and acids, each contributing to cell disruption in different ways. For instance, bases aid lipid saponification, while acids promote membrane pore formation. The excellent efficiency and purity of solvent extraction make it a popular method for producing microalgal pigments on a large scale. However, residual solvents and their possible toxicity limit their use in the food industry, given that they reduce the safety of edible pigments.
1 v/v). Ethanol can be used to extract fucoxanthin from Isochrysis galbana and leave it undamaged.54 Alternatively, the traditional methods for extracting astaxanthin from Haematococcus pluvialis employ acetone/ethyl acetate/ethanol or chloroform/methanol. Also, the appropriate temperatures should be used for solvent extraction because natural pigments are heat-sensitive. Although applying heat can increase the extraction rates, prolonged exposure at high temperatures (usually above 65 °C) can cause pigment degradation, particularly in carotenoids and chlorophylls.55 In cases where the solvent lacks sufficient cell-disruptive effects, they may be combined with other agents such as chelating agents, hypochlorite, antibiotics, detergents, chaotropic, bases, and acids, each contributing to cell disruption in different ways. For instance, bases aid lipid saponification, while acids promote membrane pore formation. The excellent efficiency and purity of solvent extraction make it a popular method for producing microalgal pigments on a large scale. However, residual solvents and their possible toxicity limit their use in the food industry, given that they reduce the safety of edible pigments.
6.2 Microwave-assisted extraction
Microwave-assisted extraction (MAE) and ultrasound-assisted extraction (UAE) have been investigated to improve cell disruption and the extraction of high-value compounds from microalgae. Acoustic cavitation is the primary mechanism, where rapid pressure changes and membrane thinning lead to cell disruption, allowing solvent penetration. The method is beneficial for pigment extraction at low temperatures (below 70 °C), and has been successfully applied to extract astaxanthin from Haematococcus pluvialis and β-carotene from Spirulina platensis. UAE has been highlighted to significantly enhance the extraction yield of PBP from Spirulina sp., though some studies have reported its negative impacts on purity, color, and antioxidant activity of C-PC.11 In the case of MAE, the application of microwave radiation induces heat shock and cell wall degradation, leading to efficient cell disruption. MAE requires much shorter thermal treatment times, reducing concerns about pigment stability under heat. A recent study showed that using vacuum conditions during MAE further lowered the required temperature. MAE has been applied to extract carotenoids, chlorophylls, PE, PC, and APC from many microalgae, such as Spirulina sp. and Scenedesmus sp. at relatively low treatment temperatures (around 55 °C). However, the investigation of MAE for the extraction of microalgal pigments is still in its early phase and requires further research.566.3 Pulse electric field
Since the 1970s, the food industry has used electric field technology, with pulsed electric field (PEF) becoming a successful extraction technique for a variety of microalgal pigments. PEF promotes electroporation and facilitates cell breakdown by delivering electrical pulses to cell membranes.57 In studies on Chlorella vulgaris, PEF showed excellent results in breaking down cells without causing significant inactivation of chlorophylls and carotenoids. To improve the extraction, particularly in the case of temperature-sensitive pigments such as lutein, the procedure can be optimized to operate at 25–30 °C. PEF has also been recommended for extracting PC from Nostoc commune, Spirulina platensis and Porphyridium cruentum. Nevertheless, it is less effective for microalgae with more resistant cell walls, such as Oscillatoria okeni.58 PEF can also be used as a pre-treatment step, as demonstrated in the solvent extraction of pigments from Nannochloropsis sp. and Haematococcus pluvialis. It is widely acknowledged as “green,” scalable, and sustainable technology for industrial extraction, requiring minimal solvent. The electric field intensity, pulse count, and time must be adjusted for the particular microalgal species and desired pigments to get the best results. For instance, cyanobacteria need more energy to electroporate than other microalgae because of their tiny cells and unique membrane structure.596.4 Enzymatic extraction
Enzymes such as glycosidase, glucanase, peptidase and lipase have been utilized widely for targeted cell lysis due to their high specificity.50 These enzymes can breakdown the cellular wall and/or membrane, thereby releasing the intracellular contents and facilitating solvent extraction. Compared to alternative techniques, enzymatic extraction has a higher extraction rate, mild reaction conditions, and reduces the necessity for downstream drying processes. For example, APC from Spirulina platensis has been successfully extracted using lysozyme, guaranteeing excellent quality and purity.60 However, for large-scale applications, it is crucial to identify enzymes capable of efficiently catalyzing cell disruption and optimize the treatment conditions that preserve pigment stability and enzyme activity.536.5 High pressure homogenization
High pressure homogenization (HPH) is considered a promising and scalable process for cell disruption, utilizing mechanical forces such as shear stress, turbulence, and cavitation. When combined with solvent extraction,61 high-quality pigments, especially those that are lipid-soluble, can be extracted from a variety of microalgae using HPH. For example, in Nannochloropsis sp. applying HPH at 1000 bar improved the bio accessibility of pigments such as antheraxanthin, violaxanthin, β-carotene and zeaxanthin in food products. However, this process also caused a reduction in pigment content due to the sharp increase in temperature, which necessitates the use of a cooling system to prevent degradation.62Another food processing technique that has recently been tested for pigment extraction is pressurized liquid extraction (PLE). High pressures (100–200 bar) and temperatures (50–200 °C) are commonly used in PLE operations, which may damage the molecular structures of the pigments. Nevertheless, some research has used PLE to efficiently extract carotenoids and chlorophylls with respectable stability. For instance, it has been shown to preserve the stability and recover lutein, β-carotene, and chlorophyll a/b from Chlorella vulgaris, β-carotene from Dunaliella salina, and carotenoids from Phormidium spp. However, more studies are needed to develop specific PLE techniques for pigments with diverse structural properties.
In addition, continuous pressurized solvent extraction (CPSE) has been introduced as a method with milder conditions (typically room temperature to 70 °C) to protect pigments from degradation. CPSE has been successfully utilized for the extraction of carotenoid from Gloeothece sp. at 60 °C, and it minimizes solvent usage by enabling efficient recirculation.63 Although pressurized systems do not require cell drying and are scalable, they are typically operated using low cell concentrations (0.01–0.85% w/w), resulting in increased energy use. Additionally, these methods lack specificity, as cell debris and unwanted compounds may enter the system, increasing the need for downstream purification.
6.6 Freezing and thawing
Freezing and thawing are commonly used in laboratories to interrupt cyanobacterial cells and extract pigments such as PC, APC, and PE, as these PBPs are best handled and preserved at low temperatures. Given that these pigments are protein-based, they are susceptible to denaturation at high temperatures, which can minimize the α-helix content. Studies have shown that performing three to four freeze–thaw cycles yields the highest purity and extraction efficiency for C-PC, with an optimal temperature of around 4 °C. Although FT delivers high-purity extracts (with yields between 0.66 and 0.87), the process is energy- and time-intensive due to the repeated freezing and thawing cycles, limiting its suitability for laboratory-scale applications.64 According to investigators, freeze-thawing at −80 °C could successfully extract B-PE in just 3 h, proving its speed and efficiency.38 Freeze-thawing at −80 °C (A545/A280 = 1.4 to 1.8) yielded the maximum purity of B-PE, surpassing the result of bead milling,65 ultrasonication, and liquid nitrogen grinding mentioned in the previous study.66 However, it was recommended that the technique be repeated with numerous freezing and thawing because it is a more gentle method for proteins and does not release any heat during the process.66 Furthermore, after taking a number of various C-PE extraction methods into consideration, they came to the realization that the freezing-thawing method was the most efficient method for causing disruptions in the cells of marine cyanobacteria.6.7 Bead milling
Bead milling (BM) employs high-speed beads constructed from steel, glass, or ceramic to disrupt cells through collisions and shear forces. This method is simple, rapid, and energy-efficient, making it appropriate for industrial-scale production. BM causes minimal harm to intracellular molecules, while achieving a high disruption efficiency. It has been successfully used to assess the overall C-PC content in cyanobacteria. However, BM lacks selectivity and typically requires the use of additional solvents for pigment extraction. When applied to species such as Chlorella sp. and S. almeriensis, BM has proven to be both effective and repeatable. The use of the correct solvent during the process can also help dissipate the thermal energy generated by the mechanical action, preventing damage to the pigment structures due to the increase in temperature.67 Table 1 presents the methods for the extraction of pigments from microalgae.| Sources | Pigments | Extraction method | Solvent used | Pressure (bar) | Temperature (°C) | Ref. |
|---|---|---|---|---|---|---|
| Haematococcus pluvialis | Carotenoids | Ultrasound-assisted extraction (UAE) | Heptane | — | 41.1 | (Poojary et al., 2016) (ref. 68) |
| Arthrospira platensis (also known as Spirulina) | β-carotene | UAE | Ethanol | — | 30 | (Giorgis et al., 2017) (ref. 69) |
| Porphyridium cruentum | Phycocyanin (PC) | Pulse electric field (PEF) | Citrate-phosphate McIlvaine | — | 20–30 | (Martínez et al., 2019) (ref. 59) |
| Nostoc commune | PC | PEF | Water | — | 40 | (Chittapun et al., 2020) (ref. 70) |
| Chlorella sp. | Carotenoids | Enzymatic high-pressure homogenization | 3% w/w NaCl | 1070 | — | (H. Sun et al., 2023) (ref. 11) |
| A. platensis | Phycobiliproteins (PBPs) | Solvent extraction | Sodium phosphate | — | 25 | (F. Wang et al., 2023) (ref. 71) |
| Chlorella vulgaris | Chlorophyll-a | Pressurized liquid extraction (PLE) | Ethanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (9:1, v/v) water (9:1, v/v) |
100 | 173 | (Poojary et al., 2022) (ref. 72) |
| Lutein | PLE | Ethanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (9 water (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) 1, v/v) |
100 | 148 | (Poojary et al., 2022) (ref. 72) | |
| Phormidium sp. | Carotenoids | PLE | Ethanol | 100 | 150 | (Pagels, Vasconcelos, et al., 2021) (ref. 73) |
| Porphyridium purpureum | Phycoerythrin (PE) | Microwave-assisted extraction (MAE) | Water | — | 40 | (Juin et al., 2015) (ref. 74) |
| H. pluvialis | Astaxanthin | Supercritical carbon dioxide (SC-CO2) | SC-CO2 + ethanol | 300 | 60 | (Chougle et al., 2016) (ref. 75) |
| Nannochloropsis gaditana | Carotenoids, chlorophylls | SC-CO2 | SC-CO2 | 400 | 60 | |
| Gloeothece sp. | Carotenoids | Continuous pressurized solvent extraction (CPSE) | Ethanol | 180 | 60 | (Amaro, Catarina Guedes, et al., 2018) (ref. 63) |
| Nannochloropsis oculata | Chlorophylls, carotenoids | High voltage electrical discharge (HVED) | Water | — | 20–30 | (Zhang et al., 2020) (ref. 41) |
7 Purification of microalgal pigments
7.1 Ammonium sulphate precipitation
The 60% ammonium sulphate precipitation method produced a purity of 2.85 and a C-PE recovery of 85.70%, which is significantly greater than the results in previous investigations.76 According to the study in,77 Nostoc muscorum precipitated with 55% ammonium sulphate produced 85% C-PE recovery with a purity of 2.85.78 According to investigators,79 three cyanobacterial species, Lyngbya sp. A09DM, Phormidium sp. A27DM, and Halomicronema sp. A32DM, demonstrated over 80% PE recovery with a purity ratio of roughly 1.5 following treatment with 70% ammonium sulphate.80 Interestingly, C-PE produced by ammonium sulphate precipitation has sufficient purity to be used in food and feed applications. Three techniques were examined to assess the partial purification of C-PE pigment including ammonium sulphate precipitation, aqueous two-phase separation, and ultrafiltration.81 The best conditions for the extraction and partial purification of C-PE from freeze-dried algae were found to be precipitation employing 80% ammonium sulphate.7.2 Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE)
Recent research has focused on R-PE purification from red algae. For example, Galland-Irmouli and colleagues described the synthesis of high-purity R-PE on a laboratory scale using preparative PAGE to extract R-PE from the red macroalga Palmaria palmata.82 Rossano and group utilized gel filtration to extract and hydroxyapatite chromatography for the extraction and purification of RPE from Corallina elongate.83 The generated R-PE had an A566/A280 purity index of greater than 5.0, and SDS-PAGE examination confirmed that it was comprised of subunits of α/β (20 kDa) and γ (30 kDa). Gracilaria corticata was purified for R-PE in a single step using a rapid chromatographic method, without affecting its structural integrity, employing PAGE.84 In the UV-Vis spectrum of pure R-PE, absorbance peaks were observed at 565, 535, and 496 nm, in addition to a fluorescence peak at 575 nm which was also observed. R-PE was produced with a purity index of 4.2 and recovery efficiency of 44.3%. SDS-PAGE examination revealed three subunits of 31 kDa, 21 kDa and 18 kDa.7.3 Ion exchange chromatography
The synthesis of R-PE from Polysiphonia urceolata has been described by Liu and colleagues employing a DEAE Sepharose Fast Flow ion exchange column, which was eluted with a pH gradient from 5.6 to 4.0. Chromatography relies on the variations in protein structure.85 According to the data presented, R-PE exhibited a noticeable decline in absorbance and fluorescent emission with pH values below 4.4. This indicates that R-PE may encounter irreversible spectroscopic changes due to associated irreversible changes in its protein structure during pH gradient elution. A single-step chromatographic procedure was used to isolate R-PE from Gracilaria gracilis using phosphate buffer (20 mM, 7.1 pH) and purify it.86 A high purity index (A565/A280 ratio) of 3.25 was obtained by purifying natural R-PE employing DEAE Sepharose Fast Flow chromatography with the fraction obtained at 200 mM sodium chloride. The emission and absorption spectra of R-PE displayed absorbance peaks at 498, 540, and 565 nm. This displayed the presence of the R-PE molecule, which weighed about 260 kDa in its original condition. The pure pigment R-PE was also comprised of four subunits, according to gel electrophoresis including 18 kDa, 21 kDa, 29 kDa, and 27 kDa. R-PE was extracted using a gel filtration method with Sephadex G-200 and Sepharose CL-4B. Then, IEC on DEAE Sepharose Fast Flow, which was made with linear ionic strength gradients, was used to purify it.87 The purified R-PE exhibited an A565 to A280 ratio of 4.89. Only one band with a pI of 4.8 was found after analysis using PAGE and isoelectric focusing.7.4 Expanded bed adsorption chromatography
During expanded-bed adsorption chromatography (EBAC), feeding organism extract causes an upward flow, which raises and expands the adsorbent bed. This happens because the upward liquid velocity balances the sedimentation rate of the adsorbent particles, allowing them to remain suspended without making contact. In this suspension state, smaller particles gather in the upper region of the column, while larger ones settle at the bottom.88 As a result, the column does not become clogged, enabling crude extracts comprising polysaccharides, cell debris, and other contaminants to move freely upward. Mariculture has been extensively used in China to cultivate the red alga Gracilaria verrucosa, primarily as a raw material for making phycocolloids and food.88 The expanded-bed column was eluted optimally with 0.1 M ammonium sulfate, and the desalted eluate was then used to purify R-PE by ion-exchange chromatography. This two-step chromatographic procedure enhanced the purity (A565/A280) of R-PE from G. verrucosa to 4.4, yielding 0.141 mg g−1 of frozen algal material. Similarly, investigators extracted and purified R-PE from Polysiphonia urceolata utilizing EBAC and phenyl-sepharose streamline, followed by chromatography with Q-Sepharose or hydroxyapatite.89 The highest A565/A280 ratios found were 3.94 for Q-Sepharose and 4.34 for hydroxyapatite. These chromatographic separations were based on hydrophobic interactions between proteins, anionic charge differences (Q-Sepharose), and site-specific ionic interactions (hydroxyapatite), rather than molecular size.7.5 Gel filtration chromatography
Using a multimodal chromatography approach that combined gel filtration chromatography (GFC) and ion-exchange chromatography (IEC), R-PE was extracted and purified at the preparatory level from the red marine macroalga H. japonica, which is recognized as a rich source of this pigment.90 Recovery of the R-PE-enriched fraction was made possible by initial gel filtration with Sepharose CL-4B, which removed particulate debris, large complexes, and small molecules from the phycobiliprotein (PBP) extract. The hexameric R-PE fraction of biliprotein was separated from trimeric R-PC and APC by further separation using Sephadex G-200. Following gel filtration, the fraction was purified by IEC on DEAE Sepharose Fast Flow. After purification, the A565/A280 ratio and absorption properties were assessed, and the results were verified using PAGE and isoelectric focusing (IEF) under both native and denatured conditions.A study compared the methods of microwave-assisted extraction (MAE), ultrasound-assisted extraction (UAE), supercritical fluid extraction (SFE) with ethanol as a co-solvent, and traditional Soxhlet extraction for the purpose of separating carotenoids and chlorophylls from the freshwater green algae Ulva flexuosa, Cladophora glomerata, and Cladophora rivularis. According to the results, MAE and UAE performed better than the conventional methods by offering greater yields at cheaper prices.91 A dioxane–water mixture was used for successive precipitation to partially purify chlorophyll, which was then purified further on a DEAE-Sepharose CL-6B column. The purity of chlorophyll a and b utilizing this approach was 30.1% and 27.5% with methanol and 44.5% and 32.4% with acetone, respectively.92
Pressurized liquid extraction (PLE) was applied to derive carotenoids and chlorophylls from the microalga Chlorella vulgaris, with the extraction performance assessed at different temperatures (50 °C, 105 °C, and 160 °C) and durations (8, 19, and 30 min). In contrast to maceration, Soxhlet extraction, and UAE, PLE displayed higher yields, with temperature being the primary parameter influencing the yield.93 Another study enhanced the extraction of carotenoids from Neochloris oleoabundans using PLE and food-grade solvents including ethanol and limonene. A factorial design was used to investigate the effects of temperature and solvent composition, and best conditions were found to be 112 °C with 100% ethanol, resulting in significantly high carotenoid yields.94
8 Stability of pigments in smart food processing and improvement strategies
In food production processes, natural pigments often degrade quickly during preservation, storage, and preparation. Maintaining the stability of pigments during these procedures is more difficult than their extraction because they include a variety of elements that can operate in concert or against one another. Several solutions have been developed to address the problem of pigment instability and maintain its colouring features.67 Chlorophylls are highly sensitive to oxygen, temperature, light, and pH fluctuations. Under unfavorable conditions, chlorophyll discoloration and degradation occur rapidly, limiting its use as a food colorant on a commercial scale. Substituting magnesium ions with more stable copper or zinc ions can produce stable blue/green metallo-chlorophylls, but the metal salt content must remain below FDA limits.62 Another approach to increase the stability of chlorophylls is physical encapsulation, in which delicate components are protected by an outer layer of protection. Protecting chlorophylls from light deterioration may ensure their excellent water solubility, antioxidant activity, and storage longevity. However, non-toxic, biocompatible, and biodegradable materials are needed for their encapsulation. The use of chlorophylls in food continues to be limited because of their susceptibility to acidic environments (pH 3.5–5.0) and light exposure, even though controlled cooking procedures and food matrices can assist in decreasing their thermal deterioration. This emphasises the need for additional research on stabilizing chlorophylls.67Anthocyanins are natural pigments found in many plants, but their stability is significantly challenged during processing and storage.95 Processing conditions such as high temperatures, oxygen, light, and variable pH levels result in the degradation of anthocyanins, which lower their food colour brilliance and antioxidant functionality.95 Thermal processing can cause the substantial breakdown and structural alteration of anthocyanins, resulting in the loss of both their visual and nutritional characteristics. Anthocyanins are mostly stable at pH of 3–4 but breakdown quickly at higher pH (neutral to alkaline), resulting in faded hues and reduced health effects. Thus, several approaches have been proposed to improve the stability of anthocyanins. Co-pigmentation, molecular structural modification (such as acylation), and most notably, microencapsulation improve their resistance to heat, oxygen, and light. Microencapsulation with carriers such as maltodextrin and cyclodextrins effectively retains the anthocyanin content during spray- or freeze-drying.96 Even under difficult processing or gastrointestinal conditions, encapsulated anthocyanins exhibit enhanced colour stability and retention, making their inclusion into functional food easier.97 Recent research has reported the use of anthocyanin-fortified smart packaging films that provide antioxidant activity, UV protection, and pH sensing capabilities, allowing for the real-time monitoring of food freshness and safeguarding items from UV-induced damage and decomposition. These sustainable biopolymer-based solutions use anthocyanins from sources such as riceberry and Clitoria ternatea for increased food safety and quality.96,97
Carotenoids, though relatively stable due to their lipid-soluble nature, can degrade during processing. High temperatures from baking, refining, and frying can cause the isomerization and degradation of carotenoids, diminishing the sensory and nutritional qualities of foods.60 Although carotenoids are resistant to extreme pH levels, bases and acids can induce their isomerization and rearrangement, and thus pH control during food processing is critical. For example, the carotenoid content decreases significantly at pH 7–8 but increases at pH 3–6. Although constant exposure to light degrades carotenoids and lowers their stability and provitamin A activity, prolonged exposure to oxygen causes oxidation and colour fading. Consequently, foods and beverages rich in carotenoids need to be stored in transparent containers to mitigate these adverse effects. Encapsulation can improve the stability of carotenoids by protecting them from light, UV rays, extreme heat, and humidity, especially when paired with spray-drying or freeze-drying.52 PBPs are susceptible to denaturation, and their stability depends on maintaining particular pH levels. For example, PC is stable in its hexameric form at pH ∼7.0, whereas PE is more appropriate for food applications because it is stable over a wider pH range (pH 4.0–10.0). PBPs degrade at high temperatures as their alpha-helix structure diminishes, and thus, they should be handled at low temperatures and stored below ambient levels to prevent their microbiological decomposition.87,98 PBPs are also sensitive to light, for instance, PC degrades after exposure to light at 100 µmoL photons m−2 s−1, eventually losing its color and stability. The use of additives is a simple and effective way to improve the stability of PBPs, especially their thermal stability. Additives such as glucose, sodium chloride, and sucrose act as protein stabilizers by protecting the chemical structure of PC and increasing the surface tension of water. Benzoic acid, with its antioxidant and antimicrobial properties, has shown great potential in preserving and enhancing the stability of PBPs, though the toxicity and flavor of thus additive must be carefully evaluated in food applications. Beet pectin has been found to protect the color of phycobilins and enhance their stability against enzymatic degradation.99 Microencapsulation has also been shown to enhance the thermal stability of PBPs and protect them from stomach acids. Variations in oxygen, light, temperature, and pH can degrade the natural pigments found in microalgae. Their color and nutritional value can be diminished by the rapid pigment breakdown caused by common cooking and storing techniques. Although a number of strategies have been established to stabilize pigments and improve their integration into food products, further study is still required to properly address these issues. Only a small number of stabilizing techniques are currently widely applied in the food industry.
9 Applications of microalgal pigments in the smart food industry
Microalgal pigments, encompassing chlorophylls, carotenoids, and PBPs, represent a versatile and multifunctional class of bioactive compounds with extensive applications across diverse industries.100 In the nutraceutical sector, these pigments demonstrate remarkable properties, including potent antioxidant and antimicrobial activities, potential for gut microbiota rebalancing, and therapeutic applications such as anemia treatment.101 Carotenoids specifically offer additional benefits such as pro-vitamin A conversion, anti-obesity effects, and protective mechanisms for retinal, dermal, and hepatic tissues.102 Pharmaceutically, these pigments show promising potential in addressing complex health challenges, ranging from neuronal damage and cardiovascular diseases to metabolic disorders, diabetes, ophthalmological conditions, viral infections, chronic inflammatory diseases, and various forms of tumors and cancers.16 PBPs contribute unique neuroprotective and hepatoprotective properties, complementing their anti-cancerous potential. The colorant applications of these pigments are equally impressive, spanning the food, beverage, cosmetic, and feed industries. They are utilized in fruit juices, dairy products, pasta, sweeteners, sausages, baked products, confectioneries, infant formulas, and even tobacco and drug coloration. Beyond their primary applications, these pigments find utility in other domains such as food preservation, dietary supplements, aquatic feed additives, and fluorescent marker development. The versatility of chlorophylls, carotenoids, and PBPs underscores their significant value across nutraceutical, pharmaceutical, and colorant markets, highlighting the immense potential of microalgal pigments as sustainable, multifunctional bioactive compounds with wide-ranging health and industrial applications (Illustrated in Fig. 6).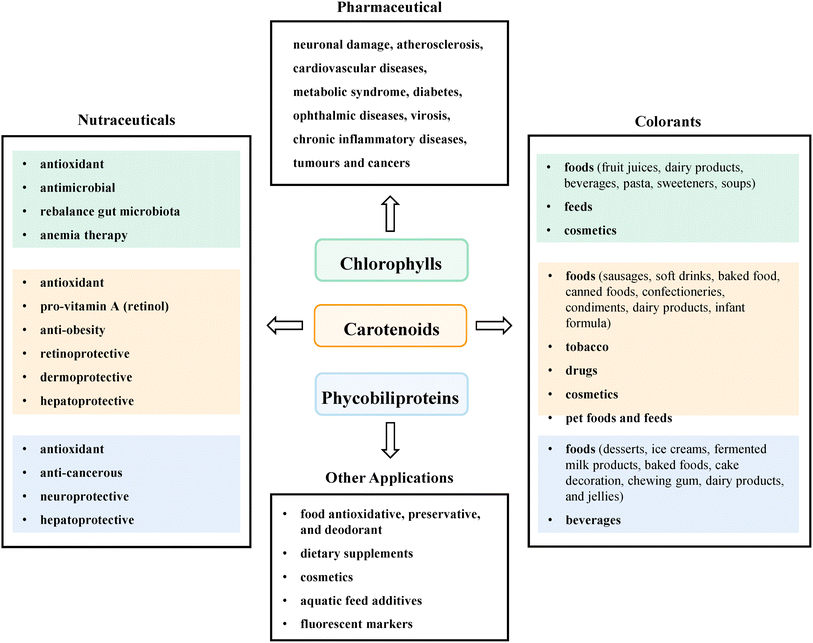 | ||
| Fig. 6 Application of microalgae pigment in the different industries (H. Sun et al., 2023).103 | ||
9.1 Phycobiliproteins (PBPs)
Research and industrial efforts are increasingly focused on developing innovative food products that leverage PBPs as alternative protein sources. Concurrently, multiple scientific studies are exploring strategies to enhance traditional food items by incorporating purified PBPs or microalgal extracts rich in these compounds. In recent years, the market has witnessed the emergence of several food products fortified with Spirulina extracts, including ice cream, milk, yogurt, cookies, juices, candies, and cheese, reflecting a growing trend towards integrating microalgal nutritional components into mainstream food offerings. A comprehensive review synthesizes critical findings regarding the multifaceted impacts of PBPs on fortified food products, examining multiple dimensions including texture, rheological characteristics, sensory perception, acidity, antioxidant capabilities, and color stability. PBPs demonstrate a nuanced dual functionality within food systems, simultaneously serving as food additives and nutritional ingredients. Their functional profile is concentration-dependent, where at lower concentrations, they predominantly act as additives, subtly modifying food properties, while at higher concentrations, their nutritional contributions become more significant, emphasizing their potential as a versatile and valuable food component. Despite the limited current research, the potential of red algae PBPs in food coloration and fortification represents an emerging and promising avenue for investigation. Given the significant annual production volumes of red macroalgae species such as Gracilaria spp. and Porphyra spp., these marine organisms offer an unexplored and potentially valuable source of PBPs for application in the food industry. The substantial biomass availability suggests considerable opportunities for developing innovative food products enriched with these unique pigment compounds.104The process of developing PBP-enriched food products involves a delicate balancing act of identifying the most appropriate concentration to enhance multiple food characteristics. This includes improving texture, rheological properties, nutritional profile, and antioxidant activity, while carefully preserving the original sensory qualities and maintaining consumer palatability. Incorporating PBPs or algae biomass can strategically enhance the functional properties of food products, specifically by positively influencing their textural characteristics, flow behavior, and overall antioxidant potential. The distinctive fishy flavor inherent in algae and cyanobacteria poses a significant obstacle to the widespread adoption of PBPs as food fortifiers, directly impacting consumer sensory perception and acceptance. Research indicates that lower concentrations of C-PC or Spirulina biomass (up to 1% by weight) generally demonstrate higher consumer palatability compared to more concentrated formulations, though some exceptions exist. To mitigate the undesirable odor associated with algal biomass, researchers have explored various strategies, including the addition of sweeteners and aromatic compounds, as comprehensively documented in recent scientific literature, with the ultimate goal of enhancing the sensory profile and marketability of PBP-enriched food products.105 An alternative method to mitigate the undesirable flavor profile of Spirulina involves strategically selecting and blending complementary spices within the enriched food product, thereby masking or neutralizing the inherent taste characteristics of the algae.106 The acceptance of Spirulina-enriched foods varies significantly depending on the specific food product type, with consumer preferences demonstrating regional variations. Research has revealed that consumers in Germany, France, and the Netherlands exhibit a notably higher preference for Spirulina-fortified pasta compared to similar enrichments in sushi and jerky products.107 Enhancing Spirulina-enriched pasta with complementary flavor profiles, such as a lemon-basil combination, has proven effective in improving consumer acceptance. Furthermore, researchers have successfully employed the response surface methodology to refine and optimize the processing parameters, ingredient formulation, and sensory characteristics of Spirulina-fortified soy yogurt (Sengupta and Bhowal 2017).108 Through advanced high moisture extrusion processing techniques, researchers successfully developed a novel meat alternative product by partially replacing soy protein with Spirulina biomass. This innovative approach resulted in a firm, fibrous plant-based protein product that effectively integrates an acceptable algal flavor profile.107 The high moisture extrusion technique demonstrates additional potential for Spirulina extracts, particularly when combined with lupin proteins, as this protein blend can generate meat analogs with enhanced physicochemical characteristics and superior nutritional value.109 Employing microencapsulation techniques with maltodextrin and gum Arabic effectively conceals the distinctive taste and aroma of Spirulina in ice cream (illustrated in Fig. 7). Additionally, encapsulating Spirulina within alginate spheres has demonstrated improved consumer acceptability, further mitigating sensory challenges associated with algal biomass incorporation.110
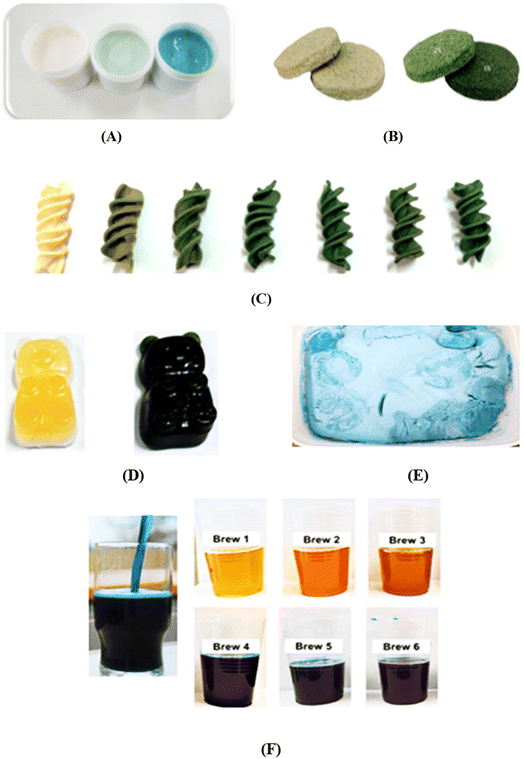 | ||
| Fig. 7 Examples of food fortification using Spirulina biomass or crude extract as additives: (A) yogurt (Nourmohammadi et al., 2020)111; (B) wheat flour cookies (Batista et al., 2017)112; (C) fusilli pasta (Nourmohammadi et al., 2020)111; (D) gummy bears (Nourmohammadi et al., 2020)111; (E) ice cream (Nourmohammadi et al., 2020)111; and (F) manufactured beers (Nourmohammadi et al., 2020).111 | ||
9.2 Chlorophylls
Chlorophyll extracts and their copper complexes have been identified as naturally occurring colorants with E-numbers E140 and E141. Chlorophylls are marketed as ‘E140i’ after being extracted from edible plants with solvents. Chlorophyllin, branded as ‘E140ii’, is generated from chlorophylls via saponification and has a steady color.113 In Europe, chlorophyll extracted from edible plants, grass, and nettles is approved as a food ingredient.114 Green food by-products contain copious chlorophylls that offer dual benefits, i.e., they provide natural coloration, while also exhibiting health-promoting aspects that can be employed throughout the food, cosmetic, and pharmaceutical industries due to their bioactive characteristics.115–117Growing consumer awareness about health issues has led to increased market demand for nutritious and safe food products (Martins and Ferreira 2017).118 Several efforts have been made to meet this demand through the development of functional foods. Enriching food with bioactive ingredients enhances their health-promoting properties.119 Research incorporated microencapsulated spirulina, a chlorophyll-rich ingredient, into pasta formulations to enhance their antioxidant capacity.120 Researchers integrated microalgae biomass, known for its high chlorophyll content, into cookie formulations to boost their bioactive compound profile.112
Studies have indicated that chlorophylls may possess significant antimicrobial properties.121 A study examined the antimicrobial properties of pigment extracts from Punica granatum L. leaves, which contained 4.9 ± 0.251 mg g−1 total chlorophyll. The findings demonstrated that 150 µL of these extracts applied for 60 min effectively inhibited various bacteria, yeasts, and fungi.122 Research showed that extracts derived from Acanthus ilicifolius L. and Heliotropium curassavicum L. plants, which contained substantial amounts of chlorophylls, demonstrated inhibitory effects against bacterial pathogens at concentrations of 50 µg mL−1.123 Research demonstrated that chlorophylls and phenolic compounds co-extracted from Pinus sylvestris L. shoots exhibited antimicrobial properties. This study found that these extracts effectively inhibited the proliferation of Gram-negative bacteria.124 Given that polyphenols are widely known for their antibacterial qualities, it is crucial to recognize that the phenolic chemicals present in the pigment extracts may work in concert to enhance their antimicrobial activity.
Naturally occurring chlorophylls, including both chlorophyll a and b, are authorized for use as additives in food.125 Adding natural, health-boosting ingredients to traditional foods can assist consumers in managing diet-related illnesses and changing to a healthier way of living. The high content of bioactive substances, such as secondary plant metabolites, in these enhanced foods, also known as functional foods, make them particularly beneficial. These substances have considerable health advantages and are thought to contribute to general well-being.126 Natural colorants can be used as food additives to enhance the nutritional content and sensory appeal of food products (Sanna and Fadda 2022).127 Chlorophyllin, a semi-synthetic, water-soluble colorant with improved stability, is created when the core magnesium in chlorophylls is replaced with copper. As a trustworthy green colouring agent, this pigment may find commercial use in the food industry. According to one study, chlorophyllin can be used to lower the microbial burden in basil.128 To improve its safety and prolong its shelf-life, they discovered that immersing basil in chlorophyllin followed by exposing it to light at 405 nm could be a useful approach.128,129
9.3 Carotenoids
Carotenoids extracted from microalgae represent a cutting-edge innovation in the food industry, offering a sustainable and nutritionally rich alternative to traditional pigments and supplement sources. These natural pigments, ranging from vibrant yellows to deep reds, are primarily derived from microalgal species such as Dunaliella salina, Haematococcus pluvialis, and Chlorella, each producing unique carotenoid compounds such as β-carotene, astaxanthin, lutein, and zeaxanthin. The significance of these microalgal carotenoids extends far beyond their visual appeal, delivering substantial health benefits including powerful antioxidant properties, vision protection, and potential immune system support.130 In the food sector, these compounds are increasingly utilized as natural colorants, replacing synthetic alternatives in products ranging from dairy and beverages to confectionery and processed foods, while simultaneously enhancing nutritional profiles.131 Table 2 includes the application of pigments from microalgae in food and allied sectors.| Microalgae | Characteristics | Ref. |
|---|---|---|
| Chlorella protothecoides | Utilized to color foods, medications, and cosmetics as well as to give fish and poultry their color. Additionally, it is used as a useful element in baby food. It also helps to prevent some types of cancer and macular degeneration | (Das Gupta & Roy, 2025) (ref. 132) |
| Dunaliella salina | Food and feed pigment, retinol (a precursor to vitamin A), a potent antioxidant used to cosmetics and health foods, an anti-inflammatory agent, an immune system modulator, a liver protector, and a component of multivitamin formulas | (Chiu et al., 2017) (ref. 133) |
| Haematococcus pluvialis | Utilized as a coloring agent in the poultry and salmon aquaculture industries. Additionally, it finds use in the culinary, cosmetics, pharmaceutical, and nutraceutical industries, especially in dietary supplements | (Zhao et al., 2025) (ref. 87) |
| Chlorella ellipsoidea | Potent anticancer and antioxidant properties | (Sruthy et al., 2025) (ref. 134) |
| Chlorella zofingiensis | Applied as a pigment to color egg yolks and chicken skin in aquaculture and poultry. Additionally, it is used in the pharmaceutical, nutraceutical, food, cosmetics, and medicine sectors (as a tanning agent) | (Unni & Karunakaran, 2025) (ref. 135) |
| Porphyridium cruentum | Encompasses a number of bioactive qualities, including as anti-inflammatory, antioxidant, antidiabetic, anti-obesity, and antiangiogenic effects, as well as anticancer effects against prostate, colon, lung, urinary, and stomach malignancies | (Giovanni Luca et al., 2024) (ref. 136) |
| Dunaliella salina | Strong antioxidant and highly prized bioproduct that is used in the food and cosmetics sectors as a natural colorant and addition | (Blanco-Llamero et al., 2025) (ref. 130) |
| Amphidinium carterae | Applications in technology and medicine as a therapeutic agent | (Kichouh-Aiadi et al., 123 C.E.) (ref. 137) |
| Phaeodactylum tricornutum and Cylindrotheca closterium | Displays strong antioxidant properties, surpassing those of β-carotene | (Lu et al., 2024) (ref. 138) |
The production of microalgal carotenoids offers remarkable advantages over traditional agricultural methods, requiring minimal land usage, consuming less water, and enabling year-round controlled production through advanced cultivation techniques.139 Extraction processes, though currently complex and expensive, are rapidly evolving with technological innovations aimed at improving efficiency and reducing costs. The commercial potential is significant, with growing consumer demand for natural, health-promoting ingredients driving research and development in this field. Challenges remain, including optimizing extraction technologies, managing production expenses, and competing with synthetic alternatives (Schoefs 2002;140 Sundararajan and Ramasamy 2024).141 However, emerging trends in genetic engineering, sustainable production methods, and expanding applications in functional foods and nutraceuticals suggest a promising future for microalgal carotenoids. As research progresses and technologies advance, these remarkable compounds are positioned to revolutionize the food industry, offering a sustainable, nutritionally dense solution that bridges the gap among natural ingredients, health benefits, and environmental consciousness.
10 Microalgal pigments: market demand, challenges and opportunities
The preference of consumers for nutritious, visually appealing, and clean-label food products has sparked significant market interest in natural ingredients, particularly within the food processing industry.6 Microalgae stand out as a primary source of natural commercial pigments, offering abundant availability and sustainable production. Numerous reports confirm the growing market interest in exploiting the potential of microalgae. Countries such as Germany, USA, and China collectively produce over 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tons of dry microalgae biomass annually, valued at approximately USD 5.7 billion. Major pigments extracted from microalgae have estimated commercial production levels of about 200 tons for Haematococcus, 1000 tons for Dunaliella, 4000 tons for Chlorella, and 10
000 tons of dry microalgae biomass annually, valued at approximately USD 5.7 billion. Major pigments extracted from microalgae have estimated commercial production levels of about 200 tons for Haematococcus, 1000 tons for Dunaliella, 4000 tons for Chlorella, and 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tons for Spirulina.6
000 tons for Spirulina.6
The commercial worth of PBPs is projected to rise rapidly in the coming years, driven by the surging demand for natural blue and green shades in the food, pharmaceutical, nutraceutical, and cosmetics industries.142 Manufacturing companies are pursuing 100% pure and natural food-grade pigments, particularly due to their antioxidant properties and vibrant colors, with Spirulina being the largest source of PBP. This microalga is among the most widely cultivated worldwide and is projected to attain a market value exceeding USD 779 million by 2026. The Asia-specific region is expected to grow significantly in this market, presenting further opportunities for manufacturers.104 The market value of PC from Spirulina was USD 112.3 million in 2018, and it is estimated to reach USD 232.9 million by the end of 2025.143 PC is already utilized in various sectors, including food, beverages, and cosmetics, with rising demand for products such as Blood Tonic, M&M chocolates, and B-blue Spirulina drinks.144
The global market value of chlorophyll is USD 300 million and is projected to exceed USD 463.7 million by 2025, with Chlorella contributing significantly and expected to achieve a market value of USD 210.15 million by 2026, particularly in Europe.100 Carotenoids are now major players in the worldwide pigment company, especially in the Americas and Europe, due to their stability, good appearance, ease of application, and increased consumer interest. The worldwide market for pigments, especially in the Americas and Europe. The global pigment market, which encompasses over 600 sources, is projected to reach USD 1.84 billion by 2026, with astaxanthin and β-carotene priced between USD 2500 and USD 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, and USD 300 and USD 3000 per kg, respectively. The market value for β-carotene is expected to reach USD 780 million by 2027, with dietary supplements accounting for over 21% of this market.145
000, and USD 300 and USD 3000 per kg, respectively. The market value for β-carotene is expected to reach USD 780 million by 2027, with dietary supplements accounting for over 21% of this market.145
The commercial production of Dunaliella salina to obtain β-carotene began in the 1980s by Western Biotechnology and Betatene (Australia), now owned by Badische Anilin-und Soda-Fabrik (BASF), the leading manufacturer of natural carotenoids derived from Dunaliella. Currently, approximately 1200 tons of Dunaliella salina are produced annually.146 Meanwhile, H. pluvialis is produced in annual quantities exceeding 300 tons in the US, India, and Israel, primarily for astaxanthin production. The Asta Real Group pioneered commercial astaxanthin manufacturing in 1994, and the Brazilian company ocean drop sells food and cosmetic products infused with astaxanthin.147 By the end of 2026, the global market value for astaxanthin could reach USD 800 million. The lutein market is also expanding, currently valued at over USD 308 million annually.148 According to future market insights and Research and Markets, it is expected to reach USD 698 million by 2035, growing at a CAGR of 5.4%. BASF, Kemin Industries, Chr. Hansen, and Anhui Ruisen are some of the major corporations involved. Europe holds the largest market share, accounting for around 34–37%. The cost of lutein decreased from USD 276 per kg in 2022 to USD 68–170 per kg in 2025, depending on its grade and supplier. Despite the continuous susceptibility to raw material and geopolitical concerns, this pattern reflects industrial advancements, diverse supply chains, and increased market competitiveness.
Microalgae have emerged as an economical and environmentally friendly source of natural pigments, and their use across various industrial sectors is expected to grow in the future. In summary, consumer aversion to synthetic food colorants has driven the growth of natural pigments in the food market.149 As natural pigments gradually replace synthetic alternatives, the demand for microalgal pigments is increasing, resulting in a significant increase in market value. Xanthophylls represent another promising category of biomolecules, with their industrial production potential enhanced by the further exploration of novel biological sources and productivity efficiency. Both microalgal astaxanthin and β-carotene are anticipated to see increased global sales, maintaining a strong presence in the market. The market value for PBPs has also experienced substantial growth in recent years, largely due to Spirulina.150 Microalgal-derived chlorophylls are becoming more popular in the food industry, and over the next five years, an important market is expected to develop. Stakeholders are actively looking for economical and effective production technologies, while promoting extensive regulatory measures to increase the market size and value of these microalgal pigments.
11 Future prospects
Microalgal pigments have tremendous potential as natural functional food ingredients due to their brilliant colouring and health-promoting characteristics. Future research should focus on developing scalable extraction and production methods that enhance yields, stability, and bioactivity while minimizing environmental impacts, in alignment with Sustainable Development Goals (SDGs) 12 and 13. Examples of these approaches include microwave-assisted, pulsed electric field, and enzymatic procedures. The goal of technological developments, such as metabolic engineering, is to boost the content of pigments and expand their uses in the food, nutraceutical, and cosmetic industries. Consumer confidence and industry adoption will be strengthened by establishing standardized extraction processes and regulatory frameworks that guarantee consistent quality, safety, and efficacy. Comprehensive techno-economic analyses combined with high-throughput, cost-effective techniques will enable widespread commercialization.Carotenoids, phycobiliproteins, PUFAs, and bioactive peptides are examples of microalgal pigments that have therapeutic effects for the prevention of chronic diseases and the promotion of health, in addition to colouring. Their bioactive properties, including antioxidant, anti-inflammatory, and metabolic-regulating effects, position them as valuable ingredients in functional foods, dietary supplements, and nutraceuticals. Their ecologically sustainable farming on non-arable land using alternative water sources supports SDGs 2 and 15. Innovation in extraction, processing, and regulation will enable them to reach their full potential as research advances, leading to the development of healthier, more sustainable food systems that are in line with the global SDGs.
12 Conclusion
Microalgae-based pigments have emerged as sustainable and health-promoting alternatives to synthetic food colorants, addressing the growing consumer demand for clean-label and environmentally friendly ingredients. Their diverse range of bioactive substances, including carotenoids, PBPs, and chlorophylls, provide natural pigments, antioxidant protection, and nutritional benefits. These characteristics make microalgae an important resource for generating functional foods and offer versatile applications in the food industry, while eliminating the safety concerns associated with synthetic dyes. The transition toward these natural colorants aligns with global sustainability goals, promoting eco-conscious production practices and reinforcing the principles of a circular economy. However, despite their potential, challenges remain in optimizing the extraction and commercialization of microalgal pigments. Current extraction methodologies, while effective, require significant refinement to enhance their efficiency, scalability, and cost-effectiveness. Future technology advances in extraction and cultivation, together with strong legal frameworks and commercial incentives, are required to fully realize their potential. Microalgae can act as carbon sinks, renewable feedstocks, and environmentally beneficial alternatives to synthetic additives, thereby promoting the ideas of a circular green economy. The incorporation of microalgal pigments into mainstream production is consistent with the UN SDGs, particularly those related to health, sustainable industry, and climate resilience. Microalgae-based pigments have the potential to revolutionize food systems in the future, assuring both ecological balance and human well-being.Conflicts of interest
There are no conflicts to declare.List of abbreviations
| PUFAs | Polyunsaturated fatty acids |
| CAGR | Compound annual growth rate |
| DMSO | Dimethyl sulfoxide |
| AMD | Age-related macular degeneration |
| PBPs | Phycobiliproteins |
| UAE | Ultrasound-assisted extraction |
| MAE | Microwave-assisted extraction |
| APC | Allophycocyanin |
| PE | Phycoerythrin |
| PEF | Pulsed electric fields |
| BM | Bead milling |
| PC | Phycocyanin |
| CPSE | Continuous pressurized solvent extraction |
| BASF | Badische Anilin-und Soda-Fabrik |
| IEC | Ion-exchange chromatography |
| DEAE | Diethylaminoethyl |
| SDGs | Sustainable development goals |
Data availability
No new data has been generated.References
- M. Vigani, C. Parisi, E. Rodr, M. J. Barbosa, L. Sijtsma and M. Ploeg, Trends Food Sci. Technol., 2015, 42, 81–92 CrossRef CAS.
- S. Mehariya, R. K. Goswami, O. P. Karthikeysan and P. Verma, Chemosphere, 2021, 280, 130553 CrossRef CAS PubMed.
- A. Khan, M. Salman, M. ansari, J. Bashir, U. Malik and M. S. Ikram, Int. J. Infect. Dis., 2018, 73, 36–37 CrossRef.
- A. Ahmad, S. W. Hassan and F. Banat, Bioengineered, 2022, 13, 9521–9547 CrossRef CAS PubMed.
- K. A. M. Andrade, C. Lauritano, G. Romano and A. Ianora, Mar. Drugs, 2018, 16 DOI:10.3390/md16050165.
- A. Kumar, F. Paolo, J. B. Albarico, P. Krishna, A. Pralhad, C. T. Nian, H. Thi, B. Chau, C. Anwar, B. Senthilkumar, Y. Tsang, C. Chen and C. Dong, Bioresour. Technol., 2022, 351, 126910 CrossRef PubMed.
- H. Begum, F. Yusoff, F. Md, S. Banerjee, H. Khatoon and M. Sariff, Crit. Rev. Food Sci. Nutr., 2016, 56, 2209–2222 CrossRef CAS PubMed.
- S. Sunoj, A. Hammed, C. Igathinathane, S. Eshkabilov and H. Simsek, Algal Res., 2021, 60, 102487 CrossRef.
- E. G. Nwoba, T. Ogbonna, C. N. Ishika and A. Vadiveloo, Microalgae Biotechnology for Food , Health and High Value Products, 2020 Search PubMed.
- M. Isabel, L. Neves, E. K. Silva and M. A. A. Meireles, Trends Food Sci. Technol., 2021, 112, 163–173 CrossRef.
- Y. Wang, L. Chen, Y. Wang, X. Wang, D. Qian, J. Yan, Z. Sun, P. Cui, L. Yu, J. Wu and Z. He, J. Nanobiotechnol., 2023, 21(1), 408 CrossRef CAS PubMed.
- V. Dolganyuk, D. Belova, O. Babich, A. Prosekov, S. Ivanova, D. Katserov, N. Patyukov and S. Sukhikh, Biomolecules, 2020, 1–24 Search PubMed.
- L. A. Singh, P. Kumari, P. Kumar, A. Yadav, R. Bhardwaj, P. Swapnil and M. Meena, Front. Sustain. Food Syst., 2025, 9, 1669731 CrossRef.
- A. K. Singh, R. K. Srivastava, P. Pal, S. Mandal, U. K. Sahoo, A. Prakash, K. Sridhar, M. Sharma, P. K. Sarangi and B. S. Inbaraj, Biocatal. Agric. Biotechnol., 2024, 58, 103192 CrossRef CAS.
- A. Aizpuru and A. González-Sánchez, World J. Microbiol. Biotechnol., 2024, 40, 1–26 CrossRef PubMed.
- S. C. Silva, I. C. F. R. Ferreira, M. M. Dias and M. F. Barreiro, Molecules, 2020, 25 DOI:10.3390/molecules25153406.
- B. Schoefs, Trends Food Sci. Technol., 2002, 13, 361–371 CrossRef CAS.
- M. Gong and A. Bassi, Biotechnol. Adv., 2016, 34, 1396–1412 CrossRef CAS PubMed.
- M. A. Yaakob, R. M. S. R. Mohamed, A. Al-Gheethi, R. A. Gokare and R. R. Ambati, Cells, 2021, 10, 1–19 CrossRef PubMed.
- A. K. Patel, R. R. Singhania, C. W. Chen, Y. S. Tseng, C. H. Kuo, C. H. Wu and C. Di Dong, Environ. Technol. Innov., 2021, 23, 101729 CrossRef CAS.
- A. G. Pereira, P. Otero, J. Echave, A. Carreira-casais, F. Chamorro, N. Collazo, A. Jaboui, C. Lourenço-lopes, J. Simal-gandara and M. A. Prieto, Mar. Drugs, 2021, 1–31 Search PubMed.
- A. T. da Silva and J. Carmo Lombardi, Pigments from Microalgae Handbook, 2020 Search PubMed.
- R. R. Ambati, D. Gogisetty, R. G. Aswathanarayana, S. Ravi, P. N. Bikkina, L. Bo and S. Yuepeng, Crit. Rev. Food Sci. Nutr., 2019, 59, 1880–1902 CrossRef CAS PubMed.
- R. George and J. A. John, Int. J. Food Sci. Technol., 2023, 513–519 CrossRef CAS.
- L. Tounsi, H. Ben Hlima, F. Hentati, O. Hentati, H. Derbel and P. Michaud, Mar. Drugs, 2023, 1–27 Search PubMed.
- F. Fitrania, D. Y. Rahman, D. Indrasi and E. Prangdimurti, Proceedings of the 10th Isibio & 13th Isism in Conjunction with the 20th Acm Meetings, 2025, 3323020019 Search PubMed.
- M. M. Maroneze, R. R. Dias, I. A. Severo and M. I. Queiroz, Pigments from Microalgae Handbook, 2020, pp. 241–264 Search PubMed.
- D. Pleissner and S. Smetana, Waste Manage., 2020, 102, 198–203 CrossRef CAS PubMed.
- S. N. A. Papadopoulou, T. Adamantidi, D. Kranas, P. Cholidis, C. Anastasiadou and A. Tsoupras, Mar. Drugs, 2025, 23, 299 CrossRef CAS PubMed.
- X. Huang, F. Wang, O. U. Rehman, X. Hu, F. Zhu, R. Wang, L. Xu, Y. Cui and S. Huo, Foods, 2025, 14, 2500 CrossRef CAS PubMed.
- J. A. M. Prates, Appl. Sci., 2025, 15, 6144 CrossRef CAS.
- D. S. Metibemu and I. V. Ogungbe, Molecules, 2022 DOI:10.3390/MOLECULES27186005.
- A. Valdez-Ortiz, K. A. Meza-Ayala, J. M. García-Padilla, R. Valdez-Ortiz and L. J. Germán-Báez, Microalgae as Promising Source of Commercial Bioproducts, 2025, pp. 153–175 Search PubMed.
- M. Dimopoulou, A. Kolonas, D. Stagos and O. Gortzi, Biomass, 2025, 5, 11 CrossRef CAS.
- L. X. Yip, J. Wang, Y. Xue, K. Xing, C. Sevencan, K. Ariga and D. T. Leong, Sci. Technol. Adv. Mater., 2024 DOI:10.1080/14686996.2024.
- V. K. Kannaujiya, P. R. Singh, D. Kumar and R. P. Sinha, Pigments from Microalgae Handbook, 2020, pp. 43–68 Search PubMed.
- L. T. Arashiro, M. Boto-Ordóñez, S. W. H. Van Hulle, I. Ferrer, M. Garfí and D. P. L. Rousseau, Bioresour. Technol., 2020, 303, 122894 CrossRef CAS PubMed.
- A. Srivastava, M. Kalwani, H. Chakdar, S. Pabbi and P. Shukla, Bioresour. Technol., 2022, 352, 127071 CrossRef CAS PubMed.
- S. J. Sim, J. Joun, M. E. Hong and A. K. Patel, Bioresour. Technol., 2019, 291, 121820 CrossRef CAS PubMed.
- A. K. Patel, J. M. Joun, M. E. Hong and S. J. Sim, Bioresour. Technol., 2019, 282, 245–253 CrossRef CAS PubMed.
- X. Wang, P. Zhang and Y. Wu, J. Appl. Phycol., 2020, 4189–4197 CrossRef.
- I. A. Borlongan, J. Appl. Phycol., 2020, 1329–1340 CrossRef CAS.
- H. Yun, Y. Kim and H. Yoon, Heliyon, 2020, 6, e04447 CrossRef PubMed.
- N. N. T. Mišić, R. J. Zemla, M. L. A. Čačković, D. K. T. Legović and P. Žutinić, J. Appl. Phycol., 2022, 243–259 Search PubMed.
- S. Kulkarni and Z. Nikolov, Algal Res., 2018, 35, 185–193 CrossRef.
- Ł. Sikorski, Water, 2021, 13 DOI:10.3390/W13182493.
- T. Ishika, N. R. Moheimani, P. A. Bahri, D. W. Laird, S. Blair and D. Parlevliet, Algal Res., 2017, 28, 66–73 CrossRef.
- N. Kalla and S. Khan, Indian J. Sci. Technol., 2016, 9, 1–7 CAS.
- K. V Ajayan, P. J. Chaithra, K. Sridharan, P. Sruthi, E. Harikrishnan and C. C. Harilal, Environ. Res., 2023, 237, 116926 CrossRef PubMed.
- P. Verma, C. Technologies and F. Outlook, Micro-algae : Next-Generation Feedstock for Biorefineries, 2022 Search PubMed.
- M. Kajikawa and H. Fukuzawa, Front. Plant Sci., 2020, 11, 1–6 CrossRef PubMed.
- A. Alam and Z. Wang, Microalgae Biotechnology for Development of Biofuel and Wastewater Treatment, 2019 Search PubMed.
- F. Pagels, R. N. Pereira, A. Vicente and A. C. Guedes, Appl. Sci., 2021, 11 DOI:10.3390/APP11115187.
- M. Gong, X. Li and A. Bassi, J. Appl. Phycol., 2018, 1617–1627 CrossRef CAS.
- D. Aguilar-machado, L. Morales-oyervides, J. C. Contreras-Esquivel, C. Aguilar, A. Méndez-Zavala, J. Raso and J. Montañez, Food Sci. Technol. Int., 2017, 23, 338–348 CrossRef CAS PubMed.
- K. L. Low, A. Idris and N. M. Yusof, Food Chem., 2020, 307, 125631 CrossRef CAS PubMed.
- A. Ahirwar, M. Jahir, K. Vandana, S. Megha, M. Anshuman and R. Benoit, Bioenergy Res., 2023, 311–324 CrossRef CAS.
- S. Kulkarni, S. L. Woodard and Z. L. Nikolov, J. Appl. Phycol., 2020, 1697–1707 Search PubMed.
- J. M. Martínez, C. Delso, I. Álvarez and J. Raso, Algal Res., 2019, 37, 51–56 CrossRef.
- H. A. Tavanandi, P. Vanjari and K. S. M. S. Raghavarao, Sep. Purif. Technol., 2019, 225, 97–111 CrossRef CAS.
- T. A. Gomes, C. M. Zanette and M. R. Spier, Prep. Biochem. Biotechnol., 2020, 50, 635–654 CrossRef CAS PubMed.
- T. M. M. Bernaerts, H. Verstreken, C. Dejonghe, L. Gheysen, I. Foubert, T. Grauwet and A. M. Van Loey, J. Funct. Foods, 2020, 65, 103770 CrossRef CAS.
- H. M. Amaro, A. C. Guedes, M. A. C. Preto, I. Sousa-Pinto and F. X. Malcata, Mar. Drugs, 2018, 16 DOI:10.3390/md16090327.
- H. Sun, Y. Wang, Y. He, B. Liu, H. Mou, F. Chen and S. Yang, Mar. Drugs, 2023, 21, 82 CrossRef CAS PubMed.
- P. Ruiz-Ruiz, T. L. Gómez-Borraz, S. Revah and M. Morales, Chemosphere, 2020, 259, 127418 CrossRef CAS PubMed.
- M. Munier, S. Jubeau, A. Wijaya, M. Morançais, J. Dumay, L. Marchal, P. Jaouen and J. Fleurence, Food Chem., 2014, 150, 400–407 CrossRef CAS PubMed.
- S. Chen, H. Zhu, E. Radican, R. Wang, X. Wang, Z. Xiao, Y. Lei, M. Qiao and Y. Luo, Compr. Rev. Food Sci. Food Saf., 2025, 24, e70308 CrossRef CAS PubMed.
- M. M. Poojary, F. J. Barba, B. Aliakbarian, F. Donsì, G. Pataro, D. A. Dias and P. Juliano, Marine Drugs, 2016, 14 DOI:10.3390/MD14110214.
- M. Giorgis, D. Garella, C. Cena, L. Boffa, G. Cravotto and E. Marini, European Food Research and Technology, 2016, 243(2), 227–237 CrossRef.
- S. Chittapun, V. Jonjaroen, K. Khumrangsee and T. Charoenrat, Algal Res., 2020, 46, 101789 CrossRef.
- F. Wang, X. Yu, Y. Cui, L. Xu, S. Huo, Z. Ding, Q. Hu, W. Xie, H. Xiao and D. Zhang, Food Chem., 2023, 406, 135005 CrossRef CAS PubMed.
- M. M. Poojary, A. Laurora, M. N. Lund and B. K. Tiwari, in Innovative and Emerging Technologies in the Bio-marine Food Sector, Applications, Regulations, and Prospects, 2022, pp. 441–479 Search PubMed.
- F. Pagels, V. Vasconcelos, A. C. Guedes, F. Pagels, V. Vasconcelos and A. C. Guedes, Biomolecules, 2021, 11 DOI:10.3390/BIOM11050735.
- C. Juin, J. R. Chérouvrier, V. Thiéry, A. L. Gagez, J. B. Bérard, N. Joguet, R. Kaas, J. P. Cadoret and L. Picot, Appl. Biochem. Biotechnol., 2014, 175(1), 1–15 CrossRef PubMed.
- J. A. Chougle, S. B. Bankar, P. V. Chavan, V. B. Patravale and R. S. Singhal, Sep. Sci. Technol., 2016, 51, 2164–2173 CrossRef CAS.
- P. Kamble, S. Cheriyamundath, M. Lopus and V. L. Sirisha, J. Appl. Phycol., 2018, 30, 1641–1653 CrossRef CAS.
- K. Ranjitha, V. R. Reddy and H. S. Oberoi, Sustainable Microbial Technologies for Valorization of Agro-Industrial Wastes, 2022, pp. 115–134 Search PubMed.
- K. Ranjitha and B. D. Kaushik, Indian J. Microbiol., 2005, 45, 67–69 Search PubMed.
- P. Parmar, R. Kumar, Y. Neha and V. Srivatsan, Front. Plant Sci., 2023, 14, 1073546 CrossRef CAS PubMed.
- A. Parmar, N. K. Singh, A. Kaushal, S. Sonawala and D. Madamwar, Bioresour. Technol., 2011, 102, 1795–1802 CrossRef CAS PubMed.
- C. Denis, C. Ledorze, P. Jaouen and J. Fleurence, Bot. Mar., 2009, 52, 278–281 CrossRef CAS.
- A. V. Galland-Irmouli, L. Pons, M. Luçon, C. Villaume, N. T. Mrabet, J. L. Guéant and J. Fleurence, J. Chromatogr. B Biomed. Sci. Appl., 2000, 739, 117–123 CrossRef CAS PubMed.
- R. Rossano, N. Ungaro, A. D'Ambrosio, G. M. Liuzzi and P. Riccio, J. Biotechnol., 2003, 101, 289–293 CrossRef CAS PubMed.
- M. Sathuvan, R. Thangam, G. Venkateshbabu, K. L. Cheong, H. Kang and Y. Liu, Int. J. Biol. Macromol., 2022, 194, 563–570 CrossRef CAS PubMed.
- L. N. Liu, X. L. Chen, X. Y. Zhang, Y. Z. Zhang and B. C. Zhou, J. Biotechnol., 2005, 116, 91–100 CrossRef CAS PubMed.
- H. P. T. Nguyen, M. Morançais, P. Déléris, J. Fleurence, C. T. Nguyen-Le, K. H. Vo and J. Dumay, J. Appl. Phycol., 2020, 32, 553–561 CrossRef.
- L. Sun, S. Wang, X. Gong, M. Zhao, X. Fu and L. Wang, Protein Expr. Purif., 2009, 64, 146–154 CrossRef CAS PubMed.
- G. Wang, Chromatographia, 2002, 56, 509–513 Search PubMed.
- J. F. Niu, G. C. Wang and C. K. Tseng, Protein Expr. Purif., 2006, 49, 23–31 CrossRef CAS PubMed.
- S. Saadi, N. Saari, F. Anwar, A. Abdul Hamid and H. M. Ghazali, Biotechnol. Adv., 2015, 33, 80–116 CrossRef CAS PubMed.
- J. Fabrowska, B. Messyasz, J. Szyling, J. Walkowiak and B. Łęska, Phycol. Res., 2018, 66, 52–57 CrossRef CAS.
- M. Page and R. Thorpe, in The Protein Protocols Handbook, Springer, 2009, pp. 1755–1756 Search PubMed.
- H. C. Kwang, H. J. Lee, S. Y. Koo, D. G. Song, D. U. Lee and C. H. Pan, J. Agric. Food Chem., 2010, 58, 793–797 CrossRef PubMed.
- M. Castro-Puyana, M. Herrero, I. Urreta, J. A. Mendiola, A. Cifuentes, E. Ibáñez and S. Suárez-Alvarez, Anal. Bioanal. Chem., 2013, 405, 4607–4616 CrossRef CAS PubMed.
- J. Song, Q. Jiao, L. Zhou, S. Peng, S. Lin, D. J. McClements and W. Liu, Food Biosci., 2025, 64, 105985 CrossRef CAS.
- S. Pandey, T. Varadavenkatesan, R. Selvaraj and R. Vinayagam, Sci. Rep., 2025, 15(1), 4309 CrossRef CAS PubMed.
- V. S. Kumar, D. J. Sarkar, B. K. Das, S. Samanta, G. Tripathi, S. Das Sarkar and S. K. Nag, Aquaculture, 2025, 599, 742121 CrossRef CAS.
- R. Chaiklahan, N. Chirasuwan and B. Bunnag, Process Biochem., 2012, 47, 659–664 CrossRef CAS.
- M. J. Selig, N. M. Malchione, S. Gamaleldin, O. I. Padilla-zakour and A. Abbaspourrad, Food Hydrocolloids, 2018, 74, 46–52 CrossRef CAS.
- K. M. Deamici, D. Figueiredo, I. Guerra, P. Letras and H. Pereira, in Algal Bioreactors: Science, Engineering and Technology of Upstream Processes, 2025, vol. 1, pp. 11–25 Search PubMed.
- R. Carmona, M. Murillo, T. Lafarga and R. Bermejo, J. Appl. Phycol., 2022, 1, 3 Search PubMed.
- T. Lafarga, Algal Res., 2019, 41, 101566 CrossRef.
- H. Sun, Y. Wang, Y. He, B. Liu, H. Mou, F. Chen and S. Yang, Marine Drugs, 2023, 21 DOI:10.3390/MD21020082.
- B. Dincoglu, G. Tensi, Z. Demirel and E. Imamoglu, Systems Microbiology and Biomanufacturing, 2024, 5, pp. 326–334 Search PubMed.
- B. S. O. Colonia, G. V. de Melo Pereira, J. C. de Carvalho, S. G. Karp, C. Rodrigues, V. T. Soccol, L. S. Fanka and C. R. Soccol, Food Chem. Adv., 2023, 2, 100270 CrossRef.
- L. M. R. Almeida, L. F. da S. Cruz, B. A. S. Machado, I. L. Nunes, J. A. V. Costa, E. de S. Ferreira, P. V. F. Lemos, J. I. Druzian and C. O. de Souza, Algal Res., 2021, 58, 102387 CrossRef.
- S. Grahl, M. Strack, R. Weinrich and D. Mörlein, J. Food Qual., 2018, 2018, 1919482 CrossRef.
- S. Sengupta and J. Bhowal, J. Microbiol. Biotechnol. Food Sci., 2017, 6, 1081–1085 CrossRef CAS.
- M. Palanisamy, S. Töpfl, R. G. Berger and C. Hertel, Eur. Food Res. Technol., 2019, 245, 1889–1898 CrossRef CAS.
- C. K. Zen, C. B. V. Tiepo, R. V. da Silva, C. O. Reinehr, L. C. Gutkoski, T. Oro and L. M. Colla, J. Sci. Food Agric., 2020, 100, 2018–2026 CrossRef CAS PubMed.
- N. Nourmohammadi, S. Soleimanian-Zad and H. Shekarchizadeh, J. Sci. Food Agric., 2020, 100, 5260–5268 CrossRef CAS PubMed.
- A. P. Batista, A. Niccolai, P. Fradinho, S. Fragoso, I. Bursic, L. Rodolfi, N. Biondi, M. R. Tredici, I. Sousa and A. Raymundo, Algal Res., 2017, 26, 161–171 CrossRef.
- S. Sarkar, M. S. Manna, T. K. Bhowmick and K. Gayen, Process Biochem., 2020, 96, 58–72 CrossRef CAS.
- B. G. Nabi, K. Mukhtar, W. Ahmed, M. F. Manzoor, M. M. A. N. Ranjha, M. Kieliszek, Z. F. Bhat and R. M. Aadil, Food Biosci., 2023, 52, 102403 CrossRef CAS.
- I. Benucci, C. Lombardelli, C. Mazzocchi and M. Esti, in Comprehensive Reviews in Food Science and Food Safety, Wiley Online Library, 2022, vol. 21, pp. 2715–2737 Search PubMed.
- C. Carrillo, G. Nieto, L. Martinez-Zamora, G. Ros, S. Kamiloglu, P. E. S. Munekata and M. Pateiro, J. Agric. Food Chem., 2022, 70, 6864–6883 CrossRef CAS PubMed.
- H. A. Tavanandi and K. S. M. S. Raghavarao, Bioresour. Technol., 2019, 271, 391–401 CrossRef CAS PubMed.
- N. Martins and I. C. F. R. Ferreira, Trends Food Sci. Technol., 2017, 62, 33–48 CrossRef CAS.
- D. Martirosyan, J. von Brugger and S. Bialow, Funct. Foods Health Dis., 2021, 11, 408–430 CAS.
- C. K. Zen, C. B. V. Tiepo, R. V. da Silva, C. O. Reinehr, L. C. Gutkoski, T. Oro and L. M. Colla, J. Sci. Food Agric., 2020, 100, 2018–2026 CrossRef CAS PubMed.
- A. Ahmadi, S. A. Shahidi, R. Safari, A. Motamedzadegan and A. Ghorbani-HasanSaraei, Food Chem. Toxicol., 2022, 163, 112980 CrossRef CAS PubMed.
- M. M. Elbatanony, A. M. El-Feky, B. A. Hemdan and M. Azab El-Liethy, Acta Ecol. Sin., 2019, 39, 89–94 CrossRef.
- C. Pothiraj, P. Balaji, R. Shanthi, M. Gobinath, R. Suresh Babu, A. A. D. Munirah, A. H. Ashraf, K. Ramesh Kumar, V. Veeramanikandan and R. Arumugam, J. Infect. Public Health, 2021, 14, 1927–1934 CrossRef PubMed.
- M. Dziedziński, J. Kobus-Cisowska, D. Szymanowska-Powałowska, K. Stuper-Szablewska and M. Baranowska, Emir. J. Food Agric., 2020, 32, 229–237 CrossRef.
- S. Sarkar, M. S. Manna, T. K. Bhowmick and K. Gayen, Process Biochem., 2020, 96, 58–72 CrossRef CAS.
- R. Klopsch, S. Baldermann, A. Voss, S. Rohn, M. Schreiner and S. Neugart, Foods, 2019, 8(10), 427 CrossRef CAS PubMed.
- D. Sanna and A. Fadda, Nutraceuticals, 2022, 2, 365–383 CrossRef.
- E. Paskeviciute, Z. Bernadeta and Z. Luksiene, Food Technol. Biotechnol., 2019, 57, 126–132 CrossRef PubMed.
- B. G. Nabi, K. Mukhtar, W. Ahmed, M. F. Manzoor, M. M. A. N. Ranjha, M. Kieliszek, Z. F. Bhat and R. M. Aadil, Food Biosci., 2023, 52, 102403 CrossRef CAS.
- C. Blanco-Llamero, P. García-García and F. J. Señoráns, Marine Molecules from Algae and Cyanobacteria: Extraction, Purification, Toxicology and Applications, 2025, pp. 229–242 Search PubMed.
- B. G. Nabi, K. Mukhtar, W. Ahmed, M. F. Manzoor, M. M. A. N. Ranjha, M. Kieliszek, Z. F. Bhat and R. M. Aadil, Food Biosci., 2023, 52, 102403 CrossRef CAS.
- P. Das Gupta and R. Roy, Environ. Sci. Eng., 2025, Part F1, 511–540 Search PubMed.
- H. F. Chiu, J. Y. Liao, Y. Y. Lu, Y. C. Han, Y. C. Shen, K. Venkatakrishnan, O. Golovinskaia and C. K. Wang, J. Food Biochem., 2017, 41, e12349 CrossRef.
- E. S. Sruthy and E. K. C. Baiju, J. Appl. Phycol., 2025, 6, 74–95 CrossRef.
- A. C. Unni and K. Karunakaran, Thalassas: An International Journal of Marine Sciences, 2025, 41(1), 53 CrossRef.
- P. De Luca, A. Di Stadio, G. Petruzzi, L. de Campora, M. Fior, C. Moretti, V. Della Peruta, F. Mazzola, L. Costarelli, R. Covello, F. Ricciardiello, G. Tortoriello, R. Pellini, M. Radici and A. Camaioni, Head Neck, 2024, 46, 2123–2131 CrossRef PubMed.
- S. Kichouh-Aiadi, J. J. Gallardo-Rodríguez, M. C. Cerón-García, L. López-Rosales, F. García-Camacho and A. Sánchez-Mirón, J. Appl. Phycol., 2024, 36(3), 1169–1179 CrossRef CAS.
- X. Lu, W. Zhao, J. Wang, Y. He, S. Yang and H. Sun, World J. Microbiol. Biotechnol., 2024, 40(7), 210 CrossRef PubMed.
- P. T. H. Nguyen, D. T. Nguyen and T. Vo, Eur. J. Med. Health. Res., 2025, 3, 21–26 CrossRef PubMed.
- B. Schoefs, Trends Food Sci. Technol., 2002, 13, 361–371 CrossRef CAS.
- D. Sundarrajan, T. Ganapathy, P. Pandian, D. Divakaran and I. Suyambulingam, Springer Proceedings in Materials, 2024, 60, 773–813 Search PubMed.
- J. Ma, J. Hu, X. Sha, D. Meng and R. Yang, Crit. Rev. Food Sci. Nutr., 2024, 64, 2999–3017 CrossRef CAS PubMed.
- T. A. Silva, J. Ferreira, J. de S. Castro, M. Q. Braga and M. L. Calijuri, Resour. Conserv. Recycl., 2022, 186, 106575 CrossRef CAS.
- A. Cavallini, S. Torre, L. Usai, M. Casula, G. Fais, P. Nieri, A. Concas and G. A. Lutzu, Sustain. Chem. Pharm., 2024, 40, 101625 CrossRef CAS.
- B. Devliya, B. Patel, A. Prajapati and H. D. Patel, Int. J. Mar. Sci., 2024, 40(2), 959–979 Search PubMed.
- J. Ferreira, M. Q. Braga, R. C. N. da Gama, I. B. Magalhães, B. B. Marangon, J. de S. Castro, J. F. Lorentz, B. S. Henriques, A. S. A. de P. Pereira, P. P. Assemany and M. L. Calijuri, J Clean Prod, 2024, 434, 140526 CrossRef CAS.
- P. Sundararajan and S. P. Ramasamy, Sustain. Chem. Pharm., 2024, 37, 101353 CrossRef CAS.
- R. L. S. Machado, D. A. Dutra, A. T. Schneider, R. R. Dias, M. C. Deprá, L. Q. Zepka and E. Jacob-Lopes, Algal Biorefinery: A Sustainable Solution for Environmental Applications, 2025, pp. 371–383 Search PubMed.
- V. Magalhães, V. Pinto, P. Sousa, J. A. Afonso, L. Gonçalves, E. Fernández and G. Minas, Sens. Actuators, B, 2025, 423, 136819 CrossRef.
- F. Jehalee, W. Maneechote, S. Srinuanpan, W. Pathom-aree, N. Phusunti, A. Mouradov and B. Cheirsilp, Biochem. Eng. J., 2024, 206, 109318 CrossRef CAS.
Footnote |
| † Authors equally contributed and treated as jointed first author. |
| This journal is © The Royal Society of Chemistry 2026 |