Open Access Article
Open Access ArticleValorization of apricot and peach pomaces as sustainable ingredients for the formulation of carotenoid-enriched puddings
Aslı Yıldırım Vardin and
Dilara Konuk Takma
and
Dilara Konuk Takma *
*
Department of Food Engineering, Faculty of Engineering, Aydın Adnan Menderes University, Aydın, 09010, Türkiye. E-mail: dilara.konuk.takma@adu.edu.tr
First published on 2nd January 2026
Abstract
Large-scale production of apricot and peach fruit juices leads to substantial amounts of pomaces, a by-product that is abundant in bioactive substances, dietary fiber, and antioxidants. This study investigated the valorization of apricot and peach pomaces for obtaining carotenoid-enriched puddings. By converting pomace into a powder form, a better flowability was achieved in the peach pomace powder than in the apricot pomace powder. Apricot pomace (AP)- and peach pomace (PP)-enriched puddings at different levels of pomace powders (0%, 10%, 20% and 30% w/w) were evaluated in terms of color, rheological behavior, total soluble solid content, pH, total phenolic content, carotenoid content, antioxidant activity and 5-hydroxymethylfurfural (HMF) content. The natural fruit sugars derived from AP and PP increased the °Brix values of the puddings. AP and PP provided higher total phenolic content (0.96–1.34 mg GAE per g dry basis), antioxidant activity (68.19–82.60%) and carotenoid content in puddings than the control. Redness and yellowness values increased with the addition of pomaces. The HMF content was the lowest (0.04 ppm) in the PP-enriched pudding at a 10% level; however, the content significantly increased in the AP-enriched puddings with increasing pomace ratio. The rheological behavior of the puddings was pseudoplastic (n < 1), and the consistency index of the puddings increased as the fruit pomace content increased. These results illustrate the applicability of incorporating by-products from fruit processing into value-added new food products, providing a sustainable approach to reducing food waste while improving functional properties.
Sustainability spotlightThis study demonstrates a sustainable approach to food production by valorizing apricot and peach pomaces, which are fruit processing by-products, into carotenoid-enriched puddings. Transforming pomace into functional ingredients reduces food waste, promotes circular bioeconomy practices, and contributes to the efficient use of natural resources. The developed puddings not only improve the nutritional quality through enhanced carotenoid and phenolic contents but also align with the global health priorities. |
1. Introduction
The global fruit-processing sector has seen impressive growth over recent decades, propelled by a rising consumer appetite for natural and nutritious beverages. Apricot (Prunus armeniaca L.) and peach (Prunus persica L.) juices are particularly well-regarded due to their delightful flavors and potential health advantages. Nonetheless, this growth has led to a considerable rise in the production of fruit pomace, a by-product of juice extraction. Around 13–15% of apricot flesh is generated as pomace during the processing of fruit juice. This pomace is rich in beneficial compounds like phenolics, dietary fibers, and carotenoids, which may offer health benefits and can be utilized in the food industry.1 Similarly, a significant amount of peach pomace, which is around 10% of the original fruit weight, is generated during peach juice production.2 Often, the disposal of these wastes includes either landfilling or using them as low-value feed for animals, both of which create environmental and economic issues. This situation highlights the importance of finding innovative approaches to repurpose these by-products, converting waste materials into valuable products. Moreover, fruit pomaces are underappreciated by-products, which are abundant in bioactive compounds, dietary fiber, vitamins, minerals, and phenolic substances.3 These constituents possess health-promoting properties, such as antioxidant activity, anti-inflammatory impact, and prebiotic effects, positioning fruit pomace as a prime candidate for functional food innovations.4Today, vitamin A deficiency disorder (VADD) has become a major public health concern. One of its main symptoms is xerophthalmia. The only way to avoid this condition, which is brought on by a lack of vitamin A in regular diets, is to take supplements. About 120 million children worldwide suffer from VADD. Carotenoids, including α-carotene, β-carotene, and β-cryptoxanthin, are essential precursors of vitamin A, and they are commonly used in the food industry as additives and supplements.5 Carotenoids must be acquired through diet because the body is unable to synthesize them. To fulfill daily vitamin A requirements, provitamin A carotenoids must be included in the diet.6 Apricot and peach fruit juice pomaces have been reported to be remarkably rich in carotenoids.7,8
Puddings are well-known food products introduced in the human diet at a very early age because of their easy and high digestion properties. The limited number of ingredients makes them particularly suitable for children.9 Since pudding is widely consumed by all age groups, improving the functionality of pudding is very important. Thus, pudding products represent an attractive and adaptable platform for incorporating fruit pomace into food products. Pudding formulations allow for a versatile medium where various ingredients can be integrated while still maintaining consumer appeal.
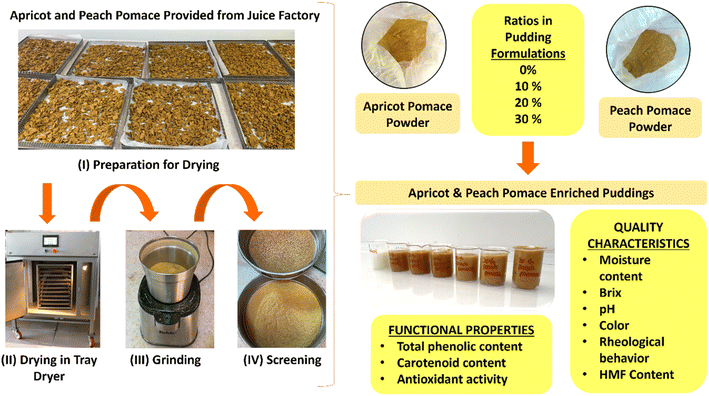
In literature, there are different studies investigating the effects of the incorporation of different wastes, including olive mill wastewater,10 banana peel,11 stevia leaf12 and wheat germ,13 into pudding formulations. However, there is no study investigating the impact of adding apricot and peach pomaces into pudding formulations. By integrating apricot and peach fruit pomaces into puddings, it will not only transform waste into high-value-added products in the market, but the product will also be enriched in terms of carotenoids. The opportunity to enhance the nutritional value of a commonly consumed product and reduce the incidence of vitamin A deficiency, particularly among at-risk groups, can be provided. Therefore, this research intends to investigate the utilization of apricot and peach pomaces as functional components in pudding formulations. The study centers on assessing nutritional profiles in terms of the carotenoid and HMF contents, the functional characteristics, such as total phenolic content and antioxidant activity, and the quality characteristics of the resulting pudding. The schematic representation of the study is presented in Fig. 1.
2. Materials and methods
2.1. Preparation of apricot and peach pomace powders
The peach and apricot pomaces were kindly supplied by DİMES Fruit Juice Factory (İzmir, Turkey). The pomaces were dried using a tray dryer at 60 °C and 1 m s−1 air speed until they reached a constant weight. Then, the samples were ground with the help of a grinder (Fakir Hausgeraete/Germany) and sieved through a 0.5 micrometer sieve, so that all samples were the same size. The samples were stored in aluminum polyethylene (ALPE) bags at +4 °C.2.2. Preparation of pudding samples
The pudding samples were prepared according to the ref. 14 with some modifications. First, the apricot or peach pomace powder (10, 20 or 30 g) was added to 100 g of water. Then, 3.75 g of non-fat dry milk, 2.25 g of saccharose, 2 g of starch and 0.25 g of xanthan gum were added to the separate mixtures. The puddings were left to boil on an electric stove (TAS electric heater) while being constantly stirred until 30 s after boiling began. A control group was produced without the addition of the pomace. The control pudding (C), apricot pomace (AP)-enriched puddings and peach pomace (PP)-enriched puddings were cooled to room temperature and then stored at +4 °C until analysis.2.3. Moisture content
The moisture content of the pudding samples was determined by the oven drying method. Samples of 2 g of pudding were placed in the sampling container and kept in the oven at 105 °C for 8 h. The containers were taken from the oven to the desiccator for cooling before weighing. The final weight, initial weight and weight of the containers were used for the calculation of the moisture content in percentages.2.4. Color
The CIE color values, including L* (lightness), a* (redness or greenness), and b* values (yellowness or blueness), of the pudding samples were determined with a color measurement device (Konica Minolta, Osaka, Japan). The hue angle was calculated by the following eqn (1).15
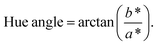 | (1) |
2.5. pH and total soluble solid content
The pH value of the pudding samples was determined using a digital pH meter probe by direct immersion (Hanna Instruments HI 8314, USA). The total soluble solid content of the pudding samples was measured, as °Brix value, using a portable refractometer (AN-KA portable refractometer, Turkey).2.6. Total phenolic content
The extract of the pudding samples was obtained by the described protocol16 with slight modifications. Ten grams of the pudding sample were combined with 20 mL of pure ethanol to achieve a 0.5 kg L−1 (pudding/ethanol) concentration. The mixture was heated in a water bath at 60 °C for 30 min. After heating, the solution was centrifuged at 4000 rpm for 5 min. The phenolic extract was then collected from the resulting supernatant. The same extract was used for the analysis of the total phenolic content and antioxidant activity.The total phenolic content of the pudding samples was determined according to a modified method.17 The extract (200 µL) was mixed with 2500 µL of Folin–Ciocalteu's reagent (10%, v/v) and 2000 µL of 7% (w/v) sodium carbonate. The mixture was incubated at room temperature for 2 h, and then, the absorbance was read at 760 nm using a spectrophotometer. The results were expressed as mg gallic acid equivalents (GAE) per g dry weight (mg GAE per g db) through the standard curve of gallic acid.
2.7. Antioxidant activity
The antioxidant activity of the pudding extracts was measured according to the method based on DPPH (2,2-diphenyl-1-picrylhydrazyl) radical scavenging.18 The extract (0.2 mL) was transferred into a test tube containing 3 mL of 6 × 10−5 M solution of DPPH. The mixture was kept in the dark for 30 min at room temperature, and then, the absorbance was measured at 515 nm using a UV-vis spectrophotometer. Antioxidant activity was calculated by the percentage inhibition, as presented in eqn (2), where Acontrol and Asample mean the absorbances of the DPPH solution and the sample, respectively. The results were also expressed as mg Trolox per g dry weight (mg Trolox per g db) through the standard curve of Trolox.
 | (2) |
2.8. Total carotenoid content
The total carotenoid amount was analyzed using previously studied spectrophotometric methods.19,20 First, the sample was mixed with an extraction solvent (hexane/acetone/ethanol; 50/25/25) and homogenized using Ultra Turrax for 30 s. Then, the mixture was centrifuged (Centurion Scientific Benchtop Centrifuges, K241). In the next step, absorbance was measured using a spectrophotometer (Shimadzu/Japan) at a wavelength of 450 nm. The carotenoid amount of the samples was determined as ppm of β-carotene according to eqn (3) which takes into account the molar absorption coefficient as E1%; E1cm = 2505.| c = (a/E × b) × 1000, | (3) |
2.9. HMF (5-hydroxymethylfurfural) content
HMF analysis was performed by the previously described high-performance liquid chromatography (HPLC, Agilent Technologies, California/USA) method.21 The mobile phase was prepared with a mixture of 80% water and 20% methanol with a flow rate of 1 mL min−1. The chromatographic separation was performed at a wavelength of 285 nm using a Diode Array Detector (DAD). The column used in the analysis was a C18 type column with a normal diameter of 3 micrometers, and the column dimensions were 150 × 4.6 mm (L × ID). The HMF content of the samples was determined in ppm using different amounts of HMF standards.2.10. Rheological measurement
The viscosity of the pudding samples was assessed using a viscometer (Fungilab Expert Series, Barcelona, Spain) with a rotating spindle (TR10) and continuous rotation speed increasing from 0 to 200 rpm between 0 and 5 min. Following production, the pudding samples were kept at 4 °C for 24 h and then subjected to viscosity analysis. Before analyzing, the samples were allowed to equilibrate to room temperature and diluted with water at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio. The relationship between shear stress (τ, in Pa) and shear rate (γ, in s−1) for the pudding samples was assessed by gradually increasing the applied shear rates. The obtained data, representing shear stress (Pa) as a function of shear rate (s−1), were modeled using the Herschel–Bulkley equation (eqn (4)). In this model, τ denotes the shear stress, K is the consistency index, γ represents the shear rate, and n is the flow behavior index. Nonlinear regression analysis was performed to fit the data to the Herschel–Bulkley model, utilizing SPSS 15 software.
1 ratio. The relationship between shear stress (τ, in Pa) and shear rate (γ, in s−1) for the pudding samples was assessed by gradually increasing the applied shear rates. The obtained data, representing shear stress (Pa) as a function of shear rate (s−1), were modeled using the Herschel–Bulkley equation (eqn (4)). In this model, τ denotes the shear stress, K is the consistency index, γ represents the shear rate, and n is the flow behavior index. Nonlinear regression analysis was performed to fit the data to the Herschel–Bulkley model, utilizing SPSS 15 software.| τ = τ0 + Kγn. | (4) |
2.11. Statistical analysis
The results were evaluated statistically using the SPSS Programme (version 22.0; SPSS Inc., Chicago, IL). The difference between the group means was determined by the analysis of variance (ANOVA). The significance of the difference was determined by the Duncan multiple comparison test (p < 0.05).3. Results & discussion
3.1. Powder properties
The physical characteristics of the apricot and peach pomace powders, such as bulk density, tapped density, and Carr's index, are presented in Table 1. These factors are essential for assessing the flowability, compressibility, and handling properties of the powders, which are significant for potential industrial uses, especially in food and pharmaceutical applications.| Apricot pomace powder | Peach pomace powder | |
|---|---|---|
| a Different letters in the same line indicate significant differences between the apricot and peach pomace powders (p < 0.05). | ||
| Bulk density (g mL−1) | 0.61 ± 0.01 | 0.62 ± 0.02 |
| Tapped density (g mL−1) | 0.81 ± 0.01 | 0.75 ± 0.02 |
| Carr's index | 24.00 ± 0.00b | 18.00 ± 0.00a |
The apricot pomace powder had a bulk density of 0.61 ± 0.01 g mL−1, which was similar to that of the peach pomace powder (0.62 ± 0.02 g mL−1). There were no significant differences between the different pomace powders (p < 0.05). Similar particle size distributions and morphological characteristics are probably the cause of these findings, which imply that both powders have comparable particle arrangements in their loose states. As opposed to the peach pomace powder (0.75 ± 0.02 g mL−1), the apricot pomace powder had a slightly higher tapped density (0.81 ± 0.01 g mL−1), but this difference was not statistically significant (p < 0.05). In powder products, compacted density is important in terms of transportability, packaging, storage and commercialization. Both powders showed similar compressibility and higher tapped density than the microencapsulated powders in the literature.22
The bulk and tapped densities of the apricot and peach pomace powders were similar, but their Carr's index values showed notable variations in flowability and compressibility. The apricot pomace powder had a significantly higher Carr's index than the peach pomace powder, which was a measure of the powder's flowability and compressibility. According to Carr's index value, low values (Carr's index ≤ 10) indicate excellent flowability, while as the values increase (11–15: good, 16–20: fair, 21–25: passable), flowability decreases; high values (Carr's index ≥ 32) indicate poor flowability.22 Comparing the peach and apricot pomace powders based on Carr's index (18 and 24) values, the flow characteristics of the peach pomace powder were superior to those of the apricot pomace powder. Relatively fine powders have increased interparticle cohesive forces; thus, they tend to exhibit poor flowability.23 That can be due to the variations in particle shape and cohesion forces, which hinder the flowability. The apricot pomace powder had a fair flowability, while the peach pomace powder presented a good flowability. The physical properties of the pomace powders had a fundamental role in their applications as functional ingredients or fillers in various food products. The high flowability of the peach pomace powders indicates that it may be relatively simple to handle and combine in dry formulations.
3.2. Color
The color parameters (L*, a* and b* values) of the pudding samples that contained the apricot and peach pomaces are presented in Table 2. The visual appearance of the pudding samples containing apricot and peach pomaces at 0%, 10%, 20% and 30% is also presented in Fig. 2. Notable differences (p < 0.05) were detected among the samples, suggesting that the addition of pomace affected the visual characteristics of the puddings. The control sample had the highest L* value (69.61 ± 0.57), indicating it had the lightest color. However, the presence of the apricot and peach pomaces led to a significant decrease in L* values, with samples containing 20% and 30% apricot pomace showing the lowest values (39.37 ± 0.21 and 39.94 ± 0.28, respectively). This reduction in lightness was likely due to the naturally dark pigment found in the apricot and peach pomaces.24 The a* values, representing the red-green spectrum, were also significantly affected by the addition of the fruit pomace. The control sample had the lowest a* value (5.66 ± 0.80), while the sample with 30% peach pomace showed the highest value (14.01 ± 0.15). This increase in the a* values can be attributed to the natural red-like color of apricot, which is linked to its high carotenoid content.24| L* | a* | b* | Hue | |
|---|---|---|---|---|
| a Different letters in the same column indicate significant differences between the pudding samples (p < 0.05). | ||||
| Control | 69.61 ± 0.57e | 5.66 ± 0.80a | 9.60 ± 0.43a | 59.58 ± 2.42d |
| 10% apricot pomace | 40.68 ± 0.04b | 11.61 ± 0.44c | 27.24 ± 0.14d | 66.93 ± 0.77a |
| 20% apricot pomace | 39.37 ± 0.21a | 12.24 ± 0.05d | 27.75 ± 0.06d | 66.19 ± 0.10a,b |
| 30% apricot pomace | 39.94 ± 0.28a | 12.37 ± 0.21d | 26.29 ± 0.43c | 64.81 ± 0.32b |
| 10% peach pomace | 43.69 ± 0.65c | 10.34 ± 0.33b | 22.25 ± 0.80b | 65.07 ± 0.22b |
| 20% peach pomace | 45.35 ± 0.20d | 11.49 ± 0.26c | 22.74 ± 0.56b | 63.19 ± 0.07c |
| 30% peach pomace | 45.60 ± 0.41d | 14.01 ± 0.15e | 27.71 ± 0.56d | 63.18 ± 0.22c |
 | ||
| Fig. 2 Visual appearance of the pudding samples, including apricot and peach pomaces at 0%, 10%, 20% and 30%. | ||
For the b* value, indicating the yellow-blue spectrum, the addition of the pomace resulted in an increase in the yellowness of the pudding samples. The control sample had the lowest b* value (9.60 ± 0.43), whereas the highest values were recorded in the samples with 20% apricot pomace (27.75 ± 0.06) and 30% peach pomace (27.71 ± 0.56). The high b* values corresponded with research conducted using apple pomace as a food fortification ingredient,25 which highlighted an increase in yellowness with the fruit pomace integration.
The ranges of the hue angle of the AP and PP-enriched puddings were 66.93–64.81 and 65.07–63.18, respectively. The average hue angle of the control pudding was 59.58, which indicated that the pomace addition significantly (p < 0.05) affected the color of the puddings. The hue angle starting from 0° represents the red-purple color, turning a yellow color by progressing toward 90°. Thus, the AP-enriched puddings indicated a more yellow color than the PP-enriched puddings. The observed color changes can greatly affect consumer acceptance, as color is crucial in food quality perception. These results are consistent with prior studies that demonstrate the contribution of fruit by-products, such as pomace, to products with dark and vibrantly colored appearances due to their inherent pigments.26 These findings may emphasize the potential of the apricot and peach pomaces as functional ingredients for creating visually attractive puddings.
3.3. °Brix and pH values
The °Brix and pH values of the pudding samples containing apricot and peach pomace are presented in Table 3. There was a significant increase in the °Brix values (p < 0.05) with the addition of both apricot and peach pomaces at different concentrations. The control sample showed the lowest °Brix value at 4.50 °Bx, whereas the sample with 30% peach pomace had the highest °Brix value at 21.00 °Bx. This considerable increase in the °Brix values with an increasing pomace level can be attributed to the natural sugar content of fruits, which can be dissolved when added to the food formulations.27 Conversely, the pH values exhibited a significant decline with increased pomace levels (p < 0.05), as was previously reported in the literature.28 The control sample had the highest pH value (7.02), while the sample with 30% peach pomace showed the lowest pH value (4.04). This reduction in pH may be associated with the acidic character of fruit pomaces due to the presence of natural acids.| °Brix (°Bx) | pH | Moisture content (%) | TPC (mg GAE per g db) | AA (% inhibition) | AA (mg Trolox per g db) | |
|---|---|---|---|---|---|---|
| a Different letters in the same column indicate significant differences between the pudding samples (p < 0.05). | ||||||
| Control | 4.50 ± 0.41a | 7.02 ± 0.00f | 91.63 ± 0.08a | 0.15 ± 0.06e | 5.64 ± 1.00d | 0.43 ± 0.08f |
| 10% apricot pomace | 9.50 ± 0.41b | 4.64 ± 0.01e | 82.74 ± 0.23b | 1.14 ± 0.02b,c | 68.19 ± 1.41c | 4.16 ± 0.09e |
| 20% apricot pomace | 13.50 ± 0.41c | 4.35 ± 0.00c | 76.97 ± 0.38c | 1.25 ± 0.10a,b | 71.98 ± 0.75c | 4.39 ± 0.04d |
| 30% apricot pomace | 20.50 ± 0.41e | 4.21 ± 0.01b | 71.42 ± 0.04e | 1.34 ± 0.02a | 76.63 ± 3.19b | 4.67 ± 0.19c |
| 10% peach pomace | 10.50 ± 0.41b | 4.57 ± 0.01d | 82.97 ± 0.11b | 1.07 ± 0.09c,d | 70.12 ± 0.75c | 4.28 ± 0.04d,e |
| 20% peach pomace | 15.50 ± 0.41d | 4.21 ± 0.00b | 76.18 ± 0.06d | 0.96 ± 0.04d | 79.02 ± 1.69a,b | 4.81 ± 0.10b |
| 30% peach pomace | 21.00 ± 0.82e | 4.04 ± 0.01a | 70.59 ± 0.25f | 1.16 ± 0.03b,c | 82.60 ± 2.44a | 5.02 ± 0.15a |
3.4. Moisture content
The control sample, without the addition of the apricot or peach pomace, presented the highest moisture content, higher than 90%. The addition of the apricot pomace resulted in the moisture content changing from 71.42% to 82.74%. Similarly, the addition of the peach pomace afforded a moisture content between 70.59% and 82.97%. The moisture content of the pudding samples enriched with both apricot and peach pomaces decreased as the percentage of the pomaces increased, and this trend was in line with the °Brix values. The significant increase in the °Brix value due to the increased pomace ratio resulted in a significant decrease in the moisture value. The moisture content plays an important role as it will also affect the quality properties of the pudding, such as color and consistency. The addition of the apricot and peach pomace, natural ingredients derived from fruit juice, into the pudding formulation enhanced its dry matter content.3.5. Total phenolic content and antioxidant activity
As the apricot and peach pomaces are rich in carotenoids, they are also abundant sources of phenolic compounds. Therefore, the control sample had the lowest total phenolic content, while the samples enriched with the pomace showed a significant (p < 0.05) increase in the total phenolic content. The total phenolic content of the pudding samples enriched with the apricot pomace was found to be higher than that of the samples enriched with the peach pomace. The highest phenolic content was determined to be 1.34 mg GAE per g dry basis in the pudding samples containing 30% apricot pomace. The phenolic contents of the pudding samples were higher compared to those of lactose-free and no-sugar-added tigernut milk pudding, which was formulated in a recent study.29 Novel tigernut milk pudding had a phenolic content ranging from 39.06 mg GAE per 100 g to 54.81 mg GAE per 100 g. The range in the presented study was between 96 mg GAE per 100 g dry basis and 134 mg GAE per 100 g dry basis for the phenolic content. The addition of the apricot and peach pomaces improved the phenolic content of the pudding. A similar trend was observed in the study incorporating lyophilized fruit powders into pudding formulations. The addition of apricot, plum–apricot, and plum powders significantly enhanced the levels of phenols in the puddings. The total phenolic content was reported to be between 0.05 and 0.37 mg GAE per g for the pudding formulated with plum and apricot powder.30The antioxidant activities of the pudding samples were evaluated by the DPPH radical scavenging method. While the inhibition value of the control sample was determined as 5.64%, those of the apricot-pulp-added puddings were found to be between 68.19% and 76.63%, and those of the peach-pulp-added puddings were found to be between 70.12% and 82.60%. The antioxidant activity results, also measured as mg Trolox per g dry basis, demonstrated a statistically significant (p < 0.05) difference in all puddings containing the apricot and peach pomaces compared with the control group. The Trolox-equivalent antioxidant activities of the pomace-enriched puddings were between 4.16 and 5.02 mg Trolox per g dry basis. While the puddings containing 10% apricot and peach pomaces were statistically in the same group, the antioxidant activities increased significantly as the pomace content increased. The highest increase, reaching a value from 4.28 to 5.02, was achieved with the addition of the peach pomace. Similar trends have been reported in another study, which reported increased antioxidant activity from 0.498 to 4.655 µM TEAC per g product in the production of puddings containing apricot and plum powders.30 It is seen that increasing the apricot and peach pomace concentrations has a positive effect on the antioxidant activity of the pudding. The addition of 30% apricot and peach pomaces to the formulation increased the inhibition values significantly. In addition, the inhibition values and total phenolic substance amounts showed the same trend. Considering that phenolic compounds are known for their free radical scavenging properties, this parallelism is an expected result. High phenolic content contributed significantly to the total antioxidant activities of the samples, and this relationship became more pronounced with increasing concentrations. The correlated results prove that the apricot and peach pomace-added puddings are rich in bioactive compounds compared with standard puddings.
3.6. Rheological properties
The Herschel–Bulkley model was used to describe the flow behavior of the newly formulated carotenoid-enriched pudding samples by obtaining determination coefficients (R2) higher than 0.90. The rheological properties of the pudding samples, including flow behaviour index (n), consistency index (K) and yield stress (τ0), are presented in Table 4. The pudding samples presented a pseudoplastic behavior, according to the Herschel–Bulkley model. The model parameter that demonstrated the pseudoplastic behavior of the pudding samples was the flow behavior index, which was found to be between 0 and 0.5 (0.000 ≤ n ≤ 0.527). The values of n lower than 1 reportedly indicate pseudoplastic behavior in pudding.31 The pudding samples with different levels of okra gum extract indicated n values in the range of 0.25–0.54. The decrease in the flow behavior index (n) with the increase in the pomace content emphasized an intense pseudoplastic nature.| Sample | τ0 (Pa) | K (Pa sn) | n | R2 |
|---|---|---|---|---|
| a τ0: yield stress, K: consistency index, n: flow behaviour index, R: regression coefficient. | ||||
| 10% apricot pomace | 0.456 ± 0.046 | 9.424 ± 0.275 | 0.527 ± 0.068 | 0.962 |
| 20% apricot pomace | 7.077 ± 0.887 | 39.208 ± 0.536 | 0.484 ± 0.026 | 0.995 |
| 30% apricot pomace | 78.597 ± 6.104 | 166.231 ± 14.946 | 0.112 ± 0.039 | 0.977 |
| 10% peach pomace | 5.744 ± 0.903 | 19.664 ± 0.646 | 0.393 ± 0.037 | 0.989 |
| 20% peach pomace | 3464.741 ± 164.790 | 3450.790 ± 104.760 | 0.001 ± 0.070 | 0.935 |
| 30% peach pomace | 5746.763 ± 119.300 | 5903.518 ± 100.180 | 0.000 ± 0.027 | 0.907 |
The pudding samples indicated an increase in the consistency index as the pomace composition increased. The consistency index ranged from 9.424 to 166.231 Pa sn and 19.664 to 5903.518 Pa sn for the apricot and peach pomace-enriched pudding samples, respectively. The pectin derived from the apricot and peach pomaces probably caused an increase in consistency. Pectin is one of the major cell wall constituents in apricot and peach fruits, followed by hemicellulose and cellulose.32 Similarly, an increase in yield stress was evaluated with increasing pomace content. The results were in accordance with the factors related to the composition of the product. The yield stress of different kinds of sauce samples increased with the content of starch. The values of the consistency index estimated by the Herschel–Bulkley model changed from 4.614 to 41.313 Pa sn, depending on the temperature, in meat sauce.33
3.7. HMF content
5-Hydroxymethylfurfural (HMF) serves as a significant marker for thermal degradation in food items, especially those that include reducing sugars. Its production is affected by processing methods, ingredient compositions and storage conditions. The 5-hydroxymethylfurfural content of the pudding samples fortified with the apricot and peach pomaces is presented in Fig. 3.While HMF was not detected in the control samples, its presence was observed in samples containing both apricot pomace (0.48–1.72 ppm) and peach pomace (0.04–0.09 ppm). The findings indicate a distinct difference in the HMF accumulation between the apricot pomace-enriched puddings and those containing peach pomace. Data indicate that the puddings containing apricot pomace showed a considerable rise in HMF levels as the pomace concentration increased. Specifically, with a 10% apricot pomace addition, the HMF concentration was 0.48 ± 0.02 ppm, rising to 1.00 ± 0.07 ppm at 20%, and peaking at 1.72 ± 0.04 ppm at 30%. This pattern implies that the apricot pomace plays a role in HMF generation, likely due to its sugar composition or its interactions with other pudding components during thermal processing. The presence of fructose may lead to higher HMF accumulation than with sucrose and glucose.34 Moreover, the fructose contents of 13 different apricot cultivars ranged between 10.61 and 44.29 g kg−1,35 while the fructose contents of 19 different peach and nectarine cultivars were between 6.76 and 12.97 g kg−1.36 Accordingly, the elevated HMF levels observed in the samples containing the apricot pomace can be ascribed to its high fructose content. However, the maximum HMF level detected in the puddings (1.72 ± 0.04 ppm) was below the standard limit (≤40 mg kg−1) specified in the Codex international standards.37 This regulation for honey indicated that appropriate HMF levels were found in the present study.
In contrast, the puddings with the peach pomace displayed substantially low HMF amounts throughout all concentration levels. The HMF content was not significantly (p > 0.05) different between the 10% PP-enriched pudding and the control pudding, in which HMF was not observed. Even at the maximum level (30%), the HMF concentration remained under 0.10 ppm. This discrepancy could be attributed to the differences in the sugar composition of the peach pomace, which may limit the degradation of sugars into HMF under the applied processing conditions. Conversely, the control samples exhibited minimal HMF presence, reinforcing that the inclusion of fruit-derived components affected HMF generation. The markedly elevated HMF levels noted in the formulations with apricot pomace suggested that this ingredient could enhance the Maillard reaction pathway, resulting in heightened HMF accumulation. While moderate HMF exposure is typically seen as safe, excessive levels have raised concerns regarding its potential cytotoxic and mutagenic effects. Therefore, it is crucial to optimize processing conditions and ingredient choices to reduce excessive HMF production in the newly formulated puddings.
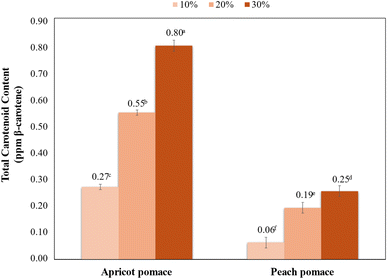
3.8. Total carotenoid content
The addition of apricot and peach pomaces to functional pudding formulations had a considerable effect on the total carotenoid levels, as indicated in Fig. 4. The findings suggest a positive relationship between the amount of fruit pomace and the total carotenoid content, expressed as ppm β-carotene. The control sample showed a low carotenoid level (0.04 ± 0.00 ppm), highlighting the significance of pomace enrichment in boosting the nutritional and functional quality of the pudding. Among the formulations examined, apricot pomace had a more significant effect on the total carotenoid content compared with peach pomace. With a 10% fortification level, the apricot pomace raised the carotenoid content to 0.27 ± 0.00 ppm, while the peach pomace at the same concentration resulted in a low increase (0.06 ± 0.01 ppm). This pattern persisted at high levels, with 20% and 30% apricot pomace producing 0.55 ± 0.00 ppm and 0.80 ± 0.00 ppm, respectively, while the corresponding peach pomace formulations achieved 0.19 ± 0.00 ppm and 0.25 ± 0.01 ppm, respectively. The total carotenoid content of peach peels was reported as 1.78–19.83 g kg−1,38 while the β-carotene content of peach peels was found between 7.5 and 16.4 µg g−1.39Our results imply that the apricot pomace is a more effective source of carotenoids. The remarkable increase in the carotenoid content found in the puddings enriched with apricot pomace showed its potential role in creating functional foods that possess enhanced bioactive properties. Carotenoids, especially β-carotene, are widely recognized for their antioxidant qualities and provitamin A activity, which are essential for human well-being. The pudding enriched with apricot pomace can attain close to 20 times greater carotenoid levels than the control sample, illustrating its effectiveness as a functional ingredient. In contrast, the comparatively modest increase observed with the peach pomace indicates that its contribution to total carotenoid levels is limited. Consequently, the apricot pomace proves to be a functional ingredient for boosting the carotenoid profile of pudding formulations, whereas the peach pomace functions as a supplementary component. However, considering that the apricot pulp causes a significant increase in the amount of HMF, the use of the peach pulp would be more appropriate.
4. Conclusion
The results highlight how apricot and peach pomaces can be used as sustainable ingredients to improve the functional properties of food products. The valorization of the peach and apricot pomaces through their addition into functional pudding formulations has shown significant potential to improve the functional and nutritional properties of the final product. The results showed that adding peach and apricot pomaces increased the total phenolic content, antioxidant activity, and carotenoid levels, offering a promising approach for the development of nutrient-enriched foods. Moreover, the changes in the pudding characteristics, including reduced L* (lightness) and increased a* and b* values (redness and yellowness), indicate their effect on the visual appearance. The apricot pomace provided carotenoid enrichment at lower concentrations than the peach pomace, and it is required to use percentages lower than 20% due to the HMF formation. In contrast, the peach pomace can be safely incorporated into puddings in a range of 0–30%. However, the concentration of carotenoids derived from the peach pomace was poor. The findings demonstrate that the apricot pomace is a more effective functional ingredient for enhancing the carotenoid profile of pudding formulations, whereas the peach pomace may act as a supplementary component. However, a limitation of the apricot pomace enrichment was HMF formation in the puddings. Alternative processing techniques, such as controlled thermal processes or enzymatic alterations, can be investigated in the next step to prevent HMF production while preserving the quality characteristics of the AP-enriched puddings. The apricot and peach pomaces were evaluated in terms of quality characteristics, functional properties and techno-functionality in pudding production for sustainable food technologies. Future research should focus on the sensory evaluation of newly formulated puddings in the appropriate range of the selected pomace content.Author contributions
The authors declare that they have contributed equally to all aspects of this study, including conceptualization, methodology, formal analysis, data curation, and writing – original draft. All authors have reviewed the manuscript.Conflicts of interest
The authors declare that there is no conflict of interest.Data availability
All relevant data and information are available within the article.Acknowledgements
The authors would like to thank the DİMES Fruit Juice Factory (İzmir, Türkiye) for providing peach and apricot pomaces.References
- E. D. Kasapoğlu, S. Kahraman and F. Tornuk, J. Food Meas. Char., 2021, 15, 5277–5287, DOI:10.1007/s11694-021-01089-0.
- C. Baltacıoğlu, H. Baltacıoğlu, I. Okur, M. Yetişen and H. Alpas, J. Food Sci., 2024, 89, 1672–1683, DOI:10.1111/1750-3841.16972.
- A. Iqbal, P. Schulz and S. S. Rizvi, Food Biosci., 2021, 44, 101384, DOI:10.1016/j.fbio.2021.101384.
- H. C. Karantonis, A. Tsoupras, D. Moran, I. Zabetakis and C. Nasopoulou, in Functional Foods and Their Implications for Health Promotion, Academic Press, 2023, pp. 131–159, DOI:10.1016/B978-0-12-823811-0.00007-9.
- M. Rodriguez-Concepcion, J. Avalos, M. L. Bonet, A. Boronat, L. Gomez-Gomez, D. Hornero-Mendez, M. C. Limon, A. J. Meléndez-Martínez, B. Olmedilla-Alonso, A. Palou, J. Ribot, M. J. Rodrigo, L. Zacarias and C. Zhu, Prog. Lipid Res., 2018, 70, 62–93, DOI:10.1016/j.plipres.2018.04.004.
- D. Dutta, A. Nayak and A. D. Dutta, Food Bioprocess Technol., 2023, 16, 467–491, DOI:10.1007/s11947-022-02888-z.
- I. Makrygiannis, V. Athanasiadis, E. Bozinou, T. Chatzimitakos, D. P. Makris and S. I. Lalas, Biomass, 2022, 2, 334–347, DOI:10.3390/biomass2040022.
- S. Lalas, A. Alibade, E. Bozinou and D. P. Makris, Beverages, 2019, 5, 43, DOI:10.3390/beverages5030043.
- D. Mihaylova, A. Popova, Z. Goranova, D. Petkova, P. Doykina and A. Lante, Foods, 2021, 10, 2563, DOI:10.3390/foods10112563.
- U. G. Spizzirri, P. Caputo, C. Oliviero Rossi, P. Crupi, M. Muraglia, V. Rago, R. Malivindi, M. L. Clodoveo, D. Restuccia and F. Aiello, Foods, 2022, 11, 158, DOI:10.3390/foods11020158.
- N. F. Sadek, IOP Conf. Ser.: Earth Environ. Sci., 2024, 1324(1), 012117, DOI:10.1088/1755-1315/1324/1/012117.
- M. Mujianto, L. Zalizar, D. Damat, R. Relawati, B. Harahap, I. Iswahyudi and S. Sustiyana, Environ. Agric. Manage., 2024, 1, 29–40, DOI:10.31102/eam.2024.1.1.29-40.
- M. Majzoobi, F. Ghiasi, M. H. Eskandari and A. Farahnaky, Foods, 2022, 11, 1815, DOI:10.3390/foods11121815.
- W. Wang, Y. Zhao, L. He, Z. Song, C. Shi, P. Jia, Q. Yu and L. Han, Food Chem.: X, 2024, 22, 101327, DOI:10.1016/j.fochx.2024.101327.
- C. Ngamlerst, P. Prangthip, B. Leelawat, S. Supawong and S. Vatthanakul, Foods, 2022, 11, 2555, DOI:10.3390/foods11172555.
- Y. Sun, S. Hayakawa, M. Ogawa and K. Izumori, Food Control, 2007, 18, 220–227, DOI:10.1016/j.foodcont.2005.09.019.
- L. A. de la Rosa, E. Alvarez-Parrilla and F. Shahidi, J. Agric. Food Chem., 2010, 59, 152–162, DOI:10.1021/jf1034306.
- S. Sangsrichan and W. Wanson, KMITL Sci. Technol. J., 2008, 8, 68–73 Search PubMed.
- K. N. Han, H. Meral and A. Demirdöven, J. Food Sci. Technol., 2024, 1–13, DOI:10.1007/s13197-024-06001-4.
- H. S. Lee and W. S. Castle, J. Agric. Food Chem., 2001, 49, 877–882, DOI:10.1021/jf000654r.
- M. Koç, PhD thesis, Ege University, Institute of Science and Technology, 2015.
- A. Goyal, V. Sharma, M. K. Sihag, S. K. Tomar, S. Arora, L. Sabikhi and A. K. Singh, Powder Technol., 2015, 286, 527–537, DOI:10.1016/j.powtec.2015.08.050.
- R. Suhag, A. Kellil and M. Razem, Powders, 2024, 3, 65–76, DOI:10.3390/powders3010006.
- I. Makrygiannis, V. Athanasiadis, T. Chatzimitakos, M. Mantiniotou, E. Bozinou and S. I. Lalas, Waste, 2024, 2, 1–28, DOI:10.3390/waste2010001.
- B. Antonic, S. Jancikova, D. Dordevic and B. Tremlova, J. Food Sci., 2020, 85, 2977–2985, DOI:10.1111/1750-3841.15449.
- D. Magalhães, R. Gonçalves, C. V. Rodrigues, H. R. Rocha, M. Pintado and M. C. Coelho, Foods, 2024, 13, 2276, DOI:10.3390/foods13142276.
- R. O. Adetunji, C. Ebute, N. O. Alamuoye and O. O. Awolu, IPS J. Nutr. Food Sci., 2024, 3, 255–261, DOI:10.54117/ijnfs.v3i4.64.
- L. Davis, J. Jung, A. Colonna, A. Hasenbeck, V. Gouw and Y. Zhao, J. Food Sci., 2018, 83, 1921–1932, DOI:10.1111/1750-3841.14196.
- S. P. Kalahal, M. Gavahian and J. Lin, Int. J. Food Sci. Technol., 2024, 59, 4593–4607, DOI:10.1111/ijfs.17183.
- A. Popova, P. Doykina, D. Mihaylova and M. Dimitrova-Dimova, Dairy, 2024, 5, 688–701, DOI:10.3390/dairy5040051.
- A. A. A. Abdo Qasem, M. S. Alamri, A. A. Mohamed, S. Hussain, K. Mahmood and M. A. Ibraheem, J. Food Process. Preserv., 2017, 41, e12931, DOI:10.1111/jfpp.12931.
- C. Kurz, R. Carle and A. Schieber, Food Chem., 2008, 106, 421–430, DOI:10.1016/j.foodchem.2007.05.078.
- V. C. Okonkwo, O. I. Mba, E. M. Kwofie and M. O. Ngadi, Food Bioprocess Technol., 2021, 14, 2146–2160, DOI:10.1007/s11947-021-02709-9.
- A. Choudhary, V. Kumar, S. Kumar, I. Majid, P. Aggarwal and S. Suri, Toxin Rev., 2021, 40, 545–561, DOI:10.1080/15569543.2020.1756857.
- E. Demiray, S. E. Karatay and G. Dönmez, Braz. Arch. Biol. Technol., 2021, 64, e21200781, DOI:10.1590/1678-4324-2021200781.
- M. Colarič, F. Štampar and M. Hudina, Acta Agric. Slov., 2004, 83, 53–61, DOI:10.14720/aas.2004.83.1.15558.
- Codex Alimentarius Commission, Revised Codex Standard for Honey Codex Stan 12-1981, Rev. 1 (1987), Rev. 2 (2001), Codex Stand., 2001, vol. 12, pp. 1–7 Search PubMed.
- H. Zhou, Z. Yu and Z. Ye, Sci. Hortic., 2018, 239, 123–132, DOI:10.1016/j.scienta.2018.05.036.
- A. F. Brown, G. G. Yousef, I. Guzman, K. K. Chebrolu, D. J. Werner, M. Parker, K. Gasic and P. Perkins-Veazie, J. Am. Soc. Hortic. Sci., 2014, 139, 676–686, DOI:10.21273/JASHS.139.6.676.
| This journal is © The Royal Society of Chemistry 2026 |