Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Intra-myocellular lipid enrichment of differentiated bovine satellite cells through beef-like fatty acid mixtures
Waris Mehmooda,
Anupam Abraham a,
Polina Rabinovich-Toidmanb,
Neta Lavonb,
Margrethe Therkildsena,
Jette Feveile Young
a,
Polina Rabinovich-Toidmanb,
Neta Lavonb,
Margrethe Therkildsena,
Jette Feveile Young a and
Martin Krøyer Rasmussen
a and
Martin Krøyer Rasmussen *a
*a
aDepartment of Food Science, Aarhus University, Denmark. E-mail: martink.rasmussen@food.au.dk
bAleph Farms Ltd, 1 Haim Holtzman Street, Rehovot, 7670401, Israel
First published on 1st December 2025
Abstract
The intramuscular fats in meat are responsible for generating taste and flavor during cooking. This study aimed to mimic the intramuscular fat in conventional beef by customizing the fatty acid composition of the media for cultivated meat. Differentiated bovine satellite cells were exposed to oleic acid, linoleic acid, linolenic acid, stearic acid, and palmitic acid, as well as designed mixtures thereof, in serum-free media. Lipid uptake and accumulation were monitored using Bodipy staining after 24, 48, and 72 hours of exposure. Concentrations up to 200 µM of individual unsaturated fatty acids and 40 µM of saturated fatty acids were not toxic to differentiated satellite cells, and intracellular lipid droplet accumulation was higher after exposure to unsaturated fatty acids (oleic, linoleic, and linolenic acids) than after exposure to saturated fatty acids (stearic and palmitic acids). Interestingly, a cocktail of saturated fatty acids (palmitic and stearic) at 80 µM demonstrated an additive effect on cell lipid uptake into droplets compared with individual exposures, whereas a cocktail of unsaturated fatty acids (linolenic, linoleic and oleic) did not induce uptake beyond that of the individual fatty acids. A mixture of fatty acids mimicking the composition of beef at a concentration of 400 µM resulted in the highest lipid droplet accumulation without compromising cell viability. In summary, lipid uptake was more pronounced when exposed to unsaturated fatty acids than when exposed to saturated fatty acids. Results presented here have implications for the future development of palatable cultivated meat products.
Sustainability spotlightCultivated meat is recognized as a sustainable alternative to conventional livestock production, offering significant reductions in land use, greenhouse gas emissions, and water consumption. A key determinant of its commercial success is the ability to replicate the flavor of traditional meat, which is largely influenced by intramuscular fat content. This study introduces a serum-free, cell-based approach to enhance the lipid accumulation in differentiated bovine satellite cells through exposure to tailored fatty acid mixtures that mimic the composition of conventional beef. By circumventing the need for adipocyte co-culture and employing defined, non-toxic lipid formulations, the method streamlines the production and minimizes the resource input. The use of serum-free media further supports sustainability by eliminating animal-derived components. The rational design of fatty acid mixtures enables controlled lipid enrichment without compromising cell viability, facilitating scalable and reproducible cultivated meat production with improved sensory attributes. |
Introduction
The emerging cultivated meat industry has gained significant attention for its potential to provide alternative meat products with reduced environmental impact.1 Cultivated meat can be produced from a combination of various cell types, primarily from muscle cells, adipocytes, and fibroblasts. Muscle cells form the bulk of the meat, adipocytes contribute to the taste and flavor, and fibroblasts provide the extracellular matrix essential for the texture development.2,3 The taste and flavor of the cultivated meat are critical drivers of consumer acceptance.4 Generally, the organoleptic properties of conventional meat are highly determined by the properties of the muscle fibers and fat.5,6 Current knowledge about the organoleptic properties of conventional meat can guide the production of cultivated meat to ensure palatable end-products.7To achieve the desired organoleptic properties in cultivated meat, both muscle and fat must be present. Myocytes and adipocytes can be co-cultured, but this process is complex due to the intercellular communication that can alter cell phenotypes and the divergent requirements for media formulation.8–10 Alternatively, adipocytes can be cultured separately and then added to the muscle cells, allowing for the manipulation of the fatty acid composition of adipocytes.11,12 Several studies have explored the production, addition, and contribution of adipocytes in the context of bovine cultivated meat.13–17 Interestingly, increasing the lipid content of myoblasts/myotubes has been proposed as a strategy to enhance the overall lipid content in the final product,7 although this is less investigated.
The aim of this study is to increase the intra-myocellular lipid content in differentiated bovine satellite cells. For this, bovine satellite cells are cultured until clear myotube formation and exposed to individual fatty acids (oleic acid, linoleic acid, linolenic acid, stearic acid, and palmitic acid) for 24, 48, and 72 hours. In addition to testing individual fatty acids, we examine mixtures consisting exclusively of saturated fatty acids (linolenic, linoleic, and oleic) and exclusively of unsaturated fatty acids (palmitic and stearic), as well as mixtures where fatty acids are provided in equal non-toxic concentrations, referred to as the “one-to-one” mixture. Finally, to mimic the fatty acid composition found in beef, we investigate lipid accumulation in differentiated satellite cells exposed to a mixture of fatty acids in concentrations reflecting their distribution in conventional beef,18 referred to as the “rational mixture.”
Materials and methods
Culturing of bovine satellite cells
Bovine satellite cells were isolated from the semimembranosus muscle of three Danish Holstein dairy cows at slaughter, all aged between 3 years 1 month and 3 years 8 months. The muscles were delivered from the slaughterhouse, on ice, within 2 h after slaughter, to the tissue sectioning lab at the Department of Food Science, Aarhus University, Denmark. Approximately 100 g of muscle tissue was excised with a biopsy rod and transferred to a 50 mL Falcon tube containing Dulbecco's phosphate-buffered saline (DPBS) (Gibco) and 1% D-glucose (Sigma). The cells were isolated following the protocol described by Skrivergaard et al.19 The isolated cells were cryopreserved in growth media with 10% v/v dimethyl sulfoxide (DMSO, Sigma).The cryopreserved cells were thawed and resuspended in growth media to remove DMSO. The cells were centrifuged at 800×g for 10 min at 4 °C. For the initial multiplication of cells, the pellet was resuspended in pre-warmed growth media and seeded in T25 flasks coated with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 Matrigel Matrix (Corning). At passage three, the cells were seeded in Matrigel-coated 96-well plates and incubated at 37 °C with 5% CO2, using serum-free growth media (SFM) (DMEM/F-12 (Gibco) containing 2 ng mL−1 FGF2, 600 µg mL−1 fetuin, 75 µg mL−1 albumin, 100 units per mL penicillin, 0.1 mg mL−1 streptomycin, 2.5 mg mL−1 amphotericin B, as well as 1× insulin-transferrin-selenium20). The media was changed every other day until clearly visible myotubes were formed. To assess the fusion index, parallel cells were grown in 96-well plates and stained with phalloidin and Hoechst following the protocol described by Skrivergaard et al.19,21
50 Matrigel Matrix (Corning). At passage three, the cells were seeded in Matrigel-coated 96-well plates and incubated at 37 °C with 5% CO2, using serum-free growth media (SFM) (DMEM/F-12 (Gibco) containing 2 ng mL−1 FGF2, 600 µg mL−1 fetuin, 75 µg mL−1 albumin, 100 units per mL penicillin, 0.1 mg mL−1 streptomycin, 2.5 mg mL−1 amphotericin B, as well as 1× insulin-transferrin-selenium20). The media was changed every other day until clearly visible myotubes were formed. To assess the fusion index, parallel cells were grown in 96-well plates and stained with phalloidin and Hoechst following the protocol described by Skrivergaard et al.19,21
Fatty acid conjugations and cocktail preparations
All fatty acids, oleic acid (Sigma Aldrich, O1383), linoleic acid (Sigma Aldrich, L1012), linolenic acid (Sigma Aldrich, L2376), palmitic acid (Sigma Aldrich, P0500), and stearic acid (Sigma Aldrich, S4751), were solubilized or diluted in 99.8% ethanol. Stock solutions were prepared at a concentration of 50 mM and stored in amber-colored glass vials at −20 °C.Fatty acid–BSA conjugates were prepared using 10% fatty acid–free bovine serum albumin (BSA; Sigma Aldrich, A1595), following the protocol described by Pappas et al.22 Individual fatty acid stocks were added dropwise to the BSA solution under constant stirring at 37 °C for unsaturated fatty acids and at 90 °C for saturated fatty acids. Deionized water was added to the mixture, which was further heated until a clear solution was obtained. Each conjugation mixture had a final fatty acid concentration of 5 mM.
The resulting fatty acid–BSA conjugates were individually mixed with SFM to obtain the desired working concentrations. Prior to cell treatment, the SFM containing fatty acid–BSA conjugates was incubated at 37 °C for 30 min and vortexed every 10 min to ensure complete solubilization.
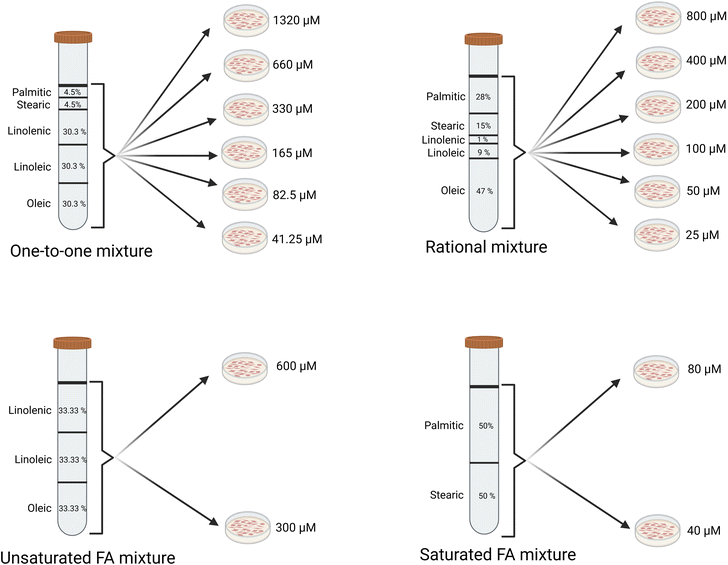
A mixture of unsaturated fatty acids was prepared by combining equal concentrations (200 µM each) of oleic, linoleic, and linolenic acid–BSA conjugates, yielding a total concentration of 600 µM (Fig. 1). This was further diluted with SFM to obtain a 300 µM working concentration. Similarly, a mixture of saturated fatty acids was prepared by mixing equal amounts of stearic and palmitic acid–BSA conjugates to achieve a final concentration of 80 µM, which was then diluted to 40 µM using SFM (Fig. 1).
Additionally, a stock of the fatty acid cocktail (one-to-one mixture) was prepared in SFM to a total fatty acid concentration of 1320 µM by combining BSA-conjugated fatty acids as follows: oleic acid, linoleic acid, and linolenic acid at 400 µM each and stearic acid and palmitic acid at 60 µM each. This cocktail was subsequently serially two-fold diluted five times, as detailed in Fig. 1. A “rational mixture” fatty acid cocktail was also prepared by mixing fatty acid–BSA conjugates in proportions resembling the fatty acid profile of beef.18 The initial total concentration was 800 µM, followed by five two-fold serial dilutions (Fig. 1).
Quantification of cellular nuclei and lipid droplet accumulation
Differentiated bovine satellite cells were treated with individual fatty acids and fatty acid cocktails in SFM for 24, 48, and 72 h at 37 °C in a 5% CO2 atmosphere. The media were replenished after 48 hours. Following treatment, cells were stained sequentially with BODIPY 493/503 to assess intracellular lipid accumulation and Hoechst 33342 to quantify the total cell number.The cells were washed with Dulbecco's phosphate-buffered saline (DPBS) and fixed in 10% neutral buffered formalin (Sigma, HT501128) for 15 min at room temperature. After fixation, the cells were washed twice with DPBS. Using BODIPY 493/503 (Invitrogen, D3922), staining was performed by incubating cells at 37 °C for 30 min with a 2 µg mL−1 DPBS solution, followed by Hoechst 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 342 staining (1
342 staining (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000 dilution in DPBS, Invitrogen, 62249) for 15 min at room temperature, as described by Grandl and Schmitz.23
1000 dilution in DPBS, Invitrogen, 62249) for 15 min at room temperature, as described by Grandl and Schmitz.23
Images were acquired using a Cytation 5 imaging system (BioTek) with a 4× objective. Hoechst fluorescence was detected using the DAPI filter, while BODIPY 493/503 fluorescence was captured using the GFP filter. Lipid content and nuclei were quantified using Gen5 Image Prime 3.15 software (Agilent BioTek). A primary mask was applied to the DAPI channel to identify and delineate cell nuclei. A secondary mask, with a diameter of 30 µm from the primary mask, was applied to the GFP channel to detect the BODIPY fluorescence corresponding to intracellular lipid accumulation. The mean fluorescence intensity within the secondary mask was used as a measure of the lipid content, with high intensity values indicating significant lipid accumulation.
Statistical analysis
Apart from the data presented in Fig. 2, the data are normalized within the donor, if not otherwise stated. Values are presented as mean ± standard deviation. For the statistical evaluation of single fatty acids, two-way ANOVA with Tukey's post-hoc test was used (Graph-pad Prism). For the statistical evaluation of the mixtures of fatty acids, one-way ANOVA with Tukey's post-hoc test was used (Graph-pad Prism). P-Values < 0.05 were regarded as significant.Results
Intracellular lipid accumulation by exposure to unsaturated fatty acids is time and dose dependent
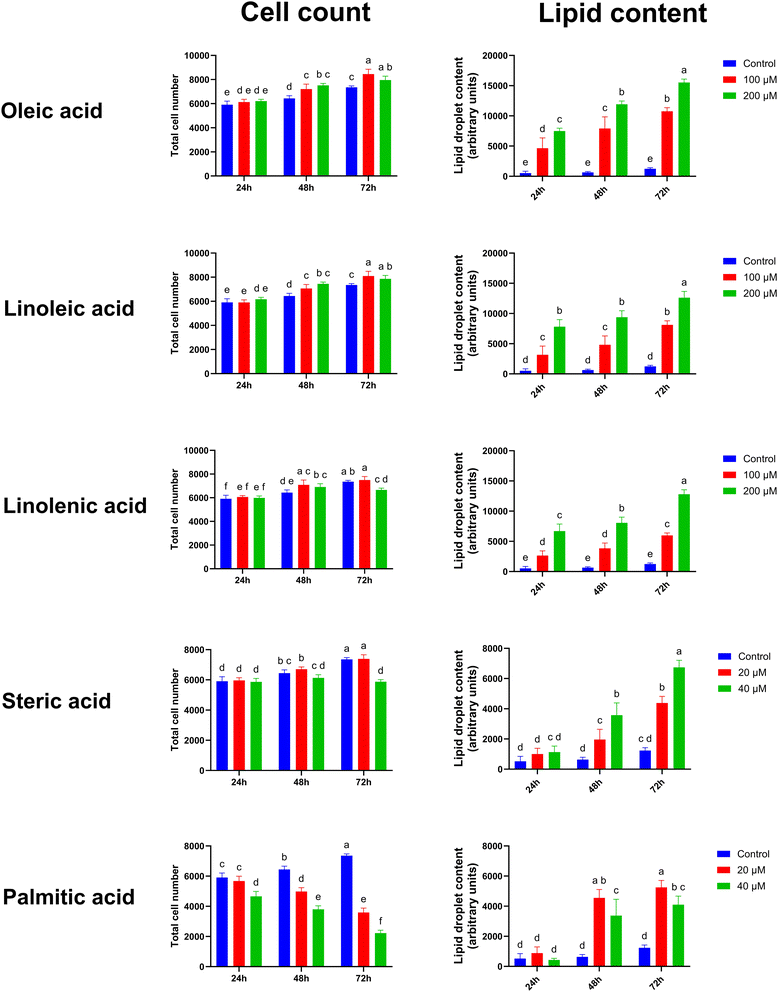
To assess the lipid accumulation capacity and cytotoxicity (defined here as significant loss in cell numbers) of individual fatty acids on differentiated satellite cells, the myotubes from a single donor were exposed to various concentrations of fatty acids for 24, 48, and 72 hours. For the unsaturated fatty acids, such as oleic acid, linoleic acid, and linolenic acid, no significant cytotoxic effects were observed at concentrations of 100 µM and 200 µM (Fig. 2A–C); contrarily, a general minor increase in the cell number was observed with the increase in exposure time. A significant dose- and time-dependent lipid accumulation was noted (Fig. 2F–H). The saturated steric acid was not toxic (Fig. 2D), whereas the saturated palmitic acid exhibited cytotoxic effects at concentrations of both 20 µM and 40 µM (Fig. 2E). Both stearic and palmitic acids induced an overall increase in lipid accumulation, but only after 48 and 72 h (Fig. 2I–J).Dose-dependent lipid accumulation induced by the fatty acid mixtures
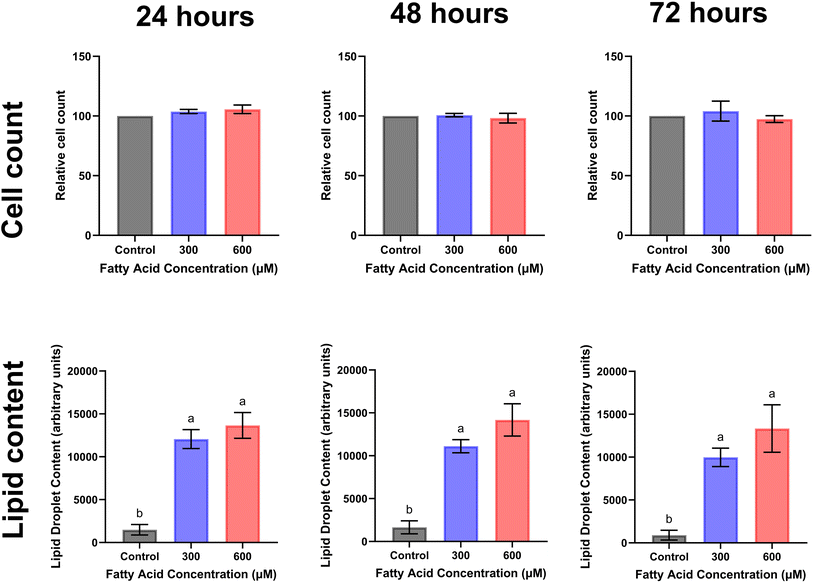
Given the observed differences in lipid accumulation and cytotoxicity between the saturated and unsaturated fatty acids, we investigated the effects of mixtures containing only saturated or unsaturated fatty acids. When the differentiated satellite cells were exposed to the “one-to-one” mixtures of unsaturated fatty acids at total concentrations of 300 µM and 600 µM, lipid accumulation was higher than that in the control, while no cytotoxic effects were detected (Fig. 3A–C). Also, no effect of time was observed (Fig. 3D–F and SI Fig. S1).For the “one-to-one” mixtures of saturated fatty acids, lipid accumulation was higher at 80 µM compared with 40 µM after 48 and 72 h (Fig. 4). At total concentrations of 40 µM and 80 µM, only minor cytotoxicity was observed after 48 h of exposure at the highest concentration (Fig. 4A–C). For both 80 µM and 40 µM, generally, lower lipid accumulation was observed after 72 h of exposure compared with 24 h and 48 h (Fig. 4D–F and SI Fig. S2).
To further test the mixtures of fatty acids, initially, cells were treated with the “one-to-one” mixtures of all fatty acids at increasing total concentrations. At a total concentration of 1320 µM, we observed cytotoxicity (Fig. 5A–C), causing very little lipid accumulation at 1320 µM (Fig. 5D–F). The lipid accumulation at 660 µM was not different from that of the cells exposed to 330 µM fatty acids, although with a large variation. For total fatty acid concentrations at 330 µM and below, significant differences in lipid accumulation from the control were observed at 24 h (Fig. 3D–F). The differences at the concentration of 330 µM at 24 hours were not observed at 48 and 72 hours. For control (no fatty acids) and 82.5 µM, lipid accumulation was increased at 72 hours compared to 48 hours (SI Fig. S3).
The lipid profile of conventional meat comprises a mixture of fatty acids. Therefore, we investigated the lipid accumulation and cytotoxicity in differentiated myotubes following exposure to the mixtures of selected fatty acids. When exposing differentiated satellite cells to fatty acid mixtures in ratios observed in meat (rational mixture), lipid accumulation increased dose-dependently (Fig. 6D–F). When cells were treated with rational mixtures at total concentrations of 400 µM or less, no cytotoxic effects were detected (Fig. 6A–C). However, at a total concentration of 800 µM, minor cytotoxicity was observed after 48 h of exposure. No consistent time-dependent effects within the dose were observed (SI Fig. S4).
Discussion
Flavor is a key attribute for the consumer acceptance of meat.24 Intramuscular fat in conventional meat, known as marbling, plays a crucial role in flavor and juiciness.25,26 In conventional meat, sensory scores are correlated with fat content, typically within the range of 3–7%,27 although other intervals have been reported.24 Previous discussions on fat incorporation in cultivated meat have highlighted the potential benefits of increasing intra-myocellular lipid content, as opposed to, e.g., the co-culturing of adipocytes and muscle cells.7 In the current study, we explored the role of single fatty acids and various mixtures in enhancing intracellular lipid accumulation in differentiated bovine satellite cells. Unlike previous studies on lipid incorporation in various cell models, e.g. C2C12 and L6 myoblasts,28,29 the primary cells used in this study were cultured using serum-free media. This makes the results applicable to future cultivated meat production.In the current study, we observed no cytotoxic effects from the unsaturated fatty acids at concentrations up to 200 µM for up to 72 h of exposure. Similar results for bovine satellite cells in the proliferative stages have previously been shown using serum-containing media.30 In that study, oleic acid and linoleic acid demonstrated no cytotoxic effect when applied at concentrations up to 100 µM for up to 48 hours. However, at 250 µM, significantly reduced cell viability was observed after just 24 hours. Additionally, it has been shown that in bovine satellite cells, proliferation following exposure to 50 mM oleic and palmitic acids for 48 h was not different from that of the control.31 In contrast, a recent study using porcine satellite cells demonstrated decreased cell viability following exposure to 100 µM oleic acid for 24 h.32 This discrepancy may be due to differences in the presence of FBS in the growth media or differences between myogenic stages (proliferating vs. differentiated cells). Indeed, differences in the cytotoxic effects of palmitate have been shown between myoblasts and differentiated C2C12 cells, with the proliferating cells being more sensitive than the differentiated ones.33
For all individually administered fatty acids, except for palmitic acid, we observed a time- and dose-dependent increase in myo-intracellular lipid accumulation. This accumulation was significantly pronounced for the unsaturated fatty acids, which could be explained by their low cytotoxicity, allowing for the use of high concentrations (200 µM for unsaturated fatty acids vs. 40 µM for saturated fatty acids). Palmitic acid at 40 µM induced even less lipid incorporation compared to that at 20 µM. Hence, for the incorporation of lipids into cultivated meat products, unsaturated fatty acids could be more favorable compared with saturated ones, as they display lower cytotoxicity and foster higher total lipid accumulation.
Interestingly, the study by Belal et al.32 also showed that at 20 µM oleic acid but not palmitic acid, the intracellular content of triglycerides increased. Although we did not assess the type of lipids accumulated in our study, our results suggest higher intracellular lipid content with both oleic acid and palmitic acid treatments compared with the control.
Among the tested unsaturated fatty acids, we examined fatty acids with different degrees of unsaturation (oleic acid, 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; linoleic acid, 18
1; linoleic acid, 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2; linolenic acid, 18
2; linolenic acid, 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3). From the data presented in Fig. 2, it appears that the lipid accumulation and cytotoxicity, following 72 h of incubation, does not differ between the unsaturated fatty acids used, suggesting that the degree of unsaturation does not dictate the lipid accumulation in differentiated bovine satellite cells. Similar results have been demonstrated in C2C12 mouse myoblasts.34 In that study, it was shown that 10 µM linoleic acid could increase cell proliferation after 4 h of incubation. In agreement, we observed increased cell numbers with both 100 µM and 200 µM oleic acid and linoleic acid compared with the control following 48 h of incubation.
3). From the data presented in Fig. 2, it appears that the lipid accumulation and cytotoxicity, following 72 h of incubation, does not differ between the unsaturated fatty acids used, suggesting that the degree of unsaturation does not dictate the lipid accumulation in differentiated bovine satellite cells. Similar results have been demonstrated in C2C12 mouse myoblasts.34 In that study, it was shown that 10 µM linoleic acid could increase cell proliferation after 4 h of incubation. In agreement, we observed increased cell numbers with both 100 µM and 200 µM oleic acid and linoleic acid compared with the control following 48 h of incubation.
To mimic conventional meat, which contains multiple fatty acids, we tested the cytotoxicity and lipid accumulation following exposure to different mixtures of fatty acids. Initially, we designed a mixture of unsaturated and saturated fatty acids based on the observed cytotoxic effects of individually applied fatty acids. This resulted in mixtures containing 200 µM of each unsaturated fatty acid and 30 µM of each saturated fatty acid. When using this mixture in a serial dilution, including up to twice the initial mixture (total concentration of 1320 µM), cytotoxicity was observed. However, at a total concentration of 330 µM, no cytotoxicity was observed. Similarly, we observed no cytotoxic effect when applying the “rational mixture,” which mimicked the balanced composition of fatty acids in bovine fat at a total concentration of 400 µM. However, at 800 µM, cytotoxicity was observed. These findings suggest that cells can tolerate relatively high total concentrations of fatty acids when exposed as a mixture without compromising cell viability, although some additive effects cannot be disregarded. For both oleic acid and linoleic acid, our results demonstrated no cytotoxic effects at 200 µM individually. However, when mixed, cytotoxic effects might occur. In the one-to-one mixture at 660 µM, which contained 200 µM of both oleic acid and linoleic acids, we observed cytotoxic effects. This suggests an additive toxic effect on differentiated satellite cells when mixing more fatty acids. Similar results have been shown by Belal et al.,30 who demonstrated that oleic acid and linoleic acid administered as single fatty acids at concentrations of 100 µM were not cytotoxic, while co-administration at a total concentration of 250 µM was toxic.
The “one-to-one” mixtures of fatty acids demonstrated a clear dose-dependent increase in cytotoxicity at high concentrations, which significantly compromised lipid accumulation. Interestingly, the “rational mixture” approach, which mimicked the fatty acid composition of conventional beef, showed minimal cytotoxicity at low concentrations and effective lipid accumulation. This approach appears promising for replicating the lipid profile of conventional meat while maintaining cell viability. Previous research has also shown that using mixtures of fatty acids (e.g., a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 ratio between unsaturated and saturated) increases the triglyceride content of C2C12 myoblasts more than pure saturated fatty acids (palmitate).35 Turner et al.28 tested mixtures of oleic acid, palmitic acid, linoleic acid, and α-linoleic acid (45%:30%:24%:1%) on lipid accumulation in 3D tissue-engineered skeletal muscles using C2C12. Although cytotoxicity was not reported, treatment with up to 800 µM of the fatty acid mixture significantly increased the number of lipid droplets, as well as droplet size, compared with the control without fatty acids. With respect to the oleic acid and palmitic acid, this mixture was comparable to our rational mixture. However, we observed toxicity with 800 µM total fatty acids, which was likely caused by our rational mixture containing 15% stearic acid. This suggests that a mixture of fatty acids, mimicking what can be expected in beef, can safely be used to increase the lipid content of differentiated bovine satellite cells.
3 ratio between unsaturated and saturated) increases the triglyceride content of C2C12 myoblasts more than pure saturated fatty acids (palmitate).35 Turner et al.28 tested mixtures of oleic acid, palmitic acid, linoleic acid, and α-linoleic acid (45%:30%:24%:1%) on lipid accumulation in 3D tissue-engineered skeletal muscles using C2C12. Although cytotoxicity was not reported, treatment with up to 800 µM of the fatty acid mixture significantly increased the number of lipid droplets, as well as droplet size, compared with the control without fatty acids. With respect to the oleic acid and palmitic acid, this mixture was comparable to our rational mixture. However, we observed toxicity with 800 µM total fatty acids, which was likely caused by our rational mixture containing 15% stearic acid. This suggests that a mixture of fatty acids, mimicking what can be expected in beef, can safely be used to increase the lipid content of differentiated bovine satellite cells.
Additionally, our investigation into the mixtures of exclusively saturated or unsaturated fatty acids revealed that unsaturated fatty acid mixtures are more favorable for lipid accumulation without cytotoxic effects compared with saturated fatty acid mixtures. Although we did not determine the resulting intra-myocellular fatty acid composition, this study demonstrated that increasing the lipid content of differentiated bovine satellite cells can be achieved by increasing the content of one or more fatty acids in the cell culture media without compromising cell viability. In this study, we focused on differentiated satellite cells, as we added fatty acids only after myotubes were visible. Interestingly, studies have demonstrated a positive effect of fatty acids in the cell culture media on muscle cell differentiation.28,31,36 This study also showed increased cell numbers upon exposure to unsaturated fatty acids, suggesting that the accumulation of intra-myocellular lipids also fostered increased myogenic capacity. However, the trans-differentiation of myocytes into adipocytes following fatty acid treatment must also be considered,37,38 as this might not be beneficial for overall cultivated meat production.
The present study demonstrates that lipid accumulation in differentiated bovine satellite cells can be modulated by the composition of the culture medium. It is plausible that the cellular lipid profile partially reflects the uptake of exogenous fatty acids provided during differentiation. This observation may have important implications for the nutritional properties of cultivated meat products. Specifically, the incorporation of health-promoting fatty acids, such as n-3 polyunsaturated fatty acids (PUFAs), represents a potential strategy to enhance the nutritional value of these products. Nevertheless, sensory attributes must be considered, as specific fatty acids can impart undesirable flavors; for example, n-3 PUFAs are known to produce a fish-like taste. Conversely, lipid supplementation may improve palatability, given that unmodified satellite cell cultures are typically perceived as dry and lacking flavor.39 These findings underscore the need to balance nutritional enhancement with sensory qualities in the development of cultivated meat. In this study, we focused on evaluating the ability of exogenous fatty acids to promote lipid accumulation within differentiated bovine satellite cells, defined as lipid droplets visualized using Bodipy staining. A key limitation of the study is that the fatty acid composition of the accumulated lipids has not been analyzed. Consequently, it remains unclear whether the lipids stored within the myotubes reflect the composition of the culture medium or whether they have undergone modification through intracellular metabolic processes. Future studies will be undertaken to elucidate that.
In conclusion, the findings of the current study provide valuable insights into the intracellular lipid accumulation and cytotoxicity effects of various concentrations of fatty acids in serum-free media in differentiated bovine satellite cells, which are crucial for the development of cultivated meat with desirable organoleptic properties. The results indicate that unsaturated fatty acids, such as oleic acid, linoleic acid, and linolenic acid, can be utilized to enhance lipid accumulation in a dose- and time-dependent manner without inducing significant cytotoxicity. This suggests that these fatty acids are suitable candidates for improving the taste and flavor of cultivated meat. In contrast, saturated fatty acids, particularly palmitic acid, exhibited cytotoxic effects at relatively low concentrations, which could pose challenges for their use in cultivated meat production. The observed cytotoxicity underscores the importance of optimizing fatty acid concentrations to balance lipid accumulation and cell viability.
Author contributions
Waris Mehmood: conceptualization, formal analysis, investigation, data curation, methodology, validation, writing original draft. Anupam Abraham: formal analysis, data curation, methodology, editing original draft. Polina Rabinovich-Toidman: conceptualization, editing original draft. Neta Lavon: conceptualization, editing original draft. Margrethe Therkildsen: conceptualization, editing original draft. Jette Feveile Young: conceptualization, editing original draft. Martin Krøyer Rasmussen: conceptualization, formal analysis, supervision, writing and editing original draft.Conflicts of interest
The authors declare no conflict of interest.Data availability
All data generated during this study are included in this published article and its supplementary information (SI) materials. Supplementary information is available. See DOI: https://doi.org/10.1039/d5fb00506j.Acknowledgements
Data were generated though accessing the research infrastructure at Aarhus University, including FOODHAY (Food and Health Open Innovation Laboratory, Danish Roadmap for Research Infrastructure). During the preparation of the manuscript, the authors utilized Copilot (Microsoft) to enhance the readability of the body text. After employing this tool, the authors reviewed and edited the content as necessary. The authors take full responsibility for the final publication.References
- H. L. Tuomisto and M. J. de Mattos, Environmental impacts of cultured meat production, Environ. Sci. Technol., 2011, 45(14), 6117–6123 CrossRef CAS PubMed.
- X. Li, X. Fu, G. Yang and M. Du, Review: Enhancing intramuscular fat development via targeting fibro-adipogenic progenitor cells in meat animals, Animal, 2020, 14(2), 312–321 CrossRef CAS PubMed.
- A. R. Weston, R. W. Rogers and T. G. Althen, Review: The Role of Collagen in Meat Tenderness, PAS, 2002, 18(2), 107–111 Search PubMed.
- M. C. Onwezen, E. P. Bouwman, M. J. Reinders and H. Dagevos, A systematic review on consumer acceptance of alternative proteins: Pulses, algae, insects, plant-based meat alternatives, and cultured meat, Appetite, 2021, 159, 105058 CrossRef CAS PubMed.
- M. S. Arshad, M. Sohaib, R. S. Ahmad, M. T. Nadeem, A. Imran and M. U. Arshad, et al., Ruminant meat flavor influenced by different factors with special reference to fatty acids, Lipids Health Dis, 2018, 17(1), 223 CrossRef CAS PubMed.
- B. Picard and M. Gagaoua, Muscle Fiber Properties in Cattle and Their Relationships with Meat Qualities: An Overview, J. Agric. Food Chem., 2020, 68(22), 6021–6039 CrossRef CAS PubMed.
- J. F. Young, A. Abraham, M. K. Rasmussen, S. Skrivergaard and M. Therkildsen, Developing cultured meat as a food product. Advances in cultured meat technology, Burleigh Dodds Ser. Agric. Sci., 2023, 299–318 Search PubMed.
- K. Seo, T. Suzuki, K. Kobayashi and T. Nishimura, Adipocytes suppress differentiation of muscle cells in a co-culture system, Anim Sci J, 2019, 90(3), 423–434 CrossRef CAS PubMed.
- Y. Takegahara, K. Yamanouchi, K. Nakamura, S. Nakano and M. Nishihara, Myotube formation is affected by adipogenic lineage cells in a cell-to-cell contact-independent manner, Exp. Cell Res., 2014, 324(1), 105–114 CrossRef CAS PubMed.
- S. Park, K. Baek and C. Choi, Suppression of adipogenic differentiation by muscle cell-induced decrease in genes related to lipogenesis in muscle and fat co-culture system, Cell Biol Int, 2013, 37(9), 1003–1009 CrossRef CAS PubMed.
- F. Louis, M. Furuhashi, H. Yoshinuma, S. Takeuchi and M. Matsusaki, Mimicking Wagyu beef fat in cultured meat: Progress in edible bovine adipose tissue production with controllable fatty acid composition, Mater Today Bio, 2023, 21, 100720 CrossRef CAS PubMed.
- S. Sugii, C. Y. Q. Wong, A. K. O. Lwin and L. J. M. Chew, Alternative fat: redefining adipocytes for biomanufacturing cultivated meat, Trends Biotechnol., 2023, 41(5), 686–700 CrossRef CAS PubMed.
- R. G. J. Dohmen, S. Hubalek, J. Melke, T. Messmer, F. Cantoni and A. Mei, et al., Muscle-derived fibro-adipogenic progenitor cells for production of cultured bovine adipose tissue, NPJ Sci Food, 2022, 6(1), 6 CrossRef PubMed.
- D. Jeong, J. W. Seo, H. G. Lee, W. K. Jung, Y. H. Park and H. Bae, Efficient Myogenic/Adipogenic Transdifferentiation of Bovine Fibroblasts in a 3D Bioprinting System for Steak-Type Cultured Meat Production, Adv. Sci., 2022, 9(31), e2202877 CrossRef PubMed.
- D. H. Kang, F. Louis, H. Liu, H. Shimoda, Y. Nishiyama and H. Nozawa, et al., Engineered whole cut meat-like tissue by the assembly of cell fibers using tendon-gel integrated bioprinting, Nat. Commun., 2021, 12(1), 5059 CrossRef CAS PubMed.
- F. Mehta, R. Theunissen and M. J. Post, in Adipogenesis from Bovine Precursors, ed. Rønning S. B., Myogenesis: Methods and Protocols. New York, NY: Springer New York; 2019. pp. 111–25 Search PubMed.
- Y. Zagury, I. Ianovici, S. Landau, N. Lavon and S. Levenberg, Engineered marble-like bovine fat tissue for cultured meat, Commun. Biol., 2022, 5(1), 927 CrossRef CAS PubMed.
- T. T. N. Dinh, K. V. To and M. W. Schilling, Fatty Acid Composition of Meat Animals as Flavor Precursors, Meat Muscle Biol., 2021, 5(1), 1–16 CAS.
- S. Skrivergaard, M. K. Rasmussen, M. Therkildsen and J. F. Young, Bovine Satellite Cells Isolated after 2 and 5 Days of Tissue Storage Maintain the Proliferative and Myogenic Capacity Needed for Cultured Meat Production, Int. J. Mol. Sci., 2021, 22(16), 8376 CrossRef CAS PubMed.
- S. Skrivergaard, J. F. Young, N. Sahebekhtiari, C. Semper, M. Venkatesan and A. Savchenko, et al., A simple and robust serum-free media for the proliferation of muscle cells, Food Res. Int., 2023, 172, 113194 CrossRef CAS PubMed.
- S. Skrivergaard, M. K. Rasmussen, M. Therkildsen and J. F. Young, High-Throughput Label-Free Continuous Quantification of Muscle Stem Cell Proliferation and Myogenic Differentiation, Stem Cell Rev Rep, 2025, 2103–2120 CrossRef CAS PubMed.
- A. Pappas, M. Anthonavage and J. S. Gordon, Metabolic fate and selective utilization of major fatty acids in human sebaceous gland, J. Invest. Dermatol., 2002, 118(1), 164–171 CrossRef CAS PubMed.
- M. Grandl and G. Schmitz, Fluorescent high-content imaging allows the discrimination and quantitation of E-LDL-induced lipid droplets and Ox-LDL-generated phospholipidosis in human macrophages, Cytometry A, 2010, 77(3), 231–242 CrossRef PubMed.
- M. Therkildsen, P. Spleth, E. M. Lange and P. I. Hedelund, The flavor of high-quality beef – a review, Acta Agric. Scand., Sect. A, 2017, 67(3–4), 85–95 CAS.
- C. H. Corbin, T. G. O'Quinn, A. J. Garmyn, J. F. Legako, M. R. Hunt and T. T. N. Dinh, et al., Sensory evaluation of tender beef strip loin steaks of varying marbling levels and quality treatments, Meat Sci, 2015, 100, 24–31 CrossRef CAS PubMed.
- J. D. Wood, in Meat Composition and Nutritional Value, ed. Toldra´ F., Lawrie's Meat Science, Woodhead Publishing, 2017. pp. 635–59 Search PubMed.
- R. K. Miller, in Chemical And Physical Characteristics Of Meat | Palatability, ed. Jensen W. K., Encyclopedia of Meat Sciences. Oxford, Elsevier; 2004. pp. 256–66 Search PubMed.
- M. C. Turner, R. P. Rimington, N. R. W. Martin, J. W. Fleming, A. J. Capel and L. Hodson, et al., Physiological and pathophysiological concentrations of fatty acids induce lipid droplet accumulation and impair functional performance of tissue engineered skeletal muscle, J. Cell. Physiol., 2021, 236(10), 7033–7044 CrossRef CAS PubMed.
- V. S. Sauro and K. P. Strickland, Changes in oleic acid oxidation and incorporation into lipids of differentiating L6 myoblasts cultured in normal or fatty acid-supplemented growth medium, Biochem. J., 1987, 244(3), 743–748 CrossRef CAS PubMed.
- S. A. Belal, A. S. Sivakumar, D. R. Kang, S. Cho, H. S. Choe and K. S. Shim, Modulatory effect of linoleic and oleic acid on cell proliferation and lipid metabolism gene expressions in primary bovine satellite cells, Anim Cells Syst., 2018, 22(5), 324–333 CrossRef CAS PubMed.
- J. Xu, D. Liu, H. Yin, H. Tong, S. Li and Y. Yan, Fatty acids promote bovine skeletal muscle satellite cell differentiation by regulating ELOVL3 expression, Cell Tissue Res., 2018, 373(2), 499–508 CrossRef CAS PubMed.
- S. A. Belal, J. Lee, J. Park, D. Kang and K. Shim, The Effects of Oleic Acid and Palmitic Acid on Porcine Muscle Satellite Cells, Foods, 2024, 13(14), 2200 CrossRef CAS PubMed.
- J. Patkova, M. Andel and J. Trnka, Palmitate-induced cell death and mitochondrial respiratory dysfunction in myoblasts are not prevented by mitochondria-targeted antioxidants, Cell. Physiol. Biochem., 2014, 33(5), 1439–1451 CrossRef CAS PubMed.
- J. H. Lee, H. Tachibana, Y. Morinaga, Y. Fujimura and K. Yamada, Modulation of proliferation and differentiation of C2C12 skeletal muscle cells by fatty acids, Life Sci., 2009, 84(13–14), 415–420 CrossRef CAS PubMed.
- S. A. Newsom, A. C. Everett, S. Park, D. W. Van Pelt, A. Hinko and J. F. Horowitz, Lipid mixtures containing a very high proportion of saturated fatty acids only modestly impair insulin signaling in cultured muscle cells, PLoS One, 2015, 10(3), e0120871 CrossRef PubMed.
- J. Zhang, Q. Li, K. M. C. Nogoy, J. Sun, B. Sun and Y. Wang, et al., Effect of palmitoleic acid on the differentiation of bovine skeletal muscle satellite cells, J Anim Sci Technol, 2021, 63(4), 919–933 CrossRef CAS PubMed.
- S. Ghnaimawi, L. Rebello, J. Baum and Y. Huang, DHA but not EPA induces the trans-differentiation of C2C12 cells into white-like adipocytes phenotype, PLoS One, 2021, 16(9), e0249438 CrossRef CAS PubMed.
- L. Wang and T. Shan, Factors inducing transdifferentiation of myoblasts into adipocytes, J. Cell. Physiol., 2021, 236(4), 2276–2289 CrossRef CAS PubMed.
- M. K. Rasmussen, R. R. Thøgersen, J. F. Young and M. Therkildsen, From Muscle Fibers to Functional Foods: Bridging Conventional and Cultivated Approaches, Berlin, Heidelberg, Springer Berlin Heidelberg, 2025, pp. 1–20 Search PubMed.
| This journal is © The Royal Society of Chemistry 2026 |