Open Access Article
Open Access ArticleValorization of brewing by-products: obtaining flavor-enhancing and antioxidant compounds from spent yeast
Antonela G. Garzón *,
Yanina Pavón,
Marilin E. Aquino and
Silvina R. Drago
*,
Yanina Pavón,
Marilin E. Aquino and
Silvina R. Drago
Instituto de Tecnología de Alimentos, CONICET, FIQ-UNL, 1° de Mayo 3250, (3000) Santa Fe, Argentina. E-mail: agarzon@fiq.unl.edu.ar; Tel: +54-342-4571164
First published on 16th December 2025
Abstract
This study optimized the recovery of flavor-enhancing and antioxidant compounds from brewing spent yeast using a two-factor (autolysis time and temperature) response surface design. Chemical composition, sensory attributes, and in vitro antioxidant activity were evaluated. Optimization aimed to maximize umami flavor, pungency, and antioxidant properties, while minimizing bitterness and yeast odor. Autolysis time strongly influenced free glutamic acid and glucose, with longer durations increasing their levels. Both time and temperature affected protein hydrolysis (PH), peptide hydrophobicity, phenolic compounds (e.g., protocatechuic and chlorogenic acids), and bioactives such as GABA and taurine. Longer autolysis enhanced umami taste, whereas shorter durations improved antioxidant activity (ABTS+, DPPH, FRAP) and reduced copper-chelating capacity. Significant correlations (p < 0.05) linked composition to functionality, including bitter taste with protein hydrolysis, umami with glutamic acid, ABTS with chlorogenic acid, and copper-chelating activity with PH or amphipathic peptides. These findings show that autolysis conditions can be strategically tailored to target specific sensory and bioactive profiles, offering a practical approach to valorize spent yeast as a functional ingredient for food applications.
Sustainability spotlightBrewer's spent yeast (BSY) represents one of the main by-products of the brewing industry, commonly discarded despite its high nutritional and functional potential. Addressing the valorization of BSY is crucial to reduce waste streams and promote resource efficiency within the food sector. This study demonstrates the recovery of natural flavour-enhancer and antioxidant compounds from BSY extracts, offering sustainable alternatives to synthetic additives while enhancing food quality. By converting an underutilized residue into value-added ingredients, the work contributes to waste minimization, circular bioeconomy, and cleaner production practices. This advancement directly aligns with the UN Sustainable Development Goals 12 (Responsible Consumption and Production) and 9 (Industry, Innovation and Infrastructure), fostering sustainable innovation in food technology. |
1. Introduction
Brewer spent yeast (BSY) is a by-product of the brewing industry generated when the yeast biomass used in fermentations is no longer useful and must be discarded. It is estimated that between 15 to 18 tons of this byproduct are generated for every 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 hL of produced beer.1 Only a small percentage of yeast is reutilized in the manufacturing process, then a large excess of biomass remains. Since it has a high chemical oxygen demand value, it cannot be disposed of in wastewater.1 This by-product is characterized by high moisture content (85–95%), high biological quality proteins, carbohydrates, fatty acids, vitamins and minerals. In addition, it contains bioactive compounds such as polyphenols, β-glucans and mannoproteins with potential beneficial health properties.2 Considering its composition, BSY is a byproduct that has many potential uses, for example to obtain extracts to be used as flavorings,3 or as bio-functional ingredients in food formulation.4
000 hL of produced beer.1 Only a small percentage of yeast is reutilized in the manufacturing process, then a large excess of biomass remains. Since it has a high chemical oxygen demand value, it cannot be disposed of in wastewater.1 This by-product is characterized by high moisture content (85–95%), high biological quality proteins, carbohydrates, fatty acids, vitamins and minerals. In addition, it contains bioactive compounds such as polyphenols, β-glucans and mannoproteins with potential beneficial health properties.2 Considering its composition, BSY is a byproduct that has many potential uses, for example to obtain extracts to be used as flavorings,3 or as bio-functional ingredients in food formulation.4
Flavor-enhancing and bioactive compounds are extracted into the soluble fraction that is recovered after the yeast cell walls have been digested or removed.5 This can be produced by autolysis, which is a self-digestion of cells generated by the action of endogenous enzymes and that occurs naturally at the end of the cell life cycle. The autolysis allows the extraction of nucleotides, free amino acids, peptides, group B vitamins, minerals and polyphenols that could provide the different properties.1,2,6 Processes with temperatures of 50 °C and 24 h are generally used to induce autolysis to extract flavor compounds.1 However, Vieira et al.7 showed that 36 °C and 6 h of autolysis after mechanical disruption of the cells was the best condition to obtain high antioxidant and antihypertensive potential.
The components of yeast extract responsible for the flavor characteristics are peptides, free amino acids (mainly glutamic and aspartic acid and their salts), and nucleotides. Umami peptides are food additives known to improve or influence the taste of foods. On the other hand, these peptides can act synergistically with ribonucleotides and amino acids.8 That is, by varying the proportion of the different compounds that provides the flavor, it is possible to obtain specific extracts to be used to improve the sensory acceptability of determined foods and beverages, depending on the target sensory profile.9 On the other hand, some studies on obtaining yeast extracts by both autolysis and hydrolysis demonstrated that the soluble fraction had bioactive compounds such as polyphenols and peptides, with antihypertensive and antioxidant activity.6,7,10
However, research to date has largely focused on either flavor or bioactivity in isolation. No systematic studies have optimized extraction conditions to maximize both properties simultaneously or established clear correlations between the compounds present and their sensory and functional effects. Addressing this gap is critical for the development of tailored BSY extracts for food applications. Therefore, this study aims to obtain natural extracts from BSY with high antioxidant and flavor-enhancing potential and to relate these properties to the specific compounds present in the extracts.
2. Materials and methods
2.1. Raw materials and reagents
Brewers' spent yeast (BSY) was supplied by Okcidenta® (Santa Fe, Argentina). It contained 22 g solids per 100 g, 38.8 g protein per 100 g dry basis (d.b.) and 6.5 g ash per 100 g d.b. Analytical standards for amino acid determination and phenolic compounds profile by HPLC, and substrates for bioactivity assays were obtained from Sigma-Aldrich (St. Louis, USA). Other reagents used were of analytical grade.2.2. Optimizing the extraction process to obtain BSY extracts
The autolysis time and temperature were optimized through a response surface central composite rotable design of two factors with three levels each (F1: time, 6, 15 or 24 h; F2: temperature, 30, 40 or 50 °C) giving a total of 11 trials (9 combinations with triplicate of the central point). The time and temperature ranges were chosen to reflect the conditions typically used to obtain yeast extracts intended for use as flavorings1 or biofunctional ingredients.7 The extraction process was carried out incubating the yeast dispersion in water (10 g solids per 100 mL) at temperatures and times indicated in Table 1, using an incubator shaker (Bioelec, Argentina). After that, all samples were treated at 90 °C for 10 min to inactivate enzyme activities. Extracts obtained (E1 to E11) were centrifuged at 3500×g for 15 min (Cavour, Argentina). The supernatant was collected, and kept at −20 °C until analysis.| Independent variable | Responses | |||||||
|---|---|---|---|---|---|---|---|---|
| Temperature (°C) | Time (h) | Solids (g per 100 mL) | Protein (g per 100 g d.b.) | DH (%) | Glutamic acid (mg per g d.b.) | RNA (g per 100 g d.b.) | FG (g per 100 g d.b.) | BG (g per 100 g d.b.) |
| a d.b. Dry basis; FAG: free amino groups; DH: degree of hydrolysis; TG: total glucose; BG: bound glucose. | ||||||||
| 30.00 | 6.00 | 4.13 | 36.4 | 10.08 | 7.21 | 4.9 | 0.30 | 3.00 |
| 25.86 | 15.00 | 4.01 | 35.9 | 8.92 | 6.65 | 5.3 | 1.28 | 3.06 |
| 30.00 | 24.00 | 5.09 | 33.5 | 27.38 | 12.46 | 4.6 | 0.40 | 2.95 |
| 40.00 | 2.27 | 3.24 | 31.0 | 3.53 | 7.20 | 5.9 | 0.25 | 3.39 |
| 40.00 | 15.00 | 4.26 | 36.4 | 30.54 | 6.37 | 4.9 | 1.06 | 2.94 |
| 40.00 | 15.00 | 4.57 | 35.5 | 21.20 | 10.61 | 6.3 | 0.76 | 3.05 |
| 40.00 | 15.00 | 4.58 | 36.4 | 35.65 | 8.09 | 5.7 | 0.91 | 2.67 |
| 40.00 | 27.73 | 4.94 | 33.7 | 32.38 | 12.77 | 6.5 | 1.15 | 2.16 |
| 50.00 | 6.00 | 4.18 | 38.3 | 17.51 | 7.18 | 5.7 | 0.46 | 3.47 |
| 54.14 | 15.00 | 4.90 | 35.5 | 37.47 | 9.79 | 5.6 | 0.63 | 2.84 |
| 50.00 | 24.00 | 4.63 | 33.9 | 42.12 | 12.49 | 4.9 | 0.97 | 2.73 |
The order of experimentation was randomized. The dependent variables or responses analyzed for each test were chemical composition, sensorial characteristics and antioxidant properties (all techniques described in the following sections). The selection of the model (linear, linear with interactions, or quadratic), for each response was made using the sum of squares of the sequential model. The model where the F tests showed significant effects (p < 0.05) was selected. The suitability of the selected model was then verified based on the lack-of-fit tests and the multiple correlation coefficients (R2). The adequacy of the models used to describe the effect of the independent variables on the responses was considered good when the R2 value was higher than 70% and the calculated F value was higher than the F value from table.11 In addition, the assumptions of the model and the presence or absence of atypical values (outliers) were verified.
2.3. Chemical composition of BSY extracts
2.4. Sensory evaluation of extracts
Flavor profile analysis of the extracts was conducted at the Sensory Analysis Laboratory of Instituto de Tecnología de Alimentos, Facultad de Ingeniería Química, Universidad Nacional del Litoral (Santa Fe, Argentina), in accordance with the guidelines established by ISO 8589 – 2007.In the first stage, an online recruitment was carried out among members of the Institute interested in forming a sensory panel for the evaluation of extracts.
A total of 13 panelists (10 women and 3 men), aged between 25 and 54 years, were selected based on achieving a minimum success rate of 80% in each test. Basic taste identification tests, odor recognition, and triangular tests using yeast extract solutions prepared under the various conditions described in the experimental design, were used as selection test. In case of triangular test, red light was used in the sensory booths to mask color differences between samples. When working with extracts, the samples were thawed in the refrigerator the day before the sensory test and subsequently resuspended at 0.5 g solids per 100 mL.
To train the sensory panel to recognize common odor and taste characteristics of the extracts, panelists underwent 10 h of training, consisting of five 2 h sessions conducted over a two-week period. During training, some extract samples corresponding to different conditions from the experimental design were presented to generate and define, by consensus, the main descriptors characterizing them (Table S1). Additionally, reference standards were used to anchor the minimum and maximum perceived intensity levels for each descriptor.
Each sample (20 mL) was served in a plastic cup with a lid and coded randomly. During individual sessions, each panelist rated the perceived intensity of each descriptor using 10 cm unstructured line scales anchored at both ends (1 and 9). Water was used as a palate cleanser. Up to three samples were served simultaneously in each session in a balanced order. The test was conducted in standardized sensory booths, at room temperature (25 °C) and under white lighting.
2.5. Determination of antioxidant properties of extracts
To estimate the antioxidant activity of extracts, four assays with different antioxidant mechanism were performed: (1) the scavenging of 2, 2′-azinobis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS•+) according to Cian et al.17 Results were expressed as mmol Trolox per g d.b. (2) Ferric reducing antioxidant power (FRAP) according to Benzie and Strain.19 Results of FRAP were expressed as mmol Trolox per g d.b. (3) The scavenging of 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical according to Cian et al.17 Results were expressed as mg ascorbic acid (AA) per g d.b. (4) Copper-chelating activity was determined by the assay of β-carotene oxidation according to Cian et al.17 The degradation of β-carotene was monitored by recording the decrease in absorbance at 470 nm.2.6. Optimization and model validation
The numerical optimization was based on the desirability function (D) of Derringer and Suich.20 The selected responses to optimize the model were: yeast smell (minimize, importance 2), umami taste (maximize, importance 5), bitter taste (maximize, importance 2), pungency (minimize, importance 1), ABTS+ scavenging activity (maximize, importance 2), DPPH scavenging activity (maximize, importance 2), copper-chelating activity (maximize, importance 5). To validate the model, a BSY extract was obtained according to the optimal conditions produced by the optimized model and the same experiments detailed above were carried out. The experimental values of the responses obtained under these conditions were compared with the predicted values by a t-test analysis of comparison between two samples using the Statgraphics Centurion XV 15.2.06 Software (Statpoint Technologies, Inc., Warrenton, Virginia, USA).2.7. Statistical analysis
The statistical software package Design Expert 7.02 (Stat-Ease Inc. Minneapolis, USA) was used to construct the experimental design as well as to analyze the data. Statgraphics Centurion XV 15.2.06 software was used to apply Pearson's correlation test to determine significant correlations between the compounds analyzed, bioactivity and sensorial characteristics, and to apply paired sample t-test to determine differences between experimental and predicted values obtained from optimization. In all cases a confidence level of 95% (p < 0.05) was used, and all determinations were performed at least by triplicate. Results were expressed as media ± standard deviation.3. Results and discussion
3.1. Chemical composition
Table 1 shows the responses related to chemical composition of BSY extracts obtained in the conditions of the experimental design. The temperature and time of autolysis were used as independent variables. Table S2 (SI file) shows the statistical results of the models fit for the responses analyzed. Solid content was in the range of 3.24–5.09 g per 100 mL, and exhibited a lineal model with time as the only significant factor (positive influence). The model did not fit the protein content of extracts, which was ∼35 g protein per 100 g d.b., in accordance with the 32.9 g per 100 g d.b. value obtained by yeast autolysis at 55 °C – 24 h.21 The DH fit with a linear model without interaction, with a positive influence of temperature and time, thus, higher temperature or higher time resulted in higher values. The highest DH obtained (42.12%) was for the sample obtained at the highest temperature (50 °C) and longer time (24 h). Tanguler and Erten22 also showed that 50 °C was an optimal temperature for yeast proteases and therefore for protein hydrolysis. On the other hand, free glucose (FG) did not adjust with any model, but bound glucose (BG) fitted with lineal model, with reverse effect of time. The lack of fit of the protein and glucose contents could be due to these compounds were used to synthesizes another molecules, like vitamins,23 and take part in Maillard reaction during enzyme inactivation (thermal treatment). Furthermore, RNA did not adjust with any model. BSY have a high content of RNA, thus they are a good source of nucleotides, which are produced during the autolysis, because of the endogenous enzymes break down nucleic acids producing 5′-nucleotides.23 Certain nucleotides (especially 5′-monophosphates) are recognized as flavor enhancers. The lack of fit between RNA content and umami flavor could be due to the detection method used to determine RNA content did not differentiate between different nucleotides released by autolysis; therefore, quantification of specific nucleotides by HPLC would be required to more accurately assess their contribution to umami perception. Results obtained for RNA (4.9–6.5 g per 100 g d.b.) are in agreement with those reported by Jacob et al.3 for a BSY extract obtained by autolysis at 50 °C for 24 h (4.9 g RNA per 100 g d.b.). On the other hand, the inclusion of yeast in food products is usually limited by the high amount of nucleic acids (4–12% dry weight), which in humans is metabolized to uric acid and may progress to kidney stones or gout.24 Taking into account that the usual percentage added to food formulation of yeast extracts is 0.5–1 g per 100 g (which implies a RNA contribution of 0.025–0.065 g per 100 g), and that 1–3 g per 100 g (dry weight) of nucleic acid is a concentration in foods without risk of increasing uric acid levels in blood and tissues in humans,24 this products are safe to be use in food formulation.3.2. Bioactive compounds
Bioactive compounds obtained in BSY extracts are shown in Table 2. Additionally, Table S3 shows the statistical results of the model fit for these responses.| Independent variable | Responses | |||||||
|---|---|---|---|---|---|---|---|---|
| Temperature (°C) | Time (h) | GABA (mg per g d.b.) | Taurine (mg per g d.b.) | PA (µg per g d.b.) | 4HBA (µg per g d.b.) | CA (µg per g d.b.) | VA (µg per g d.b.) | FA (µg per g d.b.) |
| a d.b. Dry basis; GABA: γ-aminobutyric acid; PA: protocatechuic acid; 4HBA: 4-hydroxybenzoic acid; CA: chlorogenic acid; VA: vanillic acid; FA: ferulic acid; HH: high hydrophobicity; LH: low hydrophobicity; IH: intermediate hydrophobicity; AA: ascorbic acid. | ||||||||
| 30.00 | 6.00 | 7.94 | 5.24 | 150.1 | 10.61 | 31.95 | 49.97 | 146.28 |
| 25.86 | 15.00 | 6.50 | 5.13 | 111.8 | 6.14 | 8.27 | 34.76 | 152.41 |
| 30.00 | 24.00 | 11.83 | 12.58 | 8.1 | 11.98 | 3.16 | 53.68 | 133.95 |
| 40.00 | 2.27 | 2.02 | 2.34 | 174.1 | 7.02 | 40.56 | 41.03 | 173.16 |
| 40.00 | 15.00 | 8.07 | 7.28 | 128.6 | 8.79 | 9.10 | 40.67 | 186.21 |
| 40.00 | 15.00 | 6.11 | 11.55 | 100.6 | 9.68 | 8.71 | 36.69 | 187.26 |
| 40.00 | 15.00 | 5.92 | 9.15 | 73.3 | 10.45 | 8.24 | 36.98 | 146.92 |
| 40.00 | 27.73 | 6.21 | 13.87 | 155.6 | 4.79 | 11.61 | 21.06 | 119.85 |
| 50.00 | 6.00 | 4.59 | 6.49 | 116.4 | 9.39 | 8.55 | 32.86 | 141.03 |
| 54.14 | 15.00 | 3.72 | 9.54 | 152.4 | 9.98 | 2.41 | 27.67 | 157.16 |
| 50.00 | 24.00 | 6.33 | 13.60 | 173.4 | 8.26 | 12.90 | 29.49 | 139.47 |
| Temperature (°C) | Time (h) | HH peptides (%) | LH peptides (%) | IH peptides (%) | ABTS (mM Trolox per g d.b.) | FRAP (mM Trolox per g d.b.) | DPPH (mg AA per g d.b.) | Copper- chelating (%) |
|---|---|---|---|---|---|---|---|---|
| 30.00 | 6.00 | 3.15 | 78.32 | 18.53 | 6.25 | 29.91 | 9.09 | 77.03 |
| 25.86 | 15.00 | 3.33 | 75.22 | 21.45 | 5.38 | 32.96 | 8.13 | 59.81 |
| 30.00 | 24.00 | 1.45 | 69.88 | 28.66 | 5.10 | 23.78 | 5.87 | 81.34 |
| 40.00 | 2.27 | 5.44 | 79.93 | 14.63 | 6.43 | 33.39 | 14.63 | 50.48 |
| 40.00 | 15.00 | 2.91 | 70.88 | 26.21 | 5.57 | 30.36 | 9.95 | 72.01 |
| 40.00 | 15.00 | 1.99 | 71.32 | 26.68 | 5.10 | 26.52 | 8.61 | 78.47 |
| 40.00 | 15.00 | 2.03 | 71.98 | 25.99 | 5.03 | 27.86 | 8.55 | 83.49 |
| 40.00 | 27.73 | 1.85 | 71.04 | 21.14 | 4.50 | 24.31 | 8.05 | 92.82 |
| 50.00 | 6.00 | 2.50 | 73.14 | 24.36 | 5.93 | 40.18 | 12.32 | 64.83 |
| 54.14 | 15.00 | 1.57 | 70.67 | 28.27 | 4.70 | 24.25 | 8.66 | 95.69 |
| 50.00 | 24.00 | 1.86 | 71.94 | 26.19 | 4.85 | 24.76 | 8.44 | 92.10 |
γ-Aminobutyric acid (GABA) content was in the range of 2.02 to 11.83 mg per g d.b., and fitted with a lineal model without interaction. Both factors, temperature (negative effect) and time (positive effect) were significant. In this sense, longer autolysis time increased GABA content. Yeast (Saccharomyces cerevisiae) synthesizes and accumulates GABA in vacuoles.25 It was demonstrated that during autolysis, an excess of glutamic acid as a substrate and glucose as an energy supplier can increase GABA formation at 37 °C than at 50 °C,3 which suggests an enzymatic mechanism involving the enzyme glutamate decarboxylase. The concentration of GABA in the yeast extract was reported near 10 mg per g d.b. by Jacob et al.,3 a similar value to that obtained in this experimental design. GABA is a non-proteinogenic amino acid naturally present in the brain and spinal cord of mammals and acts as an important inhibitory neurotransmitter of the central nervous system, which consumption has been demonstrated multiple health benefits.26 Inoue et al.27 have reported that a daily intake of 100 mL of fermented milk containing 10–12 mg of GABA can reduce blood pressure in patients with hypertension. Thus, the amount of GABA of the BSY extract produced at 30 °C for 24 h is sufficient to provide these functional benefits when added to food formulation at 1 g solids per 100 mL.
Taurine content was between 2.34 to 13.87 mg per g d.b. A lineal model fitted this response, with both factors (temperature and time) with positive effect. Yeasts can synthesize and store taurine, which is then extracted in autolysis processes. Agboola et al.28 reported that taurine content of three yeasts extracts (C. jadinii, B. adeninivorans and W. anomalus) ranged from 2.8 to 3.5 mg per g d.b. Likewise, Masuda et al.29 informed that Saccharomyces cerevisiae can synthesize high content of taurine. However, this is the first report of taurine produced by BSY autolysis. Moreover, the autolysis conditions studied showed that the yeast enzymes responsible of taurine metabolism resulted more active at higher temperatures (40–50 °C). It was reported that taurine acts an osmoprotectant, antioxidant, and membrane stabilizer in yeast.30 Also, taurine is preventive against hypertension, stroke and atherosclerotic arterial diseases in humans.31
Phenolic compounds identified in yeast extracts were protocatechuic acid (PA), 4-hydroxybenzoic acid (4HBA), chlorogenic acid (CA), vanillic acid (VA) and ferulic acid (FA), the last one being the majority phenolic compound in BSY extracts. It has been seen that yeast cells can adsorb phenolic compounds from malt and hops on their surfaces during beer fermentation due to the negatively charged cell walls, related to the ionization of carboxyl of cell wall proteins and phosphomannans (polysaccharides).32 All the five phenolic compounds detected were also informed in beer products and brewer spent yeast residue.1,6,33 Moreover, Viera et al.6 separated thirteen phenolic compounds from BSY by HPLC and also reported that ferulic and protocatechuic acids were the most abundant phenolic acids in BSY. Fitted models were only significant for PA and CA. Thus, 4HBA, VA and FA contents in BSY extracts were not influenced by autolysis conditions. The lineal model fitted PA content with significant interaction between factors, the highest PA content being obtained at higher temperature and time (50 °C, 24 h), but also at intermediate temperature and lower time (40 °C, 2.27 h). Additionally, a quadratic model with only significant t2 factor fitted CA content, intermediate temperature and lower time (40 °C, 2.27 h) being better for accumulate CA compound. Vieira et al.7 analyzed temperature and time autolysis conditions over total phenolic compounds (TPC), and found that the lowest temperature and time (38 °C, 3.8 h) condition was better for increased TPC in autolysates. This trend may be explained by the thermal sensitivity of certain phenolic compounds, which can undergo degradation, oxidatiolen, or structural transformation at higher temperatures or longer processing times. Consequently, milder autolysis conditions may better preserve the stability and overall concentration of phenolic compounds in the resulting autolysates. Furthermore, individual phenolic compounds could have different behavior according to the temperature and time conditions used for the autolysis.
The peptides generated during the autolysis were evaluated according to their HPLC profile, which can be used as an indicator of their hydrophobicity: 0–20 min (low hydrophobicity, LH), 20–40 min (intermediate hydrophobicity, IH), 40–60 min (high hydrophobicity, HH). Table 2 shows that most of the peptides presented LH in all the samples, and HH peptides were in a low content. Moreover, quadratic models fitted LH and IH peptides, but any model fit HH peptide content. Longer autolysis times (15–24 h) allow generating higher percentage of IH peptides and lower percentage of LH peptides. It is known that hydrophobicity plays an important role in many functional and bioactive properties of food-derived peptides.34 Intermediate hydrophobicity refers to molecules with both hydrophilic and hydrophobic parts. It has been seen that those peptides with IH and HH exhibited higher bioactive potential than LH peptides, due to these peptides could target more specific molecules.34 Peptide hydrophobicity can be affected by the degree of hydrolysis, the processing conditions, type of protease, the average hydrophobicity value of protein precursors, and the sequence of hydrophobic amino acid residues in the peptide chain.34
3.3. Antioxidant activity
Antioxidant activity obtained in BSY extracts are shown in Table 2. Additionally, Table S3 shows the statistical results of the model fit for that analyzed responses (ABTS+ and DPPH scavenging, FRAP, copper-chelating activity). Lineal model without interaction fitted ABTS+, DPPH and copper-chelating activity. For ABTS+, time negatively influenced the activity values (higher ABTS+ scavenging at 2.27–6 h); for DPPH both factors had influence (positive for temperature and negative for time, higher DPPH scavenging at 40 °C, 2.27 h); while for copper-chelating activity, only the time influenced positively (higher activity at 24 h). Additionally, the cubic model fitted FRAP results with the lowest time and the highest temperature condition (50 °C, 6 h) being the best to obtain compounds with the highest reducing power. Thus, the antioxidant compounds generated during BSY autolysis presented different antioxidant activity mechanisms. It is important to remark that for radical scavenging activity (ABTS+ and DPPH) and reducing power, longer autolysis times reduced the activity of these antioxidant compounds while for copper-chelating activity, longer times were favorable for the production of them. Moreover, the antioxidant compounds presented higher copper-chelating activity than radical scavenging or reducing power since they need to be diluted 20 times more for performing the first assay. Considering that the chelating activity was the most potent, it was compared with an EDTA solution of similar concentration (0.5 mg mL−1) to evaluate the antioxidant potential of the autolysates and their suitability for use as food ingredients. At this concentration, EDTA inhibited 80% of β-carotene oxidation in the assay, indicating that our extracts may be even more effective than a typical chelating agent commonly used in the food industry.Also, Vieira et al.7 found a reduction of antioxidant properties evaluated thorough FRAP and DPPH at longer times of autolysis attributed to the degradation of phenolic compounds, vitamins and bioactive peptides. Moreover, many S. cerevisiae peptides have been reported about their antioxidant properties assayed by radical scavenging and reducing power activity,35 or the capacity to form metal ion complexes,36 or to prevent lipid oxidation.37
Pearson correlation test showed that protein degree of hydrolysis correlated negatively with ABTS+ and FRAP (r = −0.8436 and −0.6703, respectively), but positively with copper-chelating activity (r = 0.8675). Moreover, ABTS+ inhibition correlated positively with LH and HH-peptides and with chlorogenic acid content (r = 0.8387, 0.8285, 0.7850, respectively), but negatively with IH-peptides (r = −0.8609, respectively). On the other hand, copper-chelating activity correlated positively with IH-peptides (r = 0.7375).
Higher protein degree of hydrolysis is related to the release of lower molecular weight peptides. Thus, ABTS+ and FRAP activity could be associated with peptides of intermediate molecular weight. In this sense, Amorim et al.4 showed that >3 kDa yeast peptides exhibited the highest antioxidant activity evaluated by radical scavenging activity. Moreover, copper-chelating activity could be related to low molecular weight peptides linked with the highest DH obtained in the extracts with this activity.
Regarding to peptide hydrophobicity, HH-peptides showed higher radical scavenging and reducing power potential. In this sense, according to Zou et al.38 Trp, Phe, Val, Ile, Gly, Lys, and Pro are the most established hydrophobic amino acids associated with antioxidant activities. Hydrophobic Phe and Tyr residues of peptides have aromatic rings capable of reacting with hydroxyl radical to form stable hydroxylated derivatives.34 Moreover, the fact that IH-peptides exhibited higher copper-chelating activity could be related to an amphypatic amino acid sequence. Cys, His, Asp and Glu are the most frequently reported amino acids contributing to metal chelating activity.39
Finally, chlorogenic acid was the only phenolic compound significantly correlated with antioxidant activity. This phenolic acid had proven ABTS+ radical scavenging activity.40 The absent of significant correlation between the other analyzed phenolic compounds and the antioxidant activity does not mean phenolic compound not exhibited bioactive potential, since the activity is the result of the combined effects of peptides and phenolic compounds.
3.4. Sensory properties
Sensory evaluation of BSY extracts is shown in Table 3. Additionally, Table S4 shows the statistical results of each fitted-model. Yeast smell fitted with a lineal model with interaction, the highest temperature and time being the autolysis condition with the lowest yeast smell, which is a desirable property. Color perception fitted with a quadratic model, but conditions of higher temperature and time increased the intensity of brown color, probably related with higher Maillard reaction after inactivation of enzymes at 90 °C. Umami taste model was lineal without interaction: temperature had a low negative influence and time a high positive influence, thus, higher times are preferred for generating umami compounds. Bitter taste fitted with a model of quadratic temperature factor, intermediate temperatures being the ones with increased bitter taste perception. Pungency sensation also fitted with a quadratic model, but with the quadratic factor of time being significant. The extracts obtained at intermediate times of autolysis showed the lowest pungency sensation.| Independent variable | Responses | ||||||
|---|---|---|---|---|---|---|---|
| Temperature (°C) | Time (h) | Yeast smell | Color perception | Umami taste | Bitter taste | Pungency | Residual |
| 30.00 | 6.00 | 6 | 3.4 | 3.5 | 2.0 | 0.8 | 1.9 |
| 25.86 | 15.00 | 6.4 | 2.6 | 4.6 | 1.8 | 1.6 | 1.7 |
| 30.00 | 24.00 | 6.3 | 4.0 | 5.1 | 3.7 | 2.1 | 3.8 |
| 40.00 | 2.27 | 5.8 | 3.7 | 2.9 | 3.3 | 3.9 | 4.0 |
| 40.00 | 15.00 | 5.6 | 4.3 | 4.1 | 4.3 | 1.3 | 2.5 |
| 40.00 | 15.00 | 5.1 | 4.6 | 4.3 | 3.7 | 1.0 | 2.8 |
| 40.00 | 15.00 | 4.9 | 4.4 | 4.4 | 4.0 | 0.7 | 2.9 |
| 40.00 | 27.73 | 3.2 | 5.9 | 6.0 | 5.7 | 4.4 | 5.8 |
| 50.00 | 6.00 | 4.9 | 4.2 | 3.2 | 2.6 | 2.3 | 3.4 |
| 54.14 | 15.00 | 3.6 | 5.3 | 3.7 | 2.8 | 1.4 | 2.5 |
| 50.00 | 24.00 | 2.6 | 6.2 | 4.8 | 4.0 | 2.2 | 3.7 |
Yeast flavors depends on the balance among peptides, nucleotides (guanosine monophosphate and inosine monophosphate), carbohydrates and free amino acids, released or hydrolyzed during autolysis.24 Glutamic acid is one of the predominant amino acids present in the yeast cell wall and is released during autolysis. When combined with short chain peptides, lipid derivatives and any formed 5′-nucleotide, the overall flavor is increased. Thus, the selection of a specific condition for the autolysis is important to promote the desired flavors.
Pearson correlation test showed a strong negative correlation between yeast smell and color perception (r = −0.9419), indicating that a higher content of Maillard products was favorable for reducing yeast smell. The sensory properties of the extracts are strongly affected by the processes like evaporation and drying, during which Maillard compounds are formed and which are responsible for giving cooked meat flavors.5 It was reported that the “yeast taste” in yeast extract is related to one of its main odors, which is mainly composed of propionic acid and butyric acid,41 which could be evaporated during the thermal treatments.
Related with color perception of extracts, the color correlated positively with DH (r = 0.8263), possible due to the higher free amino acid content allowed a more intense Maillard reaction.
When analyzed compounds responsible of umami taste, Pearson correlation test showed that umami correlated positively with IH-peptides (r = 0.6074) and with glutamic acid content (r = 0.7138). Thus, autolysis conditions producing higher umami taste were accompanied by higher content of IH-peptides and free glutamic acid. Umami peptides are small-molecule peptides with the molecular weights ranging from 150 to 3000 Da, and usually contain glutamate or aspartic acid residues.9 On the other hand, it is known that glutamic acid has umami taste properties. The threshold for umami taste of L-glutamic acid and its sodium salt is 0.3 mg mL−1.9
Bitter taste correlated positively with DH (r = 0.6031). Taking into account that the source of the bitterness are the peptides resulting from yeast protein hydrolysis and the intensity of the bitterness is generally proportional to the length of the peptide chain,41 this positive correlation indicates that short peptides exhibited higher bitter taste.
Additionally, residual flavor correlated positively with bitter taste (r = 0.7731), pungency (r = 0.8507) and glutamic acid (r = 0.6388). Thus, the perception of residual flavor was related with the main tastes and flavors presented in the extracts. Pungency also could be produced by nucleotides, free amino acids and hydrophobic amino acids present in peptides.42 There are a few reports of spicy flavor of yeast extracts,5 and could be an interesting parameter to select autolysis conditions to obtain extracts to be use in vegan cheese.
Although nucleotides do not correlate with any of sensory parameters evaluated, they could be making a major contribution to the taste of yeast products by interacting with other components. Nucleotides based on 5′-adenosine phosphate (AMP), 5′-inosine phosphate (IMP), and 5′-guanosine phosphate (GMP) are 100 times more taste-active than seasonings such as monosodium glutamate. Thus, nucleotides play an important role in yeast extracts as food flavoring agents.41 Studies on individual nucleotides are needed in a future to understand the influence of them on the sensory evaluation of BSY extracts.
3.5. Optimization and model validation
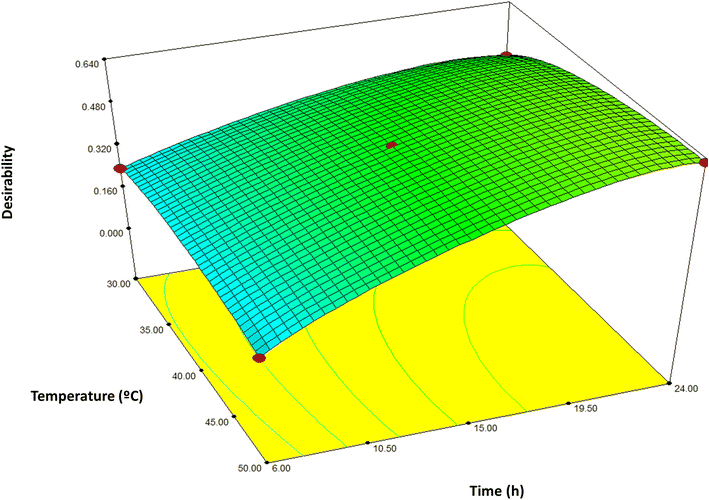
The response optimization was performed in order to obtain a yeast extract suitable for using in vegan cheese. The umami taste, pungency, and antioxidant activity were maximized, and yeast smell and bitter taste were minimized as was mentioned in Section 2.6. This was accomplished by maximizing the Derringer desirability function,20 which aims to find the experimental conditions that ensure all responses have a desirable (optimal) value. In addition, the factors temperature and time were kept within range. Responses for optimization were selected based on the fitted model obtained in the experimental designs.The desirability function graph is shown in Fig. 1. The result obtained was a desirability D of 0.631, corresponding to the condition of 47.7 °C and 23.7 h autolysis. The desirability value obtained can be considered acceptable, considering the high number of simultaneously optimized responses.
The suggested optimal conditions were subsequently corroborated by comparing the experimental values and confidence intervals (95% CI) obtained with those predicted by the model fitted with the desirability function (Table 4). The results confirmed the good predictive capacity obtained with the models.
| Predicted | Experimental | 95% CI | |
|---|---|---|---|
| a d.b. Dry basis; AA: ascorbic acid. CI: confidence interval. | |||
| Yeast smell | 2.5 | 2.6 | 1.3–3.8 |
| Umami taste | 4.9 | 4.7 | 4.3–5.5 |
| Bitter taste | 4.2 | 3.7 | 3.4–5.7 |
| Pungency | 2.5 | 2.0 | 0.9–4.1 |
| ABTS+ scavenging (mM Trolox per g d.b.) | 4.8 | 5.5 | 4.0–5.6 |
| DPPH scavenging (mg AA per g d.b.) | 7.5 | 9.8 | 5.7–9.9 |
| Copper-chelating (%) | 88.2 | 92.1 | 61.7–114.6 |
4. Conclusions
Different autolysis conditions were evaluated in order to obtain extracts intended for use in vegan cheese with high umami taste and pungency. The compounds responsible for the sensory and antioxidant properties were identified. Glutamic acid and intermediate-hydrophobicity peptides were the main compounds contributing to the umami taste, while low-molecular-weight and intermediate-hydrophobicity peptides provided greater copper-chelating capacity. Furthermore, GABA, taurine (identified for the first time in BSY extracts) and phenolic compound concentration were influenced by the autolysis conditions. The amount of GABA produced by BSY autolysis at 30 °C for 24 h is sufficient for providing functional benefits when added to a food at 1 g solids per 100 mL.This work not only allowed the determination of the autolysis conditions that provide the highest content of umami or pungent compounds to the BSY extract for use as flavoring or flavor-enhancer in plant-based foods, but also identifying those autolysis conditions that generate extracts with higher content of other compounds of interest, such as GABA and taurine.
Future work should deepen the molecular resolution of these findings by: (i) quantifying individual nucleotides via HPLC to clarify their role in umami enhancement; (ii) identifying peptide sequences responsible for antioxidant and taste properties using LC-MS/MS; (iii) evaluating the stability and functionality of the optimized extracts when incorporated into real food matrices; (iv) studying gastrointestinal digestion effects on bioactivity; and (v) assessing process scalability and cost-benefit performance for industrial adoption. These steps will further support the valorization of spent yeast as a sustainable, multifunctional ingredient for the food industry.
Author contributions
Antonela G. Garzón: methodology, investigation, formal analysis, data curation, writing – original draft, writing – review & editing, project administration, funding acquisition Yanina Pavón: investigation, formal analysis, data curation, writing – original draft. Marilin E. Aquino: investigation, formal analysis. Silvina R. Drago: writing – review & editing, validation, supervision, conceptualization.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Data availability
All the data generated or analyzed during this study are included in the manuscript.Supplementary information (SI): data about descriptors used to characterize the BSY extracts, and statistical analysis and model coefficients of experimental design. See DOI: https://doi.org/10.1039/d5fb00499c.
Acknowledgements
This work was funded by FONCyT [Grant PICT-2021-0742].References
- A. Jaeger, E. K. Arendt, E. Zannini and A. W. Sahin, Brewer's spent yeast (BSY), and underutilized brewing by-product, Fermentation, 2020, 6, 123 CrossRef CAS.
- D. San Martin, J. Ibarruri, B. Iñarra, N. Luengo, J. Ferrer, C. Alvarez-Ossorio, C. Bald, M. Gutierrez and J. Zufia, Valorization of brewer's spent yeasts' hydrolysates as high-value bioactive molecules, Sustainability, 2021, 13, 6520 CrossRef.
- F. F. Jacob, L. Striegel, M. Rychlik, M. Hutzler and F. J. Methner, Spent yeast from brewing processes: a biodiverse starting material for yeast extract production, Fermentation, 2019, 5, 51 CrossRef CAS.
- M. Amorim, J. O. Pereira, D. Gomes, C. Dias Pereira, H. Pinheiro and M. Pintado, Nutritional ingredients from spent brewer's yeast obtained by hydrolysis and selective membrane filtration integrated in a pilot process, J. Food Eng., 2016, 185, 42–47 CrossRef CAS.
- D. Tomé, Yeast extracts: nutritional and flavoring food ingredients, Food Sci. Technol., 2021, 1, 487–494 Search PubMed.
- E. F. Vieira, J. Carvalho, E. Pinto, D. Cunha, A. A. Almeida and I. Ferreira, Nutritive value, antioxidant activity and phenolic compounds profile of brewer's spent yeast extract, J. Food Compos. Anal., 2016, 52, 44–51 CrossRef CAS.
- E. F. Vieira, A. Melo and I. Ferreira, Autolysis of intracelullar content of brewer's spent yeast to maximize ACE-inhibitory and antioxidant activities, LWT, 2017, 82, 255–259 Search PubMed.
- Y. Dang, L. Hao, J. Cao, Y. Sun, X. Zeng, Z. Wu and D. Pan, Molecular docking and simulation of the synergistic effect between umami peptides, monosodium glutamate and taste receptor T1R1/T1R3, Food Chem., 2019, 271, 697–706 Search PubMed.
- Y. Zhao, M. Zhang, S. Devahastin and Y. Liu, Progress on processing methods of umami substances: A review, Trends Food Sci. Technol., 2019, 93, 125–135 CrossRef CAS.
- B. Podpora, F. Swiderski, A. Sadowska, R. Rakowska and G. Wasial-Zys, Spent brewer’s yeast extracts as a new component of functional food, Czech J. Food Sci., 2016, 34, 554–563 CrossRef CAS.
- R. B. Henika, Use of response-surface methodology in sensory evaluation, Food Technol., 1982, 36, 96–101 Search PubMed.
- O. H. Lowry, N. J. Rosenbrough, A. L. Farr and R. J. Randall, Protein measurements with the Folin phenol reagent, J. Biol. Chem., 1951, 193, 265–275 CrossRef CAS.
- M. E. Aquino, F. Sánchez de Medina, S. R. Drago, O. Martínez-Augustin and R. E. Cian, Gastrointestinal digestion and colonic fermentation of brewer's spent yeast peptides modulate their antioxidant properties and effect on intestinal barrier, Food Biosci., 2024, 60, 104294 CrossRef CAS.
- M. E. Aquino, S. R. Drago, L. P. Schierloh and R. E. Cian, Identification of bioaccessible glycosylated neuroprotective peptides from brewer's spent yeast mannoproteins by in vitro and in silico studies, Food Res. Int., 2025, 209, 116188 CrossRef CAS PubMed.
- P. M. Nielsen, D. Petersen and C. Dambann, Improved method for determining food protein degree of hydrolysis, Food Chem.Toxicol., 2001, 66, 642–646 Search PubMed.
- A. G. Garzón and S. R. Drago, Aptitude of sorghum (Sorghum bicolor (L) Moench) hybrids for brewery or bio-functional malted beverages, J. Food Biochem., 2018, 42, e12692 CrossRef.
- R. E. Cian, O. Martínez-Augustin and S. R. Drago, Bioactive properties of peptides obtained by enzymatic hydrolysis from protein byproducts of Porphyra columbina, Food Res. Int., 2012, 49, 364–372 Search PubMed.
- A. G. Garzón, R. E. Cian, M. E. Aquino and S. R. Drago, Isolation and identification of cholesterol esterase and pancreatic lipase inhibitory peptides from brewer's spent grain by consecutive chromatography and mass spectrometry, Food Funct., 2020, 11, 4994–5003 RSC.
- I. F. F. Benzie and J. J. Strain, The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay, Anal. Biochem., 1996, 239, 70–76 Search PubMed.
- G. Derringer and R. Suich, Simultaneous optimization of several response variables, J. Qual. Technol., 1980, 12, 214–219 CrossRef.
- Z. Takalloo, M. Nikkhah, R. Nemati, N. Jalilian and R. H. Sajedi, Autolysis, plasmolysis and enzymatic hydrolysis of baker's yeast (Saccharomyces cerevisiae): a comparative study, World J. Microbiol. Biotechnol., 2020, 36, 68 CrossRef CAS PubMed.
- H. Tanguler and H. Erten, Utilization of spent brewer's yeast for yeast extract production by autolysis: the effect of temperature, Food Bioprod. Process., 2008, 86, 317–321 Search PubMed.
- A. Zeko-Pivac, K. Habschied, B. Kulisic, B. Barkow and M. Tisma, Valorization od spent brewer's yeast for the production of high-value products, materials, and biofuels and environmental application, Fermentation, 2023, 9, 208 CrossRef CAS.
- G. V. Marson, R. J. Soares de Castro, M. P. Belleville and M. Dupas Hubinger, Spent brewer's yeast as a source of high added value molecules: a systematic review on its characteristics, processing and potential applications, World J. Microbiol. Biotechnol., 2020, 36, 95 CrossRef.
- B. Bach, E. Meudec, J. P. Lepoutre, T. Rossignol, B. Blondin, S. Dequin and C. Camarasa, New insights into γ-aminobutyric acid catabolism: Evidence for γ-hydroxybutyric acid and polyhydroxybutyrate synthesis in Saccharomyces cerevisiae, Appl. Environ. Microbiol., 2009, 75, 4231–4239 Search PubMed.
- E. Boonstra, R. Kieijin, L. S. Colzato, A. Alkamade, B. U. Forstmann and S. Nieuwenhuls, Neurotransmitters as food supplements: the effects of GABA on brain and behavior, Front. Psychol., 2015, 6, 1520 Search PubMed.
- K. Inoue, T. Shirai, H. Ochiai, M. Kasao, K. Hayakawa, M. Kimura and H. Sansawa, Blood-pressure-lowering effect of a novel fermented milk containing γ-aminobutyric acid (GABA) in mild hypertensives, Eur. J. Clin. Nutr., 2003, 57, 490–495 Search PubMed.
- J. O. Agboola, D. Lapeña, M. Overland, M. Overlie Arntzen, L. T. Mydland and J. O. Hansen, Yeast as a novel protein source – Effect of species and autolysis on protein and amino acid digestibility in Atlantic salmon (Salo salar), Aquaculture, 2022, 546, 737312 Search PubMed.
- Y. Masuda, S. Sakamoto and Y. Suzuki, Taurine-rich yeast and method for producing the same, Patent JP2007000037A, 2005 Search PubMed.
- A. Hébert, M. P. Forquin-Gomez, A. Roux, J. Aubert, C. Junot, J. F. Heilier, S. Landaud, P. Bonnarme and J. M. Beckerich, New insights into sulfur metabolism in yeasts as revealed by studies of Yarrowia lipolytica, Appl. Environ. Microbiol., 2012, 79, 1200–1211 Search PubMed.
- Y. Yamori, T. Taguchi, A. Hamada, K. Kunimasa, H. Mori and M. Mori, Taurine in health and diseases: consistent evidence from experimental and epidemiological studies, J. Biomed. Sci., 2009, 17, S6 Search PubMed.
- D. O. Carvalho and L. F. Guido, A review on the fate of phenolic compounds during malting and brewing: technological strategies and beer styles, Food Chem., 2022, 372, 131093 Search PubMed.
- J. Wannenmacher, M. Gasti and T. Becker, Phenolic substances in beer: structural diversity, reactive potential and relevance for brewing process and beer quality, Compr. Rev. Food Sci. Food Saf., 2018, 17, 953–988 CrossRef CAS PubMed.
- C. Acquah, E. Di Stefano and C. C. Udenigwe, Role of hydrophobicity in food peptide functionality and bioactivity, J. Food Bioact., 2018, 4, 88–98 Search PubMed.
- A. S. Oliveira, C. Ferreira, J. O. Pereira, M. E. Pintado and A. P. Carvalho, Spent brewer's yeast (Saccharomyces cerevisiae) as a potential source of bioactive peptides: an overview, Int. J. Biol. Macromol., 2022, 208, 1116–1126 Search PubMed.
- C. Ferreira, C. F. Ferreira, A. S. Oliveira, M. Faustino, A. M. Pereira, J. Durao, J. O. Pereira, M. E. Pintado and A. P. Carvalho, A step for the valorization of spent yeast thourgh production of iron-peptide complexes- A process optimization study, Processes, 2022, 10, 1464 Search PubMed.
- M. Mirzaei, A. Shavandi, S. Mirdamadi, N. Soleymanzdeh, P. Motahari, N. Mirdamadi, M. Moser, G. Subra, H. Alimoradi and S. Goriely, Bioactive peptides from yeast: a comparative review on production methods, bioactivity, structure-function relationship, and stability, Trends Food Sci. Technol., 2021, 118, 297–315 CrossRef CAS.
- T. Zou, B. Bin, T. P. He, H. Li, H. Bin, H. W. Tang and E. Q. Xia, The structure-activity relationship of the antioxidant peptides from natural proteins, Molecules, 2016, 21, 1–14 CrossRef.
- L. Guo, P. A. Harnedy, B. Li, H. Hou, Z. Zhang, X. Zhao and R. J. FitzGerald, Food protein-derived chelating peptides: Biofunctional ingredients for dietary mineral bioavailability enhancement, Trends Food Sci. Technol., 2014, 37, 92–105 CrossRef CAS.
- Y. Luo, Y. C. Li, F. B. Meng, Z. W. Wang, D. Liu, W. J. Chen and L. H. Zou, Simultaneously enhanced stability and biological activities of chlorogenic acid by covalent grafting with soluble oat β-glucan, Food Chem.:X, 2023, 17, 100546 Search PubMed.
- Z. Tao, H. Yuan, M. Liu, Q. Liu, S. Zhang, H. Liu, Y. Jiang, D. Huang and T. Wang, Yeast extract: characteristics, production, applications and future perspectives, J. Microbiol. Biotechnol., 2023, 33, 151–166 CrossRef CAS PubMed.
- Y. Huang, W. Duan, J. Xiao, H. Liu, C. Zhou, Y. Zhang, Y. Tang, B. Sun and Z. Li, Characterization of the taste compounds in 20 pungent spices by high-performance liquid chromatography, J. Food Meas. Char., 2021, 15, 1680–1692 CrossRef.
| This journal is © The Royal Society of Chemistry 2026 |