Open Access Article
Open Access ArticleGlucomannan: sources, physiological mechanisms and applications of an emerging dietary fiber
Raju Sasikumar
 *a,
Anyasha Das
a and
Selva Kumar T
ab
*a,
Anyasha Das
a and
Selva Kumar T
ab
aDepartment of Agribusiness Management and Food Technology, North-Eastern Hill University (NEHU), Tura Campus, Chasingre, Tura, WGH, 794002, Meghalaya, India. E-mail: sashibiofoodster@gmail.com
bVel Tech Rangarajan Dr. Sagunthala R&D Institute of Science and Technology, Chennai 600062, India
First published on 1st December 2025
Abstract
Glucomannan is an emerging dietary fiber from various sources such as plants including their leaves, stems, bulbs, roots, and seeds and microorganisms such as bacteria, fungi and yeasts. Its mannose and glucose ratio, degree of acetylation, and possible substitution vary depending on its origin. Owing to the properties of glucomannan such as its viscosity, bulk, and fermentation ability, it has potential uses in avoiding diet-related disorders. Herein, the use of glucomannan for treating conditions such as cardiovascular diseases, renal diseases, cancer, tumors and diabetes mellitus is discussed. The role of short chain fatty acids (SCFAs) in disease prevention and health maintenance is mainly attributed to the production of butyrate, though acetate and propionate also play other roles in different pathways. Additionally, this review discusses the role of glucomannan in novel applications such as targeted drug delivery, polyurethanes, films, paper and wastewater treatment. Also, the incorporation of other polysaccharides such as chitosan in glucomannan and the addition of various groups such as sulfate and carboxymethyl to its main structure to improve its inherent effect in the above-mentioned sectors are highlighted. This review summarizes the current research on glucomannan and its potential application as a functional food for disease prevention and as a versatile biomaterial.
Sustainability spotlightGlucomannan is a sustainable dietary fiber obtained from plants and other microorganisms, which shows innovative potential in nutrition, health, and environmental applications. Functional foods, preventing illnesses, and biodegradability are supported by its fermentability, viscosity, and structural adaptability, which help to achieve very significant UN Sustainable Development Goals (SDGs), such as SDG 3 (Good Health and Well-Being), and SDG 12 (Responsible Consumption and Production). Through the synthesis of SCFA, glucomannan can help prevent chronic illnesses and improve gut health. It can also be used for developing sustainable biomaterials including films, sheets, and wastewater treatment materials. Its functionalisation with chemical groups (such as sulphate and carboxymethyl) and polysaccharides (such as chitosan) improves its performance in the biomedical and packaging industries. As a scalable, natural alternative for better health and improved ecology, glucomannan connects the gap between food science and chemistry for sustainability, and fundamentals of the circular bioeconomy, encouraging sustainable innovation and sustainable food systems. |
1 Introduction
Dietary fibers, primarily derived from plant sources, are edible components that are resistant to digestion by human enzymes but can be fermented by the gut microbiota. The rumen microbiota secretes the β-1-4 cellulase enzyme, which is a hydrolytic enzyme for the breakdown of plant cell walls to provide energy through hydrolysis. Predominant bacteria such as Fibrobacter succinogenes, Ruminococcus flavefaciens and Ruminococcus albus are responsible for the secretion of cellulase enzyme, though a few fungi and protozoa have also been found to contribute to the digestion of dietary fibers to release 10–35% energy.1,2 However, human beings lack these bacteria, making dietary fibers indigestible. Alternatively, complete or partial fermentation takes place in the colon by the colonic microbiota to degrade them into simpler forms. For example, SCFAs have shown a positive effect on regulatory factors such as oxidative stress, reducing inflammation, and delaying gastric emptying.3,4 Mainly, there are two types of dietary fibers, namely, soluble dietary fibers (SDF) and insoluble dietary fibers (IDF). Soluble dietary fibers are fermentable fibers such as hemicellulosic glucomannans, pectin, inulin, and psyllium, and poorly or non-fermentable fibers such as cellulose and lignin, some resistant starches, and other hemicelluloses.5,6Hemicellulose, the major structural polysaccharides of angiosperms (hardwood) and gymnosperms (softwood), work in close association with lignin.7,8 They are developed during the formation of the secondary cell wall in mature plants. They are covalently cross-linked among the plant cell wall, thus making the extraction of hemicellulosic components very tricky. Hemicellulose, together with lignin, comprises 40–70% of the plant weight,9 whereas only hemicellulose comprises about 20–30% of the total plant weight.8 They are polysaccharides (complex carbohydrates) composed of either linear polymer or branched polymer derivatives. The hemicellulosic components of the cell wall are D-mannose, D-glucose, D-galactose, D-xylose and L-arabinose.10,11 Mannans are the major constituents of hemicellulose and are classified into various sub-families based on their main sugar molecules such as true mannans, galactomannan, glucomannan and galactoglucomannan. True mannans are also called mannans and made up of only β-D-mannose units (linear chain model) linked with a β-1,4-link glycosidic linkage, having a chemical formula of C24H42O21 and with no acetylation or substitution of other groups.8 Galactomannans are made up of β-D-mannose monomers with β-1,4-glycosidic linkages having a chemical formula of C18H32O16 with no occurrence of acetylation; however, having substitution with α-1,6-glycosidic linkages linking galactose residues to the main β-D-mannose backbone by β-1,4-linkages.8,12 Glucomannans are made up of β-1,4-glycosidic linkages with β-D-mannose monomers and β-D-glucose monomers. Although both mannans and glucomannans have the same empirical formula, they differ in their monomeric units. Mannans are made up of only linear chains of β-D-mannose, whereas glucomannans have β-D-mannose and β-D-glucose with 5–10% acetylation and possible substitution.13,14 Galactoglucomannans have a main backbone of β-1,4-linked β-D-glucose and β-D-mannose monomers, branching at the α-1,6-linkage, substitution with galactose residues and 0.2–0.3% acetylation.15
The various types of dietary fibers and their beneficial effect on the human body as well as industrial application in new products or technological development are still a niche subject. Only a few researchers have delved into the mechanism of action provided by each individual dietary fiber for the prevention of diseases as well as for maintaining good health. Apart from this, glucomannan, a soluble dietary fiber, plays an emerging role in various fields, and hence, research is ongoing on its possible sources, applications and involvement in providing health benefits. Thus, this review presents a summary of the different sources of glucomannan, its structural difference, its mechanism of action in physiology as well as its application in other emerging sectors.
Although a number of reviews have deliberated glucomannan primarily in terms of physiological aspects, the current review provides the different sources of glucomannan and its pharmacological mechanisms of action and modern applications. Specifically, it highlights novel aspects such as the role of glucomannan in disease prevention, and its industrial applications such as paper strengthening, film formation, and wastewater treatment. By relating insights into both its sources and applications, this review provides a broad perspective that differentiates it from the existing literature.
2 Glucomannan
Glucomannan is a common constituent of the plant cell wall. Belonging to the hemicellulose group, it is generally found in softwood and hardwood.16 Glucomannan like any other mannan, has a β-1,4-glycosidic-linked backbone made up of repeating units of β-D-mannose and β-D-glucose monomeric units. Apart from its main chain, branching also occurs at the C-3 carbon atom at 32 carbon intervals and it is linked by β-1,3-glycosidic linkage. Moreover, at the C-6 carbon atom, the acetyl group is also attached at every 9–19 repeating sugar units. Its ratio of mannose/glucose varies depending on the source from which it is extracted.10,14,17,18 Glucomannan is extracted from microorganisms such as yeasts and fungi, among which Amorphophallus konjac and Amorphophallus muelleri are the most well-known sources. However, it is not limited to these sources. It is widely distributed among angiosperms and gymnosperms, including their various sources such as leaves, stems, roots, bulbs and seeds, which are listed below in Table 1.11 Moreover, it has been reported that glucomannan is produced as a secondary metabolite by various microorganisms such as yeasts and fungi, although they are reported to have α-1,6-linkages with varying degrees of acetylation and different branching positions.19–22| Source | Parts of plants | Mannose![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) glucose ratio glucose ratio |
Degree of acetylation (%) | Linkage type | Reference |
|---|---|---|---|---|---|
| A. barbadensis | Leaf | 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
— | β-1,4 | 23 |
| C. cardiochilum | Bulbs | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
4.2 | β-1,4 | 28 |
| C. andersonii | Bulbs | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
14.6 | β-1,4 | 27 |
| N. poeticus | Bulbs | 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
— | β-1,4 | 29 |
| N. tazetta | Bulbs | 5.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
12.9 | β-1,4 | 30 |
| L. longiflorum | Bulbs | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
3.2 | β-1,4 | 31 |
| D. officinale | Stems | 6.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
— | β-1,4 | 32 |
| A. muelleri Blume | Tubers | 1.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
— | β-1,4 | 35 |
| B. striata | Tubers | 3:1–7.45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.55 2.55 |
— | β-1,4 | 37 and 38 |
| A. sphaerocephala | Seeds | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.3 1.3 |
— | β-1,4 | 39 |
| L. varius | Seeds | 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8 |
20 | β-1,4 | 41 |
| P. dactylifera | Seeds | 93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 7 |
18 | β-1,4 | 42 |
| C. utilis | Fungi | 2.73–8.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
— | α-1,6 | 21 and 43 |
2.1 Glucomannan obtained from leaves
Generally, glucomannan is obtained from the leaves of the plant species of Aloe vera, Aloe arborescens and Abelia chinensis. The polysaccharides are localized in parenchymatic cells.11 It has been reported that pure glucomannan was obtained from A. barbadensis with β-D-mannose and β-D-glucose in a molar ratio of 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.23 Alternatively, glucomannan obtained from A. barbadensis has been reported to have a molar ratio of β-D-mannose and β-D-glucose ranging from 2.8
1.23 Alternatively, glucomannan obtained from A. barbadensis has been reported to have a molar ratio of β-D-mannose and β-D-glucose ranging from 2.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 22
1 to 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.24 It has been found that acetylation of di-, tri-, tetra-methylmannose and di-methylglucose occurs at O-2,3 and O-6 D-mannose in a ratio of 1
1.24 It has been found that acetylation of di-, tri-, tetra-methylmannose and di-methylglucose occurs at O-2,3 and O-6 D-mannose in a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Acemannans have been reported to have β-1,4-D-glucomannan as their main backbone along with minor β-1,4-linked β-D-glucose units.23,25 Similarly, A. arborescens has been reported to have O-acetyl-β-1,4-linked glucomannan having an acetyl group attached at the O-2, O-3 or O-6 position.26
1. Acemannans have been reported to have β-1,4-D-glucomannan as their main backbone along with minor β-1,4-linked β-D-glucose units.23,25 Similarly, A. arborescens has been reported to have O-acetyl-β-1,4-linked glucomannan having an acetyl group attached at the O-2, O-3 or O-6 position.26
2.2 Glucomannan obtained from bulbs
Glucomannan is obtained from the bulbs of different plant species, such as Cyrtopodium cardiochilum, C. andersonii, Lilium longiflorum, Lycoris squamigera, Narcissus tazetta, and N. poeticus, belonging to the Orchidaceae family.11 C. andersonii has been reported to have β-1,4-linked glucomannan, slightly branched at the C-2, C-3 or C-4 side chain with non-reducing β-D-mannose and β-D-glucose units in a molar ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. It was also reported to have 14.6% degree of acetylation at the C-2 carbon atom with β-1,4-linked β-D-mannose units.27 Glucomannan obtained from C. cardiochilum also has a backbone of β-1,4-linked β-D-mannose and β-D-glucose in a 2
1. It was also reported to have 14.6% degree of acetylation at the C-2 carbon atom with β-1,4-linked β-D-mannose units.27 Glucomannan obtained from C. cardiochilum also has a backbone of β-1,4-linked β-D-mannose and β-D-glucose in a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio. It has also been reported to have an acetylation degree of 4.2% with substitution at the O-2 position.28 N. poeticus has been reported to have β-1,4-linked β-D-mannose and β-D-glucose units, with acetylation at its C-2, C-3 and C-6 atoms in its main backbone chain.29 N. tazetta has been reported to have glucomannan with a β-1,4-linked main backbone and β-D-mannose at the non-reducing end of its structure. It has been reported that glucomannan from N. tazetta has 12.9% acetylation and consists of a 5.6
1 molar ratio. It has also been reported to have an acetylation degree of 4.2% with substitution at the O-2 position.28 N. poeticus has been reported to have β-1,4-linked β-D-mannose and β-D-glucose units, with acetylation at its C-2, C-3 and C-6 atoms in its main backbone chain.29 N. tazetta has been reported to have glucomannan with a β-1,4-linked main backbone and β-D-mannose at the non-reducing end of its structure. It has been reported that glucomannan from N. tazetta has 12.9% acetylation and consists of a 5.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of β-D-mannose and β-D-glucose, which are required for main chain formation.30 L. longiflorum has been reported to have a glucomannan backbone consisting of β-1,4-linked β-D-mannose and β-D-glucose in a 5
1 ratio of β-D-mannose and β-D-glucose, which are required for main chain formation.30 L. longiflorum has been reported to have a glucomannan backbone consisting of β-1,4-linked β-D-mannose and β-D-glucose in a 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 molar ratio. Branching occurs at the C-2 or C-3 positions, and its degree of acetylation has been reported to be about 3.2%.31
2 molar ratio. Branching occurs at the C-2 or C-3 positions, and its degree of acetylation has been reported to be about 3.2%.31
2.3 Glucomannan obtained from stems
Glucomannan has been obtained from the stems of Dendrobium spp., such as D. densiflorum and D. officinale.11 D. officinale has been reported to have glucomannan with a backbone consisting of β-1,4-linkages of β-D-mannose and β-D-glucose units in a molar ratio of 6.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Acetylation occurs at O-2 and O-3 positions in its main backbone chain.32 D. huoshanense contains glucomannan with a similar backbone structure consisting of β-1,4-linked β-D-mannose and β-D-glucose, with β-D-mannose at its non-reducing terminal. The degree of acetylation was observed to be 25% in β-D-mannose and branching was reported to be in the O-3 position.33
1. Acetylation occurs at O-2 and O-3 positions in its main backbone chain.32 D. huoshanense contains glucomannan with a similar backbone structure consisting of β-1,4-linked β-D-mannose and β-D-glucose, with β-D-mannose at its non-reducing terminal. The degree of acetylation was observed to be 25% in β-D-mannose and branching was reported to be in the O-3 position.33
2.4 Glucomannan obtained from tubers (or roots)
Glucomannan has been obtained from tubers, generally from Amorphophallus muelleri Blume and Bletilla striata.11 A. muelleri Blume, also known as porang, belongs to the Araceae family and has been found to have a high glucomannan yield of 15 to 64% on a dry weight basis. Its glucomannan consists of a β-1,4-linked β-D-mannose and β-D-glucose backbone and branching occurs with β-1,6-linkage. Similarly, A. konjac also has reported to have β-1,4-linkage.34,35 Unlike A. muelleri, B. striata has been reported to have glucomannan consisting of β-1,2-linked β-D-mannose and β-1,4-linked β-D-glucose as the main chain; however, other studies have reported a 1,4-linked β-D-mannose and β-D-glucose backbone. The molar ratio of β-D-mannose and β-D-glucose was found to be 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; however, others have reported it to be 7.45
1; however, others have reported it to be 7.45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.55 and 2
2.55 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Acetylation was found out occur at the C-6 and C-2 positions in its main chain.36–38
1. Acetylation was found out occur at the C-6 and C-2 positions in its main chain.36–38
2.5 Glucomannan obtained from seeds
Glucomannan has been obtained from seeds generally from Artemisia sphaerocephala Krasch, Lupinus varius, and libyan dates (Phoenix dactylifera).11 A. sphaerocephala Krasch has been found to have glucomannan with a β-1,4-linked backbone and a molar ratio of β-D-mannose and β-D-glucose of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.3. Branching occurs at the O-6 position. Acetylation and substitution are also present.39,40 L. varius has reported to have glucomannan with a β-1,4-linked backbone and β-D-mannose and β-D-glucose in a molar ratio of 94
1.3. Branching occurs at the O-6 position. Acetylation and substitution are also present.39,40 L. varius has reported to have glucomannan with a β-1,4-linked backbone and β-D-mannose and β-D-glucose in a molar ratio of 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8. Its degree of acetylation is 20% and occurs at the C-6, C-2, and C-3 positions in its main chain.41 P. dactylifera has been reported to have a β-1,4-linked β-D-mannose and β-D-glucose backbone with β-D-mannose and β-D-glucose in a molar ratio of 93
8. Its degree of acetylation is 20% and occurs at the C-6, C-2, and C-3 positions in its main chain.41 P. dactylifera has been reported to have a β-1,4-linked β-D-mannose and β-D-glucose backbone with β-D-mannose and β-D-glucose in a molar ratio of 93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7. The degree of acetylation was determined to be 18%. Acetylation occurs at the C-6 and C-2,3 positions in the main chain of glucomannan.43 Given that the nutritional and physiological qualities of plant-based glucomannan have been well studied, recent studies are now beginning to explore microbial sources as potential substitutes. Unlike plant sources, these microbial forms especially glucomannan produced by fungi provides distinct metabolic pathways and potential applications. The microbial contributions to the synthesis of glucomannan are highlighted in the next section.85
7. The degree of acetylation was determined to be 18%. Acetylation occurs at the C-6 and C-2,3 positions in the main chain of glucomannan.43 Given that the nutritional and physiological qualities of plant-based glucomannan have been well studied, recent studies are now beginning to explore microbial sources as potential substitutes. Unlike plant sources, these microbial forms especially glucomannan produced by fungi provides distinct metabolic pathways and potential applications. The microbial contributions to the synthesis of glucomannan are highlighted in the next section.85
2.6 Microbial glucomannan
The majority of microbial β-mannanases are extracellular enzymes that are secreted into the environment to break down mannan. Microbial β-mannanases have a number of benefits, such as easy gene manipulation, low manufacturing costs, high stability, and ease of cultivation. Consequently, microbes constitute the source of most commercially available β-mannanases. Initially found in Bacillus sp., β-mannanases were later found in Bifidobacterium species, Alicyclobacillus species, Paenibacillus species, and other bacteria. It is well known that B. subtilis is a promising source of β-mannanase.85 B. subtilis strains US191, ATCC11774, WL-7, MA139, BE-91, MAFIC-S11, NM-39, BCC41051, B36, HM7, WL-3, TJ-102, B23, BS5, Z-2, BCC41051, TBS2, G1, and HM7 have been found to exhibit β-mannanase activity. In addition, Nocardioides sp. and Streptomyces sp. show significant mannan degradation capacity among the actinomycetes. A previous study screened Streptomyces sp. CS428 for β-mannanase. Thermophilic Thermobifida fusca BCRC19214 can generate β-mannanase when stimulated with glucomannan. Actinomycetes S. thermolilacinus NBRC14274, T. fusca NBRC1407, S. ipomoea CECT 3341, Streptomyces sp. SirexAA-E, and Streptomyces sp. S27 were also found to have β-mannanases.85 Apart from these bacterial strains, glucomannan can also be obtained from some fungal species.Candida utilis has been reported to have glucomannan with an α-1,6-D-mannose backbone. It is also reported to have a side chain with α-1,3-linkage and substitution at the α-1,2-linkage along with D-mannose and D-glucose in a ratio of 2.73–8.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.21,43 Glucomannan is produced extracellularly by C. utilis along with invertase enzyme. When 80% glucose-containing substrate is used for the growth of the fungi, it has been found that the production of glucomannan and invertase begins after 16 h. The production of glucomannan was found to be proportional to the consumption of the glucose substrate.19 Heterodermia obscurata, a lichen-forming fungi with algae, has been reported to have glucomannan with a α-1,6-D-mannose backbone; however, substitution occurs at the α-1,2-link with α-D-glucose and α-D-mannose and at the α-1,4-link with α-D-mannose.44 In 1992, a study was conducted with 18 lichens to determine the polysaccharide content produced by them. It was found that many of the mycobionts can produce glucomannan with an α-1,6-D-mannose backbone and substitution at various links with different sugar moieties, namely Ceratocystis brunnea and Saccharomyces fragilis. However, among them Tornabenia intricata, can produce glucomannan free from any residues such as D-galactose, unlike the other fungi.20 Researchers conducted another study on Physcia kalbii, a mycobiont belonging to the Physciaceae family, with a glucomannan structure similar to that from T. intricata and H. obscurata, possessing an α-1,6-D-mannose backbone but substitution only at the α-1,2-link by α-D-glucose and α-D-mannose.22
1.21,43 Glucomannan is produced extracellularly by C. utilis along with invertase enzyme. When 80% glucose-containing substrate is used for the growth of the fungi, it has been found that the production of glucomannan and invertase begins after 16 h. The production of glucomannan was found to be proportional to the consumption of the glucose substrate.19 Heterodermia obscurata, a lichen-forming fungi with algae, has been reported to have glucomannan with a α-1,6-D-mannose backbone; however, substitution occurs at the α-1,2-link with α-D-glucose and α-D-mannose and at the α-1,4-link with α-D-mannose.44 In 1992, a study was conducted with 18 lichens to determine the polysaccharide content produced by them. It was found that many of the mycobionts can produce glucomannan with an α-1,6-D-mannose backbone and substitution at various links with different sugar moieties, namely Ceratocystis brunnea and Saccharomyces fragilis. However, among them Tornabenia intricata, can produce glucomannan free from any residues such as D-galactose, unlike the other fungi.20 Researchers conducted another study on Physcia kalbii, a mycobiont belonging to the Physciaceae family, with a glucomannan structure similar to that from T. intricata and H. obscurata, possessing an α-1,6-D-mannose backbone but substitution only at the α-1,2-link by α-D-glucose and α-D-mannose.22
3 Physiological applications of glucomannan and its mechanism
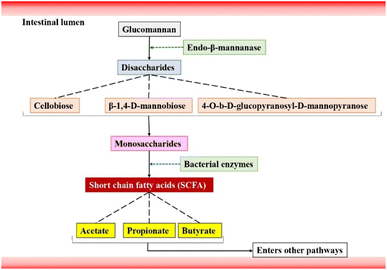
Glucomannan consisting of β-D-mannose and β-D-glucose is not digestible, unlike starches and sugars. However, intestinal bacteria such as Firmicutes and Lactobacillus can ferment it to simpler forms with C2 to C3 carbons, with the help of various enzymes. Prior to that, glucomannan gets degraded to intermediary units, as shown in Fig. 1, by the enzyme endo-β-mannanase by microorganisms such as Clostridium butyricum and C. beijerinckii. This enzyme catalyses the breakdown of glucomannan by cleaving its β-1,4-linkages. The end products, known for their vital roles in various physiological functions, are termed SCFAs.13,45 By altering epigenetic pathways, fatty acids can control gene expression, which can have either beneficial or negative effects on metabolic outcomes.86 Recent clinical investigations have shown that SCFAs affect the host metabolism and immunological regulation by modulating gene expression and by inhibiting histone deacetylase. In addition, it has been demonstrated that SCFA supplementation improves metabolic markers such as HbA1c, and LDL cholesterol, showing its significant role in metabolic health.86 Apart from these beneficial effects, SCFAs are being recognised for their therapeutic use in cardiovascular disease, where they help in improving vascular health and lowering inflammatory markers.60 Also, given that they play a role in altering the tumour microenvironment, SCFAs can be used in oncology treatment.50,513.1 Cancer prevention
The balance between cell generation and cell death is maintained by normal cells through the mechanism “homeostasis”, which was coined by Walter Bradford Cannon in 1926.46 This mechanism is essential for normal body growth and regulation. When this critical balance is broken and cells start growing without any regulation, it leads to the development of cell masses termed tumours.47 They can be either malignant tumours or benign (non-malignant) tumours. Malignant tumours invade and spread to other sites, thus resulting in the proliferation of more cells at other locations and causing diseases termed cancer. Based on the origin of the tissue cell, cancer is classified into carcinomas (epithelial origin), sarcomas (mesodermal origin) and adenocarcinomas (glandular tissue origin).48 Abnormal cell growth usually occurs due to genetic mutations, which are termed oncogenes. Many oncogenes have been reported to have carcinogenic expression such as MYC (myelocytomatosis virus MC29) in chickens.49 Dietary fibers such as inulin and β-glucans have been reported to have beneficial effects on the gut microbiome, subsequently leading to the prevention of cancers, especially colon cancer. Apart from that, researchers have also found that inflammation in the large bowel, a factor for cancer, can also be lowered by the consumption of dietary fibers.50 It has been found that the fermentation of dietary fibers at the intestinal site increases the growth of good bacteria such as bifidobacteria and decreases the growth of E. coli, Salmonella and Listeria. Dietary fibers are broken down through fermentation in the large intestine by the good bacteria. Consequently, the dietary fibers are metabolized into short chain fatty acids such as butyrate, propionate and acetate, which help in the prevention of colorectal cancer. The production of short chain fatty acids is illustrated by the following generalized equation:50| 59C6H12O6 + 38H2O → 60C2H3O2− + 22C3H5O2− + 18C4H7O2 + 96CO2 + 256H+ |
However, the complex nature and diversity of gut microbial metabolism in vivo are not completely represented by the above-mentioned equation, it only provides a simplified stoichiometric model of glucose fermentation. Also, the short chain fatty acid yields and fermentation pathway depend on the types of substrate used, microbial type, and physiology of the host.50 Soluble dietary fibers have been found to have the ability to inhibit the colony forming capacity of the tumour-causing cell lines HCT116 and HT-29. It has also been found to induce apoptosis in them, causing cell death, and leading to less proliferation of abnormal cells.51
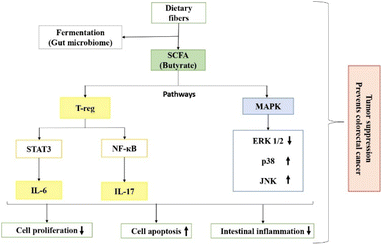
Usually, short chain fatty acids (SFCA) are the result of the saccharolytic fermentation of carbohydrates, but a minor number of amino acids has been found to have a contribution as well. Akkermansia muciniphila is the major species for propionate production and Ruminococcus bromii has been found to be the major contributor to butyrate production, although other species have been reported to have contributions as well.52 The major short chain fatty acid contributing to colorectal cancer prevention has been found to be butyrate, which enters different pathways to provide beneficial effects, as shown in Fig. 2. It prevents cell proliferation by inhibiting histone deacetylases. Histone deacetylases (HDACs) are enzymes having the role of removing the acetyl groups from histones and other proteins, performing re-modelling of chromatin and providing gene expression.53–55 They can have both positive and negative effects on inflammatory regulation in the intestine. Over activity of HDACs can suppress the natural apoptosis of cells, thus leading to unregulated cell growth and cell proliferation.54,55 It has also been reported that butyrate binds to the receptors in Treg cells and inhibits the secretion of IL-6 and IL-7 by hindering the STAT-3 and NF-κB pathways, respectively, causing a decline in the proliferation of cells and reducing inflammation, while inducing cell death (apoptosis).54 Moreover, the mitogen activated protein kinase (MAPK) pathway is responsible for the production of extracellular signal-regulated protein kinase 1/2 (ERP 1/2), which is associated with cell proliferation, differentiation and cell survival. Butyrate reduces the activation of ERK 1/2 and increases p38 and C-Jun N-terminal kinase (JNK), which are responsible for cell growth arrest and apoptosis.56
Glucomannan has been found to directly prevent the growth of tumour cells by decreasing the activity of surviving, an anti-apoptosis gene, and BLC-9, a pro-survival gene. It also controls tumour cell growth by increasing apoptosis-initiating proteins such as BAX and caspase-9. It has been also found to increase autophagy by increasing the expression of LC3-II, a biomarker.57 Glucomannan also indirectly decreases the development of tumour cells by reducing the activity of β-glucuronidase and mucinase, which are known for converting substances to carcinogens. Due to its fermentability, it promotes probiotic bacteria and indirectly aids in the hindrance of secondary bile formation, which is known to have a neoplastic effect.57
3.2 Cardiovascular disease (CVD) prevention
Cardiovascular disease is not a single disease, but a cluster of multiple conditions including coronary heart diseases (CHD), hypertension, and angina. Dietary fibers do not directly aid in the prevention of cardiovascular diseases. They get fermented by the gut bacteria to short chain fatty acids, namely propionate, acetate and butyrate usually in the ratio of approximately 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60
60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
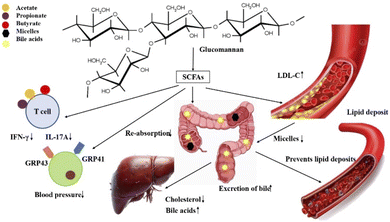
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, though there may be degrees of variation.58 Blood pressure regulation occurs via histone deacetylases (HDACs) and G protein-coupled receptors (GPCRs). SCFAs have also been reported to have vasodilation and vasocontraction properties. It has been shown that the consumption of SCFAs also contributes to hypertension regulation in the absence of probiotics.58 Acetate and propionate bind with the receptors of T cells and decrease the formation of IFN-γ and IL-17A. Similarly, their binding at the receptor sites of GRP43 and GRP41 prevents signal transmission and aids in the lowering of blood pressure.84 Notably, apart from these immune system modulator and vascular effects, propionate directly inhibits hepatic cholesterol synthesis, thus supplementing the classical fiber-mediated mechanisms. Researchers have reported that acetate and propionate have no influence on cholesterol intake. However, butyrate has been reported to show a decrease in the intake of cholesterol in mice.59 Although glucomannan has no effect on triglyceride levels, it has been reported to have a significant role in lowering the total cholesterol level. The mechanism proposed by researchers, as shown in Fig. 3, suggested that when glucomannan is fermented it has high viscosity, which helps to prevent the re-absorption of cholesterol by increasing the transit time and removing bile acids along with it. Moreover, due to the low bile acid levels, the liver is triggered to synthesize more by up taking cholesterol from the blood stream.60 Simultaneously, glucomannan prevents micelle formation. Low density lipoprotein cholesterol (LDL-C) and bile salts form micelles, which can adhere to blood vessel walls, thus constricting blood flow, a precursor of atherosclerosis. This causes the liver to produce more bile acids with the uptake of cholesterol, thus lowering the blood cholesterol levels.60 These pathways provide an improved understanding of the cholesterol-lowering effect of GM and its role in reducing cardiovascular disease when coupled with the direct inhibitory actions of SCFAs such as propionate on hepatic cholesterol production.
20, though there may be degrees of variation.58 Blood pressure regulation occurs via histone deacetylases (HDACs) and G protein-coupled receptors (GPCRs). SCFAs have also been reported to have vasodilation and vasocontraction properties. It has been shown that the consumption of SCFAs also contributes to hypertension regulation in the absence of probiotics.58 Acetate and propionate bind with the receptors of T cells and decrease the formation of IFN-γ and IL-17A. Similarly, their binding at the receptor sites of GRP43 and GRP41 prevents signal transmission and aids in the lowering of blood pressure.84 Notably, apart from these immune system modulator and vascular effects, propionate directly inhibits hepatic cholesterol synthesis, thus supplementing the classical fiber-mediated mechanisms. Researchers have reported that acetate and propionate have no influence on cholesterol intake. However, butyrate has been reported to show a decrease in the intake of cholesterol in mice.59 Although glucomannan has no effect on triglyceride levels, it has been reported to have a significant role in lowering the total cholesterol level. The mechanism proposed by researchers, as shown in Fig. 3, suggested that when glucomannan is fermented it has high viscosity, which helps to prevent the re-absorption of cholesterol by increasing the transit time and removing bile acids along with it. Moreover, due to the low bile acid levels, the liver is triggered to synthesize more by up taking cholesterol from the blood stream.60 Simultaneously, glucomannan prevents micelle formation. Low density lipoprotein cholesterol (LDL-C) and bile salts form micelles, which can adhere to blood vessel walls, thus constricting blood flow, a precursor of atherosclerosis. This causes the liver to produce more bile acids with the uptake of cholesterol, thus lowering the blood cholesterol levels.60 These pathways provide an improved understanding of the cholesterol-lowering effect of GM and its role in reducing cardiovascular disease when coupled with the direct inhibitory actions of SCFAs such as propionate on hepatic cholesterol production.
3.3 Diabetes mellitus prevention
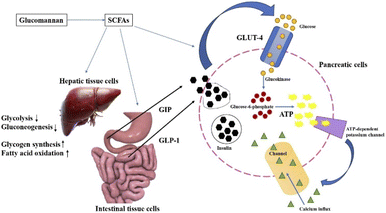
Diabetes mellitus is a condition where the body experiences high blood sugar levels, i.e., hyperglycemia. This condition can be either in fasting or post-prandial state. Diabetes mellitus (DM) is broadly classified into two types, type I DM and type II DM. Type I is known insulin-dependent diabetes mellitus.61 This is an autoimmune disorder, in which the β-cells are either dysfunctional or fail, causing little to no production of insulin, and ultimately leading to hyperglycemia. Type II DM is the most common disease, in which the body becomes resistant to insulin secretion and does not perform well in glucose storage in the liver cells. Due to the lack of glucose intake by the cells, it causes high blood sugar levels (hyperglycemia).61 In maintaining the homeostasis of glucose metabolism to release energy, the endocrine gland pancreas plays the major role. The β-cells of the pancreas, known as the islets of Langerhans, are responsible for secreting the glucose regulating hormone insulin.62,63 Insulin is a peptide hormone, which regulates the intake of glucose for energy production.62,63It has been reported that dietary fibers and their fermented constituents, SCFAs, help in reducing post-prandial glucose. Dietary fibers retain glucose within their structure after hydration.64,65 Apart from that, they compete and bind to the receptor sites of the enzymes responsible for starch metabolism. This prevents the breakdown of starch molecules into their constituents, such as glucose, ultimately reducing the level of glucose in the blood after ingestion.64,65 As shown in Fig. 4, SCFAs have been reported to be secretagogues, known for release of hormones such as glucagon-like peptide-1 (GLP-1), gastric inhibitory peptide (GIP) and peptide YY (PYY) from the small intestines of duodenal and jejunal cells.62,66 SCFAs, formed by dietary fiber fermentation, act by signalling molecules to bind with the G protein-coupled receptor (GPCR) GPR41, which is responsible for satiety and delays the movement of food to the small intestine, and GPR43, which is responsible for satiety and delayed gastric emptying, also promoting insulin secretion, in turn hindering the breakdown of starch molecules, preventing an increase in blood glucose levels. This also prevents glycolysis and gluconeogenesis in the liver cells, and also aids in the increased activity of glycogen synthesis and fatty acid oxidation.67,68
3.4 Renal disease prevention
Renal diseases are a group of diseases including glomerulonephritis, acute kidney injury (AKI), chronic kidney disease (CKD), and uremic syndrome. Glomerulonephritis is characterized by immune-mediate damage to the glomerulus in the kidney. This can be detected by several symptoms such as blood in urine (hematuria), protein in urine (proteinuria) and high levels of urea and ammonia in blood (azotemia).69 Acute kidney injury (AKI) is characterized by the rapidly declining rate of normal kidney functions, which results in the disruption of homeostasis among fluids, acid–bases and electrolytes. Chronic kidney disease occurs when the glomerular filtration rate (eGFR) is less than 60 mL/min per 1.73 m2.70Researchers have found that an imbalance in the intestinal microbiome, termed dysbiosis, is a causative factor for renal diseases, especially, chronic kidney disease (CKD). This causes epithelial modifications in the intestine and causes the movement of various substance such as lipopolysaccharides (LPS) into the blood stream by a decrease in the barrier function.71,72 These lipopolysaccharides (LPS) bind macrophages and monocytes through TLR4 by the activation of NF-κB, resulting in the onset of inflammation through various cytokines (proinflammatory gene expressors). This causes the generation of reactive oxygen species (ROS), which leads to the damage of endothelial tissues. Oxidative stress and persistent inflammation work on a positive feedback loop, causing conditions such as premature aging and kidney fibrosis by decreasing the glomerular filtration rate (GFR).72
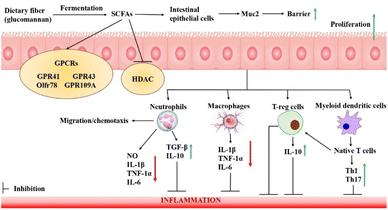
It has been found that SCFAs prevent inflammation by recruiting leukocytes and increasing the production of chemokines. They help in suppressing interleukin (IL)-6, IL-1β, and other cytokines, while simultaneously increasing the production of IL-10 (an anti-inflammatory cytokine) by G protein-coupled receptors (GPCRs) and/or inhibiting the functioning of histone deacetylases (HDACs), as shown in Fig. 5.71 Inflammatory responses create an antagonistic effect on the production of erythropoietin, a hormone stimulating the production of red blood cells. This not only causes a decrease in the sensitivity of erythrocytes towards erythropoietin but also hinders the metabolism of iron, thus, aggravating the anaemic condition.73 Nuclear factors such as NF-B, interferon, and protein activator-1 are proteins that regulate gene expression within cells. NF-κB expression is silenced when it reacts with its inhibitor (iκB); however, if its subunit p65 is exposed, degradation of iκB occurs, exacerbating inflammation. SCFAs have been shown to cause a significant decrease in NF-κBp65 subunit expression and aid in decreasing the secretion of chemokines and cytokines, thus reducing inflammation.71,73 SCFAs are produced due to the partial digestion of dietary fibers, inducing the goblet cells in the intestine to secrete mucin (Muc2), which helps in strengthening the barrier against pathogens and intestinal bacteria, thus reducing inflammation.74 It has been found that SCFAs have immune cell migration capacity, either by passive diffusion or active transporter proteins such as MCT 1/4 and SMCT 1/2, causing a decrease in immune cell migration and proliferation and cell apoptosis.75 It has also been found that SCFAs also have a significant influence on blood pressure levels. The increase in the renal blood pressure levels due to the secretion of renin hormone is counteracted by vasodilation induced by SCFAs and the GPR43 receptor.74,75
4 Applications of glucomannan
Konjac glucomannan (KGM) has been found to have various desirable properties, such as gelation property, film forming ability, water absorption capacity, high viscosity, and fermentability. Due to these properties, konjac-derived glucomannan has a positive effect on physiological ailments.76–78 Moreover, researchers have confirmed that changes in its structural composition via chemical modifications such as acetylation, sulfation, and carboxymethylation facilitate a wide range of application in various fields such as wastewater treatment, drug delivery, paper strengthening, and polymer development.76–784.1 Industrial application
Polyurethane (PU) is a synthetic polymer, which has a wide range of applications in the industrial sector owing to its versatile properties. However, in biomedical applications, it is generally used together with various polysaccharides such as chitin and cellulose.76 KGM, having unique properties, is suitable for application along with PU in the biomedical field. KGM with PU has been shown to have enhanced tensile strength, mechanical strength and enhanced cross-linkage. The affinity of glucomannan derived from A. konjac or Bletilla sp. towards carbohydrate receptors of specific cells has many future applications.76 Yang et al.87 observed a significant increase in the tensile and thermal properties of a material by blending it with waterborne polyurethane and carboxymethyl konjac glucomannan. Konjac glucomannan-based nanomaterials have been synthesized for various applications. In addition, it has been used in several commercial applications such as stabilizers, emulsifiers, and gelling agents in the food processing industry, which has been recently considered a safe food additive by the United States, Canada, and Europe.88 Purified forms of glucomannan such as E 425 i and E 425 ii have been recently recognized as food additives.894.2 Film formation
KGM is found to be a good film-forming agent. Usually, substitution is carried out with acetylation, carboxymethylation, acylation, etc. By increasing the degree of substitution in glucomannan, it was found that there was a decrease in the elongation at break and tensile strength, though it made the film transparent and decreased water adsorption, making the film water-resistant.79 However, it was also noticed that through depolymerization, a water-resistant film material could also be produced by coating with other materials on its surface to make it impermeable to moisture.79 According to a recent study, the thickness of a packaging film increased from 0.034 to 0.066 mm when the ratio of konjac glucomannan to carrageenan increased. The refined carrageenan/konjac glucomannan film had a water vapour transmission rate of 91.4–110.8 g per m2 per day, while the semi-refined carrageenan/konjac glucomannan film had a rate of 80–91.2 g per m2 per day. Konjac glucomannan with refined carrageenan and semi-refined carrageenan showed higher tensile strength and elongation at break.90Films formed using glucomannan act as good sustainable alternatives to conventional plastics, but other properties are still a challenge due to their lower tensile and mechanical strength and greater sensitive to water. Compared to other biopolymer films such as cellulose and chitosan, glucomannan-based films show good oxygen barrier properties, but at the same time less resistance to moisture. Thus, to overcome these challenges, future research should focus on chemical modifications such as blending with polymers, crosslinking with polymers, and incorporation of nanomaterials as fillers. These strategies have made GM films increasingly competitive for applications in food packaging and biomedical materials, while maintaining their biodegradability and eco-friendly profile.91
4.3 Paper strengthening
It has been reported that increasing the degree of substitution in the glucomannan chain has a positive effect on the strength of paper. Researchers have conducted an experiment where the dosage of carboxymethylated glucomannan was varied in the range of 0–1.5% for 40 min.80 It was observed that the highest modification in the paper properties was obtained at about 0.9% with an increased density of 0.53 g cm−3, tensile index of 79.0 Nm g−1, bursting strength of 7.10 kPa m2 g−1 and significant increase in folding endurance, with 996 double folds.80 Another research group conducted an experiment on the paper strengthening ability of glucomannan using cationic glucomannan. One control and four variables with different treatment times of 3, 4, 5 and 6 were employed.81 Also, 1% cationic glucomannan was used at 70 °C and 20% cationic reagent. The result showed the highest changes in 6 h treatment with an increased density of 0.59 g cm−3, tensile index of 100.4 Nm g−1, bursting strength of 7.87 kPa m2 g−1 and increase in folding endurance, with 694 double folds.814.4 Biomedical applications
Due to the physical properties of glucomannan, it has been reported to have a wide range of applications in the biomedical field as a drug delivery agent. Glucomannan in combination with acrylic acid or sodium tripolyphosphate has been used to create hydrogels for targeted drug delivery.13,78 It was found that glucomannan can be degraded by intestinal bacteria enzymes. This creates an opportunity for releasing drugs into the system at the colonic site. Oxidized glucomannan with chitosan has also been reported to have a similar function with a chitosan cross-linked hydrogel, creating better modulation for realizing the properties of the hydrogel.78 Moreover, glucomannan and chitosan microparticles have been developed for the pulmonary delivery of a cancer drug. It was found that the microparticles adhered to epithelium cells and showed a positive interaction among Calu-3 cells (human lung cancer cell line) and has scope for targeted delivery.13 Apart from that, carboxymethylated glucomannan with chitosan nanoparticles have been found to have a favourable effect on bovine serum albumin release.13,78 Researchers have developed a temperature-stable hydrocolloid-encapsulating membrane at a temperature ranging from −20 °C to 90 °C. The membrane contained a liquid material, which was able to withstand changes in temperature without bursting.79 Sulphated glucomannan was found to show similar interactions to that of anti-HIV (human immunodeficiency virus) due to the similar electrostatic interaction between the amino acid groups and glucomannan-sulphate.77,82,83Apart from these applications, many challenges remain in exploring the wider applications of glucomannan in the biomedical sector. Mass production is still a major limitation, as most of the literature reports are still at the prototype stage or not completely product based. Also, chemical modifications such as oxidation, carboxymethylation, and sulphation are not cost effective on a large scale. Although some studies have reported the good biocompatibility and long-term safety of chemically modified glucomannan, extensive assessments are still needed before translational research. By overcoming these challenges, glucomannan-based products or systems can be advanced from prototypes to commercial large-scale biomedical applications.
4.5 Waste water treatment
The application of cationic konjac glucomannan flocculant to treat wastewater has studied. It was shown that glucomannan with a graft copolymer, forming a cationic flocculant, was able to perform well. It was tested under acidic, alkaline and neutral conditions, with changes in pH, ranging from 1–9, and as well as salt treatment. It also showed a high degree of turbidity removal at more than 90% with kaolin suspension and having a 0.2% degree of substitution.515 Conclusion
In conclusion, glucomannan, a hemi-cellulosic polysaccharide, has wide range of applications. Generally, it is found in plants cell walls from different origins and parts as well as produced and secreted by a few microorganisms such as yeast and fungi. Although glucomannan cannot be digested by human beings, it is partially fermented by the microbes in the gut and the production of short chain fatty acids helps in the prevention of various diseases such as cancer, diabetes mellitus, and renal diseases. Apart from its physiological importance, glucomannan has other applications in the industrial sector as well. Recent studies have proven that glucomannan is useful in the paper industry, pharmaceutical industry, and packaging industry. Thus, future research should be focused on optimizing its extraction from various sources and other applications to prove its functioning as a bioactive polysaccharide.Author contributions
Anyasha Das: writing–original draft preparation; Raju Sasikumar: conceptualization, investigation, data curation and supervision; Selva Kumar T: writing–original draft preparation, review & editing.Conflicts of interest
There are no conflicts to declare.Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Notes and references
- G. A. Varga and E. S. Kolver, J. Nutr., 1997, 127, 819–823, DOI:10.1093/jn/127.5.819S.
- C. J. Yeoman, C. J. Fields, P. Lepercq, P. Ruiz, E. Forano, B. A. White and P. Mosoni, mBio, 2021, 12, 1–16, DOI:10.1128/mbio.03533-20.
- C. Cherbut, A. C. Aube, H. M. Blottiere and J. P. Galmiche, Scand. J. Gastroenterol., 1997, 32, 58–61, DOI:10.1080/00365521.1997.11720720.
- P. Liu, Y. Wang, G. Yang, Q. Zhang, L. Meng, Y. Xin and X. Jiang, Pharmacol. Res., 2021, 165, 1–11, DOI:10.1016/j.phrs.2021.105420.
- F. J. Dai and C. F. Chau, J. Food Drug Anal., 2017, 25, 37–42, DOI:10.1016/j.jfda.2016.09.006.
- D. Mudgil, in Dietary Fiber for the Prevention of Cardiovascular Disease, ed. R. A. Samaan, Academic Press, 2017, pp. 35–59, DOI:10.1016/B978-0-12-805130-6.00003-3.
- S. Singh, G. Singh and S. K. Arya, Int. J. Biol. Macromol., 2018, 119, 79–95, DOI:10.1016/j.ijbiomac.2018.07.130.
- R. L. Whistler, in Industrial Gums, ed. R. L. Whistler and J. N. Bemiller, Academic Press, 3rd edn, 1993, pp. 295–308, DOI:10.1016/B978-0-08-092654-4.50015-2.
- O. M. Terrett and P. Dupree, Curr. Opin. Biotechnol., 2019, 56, 97–104, DOI:10.1016/j.copbio.2018.10.010.
- L. R. S. Moreira and E. X. F. Filho, Appl. Microbiol. Biotechnol., 2008, 79, 165–178, DOI:10.1007/s00253-008-1423-4.
- X. D. Shi, J. Y. Yin, S. W. Cui, Q. Wang, S. Y. Wang and S. P. Nie, Trends Food Sci. Technol., 2020, 99, 101–116, DOI:10.1016/j.tifs.2020.02.016.
- R. F. Tester and F. H. Al-Ghazzewi, Food Res. Int., 2013, 50, 384–391, DOI:10.1016/j.foodres.2012.10.037.
- M. Alonso-Sande, D. Teijeiro-Osorio, C. Remuñán-López and M. J. Alonso, Eur. J. Pharm. Biopharm., 2009, 72, 453–462, DOI:10.1016/j.ejpb.2008.02.005.
- S. B. Widjanarko, M. Affandi and Z. Wahyuli, Food Res., 2022, 6, 425–433, DOI:10.26656/fr.2017.6(5).920.
- J. Berglund, S. Azhar, M. Lawoko, M. Lindström, F. Vilaplana, J. Wohlert and G. Henriksson, Cellulose, 2017, 26, 2155–2175, DOI:10.1007/s10570-018-1737-z.
- A. Ebringerová, Z. Hromádková and T. Heinze, in Polysaccharides I, ed. T. Heinze, Springer, Berlin, Heidelberg, 1st edn, 2005, pp. 1–67 Search PubMed.
- Y. Sun, X. Xu, Q. Zhang, D. Zhang, X. Xie, H. Zhou, Z. Wu, R. Liu and J. Pang, Polymers, 2023, 15 DOI:10.3390/polym15081852.
- S. S. Behera and R. C. Ray, Food Rev. Int., 2016, 33, 22–43, DOI:10.1080/87559129.2015.1137310.
- H. Chiura, M. Iizuka and T. Yamamoto, Agric. Biol. Chem., 1982, 46, 1723–1742, DOI:10.1080/00021369.1982.10865332.
- Z. Teixeira, M. Iacomini and P. A. Gorin, Phytochemicals, 1992, 31, 3467–3470, DOI:10.1016/0031-9422(92)83708-7.
- G. Kogan, J. Šandula and V. Šimkovicová, Folia Microbiol., 1993, 38, 219–224, DOI:10.1007/BF02814381.
- L. M. Cordeiro, V. de Fátima Reinhardt and M. Iacomini, Phytochemicals, 2012, 84, 88–93, DOI:10.1016/j.phytochem.2012.08.010.
- G. Mandal and A. Das, Carbohydr. Res., 1980, 87, 249–256, DOI:10.1016/S0008-6215(00)85211-8.
- L. H. Campestrini, J. L. M. Silveira, M. E. R. Duarte, H. S. Koop and M. D. Noseda, Carbohydr. Polym., 2013, 94, 511–519, DOI:10.1016/j.carbpol.2013.01.020.
- X. D. Shi, J. Y. Yin, X. J. Huang, Z. Q. Que and S. P. Nie, Int. J. Biol. Macromol., 2018, 120, 2373–2380, DOI:10.1016/j.ijbiomac.2018.09.005.
- X. D. Shi, J. Y. Yin, L. J. Zhang, O. Y. Li, X. J. Huang and S. P. Nie, Food Hydrocoll., 2019, 86, 50–61, DOI:10.1016/j.foodhyd.2018.01.038.
- J. P. Parente, C. R. Adao, B. P. da Silva and L. W. Tinoco, Carbohydr. Res., 2014, 391, 16–21, DOI:10.1016/j.carres.2014.03.021.
- D. W. Barreto and J. P. Parente, Carbohydr. Polym., 2006, 64, 287–291, DOI:10.1016/j.carbpol.2005.11.038.
- D. A. Rakhimov, A. S. Shashkov, K. S. Zhauynbaeva, M. K. Malikova and N. D. Abdullaev, Chem. Nat. Compd., 2004, 40, 358–361, DOI:10.1023/B:CONC.0000048247.79714.4e.
- S. Zhauynbaeva, M. K. Malikova and D. A. Rakhimov, Chem. Nat. Compd., 2003, 39, 240–243, DOI:10.1023/A:1025458115916.
- M. Tomoda, N. Satoh and C. Ohmori, Chem. Pharm. Bull., 1978, 26, 2768–2773, DOI:10.1248/cpb.26.2768.
- X. Xing, S. W. Cui, S. Nie, G. O. Phillips, H. D. Goff and Q. Wang, Bioact. Carbohydr. Diet. Fibre, 2014, 4, 74–83, DOI:10.1016/j.bcdf.2014.06.004.
- Y. S. Y. Hsieh, C. Chien, S. K. S. Liao, S. F. Liao, W. T. Hung, W. B. Yang, C. Lin, T. R. Cheng, C. Chang, J. Fang, C. Wong and C. H. Wong, Bioorg. Med. Chem., 2008, 16, 6054–6068, DOI:10.1016/j.bmc.2008.04.042.
- E. Harmayani, V. Aprilia and Y. Marsono, Carbohydr. Polym., 2014, 112, 475–479, DOI:10.1016/j.carbpol.2014.06.019.
- I. Rizoputra, S. Wahyudi, Sudarsono, D. Anggoro, N. F. Puspita, Risdiana and Darminto, Carbohydr. Polym. Technol. Appl., 2025, 9, 358–361, DOI:10.1016/j.carpta.2024.100659.
- J. Y. Liu, H. C. Wang, Y. Yin, N. Li, P. L. Cai and S. L. Yang, Carbohydr. Polym., 2012, 89, 158–162, DOI:10.1016/j.carbpol.2014.06.019.
- Y. Wang, D. Liu, S. Chen, Y. Wang, H. Jiang and H. Yin, Fitoterapia, 2014, 92, 72–78, DOI:10.1016/j.fitote.2013.10.008.
- B. Wang, H. Zhang, L. Chen, Z. Mi, Y. Xu, G. Zhao, S. Liu, H. Lei, Z. Wang and J. Niu, Carbohydr. Polym., 2020, 246, 116620, DOI:10.1016/j.carbpol.2020.116620.
- Q. Guo, S. W. Cui, Q. Wang, X. Hu, J. Kang and R. Y. Yada, Carbohydr. Res., 2012, 350, 31–39, DOI:10.1016/j.carres.2011.10.020.
- H. Li, G. Wang, X. Yan, X. Hu and J. Li, Carbohydr. Polym., 2024, 330, 121805, DOI:10.1016/j.carbpol.2024.121805.
- O. Ishurd, A. Kermagi, M. Elghazoun and J. F. Kennedy, Carbohydr. Polym., 2006, 65, 410–413, DOI:10.1016/j.carbpol.2006.01.023.
- O. Ishrud, M. Zahid, V. U. Ahmad and Y. Pan, J. Agric. Food Chem., 2001, 49, 3772–3774, DOI:10.1021/jf0103976.
- E. Ruszova, S. Pavek, V. Hajkova, S. Jandova, V. Velebny, I. Papezikova and L. Kubala, Carbohydr. Res., 2008, 343, 501–511, DOI:10.1016/j.carres.2007.11.010.
- M. I. Pereira, A. C. Ruthes, E. R. Carbonero, R. Marcon, C. H. Baggio, C. S. Freitas, A. R. S. Santos, S. Eliasaro, G. L. Sassaki, P. A. J. Gorin and M. Iacomini, Phytochemicals, 2010, 71, 2132–2139, DOI:10.1016/j.phytochem.2010.09.007.
- E. Capuano, Crit. Rev. Food Sci. Nutr., 2017, 57, 3543–3564, DOI:10.1080/10408398.2016.1180501.
- J. Davies, Mol. Aspects Med., 2016, 49, 1–7, DOI:10.1016/j.mam.2016.04.007.
- K. Kashyap and S. K. Dubey, Understanding Cancer, ed. B. Jain and S. Pandey, Academic Press, 2022, vol. 1, pp. 79–90, DOI:10.1016/B978-0-323-99883-3.00016-0.
- L. Pecorino, Molecular Biology of Cancer: Mechanisms, Targets, and Therapeutics, Oxford University Press, Great Britain, 5th edn, 2021 Search PubMed.
- S. M. Astrin and P. G. Rothberg, Cancer Invest., 1983, 1, 355–364, DOI:10.3109/07357908309063299.
- H. Zeng, D. L. Lazarova and M. Bordonaro, World J. Gastrointest. Oncol., 2014, 6, 41–51, DOI:10.4251/wjgo.v6.i2.41.
- A. Ren, L. Chen, W. Zhao, S. Shan, Z. Li and Z. Tang, J. Funct.Foods, 2023, 107, 1–15, DOI:10.1016/j.jff.2023.105659.
- D. J. Morrison and T. Preston, Gut Microbes, 2016, 7, 189–200, DOI:10.1080/19490976.2015.1134082.
- H. P. Chen, Y. T. Zhao and T. C. Zhao, Crit. Rev. Oncog., 2015, 20, 35–47, DOI:10.1615/CritRevOncog.2015012997.
- J. Chen and L. Vitetta, Clin. Colorectal Cancer, 2018, 17, 541–544, DOI:10.1016/j.clcc.2018.05.001.
- G. Milazzo, D. Mercatelli, G. Di Muzio, L. Triboli, P. De Rosa, G. Perini and F. M. Giorgi, Genes, 2020, 11, 1–49, DOI:10.3390/genes11050556.
- Y. Zhang, L. Zhou, Y. L. Bao, Y. Wu, C. L. Yu, Y. X. Huang, Y. Sun, L. H. Zheng and Y. X. Li, Chem. Biol. Interact., 2010, 185, 174–181, DOI:10.1016/j.cbi.2010.03.035.
- J. Y. Li, F. Sun, H. F. Zhou, J. Yang, C. Huang and H. Fan, Front. Pharmacol, 2019, 10, 1–9, DOI:10.3389/fphar.2019.00930.
- J. Xu, B. N. Moore and J. L. Pluznick, Hypertension, 2022, 79, 2127–2137, DOI:10.1161/HYPERTENSIONAHA.122.18558.
- Y. Chen, C. Xu, R. Huang, J. Song, D. Li and M. Xia, J. Nutr. Biochem., 2018, 56, 175–182, DOI:10.1016/j.jnutbio.2018.02.011.
- V. Musazadeh, R. Y. Rostami, A. H. Moridpour, Z. B. Hosseini, O. Nikpayam, M. Falahatzadeh and A. H. Faghfouri, BMC Cardiovasc. Disord., 2024, 24, 1–15, DOI:10.1186/s12872-024-04223-0.
- U. Alam, O. Asghar, S. Azmi and R. A. Malik, Handb. Clin. Neurol., 2014, 126, 211–222, DOI:10.1016/B978-0-444-53480-4.00015-1.
- J. E. Cade and J. Hanison, Anaesth. Intensive Care Med., 2017, 18, 527–531, DOI:10.1016/j.mpaic.2017.06.021.
- V. Gowd, L. Xie, X. Zheng and W. Chen, Crit. Rev. Biotechnol., 2019, 39, 524–540, DOI:10.1080/07388551.2019.1576025.
- M. O. Weickert and A. F. Pfeiffer, J. Nutr., 2018, 148, 7–12, DOI:10.1093/jn/nxx008.
- G. Zhang, D. Wang, Y. Ding, J. Zhang, Y. Ding and F. Lyu, Trends Food Sci. Technol., 2024, 146, 104354, DOI:10.1016/j.tifs.2024.104354.
- E. E. Canfora, J. W. Jocken and E. E. Blaak, Nat. Rev. Endocrinol., 2015, 11, 577–591, DOI:10.1038/nrendo.2015.128.
- A. Everard and P. D. Cani, Rev. Endocr. Metab. Disord., 2014, 15, 189–196, DOI:10.1007/s11154-014-9288-6.
- D. Salamone, A. A. Rivellese and C. Vetrani, Acta Diabetol., 2021, 58, 1131–1138, DOI:10.1007/s00592-021-01727-5.
- M. Kazi and M. F. Hashmi, Glomerulonephritis, 2023, https://www.ncbi.nlm.nih.gov/books/NBK560644/ Search PubMed.
- D. Makanjuola and M. Lapsley, in Clinical Biochemistry: Metabolic and Clinical Aspects, ed. A. Day, M. Lapsley, R. Ayling and W. J. Marshall, Elsevier, Churchill Livingstone, Edinburgh, 3rd edn, 2014, pp. 124–151 Search PubMed.
- M. Esgalhado, J. A. Kemp, N. R. Damasceno, D. Fouque and D. Mafra, Future Microbiol., 2017, 12, 1413–1425, DOI:10.2217/fmb-2017-0059.
- P. Stenvinkel, G. M. Chertow, P. Devarajan, A. Levin, S. P. Andreoli, S. Bangalore and B. A. Warady, Kidney Int. Rep., 2021, 6, 1775–1787, DOI:10.1016/j.ekir.2021.04.023.
- M. He, W. Wei, Y. Zhang, Z. Xiang, D. Peng, A. Kasimumali and S. Rong, J. Transl. Med., 2024, 22, 1–18, DOI:10.1186/s12967-024-04974-6.
- L. Li, L. Ma and P. Fu, Drug Des. Dev. Ther., 2017, 11, 3531–3542, DOI:10.2147/DDDT.S150825.
- A. Ramezani, Z. A. Massy, B. Meijers, P. Evenepoel, R. Vanholder and D. S. Raj, Am. J. Kidney Dis., 2016, 67, 483–498, DOI:10.1053/j.ajkd.2015.09.027.
- F. Zia, K. M. Zia, M. Zuber, H. B. Ahmad and M. Muneer, Int. J. Biol. Macromol., 2016, 87, 229–236, DOI:10.1016/j.ijbiomac.2016.02.058.
- F. Zhu, Food Chem., 2018, 256, 419–426, DOI:10.1016/j.foodchem.2018.02.151.
- S. Sharma and N. Wadhwa, J. Pharm. Res., 2022, 6, 1–5, DOI:10.18579/jopcr/v21i1.glucomannan.
- Y. Q. Zhang, B. J. Xie and X. Gan, Carbohydr. Polym., 2005, 60, 27–31, DOI:10.1016/j.carbpol.2004.11.003.
- M. Wang, W. He, S. Wang and X. Song, Carbohydr. Polym., 2015, 125, 334–339, DOI:10.1016/j.carbpol.2015.02.060.
- M. Wang, W. He, R. Yan and X. Song, J. Macromol. Sci. Pure Appl. Chem., 2017, 4, 216–220, DOI:10.1080/10601325.2017.1282232.
- E. Destandau and T. Michel, Natural Product Extraction Principles and Applications, ed. J. Prado, The Royal Society of Chemistry, 2nd edn, 2022, vol. 4, pp. 113–156, 10.1039/9781839165894-00144.
- W. J. Ren, A. Q. Zhang, S. Y. Qin and Z. K. Li, Carbohydr. Polym., 2016, 144, 238–244, DOI:10.1016/j.carbpol.2016.02.061.
- C. Xu and F. Z. Marques, Curr. Hypertens. Rep., 2022, 24, 509–521, DOI:10.1007/s11906-022-01216-2.
- P. Wang, X. Pei, W. Zhou, Y. Zhao, P. Gu, Y. Li and J. Gao, World J. Microbiol. Biotechnol., 2024, 40, 169, DOI:10.1007/s11274-024-03985-1.
- K. González-Becerra, Q. Ramos-Lopez, E. Barrón-Cabrera, J. I. Riezu-Boj, F. I. Milagro, E. Martínez-López and J. A. Martínez, Lipids Health Dis., 2019, 18, 178, DOI:10.1186/s12944-019-1120-6.
- G. Yang, Q. Huang, L. Zhang, J. Zhou and S. Gao, J. Appl. Polym. Sci., 2004, 92, 77–83, DOI:10.1002/app.13654.
- Y. F. Abbasi and H. Bera, in Biopolymer-Based Nanomaterials in Drug Delivery and Biomedical Applications, ed. H. Bera, C. M. Hossain and S. Saha, Academic Press, 2021, pp. 119–141 Search PubMed.
- G. Srzednicki and C. Borompichaichartkul, in Konjac Glucomannan: Production, Processing, and Functional Applications, CRC Press. Boca Raton, 2020 Search PubMed.
- A. R. Ganesan, M. Shanmugam, P. Ilansuriyan, R. Anandhakumar and B. Balasubramanian, Polym. Test., 2019, 78, 105936, DOI:10.1016/j.polymertesting.2019.105936.
- Y. Ni, Y. Liu, W. Zhang, S. Shi, W. Zhu, R. Wang, L. Zhang, L. Chen, J. Sun, J. Pang and J. Wang, LWT--Food Sci. Technol., 2021, 152, 112338, DOI:10.1016/j.lwt.2021.112338.
| This journal is © The Royal Society of Chemistry 2026 |