 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Valorization of chicken byproducts for bioactive coatings to extend the shelf life of okra (Abelmoschus esculentus)
Aparna Ramadoss ,
Venkata Giridhar Poosarla
,
Venkata Giridhar Poosarla *,
Ananya Kumari,
Shaik Sadiya
*,
Ananya Kumari,
Shaik Sadiya ,
Manisha Kalita and
Nagaveni Shivshetty
,
Manisha Kalita and
Nagaveni Shivshetty
Department of Life Sciences, GITAM School of Science, GITAM (Deemed to be University), Visakhapatnam, Andhra Pradesh 530045, India. E-mail: gpoosarl@gitam.edu
First published on 24th December 2025
Abstract
Environmental concerns associated with petroleum-based packaging have led to the development of sustainable alternatives and active packaging solutions. The aim of this research is to formulate and characterize bioactive packaging films based on pectin (P, 2%), sodium alginate (SA, 1.5%), and varying concentrations of chicken byproducts protein hydrolysates (CBP-PHs) that are derived from the enzymatic hydrolysis of CBPs using protease generated from Bacillus siamensis F2 and extend the shelf life of okra using coatings. The films F1, P + SA; F2, P + SA + 1%CBP-PHs; F3, P + SA + 1.5%CBP-PHs; and F4, P + SA + 2%CBP-PHs were analyzed for physical, antibacterial, antioxidant, and structural properties. Among them, the F4 film showed the highest antioxidant (diphenylpicrylhydrazyl, 53 ± 0.75%), antibacterial (Staphylococcus aureus, 24.5 ± 0.16 mm and Klebsiella pneumoniae, 21 ± 0.12 mm), and surface hydrophobicity (132°) properties due to the highest concentration of CBP-PHs. Based on this, F4 film coatings were applied to okra to evaluate their ability to extend shelf life, and F1-coating and uncoated okra were used as controls. F4-coated okra exhibited lower weight loss (78 ± 0.4%), chlorophyll loss, and ascorbic acid (7.6 ± 0.1 mg/100 g), while effectively retaining firmness (51 ± 0.8 N) and color. These findings highlight the potential of CBP-PH-impregnated active coatings as a sustainable alternative with excellent shelf life extension, supporting the development of green packaging technology that aligns with global sustainability goals. Future research will aim to optimize F4 film formulation for commercial production and investigate its application on various perishable products.
Sustainability spotlightProtein hydrolysates (PHs) obtained from chicken byproducts (CBPs) via enzymatic hydrolysis using Bacillus siamensis F2 protease were incorporated into a pectin and sodium alginate (SA) biopolymer matrix to fabricate active packaging films. These films exhibited strong antimicrobial and antioxidant properties, and the active coatings extended the postharvest shelf life of okra. This study valorises poultry byproducts into functional coatings that substitute petroleum-based polymers. Such coatings reduce food waste and advance sustainable packaging technologies aligned with the United Nations Sustainable Development Goals (SDGs) – 2, 3, 6, 9, 14, and 15, specifically targeting SDG-2 (End hunger, achieve food security, and improved nutrition). Ultimately, this study enhances food security by transforming poultry byproducts into value-added resources for sustainable packaging. |
1 Introduction
Biodegradable packaging is a sustainable alternative to conventional packaging, offering biodegradability, biocompatibility, and aesthetic appeal, and facilitates the protection and transport of food.1,2 Active packaging integrates compounds that absorb or release components from packed food or the surrounding environment. They effectively preserve food safety, quality, and sensory characteristics while enhancing shelf life.3 Various materials have been studied for active packaging; however, the focus has shifted towards naturally derived polymers like polysaccharides (cellulose, starch, pectin, and alginate), proteins (sodium caseinate, whey protein, soy protein, corn zein, and gelatin), and nanoparticles.4Among biopolymers, alginate is a linear copolymer that comprises β-D-mannuronic acid (M) residues and α-L-guluronic acid (G), arranged in homopolymeric (GG and MM) and heteropolymeric (MG) block sequences. They are naturally derived polysaccharides extracted from brown algae (Phaeophyceae), typically found as sodium, calcium, or magnesium salts of alginic acid.5 Alginate offers good film formation, flexibility, water solubility, tear resistance, tensile strength, rigidity, low water vapor and oxygen permeability, and a neutral taste and odor. Enriched with essential oils, proteins, enzymes, chelating agents, plant extracts, or metallic nanoparticles, they help retain moisture, reduce shrinkage and oxidation, and preserve color and texture. It enhances the antimicrobial activity, mechanical strength, barrier performance, and sensory quality while minimizing cooking losses.6
Pectin has excellent gelling and thickening properties and a distinct flavour, making it valuable in food packaging. It reduces the environmental footprint of conventional packaging, minimizing the use of hazardous substances, reducing food spoilage, and ultimately improving shelf life and food quality.7,8 To further optimize the performance of polysaccharides such as alginates, pectin, and biocomposites, recent research is exploring incorporating protein hydrolysates (PHs) to produce active packaging films. This innovative strategy utilises protein byproducts to improve sustainability.9,10
Protease enzymes are essential for producing peptides and amino acids by hydrolysing proteins. This releases bioactive substances and improves the valorisation of protein-rich byproducts. Several commercial proteases, such as Flavourzyme®, Pancreas Trypsin®, Alcalase®, and Novo ProD®, are used to produce hydrolysates with antioxidant activity (AA) and emulsifying properties.11 Microbial proteases have emerged as cost-effective, highly specific, and sustainable alternatives, offering excellent stability under extreme conditions, such as high salinity and pH, making them suitable for industrial protein hydrolysis.12–15 The resulting PHs, composed of peptides of varying lengths, exhibit modified structures that influence their physicochemical and functional properties.
Studies have demonstrated the strong potential of bioactive film development for packaging, offering enhanced antimicrobial, antioxidant, barrier, and mechanical properties, which leads to the extension of food shelf life.16,17 Recent studies have shown that PHs derived from poultry demonstrate superior AA, barrier properties, and antimicrobial activity against Escherichia coli and Staphylococcus saprophyticus.10,16 These PHs also extended the shelf life of sweet cherry at both room temperature and under refrigerated conditions.10 Similarly, incorporation of fish PHs has been reported to extend the shelf life of chicken fillets to 21 days.17
Okra (Abelmoschus esculentus), a nutritionally rich crop with a high water content (85%), consists of non-cellulosic and non-starch polysaccharides. It possesses several nutritional properties and serves as a source of vitamins A and C, proteins, calcium, iron, dietary fiber, and antioxidants. It helps lower serum cholesterol, thereby minimizing the risk of cardiovascular diseases, digestive disorders, and type 2 diabetes. Due to its high moisture content and respiration rate, okra is highly perishable and prone to rapid quality deterioration. It is particularly susceptible to water loss, discolouration, and textural degradation, which significantly reduces its commercial value. Therefore, effective preservation strategies are crucial for minimizing postharvest losses, preventing food waste, and contributing to improved food security.18–20
Several studies have investigated the development of packaging films using individual components such as sodium alginate (SA), pectin (P), and protein hydrolysates (PHs) from various sources. The research gap is to generate PHs from chicken byproducts (CBPs) using the protease of Bacillus siamensis F2 isolated in our laboratory and explore its potential as a functional component to extend the shelf life of okra. This research aims to impregnate chicken byproducts protein hydrolysates (CBP-PHs) into a P and SA matrix to produce active films. They were characterized for their physical, optical, antioxidant, antibacterial and structural properties. This film formulation was applied as a coating to okra to evaluate its ability to extend shelf life by studying parameters such as weight loss, pH, firmness, ascorbic acid content, antioxidant activity, color, and microbial analysis. These findings offer an innovative, eco-friendly solution for sustainable packaging and contribute to Sustainable Development Goal 2 (Zero Hunger) by valorising CBPs and reducing food spoilage.
2 Materials and methods
2.1 Materials
Extrapure SA (sodium polymannuronate) (Finar, Gujarat, India), pure pectin (high methoxyl content (7%) and a minimum of 50% galacturonic acid) and 2,2-diphenyl-1-picrylhydrazyl (DPPH) (Sisco research laboratory, Maharashtra, India), calcium chloride (CaCl2) (Emparta, Mumbai, India), glycerol (Qualigens, Maharashtra, India), and Muller Hinton agar (HiMedia, Maharashtra, India) were purchased. Chicken byproducts (CBPs) were obtained from a local poultry slaughterhouse, and okra was sourced from a local vegetable market in Visakhapatnam, India. According to our previous studies, the bacterium Bacillus siamensis F2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 21 was isolated from the water of a fish processing plant in Visakhapatnam, India, and the protease produced by this bacterium F2 was optimized and characterized.13 After obtaining promising results, this F2 protease (5.2 U mL−1)13 was used to produce CBP-PHs using CBPs to make active packaging films.
21 was isolated from the water of a fish processing plant in Visakhapatnam, India, and the protease produced by this bacterium F2 was optimized and characterized.13 After obtaining promising results, this F2 protease (5.2 U mL−1)13 was used to produce CBP-PHs using CBPs to make active packaging films.
2.2 Preparation of active films
The active packaging films were developed using the solvent casting method accordingly8,22 with slight modifications. Pectin (2 g) and SA (1.5 g) were added to distilled water (DW) (0.1 L) and heated for 25-30 min at 60 °C on a hot plate. This solution was brought to room temperature, and CBP-PHs, extracted accordingly,13 were incorporated in varying concentrations (1, 1.5, and 2%) with glycerol (2%) as a plasticizer. The obtained solution was transferred into a Petri plate (145 mm diameter) and left for drying in a biological oxygen demand (Model-925100, Labocare, India) at 30 °C. Once the films had dried, they were carefully peeled off and kept in an incubator at 35 °C for further analysis. After peeling the films off the casting plates, the dried films were labelled as F1, P + SA; F2, P + SA + 1%CBP-PHs; F3, P + SA + 1.5%CBP-PHs; and F4, P + SA + 2%CBP-PHs, as shown in Fig. 1.2.3 Characterization of films
2.3.1.1 Thickness. A digital Vernier caliper (Mitutoyo Absolute, range: 0–150 mm) with a least count of 0.00 mm at three random points was used to measure the thickness of the developed films.22
2.3.1.2 Moisture content (MC). The initial and final weights of the developed films (2 × 2 cm2) were recorded before and after drying at 105 °C for 3 h in a hot air oven.22 MC was calculated using eqn (1):
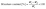 | (1) |
2.3.1.3 Water solubility (WS). The film samples (2 × 2 cm2) were weighed, placed in 10 mL of DW, and kept in a shaking incubator (Model-1254504, Venchal Scientific, India) at 180 rpm for 24 h. This solution was centrifuged at 1500 rpm for 20 min, and the undissolved pellet was dehydrated at 105 °C for 24 h using a hot air oven and then weighed.22 The WS was calculated by using eqn (2):
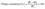 | (2) |
2.3.1.4 Swelling index (SI). 2 × 2 cm2 film samples were weighed and placed in 25 mL of DW for 2 min. Then, the surface water was discarded, and the films were reweighed.22 The SI was calculated using eqn (3):
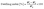 | (3) |
2.3.1.5 Water contact angle (WCA). The WCA of the fabricated films was evaluated using a contact angle meter (Acam-HSC 19, Apex Instruments, Kolkata, India) using the sessile drop technique, accordingly with slight modifications.23 A 7 µL DW was gently dispensed on the film surface using a glass syringe at 25 °C and 50% relative humidity, and the average of five replicates was reported.
2.3.1.6 Density. The developed active films were cut into 4 × 1 cm2, and the density was calculated using mass, area, and thickness22 using eqn (4):
 | (4) |
2.3.1.7 Water vapor permeability (WVP). WVP was used to determine the amount of water that diffuses within the film. The film was sealed on the mouth of the test tube containing anhydrous CaCl2. Then, these sealed test tubes were kept in a desiccator at 30 °C and 75% relative humidity.8 The samples were weighed every 24 h until the weight change was 0.001 g using eqn (5):
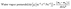 | (5) |
 | (6) |
 | (7) |
2.3.3.1 Diphenylpicrylhydrazyl (DPPH) assay. The AA of the developed films was determined by DPPH assay.22 In 3 mL of DPPH (0.1 mM) solution, 6 mg of the film was immersed for 24 h, and the optical density was measured at 517 nm using a UV-vis spectrophotometer. The DPPH solution was taken as a control, and the AA of the film samples was calculated using eqn (8):
 | (8) |
2.3.3.2 Metal chelating activity (MCA). The chelating activity of the biodegradable films on Fe2+ ions was determined.22 1 mL of the film solution was mixed with 3.7 mL of DW and maintained at room temperature. Then, 0.1 mL of 2 mM FeCl2 and 0.2 mL of 5 mM ferrozine [3-(2-pyridyl)-5,6-bis(4-phenyl-sulfonic acid)-1,2,4-triazine] were added. The mixture was incubated for 20 min, and the absorbance was measured at 562 nm using a UV-vis spectrophotometer. The chelating activity (%) was calculated using eqn (9), where 1 mL of DW served as the control.
 | (9) |
2.3.3.3 Hydroxyl radical-scavenging activity (HRSA). The hydroxyl radical-scavenging activity of the biodegradable films was determined.22 Briefly, a mixture containing 0.75 mM 1,10-phenanthroline and 0.75 mM FeSO4 was prepared in 0.1 M phosphate buffer (pH 7.4) and mixed thoroughly. To this, 0.01% H2O2 and the film solution (1.5 mg mL−1) were added, and the reaction mixture was incubated at 37 °C for 1 h. The absorbance of the resulting solution was measured at 536 nm using a UV-vis spectrophotometer. The scavenging activity was calculated using eqn (10).
 | (10) |
2.4 Shelf life studies
Shelf life refers to the duration a product remains suitable for use. Abelmoschus esculentus, commonly known as okra, was chosen for this shelf life study because it is highly perishable. It is a highly nutritious crop that can lose its quality due to respiration and moisture loss through transpiration after harvest.25
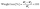 | (11) |
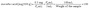 | (12) |
 | (13) |
 | (14) |
 | (15) |
 | (16) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CFU g−1).8
CFU g−1).82.5 Statistics
The statistical analysis was conducted using OriginPro 8.5. One-way ANOVA, followed by Tukey's Honestly Significant Difference (HSD) test, and two-way ANOVA, followed by the Bonferroni test, were used to evaluate differences between group means. All the analyses were conducted in triplicate, and the results were considered statistically significant at p < 0.05. Pearson correlation was performed to assess the physical, optical, antioxidant, and antibacterial properties of the films.3 Results and discussion
In this study, CBP-PHs were extracted from CBPs using the protease of the bacterium Bacillus siamensis F2 (5.2 U mL−1) accordingly.13 The produced CBP-PHs were impregnated in a pectin and SA matrix to produce active packaging films. The produced active films are characterized and applied to food as a coating to monitor their ability to extend shelf life.3.1 Developed films
The films were developed using pectin (P) (2%), sodium alginate (SA) (1.5%), and CBP-PHs of varying composition – F1, P + SA; F2, P + SA + 1%CBP-PHs; F3, P + SA + 1.5%CBP-PHs; and F4, P + SA + 2%CBP-PHs (Fig. 1). The pH of the developed film forming solutions was measured as follows – F1 (4.2), F2 (4.31), F3 (4.36), and F4 (4.4).3.2 Film characterization
| Parameters | F1 | F2 | F3 | F4 |
|---|---|---|---|---|
| a F1, P + SA; F2, P + SA + 1% CBP-PHs; F3, P + SA + 1.5%CBP-PHs; and F4, P + SA + 2%CBP-PHs. P, pectin; SA, sodium alginate; CBP-PHs, chicken byproducts protein hydrolysates; WVP, water vapor permeability. All data are averages of three measurements and reported as mean ± standard deviation (SD). Different letters (a, b, c, and d) indicate significant differences between groups at p < 0.05 according to Tukey's honestly significant difference (HSD) test. | ||||
| Density (g cm−3) | 0.10 ± 0.001a | 0.11 ± 0.005b | 0.12 ± 0.002c | 0.13 ± 0.001d |
| Moisture content (%) | 22.37 ± 0.19a | 19.48 ± 0.47b | 18.18 ± 0.43c | 15.92 ± 0.32d |
| Opacity (%) | 0.3 ± 0.001a | 0.36 ± 0.002b | 0.43 ± 0.003c | 0.54 ± 0.008d |
| Swelling index (%) | 485.7 ± 1.09a | 450.5 ± 0.25b | 416.9 ± 0.05c | 372.1 ± 0.29d |
| Transparency (%) | 5.54 ± 0.017a | 5.21 ± 0.05b | 4.82 ± 0.01c | 4.42 ± 0.02d |
| Thickness (mm) | 0.32 ± 0.015a | 0.35 ± 0.01b | 0.39 ± 0.005c | 0.42 ± 0.005d |
| Water solubility (%) | 74.11 ± 0.5a | 64.81 ± 0.8b | 61.44 ± 0.56c | 57.48 ± 0.26d |
| WVP (10−8 g (m−1 s−1 Pa−1)) | 1.99 ± 0.07a | 2.14 ± 0.05b | 2.3 ± 0.01c | 2.5 ± 0.02d |
As the concentration of CBP-PHs increases from 0 to 2% in the films, the WCA of the films shows an increase from 110° to 132°, demonstrating hydrophobicity (Fig. 1b). The films containing pectin and SA form an agglomerate when CBP-PHs are added, thereby increasing water resistance by forming a dense matrix.10,36,37 This imparts moisture resistance, leading to higher surface hydrophobicity, an increased contact angle, and reduced wettability.39 A similar increase in hydrophobicity was observed in a study where the contact angle increased from 68° to 96° with the addition of whitecheek shark PHs in whitecheek shark gelatin films.23 Another study also observed a similar increase in hydrophobicity in SA films with the addition of gold-titanium dioxide nanoparticles.40
| Parameters | F1 | F2 | F3 | F4 | Ascorbic acid standard |
|---|---|---|---|---|---|
| a F1, P + SA; F2, P + SA + 1%CBP-PHs; F3, P + SA + 1.5%CBP-PHs; and F4, P + SA + 2%CBP-PHs. P, pectin; SA, sodium alginate; CBP-PHs, chicken byproducts protein hydrolysates. DPPH, diphenylpicrylhydrazyl; MCA, metal chelating activity; HRSA, hydroxyl radical-scavenging activity. All data are averages of three measurements and reported as mean ± standard deviation (SD). Different letters (a, b, c, and d) indicate significant differences between groups at p < 0.05 according to Tukey's honestly significant difference (HSD) test. | |||||
| DPPH (%) | 39.2 ± 0.3a | 46.26 ± 0.7b | 48.42 ± 0.6c | 52.87 ± 0.75d | 92 ± 1.32e |
| MCA (%) | 21.3 ± 0.4a | 31.1 ± 0.2b | 36.4 ± 0.5c | 44.5 ± 0.7d | 82 ± 0.75e |
| HRSA (%) | 24.1 ± 0.2a | 31.7 ± 0.3b | 39.4 ± 0.4c | 48.5 ± 0.5d | 86 ± 0.87e |
| Films | Antibacterial properties (mm) | |
|---|---|---|
| Staphylococcus aureus | Klebsiella pneumoniae | |
| a F1, P + SA; F2, P + SA + 1%CBP-PHs; F3, P + SA + 1.5%CBP-PHs; and F4, P + SA + 2%CBP-PHs. P, pectin; SA, sodium alginate; CBP-PHs, chicken byproducts protein hydrolysates. All data are averages of three measurements and reported as mean ± standard deviation (SD). Different letters (a, b, and c) indicate significant differences between groups at p < 0.05 according to Tukey's HSD test. | ||
| F1 | Not detected | Not detected |
| F2 | 5.77 ± 0.12a | 5.1 ± 0.16a |
| F3 | 16.4 ± 0.24b | 15.67 ± 0.12b |
| F4 | 24.5 ± 0.16c | 21.07 ± 0.12c |
In the films containing CBP-PHs, intensified peaks were observed at 3398 cm−1 (amide A, N–H stretching), 2930 cm−1 (CH2 stretching), and 1600 cm−1 (amide I, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O vibration). A prominent peak was observed at 1413 cm−1 (amide II, C–N elongation and N–H stretching), while peaks observed at 1101 cm−1 and 1018 cm−1 corresponded to the symmetric and asymmetric stretching of S–O, which could be due to the presence of cysteine bonded by disulphide bridges (the composition of keratin in chicken feathers).38 These spectral observations support previous findings on protein–polysaccharide blend films, indicating strong biochemical interactions and compatibility between the CBP-PHs and the pectin and SA matrix.10,38,59
O vibration). A prominent peak was observed at 1413 cm−1 (amide II, C–N elongation and N–H stretching), while peaks observed at 1101 cm−1 and 1018 cm−1 corresponded to the symmetric and asymmetric stretching of S–O, which could be due to the presence of cysteine bonded by disulphide bridges (the composition of keratin in chicken feathers).38 These spectral observations support previous findings on protein–polysaccharide blend films, indicating strong biochemical interactions and compatibility between the CBP-PHs and the pectin and SA matrix.10,38,59
3.3 Shelf life studies of the developed films on okra
To evaluate the efficacy of the bioactive films in preserving the quality of okra during a storage period of 7 days, three representative groups were selected for comparative analysis: uncoated, F1-coated (P + SA without CBP-PHs), and F4-coated (P + SA + 2%CBP-PHs) (Fig. 5). This selection was made to establish a clear contrast between the untreated sample, a base composition without bioactive compounds, and the best formulation.| Films | Storage period (days) | L* | a* | b* | Browning index (BI) |
|---|---|---|---|---|---|
| a UC, uncoated okra; F1, P + SA, and F4, P + SA + 2%CBP-PHs. P, pectin; SA, sodium alginate; CBP-PHs, chicken byproducts protein hydrolysates. All data are averages of three measurements and reported as mean ± standard deviation (SD). Different letters (a, b, c, and d) indicate significant differences between groups at p < 0.05 according to the Bonferroni test. | |||||
| UC | 0 | 33.3 ± 0.2a | −4.9 ± 0.04a | 14.9 ± 0.06a | 44.9 ± 0.7a |
| 1 | 28.9 ± 0.1a | −4.6 ± 0.01a | 14.5 ± 0.02a | 55.4 ± 0.2a | |
| 2 | 26.2 ± 0.1ab | −4.1 ± 0.13a | 14.3 ± 0.02ab | 61.5 ± 0.3a | |
| 3 | 24.8 ± 0.1ab | −3.5 ± 0.01ab | 14.1 ± 0.02ab | 67.5 ± 0.2a | |
| 4 | 23.8 ± 0.1ab | −3.1 ± 0.02ab | 13.8 ± 0.1ab | 70.5 ± 0.2a | |
| 5 | 19.2 ± 0.1ab | −2.6 ± 0.06ab | 13.5 ± 0.2ab | 98.9 ± 0.6ab | |
| 6 | 16.4 ± 0.1ab | −2.1 ± 0.02ab | 13.2 ± 0.1c | 126.9 ± 0.4ab | |
| 7 | 15.4 ± 0.1ab | −1.8 ± 0.02ab | 12.9 ± 0.4d | 139 ± 0.1ab | |
| F1 | 0 | 34.5 ± 0.2a | −5.0 ± 0.05a | 14.8 ± 0.5a | 44.5 ± 0.5a |
| 1 | 30.4 ± 0.1a | −4.8 ± 0.05a | 14.6 ± 0.1a | 49.4 ± 0.1a | |
| 2 | 28.8 ± 0.3ab | −4.5 ± 0.06a | 14.4 ± 0.2ab | 53.4 ± 1.1a | |
| 3 | 27.4 ± 0.1ab | −4.1 ± 0.01ab | 14.3 ± 0.4ab | 57.5 ± 0.1a | |
| 4 | 26.8 ± 0.1ab | −3.5 ± 0.04ab | 14.1 ± 0.4ab | 60.6 ± 0.3a | |
| 5 | 23.8 ± 0.6ab | −3.1 ± 0.01ab | 13.9 ± 0.4ab | 71.5 ± 0.3ab | |
| 6 | 19.1 ± 0.1ab | −2.4 ± 0.05ab | 13.5 ± 0.1c | 99 ± 0.3ab | |
| 7 | 17.7 ± 0.1ab | −2.1 ± 0.04ab | 13.1 ± 0.1d | 113.9 ± 0.9ab | |
| F4 | 0 | 33.9 ± 0.7a | −5 ± 0.3a | 14.8 ± 0.5a | 44.8 ± 0.5a |
| 1 | 31.1 ± 0.5a | −4.9 ± 0.06a | 14.7 ± 0.1a | 48.3 ± 0.5a | |
| 2 | 30.4 ± 0.1ab | −4.7 ± 0.02a | 14.5 ± 0.2ab | 49.4 ± 0.2a | |
| 3 | 29.1 ± 0.6ab | −4.4 ± 0.07ab | 14.4 ± 0.4ab | 53 ± 0.9a | |
| 4 | 28.8 ± 0.3ab | −4.1 ± 0.01ab | 14.3 ± 0.4ab | 53.8 ± 0.2a | |
| 5 | 26.3 ± 0.1ab | −3.8 ± 0.01ab | 14 ± 0.3ab | 60.5 ± 0.3ab | |
| 6 | 24.8 ± 0.1ab | −3.1 ± 0.01ab | 13.7 ± 0.1c | 66.1 ± 0.4ab | |
| 7 | 23.9 ± 0.1ab | −2.5 ± 0.08ab | 13.4 ± 0.1d | 72 ± 0.4ab | |
The BI indicates the quality of fruits and vegetables, such as apples, avocados, and okra.31 The BI increased from 45 ± 0.5 to 72 ± 0.4 in F4-coated okra and 44 ± 0.5 to 114 ± 0.9 in F1-coated okra, and the highest increase was observed in uncoated okra, from 45 ± 0.7 to 139 ± 0.1 (Table 4). The BI increased due to the darkening of the ridges, resulting in discoloration of the okra with an increase in the storage period.31 The results are in corroboration with an increase in the BI from 85 to 144 in the coated okra using SA impregnated with basil oil nano-emulsified with Tween 20 by day 3 of storage at 24 °C and from 83 to 189 in the control.31
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CFU g−1, which increased in all the samples with the increase in the storage period (Fig. 9a). The uncoated samples showed the highest mesophilic count of 6.3 ± 0.07
CFU g−1, which increased in all the samples with the increase in the storage period (Fig. 9a). The uncoated samples showed the highest mesophilic count of 6.3 ± 0.07![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CFU g−1 on day 7, followed by F1-coated okra (6 ± 0.02
CFU g−1 on day 7, followed by F1-coated okra (6 ± 0.02![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CFU g−1). In contrast, F4-coated exhibited the lowest by maintaining the mesophilic count at 5.7 ± 0.03
CFU g−1). In contrast, F4-coated exhibited the lowest by maintaining the mesophilic count at 5.7 ± 0.03![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CFU g−1. Similarly, by day 7, the count of yeast and mold also increased from 3.6 ± 0.02
CFU g−1. Similarly, by day 7, the count of yeast and mold also increased from 3.6 ± 0.02![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CFU g−1 (day 0) to 5.8 ± 0.05
CFU g−1 (day 0) to 5.8 ± 0.05![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CFU g−1 in uncoated okra, followed by F1-coated (5.5 ± 0.06
CFU g−1 in uncoated okra, followed by F1-coated (5.5 ± 0.06![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CFU g−1) and F4-coated okra (5.3 ± 0.01
CFU g−1) and F4-coated okra (5.3 ± 0.01![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CFU g−1) (Fig. 9b). This shows that the F4 coating enhanced the shelf life of okra due to its antimicrobial properties, which effectively suppressed microbial proliferation. The bioactive peptides of CBP-PHs penetrate the microbial cell wall and alter the cell membrane structure, ultimately leading to the death of bacterial cells.10 The results are in corroboration with a study where chitosan and zinc oxide-coated okra samples restricted the bacterial and fungal cells at approximately 10 × 107 CFU mL−1 and 2 × 107 CFU mL−1, respectively.72
CFU g−1) (Fig. 9b). This shows that the F4 coating enhanced the shelf life of okra due to its antimicrobial properties, which effectively suppressed microbial proliferation. The bioactive peptides of CBP-PHs penetrate the microbial cell wall and alter the cell membrane structure, ultimately leading to the death of bacterial cells.10 The results are in corroboration with a study where chitosan and zinc oxide-coated okra samples restricted the bacterial and fungal cells at approximately 10 × 107 CFU mL−1 and 2 × 107 CFU mL−1, respectively.72
4 Conclusion
The current study aimed to develop and characterize an active packaging using pectin, SA, and CBP-PHs obtained through enzymatic hydrolysis of CBPs using the protease from Bacillus siamensis F2. Among the formulations tested, F4 (P + SA + 2%CBP-PHs) demonstrated enhanced antibacterial properties against Staphylococcus aureus (24.5 ± 0.16 mm) and Klebsiella pneumoniae (21 ± 0.12 mm) and antioxidant (DPPH, 53 ± 0.75%), surface hydrophobicity (132°) and optical and physical properties. When this coating was applied to okra, F4 effectively minimized weight loss (78 ± 0.4%), retained firmness (51 ± 0.8 N), preserved chlorophyll (8.1 ± 0.05 mg/100 g) and ascorbic acid content (7.6 ± 0.1 mg/100 g), and suppressed microbial proliferation during storage. These findings underscore the importance of bioactive CBP-PHs when incorporated into food-grade biopolymers. Beyond material innovation, this study highlights the importance of food preservation through waste valorisation (CBP-PHs from CBPs), thereby addressing food security and sustainability and reducing environmental burden by replacing conventional plastics. Future prospects of the current research include optimizing film formulation for commercial production and utilising it on various perishable products to extend their shelf life.Author contributions
AR: data curation, analysis, writing, and editing of the manuscript. VGP: funding, designing the experiments, data curation, analysis, overall supervision, writing, and editing of the manuscript. AK: data curation, analysis, writing, and editing of the manuscript. SS: data curation, analysis, and editing of the manuscript. MK: data curation and analysis. NS: data analysis and editing of the manuscript.Conflicts of interest
The authors declare that there is no conflict of interest.Data availability
Additional datasets related to the identification of bioactive peptides and possible correlations between the variables of the films are available.Supplementary information (SI) is available. See DOI: https://doi.org/10.1039/d5fb00468c.
Acknowledgements
This work was supported by projects: a UGC-BSR Start-up grant with Ref No. No. F. 30-456/2018 (BSR) and GITAM: Research Seed Grants (RSG) Ref No. F. No. 2021/0087. The authors are thankful to the “MURTI” (Multi-disciplinary Unit of Research on Translation Initiative) facility, MURTI-SAIF facility, and the Department of Mechanical Engineering of the Gandhi Institute of Technology and Management for providing instrumental facilities (FTIR, XRD, LC-MS, and the contact angle meter) for conducting experiments required for this article.References
- M. P. M. García, M. C. Gómez-Guillén, M. E. López-Caballero and G. V. Barbosa-Cánovas, Edible Films and Coatings: Fundamentals and Applications, Crc Press, 2016 Search PubMed.
- Y. Zhao, B. Li, C. Li, Y. Xu, Y. Luo, D. Liang and C. Huang, Foods, 2021, 10, 1845 CrossRef.
- C. E. Realini and B. Marcos, Meat Sci., 2014, 98, 404–419 CrossRef PubMed.
- N. Omerović, M. Djisalov, K. Živojević, M. Mladenović, J. Vunduk, I. Milenković, N. Ž. Knežević, I. Gadjanski and J. Vidić, Compr. Rev. Food Sci. Food Saf., 2021, 20, 2428–2454 CrossRef PubMed.
- R. Abka-Khajouei, L. Tounsi, N. Shahabi, A. K. Patel, S. Abdelkafi and P. Michaud, Mar. Drugs, 2022, 20, 364 CrossRef PubMed.
- M. G. Kontominas, Foods, 2020, 9, 1440 CrossRef PubMed.
- K. Y. Perera, A. K. Jaiswal and S. Jaiswal, Foods, 2023, 12, 2422 CrossRef PubMed.
- A. Ramadoss, V. G. Poosarla, S. Sadiya and N. Shivshetty, J. Food Sci., 2025, 90, e70179 CrossRef.
- J. Tkaczewska, Trends Food Sci. Technol., 2020, 106, 298–311 Search PubMed.
- O. Zinina, S. Merenkova and D. Galimov, Sustainability, 2023, 15, 15086 CrossRef.
- L. O. L. d. Rosa, M. C. Santana, T. L. Avezedo, A. I. S. BRÍGIDA, R. Godoy, S. Pacheco, C. Mellinger-Silva and L. M. C. CABRAL, Food Sci. Technol., 2018, 38, 31–36 CrossRef.
- D. S. Ningthoujam, P. Kshetri, S. Sanasam and S. Nimaichand, World Appl. Sci. J., 2009, 7, 907–916 Search PubMed.
- B. G. Rathod, V. G. Poosarla, S. K. Kuppili, K. S. Y. R. Chouhan and N. Shivshetty, Biomass Convers. Biorefin., 2024, 14, 1343–1358 CrossRef.
- S. Sankaralingam, B. Harinathan, S. Palpperumal, D. Kathiresan, S. Rajendran, T. Shankar, D. Prabhu and N. Sivakumar, Am.–Eurasian J. Agric. Environ. Sci., 2017, 17, 293–299 Search PubMed.
- M. Vidyasagar, S. Prakash and K. Sreeramulu, Lett. Appl. Microbiol., 2006, 43, 385–391 CrossRef PubMed.
- X. Chen, D. Jiang, P. Xu, Z. Geng, G. Xiong, Y. Zou, D. Wang and W. Xu, Food Chem., 2021, 343, 128417 CrossRef PubMed.
- S. Sadiya, V. G. Poosarla, A. Ramadoss, B. G. Rathod, N. Shivshetty and G. Rajagopalan, ACS Food Sci. Technol., 2025, 5, 2820–2832 CrossRef.
- H. F. Gemede, N. Ratta, G. D. Haki, A. Z. Woldegiorgis and F. Beyene, J. Food Process. Technol., 2015, 6, 2 Search PubMed.
- L. M. R. Gonzales and M. M. Benitez, Mindanao J. Sci. Technol., 2023, 21, 153–177 Search PubMed.
- M. L. Zambrano-Zaragoza, R. González-Reza, N. Mendoza-Muñoz, V. Miranda-Linares, T. F. Bernal-Couoh, S. Mendoza-Elvira and D. Quintanar-Guerrero, Int. J. Mol. Sci., 2018, 19, 705 CrossRef PubMed.
- B. G. Rathod, S. Pandala and V. G. Poosarla, Appl. Biochem. Biotechnol., 2023, 195, 4775–4795 CrossRef PubMed.
- V. G. Poosarla, S. Bisoi, A. Siripurapu, B. G. Rathod, A. Ramadoss, S. Kilaparthi, N. Shivshetty and G. Rajagopalan, J. Food Sci., 2024, 89, 6232–6252 CrossRef.
- M. Alinejad, H. Shahiri Tabarestani and A. Motamedzadegan, LWT–Food Sci. Technol., 2025, 232, 118419 CrossRef.
- S. Roytrakul, S. Charoenlappanit, S. Kittisenachai, N. Siangpro, J. Sichaem, S. Chuakrut, S. Sarin and R. Jutakanoke, PLoS One, 2024, 19, e0314482 CrossRef PubMed.
- M. R. Zare-Bavani, J. Food Sci. Technol., 2024, 20, 149–169 Search PubMed.
- D. Paulus, S. B. Ferreira and D. Becker, AIMS Agric. Food., 2021, 6, 321–336 Search PubMed.
- M. M. Shinde, M. Malik, K. Kaur, V. K. Gahlawat, N. Kumar, P. Chiraang and A. Upadhyay, Int. J. Biol. Macromol., 2024, 262, 129630 CrossRef PubMed.
- S. Sadasivam, Biochemical Methods, New age international, 1996 Search PubMed.
- R. Reis, H. Sipahi, O. Dinc, T. Kavaz, M. Charehsaz, A. Dimoglo and A. Aydın, Hum. Exp. Toxicol., 2021, 40, 452–463 CrossRef PubMed.
- S. Soiklom, W. Siri-Anusornsak, K. Petchpoung, S. Soiklom and T. Maneeboon, Foods, 2025, 14, 804 CrossRef.
- G. Gundewadi, S. G. Rudra, D. J. Sarkar and D. Singh, Food Packag. Shelf Life, 2018, 18, 1–12 CrossRef.
- D. I. Arnon, Plant Physiol., 1949, 24, 1–15 CrossRef PubMed.
- J. G. d. Oliveira Filho, J. M. Rodrigues, A. C. F. Valadares, A. B. d. Almeida, T. M. d. Lima, K. P. Takeuchi, C. C. F. Alves, H. A. d. F. Sousa, E. R. d. Silva, F. H. Dyszy and M. B. Egea, Food Hydrocolloids, 2019, 92, 267–275 Search PubMed.
- P. Raei, M. Khomeiri, A. S. Mahounak, A. Moayedi and M. Kashiri, Appl. Food Res., 2025, 5, 100727 Search PubMed.
- Z. Tahsiri, S. Hedayati and M. Niakousari, Food Hydrocolloids Health, 2025, 100199 CrossRef.
- S. Bishnoi, J. Trifol, R. Moriana and A. C. Mendes, Food Chem., 2022, 391, 133196 CrossRef PubMed.
- S. Kim, H. Lim, S. Park, D.-S. Kim and H. W. Kwak, Ind. Crops Prod., 2025, 225, 120564 Search PubMed.
- M. M. F. Santos, C. V. B. Grisi, D. A. S. Lima, G. I. B. Florentino, V. C. d. S. Ferreira, M. S. Madruga and F. A. P. d. Silva, Food Biosci., 2024, 62, 105048 Search PubMed.
- S. Rbihi, A. Aboulouard, L. Laallam and A. Jouaiti, Surf. Interfaces, 2020, 21, 100708 Search PubMed.
- S. Tang, Z. Wang, P. Li, W. Li, C. Li, Y. Wang and P. K. Chu, Nanomaterials, 2018, 8, 930 CrossRef.
- T. Dursun Capar, Food Packag. Shelf Life, 2023, 37, 101068 CrossRef.
- B. Giménez, J. Gómez-Estaca, A. Alemán, M. C. Gómez-Guillén and M. P. Montero, Food Hydrocolloids, 2009, 23, 1322–1327 Search PubMed.
- H. M. S. Akhtar, A. Riaz, Y. S. Hamed, M. Abdin, G. Chen, P. Wan and X. Zeng, Int. J. Biol. Macromol., 2018, 118, 469–477 CrossRef PubMed.
- C. Chang and M. T. Nickerson, Food Sci. Technol. Int., 2015, 21, 33–44 CrossRef.
- A. Hasanzati Rostami, A. Motamedzadegan, S. E. Hosseini, M. Rezaei and A. Kamali, J. Aquat. Food Prod. Technol., 2017, 26, 457–467 Search PubMed.
- M. M. F. Santos, C. V. B. Grisi, E. G. T. de Souza, J. d. M. Lima, V. C. d. S. Ferreira, L. E. Kurozawa, M. S. Madruga and F. A. P. da Silva, Food Biosci., 2024, 59, 103897 CrossRef.
- M. Nikoo, J. M. Regenstein and M. Yasemi, Foods, 2023, 12, 4470 Search PubMed.
- P. Andiana, M. G. M. Syahdan, A. H. Utama, K. Kasri, K. U. Al Awwaly and A. Manab, J. Ilmu dan Teknol. Has. Ternak., 2024, 19, 15–24 CrossRef.
- P. Castellano, L. Mora, E. Escudero, G. Vignolo, R. Aznar and F. Toldrá, Food Microbiol., 2016, 59, 133–141 Search PubMed.
- M. Da Rocha, A. Alemán, V. P. Romani, M. E. López-Caballero, M. C. Gómez-Guillén, P. Montero and C. Prentice, Food Hydrocolloids, 2018, 81, 351–363 CrossRef.
- M. F. Blankenvoorde, W. Van't Hof, E. Walgreen-Weterings, T. Van Steenbergen, H. Brand, E. Veerman and A. Nieuw Amerongen, Biol. Chem., 1998, 379, 1371–1376 Search PubMed.
- T. Anna, K. Alexey, B. Anna, K. Vyacheslav, T. Mikhail and M. Ulia, Curr. Res. Nutr. Food Sci., 2016, 4, 77–86 Search PubMed.
- N. Thombare, A. Mahto, D. Singh, A. R. Chowdhury and M. F. Ansari, J. Polym. Environ., 2023, 31, 3372–3380 CrossRef.
- Z. Mahcene, A. Khelil, S. Hasni, P. K. Akman, F. Bozkurt, K. Birech, M. B. Goudjil and F. Tornuk, Int. J. Biol. Macromol., 2020, 145, 124–132 CrossRef.
- Y. Roman Maldonado, S. J. Villanueva-Rodríguez, H. M. Hernández-Hernández, E. Terrés and J. Cervantes Martinez, Foods, 2024, 13, 3914 CrossRef.
- S. Azeez and R. Shenbagaraman, in Characterization Techniques in Bionanocomposites, ed. S. Ahmed and C. M. Hussain, Woodhead Publishing, United Kingdom, 2025, pp. 209–227 Search PubMed.
- M. M. Ahmad, K. Chauhan, A. Naz and M. Nayeem, Pharma Innovation, 2021, 10, 262–272 CrossRef.
- R. Pereira, A. Tojeira, D. C. Vaz, A. Mendes and P. Bártolo, Int. J. Polym. Anal. Charact., 2011, 16, 449–464 CrossRef.
- A. Bhimrao Muley, A. Bhalchandra Pandit, R. Satishchandra Singhal and S. Govind Dalvi, Ultrason. Sonochem., 2021, 71, 105385 CrossRef PubMed.
- S. Liu, Y. Li and L. Li, Carbohydr. Polym., 2017, 160, 62–70 CrossRef PubMed.
- R. K. Mishra, A. B. A. Majeed and A. K. Banthia, Int. J. Plast. Technol., 2011, 15, 82–95 CrossRef.
- M. Z. Mulla, J. Ahmed, S. F. Habeebullah and A. Vahora, J. Food Meas. Char., 2022, 16, 5058–5065 CrossRef.
- G. Babarinde and O. Fabunmi, Agric. Tropica Subtropica, 2009, 42, 151–156 Search PubMed.
- S. K. Jha, S. Sethi, M. Srivastav, A. K. Dubey, R. R. Sharma, D. V. K. Samuel and A. K. Singh, J. Food Eng., 2010, 97, 208–212 CrossRef.
- A.-H. A. H. El-Shaieny, N. A. A. Abd-Elkarim, E. M. Taha and S. Gebril, Agriculture, 2022, 12, 1699 CrossRef.
- F. Adetuyi, A. Osagie and A. Adekunle, J. Food Technol., 2008, 6, 227–230 Search PubMed.
- M. M. F. Santos, C. V. B. Grisi, D. A. S. Lima, G. I. B. Florentino, V. C. da Silva Ferreira, M. S. Madruga and F. A. P. da Silva, Food Biosci., 2024, 62, 105048 CrossRef.
- R. Kanwal, H. Ashraf, M. Sultan, I. Babu, Z. Yasmin, M. Nadeem, M. Asghar, R. R. Shamshiri, S. M. Ibrahim and N. Ahmad, Sustainability, 2020, 12, 7547 CrossRef.
- A. Abedi, L. Lakzadeh and M. Amouheydari, J. Food Process. Preserv., 2021, 45, e15284 Search PubMed.
- D. Gernah and A. Daagema, Curr. Res. J. Biol. Sci., 2012, 4, 444–448 Search PubMed.
- P. Nisha, R. S. Singhal and A. B. Pandit, J. Food Eng., 2004, 64, 135–142 CrossRef.
- L. Al-Naamani, J. Dutta and S. Dobretsov, Nanomaterials, 2018, 8, 479 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2026 |









