Open Access Article
Open Access ArticlePhytochemical improvement of sand pear cubes through impregnation of spices and tea extract: characterization using in vitro bioactivity, FTIR, ICP-AES, and SEM
Rajat Chandela,
Vikas Kumar *ab,
Ramandeep Kaura,
Satish Kumar
*ab,
Ramandeep Kaura,
Satish Kumar c,
Sandeep Janghud and
Jasleen Bhasine
c,
Sandeep Janghud and
Jasleen Bhasine
aDepartment of Food Science and Technology, Punjab Agricultural University, Ludhiana, Punjab 141004, India. E-mail: vkchoprafst@rediffmail.com
bICAR-Indian Institute of Millets Research, Rajendranagar, Hyderabad 500030, Telangana, India
cDepartment of Food Science and Technology, Dr. Y S Parmar University of Horticulture and Forestry, Nauni, Solan 173 230, HP, India
dDepartment of Food Technology, Rajiv Gandhi University, Rono Hills, Doimukh, Arunachal Pradesh 791112, India
eDepartment of Food Technology and Nutrition, School of Agriculture, Lovely Professional University, Phagwara, Punjab, India
First published on 10th December 2025
Abstract
The demand for phytochemical-enriched foods has increased in recent years; therefore, an attempt was made in the present study to improve the phytochemical properties of sand pear cubes. The effect of different variables, i.e. blanching time (0–5 min), spice extract (0–5%), and tea extract (0–5%), on the physicochemical, phytochemical, mineral and microstructural characteristics of sand pear cubes was evaluated. Compared with the unblanched sample, blanching led to a significant increase in the water activity and total soluble solids (TSS) of the sand pear cubes. The spice and tea extracts significantly enhanced the phytochemical potential of the sand pear cubes. The optimum quality attributes of the phytochemical-enriched sand pear cubes were obtained at a blanching time of 3.53 min, 2.74% spice extract concentration, and 2.44% tea extract concentration. The results for minerals and bioactive compounds indicated that the optimized samples had higher retention compared to the control. Structural characterization revealed that the optimized sample had a more porous and less crystalline surface, contributing to the soft texture of the sand pear cubes. Hence, the developed processing conditions may be useful for the confectionery industry and could serve as a promising alternative product for children.
Sustainability spotlightThis study presents a sustainable approach to enhancing the nutritional and textural quality of sand pear cubes by optimizing natural processing variables i.e. blanching time, spices, and tea extract concentration. By employing mild processing conditions and plant-based bioactive additives, the research reduces reliance on synthetic preservatives and energy-intensive methods, preserving essential minerals and bioactives. The optimized conditions offer a soft-textured, nutrient-rich product that caters to health-conscious consumers, particularly children. This innovation supports clean-label product development in the confectionery sector while promoting value addition to underutilized fruits, aligning with sustainable food processing and waste minimization goals. |
1 Introduction
Consumer lifestyle and dietary preferences have changed in the modern era as a result of increased urbanisation and globalisation. Nowadays, expectations of demand are for healthy and innovative food products with enhanced nutrition benefits.1 Fruits and vegetables are abundant in flavonoids, phenolics, antioxidants, etc. In addition to being nutritious, these functional foods aid health-beneficial functions in the human body.2,3Sand pear is a cultivar of Pyrus pyrifolia of the Rosaceae family, also popularly known as pathernakh and Akizuki.3 It is resilient to a wider range of climates and soil and thus is suitable for temperate and subtropical regions. Sand pear is distinguished by its grittiness in texture and contains carbohydrates, minerals, vitamins, phenolic acids, and flavonoids along with various antioxidants.3,4 Sand pear has a variety of health-promoting qualities due to the presence of phytochemicals, including anti-diabetic, anti-inflammatory, anti-obesity activities, and also exerts a preventive effect in cancer and cardiovascular diseases.5,6
Despite its infinitive benefits, the sand pear remains underutilised and is not commercialized due to its low palatability and high perishability. Few studies were carried out for revaluing sand pear in the form of wine, juice, candies, etc.5,7,8 However, drying and dehydration are the best and cost effective techniques to extend the shelf life of fresh fruits like this. Hot air drying is the most common method of food preservation, but it has a detrimental effect on the nutritional quality of the finished product.9 Osmotic dehydration has drawn a lot of attention recently as it retains the fruit quality by reducing the destruction of cells.10 Nowadays, dried fruit-based cubes are gaining popularity in the market among people of all ages over the confectionary products with table sugar and artificial food additives.11,12
Recently, researchers have made efforts on improving the phytochemical potential of different foods, i.e. fermented products,13 ginger candies,14 cucumber juice,15 etc. using natural extracts owing to the increased demand for nutritious food. However, no extensive investigation on the development of phytochemical enriched sand pear cubes has been reported yet. Consequently, the innovative goal of this research was to elevate the phytocompounds in sand pear by using spice and tea extracts. Spices have been playing an important role in the human dietary system since ancient times, as witnessed by their wider application in the food and pharmaceutical sectors. Numerous spices are being utilized in different food systems, including ginger candies to enhance the phytochemical potential.16,17 Black pepper (Piper nigrum) and cinnamon (Cinnamomum) are among the dominant spices, and their extracts have been used for value addition during processing and preservation15,18 and possessing various health benefits.19 Black tea and its extract, which is rich in polyphenols, is one of the most prevalent bioactive components and have been reported to exhibit antibacterial, antiviral, antioxidant, anticancer, antimutagenic, and anticarcinogenic properties.20 In the present study, optimized conditions were determined based on key physicochemical and phytochemical parameters, and the optimized product was subsequently evaluated using FTIR, ICP-AES, and SEM analyses.
2 Materials and methods
Sand pear fruits were collected from the orchard of Department of Fruit Science, Punjab Agricultural University, Ludhiana, India. Sweeteners (sucrose and stevia), spices (cardamom, cinnamon, cloves, and black pepper), and black tea were purchased from the local market in Ludhiana, India.2.1 Preparation of phytochemical enriched sand pear cubes
For the preparation of the spice extract, cardamom, cinnamon, cloves, and black pepper were used. Each spice (10 g) was added to 90 mL of distilled water and refluxed separately. The individual extracts were filtered and mixed in equal proportions (25% of each) to obtain the final spice extract. For the preparation of the tea extract, 5 g of black tea was boiled in 95 mL of distilled water for 4 minutes followed by making up to the desired volume and filtration. The extracts were stored under refrigerated conditions (4 ± 1 °C) until further use.The peeled sand pear cubes were blanched at 90 ± 2 °C and immediately cooled in cold water before dipping them in sugar syrup (40 °Bx sucrose solution and 1 g stevia to maintain the sweetness of 70 °Bx sucrose solution) containing spice (0–5%) and the tea (0–5%) extract as well (Table 1). This process proceeded at a temperature of 31 ± 2 °C until the syrup's TSS (total soluble solids) reached equilibrium. Following osmosis and diffusion, drying treatment with a tray dryer was carried out to achieve constant moisture. Samples that underwent osmotic dehydration followed by drying without blanching and incorporation of spice and tea extracts were designated as the control sand pear cubes.
| Run | Blanching time (min) | B: spice extract (%) | C: tea extract (%) | Water activity | TSS (°Bx) | L* | a* | b* | Hardness (N) | TPC (mg GAE/100 g) | TFC (mg QE/100 g) | DPPH (%) | RPA (µg AAE/100 g) | FRAP (µmol/100 g) | HRSA (%) | ABTS (%) | Antidiabetic activity (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2.50 | 2.50 | 2.50 | 0.76 | 43.2 | 38.67 | 1.79 | 6.57 | 22.8 | 160.7 | 81.23 | 44.64 | 142.12 | 2.81 | 83.85 | 83.89 | 42.54 |
| 2 | 2.50 | 2.50 | 2.50 | 0.78 | 43.4 | 34.23 | 1.88 | 6.79 | 24.18 | 162.1 | 79.64 | 41.15 | 148.87 | 2.88 | 86.78 | 82.18 | 43.37 |
| 3 | 0.00 | 5.00 | 0.00 | 0.68 | 38.2 | 30.42 | 1.97 | 7.26 | 37.13 | 177.4 | 84.9 | 36.13 | 132.62 | 2.93 | 83.69 | 87.36 | 30.65 |
| 4 | 5.00 | 5.00 | 5.00 | 0.76 | 46.8 | 32.49 | 1.52 | 7.91 | 5.29 | 185.4 | 81.48 | 53.73 | 175.37 | 3.14 | 91.12 | 90.51 | 37.85 |
| 5 | 5.00 | 2.50 | 2.50 | 0.73 | 45.4 | 34.68 | 1.56 | 6.2 | 5.13 | 150.4 | 74.53 | 42.73 | 143.37 | 2.57 | 83.63 | 80.91 | 38.12 |
| 6 | 2.50 | 2.50 | 5.00 | 0.77 | 43.5 | 24.49 | 1.63 | 6.61 | 26.74 | 188.7 | 93.91 | 46.79 | 168.75 | 3.65 | 92.33 | 90.99 | 43.63 |
| 7 | 2.50 | 2.50 | 2.50 | 0.76 | 43.2 | 33.32 | 1.82 | 6.82 | 27.4 | 159.4 | 85.36 | 42.95 | 154.12 | 2.42 | 80.65 | 84.64 | 47.36 |
| 8 | 2.50 | 0.00 | 2.50 | 0.77 | 42.8 | 36.61 | 1.44 | 5.85 | 18.68 | 158.6 | 71.28 | 38.26 | 135.57 | 2.19 | 81.1 | 83.48 | 35.47 |
| 9 | 2.50 | 5.00 | 2.50 | 0.81 | 43.6 | 26.83 | 2.56 | 8.25 | 20.73 | 182.6 | 91.55 | 53.61 | 159.25 | 3.06 | 87.41 | 96.19 | 40.89 |
| 10 | 2.50 | 2.50 | 2.50 | 0.79 | 44.4 | 35.52 | 1.84 | 6.38 | 24.88 | 165.1 | 81.6 | 48.23 | 140.25 | 2.67 | 86.48 | 84.24 | 44.61 |
| 11 | 0.00 | 2.50 | 2.50 | 0.68 | 37.2 | 34.17 | 2.35 | 6.73 | 36.28 | 164.1 | 81.3 | 35.37 | 143.62 | 2.93 | 84.25 | 93.76 | 29.65 |
| 12 | 2.50 | 2.50 | 2.50 | 0.74 | 43.3 | 34.74 | 1.93 | 6.59 | 22.44 | 168.9 | 85.1 | 41.19 | 147.12 | 2.45 | 81.07 | 81.46 | 44.88 |
| 13 | 5.00 | 0.00 | 0.00 | 0.79 | 44.6 | 32.81 | 1.11 | 4.84 | 10.25 | 138.7 | 51.06 | 33.13 | 119.12 | 2.09 | 79.94 | 80.66 | 30.12 |
| 14 | 5.00 | 5.00 | 0.00 | 0.8 | 45.2 | 20.95 | 2.58 | 6.25 | 10.74 | 160.2 | 74.86 | 42.97 | 132.37 | 2.21 | 85.05 | 79.58 | 38.45 |
| 15 | 2.50 | 2.50 | 2.50 | 0.73 | 42.2 | 35.98 | 1.78 | 6.33 | 21.58 | 161.9 | 80.31 | 41.03 | 135.25 | 2.56 | 86.05 | 92.25 | 46.53 |
| 16 | 0.00 | 0.00 | 0.00 | 0.7 | 38.4 | 33.13 | 0.99 | 5.27 | 22.44 | 154.1 | 67.42 | 31.56 | 123.87 | 2.23 | 81.46 | 88.02 | 26.2 |
| 17 | 0.00 | 5.00 | 5.00 | 0.68 | 39.6 | 25.33 | 2.5 | 8.14 | 21.82 | 194.5 | 93.34 | 56.78 | 167.12 | 3.48 | 91.25 | 96.52 | 29.24 |
| 18 | 2.50 | 2.50 | 0.00 | 0.76 | 42.2 | 21.92 | 1.39 | 4.66 | 28.61 | 159.4 | 74.37 | 42.28 | 141.37 | 2.49 | 82.18 | 87.46 | 37.73 |
| 19 | 5.00 | 0.00 | 5.00 | 0.81 | 45.4 | 26.2 | 1.13 | 6.03 | 12.75 | 169.6 | 80.87 | 39.14 | 136.37 | 2.64 | 89.03 | 82.01 | 35.37 |
| 20 | 0.00 | 0.00 | 5.00 | 0.72 | 38.8 | 31.63 | 2.33 | 6.36 | 36.49 | 182.1 | 78.28 | 40.51 | 155.62 | 2.81 | 87.86 | 89.56 | 32.21 |
2.2 Physico-chemical, phytochemical and mineral analyses
For physicochemical examination of sand pear cubes, their water activity, TSS (total soluble solids), colour, and texture were estimated. These analyses were carried out in accordance with Chandel et al. (2023).21The total phenolic content (TPC) estimation was performed using the F–C reagent.21 The gallic acid equivalent (GAE) was obtained to determine the total phenolic content. The flavonoid content was estimated by a method using aluminium chloride and potassium acetate solutions.22 Quercetin (QE) content was estimated for determining flavonoids.
Antioxidant activities were distinguished by assays such as DPPH (2,2-diphenyl-1-picryl hydrazyl), reducing power activity (RPA), ABTS (2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)), ferric reducing antioxidant power (FRAP) and hydroxyl radical scavenging activity (HRSA), and they were carried out in accordance with Barros et al., 2012,23 Singh and Rajini 2004,24 Pellegrini et al., 1999,25 and Gupta et al., 2020,26 respectively. Anti-diabetic assay was performed by estimating the capacity of 1 mL extract to inhibit the alpha amylase activity.21
2.3 Mineral analysis
Thermo-electron inductively coupled plasma atomic emission spectrometry (ICP-AES) was used to quantify the mineral content of the phytochemical enriched sand pear cubes. 0.5 g of sample was mixed with 10 mL of diacid mixture (nitric acid and perchloric acid in a 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio) and allowed to stand overnight, followed by digestion until the emission of white fumes ceased. The digested extract was diluted to a final volume of 25 mL with deionized water and was used for the mineral analysis.21
1 ratio) and allowed to stand overnight, followed by digestion until the emission of white fumes ceased. The digested extract was diluted to a final volume of 25 mL with deionized water and was used for the mineral analysis.21
2.4 FT-IR analysis
The control and optimized phytochemical enriched sand pear cubes were analysed by FT-IR spectroscopy. The FT-IR spectra were collected between 675 and 3555 cm−1, and the characteristic peaks were identified based on reference guidelines provided by Stuart (2005)27 and Kumar et al., (2018).282.5 SEM characterization
A JSM 6510 Digital Scanning Electron Microscope (JEOL) equipped with a digital image processor was utilized to examine the morphology of sand pear cubes. The sample was securely attached to an aluminium plate using electrically conductive tape, and a 10 mbar gold coating was applied for 90 s. Subsequently, each sample was transferred to a microscope slide for observation. The microscope operated at 5 kV and offered various magnification levels, including 100×, 500×, 1000×, and 2500×.52.6 Statistical analysis
All parameters were tested in triplicate, and analyses were conducted using the optimization toolbox in Design-Expert 7.1. To validate the mathematical model obtained through response surface methodology (RSM), checkpoint studies were performed. Data from mineral composition analysis were expressed as mean ± standard deviation (SD). Statistical differences among treatment means were determined using ANOVA, and when significant (p <0.05), Duncan's Multiple Range Test (DMRT) was applied. Cluster analysis was carried out using SPSS software to provide a concise summary of the data.183 Results and discussion
3.1 Preparation of phytochemical enriched sand pear cubes
Different variables i.e. blanching time, concentration of spice extract, and concentration of tea extract, and their effect on the physicochemical and phytochemical properties of sand pear are summarized in Table 1. Due to the effect of variables, water activity ranged from 0.68 to 0.81, TSS varied from 37.20 to 46.80 °Bx, L* value from 20.95 to 38.67, a* value from 0.99 to 2.58, b* value from 4.66 to 8.25, hardness from 5.13 to 37.13 N, total phenolics from 138.70 to 194.50 mg GAE/100 g, flavonoids from 51.06 to 93.91 mg QE/100 g, DPPH from 31.56 to 56.78%, RPA from 119.12 to 175.37 mg AAE (ascorbic acid equivalents)/100 g, FRAP from 2.09 to 3.65 µmol TE (Trolox equivalent)/100 g, HRSA from 79.94 to 92.53%, ABTS from 79.58 to 96.52% inhibition, and anti-diabetic activity from 26.20 to 47.36% inhibition.Respective responses were studied as linear, quadratic, and interactive values, and the F value along with the obtained R2 is presented in SI, Table 1. Depending upon the suitability, a quadratic model was selected for the statistical analysis and F-value, mean, standard deviation, R2 and lack of fit were used as measures for determining the accuracy of the model. The lack of fit for all the responses was found non-significant, indicating the significance of the models for all the attributes' R2 value was more than 0.75. The star (*) sign on the F value of the respective response indicates the significant positive or negative effect of the variable.
3.2 Physicochemical parameters
The blanching time possessed a significant effect on water activity and TSS (Table 2). Increasing the blanching time from 0 to 5 min resulted in a noticeable change in water activity. Blanching leads to an increase in porosity and a decrease in shrinkage, causing more water availability and increased water absorption during the blanching process, ultimately resulting in higher water activity in the final product.29,30 Conversely, the spice and tea extract concentrations did not significantly affect the water activity of the sand pear cubes. The increase in TSS with blanching time could be attributed to an enhancement of the diffusion rate caused by increased cell membrane permeability during blanching, which positively affected these compounds.31 In addition, the concentration of the tea extract also influenced the TSS of the product. The tea extract notably contributed to the TSS, as its infusion into the sand pear cubes enhanced solubility and promoted faster diffusion.32a  : increasing trend, : increasing trend,  : decreasing trend, : decreasing trend,  : decreasing followed by increasing trend, : decreasing followed by increasing trend,  : increasing followed by decreasing trend, : increasing followed by decreasing trend, 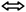 : non-significant changes. : non-significant changes. |
|---|
 |
By determining the color values, the incorporation of extracts was confirmed. As higher values of L* and b* (brightness and yellowness, respectively) correspond to greater acceptability of the sand pear cubes, there was a significant fall in the a* and b* values with increasing blanching time. Regarding the effect of spice extract, the L* value decreased, likely due to the dark color of the extract, which was confirmed by the increase in a* and b* values with higher spice extract concentrations. The tea extract possessed a positive effect on the b* value, while the a* value initially increased and then decreased with increasing tea extract concentration.
Good textural properties of food products contribute to consumer satisfaction. The results of texture profile analysis (Table 1) revealed that the hardness of the cubes was strongly influenced by the blanching time, exhibiting an inverse relationship between blanching and hardness. Previous studies have indicated that prolonged thermal blanching (>10 minutes) negatively affects plant tissues by causing degradation into soluble forms and also loss of cell turgidity.33 Blanching softens the fruit tissues, allowing the probe to easily penetrate them and confirm the softening. A similar effect of blanching on hardness was reported for carrot candies.33 Generally, tissue softening with blanching treatment is mainly due to solubilization of pectin,34 resulting in the softness of sand pear cubes. However, the spice and tea extract showed a non-significant effect on the textural properties of sand pear cubes.
3.3 Phytochemical parameters
Different phytochemical parameters of sand pear cubes were evaluated, as shown in Table 1. With the blanching treatment, a decrease was observed in total phenolic and flavonoid content, as shown in Table 2. There was more softening of tissues with 5 min blanching time compared to 2.5 min blanching time. An increase in cell permeability, aided by blanching, led to more mass flow.34 A resembling effect of blanching time was noticed in sand pear,21 berries,35,36 carrot.33 However, the increase in the polyphenol content in the 2.5 min blanched sample may be attributed to the release of the bound phenolics; during ripening, pectin gets solubilised and binds itself with phenols, and therefore blanching leads to breakage of the ester bond and phenolics get released into the cell matrix.33,37 Regarding the effect of spice and tea extract, their concentration improves the total phenols and flavonoids significantly.Sand pear, tea and spices are rich in antioxidant compounds.3 The antioxidant activities of sand pear cubes were assessed by DPPH, RPA, FRAP, HRSA and ABTS assays. In this study, blanching time had a significant effect on antioxidant activity (FRAP and ABTS), which decreased with the increase in blanching time. On the other hand, as expected with the spice and tea extract concentrations, the antioxidant activities increased except for ABTS inhibition where the effect was non-significant.
Anti-diabetic activity increased first with the increase in blanching time, and further blanching caused a reduction in anti-diabetic activity. It was found in results that there was high retention of polyphenols in sand pear at 2.5 min blanching time as compared to 0 and 5 min blanching time, which can become the factor responsible for anti-diabetic activity by inhibiting the alpha-amylase activity. Due to more availability of polyphenols in 2.5 min blanching time, there were more chances of binding of hydroxyl groups of phenols with alpha-amylase and caused more inhibition. Several studies have demonstrated a positive correlation between polyphenol content and anti-diabetic activity.38,39 The effect of the spice and tea extracts on the anti-diabetic activity was non-significant.
Cardamom, cinnamon, clove and black pepper were the spices in the spice extract (aqueous) used for the preparation of phytochemical enriched candies. From different studies, it was found that their aqueous extract possessed high phenolics, high flavonoids and exhibited high antioxidant activities in terms of DPPH, FRAP, ABTS, HRSA and RPA activity.40,41 Therefore, the increasing effect of tea extract and spice extract on TPC, TFC and antioxidant activities of sand pear cubes was due to their high bioactive profile.
3.4 Numerical optimization
Each variables and responses were chosen at different intensities to get the optimized conditions. The software suggested optimal parameters for the development of phytochemically enriched sand pear cubes, including a blanching time of 3.53 min, spice extract concentration of 2.74%, and tea extract concentration of 2.44%, with an overall desirability of 0.91 (SI Table 2).The predicted values of the responses under these optimized conditions were as follows: water activity, 0.77; TSS, 44.42 °Bx; L*, 34.89; a*, 1.80; b*, 6.55; hardness, 19.66 N; total phenols, 160.89 mg/100 g; flavonoids, 80.91 mg/100 g; DPPH inhibition, 43.88%; reducing power assay, 144.66 mg AAE/100 g; FRAP assay, 2.59 µmol TE/100 g; HRSA, 84.23%; ABTS inhibition, 83.61%; anti-diabetic activity, 45.48%. To verify the accuracy and effectiveness of the experiment, the differences between the predicted and experimental (actual) values were analyzed, revealing a close agreement. This confirmed that the optimized conditions are suitable and have strong potential for scale-up.
3.5 Cluster analysis
Cluster analysis was used to get an overview of the effect of variables on various parameters, and it was observed that major clustering was based on the blanching time. There were two major clusters initially, where the sample with 5 min blanching time without addition of any spice extract and tea extract had different and distinct attributes (SI Fig. 1). On the other hand, no clear cut clustering was further observed on the basis of variables i.e. blanching time and extracts. However, up to some extent, 2.5 and 5% spice and tea extract concentrations developed the clusters, indicating that their high concentration affects the physico-chemical and phytochemical parameters. Thus, the cluster analysis also confirmed the optimized results.Once the optimum conditions had been identified in terms of maximum retention of phytocompounds along with desirable physico-chemical parameters, the optimized sample was studied in comparison with control using different techniques.
3.6 Molecular characterization using FT-IR
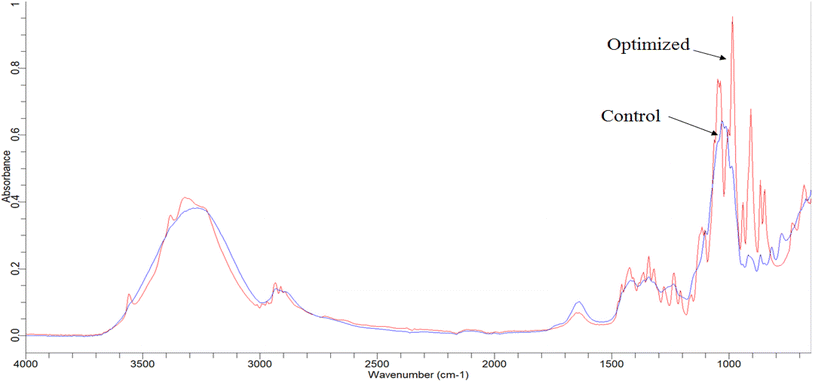
The interpretation of FTIR was done by following the data given by Stuart (2005).27 The qualitative screening of phytochemically improved optimized sand pear cubes was carried out to confirm the different functional groups as compared to the control (Fig. 1 and SI Table 3). In optimized samples, peaks at wavenumbers 1047 to 1278 cm−1 showed high intensity compared to the control, indicating the C–O stretching, which confirmed the presence of phenols. In the control sample, there was no peak around 3500–3550 cm−1, while the optimized sample showed a peak at 3555.88 cm−1, which indicates the O–H stretching of alcohol and phenol. Additionally, strong absorption peaks at 864.74, 916.62, and 905.74 cm−1 were observed in the optimized sample, indicating the presence of β-D-sucrose and β-D-glucose. This suggests higher sucrose content in the optimized sample compared to the control, likely due to enhanced osmotic exchange because of blanching.423.7 Mineral profiling
The range for mineral composition in optimized and control samples of sand pear cubes is shown in Table 3 i.e. calcium (248 ± 7.21 to 436 ± 8.43 mg kg−1), sulphur (173 ± 5.14 to 131 ± 6.12 mg kg−1), potassium (152 ± 5.63 to 222 ± 5.87 mg kg−1), sodium (122 ± 4.25 to 134 ± 3.16 mg kg−1), magnesium (88.25 ± 5.45 to 114 ± 4.49 mg kg−1), phosphorus (13.68 ± 1.02 to 15.78 ± 2.85 mg kg−1), iron (5.82 ± 2.0 to 15.98 ± 2.57 mg kg−1), boron (4.10 ± 1.01 to 2.85 ± 1.21 mg kg−1), zinc (7.90 ± 3.47 to 2.10 ± 1.49 mg kg−1), copper (0.60 ± 0.12 to 0.55 ± 0.25 mg kg−1), and manganese (2.15 ± 0.98 to 1.25 ± 0.3 mg kg−1).| Mineral composition (mg kg−1) | ||
|---|---|---|
| Elements | Control | Optimized |
| a Values are presented as mean ± standard deviation (n = 3). Different superscript letters (a, b) within a row indicate significant differences (p <0.05) according to Duncan's multiple range test. | ||
| Calcium | 248 ± 7.21a | 436 ± 8.43b |
| Sulphur | 173 ± 5.14a | 131 ± 6.12b |
| Potassium | 152 ± 5.63a | 222 ± 5.87b |
| Sodium | 122 ± 4.25a | 134 ± 3.16b |
| Magnesium | 88.25 ± 5.45a | 114 ± 4.19b |
| Phosphorus | 13.68 ± 1.02a | 15.78 ± 2.85a |
| Iron | 5.82 ± 2.00a | 15.98 ± 2.57b |
| Boron | 4.10 ± 1.01a | 2.85 ± 1.21a |
| Zinc | 7.90 ± 3.47a | 2.10 ± 1.49b |
| Copper | 0.60 ± 0.12a | 0.55 ± 0.25a |
| Manganese | 2.15 ± 0.98a | 1.25 ± 0.30a |
The optimized sample exhibited enhanced mineral content (for a majority of minerals under study) primarily due to the blanching process, which facilitated osmosis and diffusion of extracts and led to greater absorption of spice and tea extracts into the pear cubes.42 A similar effect of osmotic treatment on the infusion of anthocyanin was observed in gooseberry candies.43
3.8 Structural characterization
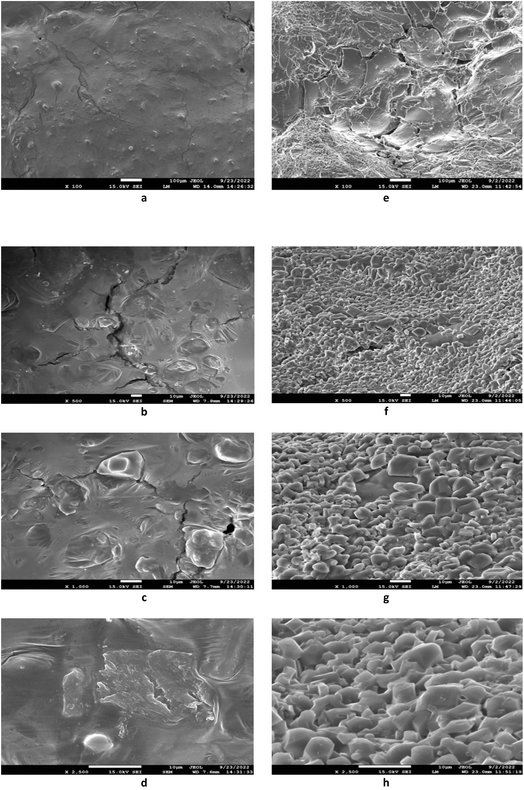
SEM analysis was employed to investigate the control and optimized sand pear cubes, with magnification levels ranging from 100× to 2500×. Fig. 2 visually demonstrates the impact of blanching on the arrangement and structure of macromolecules. Blanching caused notable changes, including cell wall deformation and structural collapse, leading to the formation of cavities (Fig. 2). The optimised sample displayed a porous surface, characterized by internal heating, resulting in temperature variations between the inner core and the surface, leading to the development of cavity. This microstructural transformation may account for the enhanced liberation, bioavailability, and concentration of bound polyphenols in blanched samples.33 The number and size of these pores influence the texture of the sand pear cubes, ultimately resulting in a softer texture compared to the control samples.4 Conclusion
The incorporation of spice and tea extracts enhanced the phytochemical profile of sand pear cubes while preserving their desirable texture. Optimisation of processing parameters like blanching time (3.53 min), spice extract concentration (2.74%), and tea extract concentration (2.44%) resulted in a product with improved and enhanced nutritional and phytochemical attributes. This study demonstrates a sustainable approach to utilizing sand pear by integrating natural extracts, leading to the development of a value-added product rich in bioactive compounds. The preparation of these phytochemical-enriched sand pear cubes represents an innovative and practical solution for the food processing industry. At an industrial scale, this strategy could help reduce post-harvest losses and provide a healthy confectionery alternative suitable for both children and adults.Author contributions
Rajat Chandel: conceptualization, formal analysis, investigation, methodology, validation, visualization, writing – original draft, writing – review and editing. Vikas Kumar, Ramandeep Kaur, Satish Kumar, and Sandeep Janghu: supervision, project administration, formal analysis, writing – original draft, writing – review and editing. Jasleen Bhasin: review and editing.Conflicts of interest
The authors declare that they have no conflict of interest.Data availability
The data pertaining to findings of this research are available in the article.Supplementary information (SI) is available. See DOI: https://doi.org/10.1039/d5fb00461f.
Acknowledgements
The authors express their sincere gratitude to the host institute for providing the necessary facilities to carry out this work.References
- P. Jnawali, V. Kumar and B. Tanwar, Food Sci. Hum. Wellness, 2016, 5, 169–176 CrossRef.
- P. Oyarzún, P. Cornejo, S. Gómez-Alonso and A. Ruiz, Plant Foods Hum. Nutr., 2020, 75, 532–539 CrossRef.
- R. Chandel, V. Kumar, R. Kaur, S. Kumar, A. Kumar, D. Kumar and S. Kapoor, Nutr. Food Sci., 2023, 53(7), 1061–1080 CrossRef.
- Y. z. Wang, M. s. Dai, D. y. Cai, S. Zhang and Z. b. Shi, Sci. Hortic., 2016, 210, 138–142 CrossRef CAS.
- P. Baniwal and B. S. Hathan, J. Food Process. Preserv., 2015, 39, 3098–3109 CrossRef CAS.
- Y. z. Wang, S. Zhang, M. s. Dai and Z. b. Shi, Plant Mol. Biol., 2014, 85, 123–134 CrossRef CAS PubMed.
- P. S. Panesar, V. K. Joshi, V. Bali and R. Panesar, Technology for Production of Fortified and Sparkling Fruit Wines, Elsevier Inc., 2017 Search PubMed.
- D. Zhang, B. Yu, J. Bai, M. Qian, Q. Shu, J. Su and Y. Teng, Sci. Hortic., 2012, 134, 53–59 CrossRef CAS.
- D. I. Onwude, N. Hashim and G. Chen, Trends Food Sci. Technol., 2016, 57, 132–145 CrossRef CAS.
- S. Dhiman, V. Kumar, R. Kaur, S. Kumar and R. Sharma, Appl. Food Res., 2022, 2, 100172 Search PubMed.
- S. Bernardini, A. Tiezzi, V. L. Masci and E. Ovidi, Nat. Prod. Res., 2017, 32, 1926–1950 CrossRef PubMed.
- N. Konar, R. Gunes, I. Palabiyik and O. S. Toker, Trends Food Sci. Technol., 2022, 123, 57–68 Search PubMed.
- L. R. Ramos, J. S. Santos, H. Daguer, A. C. Valese, A. G. Cruz and D. Granato, Food Chem., 2017, 221, 950–958 Search PubMed.
- V. Kumar, R. Kushwaha, A. Goyal, B. Tanwar and J. Kaur, Food Chem., 2018, 245, 168–177 CrossRef CAS.
- A. M. Saad, A. S. Mohamed, M. T. El-Saadony and M. Z. Sitohy, LWT--Food Sci. Technol., 2021, 148, 111668 CrossRef CAS.
- A. Kumar and H. M. Jena, Results Phys., 2016, 6, 651–658 CrossRef.
- G. Kaur, V. Kumar, A. Goyal, B. Tanwar and J. Kaur, Nutr. Food Sci., 2018, 48, 733–743 CrossRef.
- P. N. Ravindran and J. A. Kallupurackal, Black Pepper, Woodhead Publishing Limited, 2nd edn, 2012, vol. 1 Search PubMed.
- K. W. Singletary, Nutr. Today, 2018, 53, 92–97 CrossRef.
- L. A. Abdulkhaleq, M. A. Assi, M. H. M. Noor, R. Abdullah, M. Z. Saad and Y. H. Taufiq-Yap, Vet. World, 2017, 10, 869–872 CrossRef CAS PubMed.
- R. Chandel, V. Kumar, R. Kaur, S. Kumar, M. S. Gill, R. Sharma, R. V. Wagh and D. Kumar, J. Food Meas. Char., 2023, 17, 3709–3721 CrossRef.
- A. Pękal and K. Pyrzynska, Food Anal. Methods, 2014, 7, 1776–1782 CrossRef.
- H. R. De Moraes Barros, T. A. P. De Castro Ferreira and M. I. Genovese, Food Chem., 2012, 134, 1892–1898 CrossRef.
- N. Singh and P. S. Rajini, Food Chem., 2004, 85, 611–616 CrossRef CAS.
- N. Pellegrini, R. Re, M. Yang and C. Rice-Evans, Methods Enzymol., 1999, 299, 379–389 Search PubMed.
- A. Gupta, R. Kumar and A. K. Pandey, S. Afr. J. Bot., 2020, 130, 308–315 Search PubMed.
- B. H. Stuart, Infrared Spectroscopy: Fundamentals and Applications, 2005, pp. 1–224 Search PubMed.
- V. Kumar, R. Kushwaha, A. Goyal, B. Tanwar and J. Kaur, Food Chem., 2018, 245, 168–177 Search PubMed.
- A. Ciurzyńska, J. Falacińska, H. Kowalska, J. Kowalska, S. Galus, A. Marzec and E. Domian, Foods, 2021, 10, 132 CrossRef.
- M. S. Tapía, S. M. Alzamora and J. Chirife, Water Activity in Foods: Fundamentals and Applications, 2020, pp. 323–355 Search PubMed.
- M. Nowacka, L. Laghi, K. Rybak, M. D. Rosa, D. Witrowa-Rajchert and U. Tylewicz, Food Chem., 2019, 299, 125122 Search PubMed.
- W. Xiao, Y. Zhang, C. Fan and L. Han, Food Chem., 2017, 214, 242–247 CrossRef CAS PubMed.
- H. Wang, X. M. Fang, P. P. Sutar, J. S. Meng, J. Wang, X. L. Yu and H. W. Xiao, Food Chem., 2021, 338, 127799 CrossRef CAS.
- L. Z. Deng, A. S. Mujumdar, X. H. Yang, J. Wang, Q. Zhang, Z. A. Zheng, Z. J. Gao and H. W. Xiao, Food Chem., 2018, 261, 292–300 CrossRef CAS.
- S. S. Sablani, P. K. Andrews, N. M. Davies, T. Walters, H. Saez, R. M. Syamaladevi and P. R. Mohekar, J. Sci. Food Agric., 2010, 90, 769–778 Search PubMed.
- G. Giovanelli, A. Brambilla, A. Rizzolo and N. Sinelli, Food Res. Int., 2012, 49, 263–271 CrossRef CAS.
- R. F. Dibanda, E. P. Akdowa, A. Rani, Q. M. Tongwa and C. M. Mbofung, Food Chem., 2020, 302, 125308 CrossRef PubMed.
- G. J. McDougall, F. Shpiro, P. Dobson, P. Smith, A. Blake and D. Stewart, J. Agric. Food Chem., 2005, 53, 2760–2766 CrossRef CAS PubMed.
- L. Sun, F. J. Warren and M. J. Gidley, Trends Food Sci. Technol., 2019, 91, 262–273 CrossRef CAS.
- N. Chimbetete, M. Verghese, R. Sunkara, L. T. Walker, N. Chimbetete, M. Verghese, R. Sunkara and L. T. Walker, Food Nutr. Sci., 2019, 10, 266–275 CAS.
- I. Gülçin, Int. J. Food Sci. Nutr., 2009, 56, 491–499 CrossRef.
- H. W. Xiao, Z. Pan, L. Z. Deng, H. M. El-Mashad, X. H. Yang, A. S. Mujumdar, Z. J. Gao and Q. Zhang, Inf. Process. Agric., 2017, 4, 101–127 Search PubMed.
- S. R. Adsare, A. N. Bellary, H. B. Sowbhagya, R. Baskaran, M. Prakash and N. K. Rastogi, J. Food Eng., 2016, 175, 24–32 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2026 |