Open Access Article
Open Access ArticleExtraction technologies for hemp seed oil: a comparative study of yield, bioactive compound extractability, and oxidative stability
Aymane
Allay
a,
Rafika
El Ati
a,
Youssef
Rbah
a,
Marie-Laure
Fauconnier
b,
Hana
Serghini Caid
a,
Ahmed
Elamrani
 a and
Farid
Mansouri
a and
Farid
Mansouri
 *ac
*ac
aLaboratory of Agricultural Productions Improvement, Biotechnology and Environment, Faculty of Sciences, Mohammed I University, BP-717, 60000 Oujda, Morocco. E-mail: f.mansouri@ump.ac.ma
bLaboratory of Chemistry of Natural Molecules, Gembloux Agro-Bio Tech, University of Liège, Passage des Déportés, 2, 5030 Gembloux, Belgium
cHigher School of Education and Training, Mohammed I University, BP-410, Oujda 60000, Morocco
First published on 30th October 2025
Abstract
An integrated comparison of four extraction methods was carried out to evaluate their performance in terms of hemp seed oil yield, physicochemical characteristics, and bioactive compound extractability. The methods included Soxhlet extraction (SOX-HPE) using a ternary solvent system (hexane/ethyl acetate/2-propanol; 4/4/2), microwave-assisted extraction (MAE) with 7.5% ethanol, supercritical fluid extraction (SFE) with 10% ethanol, and mechanical extraction (ME). Microstructural changes in the seed powders were also examined using scanning electron microscopy to better understand the mechanisms of cell wall disruption. SOX-HPE provided the highest oil yield (33.24%), followed by MAE and SFE (30.69 and 30.51%, respectively). HPLC-DAD/ESI-MS2 analysis identified 29 phenolic compounds, with the highest total concentrations observed in oils from SOX-HPE and SFE (131.29 and 71.92 mg kg−1 oil, respectively), including elevated levels of N-trans-caffeoyltyramine (39.98 and 14.92 mg kg−1 oil), cannabisins B (10.79 and 7.63 mg kg−1 oil) and A (7.98 and 3.79 mg kg−1 oil), which contributed to excellent oxidative stability (37.77 and 28.01 hours, respectively at 100 °C). SFE oil was also rich in unsaturated fatty acids (90.03%) and exhibited favorable oil quality indices. MAE produced oil enriched in tocopherols (510.66 mg kg−1 oil), chlorophylls (99.68 mg kg−1 oil), and carotenoids (27.31 mg kg−1 oil). These findings highlight the importance of selecting appropriate extraction techniques to optimize both the nutritional quality and functional potential of hemp seed oil. In particular, SFE, especially when combined with a green polar co-solvent, emerged as an efficient and environmentally sustainable method for producing high-quality hemp seed oil. The use of less toxic polar solvents in Soxhlet extraction also demonstrated promising potential for enhancing bioactive compound recovery while reducing solvent-related hazards.
Sustainability spotlightThis research promotes sustainable food innovation by comparing extraction techniques for producing hemp seed oil. The study evaluates the use of safer polar solvents in Soxhlet extraction and alternative sustainable approaches such as supercritical CO2 and microwave-assisted extraction. Hemp seeds are valorized as a sustainable source of functional lipids and bioactive compounds. This work supports SDG 12, SDG 9, and SDG 3 by offering scalable extraction strategies that enhance the nutritional value of plant-based products while reducing environmental impact in post-harvest processing. |
1. Introduction
The growing interest in vegetable oils as raw materials for food and industrial applications has increasingly attracted scientific attention. Consequently, research efforts have been directed toward identifying, characterizing, developing, and utilizing these beneficial vegetable oils, alongside the exploration of cost-effective production and extraction methods. To meet the nutritional demands of the expanding global population, it is imperative to explore a broader spectrum of vegetable oils, including hemp seed oil.Hemp seed oil is characterized by its high nutritional value and favorable fatty acid composition, comprising mainly polyunsaturated fatty acids, particularly omega-6 linoleic and omega-3 α-linolenic acids, at a healthy ratio.1–3 This composition confers cardiovascular and anti-inflammatory benefits. It is also rich in vitamin E, which enhances its antioxidant properties and contributes to its stability.2,4 Although phenolic compounds also possess antioxidant properties, their relatively low concentration in hemp seed oil limits their effectiveness compared to tocopherols.4–6 Moderate levels of chlorophylls, carotenoids, and phytosterols further increase the health-promoting attributes of the oil.7 Its characteristic nutty, earthy flavor makes it suitable for culinary, cosmetic, and industrial uses. However, its susceptibility to oxidation necessitates careful storage under cool, dark conditions. Sustainable cultivation practices combined with its versatile application position hemp seed oil as a valuable resource in the food, health, and industrial sectors.
Different extraction methods significantly impact the yield, quality, and chemical profile of hemp seed oil. Mechanical extraction (ME) via screw pressing is favored for its operational simplicity, minimal environmental impact, and ability to produce of high-quality pressed oil, although at lower yields.8–10 Solvent extraction frequently employs hexane as a solvent, along with polar solvents such as ethanol and ethyl acetate, which results in high oil yields with varied efficiencies in extracting bioactive compounds but raises sustainability concerns due to prolonged extraction times, high energy consumption, and the use of toxic solvents.11,12 Emerging extraction techniques, such as supercritical fluid extraction (SFE) using CO2 with or without green co-solvents, and microwave-assisted extraction (MAE), combine shorter extraction durations, moderate operating conditions, high extraction efficiency, and the possibility of using greener solvents, making them particularly attractive in terms of environmental sustainability.13–17
Understanding the interactions between extraction methods and oil composition is crucial for identifying opportunities to increase oil quality, particularly through the coextraction of antioxidant compounds from seeds. Few comparative studies on hemp seed oil extraction methods exist. Some have compared solvent extraction, mechanical pressing, and SFE,13,18,19 while others, such as Rezvankhah et al.,15 have examined MAE in comparison with solvent extraction. Devi & Khanam investigated several methods, such as Soxhlet extraction (SOX), SFE, percolation (PER), ultrasonication (ULT), and pyrolysis (PYR), and evaluated yield, oil composition, and economic feasibility at an industrial scale.14
In our previous studies, we investigated and optimized each of these extraction techniques separately (SOX, MAE, SFE, and ME) to identify the optimal conditions for maximizing yield and bioactive compound extraction.20–23 The present study builds upon this body of previous work, aiming to perform a comparative evaluation of the effectiveness of these four extraction methods in coextracting antioxidant molecules with the oil and their impact on the microstructure of the seed powder, given a comprehensive comparison in terms of yield and chemical composition, especially in terms of bioactive compound extraction.
This work aims to identify the extraction method that offers the optimal balance between oil yield, nutritional quality, bioactive compound enrichment, and oxidative stability. To this end, hemp seed oil was extracted via SOX, MAE, SFE and ME under the previously optimized conditions established in our published studies. The parameters assessed included phenolic compounds, tocopherols, pigments (chlorophylls and carotenoids), color analysis (L*, a*, b*), fatty acids, peroxide value, free acidity, specific extinction coefficients (conjugated dienes and trienes), and oxidative stability. Additionally, scanning electron microscopy was employed to investigate the morphological changes in the hemp seed powder following various extraction methods. This integrated approach provides novel insights that are critical for enhancing the overall quality and commercial viability of hemp seed oil.
2. Materials and methods
2.1. Chemicals and reagents
Standards, including tocopherols (α-tocopherol, β-tocopherol, γ-tocopherol and δ-tocopherol), fatty acid methyl esters (FAME), N-trans-caffeoyltyramine, gallic, sinapic, benzoic, hydroxybenzoic, and p-coumaric acids, were supplied by Sigma-Aldrich (St. Louis, MO, USA). The extraction solvents used included n-hexane, 2-propanol, ethyl acetate and ethanol, all of analytical grade. The fluid employed for SFE was food-grade carbon dioxide (CO2), with a purity of 99.9%. In addition, all other chemicals and reagents used in this study were of analytical grade and were obtained from Merck Chemical Company (Darmstadt, Germany).2.2. Plant material
In this study, experimental trials were carried out using hemp (Cannabis sativa L.) seeds of the ‘Beldia’ ecotype grown in the Jebha region of northern Morocco. Seeds were supplied by the Agence Nationale des Plantes Médicinales et Aromatiques (ANPMA). The seed water content was measured at 5.13 ± 0.1% using the standard hot-air oven method at 105 ± 1 °C until a constant weight was obtained, following the protocol described in the literature.24 The fresh seeds were ground and sieved through a 500 μm mesh sieve for use in SOX, MAE, and SFE. Seeds were stored in plastic bags at 2–4 °C in a refrigerator to preserve their quality until use.2.3. Different hemp oil extraction methods
Four different extraction methods were used to extract hemp seed oil: SOX, MAE, SFE, and ME. The parameter levels and operating conditions for each method were previously optimized in our earlier studies.20–23 Each technique was individually optimized to identify the most effective parameters for maximizing oil yield, preserving its quality, and improving the recovery of bioactive compounds. The optimization focused respectively on the solvent type for SOX, the combination of pressure, temperature, time, and cosolvent (ethanol) percentage for SFE, the microwave power, extraction duration, and ethanol/hexane ratio (% EtOH) for MAE, and the pressing temperature for ME.In the present study, these previously determined optimal conditions were applied to ensure a rigorous and fair comparison among the four methods in terms of extraction efficiency, oil composition, antioxidant co-extraction, and impact on the microstructure of the seed powder. Each extraction was performed in triplicate, and the mean values of the obtained results were used for statistical analysis.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The seed-solvent mixture was placed in a flask, which was then inserted into the cavity of the microwave oven and connected to a cooler to condense the solvent evaporated by the radiation. Extraction was carried out under optimum conditions with a power of 800 W, an extraction time of 13.6 min and a solvent mixture containing n-hexane with 7.5% ethanol. After extraction, the liquid phase was separated from the seed powder by a filter cloth, and the remaining solids were washed twice with the extraction solvent. Then, the mixture was centrifuged for 15 min at 5000g. The collected solvent mixture was evaporated in a vacuum rotary evaporator at 40 °C. After being weighed, the resulting oil was poured into dark vials and stored under nitrogen in a freezer at −18 °C for subsequent experiments. The extraction was carried out in triplicate, and the mean values were recorded.
1. The seed-solvent mixture was placed in a flask, which was then inserted into the cavity of the microwave oven and connected to a cooler to condense the solvent evaporated by the radiation. Extraction was carried out under optimum conditions with a power of 800 W, an extraction time of 13.6 min and a solvent mixture containing n-hexane with 7.5% ethanol. After extraction, the liquid phase was separated from the seed powder by a filter cloth, and the remaining solids were washed twice with the extraction solvent. Then, the mixture was centrifuged for 15 min at 5000g. The collected solvent mixture was evaporated in a vacuum rotary evaporator at 40 °C. After being weighed, the resulting oil was poured into dark vials and stored under nitrogen in a freezer at −18 °C for subsequent experiments. The extraction was carried out in triplicate, and the mean values were recorded.
2.4. Physicochemical characterization of the extracted oils
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, v/v). The extracts were then reacted with Folin–Ciocalteu reagent, and the absorbance was measured at 760 nm. The results are expressed in mg gallic acid equivalents (GAE) per kg of oil.
20, v/v). The extracts were then reacted with Folin–Ciocalteu reagent, and the absorbance was measured at 760 nm. The results are expressed in mg gallic acid equivalents (GAE) per kg of oil.
The color of the oil samples was determined using the CIELAB parameters (L*, a*, b*) and measured with a KONICA MINOLTA CR-410 chromameter.
2.5. Scanning electron microscopy (SEM)
A Quattro ESEM scanning electron microscope (FEG-Thermo Fisher Scientific) was used to examine morphological alterations in the hemp seed samples before and after oil extraction via different methods. The samples were coated with Au/Pd before being examined by SEM. A working distance of 10.1 mm and a spot size of 3 were used with a low-vacuum secondary electron detector. SEM images were taken at an accelerating voltage of 15 kV and a pressure of 1.05 mbar.2.6. Statistical analysis
Statistical analysis of the differences between oils extracted via different methods was performed using univariate MANOVA in SPSS (v.25), with a significance threshold set at p < 0.05. The normality and homogeneity of the data were verified using the Shapiro–Wilk test and the Levene test, respectively (p > 0.05). A principal component analysis (PCA) was also performed after validating the adequacy of the data via the Kaiser–Meyer–Olkin (KMO) and Bartlett tests (p < 0.05). Finally, Pearson correlations (p < 0.05) between the main composition and quality variables of hemp seed oils obtained by different extraction methods were calculated. The results are expressed as the means ± standard deviations and were visualized using GraphPad Prism 8.0 software.3. Results and discussion
3.1. Effects of different extraction methods on oil yield
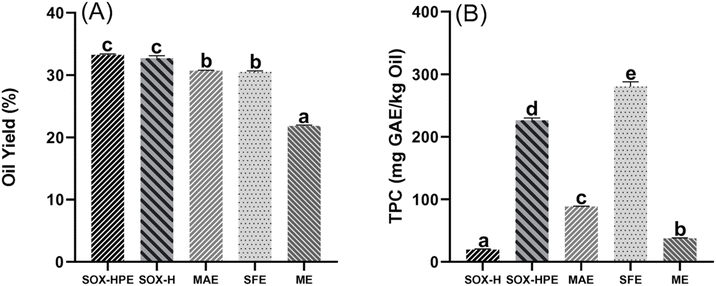
As shown in Fig. 1A, the SOX-H and SOX-HPE extractions achieved the highest yields, with values of 32.72% and 33.24%, respectively, followed by 30.69 for MAE, 30.51% (g oil per 100 g of fresh seeds) for SFE, and 21.82% for ME. Statistical analysis revealed no significant difference (p > 0.05) between the yields obtained by SOX-H and SOX-HPE, suggesting that the solvent mixture (40% n-hexane, 40% 2-propanol, and 20% ethyl acetate) is as effective as n-hexane alone for the extraction of hemp seed oil. This equivalence could be attributed to the ability of the ternary system to disrupt cell membranes and extract a broad spectrum of lipophilic compounds, including phospholipids.11,30Furthermore, the high efficiency of Soxhlet extraction can be explained by the thermally intensive operating conditions. Indeed, high temperature promotes the diffusion of lipid solutes and increases the solubility of lipophilic compounds in the solvent, leading to an increase in extraction yield.14 Moreover, the extended duration (5 hours) also contributed to this enhanced performance, enabling near-complete extraction of lipid constituents.15
These observations are perfectly consistent with the literature. Numerous studies have confirmed the superiority of the Soxhlet method for hemp seed oil extraction. For example, Devi & Khanam, after comparing five extraction methods (SOX, SFE, PER, ULT, PYR), reported that SOX offered the best yields.14 Similarly, Da Porto et al. and Aiello et al. reported that the oil yield extracted by SOX-H was significantly greater than that obtained via SFE.18,31 This trend was also confirmed by Rezvankhah et al. who highlighted a higher yield for SOX-H than for MAE.15 Finally, Crimaldi et al., Aladić et al. and Golimowski et al. reported that the SOX-H method clearly outperformed cold-press extraction in terms of yield.8,9,32
Although the SOX technique is the most efficient in terms of yield, it has significant drawbacks, notably, a long extraction time and elevated temperatures, resulting in high energy consumption, which limits its profitability. MAE, on the other hand, stands out for its much shorter extraction time, with a highly satisfactory yield of 30.69% achieved in just 13.60 min. This efficiency can be attributed to the disruption of cell walls by microwaves, favoring better exposure of intracellular materials to the solvent and thus improving extraction in a reduced time. Similar results were reported by Rezvankhah et al., who reported that MAE offers an acceptable hemp seed oil yield of 33.91% in just 7.19 min, whereas SOX-H achieves a slightly higher yield of 37.93% in 8 h.15
Overall, compared with the SOX-H method, MAE and SFE demonstrated satisfactory extraction efficiencies, reaching 93.80% and 93.24%, respectively. These results are consistent with those reported by Aiello et al.,18 who reported a recovery of 93.19% for hemp seed oil compared with SOX-H, and Rezvankhah et al. reported a recovery of 89.40% for hemp seed oil extracted by MAE with SOX-H as a ref. 15.
In contrast, ME proved to be less efficient, with an extraction efficiency of only 66.68%. Similar results were reported by Crimaldi et al.,32 who observed a maximum oil recovery of 61.52% for mechanically extracted hemp seed oil. The low oil yield obtained by ME is mainly attributed to the limited mechanical disruption of the seed microstructure and the strong retention of oil within the press cake. The applied pressure primarily ruptures the outer seed coat but only partially disrupts the internal cellular compartments, particularly the oleosomes where most of the lipids are stored.4,33 Consequently, a significant fraction of oil remains trapped within the residual matrix, leading to incomplete recovery.32 Moreover, the absence of solvent in this process prevents efficient diffusion and solubilization of lipids through the compact and fibrous seed structure, further limiting extraction efficiency. These findings are consistent with the SEM imaging results, which show a relatively intact seed powder surface after ME, in contrast to the more pronounced cellular disintegration observed in oils extracted by SOX, MAE, and SFE.
Overall, these data confirm the advantage of the SOX method in terms of absolute yield, while MAE and SFE techniques demonstrate comparable relative efficiencies with shorter extraction times and milder operating conditions, highlighting their potential as more sustainable and economically viable alternatives.
3.2. Effect of extraction methods on the phenolic composition of hemp seed oil
Owing to their substantial variation in their polarity, phenolic compounds are influenced by the extraction method used. Fig. 1B illustrates the impact of extraction methods on the TPC content of hemp seed oils. SFE extraction resulted in significantly (p < 0.05) higher TPC, reaching 294.15 mg GAE per kg oil, followed by SOX-HPE (226.43 mg GAE kg−1), MAE (88.55 mg GAE kg−1), ME (37.52 mg GAE kg−1), and SOX-H (19.43 mg GAE kg−1). This difference may be attributed to the polarity of the extraction medium. The TPC content of oil extracted by SOX-HPE with 60% polar solvents, including 40% 2-propanol and 20% ethyl acetate, and SFE with 10% ethanol as the polar solvent was almost three times greater than that obtained by MAE, where the solvent was composed of 7.5% ethanol and 92.5% n-hexane. Moreover, the use of pure n-hexane resulted in the lowest TPC content, while its combination with 2-propanol and ethyl acetate increased this content up to tenfold. In general, phenolic compounds, which are polar, are more soluble in polar solvents, which explains why increasing the polarity of the solvent facilitates the extraction of these compounds from oil.34 Furthermore, Soroush et al. reported that increasing the percentage of isopropanol to 50% compared with that of n-hexane favored an increase in the phenol content in hemp seed oil extracted via MAE.17 On the other hand, Quispe-Fuentes et al. reported that increasing the percentage of ethanol in SFE extraction of Clementina orogrande bark oil led to an increase in the TPC compared with the use of pure CO2.35In contrast, the oil obtained by ME exhibited a low TPC. Since ME relies solely on mechanical pressure without the use of solvents, it results in limited recovery of hydrophilic compounds such as phenolics. In comparison, polar solvents like ethanol and isopropanol enhance both lipid extraction and phenolic solubilization by disrupting cell wall integrity and modifying the polarity of the extraction medium. As demonstrated in various studies on oilseeds, these solvents break hydrogen bonds and hydrophobic interactions within the plant matrix, thereby facilitating the release of amphiphilic or partially hydrophilic molecules.36,37 Similar limitations in transferring phenolic compounds from seeds to oil have been reported for grape seed oil, where the extracted phenolics accounted for only 0.013–0.019% of the total seed content.38
To better assess the effect of each extraction method on the transfer of phenolic compounds from the seeds to the oil, HPLC-DAD/ESI-MS2 analysis was carried out on the oil extracts. Phenolic compounds were identified by comparing the precursor ion mass (MS), fragments (MS2) and UV-Vis spectra (SI Fig. S1) with data available in the literature.23,24,33,34 This analysis identified a total of 29 phenolic compounds: four phenolic acids (p-hydroxybenzoic, benzoic, p-coumaric, and sinapic acids), five hydroxycinnamic acid amides (HCAAs) as N-trans-caffeoyltyramine, N-trans-caffeoyltyramine isomer, N-trans coumaroyltyramine, N-feruloyl-tyramine, and tri-p-coumaroylspermidine, and twenty lignanamides as N-caffeoyltyramine dimer hydroxy derivative, cannabisins (A, B, B isomer 1, B isomer 2, C, C isomer, D, E, M, Q, F, G, and O), demethylgrossamide, 3,3-didemethylgrossamide, 3,3′-demethylheliotropamide, unnamed lignanamide, isocannabisin N, and grossamide (Table 1). Fig. 2 shows that, among the 29 phenolic compounds identified, the oils extracted by SOX-HPE and SFE contained all of them. On the other hand, 26 compounds were detected in the oil obtained by MAE. In contrast, oils extracted by SOX-H and ME contained only 3 to 4 phenolic compounds, highlighting the considerably lower efficiency of these methods in transferring these bioactive compounds.
| No. peak | RT (min) | Phenolic compounds | λ max (nm) | [M–H]− calc. (m/z) | [M–H]− found (m/z) | SOX-H | SOX-HPE | MAE | SFE | ME |
|---|---|---|---|---|---|---|---|---|---|---|
| a Data, which are the mean ± SD of three independent experiments (n = 3), were expressed as mg kg−1 oil. Mean values in the same line followed by a different letter are significantly different (p < 0.05). *Hydroxycinnamic acid amides and lignanamides are expressed in mg N-trans-caffeoyltyramine equivalent per kg of oil (mg CTE per kg oil). Nd: not detected; Tr: traces; RT: retention time; λmax: maximum absorbance peak. SOX-H: Soxhlet extraction with n-hexane; SOX-HPE: Soxhlet extraction with n-hexane, 2-propanol and ethyl acetate (40%, 40%, 20%); MAE: microwave assisted extraction (800 W, 13.6 min and 7.5% EtOH); SFE: supercritical fluid extraction (20 MPa, 50 °C, 244 min, with a mixture of 90% CO2 + 10% ethanol); ME: mechanical extraction (pressing at 100 °C). | ||||||||||
| 1 | 10.34 | Unknown 1 | 264; 292 | — | 265.002 | Nd | 2.05 ± 0.17b | Nd | 1.48 ± 0.27a | Nd |
| 2 | 13.95 | Unknown 2 | 292 | — | 325.009 | Nd | 0.47 ± 0.04a | 0.56 ± 0.05a | 1.22 ± 0.23b | Tr |
| 3 | 14.44 | p-Hydroxybenzoic acid | 288; 378 | 137.0322 | 137.0317 | Nd | 0.13 ± 0.01a | 2.70 ± 0.14c | 0.93 ± 0.16b | Tr |
| 4 | 18.99 | Benzoic acid | 280; 220 | 121.0373 | 121.0370 | Nd | 10.10 ± 0.71b | 0.27 ± 0.01a | 8.69 ± 0.12b | 0.32 ± 0.02a |
| 5 | 20.80 | p-Coumaric acid | 230; 300; 310 | 163.0478 | 163.0480 | Nd | 0.62 ± 0.01c | 0.18 ± 0.02b | 2.76 ± 0.08d | 0.03 ± 0.00a |
| 6 | 22.58 | N-Trans-caffeoyltyramine isomer | 200; 284; 315 | 298.1158 | 298.1160 | Nd | 3.92 ± 0.72c | 0.47 ± 0.04a | 0.99 ± 0.02b | Nd |
| 7 | 25.42 | N-Trans-caffeoyltyramine | 220; 250; 294; 318 | 298.1158 | 298.1160 | Nd | 39.98 ± 3.47c | 4.76 ± 0.16a | 14.92 ± 0.86b | Nd |
| 8 | 25.93 | Unknown 3 | 250; 318; 344 | — | 207.000 | Nd | 1.52 ± 0.06a | Nd | Nd | Nd |
| 9 | 26.29 | N-Caffeoyltyramine dimer hydroxy derivative | 254; 340 | 613.2269 | 613.2256 | Nd | 0.68 ± 0.02b | 0.35 ± 0.05a | 0.43 ± 0.02a | Nd |
| 10 | 27.63 | Cannabisin A | 256 | 593.2002 | 593.2016 | Nd | 7.98 ± 0.79c | 1.73 ± 0.11a | 3.79 ± 0.15b | Nd |
| 11 | 27.89 | Cannabisin B | 254; 284; 314; 334 | 595.2159 | 595.2155 | Nd | 10.79 ± 0.61c | 2.43 ± 0.18a | 7.63 ± 1.01b | Nd |
| 12 | 28.34 | N-Trans-coumaroyltyramine | 292; 308 | 282.1208 | 282.1215 | Nd | 0.64 ± 0.02a | 0.62 ± 0.01a | 0.63 ± 0.11a | Nd |
| 13 | 29.02 | Cannabisin B isomer 1 | 264; 284; 314 | 595.2159 | 595.2155 | Nd | 5.81 ± 0.08c | 0.89 ± 0.01a | 1.67 ± 0.20b | Nd |
| 14 | 29.48 | Cannabisin B isomer 2 | 268; 310 | 595.2159 | 595.2161 | Nd | 0.74 ± 0.01a | 1.34 ± 0.05b | 0.84 ± 0.18a | Nd |
| 15 | 29.73 | N-Feruloyltyramine | 256; 288; 318 | 312.1314 | 312.1320 | Nd | 10.50 ± 0.00c | 0.84 ± 0.04a | 1.19 ± 0.02b | Nd |
| 16 | 29.98 | Unknown 4 | 322 | — | 607.000 | Nd | 0.60 ± 0.06a | Nd | Tr | Nd |
| 17 | 30.41 | Demethylgrossamide | 264; 284; 314; 322 | 609.2315 | 609.2322 | Nd | 4.06 ± 0.39c | 0.25 ± 0.04a | 0.77 ± 0.09b | Nd |
| 18 | 31.24 | Cannabisin C | 260; 280 | 609.2315 | 609. 2317 | Nd | 0.38 ± 0.01a | 0.38 ± 0.02a | 0.44 ± 0.03a | Nd |
| 19 | 31.39 | Cannabisin C isomer | 284; 322 | 609.2315 | 609.2325 | Nd | 0.80 ± 0.07a | 0.77 ± 0.02a | 0.82 ± 0.08a | Nd |
| 20 | 32.20 | Unknown 5 | 284 | — | 609.168 | Nd | 0.13 ± 0.00a | 0.47 ± 0.03b | Tr | Nd |
| 21 | 32.34 | Cannabisin D | 260; 284; 308 | 623.2472 | 623.2480 | Nd | 0.13 ± 0.00a | 0.98 ± 0.02c | 0.65 ± 0.03b | Nd |
| 22 | 32.82 | 3,3-Didemethylgrossamide | 284; 324 | 595.2159 | 595.2160 | Nd | 0.63 ± 0.01b | 0.37 ± 0.04a | 1.63 ± 0.17c | Nd |
| 23 | 33.12 | Tri-p-coumaroylspermidine | 260 | 582.2688 | 582.2695 | Nd | 0.46 ± 0.07a | 0.33 ± 0.02a | 0.91 ± 0.12b | Nd |
| 24 | 33.42 | Cannabisin E | 292; 310 | 641.2577 | 641.2571 | Nd | 2.43 ± 0.52c | 0.46 ± 0.04a | 1.07 ± 0.22b | Nd |
| 25 | 33.75 | Unknown 6 | 292 | — | 612.262 | Nd | 1.04 ± 0.00a | Nd | Nd | Nd |
| 26 | 35.05 | Cannabisin M | 288; 322 | 595.2159 | 595.2155 | Nd | 3.35 ± 0.11c | 1.06 ± 0.04a | 1.95 ± 0.31b | Nd |
| 27 | 35.24 | 3,3′-Demethyl-heliotropamide | 285; 310 | 595.2159 | 595.2160 | Nd | 4.01 ± 0.63b | 0.17 ± 0.03a | 3.19 ± 0.14b | Nd |
| 28 | 35.55 | Unnamed lignanamide | 230–312 | — | 589.2661 | Nd | 0.51 ± 0.01b | Nd | 0.25 ± 0.05a | Nd |
| 29 | 35.72 | Unknown 7 | 284 | — | 591.539 | Nd | 0.96 ± 0.02a | Nd | Tr | Nd |
| 30 | 36.05 | Cannabisin Q | 284; 308 | 595.2159 | 595.2150 | Nd | 0.96 ± 0.08a | 1.31 ± 0.02b | 1.39 ± 0.08b | Nd |
| 31 | 36.72 | Cannabisin F | 288; 312 | 623.2472 | 623.2470 | Nd | 1.03 ± 0.08c | 0.20 ± 0.02a | 0.85 ± 0.01b | Nd |
| 32 | 37.06 | Isocannabisin N | 284; 324 | 609.2315 | 609.2306 | Nd | 0.47 ± 0.00b | 0.26 ± 0.03a | 1.88 ± 0.01c | Nd |
| 33 | 37.65 | Grossamide | 250; 288; 320 | 623.2472 | 623.2481 | Nd | 8.74 ± 0.78c | 0.22 ± 0.01a | 2.47 ± 0.10b | Nd |
| 34 | 37.93 | Cannabisin G | 290; 312 | 623.2477 | 623.2475 | Nd | 0.37 ± 0.33a | Nd | 0.66 ± 0.02b | Nd |
| 35 | 38.11 | Cannabisin O | 288; 312 | 934.3629 | 934.3635 | Nd | 2.51 ± 0.38b | Tr | 0.17 ± 0.01a | Nd |
| 36 | 48.31 | Unknown 8 | 278 | — | 652.354 | 0.61 ± 0.03b | 0.64 ± 0.06b | 0.28 ± 0.02a | 2.45 ± 0.08c | 0.58 ± 0.05b |
| 37 | 48.83 | Sinapic acid | 276 | 223.0690 | 223.0682 | 1.71 ± 0.13b | 1.14 ± 0.16a | 2.36 ± 0.12c | 3.20 ± 0.31d | 1.94 ± 0.23b |
| Hydroxybenzoic acid (HBA) | Nd | 14.23 ± 0.70d | 2.98 ± 0.15b | 9.62 ± 0.35c | 0.32 ± 0.02a | |||||
| Hydroxycinnamic acid (HCA) | 1.71 ± 0.13a | 1.76 ± 0.17a | 2.54 ± 0.10b | 5.96 ± 0.35c | 1.97 ± 0.23a | |||||
| Phenolic acids | 1.71 ± 0.13a | 15.99 ± 0.53d | 5.52 ± 0.05c | 15.58 ± 0.51d | 2.29 ± 0.24b | |||||
| Hydroxycinnamic acid amide (HCAA) | Nd | 28.63 ± 0.89c | 8.79 ± 0.22a | 18.64 ± 1.17b | Nd | |||||
| Lignanamides | Nd | 53.55 ± 0.95c | 15.53 ± 0.10a | 32.55 ± 1.12b | Nd | |||||
| Phenylpropanoids | Nd | 82.18 ± 1.84c | 24.32 ± 0.33a | 51.19 ± 2.85b | Nd | |||||
| Total phenolic compounds | 2.32 ± 0.10a | 131.29 ±4.62e | 27.01 ± 0.59c | 71.92 ± 3.91d | 2.87 ± 0.21b | |||||
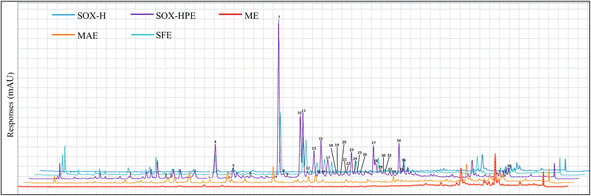 | ||
| Fig. 2 HPLC-DAD chromatograms recorded at 280 nm correspond to hemp seed oil extracted using different methods, SOX-H: Soxhlet extraction with n-hexane; SOX-HPE: Soxhlet extraction with n-hexane, 2-propanol and ethyl acetate (40%, 40%, 20%); MAE: microwave assisted extraction (800 W, 13.6 min and 7.5% EtOH); SFE: supercritical fluid extraction (20 MPa, 50 °C, 244 min, with a mixture of 90% CO2 + 10% ethanol); ME: mechanical extraction (pressing at 100 °C). The names of the peaks correspond to the phenolic compounds in Table 1. | ||
The quantification results (Table 1) revealed a significant abundance of phenylpropanoids, a phenolic subgroup comprising HCAAs and lignanamides, in the extracted oils. The oils obtained by SOX-HPE had total HCAA and lignanamide contents of 28.63 and 53.55 mg kg−1 oil, respectively, the highest of all the methods studied, representing 62% of the phenolic compounds identified. These results are in line with previous studies on hemp seeds, which also reported a predominance of phenylpropanoids,26,27,39 confirming the efficient transfer of these compounds from seeds to oil.
Among the HCAAs, N-trans-caffeoyltyramine stood out as the most abundant compound, with concentrations of 39.98 mg kg−1 in oil extracted by SOX-HPE, 14.92 mg kg−1 by SFE and 4.76 mg kg−1 by MAE. In addition, N-feruloyltyramine and N-trans-caffeoyltyramine isomer were also detected in significant quantities, reaching 10.50 and 3.92 mg CTE kg−1 respectively, in the SOX-HPE oil. For lignanamides, cannabisins A and B were the most widespread, with maximum concentrations in SOX-HPE (7.98 and 10.79 mg CTE kg−1), followed by SFE (3.79 and 7.63 mg CTE kg−1) and MAE (1.73 and 2.43 mg CTE kg−1). These results corroborate observations in the literature, where these compounds dominate the phenolic profiles of hemp seeds.26,27,39 Other lignanamides, such as grossamide, demethylgrossamide, 3,3′-demethylheliotropamide, and cannabisin M, were particularly abundant in SOX-HPE oil, whereas compounds such as Tri-p-coumaroylspermidine and cannabisins C, D, G and O were present in low quantities, or even absent, in oils extracted by SFE and MAE.
In terms of phenolic acids, the oil extracted by SOX-HPE presented the highest total content of hydroxybenzoic acids (HBAs), reaching 14.23 mg kg−1. On the other hand, the oil obtained by SFE showed the highest concentration of hydroxycinnamic acids (HCAs), totaling 5.96 mg kg−1. Among the phenolic acids identified, benzoic acid was the most abundant, with concentrations of 10.10 mg kg−1 and 8.69 mg kg−1 in the SOX-HPE and SFE oils, respectively. p-Hydroxybenzoic acid was particularly enriched in the oil extracted by MAE (2.70 mg kg−1), whereas sinapic acid was detected in all oils, with the highest level observed in the SFE oil (3.20 mg kg−1).
Overall, the total phenolic compounds quantified by HPLC-DAD in the oil extracted by SOX-HPE reached 131.29 mg kg−1, which was significantly higher than those obtained with SFE (71.92 mg kg−1), MAE (27.01 mg kg−1), and the negligible amounts observed for ME and SOX-H (2.87 and 2.32 mg kg−1, respectively). However, the TPC values obtained via the Folin–Ciocalteu method showed a different trend, with a higher content for SFE oil (294.15 mg GAE kg−1) than for SOX-HPE (226.43 mg GAE kg−1). This discrepancy can be attributed to the limited specificity of the Folin–Ciocalteu reagent, which also reacts with nonphenolic compounds such as chlorophylls.40,41 Consequently, the Folin–Ciocalteu results expressed in mg GAE kg−1 may overestimate the phenolic content.
3.3. Effect of extraction methods on the tocopherol composition of hemp seed oil
In hemp seed oil, tocopherols are the dominant antioxidant components that can stabilize the oil against oxidation. Table 2 shows the total tocopherol content of the oils extracted via the different methods. The tocopherol content of the oils is clearly affected by the extraction method. The total tocopherol content ranged from 408.98 mg kg−1 oil for oil extracted by SOX-H to 510.66 mg kg−1 for oil extracted by MAE. γ-Tocopherol was the predominant isomer in all hemp seed oils, irrespective of the extraction method, with a maximum value of 435.13 mg kg−1 observed in oil extracted via MAE. These results concur with those reported by Farinon et al., who reported that the tocopherol content of hemp seed oil ranged from 143 to over 900 mg kg−1, mainly in the form of γ-tocopherol.4| Parameters | SOX-H | SOX-HPE | MAE | SFE | ME |
|---|---|---|---|---|---|
| a Mean ± SD of three independent experiments (n = 3). Different letters (a, b, c, d, e) indicate significant differences (p < 0.05) within rows. SOX-H: Soxhlet extraction with n-hexane; SOX-HPE: Soxhlet extraction with n-hexane, 2-propanol and ethyl acetate (40%, 40%, 20%); MAE: microwave assisted extraction (800 W, 13.6 min and 7.5% EtOH); SFE: supercritical fluid extraction (20 MPa, 50 °C, 244 min, with a mixture of 90% CO2 + 10% ethanol); ME: mechanical extraction (pressing at 100 °C). | |||||
| Tocopherols (mg kg −1 oil) | |||||
| γ-Tocopherol | 377.74 ± 11.03a | 418.46 ± 19.41 ab | 435.13 ± 12.49b | 412.61 ± 28.5 ab | 402.80 ± 27.60 ab |
| α-Tocopherol | 21.66 ± 0.93a | 31.18 ± 1.19b | 33.12 ± 1.72b | 39.68 ± 2.36c | 34.28 ± 1.04bc |
| δ-Tocopherol | 9.57 ± 0.94a | 8.97 ± 0.56a | 42.41 ± 1.07c | 30.25 ± 1.67b | 29.17 ± 0.16b |
| Total tocopherols | 408.98 ± 11.02a | 458.61 ± 20.44 ab | 510.66 ± 14.97b | 482.54 ± 32.52b | 466.25 ± 27.69 ab |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Pigments (mg kg −1 oil) | |||||
| Total carotenoid | 1.78 ± 0.03a | 19.92 ± 1.39d | 27.31 ± 0.83e | 14.66 ± 0.17c | 12.96 ± 0.04b |
| Total chlorophyll | 8.03 ± 0.50a | 66.59 ± 4.50b | 99.68 ± 0.73d | 61.03 ± 0.85b | 76.15 ± 0.13c |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Color | |||||
| L* | 36.21 ± 0.09b | 33.33 ± 0.10a | 33.54 ± 0.63a | 34.97 ± 0.45 ab | 35.73 ± 0.66 ab |
| a* | −1.25 ± 0.16a | −4.28 ± 0.26b | −4.01 ± 0.23b | −3.96 ± 0.16b | −6.96 ± 0.11c |
| b* | 8.39 ± 0.14c | 2.80 ± 0.14a | 3.17 ± 0.08a | 3.75 ± 0.21b | 3.44 ± 0.06d |
On the other hand, statistical analysis revealed a notable difference between methods, particularly for the δ-tocopherol isomer. The lowest content was observed in oils extracted by SOX-HPE and SOX-H (8.97 and 9.57 mg kg−1, respectively), whereas the maximum value of 42.41 mg kg−1 was obtained with MAE. For the α-tocopherol isomer, the oil extracted by SFE had the highest content, reaching 39.68 mg kg−1.
MAE and SFE extraction methods demonstrate superior efficiency for tocopherol recovery compared to SOX-H and ME techniques. This enhanced performance can be explained by the specific mechanisms underlying each process. MAE uses electromagnetic radiation to induce rapid disruption of cell walls, thereby increasing the exposure of intracellular compounds to the solvent. Combined with its short extraction duration (7.5 min), this approach greatly limits the thermal degradation of tocopherols. This efficiency is supported by the findings of Rezvankhah et al.,15 who reported a tocopherol content of 929.67 mg kg−1 for MAE compared to 832.61 mg kg−1 for the SOX-H method.
SFE, on the other hand, exploits the unique properties of supercritical CO2, particularly when a co-solvent such as ethanol is added to enhance the solubilization of tocopherols. Its operation in a closed, oxygen-free system under moderate temperatures creates a protective environment against oxidative degradation, thereby preserving the integrity of thermolabile compounds. A study by Aladić et al.13 demonstrated that SFE can yield tocopherol contents two to three times higher than those obtained by cold pressing or SOX-H extraction.
In contrast, conventional methods exhibit significant limitations. The SOX-H technique, with its prolonged extraction time (5 hours), and the ME method, involving high temperatures (100 °C), lead to greater tocopherol degradation, as reported by Rezvankhah et al.15 These harsh conditions accelerate the decomposition of these sensitive molecules, thus reducing their concentration in the final oil.
Moreover, the superior efficiency observed for MAE and SFE can also be attributed to the use of ethanol, which plays a crucial role in the extraction process. This polar solvent deeply disrupts the architecture of cell walls and membranes by acting on macromolecular structures and breaking the bonds that stabilize interactions between bioactive compounds and the cellular matrix.30,42 Similar effects have been reported in oilseeds and cereals, where ethanol and isopropanol disrupt hydrogen bonds and hydrophobic interactions within polysaccharide–protein matrices, thereby promoting the release of lipids and tocopherols.36,37
3.4. Effect of extraction methods on the pigment content and color of hemp seed oil
Pigments, notably chlorophylls and carotenoids, were analyzed in hemp seed oils extracted via different methods using a spectrophotometer. The absorbances were measured at 640 and 663 nm to determine the total chlorophyll content and at 470 nm to determine the total carotenoid content. As shown in Table 2, the total chlorophyll and carotenoid contents of the oil extracted by SOX-H were significantly lowest at 8.03 and 1.78 mg kg−1 oil, respectively, whereas the highest contents at 99.68 and 27.31 mg kg−1, respectively, were observed in the oil extracted by MAE.Methods using mixtures of polar and nonpolar solvents, such as SOX-HPE, MAE, and SFE, presented the highest pigment concentrations, suggesting that these combinations offer favorable conditions for extraction. Although pigments are predominantly apolar, several studies have confirmed the efficiency of moderately polar mixtures, such as n-hexane with 2-propanol or ethanol, to extract chlorophylls and carotenoids from hemp seeds or other plant matrices.17,43 This efficiency is often attributed to the ability of mixed solvents to penetrate plant cells, enabling optimal extraction of their contents.
The oil extracted by MAE had the highest pigment content compared with that extracted by SOX-HPE and SFE. This is could be due to the combined effect of solvent mixtures (hexane–ethanol) and the use of microwaves, which promote cell wall rupture and thereby increase pigment release. Furthermore, even without the use of solvents, the oil obtained by ME presented significant levels of chlorophylls (76.15 mg kg−1), which were higher than those extracted by SOX-H, SOX-HPE, and SFE.
The high chlorophyll content observed in the oil obtained by ME can be attributed to the direct release of these lipophilic pigments, mainly localized and freely available in the seed coat. Unlike tocopherols and phenolic compounds, which are often bound to macromolecules such as proteins or polysaccharides and require the use of solvents to break these bonds, chlorophylls are present in the chloroplasts of the green outer layers of the seed and require no solvents for extraction.44–46
Mechanical pressure, combined with temperatures of up to 100 °C, causes structural disruption of the cells and degradation of the chloroplasts, resulting in the direct transfer and accumulation of easily mobilized chlorophylls in the oil phase, a phenomenon amplified by their lipophilic nature. These observations are consistent with data in the literature: a comparative study by Aladić et al. showed that hemp seed oils obtained by ME had the highest chlorophyll concentrations, followed by those extracted by SFE and SOX-H.13
Colorimetric parameters obtained by CIELAB analyses performed on hemp seed oils extracted via different methods, expressed as L* (lightness), a* (green–red), and b* (blue–yellow), are presented in Table 2. Overall, all samples fall within the second quadrant, characterized by negative values of a* (indicating a green hue) and positive values of b* (indicating a yellow hue).
Among the different methods, the oil extracted by SOX-H presented the highest values of L* (36.21) and b* (8.39), reflecting a lighter and slightly more yellow oil. In contrast, the oil extracted by SOX-HPE presented the lowest values for L* (33.33) and b* (2.80), indicating a darker, less yellow hue. For a*, the oil extracted by SOX-H presented the least negative value (−1.25), indicating a less green hue, whereas the oil extracted by ME presented the most negative value (−6.96), corresponding to a strongly green hue.
In the absence of official colorimetric standards for unrefined oils such as hemp seed oil, the values measured using the CIELAB system were interpreted by comparison with data reported in the scientific literature. Our results fall within the typical ranges generally observed for cold-pressed and commercial hemp seed oils, the most common extraction approach for premium-quality products, with L* values between 25 and 54, a* between −1 and 12, and b* between 1 and 88.47,48 These intervals reflect the natural variability associated with the extraction method, pigment content of the seeds, and genetic and agronomic factors.
These differences can be mainly attributed to the chlorophyll content, the primary pigment responsible for the green hue of hemp seed oil. Moyano et al. also reported a strong positive correlation between pigment concentration and a* values, as well as a negative correlation with L* and b* in virgin olive oils.49 Moreover, a comparative study by Mansouri et al.,50 on oils extracted from roasted and unroasted hemp seeds showed that the increase in b* value in roasted seed oils could be linked to a decrease in chlorophyll content, compensated by a higher concentration of carotenoids. These findings confirm the major influence of pigments on the colorimetric properties of vegetable oils.
Thus, color variations are not merely analytical observations but also key indicators guiding the selection of oils for specific applications. Oils exhibiting negative a* values with low L*, obtained through SOX-HPE, MAE, SFE, and ME, are particularly suitable for cosmetic and nutraceutical uses due to their rich profile in bioactive compounds. In contrast, lighter-colored oils, characterized by higher L* and lower a* values such as those produced by the conventional SOX-H method are more appropriate for culinary purposes, offering a neutral flavor, moderate thermal stability, and a visual appearance perceived by consumers as a sign of quality and purity.
3.5. Effect of extraction methods on the fatty acid composition of hemp seed oil
The fatty acid composition of hemp seed oil is presented in Table 3. Regardless of the extraction method used, the fatty acid profile of the oils remains broadly similar, with ten fatty acids identified.| Parameters | SOX-H | SOX-HPE | MAE | SFE | ME |
|---|---|---|---|---|---|
| a Mean ± SD of three independent experiments (n = 3). Different letters (a, b, c, d) indicate significant differences (p < 0.05) within rows. SOX-H: Soxhlet extraction with n-hexane; SOX-HPE: Soxhlet extraction with n-hexane, 2-propanol and ethyl acetate (40%, 40%, 20%); MAE: microwave assisted extraction (800 W, 13.6 min and 7.5% EtOH); SFE: supercritical fluid extraction (20 MPa, 50 °C, 244 min, with a mixture of 90% CO2 + 10% ethanol); ME: mechanical extraction (pressing at 100 °C). | |||||
| Fatty acids (%) | |||||
| Palmitic acid (C16:0) | 10.43 ± 0.59a | 10.05 ± 1.34a | 10.30 ± 1.24a | 6.39 ± 0.46b | 10.06 ± 0.59a |
| Stearic acid (C18:0) | 3.23 ± 0.55a | 2.93 ± 0.21a | 3.09 ± 0.50a | 1.26 ± 0.01b | 2.80 ± 0.11a |
| Oleic acid (C18:1) | 18.50 ± 0.66a | 18.69 ± 0.58a | 18.23 ± 1.33a | 19.12 ± 1.44a | 18.76 ± 0.28a |
| Linoleic acid (C18:2n6) | 51.32 ± 0.33a | 51.13 ± 0.49a | 51.04 ± 1.54a | 50.76 ± 0.71a | 51.27 ± 0.27a |
| γ-Linolenic acid (C18:3n6) | 1.02 ± 0.01a | 1.06 ± 0.03a | 0.98 ± 0.02a | 0.95 ± 0.10a | 1.02 ± 0.01a |
| α-Linolenic acid (C18:3n3) | 14.52 ± 0.54a | 14.70 ± 0.44a | 15.09 ± 0.62a | 17.17 ± 0.34b | 14.87 ± 0.20a |
| Arachidic acid (C20:0) | 0.39 ± 0.01a | 0.69 ± 0.05b | 0.62 ± 0.20b | 1.16 ± 0.09c | 0.63 ± 0.05b |
| Eicosenoic acid (C20:1) | 0.16 ± 0.03a | 0.22 ± 0.02a | 0.18 ± 0.01a | 2.03 ± 0.17b | 0.18 ± 0.02a |
| Behenic acid (C22:0) | 0.28 ± 0.03a | 0.39 ± 0.08a | 0.34 ± 0.08a | 0.86 ± 0.07b | 0.27 ± 0.04a |
| Lignoceric acid (C24:0) | 0.15 ± 0.01a | 0.14 ± 0.05a | 0.13 ± 0.09a | 0.30 ± 0.00b | 0.14 ± 0.01a |
| SFA | 14.48 ± 0.18a | 14.20 ± 0.70a | 14.48 ± 0.36a | 9.97 ± 0.69b | 13.90 ± 0.79a |
| MUFA | 18.66 ± 0.67a | 18.91 ± 0.60a | 18.41 ± 1.34a | 21.15 ± 0.57b | 18.94 ± 0.26b |
| PUFA | 66.86 ± 0.87 ab | 66.89 ± 0.38 ab | 67.11 ± 0.50a | 68.88 ± 0.67b | 67.16 ± 0.48 ab |
| UFA | 85.52 ± 0.80a | 85.80 ± 0.98a | 85.52 ± 0.84a | 90.03 ± 0.60b | 86.10 ± 0.74a |
| n-6 | 52.34 ± 0.32a | 52.19 ± 0.46a | 52.02 ± 1.52a | 51.71 ± 0.81a | 52.29 ± 0.28a |
| n-3 | 14.52 ± 0.54a | 14.70 ± 0.84a | 15.09 ± 1.02a | 17.17 ± 0.14b | 14.87 ± 0.20a |
| n-6/n-3 | 3.60 ± 0.11a | 3.55 ± 0.24a | 3.45 ± 0.37a | 3.01 ± 0.07b | 3.52 ± 0.13a |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Oil quality indices | |||||
| Conjugated dienes (K232) (CD) | 1.96 ± 0.15b | 2.06 ± 0.12b | 2.24 ± 0.16b | 1.52 ± 0.19a | 1.77 ± 0.12 ab |
| Conjugated trienes (K270) (CT) | 0.55 ± 0.02 ab | 0.58 ± 0.02b | 0.51 ± 0.01a | 0.48 ± 0.08a | 0.40 ± 0.06a |
| Peroxide value (meq O2 per kg oil) | 20.80 ± 2.77d | 12.56 ± 1.06c | 7.68 ± 0.34b | 4.67 ± 0.06a | 8.30 ± 0.47b |
| Acid value (mg KOH per g oil) | 3.91 ± 0.05c | 3.09 ± 0.06b | 0.26 ± 0.02a | 0.22 ± 0.02a | 0.18 ± 0.01a |
Polyunsaturated fatty acids (PUFAs) are predominant in all the oil samples, accounting for 66.86% to 68.88%, mainly in the form of linoleic (50.76–51.32%), α-linolenic (14.52–17.17%), and γ-linolenic acids (0.95–1.06%). Monounsaturated fatty acids (MUFAs) followed, with proportions ranging from 18.41% to 21.15%, and were dominated by oleic (18.23–19.12%) and eicosenoic acids (0.16–2.03%). Finally, saturated fatty acids (SFAs) accounted 9.97% to 14.48% of the total fatty acid content and were mainly composed of palmitic (6.39–10.43%), stearic (1.26–3.23%), behenic (0.28–0.86%), and lignoceric acids (0.13–0.30%). The proportions of omega-6 and omega-3 individuals vary from 51.71% to 52.34% and from 14.52% to 17.17%, respectively, with an optimal, well-balanced omega-6/omega-3 ratio of 3.01 to 3.60.
Statistical analysis revealed no significant differences (p > 0.05) in fatty acid composition between oils extracted by SOX-H, SOX-HPE, MAE and ME. However, the oil extracted by SFE was distinguished by significantly higher proportions of α-linolenic, γ-linolenic, eicosenoic, behenic and lignoceric acids, as well as lower proportions of palmitic and stearic acids. This composition resulted in an oil that was particularly rich in UFAs (90.03%) and low in SFAs (9.97%) compared with other oils.
These results were consistent with the data available in the literature. Devi & Khanam compared five extraction methods (SOX, SFE, PER, ULT, and PYR),14 whereas Aladić et al. analyzed the differences among SFE, SOX-H and cold pressing.13 Similarly, Rezvankhah et al. compared SOX-H and MAE.15 These studies show that there is no significant difference, or only minor deviations, in fatty acid composition depending on the extraction method used. On the other hand, some research has reported higher proportions of linoleic acid (up to 59%), α-linolenic acid (up to 18%) and γ-linolenic acid (up to 3%), and lower proportions of oleic acid (∼10%). These findings suggest that the oil's fatty acid composition is primarily influenced by genotype and climatic conditions, as confirmed by several studies that have shown that the fatty acid composition of hemp oils is strongly influenced by these factors.1,2
3.6. Effect of extraction methods on the quality indices and oxidative stability of hemp seed oil
The quality indices peroxide value (PV), acidity value (AV) and specific extinction coefficients (conjugated dienes (CD) and trienes (CT)) were used to compare the oils extracted via the different methods. The results of these indices are shown in Table 3.The oil extracted by SFE was the best quality, with the lowest values (p < 0.05), a PV of 4.67 meq O2 kg−1, an AV of 0.26 mg KOH g−1, a CD of 1.52 and a CT of 0.48, followed by oils obtained by MAE and ME, which had respective values for PV (7.68 and 8.30 meq O2 kg−1), AV (0.26 and 0.18 mg KOH g−1), CD (2.24 and 1.77) and CT (0.51 and 0.40). In contrast, oils obtained via the Soxhlet method (SOX-H and SOX-HPE) presented the highest values (p < 0.05), PV (20.80 and 12.56 meq O2 kg−1), AV (3.91 and 3.09 mg KOH g−1), CD (1.96 and 2.06) and CT (0.55 and 0.58).
Despite these variations, almost all the oils extracted by the various methods comply with the Codex Alimentarius recommendations for crude vegetable oils,51 which set a maximum AV of 4.0 mg KOH g−1 and a maximum PV of 15 meq O2 kg−1. Only SOX-H-extracted oil exceeds these limits, with a PV of 20.80 meq O2 kg−1.
The high values of quality indices observed in hemp seed oil obtained by SOX-H could be attributed to the high temperature and long extraction time characteristic of this method. Several studies have confirmed these observations, showing that oil extracted by SOX-H exhibited higher values of PV, AV, CD and CT than those obtained by methods such as MAE, SFE or ultrasonic-assisted extraction.15,52,53 In contrast, the combination of solvents such as n-hexane, 2-propanol and ethyl acetate in the SOX-HPE method reduced fatty acid oxidation. This can be explained by the extraction of phenolic compounds that neutralize free radicals, thus limiting the formation of peroxides and hydroperoxides.20
The oil extracted by SFE was distinguished by its higher quality owing to moderate extraction temperatures (50 °C) and the use of supercritical CO2. The latter creates an oxygen-free environment, minimizing the degradation of fatty acids into peroxides and hydroperoxides. These results were consistent with those reported by Aiello et al.,18 who reported that hemp seed oil extracted via SFE had significantly lower PV (5.50 meq O2 kg−1), CD (3.81) and CT (1.20) values than oil extracted via SOX-H (PV 19.50 meq O2 kg−1, CD 6.32 and CT 1.25). In addition, Liu et al. demonstrated that pomegranate seed oil extracted via a subcritical extraction method with n-butane presented lower PV and AV values than oils obtained via MAE, UAE or shaking extraction with n-hexane and ether petroleum.54
In addition to oil quality indices, the Rancimat accelerated oxidation test was used to evaluate and compare the oxidative stability of hemp seed oils extracted via different methods. This technique is based on the measurement of the induction time, which reflects the resistance of lipids to oxidation when exposed to high temperature (100 °C) and constant air flow (20 L h−1).
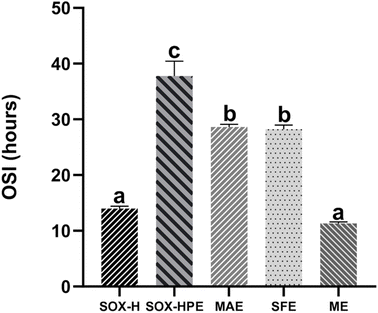
The induction time results in hours (h), presented in Fig. 3, show that the oil extracted by SOX-HPE has the best oxidative stability, with an induction time of 37.77 h, followed by oils extracted by MAE (28.60 h) and SFE (28.01 h). On the other hand, the lowest induction times were observed for oils extracted by SOX-H (13.97 h) and ME (11.70 h). Statistical analysis revealed no significant differences (p > 0.05) between the induction times of oils extracted by MAE and SFE or between those obtained by SOX-H and ME.
The oil extracted by ME was the most sensitive to oxidation, which could be explained by the high temperature reached during extraction by the screw press (100 °C). The oil extracted by SOX-H was also highly sensitive to oxidation. This could be explained by the high SOX-H temperature and the long duration of the process.15 In contrast, the oil extracted using the SOX-HPE method exhibited oxidative stability more than three times greater than that of SOX-H oil. This shows the effect of the extraction solvent, where the mixture of n-hexane, 2-propanol and ethyl acetate extracted a large quantity of phenolic compounds (Table 1), which are very strong antioxidants that play a protective role against the formation of free radicals and the degradation of fatty acids.4 In addition, the use of a polar co-solvent such as ethanol with n-hexane in MAE or with supercritical CO2 in SFE enabled the extraction of high quantities of phenolic compounds (Table 1), which slowed the oxidation rate and increased the induction time by more than 2-fold compared with oils extracted by SOX-H and ME. However, SFE resulted in higher phenolic compound contents (70.84 mg kg−1) than did MAE (28.09 mg kg−1) (Table 1), but the same induction time was used. This can be explained by the high unsaturated fatty acid content of the oil extracted by SFE (Table 3). The degree of fatty acid unsaturation is the main factor affecting oil stability. The richer oils are in unsaturated fatty acids, the more sensitive they are to oxidation.4
3.7. Principal component analysis
PCA was performed to visualize and interpret the variability of oils extracted via different methods in terms of yield, composition, and quality.Before performing the PCA, pre-suitability tests were conducted to verify the statistical validity of the data. The analysis was conducted on the following variables: oil yield, total tocopherols, γ-tocopherol, total chlorophylls, total carotenoids, peroxide value, acidity value, oxidative stability index, phenolic acids, hydroxycinnamic acid amides, lignanamides, phenylpropanoids, and total phenolic compounds. The KMO index gave a score of 0.621, indicating a satisfactory partial correlation between the variables and therefore a good fit of the sample to PCA. In addition, Bartlett's sphericity test was highly significant (p < 0.001), confirming that the correlation matrix is not an identity matrix, which validates the existence of significant relationships between the variables.
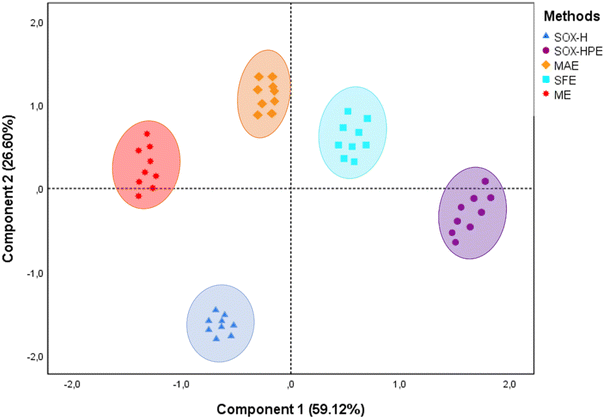
The PCA showed that the first two principal components (PC1 and PC2) together explained 85.72% of the total variance, with 59.12% for PC1 and 26.60% for PC2, justifying their use for data interpretation. The correlation circle analysis (Fig. 4) provides a more detailed understanding of the contributions of the variables. Total tocopherols, γ-tocopherol, total chlorophylls, total carotenoids, OSI, phenolic acids, hydroxycinnamic acid amides, lignanamides, phenylpropanoids, and total phenolics are oriented in a similar direction (positive PC1 and PC2), indicating that they are strongly correlated with each other. This positive association shows that methods favoring the extraction of one group of antioxidant compounds tend to enrich the others as well. Their strong projection onto the circle attests to their major contribution to the structuring of the data. In addition, there is a strong positive and significant correlation (p < 0.05) between these variables (SI Table S1), indicating that methods favoring one of these groups of compounds tend to favor the others as well, probably due to similar extraction mechanisms (interaction with plant membranes, solvent/compound affinity, etc.).
In contrast, the PV and AV variables are orthogonal to those of the bioactive compounds, suggesting that the richer the oil is in antioxidants, the less it is oxidized. This was confirmed by the strong significant positive correlation between these different bioactive compounds and the OSI. This relationship is consistent with the well-established protective role of polyphenols and tocopherols against oxidation.
The oil yield variable is projected intermediately. It is positively oriented on PC2 but negatively oriented on PC1. According to the correlation matrix (SI Table S1), oil yield is positively and significantly correlated (p < 0.05) with phenolic compounds such as HCAAs, lignanamides, and phenylpropanoids. These findings indicate that certain methods simultaneously improve the extraction of oil and these compounds, suggesting better extractability of phenolic compounds in the presence of high oil yield.
The projection of the samples on the factorial plane (Fig. 5) reveals a clear separation between the different extraction methods, illustrating the diversity of physicochemical and bioactive profiles of the oils obtained. The oils extracted by SOX-H are clearly grouped in the negative quadrants of PC1 and PC2. This position is strongly correlated with the PV and, to a lesser extent, with the oil yield and AV, reflecting quantitatively efficient extraction, but it is also associated with high levels of primary and secondary oxidation. This profile suggests that while this method maximizes lipid recovery, it compromises the oxidative stability and functional quality of the oil, as indicated by the distance of these samples from the vectors associated with bioactive compounds.
Conversely, oils extracted by SOX-HPE appear to be grouped in a quadrant characterized by high yields, phenolic compounds, and OSI. This grouping indicates that this method, which combines prolonged extraction and the use of polar solvents, promotes not only high oil extractability but also significant enrichment of antioxidant compounds. These compounds, particularly phenolic compounds, directly contribute to improving the resistance of the oil to oxidation, as evidenced by their correlation with the OSI. The position of these samples on the factorial plane therefore highlights a dual performance: a very high yield and a high content of functional compounds.
The oil obtained by SFE is positioned in the positive quadrant of PC1 and PC2, in association with tocopherols, pigments (chlorophylls, carotenoids), and low AV and PV. This distribution reflects a gentle extraction method, limiting oxidation phenomena while allowing good preservation of sensitive compounds. Although the yield is moderate, the nutritional quality and stability of the oil obtained by SFE make it a particularly interesting technological alternative.
The samples obtained via MAE occupy an intermediate position on the factorial plane, with a more marked contribution from PC2. This location reflects a high extraction efficiency of tocopherols and pigments while revealing a lower content of phenolic compounds. This positioning reflects a targeted extraction capacity for certain compounds while revealing certain limitations in terms of yield and extractability.
Finally, oils extracted by ME are at the opposite end of the spectrum of the most effective methods. Their distance from vectors associated with bioactive compounds, yield, and oxidative stability reflect their limited overall efficiency. These samples are characterized by low concentrations of natural antioxidants, limited yield, and increased vulnerability to oxidation.
3.8. SEM analysis of the seed powder surfaces
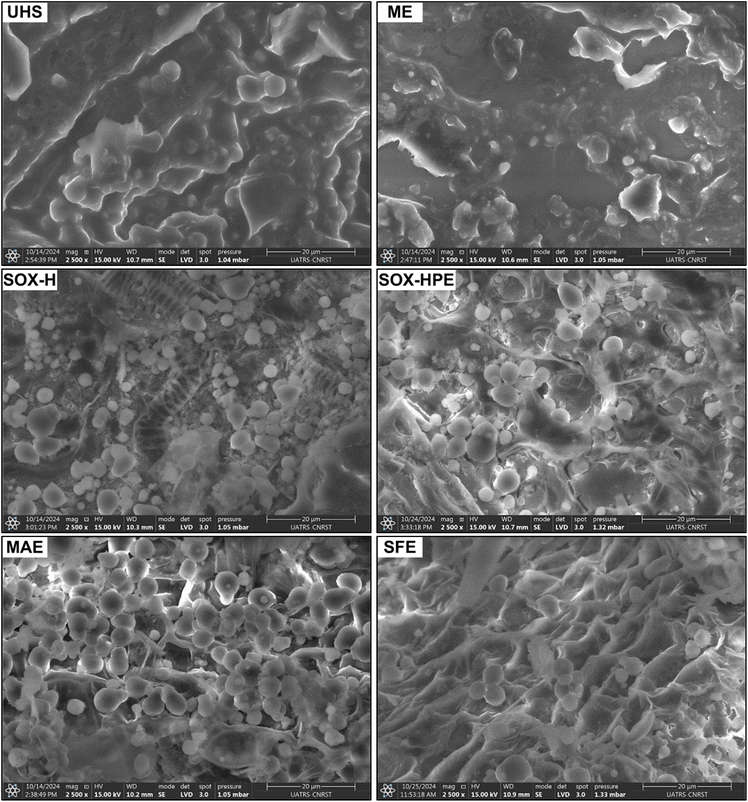
SEM analysis revealed clear morphological differences between untreated hemp seed powder (UHS) and the residues obtained after the various extraction methods (Fig. 6). These micrographs provide valuable insights into how each extraction process affects the microstructure of the seed matrix, thereby influencing oil release and the removal of associated lipid components.The UHS exhibited a compact and granular surface with well-defined spherical structures dispersed within the cellular matrix. These spheres most likely correspond to intact lipid droplets confined within undamaged cells, representing the natural distribution of lipids in hemp seeds prior to extraction.55–57 After ME, the surface appeared smoother, flattened, and densified, with the complete disappearance of the spherical lipid structures observed in the control. This transformation suggests that during pressing at 100 °C, partial fusion and coalescence of the lipid phases occurred under the combined effects of pressure and heat. The dense structure observed indicates residual oil entrapment within the compressed matrix, resulting from limited cell rupture and incomplete lipid release. Similar observations were reported by Gharsallah et al. who found that ground Moringa oleifera seeds exhibited swollen lipid cells prior to extraction, which became defatted and flattened after pressing.56
In contrast, the SOX-H, SOX-HPE, MAE, and SFE methods induced pronounced structural damage, though with distinct morphological characteristics. The surfaces appeared fractured, porous, and exhibited significant loss of structural integrity. These findings are consistent with those of Devi & Khanam,14 who also reported the formation of deep cavities on the surfaces of hemp seeds following extraction by SOX, SFE, and ULT, resulting from complete lipid depletion.
The SOX-H and SOX-HPE powders exhibited extensive matrix fragmentation associated with high porosity and irregular cavities. In the case of SOX-H, the surface appeared highly heterogeneous, composed of lamellar fragments and dispersed cell debris, reflecting deep cell wall disorganization caused by the combined effects of heat and solvent action, which facilitated lipid migration into the solvent phase. Conversely, the SOX-HPE powder displayed more homogeneous porosity with well-defined cavities. The use of a mixture of polar and non-polar solvents appears to promote more complete solubilization of lipido-protein components while enhancing the disintegration of the cellulosic network.58
The powder obtained by MAE exhibited a highly altered morphology, characterized by the formation of deep cavities and marked surface agglomeration. These transformations result from the action of the high-power microwave field (800 W), which causes rapid dehydration of cellulose and internal vaporization of the cellular content. This phenomenon weakens the mechanical strength of the cell walls, leading to their rupture and thereby facilitating the release and extraction of intracellular constituents.59,60
Finally, the powder obtained after SFE extraction showed a highly disrupted structure characterized by broken and porous layers, visibly deflated and oil-free surfaces, and the presence of longitudinal channels and deep fissures across the surface. These alterations reflect the mechanical stress induced by the depressurization of supercritical CO2 on the cellular matrix, demonstrating the efficiency of this process in achieving complete oil removal.61,62
These structural changes, in addition to reflecting the impact of each extraction method, also suggest the breakdown of specific chemical bonds between bioactive compounds and various intracellular constituents. In the case of the SOX-HPE, SFE, and MAE methods, the use of 2-propanol and ethanol plays an essential role in this process. These solvents have the ability to profoundly disrupt the architecture of cell walls and membranes by acting directly on macromolecular structures and causing the breakdown of bonds that stabilize interactions between bioactive compounds and the cellular matrix.30,42 Their action thus promotes the dissociation of protein–phenol complexes, interactions with polysaccharides, and the extraction of bound compounds.63,64
Consequently, the degree of cellular disorganization induced by these solvents, combined with the effects of microwaves, pressure, or heat from the extraction processes, strongly influences not only the oil yield but also the extractability of bioactive compounds.
4. Conclusion
Various extraction methods, including Soxhlet (SOX-H and SOX-HPE), MAE, SFE and ME, were used to extract oil from hemp seeds. Among these methods, SOX-HPE presented the highest oil yield, reaching 33.24%. This efficiency was confirmed by SEM observation, which revealed a more complete extraction of lipid components.In terms of composition, the oil obtained by MAE was the richest in tocopherols and pigments (chlorophylls and carotenoids), resulting in a characteristic green color with low lightness, low yellowness, and high greenness. On the other hand, the SOX-HPE and SFE methods stood out for their efficiency in extracting phenolic compounds. These oils were particularly rich in N-trans-caffeoyltyramine and Cannabisins A and B, phenolic compounds specific to hemp seeds. This high antioxidant content confers increased oxidative stability to the oil, surpassing that of oils obtained by other methods. In terms of fatty acid composition, the oil extracted by SFE was particularly rich in PUFAs, notably α-linolenic acid, and presented the lowest oil quality indices, indicating superior quality.
Author contributions
Aymane Allay: experiment, formal analysis, investigation, software, writing – original draft. Rafika El Ati: formal analysis, validation. Youssef Rbah: investigation, data curation. Marie-Laure Fauconnier: project administration, visualization. Hana Serghini Caid: validation, supervision. Ahmed Elamrani: funding acquisition, project administration, visualization. Farid Mansouri: conceptualization, methodology, writing – review & editing, supervision.Conflicts of interest
There are no conflicts to declare.Data availability
The data that support this study's findings are available from the corresponding author upon request.Supplementary information: MS2 fragmentation spectra of the phenolic compounds identified in hemp (Cannabis sativa L.) seed oil, obtained by different methods as well as a Pearson correlation matrix between the main composition and quality variables of hemp seed oils obtained by different extraction methods. See DOI: https://doi.org/10.1039/d5fb00424a.
Acknowledgements
The authors gratefully acknowledge the support of the Moroccan Ministry of Higher Education, Scientific Research and Innovation through the VPMA2/ref.2020/1 project. This work also benefited from the support of the Chair “Sustainable Energy” led by Mohammed VI Polytechnic University, sponsored by OCP.References
- Y. Taaifi, A. Benmoumen, K. Belhaj, S. Aazza, M. Abid and E. Azeroual, et al., Seed composition of non-industrial hemp (Cannabis sativa L.) varieties from four regions in northern Morocco, Int. J. Food Sci. Technol., 2021, 56, 1–2 CrossRef.
- M. Irakli, E. Tsaliki, A. Kalivas, F. Kleisiaris and E. Sarrou, et al., Effect of Genotype and Growing Year on the Nutritional, Phytochemical, and Antioxidant Properties of Industrial Hemp (Cannabis sativa L.) Seeds, Antioxidants, 2019, 8, 491 CrossRef CAS PubMed.
- K. Belhaj, F. Mansouri, Y. Rbah, A. Allay, A. Tikent and Y. Taaifi, et al., Carcass and meat quality characteristics of Beni-Guil autochthonous ovine breed: effect of live weight at slaughter, E3S Web Conf., 2022, 337, 04003 CrossRef.
- B. Farinon, R. Molinari, L. Costantini and N. Merendino, et al., The seed of industrial hemp (Cannabis sativa L.): nutritional quality and potential functionality for human health and nutrition, Nutrients, 2020, 12, 1935 CrossRef CAS PubMed.
- J. Nkengurutse, F. Mansouri, O. Bekkouch, A. Ben Moumen, T. Masharabu and G. Gahungu, et al., Chemical composition and oral toxicity assessment of Anisophyllea boehmii kernel oil: potential source of new edible oil with high tocopherol content, Food Chem., 2019, 278, 795–804 CrossRef CAS PubMed.
- Y. Rbah, K. Belhaj, Y. Taaifi, A. Allay, R. Melhaoui and H. S. Caid, et al., Nutritional Composition and Functional Properties of 'Beldiya' Hemp Seed and Oil: A Sustainable Local Resource from Northern Morocco for Health and Nutrition, J. Oleo Sci., 2025, 74, 533–542 CrossRef CAS.
- J. Liang, A. Appukuttan Aachary and U. T. Hollader, et al., Hemp seed oil: minor components and oil quality, Lipid Technol., 2015, 27, 231–233 CrossRef CAS.
- K. Aladić, S. Jokić, T. Moslavac, S. Tomas, S. Vidović and J. Vladić, et al., Cold Pressing and Supercritical CO2 Extraction of Hemp (Cannabis sativa) Seed Oil, Ind. Crops Prod., 2014, 28, 481–490 Search PubMed.
- W. Golimowski, M. Teleszko, D. Marcinkowski, D. Kmiecik, A. Grygier and A. Kwaśnica, et al., Quality of Oil Pressed from Hemp Seed Varieties: ‘Ealina 8FC’, ‘Secueni Jubileu’ and ‘Finola’, Molecules, 2022, 27, 3219 CrossRef.
- C. Occhiuto, G. Aliberto, M. Ingegneri, D. Trombetta, C. Circosta and A. Smeriglio, et al., Comparative Evaluation of the Nutrients, Phytochemicals, and Antioxidant Activity of Two Hempseed Oils and Their Byproducts after Cold Pressing, Molecules, 2022, 27, 3431 CrossRef CAS.
- G. S. Martins, R. G. Cândido, D. H. P. Guimaraes, E. R. Triboni, C. E. C. Rodrigues and F. R. M. Batista, et al., Hemp seed oil: extraction conditions, characterization and density and viscosity temperature profile, Braz. J. Chem. Eng., 2023, 40, 1177–1190 Search PubMed.
- M. D. Kostić, N. M. Joković, O. S. Stamenković, K. M. Rajković, P. S. Milić and V. B. Veljković, et al., Optimization of hempseed oil extraction by n-hexane, Ind. Crops Prod., 2013, 48, 133–143 CrossRef.
- K. Aladić, K. Jarni, T. Barbir, S. Vidović, J. Vladić and M. Bilić, et al., Supercritical CO2 extraction of hemp (Cannabis sativa L.) seed oil, Ind. Crops Prod., 2015, 76, 472–478 CrossRef.
- V. Devi and S. Khanam, et al., Comparative study of different extraction processes for hemp (Cannabis sativa) seed oil considering physical, chemical and industrial-scale economic aspects, J. Cleaner Prod., 2019, 207, 645–657 CrossRef CAS.
- A. Rezvankhah, Z. Emam-Djomeh, M. Safari, G. Askari and M. Salami, et al., Microwave-assisted extraction of hempseed oil: studying and comparing of fatty acid composition, antioxidant activity, physiochemical and thermal properties with Soxhlet extraction, J. Food Sci. Technol., 2019, 56, 4198–4210 CrossRef CAS.
- R. Muangrat and A. Kaikonjanat, et al., Comparative evaluation of hemp seed oil yield and physicochemical properties using supercritical CO2, accelerated hexane, and screw press extraction techniques, J. Agric. Food Res., 2025, 19, 101618 CAS.
- D. R. Soroush, S. Solaimanimehr, M. Azizkhani, R. E. Kenari, B. Dehghan and G. Mohammadi, et al., Optimization of microwave-assisted solvent extraction of hemp (Cannabis sativa L.) seed oil using RSM: evaluation of oil quality, J. Food Meas. Charact., 2021, 15, 5191–5202 Search PubMed.
- A. Aiello, F. Pizzolongo, G. Scognamiglio, A. Romano, P. Masi and R. Romano, et al., Effects of supercritical and liquid carbon dioxide extraction on hemp (Cannabis sativa L.) seed oil, Int. J. Food Sci. Technol., 2020, 55, 2472–2480 CrossRef CAS.
- T. Senphan, N. Mungmueang, C. Sriket, P. Sriket, S. Sinthusamran and P. Narkthewan, et al., Influence of extraction methods and temperature on hemp seed oil stability: a comprehensive quality assessment, Appl. Food Res., 2025, 5, 100702 Search PubMed.
- A. Allay, C. Benkirane, A. Ben Moumen, Y. Rbah, M. L. Fauconnier and H. S. Caid, et al., Enhancing bioactive compound extractability and antioxidant properties in hemp seed oil using a ternary mixture approach of polar and non-polar solvents, Ind. Crops Prod., 2024, 219, 119090 Search PubMed.
- A. Allay, C. Benkirane, A. Ben Moumen, Y. Rbah, M. L. Fauconnier and H. S. Caid, et al., Microwave-Assisted Extraction of Hemp Seed Oil: Process Optimization for Enhancing Oil Yield and Bioactive Compound Extractability, Int. J. Food Sci., 2025, 2025, 7381308 CrossRef CAS PubMed.
- A. Allay, A. Ben Moumen, Y. Rbah, M. L. Fauconnier, J. Nkengurutse and H. S. Caid, et al., Effect of screw pressing temperature on yield, bioactive compounds, and quality of hemp (Cannabis sativa L.) seed oil, J. Cannabis Res., 2025, 7, 13 CrossRef PubMed.
- A. Allay, C. Benkirane, A. Ben Moumen, M. L. Fauconnier, H. Bouakline and J. Nkengurutse, et al., Optimizing ethanol-modified supercritical CO2 extraction for enhanced bioactive compound recovery in hemp seed oil, Sci. Rep., 2025, 15, 11055 CrossRef PubMed.
- F. Mansouri, A. Ben Moumen, G. Richard, M. L. Fauconnier, M. Sindic and A. Elamrani, et al., Proximate composition, amino acid profile, carbohydrate and mineral content of seed meals from four safflower (Carthamus tinctorius L.) varieties grown in north-eastern Morocco, OCL: Oilseeds Fats, Crops Lipids, 2018, 25, D205 CrossRef.
- F. Mansouri, A. Ben Moumen, G. Richard, M. L. Fauconnier, M. Sindic and H. S. Caid, et al., Flavor profiles of monovarietal virgin olive oils produced in the Oriental region of Morocco, OCL: Oilseeds Fats, Crops Lipids, 2017, 24, D607 CrossRef.
- C. Benkirane, A. Ben Moumen, M. L. Fauconnier, K. Belhaj, M. Abid and H. S. Caid, et al., Bioactive compounds from hemp (Cannabis sativa L.) seeds: optimization of phenolic antioxidant extraction using simplex lattice mixture design and HPLC-DAD/ESI-MS2 analysis, RSC Adv., 2022, 12, 25764–25777 RSC.
- E. Nigro, G. Crescente, M. Formato, M. T. Pecoraro, M. Mallardo and S. Piccolella, et al., Hempseed lignanamides rich-fraction: chemical investigation and cytotoxicity towards U-87 glioblastoma cells, Molecules, 2020, 25, 1049 CrossRef CAS PubMed.
- A. Ben Moumen, F. Mansouri, G. Richard, M. Abid, M. L. Fauconnier and M. Sindic, et al., Biochemical characterisation of the seed oils of four safflower (Carthamus tinctorius) varieties grown in north-eastern of Morocco, Int. J. Food Sci. Technol., 2015, 50, 804–810 CrossRef CAS.
- F. Mansouri, A. Ben Moumen, S. Aazza, K. Belhaj, M. L. Fauconnier and M. Sindic, et al., Quality and chemical profiles of virgin olive oils of three European cultivars suitable for super-high-density planting conditions in eastern Morocco, Mater. Today: Proc., 2019, 13, 998–1007 CAS.
- R. Tir, P. C. Dutta and A. Y. Badjah-Hadj-Ahmed, et al., Effect of the extraction solvent polarity on the sesame seeds oil composition, Eur. J. Lipid Sci. Technol., 2012, 114, 1427–1438 CrossRef CAS.
- C. Da Porto, D. Decorti and F. Tubaro, et al., Fatty acid composition and oxidation stability of hemp (Cannabis sativa L.) seed oil extracted by supercritical carbon dioxide, Ind. Crops Prod., 2012, 36, 401–404 CrossRef CAS.
- M. Crimaldi, S. Faugno, M. Sannino and L. Ardito, et al., Optimization of hemp seeds (Cannabis Sativa L.) oil mechanical extraction, Chem. Eng. Trans., 2017, 58, 373–378 Search PubMed.
- R. Savoire, J. L. Lanoisellé and E. Vorobiev, et al., Mechanical Continuous Oil Expression from Oilseeds: A Review, Food Bioprocess Technol., 2013, 6, 1–16 CrossRef CAS.
- L. O. de Silva, V. N. Castelo-Branco, A. G. Guimarães, M. C. Monteiro, D. Perrone and A. G. Torres, et al., Ethanol extraction renders a phenolic compounds-enriched and highly stable jussara fruit (Euterpe edulis M.) oil, Eur. J. Lipid Sci. Technol., 2017, 119, 1700095 Search PubMed.
- I. Quispe-Fuentes, E. Uribe, J. López, D. Contreras and J. Poblete, et al., A study of dried mandarin (Clementina orogrande) peel applying supercritical carbon dioxide using co-solvent: influence on oil extraction, phenolic compounds, and antioxidant activity, J. Food Process. Preserv., 2022, 46, e16116 CAS.
- M. Naczk and F. Shahidi, et al., Phenolics in cereals, fruits and vegetables: occurrence, extraction and analysis, J. Pharm. Biomed. Anal., 2006, 41, 1523–1542 CrossRef CAS.
- B. J. Xu and S. K. C. Chang, et al., A comparative study on phenolic profiles and antioxidant activities of legumes as affected by extraction solvents, J. Food Sci., 2007, 72, S159–S166 CAS.
- T. Maier, A. Schieber, D. R. Kammerer and R. Carle, et al., Residues of grape (Vitis vinifera L.) seed oil production as a valuable source of phenolic antioxidants, Food Chem., 2009, 112, 551–559 CrossRef CAS.
- W. Leonard, P. Zhang, D. Ying, Y. Xiong and Z. Fang, et al., Extrusion improves the phenolic profile and biological activities of hempseed (Cannabis sativa L.) hull, Food Chem., 2021, 346, 128976 CrossRef.
- J. O. Kuti and H. B. Konuru, et al., Antioxidant capacity and phenolic content in leaf extracts of tree spinach (Cnidoscolus spp.), J. Agric. Food Chem., 2004, 52, 117–121 CrossRef CAS.
- H. Chodar Moghadas and K. Rezaei, et al., Laboratory-Scale Optimization of Roasting Conditions Followed by Aqueous Extraction of Oil from Wild Almond, J. Am. Oil Chem. Soc., 2017, 94, 867–876 CrossRef CAS.
- A. S. Bhatnagar and A. G. Gopala Krishna, et al., Effect of extraction solvent on oil and bioactives composition of commercial Indian Niger (Guizotia abyssinica (L.f.) Cass.) seed, J. Am. Oil Chem. Soc., 2013, 90, 1203–1212 CrossRef CAS.
- B. Chand, M. Kumar, S. Prasher, A. Sharma and M. Kumar, et al., Aprotic and protic solvent for extraction of chlorophyll from various plants: chemical characteristic and analysis, J. Phys.: Conf. Ser., 2022, 2267, 012078 CrossRef.
- M. Grulichová, P. Mendel, V. Trojan, T. Vyhnánek, et al., Determination of the content of pigments in seeds, in MendelNet, 2018, pp. 620–625 Search PubMed.
- V. Tănase Apetroaei, E. M. Pricop, D. I. Istrati and C. Vizireanu, et al., Hemp Seeds (Cannabis sativa L.) as a Valuable Source of Natural Ingredients for Functional Foods-A Review, Molecules, 2024, 29, 2117 CrossRef PubMed.
- A. H. Banskota, A. Jones, J. P. M. Hui and R. Stefanova, et al., Triacylglycerols and Other Lipids Profiling of Hemp By-Products, Molecules, 2022, 27, 2239 CrossRef PubMed.
- M. Spano, G. Di Matteo, M. Rapa, S. Ciano, C. Ingallina and S. Cesa, et al., Commercial hemp seed oils: a multimethodological characterization, Appl. Sci., 2020, 10, 6932 CrossRef.
- M. Islam, Y. V. Rajagukguk, A. Siger and J. Tomaszewska-Gras, et al., Assessment of Hemp Seed Oil Quality Pressed from Fresh and Stored Seeds of Henola Cultivar Using Differential Scanning Calorimetry, Foods, 2023, 12, 135 CrossRef CAS PubMed.
- M. J. Moyano, A. J. Meléndez-Martínez, J. Alba and F. J. Heredia, et al., A comprehensive study on the colour of virgin olive oils and its relationship with their chlorophylls and carotenoids indexes (II): CIELUV and CIELAB uniform colour spaces, Food Res. Int., 2008, 41, 513–521 CrossRef CAS.
- F. Mansouri, A. Allay, A. Ben Moumen, C. Benkirane, Y. Taaifi and K. Belhaj, et al., Laboratory-Scale Optimization of Hemp Seed Roasting Temperature and Time for Producing a High-Quality Pressed Oil, J. Food Process. Preserv., 2023, 2023, 6689667 Search PubMed.
- Codex Alimentarius, Codex Standards for Fats and Oils from Vegetable Sources, Codex Stan, pp. , pp. 210–1999 Search PubMed.
- A. Rezvankhah, Z. Emam-Djomeh, M. Safari, G. Askari and M. Salami, et al., Investigation on the extraction yield, quality, and thermal properties of hempseed oil during ultrasound-assisted extraction: a comparative study, J. Food Process. Preserv., 2018, 42, e13765 CrossRef.
- C. Da Porto, A. Natolino and D. Decorti, et al., Effect of ultrasound pre-treatment of hemp (Cannabis sativa L.) seed on supercritical CO2 extraction of oil, J. Food Sci. Technol., 2015, 52, 1748–1753 CrossRef CAS PubMed.
- N. Liu, G. Ren, M. Faiza, D. Li, J. Cui and K. Zhang, et al., Comparison of conventional and green extraction methods on oil yield, physicochemical properties, and lipid compositions of pomegranate seed oil, J. Food Compos. Anal., 2022, 114, 104788 CrossRef.
- Y. Huang, C. Liu, Z. Ge, F. Huang, H. Tang and Q. Zhou, et al., Influence of different thermal treatment methods on the processing qualities of sesame seeds and cold-pressed oil, Food Chem., 2023, 404, 134677 Search PubMed.
- K. Gharsallah, L. Rezig, F. B'chir, S. Bourgou, N. Ben Achour and C. Jlassi, et al., Composition and Characterization of Cold Pressed Moringa oleifera Seed Oil, J. Oleo Sci., 2022, 71, 1263–1273 CrossRef CAS PubMed.
- G. Sodeifian, N. S. Ardestani, S. A. Sajadian and K. Moghadamian, et al., Properties of Portulaca oleracea seed oil via supercritical fluid extraction: experimental and optimization, J. Supercrit. Fluids, 2018, 135, 34–44 CrossRef CAS.
- E. Aspé and K. Fernández, et al., The effect of different extraction techniques on extraction yield, total phenolic, and anti-radical capacity of extracts from Pinus radiata Bark, Ind. Crops Prod., 2011, 34, 838–844 CrossRef.
- X. Sun, W. Li, J. Li, Y. Zu, C. Y. Hse and J. Xie, et al., Process optimisation of microwave-assisted extraction of peony (Paeonia suffruticosa Andr.) seed oil using hexane-ethanol mixture and its characterisation, Int. J. Food Sci. Technol., 2016, 51, 2663–2673 CrossRef CAS.
- Z. Ciğeroğlu, M. Bayramoğlu, Ş. İ. Kırbaşlar and S. Şahin, et al., Comparison of microwave-assisted techniques for the extraction of antioxidants from Citrus paradisi Macf. biowastes, J. Food Sci. Technol., 2021, 58, 1190–1201 CrossRef.
- G. L. Teixeira, L. G. Maciel, S. Mazzutti, R. C. T. Barbi, R. H. Ribani and S. R. S. Ferreira, et al., Sequential green extractions based on supercritical carbon dioxide and pressurized ethanol for the recovery of lipids and phenolics from Pachira aquatica seeds, J. Cleaner Prod., 2021, 306, 127223 CrossRef CAS.
- F. M. Barrales, C. A. Rezende and J. Martínez, et al., Supercritical CO2 extraction of passion fruit (Passiflora edulis sp.) seed oil assisted by ultrasound, J. Supercrit. Fluids, 2015, 104, 183–192 CrossRef CAS.
- L. Jakobek, Interactions of polyphenols with carbohydrates, lipids and proteins, Food Chem., 2015, 175, 556–567 CrossRef CAS PubMed.
- X. Liu, C. Le Bourvellec and C. M. G. C. Renard, et al., Interactions between cell wall polysaccharides and polyphenols: effect of molecular internal structure, Compr. Rev. Food Sci. Food Saf., 2020, 19, 3574–3617 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2026 |