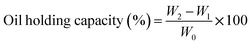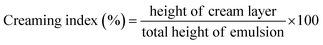Open Access Article
Open Access ArticleUltrasound-assisted microalgal docosahexaenoic acid (DHA) nanoemulsion preparation using casein, chitosan, and pectin as emulsifiers for enhanced oxidative stability and shelf life for food fortification
Sirajdeen Asmath
Mubeena
and
Radhakrishnan
Preetha
 *
*
Department of Food Technology, School of Bioengineering, Faculty of Engineering and Technology, SRM Institute of Science and Technology, Kattankulathur, Tamilnadu 603203, India. E-mail: preethar@srmist.edu.in
First published on 24th November 2025
Abstract
The current work examines the effect of three distinct emulsifiers, such as casein, chitosan, and pectin, on the oxidative stability and physicochemical characteristics of docosahexaenoic acid (DHA) nanoemulsions. The DHA nanoemulsion formulated using chitosan (CHDN) had a greater zeta potential value of 42.2 ± 0.75 mV with a particle size of 122.5 ± 2.5 nm initially and maintained electrostatic stability (>30 mV) throughout storage (28 days). The DHA nanoemulsions were further characterized using SEM, FTIR-ATR spectroscopy, and micro-Raman spectroscopy to confirm the presence of DHA in the nanoemulsions. Rheological measurements revealed shear-thinning behavior among nanoemulsions with an increase in shear rate. From the shelf life studies (28 days), it was evident that the DHA nanoemulsion formulated using chitosan (4.94 ± 0.1 Meq O2 per kg) had significantly (p < 0.05) higher oxidative stability compared to casein (6.4 ± 0.2 Meq O2 per kg) and pectin (5.7 ± 0.12 Meq O2 per kg). Furthermore, the encapsulation efficiency of CHDN was found to be 87.33%, as confirmed by GC-FID. Kalakand (a dairy confectionary) fortified with CHDN retained a higher concentration of DHA (92%) compared to neat DHA oil-incorporated kalakand (20%) after 10 days of storage. The physicochemical and textural properties of CHDN-fortified kalakand were significantly higher (p < 0.05) than plain kalakand. Moreover, CHDN-incorporated kalakand had higher sensory attributes. These results demonstrate the successful use of DHA nanoemulsions in food matrices and thereby offer a unique nanoencapsulated DHA-fortified food with increased DHA retention.
Sustainability spotlightThe study offers a promising and sustainable solution to the worldwide problem of omega-3 fatty acid deficiency by employing a green nanoencapsulation method for algal-derived docosahexaenoic acid (DHA). Utilizing microalgal oil, a plant-based, renewable, and ecologically friendly source of DHA, the study supports international initiatives to reduce reliance on overfished marine resources while maintaining nutritional equity. Casein, chitosan, and pectin, which are non-toxic and biodegradable natural polymers, are used as emulsifiers. By extending DHA's shelf life and sensory compatibility, the nanoemulsion technology used here makes it possible to include it into the dairy confectionary, kalakand. The study aligns with UN Sustainable Development Goals such as zero hunger, good health and well-being, responsible consumption and production, and life below water. |
1. Introduction
Docosahexaenoic acid (DHA) is an omega-3 fatty acid important for brain function and visual development.1 DHA is primarily sourced from fatty fish, cod liver, krill, tuna, and sharks. Each source varies in omega-3 composition and intestinal absorption. The alternatives, such as algal oil, chia seeds, and walnuts, are gaining popularity nowadays.2 DHA from algal oil provides a safe substitute for fish oil without compromising its bioavailability. Algal oil is a sustainable source of DHA, especially in the context of growing concerns about heavy metal poisoning in fish and overfishing.3 Dietary intake of DHA is often insufficient, especially in populations with low fish consumption. Fortifying everyday food products, such as dairy, bread, beverages, and snacks, ensures that a larger segment of the population can meet recommended intake levels. Unfortunately, DHA is quite vulnerable to oxidative breakdown, which can reduce its nutritional benefits and also result in the development of an unpleasant aroma and taste.4 The stability of the DHA can be improved by encapsulation using different wall materials and emulsifiers by various technologies, including ultrasonication, microfluidization, freeze-drying, and high-pressure homogenization.5 The ultrasonication process involves the use of high-frequency sound waves, which create intense shear forces and cavitation within the liquid medium, breaking the oil droplets into nanoscale sizes and forming a stable emulsion. It is a commonly utilized encapsulation method that may produce tiny droplet sizes.6Nanoemulsions, colloidal particulate systems, are widely used for the encapsulation and protection of bioactive compounds with droplet sizes between 20 and 200 nm.7 Nanoemulsions have a high surface area and greater kinetic stability compared to microemulsions. Nanoemulsions prevent oxidative degradation of DHA by preventing lipid oxidation, which is a major challenge with traditional formulations. Encapsulation of DHA in nanoemulsions improves its sensory characteristics by masking the unpleasant odor, which is a limiting factor in food fortification.8 Protein and polysaccharide-based materials are extensively used for their stabilizing properties.9 Casein is an amphiphilic protein without a fixed secondary structure. It is composed of phosphoproteins with four sub-fractions, namely αS1-, αS2-, β-, and κ-caseins.10 All these protein fractions have an isoelectric point between 4.1 and 5.3. Due to the presence of exposed hydrophobic regions in its structure, casein adsorbs quickly to the surface of droplets.11 κ-Casein is a special stabilizing protein essential for preserving the stability and integrity of casein micelles. It creates stearic hindrance, which prevents the mixing of oil and water droplets in an emulsion.12
Polysaccharides such as chitosan and pectin migrate slowly in a solution, as they are more rigid and larger in size.12 Chitosan, a natural polysaccharide, is extensively used as an encapsulating material known for its polycationic properties and can form electrostatic interactions with negatively charged components.13 Chitosan is composed of β-(1→4)- linked D-glucosamine and N-acetyl-D-glucosamine units. Chitosan stabilizes emulsions by providing electrostatic repulsion and steric hindrance when the pH of the solution is below its pKa for the amine group (–NH2).14 The concentration, viscosity and degree of deacetylation are the important factors that enhance the emulsifying properties and reduce droplet aggregation and creaming.15 Usage of chitosan as an emulsifier enhanced the stability of DHA-enriched fish oil along with sodium tripolyphosphate by limiting the production of lipid peroxides, aldehydes (4-hydroxy-2-hexenal), and secondary oxidation products of fish oil.16 Pectin, a plant-based polysaccharide, is composed of 1,4-linked α-D-galacturonic acid units. It stabilizes the nanoemulsion by increasing viscosity and preventing phase separation.17 Pectin contributes a negative charge, especially when the pH of the solution is above its pKa for the carboxyl group (–COOH). Pectin as an emulsifier exhibits good electrostatic stabilization if the pH of the solution is above its pKa.18 Pectin containing ferulic acid and a higher degree of methoxy and acetyl groups is more efficient in reducing interfacial tension.12
Milk and milk-based products are widely consumed foods that can be the right medium for the delivery of bioactive compounds.19 Kalakand is one of the significant traditional milk products made by adding sugar and the coagulant to the hot milk. Kalakand is an excellent source of lactose, fat, minerals, and proteins.20 In the present study, kalakand was selected as a food matrix for DHA fortification because of its favorable composition. Milk proteins, particularly casein found in kalakand, maintain the stability of the DHA nanoemulsion by inhibiting lipid oxidation and enhancing its bioavailability.11 Since kalakand doesn't require additional heat treatment during fortification, its structural integrity is maintained while preserving DHA's stability. DHA-fortified kalakand serves as a nutritious snack and mitigates omega-3 deficiencies, especially in populations with low fish consumption.21
Fortification of DHA in a food matrix in its encapsulated form, such as nanoemulsion, protects it from oxidative degradation, masks its unpleasant odor, and increases its bioavailability.22 In recent research, Estrada et al. fortified microencapsulated salmon oil in yogurt to develop an omega-3-fortified dairy product. The above fortified product was supplemented to pregnant and breastfeeding women, which resulted in increased PUFA (polyunsaturated fatty acid) content in the blood.23 Similarly, flaxseed oil emulsions were fortified in butter to enhance its nutritional profile.24 Other food products such as spaghetti, cheese, low-fat probiotic fermented milk, probiotic soymilk, and apple juice were fortified with omega-3 fatty acids, particularly DHA and EPA.21 The novelty of the current study lies in the incorporation of a chitosan-stabilized DHA nanoemulsion in kalakand, a dairy-based confectionary. Even though fortification of nanoencapsulated omega-3 fatty acids has been widely studied in beverages and dairy matrices such as yogurt and milk, the application of DHA, especially as a nanoemulsion in dairy-based confectionary, remains unexplored. This innovation transforms conventional dairy products into functional foods.
In the current research, DHA nanoemulsions were prepared using three different emulsifiers: casein, chitosan, and pectin. The study investigates the impact of different emulsifier concentrations on the zeta potential, droplet size, morphology, and oxidative stability of DHA nanoemulsions and the fortification of DHA nanoemulsions in dairy food products. The findings aim to bridge the gap between nanoemulsion technology and traditional food fortification, contributing to both scientific knowledge and consumer health.
2. Materials and methods
Algal DHA oil (50% purity) was purchased from Ovegha Nutraceuticals Pvt Ltd, India. Casein and pectin were procured from Saatvic Foods, India. Tween 80 was procured from Sigma-Aldrich, USA. Food-grade chitosan with 90% deacetylation was purchased from Mark Nature, USA.2.1. Oil holding capacity of emulsifiers
The oil-holding capacity of the emulsifiers was determined along with neat DHA oil to assess their ability to emulsify and retain DHA oil as described below.25 The emulsifiers, such as casein, chitosan, and pectin, were individually measured (1 g, w/v) and added to 10 ml of neat DHA oil. The mixture was vortexed and centrifuged at 1600 g for 10 minutes. The oil-holding capacity of the emulsifiers after removing the supernatant was measured using the following equation, as per the literature.25where W0 is the weight of the dry emulsifier (g), W1 is the weight of the centrifuge tube containing the dry emulsifier (g), and W2 is the weight of the centrifuge tube containing sediment after centrifugation (g).
2.2. Formulation of DHA nanoemulsions
The concentration of the emulsifiers was fixed based on the preliminary studies (SI Table 1), and the range of concentration to be studied was fixed based on the literature. The oil-in-water nanoemulsion was prepared by dissolving casein (5, 10, and 15% w/v),26 chitosan (1, 1.5, and 3% w/v),16 and pectin (3, 3.5, and 6% w/v)27 at different concentrations along with Tween 80 (1%) for the encapsulation of DHA (Table 1). Casein and pectin were dissolved individually in distilled water at 80 °C, and chitosan was dissolved in 1% acetic acid (w/v) at 800 rpm using a magnetic stirrer. Emulsifiers were allowed to hydrate overnight, which served as an aqueous phase. After complete hydration, DHA was added dropwise along with Tween 80 under stirring at 800 rpm for 40 to 60 minutes. The oil-to-aqueous phase ratio was maintained at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20. The emulsions formulated were sonicated (500 W) at 30 kHz using a probe sonicator with a 25 mm diameter probe size (VC-505-220, Sonics Materials, Portugal) for 10 minutes with a cycle of 10 s on and 10 s off. The formulated emulsion was stored under refrigerated conditions (4 °C) until analysis. All the experiments were carried out at ambient temperature (25 ± 2 °C) unless otherwise stated.
20. The emulsions formulated were sonicated (500 W) at 30 kHz using a probe sonicator with a 25 mm diameter probe size (VC-505-220, Sonics Materials, Portugal) for 10 minutes with a cycle of 10 s on and 10 s off. The formulated emulsion was stored under refrigerated conditions (4 °C) until analysis. All the experiments were carried out at ambient temperature (25 ± 2 °C) unless otherwise stated.
| Type of emulsifier | Emulsifier concentration% | T80% | DHA oil% | Water% |
|---|---|---|---|---|
| Casein | 5 | 1 | 5 | 95 |
| 10 | 1 | 5 | 95 | |
| 15 | 1 | 5 | 95 | |
| Chitosan | 1 | 1 | 5 | 95 |
| 1.5 | 1 | 5 | 95 | |
| 3 | 1 | 5 | 95 | |
| Pectin | 3 | 1 | 5 | 95 |
| 3.5 | 1 | 5 | 95 | |
| 6 | 1 | 5 | 95 |
2.3. Stability studies of DHA nanoemulsions with various emulsifier concentrations
2.4. Characterization of stable DHA nanoemulsions with selected emulsifier concentration
2.5. Storage stability of DHA in nanoemulsions
The DHA nanoemulsions were developed and stored at 4 °C for 28 days. During storage, droplet size, electrostatic stability, oxidative stability, and concentration of DHA in nanoemulsions were measured.2.6. Fortification of DHA nanoemulsions in kalakand
The kalakand was prepared using the standardized milk with 3 g total fat, 4.7 g carbohydrates, and 3.4 g protein procured from a local market in Chennai, Tamil Nadu, India. Kalakand was prepared according to the method described by Jain et al.33 The preparation was divided into three groups consisting of a negative control (plain kalakand, KCON), a positive control (kalakand with neat DHA oil, KDHO), and a test group (kalakand with DHA oil nanoemulsions, KCDN). Neat DHA oil and DHA nanoemulsions were added to the kalakand formulation at 40–45 °C and were immediately allowed to set at room temperature. The concentration of DHA present in the kalakand samples was 0.5 g per 100 g. CHDN was selected for food fortification based on its higher stability throughout storage. A detailed flowchart illustrating the preparation of kalakand is provided in the SI (SI Fig. 1).2.6.2.1. Physicochemical analysis. The prepared kalakand samples were analyzed for moisture, ash, protein, fat, and carbohydrates using the AOAC (2000) method immediately after preparation and throughout storage (day 10). The color, pH (AOAC, 2000), acidity (AOAC, 2000), and peroxide value (AOAC, 2000) of the samples were monitored throughout storage (day 10) as described earlier. The water activity (aw) of kalakand samples was determined using a water activity meter (AquaLab 3 TE, USA).
2.6.2.2. Concentration of DHA in kalakand. The concentration of DHA present in the fortified kalakand samples was measured using GC-FID (Agilent 7890B, US). The analysis was performed as described in Section 2.5.3.
2.6.2.3. Texture profile analysis. A texture analyzer (Stable Micro Systems, UK) was used to characterize the texture of kalakand samples (1.5 cm3 size pieces) using probe P/75 compression platen with the speed of 5 mm s−1.
2.6.2.4. Microbial analysis. The microbiological analysis of samples was performed to assess their safety for consumption and to ensure the absence of microbial contamination. All the prepared samples were tested for yeast and mold count (Y&M), as well as total plate count (TPC). Kalakand samples (1 g) were homogenized using a mortar and pestle after adding sterile saline (9 ml). Then, 1 ml of the homogenized sample was taken, and serial dilution was performed using sterile saline. The homogenized kalakand sample, as such and after different dilutions, was plated on plate count agar (TPC) and potato dextrose agar (Y&M). For TPC, the plates were incubated at 37 ± 1 °C for 48 h, and for Y&M at 25 ± 1 °C for 3 to 5 days. 1 g of DHA nanoemulsion after preparation was also analyzed for TPC and Y&M, to check for any microbial contamination. The samples were analyzed throughout the storage period (10 days).
2.7. Statistical analysis
Statistical analyses were conducted using IBM SPSS Statistics 20. One-way ANOVA followed by Tukey's multiple comparison test was used to identify significant differences.3. Results and discussion
3.1. Oil holding capacity of different emulsifiers
The oil holding capacity (OHC) of the emulsifiers such as casein, chitosan, and pectin was determined initially before the preparation of the nanoemulsion to determine its efficiency. The OHC of casein, chitosan, and pectin was found to be 19.3 ± 0.57%, 34.5 ± 0.68%, and 17.2 ± 0.57%, respectively. The oil-holding capacity of chitosan was significantly (p < 0.05) higher compared to casein and pectin.3.2. Physical stability (creaming index%) of DHA nanoemulsions with various emulsifier concentrations
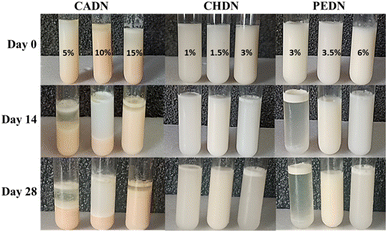
The casein DHA nanoemulsion (CADN), chitosan DHA nanoemulsion (CHDN), and pectin DHA nanoemulsion (PEDN) prepared at different concentrations of emulsifiers (10–20%, 1–3%, and 3–6%, respectively) were analyzed for phase separation over storage for 28 days at 4 °C (Fig. 1). The creaming index evaluates the physical stability of the nanoemulsion, as it is indirectly related to droplet aggregation.28 All the prepared nanoemulsions were stable, and no phase separation was observed immediately after the preparation (Table 2). However, CHDN (1.5%) and PEDN (3.5%) exhibited higher physical stability without phase separation for up to 28 days compared to other combinations of nanoemulsions. The separation of the cream and serum layers occurred due to the Ostwald ripening effect.34 Phase separation can also happen due to the difference in density of the oil and aqueous phase, coalescence, increased particle size, and flocculation in the emulsion.28| DHA nanoemulsion | Emulsifier concentration (%) | Creaming index (%) | ||||
|---|---|---|---|---|---|---|
| Day 1 | Day 7 | Day 14 | Day 21 | Day 28 | ||
| a CADN – casein DHA nanoemulsion; CHDN – chitosan DHA nanoemulsion; PEDN – pectin DHA nanoemulsion. * indicates a stable nanoemulsion without phase separation. Data are articulated as mean ± standard deviation (n = 3). In an individual column, each value tailed by a different letter is significantly different (p ≤ 0.05) as determined by Tukey's test. | ||||||
| CADN | 10 | * | 25 ± 0.01c | 47 ± 0.01b | 48 ± 0.05b | 50 ± 0.05a |
| 15 | * | 35 ± 0.07c | 69.2 ± 0.05b | 71.8 ± 0.07a | 71.8 ± 0.07a | |
| 5 | * | 10 ± 0.05c | 20.6 ± 0.01b | 25 ± 0.05a | 25 ± 0.01a | |
| CHDN | 1 | * | * | 50 ± 0.03c | 70 ± 0.07b | 75 ± 0.05a |
| 1.5 | * | * | * | * | * | |
| 3 | * | * | * | 5.5 ± 0.03b | 9.09 ± 0.03a | |
| PEDN | 3 | * | * | 12.5 ± 0.07a | 12.5 ± 0.01a | 12.5 ± 0.05a |
| 3.5 | * | * | * | * | * | |
| 6 | * | * | * | 5 ± 0.05b | 10 ± 0.07a | |
3.3. Color stability of DHA nanoemulsions with various emulsifier concentrations
The color analysis was performed for all the samples considering the L*, a*, and b* parameters. All the DHA nanoemulsion samples were analyzed for color during storage (28 days) (Table 3). L*, a*, and b* indexes significantly varied (p < 0.05) across samples immediately after preparation. All combinations of casein-based nanoemulsions showed a slight shift towards red. After storage (28 days), all the casein and pectin-based nanoemulsions varied significantly (p < 0.05) in lightness and yellowness index with time, which may be due to particle aggregation.29 Chitosan-based nanoemulsions showed a greenish tint that decreased over time. There was a continuous increase in the yellowness of all the nanoemulsions throughout storage. Among the formulations, CHDN1.5, PEDN6, and CADN5 showed significantly lesser (p < 0.05) variations in color (ΔE <3) upon storage. Major deviation in color was observed with PEDN3. Interestingly, the yellowness of the nanoemulsion with a chitosan concentration of 1.5% does not have much variation in yellow index from day 1 (14.6 ± 0.1) to day 28 (14.8 ± 0.1). This may be due to the uniform dispersion of particles in the nanoemulsion, which prevented particle aggregation and oxidation of the DHA oil. The particle size influences the color of the nanoemulsion, which occurs due to Ostwald ripening.29 Decreased particle size with a higher zeta potential value increases the stability of the emulsion.34 The DHA nanoemulsions with emulsifier concentrations of CADN (10%), CHDN (1.5%), and PEDN (3.5%) were selected for further analysis because of higher physical and electrostatic stability.| Number of days | Color parameters | CADN5 | CADN10 | CADN15 | CHDN1 | CHDN1.5 | CHDN3 | PEDN3 | PEDN3.5 | PEDN6 |
|---|---|---|---|---|---|---|---|---|---|---|
| a CADN – casein DHA nanoemulsion; CHDN – chitosan DHA nanoemulsion; PEDN – pectin DHA nanoemulsion. The numbers next to the sample code denote the percentage of emulsifier used. Data are articulated as mean ± standard deviation (n = 3). In an individual row, each value tailed by a different letter is significantly different (p ≤ 0.05) as determined by Tukey's test. | ||||||||||
| Day 1 | L* | 67.7 ± 0.5a | 65.7 ± 0.03ab | 63.9 ± 0.9c | 65.9 ± 0.1ab | 78.9 ± 0.2a | 72.3 ± 0.2a | 59.2 ± 1.07b | 68.7 ± 0.6c | 69.6 ± 0.7b |
| a* | 0.66 ± 0.01a | 4.06 ± 0.05a | 1.42 ± 0.1c | −2.09 ± 0.1b | −1.75 ± 0.2bc | −1.9 ± 0.09c | −0.9 ± 0.2a | 0.32 ± 0.28a | −0.98 ± 0.1ab | |
| b* | 18.6 ± 0.1a | 19.8 ± 0.04a | 14.01 ± 0.7d | 11.03 ± 0.2d | 14.6 ± 0.1b | 9.81 ± 0.2c | 9.35 ± 0.3ab | 14.05 ± 0.2b | 8.69 ± 0.2ab | |
| Day 7 | L* | 67.2 ± 3.4a | 67.9 ± 0.9a | 72 ± 0.06a | 69 ± 0.5a | 77.5 ± 0.8b | 71.4 ± 0.01a | 63.5 ± 0.9a | 77 ± 0.09a | 71.8 ± 0.06a |
| a* | 0.65 ± 0.1a | 3.4 ± 0.05a | 0.41 ± 0.09d | −2.1 ± 0.2b | −1.8 ± 0.04c | −2.2 ± 0.04c | −1 ± 0.05a | 0.01 ± 0.09a | −1.3 ± 0.1c | |
| b* | 18.6 ± 0.5a | 19.04 ± 0.5a | 15.6 ± 0.08c | 11.8 ± 0.4c | 14.3 ± 0.3c | 10.1 ± 0.05c | 8.6 ± 0.2bc | 14.7 ± 0.03a | 8.37 ± 0.1b | |
| Day 21 | L* | 66.1 ± 1.3a | 63.01 ± 0.2c | 67.04 ± 0.8b | 63.7±2b | 77.2 ± 2.6a | 69.9 ± 1.5b | 65.04 ± 0.2b | 71.7 ± 1.4b | 68.9 ± 0.4b |
| a* | 0.9 ± 0.09a | 2.3 ± 0.2ab | 4.2 ± 0.2b | −1.7 ± 0.07b | −1.9 ± 0.2b | −1.5 ± 0.1b | −0.8 ± 0.2a | 0.16 ± 0.3a | −0.7 ± 0.04a | |
| b* | 15.4 ± 0.3c | 18.5 ± 0.16a | 20.2 ± 0.14a | 13.8 ± 0.2b | 14.7 ± 0.4b | 10.9 ± 0.4b | 8.1 ± 0.4c | 14.4 ± 0.08ab | 8.9 ± 0.07a | |
| Day 28 | L* | 67.4 ± 0.2a | 64.9 ± 1.6c | 61.9 ± 0.7d | 63 ± 0.9ab | 77.3 ± 0.1c | 68.7 ± 0.2b | 68.8 ± 0.3b | 65.7 ± 0.4d | 72.2 ± 0.05a |
| a* | 1.5 ± 0.4b | 1.2 ± 1.5b | 3.9 ± 0.08b | −1.9 ± 0.2a | −1.8 ± 0.1a | −1.4 ± 0.2a | −1 ± 0.2a | 0.1 ± 0.1a | −1.1 ± 0.1bc | |
| b* | 16.8 ± 0.4b | 18.7 ± 1.5a | 18.1 ± 0.2a | 13.6±1a | 14.8 ± 0.1a | 12.1 ± 0.5a | 9.6 ± 0.5c | 14.5 ± 0.2ab | 8.8 ± 0.3ab | |
| ΔE | 2.01 ± 0.1c | 3.31 ± 0.06b | 3.6 ± 0.08b | 2.97 ± 0.1bc | 1.59 ± 0.2c | 3.91 ± 0.08b | 9.05 ± 0.09a | 3.48 ± 0.5b | 2.62 ± 0.41bc | |
3.4. Droplet size and electrostatic stability of DHA nanoemulsions with various emulsifier concentrations
DHA nanoemulsions prepared using various concentrations of casein (CADN), chitosan (CHDN), and pectin (PEDN) as emulsifiers had zeta potential values in the range from −31.33 ± 0.58 to −16.37 ± 0.55 mV, 42.2 ± 0.75 to 11.5 ± 0.44 mV, and −38.63 ± 1 to −29.17 ± 0.29 mV, respectively (Table 4). Zeta potential measures the electrophoretic mobility and characterizes the surface charge.35 Zeta potential values above 30 mV indicate higher electrostatic stability.36 The droplet size of CADN, CHDN, and PEDN varied from 192.3 ± 7.51 to 225 ± 5 nm, 185.1 ± 4.46 to 258.6 ± 2.28 nm, and 122.5 ± 2.5 to 235.43 ± 4 nm, respectively. CHDN had a higher zeta potential value next to PEDN. The increased zeta potential of chitosan is due to its low pH and polycationic nature. In an acidic environment, the amino groups (–NH2) become protonated, forming –NH3+ groups, which leads to an overall positive surface charge, which in turn increases the zeta potential value. The polycationic nature of the chitosan enhances the electrostatic repulsion between the particles, which is one of the major reasons for its increased stability.37 In a study conducted by Esquerdo et al.,32 a food-grade nanoemulsion was prepared using chitosan and gelatin as wall materials to encapsulate PUFA from carp oil. An increase in the zeta potential value (26.5 to 31 mV) was observed with an increased concentration of chitosan.32 In a similar study, a double-layer emulsion was prepared to encapsulate capsaicin using chitosan and alginate, which had zeta potential values of 26.3 ± 12.7 mV and −8.97 ± 0.43 mV, respectively.38 The increased stability of nanoemulsions improves the absorption of DHA in the intestinal membrane and also protects DHA from degradation during the gastric digestion.38| DHA nanoemulsion | Emulsifier concentration (%) | Zeta potential (mV) | Droplet size (nm) | Polydispersity index (PDI) |
|---|---|---|---|---|
| a CADN – casein DHA nanoemulsion; CHDN – chitosan DHA nanoemulsion; PEDN – pectin DHA nanoemulsion. Data are articulated as mean ± standard deviation (n = 3). In an individual column, each value tailed by a different letter is significantly different (p ≤ 0.05) as determined by Tukey's test. | ||||
| CADN | 5 | −31.3 ± 0.58abc | 192.27 ± 7.51bc | 0.4 ± 0.06de |
| 10 | −29 ± 1abc | 225 ± 5abc | 0.34 ± 0.05e | |
| 15 | −16.37 ± 0.55c | 247.33 ± 5.5ab | 0.59 ± 0.04c | |
| CHDN | 1 | 38.33 ± 0.5a | 213.97 ± 4.3abc | 0.83 ± 0.06b |
| 1.5 | 42.2 ± 0.75a | 185.1 ± 4.46c | 0.5 ± 0.05cd | |
| 3 | 11.5 ± 0.44c | 258.63 ± 2.2a | 0.91 ± 0.07ab | |
| PEDN | 3 | −32.7 ± 1.13ab | 198.33 ± 1.53bc | 0.61 ± 0.06c |
| 3.5 | −38.6 ± 1ab | 122.47 ± 2.5d | 1 ± 0.04a | |
| 6 | −29.17 ± 0.29abc | 235.43 ± 4ab | 0.52 ± 0.05cd | |
3.5. Morphological measurements of DHA nanoemulsions
The morphology and shape of the nanoemulsion were analyzed using SEM (Fig. 2). Among DHA nanoemulsions, CADN and CHDN exhibited a smooth and uniform microstructure, while PEDN showed an irregular shape with poor distribution. The surface morphology of the emulsions can be correlated with their particle size and polydispersity index (PDI), as a low PDI value indicates uniform dispersion and high homogeneity. The PDI values of CADN, CHDN, and PEDN were 0.4, 0.5, and 1, respectively. Even though PEDN has increased electrostatic stability (−38.6 ± 1 mV) and smaller droplet size (122.47 ± 2.5 nm) compared to CADN (−31.3 ± 0.58 mV; 192.27 ± 7.51 nm), a spherical shape with uniform droplet distribution was attained in CADN. The morphology of CHDN aligns well with its electrostatic stability (42.2 ± 0.75 mV) and droplet size (185.1 ± 4.46 nm). Overall, from the morphological studies, it was evident that CHDN and CADN had uniform dispersion, which may be due to their ability to prevent Ostwald ripening and coalescence of droplets.3.6. Chemical characterization
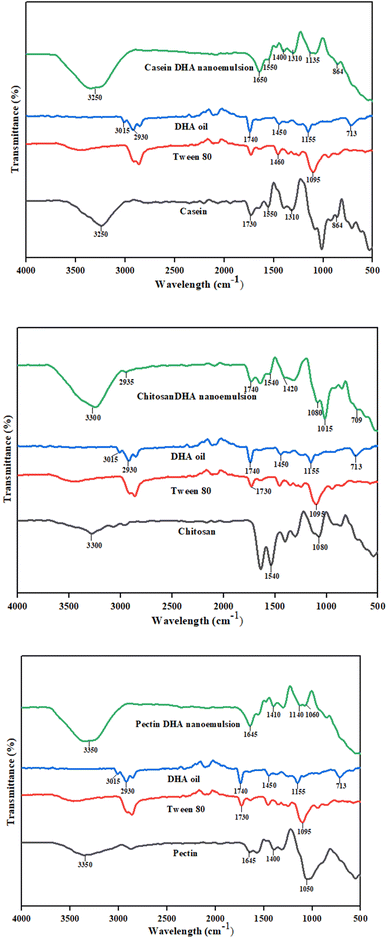
The FTIR spectra were analyzed to study the chemical interaction between DHA and emulsifiers within the nanoemulsion (Fig. 3). In neat DHA oil, the characteristic peaks of DHA were found around 1740 cm−1, 2930 cm−1, 1160 cm−1, 1450 cm−1 and 713 cm−1.39 A sharp peak at 1740 cm−1 corresponds to the carbonyl (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) stretching vibration of ester functional groups present in fatty acids. Peaks around 2800–3000 cm−1 in neat DHA oil correspond to C–H stretching vibration of the –CH2– and CH3– groups of the lipid backbone, indicating its aliphatic nature.28 The peak at 1160 cm−1 was due to C–O stretching. The appearance of a peak at 1450 cm−1 was due to the bending vibration of methylene groups, and a weak band around 713 cm−1 occurred due to the vibration of methylene groups present in long-chain fatty acids.39 The above functional groups confirm the structural integrity of neat DHA oil.
O) stretching vibration of ester functional groups present in fatty acids. Peaks around 2800–3000 cm−1 in neat DHA oil correspond to C–H stretching vibration of the –CH2– and CH3– groups of the lipid backbone, indicating its aliphatic nature.28 The peak at 1160 cm−1 was due to C–O stretching. The appearance of a peak at 1450 cm−1 was due to the bending vibration of methylene groups, and a weak band around 713 cm−1 occurred due to the vibration of methylene groups present in long-chain fatty acids.39 The above functional groups confirm the structural integrity of neat DHA oil.
 | ||
| Fig. 3 FTIR spectra showing the chemical characterization of DHA nanoemulsions formulated with different emulsifiers. | ||
In neat casein, the bands between 3300 cm−1 and 3000 cm−1 indicate N–H stretching of the amide group, and the bands around 1730 cm−1 represent the vibration in the carbonyl (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) group of ester groups.40 The band around 1550 cm−1 (amide I) indicates the presence of N–H group vibrations.40 A shift in peak 1650 cm−1 was observed in CADN, which may be due to the interaction of ester carbonyl groups present in casein with DHA, causing changes in its secondary structure.41 In neat pectin, the bands around 1645 cm−1 represent COO− asymmetric stretching.42 The bands around 1400 cm−1 represent the stretching of CH3.43 The bands between 1200 cm−1 and 950 cm−1 indicate the presence of carbohydrates from polysaccharides.42 Bands between 1000 cm−1 and 1150 cm−1 indicate the presence of homogalacturonan, a structural component present in pectin.44 The bands between 3500 cm−1 and 3000 cm−1 represent galacturonic acid present in pectin.44 The peak at 3313 cm−1 (O–H stretching) in the pectin nanoemulsion represents strong hydrogen bonding between DHA and the hydroxyl (–OH) groups present in pectin, leading to a change in vibrational frequency.44
O) group of ester groups.40 The band around 1550 cm−1 (amide I) indicates the presence of N–H group vibrations.40 A shift in peak 1650 cm−1 was observed in CADN, which may be due to the interaction of ester carbonyl groups present in casein with DHA, causing changes in its secondary structure.41 In neat pectin, the bands around 1645 cm−1 represent COO− asymmetric stretching.42 The bands around 1400 cm−1 represent the stretching of CH3.43 The bands between 1200 cm−1 and 950 cm−1 indicate the presence of carbohydrates from polysaccharides.42 Bands between 1000 cm−1 and 1150 cm−1 indicate the presence of homogalacturonan, a structural component present in pectin.44 The bands between 3500 cm−1 and 3000 cm−1 represent galacturonic acid present in pectin.44 The peak at 3313 cm−1 (O–H stretching) in the pectin nanoemulsion represents strong hydrogen bonding between DHA and the hydroxyl (–OH) groups present in pectin, leading to a change in vibrational frequency.44
A broad band around 3300 cm−1 represents stretching of O–H in chitosan. The broadening of the peak around 3300 cm−1 (OH band) indicates hydrogen bonding and interaction of chitosan with DHA in nanoemulsions.45 The occurrence of a band around 1540 cm−1 in chitosan indicates N–H bending of amino groups –NH2 and –NH3+ (amide II) found in chitosan. There was a shift in the peak at 1540 cm−1 (chitosan) to a lower wave number of 1420 cm−1 in CHDN, indicating interaction of DHA with amine groups present in chitosan.46 But there was no formation of new covalent bonds, indicating physical interaction between DHA and chitosan at the interface.47 Additionally, there might be a formation of a hydrogen bond between DHA (carboxyl or hydroxyl group) and chitosan (amide or carboxyl group) in CHDN. In similar research, Liu et al. observed a shift in the amide II band of DHA-loaded nanoparticles due to the electrostatic interaction of DHA with zein and PLGA.48 Therefore, in the current study, the shift in amide II band was due to electrostatic interaction between chitosan (–NH3+) and DHA (–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and –COOH groups). The occurrence of bands around 1000 cm−1 indicates C–O stretching skeletal vibrations, and around 1100 cm−1 indicates C–O–C antisymmetric and C–N stretching. The shift observed from 1080 cm−1 to 1015 cm−1 may be due to the adsorption of chitosan with DHA oil.46 These interactions confirm the formation of nanoemulsions only by physical adsorption without the formation of additional new bands.
O and –COOH groups). The occurrence of bands around 1000 cm−1 indicates C–O stretching skeletal vibrations, and around 1100 cm−1 indicates C–O–C antisymmetric and C–N stretching. The shift observed from 1080 cm−1 to 1015 cm−1 may be due to the adsorption of chitosan with DHA oil.46 These interactions confirm the formation of nanoemulsions only by physical adsorption without the formation of additional new bands.
Raman spectroscopy was carried out to analyze the nonpolar molecular interactions between DHA and emulsifiers in the nanoemulsions (Fig. 4). DHA in CADN, CHDN, and PEDN was confirmed by its characteristic peaks around 1650 cm−1, representing the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching vibrations of unsaturated fatty acids.49 An intense peak around 2850 cm−1 (C–H stretching) was also observed in all the DHA nanoemulsions (CADN, CHDN, and PEDN). These results are in agreement with the findings of Killeen et al., where intense bands were observed around 1660 cm−1 (C
C stretching vibrations of unsaturated fatty acids.49 An intense peak around 2850 cm−1 (C–H stretching) was also observed in all the DHA nanoemulsions (CADN, CHDN, and PEDN). These results are in agreement with the findings of Killeen et al., where intense bands were observed around 1660 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching) and 2839 cm−1 (C–H stretching) in oil samples rich in omega-3 fatty acids.50 The CADN spectrum confirms the presence of casein (α helix structure) by exhibiting its characteristic bands around 1650 cm−1 (amide I C
C stretching) and 2839 cm−1 (C–H stretching) in oil samples rich in omega-3 fatty acids.50 The CADN spectrum confirms the presence of casein (α helix structure) by exhibiting its characteristic bands around 1650 cm−1 (amide I C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretch). The bands between 1200 and 1300 cm−1 represent the (amide III) C–N stretch and N–H bending of casein in CADN.51 The characteristic bands between 1600 and 1750 cm−1 correspond to C
O stretch). The bands between 1200 and 1300 cm−1 represent the (amide III) C–N stretch and N–H bending of casein in CADN.51 The characteristic bands between 1600 and 1750 cm−1 correspond to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibrations of pectin.52 The bands occurring around 850 cm−1 indicate the presence of α-glycosidic bonds in PEDN and pectin. Another characteristic band around 478 cm−1 was observed in pectin as well as in PEDN, corresponding to galacturonic acid present in pectin.53 The characteristic spectra of chitosan were observed around 1670 cm−1, 1570 cm−1 and 1440 cm−1 corresponding to amide I, amide II, and amide III bands, respectively.54 Additional bands between 1320 and 1200 cm−1 (C–N bending) and around 1100 cm−1 (C–C) confirm the presence of chitosan in CHDN.54
O stretching vibrations of pectin.52 The bands occurring around 850 cm−1 indicate the presence of α-glycosidic bonds in PEDN and pectin. Another characteristic band around 478 cm−1 was observed in pectin as well as in PEDN, corresponding to galacturonic acid present in pectin.53 The characteristic spectra of chitosan were observed around 1670 cm−1, 1570 cm−1 and 1440 cm−1 corresponding to amide I, amide II, and amide III bands, respectively.54 Additional bands between 1320 and 1200 cm−1 (C–N bending) and around 1100 cm−1 (C–C) confirm the presence of chitosan in CHDN.54
 | ||
| Fig. 4 Raman spectra showing the chemical characterization of DHA nanoemulsions formulated with different emulsifiers. | ||
3.7. Viscosity measurement
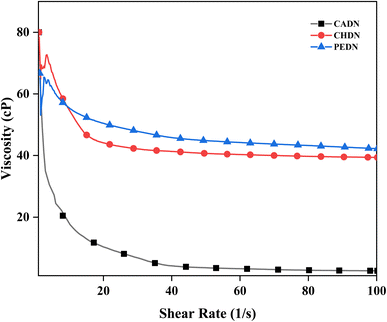
The DHA nanoemulsions were evaluated for viscosity measurements. The impact of the type of emulsifiers on the nanoemulsion's viscosity with respect to different shear rates is represented in Fig. 5. Along with CHDN, PEDN also exhibited favorable shear-thinning behavior, contributing to improved emulsion stability under varying shear rates. Significant changes (p < 0.05) in the viscosity of CADN, CHDN, and PEDN were detected with an increase in shear rate. The initial viscosity (shear rate 1 s−1) of CADN, CHDN, and PEDN was 80, 80 and 70.6 cP, which was decreased to 2.58, 39.38 and 42.23 cP, respectively, when the shear rate increased to 100 s−1, showing shear thinning behavior.55 Compared to casein, nanoemulsions containing chitosan and pectin had a higher viscosity, which correlates with the findings of Tavares et al.55 where garlic extract was encapsulated using whey protein isolate and chitosan. The viscosity of the coacervates showed non-Newtonian (shear thinning) behavior. The same behavior from the previous study was observed in chitosan with increased shear rate due to structural rearrangement or breakdown of molecules.56 In a similar study, nanoemulsions were prepared using chitosan and alginate as wall materials using the self-assembly method.57 It was found that the increase in chitosan concentration increased the viscosity of the nanoemulsions. The increase in viscosity is due to interactions in the hydrogen bonds, electrostatic bonds, and polymer chain. The addition of food hydrocolloids such as chitosan and pectin increases the viscosity of the emulsion, whereas ionic strength, size of particles, molecular weight, charge of particles, temperature, and particle interactions are the factors affecting viscosity.563.8. Influence of emulsifiers on electrostatic stability over storage
Zeta potential is a critical component in determining the electrostatic stability of colloidal systems.29 All three nanoemulsions (CADN, CHDN, and PEDN) had initial zeta potential values >30 mV, suggesting that there is greater electrostatic repulsion to keep particles from aggregation (Fig. 6A). CHDN had an initial zeta potential value of 42.2 ± 0.75 mV. The zeta potential value of CHDN after 28 days of storage was 34 ± 1 mV, which indicates higher stability. Higher levels of zeta potential often indicate more electrostatic repulsion between particles, which helps to avoid aggregation and preserve stability. The zeta potential may decrease with time due to the disintegration of the emulsifier, desorption, or rearrangement. The zeta potential of CADN dropped dramatically, indicating that emulsifier desorption may have happened more widely. Despite this reduction, CHDN maintained a zeta potential above 30 mV, suggesting it remains relatively stable. Pan et al.58 prepared nanocapsules using spray-dried technology with sodium caseinate to encapsulate curcumin, which had stable particles at a pH around 6.8 (−31.1 ± 0.9 mV) with a particle size of 168.7 ± 10.2 nm. Furthermore, casein was studied for its stability at various pH ranges from 4 to 8 to encapsulate β-carotene using spray-drying technology.59 It was found that, between pH 5 and 7, casein had a negative charge and had an isoelectric point at pH 4.4. In the above study, there was a noticeable increase in the stability of the spray-dried powders with a decrease in pH. However, below the isoelectric point, precipitation of casein occurred due to aggregation in particles. At the same time, β-carotene was well preserved at pH 5.5 at different concentrations of casein: 0.5% w/v (−20 to −30 mV), 1% w/v (−25 to −30 mV), and 5% w/v (around −20 mV). In a recent study, chitosan was modified using octenyl succinic anhydride to increase the stability of fish oil DHA nanoemulsions. The prepared nanoemulsion had an initial zeta potential value of 36 mV, which decreased to 32 mV upon storage (23 days).60 However, in the current study, formulation of DHA nanoemulsions using chitosan along with Tween 80 as an emulsifier improved the stability of DHA (42 mV) without any modification in the structure of chitosan.61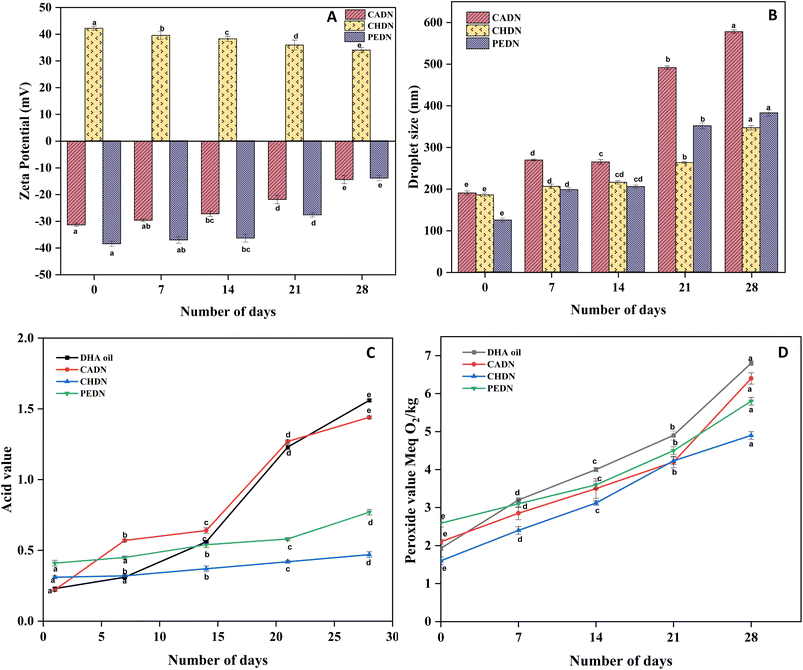 | ||
| Fig. 6 Storage stability of the DHA nanoemulsion. Change in (A) zeta potential, (B) droplet size, (C) acid value, and (D) peroxide value of the DHA nanoemulsion. | ||
3.9. Influence of droplet size on emulsifiers over storage
Over storage (28 days), the droplet size of chitosan nanoemulsion (346.7 ± 5.1 nm) was stable. For casein and pectin nanoemulsions, the droplet size was increased (p < 0.05) to 578 ± 4.16 nm and 382.3 ± 7.56 nm, respectively, over storage (Fig. 6B). This can be correlated with the study published by Guerra-Rosas et al.62 where the phase separation occurred with an increase in the droplet size of the emulsion prepared using pectin as an emulsifier. The significant increase in size suggests that factors such as pH changes, ionic interactions, or the presence of counter ions influence the stability, leading to droplet coalescence.63 Pectin and Tween 80 were used to encapsulate essential oils using the micro fluidization technique.62 The synthesized particles had a droplet size of less than 50 nm, which increased up to 1000 nm upon storage for 56 days. However, the zeta potential of the particles was very low, between −6 and −15 mV, depending on the type of essential oil used. The particle size affects the viscosity of an emulsion. Because the droplets in the nanoemulsion break with a higher shear rate, which in turn changes their molecular conformation. High shear rate, high-frequency vibration, and cavitation forces are some of the reasons for decreased viscosity.313.10. Oxidative stability of DHA nanoemulsions
The type of emulsifiers used in the emulsion influenced the final pH of the emulsion. The pH of casein, chitosan, and pectin immediately after preparation was 6.75 ± 0.02, 8.05 ± 0.04, and 2.16 ± 0.02, respectively. The pH of the neat DHA oil was 4.7 ± 0.01. The pH of DHA nanoemulsions varied significantly over storage (p < 0.05). The acid value of DHA nanoemulsions was determined to measure the rancidity. Free fatty acids are formed during storage, which is due to the decomposition of triglycerides present in the samples.32 The acid value of neat DHA oil was 0.23 ± 0.01 at the time of preparation. Geng et al.,64 used various heat treatments to study the stability of various cooking oils at different intervals of time (1 to 5 minutes). The acid value of the above study varied between 1.09 ± 0.13 and 3.48 ± 0.74 g, indicating lesser stability at higher temperatures for prolonged durations. The method of emulsion formulation and storage temperature affects the stability of DHA oil. Higher stability was found in CHDN (0.48 ± 0.03) at day 28, with the least stability found in CADN (1.45 ± 0.04) over storage (Fig. 6C).CADN, CHDN, and PEDN were determined for their primary oxidative products during storage. Lipid hydroperoxides produced by oil droplets quantify the extent of oxidation.32 During storage, CHDN was found to have higher oxidative stability (4.94 ± 0.1 Meq O2 per kg) compared to PEDN and CADN (Fig. 6D). This could be achieved due to the strong electrostatic repulsion between the DHA droplets and emulsifiers in nanoemulsions.60 Moreover, the oil becomes unacceptable (rancid) when the peroxide value is above 20 Meq O2 per kg. A peroxide value below 10 Meq O2 per kg indicates that the sample is fresh and acceptable for consumption. In a similar study, a peroxide value of 4.8 Meq O2 per kg was obtained after 7 days of nanoemulsion preparation formulated using chitosan and gelatin to encapsulate carp oil.32 Taken together, the higher oxidative stability attained by CHDN throughout storage was due to the antioxidant properties of chitosan to quench reactive oxygen species (ROS). The functional groups (–NH2 and –OH) present in chitosan are responsible for maintaining oxidative stability by neutralizing ROS.65 Encapsulation of DHA using chitosan forms a strong coating around DHA further protecting it from oxidation.66 Moreover, these results correlate with the electrostatic stability of DHA nanoemulsions stabilized by chitosan (CHDN). In the current study, higher electrostatic stability was maintained in CHDN (34 ± 1 mV) even after 28 days of storage. This could be possibly due to strong electrostatic repulsion between droplets, which prevents coalescence and exposure of the DHA droplet's surface to oxygen.66
3.11. Concentration of DHA in nanoemulsions over storage
From the shelf life studies it was evident that CHDN had greater oxidative stability over storage and was further studied (SI Fig. 2A–C). The encapsulation efficiency of the CHDN nanoemulsion on day 1 and day 28 was found to be 87.33% and 78.6%, respectively. About 90% of the DHA was preserved in the nanoemulsion from day 1, even after storage of 28 days. The initial (day 1) concentration of DHA in CHDN was 2 g per 100 g. At the end of storage period (28 days), the concentration of DHA was 1.8 g per 100 g. The other predominant fatty acids present in CHDN were oleic acid (2.82 g per 100 g), palmitic acid (3.9 g per 100 g) and myristic acid (0.14 g per 100 g). The concentrations of the above-mentioned fatty acids at the end of storage were 1.55 g per 100 g, 1.81 g per 100 g and 0.1 g per 100 g respectively. Interestingly, the retention of 90% of DHA during storage suggests higher oxidative stability. The encapsulation of DHA in a chitosan matrix and storage of the DHA nanoemulsion under refrigerated condition (4 °C) prevented oxidative degradation, even without the use of additional antioxidants. The droplet size of the DHA nanoemulsion stabilized by chitosan (185.1 ± 4.46 nm) was also an important parameter in maintaining the stability of DHA. The encapsulation of DHA using the chitosan-stabilized nanoemulsion demonstrated improved shelf life compared to unencapsulated DHA oil.The increased encapsulation efficiency of chitosan, the integrity of the interfacial layer created by chitosan and Tween 80, and the storage of DHA nanoemulsions in cold temperature (4 °C) were the factors that protected DHA from oxidation and reduced the formation of free radicals.67 Similar results were observed by Rao et al.8 where nanoemulsions containing omega-3 fatty acids were prepared, which had an encapsulation efficiency >80% with no phase separation for 4 weeks. Zeng et al. has demonstrated that nanoencapsulation of DHA using zein (a corn protein) creates a stable core–shell structure, mimicking milk fat globules. This structure not only protected DHA during digestion but also facilitated its absorption across the blood–brain barrier and increased its bioavailability.68 Nanoemulsions formulated using chitosan may improve DHA's bioaccessibility by enhancing lipid digestion and micelle formation, which are the key steps in the in vivo absorption process. The current research demonstrates that chitosan is the most effective emulsifier for producing stable DHA nanoemulsions, positioning it as a promising option for food applications.
3.12. Physicochemical analysis of kalakand
The chitosan-stabilized DHA nanoemulsion (CHDN) was fortified into kalakand to evaluate its stability in a dairy matrix during storage. Sensory analysis was performed immediately after the preparation of kalakand samples. Kalakand fortified with CHDN (KCDN) scored higher in overall acceptability compared to kalakand fortified with neat DHA oil (KDHO) in sensory analysis (Fig. 7A). Incorporation of DHA in the nanoemulsion masked the unpleasant fishy odor of DHA, whereas kalakand fortified with neat DHA oil had the fishy odor of DHA, making it unacceptable for consumption. In addition, KCDN showed a creamy mouthfeel due to the addition of the nanoemulsion and exhibited significantly higher (p < 0.05) cohesiveness (discussed below in Section 3.15). These results correlate with recent research where lipid vesicles loaded with omega-3 fatty acids were prepared to mask their unpleasant fishy odor.69 The formulated omega-3 lipid vesicles demonstrated higher sensory acceptance compared to the unencapsulated product. The nanoencapsulation process enhances the palatability of DHA and other omega 3 fatty acids, making them suitable candidates for food fortification.70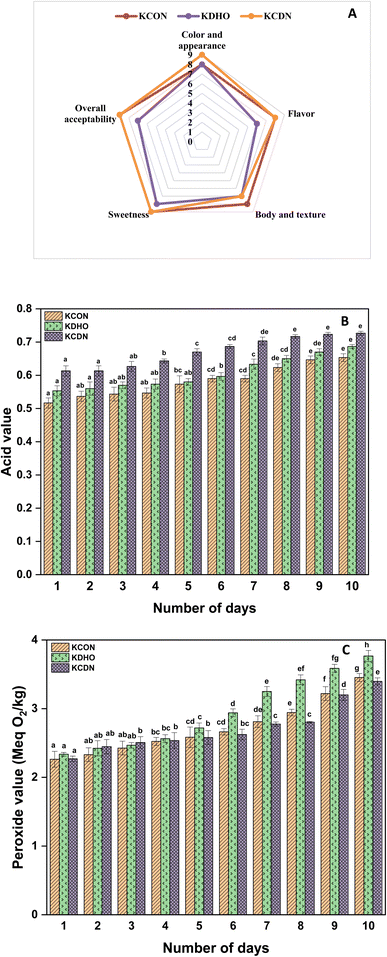 | ||
| Fig. 7 Sensory and storage study of kalakand fortified with the chitosan DHA nanoemulsion. (A) Sensory analysis, (B) acid value, and (C) peroxide value of kalakand over storage. | ||
The moisture content of food products affects various biochemical and microbiological activities.71 The moisture content of KCON has significantly decreased (p < 0.05) on day 10 (47.41 ± 0.06) compared to day 1 (48.57 ± 0.08). KDHO showed a similar trend (48.26 ± 0.2 on day 1 to 47.44 ± 0.08 on day 10) indicating minor dehydration over time (Table 5). KCDN exhibited the highest moisture retention over storage (49.57 ± 0.28 on day 1 and 48.58 ± 0.05 on day 10). This implies that the increased emulsification stability and reduced droplet size of the DHA nanoemulsion led to an increase in water-holding capacity. The initial water activity of KCON, KDHO and, KCDN was same (0.77 ± 0.01). There was no significant difference (p > 0.05) in the water activity across samples. The higher water activity (>0.95) supports the growth of spoilage microorganisms. Water activity impacts lipid oxidation, which alters the flavor and appearance of food products over storage and shortens their shelf life.72
| KCON | KDHO | KCDN | ||||
|---|---|---|---|---|---|---|
| Day 1 | Day 10 | Day 1 | Day 10 | Day 1 | Day 10 | |
| a KCON – kalakand control; KDHO – kalakand with neat DHA oil; KCDN – kalakand with the DHA nanoemulsion. Data are articulated as mean ± standard deviation (n = 3). In an individual row, each value tailed by a different letter is significantly different (p ≤ 0.05) between samples at day 1 and 10 as determined by Tukey's test. | ||||||
| Physicochemical analysis | ||||||
| Moisture | 48.57 ± 0.08b | 47.41 ± 0.06b | 48.26 ± 0.2b | 47.44 ± 0.08b | 49.57 ± 0.28a | 48.58 ± 0.05a |
| Ash | 2.15 ± 0.01a | 2.15 ± 0.02a | 2.15 ± 0.01a | 2.15 ± 0.01a | 1.97 ± 0.01b | 1.96 ± 0.01b |
| Protein | 19.64 ± 0.03a | 19.16 ± 0.03a | 18.21 ± 0.02c | 17.75 ± 0.05c | 18.51 ± 0.02b | 18.18 ± 0.05b |
| Fat | 23.64 ± 0.04b | 23.5 ± 0.05c | 23.86 ± 0.03a | 23.71 ± 0.02b | 23.98 ± 0.12a | 23.87 ± 0.02a |
| Total carbohydrates | 1.22 ± 0.01b | 1.21 ± 0.02a | 1.22 ± 0.01b | 1.21 ± 0.01a | 1.26 ± 0.01a | 1.26 ± 0.01b |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||
| Textural properties | ||||||
| Hardness (g) | 270.88 ± 2.49a | 553.95 ± 4.12a | 272.56 ± 2.02ab | 557.99 ± 1.68a | 285.13 ± 3.65b | 542.98 ± 2.55a |
| Springiness | 0.73 ± 0.01ab | 0.72 ± 0.01a | 0.61 ± 0.09b | 0.52 ± 0.01a | 0.79 ± 0.07a | 0.77 ± 0.01a |
| Cohesiveness | 0.68 ± 0.06a | 0.7 ± 0.01ab | 0.67 ± 0.02a | 0.66 ± 0.02b | 0.75 ± 0.04a | 0.77 ± 0.01a |
| Gumminess | 137 ± 2.19a | 137.65 ± 4.6b | 140.15 ± 1.67a | 141.09 ± 6.63ab | 156.25 ± 2.86b | 155.12 ± 2a |
| Chewiness | 343.89 ± 2.66b | 344.79 ± 4.6b | 356.65 ± 2a | 358.65 ± 6.63ab | 373.08 ± 2.48a | 374 ± 2a |
Ash content remains consistent across KCON and KDHO samples over storage, indicating no significant mineral loss (Table 5). KCDN samples consistently show the highest carbohydrate content (1.26 ± 0.01), followed by KCON (1.22 ± 0.01) and KDHO (1.22 ± 0.01). This may indicate the interaction of the nanoemulsion with residual sugars, promoting structural integrity, or because of the presence of chitosan, which is a polysaccharide.73
KCON showed higher protein content (19.64 ± 0.03 on day 1 and 19.16 ± 0.03 on day 10) compared to KDHO and KCDN. Protein content decreased significantly over time in all samples due to enzymatic or chemical reactions during storage (Table 5). KCON has the lowest fat content (23.64 ± 0.04 on day 1 and 23.5 ± 0.05 on day 10), reflecting the absence of DHA (Table 5). Higher fat content was observed in the KCDN sample (23.98 ± 0.12 on day 1 and 23.87 ± 0.02 on day 10), likely because nanoemulsions increase the dispersion of DHA, enhancing fat incorporation. Fat stability between day 1 and day 10 in KDCN suggests that DHA in the nanoemulsion form improved lipid stability compared to oil form.
The pH of KCON, KDHO, and KCDN on day 1 was found to be 6.56 ± 0.01, 6.53 ± 0.01, and 6.39 ± 0.01 respectively. The pH and acid value of kalakand varied significantly (p < 0.05) over storage (Fig. 7B). All the kalakand samples had good oxidative stability with a peroxide value <20 Meq O2 per kg. KDHO had a significantly higher peroxide value of 3.77 ± 0.08 (day 10) compared to KCON and KCDN (Fig. 7C).
3.13. Color analysis of kalakand
KCDN has a significantly higher L* index compared to KCON and KDHO on day 1 due to the incorporation of the DHA nanoemulsion (SI Table 2). Over storage (10 days), the lightness of the samples increased significantly (p < 0.05). The increase in lightness of kalakand over storage is likely due to a combination of factors primarily related to moisture loss, crystallization of sugar, and potential changes in the texture of the product.74 The increase in a* and b* over time for all three kalakand samples indicates a gradual shift towards a red hue, and yellow hue respectively. KCDN showed a similar increase in b*, but the rate of increase is significantly less (p < 0.05) compared to KCON and KDHO. All the kalakand samples showed a significant (p < 0.05) color change upon storage. Compared to KCDN (ΔE 3.95 ± 0.1), KDHO (ΔE 4.41 ± 0.1) had significantly higher (p < 0.05) color variation upon storage. The above findings show that chitosan nanoemulsion-incorporated kalakand was well preserved throughout storage.3.14. Concentration of DHA in fortified kalakand
The initial concentration of DHA in KCDN and KDHO on day 1 was 0.5 g per 100 g. The concentration of DHA in KCDN and KDHO on day 30 was 0.46 g per 100 g and 0.1 g per 100 g, respectively (SI Fig. 3A and B). About 92% of DHA was preserved in KCDN, and 20% of DHA was preserved in KDHO over storage (10 days). The incorporation of DHA in the nanoemulsion form protected DHA from degradation. Compared to neat DHA oil-incorporated kalakand (KDHO), kalakand with DHA-encapsulated nanoemulsions (KCDN) had a 72% higher DHA retention.In a study conducted by Ojagh et al.,75 nano-liposomes encapsulating fish oil were produced and fortified in bread. It was observed that fish oil nano-liposomes significantly increased the volume of bread loaf with good texture compared to free fish oil fortified bread.75 Moghadam et al. used gum Arabic to encapsulate fish oil to fortify fermented milk. It was found in the above study that the incorporation of nanoencapsulated fish oil increased the Lactobacillus plantarum count (8.41 log CFU mL−1), and the concentrations of DHA and EPA were higher compared to free fish oil.76 In another study, flaxseed oil and flaxseed oil nanoemulsion formulated using whey protein concentrate were fortified in butter. The fortified butter had 3.7 times more α-linolenic acid compared to free flaxseed oil with good spreadability.77 DHA-enriched egg products with unique compositions and varying structures (mousse, omelet, and hard-boiled egg) were developed to assess the recovery of DHA after preparation.78 DHA transportation through the duodenum was faster in mousse compared to omelet and hard-boiled egg. The recovery of DHA in mousse after the heat treatment at the time of preparation was 91.7 ± 3.6. Rao et al.8 fortified a rose hip essential oil nanoemulsion in yogurt and studied its stability over storage. From the above study, it was evident that yogurt fortified with a nanoemulsion was able to preserve omega-3 fatty acids (linoleic acid and oleic acid) compared to neat rosehip oil-fortified yogurt.8 In the current study, the DHA nanoemulsion was effectively fortified in kalakand with higher retention of DHA (92%) at the end of storage (10 days).
3.15. Texture profile analysis of kalakand
Texture is an important parameter determining the acceptability and quality of products. Hardness measures the force required to compress the food sample to a certain extent.71 During storage of kalakand samples (10 days), the hardness increased significantly (p < 0.05) with time, depending on their moisture content. KCDN (553.95 ± 4.12) had significantly reduced (p < 0.05) hardness over storage compared to KCON (557.99 ± 1.68) and KDHO (542.98 ± 2.55) due to its better moisture retention.The kalakand samples showed no significant difference (p > 0.05) in springiness throughout storage. Jain et al.79 reported similar observations in kalakand samples stored under modified atmosphere packaging conditions for 10 days. Cohesiveness measures the extent to which the food holds together during chewing.33 KCDN (0.75 ± 0.04) had significantly higher cohesiveness compared to KCON and KDHO (Table 5). The incorporation of the DHA nanoemulsion improved the texture by well binding with the kalakand, with no significant difference (p > 0.05) throughout storage, indicating retention of moisture. Furthermore, the gumminess of the kalakand fortified with the DHA nanoemulsion significantly increased (p < 0.05) over storage. The chewiness of the kalakand samples didn't vary significantly (p > 0.05) over storage.
3.16. Microbial analysis of kalakand
The total plate count (TPC) and yeast and mold count (YMC) of the chitosan-stabilized DHA nanoemulsion (CHDN), plain kalakand (KCON), kalakand fortified with neat DHA oil (KDHO), and kalakand fortified with CHDN (KCDN) were analyzed over storage. The TPC count of CHDN on day 1 of preparation was <10 CFU g−1. At the same time, on day 1 of storage, the TPC of KCON, KDHO, and KCDN was 2.9 log10 CFU g−1, 2.8 log10 CFU g−1, and 2.9 log10 CFU g−1, respectively. At the end of the storage period (10 days), the TPC of KCON, KDHO, and KCDN was found to be 4.27 log10 CFU g−1, 4.19 log10 CFU g−1, and 4.25 log10 CFU g−1, respectively. Before and after storage, there was no significant difference (p < 0.05) observed among KCON, KDHO, and KCDN. As per the FSSAI standards, the TPC and Y&M counts of paneer (cottage cheese)-based confectionaries should be less than 5.54 log10 CFU g−1 and less than 150 CFU g−1, respectively, for safer consumption. In the current study, all the samples had a TPC count of less than 5.54 log10 CFU g−1, which is under the recommended (FSSAI, 2015) microbial standard for paneer (cottage cheese)-based confectionaries. The Y&M count of KCON, KDHO, and KCDN at the end of storage was 13 CFU g−1, 9 CFU g−1, and 10 CFU g−1, respectively. Similar results were observed in a study where the TPC count of a kalakand sample on day 0 and day 7 of storage was 3.2 log10 CFU g−1 and 3.75 log10 CFU g−1, respectively.80 The observed increase in microbial count during storage may be attributed to the initial microbial load in the kalakand samples. Preparation of kalakand under sterile industrial conditions may increase its shelf life further.81 Overall, the findings from sensory, physicochemical, and shelf-life analyses indicate that the DHA nanoemulsion-fortified kalakand had higher acceptability compared to the kalakand fortified with neat DHA oil.4. Conclusion
The current study presents the importance of choosing appropriate emulsifiers for improving the stability of the DHA nanoemulsion using ultrasound, a green technology, and its effective application in a dairy confectionary, kalakand. Among the three emulsifiers—casein, chitosan, and pectin—the DHA nanoemulsion stabilized using chitosan (CHDN) showed enhanced oxidative stability with a higher zeta potential (42.2 ± 0.75 mV) and smaller droplet size (122.5 ± 2.5 nm). This strong electrostatic repulsion of chitosan prevented droplet aggregation throughout the storage (28 days). The polycationic nature and enhanced oil-holding capacity of chitosan improved the emulsification and protected DHA against oxidative degradation with high encapsulation efficiency (87.3%). Furthermore, fortification of kalakand with CHDN (KCDN) enhanced the sensory and nutritional properties of kalakand and masked DHA's unpleasant odor compared to neat DHA oil-incorporated kalakand. At the end of the storage study, kalakand with CHDN retained the higher concentration of DHA (92%) with good textural properties under refrigerated conditions (4 °C). The above findings confirm the use of the DHA nanoemulsion as an efficient approach to improve the stability and retention of DHA in dairy products such as kalakand. In addition, a detailed investigation into the bioavailability of the DHA nanoemulsion and the cost feasibility of producing DHA nanoemulsion-fortified food on an industrial scale remains scope for future studies. Furthermore, upcoming research should focus on expanding the application of nanoencapsulated DHA in other food matrices such as beverages, bakery products, and infant formulas. Fortification of DHA nanoemulsion in food matrices opens the door for transforming conventional food products into functional foods.Ethical statement
No formal approval was required for conducting sensory analysis, whilst appropriate protocols were still followed for protecting the rights and privacy of all participants involved in the research. Informed consent was obtained from the panelists before performing the experiment.Author contributions
Sirajdeen Asmath Mubeena: conceptualisation, methodology, investigation, validation, visualisation, formal analysis, project administration writing – original draft; Radhakrishnan Preetha: conceptualisation, supervision, writing – review & editing.Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included as part of the supplementary information (SI). Supplementary information is available. See DOI: https://doi.org/10.1039/d5fb00388a.Acknowledgements
The authors deeply acknowledge the Research Facility, School of Bioengineering, Department of Chemistry and Physics, C. V. Raman Research Centre, and Nano-technology Research Centre (NRC) at the SRM Institute of Science and Technology (SRMIST). The authors also thank Prof. C. Muthamizchelvan, VC, SRMIST, Kattankulathur and Dr Parani Madasamy, Chairperson, School of Bioengineering, SRMIST, for their support.References
- J. Wang, J. Ossemond, J. Jardin, V. Briard-Bion, G. Henry, Y. Le Gouar, O. Ménard, S. Lê, A. Madadlou, D. Dupont and F. Pédrono, Encapsulation of DHA oil with heat-denatured whey protein in Pickering emulsion improves its bioaccessibility, Food Res. Int., 2022, 162, 112112 CrossRef CAS PubMed.
- A. F. Yeşilsu, G. Alak, E. Alp Erbay, O. Sağdıç, F. Özoğul, İ. Demirkesen, N. B. Rathod, F. Bozkurt, C. Alasalvar, S. Benjakul, A. Rezek Jambrak and İ. Aydın, Sustainable Horizons: A Review on Sustainable Processing, Quality Enhancement, and Safety Assurance in Aquatic Food Products, Food Rev. Int., 2025, 41, 1709–1737 CrossRef.
- R. Katiyar and A. Arora, Health promoting functional lipids from microalgae pool: A review, Algal Res., 2020, 46, 101800 CrossRef.
- E. Ahonen, A. Damerau, J.-P. Suomela, M. Kortesniemi and K. M. Linderborg, Oxidative stability, oxidation pattern and α-tocopherol response of docosahexaenoic acid (DHA, 22:6n–3)-containing triacylglycerols and ethyl esters, Food Chem., 2022, 387, 132882 CrossRef CAS.
- A. Gattuso, C. Giacondino, S. Santacaterina, A. Piscopo and A. De Bruno, Freeze-dried microencapsulation of bergamot pomace extract: stability and antioxidant performance in hydrophilic and lipophilic systems, Sustainable Food Technol., 2025, 3, 1405–1415 RSC.
- M. Ashokkumar, Applications of ultrasound in food and bioprocessing, Ultrason. Sonochem., 2015, 25, 17–23 CrossRef CAS.
- T. N. Tan, Y. P. A. Mnocaran, M. E. Ramli, U. Utra, F. Ariffin and N. S. Yussof, Physical Properties, Antioxidant Activity, and In Vitro Digestibility of Essential Oil Nanoemulsions of Betel and Pandan Leaves, ACS Food Sci. Technol., 2023, 3, 150–160 Search PubMed.
- S. Rao, P. Radhakrishnan, S. Valiathan and S. M, Rosehip oil nanoemulsion as a stable delivery system for omega-3 fatty acids to enhance the nutritional value of yogurt, Food Chem. Adv., 2023, 3, 100545 CrossRef.
- A. G. Abdel-Razek, M. M. Hassanein, B. Ozçelik, D. A. Baranenko and T. M. El-Messery, Omega fatty acid-balanced oil formula and enhancing its oxidative stability by encapsulation with whey protein concentrate, Food Biosci., 2022, 50, 101975 Search PubMed.
- C. G. De Kruif and C. Holt, in Advanced Dairy Chemistry—1 Proteins, Springer US, Boston, MA, 2003, pp. 233–276 Search PubMed.
- C. Broyard and F. Gaucheron, Modifications of structures and functions of caseins: a scientific and technological challenge, Dairy Sci. Technol., 2015, 95, 831–862 Search PubMed.
- L. L. Wusigale and Y. Luo, Casein and pectin: Structures, interactions, and applications, Trends Food Sci. Technol., 2020, 97, 391–403 Search PubMed.
- C. Demetgül and N. Beyazit, Synthesis, characterization and antioxidant activity of chitosan-chromone derivatives, Carbohydr. Polym., 2018, 181, 812–817 Search PubMed.
- C. Branca, G. D'Angelo, C. Crupi, K. Khouzami, S. Rifici, G. Ruello and U. Wanderlingh, Role of the OH and NH vibrational groups in polysaccharide-nanocomposite interactions: A FTIR-ATR study on chitosan and chitosan/clay films, Polymer, 2016, 99, 614–622 Search PubMed.
- S. Kou, L. M. Peters and M. R. Mucalo, Chitosan: A review of sources and preparation methods, Int. J. Biol. Macromol., 2021, 169, 85–94 Search PubMed.
- P.-K. Chang, M.-F. Tsai, C.-Y. Huang, C.-L. Lee, C. Lin, C.-J. Shieh and C.-H. Kuo, Chitosan-Based Anti-Oxidation Delivery Nano-Platform: Applications in the Encapsulation of DHA-Enriched Fish Oil, Mar. Drugs, 2021, 19, 470 Search PubMed.
- J. Leroux, V. Langendorff, G. Schick, V. Vaishnav and J. Mazoyer, Emulsion stabilizing properties of pectin, Food Hydrocoll., 2003, 17, 455–462 Search PubMed.
- A. Sanchez, M. C. García, M. J. Martín-Piñero, J. Muñoz and M. Alfaro-Rodríguez, Elaboration and characterization of nanoemulsion with orange essential oil and pectin, J. Sci. Food Agric., 2022, 102, 3543–3550 Search PubMed.
- F. Adinepour, S. Pouramin, A. Rashidinejad and S. M. Jafari, Fortification/enrichment of milk and dairy products by encapsulated bioactive ingredients, Food Res. Int., 2022, 157, 111212 Search PubMed.
- R. Kumar, D. Mishra, H. Sutariya, M. B. Chaudhary and K. J. Rao, Effect of different coagulants on the yield, sensory, instrumental colour and textural characteristics of cow's milk Paneer, Int. J. Dairy Technol., 2019, 72, 617–625 CAS.
- A. Patel, S. S. Desai, V. K. Mane, J. Enman, U. Rova, P. Christakopoulos and L. Matsakas, Futuristic food fortification with a balanced ratio of dietary ω-3/ω-6 omega fatty acids for the prevention of lifestyle diseases, Trends Food Sci. Technol., 2022, 120, 140–153 CAS.
- T. N. Tan, Y. P. A. Mnocaran, M. E. Ramli, U. Utra, F. Ariffin and N. S. Yussof, Physical Properties, Antioxidant Activity, and In Vitro Digestibility of Essential Oil Nanoemulsions of Betel and Pandan Leaves, ACS Food Sci. Technol., 2023, 3, 150–160 Search PubMed.
- J. D. Estrada, C. Boeneke, P. Bechtel and S. Sathivel, Developing a strawberry yogurt fortified with marine fish oil, J. Dairy Sci., 2011, 94, 5760–5769 Search PubMed.
- S. Torres-Giner, A. Martinez-Abad, M. J. Ocio and J. M. Lagaron, Stabilization of a Nutraceutical Omega-3 Fatty Acid by Encapsulation in Ultrathin Electrosprayed Zein Prolamine, J. Food Sci., 2010, 75(6), N69–N79 Search PubMed.
- M. Ahmedna, W. Prinyawiwatkul and R. M. Rao, Solubilized Wheat Protein Isolate: Functional Properties and Potential Food Applications, J. Agric. Food Chem., 1999, 47, 1340–1345 CAS.
- L. Wang, S. Zhang, W. Jiang, H. Zhao and J. Fu, Ability of casein hydrolysate-carboxymethyl chitosan conjugates to stabilize a nanoemulsion: Improved freeze-thaw and pH stability, Food Hydrocoll., 2020, 101, 105452 Search PubMed.
- Z. Yu, L. Zhou, Z. Chen, L. Chen, K. Hong, D. He and F. Lei, Fabrication and Characterization of Docosahexaenoic Acid Algal Oil Pickering Emulsions Stabilized Using the Whey Protein Isolate–High-Methoxyl Pectin Complex, Foods, 2024, 13, 2159 CAS.
- H. Singh, C. Kumar, N. Singh, S. Paul and S. K. Jain, Nanoencapsulation of docosahexaenoic acid (DHA) using a combination of food grade polymeric wall materials and its application for improvement in bioavailability and oxidative stability, Food Funct., 2018, 9, 2213–2227 CAS.
- G. Himanath, R. Shruthy, R. Preetha and V. Sreejit, Nanoemulsion with Coconut Oil and Soy Lecithin as a Stable Delivery System for Lycopene and Its Incorporation into Yogurt to Enhance Antioxidant Properties and Maintain Quality, ACS Food Sci. Technol., 2021, 1, 1538–1549 Search PubMed.
- R. Geetanjali, V. Sreejit, P. Sandip and R. Preetha, Preparation of aloe vera mucilage- ethyl vanillin Nano-emulsion and its characterization, Mater. Today: Proc., 2021, 43, 3766–3773 CAS.
- M. Alfaro-Rodríguez, P. Prieto, M. C. García, M. J. Martín-Piñero and J. Muñoz, Influence of nanoemulsion/gum ratio on droplet size distribution, rheology and physical stability of nanoemulgels containing inulin and omega-3 fatty acids, J. Sci. Food Agric., 2022, 102, 6397–6403 Search PubMed.
- V. M. Esquerdo, P. P. Silva, G. L. Dotto and L. A. A. Pinto, Nanoemulsions From Unsaturated Fatty Acids Concentrates of Carp Oil Using Chitosan, Gelatin, and Their Blends as Wall Materials, Eur. J. Lipid Sci. Technol., 2018, 120(2), 1700240 Search PubMed.
- V. Jain, P. Rasane, A. Jha, N. Sharma and A. Gautam, Effect of modified atmospheric packaging on the shelf life of Kalakand and its influence on microbial, textural, sensory and physico-chemical properties, J. Food Sci. Technol., 2015, 52, 4090–4101 Search PubMed.
- G. T. Karsli, S. Sahin and M. H. Oztop, High-Pressure-Homogenized Clove and Thyme Oil Emulsions: Formulation, Stability, and Antioxidant Capacity, ACS Food Sci. Technol., 2022, 2, 1832–1839 Search PubMed.
- G. Himanath, R. Shruthy, R. Preetha and V. Sreejit, Nanoemulsion with Coconut Oil and Soy Lecithin as a Stable Delivery System for Lycopene and Its Incorporation into Yogurt to Enhance Antioxidant Properties and Maintain Quality, ACS Food Sci. Technol., 2021, 1, 1538–1549 Search PubMed.
- J. F. Fangueiro, T. Andreani, M. A. Egea, M. L. Garcia, S. B. Souto, A. M. Silva and E. B. Souto, Design of cationic lipid nanoparticles for ocular delivery: Development, characterization and cytotoxicity, Int. J. Pharm., 2014, 461, 64–73 CrossRef CAS PubMed.
- V. P. Hoven, V. Tangpasuthadol, Y. Angkitpaiboon, N. Vallapa and S. Kiatkamjornwong, Surface-charged chitosan: Preparation and protein adsorption, Carbohydr. Polym., 2007, 68, 44–53 CrossRef CAS.
- N. A. Alhakamy, H. M. Aldawsari, K. M. Hosny, J. Ahmad, S. Akhter, A. K. Kammoun, A. F. Alghaith, H. Z. Asfour, M. W. Al-Rabia and S. Md, Formulation design and pharmacokinetic evaluation of docosahexaenoic acid containing self-nanoemulsifying drug delivery system for oral administration, Nanomater. Nanotechnol., 2020, 10, 184798042095098 CrossRef.
- R. A. Holser, Docosahexaenoic Acid Ester Degradation Measured by FTIR-ATR with Correlation Spectroscopy, Am. J. Anal. Chem., 2014, 05, 373–377 CrossRef.
- K. E. Orskov, L. B. Christensen, L. Wiking, T. Hannibal and M. Hammershoj, Detecting interactions between starch and casein in imitation cheese by FTIR and Raman spectroscopy, Food Chem. Adv., 2023, 2, 100322 CrossRef.
- T. Markoska, D. Daniloski, T. Vasiljevic and T. Huppertz, Structural Changes of β-Casein Induced by Temperature and pH Analysed by Nuclear Magnetic Resonance, Fourier-Transform Infrared Spectroscopy, and Chemometrics, Molecules, 2021, 26, 7650 CrossRef CAS PubMed.
- D. Demir, S. Ceylan, D. Göktürk and N. Bölgen, Extraction of pectin from albedo of lemon peels for preparation of tissue engineering scaffolds, Polym. Bull., 2021, 78, 2211–2226 CrossRef CAS.
- A. Fellah, P. Anjukandi, M. R. Waterland and M. A. K. Williams, Determining the degree of methylesterification of pectin by ATR/FT-IR: Methodology optimisation and comparison with theoretical calculations, Carbohydr. Polym., 2009, 78, 847–853 CrossRef CAS.
- E. E. Santos, R. C. Amaro, C. C. C. Bustamante, M. H. A. Guerra, L. C. Soares and R. E. S. Froes, Extraction of pectin from agroindustrial residue with an ecofriendly solvent: use of FTIR and chemometrics to differentiate pectins according to degree of methyl esterification, Food Hydrocoll., 2020, 107, 105921 CrossRef CAS.
- E. Alehosseini, H. Shahiri Tabarestani, M. S. Kharazmi and S. M. Jafari, Physicochemical, Thermal, and Morphological Properties of Chitosan Nanoparticles Produced by Ionic Gelation, Foods, 2022, 11, 3841 CrossRef CAS PubMed.
- I. Dziedzic and A. Kertmen, Methods of chitosan identification: history and trends, Lett. Appl. Nanobioscience, 2023, 12(4), 94 Search PubMed.
- M. Ruffo, O. I. Parisi, F. Patitucci, M. Dattilo, R. Malivindi, F. Amone, C. Morelli, A. Nigro, D. Sisci and F. Puoci, Controlled Release of 5-FU from Chi–DHA Nanoparticles Synthetized with Ionic Gelation Technique: Evaluation of Release Profile Kinetics and Cytotoxicity Effect, J. Funct. Biomater., 2020, 11, 48 CrossRef CAS PubMed.
- E. Liu, Z. Su, C. Yang, Y. Ji, B. Liu and X. Meng, Fabrication, characterization and properties of DHA-loaded nanoparticles based on zein and PLGA, Food Chem., 2021, 360, 129957 CrossRef CAS.
- K. Esmonde-White, M. Lewis, T. Perilli, T. Della Vedova and I. Lewis, Raman Spectroscopy in Analyzing Fats and Oils in Foods, Spectroscopy, 2022, 34–45 Search PubMed.
- D. P. Killeen, S. N. Marshall, E. J. Burgess, K. C. Gordon and N. B. Perry, Raman Spectroscopy of Fish Oil Capsules: Polyunsaturated Fatty Acid Quantitation Plus Detection of Ethyl Esters and Oxidation, J. Agric. Food Chem., 2017, 65, 3551–3558 CrossRef CAS PubMed.
- R. R. Panthi, S. N. Shibu, T. J. Ochalski and J. A. O'Mahony, Raman spectra of micellar casein powders prepared with wet blending of glycomacropeptide and micellar casein concentrate, Int. J. Dairy Technol., 2023, 76, 429–435 CrossRef CAS.
- H. Schulz and M. Baranska, Identification and quantification of valuable plant substances by IR and Raman spectroscopy, Vib. Spectrosc., 2007, 43, 13–25 CrossRef CAS.
- M. Chylińska, M. Szymańska-Chargot and A. Zdunek, Imaging of polysaccharides in the tomato cell wall with Raman microspectroscopy, Plant Methods, 2014, 10, 14 CrossRef PubMed.
- R. Nirmala, B. W. Il, R. Navamathavan, M. H. El-Newehy and H. Y. Kim, Preparation and characterizations of anisotropic chitosan nanofibers via electrospinning, Macromol. Res., 2011, 19, 345–350 CrossRef CAS.
- L. Tavares and C. P. Zapata Noreña, Encapsulation of garlic extract using complex coacervation with whey protein isolate and chitosan as wall materials followed by spray drying, Food Hydrocoll., 2019, 89, 360–369 Search PubMed.
- M. Glicksman, Functional Properties of Hydrocolloids, CRC Press, 1982 Search PubMed.
- A.-J. Choi, C.-J. Kim, Y.-J. Cho, J.-K. Hwang and C.-T. Kim, Characterization of Capsaicin-Loaded Nanoemulsions Stabilized with Alginate and Chitosan by Self-assembly, Food Bioprocess Technol., 2011, 4, 1119–1126 CrossRef CAS.
- K. Pan, Q. Zhong and S. J. Baek, Enhanced Dispersibility and Bioactivity of Curcumin by Encapsulation in Casein Nanocapsules, J. Agric. Food Chem., 2013, 61, 6036–6043 Search PubMed.
- T. Jarunglumlert and K. Nakagawa, Spray Drying of Casein Aggregates Loaded with β-Carotene: Influences of Acidic Conditions and Storage Time on Surface Structure and Encapsulation Efficiencies, Drying Technol., 2013, 31, 1459–1465 CrossRef CAS.
- Z. Yan, X. Wang, P. Zhao, Y. He, X. Meng and B. Liu, The effect of octenyl succinic anhydride-modified chitosan coating on DHA-loaded nanoemulsions: Physichemical stability and in vitro digestibility, Food Chem., 2024, 441, 138289 Search PubMed.
- Q. Zhou, K. E. Lane and W. Li, Evaluating the Stability and Digestibility of Long-Chain Omega-3 Algal Oil Nanoemulsions Prepared with Lecithin and Tween 40 Emulsifiers Using an In Vitro Digestion Model, Foods, 2024, 13, 2407 CrossRef CAS PubMed.
- M. I. Guerra-Rosas, J. Morales-Castro, L. A. Ochoa-Martínez, L. Salvia-Trujillo and O. Martín-Belloso, Long-term stability of food-grade nanoemulsions from high methoxyl pectin containing essential oils, Food Hydrocoll., 2016, 52, 438–446 CrossRef CAS.
- M. S. Algahtani, M. Z. Ahmad and J. Ahmad, Investigation of Factors Influencing Formation of Nanoemulsion by Spontaneous Emulsification: Impact on Droplet Size, Polydispersity Index, and Stability, Bioengineering, 2022, 9, 384 CrossRef CAS PubMed.
- L. Geng, W. Zhou, X. Qu, R. Sa, J. Liang, X. Wang and M. Sun, Iodine values, peroxide values and acid values of Bohai algae oil compared with other oils during the cooking, Heliyon, 2023, 9, e15088 CrossRef CAS PubMed.
- Z. Wang, Y. Yan, Z. Zhang, C. Li, L. Mei, R. Hou, X. Liu and H. Jiang, Effect of Chitosan and Its Water-Soluble Derivatives on Antioxidant Activity, Polymers, 2024, 16, 867 CrossRef CAS PubMed.
- P. Inthamat and U. Siripatrawan, Influence of chitosan encapsulation on functionality and stability of astaxanthin nanoemulsion fabricated using high pressure homogenization, Int. J. Biol. Macromol., 2025, 303, 140379 CrossRef CAS PubMed.
- P. Zhao, Y. Ji, H. Yang, X. Meng and B. Liu, Soy Protein Isolate–Chitosan Nanoparticle-Stabilized Pickering Emulsions: Stability and In Vitro Digestion for DHA, Mar. Drugs, 2023, 21, 546 CrossRef CAS PubMed.
- J. Zeng, W. Yu, X. Dong, S. Zhao, Z. Wang, Y. Liu, M.-S. Wong and Y. Wang, A nanoencapsulation suspension biomimetic of milk structure for enhanced maternal and fetal absorptions of DHA to improve early brain development, Nanomedicine, 2019, 15, 119–128 CrossRef CAS.
- S. Shariat, V. Hakimzadeh and A. Pardakhty, The physicochemical and organoleptic evaluation of the nano/micro encapsulation of Omega-3 fatty acids in lipid vesicular systems, Nanomed. J., 2020, 7, 80–86 CAS.
- G. Rathod and N. Kairam, Preparation of omega 3 rich oral supplement using dairy and non-dairy based ingredients, J. Food Sci. Technol., 2018, 55, 760–766 CrossRef CAS.
- R. Kapoor, A. Jash and S. S. H. Rizvi, Shelf-life extension of Paneer by a sequential supercritical-CO2-based process, LWT, 2021, 135, 110060 CrossRef CAS.
- Q. Rao, M. C. Fisher, M. Guo and T. P. Labuza, Storage Stability of a Commercial Hen Egg Yolk Powder in Dry and Intermediate-Moisture Food Matrices, J. Agric. Food Chem., 2013, 61, 8676–8686 CrossRef CAS PubMed.
- S. Kou, L. M. Peters and M. R. Mucalo, Chitosan: A review of sources and preparation methods, Int. J. Biol. Macromol., 2021, 169, 85–94 CrossRef CAS PubMed.
- R. Kumar, D. Mishra, H. Sutariya, M. B. Chaudhary and K. J. Rao, Effect of different coagulants on the yield, sensory, instrumental colour and textural characteristics of cow's milk Paneer, Int. J. Dairy Technol., 2019, 72, 617–625 CrossRef CAS.
- S. M. Ojagh and S. Hasani, Characteristics and oxidative stability of fish oil nano-liposomes and its application in functional bread, J. Food Meas. Char., 2018, 12, 1084–1092 CrossRef.
- F. V. Moghadam, R. Pourahmad, A. Mortazavi, D. Davoodi and R. Azizinezhad, Use of Fish Oil Nanoencapsulated with Gum Arabic Carrier in Low Fat Probiotic Fermented Milk, Food Sci. Anim. Resour., 2019, 39, 309–323 CrossRef PubMed.
- V. S. Pandule, M. Sharma, D. HC and S. N. B, Omega-3 fatty acid-fortified butter: Preparation and characterisation of textural, sensory, thermal and physico-chemical properties, Int. J. Dairy Technol., 2021, 74, 181–191 CrossRef CAS.
- C. Pineda-Vadillo, F. Nau, C. Guérin-Dubiard, C. Bourlieu, F. Capozzi, A. Bordoni and D. Dupont, In Vivo Digestion of Egg Products Enriched with DHA: Effect of the Food Matrix on DHA Bioavailability, Foods, 2020, 10, 6 CrossRef PubMed.
- V. Jain, P. Rasane, A. Jha, N. Sharma and A. Gautam, Effect of modified atmospheric packaging on the shelf life of Kalakand and its influence on microbial, textural, sensory and physico-chemical properties, J. Food Sci. Technol., 2015, 52, 4090–4101 CrossRef CAS PubMed.
- H. M. Gawande, S. Arora, V. Sharma and B. K. Wadhwa, Aspartame: safety and stability in kalakand, J. Food Sci. Technol., 2015, 52, 2373–2379 CrossRef CAS.
- B. C. Viljoen, The interaction between yeasts and bacteria in dairy environments, Int. J. Food Microbiol., 2001, 69, 37–44 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2026 |