Open Access Article
Open Access ArticleGreen extraction of phenolic compounds and carotenoids from the pulp and peel of mango criollo by sustainable emerging technologies
Elena
Rodríguez-Rodríguez
 *a,
Gonzalo Adrián
Ojeda
*a,
Gonzalo Adrián
Ojeda
 b,
Sonia Cecilia
Sgroppo
b,
Begoña
Olmedilla-Alonso
b,
Sonia Cecilia
Sgroppo
b,
Begoña
Olmedilla-Alonso
 c,
Concepción
Sánchez-Moreno
c,
Concepción
Sánchez-Moreno
 d,
Milagros
Sánchez-Prieto
c,
Lucía
Giménez
d,
Ruth
Núñez-Flores
d and
Begoña
De Ancos
*d
d,
Milagros
Sánchez-Prieto
c,
Lucía
Giménez
d,
Ruth
Núñez-Flores
d and
Begoña
De Ancos
*d
aDepartment of Chemistry in Pharmaceutical Sciences, Analytical Chemistry, Faculty of Pharmacy, Complutense University of Madrid (UCM), VALORNUT Research Group (920030-UCM), Madrid, Spain. E-mail: elerodri@ucm.es
bUniversidad Nacional del Nordeste (UNNE), Instituto de Química Básica y Aplicada del Nordeste Argentino (IQUIBA NEA-CONICET-UNNE), Corrientes, Argentina
cDepartment of Metabolism and Nutrition, Institute of Food Science, Technology and Nutrition (ICTAN-CSIC), Madrid, Spain
dDepartment of Characterization, Quality and Safety, Institute of Food Science, Technology and Nutrition (ICTAN-CSIC), Madrid, Spain. E-mail: ancos@ictan.csic.es
First published on 21st October 2025
Abstract
The present study investigates the recovery of bioactive compounds from underutilized and non-commercial mango criollo by using sustainable technologies. Deep eutectic solvents (DES) [β-alanine (β-Ala) with malic acid (DES-1) and citric acid (DES-2) and choline chloride (ChCl) with ethylene glycol (DES-3) and glycerol (DES-4)] combined with ultrasound-assisted extraction (UAE) [500 W–20 kHz at 36 μm/6 min for peel and at 45 μm/30 min for pulp] (DES-UAE) successfully isolated phenolic compounds, carotenoids, and terpenoids from mango criollo pulp and peel. Conventional extraction yielded 10 times more phenolic compounds and 3 times more carotenoids in the peel than in the pulp. ChCl-based DES significantly improved the extraction of phenolic compounds and antioxidant activity in both pulp and peel compared to conventional extraction (stirring with aqueous ethanol). Total phenolic compounds in peel with DES-4 (4.71 mg g−1 DW) and in pulp with DES-2 (0.87 mg g−1 DW) represented the best results with increases of 29% and 72%, respectively, compared to conventional extraction. Carotenoid recovery in pulp was similar between methods, while in the peel, the conventional extraction (stirring with diethyl ether/petroleum ether) was superior. All DES in pulp recovered ∼85% of the carotenoids that conventional methods yielded (42.74 μg g−1 DW), but DES in peel were less effective than conventional extraction (129.70 μg g−1 DW), where the best result was with β-Ala-based DES-2 that extracted 71% of the carotenoids obtained by conventional extraction. DES-UAE presents a promising, eco-friendly alternative for extracting valuable compounds from mango waste, supporting applications in the food industry while reducing environmental impact.
Sustainability spotlightThe research on “Green extraction of phenolic compounds and carotenoids from the pulp and peel of mango criollo by sustainable emerging technologies” presents a significant sustainable advance by developing an eco-friendly method for extracting valuable bioactive compounds from mango by-products. This work utilizes deep eutectic solvents (DES) and ultrasound-assisted extraction (UAE), offering a greener, safer alternative to conventional methods that rely on harmful organic solvents, reducing environmental impact and energy consumption. This innovation directly aligns with SDG 12: Responsible Consumption and Production, by transforming agricultural waste into beneficial resources, thus minimizing environmental impact and promoting circular economy principles; SDG 9: Industry, Innovation, and Infrastructure, through the development and implementation of sustainable, cutting-edge extraction technologies in the food industry; and SDG 13: Climate Action, by promoting sustainable resource use and reducing agro-industrial waste. |
1 Introduction
Mango (Mangifera indica L.) is widely cultivated in tropical and subtropical areas. Global mango production in 2023 was around 59.3 million tonnes (including mangosteens and guavas), with India, China and Thailand being the largest producers in the world, although India's production represents around 40% globally.1 Mango consumption has increased worldwide as fresh fruit and in derived products (juice, nectars, smoothies, concentrate, jams, dried fruit, etc.) due to its sensory characteristics and nutrient richness, including carbohydrates, lipids, vitamins, proteins, minerals, and bioactive compounds like dietary fiber, polyphenols (phenolic acids, gallotannins, flavonols, flavanols, benzophenones and xanthones such as mangiferin), and carotenoids (β-carotene and β-cryptoxanthin, among others). These compounds have potential health benefits against major global health problems.2–5 Studies suggest that mango extracts possess antidiabetic, anticancer, anti-inflammatory, antioxidant, and antibacterial activities.6–10The agro-food industry generates significant by-products (and waste) like peels and seeds from mango processing (35–60% of the fruit), which are rich in bioactive compounds.11,12 Whole mangoes are also discarded due to factors like size and appearance,13 as is the case with mango criollo in Argentina, valued for its phenolic compounds and carotenoids despite its low commercial value due to high fiber content and small size. Mango peel contains more polyphenols than pulp and is a significant source of carotenoids, mainly all-trans-β-carotene (around 60% of total carotenoids). The content of these compounds varies by cultivar, location, and maturity.2,4
The valorisation of mango by-products and waste must be updated to use green extraction techniques that replace traditional methods that use harmful solvents, reducing processing costs and energy.14
Alternatives include heterogeneous catalysts, water, supercritical fluids, ionic liquids,15 nanoparticles16,17 and deep eutectic solvents (DES) for extracting carotenoids and polyphenols.18,19 DES are biocompatible, low-cost, and biodegradable,20,21 formed by mixing a hydrogen bond acceptor (HBA), like choline chloride (ChCl) or β-alanine (β-Ala) with a hydrogen bond donor (HBD) such as amino acids or sugars.22,23 Natural deep eutectic solvents (NaDES) use naturally occurring components and can extract polar bioactive compounds by interacting with them or by disrupting cell walls.24,25
While most DES are used for hydrophilic compounds, some hydrophilic NaDES can dissolve lipophilic compounds like carotenoids.26 Sustainable assisted extraction methods using microwave, high-hydrostatic pressure, pulsed electric field, and ultrasound-assisted extraction (UAE) are being investigated to improve efficiency and reduce time, energy, and solvent use.
UAE is based on the acoustic cavitation phenomenon that is the formation and growth of microbubbles during the period of negative pressure; these microbubbles are compressed and collapsed producing damage in the cell wall that allows better solvent penetration and the release of bioactive compounds. The combination of deep eutectic solvents (DES) with ultrasound-assisted extraction (UAE) could be more effective than other green extraction technologies because, on one hand, UAE enhances the extraction efficiency by improving the mass transfer and reducing the extraction times, energy consumption and solvent volume, and on the other hand, DES are biodegradable, non-toxic, and low-cost, making them a sustainable alternative to conventional organic solvents. For these reasons, the combination of DES + UAE is a highly effective and environmentally friendly method for extracting compounds from fruit by-products aligning with green chemistry principles.2,14,27,28
This study aimed to explore the simultaneous extraction of phenolic and carotenoid compounds from mango criollo pulp and peel using DES (ChCl and β-Ala) combined with UAE, as these DES components are used in food supplements, allowing potential direct application of the extracts (without requiring the isolation of bioactive molecules from the solvent).
2 Materials and methods
2.1 Sample preparation
Mango criollo fruits (50 units, totaling six kilograms) at full maturity were collected from trees located in Corrientes, Argentina (latitude − 27.510501795690335, longitude − 58.82515496578836). Immediately post-harvest, the fruits underwent a washing step with water, followed by drainage on paper. Subsequently, the peel was manually separated using a sharp knife, and the remaining pulp was obtained by gentle scraping with a blunt edge, yielding two fractions: peel and pulp. These fractions were then freeze-dried for 48 hours at −58 °C and 0.035 mbar using a Christ Alpha 1–4 LD lyophilizer (Osterode, Germany) before being ground to a particle size of less than 0.25 mm (60 mesh) with an Arcano FW 100 industrial grinder (Beijing, China). The resulting dry powders were stored in airtight, opaque black PVC containers without air space and kept at −20 °C. These samples were then transported by air to ICTAN in Madrid, Spain, within 48 hours and stored again at −20 °C until experimentation.2.2 Chemical and reagents
Absolute ethanol (ETOH), methanol (MeOH) and acetonitrile (HPLC-grade) were provided by Lab-Scan (Dublin, Ireland). Analytical grade formic acid (98%) was purchased from Panreac Química (Barcelona, Spain). Methyl tert-butyl ether (MTBE), tetrahydrofuran (THF), petroleum ether (PE) and diethyl ether (DE), were obtained from Análisis Vínicos (Tomelloso, Spain). Deionised water of 18 MΩ cm purified with a Milli-Q system (Millipore, Bedford, USA), was used. Folin Ciocalteu reagent, 2,2-diphenyl-1-picrylhydrazyl (DPPH) and 2,2′-azinobis(3-ethylbenzothiazoline)-6-sulphonate (ABTS), gallic acid, quercetin, maclurin, mangiferin, quercitrin, quercetin-3-O-galactoside, quercetin-3-O-rhamnoside, ellagic acid and (−) catechin, lutein (xanthophyll from marigold), zeaxanthin, α- and β-carotene, β-cryptoxanthin, tocopherol acetate, potassium hydroxide (KOH), pyrogallic acid and trimethylamine were obtained from Sigma Aldrich (Madrid, Spain).2.3 Deep eutectic solvent (DES) preparation and characterization
| DES-1 | DES-2 | DES-3 | DES-4 | |
|---|---|---|---|---|
| a HBA: hydrogen bond acceptors, HBD, hydrogen bond donors, Td: degradation temperature, and ENR = molar transition energy. | ||||
| HBA | β-Alanine | β-Alanine | Choline chloride | Choline chloride |
| HBD | DL-Malic acid | Citric acid | Ethylene glycol | Glycerol |
| Other | H2O | H2O | — | — |
| Molar ratio | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
| Colour of solution | Light yellow | Light yellow | Colourless | Colourless |
| Viscosity (cps) | 750 ± 30 | 2350 ± 25 | 150 ± 15 | 450 ± 20 |
| Td °C | 148.54 ± 0.20 | 149.59 ± 0.05 | 203.58 ± 0.86 | 182.48 ± 0.07 |
| Polarity ENR (kcal mol−1) | 48.08 ± 0.34 | 47.89 ± 0.43 | 50.53 ± 0.17 | 50.13 ± 0.42 |
The molar transition energy (ENR) (kcal mol−1) of the solvents employed in the extraction process was calculated using the equation ENR (kcal mol−1) = hc/λmax = 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 591/λmax. Lower ENR values indicate higher polarity because solvents with higher polarity exhibit higher shifts in λmax.31 Thus, ENR values (kcal mol−1) for water (48.46 ± 0.30), ETOH (52.05 ± 0.11), MeOH (51.75 ± 0.17), THF (55.15 ± 0.18), DE (55.29 ± 0.40), PE (56.12 ± 0.50), a mix of DE
591/λmax. Lower ENR values indicate higher polarity because solvents with higher polarity exhibit higher shifts in λmax.31 Thus, ENR values (kcal mol−1) for water (48.46 ± 0.30), ETOH (52.05 ± 0.11), MeOH (51.75 ± 0.17), THF (55.15 ± 0.18), DE (55.29 ± 0.40), PE (56.12 ± 0.50), a mix of DE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PE (50
PE (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) (55.82 ± 0.30), DES-1 (48.08 ± 0.34), DES-2 (47.89 ± 0.43), DES-3 (50.53 ± 0.17) and DES-4 (50.13 ± 0.42) were calculated (Table 1).
50) (55.82 ± 0.30), DES-1 (48.08 ± 0.34), DES-2 (47.89 ± 0.43), DES-3 (50.53 ± 0.17) and DES-4 (50.13 ± 0.42) were calculated (Table 1).
2.4 Extraction procedures
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 (g mL−1) for peel and 1
30 (g mL−1) for peel and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 (g mL−1) for pulp with an ETOH/water ratio of 46
15 (g mL−1) for pulp with an ETOH/water ratio of 46![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 54 (v/v) for peel and 25
54 (v/v) for peel and 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 75 (v
75 (v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v) for pulp. The mixtures were homogenized for 3 min at 9500 rpm using an Ultra-Turrax homogenizer (T-18 Digital, IKA Works Inc, Breisgau, Germany). Then, the mixtures were magnetically stirred (800 rpm) for 1 h at room temperature and the extracts were centrifuged in a Sorvall Evolution RC centrifuge (Thermo Fisher Scientific Inc, USA) at 16
v) for pulp. The mixtures were homogenized for 3 min at 9500 rpm using an Ultra-Turrax homogenizer (T-18 Digital, IKA Works Inc, Breisgau, Germany). Then, the mixtures were magnetically stirred (800 rpm) for 1 h at room temperature and the extracts were centrifuged in a Sorvall Evolution RC centrifuge (Thermo Fisher Scientific Inc, USA) at 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g at 4 °C for 15 min and the supernatants were separated and concentrated at 40 °C in a rotary evaporator (Büchi model R111 Evaporator Lab), made up to 10 mL with ETOH and stored at −20 °C until analysis. Extraction was performed in duplicate (n = 2).
000g at 4 °C for 15 min and the supernatants were separated and concentrated at 40 °C in a rotary evaporator (Büchi model R111 Evaporator Lab), made up to 10 mL with ETOH and stored at −20 °C until analysis. Extraction was performed in duplicate (n = 2).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 (g mL−1) for peel and 1
30 (g mL−1) for peel and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 (g mL−1) for pulp. The mixtures were previously homogenized for 3 min at 9500 rpm by using an Ultra-Turrax homogenizer (T-18 Digital, IKA Works Inc, Breisgau, Germany). Ultrasound-assisted extraction (UAE) was performed using a 19 mm solid tip sonotrode (maximum amplitude of 60 μm) connected to a Fisherbrand Model 505 Sonic Dismembrator ultrasound generator (500 watts, 20 kHz, Fisher Scientific™). The sonication was conducted at 36 μm amplitude for a total of 12 min in pulsed mode with cycles of 1 second on and 1 second off. To prevent overheating, the generator was cooled by circulating compressed air at a pressure of 5 psi. The heat generated during the sonication of the samples was offset by using an ice bath to avoid temperatures over 30 °C. After the UAE process, the samples were centrifuged at 20
15 (g mL−1) for pulp. The mixtures were previously homogenized for 3 min at 9500 rpm by using an Ultra-Turrax homogenizer (T-18 Digital, IKA Works Inc, Breisgau, Germany). Ultrasound-assisted extraction (UAE) was performed using a 19 mm solid tip sonotrode (maximum amplitude of 60 μm) connected to a Fisherbrand Model 505 Sonic Dismembrator ultrasound generator (500 watts, 20 kHz, Fisher Scientific™). The sonication was conducted at 36 μm amplitude for a total of 12 min in pulsed mode with cycles of 1 second on and 1 second off. To prevent overheating, the generator was cooled by circulating compressed air at a pressure of 5 psi. The heat generated during the sonication of the samples was offset by using an ice bath to avoid temperatures over 30 °C. After the UAE process, the samples were centrifuged at 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g (Sorvall Evolution RC centrifuge, Thermo Fisher Scientific Inc, USA) for 30 min, and the supernatants were stored at −20 °C until analysis. Extraction was performed in duplicate (n = 2).
000g (Sorvall Evolution RC centrifuge, Thermo Fisher Scientific Inc, USA) for 30 min, and the supernatants were stored at −20 °C until analysis. Extraction was performed in duplicate (n = 2).
2.4.2.1 Preparation of DES-US extracts for analysis. For the release of phenolic compounds from DES, samples of 1 g of the DES-supernatant were heated up to 35 °C in a water bath, dissolved with 4 mL of distilled water, vortexed for 2 min and slowly loaded on a Sep-Pack cartridge (1000 mg, silica-based C18 sorbent, 55–105 μm particle size) (PurpleSeries, Análisis Vínicos, Tomelloso, and España) which had been previously equilibrated with 4 mL of MeOH followed by 4 mL of water. After loading 4 mL of the aqueous supernatant, the column was washed with 4 mL of deionized water, and the phenolic compounds were recovered from the column by elution with 4 mL of MeOH. The extracts were concentrated at 40 °C in a rotary evaporator (Büchi model R111 Evaporator Lab) to dryness and stored under a nitrogen atmosphere in amber vials with screw caps at −20 °C until analysis. The extraction was performed in duplicate (n = 2).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PE (1
PE (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v), followed by vortexing for 2 min, and centrifugation at 3500 rpm for 3 min at 4 °C. The supernatant was collected, and the pellet was re-extracted with DE
1, v/v), followed by vortexing for 2 min, and centrifugation at 3500 rpm for 3 min at 4 °C. The supernatant was collected, and the pellet was re-extracted with DE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PE until colorless supernatants were obtained. The supernatants were dried with nitrogen and reconstituted with 1.5 mL of MTBE
PE until colorless supernatants were obtained. The supernatants were dried with nitrogen and reconstituted with 1.5 mL of MTBE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeOH (1
MeOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) for HPLC injection.
1, v/v) for HPLC injection.
For saponification of xanthophyll fatty acid esters (adapted from Granado et al., 2001),33 400–600 μL of extracted carotenoids were mixed with an equal volume of pyrogallic acid (0.1 M in ETOH) and KOH (30% in MeOH) and subjected to ultrasonic bath treatment in darkness for 7 minutes. Subsequently, 800–1200 μL of distilled water and 1600–2400 μL of DE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PE were added. After vortexing for 1 min and centrifugation at 3500 rpm for 3 min, the upper organic layer was transferred. This liquid–liquid extraction was repeated twice. The combined organic extracts were dried under nitrogen and reconstituted in 150–200 μL of MTBE
PE were added. After vortexing for 1 min and centrifugation at 3500 rpm for 3 min, the upper organic layer was transferred. This liquid–liquid extraction was repeated twice. The combined organic extracts were dried under nitrogen and reconstituted in 150–200 μL of MTBE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeOH (1
MeOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) for HPLC analysis.
1, v/v) for HPLC analysis.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PE (50
PE (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50).
50).
For SPE, 1 g of the DES extract was heated up to 35 °C, dissolved in 2 mL of water, vortexed and loaded on an equilibrated SPE cartridge. After loading 3 mL of the aqueous extract, the column was washed with 4 mL of water, and carotenoids were eluted with 3 mL of ETOH (first 20 drops were discarded) followed by another 3 mL ETOH. Combined extracts were dried with nitrogen, stored at −20 °C, and finally reconstituted in 300 μL of HPLC solvent.
For LLE, 1 g of the DES extract was heated to 35 °C, dissolved in 1 mL water, and vortexed. After one minute, 2 mL of DE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PE (1
PE (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) or THF was added, followed by vortexing and centrifugation at 3500 rpm for 3 min at 4 °C. The supernatant was collected, and the pellet was re-extracted. Combined supernatants (THF extracts were washed with PE) were dried under nitrogen and reconstituted in 200 μL of MeOH
1) or THF was added, followed by vortexing and centrifugation at 3500 rpm for 3 min at 4 °C. The supernatant was collected, and the pellet was re-extracted. Combined supernatants (THF extracts were washed with PE) were dried under nitrogen and reconstituted in 200 μL of MeOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MTBE (1
MTBE (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) for HPLC analysis.
1, v/v) for HPLC analysis.
2.5 Analysis
The separation and identification of major phenolic compounds in the ETOH-MS extract of pulp and peel of mango criollo were carried out by HPLC-DAD-ESI-QTOF-MS/MS according to the procedure described by Ojeda et al. (2022)32 by using an Agilent 1200 series high pressure liquid chromatography system coupled to a quadrupole mass time-of-flight spectrometer with an electrospray ionization source (ESI) via Jet Stream Technology (Agilent G6530A Accurate Mass Q-TOF MS/MS). The major phenolic compounds present in ethanol-based (ETOH-MS) and deep eutectic solvent–ultrasound assisted (DES-US) extracts of mango criollo pulp and peel were quantified using HPLC with diode array detection (DAD). The analysis was performed on an Agilent 1100 series HPLC system (Agilent Technologies, Santa Clara, CA, USA), which included a quaternary pump, an integrated degasser, and a DAD. Separation was achieved using a Poroshell C18 column (4.6 × 250 mm internal diameter, 4.0 μm particle size, Agilent Technologies, Santa Clara, CA, USA). The mobile phase, identical to that used in HPLC-MS/MS, consisted of a linear gradient of 0.1% formic acid in Milli-Q water (solvent A) and 0.1% formic acid in acetonitrile (solvent B) with the following profile: 95% A at 0 min, held at 95% A for 5 min, decreased to 85% A by 10 min, further reduced to 75% A by 20 min, then to 70% A by 30 min, and finally to 60% A by 60 min, followed by a return to 95% A at 65 min and held at 95% A until 70 min. The flow rate was maintained at 0.7 mL min−1, and the injection volume was 20 μL. UV detection was carried out at 280 nm (gallic acid and derivatives), 320 nm (xanthones), 360 nm (flavonols) and 255 nm (ellagic acid). Commercial standards of gallic acid, catechin, mangiferin, maclurin, quercetin-3-O-galactoside, quercetin-3-O-glucoside and ellagic acid were used to build calibration curves in the range of 0.05 to 50 μg mL−1.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 (start), 70
5 (start), 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 (25 min), 35
30 (25 min), 35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 65 (55 min), and back to 95
65 (55 min), and back to 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 (60 min). Carotenoids were detected at 450 nm, while tocopherol acetate (the internal standard) was detected at 285 nm. Empower 2 software (Waters, Milford, MA, USA) was used for chromatogram processing. A calibration (0.6–12.8 ng μL−1 range) was built using commercial standards for lutein, zeaxanthin, β-cryptoxanthin, α-carotene and β-carotene.
5 (60 min). Carotenoids were detected at 450 nm, while tocopherol acetate (the internal standard) was detected at 285 nm. Empower 2 software (Waters, Milford, MA, USA) was used for chromatogram processing. A calibration (0.6–12.8 ng μL−1 range) was built using commercial standards for lutein, zeaxanthin, β-cryptoxanthin, α-carotene and β-carotene.
2.6 Statistical analysis
Data are expressed as the mean ± standard deviation (SD). The normality of the variables was assessed using the Shapiro–Wilk test, confirming that the data followed a normal distribution. To compare the means between two groups, Student's t-test was employed. For comparisons involving more than two groups, a one-way Analysis of Variance (ANOVA) was conducted. Statistical significance was set at p < 0.05. SPSS Statistics Editor (IBM SPSS Statistics, v.29) was used for all statistical calculations.3 Results and discussion
3.1 Conventional extraction of phenolic compounds and terpenoids from mango criollo pulp and peel
The extracts of pulp and peel of mango criollo obtained by magnetic stirring with aqueous ETOH as solvent [ETOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water, 46
water, 46![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 54 (v/v) for peel and 25
54 (v/v) for peel and 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 75 (v
75 (v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v) for pulp according to Ojeda et al. (2022)32] were analyzed using HPLC-ESI-QTOF-MS/MS (Table S1) and 42 compounds from different families of compounds were tentatively identified following the procedure previously described:32 organic acids (quinic acid and citric acid); phenolic acids derivatives (p-hydroxibenzoic acid glucoside, vanillic acid glucoside, valonic acid dilactone, and ellagic acid); benzophenones (maclurin derivatives and iriflophenone-3-C-(2-O-galloyl)-β-D-glucoside); xanthones (mangiferin and derivatives); different classes of flavonoids such as flavonols (quercetin derivatives), flavanols (catechin), different gallates and gallotanins (pentagalloylglucose) and terpenoids (dihydrophaseic acid 4-O-beta-D-glucoside, jasminoside R, and abscisic acid).
v) for pulp according to Ojeda et al. (2022)32] were analyzed using HPLC-ESI-QTOF-MS/MS (Table S1) and 42 compounds from different families of compounds were tentatively identified following the procedure previously described:32 organic acids (quinic acid and citric acid); phenolic acids derivatives (p-hydroxibenzoic acid glucoside, vanillic acid glucoside, valonic acid dilactone, and ellagic acid); benzophenones (maclurin derivatives and iriflophenone-3-C-(2-O-galloyl)-β-D-glucoside); xanthones (mangiferin and derivatives); different classes of flavonoids such as flavonols (quercetin derivatives), flavanols (catechin), different gallates and gallotanins (pentagalloylglucose) and terpenoids (dihydrophaseic acid 4-O-beta-D-glucoside, jasminoside R, and abscisic acid).
HPLC-DAD chromatograms (280 nm) showed that the qualitative phenolic composition of peel and pulp was very similar although the peel exhibited more peaks corresponding to gallotannins of high molecular weight (peaks 39–41) (Fig. S1).
The tentative quantification by HPLC-DAD of major phenolic compounds in the pulp and peel of mango criollo is shown in Table 2. Total phenolic compounds (TPC-HPLC) in peel (3646 ± 510.37 μg g−1 DW) were 9.7 times higher than in pulp (374.55 μg g−1 DW) (Table 2). Similar results were found for the peel and pulp of other mango cultivars.13 Gallates and gallotannins constituted the main phenolic family, accounting for 75% and 85% of the total phenolic compounds in peel and pulp, respectively (Table 2). Gallotannins are hydrolysable tannins composed of polymeric chains of gallic acid esterified with glucose. The main gallotannins found in peel and pulp (Tables S1 and 2) were heptagalloyl glucoside (compounds 36–38), hexagalloyl glucoside (compounds 32–35), pentagalloyl glucoside (compound 27), tetragalloyl glucoside (compound 21) and trigalloyl glucoside (compound 12). Also, some gallic acid derivatives were quantified such as ethyl gallate (compound 22), galloyl glucoside (compound 5) and digalloyl glucoside (compound 7) (Table 2). These compounds have been previously described in mango peel.35
| No. | Compounds | Peel (μg g−1 DW) | Pulp (μg g−1 DW) |
|---|---|---|---|
| Phenolic compounds (total) | 3646.00 ± 374.55b | 510.37 ± 58.46a | |
| Gallates and gallotannins (total) | 2733.50 ± 306.17b | 436.30 ± 49.11a | |
| a Different small letters mean statistically significant differences (p < 0.05) between peel and pulp of the same compound. | |||
| 5 | Galloyl glucoside | 21.73 ± 2.81b | 15.24 ± 1.86a |
| 7 | Di-galloyl glucoside | 44.05 ± 4.29b | 9.30 ± 1.26a |
| 12 | Tri-galloyl glucoside | 133.61 ± 5.06 | nq |
| 21 | Tetra-O-galloyl glucoside | 32.03 ± 4.62b | 17.32 ± 2.97a |
| 22 | Ethyl gallate | 99.61 ± 13.26b | 8.74 ± 1.34a |
| 27 | Penta-O-galloyl glucoside | 531.22 ± 15.39b | 102.96 ± 3.22a |
| 32–35 | Hexagalloyl glucoside | 628.08 ± 87.10b | 74.51 ± 7.50a |
| 36–38 | Heptagalloyl glucoside | 1234.90 ± 173.60b | 180.67 ± 25.42a |
| Benzophenones (total) | 207.22 ±12.50b | 32.25 ± 4.42a | |
| 8 | Maclurin-3-C-β-D-glucoside I | 32.95 ± 1.65b | 3.71 ± 1.30a |
| 9 | Maclurin-3-C-β-D-glucoside II | 107.36 ± 1.38b | 26.03 ± 3.11a |
| 10 | Maclurin-3-C-(2-O-galloyl)-β-D-glucoside | 28.97 ± 0.53b | 2.32 ± 0.27a |
| 15 | Iriflophenone-3-C-(2-O-galloyl)-β-D-glucoside | 6.99 ± 3.38b | 1.28 ± 0.15a |
| 17 | Maclurin-3-C-(2,3-di-O-galloyl)-β-D-glucoside | 30.94 ± 5.55 | Nq |
| Xanthones (total) | 261.24 ±48.87b | 13.67 ±2.54a | |
| 14 | Mangiferin | 247.97 ± 45.60b | 2.17 ± 0.43a |
| 18 | Mangiferin-6-O-gallate | 13.27 ± 3.29a | 11.56 ± 2.12a |
| Flavonols (total) | 441.58 ±6.95b | 7.34 ±0.23a | |
| 20 | Quercetin-3-diglucoside | 30.99 ± 0.15b | 0.532 ± 0.003a |
| 25 | Quercetin-3-O-galactoside | 280.59 ± 4.91b | 2.928 ± 0.004a |
| 26 | Quercetin-3-O-glucoside | 30.99 ± 0.149b | 2.162 ± 0.002a |
| 29 | Quercetin-pentoside I | 25.80 ± 1.62b | 0.718 ± 0.085a |
| 30 | Quercetin-3-O-rhamnoside | 73.22 ± 0.12b | 0.493 ± 0.057a |
| Phenolic acid derivatives | 2.46 ±0.06a | 20.76 ±2.16b | |
| 4 | Vanillic acid glucoside | ||
| Terpenoids (total) | 209.48 ±24.20b | 127.83 ±7.74a | |
| 16 | Dihydrophaseic acid 4-0-β-D-glucoside | 147.26 ± 24.20b | 33.52 ± 3.30a |
| 11 | Jasminoside R | 62.22 ± 12.59a | 54.85 ± 2.01a |
| 42 | Abscisic acid | Nd | 39.47 ± 2.43 |
Gallotannins are recognized as important mango bioactive compounds for their functional properties such as antibacterial, antioxidant and antiinflammatory characteristics and positive effects on human health.8,13,36,37 It is remarkable that vanillic acid glucoside (compound 4) was present at 8.4 times higher concentration in pulp (20.76 μg g−1 DW) than in peel.
Flavonols in the peel (441.58 μg g−1 DW), mainly quercetin derivatives, accounted for 12% of the total phenolic compounds (Table 2). However, the concentration of flavonols in the pulp (7.34 μg g−1 DW) was 60 times lower than in the peel. The main flavonol was quercetin-3-galactoside, accounting for 65% and 40% of the total flavonols in peel and pulp, respectively, followed by quercetin-3-glucoside (Table 2). Quercetin and quercetin glycosides have demonstrated antioxidant, anti-inflammatory, antitumor, anti-proliferative, and apoptotic properties in numerous in vitro and in vivo assays. There is a growing body of evidence showing that quercetin and quercetin glycosides have great potential in the prevention and treatment of different chronic diseases, including cardiovascular and neurodegenerative diseases, as well as cancer.38–40
Xanthones, mainly mangiferin (compound 14), were present at 5.3 times higher concentration in the peel (261.24 μg g−1 DW) than in the pulp (Table 2).
Another important group was benzophenones, primarily maclurin-3-C-β-D-glucoside (compound 9), followed by maclurin-3-C-(2-O-galloyl)-β-D-glucoside (compound 17). Total benzophenone concentration in peel (207.22 μg g−1 dw) was 5.3 times higher than in the pulp (Table 2).
In the present study, neither catechin nor ellagic acid were quantified since they were below the detection limit of the HPLD-DAD system, in contrast to those described by Ojeda et al. (2022).32
Different terpenoids, compounds 11, 16 and 42 (Table S1), were tentatively identified for the first time in the mango criollo extracts. Compound 11 was consistent with the monocyclic monoterpenoid jasminoside R. Abscisic acid (ABA) (compound 42), a sesquiterpenoid plant hormone formed from three isoprene units, was tentatively identified, and quantified in the mango criollo pulp (39.47 μg g−1 DW) but not in the peel. Abscisic acid plays a fundamental role in regulating the ripening of mango fruit. Thus, abscisic acid helps in the degradation of starch present in the fruit cells, increases the accumulation of total sugars, reduces total organic acids, and facilitates the transformation of chloroplasts into chromoplasts containing red or yellow carotenoid pigments.37 Another plant hormone identified in peel and pulp of mango criollo was the apocarotenoid sesquiterpenoid dihydrophaseic acid 4-O-β-D-glucoside (compound 16). Apocarotenoids are synthesized via the oxidative cleavage of carotenoids and play key roles in regulating plant growth and development as well as responses to environmental changes and stress factors. Compound 11, 16 and 42 have been previously identified in mango peel.35
The discrepancies between the phenolic compound profile of the criollo extracts analysed in this study and those reported by Ojeda et al. (2022),32 could be attributed to variations in fruit, harvesting area, ripeness, and the chromatographic system employed.2
In summary, 42 compounds were tentatively identified in the pulp and peel of mango criollo by HPLC-ESI-QTOF-MS/MS such as penta-galloyl glucoside (gallotannins), quercetin-3-O-galactoside, (quercetin derivatives), mangiferin (xanthones), among others. The total phenolic content quantified by HPLC-DAD (TPC-HPLC) was 10 times higher in the peel than in pulp. Also, terpenoids (dihydrophaseic acid 4-O-beta-D-glucoside, jasminoside R, and abscisic acid) were described for the first time in mango criollo.
3.2 Conventional extraction of carotenoids from mango criollo pulp and peel
The total carotenoid concentration was three times higher in mango peel than in the pulp (129.7 μg g−1 and 42.7 μg g−1 DW, respectively). Consistent with previous studies,41,42 β-carotene was the predominant carotenoid in both tissues (87.6% in the pulp and 53.0% in the peel), while lutein was also notable in the peel (27.6%). Zeaxanthin and β-cryptoxanthin were only identified in the peel (Fig. 1 and Table 3). The total carotenoid concentration in criollo mango peel was higher than in other cultivars like Tommy Atkins, Haden, and Kent,41 while the pulp content was lower.42–44 These variations are common among cultivars due to genetic, maturity, and agronomic factors.45,46 Notably, this study used HPLC-DAD for quantification, unlike some previous studies which used spectrophotometry. | ||
| Fig. 1 Carotenoid content in conventional extracts of pulp and peel of mango criollo. Different small letters mean statistically significant differences (p < 0.05) between in peel and pulp extracts. | ||
| Extraction type | β-Carotene | α-Carotene | Lutein | Zeaxanthin | β-Cryptoxanthin | Total carotenoids | % extraction (of total carotenoids) | |
|---|---|---|---|---|---|---|---|---|
| a BLQ: below the limit of quantitation; a: difference between conventional and DES (SPE); b: differences with respect to DES-2. | ||||||||
| Pulp | Conventional | 37.4 ± 1.2 | 5.3 ± 0.1 | BLQ | BLQ | BLQ | 42.7 | |
| DES-1 | 31.4 ± 3.3 | 4.3 ± 0.3 | BLQ | BLQ | BLQ | 35.7 | 83.4 | |
| DES-2 | 30.5 ± 2.3 | 5.0 ± 0.3 | BLQ | BLQ | BLQ | 35.5 | 83.0 | |
| DES-3 | 30.8 ± 2.2 | 4.5 ± 0.3 | BLQ | BLQ | BLQ | 35.3 | 82.5 | |
| DES-4 | 33.0 ± 5.9 | 5.3 ± 0.7 | BLQ | BLQ | BLQ | 38.3 | 89.5 | |
| Peel | Conventional | 68.3 ± 3.9b | 15.2 ± 0.7 | 35.9 ± 4.9 | 7.3 ± 1.2 | 3.1 ± 0.4 | 129.7 | |
| DES-1 | 30.2 ± 6.4ab | 4.9 ± 1.8a | 17.9 ± 2.0a | 3.4 ± 0.3a | 1.6 ± 0.5ab | 58.1ab | 44.8 | |
| DES-2 | 48.1 ± 0.4a | 8.2 ± 2.3a | 27.0 ± 1.1 | 5.8 ± 0.5 | 3.0 ± 0.4 | 92.0a | 71.0 | |
| DES-3 | 28.2 ± 0.4ab | 4.6 ± 0.0a | 18.5 ± 4.0a | 3.2 ± 0.8a | 1.5 ± 0.3ab | 56.0ab | 43.1 | |
| DES-4 | 25.0 ± 1.6ab | 4.8 ± 0.2a | 15.0 ± 2.2ab | 2.9 ± 0.5ab | 1.5 ± 0.3ab | 49.0ab | 37.8 | |
In summary, mango peel contains a significantly higher amount of carotenoids than the pulp, making it a valuable source of β-carotene and lutein.12,42
3.3 Extraction of phenolic compounds and carotenoids from mango criollo pulp and peel using deep eutectic solvents combined with ultrasound-assisted extraction (DES-UAE)
In the context of developing sustainable extraction methods, deep eutectic solvents (DES) were chosen for this study due to their favorable properties, including non-volatility, non-flammability, and derivation from renewable sources.17,47 We combined DES with ultrasound-assisted extraction,28 a method known for its sustainability.Based on previous work demonstrating the successful extraction of phenolic compounds from mango peel and seeds,48 we selected and prepared four specific DES to extract both phenolic compounds and carotenoids. The physicochemical properties of these DES, which were composed of various hydrogen bond acceptors and donors (Table 1), were expected to influence their extraction efficiency.
Although these DES are typically suitable for hydrophilic compounds, this study investigates the possibility of using a single DES to simultaneously extract both hydrophilic phenolic compounds and lipophilic carotenoids from mango peel and pulp. The polarity values of the selected DES (Table 1) were closer to those of ETOH and MeOH (∼52 kcal mol−1) than to solvents typically used for carotenoid extraction, such as DE or PE (∼55 kcal mol−1).
| Compounds | Conventional | DES-1 | DES-2 | DES-3 | DES-4 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pulp | Peel | Pulp | Peel | Pulp | Peel | Pulp | Peel | Pulp | Peel | |
| a Different small letters mean statistically significant differences (p < 0.05) between different solvents (conventional and DES) for each compound and mango product (peel or pulp). | ||||||||||
| Gallates and gallotannins | 436.30 ± 49.11a | 2733.50 ± 306.20c | 695.02 ± 36.21c | 2067.65 ± 197.70b | 736.24 ± 54.56d | 1766.26 ± 242.80a | 452.76 ± 10.37a | 1891.10 ± 222.70a | 511.97 ± 21.20b | 3434.33 ± 282.50d |
| Benzophenones | 32.25 ± 4.42d | 207.22 ± 12.50e | 1.21 ± 0.11a | 79.35 ± 3.95b | 18.23 ± 0.76c | 33.30 ± 6.93a | 18.78 ± 1.33c | 133.73 ± 18.79c | 11.32 ± 3.69b | 172.35 ± 41.68d |
| Xanthones | 13.72 ± 2.54a | 261.24 ± 48.87c | 16.27 ± 1.09a | 166.32 ± 10.81b | 86.68 ± 5.12d | 138.81 ± 10.48a | 18.40 ± 1.91b | 153.10 ± 15.47ab | 37.90 ± 3.04c | 275.81 ± 20.31c |
| Flavonols | 7.34 ± 0.23b | 441.59 ± 6.95a | 4.00 ± 0.05a | 754.29 ± 96.89c | 4.76 ± 0.02a | 545.03 ± 65.27b | 9.14 ± 0.35c | 1014.97 ± 121.60d | 7.49 ± 0.12b | 824.39 ± 96.27c |
| Phenolic acid derivatives | 20.76 ± 2.16a | 2.46 ± 0.06a | 29.95 ± 1.33b | 9.11 ± 0.42b | 31.38 ± 0.79b | 10.60 ± 0.51b | nd | nd | 20.32 ± 0.19a | nd |
| Total phenolic compounds | 510.38 ± 58.46ab | 3646.00 ± 374.50b | 746.46 ± 38.80c | 3076.72 ± 309.70ab | 877.29 ± 61.26d | 2494.01 ± 326.00a | 499.09 ± 13.97a | 3192.91 ± 378.60ab | 588.96 ± 28.25b | 4708.89 ± 440.70c |
| Total terpenoids | 127.83 ± 7.74b | 209.48 ± 36.83b | 117.41 ± 1.15a | 331.81 ± 46.44c | 131.62 ± 2.63b | 138.05 ± 7.53a | 145.46 ± 6.97c | 301.71 ± 27.44c | 199.40 ± 3.90d | 307.91 ± 28.40c |
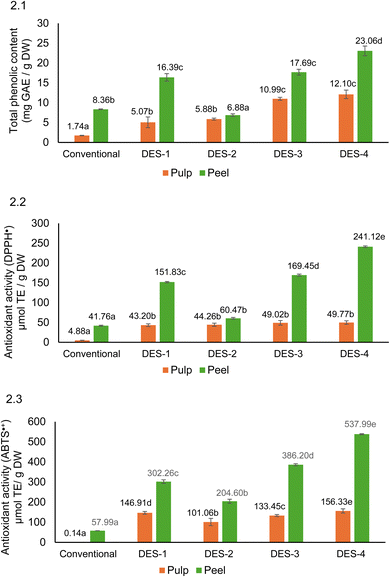
In addition, the antioxidant activity was studied by three methods: the reducing capacity of the extracts was determined by the analysis of total phenolic compounds using the Folin–Ciocalteu (TPC-FC) method and the radical scavenging capacity was evaluated by the DPPH˙ and ABTS˙+ methods. The data obtained with the four DES-UAE systems studied were compared with those obtained with conventional magnetic stirring extraction using aqueous ethanol as the solvent. In all cases, the release of phenolic compounds from DES was carried out by using solid phase extraction (SPE) cartridges.
3.3.1.1 Antioxidant activity: total phenolic content using Folin–Ciocalteu (TPC-FC), DPPH˙ and ABTS˙+ assays. The results of TPC-FC, DPPH˙ and ABTS˙+ assays are shown in Fig. 2. The TPC-FC values of the peel DES-extracts were 3 times (DES-1), 1.2 times (DES-2), 1.6 times (DES-3) and 2 times (DES-4) higher than their respective values in pulp (Fig. 2).
DES-4 showed the highest TPC-FC (23.06 mg GAE g−1 DW) value in the peel followed by DES-3 (17.69 mg GAE g−1 DW) and DES-1 (16.39 mg GAE g−1 DW). Thus, DES-1, DES-3 and DES-4 significantly increased (∼2.2 times, p < 0.05) the TPC-FC value in the peel compared to conventional extraction with aqueous ETOH (8.36 mg GAE g−1 DW). However, the TPC-FC value obtained with DES-2 was 18% lower than that of the conventional extract, perhaps due to DES-2 having the highest viscosity of the four DES studied (Table 1).
In the pulp, all four DES significantly increased the TPC-FC value compared to the conventional extract (1.74 mg GAE g−1 DW). In fact, ChCl-based solvents (DES-3 and DES-4), showed a higher increase (∼6.6 times) than β-Ala-based solvents (DES-1 and DES-2) (∼3.2 times) compared to the conventional extract (Fig. 2).
Generally, the composition and viscosity of the DES influenced the TPC-FC values. In peel, DES-4 showed the highest TPC-FC value followed by DES 3. Both DES-3 and DES-4, were Ch–Cl-based solvents with the lowest viscosity (Table 1).
Antioxidant activity in the peel, as determined by DPPH• and ABTS˙+ showed the same trend as TPC-FC. Thus, ChCl-based solvents, DES-4 and DES-3, presented ∼7.5 times and ∼5 times higher antioxidant activity (ABTS˙+ and DPPH˙) than conventional extraction (Fig. 2).
Also, all four DES significantly increased antioxidant activity in the pulp by ∼10 times (DPPH˙ and ABTS˙+) compared to the conventional extracts.
Similar results have been reported for other fruit by-products.
The fact that certain DES mainly ChCl-based solvents, increased the TPC-FC value and the antioxidant activity of fruit by-products compared to traditional extraction, has been also reported for olive pomace,14 apple pomace,49 and mango peel,29 among others.
Pearson correlation analysis indicated that in both peel and pulp, there was a very high correlation between the three methods used to determine the hydrophilic antioxidant activity (TPC-FC, DPPH˙ and ABTS˙+). Thus, in peel, DPPH˙ showed a high correlation with TPC-FC (r = 0.99) and ABTS˙+ (r = 0.97), with similar trends observed in the pulp (TPC-FC, r = 0.80; ABTS˙+, r = 0.77).
In conclusion, the DES-UAE systems assayed, mainly CHCl-based solvents in the peel, are useful for the valorization of mango by-products as they significantly increase the antioxidant activity values compared to traditional stirring with aqueous ethanol.
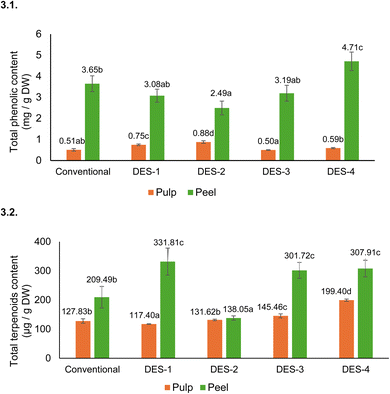
3.3.1.2 Phenolic compounds and terpenoids by HPLC-DAD. The major individual phenolic compounds of pulp and peel of mango criollo were identified and quantified by HPLC-DAD and grouped by families (Tables 2 and 4).
The TPC-HPLC contents determined in DES-1, DES-2, DES-3 and DES-4 peel extracts were 4, 3, 6 and 8 times higher than their counterparts in pulp (Fig. 3.1). DES-4 showed the highest TPC-HPLC (4.71 mg g−1 DW) in peel which represented an increase of 29% compared to conventional extraction (Fig. 3.1). In contrast, DES-2 recovered the lowest TPC-HPLC in the peel (2.41 mg g−1 DW) which was 32% less than that obtained with conventional extraction, followed by DES-1 and DES-3 although no statistically significant differences were found among these three solvents. In fact, the TPC-HPLC values of DES-1 and DES-3 were statistically similar to that of conventional extraction.
Different trends were found in the pulp, where the TPC-HPLC values of DES-2 (0.88 mg g−1 DW) and DES-1 (0.75 mg g−1 DW) increased by 72% and 47% compared to conventional extraction. On the other hand, DES-3 and DES-4 showed concentrations similar to conventional extraction (0.51 mg g−1 DW) (Fig. 3.1).
These results showed that ChCl-based solvents (DES-3 and DES-4) were more effective for the extraction of phenolic compounds in the peel, while β-ALA-based solvents (DES-1 and DES-3), which have higher viscosity and polarity (Table 1), were more efficient in extracting phenolic compounds from the pulp. It appears that the more viscous DES-1 and DES-2 have greater difficulty than DES-3 and DES-4 entering the fibrous tissue of the peel compared with the less structured and softer tissue of the pulp.
The 29% increase in TPC-HPLC extraction from the peel using DES-4, compared to conventional extraction, could be related to the higher levels of total gallates, gallotannins, and flavonols (Table 4). In fact, the four DESs assayed increased the extraction of all the individual flavonols in peel (mainly quercetin-3-O-galactoside and quercetin-3-O-glucoside) compared to conventional extraction (Table 2). Thus, DES-3 and DES-4 increased 2.3 times and 1.8 times, respectively, the extraction of flavonols compared with the conventional extraction. However, xanthones (mangiferin) and benzophenones (maclurin derivatives) in peel were extracted 2–5 times less efficiently with DES than with conventional extraction.
In the pulp, the increase in TPC-HPLC with DES-1 and DES-2 compared to conventional extraction has been related with the increase in the recovery of gallates and gallotannins and bezophenones (Tables 2 and 4).
Terpenoids determined by HPLC-DAD (Table 2) in the conventional extracts were also extracted using DES (Fig. 3.2), but only jasminoside R (compound 11) and dihydrophaseic acid 4-O-β-D-glucoside (compound 16). Abscisic acid was not extracted with DES.
DES-1, DES-3 and DES-4 increased the extraction of total terpenoids by approximately 49% compared to the conventional extraction. The most viscous solvent DES-2 showed the lowest total terpenoid content extracted as also observed with phenolic compounds.50,51
Pearson correlation analysis indicated a positive correlation between TPC-HPLC and TPC-FC (r = 0.68), DPPH˙ (r = 0.63), and ABTS˙+ (r = 0.53) in the peel. Also in the pulp, positive correlations were found between TPC-HPLC and DPPH˙ (r = 0.34) and ABTS˙+ (r = 0.25).
In conclusion, ChCl-based solvents, DES-3 and DES-4, showed the better results in the peel, while in the pulp, β-Ala-based solvents, DES-1 and DES-2, gave the best results. In both, the increase in TPC-HPLC was related with the increase in total gallates and gallotannins and total flavonols. Xanthones (mangiferin) and benzophenones were extracted with DES but in lower amounts than with a traditional process. DES-UAE is a sustainable system to increase the extraction of phenolic compounds from mango by-products compared to conventional extraction.
The results show that DES-UAE was more effective in the pulp than in the peel (Table 3, Fig. 4.1 and 4.2), likely due to the pulp's homogeneity,50 which led to more consistent recovery across DES.
Most DES are water-soluble, which limits their applicability for hydrophobic compounds like carotenoids.31 This is consistent with previous literature, where DES did not enhance the extraction of carotenoids from materials like apricot waste Buriti fruit.52 In the present study, DES-UAE/SPE extraction (β-Ala and Ch–Cl based) was less effective than the conventional method (Table 3), especially in the peel. Given that this study aimed to extract both carotenoids and polyphenols, it is possible that hydrophobic DES might be suitable for carotenoid-specific extractions.
Regarding the release of the compounds, LLE-THF and SPE yielded similar results for pulp carotenoids. However, DE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PE for the peel proved to be more efficient than SPE (Fig. 4.2), due to faster drying and greater efficiency than SPE,53 and was chosen due to THF's toxicity and long drying times.54
PE for the peel proved to be more efficient than SPE (Fig. 4.2), due to faster drying and greater efficiency than SPE,53 and was chosen due to THF's toxicity and long drying times.54
Among the different deep eutectic solvents, DES-2 (β-Ala-based) showed the highest peel extraction, despite viscosity25 challenges which were mitigated with heat and water.55,56 The use of β-Ala-based DES for non-polar carotenoids is a novel finding, as its application has previously been limited to polar compounds,57 highlighting its potential, even though its effectiveness remained lower than that of the conventional method.
In summary, DES-UAE extraction was more efficient in mango pulp (∼85% recovery) than in peel (∼40% for DES-1/3/4; ∼71% for DES-2). While conventional methods remain superior for the peel, DES-UAE offers a sustainable alternative, and β-Ala-based DES showed potential for carotenoids despite viscosity-related limitations. Future studies should explore hydrophobic DES and optimize extraction conditions.
4 Conclusion
This study demonstrates that combining DES with UAE is a sustainable and effective method for recovering bioactive compounds from underutilized mango criollo by-products. Both pulp and peel yielded significant concentrations of polyphenols and carotenoids, with promising implications for the food industry. The DES-UAE method notably enhanced polyphenol extraction, outperforming conventional aqueous ethanol extraction. DES-2 in pulp and DES-4 in peel achieved increases of 72% and 29%, respectively. Peel samples contained up to 3646 μg g−1 DW of total phenolics, approximately ten times more than in pulp. ChCl-based DES (DES-3 and DES-4) also boosted antioxidant activity, with up to 7.5-fold increases in TPC-FC values. For carotenoids, DES-UAE matched conventional extraction in pulp, recovering ∼85% (42.74 μg g−1 DW), while DES-2 achieved the highest recovery in peel (∼71%). Peel was identified as a richer source of carotenoids (129.7 μg g−1 DW), with β-carotene as the predominant compound and lutein as a key pigment.The extracts, rich in gallotannins, xanthones (e.g., mangiferin), and carotenoids, offer potential as natural antioxidants, dietary supplement ingredients, and food colorants. The safety and biodegradability of DES may allow direct use of these extracts without further purification.
Future research should explore hydrophobic DES to improve non-polar carotenoid extraction, optimize conditions for fibrous matrices like peel, and assess extract stability and functionality in real food systems. Additionally, further investigation into the newly identified terpenoids may uncover novel applications.
Author contributions
Elena Rodríguez-Rodríguez: data curation, formal analysis, investigation, writing – original draft, writing – review & editing. Gonzalo Adrián Ojeda: investigation. Sonia Cecilia Sgroppo: resources, writing – review & editing. Begoña Olmedilla-Alonso: conceptualization, methodology, resources, writing – review & editing. Concepción Sánchez-Moreno: conceptualization, funding acquisition, methodology, project administration, resources. Milagros Sánchez-Prieto: investigation. Lucía Giménez: investigation. Ruth Núñez-Flores: investigation. Begoña De Ancos: conceptualization, data curation, formal analysis, funding acquisition, methodology, project administration, resources, writing – original draft, writing – review & editing.Conflicts of interest
There are no conflicts to declare.Data availability
Further inquiries can be directed to the corresponding author(s). The accepted and final version of the manuscript will be deposited in Docta Complutense, the institutional repository of the Universidad Complutense de Madrid; in DIGITAL.CSIC, the institutional repository of the Spanish National Research Council (CSIC); in the Repositorio Institucional de CONICET (CONICET Digital), a public, open-access platform that gathers the scientific and technological output of researchers from the National Scientific and Technical Research Council of Argentina (CONICET); and in the Repositorio Digital de la Universidad Nacional del Nordeste. The datasets will be accessible at Docta Complutense: https://docta.ucm.es/home DIGITAL.CSIC: https://digital.csic.es. CONICET Digital: https://ri.conicet.gov.ar/. Repositorio Digital de la Universidad Nacional del Nordeste: https://repositorio.unne.edu.ar/.The original contributions presented in this study are included in the article/supplementary information (SI). Supplementary information: Table S1 tentative identification of major phenolic compounds in mango criollo peel and pulp by HPLC-DAD-ESI-QTOF-MS/MS. Fig. S1 HPLC chromatogram register at λ280 nm of conventional extract of peel and pulp of mango criollo. The identification of the peaks (Table S1). See DOI: https://doi.org/10.1039/d5fb00383k.
Acknowledgements
The authors acknowledge Grant PID2019-107980RB-I00 funded by MICIU/AEI/10.13039/501100011033 and Grant PID2023-147025NB-I00 funded by MICIU/AEI/10.13039/501100011033 and by “ERDF/EU”. The authors are grateful to the staff of the Analysis Service Unit at ICTAN for assistance with chromatography and mass spectrometry analyses. B, Olmedilla-Alonso and E. Rodríguez-Rodríguez are members of the Spanish Carotenoid Network (CaRed), grant RED2022-134577-T, funded by MCIN/AEI/10.13039/501100011033. G. A. Ojeda thanks the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) of Argentina.References
- FAO, Faostat Database, 2025, https://www.fao.org/faostat/es/#data/QCL, accessed 2nd April 2025 Search PubMed.
- A. G. Gupta, P. S. Gurjar, K. Beer, A. Pongener, S. C. Ravi, S. Singh, A. Verma, A. Singh, M. Thakur, S. Tripathy and D. K. Verma, Food Biosci., 2022, 48, 101783, DOI:10.1016/j.fbio.2022.101783.
- M. Majdan and B. Bobrowska-Korczak, Nutrients, 2022, 14, 2496, DOI:10.3390/nu14122496.
- E. M. Yahia, J. D. J. Ornelas-Paz, J. K. Brecht, P. García-Solís and M. E. M. Celis, Arabian J. Chem., 2023, 16, 104860, DOI:10.1016/j.arabjc.2023.104860.
- A. Wall-Medrano, F. J. Olivas-Aguirre, J. F. Ayala-Zavala, J. A. Domínguez-Avila, G. A. Gonzalez-Aguilar, L. A. Herrera-Cazares and M. Gaytan-Martinez, in Food Wastes and By-Products, ed. R. Campos-Vega, B. D. Oomah and H. A. Vergara-Castaneda, Blackwell Publishing, 2020, pp. 159–191 Search PubMed.
- G. D. Noratto, M. C. Bertoldi, K. Krenek, S. T. Talcott, S. T. Stringheta and S. U. Mertens-Talcott, J. Agric. Food Chem., 2010, 58(7), 4104–4112, DOI:10.1021/jf903161g.
- C.-Y. Huang, C.-H. Kuo, C.-H. Wu, A.-W. Kuan, H.-R. Guo, Y.-H. Lin and P.-K. Wang, J. Food Qual., 2018, 1–13, DOI:10.1155/2018/1025387.
- H. Kim, M. J. Castellon-Chicas, S. Arbizu, S. T. Talcott, N. L. Drury, S. Smith and S. U. Mertens-Talcott, Molecules, 2021, 26, 2732, DOI:10.3390/molecules26092732.
- D. I. Zafra Ciprián, G. V. Nevárez Moorillón, S. Soto Simental, L. E. Guzmán-Pantoja, L. H. López Hernández, J. T. Santiago Castro and L. H. Villalobos Delgado, Processes, 2023, 11, 1772, DOI:10.3390/pr11061772.
- S. A. Zarasvanda, A. P. Mullins, B. Arjmandi and V. Haley-Zitlina, Nutr. Res., 2023, 111(7), 73–89, DOI:10.1016/j.nutres.2023.01.003.
- M. H. A. Jahurul, I. S. M. Zaidul, K. Ghafoor, Y. Fahad, F. Norulaini, A. K. Sahena and A. K. Mohd Omar, Food Chem., 2015, 184, 173–180, DOI:10.1016/j.fbio.2022.101783.
- L. Serna-Cock, E. García-Gonzales and C. Torres-León, Food Rev. Int., 2016, 32, 364–376, DOI:10.1080/87559129.2015.1094815.
- M. E. Alañón, S. Pimentel-Moral, D. Arráez-Román and A. Segura-Carretero, Food Rev. Int., 2021, 140, 109852, DOI:10.1016/j.foodres.2020.109852.
- S. Chanioti and C. Tzia, Innovative Food Sci. Emerging Technol., 2018, 48, 228–239, DOI:10.1016/j.ifset.2018.07.001.
- R. D. Rogers and K. R. Seddon, Science, 2003, 302(5646), 792–793, DOI:10.1126/science.1090313.
- M. Ahmadi, H. Elmongy, T. Madrakian and M. Abdel-Rehim, Anal. Chim. Acta, 2017, 958, 1–21, DOI:10.1016/j.aca.2016.11.062.
- K. Murtada, Trends Environ. Anal. Chem., 2020, 25, e00077, DOI:10.1016/J.TEAC.2019.E00077.
- S. Koutsoukos, T. Tsiaka, A. Tzani, P. Zoumpoulakis and A. Detsi, J. Cleaner Prod., 2019, 241, 118384, DOI:10.1016/j.jclepro.2019.118384.
- Q. Luo, J.-R. Zhang, H.-B. Li, D.-T. Wu, F. Geng, H. Corke, X.-L. Wei and R.-Y. Gan, Antioxidants, 2020, 9, 785, DOI:10.3390/antiox9090785.
- A. Thongsaw, Y. Udnan, G. M. Ross and W. C. Chaiyasith, Talanta, 2019, 197, 310–318, DOI:10.1016/j.talanta.2019.01.018.
- H. Aktas and M. A. Kurek, Food Chem., 2024, 444(30), 138629, DOI:10.1016/j.foodchem.2024.138629.
- A. Saini, A. Kumar, P. S. Panesar and A. Thakur, Appl. Food Res., 2022, 2(2), 100211, DOI:10.1016/j.afres.2022.100211.
- C. Ferreira and M. A. Sarraguça, Pharmaceuticals, 2024, 17, 124, DOI:10.3390/ph17010124.
- P. Kalhor and K. Ghandi, Molecules, 2019, 24(22), 4012, DOI:10.3390/molecules24224012.
- T. G. S. Guimarães, D. F. Andrade, A. P. R. Santana, P. Moser, S. S. Ferreira, J. M. N. R. Menezes, C. D. B. Amaral, A. Oliveira and M. H. Gonzalez, J. Mol. Liq., 2022, 345, 117887, DOI:10.1016/j.molliq.2021.117887.
- Y. Liu, J. B. Friesen, J. B. McAlpine, D. C. Lankin, S.-H. Chen and G. F. Pauli, J. Nat. Prod., 2018, 81, 679–690, DOI:10.1021/acs.jnatprod.7b00945.
- E. Kultys and M. A. Kurek, Molecules, 2022, 27(2), 518, DOI:10.3390/molecules27020518.
- O. Zannou, K. F. Oussou, S. Mohammed, I. B. Chabi, Y. E. Kpoclou, B. Tekgüler, D. S. Dabadé, I. K. Koca and T. Esatbeyoglu, Biomass Convers. Biorefin., 2025, 15, 8253–8265, DOI:10.1007/s13399-024-05674-3.
- C. B. T. Pal and G. C. Jadeja, Food Sci. Technol. Int., 2020, 26(1), 78–92, DOI:10.1177/1082013219870010.
- K. M. Jeong, J. Ko, J. Zhao, Y. Jin, D. E. Yoo, S. Y. Han and J. Lee, J. Cleaner Prod., 2017, 151(10), 87–95, DOI:10.1016/j.jclepro.2017.03.038.
- A. Viñas-Ospino, D. López-Malo, M. J. Esteve, A. Frígola and J. Blesa, Foods, 2023, 12(4), 863, DOI:10.3390/foods12040863.
- G. A. Ojeda, S. C. Sgroppo, C. Sánchez-Moreno and B. De Ancos, Food Chem., 2022, 396, 133738, DOI:10.1016/j.foodchem.2022.133738.
- F. Granado, B. Olmedilla-Alonso, E. Gil-Martinez and I. Blanco, J. Food Compos. Anal., 2001, 14, 479–489, DOI:10.1006/jfca.2001.0989.
- D. González-Peña, D. Dudzik, C. Colina-Coca, B. De Ancos, A. García, C. Barbas and C. Sánchez-Moreno, J. Funct. Foods, 2015, 19, 363–375, DOI:10.1016/j.jff.2015.09.033.
- A. García-Villegas, A. Fernández-Ochoa, A. Rojas-García, M. E. Alañón, D. Arráez-Román, M. L. Cádiz-Gurrea and A. Segura-Carretero, Antioxidants, 2023, 12(10), 1892, DOI:10.3390/antiox12101892.
- R. C. Barnes, K. A. Krenek, B. Meibohm, S. U. Mertens-Talcott and S. T. Talcott, Mol. Nutr. Food Res., 2016, 60(3), 542–550, DOI:10.1002/mnfr.201500706.
- R. C. Barnes, H. Kim, C. Fang, W. Bennett, M. Nemec, M. A. Sirven, J. S. Suchodolski, N. Deutz, R. A. Britton, S. U. Mertens-Talcott and S. T. Talcott, Mol. Nutr. Food Res., 2019, 63(2), e1800512, DOI:10.1002/mnfr.201800512.
- M. Lesjak, I. Beara, I. Simin, D. Pintać, T. Majkić, K. Bekvalac, D. Orčić and N. Mimica-Dukić, J. Funct. Foods, 2018, 40, 68–75, DOI:10.1016/j.jff.2017.10.047.
- N. A. Alsharairi, Int. J. Mol. Sci., 2023, 24, 15208, DOI:10.3390/ijms242015208.
- G. Minniti, L. F. Laurindo, N. M. Machado, L. G. Duarte, E. L. Guiguer, A. C. Araujo, J. A. Dias, C. B. Lamas, Y. C. Nunes and M. D. Bechara, et al. , Life, 2023, 13, 2270, DOI:10.3390/life13122270.
- V. Marcillo-Parra, M. Anaguano, M. Molina, D. S. Tupuna-Yerovi and J. Ruales, NFS J., 2021, 23, 1–7, DOI:10.1016/j.nfs.2021.02.001.
- M. Liang, X. Su, Z. Yang, H. Deng, Z. Yang, R. Liang and J. Huang, Sci. Hortic., 2020, 63, 109072, DOI:10.1016/j.scienta.2019.109072.
- J. A. Manthey and P. Perkins-Veazie, J. Agric. Food Chem., 2009, 57, 10825–10830, DOI:10.1021/jf902606h.
- F. X. Liu, X. F. Fu, F. Chen, X. J. Liao, X. S. Hu and J. H. Wu, Food Chem., 2013, 138, 396–405, DOI:10.1016/j.foodchem.2012.09.111.
- G. R. Karanjalker, K. V. Ravishankar, K. S. Shivashankara, M. R. Dinesh, T. K. Roy and R. D. V. Sudhakar, Appl. Biochem. Biotechnol., 2018, 184, 140–154, DOI:10.1007/s12010-017-2529-x.
- D. B. Rodriguez-Amaya and M. Kimura, HarvestPlus Handbook For Carotenoid Analysis, Harvest Plus Technical Monographs, series 2, 2024, p. 59 Search PubMed.
- F. M. Fuad, M. M. Nadzir and A. H. Kamaruddin, J. Mol. Liq., 2021, 339, 116923, DOI:10.1016/j.molliq.2021.116923.
- G. A. Ojeda, M. M. Vallejor, S. C. Sgroppo, C. Sánchez-Moreno and B. De Ancos, Heliyon, 2023, 9, e16912, DOI:10.1016/j.heliyon.2023.e16912.
- R. Rashid, S. M. Wani, S. Manzoor, F. A. Masoodi and M. M. Dar, Food Chem., 2023, 398, 133871, DOI:10.1016/j.foodchem.2022.133871.
- P. Gullón, B. Gullón, A. Romaní, G. Rocchetti and J. M. Lorenzo, Trends Food Sci. Technol., 2020, 101, 182–197, DOI:10.1016/j.tifs.2020.05.007.
- R. Manurung and A. G. A. Siregar, Int. J. GEOMATE, 2020, 19, 131–137, DOI:10.21660/2020.74.96998.
- P. I. P. Leite, C. F. De Assis, E. Silvino dos Santos, C. E. Araújo Padilha, M. Ferrari and F. C. Sousa Jr, Sustainable Chem. Pharm., 2021, 20, 100375, DOI:10.1016/j.scp.2021.100375.
- E. Rodríguez-Rodríguez, B. Beltrán-de-Miguel, K. X. Samaniego-Aguilar, M. Sánchez-Prieto, R. Estévez-Santiago and B. Olmedilla-Alonso, Antioxidants, 2020, 9(6), 484, DOI:10.3390/antiox9060484.
- L. M. Schüler, K. N. Gangadhar, P. Duarte, C. Placines, A. M. Molina-Márquez, R. Léon-Bañares and L. Barreira, Bioprocess Biosyst. Eng., 2020, 43(5), 785–796, DOI:10.1007/s00449-019-02273-9.
- M. Panić, M. Radić Stojković, K. Kraljić, D. Škevin, I. Radojčić Redovniković, V. Gaurina Srček and K. Radošević, Food Chem., 2019, 283, 628–636, DOI:10.1016/j.foodchem.2019.01.061.
- W. W. Oomen, P. Begines, N. Rianika Mustafa, E. G. Wilson, R. Verpoorte and Y. H. Choi, Molecules, 2020, 25, 617, DOI:10.3390/molecules25030617.
- A. V. Gómez, C. C. Tadini, A. Biswas, M. Buttrum, S. Kim, V. M. Boddu and H. N. Cheng, LWT--Food Sci. Technol., 2019, 107, 79–88, DOI:10.1016/j.lwt.2019.02.052.
| This journal is © The Royal Society of Chemistry 2026 |