Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Carbonylation with 3d metal hydrides: expanding the potential for industrial applications
Zhi-Peng Bao
ab,
Le-Cheng Wang
ab and
Xiao-Feng Wu
 *ab
*ab
aDalian National Laboratory for Clean Energy, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, 116023, Dalian, Liaoning, China. E-mail: xwu2020@dicp.ac.cn
bLeibniz-Institut für Katalyse e.V., Albert-Einstein-Straße 29a, 18059, Rostock, Germany. E-mail: Xiao-Feng.Wu@catalysis.de
First published on 22nd December 2025
Abstract
Metal–hydride-mediated carbonylation toward value-added products has found broad applications in synthetic chemistry, materials science, pharmaceuticals, and industrial catalysis, exemplified by hydroformylation and Reppe-type carbonylation. However, most of these applications rely on highly active but precious metal catalysts. Therefore, the development of novel, practically useful, and inexpensive catalytic systems based on earth-abundant metals is highly desirable. On the other hand, olefin isomerization–carbonylation tandem processes can assist in converting mixed olefins into single products, which is of relevance to chemical industry applications. In this review, we summarize and discuss recent advances in abundant metal–hydride-mediated carbonylation and its isomerization–carbonylation tandem process (chain-walking carbonylation), with the aim of providing insights to researchers in both organic chemistry and industrial catalysis for future reaction design.
Broader contextCarbon monoxide based C1 chemistry has a significant influence on economics based on its origin and consumption. Over 80% of global acetic acid production was achieved by methanol carbonylation. And of course, hydroformylation is mediated by metal hydride. The produced products have found broad applications in synthetic chemistry, materials science, pharmaceuticals, and industrial catalysis. However, most of these applications rely on highly active but precious metal catalysts. Therefore, the development of novel, practically useful, and inexpensive catalytic systems based on earth-abundant metals is highly desirable. On the other hand, olefin isomerization–carbonylation tandem processes can assist in converting mixed olefins into single products, which is of relevance to chemical industry applications. In this review, we summarize and discuss recent advances in abundant metal–hydride-mediated carbonylation and its isomerization–carbonylation tandem process (chain-walking carbonylation), with the aim of providing insights to researchers in both organic chemistry and industrial catalysis for future reaction design. |
1. Introduction
Carbon monoxide, a cost-effective and accessible C1 source, has been central to industrial and energy chemistry for nearly a hundred years of continuous development.1–3 The development of carbonylation chemistry has been closely intertwined with the evolution of industrial catalysis. As early as 1925, the Fischer–Tropsch process was established, enabling the conversion of coal or natural gas into synthesis gas (CO and H2) through the water–gas shift reaction. Employing transition-metal catalysts such as Fe, Co, Ni, and Ru, synthesis gas could be transformed into fuels and lubricants, representing one of the most important routes toward energy transition and clean fuel production.4 In 1938, German chemist Otto Roelen discovered the formation of n-propionaldehyde during the Fischer–Tropsch reaction of ethylene over cobalt catalysts – marking the birth of the hydroformylation reaction.5 Subsequently, in 1941, Walter Reppe developed the carbonylation of methanol, which proceeded under extremely harsh conditions (500–700 bar, 250–270 °C). After significant improvement by BASF, the cobalt-catalyzed methanol carbonylation was commercialized in 1960, representing the first industrial application of carbonylation chemistry.6 A breakthrough came in 1967, when Monsanto developed a rhodium-catalyzed process for methanol carbonylation to acetic acid, operating under much milder conditions (180–220 °C, 30–40 bar) with excellent selectivity.7 Building on Roelen's work, Geoffrey Wilkinson reported a rhodium-catalyzed hydroformylation process with remarkable activity and regioselectivity in 1968, firmly establishing the industrial significance of hydroformylation.8 In 1996, BP Chemicals introduced the Cativa process, employing an iridium-based catalyst [Ir(CO)2I2]−, which surpassed the Monsanto process in both efficiency and environmental performance.9 Later, in 2008, Mitsubishi Chemical developed a two-step process for methyl methacrylate (MMA) production via ethylene carbonylation and subsequent condensation. This new route offered competitive economics and a significantly reduced environmental impact compared with the conventional acetone cyanohydrin (ACH) and isobutylene (C4) processes.10 More recently, in 2019, the Beller group achieved the double carbonylation of 1,3-butadiene to directly produce dimethyl adipate, a key nylon-6,6 precursor, in a one-step, highly active, and selective catalytic system with strong potential for industrial application.11 In 2023, the Liu group realized the industrial production of ethanol via dimethyl ether carbonylation, marking another milestone in the evolution of carbonylation chemistry (Scheme 1A).12 | ||
| Scheme 1 Metal–hydride-mediated carbonylation toward value-added products from noble metals to earth-abundant metals. | ||
Among the diverse industrial applications of carbonylation chemistry, metal-hydride-mediated carbonylation reactions hold a particularly important position. A notable example is the rhodium-catalyzed hydroformylation reaction. The Oxo Alcohol Process, developed and commercialized by Johnson Matthey plc, utilizes a rhodium-based catalytic system to take hydroformylation efficiently of long-chain alkenes, representing a milestone in the large-scale application of carbonylation technology.13 The ExxonMobil Oxo process employs rhodium-catalyzed hydroformylation to produce highly branched long-chain alcohols.14 Ding and co-workers achieved a rhodium-catalyzed heterogeneous hydroformylation process through the utilization of porous organic ligands (POLs), providing an efficient and recyclable system for industrially relevant carbonylation reactions.15 Cobalt-catalyzed systems, owing to their low cost and catalyst stability, continue to offer advantages in the production of high-carbon alcohols, as exemplified by industrial processes at BASF, Sasol, and Shell. Pd–H intermediates are employed in processes such as the Lucite Alpha process and the conversion of 1,3-butadiene toward Nylon 66. Notably, the Lucite Alpha process has achieved an annual production capacity of approximately 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tons of MMA per year.10 Another notable example of metal-hydride chemistry in industrial applications is isomerization–hydroformylation tandem reactions (known as chain-walking carbonylation). In industrial production, chemists and engineers aim to avoid generating mixtures of isomeric products. To achieve this, hydroformylation of mixed alkenes under appropriate reaction conditions can yield a single, well-defined product while suppressing isomer formation. This is particularly important because most industrial feedstocks or by-products contain internal alkenes; utilizing such mixed alkenes as starting materials, obtaining a single product is a highly desirable goal. The process is accomplished via isomerization of the double bond to a terminal alkenyl unit prior to hydroformylation, and the overall methodology is referred to as isomerization–hydroformylation (Scheme 1B2). The relevant industrial applications operate on a 10
000 tons of MMA per year.10 Another notable example of metal-hydride chemistry in industrial applications is isomerization–hydroformylation tandem reactions (known as chain-walking carbonylation). In industrial production, chemists and engineers aim to avoid generating mixtures of isomeric products. To achieve this, hydroformylation of mixed alkenes under appropriate reaction conditions can yield a single, well-defined product while suppressing isomer formation. This is particularly important because most industrial feedstocks or by-products contain internal alkenes; utilizing such mixed alkenes as starting materials, obtaining a single product is a highly desirable goal. The process is accomplished via isomerization of the double bond to a terminal alkenyl unit prior to hydroformylation, and the overall methodology is referred to as isomerization–hydroformylation (Scheme 1B2). The relevant industrial applications operate on a 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–100
000–100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000-ton scale.16
000-ton scale.16
Although impressive progress has been achieved with noble metal catalysts in hydrocarbonylation, their high cost together with the even more expensive combined phosphine ligands making alternative, cheaper catalytic systems attractive. Among the candidates, 3d metals are meaningful due to their abundance and low toxicity. This idea has also been recognized by chemists and carbonylation reactions catalyzed by earth-abundant metal hydrides, including iron-, cobalt-, nickel-, and copper, were achieved during the past decades. While these earth-abundant metal systems offer the advantage of low cost, their catalytic activity is generally lower. This contrast is well illustrated by hydroformylation processes catalyzed by rhodium versus cobalt. Therefore, enhancing the reactivity of earth-abundant metal catalysts remains a key challenge that chemists must address. In this review, we summarize some interesting strategies developed over the past decade by organic chemists to achieve efficient carbonylation reactions mediated by earth-abundant metal hydrides,17–21 which have demonstrated certain industrial potential. These include both carbonylation reactions and isomerization–carbonylation tandem reactions (Scheme 1D).
2. Carbonylation involving Fe–H species
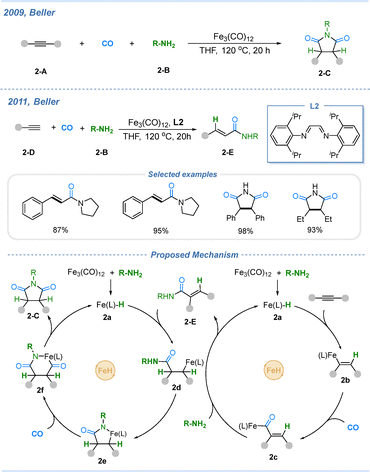
Iron is one of the most abundant metals in the Earth's crust, accounting for approximately 5% of its composition. The oxidation states of iron range from −II to +VI, and its oxides, as well as the various salts derived from them, are generally nontoxic. Moreover, regulatory authorities classify iron as a metal with very low safety risks, allowing residual levels of iron in pharmaceuticals as high as 1300 ppm.22 In recent years, iron-catalyzed carbonylation reactions have been actively developed, including the carbonylation of halides,23,24 unsaturated compounds,25 inert gases,26 Suzuki-type carbonylative coupling,27 and so on. Among these, Fe–H-mediated carbonylation transformations have been developed by the Beller group. For instance, in 2009, the Beller group reported the first example of an iron-catalyzed double carbonylation of alkynes with amines to afford succinimides with good functional-group tolerance, both aryl-, alkyl-, and internal alkynes reacted efficiently.28 In 2011, Beller and co-workers further achieved the selective carbonylation of terminal alkynes to give linear α,β-unsaturated amides.29 The corresponding mechanism is illustrated in Scheme 2. Initially, Fe3(CO)12 reacts with amines to generate Fe–H species 2a. Hydride species 2a undergoes hydroironation of the alkyne to afford intermediate 2b, which subsequently captures carbon monoxide to form the acyl intermediate 2c. Upon nucleophilic attack by the amine, the monocarbonylated product 2-E is obtained. A second Fe–H addition to the alkyne furnishes intermediate 2d, which then undergoes intramolecular cyclization to form 2e. Following CO coordination and migratory insertion, the acyl intermediate 2f is generated. Finally, reductive elimination from 2f delivers the double carbonylation product 2-C.3. Carbonylation involving Co–H species
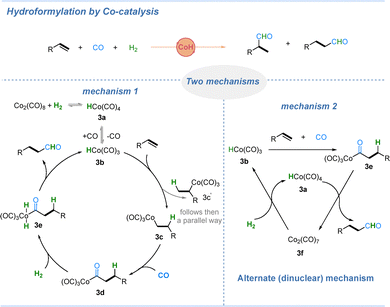
CoH-mediated carbonylation was first exemplified in hydroformylation reactions.30 In this process, molecular hydrogen serves as the hydrogen source. Cobalt-catalyzed hydroformylation typically affords a mixture of linear and branched aldehydes, and compared to rhodium-catalyzed systems, selectivity control is more challenging, primarily because cobalt catalysts exhibit lower sensitivity toward ligand modification.31 As illustrated in Scheme 3, the cobalt hydride species [Co(H)(CO)4] and [Co(H)(CO)3] are recognized as key reactive intermediates. Two main mechanistic pathways have been proposed. In the first mechanism, Co2(CO)8 reacts with H2 to generate intermediate 3a, which can lose one ligand of CO to form 3b. Subsequent olefin coordination and insertion afford 3c, followed by CO migratory insertion to generate 3d. Addition of hydrogen to 3d yields 3e, which then undergoes reductive elimination to produce the corresponding aldehyde. The second mechanism involves an alternating (binuclear) pathway, in which, under stoichiometric cobalt conditions, [Co(H)(CO)4] (3a) directly reacts with the acyl intermediate 3e to form the aldehyde product and [Co2(CO)7]. The latter unsaturated dimer can coordinate with H2, regenerating the two cobalt hydride species [Co(H)(CO)4] and [Co(H)(CO)3]. However, this binuclear pathway is generally operative only at high cobalt concentrations in the catalytic solution.31In 2017, the Alexanian group reported a cobalt-catalyzed silylcarbonylation of secondary alkyl tosylates under mild conditions (Scheme 4).32 As illustrated in Scheme 4, the reaction begins with a nucleophilic substitution of the alkyl tosylate by the tetracarbonylcobalt anion, Co(CO)4− (intermediate 4a), to generate an alkyl-cobalt species. Insertion of carbon monoxide then furnishes the acyl cobalt intermediate 4b. Subsequently, oxidative addition of triethylsilane (Et3SiH) to 4b produces intermediate 4c, which undergoes 1,3-silyl migration to form the cobalt–carbene intermediate 4d. A following 1,2-hydride shift yields intermediate 4e, and finally, β-hydride elimination affords the silyl enol ether product along with regeneration of the cobalt species 4f. The reaction performs poorly with primary alkyl tosylates, likely because less substituted alkyl-cobalt intermediates undergo β-hydride elimination at a significantly slower rate, thus reducing the overall efficiency of the transformation.
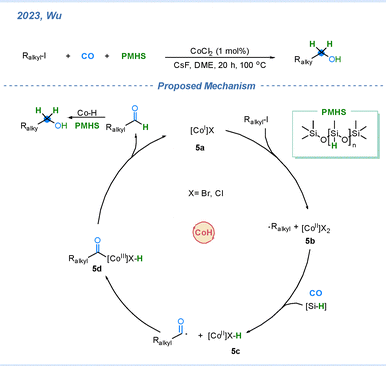
In 2023, Wu and co-workers reported the cobalt-catalyzed hydroxymethylation of alkyl halides by using PMHS as the H source.33 The proposed reaction mechanism is depicted in Scheme 5. Initially, cobalt(I) species 5a undergoes single-electron transfer (SET) to the alkyl iodide, generating an alkyl radical and the cobalt(II) intermediate 5b. In the presence of Et3SiH and CO, the alkyl radical is trapped by CO to afford an acyl radical, which then associates with the cobalt center to form intermediate 5c. Subsequent radical recombination produces the acyl–cobalt intermediate 5d, which undergoes reductive elimination to deliver the corresponding aldehyde and regenerate the active Co(I) catalyst (5a). Finally, the aldehyde is reduced by the Cu–H species to furnish the alcohol product bearing one additional –CH2 moiety.
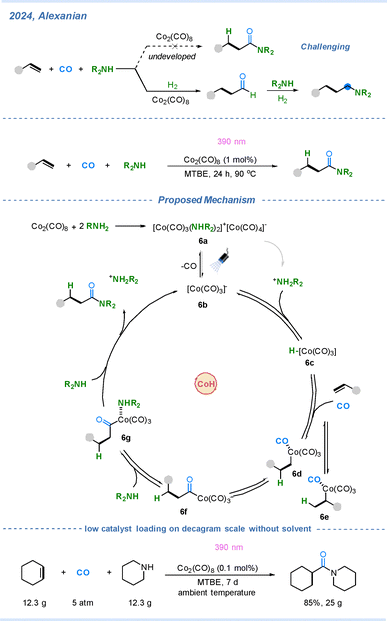
In 2024, the Alexanian group reported the light-promoted cobalt-catalyzed carbonylation of alkenes and amines toward amides, representing a significant breakthrough in the field.34 Since the discovery of the cobalt-catalyzed hydroformylation reaction in 1938 and Otto Reppe's development of Ni(CO)4-mediated acetylene carbonylation (Reppe carbonylation) in the 1930s, Pd–H-mediated carbonylation has been extensively explored and well-established.35 However, CoH-mediated Reppe-type carbonylation had not been realized due to several intrinsic challenges: the relatively low activity of cobalt catalysts, the difficulty in controlling alkene selectivity through ligands, and the strong influence of Lewis-basic amines on the catalyst's reactivity. To address these challenges, Alexanian and co-workers developed a Reppe-type carbonylation with the combination of photochemistry, and thus the long-standing limitations of Co–H-mediated systems were effectively overcome. As illustrated in Scheme 6, complex 6a is formed by the reaction of Co2(CO)8 with an amine. Under irradiation with 390 nm LED light, one CO ligand dissociates to generate the active cobalt species 6b, [Co(CO)3]−. Subsequent protonation of 6b affords intermediate 6c HCo(CO)3, which undergoes hydrocobaltation with the alkene to form linear and branched intermediates (6d and 6e, respectively; 6d being the major species). Coordination and migratory insertion of CO then lead to intermediate 6f. Nucleophilic coordination of the amine to the cobalt center produces 6g, which undergoes concerted addition and reductive elimination to afford the amide product along with regeneration of 6b, [Co(CO)3]−, thereby completing the catalytic cycle.
In this hydroaminocarbonylation reaction, a high quantum yield (Φ = 15.7) was observed, indicating that after the initial photolytic CO dissociation, the catalytic cycle can proceed through multiple turnovers without the need for further photoexcitation. This efficient catalytic turnover is likely attributed to the rapid protonation of [Co(CO)3]− under low CO pressure, which outcompetes CO re-coordination and thereby facilitates the formation of the stable cobalt hydride intermediate 6c HCo(CO)3. Notably, this hydride species 6c exhibits remarkable tolerance toward Lewis-basic amines and remains catalytically competent for alkene coordination and activation, enabling the reaction to proceed efficiently under mild conditions.
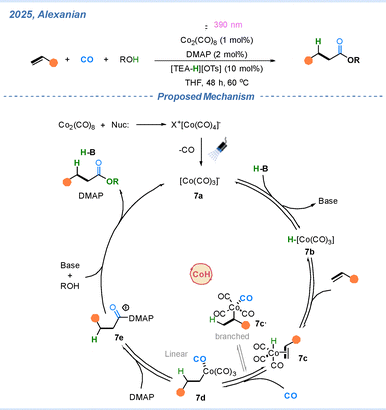
In 2025, the Alexanian group further extended their cobalt hydride catalytic system to achieve a hydroesterification reaction, representing the Reppe-type carbonylation of alcohols.36 As illustrated in Scheme 7, intermediate 7a is generated from the reaction of Co2(CO)8 with a nucleophile under irradiation with 390 nm LED light. In the presence of triethylamine sulfonate, the cobalt hydride species 7b is formed. Subsequently, 7b coordinates to the alkene, producing intermediate 7c, which can evolve into either the linear or branched cobalt intermediates (7d or 7c′), with the linear intermediate 7d being predominant. Under the promotion of DMAP, 7d is converted into an acyl–DMAP salt intermediate 7e. Finally, nucleophilic attack by an alcohol affords the desired ester product, while regenerating 7a to complete the catalytic cycle.
In 2024, the Cheng group developed the cobalt-catalyzed intramolecular Markovnikov hydrocarbonylation of unactivated alkenes.37 In this reaction, mechanistic investigations revealed that the transformation proceeds via a CO-mediated hydrogen atom transfer (HAT) and radical-polar crossover (RPC) process, in which an acyl–Co(IV) complex serves as the key intermediate. As illustrated in Scheme 8, the reaction begins with the formation of cobalt hydride species 8b from Co(II) precursor 8a in the presence of an oxidant and a silane. The Co–H species then adds to the alkene, generating radical intermediate 8c along with a Co(II) species. Under a CO atmosphere, the resulting radical captures CO to form acyl radical 8d. Subsequent radical-polar crossover process between the acyl radical and Co(II) affords the acyl–Co(III) intermediate 8e. Intermediate 8e undergoes oxidation or disproportionation to yield the crucial acyl–Co(IV) species 8f, which then undergoes reductive elimination to furnish the desired lactone products while regenerating the Co(II) catalyst, thereby completing the catalytic cycle (Scheme 8).
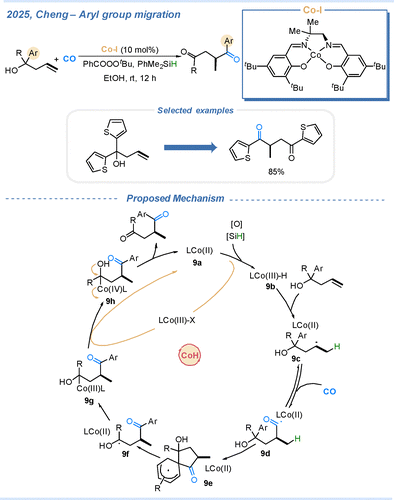
In 2025, the Cheng group developed a Co–H-mediated hydroaryloxycarbonylation of unactivated alkenes, enabling remote aryl migration.38 Mechanistic studies revealed that the acyl radical species is generated under a CO atmosphere via a hydrogen atom transfer (HAT) process. The driving force for the aryl migration arises from the formation of a thermodynamically favourable five-membered cyclic transition state. The detailed reaction mechanism is illustrated in Scheme 9. Initially, Co(II) species 9a reacts with an oxidant and a silane to generate the Co(III)–H species 9b and a Co(III) complex. The Co(III)–H species then undergoes a hydrogen atom transfer process to the alkene, producing the alkyl radical intermediate 9c. Under a CO atmosphere, this alkyl radical is readily trapped by CO to form the acyl radical 9d. Owing to the thermodynamic stability of the five-membered cyclic transition state, 9d readily undergoes intramolecular cyclization to afford intermediate 9e. Subsequently, aryl migration occurs via homolytic C–C bond cleavage, generating a more stable hydroxyalkyl radical intermediate, 9f. This radical then reacts with Co(II) species to form a Co(III) complex 9g. Finally, 9g is oxidized by either the Co(III) complex or an external oxidant to yield a highly electrophilic Co(IV) intermediate 9h, which undergoes reductive elimination to deliver the aryl migration product while regenerating Co(II) 9a, completing the catalytic cycle. Alternatively, the hydroxyalkyl radical 9f may be directly oxidized by the Co(III) complex or oxidant, followed by deprotonation to furnish the product and regenerate Co(II) catalyst 9a.
In 2025, the Wu group reported the Co–H-mediated hydroamination of bicyclo[1.1.0]butanes (BCBs) with aliphatic amines.39 As illustrated in Scheme 10, the HCo(CO)4 intermediate is first generated via a disproportionation reaction between Co2(CO)8 and the amine. The HCo(CO)4 species then inserts into the C–C bond of the BCB, forming intermediate 10b. Subsequently, migratory insertion of a CO ligand occurs to produce intermediate 10c. This step is reversible, and applying higher CO pressure drives the equilibrium toward the formation of 10c. Under a CO atmosphere, 10c coordinates an additional CO molecule to regenerate the tetracarbonyl cobalt complex 10d. Finally, nucleophilic attack by the amine on the carbonyl group affords the desired product, while regenerating the HCo(CO)4 species 10a, thereby completing the catalytic cycle.
In 2025, the Wu group developed the photoredox-cobalt co-catalyzed Markovnikov-selective hydrocarboxylation of unactivated alkenes under blue-light irradiation.40 As illustrated in Scheme 11, upon exposure to blue light, the photocatalyst (PC) is excited to its active state (PC*). The excited PC* then reduces the Co(II) intermediate 11a to generate Co(I) species 11b and the oxidized PC˙+. Subsequently, protonation of Co(I) 11b by a Brønsted acid affords the Co(III)–H intermediate 11c. This Co(III)–H species undergoes a hydrogen atom transfer (HAT) process with the alkene substrate, forming a metal–alkyl radical pair 11d. Under a CO atmosphere, the alkyl radical captures a molecule of CO to generate the metal–acyl radical pair 11e, which exists in reversible equilibrium with the acyl–Co(III) intermediate 11f. The acyl–Co(III) species 11f is then oxidized by PC˙+ via a single-electron transfer (SET) process to produce the high-valent cationic acyl–Co(IV) intermediate 11g. Finally, in the presence of a base, the Co(IV) intermediate 11g undergoes nucleophilic substitution with an alcohol, yielding the ester product while regenerating the Co(II) catalyst, thereby completing the catalytic cycle.
4. Carbonylation involving Ni–H species
Nickel is an earth-abundant and inexpensive metal that exhibits similar catalytic properties to palladium in coupling reactions.41 Due to its smaller atomic radius, nickel demonstrates strong oxidative insertion ability but weaker reductive elimination capability. In recent years, there has been growing interest in developing nickel-catalyzed carbonylation reactions, driven by the need for more sustainable and cost-effective catalytic systems.42 Carbon monoxide binds strongly to nickel, and this property is exploited industrially in the Mond process.43 In this process, impure nickel reacts with CO at 50–60 °C to form the volatile compound nickel tetracarbonyl, Ni(CO)4. The gaseous Ni(CO)4 is then decomposed at 180–200 °C, yielding high-purity nickel metal. Therefore, when employing nickel catalysts in CO-involved carbonylation reactions, catalyst poisoning often occurs due to the strong coordination between CO and nickel.44,45 As a result, the development of nickel-catalyzed carbonylation reactions has been relatively slow. In recent years, however, several research groups have focused on using CO surrogates or designing pincer-type NN2 nickel catalysts to effectively avoid the formation of Ni(CO)4 and thus overcome this challenge.46–48 This review primarily focuses on the recent advances in Ni–H-mediated carbonylation reactions employing CO surrogates.In 2021, the Zhu group reported a nickel-catalyzed multicomponent regio- and enantioselective hydrocarbonylative coupling reaction.49 This method features a broad substrate scope and excellent enantioselectivity. The proposed reaction mechanism is shown in Scheme 12: first, the nickel catalyst 12a undergoes oxidative addition to the chloroformate reagent, generating the Ni(II) intermediate 12b. In the presence of a silane and a base, 12b is converted into the Ni–H species 12c, which then adds across the alkene to form 12d. Subsequent migratory insertion of the CO ligand affords intermediate 12e. At this stage, nickel remains in the +2 oxidation state. Oxidative addition of the electrophile then produces the possible Ni(IV) intermediate 12f. Finally, reduction by the silane yields the desired product and regenerates catalyst 12a, completing the catalytic cycle.
In 2022, the Fang group developed the first nickel-catalyzed asymmetric Reppe-type carbonylation of cyclopropenes.50 This method enables the synthesis of a variety of highly substituted α-chiral cyclopropanecarboxylates and bicyclic lactones under CO-free conditions or using only a CO balloon, delivering excellent diastereo- and enantioselectivities, respectively. The proposed reaction mechanism, shown in Scheme 13, proceeds as follows: The nickel catalyst 13a undergoes oxidative addition to a formate ester to produce intermediate 13b, or alternatively, under CO atmosphere, oxidative addition to phenol occurs to give intermediate 13c, which then undergoes migratory insertion of CO to furnish 13b. Intermediate 13b adds to the cyclopropene derivative, generating 13d. Finally, reductive elimination affords the desired product and regenerates the Ni(0) species 13a, thereby completing the catalytic cycle.
In 2025, the Shu group developed nickel-catalyzed asymmetric cross-hydrocarbonylation between aryl olefins and alkyl olefins.51 A key feature of this reaction is that two different alkenes undergo Ni-catalyzed hydrocarbonylation with distinct regioselectivities. This method shows excellent chemoselectivity and regioselectivity, providing an efficient synthetic pathway to asymmetric dialkyl ketones from simple and readily available starting materials. As shown in Scheme 14, the reaction mechanism proceeds as follows: first, Ni(0) species 14a undergoes oxidative addition to chloroformate, generating 14b. In the presence of a silane and base, a metal exchange process affords the Ni(II)–H intermediate 14c. Intermediate 14c then selectively undergoes migratory insertion with the alkyl olefin, forming the alkyl–Ni(II) species 14d. Subsequent ligand dissociation and migratory CO insertion generate the acyl–Ni(II) intermediate 14e. Next, 14e undergoes another metal exchange process with a silane and base to form a second Ni(II)–H species, 14f. This Ni–H intermediate selectively inserts into the aryl olefin through a hydrometallation step to afford 14g. Reductive elimination from 14g delivers the final product and regenerates the Ni(0) catalyst 14a to complete the catalytic cycle. A possible side pathway involves homolytic cleavage of the Ni–C bond in 14g, forming a Ni(I)/benzyl-radical pair.
In 2025, the Shu group developed another nickel-catalyzed reductive carbonylation that couples two different olefins – an unactivated alkene and an N-alkenyl heteroarene – to afford chiral α-N-heteroaryl ketones (Scheme 15).52 The reaction proceeds with a similar mechanism and starts with Ni(0) species 15a. After the acyl–Ni(II)–H species 15f as the key intermediate was generated, a regio- and enantioselective migratory hydrometallation of the N-alkenyl heteroarene with 15f furnishes the acyl–Ni(II)–alkyl intermediate 15g, which then delivers the desired product after reductive elimination and regenerates the Ni(0) species 15a for the next catalytic cycle.
5. Carbonylation involving Cu–H species
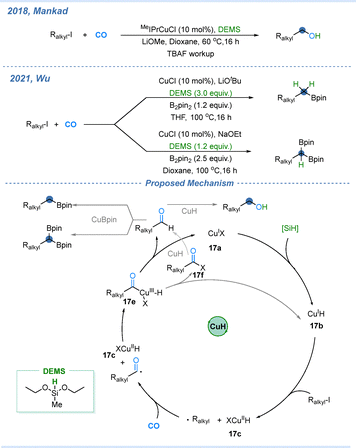
Copper-catalyzed carbonylative transformations have developed rapidly over the past decade, largely due to the versatile reactivity of Cu–H, Cu–B, and Cu–Si species in both hydrofunctionalization of unsaturated bonds and single-electron reduction of alkyl halides.53,54 In 2017 and 2018, Mankad and co-workers reported the copper-catalyzed hydrocarbonylation of terminal alkynes for the construction of dialkyl ketones and allylic alcohol derivatives.55,56 By controlling the temperature of this reaction, they further achieved either 1,4- or 1,2-addition of Cu–H species to the resulting enone intermediate, leading to two types of products. As described in Scheme 16, IPrCuCl first reacts with a base to form IPrCuOMe, which then undergoes transmetallation with a silane to yield the active Cu–H species 16a. This species adds across the terminal alkyne to give intermediate 16b. Subsequently, 16b engages in a single-electron process of the alkyl iodide, giving intermediate 16c and an alkyl radical. The alkyl radical rapidly adds CO to produce an acyl radical, which then combines with 16c to yield the Cu(III) intermediate 16d. Reductive elimination from 16d affords the unsaturated ketone 16e and reproduces the Cu(I) salts, which re-enter the next catalytic cycle via conversion with a base and silane to get IPrCuH. Then 16e undergoes further functionalization. It undergoes 1,4-addition with Cu–H at 60 °C, followed by quenching of [Si]–H compounds to obtain dialkyl ketones. In another pathway, 16e intermediate undergoes 1,2-addition with Cu–H at room temperature, and subsequent quenching with [Si]–H compounds to obtain allylic alcohols.In 2018, the Mankad group reported the copper-catalyzed hydroxymethylation of unactivated alkyl iodides.57 This transformation represents an intriguing homologation strategy: alkyl iodides, readily prepared from the corresponding alcohols, are converted into alcohols extended by one carbon. The newly formed alcohols can be further transformed back into alkyl iodides, enabling iterative chain elongation to access progressively longer alkyl iodides and alcohols. Later in 2021, Wu and co-workers developed a borylative methylation that simultaneously engages both Cu–Bpin and Cu–H intermediates (Scheme 17).58 In this catalytic system, the reaction of Cu–H with alkyl iodides is significantly faster than that of Cu–Bpin. The proposed mechanism is outlined as follows: in the presence of a silane, the copper catalyst 17a is converted into the Cu–H species 17b, which reduces the alkyl iodide via a single-electron process to form a carbon-centered radical and copper intermediate 17c. Under a CO atmosphere, the carbon radical rapidly captures CO to generate an acyl radical that then couples with 17c, producing the acyl–copper intermediate 17d. Reductive elimination from 17d affords either an acyl halide or an aldehyde, while regenerating the active copper species (17a or 17b). The acyl halide can be further reduced by Cu–H to the corresponding aldehyde, which may then undergo subsequent transformations, including reduction to one-carbon-extended alcohols, borylmethylation to give boronates, or diborylmethylation to yield diboron-containing products.
In copper-catalyzed carbonylation chemistry, particularly over the past five years, CuH-mediated asymmetric carbonylation has emerged as one of the most efficient strategies for constructing chiral carbonyl compounds. In 2020, Wu and co-workers developed the first Cu-catalyzed carbonylative hydroamidation of aryl alkenes and its related asymmetric variants, enabling the synthesis of chiral amides in good yields and excellent enantioselectivities (up to 99% ee).59 In 2023, Wu and co-workers reported the Cu-catalyzed carbonylative hydroamidation of unactivated alkenes.60 A key to this transformation was the use of the highly sterically demanding bidentate phosphine ligand (R)-DTBM-Segphos, which significantly enhanced the hydrometallation capability of the Cu–H species toward unactivated C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds. β-Chiral amides were obtained when 1,1-disubstituted alkenes were employed. In 2024, while existing methods for synthesizing chiral amides primarily focused on accessing α- and β-substituted amides, controlling remote stereocenters in γ-chiral amides typically required harsh reaction conditions. To address this challenge, the Wu group developed a general Cu-catalyzed strategy for the enantioselective synthesis of γ-chiral amides through a reductive relay hydroamidation process.61 This method exhibited a broad substrate scope and delivered products with excellent enantioselectivities (up to 99% ee). In the same year, under similarly mild conditions, they also realized an asymmetric carbonylation of internal alkenes.62 In the asymmetric carbonylation reactions mentioned above, the electrophiles were nitrogen-based reagents. In 2025, Wu and co-workers reported a novel carbonylation example on employing a carbon-based electrophile, 2-methylallyl diphenyl phosphate, to furnish chiral α,β-unsaturated ketones (Scheme 18).63
C bonds. β-Chiral amides were obtained when 1,1-disubstituted alkenes were employed. In 2024, while existing methods for synthesizing chiral amides primarily focused on accessing α- and β-substituted amides, controlling remote stereocenters in γ-chiral amides typically required harsh reaction conditions. To address this challenge, the Wu group developed a general Cu-catalyzed strategy for the enantioselective synthesis of γ-chiral amides through a reductive relay hydroamidation process.61 This method exhibited a broad substrate scope and delivered products with excellent enantioselectivities (up to 99% ee). In the same year, under similarly mild conditions, they also realized an asymmetric carbonylation of internal alkenes.62 In the asymmetric carbonylation reactions mentioned above, the electrophiles were nitrogen-based reagents. In 2025, Wu and co-workers reported a novel carbonylation example on employing a carbon-based electrophile, 2-methylallyl diphenyl phosphate, to furnish chiral α,β-unsaturated ketones (Scheme 18).63
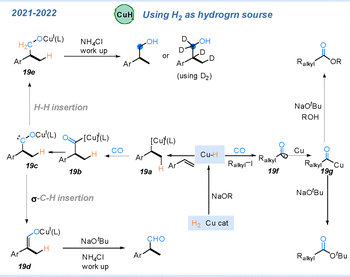
Hydrogen gas, as an inexpensive and readily available hydrogen source, is widely favoured in metal–hydride chemistry.64 As described in Scheme 19, the Cu–H species can add to alkenes to form intermediate 19a, which can then capture CO to afford the acyl–copper intermediate 19b. Intermediate 19b undergoes isomerization to form carbene species 19c. Hydrogenation of 19c gives 19e, after quenching with ammonium chloride, yields the corresponding alcohols. Alternatively, α-C–H insertion of 19c forms intermediate 19d, which, after quenching with a base and ammonium chloride, provides the corresponding aldehydes. When D2 is used instead of H2, various deuterated compounds can be obtained. In addition, the Cu–H intermediate can reduce alkyl iodides via a single-electron process to generate alkyl radicals, which capture CO to form acyl radicals. Trapping of these radicals by copper gives acyl–copper intermediates 19g, which deliver esters upon nucleophilic quenching.65–67
In 2025, the Wu group developed the highly selective copper-catalyzed carbonylation of methylenecyclopropanes, enabling the construction of chiral δ-boryl ketones.68 This methodology allows three structurally distinct chiral organoboron ketones to be selectively synthesized from a common set of starting materials, and diverse downstream derivatizations further enrich the chemical space of chiral ketones. As illustrated in Scheme 20, the reaction begins with the formation of the active LCuH species 20b through the reaction of the copper catalyst with a silane. Hydrometalation of the methylenecyclopropane affords intermediate 20c, followed by β-C scission to generate 20d. Trapping of 20d by a boron reagent yields the key homoallyl boronate intermediate 20e while regenerating LCuH. Subsequently, intermediate 20e undergoes hydrometalation with LCuH to form the chiral copper species 20f. Coordination of an allyl phosphate and CO to 20f gives intermediate 20g, which then undergoes β-halogen elimination to deliver product 20A. In the presence of an appropriate base, product 20A can be further isomerized or reduced to give product 20B.
 | ||
| Scheme 20 CuH-mediated asymmetric carbonylative coupling between strained alkenes and electrophiles. | ||
6. Isomerization–carbonylation tandem reactions
In metal-hydride-mediated hydroformylation reactions, isomerization–carbonylation tandem reactions have long been among the most intriguing and valuable transformations. Their greatest advantage is the ability to convert a mixture of starting olefins into a single carbonylated product. This is particularly important in the chemical industry, where mixtures of olefins that are difficult to separate can be further transformed via this tandem process. The isomerization–carbonylation sequence can convert such mixed olefins into terminal esters, which can serve as valuable single-component products, for example, as plasticizers.696.1 Isomerization–hydroformylation tandem reactions
In isomerization–carbonylation tandem reactions, isomerization–hydroformylation tandem processes have already been applied in the chemical industry on a 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–100
000–100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000-ton scale.16 The isomerization–carbonylation tandem reactions generally proceed via two mechanistic pathways, as illustrated in Scheme 21. In the first pathway, 21a undergoes M–H addition to form 21b, followed by β-hydride elimination to yield 21c. The second pathway involves oxidative addition of an allylic C–H bond to generate 21e, which then forms the π–allyl intermediate 21f. Subsequent isomerization affords 21g, and finally reductive elimination gives 21h. Moreover, comparisons of catalysts based on different metals (Rh, Ru, and Pt) in isomerization–carbonylation tandem reactions reveal that rhodium, as a noble metal catalyst, generally provides superior selectivity.
000-ton scale.16 The isomerization–carbonylation tandem reactions generally proceed via two mechanistic pathways, as illustrated in Scheme 21. In the first pathway, 21a undergoes M–H addition to form 21b, followed by β-hydride elimination to yield 21c. The second pathway involves oxidative addition of an allylic C–H bond to generate 21e, which then forms the π–allyl intermediate 21f. Subsequent isomerization affords 21g, and finally reductive elimination gives 21h. Moreover, comparisons of catalysts based on different metals (Rh, Ru, and Pt) in isomerization–carbonylation tandem reactions reveal that rhodium, as a noble metal catalyst, generally provides superior selectivity.
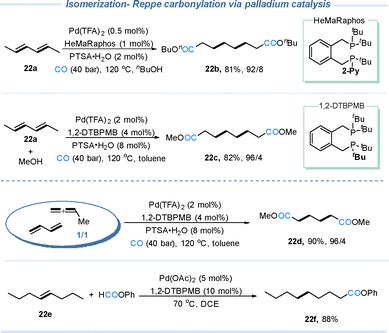
6.2 PdH-mediated isomerization–carbonylation tandem reactions
In isomerization–carbonylation tandem reactions, palladium-catalyzed migratory hydrocarbonylation is the most fully developed case. As shown in Scheme 22, the HeMaRaphos ligand developed by the Beller group enables terminal-selective migratory carbonylation with high selectivity.11 In addition, the commercially available 1,2-DTBPMB also provides excellent selectivity. Using mixed allenes and 1,3-butadiene in this migratory carbonylation process affords the single diester product 22d (Nylon-66 monomer) in high yield.70 Moreover, when phenyl formate is employed as an alternative carbonyl source, the terminally migrated carbonylation product 22f can also be obtained.716.3 Earth abundant metal–hydride-mediated isomerization–carbonylation tandem reactions
In the cobalt-catalyzed hydroamidation reported by the Alexanian group in 2024, the reaction can be applied to isomerization–carbonylation, achieving a linear-to-branched selectivity of up to 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The corresponding mechanism is illustrated in Scheme 23. First, 23a reacts with the ammonium salt to generate the cobalt–hydride intermediate 23b, which can subsequently add to the mixed olefins to form 23c and 23d. These intermediates then undergo chain-walking to reach the terminal position, giving 23f. Coordination of CO and the amine leads to intermediate 23g, which finally delivers the amide product under the action of the amine, along with regeneration of the ammonium salt and 23a, thereby completing an isomerization–carbonylation catalytic cycle.34
1. The corresponding mechanism is illustrated in Scheme 23. First, 23a reacts with the ammonium salt to generate the cobalt–hydride intermediate 23b, which can subsequently add to the mixed olefins to form 23c and 23d. These intermediates then undergo chain-walking to reach the terminal position, giving 23f. Coordination of CO and the amine leads to intermediate 23g, which finally delivers the amide product under the action of the amine, along with regeneration of the ammonium salt and 23a, thereby completing an isomerization–carbonylation catalytic cycle.34
In 2025, Shu and co-workers reported a nickel-catalyzed migratory carbonylation reaction involving two different alkenes. The substrates included two alkyl-substituted alkenes and a chloroformate derivative, with one of the alkenes bearing a terminal aryl group. This migratory carbonylation enabled the synthesis of benzyl-position chiral ketones. Unlike previous examples, in this reaction, one alkene undergoes migration toward the internal position (benzyl site), while the other alkene migrates to the terminal position of the alkyl-substituted alkene. The mechanism is illustrated in Scheme 24. First, Ni(0) species 24a undergoes oxidative addition with the chloroformate derivatives to form 24b, which, in the presence of a silane and base, is converted to 24c. Intermediate 24c adds across the alkene to give 24d. Under the influence of ligand L15, it undergoes migration to form the terminal organonickel intermediate 24e. Subsequent CO migratory insertion yields the acyl–nickel intermediate 24f. Treatment with a silane and base regenerates the Ni–H species 24g, which adds to the aryl-substituted alkene to form 24h. Ligand-controlled migration then affords the chiral intermediate 24i, which undergoes reductive elimination to give the target product and regenerates Ni(0) intermediate 24a, completing the catalytic cycle.72
7. Conclusions and outlook
In summary, carbonylation has become a powerful strategy for constructing value-added chemicals across organic synthesis, materials science, pharmaceuticals, and industrial processes.73–78 Hydrocarbonylation, exemplified by hydroformylation and Reppe-type carbonylation, plays an especially prominent role. While traditional carbonylation technologies rely heavily on noble-metal catalysts, growing attention is now being directed toward developing efficient, practical, and cost-effective catalytic systems based on earth-abundant metals. In this review, we have summarized recent advances in Fe-, Co-, Ni-, and Cu-catalyzed hydroesterification and hydroamidation reactions, and we anticipate that these transformations will find increasing relevance in industrial applications.Furthermore, olefin isomerization–carbonylation tandem processes (chain-walking carbonylation) provide a valuable approach for converting complex or mixed olefin feedstocks into single products, an aspect of particular interest to the chemical industry. This review also highlights recent progress in earth-abundant-metal-hydride-mediated carbonylation and isomerization–carbonylation tandem processes. Looking forward, we expect that even more economical metal catalysts, such as iron or copper, will continue to expand the scope of migratory hydrocarbonylation chemistry.
Conflicts of interest
There are no conflicts to declare.Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Acknowledgements
We are grateful for the financial support provided by the National Key R&D Program of China (2023YFA1507500), the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB1530000), the National Natural Science Foundation of China (22571291, 22302198, and 22572190), and the Dalian Institute of Chemical Physics (DICP, CAS).References
- X. Ma, J. Albertsma, D. Gabriels, R. Horst, S. Polat, C. Snoeks, F. Kapteijn, H. B. Eral, D. A. Vermaas, B. Mei, S. de Beer and M. A. van der Veen, Carbon monoxide separation: past, present and future, Chem. Soc. Rev., 2023, 52, 3741–3777 RSC.
- J.-B. Peng, F. P. Wu and X.-F. Wu, First-row transition-metal-catalyzed carbonylative transformations of carbon electrophiles, Chem. Rev., 2019, 119, 2090–2127 Search PubMed.
- L.-C. Wang and X.-F. Wu, Single-electron-transfer-mediated carbonylation reactions, Acc. Chem. Res., 2025, 58, 1036–1050 CrossRef PubMed.
- X. Pan, F. Jiao, D. Miao and X. Bao, Oxide–zeolite-based composite catalyst concept that enables syngas chemistry beyond Fischer–Tropsch synthesis, Chem. Rev., 2021, 121, 6588–6609 CrossRef.
- F. Hebrard and P. Kalck, Cobalt-catalyzed hydroformylation of alkenes: Generation and recycling of the carbonyl species, and catalytic cycle, Chem. Rev., 2009, 109, 4272–4282 CrossRef PubMed.
- J.-B. Peng, H.-Q. Geng and X.-F. Wu, The chemistry of CO: Carbonylation, Chem., 2019, 5, 526–552 Search PubMed.
- T. W. Dekleva and D. Forster, The Rhodium-catalyzed carbonylation of linear primary alcohols, J. Am. Chem. Soc., 1985, 107, 3565–3567 Search PubMed.
- R. Franke, D. Selent and A. Börner, Applied hydroformylation, Chem. Rev., 2012, 112, 5675–5732 Search PubMed.
- A. Haynes, P. M. Maitlis, G. E. Morris, G. J. Sunley, H. Adams, P. W. Badger, C. M. Bowers, D. B. Cook, P. I. P. Elliott, T. Ghaffar, H. Green, T. R. Griffin, M. Payne, J. M. Pearson, M. J. Taylor, P. W. Vickers and R. J. Watt, Promotion of Iridium-catalyzed methanol carbonylation: mechanistic studies of the cativa process, J. Am. Chem. Soc., 2004, 126, 2847–2861 CrossRef PubMed.
- J. Vondran, M. R. L. Furst, G. R. Eastham, T. Seidensticker and D. J. Cole-Hamilton, Magic of Alpha: The chemistry of a remarkable bidentate phosphine, 1,2-bis(di-tert-butylphosphinomethyl) benzene, Chem. Rev., 2021, 121, 6610–6653 CrossRef PubMed.
- J. Yang, J. Liu, H. Neumann, R. Franke, R. Jackstell and M. Beller, Direct synthesis of adipic acid esters via palladium-catalyzed carbonylation of 1,3-dienes, Science, 2019, 366, 1514–1517 CrossRef PubMed.
- R. Liu, B. Fan, Y. Zhi, C. Liu, S. Xu, Z. Yu and Z. Liu, Dynamic evolution of aluminum coordination environments in mordenite zeolite and their role in the dimethyl ether (DME) carbonylation reaction, Angew. Chem., Int. Ed., 2022, 61, e202210658 CrossRef PubMed.
- Nexant Inc. (2012) Oxo alcohols-report abstract. PERP 2011-2, May 2012, Nexant Inc.
- S. W. Beadle, R. D. Garton, H. J. Beckers, R. F. Caers, A. Van Vliet and J. J. Houben. (to ExxonMobil Chemical Company) ( 2008).
- Y. Wang, L. Yan, C. Li, M. Jiang, Z. Zhao, G. Hou and Y. Ding, Heterogeneous Rh/CPOL-BP&P(OPh)3 catalysts for hydroformylation of 1-butene: The formation and evolution of the active species, J. Catal., 2018, 368, 197–206 Search PubMed.
- M. Vilches-Herrera, L. Domke and A. Börner, Isomerization–hydroformylation tandem reactions, ACS Catal., 2014, 4, 1706–1724 Search PubMed.
- Y. Wang, Y. He and S. Zhu, NiH-Catalyzed Functionalization of Remote and Proximal Olefins: New Reactions and Innovative Strategies, Acc. Chem. Res., 2022, 55, 3519–3536 Search PubMed.
- F. Zhou, J. Zhu, Y. Zhang and S. Zhu, NiH-Catalyzed Reductive Relay Hydroalkylation: A Strategy for the Remote C(sp3)-H Alkylation of Alkenes, Angew. Chem., Int. Ed., 2018, 57, 4058–4062 CrossRef PubMed.
- J. Liu, H. Gong and S. Zhu, Nickel-Catalyzed, Regio- and Enantioselective Benzylic Alkenylation of Olefins with Alkenyl Bromide, Angew. Chem., Int. Ed., 2021, 60, 4060–4064 Search PubMed.
- A. J. Jordan, G. Lalic and J. P. Sadighi, Coinage Metal Hydrides: Synthesis, Characterization, and Reactivity, Chem. Rev., 2016, 116, 8318–8372 CrossRef PubMed.
- J. R. Norton and J. Sowa, Introduction: Metal Hydrides, Chem. Rev., 2016, 116, 8315–8317 CrossRef PubMed.
- K. Sun, G.-P. Lu, W. Han and M. Beller, Iron-catalyzed carbonylation reactions with carbon monoxide, Angew. Chem., Int. Ed., 2025, 64, e202512346 CrossRef PubMed.
- H. J. Ai, B. N. Leidecker, P. Dam, C. Kubis, J. Rabeah and X.-F. Wu, Iron-catalyzed alkoxycarbonylation of alkyl bromides via a two-electron transfer process, Angew. Chem., Int. Ed., 2022, 61, e202211939 CrossRef PubMed.
- S. Shao, Z.-P. Bao and X.-F. Wu, Iron-catalyzed carbonylative synthesis of tert-alkyl thioesters, Green Synth. Catal., 2025, 6, 449–452 Search PubMed.
- Z. Huang, J. Tang, X. Jiang, T. Xie, M. Zhang, D. Lan, S. Pi, Z. Tan, B. Yi and Y. Li, Iron-catalyzed hydroaminocarbonylation of alkynes: Selective and efficient synthesis of primary α,β-unsaturated amides, Chin. Chem. Lett., 2022, 33, 4842–4845 CrossRef.
- H. Pan, Q. An, B. K. Mai, Y. Chen, P. Liu and Z. Zuo, Iron-catalyzed aerobic carbonylation of methane via ligand-to-metal charge transfer excitation, J. Am. Chem. Soc., 2025, 147, 1440–1447 CrossRef PubMed.
- N. Xu, J. J. Chen, K. K. Sun and W. Han, Ligand-free Iron-catalyzed carbonylation of aryl iodides with alkenyl boronic acids: Access to α,β-unsaturated ketones, Org. Lett., 2024, 26, 9460–9465 CrossRef PubMed.
- K. M. Driller, H. Klein, R. Jackstell and M. Beller, Iron-catalyzed carbonylation: Selective and efficient synthesis of succinimides, Angew. Chem., Int. Ed., 2009, 48, 6041–6044 CrossRef PubMed.
- K. M. Driller, S. Prateeptongkum, R. Jackstell and M. Beller, A general and selective iron-catalyzed aminocarbonylation of alkynes: Synthesis of acryl- and cinnamides, Angew. Chem., Int. Ed., 2011, 50, 537–541 CrossRef.
- O. Roelen, Über Aldehyd-Synthesen, Angew. Chem., 1948, 60, 213 Search PubMed.
- F. Hebrard and P. Kalck, Cobalt-catalyzed hydroformylation of alkenes: Generation and recycling of the carbonyl species, and catalytic cycle, Chem. Rev., 2009, 109, 4272–4282 CrossRef.
- J. E. Roque Pena and E. J. Alexanian, Cobalt-catalyzed silylcarbonylation of unactivated secondary alkyl tosylates at low pressure, Org. Lett., 2017, 19, 4413–4415 CrossRef.
- Y. Yuan, N. X. Sun, C. S. Wang, K. Guo and X.-F. Wu, Cobalt-catalyzed hydroxymethylation of alkyl halides with CO as the C1 source, Org. Lett., 2023, 25, 5084–5088 CrossRef.
- M. S. Faculak, A. M. Veatch and E. J. Alexanian, Cobalt-catalyzed synthesis of amides from alkenes and amines promoted by light, Science, 2024, 383, 77–81 CrossRef.
- R. Sang, Y. Hu, R. Razzaq, R. Jackstell, R. Franke and M. Beller, State-of-the-art palladium-catalyzed alkoxycarbonylations, Org. Chem. Front., 2021, 8, 799–811 RSC.
- M. S. Faculak, M. R. Rodriguez and E. J. Alexanian, Cobalt-catalyzed syntheses of esters and carboxylic acids from alkenes promoted by light, J. Am. Chem. Soc., 2025, 147, 14060–14064 CrossRef.
- D. Jiang, X. Li, M. Xiao and L. J. Cheng, Cobalt-catalyzed intramolecular Markovnikov hydrocarbonylation of unactivated alkenes via hydrogen atom transfer, Angew. Chem., Int. Ed., 2024, 63, e202412828 CrossRef PubMed.
- M. Xiao, W. Li, D. Jiang and L.-J. Cheng, Cobalt-catalyzed Markovnikov hydroarylcarbonylation of unactivated alkenes via distal aryl migration, CCS Chem., 2025 DOI:10.31635/ccschem.025.202505922.
- P. Yang, Y.-H. Zhao, T. Brockmann, Y.-K. Liu and X.-F. Wu, Cobalt-catalyzed hydroaminocarbonylation of C-C σ bond of bicyclo[1.1.0]butanes toward cyclobutanecarboxamides, Cell Rep. Phys. Sci., 2025, 6, 102346 CrossRef.
- X. Mao, M. Hou, Y. Wang and X.-F. Wu, Cobalt/Brønsted acid co-catalyzed visible light-induced intermolecular Markovnikov hydrocarboxylation of unactivated alkenes, J. Catal., 2026, 453, 116535 CrossRef.
- R. Shi, Z. Zhang and X. Hu, Nickamine and analogous Nickel pincer catalysts for cross-coupling of alkyl halides and hydrosilylation of alkenes, Acc. Chem. Res., 2019, 52, 1471–1483 CrossRef CAS PubMed.
- X. Chen, G. Chen and Z. Lian, Recent advances in nickel catalyzed carbonylative reactions via the insertion of carbon monoxide, Chin. J. Chem., 2024, 42, 177–189 CrossRef CAS.
- The extraction of nickel from its ores by the Mond process, Nature, 1898, 59, 63–64 CrossRef.
- X. Qi, Z.-P. Bao, X.-T. Yao and X.-F. Wu, Nickel-catalyzed thiocarbonylation of arylboronic acids with sulfonyl chlorides for the synthesis of thioesters, Org. Lett., 2020, 22, 6671–6676 CrossRef CAS.
- P. Ji, C.-Y. Hou, B. Yao, T. Li, X. Qi and X.-F. Wu, Nickel-catalyzed carbonylation reaction of aryl N-tosylaziridines with arylboronic acids toward β-amino ketones, ACS Catal., 2025, 15, 13316–13321 CrossRef CAS.
- T. L. Andersen, A. S. Donslund, K. T. Neumann and T. Skrydstrup, Carbonylative coupling of alkyl zinc reagents with benzyl bromides catalyzed by a Nickel/NN2
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) pincer ligand complex, Angew. Chem., Int. Ed., 2018, 57, 800–804 CrossRef CAS.
pincer ligand complex, Angew. Chem., Int. Ed., 2018, 57, 800–804 CrossRef CAS. - A. S. Donslund, S. S. Pedersen, C. Gaardbo, K. T. Neumann, L. Kingston, C. S. Elmore and T. Skrydstrup, Direct access to isotopically labeled aliphatic ketones mediated by Nickel(I) activation, Angew. Chem., Int. Ed., 2020, 59, 8099–8103 CrossRef CAS.
- S. J. Ton, K. T. Neumann, P. Nørby and T. Skrydstrup, Nickel-mediated alkoxycarbonylation for complete carbon isotope replacement, J. Am. Chem. Soc., 2021, 143, 17816–17824 CrossRef CAS.
- J. Chen and S. Zhu, Nickel-catalyzed multicomponent coupling: Synthesis of alpha-chiral ketones by reductive hydrocarbonylation of alkenes, J. Am. Chem. Soc., 2021, 143, 14089–14096 CrossRef CAS.
- R. Yu, S. Z. Cai, C. Li and X. Fang, Nickel-catalyzed asymmetric hydroaryloxy- and hydroalkoxycarbonylation of cyclopropenes, Angew. Chem., Int. Ed., 2022, 61, e202200733 CrossRef CAS.
- Y. M. Du, X. Y. Chen, Y. Li, M. J. Koh and W. Shu, Rapid assembly of enantioenriched alpha-arylated ketones via Ni-catalyzed asymmetric cross-hydrocarbonylation enabled by alkene sorting, Nat. Commun., 2025, 16, 4163 CrossRef CAS PubMed.
- X. Y. Chen, Q. Yu and W. Shu, Synthesis of alpha-N-heteroaryl ketones by nickel-catalyzed chemo-, regio- and enantioselective carbonylation of alkenes and N-alkenyl heteroarenes, Angew. Chem., Int. Ed., 2025, 64, e202423426 CrossRef CAS.
- L.-J. Cheng and N. P. Mankad, Copper-catalyzed carbonylative coupling of alkyl halides, Acc. Chem. Res., 2021, 54, 2261–2274 CrossRef CAS.
- P. Yang, Y.-H. Zhao and X.-F. Wu, Pd/Cu-catalyzed regiodivergent silylcarbonylation of alkynes toward β-silylenones, ACS Catal., 2025, 15, 14423–14431 CrossRef CAS.
- L.-J. Cheng and N. P. Mankad, Cu-catalyzed hydrocarbonylative C–C coupling of terminal alkynes with alkyl iodides, J. Am. Chem. Soc., 2017, 139, 10200–10203 CrossRef CAS.
- L.-J. Cheng, S. M. Islam and N. P. Mankad, Synthesis of allylic alcohols via Cu-catalyzed hydrocarbonylative coupling of alkynes with alkyl halides, J. Am. Chem. Soc., 2018, 14, 1159–1164 CrossRef.
- S. Zhao and N. P. Mankad, Cu-catalyzed hydroxymethylation of unactivated alkyl iodides with CO to provide one carbon-extended alcohols, Angew. Chem., Int. Ed., 2018, 57, 5867–5870 CrossRef CAS.
- F.-P. Wu and X.-F. Wu, Copper-catalyzed borylative methylation of alkyl iodides with CO as the C1 source: Advantaged by faster reaction of CuH over CuBpin, Angew. Chem., Int. Ed., 2021, 60, 11730–11734 CrossRef CAS.
- Y. Yuan, F.-P. Wu, C. Schunemann, J. Holz, P. C. J. Kamer and X.-F. Wu, Copper-catalyzed carbonylative hydroamidation of styrenes to branched Amides, Angew. Chem., Int. Ed., 2020, 59, 22441–22445 CrossRef CAS PubMed.
- Y. Yuan, Y. Zhang, W. Li, Y. Zhao and X.-F. Wu, Regioselective and Enantioselective Copper-catalyzed hydroaminocarbonylation of unactivated alkenes and alkynes, Angew. Chem., Int. Ed., 2023, 62, e202309993 CrossRef CAS.
- Y. Yuan, Y. Zhang and X.-F. Wu, Enantioselective synthesis of g-chiral amides via copper-catalyzed reductive relay hydroaminocarbonylation, Nat. Commun., 2024, 15, 6705 CrossRef CAS PubMed.
- Y. Yuan, S. Shao, M. Hu, J. Zhu, Y. Zhao and X.-F. Wu, Copper-catalyzed asymmetric hydroaminocarbonylation of unactivated internal alkenes, CCS Chem., 2024, 7, 965–972 CrossRef.
- S. Shao, Y. Yuan, A. Schmoll and X.-F. Wu, Copper-catalyzed asymmetric carbonylative hydroallylation of vinylarenes, Chem. Sci., 2025, 16, 10951–10956 RSC.
- R. Franke, D. Selent and A. Börner, Applied hydroformylation, Chem. Rev., 2012, 112, 5675–5732 CrossRef CAS.
- H.-Q. Geng and X.-F. Wu, Copper-catalyzed alkoxycarbonylation of alkyl iodides for the synthesis of aliphatic esters: Hydrogen makes the difference, Org. Lett., 2021, 23, 8062–8066 Search PubMed.
- H.-Q. Geng, T. Meyer, R. Franke and X.-F. Wu, Copper-catalyzed hydroformylation and hydroxymethylation of styrenes, Chem. Sci., 2021, 12, 14937–14943 Search PubMed.
- H.-Q. Geng, R. Franke and X.-F. Wu, Copper-catalyzed synthesis of multideuterium-labeled alcohols from styrenes with CO and D2 as the source of the hydroxymethyl group, Org. Lett., 2022, 24, 7993–7996 Search PubMed.
- Y. Yuan, F. Ge, T. Yang and X.-F. Wu, Divergent synthesis of chiral δ-boryl ketones via copper-catalyzed carbonylative 1,4-borylallylation, ACS Catal., 2025, 15, 17614–17624 Search PubMed.
- Y. Hu, R. Sang, R. Vroemans, G. Mollaert, R. Razzaq, H. Neumann, H. Junge, R. Franke, R. Jackstell, B. U. W. Maes and M. Beller, Efficient synthesis of novel plasticizers by direct palladium-catalyzed di- or multi-carbonylations, Angew. Chem., Int. Ed., 2023, 62, e202214706 CrossRef CAS.
- J. Yang, J. Liu, Y. Ge, W. Huang, F. Ferretti, H. Neumann, H. Jiao, R. Franke, R. Jackstell and M. Beller, Efficient palladium-catalyzed carbonylation of 1,3-dienes: Selective synthesis of adipates and other aliphatic diesters, Angew. Chem., Int. Ed., 2021, 60, 9527–9533 CrossRef CAS.
- J. Li, T. Shen, Y. Zhuang, Y. Fu and Y. Shi, Pd-catalyzed highly regioselective migratory hydroesterification of internal olefins with formates, Chin. Chem. Lett., 2025, 36, 110599 CrossRef.
- Y.-M. Du, J.-N. Lin, Y.-L. Li, Q. Yu and W. Shu, Nickel-catalyzed adaptive migration-enabled asymmetric cross-hydrocarbonylation of unactivated alkenes, J. Am. Chem. Soc., 2025, 147, 18944–18952 CrossRef PubMed.
- Z.-P. Bao, L.-C. Wang and X.-F. Wu, Carbonylation: Unlocking opportunities for bioactive molecule and pharmaceutical development, ACS Catal., 2025, 15, 19580–19606 CrossRef.
- Z.-P. Bao and X.-F. Wu, Palladium-catalyzed direct carbonylation of bromoacetonitrile to synthesize 2-cyano-N-acetamide and 2-cyanoacetate compounds, Angew. Chem., Int. Ed., 2023, 62, e202301671 CrossRef.
- J. Yang, P. Wang, H. Neumann, R. Jackstell and M. Beller, Industrially applied and relevant transformations of 1,3-butadiene using homogeneous catalysts, Ind. Chem. Mater., 2023, 1, 155–174 RSC.
- Y. Wang, H. Yang, Y. Zheng, M. Hu, J. Zhu, Z.-P. Bao, Y. Zhao and X.-F. Wu, Carbon monoxide enabling synergistic carbonylation and (hetero)aryl migration, Nat. Catal., 2024, 7, 1065–1075 Search PubMed.
- H. C. D. Hammershøj, H. G. Gudmundsson, S. Kjærsgaard, J. Bønnelykke, J. Kolodiazhnaia and T. Skrydstrup, Multi-carbon labelling of active pharmaceutical ingredients enabled by a three-gas surrogate hydroformylation, Nat. Synth., 2023, 2, 243–250 CrossRef.
- L.-C. Wang, H. Yang, Z.-W. Liu, R.-G. Miao, M. Hou and X.-F. Wu, Recent advances in Single-Electron-Transfer-mediated carbonylation, Chem. Rev., 2026 DOI:10.1021/acs.chemrev.5c00664.
| This journal is © The Royal Society of Chemistry 2026 |