Open Access Article
Open Access ArticleTailoring penta-coordinated aluminium species on silica–alumina via flame spray pyrolysis for enhanced arene benzylation
Xingxu Liu a,
Wenjie Yanga,
Luke A. O’Dell
a,
Wenjie Yanga,
Luke A. O’Dell b,
Haipeng Licd,
Qinfen Gu
b,
Haipeng Licd,
Qinfen Gu e,
Suman Pokhrel
e,
Suman Pokhrel *cdf,
Lutz Mädler
*cdf,
Lutz Mädler *cdf and
Jun Huang
*cdf and
Jun Huang *a
*a
aLaboratory for Catalysis Engineering, School of Chemical and Biomolecular Engineering, Sydney Nano Institute, the University of Sydney, NSW 2006, Australia. E-mail: jun.huang@sydney.edu.au
bInstitute for Frontier Materials, Deakin University, Geelong, VIC 3220, Australia
cFaculty of Production Engineering, University of Bremen, Badgasteiner Straße 1, 28359, Bremen, Germany. E-mail: spokhrel@iwt.uni-bremen.de; Imaedler@iwt.uni-bremen.de
dLeibniz Institute for Materials Engineering IWT, Badgasteiner Straße 3, 28359 Bremen, Germany
eAustralian Synchrotron, ANSTO, 800 Blackburn Rd, Clayton, VIC 3168, Australia
fMAPEX Center for Materials and Processes, University of Bremen, 28359 Bremen, Germany
First published on 3rd December 2025
Abstract
Flame spray pyrolysis (FSP) was employed to tailor the coordination environment of aluminum species on amorphous silica–alumina (ASA) for enhanced solid acidity. By regulating the oxygen flow, the formation of penta-coordinated Al (AlV) species was promoted, especially the surface-distorted AlVb type. Advanced solid-state NMR and XAS analyses confirmed that AlV species incorporate into the silica network and interact with nearby silanol groups to form strong Brønsted acid sites (BASs) through pseudo-bridging structures. The resulting ASA catalysts exhibited increased acid strength, with TMPO-31P NMR shifts up to 67 ppm, comparable to zeolite-like acidity. These structural features correlate with significantly improved catalytic activity and selectivity in arene benzylation. This study establishes a clear synthesis–structure–acidity–function relationship and offers a rational strategy for designing high-performance ASA-based solid acids.
Broader contextThe sustainable transformation of chemicals and fuels requires replacing corrosive liquid acids, such as HF and H2SO4, with environmentally benign and recyclable solid acids. However, controlling the strength and density of acid sites in solid materials remains a major challenge. This study introduces a scalable flame spray pyrolysis method to tailor the coordination of aluminium species on amorphous silica–alumina, generating highly active penta-coordinated Al sites that form strong Brønsted acidity comparable to zeolites. The resulting catalysts enable efficient benzylation of arenes under mild, solvent-free conditions. Beyond this specific reaction, the strategy provides a general framework for designing solid acids with tunable acidity, bridging the gap between conventional silica–alumina and zeolitic materials. This approach offers both environmental and economic benefits by enabling cleaner, safer, and energy-efficient pathways for sustainable chemical manufacturing. |
1. Introduction
Silicon- and aluminum-based mixed oxides like amorphous silica–alumina (ASA) have become pivotal in modern catalysis due to their moderate and strong Brønsted acid sites (BASs) generated by the aluminium species in the silica network, making them versatile solid acids in various chemical processes.1,2 In addition to Brønsted acidity, ASA also provides Lewis acid sites (LASs) formed by unsaturated aluminum species, which can initiate reactions requiring electron-pair acceptors, like alcohol dehydration.3–5 LASs are also present and contribute significantly to the overall catalytic activity and selectivity of ASAs. The interplay between LASs and BASs enables a synergistic effect, optimizing reaction pathways and enhancing product yields.6–8 ASA is also widely used as an acid support for metal nanoparticles, enabling unconstrained mass transfer and delivering bi-functional catalytic performance due to the combination of acid sites and metal active species.9 The above properties of ASAs have enabled their widespread use in catalytic processes like hydrocarbon transformation, biomass conversion, hydrocracking, chemoselective hydrogenation, etc.10,11For the sustainable chemical industry, many chemical processes that currently rely on corrosive and poisonous liquid acids (e.g., HF and HCl) must be replaced with clean and safe solid acids. However, the flexible tuning of acidity in solid acids remains a challenge. Unlike the easily tuned acidity of liquid acids, which can range from weak to superacids for the target reaction steps, the acidity of solid acids is limited by their local structure and overall electronegativity. On ASA, the unsaturated tetra-coordinated aluminum (AlIV) species are generally regarded as key sites to replace tetra-coordinated silicon in the silica network for generating catalytically active Brønsted acid sites (BASs, Si–O(H+)–Al).12 It requires the atomic dispersion of Al species in the silica network and a lower Al fraction to avoid the generation of the low-dispersed alumina phase. Thus, both the density and strength of acid sites on ASA were not good enough to be tuned in a wide range from weak to strong acidity, although many traditional synthesis methods, including co-gelation, co-precipitation, and grafting, have been applied to improve the AlIV species in the silica framework. Recently, another active aluminum species, the penta-coordinated aluminum species (AlV), has been synthesized on silica–alumina and found to contribute to the formation of Brønsted acidity.13–15 The majority of AlV species are located on the accessible surface on ASA rather than the limited area at the interface.13,14,16,17 The AlV species is located near the surface SiOH group to form BAS via SiOHAlV. This AlV based BAS can be co-existing with AlIV based BAS, rather than replacing each other. Thus, the density of acid sites is obviously enhanced.13,14,17,18 In addition, free AlV species nearby BAS can interact with the O atom in BAS to further enhance BAS acidity or form the zeolite type of the bridging OH group.13,14,17,19 These strong acid sites on ASA can provide much lower activation energy in hydrocarbon activation via H/D exchange, compared to the most popular strong solid acid H-ZSM-5 zeolite.20 It indicates the promising future of AlV species based catalysts in chemical reactions, while the controlled synthesis of AlV species remains challenging due to their metastable nature, as they tend to transform into the more stable AlIV or AlVI coordinations. Among the various approaches for synthesizing AlV-enriched amorphous silica–alumina (ASA), flame spray pyrolysis (FSP) emerges as a highly promising technique, enabling the rapid and scalable production of ASA catalysts within microseconds.21 The oxygen flow not only provides oxygen for flame but also provides O atoms for Si and Al oxides. In this research, we will tune the oxygen flow to adjust O atom coordination with Al species and then the local structure will be tuned for further tuning the surface acidity. These observations are consistent with recent reports showing that cooperative interactions between adjacent aluminum centers can significantly modulate both Brønsted acid strength and catalytic behavior in silica–alumina systems.22
Advanced characterization techniques are required to monitor and investigate the proposed synthesis–structure–function correlations. For ASA characterization in this research, solid state NMR is a powerful spectroscopic technology. 2D 27Al multiple quantum (MQ) magic-angle spinning (MAS) NMR is the most powerful method to distinguish the various Al species in ASA.23 Two-dimensional 27Al through-space double-quantum to single-quantum (DQ-SQ) homonuclear correlation MAS NMR will describe the interaction between nearby Al species.24,25 1H-27Al 2D heteronuclear correlation (HETCOR) NMR is used to detect the interaction and bridging between Al species with surface SiOH for the formation of BAS.26 29Si MAS NMR will monitor the distribution of Al species into the silica network, which can be double confirmed by near-edge X-ray absorption fine structure (NEXAFS).27,28 The acidity of ASAs will be evaluated through 31P MAS NMR investigation, following the adsorption of trimethylphosphine oxide (TMPO) as a probe molecule into dehydrated ASA samples.29 The catalytic evaluation of ASA's acidity will be performed through the benzylation of various arenes, thus to complete the synthesis–structure–function correlation.
2. Experimental
2.1. Preparation of ASAs
ASA catalysts were synthesized using a gas phase flame aerosol technique. For the synthesis of amorphous aluminium–silica catalysts (ASA/50), 50 mL of aluminium-tri-sec-butoxide (97%, Sigma-Aldrich) and 50 mL of TEOS (≥99%, Sigma-Aldrich) dissolved in xylene (99% purity, VWR) were mixed together to obtain a total concentration of 0.5 M. Similarly, for the synthesis of aluminium–silica catalysts (ASA/70), 70 mL of aluminium-tri-sec-butoxide and 30 mL of TEOS dissolved in xylene were mixed together (a total concentration of 0.5 M). In this study, catalysts are denoted as ASA/x_y, where x = nAl/(nAl + nSi) × 100 represents the mol% of the Al precursor in the Al–Si precursor mixture. The second number y refers to the dispersed O2 flow rate (2.5, 5, or 7 L min−1) used during flame spray pyrolysis. The precursor–solvent combination was fed into a two-fluid nozzle to form a spray flame combustion with constant premixed gas flows of 1.5 and 3.2 L min of CH4 and O2, respectively.30,31 During the flame synthesis of ASA catalysts, the precursor–solvent flow was kept constant at 5 mL min−1, while the dispersed O2 flow was largely varied from 2.5 to 7 L min−1 with a constant pressure drop of 1.5 bar at the nozzle. The particles were collected on a glass fiber filter with 257 mm in diameter (Pall Laboratory A/F Glass) placed at a distance of 60 cm above the nozzle.322.2. Characterization
For the acidity tests, trimethylphosphine oxide (TMPO, 5 wt%) was introduced as a probe molecule into dehydrated samples. The mixtures were subsequently heated to 160 °C to remove physically adsorbed TMPO. After weighing, the samples were loaded into MAS rotors under an inert atmosphere inside a nitrogen-filled glovebox. All MAS NMR experiments were performed using a 4 mm rotor at a spinning frequency of 12 kHz.
Two-dimensional 27Al MQMAS NMR spectra were acquired employing a z-filtered three-pulse sequence, utilizing pulse widths of 3.0, 1.25, and 12 µs, along with a 200 ms recycle delay.33 The experimental settings from the MQMAS analysis were subsequently applied to reconstruct the corresponding one-dimensional 27Al MAS NMR spectra through simulation.
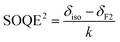 | (1) |
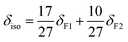 | (2) |
 | (3) |
 | (4) |
The second-order quadrupolar effect (SOQE) parameter is calculated based on the central positions of the contours in the F1 and F2 dimensions, represented by δF1 and δF2. In this context, δiso indicates the isotropic chemical shift associated with the aluminum coordination environment, v0 corresponds to the Larmor frequency of the quadrupolar 27Al nucleus (130.3 MHz), and I signifies its nuclear spin value of 5/2. Using the determined SOQE along with the asymmetry parameter (η), the quadrupolar coupling constant (CQCC) can be subsequently extracted.
Two-dimensional 27Al DQ–SQ MAS NMR experiments were carried out using a central transition (CT)-selective pulse sequence, employing π/2 and π pulses of 8 and 16 µs duration, respectively, under a radiofrequency (rf) field of approximately 10 kHz9. The generation and reconversion of double-quantum coherences were achieved via a BR recoupling sequence with durations ranging from 800 to 1200 µs and a rf amplitude of 6.6 kHz. To enhance polarization of the CT by suppressing satellite transitions, a hyperbolic secant (HS) saturation pulse (4 ms, 16 kHz rf, 20 kHz sweep width, and 200 kHz offset) was applied prior to the excitation. Autocorrelation signals located at coordinates (2ν, ν) in the resulting spectra indicate homonuclear interactions between identical aluminum sites, whereas cross-peaks at (νa + νb, νa) and (νa + νb, νb) reflect spatial proximities between chemically distinct Al species. For the ASA/70 sample, 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 and 3200 transients were collected for the DQ and SQ dimensions, respectively, using a 0.2 s recycle delay, resulting in total acquisition times of 25.3 and 5.7 hours. Chemical shifts for 27Al were externally referenced to a 1 M aqueous solution of Al(NO3)3. Subsequently,1H DQ–SQ spectra were recorded to investigate short-range dipolar interactions between protons. A symmetry-based recoupling scheme was used to reintroduce 1H–1H dipolar couplings under MAS conditions. π/2 pulses were applied at a radiofrequency amplitude of 75 kHz, while recoupling pulses were set at 60 kHz. The durations for excitation and reconversion ranged between 250 and 300 µs. Depending on the signal-to-noise requirement, 32 to 128 transients were averaged per spectrum with recycle delays varying from 1 to 5 s. Proton chemical shifts were calibrated to TMS using adamantane as a secondary reference (1.83 ppm).
400 and 3200 transients were collected for the DQ and SQ dimensions, respectively, using a 0.2 s recycle delay, resulting in total acquisition times of 25.3 and 5.7 hours. Chemical shifts for 27Al were externally referenced to a 1 M aqueous solution of Al(NO3)3. Subsequently,1H DQ–SQ spectra were recorded to investigate short-range dipolar interactions between protons. A symmetry-based recoupling scheme was used to reintroduce 1H–1H dipolar couplings under MAS conditions. π/2 pulses were applied at a radiofrequency amplitude of 75 kHz, while recoupling pulses were set at 60 kHz. The durations for excitation and reconversion ranged between 250 and 300 µs. Depending on the signal-to-noise requirement, 32 to 128 transients were averaged per spectrum with recycle delays varying from 1 to 5 s. Proton chemical shifts were calibrated to TMS using adamantane as a secondary reference (1.83 ppm).
The spatial interactions between 1H and 27Al nuclei were investigated using a two-dimensional heteronuclear correlation (HETCOR) NMR experiment.13 Samples were packed into 2.5 mm MAS rotors and spun at 25 kHz. Cross-polarization contact times of 0.5 ms and 1 ms were employed to probe dipolar couplings. For each domain increment, 2500 scans were averaged with a 1 s recycle delay. A total of 16 increments were recorded to construct the full two-dimensional HETCOR spectra.
All optical components—including three fixed-angle mirrors set at a 1° glancing incidence and coated with 30 nm of gold—were optimized to ensure stable reflectivity across the full working range. During measurements, the vertical exit slit width was maintained at 20 µm, yielding an estimated photon energy resolution of ∼0.07–0.08 eV based on prior calibration using the N2 1s → π* transition and the Fermi edge of a gold foil. The X-ray beam was directed onto the sample using a series of five optical components, and spectral data were collected in either total electron yield (TEY) or fluorescence yield (FY) mode, selected based on the sample's electrical conductivity and the specific detection needs.
2.3. Catalyst performance test
The liquid-phase benzylation reaction between mesitylene and benzyl alcohol was carried out in a 25 mL three-neck round-bottom flask placed in a thermostatically controlled oil bath. The reaction mixture was preheated to 373 K and held at this temperature for 30 minutes to ensure thermal equilibration. Following this, 4 mmol of benzyl alcohol was rapidly introduced into the flask, marking the start of the reaction time. Reaction samples were periodically collected and analyzed using a Shimadzu GC-2010 gas chromatograph equipped with a Rtx-5 capillary column.3. Results and discussion
3.1. Physical characterization
In this research, a series of ASA catalysts were prepared using the flame spray pyrolysis (FSP) process, yielding thermally stable, dry, and high-purity ASA catalysts in a single step. XRD patterns confirmed the amorphous structure of all catalysts (Fig. S1). Nitrogen adsorption/desorption isotherms (Fig. S2) indicated typical characteristics of dispersed nonporous particles, with surface areas summarized in Table 1. As the oxygen flow rate increased, the BET surface areas of ASA/50 and ASA/70 catalysts increased from 98 to 242 m2 g−1 and 84 to 198 m2 g−1, respectively, with the average particle sizes decreased from 17.7 to 7.1 nm and 20.6 to 8.6 nm, due to higher oxygen flow promoting better dispersion of silicon/Al atoms and inhibiting coagulation.16| Catalysts | SSAa (m2 g−1) | dBETa (nm) | AlIVa (%) | AlIVb (%) | AlVa (%) | AlVb (%) | AlVI (%) | BAS (×10−2 mmol g−1) | LAS (×10−2 mmol g−1) | B/L |
|---|---|---|---|---|---|---|---|---|---|---|
| a Specific surface area, dBET: primary particle size obtained from BET. | ||||||||||
| ASA/50_2.5 | 97.9 | 17.7 | 26.35 | 10.28 | 29.16 | 13.47 | 20.64 | 4.0 | 2.5 | 1.60 |
| ASA/50_5 | 177.8 | 9.6 | 25.60 | 17.31 | 23.75 | 14.32 | 18.94 | 4.6 | 2.5 | 1.84 |
| ASA/50_7 | 242.0 | 7.1 | 21.70 | 17.49 | 21.14 | 21.53 | 18.14 | 5.1 | 3.0 | 1.71 |
| ASA/70_2.5 | 83.9 | 20.6 | 25.67 | 17.58 | 21.86 | 23.28 | 11.62 | 7.2 | 3.8 | 1.92 |
| ASA/70_5 | 140.6 | 12.2 | 26.31 | 23.13 | 18.99 | 24.23 | 7.34 | 7.8 | 4.1 | 1.90 |
| ASA/70_7 | 198.0 | 8.6 | 22.03 | 24.16 | 17.08 | 30.15 | 6.57 | 8.5 | 4.1 | 2.07 |
3.2. Local structure of catalysts by ssNMR spectroscopy and XAS investigation
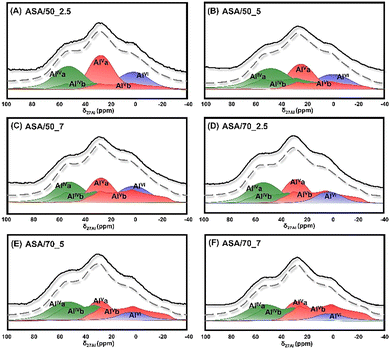
To overcome the significant line broadening typically associated with quadrupolar 27Al nuclei, two-dimensional multiple-quantum magic-angle spinning (MQMAS) NMR spectroscopy was employed to resolve and differentiate the locally distorted environments of aluminum species.13,35 For the ASA/50 and ASA/70 catalysts, as shown in Fig. 1A–F, three distinct contours were identified in the spectrum, corresponding to four-coordinated (a: δiso = 56 ppm and b: δiso = 41 ppm), five-coordinated (a: δiso = 33 ppm and b: δiso = 18 ppm), and six-coordinated (δiso = 3–9 ppm) aluminum species. A comparison of quadrupole coupling constant (CQCC) values revealed that AlIVb and AlVb exhibit significantly higher values than AlIVa and AlVa, indicating a more distorted local structure,36 which may be caused by the nearby AlIV and AlV species interacting each other. Both AlIV and AlV species can be located adjacent to surface silanol (SiOH) groups, generating bridging or pseudo-bridging SiOHAl groups to form Brønsted acid sites (BASs).13,37 | ||
| Fig. 1 2D 27Al multiple quantum MAS NMR spectra of (A) ASA/50_2.5, (B) ASA/50_5, (C) ASA/50_7, (D) ASA/70_2.5, (E) ASA/70_5, and (F) ASA/70_7 catalysts. | ||
Using the MQMAS spectral data and the extracted quadrupolar parameters summarized in Fig. 1 and Table S1, the one-dimensional 27Al single-pulse MAS NMR spectra were simulated, as shown in Fig. 2. AlV species is dominant in all samples at a fraction from 36.63% to 49.19% with AlIV and AlVI species from 42.63% to 48.23%, when the oxygen flow increases from 2.5 to 7 mL min−1. Among these species on ASA/50_2.5, ASA/50_5, ASA/50_7, ASA/70_2.5, ASA/70_5, and ASA/70_7, both AlIVa and AlVa mole fractions decreased from 26.4 to 22.0% and 29.2 to 17.1%, respectively. However, the mole fraction of AlIVb and AlVb species increased from 10.3 to 24.2% and 13.5 to 30.2%, respectively. All AlIV and AlV species are incorporated into the silica network. However, the AlIVb and AlVb species exhibit a more highly distorted coordination environment and stronger interaction with the silica network, as indicated by 29Si cross-polarization (CP) MAS NMR investigations of dehydrated ASA samples prepared by the increased oxygen flow. 29Si CP MAS NMR only shows the local environment of surface Si species with OH groups; as shown in Fig. 3A and B, the peak slightly shifts to a lower field with an increasing oxygen flow rate both in the ASA/50 and ASA/70 catalysts. This indicates that more Al species incorporate into the surface silica network under the increased oxygen flow rate during FSP synthesis.5,38 It correlates well with the above 27Al MAS NMR study that more AlIVb and AlVb incorporate with nearby SiOH.
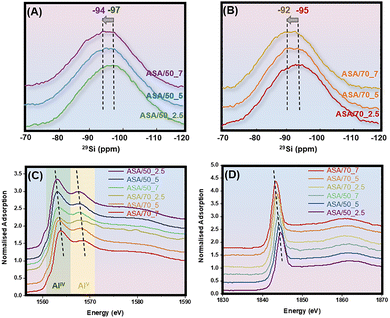 | ||
| Fig. 3 29Si cross-polarization MAS NMR spectra of ASA/50 (A) and ASA/70 (B) with O2 flow rates of 2.5, 5, and 7 L min−1. Al K-edge (C) and Si K-edge (D) for all ASA catalysts. | ||
Al and Si K-edge XANES investigation also confirmed that more Al species incorporate into the surface silica network with an increased oxygen flow rate during FSP synthesis. From XANES spectra, it is possible to predict the coordination environment of Al from the energy position of the K absorption edge; the exact energy of this 1s to 3p electron transition depends on the coordination number of the coordination sphere around the atom.39 As shown in Fig. 3C, Al K-edge XAS spectra exhibit two dominant broad peaks at 1563–1564 eV (a) and 1568–1569 eV (b) assigned to AlIV and AlV species, respectively, and one broad peaks at around 1580 eV (c) may be caused by AlVI species.40–42 In general, the Al K-edge shifts to higher energy with increase in the numbers of aluminium coordination.42 With the oxygen flow rate increasing for the ASA synthesis, both peaks for AlIV and AlV species shift to high energy, indicating the strongly distorted Al environments of both species.43 This observation corelates very well with the 27Al MAS NMR spectra.
Fig. 3D shows the Si K-edge XANES spectra of the prepared ASA catalysts. The strong dominant peak observed between 1843 and 1844 eV corresponds to the electronic transition of Si 1s to 3p.44 The broader peaks appearing between 1857 and 1865 eV are attributed to higher-energy shape resonances.45 With increasing oxygen flow and more Al fraction during synthesis, the Si K-edge shifts to lower energies from 1844 eV in ASA/50_2.5 to 1843 eV in ASA/70_7. It is reported that the substitution of Al for Si lowers the effective charge on the silicon atoms because the Al–O bond is weaker than the Si–O bond, facilitating electron density redistribution and causing the Si K-edge shift to lower energy.44 This observation also correlates very well with 29Si CP MAS NMR spectra that aluminum species become incorporated into silica frameworks, modifying local silicon environments.
3.3. ssNMR investigation of BAS formation and the surface acidity
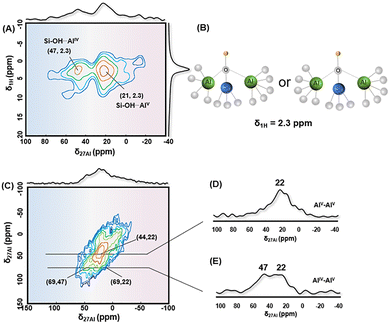
1H MAS NMR spectra of ASA samples in Fig. S3 are dominated by a strong signal at δ1H = 2.3 ppm, which is caused by the surface SiOH groups on ASAs.16 The above observed incorporation of AlIV and AlV species into silica frameworks can generate the surface Si–OH⋯AlIV/V groups, which is BAS on ASA.46 This kind of incorporation will induce a dipolar coupling between 27Al and 1H nuclei, which can be effectively probed through two-dimensional 1H–27Al CP/MAS HETCOR NMR investigation. As shown in Fig. 4a, the correlation at δ27Al = 47 ppm of AlIV species and δ27Al = 21 ppm of AlV species with δ1H = 2.3 ppm in the 1H–27Al CP/MAS HETCOR NMR spectrum of dehydrated ASA/70_7 indicates the formation of Si–OH⋯AlIV/V groups, which is the typical BAS-AlIV or BAS-AlV structure for acid ASA.18 They are not bridging SiOHAl groups like zeolites, as δ1H is around 3.6–5.2 ppm for bridging OH groups or disturbed bridging OH groups. Si–OH⋯AlIV/V are pseudo-bridging groups between Al species and nearby silanols.37,47 The electron-withdrawing nature of Al species to nearby surface SiOH groups would generate the acidic proton on Si–OH⋯AlIV/V as Brønsted acid sites.18,48 A very weak correlation at δ27Al = 0 ppm of AlVI species and δ1H = 1.1 ppm of AlOH groups is assigned to non-acidic terminal AlVIOH groups, which indicates that AlVI species do not incorporate with the silica network to form the acid sites. Fig. 4A displays a dominant contour centered at (δ1H = 2.3 ppm and δ27Al = 21 ppm), indicating that the mainly Brønsted acid sites on ASA/70_7 are associated with Si–OH⋯AlV groups at the surface. The density of BASs increases with the increased fraction of AlVb species on ASAs. As shown in Table 1, the mole fractions of AlVb of 23.3%, 24.2%, and 30.2% for ASA/70_2.5, ASA/70_5, and ASA/70_7, respectively, correlate with the increase in surface BAS concentrations of 7.2 × 10−2 mmol g−1, 7.8 × 10−2 mmol g−1, and 8.5 × 10−2 mmol g−1 for these ASA samples.Since the mole fraction of Al species is higher than that of Si species, there should be existing two Al species nearby one Si species. Spatial proximities among 27Al nuclei were examined using two-dimensional through-space 27Al DQ–SQ homonuclear correlation MAS NMR, as illustrated in Fig. 4C. The most prominent feature is a diagonal peak located at (44, 22) ppm, corresponding to the AlV site in the ASA/70_7 sample (Fig. 4C and its associated projection in Fig. 4D). This strong autocorrelation signal signifies the presence of adjacent AlV sites, suggesting their uniform distribution and high surface concentration. Additionally, two intense off-diagonal cross-peaks at (69, 47) and (69, 22) ppm confirm the close spatial relationship between AlV species (δ = 22 ppm) and AlIV centers (δ = 47 ppm), as supported by Fig. 4C and the corresponding slice in Fig. 4E. Furthermore, the two-dimensional 1H DQ–SQ spectrum (Fig. S4) reveals a dominant correlation at (3.4, 1.7) ppm and a weaker one at (4.2, 2.1) ppm, indicating the presence of spatially adjacent silanol groups across the surface.
To elucidate the relationship between structure and acidity in ASA materials,31P MAS NMR spectroscopy was performed using trimethylphosphine oxide (TMPO) as a molecular probe.49–51 This technique enables the differentiation of acid sites with varying strengths by analyzing the chemical shifts of adsorbed or reacted TMPO species. As depicted in Fig. 5A–F, shifts in the 31P resonance positions provide insight into the type and strength of surface acid sites.52–54 Typically, downfield shifts in the 31P signal are indicative of stronger Brønsted acidity, as these sites withdraw more electron density from the phosphorus nucleus, resulting in reduced shielding.55,56 Based on peak deconvolution (Fig. 5), the dominant signal in the 65–67 ppm range corresponds to TMPO coordinated with Brønsted acid sites (BASs). The peaks located between 47 and 51 ppm in Fig. 5A–F are assigned to TMPO interacting with Lewis acid sites (LAS), which are typically associated with trigonal (3-coordinate) or distorted/penta-coordinate aluminum species, rather than fully coordinated octahedral Al.55,57,58 A separate resonance at approximately 42 ppm arises from physisorbed TMPO molecules.48,59 Quantitative analysis of the signal intensities allows for the estimation of the relative populations of BAS and LAS, as shown in Fig. 5, with the calculated molar fractions summarized in Table 1. Both Brønsted and Lewis acid site densities increase progressively from ASA/50 to ASA/70 as the oxygen flow rate during synthesis is elevated. This trend aligns with the growing presence of Si–OH–AlIVb or AlVb, suggesting that these aluminum environments are closely associated with surface silanol groups and function as catalytically active acid sites in ASA catalysts.
In most ASAs synthesized using traditional methods, only moderate Brønsted acid site (BAS) strength (δ31P = 61–62 ppm, as probed by TMPO) has been detected.13 This moderate acidity is thought to result from a single aluminum center interacting with a neighboring silanol group, thereby reducing the electron density of the oxygen atom and forming an acidic SiOH site. This configuration is comparable to the pseudobridging silanol (PBS) model proposed in previous theoretical studies, where a SiOH group electrostatically interacts with an Al center (AlIV or AlV) without forming a covalent bond, unlike the bridging OH groups typically found in zeolites.37,60
In contrast to conventional ASAs, those synthesized via flame spray pyrolysis (FSP), such as FSP ASA/50 and ASA/70, exhibit the unique structural feature wherein two aluminum species, either both AlV or a combination of AlV and AlIV, are positioned in close proximity to a single surface SiOH group. A study by Valla et al., which integrated dynamic nuclear polarization (DNP) NMR with first-principles calculations, proposed structural motifs for ASA materials that support this arrangement.61 Their models, developed for ASAs prepared by chemical liquid deposition of SiO2 on Al2O3, suggest that multiple Al centers can cluster near a silanol group. This hypothesis is further corroborated by radial distribution function (RDF) analyses, which reveal a tendency for Al species to aggregate, particularly in high-Al-content ASAs such as ASA/50.14 Consequently, it is reasonable to expect the coexistence of multiple Al centers near a single SiOH moiety in both ASA/50 and ASA/70.
Building on these insights, we propose a structural model for Brønsted acid site (BAS) formation in ASA/50 and ASA/70 (Fig. 4b). In this model, the presence of a second, unsaturated neighboring aluminum atom plays a dual role: it acts as an auxiliary Lewis acid site, stabilizing the deprotonated silanolate intermediate, and it enhances electron withdrawal from the bridging oxygen of the SiOH group.47,60 This dual-Al environment significantly amplifies the proton-donating capability of the site, resulting in an observed BAS strength as high as δ31P = 67 ppm. This value exceeds the Brønsted acid strength reported for zeolite H–Y (δ31P = 63–65 ppm)62,63 and aligns closely with highly active systems like H-MCM-22 (δ31P = 66 ppm)64 and H-ZSM-5 (δ31P = 64–73 ppm),55,65,66 as summarized in Table 2.
| Acids | δ31P (ppm) | BAS structure | Ref. |
|---|---|---|---|
| HY | 63–65 | 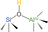 |
62 and 63 |
| H-MCM-22 | 66 | 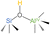 |
64 |
| H-ZSM-5 | 64–73 | 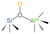 |
55, 65 and 66 |
| ASAs | 61–62 | 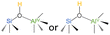 |
13 |
| PBS (one Al center with SiOH) | |||
| FSP ASAs | 65–67 | — | This work |
This proposed model mirrors mechanisms observed in zeolitic frameworks, where extra-framework Al species enhance Brønsted acidity via an electrostatic effect. Accordingly, the cooperative interaction between two adjacent aluminum centers, whether AlV–AlV or AlIV–AlV, is anticipated to substantially elevate the acid strength of BAS in ASA materials, contributing to their catalytic performance (Fig. 5).
4. Catalyst performance test
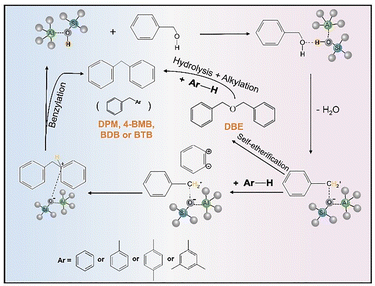
To evaluate the catalytic performance of ASA materials in terms of both activity and selectivity, benzylation reactions of arenes including benzene, toluene, p-xylene, and mesitylene with benzyl alcohol were conducted at 353 K for 4 hours (Fig. 6A). As presented in Fig. 6, the conversion of benzyl alcohol over different ASA catalysts varied with time, following the trend ASA/70_7 > ASA/70_5 > ASA/70_2.5 > ASA/50_7 > ASA/50_5 > ASA/50_2.5, which closely mirrors the Brønsted acid site concentrations summarized in Table 1. This observation supports previous findings that higher Brønsted acid densities promote more efficient activation of benzyl alcohol.67 In this context, increasing the aluminum content and oxygen flow rate during FSP synthesis led to a greater concentration of Brønsted acid sites, thereby enhancing overall catalytic activity. In terms of selectivity, Table 3 shows that the production of monobenzylated compounds followed the sequence ASA/50_2.5 < ASA/50_7 < ASA/50_5 < ASA/70_5 < ASA/70_2.5 < ASA/70_7, which is consistent with the increasing Brønsted-to-Lewis acid site ratios reported in Table 1. These findings highlight the critical role of acid site balance,68 where Brønsted sites promote electrophilic substitution while Lewis sites suppress the self-condensation of benzyl alcohol.69 This synergistic interaction between acid site types ultimately governs the product distribution, as illustrated in Scheme 1.70 The major reaction products include dibenzyl ether (DBE), diphenylmethane (DPM), benzyl-4-methylbenzene (4-BMB), 2-benzyl-1,4-dimethylbenzene (BDB), and 2-benzyl-1,3,5-trimethylbenzene (BTB), which originate from two competing pathways. DBE is formed through the self-condensation of benzyl alcohol, while the monobenzylated products result from direct electrophilic benzylation of the aromatic ring. In comparison to ZSM-5 catalysts (Fig. 6B–F), where the catalytic efficiency decreases with increasing molecular size due to diffusion limitations in microporous channels.71 ASA catalysts alleviate these issues through their mesoporous structure, thus enabling improved performance across a broader range of substrates.| Arenes | Products | Conversion% (selectivity%) | |||||
|---|---|---|---|---|---|---|---|
| ASA/50_2.5 | ASA/50_5 | ASA/50_7 | ASA/70_2.5 | ASA/70_5 | ASA/70_7 | ||
| a Conditions: 25 mg catalysts, 3.5 mL arenes, 0.25 mL benzyl alcohol, reacted at 353 K for 4 h. DPM, BMB, BDB and BTB are for diphenylmethane, benzyl-methylbenzene, 2-benzyl-1,4-dimethylbenzene and 2-benzyl-1,3,5-trimethylbenzene. | |||||||
| Benzene | DPM | 41.2 (68.8) | 50.4 (72.1) | 55.2 (70.6) | 65.7 (85.6) | 78.1 (78.2) | 90.5 (86.3) |
| Toluene | 4-BMB | 40.5 (65.7) | 48.5 (70.4) | 55.2 (66.1) | 64.3 (79.0) | 81.8 (73.6) | 90.0 (79.3) |
| p-Xylene | BDB | 33.0 (73.8) | 40.5 (77.1) | 49.2 (76.9) | 65.4 (89.1) | 75.4 (86.4) | 88.2 (90.2) |
| Mesitylene | BTB | 27.2 (76.4) | 41.1 (80.2) | 49.8 (80.0) | 63.8 (92.5) | 73.0 (88.3) | 84.7 (94.1) |
To assess the long-term usability of the FSP-derived ASAs, recyclability tests were performed using the most active catalyst, ASA/70_7, in the benzylation of arenes (Fig. S5). After each reaction, the catalyst was recovered by centrifugation, washed with ethanol, dried at 373 K, and reused without further treatment. The conversion of benzyl alcohol still showed very stable performance, while the selectivity toward aimed products remained above 80% throughout all cycles. These results confirm the high structural stability of the surface AlV–SiOH Brønsted acid sites generated via FSP and demonstrate that the catalysts possess excellent recyclability suitable for practical applications.
Conclusions
In this work, we have demonstrated that tailoring the coordination environment of aluminum species on amorphous silica–alumina (ASA) via flame spray pyrolysis (FSP) can effectively enhance surface acidity and catalytic performance in arene benzylation. By tuning the oxygen flow during synthesis, we promoted the formation of penta-coordinated aluminum (AlV) species, particularly the surface distorted AlVb type, which were found in close proximity to SiOH groups and acted as key contributors to strong Brønsted acid sites (BASs). Advanced solid-state NMR and XAS analyses confirmed the incorporation of AlV into the silica network and its role in forming pseudo-bridging acidic sites. The resulting ASA catalysts exhibited improved acid strength, as shown by 31P MAS NMR, and superior activity and selectivity in benzylation reactions. This study establishes a clear synthesis–structure–acidity–function relationship, demonstrating that flame-made ASAs achieve zeolite-like acidity through cooperative interactions between AlV species and neighboring Al sites near silanols, rather than through conventional bridging hydroxyls. The combination of advanced NMR techniques provides compelling evidence for this unique dual-Al-site mechanism of acidity enhancement.Author contributions
J. Huang. designed the study. Haipeng Li. prepared the catalysts. X. Liu., L. O’Dell. and W. Yang performed the NMR experiments. X. Liu performed the structural assignment. Q. Gu performed the NEXAFS experiments. X. Liu contributed to the catalytic reaction test. J. Huang. supervised scientific work. X. Liu contributed to writing the paper, and X. Liu., S. Pokhrel, L. Mädler and J. Huang revised it.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Data availability
The data supporting this article have been included as part of the supplementary information (SI). The supplementary information includes additional structural and spectroscopic characterization data, including XRD patterns, N2 adsorption–desorption isotherms, detailed 27Al NMR fitting parameters, 1H MAS and DQ–SQ NMR spectra, and catalyst regeneration performance data. See DOI: https://doi.org/10.1039/d5ey00312a.Acknowledgements
This work is financially supported by the Australian Research Council Discovery Project (DP220102851) and the ARC Future Fellowships (FT220100601). Authors acknowledge facilities of Sydney Analytical, a core research facility at the University of Sydney, and NMR facility of Deakin University. Part of the experiment was conducted at SXR beamline, Australian Synchrotron, ANSTO.References
- D. Yun, Y. S. Yun, T. Y. Kim, H. Park, J. M. Lee, J. W. Han and J. Yi, J. Catal., 2016, 341, 33–43 CrossRef CAS.
- G. Busca, Chem. Rev., 2007, 107, 5366–5410 CrossRef CAS PubMed.
- J. Bao, Q.-h Yang, S.-q Zeng, X.-y Sang, W.-m Zhai and H. Nie, Microporous Mesoporous Mater., 2022, 337, 111897 CrossRef CAS.
- J. Ge, J. Sun, P. Zhang, Z. Xie, Z. Wu and B. Liu, Catal. Sci. Technol., 2022, 12, 3695–3705 RSC.
- K. D. Kim, S. Pokhrel, Z. Wang, H. Ling, C. Zhou, Z. Liu, M. Hunger, L. Mädler and J. Huang, ACS Catal., 2016, 6, 2372–2381 CrossRef CAS.
- J. Horlyck, S. Pokhrel, E. Lovell, N. M. Bedford, L. Mädler, R. Amal and J. Scott, Catal. Sci. Technol., 2019, 9, 4970–4980 RSC.
- N. Fang, K. Huo, Y. Jin, D. Liu, H. Lin, H. Wu, X. Liu, Y. Liu and M. He, ACS Catal., 2024, 14, 4786–4790 CrossRef CAS.
- G. Crépeau, V. Montouillout, A. Vimont, L. Mariey, T. Cseri and F. Maugé, J. Phys. Chem. B, 2006, 110, 15172–15185 CrossRef PubMed.
- Z. Wang, Y. Jiang, F. Jin, C. Stampfl, M. Hunger, A. Baiker and J. Huang, J. Catal., 2019, 372, 1–7 CrossRef CAS.
- A. Ishihara, Catal. Surv. Asia, 2012, 16, 36–47 CrossRef CAS.
- T. Li, L. Zhang, Z. Tao, C. Hu, C. Zhao, F. Yi, X. Gao, X. Wen, Y. Yang and Y. Li, Fuel, 2020, 279, 118487 CrossRef CAS.
- M. Trombetta, G. Busca, S. Rossini, V. Piccoli, U. Cornaro, A. Guercio, R. Catani and R. J. Willey, J. Catal., 1998, 179, 581–596 CrossRef CAS.
- W. Yang, X. Liu, L. A. O’Dell, L. Wang, Y. Jiang and J. Huang, J. Phys. Chem. C, 2023, 127, 23212–23222 CrossRef CAS.
- Z. Wang, T. Li, Y. Jiang, O. Lafon, Z. Liu, J. Trébosc, A. Baiker, J.-P. Amoureux and J. Huang, Nat. Commun., 2020, 11, 225 CrossRef CAS PubMed.
- W. Liu, H. Zhao, X. Wu, G. Dury, W. Hu and W. Liu, J. Energy Chem., 2025, 111, 178–189 CrossRef CAS.
- Z. Wang, Y. Jiang, X. Yi, C. Zhou, A. Rawal, J. Hook, Z. Liu, F. Deng, A. Zheng and M. Hunger, Sci. Bull., 2019, 64, 516–523 CrossRef CAS PubMed.
- W. Yang, X. Liu, L. A. O’Dell, X. Liu, L. Wang, W. Zhang, B. Shan, Y. Jiang, R. Chen and J. Huang, JACS Au, 2023, 3, 2586–2596 CrossRef CAS PubMed.
- Z. Wang, Y. Jiang, O. Lafon, J. Trébosc, K. Duk Kim, C. Stampfl, A. Baiker, J.-P. Amoureux and J. Huang, Nat. Commun., 2016, 7, 13820 CrossRef CAS PubMed.
- Z. Wang, R. Buechel, Y. Jiang, L. Wang, H. Xu, P. Castignolles, M. Gaborieau, O. Lafon, J.-P. Amoureux and M. Hunger, JACS Au, 2021, 1, 262–271 CrossRef CAS PubMed.
- Z. Wang, Y. Jiang, A. Baiker, M. Hunger and J. Huang, J. Phys. Chem. Lett., 2022, 13, 486–491 CrossRef CAS PubMed.
- H. K. Kammler, L. Mädler and S. E. Pratsinis, Chem. Eng. Technol., 2001, 24, 583–596 CrossRef CAS.
- L. Chen, W. Wang, T. Lu, X. Luo, X. Xie, K. Huang, S. Qin, T. Su, Z. Qin and H. Ji, Acta Phys.-Chim. Sin., 2025, 41, 100054 CrossRef.
- J. Ashenhurst, S. Wang and G. Wu, J. Am. Chem. Soc., 2000, 122, 3528–3529 CrossRef CAS.
- M. Zheng, Q. Wang, Y. Chu, X. Tan, W. Huang, Y. Xi, Y. Wang, G. Qi, J. Xu and S. B. Hong, J. Am. Chem. Soc., 2024, 146, 29417–29428 CrossRef CAS PubMed.
- F. A. Perras, Z. Wang, T. Kobayashi, A. Baiker, J. Huang and M. Pruski, Phys. Chem. Chem. Phys., 2019, 21, 19529–19537 RSC.
- R. Verma, C. Singhvi, A. Venkatesh and V. Polshettiwar, Nat. Commun., 2024, 15, 6899 CrossRef PubMed.
- C. J. Heard, L. Grajciar, C. M. Rice, S. M. Pugh, P. Nachtigall, S. E. Ashbrook and R. E. Morris, Nat. Commun., 2019, 10, 4690 CrossRef PubMed.
- J. A. van Bokhoven, A. M. Van der Eerden and D. C. Koningsberger, J. Am. Chem. Soc., 2003, 125, 7435–7442 CrossRef CAS PubMed.
- A. Zheng, H. Zhang, X. Lu, S.-B. Liu and F. Deng, J. Phys. Chem. B, 2008, 112, 4496–4505 CrossRef CAS PubMed.
- M. Minnermann, S. Pokhrel, K. Thiel, R. Henkel, J. Birkenstock, T. Laurus, A. Zargham, J.-I. Flege, V. Zielasek and E. Piskorska-Hommel, J. Phys. Chem. C, 2011, 115, 1302–1310 CrossRef CAS.
- M. Gäßler, J. Stahl, M. Schowalter, S. Pokhrel, A. Rosenauer, L. Mädler and R. Güttel, ChemCatChem, 2022, 14, e202200286 CrossRef.
- H. Li, C. Erinmwingbovo, J. Birkenstock, M. Schowalter, A. Rosenauer, F. La Mantia, L. Mädler and S. Pokhrel, ACS Appl. Energy Mater., 2021, 4, 4428–4443 CrossRef CAS PubMed.
- C. A. Fyfe, J. L. Bretherton and L. Y. Lam, J. Am. Chem. Soc., 2001, 123, 5285–5291 CrossRef CAS PubMed.
- B. C. C. Cowie, A. Tadich and L. Thomsen, AIP Conf. Proc., 2010, 1234, 307–310 CrossRef.
- Z. Wang, K. Chen, Y. Jiang, J. Trébosc, W. Yang, J.-P. Amoureux, I. Hung, Z. Gan, A. Baiker and O. Lafon, J. Phys. Chem. Lett., 2021, 12, 11563–11572 CrossRef CAS PubMed.
- T.-H. Chen, K. Houthoofd and P. J. Grobet, Microporous Mesoporous Mater., 2005, 86, 31–37 CrossRef CAS.
- C. Chizallet and P. Raybaud, Angew. Chem., 2009, 121, 2935–2937 CrossRef.
- B. Sun, S. Pokhrel, D. R. Dunphy, H. Zhang, Z. Ji, X. Wang, M. Wang, Y.-P. Liao, C. H. Chang and J. Dong, ACS Nano, 2015, 9, 9357–9372 CrossRef CAS PubMed.
- A. Omegna, R. Prins and J. A. Van Bokhoven, J. Phys. Chem. B, 2005, 109, 9280–9283 CrossRef CAS PubMed.
- J. Wong, Z. Rek, M. Rowen, T. Tanaka, F. Schäfers, B. Müller, G. George, I. Pickering, G. Via and B. DeVries, Phys. B, 1995, 208, 220–222 CrossRef.
- M. Fröba, J. Wong, M. Rowen, G. Brown Jr, T. Tanaka and Z. Rek, Phys. B, 1995, 208, 555–556 CrossRef.
- D. Li, G. Bancroft, M. Fleet, X. Feng and Y. Pan, Am. Mineral., 1995, 80, 432–440 CAS.
- J. L. Fulton, N. Govind, T. Huthwelker, E. J. Bylaska, A. Vjunov, S. Pin and T. D. Smurthwaite, J. Phys. Chem. B, 2015, 119(26), 8380–8388 CrossRef CAS PubMed.
- D. Li, G. Bancroft, M. Fleet and X. Feng, Phys. Chem. Miner., 1995, 22, 115–122 CAS.
- J. L. Dehmer, J. Chem. Phys., 1972, 56, 4496–4504 CrossRef CAS.
- Z. Wang, S. Pokhrel, M. Chen, M. Hunger, L. Mädler and J. Huang, J. Catal., 2013, 302, 10–19 CrossRef CAS.
- C. Chizallet and P. Raybaud, ChemPhysChem, 2010, 11, 105–108 CrossRef CAS PubMed.
- Z. Wang, Y. Jiang, A. Baiker and J. Huang, ACS Catal., 2013, 3, 1573–1577 CrossRef CAS.
- C. Wang, W. Dai, G. Wu, N. Guan and L. Li, Magn. Reson. Lett., 2022, 2, 28–37 CrossRef.
- W. Yang, Z. Wang, J. Huang and Y. Jiang, J. Phys. Chem. C, 2021, 125, 10179–10197 CrossRef CAS.
- Z. Wang, Y. Jiang, C. Stampfl, A. Baiker, M. Hunger and J. Huang, ChemCatChem, 2020, 12, 287–293 CrossRef CAS.
- L. Lakiss, A. Vicente, J. P. Gilson, V. Valtchev, S. Mintova, A. Vimont, R. Bedard, S. Abdo and J. Bricker, ChemPhysChem, 2020, 21, 1873–1881 CrossRef CAS PubMed.
- C. Bornes, M. Sardo, Z. Lin, J. Amelse, A. Fernandes, M. F. Ribeiro, C. Geraldes, J. Rocha and L. Mafra, Chem. Commun., 2019, 55, 12635–12638 RSC.
- R. Zhao, Z. Zhao, S. Li and W. Zhang, J. Phys. Chem. Lett., 2017, 8, 2323–2327 CrossRef CAS PubMed.
- Y. Jiang, J. Huang, W. Dai and M. Hunger, Solid State Nucl. Magn. Reson., 2011, 39, 116–141 CrossRef CAS PubMed.
- W.-H. Chen, H.-H. Ko, A. Sakthivel, S.-J. Huang, S.-H. Liu, A.-Y. Lo, T.-C. Tsai and S.-B. Liu, Catal. Today, 2006, 116, 111–120 CrossRef CAS.
- W. Hu, Q. Luo, Y. Su, L. Chen, Y. Yue, C. Ye and F. Deng, Microporous Mesoporous Mater., 2006, 92, 22–30 CrossRef CAS.
- N. Feng, A. Zheng, S.-J. Huang, H. Zhang, N. Yu, C.-Y. Yang, S.-B. Liu and F. Deng, J. Phys. Chem. C, 2010, 114, 15464–15472 CrossRef CAS.
- J. Huang, Y. Jiang, N. Van Vegten, M. Hunger and A. Baiker, J. Catal., 2011, 281, 352–360 CrossRef CAS.
- F. Leydier, C. Chizallet, A. Chaumonnot, M. Digne, E. Soyer, A.-A. Quoineaud, D. Costa and P. Raybaud, J. Catal., 2011, 284, 215–229 CrossRef CAS.
- M. Valla, A. J. Rossini, M. Caillot, C. Chizallet, P. Raybaud, M. Digne, A. Chaumonnot, A. Lesage, L. Emsley and J. A. Van Bokhoven, J. Am. Chem. Soc., 2015, 137, 10710–10719 CrossRef CAS PubMed.
- E. F. Rakiewicz, A. W. Peters, R. F. Wormsbecher, K. J. Sutovich and K. T. Mueller, J. Phys. Chem. B, 1998, 102, 2890–2896 CrossRef CAS.
- M. D. Karra, K. J. Sutovich and K. T. Mueller, J. Am. Chem. Soc., 2002, 124, 902–903 CrossRef CAS PubMed.
- A. Zheng, L. Chen, J. Yang, M. Zhang, Y. Su, Y. Yue, C. Ye and F. Deng, J. Phys. Chem. B, 2005, 109, 24273–24279 CrossRef CAS PubMed.
- S. Zhao, W. Yang, K. D. Kim, L. Wang, Z. Wang, R. Ryoo and J. Huang, J. Phys. Chem. C, 2021, 125, 11665–11676 CrossRef CAS.
- X. Yi, H.-H. Ko, F. Deng, S.-B. Liu and A. Zheng, Nat. Protoc., 2020, 15, 3527–3555 CrossRef CAS PubMed.
- H. Jin, M. B. Ansari, E.-Y. Jeong and S.-E. Park, J. Catal., 2012, 291, 55–62 CrossRef CAS.
- J. Ma, W. Luo, X. Wang, X. Yu, J. J. Wang, H. Hu, H. Du, J. Zeng, W. Chen and M. Yang, Nano-Micro Lett., 2026, 18, 39 CrossRef CAS PubMed.
- M. Milina, S. Mitchell, Z. D. Trinidad, D. Verboekend and J. Pérez-Ramírez, Catal. Sci. Technol., 2012, 2, 759–766 RSC.
- B. Yuan, Y. Li, Z. Wang, F. Yu, C. Xie and S. Yu, Mol. Catal., 2017, 443, 110–116 CAS.
- S. Zhao, W. D. Wang, L. Wang, W. Schwieger, W. Wang and J. Huang, ACS Catal., 2019, 10, 1185–1194 CrossRef.
| This journal is © The Royal Society of Chemistry 2026 |