 Open Access Article
Open Access ArticleDesaturation and chemical recovery from desalination concentrates using ion exchange and bipolar membrane electrodialysis as a zero liquid discharge process
Luis Salas *ab,
Alex Schwarz
*ab,
Alex Schwarz b and
Alvaro Gonzalez-Vogel
b and
Alvaro Gonzalez-Vogel a
a
aBioforest SpA, Camino Coronel Km 15, Coronel, 4190000, Chile
bDepartment of Civil Engineering, Universidad de Concepción, P.O. Box 160-C, Concepción, 4030000, Chile. E-mail: lusalas@udec.cl
First published on 10th December 2025
Abstract
The cellulose pulp industry still consumes high amounts of water, making water recovery essential. To address this, pulsed electrodialysis reversal (pEDR) has been proposed; however, the remaining concentrate management is still a challenge. Thus, this study evaluates an integrated system combining ion exchange, pEDR and bipolar membrane electrodialysis for concentrate desaturation and simultaneous recovery of caustics and acids, as an alternative to conventional zero liquid discharge systems, such as evaporation and crystallization. Experiments with ion exchange resins assessed their capacity with industrial effluents, while bipolar membrane electrodialysis was tested at different voltages and (synthetic) reject stream concentrations. A nine-step setup simulated industrial performance, achieving 0.67 M NaOH with 73% efficiency and an energy consumption of 4.57 kWh kg−1 NaOH. Economic analysis showed that integrating pEDR with evaporation and crystallization in an industrial scale system requires nearly 38% more in capital cost than integrating pEDR with the desaturation system. The operational cost for evaporation–crystallization with pEDR is 0.43 USD per m3, while desaturation with pEDR costs 0.34 USD per m3 and decreases to 0.20 USD per m3 with soda valorization. These results show a more sustainable and cost-effective alternative for zero liquid discharge in the cellulose pulp industry.
Water impactThis study demonstrates a cost-effective alternative to conventional zero liquid discharge systems in pulp mills, reducing capital costs by 38% and operational costs by up to 53% through chemical recovery. The integrated pulsed electrodialysis and bipolar membrane system enables water recycling while converting waste streams into reusable caustics and acids, advancing circular economy principles in water-intensive industries. |
1. Introduction
The cellulose pulp industry is a key global sector, producing items like toilet paper, printing paper, and packaging. Globalization has driven major changes, increasing competition and output, especially in Asia and South America.5 Pulp production rose from 120 million tons in 1982 to nearly 190 million in 2021.9A major environmental concern is high-water consumption and liquid discharge. Bleaching is the most water-intensive stage, consuming up to 50% of a plant's water and generating 80% of its effluent,24,28 due to pulp washing after each step to remove oxidized materials.23 A kraft pulp mill producing one million tons annually consumes water comparable to a city of 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 habitants.22 Efficiency improvements have reduced water consumption from 200 m3 per ton of air-dry pulp (ADt) in the 1960s to below 25 m3 ADt−1 in modern mills.18 However, further reductions remain challenging and costly, especially when closing water loops.
000 habitants.22 Efficiency improvements have reduced water consumption from 200 m3 per ton of air-dry pulp (ADt) in the 1960s to below 25 m3 ADt−1 in modern mills.18 However, further reductions remain challenging and costly, especially when closing water loops.
Desalination technologies such as evaporation, reverse osmosis and electrodialysis are commercially available on a large scale14 but are unsuitable for pulp mill effluents. Evaporation is highly energy-intensive, while membrane-based processes suffer from organic and inorganic fouling. As an alternative, variations of electrodialysis, such as electrodialysis reversal (EDR)1 and pulsed electrodialysis,13 have been proposed. EDR reduces the need for pretreatment and cleaning-in-place procedures compared to conventional electrodialysis, but this is achieved at the expense of lower production rates. On the other hand, in pulsed electrodialysis, membrane fouling remains a significant issue, limiting its viability by reducing process efficiency and membrane lifespan.1 A promising solution to these limitations is pulsed electrodialysis reversal (pEDR),14 which has been proposed as a key technology to at least partially close the water loop in bleached kraft pulp mills.
Although pEDR maximizes water recovery, the concentrate loop accumulates inorganic and organic ions that must be removed. Typically, reject is recirculated near saturation, with make-up water added to prevent exceeding solubility limits. Some of the concentrate is discharged, but due to its high salinity, discharge could be restricted, making its treatment a significant challenge.
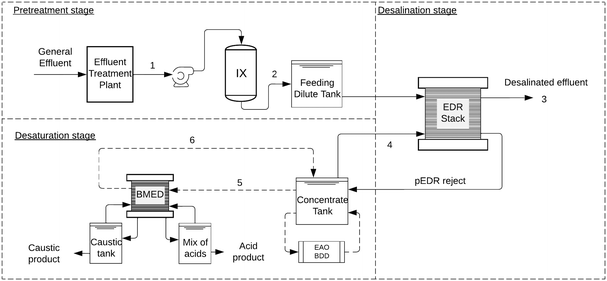
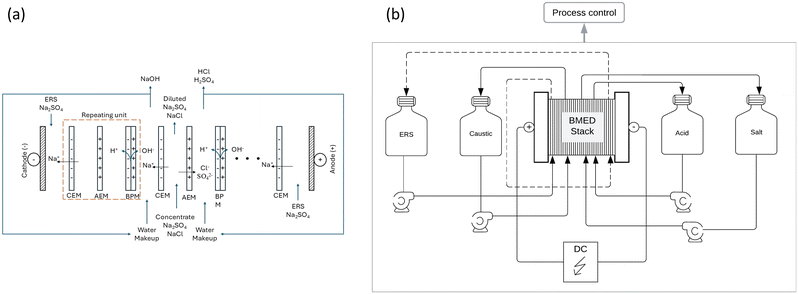
The most used concentrate treatment is evaporation/crystallization, a zero liquid discharge (ZLD) method that recovers water and produces solid salts via thermal processes. However, this method is energy-intensive and costly, and lacks circularity. To address this, we investigate bipolar membrane electrodialysis (BMED) to desaturate the concentrate and ion exchange (IX) as a pretreatment to protect the membranes (Fig. 1). BMED shows potential in ZLD and resource recovery due to its unique membrane structure,42 consisting of cation/anion exchange layers and an interfacial layer.25 Under direct current fields, water in the interfacial layer splits into H+ and OH− ions,20 enabling salt conversion into acids and caustics without chemicals, reducing pollution and generating valuable by-products.30
However, BMED performance can be adversely affected by contaminants, such as organic matter and multivalent metal ions. Organic pollutants in pulp effluents include carbohydrates, extractives, lignans, lignin-derived phenolics, and low molecular weight acids.32 Humic and fulvic acids and other lignin derivatives are also present.27 Here, we proposed for the first time pEDR as a pretreatment stage of bipolar membranes, as most of the organics in the effluents cannot cross the ion exchange membranes of electrodialysis. On the other hand, the problematic multivalent metal ions are hardness ions Ca2+ and Mg2+ which can form precipitates, blocking cation exchange membranes, increasing electrical resistance, and reducing efficiency and equipment lifetime.39,43 IX columns effectively remove hardness ions by exchanging them with resin-bound ions.19 IX is highly selective and efficient at removing metal ions, even at low concentrations.26 To complete the loop, IX regeneration stream, rich in calcium and magnesium, can be treated by chemical precipitation. Magnesium and calcium hydroxides can be selectively precipitated by adding alkaline solution in two consecutive steps while controlling the pH.37 These ions are valuable for the forestry industry and can be recycled to the forests if necessary.
This research evaluates an alternative ZLD system to traditional evaporation and crystallization in the kraft pulp industry based on IX as a pretreatment, pEDR as an isolation stage and BMED for desaturation of concentrates. The proposed technologies are evaluated experimentally and compared to conventional solutions in terms of performance, costs and sustainability.
2. Materials and methods
2.1 Characterization of the solutions
The schematic of the proposed solution is illustrated in Fig. 1, integrating pEDR desalination, IX pretreatment and BMED desaturation. Here, we evaluate IX and BMED, since pEDR desalination was already evaluated in ref. 14.For the IX pretreatment, the influent stream corresponds to the tertiary effluent from the pulp mill, previously treated using conventional methods. Tertiary effluent and pEDR (2 stages) dilute characterization results are presented in Table 1. Samples correspond to data obtained from the operation of a pEDR pilot plant. The pulp mill effluent refers to the characterization after ultrafiltration and before pEDR. The data correspond to a 45 day discontinuous operation period, with a total of 18 measurements for each parameter.
| Parameter | Pulp mill effluent | pEDR dilute | Unit |
|---|---|---|---|
| Aluminum | 0.04 ± 0.02 | 0.045 ± 0.026 | mg l−1 |
| Calcium | 3.89 ± 2.78 | 0.42 ± 0.85 | mg l−1 |
| Chloride | 63.71 ± 25.62 | 6.74 ± 4.013 | mg l−1 |
| COD | 31.22 ± 7.59 | 24.78 ± 10.61 | mg l−1 |
| Iron | 0.018 ± 0.006 | 0.018 ± 0.011 | mg l−1 |
| Magnesium | 1.10 ± 0.41 | 0.083 ± 0.078 | mg l−1 |
| Manganese | 0.003 ± 0.0019 | <0.002 | mg l−1 |
| Potassium | 5.15 ± 2.69 | 2.40 ± 2.86 | mg l−1 |
| Silica | 8.51 ± 4.03 | 10.58 ± 1.36 | mg l−1 |
| Sodium | 91.64 ± 45.75 | 34.74 ± 17.84 | mg l−1 |
| Sulfate | 138.05 ± 65.68 | 51.59 ± 20.26 | mg l−1 |
| Conductivity | 665.67 ± 235.16 | 157.57 ± 49.78 | mS cm−1 |
| pH | 7.44 ± 0.43 | 6.11 ± 0.27 | − |
In BMED experiments, a synthetic solution of pEDR concentrate was prepared, considering the main salts identified through the characterization of the concentrate in the pilot test (NaCl and Na2SO4) as they account for more than 85% of the total dissolved solids in the effluent. To evaluate the salt concentration effect on BMED performance, we tested three different synthetic solutions within the concentrate operational range observed in the pilot tests. These solutions are presented in Table 2.
| Conductivity (mS cm−1) | NaCl [mM] | Na2SO4 [mM] |
|---|---|---|
| 10 | 42.5 | 42.7 |
| 14 | 62.5 | 62.7 |
| 18 | 82.5 | 82.7 |
2.2 Experimental procedures
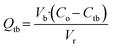 | (1) |
| Type | Electrical resistance (Ω cm−2) | Membrane thickness (mm) | Thermal stability (°C) | pH | Burst strength (MPa) | |
|---|---|---|---|---|---|---|
| CMB | Cation exchange | 4.5 | 0.21 | ≤60 | 0–14 | ≥0.40 |
| AHA | Anion exchange | 4.1 | 0.22 | ≤60 | 0–14 | ≥0.90 |
| BP-1 | Bipolar membrane | — | 0.28 | — | — | ≥0.70 |
The experiments were conducted under constant voltage (potentiostatic mode) across the electrodes. Four pumps controlled the flow in the BMED system. The flow rate in the three compartments (salt, acid and base) was maintained at a constant value of 10 L h−1 for all experiments, while the electrode flow rate was set at 30 L h−1. Throughout the experiments, the temperature, conductivity, voltage and current were constantly measured in all three compartments. Additionally, for the acid compartment, the pH was constantly measured. The temperature was maintained in the same range of IX, 26 °C to 29 °C, with a water jacket in all the compartments, according to the data obtained from the pulp mill effluent. The experiments were conducted over the range of 10 mS cm−1 to 2 mS cm−1, 14 mS cm−1 to 6.30 mS cm−1, and 18 mS cm−1 to 9.90 mS cm−1, to achieve the required desaturation rate.
Energy consumption (Ec, kWh kg−1 NaOH) and current efficiency (Ie, %) were calculated using eqn (2) and (3), respectively, as described by ref. 33. The desaturation rate was calculated using eqn (4).
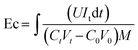 | (2) |
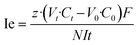 | (3) |
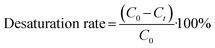 | (4) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 485 C mol−1). C0 and Ct (mol l−1) represent the alkali concentration at times 0 and t, respectively. V0 and Vt (L) correspond to the volume of the alkali solution at times 0 and t, respectively. N is the number of repetitive units in the BMED cell (4 in this study). t is the time (s) to complete the desaturation level. U (V) and It (A) represent the loading voltage and actual current of the BMED stack, respectively. M (kg mol−1) represents the molar mass per unit NaOH.
485 C mol−1). C0 and Ct (mol l−1) represent the alkali concentration at times 0 and t, respectively. V0 and Vt (L) correspond to the volume of the alkali solution at times 0 and t, respectively. N is the number of repetitive units in the BMED cell (4 in this study). t is the time (s) to complete the desaturation level. U (V) and It (A) represent the loading voltage and actual current of the BMED stack, respectively. M (kg mol−1) represents the molar mass per unit NaOH.
The costs of bipolar electrodialysis were estimated based on the total investment cost of the equipment. The equipment requirements were determined with the mass transfer requirements according to the desired conversion of salt input into acid and base. The mass transfer was estimated by transferring sodium moles from the salt compartment to the alkaline compartment, using a previously calibrated concentration and conductivity curve of the soda solution. The total capital cost was obtained from the equipment investment cost using previously published economic scaling models.34
For the economic analysis of BMED, the normalized energy consumption per kilogram of soda produced was considered. The total production cost of BMED can be defined as the sum of fixed, related to capital cost amortization, and operating costs (e.g., energy and maintenance).2
| CPro,BMED = Cfixed + Coperating | (5) |
On the other hand, the base case of analysis corresponds to concentrate evaporation and crystallization, evaluated using cost models obtained from the literature.17 The cost was updated using the Consumer Price Index factor for the years 2008–2025. The technical capability of this equipment is well-validated even for the treatment of highly concentrated streams.17 The cost model depends on the treatment flow rate and has different cases based on the salt concentration in the stream to be treated.
3. Results
3.1 Desaturation process
The influent of the treatment system is the tertiary effluent. Hardness ions are first removed by IX and the remaining ions by pEDR. The pEDR dilute stream serves as the recovered water for the pulp mill. Since IX capacity decreases at high salinity,7 placing IX before pEDR optimizes performance and reduces regeneration chemical use, treating a lower salt concentration in the concentrate loop. This configuration was validated via WAVE simulations and improves both pEDR and BMED membrane performance and lifespan (Fig. 1).Electrodialysis is designed for removal of small ions. Therefore, in this case it is also seen as a pretreatment and isolation stage for BMED, as it decreases the occurrence of organics in the concentrate. The subsequent treatment of this organic matter is critical for industrial implementation. One of the most promising technologies is electrochemical advanced oxidation with boron-doped diamond anodes.30 BDD electrodes have been considered as an optimal electrode material due to their favourable physical and chemical properties. The feasibility of the EAOP with BDD for this application is robustly supported by studies demonstrating its efficiency in high salinity/conductive media, such as treatment of reverse osmosis concentrates and high-salinity industrial wastewater.16 While detailed analysis of this specific downstream treatment is outside the scope of the present study, the literature confirms the BDD/EAOP pathway as an effective strategy for the complete oxidation of organic matter in the resulting concentrate.16,30
A fraction of the pEDR concentrate is recirculated, while the rest is desaturated in BMED. BMED dilute (Fig. 2) is only partially desalinated and recirculated back into a concentrate tank, which acts as a buffer. At steady state, the flow of ions from the dilute to the concentrate in pEDR is equal to the flow of ions that are converted into BMED products in the desaturation circuit. That is, the objective of the desaturation stage is to maintain a balanced ion transfer from the pEDR dilute into BMED products.
As mentioned above, the main salts identified in the concentrate are NaCl and Na2SO4. Therefore, the focus of BMED is to transform these salts into acid and base products. Regarding other elements, multivalent cations, mainly calcium and magnesium, were removed during pretreatment by ion exchange. These cations were subsequently transformed into sodium hydroxide and calcium carbonate through chemical precipitation from the IX regeneration stream, which was validated by modelling in PHREEQC. Moreover, potassium does not represent a problem for the BMED system, like sodium, it can be easily transferred to the alkaline compartment and transformed into potassium hydroxide.
For the industrial scale-up economic evaluations, a pulp mill effluent flow rate of 2500 m3 h−1 was considered. Based on the input salts to BMED and the ion transfer from the dilute to the concentrate in pEDR, a mass balance estimation determined that for a feed flow rate of 200 m3 h−1 in BMED, desaturation rates of 80%, 55%, and 45% were required for input conductivity levels of 10 mS cm−1, 14 mS cm−1, and 18 mS cm−1, respectively. This transfer allows steady state operation between the desalination system and the desaturation system to be maintained. A higher desaturation rate is required for lower input concentrations to ensure the same mass transfer in all three cases. For practical purposes in the BMED experiments, the desaturation rate for the identified salts was considered equivalent to the percentage reduction in conductivity. Therefore, BMED was operated until the final conductivity of the salt compartment reached 2.00 mS cm−1, 6.30 mS cm−1, and 9.90 mS cm−1, respectively.
3.2 Ion exchange column and chemical precipitation modelling
Sorption mechanisms depend on solid–liquid interactions and system conditions. Therefore, sorption capacity and required contact time are key parameters to assess.3 Ion exchange (IX) is commonly studied through kinetics, equilibrium, and column operation experiments,21 all of which apply models that describe adsorption or ion exchange processes. While batch studies offer valuable data, they are not directly applicable to continuous systems.3 For this reason, fixed-bed column experiments were performed to gain practical operational insights.The aim of the IX experiments was to determine the breakthrough point of resins treating real effluent. Breakthrough curves provide critical data for scaling up.11 These curves were built by monitoring the effluent concentration as a function of the treated volume. Although 5% of the influent is a common definition for breakthrough, in this study, it was set at 10% due to the low inlet concentrations of calcium and magnesium. This corresponds to less than 1 mg L−1, below the 5 mg L−1 threshold to avoid scaling in BMED.12
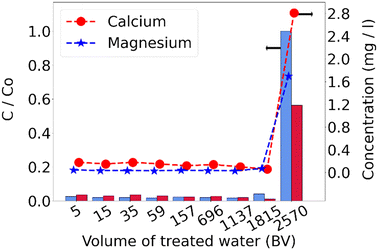
Fig. 3 shows the saturation curves for calcium and magnesium. Adsorption was effective, keeping calcium below 0.18 mg L−1 and magnesium below 0.05 mg L−1 in the effluent. As exchange sites are gradually occupied, concentrations increase until the resin exhaustion. However, the process was stopped before that point, focusing instead on the resin's capacity up to the predefined breakthrough.
The breakthrough was identified at the intersection between the data projection and the threshold line (Ctb). For calcium, it occurred at 1937 bed volumes (BV), and for magnesium at 1861 BV. Since magnesium broke through first, the resin's exchange capacity was calculated up to this point, resulting in 0.68 eq L−1. The capacity was lower than that reported by the supplier; this could be due to organic fouling caused by humic and fulvic acids,7 and to the breakthrough point defined based on magnesium concentration, meaning that the column did not reach its full capacity.
Weak acid resins have a high affinity for hardness ions, making regeneration with salt ineffective. Instead, diluted acid is used to regenerate the resin to its hydrogen form, leveraging its strong affinity for hydrogen ions. The conversion to the sodium form is then achieved by neutralization with diluted sodium hydroxide.7
The IX regeneration stream was modeled using WAVE software, with the effluent characterization from Table 1 and a selected weak acid resin. Regeneration was simulated using 4% HCl and 4% NaOH. Although the aim is to use BMED products for regeneration, only soda is suitable. The acid stream from BMED has a high sulfate content, which could cause calcium sulfate precipitation. To avoid this, the BMED acid stream must be diluted or replaced with HCl. For simplicity, regeneration with HCl was considered, allowing the BMED acid to be used elsewhere.
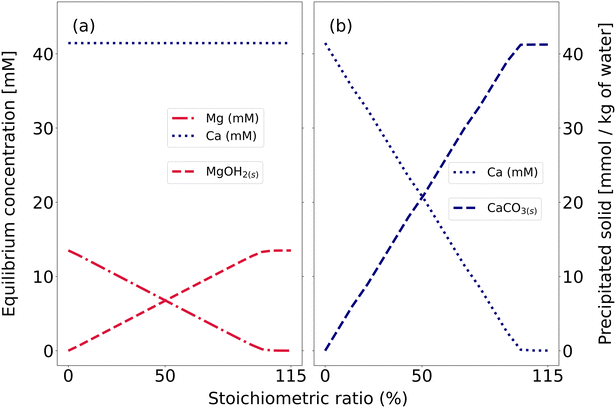
The regeneration stream obtained from WAVE contained 1898 mg l−1 Na+, 1644 mg l−1 Ca2+, 325 mg l−1 Mg2+, 6058 mg l−1 Cl−, and 285 mg l−1 SO42−. This was entered into PHREEQC to model sequential reactions with NaOH and Na2CO3 up to 1.15 times stoichiometry as shown in Fig. 4. NaOH led to Mg(OH)2(s) precipitation, reducing Mg2+ to 4.86 mg l−1. Then, CaCO3(s) formed, reducing Ca2+ to 5.21 mg l−1. Depending on requirements, additional steps could enable further valorization. Magnesium may be used as a fertilizer, and calcium carbonate as a raw material in pulp mills.
3.3 BMED
The current efficiency results obtained from the experiments are shown in Table 4, along with the values adjusted by the regression model. The modeled values closely match those obtained in the experiments, with a maximum difference of 3.98%.
| Conductivity (mS cm−1) | Voltage (V) | Experimental Ie (%) | Model predicted Ie (%) | Residual (%) |
|---|---|---|---|---|
| 10 | 14 | 78.70 | 78.38 | 0.32 |
| 10 | 16 | 79.05 | 79.08 | −0.03 |
| 10 | 18 | 79.75 | 78.97 | 0.78 |
| 10 | 20 | 76.15 | 78.05 | −1.90 |
| 10 | 22 | 93.70 | 94.44 | −0.74 |
| 14 | 14 | 94.75 | 95.14 | −0.39 |
| 14 | 16 | 95.40 | 95.03 | 0.37 |
| 14 | 18 | 94.65 | 94.11 | 0.54 |
| 14 | 20 | 90.40 | 91.66 | −1.26 |
| 14 | 22 | 95.50 | 92.36 | 3.14 |
| 18 | 14 | 93.05 | 92.25 | 0.80 |
| 18 | 16 | 87.35 | 91.33 | −3.98 |
| 18 | 18 | 77.15 | 76.32 | 0.83 |
| 18 | 20 | 92.60 | 92.38 | 0.22 |
| 18 | 22 | 90.90 | 89.60 | 1.30 |
The regression model was applied using the least squares method, and the regression equation is shown in eqn (6), where V is the applied voltage, C is the feed salt conductivity and Ie (%) is the current efficiency.
| Ie (%) = −71.66 + 18.15·C + 3.37·V − 0.59·C2 − 0.10·V2 | (6) |
Higher voltage improves current efficiency and acid/base concentration but increases energy use and heat generation, risking membrane damage.20,41 Also note that while moderate voltage enhances demineralization, excessive levels cause energy losses and reduce membrane lifespan.
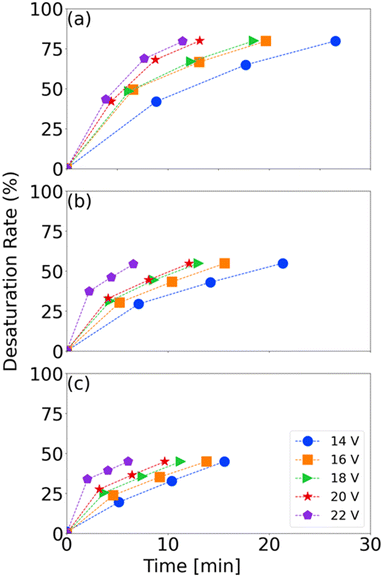
On the other hand, high demineralization rates lead to a higher resistance (lower conductivity) of the dilute compartment, decreasing the efficiency of the process in the long term. This can be observed in Fig. 6(a), where the energy efficiency improves progressively from 93.7% to 95.4% at 18 V but slightly decreases at 20 V (94.7%) and 22 V (92.6%). At 14 mS cm−1, the peak efficiency occurs at 18 V, though differences across voltages are minimal. Thus, voltage selection should consider its overall impact on BMED costs, as analysed in later sections.
As the feed salt concentration increases, the membrane stack resistance decreases, allowing higher current density at constant voltage. Greater conductivity also lowers ohmic resistance, enhancing ion flux and H+/OH− generation. Fig. 6(b) shows that, at 18 V, the energy consumption per unit of soda decreases as the conductivity rises from 10 to 14 mS cm−1, but it slightly increases at 18 mS cm−1. This is due to higher salt concentrations raising current and energy use, generating more heat and potential losses. The efficiency also improves from 10 to 14 mS cm−1, and then decreases beyond that point. Elevated salt concentrations increase osmotic pressure on the bipolar membrane, limiting H+ and OH− production from water dissociation.20 Therefore, both voltage and feed concentration must be carefully optimized to enhance BMED performance while minimizing energy consumption and membrane degradation.
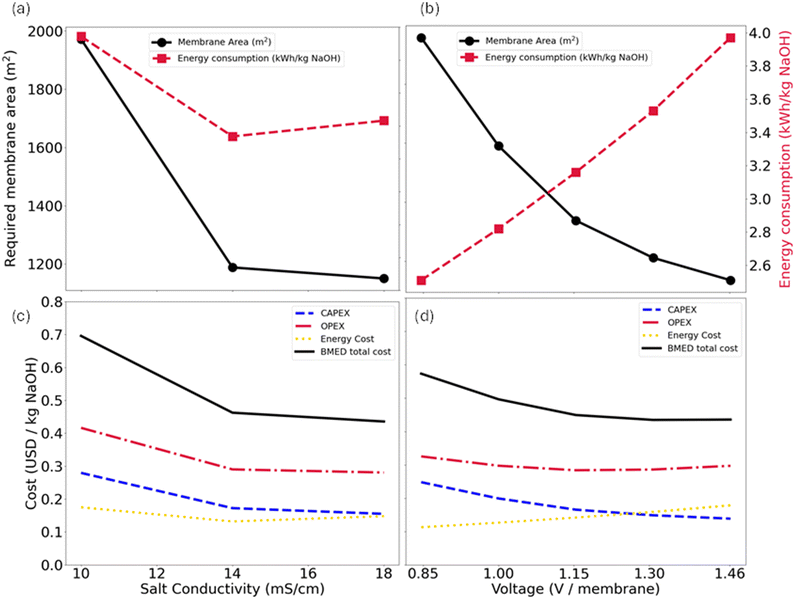
An initial evaluation was performed based on the feed salt concentration. As shown in Fig. 7(a), increasing salt concentration initially reduced the membrane area and energy consumption due to lower electrical resistance and improved mass transfer. However, beyond 14 mS cm−1, further increases showed diminishing returns in the membrane area reduction and led to higher specific energy consumption.
Fig. 7(c) shows that increasing the conductivity from 14 to 18 mS cm−1 reduces capital costs, as higher current densities allow for a smaller membrane area. This benefit offsets the increase in energy consumption, resulting in a slightly lower total cost at 18 mS cm−1 (0.44 vs. 0.46 USD per kg NaOH). However, the cost difference is marginal. Additional factors must be considered when integrating the desaturation system with pEDR, such as water transport through membranes by osmosis and electroosmosis, which intensify with higher reject concentrations.22 Considering these aspects, a feed salt concentration of 14 mS cm−1 was selected for further analysis on operating voltage.
Then, an economic analysis was conducted by setting the inlet salt concentration at 14 mS cm−1 and varying the operating voltage. As shown in Fig. 7(b), increasing the voltage reduces the required membrane area due to increased ion migration. However, it also can increase energy losses due to heat generation, decreasing energy efficiency and resulting in a near linear rise in energy consumption.
Fig. 7(d) shows the costs for the 14 mS cm−1 case as a function of operating voltage, normalized by the total number of membranes. Although increasing the voltage increases the energy cost, it reduces the capital cost by reducing the membrane area. This also decreases associated maintenance and replacement costs.
The total costs include all previously discussed factors. As the voltage increases, costs decrease from 0.57 USD per kg NaOH at 0.85 V per membrane to 0.43 USD per kg NaOH at 1.31 V per membrane. However, at 1.46 V per membrane, the total cost slightly increases to 0.44 USD per kg NaOH. This indicates that beyond 1.31 V per membrane, the rise in energy costs outweighs the reduction in capital expenses.
Preliminary tests at 1.62 V per membrane (24 V) reached 91.12 mA cm−2 at 14 mS cm−1. This is impractical for semi-continuous operation, as acid and soda concentration increases reduce resistance and raise current, potentially exceeding the typical BMED range of 10–100 mA cm−2,38 depending on the manufacturer. Therefore, the case of 14 mS cm−1 and 1.31 V per membrane (20 V) was selected for a semi-continuous experiment, representing a larger-scale operation.
A nine-step experiment was performed, with the final step selected as the representative of industrial operation. In this stage, once acid and base concentrations reached target values, both streams operated in feed-and-bleed mode, purging some of the solution and adding make-up water to maintain stable concentrations.
The main parameters from the multistep experiment are shown in Fig. 8. The NaOH concentration reached 0.67 M. The efficiency in the final step was 73%, with an energy consumption of 4.57 kWh kg−1 NaOH. The NaOH flux achieved was 6.27 × 10−4 mmol cm−2 s−1.
The NaOH concentrations reached at each step, along with the initial and final conductivity of the saline compartment, are shown in Fig. 8(a). The initial conductivity was 14 mS cm−1, and each step ended when it dropped to 6.30 mS cm−1, achieving 55% desalination, required to maintain steady ion transfer in pEDR.
The current density increased from 60.63 mA cm−2 in the first step to 83.03 mA cm−2 in the final one, peaking at 90.63 mA cm−2. This rise is due to the accumulation of acid and base, which lowers stack resistance. No additional stage was performed beyond this point to avoid exceeding the typical BMED upper limit (100 mA cm−2), which causes membrane damage.10 For sustainable long-term operations, a feed-and-bleed mode would maintain appropriate current density levels.
At each step, the current initially starts low due to high resistance from low acid/base concentrations. As concentrations rise, the resistance decreases, increasing the current. Then, as the salt solution becomes desaturated, the ion content drops, the resistance increases again, and the current decreases. This phenomenon has been previously observed by other researchers.29,41
The energy consumption rose from 3.43 to 4.57 kWh kg−1 NaOH across the steps, while the efficiency decreased from 97% to 73% (Fig. 8(b)). This is attributed to intensified H3O+ and OH− leakage at higher concentrations, which reduces efficiency.41 Additionally, higher osmotic pressure hinders water migration to the bipolar membrane interface, limiting H3O+ and OH− generation.40 Thus, lower initial acid/base concentrations favor lower energy consumption and higher efficiency compared to later stages.
3.4 Cost comparison with the conventional ZLD system
Using the parameters from the multistep operation, an economic evaluation of the proposed system was performed. The BMED design flowrate is 200 m3 h−1. Membrane costs were obtained from suppliers: 600 USD per m2 for bipolar membranes and 100 USD per m2 for ion exchange membranes.For ion exchange (IX), the design flowrate is 2500 m3 h−1, corresponding to the effluent desalinated by pEDR. The chemical precipitation flowrate is 14 m3 h−1, based on the IX regeneration stream modeled with WAVE. The chemical precipitation evaluation includes chemical feed, rapid mixing, and sedimentation. A sludge drying or conditioning stage may be added depending on valorization needs and industry infrastructure.
Previous studies reported pEDR water recovery rates above 95%.14 This study assumes 98% recovery to compare desaturation against a minimum-cost ZLD scenario. The evaporation and crystallization flowrate was set at 50 m3 h−1 (2% of the effluent). ZLD systems typically generate a solid salt mixture that is difficult to valorize and is usually disposed of as waste. Therefore, landfill disposal costs were included.
Table 5 presents the disposal costs and main parameters of the cost model, along with estimated CAPEX and OPEX values for each process.
| CAPEX (USD) | OPEX (USD per year) | |
|---|---|---|
| Desaturation system | ||
| IX | $7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 655 655![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 354 354 |
138![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 121 121 |
| BMED | $10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 978 978![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 554 554 |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 530 530![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 618 618 |
| CP | $1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 798 798![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 518 518 |
182![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 062 062 |
| Total | $20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 432 432![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 425 425 |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 850 850![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
| Conventional ZLD | ||
| Evaporation/crystallization | $28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 179 179![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 167 167 |
$1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 954 954![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 599 599 |
| Solid disposal | $2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 943 943![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 360 360 |
|
| Total | $28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 179 179![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 167 167 |
$4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 897 897![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 959 959 |
| Parameters | ||
| BM | 600 | USD per m2 |
| CEM | 100 | USD per m2 |
| AEM | 100 | USD per m2 |
| Specific solid disposal cost | 288 | USD per ton |
| Energy cost | 45 | USD per MWh |
| Soda price for valorization | 500 | USD per ton |
As shown in Table 5, the CAPEX of the evaporation/crystallization is about 38% higher than that of the desaturation system. Fig. 9 shows that the OPEX of the desaturation system is slightly higher than the OPEX of the conventional ZLD system. Although the specific energy consumption of the desaturation system (19 kWh m−3) is lower than the evaporation and crystallization energy consumption (62.6 kWh m−3), the higher flowrate of the BMED process explains the OPEX difference when normalized by the total treated water flow incorporating pEDR. However, the evaporation system requires the disposal of solids as waste, which has both a negative environmental impact and an additional cost. In contrast, the desaturation system considers the valorization of by-products, generating a positive impact on the overall cost balance.
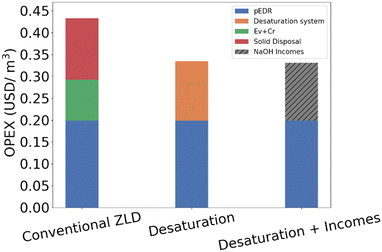 | ||
| Fig. 9 OPEX comparison for both scenarios, including NaOH valorization (hatched line) in the third case. | ||
Fig. 9 shows the OPEX for each stage, normalized per cubic meter of treated water (2500 m3 h−1). The OPEX of pEDR was estimated at 0.20 USD per m3, the desaturation system (IX + BMED + CP) at 0.14 USD per m3, and the conventional ZLD at 0.09 USD per m3. Solid disposal in ZLD adds 0.14 USD per m3. The desaturation system enables NaOH valorization, with a recovery of 5530 tons per year. At 500 USD per ton, this results in 2.77 MUSD per year. Accounting for this benefit, the combined OPEX of pEDR and desaturation drops to 0.20 USD per m3, less than half of the 0.43 USD per m3 for pEDR plus conventional ZLD with disposal. Additionally, desaturation reduces pEDR operating costs, as it avoids near-saturation conditions in the reject stream, lowering chemical use for pH and scaling control. This advantage may vary depending on the pEDR water recovery.
4. Conclusions
A techno-economic optimization of BMED was performed based on input voltage and salt concentration. The optimum conditions found were 14 mS cm−1 and 20 V. Under these conditions, a multistep operation was conducted to simulate industrial-scale performance, achieving 73% energy efficiency, 4.57 kWh kg−1 NaOH energy consumption, and a NaOH flux of 6.27 × 10−4 mmol cm−2 s−1.In IX, the breakthrough point was reached at 1861 BV, with an exchange capacity of 0.67 eq L−1. Modeling confirmed the selective separation of calcium and magnesium via precipitation of calcium carbonate and magnesium hydroxide in the regeneration stream.
The desaturation system outperforms conventional ZLD in CAPEX, OPEX, and environmental impact. Validated through experiments (IX and BMED) and modeling (CP), the total OPEX is 0.34 USD per m3, reduced to 0.20 USD per m3 when accounting for soda valorization. In contrast, ZLD has a total cost of 0.43 USD per m3.
The desaturation system addresses the issue of reject concentrate in desalination of pulp industry effluent with a holistic approach, solving the waste disposal problem by valorizing components in the wastewater stream. This approach could become the standard in the water and wastewater industry across different sectors, as regulations become more restrictive, and water becomes scarcer.
Next steps include evaluating organic fouling in BMED using real wastewater. While electrochemical advanced oxidation is proposed as an alternative for organic desaturation, its feasibility must be further assessed. Additionally, applying pulsed electric fields could enhance BMED performance and mitigate fouling.
Author contributors
L. Salas: writing – original draft, methodology, investigation, formal analysis. A. Schwarz: supervision, methodology, investigation, formal analysis. A. Gonzalez-Vogel: validation, supervision, resources, methodology, formal analysis, conceptualization.Conflicts of interest
There are no conflicts of interest to declare.Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.Acknowledgements
The authors are grateful to Arauco Bioforest SpA for funding this research and giving the rights to use the presented results.References
- M. Akhter, G. Habib and S. U. Qamar, Application of electrodialysis in waste water treatment and impact of fouling on process performance, J. Membr. Sci. Technol., 2018, 8(2), 1000182 CrossRef.
- A. Basem, D. J. Jasim, P. Ghodratallah, S. AbdulAmeer, A. M. Mahmood and W. J. Khudhayer, et al., Technical and financial feasibility of a chemicals recovery and energy and water production from a dairy wastewater treatment plant, Sci. Rep., 2024, 14(1), 11143 CrossRef CAS PubMed.
- L. Bibiano-Cruz, J. Garfias, J. Salas-García, R. Martel and H. Llanos, Batch and column test analyses for hardness removal using natural and homoionic clinoptilolite: breakthrough experiments and modeling, Sust. Water Resour. Manag., 2016, 2, 183–197 CrossRef.
- J. C. Bui, K. R. M. Corpus, A. T. Bell and A. Z. Weber, On the nature of field-enhanced water dissociation in bipolar membranes, J. Phys. Chem. C, 2021, 125(45), 24974–24987 CrossRef CAS.
- M. N. Cabrera, Pulp mill wastewater: characteristics and treatment, Biol. Wastewater Treat. Resour. Recovery, 2017, 2, 119–139 Search PubMed.
- T. Chen, J. Bi, Y. Zhao, Z. Du, X. Guo and J. Yuan, et al., Carbon dioxide capture coupled with magnesium utilization from seawater by bipolar membrane electrodialysis, Sci. Total Environ., 2022, 820, 153272 CrossRef CAS PubMed.
- Dow Water Solutions, Ion exchange resins Water Conditioning Manual, The Dow Chemical Company, Form No. 177-01766-1105.
- Dupont, Wave: Water application value engine (Version 1.82) [Software], Dupont Water Solutions, 2021 Search PubMed.
- Food and Agriculture Organization of the United Nations [Internet], [cited 2023 Mar 26], available from: http://www.fao.org/.
- R. Fu, H. Wang, J. Yan, R. Li, B. Wang and C. Jiang, et al., Ion injection bipolar membrane electrodialysis realizes over 8 mol/L NaOH conversion from a brine stream, AIChE J., 2024, 70(4), e18345 CrossRef CAS.
- D. Geraud, Ion Exchange Experiments – Water Softening and Deionization, Bachelor thesis, Williams Honors College, Honors Research Projects, 2016, p. 345, https://ideaexchange.uakron.edu/honors_research_projects/345 Search PubMed.
- K. Ghyselbrecht, M. Huygebaert, B. Van der Bruggen, R. Ballet, B. Meesschaert and L. Pinoy, Desalination of an industrial saline water with conventional and bipolar membrane electrodialysis, Desalination, 2013, 318, 9–18, DOI:10.1016/j.desal.2013.03.020.
- A. Gonzalez-Vogel and O. J. Rojas, Exploiting electroconvective vortices in electrodialysis with high-frequency asymmetric bipolar pulses for desalination in overlimiting current regimes, Desalination, 2020, 474, 114190 CrossRef CAS.
- A. Gonzalez-Vogel, J. J. Moltedo and O. J. Rojas, Desalination by pulsed electrodialysis reversal: Approaching fully closed-loop water systems in wood pulp mills, J. Environ. Manage., 2021, 298, 113518, DOI:10.1016/j.jenvman.2021.113518.
- R. Gumerman, R. Culp and S. Hansen, Estimating Water Treatment Costs. Volume 2. Cost Curves Applicable to 1. WATER or Water Treatment Estimation Routine, 1979, vol. 2, p. 542 Search PubMed.
- Y. He, H. Lin, Z. Guo, W. Zhang, H. Li and W. Huang, Recent developments and advances in boron-doped diamond electrodes for electrochemical oxidation of organic pollutants, Sep. Purif. Technol., 2019, 212, 802–821 CrossRef CAS.
- G. Juby, A. Zacheis, W. Shih, P. Ravishanker, B. Mortazavi and M. D. Nusser, Evaluation and selection of available processes for a zero-liquid discharge system for the Perris, California, Ground Water Basin (Desalination and Water Purification Research and Development Program Report No. 149), RECLAMATION Managing Water in the West, 2008.
- A. Kiperstok and C. M. Silva, The responsibility of the pulp and paper sector with regard to sustainable development: how much is enough?, Water Sci. Technol., 2007, 55(6), 65–71 CrossRef CAS PubMed.
- Y. A. R. Lebron, V. R. Moreira and M. C. S. Amaral, Metallic ions recovery from membrane separation processes concentrate: A special look onto ion exchange resins, Chem. Eng. J., 2021, 425, 131812, DOI:10.1016/j.cej.2021.131812.
- Y. Liu, Y. Sun and Z. Peng, Evaluation of bipolar membrane electrodialysis for desalination of simulated salicylic acid wastewater, Desalination, 2022, 537, 115866 CrossRef CAS.
- G. J. Millar, S. Papworth and S. J. Couperthwaite, Exploration of the fundamental equilibrium behaviour of calcium exchange with weak acid cation resins, Desalination, 2014, 351, 27–36 CrossRef CAS.
- A. Moura Bernardes, M. A. Rodrigues and J. Z. Ferreira, General aspects of electrodialysis, in Electrodialysis and Water Reuse: Novel Approaches, Springer Berlin Heidelberg, Berlin, Heidelberg, 2013, pp. 11–23 Search PubMed.
- E. Nascimben Santos, C. M. Silva, J. L. Colodette, S. B. Z. de Almeida, A. J. V. Zanuncio and T. O. de Souza, et al., Recirculation of treated effluent in the bleaching of kraft pulp, BioResources, 2020, 15(4), 8944–8964 Search PubMed.
- National Council for Air and Stream Improvement (NCASI), Environmental footprint comparison tool, A tool for understanding environmental decisions related to the pulp and paper industry, Washington, D.C., 2009.
- R. Pärnamäe, S. Mareev, V. Nikonenko, S. Melnikov, N. Sheldeshov and V. Zabolotskii, et al., Bipolar membranes: A review on principles, latest developments, and applications, J. Membr. Sci., 2021, 617, 118538 CrossRef.
- E. Pehlivan and T. Altun, The study of various parameters affecting the ion exchange of Cu2+, Zn2+, Ni2+, Cd2+, and Pb2+ from aqueous solution on Dowex 50W synthetic resin, J. Hazard. Mater., 2006, 134(1–3), 149–156 CrossRef CAS PubMed.
- Y. Qiu, S. Wu, L. Xia, L. F. Ren, J. Shao and J. Shen, et al., Ionic resource recovery for carbon neutral papermaking wastewater reclamation by a chemical self-sufficiency zero liquid discharge system, Water Res., 2023, 229, 119451 CrossRef CAS PubMed.
- D. Reeve and C. M. Silva, Closed cycle systems for manufacture of bleached chemical wood pulp, Chemical pulping, 2000,(6), 440–473 Search PubMed.
- H. Ruan, S. Wu, X. Chen, J. Zou, J. Liao and H. Cui, et al., Capturing CO2 with NaOH solution from reject brine via an integrated technology based on bipolar membrane electrodialysis and hollow fiber membrane contactor, Chem. Eng. J., 2022, 450, 138095 CrossRef CAS.
- C. Salazar, I. Sirés, R. Salazar, H. D. Mansilla and C. A. Zaror, Treatment of cellulose bleaching effluents and their filtration permeates by anodic oxidation with H2O2 production, J. Chem. Technol. Biotechnol., 2015, 90(11), 2017–2026 CrossRef CAS.
- A. Schwarz, L. Salas, I. Nancucheo and A. González-Vogel, Zero liquid discharge recovery system for maximized tailings water reuse, J. Environ. Manage., 2025, 394, 127272 CrossRef CAS PubMed.
- J. Shen, J. Huang, L. Liu, W. Ye, J. Lin and B. Van der Bruggen, The use of BMED for glyphosate recovery from glyphosate neutralization liquor in view of zero discharge, J. Hazard. Mater., 2013, 260, 660–667, DOI:10.1016/J.JHAZMAT.2013.06.028.
- H. Tang, X. Wang, X. Zhao, Y. Dong, B. Xu and L. Wang, Ion migration characteristics during the bipolar membrane electrodialysis treatment of concentrated reverse osmosis brine, Desalination, 2023, 561, 116660 CrossRef CAS.
- R. Thompson, M. Paleologou and R. M. Berry, Caustic soda and sulfuric acid production from sodium sulfate by-product of chlorine dioxide generation - economics, Tappi J., 1995, 78(6), 127–134 Search PubMed.
- U.S. Bureau of Labor Statistics [Internet], [cited 2023 Dec], available from: https://www.bls.gov/.
- U.S. Geological Survey, PHREEQC (Version 3) [Software], 2021, available from: https://www.usgs.gov/software/phreeqc-version-3 Search PubMed.
- F. Vassallo, D. La Corte, N. Cancilla, A. Tamburini, M. Bevacqua and A. Cipollina, et al., A pilot-plant for the selective recovery of magnesium and calcium from waste brines, Desalination, 2021, 517, 115231 CrossRef CAS.
- G. Virruso, C. Cassaro, A. Culcasi, A. Cipollina, A. Tamburini and I. D. L. Bogle, et al., Multi-scale modelling of an electrodialysis with bipolar membranes pilot plant and economic evaluation of its potential, Desalination, 2024, 583, 117724 CrossRef CAS.
- Y. Wang, X. Zhang and T. Xu, Integration of conventional electrodialysis and electrodialysis with bipolar membranes for production of organic acids, J. Membr. Sci., 2010, 365(1–2), 294–301 CrossRef CAS.
- Y. Wei, Y. Wang, X. Zhang and T. Xu, Treatment of simulated brominated butyl rubber wastewater by bipolar membrane electrodialysis, Sep. Purif. Technol., 2011, 80(2), 196–201 CrossRef CAS.
- H. R. Yang, B. Li, C. Q. Zhang, J. C. Yang, Y. M. Zheng and M. Younas, et al., Bipolar membrane electrodialysis for sustainable utilization of inorganic salts from the reverse osmosis concentration of real landfill leachate, Sep. Purif. Technol., 2023, 308, 122898 CrossRef CAS.
- C. Yu, Development and application of new electrodialysis technologies, 2021 Search PubMed.
- X. Zhang, C. Ye, K. Pi, J. Huang, M. Xia and A. R. Gerson, Sustainable treatment of desulfurization wastewater by ion exchange and bipolar membrane electrodialysis hybrid technology, Sep. Purif. Technol., 2019, 211, 330–339, DOI:10.1016/j.seppur.2018.10.003.
| This journal is © The Royal Society of Chemistry 2026 |


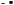

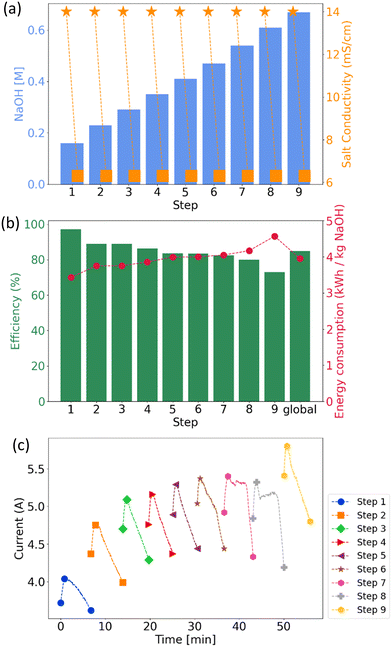
![[rectangle open, vertical]](https://www.rsc.org/images/entities/char_25af.gif) ) and final (■) conductivity in the salt compartment, as well as the NaOH concentration reached in each step (bar); (b) energy efficiency (bar) and energy consumption (●) of each step, along with the average across all steps (global); (c) current along each step.
) and final (■) conductivity in the salt compartment, as well as the NaOH concentration reached in each step (bar); (b) energy efficiency (bar) and energy consumption (●) of each step, along with the average across all steps (global); (c) current along each step.