Open Access Article
Open Access ArticleComparing the microplastic content in biosolid-amended and non-amended agricultural soils
Nicholas V. Letwin,
Adam W. Gillespie,
Joel D. Csajaghy,
Yaryna M. Kudla,
Moira M. Ijzerman and
Ryan S. Prosser *
*
School of Environmental Sciences, University of Guelph, 50 Stone Rd E, Guelph, ON N1G4W2, Canada. E-mail: prosserr@uoguelph.ca
First published on 23rd December 2025
Abstract
Biosolids have been identified as a major source of microplastics (MP) to the environment. While they have been heavily studied, the impacts biosolids have following their amendment to agricultural soils on the MP content of these soils is poorly understood. Eleven biosolid-amended and nine non-amended agricultural fields in Southern Ontario were sampled to compare the MP content between them. Biosolid-amended fields averaged 2441.82 ± 268.03 MP kg−1, while non-amended fields averaged 775 ± 50.97 MP kg−1. Additionally, MP abundance was correlated with the type of biosolid applied, with fields that received a single application of dewatered biosolids averaging 2412.14 ± 174.81 MP kg−1, whereas fields that received a single application of liquid biosolids averaged 1689.83 ± 225.81 MP kg−1. However, differences in MP abundance were primarily dictated by differences in application rate between dewatered and liquid biosolids. In addition to increasing overall MP content, biosolid amendments influenced MP composition. Biosolid amendment increased soil fibre content, as biosolids are rich in textile fibres derived from the laundering process. As a result, biosolid-amended soils primarily contained polyester, while unamended soils primarily contained polypropylene. Quantifying and characterizing MP content in biosolid-amended fields, and understanding how it differs from unamended fields, is crucial for accurately assessing the risks microplastics pose to terrestrial ecosystems.
Environmental significanceBiosolids are commonly applied to agricultural soils, yet their role in microplastic (MP) contamination is not fully understood. This is critical to assess, as MPs in soil can potentially alter ecosystem function and soil structure. We compared MP abundance and composition in eleven biosolid-amended and nine non-amended fields in Southern Ontario. Biosolid-amended soils had more than three times as many MPs on average, with concentrations influenced by biosolid type and number of applications. Amendments also shifted MP composition, increasing textile-derived polyester fibres. These findings highlight biosolids as a significant vector of MPs to terrestrial systems. Understanding this pathway is essential for generalizing MP pollution risks beyond aquatic environments and informing policies on land-based waste management and soil health protection. |
1 Introduction
The widespread use of plastic, coupled with poor waste management, has led to extensive pollution, making it a significant environmental concern. It is estimated that of all generated plastics, 9% is recycled, 12% is incinerated, and 79% is accumulated within landfills or the environment.1 Furthermore, estimations indicate that plastic contamination in soil is 40 times greater than in water.2 Microplastics (MPs, plastic particles <5 mm) are primarily derived from the fragmentation of larger plastic materials,3 and are an emerging contaminant of concern. There has been an increased focus on the research of MPs as they are ubiquitous, resist degradation, and their interactions with other components in the environment are relatively unknown. Sources of MP pollution to soils are numerous, including improper waste disposal, plastic mulching, urban and road runoff, aerial deposition, wastewater irrigation, and land application of biosolids.4–6 One of the main concerns of MPs is the risks they could pose to organisms through their uptake.7 Recent findings indicate that MPs can reduce reproduction in soil biota, such as nematodes, earthworms, and springtails, and can also cause shifts in the soil microbiome.8Additionally, there is evidence that MPs can alter soil structure, nutrient availability, and soil fertility.9 These risks associated with MPs are strongly influenced by their physicochemical properties, such as variations in size, morphology, polymer type, and chemical additives.10 As such, it is essential to characterize the types, amounts, and shapes of MPs accumulating in soil ecosystems.
Biosolids (treated sewage sludge) are a byproduct of wastewater treatment. They are often used as an agricultural fertilizer amendment because they supply organic matter, nitrogen, and phosphorus to soils.11 In Ontario, approximately 300![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 dry tons of biosolids are produced annually. Of this amount, about 40% is applied to agricultural land, 40% is deposited in landfills, and 20% is incinerated.12 As biosolids are derived from human waste and urban wastewater, they may contain several contaminants, including metals, pharmaceuticals, personal care products, polycyclic aromatic hydrocarbons (PAHs), and per- and polyfluoroalkyl substances (PFAS).13–17
000 dry tons of biosolids are produced annually. Of this amount, about 40% is applied to agricultural land, 40% is deposited in landfills, and 20% is incinerated.12 As biosolids are derived from human waste and urban wastewater, they may contain several contaminants, including metals, pharmaceuticals, personal care products, polycyclic aromatic hydrocarbons (PAHs), and per- and polyfluoroalkyl substances (PFAS).13–17
In some cases, these contaminants have been identified, and regulatory tools are in place to mitigate their transfer to agricultural lands.18 Biosolids have also been identified as potentially containing a significant source of MPs, which could be transferred to agricultural soils.19 Studies have shown that up to 99% of MPs entering wastewater treatment plants (WWTPs) are removed from the effluent and deposited in biosolids.20,21 Current reported estimates of MPs within Canadian biosolids range from 8000 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 350
350![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 MPs kg−1 dry weight.5,22,23 MPs in biosolids are predominantly sourced from personal care products and textile fibres released during laundering.24 As a result, biosolids contain high levels of plastics commonly associated with textile fibres, such as polyester, polyacrylamide, and polyamide.23
000 MPs kg−1 dry weight.5,22,23 MPs in biosolids are predominantly sourced from personal care products and textile fibres released during laundering.24 As a result, biosolids contain high levels of plastics commonly associated with textile fibres, such as polyester, polyacrylamide, and polyamide.23
While biosolids have been identified as a significant potential source of MPs in agroecosystems, conflicting results exist regarding whether MPs are incorporated into soils.5,25 In addition, it is uncertain if biosolids contribute more MPs than other fertilizers. Similar to biosolids, compost has been identified as a notable contributor of MPs to agricultural fields.26 Inorganic fertilizers, including slow- or controlled-release fertilizers, are often coated with synthetic polymers such as urea-based resins and polyurethanes, which gradually break down into MPs over time.27 Additionally, manure may introduce MPs indirectly through contaminated feed, equipment, or handling tools.28 Evidence suggests that runoff from biosolid-amended fields contains higher MP concentrations than runoff from manure-amended fields, yet there is limited research comparing MP concentrations within the soil itself.29 Furthermore, the fate and accumulation of MPs in soils following biosolid application remain largely unknown. Previous studies looking at Ontario biosolid-amended soils reported that only 1–7% of the biosolid-derived MPs are retained within the surface levels of the soil, with the majority hypothesized to be lost to wind erosion or surface runoff.5,30 In addition, natural processes such as bioturbation and water infiltration can move MPs to deeper soil horizons, removing them from topsoil.31 Currently, the highest reported average MP concentration found in Canadian biosolid-amended soils is 6870 MP kg−1 dry weight.30 Nevertheless, it is estimated that Ontario biosolids can deposit between 4.1 × 1011 and 1.3 × 1012 MP particles into agricultural fields per year.5 Further MP loss may be attributed to movement into deeper soil layers via bioturbation or water infiltration, potentially reaching groundwater.32 Furthermore, physical properties of an agricultural field, such as slope and moisture redistribution, are hypothesized to affect the long-term retention of MPs in the soil column.33,34
This study aimed to contribute to understanding MP presence in agroecosystems. The key objectives of this study were to (1) enumerate and characterize MP content within biosolid-amended soils, (2) compare the MP content in biosolid-amended soils with fields that have never received biosolid treatment, and (3) determine if the physical properties of an agricultural field can predict long-term MP retention.
2 Materials and methods
2.1 Field selection
This study was conducted in Wellington County, located in southern Ontario, Canada. This region experiences a humid continental climate with cold winters, warm summers, and annual precipitation of 800–1000 mm.35 Land use is predominantly agricultural, as Wellington County covers only 0.2% of Ontario's total land area but provides 5% of the province's field crops.36 A 2021 census estimates a population of ∼240![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 residents.37
000 residents.37
Twenty agricultural fields across three locations were selected for sampling (Fig. 1). Fields 1–12 are in Location 1, Fields 14,15 are in Location 2, and Fields 16–20 are in Location 3. None of the twenty fields practice tilling. Fields 1, 2, and 5–10 are classified as silt loams, Fields 3, 4, 7, and 12 are loams, and Field 13 is a gravelly sandy loam. Furthermore, fields in Locations 2 and 3 are all classified as loam. Detailed information on cation exchange capacity, pH, particle size distribution, and levels of phosphorus, inorganic and organic carbon, organic matter, and heavy metals can be found in the supplementary information (Tables S1–S8). Eleven of these fields received biosolid amendments, while the remaining nine were fertilized with manure. All 20 fields are on a three-crop rotation of corn–soybeans–wheat. At the time of sampling, Field 15 had received four biosolid applications between 2015 and 2021. All other fields amended with biosolids had only one application, conducted between 2019 and 2021. Fields 1–9 and 15–18 were sampled in May 2022, while Fields 10–14, 19, and 20 were sampled in May 2023. A summary of each field is provided in Table 1.
| Field | Amendment | Year(s) of biosolid application | Biosolid type | Application rate (m3 hectare−1) | Field size (hectare) | Crop type |
|---|---|---|---|---|---|---|
| Field 1 | Biosolids | 2019 | Dewatered | 19.77 | 15.53 | Corn |
| Field 2 | Biosolids | 2019 | Dewatered | 19.77 | 7.48 | Corn |
| Field 3 | Biosolids | 2019 | Dewatered | 19.77 | 26.12 | Corn |
| Field 4 | Biosolids | 2019 | Dewatered | 19.77 | 3.90 | Corn |
| Field 5 | Other | N/A | N/A | N/A | 3.51 | Corn |
| Field 6 | Biosolids | 2020 | Dewatered | 31.15 | 16.66 | Corn |
| Field 7 | Other | N/A | N/A | N/A | 20.28 | Corn |
| Field 8 | Biosolids | 2019 | Dewatered | 63.71 | 46.24 | Corn |
| Field 9 | Biosolids | 2019 | Dewatered | 63.71 | 18.45 | Corn |
| Field 10 | Other | N/A | N/A | N/A | 37.57 | Corn |
| Field 11 | Other | N/A | N/A | N/A | 37.44 | Corn |
| Field 12 | Other | N/A | N/A | N/A | 3.82 | Soybeans |
| Field 13 | Other | N/A | N/A | N/A | 52.11 | Corn |
| Field 14 | Other | N/A | N/A | N/A | 28.67 | Corn |
| Field 15 | Biosolids | 2015 | Liquid | 105.80 (2015) | 31.54 | Corn (2015) |
| 2017 | 95.16 (2017) | Soybeans (2017) | ||||
| 2019 | 107.91 (2019) | Corn (2019) | ||||
| 2021 | 100.66 (2021) | Corn (2021) | ||||
| Field 16 | Biosolids | 2021 | Dewatered, liquid | 8.15 | 9.70 | Corn |
| 80.13 | ||||||
| Field 17 | Biosolids | 2021 | Liquid | 100.00 (±5%) | 10.56 | Corn |
| Field 18 | Biosolids | 2021 | Liquid | 100.00 (±5%) | 9.64 | Corn |
| Field 19 | Other | N/A | N/A | N/A | 18.00 | Wheat |
| Field 20 | Other | N/A | N/A | N/A | 11.51 | Wheat |
2.2 Sample collection
To eliminate potential bias from MP horizontal movement within an agricultural field, a conditioned Latin hypercube sampling approach was employed.38 First, polygons representing each field were created in Google Earth and then overlaid onto digital elevation models (DEMs) provided by the Ontario Ministry of Natural Resources and Forestry within ArcMap 10.0.39 Using the “Extract by Mask” function, DEMs for each polygon were generated. Estimates of slope, LS-factor (a measure of slope length and steepness used to estimate soil erosion risk), and topographic wetness index were derived from these DEMs using the System for Automated Geoscientific Analyses (SAGA GIS). R packages “raster”, “terra”, and “clhs” were then used to generate 20 sampling points for each field, with DEM, slope, LS-factor, and topographic wetness index serving as covariates.A T-handled stainless-steel soil auger with a tip that was 5 cm in diameter and 15 cm long was used to sample soil up to 30 cm in depth. Although MP concentrations are highest within the top 10 cm of soil, evidence suggests that approximately 70% of MPs within the soil column are found in the first 30 cm.40 Upon site arrival, debris on the top of the soil was removed. To minimize MP contamination, soil samples were wrapped in aluminum foil immediately following collection. Samples were held at 4 °C until further subsampling.
2.3 Microplastic extraction
Each soil sample was transferred from aluminum foil to a stainless-steel mixing bowl and thoroughly mixed until homogenized, yielding 20 homogenized samples per field. A 100 g subsample was transferred to a 250 mL glass beaker and dried in a drying oven set to 65 °C. Following this, a 10 g subsample of dried soil was placed into a 300 mL glass beaker. Soil samples were digested using Fenton's reagent, with a 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of 30% w/w H2O2, 20 mmol L−1 iron(II) sulfate heptahydrate solution, and 20 mmol L−1 protocatechuic acid solution. Digestions were conducted on shaker tables set to 175 rpm in a fume hood for 24 h. Afterward, the samples were returned to a drying oven set to 65 °C to remove any residual H2O2. Once dried, density separation was performed by adding 100 mL of 905 g L−1 NaBr, a solution with a specific gravity of approximately 1.45 g cm−3, to each sample. The samples were mixed with a stir bar for 5 min, then allowed to separate for 24 hours. Following separation, the top 50 mL was transferred to a secondary beaker using a 10 mL stainless steel ladle. Vacuum filtration was then carried out using 0.45-µm Durapore™ polyvinylidene fluoride membrane filters.
1 ratio of 30% w/w H2O2, 20 mmol L−1 iron(II) sulfate heptahydrate solution, and 20 mmol L−1 protocatechuic acid solution. Digestions were conducted on shaker tables set to 175 rpm in a fume hood for 24 h. Afterward, the samples were returned to a drying oven set to 65 °C to remove any residual H2O2. Once dried, density separation was performed by adding 100 mL of 905 g L−1 NaBr, a solution with a specific gravity of approximately 1.45 g cm−3, to each sample. The samples were mixed with a stir bar for 5 min, then allowed to separate for 24 hours. Following separation, the top 50 mL was transferred to a secondary beaker using a 10 mL stainless steel ladle. Vacuum filtration was then carried out using 0.45-µm Durapore™ polyvinylidene fluoride membrane filters.
To validate the method, a spike-recovery test was performed with seven replicates. MP recovery was assessed using white polyethylene spheres (962.75 ± 21.83 µm), red polyester fibres (860.65 ± 129.65 µm), and blue polypropylene fragments (508.82 ± 56.91 µm). The white polyethylene spheres were purchased from Cospheric, while the red polyester fibres and blue polypropylene fragments were produced by freezing and bead milling their respective macroplastics. Ten individual MPs of each MP type was measured and added to 1 g of dried reference soil. The reference soil was a loam with approximately 2% organic carbon. Three method blanks were included in the spike-recovery test, and no contamination of the spiked MPs was observed. Results showed >70% recovery for all three MPs (Table S9).
2.4 Microplastic quantification
MPs were visually quantified using a Nikon® SMZ18 stereomicroscope paired with a Nikon® DS-Ri2 camera. Each particle was categorized by colour, size, and morphology (fragment, sphere, fibre, film, or foam). Size was determined by measuring the longest dimension of each MP. For fibres, length was measured as the distance between the two endpoints of the fibre. To simplify colour classification, similar shades were grouped together. Particles that appeared white, clear, or grey were classified as “clear.” Those that appeared black or dark grey were grouped as “black.” Finally, particles in shades of brown or orange were categorized as “orange.”2.5 Microplastic characterization
MP polymers were identified using a Bruker LUMOS II FT-IR microscope. Individual MPs were transferred to a Thermo Scientific™ Micro Compression Cell™ with KBr windows and analyzed through transmission FT-IR spectroscopy with a mercury–cadmium–telluride detector. In each field, 50 MPs were analyzed, representing 5.64–51.00% (mean = 22.12%) of all individual MPs quantified in that field. To accurately represent MP content, MPs were selected based on the calculated proportions of their morphology and colour found within the field. The resulting spectra were compared with the open-source Open Specy FT-IR spectral library,41 chosen for its extensive database of weathered MPs. Environmental MP samples often show altered spectra due to surface degradation caused by factors such as bacterial digestion, heat, ozone, and UV radiation. As such, spectra from pristine plastics may not match those of weathered MPs.42 Therefore, MPs were considered a positive match if they exhibited a similarity score of 60% or higher.2.6 Quality assurance and quality control
Plastic use was minimized as much as possible during sample processing and collection. Initially, soil samples were wrapped in aluminum foil during collection. While processing, samples were covered with an aluminum foil lid at all times, except during chemical addition. Furthermore, hands, surfaces, and glassware were thoroughly rinsed with deionized (DI) water between samples. When handling samples, emphasis was placed on wearing bright-coloured, non-synthetic clothing to identify contaminated fibres easily.Samples were processed in batches of 10, with one method blank included per batch, resulting in a total of 40 method blanks and 400 soil samples. Method blanks followed the same procedures as the soil samples for collection, storage, and processing. MPs detected in the method blanks were subtracted from the final estimates of the 10 soil samples within their batch. Method blank analysis showed a range of 2–9 particles per filter (mean = 5.28), with 83.89% of them being fibres.
2.7 Data analysis
Twenty samples and two method blanks were analyzed for each agricultural field. Results are reported in MPs per kilogram of soil (dry wt). Standard errors were calculated and reported for each sample.The Shapiro–Wilk and Bartlett's tests were used to assess normality and homogeneity of variances. Once these assumptions were met, independent t-tests (α = 0.05) were used to compare differences across amendments and biosolid types. An F-test was performed to compare the variances of biosolid-amended and unamended soils.
To estimate MP load from biosolids in soil, the following equations were used:
| VBiosolid = Application rate × 1 hectare | (1) |
| MBiosolid = VBiosolid × ρBiosolid | (2) |
| MP = [Biosolid] × MBiosolids | (3) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 331 MP kg−1 were derived from Letwin et al., 2023 as fields in this study were amended with biosolids sourced from the same locations described in that work.23 While concentrations may have varied over time, these values provide a reasonable estimate of the MP load associated with the biosolids applied.
331 MP kg−1 were derived from Letwin et al., 2023 as fields in this study were amended with biosolids sourced from the same locations described in that work.23 While concentrations may have varied over time, these values provide a reasonable estimate of the MP load associated with the biosolids applied.| MSoil = VSoil × ρSoil | (4) |
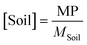 | (5) |
To create a predictive model of MP size, the data were grouped into size classes with 25 µm intervals. The midpoint of each class was calculated as the average of the lower and upper bounds. Due to uncertainties in quantifying MPs within the smallest size ranges, any MPs smaller than 50 µm were excluded from the analysis. A series of regression models, including Poisson, Negative Binomial, and Log-Normal, were fitted to examine the relationship between the midpoints of the size classes and the frequency of occurrences. Model fit was assessed using Akaike Information Criterion (AIC) and deviance scores. Additionally, visual inspection of the models was conducted to evaluate their effectiveness.
The data for this study are available on the University of Guelph's Research Data Repository at: https://doi.org/10.5683/SP3/HEVKKP.
3 Results and discussion
3.1 Microplastic concentrations
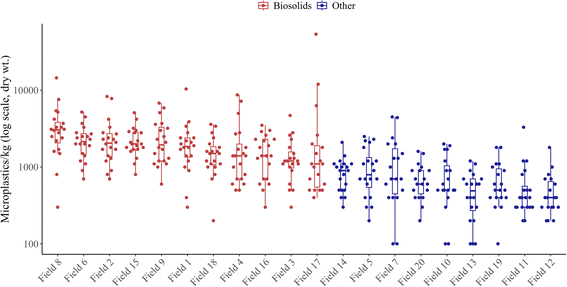
MP concentrations within biosolid-amended fields ranged from 1450 ± 226 MP kg−1 to 3610 ± 684 MP kg−1, with an average of 2442 ± 268 MP kg−1. These levels are slightly elevated compared to global concentrations of MPs in biosolid-amended soils, where most studies report values between 532 MP kg−1 and 2263 MP kg−1.47 In addition, MP concentrations in fields amended with Canadian biosolids exhibited considerable variability, ranging from 187 to 6870 MP kg−1.5,30MP levels in biosolid-amended soils can be influenced by several factors, including biosolid application rate, number of applications, biosolid type, and biosolid source.48 Furthermore, environmental factors such as temperature and precipitation can affect the transport, retention, and breakdown of MPs within soil.33,49 Additionally, soil properties such as texture, organic matter content, and porosity influence MP movement and aggregation.34,50 All fields chosen for this study received biosolids sourced from the same region, suggesting that MP concentrations in these biosolids may be higher than global averages. However, MP loading in the soils could vary depending on the application rate, number of applications, and cumulative amendments, highlighting the difficulties of comparing MP concentrations across studies. Furthermore, variations in extraction methods make it challenging to compare MP concentrations across studies globally.51 Nonetheless, the findings of this study are consistent with estimates of MP concentrations within Canada and worldwide.
Of the twenty fields analyzed, all eleven with the highest MP concentrations were biosolid-amended (Fig. 2). MP concentrations within fields that have never received biosolids ranged from 490 ± 87 MP kg−1 to 1250 ± 287 MP kg−1, with an average of 775 ± 50 MP kg−1. An independent t-test (α = 0.05) comparing the average MP concentrations between biosolid-amended and non-amended soils revealed significant differences across locations 1, 2, and 3 (p < 0.05). These results suggest that biosolid amendments significantly increase the average MP concentrations within agricultural soils.
Individual samples from biosolid-amended fields varied widely, from 0 to 53![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 MP kg−1, with the highest concentration (53
800 MP kg−1, with the highest concentration (53![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 MP kg−1) far exceeding the next-highest value (14
800 MP kg−1) far exceeding the next-highest value (14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 MP kg−1), whereas non-amended fields had a more limited range from 0 to 4500 MP kg−1. Additionally, an F-test showcased a significant difference in variance between the biosolid-amended and non-amended fields (F(219,179) = 33.79, p < 2.2 × 10−16). This suggests that the distribution of biosolid-derived MPs within the soil matrix is likely to exhibit significant variability. This is likely due to the heterogeneity of biosolids composition and inconsistencies during the spreading process.48,52 In contrast, when comparing variances between fields that received one application of dewatered biosolid and those that received one application of liquid biosolid, no significant difference was observed (F(139,58) = 1.42, p = 0.129), indicating that the type of biosolid applied does not appear to substantially influence variability in MP concentrations. MPs are hydrophobic particles with large surface areas, so they tend to interact with one another.53 This, combined with their interactions with organic matter, can lead to pockets of increased MP density within biosolids.6 This will further contribute to the uneven distribution of MPs in biosolid-amended fields.
500 MP kg−1), whereas non-amended fields had a more limited range from 0 to 4500 MP kg−1. Additionally, an F-test showcased a significant difference in variance between the biosolid-amended and non-amended fields (F(219,179) = 33.79, p < 2.2 × 10−16). This suggests that the distribution of biosolid-derived MPs within the soil matrix is likely to exhibit significant variability. This is likely due to the heterogeneity of biosolids composition and inconsistencies during the spreading process.48,52 In contrast, when comparing variances between fields that received one application of dewatered biosolid and those that received one application of liquid biosolid, no significant difference was observed (F(139,58) = 1.42, p = 0.129), indicating that the type of biosolid applied does not appear to substantially influence variability in MP concentrations. MPs are hydrophobic particles with large surface areas, so they tend to interact with one another.53 This, combined with their interactions with organic matter, can lead to pockets of increased MP density within biosolids.6 This will further contribute to the uneven distribution of MPs in biosolid-amended fields.
While it is clear that biosolids introduce MPs to the soil matrix, factors such as the type of biosolid and the frequency of application can influence MP concentrations within fields (Fig. 3).
For example, fields with a single application of dewatered biosolid had an average MP concentration of 2412 ± 175 MP kg−1, whereas fields with a single application of liquid biosolid had an average MP concentration of 1690 ± 226 MP kg−1. An independent t-test (α = 0.05) comparing these two groups revealed a significant difference in MP concentrations (p = 0.0126). Although the application rate of liquid biosolid (80.13–100.00 m3 hectare−1) was higher than that of dewatered biosolid (19.77–63.71 m3 hectare−1), this did not correspond to higher MP levels (Table 1). Liquid biosolids have a solids content of 2–7%, while dewatered biosolids exhibit a broader range, with solids content between approximately 20–80%.54,55 Evidence suggests that MPs are primarily retained within the solids portion of biosolids, indicating minimal loss of MPs during the dewatering process.56 As a result, MP density is much greater in dewatered biosolids. While the application rate of liquid biosolids is greater, it is not high enough to offset the MP content of dewatered biosolids, resulting in increased MP levels in fields spread with dewatered biosolids.
Field 15, amended with liquid biosolids, exhibited an average MP concentration of 2300.00 ± 232 MP kg−1. This elevated concentration is likely due to the field receiving a total of four applications (i.e., 2015, 2017, 2019, 2021) (Table 1). While this concentration was not statistically different from fields that received a single biosolid amendment (p = 0.0645), Field 15 has a higher MP concentration compared to fields that received a single application of liquid biosolid (Fields 16, 17, and 18) (Fig. 3). Previous evidence suggests that successive applications of biosolids contribute to elevated MPs accumulation.57 However, although all four fields received liquid biosolids from the same source and had similar application rates, the observed MP concentrations in Field 15 are not proportionally higher. This provides evidence of a partial loss or redistribution of MPs in the period following the application.
Estimates of MP load introduced through biosolid application vary widely based on factors such as application rate, biosolid type, and biosolid density (eqn (1)–(5)). For example, Fields 16, 17, and 18, which were amended with liquid biosolids, are estimated to have received 151–188 MP kg−1 of soil (Table S26). In contrast, Fields 1–4, 6, 8, and 9, which received dewatered biosolids, are estimated to have received 438–1674 MP kg−1 of soil, with large variations being reliant on the application rate (Table S26). Field 15, which received four applications of liquid biosolid, is estimated to have additional MP concentrations in soil of 771 MP kg−1. With these estimations, four applications of liquid biosolids (application rate of approx. 100 m3 ha−1) are equal to one singular application of dewatered biosolid at an application rate of 29.33 m3 ha−1. Furthermore, the estimated MP load from biosolid applications highlights that differences between amended and non-amended fields reflect the addition of biosolids rather than external factors (Fig. 2).
3.2 Microplastic morphology
MPs in biosolid-amended fields were 46.36 ± 3.83% fragments, 49.50 ± 4.11% fibres, 2.19 ± 0.33% films, 1.42 ± 0.04% foams, and 1.87 ± 0.35% spheres (n = 5596). In contrast, MPs in unamended fields were 67.42 ± 2.63% fragments, 29.55 ± 2.40% fibres, 1.88 ± 0.54% films, 0.94 ± 0.34% foams, and 0.21 ± 0.21% spheres (n = 1174, Table S11). Some key differences between these fields include an increase in fibre content in biosolid-amended fields (p = 0.000715), a decrease in fragments (p = 0.000295) (Fig. 4).While the reduction in biosolid fragment % is significant, it is likely a consequence of the increased fibre content introduced into the soil through biosolid application. It is widely documented that fibres are the most common MP found within biosolids globally; however, interpreting these patterns is constrained, as microplastics tend to lose their distinct morphologies as particle size decreases.58 Large proportions of synthetic fibres such as polyester, polyacrylamide, and nylon are deposited in biosolids via the laundering of textile materials.59 The fibre content in biosolids is so great that the detectability of synthetic fibres can serve as a reliable indicator of historical biosolid amendment in agricultural soils.60 In addition to fibres, spheres showed a significant increase (p = 0.00477) in biosolid-amended fields. Spheres are a type of primary MP, manufactured to a small size rather than resulting from the breakdown of larger plastic items. Notably, of the nine unamended fields, eight contained no spheres at all. The majority of spheres found in biosolids are derived from microbeads used in personal care products such as exfoliating scrubs, toothpaste, and cosmetics.61 As of 2018, microbeads were classified as a Schedule 1 toxic substance under the Canadian Environmental Protection Act, 1999,62 leading to a ban on their manufacture and use in personal care products. With the exception of Field 15, all biosolid-amended fields received biosolids generated between 2019 and 2021, suggesting that the microbeads detected in these fields are possibly from personal care products. However, over time, it is expected that the abundance of microbeads in newly amended fields will decline as compliance with the ban increases.
While this study found that the majority of MPs in biosolid-amended soils are fibres, there is considerable variation in the literature. For example, Ziajahromi et al.25 examined the MP content in two biosolid-amended fields in Queensland, Australia, where 1 to 3 applications had been made to specific areas, and found that only 15–30% of the MPs were fibres. In contrast, Adhikari et al.48 investigated a biosolid-amended field in Washington, USA that had received 6 applications and identified that greater than 50% of the detected MPs were fibres. Furthermore, a study by Corradini et al.,57 which sampled 31 fields in Mellipillia County, Chile, with 1 to 5 biosolid applications per field, revealed a staggering 97% abundance of fibres. This inconsistency across studies highlights the numerous confounding variables involved in assessing MP concentrations in biosolid-amended soils. Factors such as the type, source, application frequency, and application rate of biosolids, along with variations in extraction methods and potential personal biases, all could potentially contribute to the wide discrepancies observed between studies.
Classification of MP morphology is crucial for the understanding of the risk associated with MPs. Within soil, different MP morphologies have been shown to influence various soil properties, such as aggregate formation, bulk density, aeration, and microbial activity.63 Furthermore, the potential toxicity of MPs to soil biota is closely tied to their surface area.64 MP morphologies with larger surface areas pose a greater risk due to their increased capacity to interact with other pollutants, including metals, organic pollutants, and antibiotics.65
3.3 Microplastic size
Of the 6770 particles categorized in this study, 6759 were analyzed for size, while 11 particles were excluded because they were fibres that were entangled or overlapping, making accurate measurement impossible. Following analysis, it was determined that detected MP sizes ranged from 10.81 to 4909.65 µm, with an average size of 442.88 ± 6.55 µm, and a median size of 240.13 µm. A well-established trend emerged showing an increase in MP frequency as particle size decreased.66,67 Specifically, 57.5% of all MPs identified were smaller than 300 µm (Table S17). This trend was consistent across different MP morphologies, with fragments (76.5%), films (57.8%), foams (74%), and spheres (100%) showing similar patterns (Table S14). However, only 28.0% of fibres were less than 300 µm. Fibres dominated the larger MPs, comprising 89.7% of MPs above 500 µm (Tables S14 and S15). Interestingly, 90.3% of all MPs below 100 µm were considered fragments (Table S15). This is likely due to the challenges associated with visually quantifying particles at smaller sizes, as determining MP morphology becomes increasingly difficult. This often leads to a higher inclination to classify most MPs as fragments. Additionally, identifying MPs smaller than 50 µm proved challenging, which likely contributed to an underestimation of MPs in this size range. Finally, there was no apparent difference in MP size between biosolid-amended and unamended fields (Table S16).The trend of increased agricultural soil MP frequency at smaller sizes is widely reported in the literature.68–71 The degradation of MPs is controlled by factors such as UV radiation, heat, microbial activity, and physical abrasion.42 As these processes break MPs into smaller pieces, their frequency within smaller size classes increases. Research shows that smaller MPs are more likely to undergo both horizontal and vertical movement within soil.33 For example, they are more likely to penetrate deeper into the soil column, raising the risk of entering groundwater.72 In agricultural fields, smaller MPs are more prone to migration during rainfall events, increasing their likelihood of reaching freshwater systems.33 They are also more susceptible to wind, which enhances their potential for long-term transport.73 Additionally, smaller MPs are more likely to be ingested by soil biota, potentially leading to further horizontal or vertical movement through bioturbation, as well as adverse effects on the organisms that ingest them.74
Due to challenges in accurately quantifying very small MPs, a Poisson regression model (Pseudo R2 = 0.93) was applied to estimate the total MPs within the smallest size classes (<25 µm and 26–50 µm) (Fig. 5).
This model was used under the assumption that MP frequency continuously increases as size decreases. The observed values for these size classes were 80 MPs (<25 µm) and 361 MPs (26–50 µm). However, according to the predictive model, the estimated counts were significantly higher, with 7260 MPs expected in the <25 µm range and 1799 MPs in the 26–50 µm range. It is important to note that these predictions represent the total MPs counted across all 20 agricultural fields. Estimates for individual fields would be considerably lower. Nonetheless, this model highlights the potential for error in visually quantifying MPs as the size decreases, suggesting that the actual MP counts in this study are likely much higher than reported.
3.4 Microplastic polymers
A total of 1000 particles were identified using FT-IR microscopy, including 550 from biosolid-amended fields and 450 from non-amended fields. In biosolid-amended fields, 63.1 ± 3.21% of particles were confirmed plastic (specific identifications are below), while 9.60 ± 1.60% were organic matter, 13.5 ± 2.89% were natural or semi-synthetic fibres, 10.0 ± 2.07% were minerals, and 5.78 ± 1.65% were inconclusive. Similarly, in non-amended fields, 58.4 ± 1.79% of particles were confirmed plastic, with 17.1 ± 2.19% classified as organic matter, 8.00 ± 1.20% as natural or semi-synthetic fibres, 10.2 ± 1.71% as minerals, and 6.22 ± 1.08% as inconclusive (Table S18). Additionally, no individual field had a plastic confirmation rate lower than 50%. Difficulties arose during FT-IR analysis as particles had to be large enough to transfer from the sample to a compression cell. Additionally, the Bruker LUMOS II FT-IR microscope used had a sensitivity of ∼100 µm. These two factors made the analysis of particles below 100 µm extremely difficult.The most prominent MPs found in biosolid-amended fields included polyester (25.3%), polyethylene (22.4%), and polypropylene (17.9%) (Tables S22–S24 and Fig. 6). These polymers dominated the composition of microplastics in biosolids, which is consistent with global trends observed in various studies on the MP content of biosolids.23,29,58,75 In contrast, the dominant microplastics in non-amended fields were polypropylene (19.8%), polyester (16.0%), polyvinyl chloride (12.2%), polyethylene (12.2%), and polystyrene (10.6%) (Tables S22–S24 and Fig. 6). The majority of polyvinyl chloride particles were identified in Fields 10–13, all of which are owned and cultivated by the same farmer. This suggests that previous agricultural practices or land use may have contributed to the elevated levels of polyvinyl chloride observed in these fields, making them unique compared to the others. A possible explanation could be the usage of plastic tubing for drainage, as drainage tile is primarily composed of high-density polyethylene or polyvinyl chloride.76 While some overlap with the biosolid-amended fields exists, the relative abundances differ, particularly with a higher prevalence of polyester and polyethylene in biosolid-amended soils. These differences are likely as a result of the high prevalence of polyester and polyethylene within biosolids, further providing evidence that the MPs being applied to soils through biosolids are incorporated into the soil matrix.
3.5 Field characteristics and microplastic retention
Analysis of microplastic (MP) counts in relation to the physical characteristics of each biosolid-amended field revealed that LS-factor (R2 = 0), slope (R2 = 0.004), topographic wetness index (R2 = 0.001), and elevation (R2 = 0.05) were not reliable factors for predicting MP deposition (Fig. 7). The data for this analysis were separated between biosolid-amended and unamended fields, as biosolid application was found to be a strong confounding variable in MP concentration. Similarly, analysis of physical characteristics in unamended fields showed no predictive relationship, with LS-factor (R2 = 0.001), slope (R2 = 0.001), topographic wetness index (R2 = 0.003), and elevation (R2 = 0) also demonstrating no capability for predicting MP deposition (Fig. S1).While these factors were hypothesized to influence MP movement within agricultural soils, the data provide little support for this hypothesis. Some studies suggest that microplastic abundance may be inversely correlated with soil moisture content, and slope, LS-factor, and elevation all play a role in water redistribution.77 Heerey et al.78 examined MP concentrations along slopes within biosolid-amended fields by measuring MP levels at different transects, finding that MP abundance remained relatively unchanged throughout the hill. Additionally, it is essential to note that the movement of MPs within soil is mainly dependent on soil texture.79 Of the 20 fields sampled in this study, 13 are classified as silty loams, while the remaining seven are classified as loams. This subtle difference in soil texture could influence the long-term movement and behavior of MPs within these fields. It is also important to note that 10 of the 11 biosolid-amended fields received their first application within 3 years of sampling, suggesting that these factors may become more reliable predictors over time as MPs have more opportunities to travel within the field.
Although no significant relationships were found between the four factors (elevation, LS-factor, slope, and topographic wetness index) and MP counts, using Latin hypercube sampling remained a valuable approach. This method reduces bias by avoiding over- or undersampling specific areas and ensures that many possible environmental conditions are considered. Additionally, this approach allows for further exploration of other physical characteristics of fields, such as land cover, proximity to water sources, and soil permeability, which may more effectively influence microplastic movement and deposition. In addition, further analysis of factors such as time since amendment and spatially resolved soil chemistry (e.g., organic carbon and cation exchange capacity) may better explain within-field dispersion patterns and provide additional insight into the mechanisms driving MP distribution.
4 Conclusion
This study provides further evidence that biosolid applications significantly increase MP concentrations in agricultural soils. On average, MP levels in biosolid-amended fields were 3.15 times higher than in non-amended fields, with considerable variability observed both within and between fields. The influence of biosolid type was also evident, with dewatered biosolids contributing significantly higher MP loads compared to liquid biosolids, despite differences in application rates. Furthermore, MP morphology in biosolid-amended soils was distinct from that in unamended soils, with fibres dominating in biosolid-treated fields. The high fibre content aligns with previous research, highlighting the role of biosolids as a key pathway for synthetic fibres into the soil. In turn, this led to a shift in the distribution of MP polymers found within biosolid-amended fields, as polyester and polyethylene were more abundant. Additionally, while MPs were detected across a broad size range, smaller MPs were more prevalent, which is consistent with trends observed in other MP studies. Finally, MP retention was unaffected by topographical variation. However, this could change over time. Overall, this study contributes to the growing body of evidence on MP contamination in agricultural soils, reinforcing the impact of biosolids as a primary source of MPs. Future research should focus on long-term monitoring of MP accumulation and the potential ecological effects of MPs in soil ecosystems.Conflicts of interest
There are no conflicts to declare.Data availability
Data for this article are available at the University of Guelph's Research Data Repository at https://doi.org/10.5683/SP3/HEVKKP.Supplementary information (SI) is available. See DOI: https://doi.org/10.1039/d5em00431d.
Acknowledgements
Funding for this project came from the Natural Sciences and Engineering Research Council of Canada's (NSERC) “Plastics Science for a Cleaner Future” grant. Thank you to Mark Janiec for their help in providing agricultural fields. We thank Pamela Kitching, Tyler Black, Jenna Anderson, Karen Lemon, Daniel Stein, and Nicole Wardell for their contributions throughout this project.References
- R. Geyer, J. Jambeck and K. Law, Production, use, and fate of all plastics ever made, Sci. Adv., 2017, 3, e1700782 CrossRef PubMed.
- D. Kawecki and B. Nowack, Polymer-Specific Modeling of the Environmental Emissions of Seven Commodity Plastics As Macro- and Microplastics, Environ. Sci. Technol., 2019, 53(16), 9664–9676 CrossRef CAS.
- K. Zhang, A. H. Hamidian, A. Tubic, Y. Zhang, J. K. H. Fang and C. Wu, et al., Understanding plastic degradation and microplastic formation in the environment: A review, Environ. Pollut., 2021, 274, 116554 CrossRef CAS.
- T. K. A. Tran, S. Raju, A. Singh, K. Senathirajah, G. Bhagwat-Russell and L. Daggubati, et al., Occurrence and distribution of microplastics in long-term biosolid-applied rehabilitation land: An overlooked pathway for microplastic entry into terrestrial ecosystems in Australia, Environ. Pollut., 2023, 336, 122464 CrossRef CAS PubMed.
- J. Crossman, R. R. Hurley, M. Futter and L. Nizzetto, Transfer and transport of microplastics from biosolids to agricultural soils and the wider environment, Sci. Total Environ., 2020, 724, 138334 CrossRef CAS.
- J. J. Guo, X. P. Huang, L. Xiang, Y. Z. Wang, Y. W. Li and H. Li, et al., Source, migration and toxicology of microplastics in soil, Environ. Int., 2020, 137, 105263 CrossRef CAS.
- M. Chen, B. Coleman, L. Gaburici, D. Prezgot, Z. J. Jakubek and B. Sivarajah, et al., Identification of microplastics extracted from field soils amended with municipal biosolids, Sci. Total Environ., 2024, 907, 168007 CrossRef CAS.
- M. Liu, J. Feng, Y. Shen and B. Zhu, Microplastics effects on soil biota are dependent on their properties: A meta-analysis, Soil Biol. Biochem., 2023, 178, 108940 CrossRef CAS.
- U. Surendran, M. Jayakumar, P. Raja, G. Gopinath and P. V. Chellam, Microplastics in terrestrial ecosystem: Sources and migration in soil environment, Chemosphere, 2023, 318, 137946 Search PubMed.
- N. Gao, L. Yang, X. Lu, Z. Duan, L. Zhu and J. Feng, A review of interactions of microplastics and typical pollutants from toxicokinetics and toxicodynamics perspective, J. Hazard. Mater., 2022, 432, 128736 CrossRef CAS PubMed.
- N. D. R. Klemmensen, R. Chand, M. S. Blanco and J. Vollertsen, Microplastic abundance in sludge-treated fields: Variance and estimated half-life, Sci. Total Environ., 2024, 922, 171394 CrossRef CAS PubMed.
- CIEFP, Brief on Biosolids Management in Ontario: Canadian Institute for, EPL, 2009 Search PubMed https://www.cielap.org/pdf/Brief_Biosolids.pdf.
- Y. Liu, G. Liu, J. Zhang, H. Li and J. Wu, Effects of biosolid biochar on crop production and metal accumulation through a rice-wheat rotation system in fields, Environ. Pollut. Bioavailability, 2023, 35(1), 2240016 CrossRef.
- C. A. Kinney and B. V. Heuvel, Translocation of pharmaceuticals and personal care products after land application of biosolids, Curr. Opin. Environ. Sci. Health, 2020, 14, 23–30 Search PubMed.
- R. S. Prosser and P. K. Sibley, Human health risk assessment of pharmaceuticals and personal care products in plant tissue due to biosolids and manure amendments, and wastewater irrigation, Environ. Int., 2015, 75, 223–233 Search PubMed.
- C. F. Chen, Y. R. Ju, Y. C. Lim, S. L. Hsieh, M. L. Tsai and P. P. Sun, et al., Determination of Polycyclic Aromatic Hydrocarbons in Sludge from Water and Wastewater Treatment Plants by GC-MS, Int J Environ Res Public Health, 2019, 16(14), 2604 CrossRef.
- S. B. Gewurtz, A. S. Auyeung, A. O. De Silva, S. Teslic and S. A. Smyth, Per- and polyfluoroalkyl substances (PFAS) in Canadian municipal wastewater and biosolids: Recent patterns and time trends 2009 to 2021, Sci. Total Environ., 2024, 912, 168638 CrossRef PubMed.
- OMAFRA. Nutrient Management Act, 2002. 2022 Search PubMed.
- A. Hooge, K. Syberg and T. Walker, Ecological risk assessment framework for microplastics in agricultural soils amended with biosolids, J. Hazard. Mater. Adv., 2024, 15, 100445 Search PubMed.
- S. Ziajahromi, P. A. Neale, I. Telles Silveira, A. Chua and F. D. L. Leusch, An audit of microplastic abundance throughout three Australian wastewater treatment plants, Chemosphere, 2021, 263, 128294 CrossRef PubMed.
- M. Lares, M. C. Ncibi, M. Sillanpaa and M. Sillanpaa, Occurrence, identification and removal of microplastic particles and fibers in conventional activated sludge process and advanced MBR technology, Water Res., 2018, 133, 236–246 CrossRef PubMed.
- B. Sivarajah, D. R. Lapen, S. B. Gewurtz, S. A. Smyth, J. F. Provencher and J. C. Vermaire, How many microplastic particles are present in Canadian biosolids?, J. Environ. Qual., 2023, 1037–1048 Search PubMed.
- N. V. Letwin, A. W. Gillespie, M. M. Ijzerman, Y. M. Kudla, J. D. Csajaghy and R. S. Prosser, Characterizing the Microplastic Content of Biosolids in Southern Ontario, Canada, Environ. Toxicol. Chem., 2024, 43(4), 793–806 Search PubMed.
- A. S. Reddy and A. T. Nair, The fate of microplastics in wastewater treatment plants: An overview of source and remediation technologies, Environ. Technol. Innovation, 2022, 28, 102815 Search PubMed.
- S. Ziajahromi, H. C. Lu, J. Dwyer, M. Fernandes, M. Griffith and F. D. Leusch, Transport and Accumulation of Microplastics from Biosolids to Australian Agricultural Soils: Detection of Microplastics Down to 1 mum, Environ. Sci. Technol., 2024, 17048–17057 Search PubMed.
- N. Nourozi, T. Massahi, M. Nouri, M. Mardani and H. Hossini, A systematic review of the occurrence of microplastics in compost: Understanding the abundance, sources, characteristics and ecological risk, Results Eng., 2024, 24, 103639 Search PubMed.
- V. Isakov, E. Vlasova, V. Forer, J. Kenny and S. Lyulin, Analysis of Slow-Released Fertilisers as a Source of Microplastics, Land, 2024, 14(1), 38 Search PubMed.
- S. J. Cusworth, W. J. Davies, M. R. McAinsh, A. S. Gregory, J. Storkey and C. J. Stevens, Agricultural fertilisers contribute substantially to microplastic concentrations in UK soils, Commun. Earth Environ., 2024, 5(1), 7 Search PubMed.
- B. N. Naderi, S. Karimifard, J. Gilley, T. Messer, A. Schmidt and S. Bartelt-Hunt, Higher concentrations of microplastics in runoff from biosolid-amended croplands than manure-amended croplands, Commun. Earth Environ., 2023, 4(1), 42 CrossRef.
- H. Walker and J. Aherne, Microplastic fate in a chronosequence of biosolid-amended agricultural soil in Southern Ontario, Canada, Eur. J. Soil Sci., 2024, 75(5), 13592 Search PubMed.
- H. Luo, L. Chang, T. Ju and Y. Li, Factors Influencing the Vertical Migration of Microplastics up and down the Soil Profile, ACS Omega, 2024, 9(51), 50064–50077 Search PubMed.
- M. Sajjad, Q. Huang, S. Khan, M. A. Khan, Y. Liu and J. Wang, et al., Microplastics in the soil environment: A critical review, Environ. Technol. Innovation, 2022, 27, 102408 CrossRef.
- X. Zhang, Y. Chen, X. Li, Y. Zhang, W. Gao and J. Jiang, et al., Size/shape-dependent migration of microplastics in agricultural soil under simulative and natural rainfall, Sci. Total Environ., 2022, 815, 152507 CrossRef.
- Z. Guo, P. Li, X. Yang, Z. Wang, B. Lu and W. Chen, et al., Soil texture is an important factor determining how microplastics affect soil hydraulic characteristics, Environ. Int., 2022, 165, 107293 CrossRef PubMed.
- Climate & Weather Averages in Wellington County, Ontario, Canada: Time and Date AS; 2025 https://www.timeanddate.com/weather/@6177913/climate.
- WFA. Wellington County Emerges as an Agri-Food Powerhouse: Leading the Way in Crop and Livestock Production: Wellington Federation of Agriculture; 2023 https://www.wfofa.on.ca/newsroom/wfa-announcements/228-wellington-county-emerges-as-an-agri-food-powerhouse-leading-the-way-in-crop-and-livestock-production.
- StatsCan. Focus on Geography Series: Wellington (County), Ontario, Statistic Canada, 2022 https://www12.statcan.gc.ca/census-recensement/2021/as-sa/fogs-spg/page.cfm?topic=1%26dguid=2021A00033523%26lang=e Search PubMed.
- B. Minasny and A. B. McBratney, A conditioned Latin hypercube method for sampling in the presence of ancillary information, Comput. Geosci., 2006, 32(9), 1378–1388 CrossRef.
- OMNRF. Ontario Digital Elevation Model, (Imagery-Derived) Ontario Ministry of Natural Resources and Forestry, 2024 https://geohub.lio.gov.on.ca/maps/mnrf::ontario-digital-elevation-model-imagery-derived/explore.
- Y. Qiu, S. Zhou, C. Zhang, L. Chen, W. Qin and Q. Zhang, Vertical distribution and weathering characteristic of microplastics in soil profile of different land use types, Sci. Total Environ., 2023, 905, 166902 CrossRef PubMed.
- W. Cowger, Z. Steinmetz, A. Gray, K. Munno, J. Lynch and H. Hapich, et al., Microplastic Spectral Classification Needs an Open Source Community: Open Specy to the Rescue, Anal. Chem., 2021, 93(21), 7543–7548 CrossRef PubMed.
- C. Campanale, C. Massarelli, I. Savino, V. Locaputo and V. F. Uricchio, A Detailed Review Study on Potential Effects of Microplastics and Additives of Concern on Human Health, Int J Environ Res Public Health, 2020, 17(4), 1212 CrossRef PubMed.
- B. C. O'Kelly, Mechanical properties of dewatered sewage sludge, Waste Manag, 2005, 25(1), 47–52 CrossRef.
- E. E. Dammel and E. D. Schroeder, DENSITY OF ACTIVATED SLUDGE SOLIDS, Water Res., 1991, 25(7), 841–846 CrossRef.
- L. A. Morris and R. F. Lowery, Influence of Site Preparation on Soil Conditions Affecting Stand Establishment and Tree Growth, South. J. Appl. For., 1988, 12(3), 170–178 CrossRef.
- G. R. Saini, Organic Matter as a Measure of Bulk Density of Soil, Nature, 1966, 210, 1295–1296 CrossRef.
- M. Kedzierski, D. Cirederf-Boulant, M. Palazot, M. Yvin and S. Bruzaud, Continents of plastics: An estimate of the stock of microplastics in agricultural soils, Sci. Total Environ., 2023, 880, 163294 CrossRef PubMed.
- K. Adhikari, C. I. Pearce, K. A. Sanguinet, A. I. Bary, I. Chowdhury and I. Eggleston, et al., Accumulation of microplastics in soil after long-term application of biosolids and atmospheric deposition, Sci. Total Environ., 2024, 912, 168883 CrossRef PubMed.
- F. Guo, B. Liu, J. Zhao, Y. Hou, J. Wu and H. Hu, et al., Temperature-dependent effects of microplastics on sediment bacteriome and metabolome, Chemosphere, 2024, 350, 141190 CrossRef PubMed.
- F. M. Ivanic, G. Guggenberger, S. K. Woche, J. Bachmann, M. Hoppe and J. F. Carstens, Soil organic matter facilitates the transport of microplastic by reducing surface hydrophobicity, Colloids Surf., A, 2023, 676, 132255 CrossRef.
- M. Rani, S. Ducoli, L. E. Depero, M. Prica, A. Tubic and Z. Ademovic, et al., A Complete Guide to Extraction Methods of Microplastics from Complex Environmental Matrices, Molecules, 2023, 28(15), 5710 CrossRef PubMed.
- A. A. Keller, W. Li, Y. Floyd, J. Bae, K. M. Clemens and E. Thomas, et al., Elimination of microplastics, PFAS, and PPCPs from biosolids via pyrolysis to produce biochar: Feasibility and techno-economic analysis, Sci. Total Environ., 2024, 947, 174773 Search PubMed.
- A. Prajapati, A. Narayan Vaidya and A. R. Kumar, Microplastic properties and their interaction with hydrophobic organic contaminants: a review, Environ Sci Pollut Res Int, 2022, 29(33), 49490–49512 CrossRef.
- Q. Le and G. W. Price, A review of the influence of heat drying, alkaline treatment, and composting on biosolids characteristics and their impacts on nitrogen dynamics in biosolids-amended soils, Waste Manag, 2024, 176, 85–104 CrossRef.
- S. D. Chowdbury, R. Bandyopadhyay and P. Bhunia, Reutilization of sludge as fertilizer: Wastewater Treatment Plants as Biorefineries, Clean Energy and Resource Recovery, 2022, 2, 423–434 Search PubMed.
- D. Harley-Nyang, F. A. Memon, N. Jones and T. Galloway, Investigation and analysis of microplastics in sewage sludge and biosolids: A case study from one wastewater treatment works in the UK, Sci. Total Environ., 2022, 823, 153735 CrossRef PubMed.
- F. Corradini, P. Meza, R. Eguiluz, F. Casado, E. Huerta-Lwanga and V. Geissen, Evidence of microplastic accumulation in agricultural soils from sewage sludge disposal, Sci. Total Environ., 2019, 671, 411–420 CrossRef PubMed.
- D. Harley-Nyang, F. A. Memon, A. Osorio Baquero and T. Galloway, Variation in microplastic concentration, characteristics and distribution in sewage sludge & biosolids around the world, Sci. Total Environ., 2023, 891, 164068 CrossRef PubMed.
- I. E. Napper and R. C. Thompson, Release of synthetic microplastic plastic fibres from domestic washing machines: Effects of fabric type and washing conditions, Mar. Pollut. Bull., 2016, 112(1–2), 39–45 CrossRef PubMed.
- K. A. Zubris and B. K. Richards, Synthetic fibers as an indicator of land application of sludge, Environ. Pollut., 2005, 138(2), 201–211 CrossRef.
- C. M. Rochman, S. M. Kross, J. B. Armstrong, M. T. Bogan, E. S. Darling and S. J. Green, et al., Scientific Evidence Supports a Ban on Microbeads, Environ. Sci. Technol., 2015, 49(18), 10759–10761 CrossRef PubMed.
- ECCC. Microbeads in Toiletries Regulations. 2017 Search PubMed.
- Y. M. Lozano, T. Lehnert, L. T. Linck, A. Lehmann and M. C. Rillig, Microplastic Shape, Polymer Type, and Concentration Affect Soil Properties and Plant Biomass, Front. Plant Sci., 2021, 12, 616645 CrossRef.
- N. Bostan, N. Ilyas, N. Akhtar, S. Mehmood, R. U. Saman and R. Z. Sayyed, et al., Toxicity assessment of microplastic (MPs); a threat to the ecosystem, Environ. Res., 2023, 234, 116523 CrossRef CAS.
- N. Rafa, B. Ahmed, F. Zohora, J. Bakya, S. Ahmed and S. F. Ahmed, et al., Microplastics as carriers of toxic pollutants: Source, transport, and toxicological effects, Environ. Pollut., 2024, 343, 123190 Search PubMed.
- A. M. Mahon, B. O'Connell, M. G. Healy, I. O'Connor, R. Officer and R. Nash, et al., Microplastics in Sewage Sludge: Effects of Treatment, Environ. Sci. Technol., 2017, 51(2), 810–818 CrossRef CAS PubMed.
- W. Zhao, J. Li, M. Liu, R. Wang, B. Zhang and X. Z. Meng, et al., Seasonal variations of microplastics in surface water and sediment in an inland river drinking water source in southern China, Sci. Total Environ., 2024, 908, 168241 CrossRef CAS PubMed.
- J. Zhang, G. Zou, X. Wang, W. Ding, L. Xu and B. Liu, et al., Exploring the Occurrence Characteristics of Microplastics in Typical Maize Farmland Soils With Long-Term Plastic Film Mulching in Northern China, Front. Mar. Sci., 2021, 8, 800087 CrossRef.
- P. He, L. Chen, L. Shao, H. Zhang and F. Lu, Municipal solid waste (MSW) landfill: A source of microplastics? -Evidence of microplastics in landfill leachate, Water Res., 2019, 159, 38–45 CrossRef CAS.
- G. S. Zhang and Y. F. Liu, The distribution of microplastics in soil aggregate fractions in southwestern China, Sci. Total Environ., 2018, 642, 12–20 CrossRef CAS PubMed.
- F. Büks and M. Kaupenjohann, Global concentrations of microplastics in soils – a review, Soil, 2020, 6(2), 649–662 CrossRef.
- A. Ameen, M. E. Stevenson, A. K. T. Kirschner, S. Jakwerth, J. Derx and A. P. Blaschke, Fate and transport of fragmented and spherical microplastics in saturated gravel and quartz sand, J. Environ. Qual., 2024, 53(5), 727–742 CrossRef CAS PubMed.
- S. Illuminati, V. Notarstefano, C. Tinari, M. Fanelli, F. Girolametti and B. Ajdini, et al., Microplastics in bulk atmospheric deposition along the coastal region of Victoria Land, Antarctica, Sci. Total Environ., 2024, 949, 175221 CrossRef CAS PubMed.
- D. Kim, S. A. Kim, S. H. Nam, J. I. Kwak, L. Kim and T. Y. Lee, et al., Microplastic ingestion in aquatic and soil biota: A comprehensive review of laboratory studies on edible size and intake pattern, Mar. Pollut. Bull., 2024, 200, 116056 CrossRef CAS PubMed.
- Q. Li, J. Wu, X. Zhao, X. Gu and R. Ji, Separation and identification of microplastics from soil and sewage sludge, Environ. Pollut., 2019, 254(Pt B), 113076 CrossRef CAS PubMed.
- R. K. Gupta, I. P. Abrol, C. W. Finkl, M. B. Kirkham, M. C. Arbestain and F. Macías, et al., Soil drainage, ed Chesworth W., in Encyclopedia of Soil Science. Dordrecht, Springer Netherlands, 2008. pp. 643–646 Search PubMed.
- E. Tiwari and S. Sistla, Agricultural plastic pollution reduces soil function even under best management practices, PNAS Nexus, 2024, 3(10), 433 CrossRef PubMed.
- L. Heerey, J. J. O'Sullivan, M. Bruen, J. Turner, A. M. Mahon and S. Murphy, et al., Export pathways of biosolid derived microplastics in soil systems - Findings from a temperate maritime climate, Sci. Total Environ., 2023, 888, 164028 CrossRef CAS PubMed.
- R. Rehm, T. Zeyer, A. Schmidt and P. Fiener, Soil erosion as transport pathway of microplastic from agriculture soils to aquatic ecosystems, Sci. Total Environ., 2021, 795, 148774 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2026 |