Unsaturated coordination-regulated high-spin nickel sites for selective solar-driven carbon dioxide conversion in pure water
Guixiang
Ding
a,
Zhaoqiang
Wang
a,
Zihe
Chen
a,
Yin
Xiao
a,
Xin
Liu
a,
Li
Shuai
a,
Lihui
Chen
 *a,
Hongwei
Huang
*a,
Hongwei
Huang
 *b and
Guangfu
Liao
*b and
Guangfu
Liao
 *a
*a
aNational Forestry and Grassland Administration Key Laboratory of Plant Fiber Functional Materials, College of Materials Engineering, Fujian Agriculture and Forestry University, Fuzhou 350002, China. E-mail: lihuichen@fafu.edu.cn; liaogf@fafu.edu.cn
bBeijing Key Laboratory of Materials Utilization of Nonmetallic Minerals and Solid Wastes, National Laboratory of Mineral Materials, School of Materials Science and Technology, China University of Geosciences, Beijing, 100083, P. R. China. E-mail: hhw@cugb.edu.cn
First published on 5th December 2025
Abstract
Achieving efficient photocatalytic carbon dioxide (CO2) reduction is crucial for sustainable energy and carbon neutrality. However, a fundamental challenge resides in the rational design and fine-tuning of catalyst active sites. Here, we construct edge-rich nickel–aluminum layered double hydroxide (ED-NiAl-LDH) nanoflakes with abundant lattice O defects for effective and selective solar-driven CO2 conversion in a pure water system. The ED-NiAl-LDH exhibits an excellent carbon monoxide (CO) production rate of 773.4 µmol g−1 h−1 with a high selectivity of 98.5%, surpassing that of state-of-the-art photocatalysts reported in recent years. Outdoor tests also demonstrate an impressive CO2-to-CO photo-conversion rate of 500 µmol g−1 h−1, with stable activity over an 80-hour period. In situ characterization methods and theoretical calculations confirm that the edge-rich structure provides abundant unsaturated coordination-regulated high-spin Ni active sites. The high-spin Ni active sites possess half-filled degenerate eg orbitals in the octahedral field, which significantly accelerates the migration of photogenerated electrons from Ni to CO2 molecules while inhibiting other competitive reactions, thereby enabling the observed exceptional performance. This work establishes edge engineering as a general strategy to unlock high-spin catalytic centers in LDHs, advancing the design of efficient solar fuel systems.
Broader contextUnsustainable fossil fuel consumption results in steadily rising atmospheric carbon dioxide (CO2) concentrations, contributing critically to global energy scarcity and climate change impacts. Efficient photocatalytic CO2 reduction is paramount for sustainability, yet remains a formidable challenge due to stringent catalyst requirements. This work constructs edge-rich nickel–aluminum layered double hydroxide (ED-NiAl-LDH) nanoflakes with abundant lattice oxygen defects for highly effective solar-driven CO2 conversion in pure water. The ED-NiAl-LDH catalyst demonstrates exceptional performance, achieving a remarkable carbon monoxide (CO) production rate of 773.4 µmol g−1 h−1 with 98.5% selectivity, surpassing recent benchmarks. Outdoor testing confirms practical viability, yielding a CO2-to-CO conversion rate of 500 µmol g−1 h−1 while maintaining stable activity over 80 hours. Mechanistic studies reveal that the engineered edge-rich structure creates numerous unsaturated high-spin Ni active sites. These unsaturated high-spin Ni sites possess half-filled degenerate eg orbitals within the octahedral crystal field. This unique electronic configuration critically accelerates the transfer of photogenerated electrons from the Ni sites to adsorbed CO2 molecules. Simultaneously, it suppresses competing side reactions, enabling both the high activity and selectivity observed. This work establishes edge engineering as a powerful strategy to unlock highly active high-spin metal active centers within LDHs. |
Introduction
Excessive consumption of fossil fuels leads to a persistent rise in carbon dioxide (CO2) concentrations in the atmosphere, which has caused global energy depletion and climate change.1,2 In this context, photocatalytic CO2 reduction stands out as a promising artificial photosynthesis technology, as it directly converts CO2 and H2O into valuable chemicals using only solar light as the energy input, without the need for an external electrical bias or a complex electrochemical cell.3 Among various reduction pathways, selective photocatalytic reduction of CO2 to carbon monoxide (CO) holds significant promise for sustainable fuel synthesis, as CO serves as a key feedstock for Fischer–Tropsch processes and methanol production.4,5 Achieving high CO selectivity (>90%) remains challenging due to competitive pathways toward methane and multi-carbon product formation.6–8 Critical to CO selectivity is the ability of a photocatalyst to stabilize the CO intermediates (*COOH/*CO2)9 while facilitating CO desorption.10 Consequently, engineering catalysts with tailored adsorption sites11 for preferential CO stabilization is essential to suppress over-reduction and enhance CO yield.The practical application of photocatalytic CO2 reduction is impeded by several fundamental challenges, including inefficient charge dynamics, limited electron transfer efficiency, insufficient active site density, and poor solar-to-chemical energy conversion.12,13 These limitations arise from rapid recombination of photogenerated electron–hole pairs, the weak interaction between catalysts and CO2 molecules, and the limited accessibility of active sites.14 To address these issues, engineering catalysts with tailored adsorption sites has emerged as an important strategy.15 For instance, heterojunction design, defect engineering, structure engineering, and interface modification have been widely explored to improve charge transfer kinetics and adsorption selectivity.16–18 Layered double hydroxides (LDHs) are a class of two-dimensional (2D) materials characterized by alternately stacked metal cation and exchangeable anion layers, which endow them with superior structural tunability and functionalization potential. In addition, the abundant hydroxyl groups (–OH) on the LDH layers enhance the CO2 adsorption, while their structural tunability and functionalization versatility improve their light-harvesting capability. Consequently, LDHs exhibit great potential for application in photocatalytic CO2 reduction.19,20 However, their inherent layered stacking structure often encapsulates metal active sites within interlayers, creating a physical barrier that hinders direct interaction with CO2 molecules and their reaction intermediates. This structural limitation reduces the accessibility of active sites and impedes efficient electron transfer.21 Therefore, rational design of LDH architectures, such as expanding interlayer spacing through anion exchange,22–24 exposing edge sites via exfoliation or etching, or hybridizing with conductive substrates,25,26 is critical to fully exploit their catalytic potential.
Herein, we synthesized edge-rich NiAl-LDH (ED-NiAl-LDH) nanoflakes with abundant lattice O defects, enabling the exposure of a large number of high-spin-state unsaturated-coordinated Ni active sites, thereby significantly enhancing the CO2-to-CO activity and selectivity. State-of-the-art characterization methods and theoretical calculations demonstrated that high-spin-state unsaturated-coordinated Ni active sites lowered the energy barrier for CO2 reduction while facilitating efficient charge transfer dynamics. Furthermore, the increased exposure of Ni active sites significantly enhanced the photocatalytic activity and selectivity. As a result, the solar-driven CO2-to-CO activity was significantly improved, achieving laboratory and outdoor CO production rates of 773.4 µmol g−1 h−1 and 500 µmol g−1 h−1, respectively. Moreover, the CO2-to-CO selectivity reached an impressive 98.5%, surpassing that of state-of-the-art photocatalysts reported in recent years. Edge engineering of NiAl-LDH produces abundant exposed metal sites, providing an effective strategy to activate high-spin Ni catalytic centers for highly efficient and selective solar-driven CO2-to-CO conversion.
Experimental section
Synthesis of NiAl-LDH
0.38 g of Al(NO3)3 9H2O and 0.87 g of Ni(NO3)2 6H2O were dissolved in 60 mL of pure H2O to obtain the NiAl-LDH precursor solution. Afterward, 0.296 g of urea and 1.2012 g of NH4F were dissolved in the mixed solution and stirred for another 0.5 h. The resulting solution was transferred into a 100 mL sealed Teflon-lined autoclave and hydrothermally treated at 120 °C for 24 h. The product was centrifuged, filtered, rinsed in water and ethanol several times, and dried in an oven at 80 °C for 24 h to obtain the green powder of NiAl-LDH.Synthesis of E-NiAl-LDH
1.50 g of Al(NO3)3 9H2O and 1.74 g of Ni(NO3)2 6H2O were dissolved in a mixture of 30 mL of distilled water and 30 mL of methanol to prepare the edge-rich NiAl-LDH (E-NiAl-LDH) nanoflake precursor solution. Subsequently, 1.892 g of urea was added to the solution and stirred for an additional 0.5 h. The mixture was then transferred to a 100 mL Teflon-lined autoclave and subjected to hydrothermal treatment at 150 °C for 12 h. After treatment, the product was separated by centrifugation, washed multiple times with water and ethanol, and finally dried at 80 °C for 24 h to yield the green E-NiAl-LDH powder.Synthesis of ED-NiAl-LDH
The as-synthesized E-NiAl-LDH colloid was dispersed in a buffer solution at pH 5.0–6.0 for 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) h. The resultant ED-NiAl-LDH was then collected by centrifugation (6000 × g, 5
h. The resultant ED-NiAl-LDH was then collected by centrifugation (6000 × g, 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) min) and redispersed in cooled deionized water.
min) and redispersed in cooled deionized water.
Photocatalytic CO2 reduction
5 mg of the powder sample was uniformly dispersed on the hydrophilic microporous filter membrane, which floated on the water surface (Fig. S8). The solar-driven CO2 reduction reaction was performed at 0.1 MPa in a sealed reactor, which had been previously purged with high-purity CO2 (99.999%) via continuous bubbling to ensure complete gas exchange. The reaction mixture was irradiated using a 300 W xenon lamp (Microsolar300, Beijing Perfectlight) as the light source. The full spectrum located in the UV-visible light region (325–2000 nm). The C-containing gases produced were analyzed using a gas chromatography analyzer (GC-9790II (PLF-01)) equipped with flame ionization detectors (FIDs), and H2 was detected using a thermal conductivity detector (TSD). The selectivity of CO calculation follows the equation:| Selectivity (CO) = n(CO)/(n(CO) + n(H2) + n(CH4)) × 100% |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 mL reactor under solar irradiation. 350 mg of the powder sample was uniformly dispersed on the hydrophilic microporous filter membrane, which floated on the water surface (Fig. S13). The solar-driven CO2 reduction reaction was performed under 0.1 MPa of a certain CO2 (99.999%) atmosphere. Stability testing was assessed through an 80-hour outdoor experiment. The test was conducted over 10 days, from 9
500 mL reactor under solar irradiation. 350 mg of the powder sample was uniformly dispersed on the hydrophilic microporous filter membrane, which floated on the water surface (Fig. S13). The solar-driven CO2 reduction reaction was performed under 0.1 MPa of a certain CO2 (99.999%) atmosphere. Stability testing was assessed through an 80-hour outdoor experiment. The test was conducted over 10 days, from 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 00 to 17
00 to 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 00 each day, totaling 8 h per day. The apparent quantum yield (AQY) was measured under a 300 W xenon lamp (Microsolar300, Beijing Perfectlight Technology, China) with narrowband filters of 365, 420, 450, 500, 550 and 650 nm; the AQY was calculated using the following equation:27–29
00 each day, totaling 8 h per day. The apparent quantum yield (AQY) was measured under a 300 W xenon lamp (Microsolar300, Beijing Perfectlight Technology, China) with narrowband filters of 365, 420, 450, 500, 550 and 650 nm; the AQY was calculated using the following equation:27–29| AQY = (number of reacted electrons/number of incident photons) × 100% |
The 13C isotopic labeling experiment was also carried out in pure water and under a high-purity 13CO2 atmosphere. A 300 W Xe lamp was used as the visible light source. After irradiation, gas products were analyzed using gas chromatography mass spectrometry with an HP-MOLESIEVE column (30 m × 0.32 mm × 25 µm, Agilent Technologies, USA) to detect 13CO and 13CH4.
Results and discussion
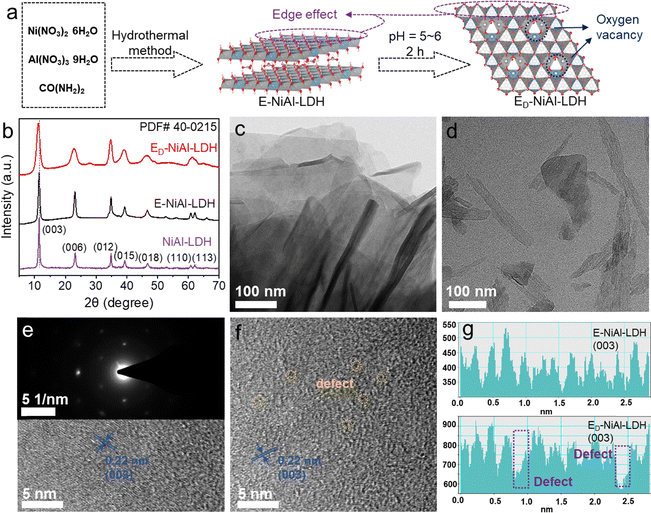
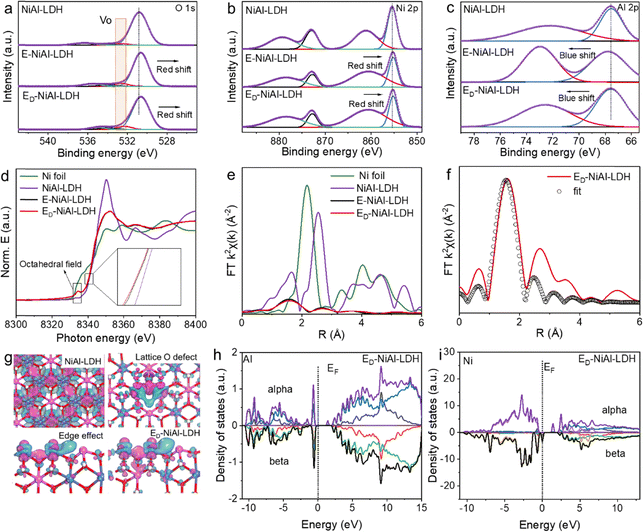
The NiAl-LDH and E-NiAl-LDH were prepared using a straightforward hydrothermal method. ED-NiAl-LDH was prepared via a facile acid etching method by adding E-NiAl-LDH to buffer solution with pH = 5–6 (Fig. 1(a)). As presented in the X-ray diffraction (XRD) patterns (Fig. 1(b)), typical characteristic peaks of all samples closely matched the standard pattern of NiAl-LDH (PDF# 40-0215). E-NiAl-LDH and ED-NiAl-LDH showed a stronger (0 0 6) crystal plane peak than NiAl-LDH, indicating a higher exposure of the (0 0 6) plane and a pronounced edge effect on the surface properties. In addition, the characteristic peak for the (0 0 3) crystal plane in the XRD pattern of ED-NiAl-LDH shifted to the left, which indicated the expanded (0 0 3) crystal plane.30 Transmission electron microscopy (TEM) (Fig. 1(c), (d) and Fig. S1) and high-resolution TEM (HR-TEM) (Fig. 1(e) and (f)) showed the urchin-like NiAl-LDH with a size of approximately 2 µm and E-NiAl-LDH and ED-NiAl-LDH were both edge-rich nanoflake structures, which suggested that the edge-to-bulk ratio of the E-NiAl-LDH and ED-NiAl-LDH increased. The lattice fringes of NiAl-LDH, E-NiAl-LDH, and ED-NiAl-LDH corresponded to the (0 0 3) crystal plane with 0.22 nm spacing, and the selected-area electron diffraction (SAED) pattern of E-NiAl-LDH confirmed the hexagonal closest packing structure, which indicated that E-NiAl-LDH and NiAl-LDH shared the same crystalline structure. In addition, the line profile along the white line for the E-NiAl-LDH and ED-NiAl-LDH (Fig. 1(g)) suggested that the defect was introduced into the (0 0 3) crystal plane of ED-NiAl-LDH via the acid etching method. In a word, structural characterization suggested the successful synthesis of ED-NiAl-LDH with a high edge-to-bulk ratio structure and oxygen vacancy.To elucidate the changes in the surface chemical state of ED-NiAl-LDH, X-ray photoelectron spectroscopy (XPS) was employed. The position of peaks in the samples should be calibrated using the C 1s peak, which has a binding energy of 284.8 eV. The diffraction peaks of O, Ni, and Al could be observed in the XPS spectra of NiAl-LDH, E-NiAl-LDH, and ED-NiAl-LDH (Fig. S2). In the high-resolution spectrum of O 1s of NiAl-LDH (Fig. 2(a)), the peaks at 530.8, 532.5, 534.1, and 536.4 eV corresponded to oxygen species with the Ni and Al metal–oxygen (M–O) bond, O defects, hydroxyl group (–OH), and adsorbed water states, respectively. The Ni 2p XPS analysis of NiAl-LDH (Fig. 2(b)) showed Ni 2p1/2 and Ni 2p3/2 at 873.0 and 855.4 eV, respectively, indicating that Ni ions existed as Ni–OH in the structure. Compared with NiAl-LDH, the peaks of O 1s and Ni 2p for E-NiAl-LDH and ED-NiAl-LDH displayed redshifts. The red shift suggested the reduction in the oxidation state of the Ni element, attributed to the unsaturated coordination around the Ni2+ center. In addition, the Al 2p XPS spectrum of NiAl-LDH at 67.5 and 72.6 eV (Fig. 2(c)) clarified that Al ions in all the samples were in the trivalent oxidation state. The peaks of Al 2p for E-NiAl-LDH and ED-NiAl-LDH showed slight blue shifts, indicating an increase in the oxidation state of the Al element, which could improve the stability of the E-NiAl-LDH and ED-NiAl-LDH framework.31
The chemical structures of NiAl-LDH, E-NiAl-LDH, and ED-NiAl-LDH were further investigated using the X-ray absorption fine structure (XAFS) technique. The normalized Ni K-edge X-ray absorption near-edge structure (XANES) for ED-NiAl-LDH exhibited a pre-edge peak at 8333 eV and similar absorption edge features to those of Ni-foil (Fig. 2(d)). The pre-edge peaks of E-NiAl-LDH (∼8336.042 eV) and ED-NiAl-LDH (∼8335.794 eV) were between those of Ni foil and NiAl-LDH, revealing a valence between 0 and +2; the lower photon energy also verified the lower Ni oxidation state in the ED-NiAl-LDH samples.32 In addition, the octahedral structures and the coordination environment of the 3d transition metal (Ni) have been studied using extended X-ray absorption fine structure (EXAFS) as well as XANES. Our analyses suggested that Ni ions in NiAl-LDH coordinated with six O atoms, which was in accordance with the previous crystallographic study. E-NiAl-LDH and ED-NiAl-LDH maintained the original octahedral field coordination according to the XANES pattern. The prominent peak for the Ni–O shell was found at 1.65, 1.59 and 1.56 Å for NiAl-LDH, E-NiAl-LDH, and ED-NiAl-LDH, respectively (Fig. 2(e) and Fig. S3). The shorter Ni–O distance was relevant to the unsaturated coordination of Ni atoms, which led to a strong interaction between the central atom and its ligands. The fitted coordination numbers (CNs) of O and Ni were 5.65 and 5.17 for E-NiAl-LDH and ED-NiAl-LDH, respectively (Fig. 2(f), Fig. S4 and Table S1). Therefore, the higher edge-to-bulk ratio corresponded to lower CNs. Theoretical calculation was carried out to further reveal the configuration of electrons in NiAl-LDH, E-NiAl-LDH and ED-NiAl-LDH samples. Electron density difference images of Ni atoms in NiAl-LDH, O defects, and edges were obtained (Fig. 2(g)). The electron cloud density around unsaturated Ni atoms was significantly increased compared to that around Ni atoms in NiAl-LDH, and the high-binding-energy Al atoms play a crucial role in the distribution of the electron cloud, which facilitated electron delocalization around unsaturated Ni atoms. In addition, calculated density of states (DOS) patterns of Ni and Al atoms for NiAl-LDH, E-NiAl-LDH, and ED-NiAl-LDH (Fig. 2(h), (i) and Fig. S5) suggested that the 3d orbitals of Ni atoms contained half-filled spin-up electrons. In the octahedral field, O atoms acted as a weak ligand for Ni, which meant the degenerate eg orbitals of Ni host two half-filled high-spin-up state electrons, thereby providing a favorable pathway for migration of photogenerated electrons. Moreover, electrons in the 3d orbital of unsaturated coordination high-spin Ni active sites were distributed on the Fermi level, which also improved the adsorption of CO2. Consequently, O defects and the edge effect provided abundant unsaturated coordination high-spin Ni active sites. The complex electron configuration in the 3d orbital was the pivotal factor for effective and selective solar-driven CO2 conversion.
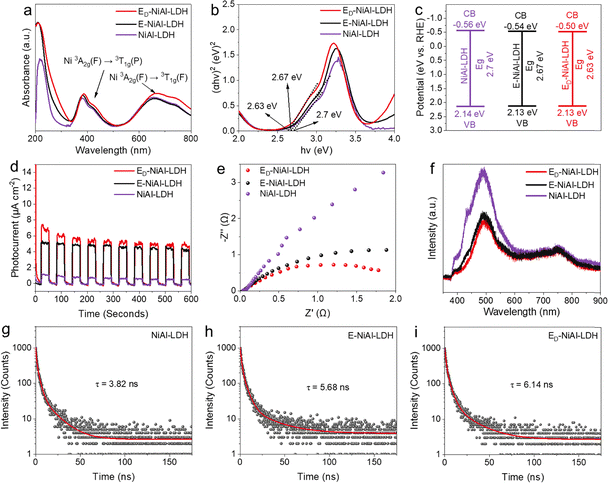
To investigate the optoelectronic characteristics, analyses including UV-visible spectroscopy, electrochemical impedance spectroscopy (EIS), photocurrent–time tests and photoluminescence (PL) spectroscopy were performed. UV-visible spectra of NiAl-LDH, E-NiAl-LDH, and ED-NiAl-LDH were obtained to assess their light absorption abilities for subsequent photocatalytic studies (Fig. 3(a)). The 3A2g(F) → 3T1g(P) transition gives rise to an absorption band at ∼390 nm. The absorption band at 645–710 nm, obtained as a result of the spin–orbit coupling, corresponds to the 3A2g(F) → 3T1g(F) transition. The band gap energy (Eg) of the prepared catalysts was estimated based on the equation of a direct semiconductor αhν = A(hν − Eg)1/2 (Fig. 3(b) and Fig. S7). As shown in the Tauc diagram, the band gaps of NiAl-LDH, E-NiAl-LDH, and ED-NiAl-LDH were 2.70, 2.67 and 2.63 eV, respectively. The valence band (VB) potential of NiAl-LDH, E-NiAl-LDH, and ED-NiAl-LDH was obtained through Mott–Schottky (M–S) plots with values of 2.04, 2.03 and 2.03eV, respectively (Fig. S6). Based on the Eg value and VB edge potential, the conduction band (CB) edge potentials were obtained using the formula Eg = EVB − ECB, and the band structure alignments of NiAl-LDH, E-NiAl-LDH and ED-NiAl-LDH were established (Fig. 3(c)). Therefore, unsaturated coordination high-spin Ni enhanced the light absorption of ED-NiAl-LDH and played an important role in modulating the band structure. The photocurrent was consistently higher in ED-NiAl-LDH than in NiAl-LDH or E-NiAl-LDH (Fig. 3(d)), which indicated the better light responsiveness of ED-NiAl-LDH under light illumination. The EIS data shown in Fig. 3(e) demonstrated that ED-NiAl-LDH markedly reduced charge-transfer resistance in comparison with NiAl-LDH and E-NiAl-LDH, facilitating the movement of photogenerated electrons. The enhanced conductivity was ascribed to the exposure of unsaturated coordinated high-spin Ni sites caused by O defects and the edge effect. The photoluminescence (PL) results of ED-NiAl-LDH showcased the lowest intensity, further suggesting the most efficient separation of photogenerated electron–hole pairs (Fig. 3(f)). According to time-resolved fluorescence decay spectroscopy (Fig. 3(g)–(i) and Table S2), the average emission lifetimes of NiAl-LDH, E-NiAl-LDH, and ED-NiAl-LDH were 3.82, 5.68, and 6.14 ns, respectively. Among these materials, the photogenerated electrons in ED-NiAl-LDH had the longest lifetime, which demonstrated that the unsaturated coordinated high-spin Ni sites suppressed the recombination of photogenerated electron–hole pairs.
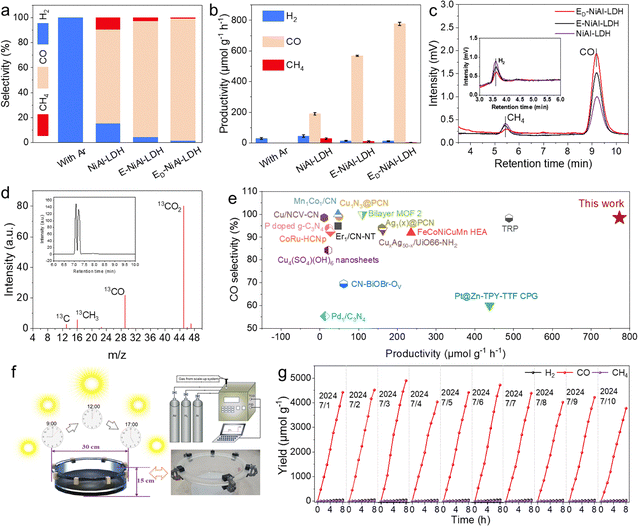
The performance of solar-driven CO2 reduction in a pure water and CO2 atmosphere tri-phase system under 300 W Xe lamp irradiation was subsequently evaluated (Fig. S8 and S9). As shown in Fig. S10, the photocatalytic activity of converting CO2 to CO increases with the rise in catalyst dosage. The catalytic activity reaches an optimal value when the catalyst dosage is 5 mg. The control experiments were carried out in pure water and under an Ar atmosphere, and no C-containing products were detected, which indicated that CO2 was the sole carbon source for the solar-driven conversion to C-containing products (Fig. S12a). As shown in Fig. 4(a), the CO2-to-CO selectivity evidently improved from 75.2% for NiAl-LDH to an unprecedented 98.5% for ED-NiAl-LDH, while the selectivity of by-product H2 was suppressed from 15.5% for NiAl-LDH to 1.2% for ED-NiAl-LDH. Moreover, electron paramagnetic resonance (EPR) analysis revealed that ED-NiAl-LDH exhibits the highest g-value, suggesting the greatest exposure of high-spin Ni sites (Fig. S14), which indicates that the exposure of unsaturated Ni sites increases the selectivity for photocatalytic reduction of CO2 to CO. An impressive solar-driven CO2-to-CO activity of 773.4 µmol g−1 h−1 was observed for ED-NiAl-LDH, which was 4 times higher than that of NiAl-LDH (Fig. 4(b), (c) and Fig. S12b–d). The oxidation products primarily comprised O2 with a small amount of H2O2, and no C2 products were detected (Fig. S13 and S15). Moreover, photocatalytic CO2 reduction at a single wavelength was investigated to further confirm the excellent photocatalytic activity of ED-NiAl-LDH by calculating the AQY at 365, 420, 450, 500, 550, 600 and 650 nm. The ED-NiAl-LDH sample possesses an apparent quantum yield of 0.08% at 365 nm and even 0.17% at a higher wavelength (420 nm) (Fig. S17a).33 The photocurrent responses under different monochromatic wavelengths indicated irradiation at 365, 420, and 450 nm generated significantly higher photocurrent. Therefore, higher-energy light is more effective in generating high-kinetic electrons, which contribute to improved photocatalytic CO2 reduction (Fig. S17b). Furthermore, isotopically labeled 13CO2 experiments were also carried out by gas chromatography-mass spectrometry (GC-MS). As shown in Fig. 4(d) and Fig. S16, the ion fragmentation peaks corresponding to 13CO and 13CO2 are significantly detected, verifying that the generated CO and hydrocarbons on ED-NiAl-LDH are indeed derived from the reduction of CO2. Additionally, in comparison with recently reported state-of-the-art photocatalysts (Fig. 4(e) and Tables S4, S5), the ED-NiAl-LDH maintained an impressive CO production rate and selectivity.30,31,34–47 To evaluate the practical solar-driven CO2-to-CO performance, a scale-up experiment was carried out under genuine sunlight. As shown in Fig. 4(f), (g), Fig. S18 and Table S3, the ED-NiAl-LDH exhibited a stable solar-driven CO2-to-CO activity of approximately 500 µmol g−1 h−1 within 80 hours. Besides, there was no significant phase change in the catalyst structure before and after 80-hours of reaction (Fig. S19 and S20). Thus, the customized ED-NiAl-LDH displayed ultra-high photocatalytic activity, selectivity, and stability for CO production under both laboratory and outdoor conditions, showcasing its great potential for sustainable and scalable solar-driven CO2 reduction towards specific products.
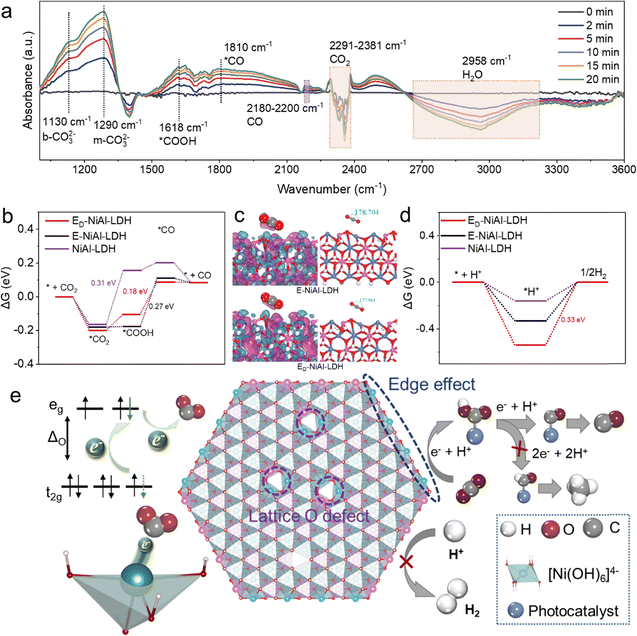
In situ diffuse reflectance infrared Fourier transform spectroscopy (in situ-DRIFTS) was carried out to investigate the pathways of CO2 activation on ED-NiAl-LDH. ED-NiAl-LDH was exposed to a CO2/H2O gas mixture and illuminated by light for 20 min, during which the DRIFTS spectra were recorded at 0, 2, 5, 10, 15, and 20 min. The evolution of surface species under continuous irradiation is shown in Fig. 5(a). The absorption peak between 2291 and 2381 cm−1 suggested the consumption of CO2. Furthermore, the peak at 1130 cm−1 was attributed to the bi-dentate bicarbonate (b-CO32−), and the peak at 1290 cm−1 belonged to mono-dentate bicarbonate (m-CO32−), all originating from CO2 adsorption, highlighting the good CO2 uptake ability. The peak around 1618 cm−1 was attributed to carboxylate species (*COOH), which was the key intermediate for CO products and originated the protonation step of *CO2. Noteworthily, the main product CO (2180–2200 cm−1) was also observed during solar-driven CO2 reduction. Thus, the possible pathways for solar-driven conversion of CO2 to CO using ED-NiAl-LDH were speculated as shown in Fig. S22–S26: CO2 → *COOH → *CO → CO. To further elucidate the plausible pathways for the highly selective conversion of CO2 to CO, the Gibbs free energy diagram for the adsorption and reduction of CO2 on NiAl-LDH, E-NiAl-LDH, and ED-NiAl-LDH surfaces was obtained and is shown in Fig. 5(b). The rate-determining step for NiAl-LDH was the protonation of *CO2 to *COOH, with an energy barrier of 0.31 eV, while for E-NiAl-LDH and ED-NiAl-LDH, it was the protonation and dehydration of *COOH to CO, with energy barriers of 0.27 eV and 0.18 eV, respectively, which indicated the excellent photocatalytic activity of ED-NiAl-LDH. In addition, the adsorption state of CO2 on the different Ni active sites was directly visualized in the charge density difference images and the side views (Fig. 5(c), Fig. S27, S28 and Table S8). As a result, the activated CO2 molecules underwent angular deformations of 179.88°, 176.93°, and 178.7° for the Ni located NiAl-LDH layer, O defect (Vo), and edge, respectively. The Ni atom located Vo and edge of NiAl-LDH facilitated the transfer of electrons onto the CO2 molecule. Thus, unsaturated coordinated high-spin Ni atoms exhibited excellent adsorption and activation capacities for CO2. H2 evolution of ED-NiAl-LDH was a competitive step for solar-driven CO2-to-CO reduction, which needed to overcome a higher energy barrier of 0.15 eV than solar-driven CO2-to-CO reduction, suggesting an ideal selectivity toward *CO over ED-NiAl-LDH (Fig. 5(d)). In addition, compared to the desorption of adsorbed *CO to form gaseous CO, the formation of *CHO on ED-NiAl-LDH by gaining one electron and one proton needed to overcome a high energy barrier, which indicated that the pathway toward CH4 production was very challenging (Fig. S29).48,49
According to the above results, a possible mechanism could be proposed as follows: defect-rich E-NiAl-LDH exposed abundant unsaturated coordinated high-spin Ni sites, which was pivotal to the enhancement of CO2-to-CO selectivity and activity (Fig. 5(e)). In a high-spin state, the degenerate eg orbitals of the Ni atom remained half-filled. Upon illumination, the excited electrons migrated to the degenerate eg orbitals and subsequently combined with the reactants (e.g., CO2, H+, and intermediates), finally generating massive CO and a small amount of H2 and CH4. The remarkable photocatalytic activity and CO selectivity of ED-NiAl-LDH were ascribed to the following: (i) edge effect and O defect evidently improved the photoelectrochemical properties of ED-NiAl-LDH, primarily by inhibiting the recombination of photogenerated carriers, and thus increasing the utilization efficiency of solar light. (ii) The unsaturated coordinated high-spin Ni sites exhibited improved adsorption of CO2 molecules and suppressed adsorption of H+. The degenerate eg orbitals of Ni sites were no longer hindered by OH−, which enabled CO2 molecules to interact more effectively with the Ni atom and receive photogenerated electrons from it. (iii) The unsaturated coordinated high-spin Ni sites lowered the energy barrier for the conversion of *CO2 to *COOH, thereby enhancing both CO2-to-CO activity and selectivity.
Conclusion
In summary, we constructed edge-rich NiAl-LDH (ED-NiAl-LDH) nanoflakes with abundant lattice O defects for effective and selective solar-driven CO2 conversion in a pure water system. The ED-NiAl-LDH exhibited an impressive CO selectivity of 98.5%. The laboratory and outdoor CO production rates also reached 773.4 µmol g−1 h−1 and 500 µmol g−1 h−1, respectively, surpassing those of state-of-the-art photocatalysts in recent years. State-of-the-art characterization methods and theoretical calculations demonstrated that high-spin-state unsaturated-coordinated Ni active sites lower the energy barrier for CO2 reduction while facilitating efficient charge transfer dynamics. Furthermore, the increased exposure of Ni active sites significantly enhanced the photocatalytic activity. This study offers a novel perspective on achieving the selective solar-driven CO2 reduction, which paves the way for large-scale fuel production and broader sustainable applications.Conflicts of interest
The authors declare that they have no competing interests.Data availability
All the data for this article are available in the main text and the supplementary information (SI), or upon reasonable request from the corresponding author. Supplementary information is available. See DOI: https://doi.org/10.1039/d5ee04331j.Acknowledgements
This work was supported by the National Natural Science Foundation of China (grant no. 22578059), the Youth Talent Support Program of China Association for Science and Technology (grant no. 2023QNRC0692), and the Natural Science Foundation of Fujian Province (grant no. 2024J01403). We would be grateful to Prof. Ruquan Ye from City University of Hong Kong for the helpful discussion and revision on this work. The authors also extend their gratitude to Scientific Compass (https://www.shiyanjia.com) for providing invaluable assistance with the XPS analysis.References
- Y. Xiao, G. Ding, J. Tao, Z. Wang, Z. Chen, L. Chen, L. Shuai and G. Liao, Nat. Commun., 2025, 16, 7476 CrossRef CAS PubMed.
- J. Zhang, D. Yan, G. Ding, X. Wang, C. Li, S. Zhong, Y. Yu, L. Shuai and G. Liao, Angew. Chem., Int. Ed., 2025, 64, e202511448 CrossRef CAS PubMed.
- S. Fang, M. Rahaman, J. Bharti, E. Reisner, M. Robert, G. A. Ozin and Y. H. Hu, Nat. Rev. Methods Primers, 2023, 3, 61 CrossRef CAS.
- X. Liu, G. Ding, X. Guo, X. Wang, Z. Wang, Z. Chen, Y. Xiao, L. Shuai and G. Liao, Adv. Mater., 2026 DOI:10.1002/adma.202520384.
- C. Ban, Y. Wang, Y. Feng, Z. Zhu, Y. Duan, J. Ma, X. Zhang, X. Liu, K. Zhou, H. Zou, D. Yu, X. Tao, L. Gan, G. Han and X. Zhou, Energy Environ. Sci., 2024, 17, 518–530 RSC.
- G. Ding, C. Li, L. Chen and G. Liao, Energy Environ. Sci., 2024, 17, 5311–5335 RSC.
- M. Bonchio, J. Bonin, O. Ishitani, T.-B. Lu, T. Morikawa, A. J. Morris, E. Reisner, D. Sarkar, F. M. Toma and M. Robert, Nat. Catal., 2023, 6, 657–665 CrossRef.
- G. Liao, G. Ding, B. Yang and C. Li, Precis. Chem., 2024, 2, 49–56 CrossRef CAS.
- H. Liu, Y. Chen, H. Li, G. Wan, Y. Feng, W. Wang, C. Xiao, G. Zhang and Y. Xie, Angew. Chem., Int. Ed., 2023, 62, e202304562 CrossRef PubMed.
- J. Li, T. Xiang, X. Liu, M. N. Ghazzal and Z.-Q. Liu, Angew. Chem., Int. Ed., 2024, 63, e202407287 CrossRef CAS PubMed.
- B. Zhou, Y. Ma, P. Ou, Z. Ye, X.-Y. Li, S. Vanka, T. Ma, H. Sun, P. Wang, P. Zhou, J. K. Cooper, Y. Xiao, I. A. Navid, J. Pan, J. Song and Z. Mi, Nat. Catal., 2023, 6, 987–995 CrossRef CAS.
- Z. Chen, G. Ding, Z. Wang, Y. Xiao, X. Liu, L. Chen, C. Li, H. Huang and G. Liao, Adv. Funct. Mater., 2025, 35, 2423213 CrossRef CAS.
- H. Jung, C. Kim, H.-W. Yoo, J. You, J. S. Kim, A. Jamal, I. Gereige, J. W. Ager and H.-T. Jung, Energy Environ. Sci., 2023, 16, 2869–2878 RSC.
- Y.-S. Xia, L. Zhang, J.-N. Lu, X.-H. Zhao, L.-Z. Dong, J. Liu and Y.-Q. Lan, Nat. Synth., 2024, 3, 406–418 CrossRef CAS.
- S. Mohata, R. Das, K. Koner, M. Riyaz, K. Das, S. Chakraborty, Y. Ogaeri, Y. Nishiyama, S. C. Peter and R. Banerjee, J. Am. Chem. Soc., 2023, 145, 23802–23813 CrossRef CAS PubMed.
- Y. Tan, J. Feng, H. Dong, L. Liu, S. Zhao, F. Lai, T. Liu, Y. Bai, I. P. Parkin and G. He, Adv. Funct. Mater., 2023, 33, 2209967 CrossRef CAS.
- K. Kim, J. Lee, O. K. Park, J. Kim, J. Kim, D. Lee, V. K. Paidi, E. Jung, H. S. Lee, B. Lee, C. W. Lee, W. Ko, K. Lee, Y. Jung, C. Lee, N. Lee, S. Back, S. H. Choi and T. Hyeon, Adv. Mater., 2023, 35, 2207666 CrossRef CAS PubMed.
- Q. Liu, H. Cheng, T. Chen, T. W. B. Lo, Z. Xiang and F. Wang, Energy Environ. Sci., 2022, 15, 225–233 RSC.
- T. Low, A. Chaves, J. D. Caldwell, A. Kumar, N. X. Fang, P. Avouris, T. F. Heinz, F. Guinea, L. Martin-Moreno and F. Koppens, Nat. Mater., 2017, 16, 182–194 CrossRef CAS PubMed.
- G. Ding, C. Li, Y. Ni, L. Chen, L. Shuai and G. Liao, EES Catal., 2023, 1, 369–391 RSC.
- K. Teramura, S. Iguchi, Y. Mizuno, T. Shishido and T. Tanaka, Angew. Chem., Int. Ed., 2012, 51, 8008–8011 CrossRef CAS PubMed.
- S. Li, Z. Li, J. Yue, H. Wang, Y. Wang, W. Su, G. I. N. Waterhouse, L. Liu, W. Zhang and Y. Zhao, Angew. Chem., Int. Ed., 2024, 63, e202407638 CrossRef CAS PubMed.
- C. Ning, J. Yang, S. Bai, G. Chen, G. Liu, T. Shen, L. Zheng, S.-M. Xu, X. Kong, B. Liu, Y. Zhao and Y.-F. Song, Adv. Funct. Mater., 2023, 33, 2300365 CrossRef CAS.
- Y. Lv, X. Deng, J. Ding and Y. Zhou, Sci. Rep., 2024, 14, 902 CrossRef CAS PubMed.
- Y. Tian, M. Li, Z. Wu, Q. Sun, D. Yuan, B. Johannessen, L. Xu, Y. Wang, Y. Dou, H. Zhao and S. Zhang, Angew. Chem., Int. Ed., 2022, 61, e202213296 CrossRef CAS PubMed.
- Z. Su, X. Chen, M. Sun, X. Yang, J. Kang, Z. Cai and L. Guo, Angew. Chem., Int. Ed., 2024, 137, e202416878 CrossRef.
- Y. Ren, Y. Fu, N. Li, C. You, J. Huang, K. Huang, Z. Sun, J. Zhou, Y. Si, Y. Zhu, W. Chen, L. Duan and M. Liu, Nat. Commun., 2024, 15, 4675 CrossRef CAS PubMed.
- Q. Wang, J. Warnan, S. Rodríguez-Jiménez, J. J. Leung, S. Kalathil, V. Andrei, K. Domen and E. Reisner, Nat. Energy, 2020, 5, 703–710 CrossRef CAS.
- Q. Wang, S. Kalathil, C. Pornrungroj, C. D. Sahm and E. Reisner, Nat. Catal., 2022, 5, 633–641 CrossRef CAS.
- S. Ji, Y. Qu, T. Wang, Y. Chen, G. Wang, X. Li, J. Dong, Q. Chen, W. Zhang, Z. Zhang, S. Liang, R. Yu, Y. Wang, D. Wang and Y. Li, Angew. Chem., Int. Ed., 2020, 59, 10651–10657 CrossRef CAS PubMed.
- P. Liu, Z. Huang, X. Gao, X. Hong, J. Zhu, G. Wang, Y. Wu, J. Zeng and X. Zheng, Adv. Mater., 2022, 34, 2200057 CrossRef CAS.
- Q. Hao, H.-x Zhong, J.-z Wang, K.-h Liu, J.-m Yan, Z.-h Ren, N. Zhou, X. Zhao, H. Zhang, D.-x Liu, X. Liu, L.-w Chen, J. Luo and X.-b Zhang, Nat. Synth., 2022, 1, 719–728 CrossRef CAS.
- A. Prajapati, N. C. Kani, J. A. Gauthier, R. Sartape, J. Xie, I. Bessa, M. T. Galante, S. L. Leung, M. H. S. Andrade, R. T. Somich, M. V. Rebouças, G. T. Hutras, N. Diniz and M. R. Singh, Cell Rep. Phys. Sci., 2022, 3, 101053 CrossRef CAS.
- L. Cheng, X. Yue, L. Wang, D. Zhang, P. Zhang, J. Fan and Q. Xiang, Adv. Mater., 2021, 33, 2105135 CrossRef CAS PubMed.
- Y. Duan, Y. Wang, W. Zhang, J. Zhang, C. Ban, D. Yu, K. Zhou, J. Tang, X. Zhang, X. Han, L. Gan, X. Tao and X. Zhou, Adv. Funct. Mater., 2023, 33, 2301729 CrossRef CAS.
- S. Hu, P. Qiao, X. Yi, Y. Lei, H. Hu, J. Ye and D. Wang, Angew. Chem., Int. Ed., 2023, 62, e202304585 CrossRef CAS PubMed.
- X. Li, L. Li, G. Chen, X. Chu, X. Liu, C. Naisa, D. Pohl, M. Löffler and X. Feng, Nat. Commun., 2023, 14, 4034 CrossRef CAS.
- H. Ou, S. Ning, P. Zhu, S. Chen, A. Han, Q. Kang, Z. Hu, J. Ye, D. Wang and Y. Li, Angew. Chem., Int. Ed., 2022, 61, e202206579 CrossRef CAS PubMed.
- P. Verma, A. Singh, F. A. Rahimi, P. Sarkar, S. Nath, S. K. Pati and T. K. Maji, Nat. Commun., 2021, 12, 7313 CrossRef CAS PubMed.
- S. Xie, C. Deng, Q. Huang, C. Zhang, C. Chen, J. Zhao and H. Sheng, Angew. Chem., Int. Ed., 2023, 62, e202216717 CrossRef CAS.
- Z. Zhou, H. Zeng, C. Feng, L. Li, R. Tang, W. Li, Y. Huang and Y. Deng, Energy Environ. Sci., 2024, 17, 5627–5638 RSC.
- H. Huang, J. Zhao, H. Guo, B. Weng, H. Zhang, R. A. Saha, M. Zhang, F. Lai, Y. Zhou, R.-Z. Juan, P.-C. Chen, S. Wang, J. A. Steele, F. Zhong, T. Liu, J. Hofkens, Y.-M. Zheng, J. Long and M. B. J. Roeffaers, Adv. Mater., 2024, 36, 2313209 CrossRef CAS PubMed.
- L. Jiang, D. Chen, Z. Hao, D. Cao, R. Liu, J. Cheng, L. Chen, X. Liu, B. Jia and D. Liu, Energy Environ. Sci., 2024, 17, 8228–8242 RSC.
- Z. Jiang, H. Sun, T. Wang, B. Wang, W. Wei, H. Li, S. Yuan, T. An, H. Zhao, J. Yu and P. K. Wong, Energy Environ. Sci., 2018, 11, 2382–2389 RSC.
- J. Liang, H. Yu, J. Shi, B. Li, L. Wu and M. Wang, Adv. Mater., 2023, 35, 2209814 CrossRef CAS PubMed.
- D. Liu, D. Chen, N. Li, Q. Xu, H. Li, J. He and J. Lu, Angew. Chem., Int. Ed., 2020, 59, 4519–4524 CrossRef CAS PubMed.
- F. Wang, T. Hou, X. Zhao, W. Yao, R. Fang, K. Shen and Y. Li, Adv. Mater., 2021, 33, 2102690 CrossRef CAS PubMed.
- R. Tan, A. Wang, R. Malpass-Evans, R. Williams, E. W. Zhao, T. Liu, C. Ye, X. Zhou, B. P. Darwich, Z. Fan, L. Turcani, E. Jackson, L. Chen, S. Y. Chong, T. Li, K. E. Jelfs, A. I. Cooper, N. P. Brandon, C. P. Grey, N. B. McKeown and Q. Song, Nat. Mater., 2020, 19, 195–202 CrossRef CAS PubMed.
- R. Kortlever, J. Shen, K. J. P. Schouten, F. Calle-Vallejo and M. T. M. Koper, J. Phys. Chem. Lett., 2015, 6, 4073–4082 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2026 |