Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Acetyl peroxy radical-initiated oxidation of oxygenated monoterpenes: functional group effects on reaction pathways
Ida
Karppinen
 a,
Dominika
Pasik
ab and
Nanna
Myllys
a,
Dominika
Pasik
ab and
Nanna
Myllys
 *ab
*ab
aDepartment of Chemistry, University of Helsinki, Helsinki, 00014, Finland. E-mail: nanna.myllys@helsinki.fi
bInstitute for Atmospheric and Earth System Research, University of Helsinki, Helsinki, 00014, Finland
First published on 1st December 2025
Abstract
Biogenic volatile organic compounds are emitted into the atmosphere where they can oxidize forming compounds with lower volatilities. These low-volatility compounds can participate in the formation of secondary organic aerosols (SOAs) that affect human health and the climate in various ways. We studied the oxidation pathways initiated by the acetyl peroxy radical (APR) of less frequently studied oxygenated monoterpenes (monoterpenoids) using computational methods. The studied reactions included APR-addition, ring-opening reactions and C–C bond scissions. Ring-rearrangement after APR-addition was shown to be significant (43% yield) for one bicyclic monoterpenoid, sabinol. All other studied monoterpenoids will mostly react only with O2 forming a peroxy radical which will go on to form an alkoxy radical. Alkoxy radical β-scissions lead to different kinds of products depending on the reacting monoterpenoid. Verbenol and α-terpineol will mostly form closed-shell species through alkoxy radical C–C bond scissions leading to low SOA yields. The fate of carveol depends strongly on the stereoisomer. The S-isomer will form a closed-shell species with a 50% yield, whereas the R-isomer will form an alkyl radical with a 100% yield capable of further oxidation. Therefore, a significant SOA yield could be expected for carveol depending on the stereoisomer. For sabinol, umbellulone and carvone, high SOA yields are expected as the majority of them will form an alkyl radical that reacts with O2 forming a new peroxy radical. In the case of umbellulone and carvone, the formed peroxy radical is an acyl peroxy radical which can undergo rapid unimolecular reactions initiating fast autoxidation. Compared to the monoterpene counterparts of the studied monoterpenoids, significant differences were observed in reaction pathways and SOA yields. While APR-initiated oxidation serves as a minor pathway in the atmosphere, the studied reactions could have an impact on the production of low-volatility compounds.
Environmental significanceWe demonstrate that acyl peroxy radical-initiated oxidation of monoterpenoids is a relatively fast reaction in the atmosphere. Our results showcase that depending on the monoterpenoid structure, its oxidation can lead to a rapid termination reaction or further autoxidation and highly oxygenated organic molecule formation. This means the biogenic secondary organic aerosol (BSOA) yield is highly dependent on the exact monoterpenoid structure. Emissions of sabinol, umbellulone, carvone and carveol are likely to lead to high BSOA yield which is known to have negative radiative forcing. This is an important finding to improve existing models and also for policy makers to regulate biogenic emissions and further BSOA formation. |
1 Introduction
Volatile organic compounds (VOCs) are emitted into the atmosphere, the majority of which are from biogenic sources.1 Most of the emitted VOCs undergo degradative oxidation initiated by hydroxyl radicals (as well as NO3 and O3) leading to the formation of CO2 and water.2 However, a small fraction will oxidize forming larger compounds with low volatilities. These low-volatility organic compounds (LVOCs) can condense onto already existing particles or create new ones through new particle formation (NPF) and participate in the formation of secondary organic aerosols (SOAs).3,4 Depending on the location, 20–90% of the aerosol mass is organic matter of which the largest fraction is usually secondary in nature.5–7 Aerosols are known to have impacts on human health, air quality as well as climate, including a cooling role in radiative forcing through cloud formation.8–11 The magnitude of the effect of aerosols on radiative forcing is however very uncertain.11 Therefore, the understanding of the small fraction of VOCs that oxidizes forming larger molecules is important.A significant portion (up to 50%) of global SOA formation could be attributed to biogenic VOC (BVOC) oxidation.12 Approximately 15% of the global BVOC emissions comprise monoterpenes of which a large portion constitutes α-pinene, β-pinene and limonene.1,13 However, regional emissions vary and some globally negligible monoterpenes, e.g. oxygenated monoterpenes, could have significant effects on local SOA production.14,15 Moreover, climate change and the increase in temperature can lead to an enhancement of BVOC emissions from plants and even changes in BVOC profiles.16,17 This increases the need to understand the oxidation processes of less abundant but possibly more reactive monoterpenes. The SOA yields of oxygenated monoterpenes (from here on called monoterpenoids) have been the focus of a few studies.15,18–23 The presence of oxygen containing functional groups in monoterpenoids can lead to significant differences in SOA yields compared to monoterpenes which is why there is need for more studies surrounding this topic.
Acyl peroxy radicals have been shown to react fast enough with unsaturated hydrocarbons, e.g. monoterpenes, and these reactions can occur under atmospheric conditions.24,25 Although this is a minor pathway, these reactions could lead to the formation of low vapor pressure compounds. Reactions between acyl peroxy radicals and monoterpenes lead to the formation of an alkyl radical that rapidly reacts with O2 forming a peroxy radical.26 This peroxy radical can react further unimolecularly forming more oxidizied products or bimolecularly with radicals such as RO2 or HO2 forming an alkoxy radical. Alkoxy radical C–C bond scissions (β-scissions) can initiate autoxidation or the formation of closed-shell products that terminate the oxidation channel. Autoxidation is a key mechanism in which peroxy radicals react rapidly through subsequent intramolecular H-shift reactions to form highly oxygenated organic molecules (HOMs) with very low volatilities.27–29 Numerous studies have shown that HOMs are important for SOA growth.30–32 It is highly dependent on the alkoxy radical structure, in which the β-scission channel is the most favorable.33–35 Thus, the SOA yields from these different channels differ greatly depending on the parent alkoxy radical, i.e. the initial reacting monoterpene.
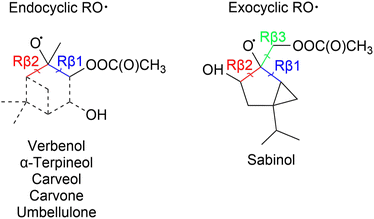
Draper et al.34 studied the oxidation pathways of monoterpenes initiated by the NO3 radical, and later on Pasik et al.35 studied similar oxidation pathways initiated by the acetyl peroxy radical (from here on abbreviated APR) of 7 different monoterpenes. Their studies showed significant differences in reaction pathways and SOA yields depending on the reacting monoterpene. This study builds upon the work by Pasik et al.35 to monoterpenoids, where oxygen containing functional groups are present. Using computational methods, we investigated the APR-initiated oxidation of six monoterpenoids: umbellulone, carvone, carveol, sabinol, verbenol and α-terpineol. The structures of the studied monoterpenoids are shown in Fig. 1 alongside their monoterpene counterparts that were studied by Pasik et al.35 Addition of APR is often followed by O2 addition, however, in the case of bicyclic monoterpenoids, alkyl-radical ring-opening reactions can be competitive.34,35 Therefore, the studied reactions included APR-addition, ring rearrangement and β-scission reactions of the alkoxy radical that forms after O2 addition as shown in Fig. 2. The goal of this study was to determine how the APR-initiated oxidation pathways and possible SOA yields are affected by functional groups, and how these compare to the monoterpene counterparts.
 | ||
| Fig. 1 Structures of monoterpenoids investigated in this study. In brackets are the monoterpene counterparts of each monoterpenoid that were studied by Pasik et al.35 | ||
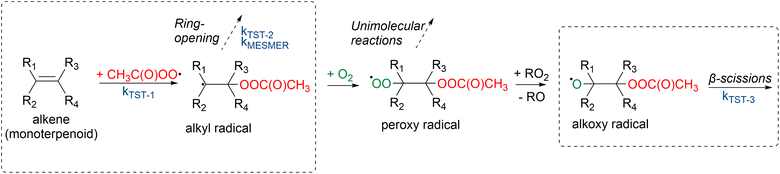 | ||
| Fig. 2 Monoterpenoid + APR reactions. Reaction rate coefficients were calculated for the circled reactions. | ||
2 Methods
The methodology described here is adopted from the work by Pasik et al.,36 where a cost-effective and accurate methodology was developed to study reactions between unsaturated hydrocarbons and oxygenated organic radicals. Transition state (TS) structures corresponding to addition reactions between APR and monoterpenoids as well as subsequent ring-opening and β-scission reactions were located using the relaxed potential energy surface (PES) scan using density functional theory (DFT). All reactant, TS and product structures were optimized using DFT. TS structures were identified as correct with the presence of one imaginary frequency and by performing intrinsic reaction coordinate (IRC) calculations to verify that the TS connects to the correct reactant and product wells. All DFT calculations were carried out at the ωB97X-D/6-31+G* level of theory.37–40 Conformational sampling of reactant, TS and product structures was conducted using Conformer-Rotamer Ensemble Sampling Tool (CREST) at the GFN2-xTB level.41,42 Conformational sampling of TS structures included constraining the bonds being formed/broken. Conformers were optimized with DFT using a 2.5 kcal mol−1 cutoff after CREST, as xTB level electronic energies have shown to correlate fairly well with DFT energies.36,43 TS conformers were first optimized keeping the bond being formed/broken frozen, followed by full TS optimization and frequency calculation. Duplicates were removed based on electronic energy and dipole moment. For the lowest energy reactant, TS and product conformers, single-point energies were calculated on top of the ωB97X-D/6-31+G*-optimized structures at the DLPNO-CCSD(T)/aug-cc-pVTZ level of theory for more accurate estimation of reaction energy barriers.44–47 DFT calculations were performed using Gaussian 16 revision C.02, and single-point energies were calculated using ORCA version 5.0.3.48,49Reaction rate coefficients were calculated using Multi-Conformer Transition State Theory (MC-TST).50 Rate coefficients for bimolecular addition reactions between APR and monoterpenoids were calculated using eqn (1):51,52
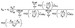 | (1) |
Reaction rate coefficients for unimolecular ring-opening and β-scission reactions were calculated using eqn (2):53
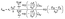 | (2) |
The dynamics of monoterpenoid-derived alkyl radical ring-opening reactions were studied using the methodology described in the work by Draper et al.,34 and later also used to study APR + monoterpene-derived alkyl radical ring-opening reactions in the work by Pasik et al.35 For two bicyclic monoterpenoids (sabinol and verbenol), the ring-opening reactions were simulated using the Master Equation Solver for Multi Energy-Well Reactions (MESMER) software.54 Vibrational frequencies and rotational constants were calculated using DFT level ωB97X-D/6-31+G*. The zero-point corrected energies were calculated at the DLPNO-CCSD(T)/aug-cc-pVTZ//ωB97X-D/6-31+G* level. APR-addition reactions were modeled assuming a barrierless association using the Inverse Laplace Transform (ILT) method. MC-TST calculated reaction rate coefficients for APR-addition reactions were used as a pre-exponential factor in the ILT calculations. O2-addition reactions were treated as bimolecular sinks using a bimolecular rate coefficient of 6 × 10−12 cm3 molecule−1 s−1 and O2 concentration of 5.16 × 1018 mol cm−3, values previously used in other studies.33,35,55 Ring-opening reactions were modeled using standard Rice–Ramsperger–Kassel–Marcus (RRKM) theory with Eckart tunneling correction. RRKM theory takes into account the excess energy released in the APR-addition reaction which affects the reaction rate coefficient of the ring-opening. An exponential-down parameter of Edown = 225 cm−1 was used to model collisional energy transfer. The bath gas used was N2 with Lennard–Jones parameters ε/kB = 91.85 K and σ = 3.919 Å. The Lennard–Jones parameters for monoterpenoid-derived alkyl radicals and ring-opened products, which are available in the SI (Table S1), were obtained based on the group-additivity method applied to pure-compound critical properties by Joback and Reid,56 a procedure described in Gao et al.57 and Tee et al.58 A concentration of 1015 mol cm−3 was used for APR to guarantee the rapid formation of the modeled monoterpenoid-APR intermediate. A grain size of 30 cm−1 was used, and the simulated energy grains spanned 50kBT above the highest TS.
3 Results
3.1 Acetyl peroxy radical addition reactions
We started by investigating the kinetics of reactions between APR and monoterpenoids shown in Fig. 1. We considered all relevant APR-addition sides and calculated rates for addition reactions to double bonds. The rate is affected by stereochemistry, which was also considered. For umbellulone, carveol, sabinol and verbenol, we calculated APR-addition rates for different sides, where the attacking APR can either be on the same side of the secondary ring structure/OH-group, or on the opposite side. It was previously shown that it indeed affects the rate when considering different sides of APR attack relative to the secondary ring structure.35 The fastest calculated reaction rate coefficients for APR-addition reactions are listed in Table 1. Other calculated rate coefficients can be found in the SI (Table S2). Addition reactions are labeled according to the side of the attacking APR: (a) APR attack from the secondary ring side, (b) attack from the methyl group side, (c) attack from the OH-group side, and (d) attack from the side opposite to the OH-group. In cases where both the secondary ring and the OH-group are present, combinations such as (a,d) indicate that attack occurs from the same side of the secondary ring but from the opposite side of the OH-group.| Monoterpenoid | ΔE1 | k TST-1 | ΔEex | ΔE2 | k TST-2 | k MESMER | %TST-2 | %MESMER |
|---|---|---|---|---|---|---|---|---|
| α-Terpineol | 0.9 | 7.9 × 10−17 | 16.8 | ✗ | ✗ | ✗ | ✗ | ✗ |
| Umbellulone (b) | 1.7 | 9.5 × 10−18 | 16.5 | ✗ | ✗ | ✗ | ✗ | ✗ |
| Carveol (c) | −0.1 | 1.3 × 10−15 | 15.8 | ✗ | ✗ | ✗ | ✗ | ✗ |
| Carvone | 1.5 | 8.0 × 10−17 | 17.5 | ✗ | ✗ | ✗ | ✗ | ✗ |
| Sabinol (b,c) | −0.8 | 4.9 × 10−16 | 17.6 | 10.0 | 7.1 × 106 | 6.4 × 105 | 19 | 43 |
| Verbenol (a,c) | 1.3 | 1.1 × 10−16 | 14.6 | 14.0 | 2.3 × 102 | 2.4 × 102 | ∼ 0 | ∼ 0 |
| Verbenol (a,d) | 1.9 | 1.2 × 10−16 | 14.7 | 13.2 | 1.7 × 103 | 5.3 × 103 | ∼ 0 | 1 |
Considering the stereochemistry has a substantial impact on the calculated barriers. The rate is affected by both the attack side relative to the secondary ring side and OH-group side. For carveol, the APR attack happening from the OH-group side exhibits 1.8 kcal mol−1 lower barrier than the APR attack happening from the opposite side of the OH-group. When the OH-group lies on the same side of the attacking APR, hydrogen bonding can occur, lowering the reaction barrier. The same is observed for sabinol, where the attack happening from the same side of the OH-group and methyl group has the lowest barrier.
For verbenol, attacks from both the OH-group side (c) and the opposite side (d) yield similar reaction rate coefficients, even though attack occurring from the OH-group side has a 0.6 kcal mol−1 lower barrier. This results from a different amount of unique TS conformers: the OH-group side has 3 unique TS conformers, whereas the opposite side has 18. The larger number of conformers increases the overall partition function sum in eqn (1), compensating for the barrier difference. Consequently, the rate coefficients for different sides of APR attack are similar even though the reaction barriers differ. The same was also observed for verbenol in the APR-addition reaction that produces a secondary alkyl radical. Attack happening from the OH-group side exhibited 1.9 kcal mol−1 lower barrier than attack happening from the opposite side, however, the rates for these reactions were of the same order of magnitude (see SI Table S2). This was again due to different amounts of unique TS conformers (2 for reaction c and 15 for reaction d).
The fastest addition reaction results in the formation of a tertiary radical for all monoterpenoids studied, except for umbellulone. For umbellulone, the fastest addition reaction involves the formation of a secondary alkyl radical that is stabilized by the adjacent carbonyl group. The formation of a tertiary radical would hinder the conjugation in umbellulone which makes this reaction pathway less favorable. For the pathway that involves the formation of a secondary radical, addition b is favored. However, for the pathway involving the formation of a tertiary radical, addition a is favored (see SI Table S2). For this secondary radical forming pathway, addition to the cyclopropyl side (a) has a barrier as high as 7.1 kcal mol−1. We conducted additional conformer sampling for the TS using the new Global Optimizer GOAT in Orca 6.0.59 However, conformer search using GOAT resulted in the same lowest energy TS conformer as configurational search using CREST. Further details and discussion are provided in the SI.
Fastest addition reactions are observed for carveol, sabinol and verbenol which have an OH-group in the structure. When compared to results obtained by Pasik et al.35 on APR-initiated monoterpene oxidation, sabinol and carveol exhibit lower barriers than their monoterpene counterparts sabinene and limonene, respectively. The reaction between APR and α-terpineol has a slightly lower barrier than the APR + limonene reaction. However, in the case of limonene, the reaction rate coefficient is approximately an order of magnitude higher. Verbenol and its monoterpene counterpart α-pinene have similar barriers and reaction rate coefficients. Contrastingly, carvone and umbellulone exhibit higher barriers and slower reactions than their monoterpene counterparts limonene and α-thujene, respectively. Evidently, the OH-group near the APR attack site lowers the reaction barrier, whereas a carbonyl group near the APR attack site increases the barrier. Altogether, reaction barriers were low and all reaction rate coefficients in Table 1 would be considered high enough for these reactions to be feasible under atmospheric conditions.
There is very limited data available for the OH-initiated oxidation of the investigated monoterpenoids. The gas-phase reaction between α-terpineol and OH has been studied experimentally and a rate of 1.9 ± 0.5 × 10−10 cm3 molecule−1 s−1 was reported.60 With an average atmospheric OH concentration of 106 mol cm−3,61 this rate would correspond to a pseudo-first-order reaction rate of 1.9 × 10−4 s−1. The total reaction rate coefficient for α-terpineol + APR reaction is a sum of the two rates calculated here (see Tables 1 and S2) and gives a rate of 9.1 × 10−17 cm3 molecule−1 s−1. Using a concentration of 108 mol cm−3 for APR,62 a pseudo-first-order reaction rate of 9.1 × 10−9 s−1 is derived for the α-terpineol + APR reaction. Thus, the APR-initiated oxidation would correspond to a negligible percentage of the OH-initiated oxidation of α-terpineol. For the other monoterpenoids, such a comparison is not possible due to the lack of experimental data on OH-initiated oxidation of said structures.
There is uncertainty in our estimation of absolute rate coefficients that could lead to underestimation of rates of the APR-initiated oxidation of monoterpenoids. In our previous study,63 we investigated the reaction between APR and isoprene and comparison to experimental data presented by Nozière and Fache24 and SAR predictions by Stark64 showed an underestimation of one to two orders of magnitude in our calculated rate. Accordingly, we are assuming a similar underestimation of the absolute rates of reactions between APR and monoterpenoids reported here. Theoretical reaction rates are usually associated with an uncertainty of approximately a factor of five arising from the sensitivity analysis where barrier heights are systematically varied by ± 1 kcal mol−1. Even with this uncertainty, the computed rates for isoprene + APR reactions remain lower than the experimental values. Sources of uncertainty could arise from the larger uncertainties introduced by approximations used in the DLPNO-CCSD(T) approach compared to canonical CCSD(T). However, for the large systems studied here, canonical CCSD(T) is too expensive. The uncertainties discussed here could lead to an underestimation of the contribution of APR-initiated oxidation in monoterpenoid oxidation. Recent experiments have verified that APR + double bond chemistry indeed occurs under laboratory conditions.25,65 However, more experimental data would be needed to confirm reaction kinetics and branching ratios.
3.2 Monoterpenoid-derived alkyl radical ring-opening reactions
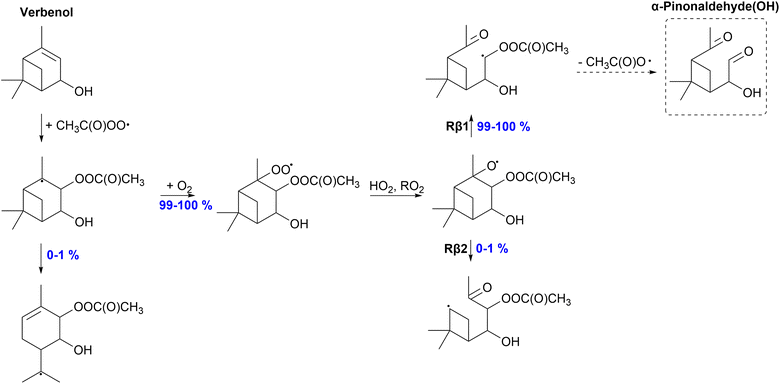
After APR-addition, O2-addition to the alkyl radical center is expected due to its high concentration in the atmosphere. However, the excess energy released in the APR-addition reaction can help overcome the barrier for subsequent ring-opening reactions. Thus, we further studied the ring-opening reactions following APR-addition for sabinol and verbenol using two different methods, MC-TST and RRKM simulations. No ring-opening reaction is expected for umbellulone as the radical center is too far from the secondary ring structure. Verbenol exhibits slow ring-opening reactions and the ring-opening is a minor (1%) pathway for the verbenol + APR d-adduct and negligible for the c-adduct. This was expected as the secondary ring was a four-membered ring, and ring-opening reactions have shown to become more competitive as the ring size decreases.34,35 Compared to α-pinene, the monoterpene counterpart of verbenol studied by Pasik et al.,35 the yield of the alkyl radical ring-opening reaction is enhanced only slightly (0% for α-pinene and 0–1% for verbenol).For sabinol, which has a three-membered secondary ring, the ring-opening reaction becomes much more significant due to the small and strained ring-structure. RRKM simulations show a 43% yield for the ring-opening reaction, whereas MC-TST calculations predict a significantly lower yield of 19%. Interestingly, the MESMER-derived rate coefficient is lower than the MC-TST rate coefficient. MESMER-derived rates only account for one conformer which could explain the lower rate coefficient. Despite the lower rate coefficient, RRKM simulations still result in a larger yield for ring-opening reaction for sabinol. The simulated time profile of species distribution for sabinol-derived alkyl radical ring-opening versus O2 addition is shown in Fig. 3. The yield of the sabinol-derived alkyl radical ring-opening reaction is also larger compared to the yield of its monoterpene counterpart sabinene (31%) studied by Pasik et al.35
3.3 Monoterpenoid-derived alkoxy radical β-scission reactions
After the addition of APR to the monoterpenoid, the formed product can rapidly react with O2 to form a peroxy radical. The peroxy radical can again react to form an alkoxy radical as shown in Fig. 2. This alkoxy radical can undergo β-scission reactions that were studied for all monoterpenoids. “Right” (Rβ1) and “left” (Rβ2) β-scissions were studied for the endocyclic alkoxy radicals and “right”, “left” and “top” (Rβ3) β-scissions were studied for the exocyclic alkoxy radical as shown in Fig. 4. Stereochemistry was also taken into account according to Fig. S4. Calculated energy barriers, MC-TST rate coefficients and product yields for the different β-scission channels are given in Table 2.| Monoterpenoid | ΔE | k TST-3 | Yield (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Rβ1 | Rβ2 | Rβ3 | Rβ1 | Rβ2 | Rβ3 | Rβ1 | Rβ2 | Rβ3 | |
| Carveol (R) | 9.5 | 2.3 | ✗ | 9.5 × 105 | 1.0 × 1011 | ✗ | 0 | 100 | ✗ |
| Carveol (S) | 4.9 | 4.3 | ✗ | 2.0 × 109 | 2.0 × 109 | ✗ | 50 | 50 | ✗ |
| Carvone (R,R) | 6.3 | 1.3 | ✗ | 1.0 × 108 | 4.4 × 1011 | ✗ | 0 | 100 | ✗ |
| Carvone (R,S) | 7.6 | 0.8 | ✗ | 1.3 × 107 | 2.0 × 1012 | ✗ | 0 | 100 | ✗ |
| Carvone (S,R) | 6.9 | 0.1 | ✗ | 1.2 × 107 | 3.5 × 1012 | ✗ | 0 | 100 | ✗ |
| Carvone (S,S) | 3.7 | −0.6 | ✗ | 2.9 × 109 | 7.0 × 1012 | ✗ | 0 | 100 | ✗ |
| α-Terpineol (R,R) | 10.2 | 11.4 | ✗ | 4.8 × 105 | 3.3 × 104 | ✗ | 93 | 7 | ✗ |
| α-Terpineol (R,S) | 6.3 | 11.9 | ✗ | 5.1 × 107 | 1.7 × 104 | ✗ | 100 | 0 | ✗ |
| α-Terpineol (S,R) | 6.4 | 10.5 | ✗ | 1.7 × 107 | 3.5 × 104 | ✗ | 100 | 0 | ✗ |
| α-Terpineol (S,S) | 8.1 | 12.6 | ✗ | 2.2 × 106 | 3.9 × 103 | ✗ | 100 | 0 | ✗ |
| Umbellulone (R) | 7.9 | 1.5 | ✗ | 3.0 × 107 | 1.1 × 1012 | ✗ | 0 | 100 | ✗ |
| Umbellulone (S) | 7.8 | 1.7 | ✗ | 1.5 × 107 | 1.1 × 1012 | ✗ | 0 | 100 | ✗ |
| Sabinol (R,R) | 0.7 | 17.5 | 10.3 | 4.7 × 1011 | 2.5 × 100 | 4.9 × 104 | 100 | 0 | 0 |
| Sabinol (R,S) | 2.2 | 15.0 | 9.8 | 4.6 × 1010 | 4.8 × 102 | 4.7 × 105 | 100 | 0 | 0 |
| Sabinol (S,R) | 0.8 | 16.3 | 6.9 | 7.8 × 1011 | 2.1 × 102 | 2.0 × 108 | 100 | 0 | 0 |
| Sabinol (S,S) | 0.03 | 12.8 | 7.3 | 2.9 × 1012 | 1.4 × 104 | 3.5 × 107 | 100 | 0 | 0 |
| Verbenol (R,R) | 8.5 | 11.5 | ✗ | 7.2 × 106 | 4.0 × 104 | ✗ | 99 | 1 | ✗ |
| Verbenol (R,S) | 8.9 | 12.2 | ✗ | 1.8 × 106 | 1.3 × 104 | ✗ | 99 | 1 | ✗ |
| Verbenol (S,R) | 6.7 | 12.6 | ✗ | 4.1 × 107 | 6.7 × 103 | ✗ | 100 | 0 | ✗ |
| Verbenol (S,S) | 6.8 | 11.2 | ✗ | 9.4 × 107 | 8.0 × 104 | ✗ | 100 | 0 | ✗ |
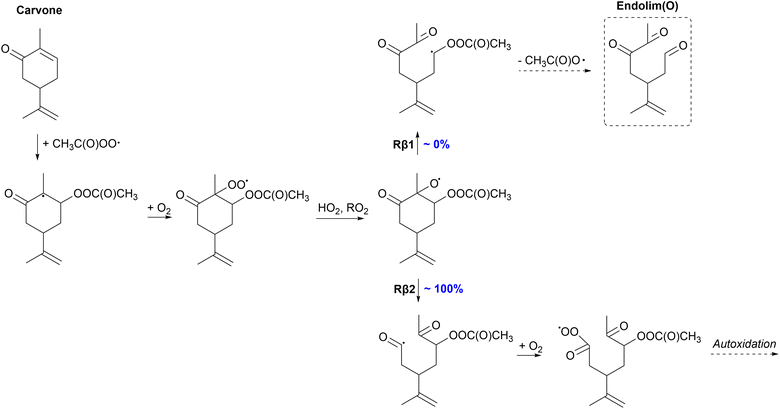
β-Scission channels resulting in the formation of a radical center on a three- or four-membered ring structure have the highest energy barriers which has also been observed for monoterpene derived nitrooxy- and acetyl-alkoxyl β-scissions.34,35 High barriers are also observed for the α-terpineol Rβ2 channel which forms a primary alkyl radical. Channels leading to the formation of an acyl radical or α-OH alkyl radical center exhibit the lowest barriers (as low as −0.6 kcal mol−1). The channel where an acyl radical forms is especially interesting as rapid reaction with O2 leads to the formation of a new acyl peroxy radical that can undergo fast unimolecular reactions initiating possible further autoxidation. Each monoterpenoid has its own unique reactivity and although most stereoisomers of the parent monoterpenoid exhibit similar reactivity, some differences are also observed between different isomers. As a result, each monoterpenoid has a different potential to contribute to the formation of SOA. A more detailed description of each monoterpenoid's reactivity and a proposed fate is given in the following sections.
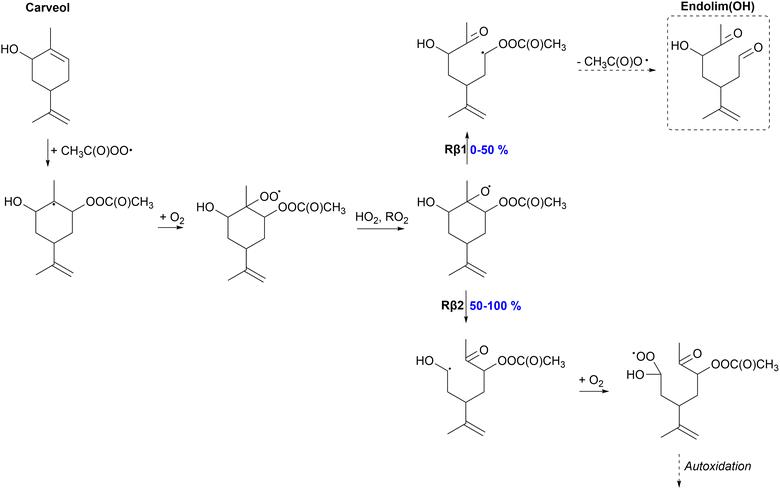 | ||
| Fig. 5 Proposed fate of carveol + CH3C(O)OȮ. Dashed box indicates termination of the oxidation pathway through the formation of a closed-shell product. | ||
Unlike other monoterpenoids, carveol shows significant differences in the preferred β-scission pathways depending on the stereoisomer. Of the R-isomer, 100% will react through the left C–C scission (Rβ2) forming the α-OH alkyl radical. The yields of Rβ1 and Rβ2 channels for the S-isomer are both 50%. Right C–C scission (Rβ1) leads to the formation of a α-QOOR alkyl radical which is unstable and undergoes decomposition as shown in other studies.28,67,68 Therefore, this pathway will lead to the formation of a closed-shell species, endolim(OH), and termination of the oxidation chain. The formed α-OH alkyl radical in the Rβ2 channel could add O2 and react unimolecularly leading to more oxidized products, and eventually HOMs, exhibiting higher SOA yields. In the APR-initiated oxidation of limonene, the formed alkoxy radical reacts 100% through the Rβ1 channel leading to the termination of the oxidation chain and low SOA yields.35 The SOA yield is possibly enhanced for carveol compared to limonene but it strongly depends on the stereoisomer.
 | ||
| Fig. 9 Proposed fate of sabinol + CH3C(O)OȮ. Dashed box indicates termination of the oxidation pathway through the formation of a closed-shell product. | ||
57% of the formed alkyl radical will react through O2 addition eventually forming an alkoxy radical. In addition to left and right C–C scissions, top C–C scission (Rβ3) is possible for the sabinol-derived exocyclic alkoxy radical. However, according to our calculations, 100% of the alkoxy radical will react through the right C–C scission (Rβ1) forming an α-OH alkyl radical. Left-sided scission would form a radical center on a three-membered ring structure, whereas the top C–C bond scission terminates the oxidation chain through the formation of sabinaketone(OH). The α-OH alkyl radical formed in the Rβ1 channel can react with O2 and further oxidize, leading to low-volatility products. Both the ring-opening rearrangement and reaction with O2 of the initial alkyl radical can lead to the formation of HOMs predicting high SOA yields for the APR-initiated oxidation of sabinol. High SOA yield was also expected for the APR-initiated oxidation of sabinene by Pasik et al.,35 however, a significant percentage (31–63%) of the sabinene-derived alkoxy radical is shown to react through the top C–C scission leading to the termination of the oxidation chain. 100% of the sabinol-derived alkoxy radical will react through the Rβ1 channel that is most likely to initiate further oxidation and therefore higher SOA yields would be expected for sabinol than sabinene.
3.4 Atmospheric implications
The end products and expected SOA yields of all studied monoterpenoids are summarized in Table 3. The table includes the most likely oxidation products of each monoterpenoid and SOA yields predicted according to major oxidation pathways. Oxidation of carveol, carvone, umbellulone, and sabinol leads to the formation of peroxy radicals, which have potential to begin the autoxidation process that leads to the rapid formation of highly oxygenated organic molecules. These compounds tend to be very low volatile, thus capable of participating in secondary organic aerosol formation. In warm climates, biogenic VOC emissions increase. Depending on the type of emitting monoterpene or monoterpenoid, SOA yield can vary a lot. In the case of C10H16 monoterpenes, exocyclic monoterpenes lead to a higher SOA yield than endocyclic monoterpenes.35 For monoterpenoids, the functional group can lead to different branching of autoxidation/termination pathways. The more VOCs capable of autoxidizing are emitted, the more biogenic SOA is formed creating a negative climate feedback mechanism.70 Thus, emissions of α-terpineol or verbenol are likely to have minor impact to the climate forcing via SOA formation whereas, carveol, carvone, umbellulone, and sabinol could have larger impacts.| Monoterpenoid | End product | SOA yield |
|---|---|---|
| Carveol | Endolim(OH) (0–50%)/HOM (50–100%) | Moderate/high |
| Carvone | HOM | High |
| α-Terpineol | Endolim(OH) | Low |
| Umbellulone | HOM | High |
| Sabinol | HOM (via two main pathways) | High |
| Verbenol | α-Pinonaldehyde(OH) | Low |
Other acyl peroxy radicals (for which unimolecular reactions are slow) are known to react in a similar manner to the acetyl peroxy radical used as a representative APR in this study. Reactions of larger acyl peroxy radicals with unsaturated hydrocarbons are often faster compared to those of the acetyl peroxy radical,25,63 but the concentrations of these acyl peroxy radicals are even less well known. However, the larger acyl peroxy radicals, such as the benzoyl peroxy radical, when reacting with monoterpenoids can form compounds with more than 15 carbon atoms. These compounds (when highly oxygenated) can be classified as ultra-low-volatility organic compounds that can nucleate under atmospheric conditions.71,72 In contrast, self- and cross-reactions of RO2, where a ROOR dimer forms, are needed to form >C15 compounds from the OH-initiated oxidation. Thus, while APR-initiated oxidation serves as a minor pathway in the atmosphere, its impact on the production of low vapor pressure compounds should not be neglected. Moreover, the relevance of these reactions could be substantially enhanced in environments where reactions with OH are limited, e.g. at nighttime with low-NOx and high-VOC conditions.
4 Conclusions
We investigated the oxidation pathways of six different oxygenated monoterpenes initiated by the acetyl peroxy radical. Our goal was to determine how oxygen containing functional groups affect the reaction pathways and SOA yields and compare our results with results obtained by Pasik et al.35 on APR-initiated oxidation of monoterpenes.According to our calculations, APR-addition is enhanced by an OH-group near the APR attack site, whereas a carbonyl group will inhibit the reaction. The formation of a tertiary alkyl radical was favored for all but one monoterpenoid, umbellulone. For umbellulone, the APR-addition that forms a secondary alkyl radical is stabilized by the adjacent carbonyl group making it faster than the tertiary radical forming pathway. Ring rearrangement reactions were studied for verbenol- and sabinol-derived alkyl radicals. RRKM simulations showed that the yields of ring-opening reactions were negligible (0–1%) for verbenol, but significant (43%) for sabinol. Ring-opening yields were similar for verbenol and its monoterpene counterpart α-pinene35 (negligible for both), but notably enhanced for sabinol compared to the monoterpene counterpart sabinene (31%35). Sabinol-derived alkyl radical rearrangement could open up additional pathways for further oxidation leading to the formation of low-volatility compounds.
Each monoterpenoid has unique reactivity toward different C–C bond scission channels, leading to varying possibilities to initiate SOA formation. The formation of a closed-shell species in the APR-initiated oxidation is very likely for α-terpineol and verbenol suggesting low SOA yields. For carveol, the preferred β-scission depends strongly on the stereoisomer. The formation of a closed-shell species, endolim(OH), is negligible (0%) for the R-isomer but significant for the S-isomer (50%). 50–100% of carveol will react through the Rβ2 channel that forms an α-OH alkyl radical that could further oxidize. This would mean non-negligible SOA yield for carveol, but it is strongly dependent on the stereoisomer. High SOA yields would be expected for carvone and umbellulone through the formation of an acyl peroxy radical that could initiate fast autoxidation. Also, sabinol is expected to have high SOA yield in the APR-initiated oxidation. The SOA yield expectancy is similar for verbenol and α-terpineol compared to their monoterpene counterparts α-pinene and limonene, respectively, moderately higher for carveol compared to limonene and significantly higher for carvone, umbellulone and sabinol compared to their monoterpene counterparts limonene, α-thujene and sabinene, respectively.
Our study showcased significant differences in the oxidation mechanisms between monoterpenes and oxygenated monoterpenes. Oxygenated monoterpenes could have crucial effects on local SOA formation through enhanced reactivity toward oxidants. Consequently, understanding the oxidation mechanisms and SOA formation from these oxygenated monoterpenes is important.
Author contributions
IK performed the calculations and wrote the manuscript; DP assisted with the calculations; DP and NM contributed to the analysis. The study was designed and supervised by NM. All authors proofread the manuscript.Conflicts of interest
The contact author has declared that none of the authors have any competing interests.Data availability
The optimized structures and calculation output files of all relevant compounds that support the findings of this manuscript will be available in the Zenodo repository: https://doi.org/10.5281/zenodo.17732259.Supplementary information (SI) is available. See DOI: https://doi.org/10.1039/d5ea00117j.
Acknowledgements
This research has been supported by the Research Council of Finland (grant no. 347775), European Union (ERC, AtmosCOC, project no 101161989), and the Doctoral Programme in Atmospheric Sciences (ATM-DP), University of Helsinki. We acknowledge the CSC-IT Center for Science in Espoo, Finland, for the computational resources. Views and opinions expressed are however those of the authors only and do not necessarily reflect those of the European Union or the European Research Council Executive Agency. Neither the European Union nor the granting authority can be held responsible for them.Notes and references
- A. Guenther, C. N. Hewitt, D. Erickson, R. Fall, C. Geron, T. Graedel, P. Harley, L. Klinger, M. Lerdau, W. A. Mckay, T. Pierce, B. Scholes, R. Steinbrecher, R. Tallamraju, J. Taylor and P. Zimmerman, J. Geophys. Res.: Atmos., 1995, 100, 8873–8892 CrossRef CAS.
- J. Williams and R. Koppmann, in Volatile Organic Compounds in the Atmosphere: an Overview, ed. R. Koppmann, John Wiley & Sons, Chichester, 2007, pp. 1–32 Search PubMed.
- C. D. O'Dowd, P. Aalto, K. Hmeri, M. Kulmala and T. Hoffmann, Nature, 2002, 416, 497–498 CrossRef PubMed.
- M. Kulmala, Science, 2003, 302, 1000–1001 CrossRef CAS PubMed.
- M. Kanakidou, J. H. Seinfeld, S. N. Pandis, I. Barnes, F. J. Dentener, M. C. Facchini, R. Van Dingenen, B. Ervens, A. Nenes, C. J. Nielsen, E. Swietlicki, J. P. Putaud, Y. Balkanski, S. Fuzzi, J. Horth, G. K. Moortgat, R. Winterhalter, C. E. L. Myhre, K. Tsigaridis, E. Vignati, E. G. Stephanou and J. Wilson, Atmos. Chem. Phys., 2005, 5, 1053–1123 CrossRef CAS.
- M. Crippa, F. Canonaco, V. A. Lanz, M. Äijälä, J. D. Allan, S. Carbone, G. Capes, D. Ceburnis, M. Dall'Osto, D. A. Day, P. F. DeCarlo, M. Ehn, A. Eriksson, E. Freney, L. Hildebrandt Ruiz, R. Hillamo, J. L. Jimenez, H. Junninen, A. Kiendler-Scharr, A.-M. Kortelainen, M. Kulmala, A. Laaksonen, A. A. Mensah, C. Mohr, E. Nemitz, C. O'Dowd, J. Ovadnevaite, S. N. Pandis, T. Petäjä, L. Poulain, S. Saarikoski, K. Sellegri, E. Swietlicki, P. Tiitta, D. R. Worsnop, U. Baltensperger and A. S. H. Prévôt, Atmos. Chem. Phys., 2014, 14, 6159–6176 CrossRef.
- G. Chen, F. Canonaco, A. Tobler, W. Aas, A. Alastuey, J. Allan, S. Atabakhsh, M. Aurela, U. Baltensperger, A. Bougiatioti, J. F. De Brito, D. Ceburnis, B. Chazeau, H. Chebaicheb, K. R. Daellenbach, M. Ehn, I. El Haddad, K. Eleftheriadis, O. Favez, H. Flentje, A. Font, K. Fossum, E. Freney, M. Gini, D. C. Green, L. Heikkinen, H. Herrmann, A.-C. Kalogridis, H. Keernik, R. Lhotka, C. Lin, C. Lunder, M. Maasikmets, M. I. Manousakas, N. Marchand, C. Marin, L. Marmureanu, N. Mihalopoulos, G. Močnik, J. Nęcki, C. O'Dowd, J. Ovadnevaite, T. Peter, J.-E. Petit, M. Pikridas, S. Matthew Platt, P. Pokorná, L. Poulain, M. Priestman, V. Riffault, M. Rinaldi, K. Różański, J. Schwarz, J. Sciare, L. Simon, A. Skiba, J. G. Slowik, Y. Sosedova, I. Stavroulas, K. Styszko, E. Teinemaa, H. Timonen, A. Tremper, J. Vasilescu, M. Via, P. Vodička, A. Wiedensohler, O. Zografou, M. Cruz Minguillón and A. S. Prévôt, Environ. Int., 2022, 166, 107325 CrossRef CAS PubMed.
- J. A. Bernstein, N. Alexis, C. Barnes, I. L. Bernstein, A. Nel, D. Peden, D. Diaz-Sanchez, S. M. Tarlo and P. B. Williams, J. Allergy Clin. Immunol., 2004, 114, 1116–1123 CrossRef PubMed.
- V.-M. Kerminen, H. Lihavainen, M. Komppula, Y. Viisanen and M. Kulmala, Geophys. Res. Lett., 2005, 32, L14803 CrossRef.
- U. Pöschl, Angew. Chem., Int. Ed., 2005, 44, 7520–7540 CrossRef PubMed.
- P. Forster, T. Storelvmo, K. Armour, W. Collins, J.-L. Dufresne, D. Frame, D. Lunt, T. Mauritsen, M. Palmer, M. Watanabe, M. Wild and H. Zhang, in The Earth's Energy Budget, Climate Feedbacks, and Climate Sensitivity, ed. V. Masson-Delmotte, P. Zhai, A. Pirani, S. Connors, C. Péan, S. Berger, N. Caud, Y. Chen, L. Goldfarb, M. Gomis, M. Huang, K. Leitzell, E. Lonnoy, J. Matthews, T. Maycock, T. Waterfield, O. Yelekçi, R. Yu and B. Zhou, Cambridge University Press, Cambridge and New York, 2021, pp. 923–1054 Search PubMed.
- S. H. Chung and J. H. Seinfeld, J. Geophys. Res.: Atmos., 2002, 107, 14–33 Search PubMed.
- A. B. Guenther, X. Jiang, C. L. Heald, T. Sakulyanontvittaya, T. Duhl, L. K. Emmons and X. Wang, Geosci. Model Dev., 2012, 5, 1471–1492 CrossRef.
- D. R. Gentner, E. Ormeño, S. Fares, T. B. Ford, R. Weber, J.-H. Park, J. Brioude, W. M. Angevine, J. F. Karlik and A. H. Goldstein, Atmos. Chem. Phys., 2014, 14, 5393–5413 CrossRef.
- A. Mehra, J. E. Krechmer, A. Lambe, C. Sarkar, L. Williams, F. Khalaj, A. Guenther, J. Jayne, H. Coe, D. Worsnop, C. Faiola and M. Canagaratna, Atmos. Chem. Phys., 2020, 20, 10953–10965 CrossRef CAS.
- J. Peñuelas and M. Staudt, Trends Plant Sci., 2010, 15, 133–144 CrossRef PubMed.
- M. Kivimäenpää, R. P. Ghimire, S. Sutinen, E. Häikiö, A. Kasurinen, T. Holopainen and J. K. Holopainen, Eurasian J. For. Res., 2016, 135, 343–360 Search PubMed.
- C. Coeur, V. Jacob, P. Foster and P. Baussand, WIT Trans. Ecol. Environ., 1999, 35, 612–617 Search PubMed.
- A. Lee, A. H. Goldstein, J. H. Kroll, N. L. Ng, V. Varutbangkul, R. C. Flagan and J. H. Seinfeld, J. Geophys. Res.: Atmos., 2006, 111, D17305 Search PubMed.
- X. Chen and P. K. Hopke, Atmos. Environ., 2009, 43, 3935–3940 CrossRef CAS.
- Y. Iinuma, M. Keywood, T. Gnauk and H. Herrmann, Environ. Sci. Technol., 2009, 43, 280–285 CrossRef CAS PubMed.
- S. Gu, F. Khalaj, V. Perraud and C. L. Faiola, Environ. Sci.: Processes Impacts, 2024, 26, 1156–1170 RSC.
- F. Khalaj, S. Gu, A. Mehra, L. R. Williams, J. Krechmer, A. Lambe, V. Perraud and C. L. Faiola, Aerosol Sci. Technol., 2025, 59, 470–486 CrossRef CAS.
- B. Nozière and F. Fache, Chem. Sci., 2021, 12, 11676–11683 RSC.
- D. Pasik, B. N. Frandsen, M. Meder, S. Iyer, T. Kurtén and N. Myllys, J. Am. Chem. Soc., 2024, 146, 13427–13437 CrossRef CAS PubMed.
- B. Nozière, O. Durif, E. Dubus, S. Kylington, A. Emmer, F. Fache, F. Piel and A. Wisthaler, Phys. Chem. Chem. Phys., 2023, 25, 7772–7782 RSC.
- J. D. Crounse, L. B. Nielsen, S. Jørgensen, H. G. Kjaergaard and P. O. Wennberg, J. Phys. Chem. Lett., 2013, 4, 3513–3520 CrossRef CAS.
- M. P. Rissanen, T. Kurtén, M. Sipilä, J. A. Thornton, J. Kangasluoma, N. Sarnela, H. Junninen, S. Jørgensen, S. Schallhart, M. K. Kajos, R. Taipale, M. Springer, T. F. Mentel, T. Ruuskanen, T. Petäjä, D. R. Worsnop, H. G. Kjaergaard and M. Ehn, J. Am. Chem. Soc., 2014, 136, 15596–15606 CrossRef CAS PubMed.
- S. Iyer, M. P. Rissanen, R. Valiev, S. Barua, J. E. Krechmer, J. Thornton, M. Ehn and T. Kurtén, Nat. Commun., 2021, 12, 878 CrossRef CAS PubMed.
- M. Ehn, J. A. Thornton, E. Kleist, M. Sipilä, H. Junninen, I. Pullinen, M. Springer, F. Rubach, R. Tillmann, B. Lee, F. Lopez-Hilfiker, S. Andres, I.-H. Acir, M. Rissanen, T. Jokinen, S. Schobesberger, J. Kangasluoma, J. Kontkanen, T. Nieminen, T. Kurtén, L. B. Nielsen, S. Jørgensen, H. G. Kjaergaard, M. Canagaratna, M. D. Maso, T. Berndt, T. Petäjä, A. Wahner, V.-M. Kerminen, M. Kulmala, D. R. Worsnop, J. Wildt and T. F. Mentel, Nature, 2014, 506, 476–479 CrossRef CAS PubMed.
- E. Öström, Z. Putian, G. Schurgers, M. Mishurov, N. Kivekäs, H. Lihavainen, M. Ehn, M. P. Rissanen, T. Kurtén, M. Boy, E. Swietlicki and P. Roldin, Atmos. Chem. Phys., 2017, 17, 8887–8901 CrossRef.
- J. Brean, R. M. Harrison, Z. Shi, D. C. S. Beddows, W. J. F. Acton, C. N. Hewitt, F. A. Squires and J. Lee, Atmos. Chem. Phys., 2019, 19, 14933–14947 CrossRef CAS.
- T. Kurtén, K. H. Møller, T. B. Nguyen, R. H. Schwantes, P. K. Misztal, L. Su, P. O. Wennberg, J. L. Fry and H. G. Kjaergaard, J. Phys. Chem. Lett., 2017, 8, 2826–2834 CrossRef PubMed.
- D. Draper, T. G. Almeida, S. Iyer, J. N. Smith, T. Kurtén and N. Myllys, J. Aerosol Sci., 2024, 179, 106379 CrossRef CAS.
- D. Pasik, T. Golin Almeida, E. Ahongshangbam, S. Iyer and N. Myllys, Atmos. Chem. Phys., 2025, 25, 4313–4331 CrossRef CAS.
- D. Pasik, S. Iyer and N. Myllys, Phys. Chem. Chem. Phys., 2024, 26, 2560–2567 RSC.
- J.-D. Chai and M. Head-Gordon, J. Chem. Phys., 2008, 128, 084106 CrossRef PubMed.
- J.-D. Chai and M. Head-Gordon, Phys. Chem. Chem. Phys., 2008, 10, 6615–6620 RSC.
- W. J. Hehre, R. Ditchfield and J. A. Pople, J. Chem. Phys., 1972, 56, 2257–2261 CrossRef CAS.
- T. Clark, J. Chandrasekhar, G. W. Spitznagel and P. V. R. Schleyer, J. Comput. Chem., 1983, 4, 294–301 CrossRef CAS.
- P. Pracht, F. Bohle and S. Grimme, Phys. Chem. Chem. Phys., 2020, 22, 7169–7192 RSC.
- C. Bannwarth, S. Ehlert and S. Grimme, J. Chem. Theory Comput., 2019, 15, 1652–1671 CrossRef CAS PubMed.
- J. Kubečka, V. Besel, T. Kurtén, N. Myllys and H. Vehkamaki, J. Phys. Chem. A, 2019, 123, 6022–6033 CrossRef PubMed.
- C. Riplinger and F. Neese, J. Chem. Phys., 2013, 138, 034106 CrossRef PubMed.
- C. Riplinger, B. Sandhoefer, A. Hansen and F. Neese, J. Chem. Phys., 2013, 139, 134101 CrossRef PubMed.
- T. H. Dunning Jr, J. Chem. Phys., 1989, 90, 1007–1023 CrossRef.
- R. A. Kendall, T. H. Dunning and R. J. Harrison, J. Chem. Phys., 1992, 96, 6796–6806 CrossRef CAS.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, G. A. Petersson, H. Nakatsuji, X. Li, M. Caricato, A. V. Marenich, J. Bloino, B. G. Janesko, R. Gomperts, B. Mennucci, H. P. Hratchian, J. V. Ortiz, A. F. Izmaylov, J. L. Sonnenberg, D. Williams-Young, F. Ding, F. Lipparini, F. Egidi, J. Goings, B. Peng, A. Petrone, T. Henderson, D. Ranasinghe, V. G. Zakrzewski, J. Gao, N. Rega, G. Zheng, W. Liang, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, K. Throssell, J. A. Montgomery, Jr., J. E. Peralta, F. Ogliaro, M. J. Bearpark, J. J. Heyd, E. N. Brothers, K. N. Kudin, V. N. Staroverov, T. A. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. P. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, J. M. Millam, M. Klene, C. Adamo, R. Cammi, J. W. Ochterski, R. L. Martin, K. Morokuma, O. Farkas, J. B. Foresman and D. J. Fox, Gaussian 16 Revision C.02, Gaussian Inc, 2016, Wallingford CT Search PubMed.
- F. Neese, Wiley Interdiscip. Rev.:Comput. Mol. Sci., 2012, 2, 73–78 CAS.
- L. Vereecken and J. Peeters, J. Chem. Phys., 2003, 119, 5159–5170 CrossRef CAS.
- L. P. Viegas, J. Phys. Chem. A, 2018, 122, 9721–9732 CrossRef CAS PubMed.
- L. P. Viegas, J. Phys. Chem. A, 2021, 125, 4499–4512 CrossRef CAS PubMed.
- K. H. Møller, R. V. Otkjær, N. Hyttinen, T. Kurtén and H. G. Kjaergaard, J. Phys. Chem. A, 2016, 120, 10072–10087 CrossRef PubMed.
- D. R. Glowacki, C.-H. Liang, C. Morley, M. J. Pilling and S. H. Robertson, J. Phys. Chem. A, 2012, 116, 9545–9560 CrossRef CAS PubMed.
- T. Berndt, S. Richters, R. Kaethner, J. Voigtländer, F. Stratmann, M. Sipilä, M. Kulmala and H. Herrmann, J. Phys. Chem. A, 2015, 119, 10336–10348 CrossRef CAS PubMed.
- K. Joback and R. Reid, Chem. Eng. Commun., 1987, 57, 233–243 CrossRef CAS.
- C. W. Gao, J. W. Allen, W. H. Green and R. H. West, Comput. Phys. Commun., 2016, 203, 212–225 CrossRef CAS.
- L. S. Tee, S. Gotoh and W. E. Stewart, Ind. Eng. Chem. Fundam., 1966, 5, 356–363 CrossRef.
- B. de Souza, Angew. Chem., Int. Ed., 2025, 64, e202500393 CrossRef CAS PubMed.
- J. R. Wells, Environ. Sci. Technol., 2005, 39, 6937–6943 CrossRef CAS PubMed.
- M. A. Pimlott, R. J. Pope, B. J. Kerridge, B. G. Latter, D. S. Knappett, D. E. Heard, L. J. Ventress, R. Siddans, W. Feng and M. P. Chipperfield, Atmos. Chem. Phys., 2022, 22, 10467–10488 CrossRef CAS.
- E. Villenave, R. Lesclaux, S. Seefeld and W. R. Stockwell, J. Geophys. Res.: Atmos., 1998, 103, 25273–25285 CrossRef CAS.
- I. Karppinen, D. Pasik, E. Ahongshangbam and N. Myllys, Aerosol Res., 2025, 3, 175–183 CrossRef.
- M. S. Stark, J. Phys. Chem. A, 1997, 101, 8296–8301 CrossRef CAS.
- B. N. Frandsen, L. Franzon, M. Meder, D. Pasik, E. Ahongshangbam, N. Vinkvist, N. Myllys, S. Iyer, M. P. Rissanen and M. Ehn, et al. , ACS Earth Space Chem., 2025,(6), 1322–1337 CrossRef CAS.
- K. H. Møller, R. V. Otkjær, J. Chen and H. G. Kjaergaard, J. Phys. Chem. A, 2020, 124, 2885–2896 CrossRef PubMed.
- L. Vereecken, T. L. Nguyen, I. Hermans and J. Peeters, Chem. Phys. Lett., 2004, 393, 432–436 CrossRef CAS.
- J. M. Anglada, R. Crehuet and J. S. Francisco, Chem.–Eur. J., 2016, 22, 18092–18100 CrossRef CAS PubMed.
- L. Vereecken and B. Nozière, Atmos. Chem. Phys., 2020, 20, 7429–7458 CrossRef CAS.
- T. Yli-Juuti, T. Mielonen, L. Heikkinen, A. Arola, M. Ehn, S. Isokääntä, H.-M. Keskinen, M. Kulmala, A. Laakso and A. Lipponen, et al. , Nat. Commun., 2021, 12, 5637 CrossRef CAS PubMed.
- M. Schervish and N. M. Donahue, Atmos. Chem. Phys., 2020, 20, 1183–1199 CrossRef CAS.
- L. Dada, D. Stolzenburg, M. Simon, L. Fischer, M. Heinritzi, M. Wang, M. Xiao, A. L. Vogel, L. Ahonen, A. Amorim, R. Baalbaki, A. Baccarini, U. Baltensperger, F. Bianchi, K. R. Daellenbach, J. DeVivo, A. Dias, J. Dommen, J. Duplissy, H. Finkenzeller, A. Hansel, X.-C. He, V. Hofbauer, C. R. Hoyle, J. Kangasluoma, C. Kim, A. Kürten, A. Kvashnin, R. Mauldin, V. Makhmutov, R. Marten, B. Mentler, W. Nie, T. Petäjä, L. L. J. Quéléver, H. Saathoff, C. Tauber, A. Tome, U. Molteni, R. Volkamer, R. Wagner, A. C. Wagner, D. Wimmer, P. M. Winkler, C. Yan, Q. Zha, M. Rissanen, H. Gordon, J. Curtius, D. R. Worsnop, K. Lehtipalo, N. M. Donahue, J. Kirkby, I. E. Haddad and M. Kulmala, Sci. Adv., 2023, 9, eadi5297 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2026 |