Open Access Article
Open Access ArticleStructural diversity in Zn(II)/Cu(II) complexes with salicylic/acetylsalicylic acids and bis(4-pyridylthio)methane: coordination polymers, one organic cocrystal, and one organic salt
Olaya
Gómez-Paz
 ab,
Rosa
Carballo
ab,
Rosa
Carballo
 *ab,
Ana B.
Lago
*ab,
Ana B.
Lago
 *c,
Ezequiel M.
Vázquez-López
*c,
Ezequiel M.
Vázquez-López
 ab and
Berta
Covelo
d
ab and
Berta
Covelo
d
aUniversidade de Vigo, Departamento de Química Inorgánica, Facultade de Química, 36310 Vigo, Spain. E-mail: alagobla@ull.edu.es
bMetallosupramolecular Chemistry Group, Galicia Sur Health Research Institute (IIS Galicia Sur). SERGAS-UVIGO, Galicia, Spain
cDepartamento de Química, Facultad de Ciencias, Sección Química Inorgánica, Universidad de la Laguna, 38206 La Laguna, Spain
dServizo de Determinación Estructural, Proteómica e Xenómica, CACTI Universidade de Vigo, Spain
First published on 15th December 2025
Abstract
The solid-state chemistry of Zn(II)/Cu(II) salts with salicylic and acetylsalicylic acids and bis(4-pyridylthio)methane (SCS) was explored using various synthetic methods, leading to the isolation of several crystalline materials. These included three polymorphic forms of the one-dimensional coordination polymer 1∞Zn(Hsal)2(SCS) (1m, 1m′ and 1o), as well as the hydrated pseudopolymorph 1∞Zn(Hsal)2(SCS)·H2O (1w). Additionally, a two-dimensional polymer 2∞Cu(Hsal)2(SCS)·CH3CN (2·CH3CN), a three-dimensional salicylate polymer 3∞Cu5(Hsal)6(sal)2(SCS)4(H2O)2 (4·solv), and a two-dimensional aspirinate polymer 2∞Cu3(CH3CO2)4asp2(SCS)3(H2O)2 (3·solv), which also contain a coordinated acetate anion, were obtained. In most systems, the crystallization of the organic salt [(H-SCS)(Hsal)] (5) and the organic cocrystal [(SCS)(H2sal)2] (6) was also observed. All materials were structurally characterized. Stability studies of compound 1m were conducted in water, under acidic conditions and in physiological PBS buffer. Furthermore, the cytotoxicity of the zinc polymorphic polymers was evaluated against non-tumoral human cells (MRC-5) and tumour cell lines (NCI-H460, A549 and MDA-MB231).
Introduction
Bioactive organic molecules, such as drugs and biomolecules, contain functional groups (carboxylate, phenol, thiol, heterocyclic nitrogen, etc.) that can act as ligands towards metal cations to form metal complexes. The coordination chemistry of these molecules may have some biological and pharmacological implications, for example in metalloenzymes mimicking biological functions or in metallodrugs with enhanced drug activity. Metallodrugs, like other metal complexes, can exhibit sensitivity to various stimuli like pH changes, light exposure, ion activation, or redox conditions so that they could result in efficient systems for the controlled release of a certain drug.1 In this field, coordination polymers are currently attractive host materials for drug incorporation.2,3Among bioactive organic ligands are salicylates, highlighting salicylic acid (H2sal) which is the principal metabolite of acetylsalicylic acid (Aspirin®, Hasp) and responsible for the anti-inflammatory, antipyretic and analgesic effects of aspirin. On the other hand, salicylic acid and salicylates are naturally present in various plants, fruits, vegetables and spices and it has been suggested that a diet rich in these compounds helps to reduce the risk of suffering from some diseases such as colorectal cancer.4 In addition, aspirin has an antiplatelet effect and is therefore used at low but prolonged doses to prevent heart attacks, ischemic strokes and blood clots in high-risk people.
To coordinate metal cations, aspirin typically involves the carboxylate group, as exemplified by Cu2(asp)4, which has demonstrated antioxidative properties.5 After the hydrolysis of aspirin, the resulting salicylic acid can coordinate to metal cations through the carboxylate and phenol/phenolate groups, yielding structurally different metal salicylates.6 The combination of metal cations, aspirin/salicylic acid and an N,N′-divergent ligand as a linker between metal cations offers the possibility of obtaining coordination polymers hosting the bioactive molecules. This type of strategy was used with several carboxylate-based drugs such as nonsteroidal anti-inflammatory drugs (NSAIDs): naproxen, ketoprofen, ketorolac, biotin, levodopa, indomethacin, carbocysteine,2 ibuprofen2,7 and diclofenac.2,3
We are interested in the preparation and study of organized solid-state materials based on coordination polymers containing a coordinated drug or active pharmaceutical ingredient (API). In this way, we have previously explored the Zn(II)/ibuprofen/bis(4-pyridylthio)methane (SCS) system,7 finding that the resulting 1D polymeric material shows a high drug content and stability and that the N,N′-divergent ligand SCS is very useful for the preparation of such coordination polymers. More recently, Murphy and colleagues3 introduced the concept of Therapeutic Coordination Polymers (TCPs), non-porous coordination polymers that utilize metal–ligand interactions to achieve degradation-based drug release. Building on these works, we have considered exploring the incorporation of aspirin or salicylic acid in Zn(II)/Cu(II) coordination polymers facilitated by the linking role of SCS.
A bibliographic search on crystalline materials based on Zn(II) or Cu(II) coordination compounds with salicylate and an N,N′-divergent ligand yielded the information summarized in Table S1.8–12 From this information several observations can be drawn: (i) the compounds have been prepared by hydro/solvothermal or diffusion methodologies; (ii) when aspirin (acetylsalicylic acid, Hasp) is used as a reagent, hydrolysis occurs, and the resulting metal complex incorporates salicylate (Hsal−); (iii) the dimensionality of the metal complexes ranges from 0D to 2D, but the N,N′-divergent ligand does not always yield coordination polymers,8,10 and the salicylate (Hsal−) contributes to an increase in dimensionality in one case10; (iv) in all cases, the salicylate ligand uses the carboxylate group to coordinate the metal cations showing monodentate, chelating and bis-monodentate bridging coordination modes; and (v) in the Cu(II)/Hsal−/4,4′-bipy system,10,11 the synthesis by diffusion yields different molecular and polymeric crystalline species, showing that under very similar synthetic conditions, different metal complexes can be stabilized. These observations have aroused our interest in exploring the solid-state coordination chemistry of the Cu(II) and Zn(II)/aspirin and salicylic acid/bis(4-pyridylthio)methane (SCS) system using different synthetic methods to investigate the possibilities of obtaining various crystalline species. As a result, we report here the preparation and structural characterization of three polymorphic forms and a pseudopolymorph of a 1D Zn(II) coordination polymer, two 2D and one 3D Cu(II) coordination polymers, one organic cocrystal and one organic salt.
Results and discussion and experimental
Synthesis and characterization
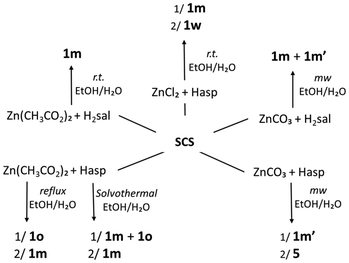
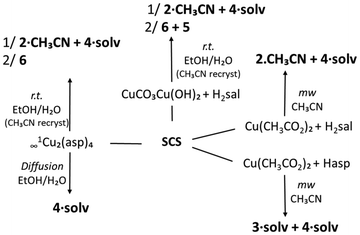
To study the reactivity of the Zn(II)/Cu(II)–SCS–salicylic/acetylsalicylic acid system, several synthetic methods were used: stirring at room temperature, heating under reflux, diffusion techniques, solvothermal conditions and reactions under microwave irradiation. Also, different metal cation precursors have been tested: for the Zn(II) compounds, ZnCl2, ZnCO3 and Zn(OAc)2 salts and for the Cu(II) complexes, Cu(OAc)2, CuCO3 Cu(OH)2 and the previously prepared Cu2asp4 (asp = acetylsalicylate/aspirinate).5 Most of the reactions were carried out in an EtOH/H2O mixture, except for those with Cu(II) performed under microwave irradiation using CH3CN.As a result of the synthetic work, the compounds listed in Scheme 1 have been isolated and characterized.
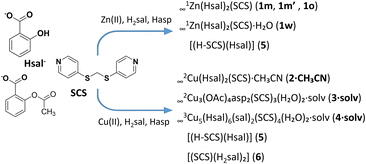 | ||
| Scheme 1 Compounds isolated from the reaction of SCS, H2sal or Hasp and different zinc and copper salts. | ||
The resulting Zn(II) salicylate complexes are 1D polymers crystallized with different morphologies corresponding to three different polymorphs: two monoclinic forms (1m and 1m′) and a third orthorhombic form (1o), and also the pseudopolymorph 1w containing crystallization water. The isolated Cu(II) compounds are two salicylate coordination polymers (the 2D 2·CH3CN and the 3D 4·solv) and the 2D aspirinate polymer 3·solv which also contain a coordinated acetate anion. This last compound is the only one that contains an aspirinate ligand; in all other cases where acetylsalicylic acid or Cu2asp4 was used as a precursor, a deacetylation process occurred, producing a salicylate ligand.
Although simultaneous crystallization of the Zn(II) polymorphs is observed, some synthetic aspects can be highlighted. As shown in Scheme 2, the polymorph 1m is obtained under all the synthetic conditions tested, showing that it is the more stable species. For the preparation of the polymorph 1m′, the use of ZnCO3 under microwave irradiation is required and in consequence, the key factor seems to be the metal salt. The polymorph 1o was isolated only when Zn(CH3CO2)2 and aspirin were used under reflux or solvothermal conditions, suggesting that the combination of the two reagents is important. The pseudopolymorph 1w has been isolated only once, in low yield, from a reaction using ZnCl2 at room temperature, and again, the metal salt seems to be the key factor.
The synthetic results for the Cu(II) compounds (Scheme 3) show that the salicylate coordination polymers 2·CH3CN and 4·solv consistently precipitate or crystallize simultaneously, regardless of the metal precursor or synthetic method used. For the crystallization of 2·CH3CN, the presence of CH3CN is essential; even when the reaction is carried out in EtOH/H2O, recrystallization in CH3CN is required to obtain the crystalline material. Additionally, the synthetic conditions associated with the diffusion technique using Cu2asp4 appear to favour the crystallization of 4·solv. The aspirinate coordination polymer 3·solv was obtained in low yield only under microwave irradiation of a CH3CN solution containing acetylsalicylic acid and Zn(CH3COO)2, again indicating that the presence of CH3CN is a determining factor in the crystallization process.
The salt [(H-SCS)(Hsal)] (5) and the cocrystal [(SCS)(H2sal)2] (6) have been obtained in the final phases of crystallization in several of the synthetic processes previously described (Schemes 2 and 3). Compound 5 resulted from the reaction of ZnCO3 with aspirin and was also obtained, mixed with 6, from the reaction of CuCO3Cu(OH)2 with salicylic acid. Compound 6 crystallized from the medium of the reaction with Cu2asp4 that suffers decomposition and deacetylation of the coordinated aspirinate. Compounds 5 and 6 could also be crystallized, together from an EtOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O solution of SCS and aspirin, and consecutively (first 6, second 5) from an EtOH
H2O solution of SCS and aspirin, and consecutively (first 6, second 5) from an EtOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O solution of SCS and salicylic acid.
H2O solution of SCS and salicylic acid.
The diffractograms (XRD) of the compounds are in good agreement with the simulated patterns generated from single-crystal diffraction data (Fig. S4–S8).
The infrared spectra of the compounds (Fig. S1 and S2) present the characteristic bands of SCS, sometimes slightly displaced due to the coordination to the metal cations. The bands observed in the range 3400–3050 cm−1 are due to the OH vibrations from the hydroxyl groups and coordinated water molecules in compounds 3·solv and 4·solv. The spectra also show bands close to 1600 cm−1 and between 1330 and 1390 cm−1, attributable to νas(OCO) and νs(OCO), respectively. In compound 6, the intense band at 1736 cm−1 (absent in the other compounds), assignable to the ν(CO) vibration mode, agrees with the presence of salicylic acid (H2sal). In the spectra of 2·CH3CN and 3·solv, the weak band around 2300 cm−1 suggests the presence of CH3CN in the second coordination sphere. In 5, the broad band centered at 3370 cm−1 agrees with the presence of a protonated SCS pyridine in the salt.
The diffuse reflectance spectra (Fig. S3) of the Cu(II) compounds show broad bands between 640 and 680 nm attributable to d–d transitions, additional bands around 400 nm corresponding to ligand-to-metal charge transfer and bands between 309 and 323 nm due to intraligand transitions.
Crystal structures of polymers containing salicylate (Hsal−/sal−2) ligands
Crystal data and structure refinement parameters are reported in Tables S2 and S3, and selected bond lengths and angles are listed in Tables S4–S6. Table 1 shows the coordination modes adopted by the salicylate Hsal−/sal−2 ligands in the coordination polymers 1m, 1m′, 1o, 1w, 2·CH3CN and 4·solv.Fig. 1 shows the 1D structures and metal coordination environments of the three polymorphic forms of 1∞Zn(Hsal)2(SCS) (1m, 1m′ and 1o) and of the pseudopolymorph 1∞Zn(Hsal)2(SCS)·H2O (1w). 1∞Zn(Hsal)2(SCS) crystallizes in three different phases: the centrosymmetric prismatic 1m and lamellar 1m′ crystals (P21/c) and the orthorhombic acentric non-polar 1o (P212121) phase. All the compounds are 1D coordination polymers due to the bridging role of the SCS ligand.
 | ||
| Fig. 1 Crystal structures and coordination environments of 1D polymers 1m, 1m′, 1o and 1w and superposition of the SCS ligand in the four polymers (bottom). | ||
In 1m, 1m′ and 1w, the Zn cations are in an N2O2 environment, involving two nitrogen atoms of two bridging SCS ligands and two oxygen carboxylate atoms of two monodentate salicylate (Hsal−) ligands (Table 1). The coordination geometry around the Zn cation can be evaluated using the τ4 parameter15 so that a value of 1 corresponds to a regular tetrahedral (Td) geometry, a value of 0 indicates a regular square-planar geometry (D4h), and a value of 0.85 is due to a trigonal pyramidal geometry (C3v). Compounds 1m and 1m′ present τ4 parameters of 0.88 and 0.89, respectively, indicating coordination geometries close to trigonal pyramidal. Compound 1w presents two tetracoordinated Zn cations with coordination geometries slightly different: close to trigonal pyramidal around Zn1 (τ4 = 0.87) and close to tetrahedral around Zn2 (τ4 = 0.92). Compound 1o presents the cation in an N2O3 environment with a monodentate Hsal− and an anisobidentate chelating carboxylate of another Hsal−1 ligand (Fig. 1). The geometry around the pentacoordinated cation can be evaluated through the τ5 parameter16 (τ5 = 0, square pyramidal; τ5 = 1, trigonal bipyramidal), so a value of 0.22 indicates a distorted square pyramidal geometry. The polymorphism of 1∞Zn(Hsal)2(SCS) is due to the flexibility of the SCS ligand which adopts different conformations in each polymorph:17 GA in 1m, AA in 1m′ and G+G+ in 1o. Fig. 1 illustrates this aspect by showing a superposition of the SCS ligands in the four polymers. In 1w, 1∞Zn(Hsal)2(SCS) crystallizes including water in the lattice, and in this case, the SCS ligand presents two different conformations: GA and G+G+. The four 1D polymers present intrachain hydrogen bonds (Tables S7 and S8) between the hydroxyl group and the uncoordinated oxygen atom of the carboxylate group of each salicylate ligand. In 1o, the bidentate salicylate is also involved in hydrogen bonding with the hydroxyl group (Table S7). The packing of the chains of the four compounds is mainly due to C–H⋯O hydrogen bonds. In 1m′ and 1o, the packing is reinforced by π–π interactions between the SCS pyridine rings with centroid–centroid distances of 3.546 and 3.493 Å, respectively. In 1w, the water molecule in the second coordination sphere is mainly responsible for the packing, acting as a hydrogen donor and an acceptor between four chains. These interactions produce efficient packings as shown by the corresponding Kitaigorodskii packing indices:18 71% for 1m and 1m′, 67% for 1o and 72% for 1w.
Fig. 2 and 3 show the 2D and 3D structures and the metal coordination environments of 2∞Cu(Hsal)2(SCS)·CH3CN (2·CH3CN) and 3∞Cu5(Hsal)6(sal)2(SCS)4(H2O)2 (4·solv), respectively.
 | ||
| Fig. 3 Crystal structure and coordination environments of the 3D polymer 4·solv. Hydrogen atoms are omitted to improve visualization. | ||
The 2D polymerization in 2·CH3CN is achieved by the coordination bridging role of the SCS and Hsal− ligands (Fig. 2). The metal cation is coordinated to three oxygen atoms of three salicylate anions and to two nitrogen atoms of two molecules of SCS with a G+G+ configuration.17 The Addison parameter16 of 0.01 indicates a square-based pyramidal geometry. In the structure, each salicylate ligand behaves in a different way (Table 1): one acts as a terminal monodentate by the carboxylate group, while the other acts as a bis-monodentate bridge through the carboxylate and the hydroxyl groups. In the base of the pyramid, the Cu–O and Cu–N distances range between 1.949 and 2.022 Å, and at the apical position, occupied by the hydroxyl group, the distance increases to 2.345 Å. In the 2D structure, rectangular metallocycles are formed involving four Cu(II) cations, two SCS molecules and two Hsal− ligands (Fig. 2) with Cu⋯Cu distances of 7.159 Å through the Hsal−1 ligand and 12.340 Å through the SCS ligand. As in the previously described 1D coordination polymers, the Hsal− ligands establish intramolecular OH⋯Ocarb hydrogen bonds. The packing of the sheets involves the CH3CN of crystallization through CHSCS⋯N (C⋯N distances between 3.501 and 3.730 Å) (Table S9) and CH(CH3CN)⋯π(Hsal− ring) (C⋯centroid distance, 3.550 Å) interactions. The packing is reinforced by the contribution of a π(SCS ring)⋯π(Hsal− ring) interaction (centroid–centroid distance = 3.594 Å), leading to a Kitaigorodskii packing index18 of 66.7%.
The coordinative bridging role of the SCS, Hsal− and sal−2 ligands gives rise to the 3D coordination polymer 4·solv. As shown in Table 1, the salicylate ligands take four different coordination modes: Hsal− acts as monodentate, bidentate chelate and bis-monodentate through the carboxylate group, and sal−2 acts as a bis-bidentate chelate bridging two metal centres (Fig. 3). The structure of 4·solv is based on two types of metal nodes, Cu2O8 and Cu3O10, connected by SCS ligands. In the dinuclear Cu2O8 node (Fig. 3, left), the metal cations are in a distorted octahedral environment involving two oxygen atoms of a bidentate chelating Hsal−, two oxygen atoms of a bis-monodentate bridging Hsal−, and, at the axial positions, two nitrogen atoms of two SCS ligands. In the trinuclear Cu3O10 node (Fig. 3, right), the central metal cation (Cu3) is octahedrally coordinated by two SCS ligands and four carboxylate oxygen atoms belonging to two sal−2 ligands that coordinate also to two lateral metal cations (Cu2). These metal cations are in a square-pyramidal geometry (τ5 = 0 (ref. 16)) based on two oxygen atoms (carboxylate and deprotonated hydroxyl groups) of the sal−2 ligand, one carboxylate oxygen atom of a monodentate Hsal− ligand, one water molecule and one SCS ligand. The SCS ligands connecting the nodes present two different conformations: GA and G+G+.17 The Kitaigorodskii packing index18 of 62.5% is lower than those calculated for the M/SCS/salicylate coordination polymers of lower dimensionality discussed above.
Crystal structure of the polymer containing aspirinate, 2∞Cu3(OAc)4asp2(SCS)3(H2O)2 (3·solv)
3·solv is the only compound obtained with acetylsalicylate (asp−) coordinated without undergoing de-acetylation and the acetate ligand from the metal precursor. The 2D structure of this compound contains three different octahedral metal centres (Fig. 4). A planar Cu2O6 node, with four oxygen atoms belonging to two anisobidentate chelating acetate ligands and other two oxygen atoms of two bis-monodentate bridging acetate ligands, is connected by SCS ligands to two different octahedral Cu(II) centres (Cu2 and Cu3 in Fig. 4). The octahedral Cu2 center is coordinated by two anisobidentate chelating acetate ligands and two bridging SCS ligands at the axial positions. The Cu3 center is the only metal cation in the structure involved in the coordination to two monodentate acetylsalicylate (asp−) ligands. The octahedral environment around Cu3 is completed with two water molecules and two bridging SCS ligands (Fig. 4). All the SCS ligands in 3·solv present the conformation G+G+.17 The highly disordered solvent component, which represents a potential solvent area of 723.3 Å3, could not be taken into account for the determination of the model and the Kitaigorodskii packing index18 under these conditions is 55.5%. | ||
| Fig. 4 Crystal structure and coordination environments of the 2D polymer 3·solv. Hydrogen atoms are omitted to improve visualization. | ||
Crystal structure of the salt [(H-SCS)(Hsal)] (5) and the cocrystal [(SCS)(H2sal)2] (6)
Selected bond lengths and angles and hydrogen-bond distances are listed in Tables S6 and S10, respectively. The prismatic crystals of 5 and the needle-shaped crystals of 6 crystallize in the monoclinic P21/c and the orthorhombic P212121 space groups, respectively. The structural resolution shows the ionic nature of 5 stabilized by the charge-assisted pyridinium-carboxylate N–H+⋯O− synthon and the neutral nature of 6 based on the O–H⋯N hydrogen bond synthon (Fig. 5). The stoichiometric ratio is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in 5, involving the protonation of one of the two pyridyl groups of one SCS molecule in a GA conformation.17 The stoichiometry in 6 is 2
1 in 5, involving the protonation of one of the two pyridyl groups of one SCS molecule in a GA conformation.17 The stoichiometry in 6 is 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 with the two pyridyl groups of one SCS molecule in a conformation G+G+
1 with the two pyridyl groups of one SCS molecule in a conformation G+G+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17 involved in hydrogen bonds with two carboxylic groups of two salicylic acid molecules. The C–O bond length analysis allows differentiating between salt and the cocrystal.19 In 5, a difference of 0.020 Å between the C–O distances agrees with the salt nature of the compound, and in 6, the differences of 0.066 and 0.076 Å correspond to a cocrystal compound.
17 involved in hydrogen bonds with two carboxylic groups of two salicylic acid molecules. The C–O bond length analysis allows differentiating between salt and the cocrystal.19 In 5, a difference of 0.020 Å between the C–O distances agrees with the salt nature of the compound, and in 6, the differences of 0.066 and 0.076 Å correspond to a cocrystal compound.
The packing of the salt 5 (Fig. 5) is mainly due to the contributions of the following interactions: CHpy⋯Nunprotonated (distance C⋯N = 3.327 Å), CHpy⋯πHsal (distance C⋯Hsal− centroid = 3.468 Å) and π(SCS ring)–π(SCS ring) with a distance between pyridine centroids of 3.602 Å. The packing of the cocrystal 6 (Fig. 5) is organized mainly by interactions involving the thioether sulfur atoms of SCS: OH⋯S (distance O⋯S = 3.220 Å), CH (methylene group of SCS)⋯S (distance C⋯S = 3.452 Å) and CHpy⋯S (distance C⋯S = 3.629 Å).
Thermal stability
The thermal stability of the compounds was investigated by thermogravimetric analysis (Fig. S11). Different thermal behaviours were observed for the unsolvated and the solvated compounds. In the solvated compounds 2·CH3CN, 3·solv and 4·solv, the first weight loss, at 150 °C, 130 °C and 100 °C, respectively, corresponds to the solvent loss. After this first step, the metal–organic framework begins its decomposition, which is completed around 300 °C. The unsolvated polymers are stable until 200 °C and a weight loss of around 68%, corresponding to the organic component, occurs between 200 °C and 400 °C.Despite their different natures, ionic and neutral, compounds 5 and 6 present a similar thermal behaviour. Both compounds are stable up to 150 °C where their thermal degradation begins, which is completed at 350 °C.
Chemical stability
The ability of 1m (1D), 3·solv (2D), and 4·solv (3D) to preserve their long-range ordered structure and release their components under various chemical conditions was evaluated. The release of SCS or salicylate ligands was examined in water, acidic environments, and simulated physiological conditions using a phosphate-buffered solution (PBS, pH = 7.4).Generally, the behaviour of copper compounds 3·solv and 4·solv was consistent across the different media tested, whereas the behaviour of the Zn compound 1m was different. The supernatants of the solutions were analysed using UV-Vis spectroscopy after the compounds were immersed in water for 7, 14, and 21 days. No significant temporal differences were observed in the bands; however, the bands varied depending on the initial compound. A single band was observed for 1m (275 nm) and 3·solv (300 nm), while two bands were noted for 4·solv (275 and 295 nm). These observations are likely associated with a higher concentration of SCS in the water solution of 1m and a mixture of ligands in 4·solv.
A similar behaviour was observed for 1m when the study was conducted in PBS solution, with aliquots collected from 4 hours to 26 days, revealing a single band oscillating between 266–273 nm (Fig. S21). Conversely, 3·solv and 4·solv exhibited similar behaviours, with a band at 266 nm and two shoulders (at 240 and 296 nm) in the first 24 hours, which increased in intensity over time until the band at 295 nm became the most intense (approximately after 7 days). Ultimately, a single, intense band was observed at 272 nm. The PXRD pattern of water-treated 1m indicates a transformation into a structure distinct from any of the polymorphs or salts isolated in this study (Fig. S14 and S15). The diffraction patterns of the solids remaining after water treatment of 3·solv and 4·solv demonstrate that both systems are crystalline and correspond to a pattern attributed to the same unidentified structure.
During the studies conducted in an acidic environment, it was observed that the pH stabilised in 30 minutes after the acid was introduced (Table S11). The behaviour was consistent across all compounds: the lower-intensity bands (ranging from 240–300 nm) shifted to bands of higher intensity and wavelength, specifically 312 nm when the pH was approximately 5 and 318 nm when the pH was approximately 3 (Fig. S16–S18). This behaviour is probably associated with an initial release of the SCS ligand, with a greater release of salicylic acid as the pH of the medium decreases. Regarding the solids resulting from these tests, 3·solv exhibited considerable amorphous characteristics, 4·solv retained good crystallinity, despite the inability to identify the phase, and the compound resulting from the acid treatment of 1m exhibited the structure of [HSCS][ZnCl4].7
In total, the analysis of the various UV-Vis spectra revealed the release of the SCS ligand and salicylic acid, as both combined bands or isolated SCS or salicylic bands were observed. However, the precise assignment of each analyte was not feasible due to the proximity and potential overlap of the bands. Additionally, these bands are likely to undergo bathochromic shifts upon protonation.7 The substantial differences in the release of ligands from these three compounds demonstrate the significant effect that structure and the metal ion could have on the release of salicylic acid from these materials. These findings suggest the potential to modulate and control the presence of the bioactive molecule, salicylic acid, using the compounds developed in this study as drug carriers.
Cytotoxicity
The procedure followed for the cytotoxicity experiments is detailed in the SI. The cytotoxicity levels of polymorphs 1m and 1w were evaluated against both normal cells (MRC-5, non-tumour human fibroblasts) and tumour cells (NCI-H460, A549, and MDA-MB231). This comprehensive approach to testing allows for a thorough understanding of the compounds’ effects on various cell types, providing crucial information about their selectivity and potential side effects.The selection of these polymers for this study was based on several considerations: firstly, the drug content is notably high, with salicylic content of approximately 48 wt% in 1m, 1m′, and 1o, and reaching 46 wt% in 1w. Secondly, the structural simplicity is characterized by a lower number of components. Lastly, the synthetic procedure employed is notably straightforward, yielding higher outputs. The ability to efficiently synthesize these compounds in substantial quantities is crucial for guaranteeing large-scale production.
The cytotoxicity levels of 1m and 1w against normal cells (MRC-5, non-tumour human fibroblasts) were determined and compared with the SCS or ZnCl2(SCS) compounds.7 The results of these experiments are included in Table S12. All the compounds exhibited low growth inhibition at the maximum concentration assayed (10 ± 3% for 100 μM) and the growth inhibition curve was not calculated. These results suggest that the introduction of high concentrations of SCS and Zn compounds barely alters the cytotoxicity profile of SCS and Zn compounds on MRC-5 cells. The cytotoxicity of the polymeric compounds 1 and 1w is related to the toxicity arising from the interactions between salicylic acid, the SCS ligand and Zn ions of the polymeric compounds with MRC-5 cells. The data obtained for compounds 1m and 1w were considered interesting for expanding cytotoxicity studies with three more cell lines.
The MTT assay was employed to evaluate the in vitro cytotoxicity of polymorphs 1m and 1m′ and pseudopolymorph 1w against multiple cancer cell lines, including MDA-MB-231 (triple-negative breast cancer) and A549 and NCI–H460 (lung cancer). The results of the MTT assay are presented in Table 2. While they were less cytotoxic than cisplatin, they showed cytotoxicity in all the tested cells. Notably, compound 1w exhibits the highest cytotoxicity against all the tested cells. Within the series, 1w showed almost double cytotoxicity effects in A549 cells, while all the compounds exhibited similar cytotoxicity against MDA-MB231 cells. These results could be very helpful for a thorough understanding of the crystal structure effects on various cell types, providing information about their selectivity and potential side effects.
| Compounds | Cell line | ||
|---|---|---|---|
| NCI-H460 | A549 | MDA-MB231 | |
| 1m | 35 ± 1 | 28 ± 1 | 43 ± 2 |
| 1m′ | 35 ± 1 | 26 ± 2 | 42 ± 1 |
| 1o | 38 ± 1 | 26 ± 1 | 41 ± 1 |
| 1w | 50 ± 2 | 44 ± 1 | 47 ± 1 |
| Reference (cisplatin) | 67 ± 2 | 78 ± 1 | 78 ± 1 |
The selective effect of the drug could be evaluated by comparing the cytotoxic activity of each compound against cancer and normal cells. The % growth inhibition suggests that compound 1w could selectively target breast and lung cancer cells while exerting minimal effects on normal cells, as shown by the % growth inhibition observed with MRC-5. This is particularly relevant given that breast and lung cancers are among the most prevalent and clinically challenging cancers worldwide, underscoring the importance of developing effective therapeutic agents for these types.20
Conclusions
The implementation of diverse synthetic methodologies within the Zn(II)/Cu(II)–SCS–salicylic/acetylsalicylic acid system enables the formation and isolation of multiple crystalline species, occasionally occurring simultaneously. This approach has yielded 1D Zn(II) coordination polymers, as well as 2D and 3D Cu(II) coordination frameworks, alongside the salt [(H-SCS)(Hsal)] and the cocrystal [(SCS)(H2sal)2]. In all metal complexes, the N,N′-divergent SCS ligand acts as a bridging unit, promoting 1D polymerization. Its conformational flexibility also accounts for the formation of three polymorphic forms of 1∞Zn(Hsal)2(SCS). In most cases, when aspirin or the Cu2asp4 complex was used as a reactant, a deacetylation process occurred, yielding salicylate ligands. Only in one instance was it possible to isolate the Cu(II) aspirinate compound 2∞Cu3(CH3CO2)4asp2(SCS)3(H2O)2. In these compounds, the salicylate ligands exhibit distinct coordination behaviours. The common monoanionic form, Hsal−, coordinates either to a single metal centre or bridges two metal ions, thereby contributing to the formation of coordination polymers. In contrast, the dianionic form, sal2−, binds to two metal centres through both carboxylate oxygen atoms and the phenoxide oxygen, facilitating the construction of a 3D coordination framework. Notably, the 3D polymer 3Cu5(Hsal)6(sal)2(SCS)4(H2O)2 incorporates both anionic forms of salicylate: Hsal− adopts two distinct coordination modes, while sal−2 is responsible for driving the 3D polymerization. Compounds 1m, 3·solv, and 4·solv demonstrated distinct release patterns of SCS and salicylate ligands in water, acidic environments, or simulated physiological conditions. These findings highlight the potential of these compounds as drug carriers for controlled release of bioactive molecules, specifically salicylic acid. Compounds 1m and 1w demonstrated low cytotoxicity against normal cells while exhibiting cytotoxic effects on various cancer cell lines. Notably, compound 1w showed the highest cytotoxicity against all tested cancer cells, with particularly strong effects on A549 lung cancer cells. These findings underscore the importance of the crystal structure in determining biological activity and pave the way for future research into the optimization and application of these zinc-based polymeric compounds in therapeutic applications.Experimental
Materials and methods
The ligand bis(4-pyridylthio)methane (SCS) was prepared as described previously.21 The precursor 1∞Cu2asp4 (Hasp = aspirin) was prepared by reaction of Cu(CH3CO2)2·H2O with acetylsalicylic acid in H2O.13,14 The other starting materials and solvents were obtained commercially and used as supplied. Elemental analysis (C, H, N) was carried out on a Fisons EA-1108 microanalyser. Infrared spectra were recorded in the solid phase by ATR on a Jasco FT/IR-6100 spectrophotometer. Solid state and solution UV-vis spectra were recorded on a Jasco V670 spectrophotometer over the range of 1200–200 nm. TGA/DSC analysis profiles were obtained with the thermal analyser TGA-ATD/DSC, SETSYS Evolution 1750 (Setaram).Synthesis and crystallization of the complexes
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) was stirred at room temperature for 24 h. The resulting white precipitate of 1m was filtered off and dried under vacuum. Colourless single crystals of 1m (m = monoclinic crystal system) were obtained by slow evaporation of the mother liquor.
2) was stirred at room temperature for 24 h. The resulting white precipitate of 1m was filtered off and dried under vacuum. Colourless single crystals of 1m (m = monoclinic crystal system) were obtained by slow evaporation of the mother liquor.
Data for 1m. Yield 37%. Anal. calcd for C25H20N2O6S2Zn: N 4.9%, C 52.3%, H 3.5%. Found: N 4.9%, C 52.1%, H 3.5%. IR (cm−1): 3062w, ν(OH); 1629w, 1596s, νas(OCO); 1564s, 1535w, 1483s, ν(ring); 1393vs, νs(OCO); 1255s, 1226m, 1208w, ω(CH, CH2); 1025s (ring breathing); 769s, γ(CH); 705s, 639vs, ν(C–S).
Single crystals of 1m were also obtained from the reaction at room temperature of ZnCl2, acetylsalicylic acid and SCS in EtOH/H2O.
Method 1: reflux. A mixture of 0.094 g (0.43 mmol) of Zn(OAc)2·2H2O, 0.101 g (0.43 mmol) of SCS and 0.154 g (0.86 mmol) of acetylsalicylic acid in 20 mL of H2O/EtOH (1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) was heated under reflux for 8 h. After 4 days, the evaporation of the resulting colourless solution yielded colourless cubic crystals of 1o (o = orthorhombic crystal system, yield 49%). After 15 days of evaporation, single crystals of 1m were also isolated.
2) was heated under reflux for 8 h. After 4 days, the evaporation of the resulting colourless solution yielded colourless cubic crystals of 1o (o = orthorhombic crystal system, yield 49%). After 15 days of evaporation, single crystals of 1m were also isolated.
Method 2: solvothermal. A mixture of a solution of 0.222 g (1.01 mmol) Zn(OAc)2·2H2O in 5 mL H2O and a suspension of 0.244 g (1.04 mmol) of SCS in 5 mL of EtOH was stirred for 30 minutes at room temperature. Then, a solution of 0.375 g (2.08 mmol) of acetylsalicylic acid in 5 mL EtOH was added. The resulting mixture with pH 5 was treated with NaOH 1 M until reaching pH = 7. The resulting suspension was sealed in a 20 mL Teflon-lined autoclave, heated to 120 °C for 3 days, and then slowly cooled to room temperature at a rate of 7 °C h−1. The resulting crystalline white precipitate of 1m and 1o was filtered off and dried under vacuum. Some single crystals of 1m were isolated by slow evaporation of the mother liquor.
Data for 1o. Anal. calcd for C25H20N2O6S2Zn: N 4.8%, C 52.3%, H 3.5%. Found: N 5.0%, C 51.9%, H 3.7%. IR (cm−1): 3050w, ν(OH); 1627w, 1594s, νas(OCO); 1551m, 1537w, 1481m, ν(ring); 1392s, νs(OCO); 1250s, 1223m, 1154w, ω(CH, CH2); 1024s (ring breathing); 751s, γ(CH); 702s, 668s, ν(C–S).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was irradiated for 10 min in a modified conventional microwave oven.22 After 3 days, the unreacted ligand and metal salt were filtered off and after 2 months, the resulting colourless solution yielded single crystals of 1m (distorted cubic shape) and 1m′ (lamellar shape) which were separated manually.
1) was irradiated for 10 min in a modified conventional microwave oven.22 After 3 days, the unreacted ligand and metal salt were filtered off and after 2 months, the resulting colourless solution yielded single crystals of 1m (distorted cubic shape) and 1m′ (lamellar shape) which were separated manually.
Another synthetic option is the reaction under microwave irradiation (10 minutes) of 0.113 g (0.90 mmol) of ZnCO3, 0.204 g (0.87 mmol) of SCS and 0.161 g (0.89 mmol) of acetylsalicylic acid in 20 mL EtOH/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). After the filtration of the resulting suspension, the evaporation of the mother liquor (3 days) allowed the isolation of single crystals of 1m′ (47%). The evaporation of the remaining mother liquor for 15 days gave rise to the crystallization of [(H-SCS)(Hsal)] (5).
1). After the filtration of the resulting suspension, the evaporation of the mother liquor (3 days) allowed the isolation of single crystals of 1m′ (47%). The evaporation of the remaining mother liquor for 15 days gave rise to the crystallization of [(H-SCS)(Hsal)] (5).
Data for 1m′. Anal. calcd for C25H20N2O6S2Zn: N 4.9%, C 52.3%, H 3.5%. Found: N 5.3%, C 52.9%, H 3.5%. IR (cm−1): 3360w, ν(OH); 1627w, 1596m, νas(OCO); 1567s, 1538w, 1482m, ν(ring); 1392vs, νs(OCO); 1254s, 1227m, 1190w, ω(CH, CH2); 1025s (ring breathing); 755s, γ(CH); 705vs, 668vs, ν(C–S).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and a solution of 0.083 g (0.46 mmol) of acetylsalicylic acid with 0.027 g (0.68 mmol) of NaOH in 10 mL of EtOH/H2O (2
1) and a solution of 0.083 g (0.46 mmol) of acetylsalicylic acid with 0.027 g (0.68 mmol) of NaOH in 10 mL of EtOH/H2O (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was stirred at room temperature for 16 h. A white precipitate of 1m (90%) was filtered off and dried under vacuum. The evaporation of the colourless mother liquor yielded single crystals of 1w (8%).
1) was stirred at room temperature for 16 h. A white precipitate of 1m (90%) was filtered off and dried under vacuum. The evaporation of the colourless mother liquor yielded single crystals of 1w (8%).
Data for 1w. Anal. calcd for C25H21N2O6.5S2Zn: N 4.8%, C 51.6%, H 3.6%. Found: N 4.7%, C 50.8%, H 3.4%. IR (cm−1): 3080w, ν(OH); 1628m, 1597vs, νas(OCO); 1562m, 1538w, 1483w, ν(ring); 1390s, νs(OCO); 1251vs, 1224m, 1192m, ω(CH, CH2); 1024s (ring breathing); 753s, γ(CH); 701vs, 669vs, ν(C–S).
Copper(II) complexes 2·CH3CN, 3·solv and 4·solv
Method 1: room temperature. A mixture of 0.190 g (0.23 mmol) of 1∞Cu2(asp)45 and 0.102 g (0.44 mmol) of SCS in 20 mL EtOH/H2O (2
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was stirred at room temperature for 24 h. After the filtration of the resulting suspension, the evaporation of the mother liquor produced a green crystalline material that was dissolved in 20 mL of CH3CN/iPrOH (1
1) was stirred at room temperature for 24 h. After the filtration of the resulting suspension, the evaporation of the mother liquor produced a green crystalline material that was dissolved in 20 mL of CH3CN/iPrOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The slow evaporation of this solution yielded a green solid corresponding to a mixture of 4·solv and 2·CH3CN. The evaporation of the remaining solution produced colourless single crystals of [(SCS)(H2sal)2] (6).
1). The slow evaporation of this solution yielded a green solid corresponding to a mixture of 4·solv and 2·CH3CN. The evaporation of the remaining solution produced colourless single crystals of [(SCS)(H2sal)2] (6).
Also, at room temperature, by reaction of 0.083 g (0.38 mmol) of CuCO3·Cu(OH)2·0.5H2O, 0.205 g (0.88 mmol) of SCS and 0.135 g (0.98 mmol) of salicylic acid in 30 mL EtOH/H2O (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) a green precipitate was formed which after recrystallization in CH3CN showed a diffractogram corresponding to a mixture of 4·solv and 2·CH3CN. After filtration, the resulting colourless solution yielded colourless single crystals of 5 and 6.
1) a green precipitate was formed which after recrystallization in CH3CN showed a diffractogram corresponding to a mixture of 4·solv and 2·CH3CN. After filtration, the resulting colourless solution yielded colourless single crystals of 5 and 6.
Method 2: microwave irradiation. A mixture of 0.095 g (0.476 mmol) of Cu(OAc)2·H2O, 0.100 g (0.427 mmol) of SCS and 0.137 g (1 mmol) of salicylic acid in 20 mL of CH3CN was irradiated for 10 min in a microwave oven. Upon cooling, some single crystals of 4·solv immediately formed, and after slow evaporation for 3 days, blue single crystals of 2·CH3CN were obtained (20%).
Data for 2·CH3CN. Anal. calcd for C27H23N3O6S2Cu: N 6.9%, C 52.9%, H 3.7%. Found: N 6.6%, C 52.4%, H 3.2%. IR (cm−1): 3070w, ν(OH); 2369w, ν(CN); 1625w, 1598s, νas(OCO); 1559m, 1540w, 1481s, ν(ring); 1389s, νs(OCO); 1254s, 1223m, 1193m, ω(CH, CH2); 1026s (ring breathing); 755s, 728s, γ(CH); 703vs, 667vs, ν(C–S).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); the evaporation of this solution yielded green single crystals of 4.
1); the evaporation of this solution yielded green single crystals of 4.
Data for 3·solv. Anal. calcd for C43H51N7O13S4Cu2 (solv = 3CH3CN + 2H2O): N 8.7%, C 45.8%, H 4.5%. Found: N 8.1%, C 45.9%, H 4.2%. IR (cm−1): 3640, 3070w, ν(OH); 2324w, ν(CN); 1624w, 1596s, νas(OCO); 1559m, 1505w, 1483s, ν(ring); 1391m, νs(OCO); 1253vs, 1222m, ω(CH, CH2); 2025s (ring breathing); 756s, γ(CH); 704s, 669s, ν(C–S).
Data for 4·solv. Anal. calcd for C100H86N8O28S8Cu5 (solv = 2H2O): N 4.6%, C 49.6%, H 3.5%. Found: N 4.6%, C 50.3%, H 3.5%. IR (cm−1): 3060w, ν(OH); 1623w, 1596s, νas(OCO); 1560m, 1506w, 1482s ν(ring); 1389s, νs(OCO); 1252s, 1221s, 1195s, ω(CH, CH2); 1066m (ring breathing); 805s, 758vs, γ(CH); 703vs, ν(C–S).
Synthesis and crystallization of [(H-SCS)(Hsal)] (5) and [(SCS)(H2sal)2] (6)
Compounds 5 and 6 crystallized in several of the synthetic processes described above. They can be prepared by the direct synthesis described below.A solution of 0.202 g (0.86 mmol) of SCS and 0.160 g (0.89 mmol) of acetylsalicylic acid in 30 mL of EtOH/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) was stirred for 48 h at room temperature. The slow evaporation of the resulting colourless solution yielded a mixture of single crystals of 5 and 6.
2) was stirred for 48 h at room temperature. The slow evaporation of the resulting colourless solution yielded a mixture of single crystals of 5 and 6.
Under the same conditions and solvent, but using a mixture of 0.207 g (0.88 mmol) of SCS and 0.13 g (0.94 mmol) of salicylic acid, the resulting colourless solution afforded single crystals of 6 (80%) after 1 month and single crystals of 5 (10%) after two weeks.
Data for 5. Anal. calcd for C18H16N2O3S2: N 7.5%, C 58.1%, H 4.3%. Found: N 7.5%, C 58.3%, H 4.5%. IR (cm−1): 3370w,b, ν(NH); 3080w, ν(OH); 1608m, 1594m, νas(OCO); 1577s, 1538w (1481f, 1445d) ν(ring); 1363m, νs(OCO); 1257m, 1219m, 1197m, ω(CH, CH2); 1028m (ring breathing); 764vs, γ(CH); 703vs, ν(C–S).
Data for 6. Anal. calcd for C25H22N2O6S2: N 5.5%, C 58.8%, H 4.3%. Found: N 6.1%, C 58.4%, H 4.1%. IR (cm−1): 3030w, ν(OH); 1736m, ν(CO); 1582s, 1481s, 1446m, ν(ring); 1246s, 1217vs, ω(CH, CH2); 1017s (ring breathing); 736vs, γ(CH); 697vs, ν(C–S).
Crystallography
Crystallographic data were collected at 100 K using a Bruker D8 Venture diffractometer with a Photon II CMOS detector and Mo-Kα radiation (λ = 0.71073 Å) generated by an Incoatec high-brilliance microfocus source equipped with Incoatec Helios multilayer optics. The software APEX3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 23 was used for collecting frames of data, indexing reflections, and the determination of lattice parameters, SAINT23 for integration of intensity of reflections, and SADABS24 for scaling and empirical absorption correction. The structures were solved using a dual-space algorithm using the program SHELXT.25 All non-hydrogen atoms were refined with anisotropic thermal parameters by full-matrix least-squares calculations on F2 using the program SHELXL26 with OLEX2.27 Hydrogen atoms were inserted at calculated positions and constrained with isotropic thermal parameters. In 2·CH3CN, the crystal was twinned by inversion (BASF parameter, 0.350). In 3·solv and 4·solv, the OLEX2
23 was used for collecting frames of data, indexing reflections, and the determination of lattice parameters, SAINT23 for integration of intensity of reflections, and SADABS24 for scaling and empirical absorption correction. The structures were solved using a dual-space algorithm using the program SHELXT.25 All non-hydrogen atoms were refined with anisotropic thermal parameters by full-matrix least-squares calculations on F2 using the program SHELXL26 with OLEX2.27 Hydrogen atoms were inserted at calculated positions and constrained with isotropic thermal parameters. In 2·CH3CN, the crystal was twinned by inversion (BASF parameter, 0.350). In 3·solv and 4·solv, the OLEX2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 26 solvent mask routine was used to remove the intensity contributions from the highly disordered solvent molecules. In 6, one H2sal molecule is disordered over two positions, and the site occupation factors were refined converging to 71
26 solvent mask routine was used to remove the intensity contributions from the highly disordered solvent molecules. In 6, one H2sal molecule is disordered over two positions, and the site occupation factors were refined converging to 71![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 29. Drawings were produced with Mercury.28 Special computations for the crystal structure discussion were carried out with PLATON.29 Crystal data and structure refinement parameters are reported in Tables S2 and S3, where deposition reference numbers at the Cambridge Crystallographic Data Centre (CCDC) are also included. Selected bond lengths and angles and hydrogen bond distances are listed in Tables S4–S10.
29. Drawings were produced with Mercury.28 Special computations for the crystal structure discussion were carried out with PLATON.29 Crystal data and structure refinement parameters are reported in Tables S2 and S3, where deposition reference numbers at the Cambridge Crystallographic Data Centre (CCDC) are also included. Selected bond lengths and angles and hydrogen bond distances are listed in Tables S4–S10.
X-ray powder diffraction (PXRD) was performed using an X'pert Pro 3 (PANalytical) diffractometer with Cu-Kα radiation (λ = 1.5406 Å) over the range 5 to 50° in steps of 0.026° (2θ). The program MERCURY27 was employed to obtain theoretical powder diffractograms from single crystal data. The program FULL PROF SUITE30 and the tool WINPLOTR31 were used to perform profile matching.
Stability and degradation of the compounds
The stability and degradation of 1m, 3·solv and 4·solv were investigated using UV-Vis and PXRD techniques. The samples were exposed to water for a duration of 21 days (Scheme S1), and their progression was monitored using UV-Vis at intervals of 7, 14, and 21 days. Under acidic conditions, 100 μL, 300 μL, and 1000 μL of 0.1 M HCl were added to the suspension of each compound (Scheme S2). These suspensions were stirred at room temperature for 24 hours, during which pH measurements were taken initially, after 30 minutes, and at the conclusion of the experiment (Table S11). Each compound (20 mg of 1m, 3·solv and 4·solv) was immersed in 10 mL of 0.01 M PBS (phosphate-buffered solution) for 30 days in a sand bath maintained at 35–40 °C (Scheme S3). Changes were monitored through solution analysis by UV-Vis at 4 and 24 hours, and on days 5, 7, 8, 12, 13, 15, 19, and 26. UV spectrophotometry was conducted at room temperature in aqueous, acidic, or PBS solutions to confirm the presence of SCS or salicylate in solution. Two absorption bands were observed in the spectrum of salicylic acid in aqueous solution: a strong band at 320 nm and a weaker band at 252 nm. The absorption maximum of SCS was observed at 284 nm. The UV-Vis spectra of the polymeric compounds 1m, 3·solv and 4·solv exhibited different bands depending on the media (see the SI). The PXRD patterns of the remaining solids after the various treatments were collected and compared with the theoretical patterns (SI).Cytotoxicity assays
Cytotoxicity studies of some compounds were carried out on the MRC-5 (non-tumor human fibroblasts), NCI-H460 (human lung carcinoma), A549 (human lung carcinoma), and MDA-MB231 (human breast adenocarcinoma) cell lines obtained from the American Type Culture Collection (ATCC). The conditions of the experiments with cell lines are reported in Table S13.Author contributions
Olaya Gómez-Paz: conceptualization, formal analysis, and investigation. Rosa Carballo: conceptualization, formal analysis, funding acquisition, methodology, and writing – original draft. Ana Belén Lago: formal analysis, investigation, and writing – original draft. Ezequiel M. Vázquez-López: conceptualization, formal analysis, funding acquisition, and writing – review. Berta Covelo: formal analysis, investigation, and writing – review. All authors have read and agreed to the published version of the manuscript.Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included as part of the supplementary information (SI). Supplementary information: data and literature references regarding the crystal structures of Zn/Cu salicylate coordination polymers with N,N′-divergent ligands. Selected crystal data and structure refinement, selected bond lengths and angles, main hydrogen bonds, IR and UV-Vis-diffuse reflectance spectra and TGA curves of the complexes. Experimental procedure schemes, PXRD spectra and UV-Vis absorption spectra of the solutions for studies of stability in water, under acidic conditions and in PBS. Experimental conditions for the experiments with cell lines. See DOI: https://doi.org/10.1039/d5dt02303c.CCDC 2487455–2487463 (1m, 1m′, 1o, 1w, 2·CH3CN, 3·solv, 4·solv, 5 and 6) contain the supplementary crystallographic data for this paper.32a–i
Acknowledgements
This work was developed within the scope of the project ref. ED431C 2024/28 funded by Xunta de Galicia and ref. CNS2022-135453 funded by the MCIN/AEI/10.13039/501100011033 and by the “European Union NextGenerationEU”/PRTR. We thank the Structural Determination Service of the Universidade de Vigo-CACTI for X-ray diffraction measurements.References
- S. Kothawade and P. Shende, Coord. Chem. Rev., 2024, 510, 215851 CrossRef CAS.
- V. N. Vukotic, J. N. Murphy, J. Kobti and M. Dao, 2023, U.S. Patent and Trademark Office, filed on August 6th, 2021.
- J. N. Murphy, J. L. Kobti, M. Dao, D. Wear, M. Okoko, S. Pandey and V. N. Vukotic, Chem. Sci., 2024, 15, 7041–7050 RSC.
- J. R. Paterson and J. R. Lawrence, J. Med., 2001, 94, 445–448 CAS.
- T. Fujimori, S. Yamada, H. Yasui, H. Sakurai and Y. T. Ishida, J. Biol. Inorg. Chem., 2005, 10, 831–841 CrossRef CAS PubMed.
- A. Gusev, Y. Baluda, E. Braga, M. Kryukova, M. Kiskin, E. Chuyan, M. Ravaeva, I. Cheretaev and W. Linert, Inorg. Chim. Acta, 2021, 528, 120606 CrossRef CAS.
- A. B. Lago, A. Pino-Cuevas, R. Carballo and E. M. Vázquez-López, Dalton Trans., 2016, 45, 1614–1621 RSC.
- S. Chooset, A. Kantacha, K. Chainok and S. Wongnawa, Inorg. Chim. Acta, 2018, 471, 493–501 CrossRef CAS.
- Y.-Y. Liu, Y.-Y. Jiang, J. Yang, Y.-Y. Liu and J.-F. Ma, CrystEngComm, 2011, 13, 6118–6129 RSC.
- L.-G. Zhu and S. Kitagawa, Inorg. Chem. Commun., 2003, 6, 1051–1055 CrossRef CAS.
- L.-G. Zhu, S. Kitagawa, H. Miyasaka and H.-C. Chang, Inorg. Chim. Acta, 2003, 355, 121–126 CrossRef CAS.
- S.-R. Zheng, X.-L. Wen, Z.-M. Liu, T. Xie, J.-B. Tan, J. Fan, S.-S. Hou and W.-G. Zhang, Z. Anorg. Allg. Chem., 2014, 640, 2057–2061 CrossRef CAS.
- B. Viossat, J.-C. Daran, G. Savouret, G. Morgant, F. T. Greenaway, N.-H. Dung, V. A. Pham-Tran and J. R. J. Sorenson, J. Inorg. Biochem., 2003, 96, 375–385 CrossRef CAS PubMed.
- N. Bouhmaida, M. A. Méndez-Rojas, A. Pérez-Benítez, G. Merino, B. Fraisse and N. E. Ghermani, Inorg. Chem., 2010, 49, 6443–6452 CrossRef CAS PubMed.
- L. Yang, D. R. Powell and R. P. Houser, Dalton Trans., 2007, 955–964 RSC.
- A. W. Addison, T. N. Rao, J. Reedij, J. van Rijn and G. C. Verschoor, J. Chem. Soc., Dalton Trans., 1984, 1349–1356 RSC.
- E. M. Page, D. A. Rice, K. Aarset, K. Hagen and R. J. Genge, J. Phys. Chem. A, 2000, 104, 6672–6676 CrossRef CAS.
- A. I. Kitaigorodsky, Molecular Crystals and Molecules, Academic Press, New York, 1973, vol. 29 Search PubMed.
- S. L. Childs, G. P. Stahly and A. Park, Mol. Pharm., 2007, 4, 323–338 CrossRef CAS PubMed.
- I. Passi, L. M. Abraham, A. S. W. W. Raj, M. Varadhan, S. Muthuraman, M. P. Sivaramakrishnan, P. Vadivelu, R. Manoharan, M. Velusamy and V. Rajendiran, Inorg. Chem., 2025, 54, 14073–14090 CrossRef PubMed.
- R. Carballo, B. Covelo, E. García-Martínez, A. B. Lago and E. M. Vázquez-López, Z. Anorg. Allg. Chem., 2007, 633, 780–782 CrossRef CAS.
- M. Ardon, P. D. Hayes and G. Hogarth, J. Chem. Educ., 2002, 79, 1249–1251 CrossRef CAS.
- Bruker, APEX3 and SAINT, Bruker AXS Inc., Madison, Wisconsin, USA, 2019 Search PubMed.
- L. Krause, R. Herbst-Irmer, G. M. Sheldrick and D. Stalke, J. Appl. Crystallogr., 2015, 48, 3–10 CrossRef CAS PubMed.
- G. M. Sheldrick, SHELXT – Integrated space-group and crystal structure determination, Acta Crystallogr., Sect. A, 2015, 71, 3–8 CrossRef PubMed.
- G. M. Sheldrick, Crystal Structure Refinement with SHELXL, Acta Crystallogr., Sect. C, 2015, 71, 3–8 CrossRef PubMed.
- O. V. Dolomanov, L. J. Bourhis, R. J. Gildea, J. A. K. Howard and H. Puschmann, J. Appl. Crystallogr., 2009, 42, 339–341 CrossRef CAS.
- C. F. Macrae, I. Sovago, S. J. Cottrell, P. T. A. Galek, P. McCabe, E. Pidcock, M. Platings, G. P. Shields, J. S. Stevens, M. Towler and P. A. Wood, J. Appl. Crystallogr., 2020, 52, 226–235 CrossRef PubMed.
- A. L. Spek, Acta Crystallogr., Sect. D:Biol. Crystallogr., 2009, 65, 148–155 CrossRef CAS PubMed.
- J. Rodriguez-Carvajal, Commission on Powder Diffraction (CPD), 2001, 12–19.
- T. Roisnel and J. Rodríguez-Carvajal, in Proceeding of the Seventh European Powder Diffraction Conference (EPDIC 7); 2000, pp. 118–123.
- (a) CCDC 2487455: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2phdh3; (b) CCDC 2487456: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2phdj4; (c) CCDC 2487457: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2phdk5; (d) CCDC 2487458: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2phdl6; (e) CCDC 2487459: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2phdm7; (f) CCDC 2487460: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2phdn8; (g) CCDC 2487461: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2phdp9; (h) CCDC 2487462: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2phdqb; (i) CCDC 2487463: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2phdrc.
| This journal is © The Royal Society of Chemistry 2026 |