Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Interzeolite-type transformation between microporous titanosilicates
Stanislav Ferdov ab
ab
aPhysics Centre of Minho and Porto Universities (CF-UM-UP), University of Minho, 4710-057 Braga, Portugal. E-mail: sferdov@fisica.uminho.pt
bLaboratory of Physics for Materials and Emergent Technologies, LaPMET, University of Minho, 4710-057, Braga, Portugal
First published on 15th January 2026
Abstract
This work reveals the first example of an interzeolite-type transformation between microporous heteropolyhedral silicates. In KOH solution, preformed spherulitic particles of the microporous titanosilicate ETS-4 (Engelhard Titanium Silicate-4) transform into single crystals of GTS-1 (Grace Titanium Silicate-1). Mechanistically, the transformation follows a distinct reversed crystallization pathway, beginning on the surface of a seed particle and ending in the reconstructive self-assembly of multiple single crystals. In addition to advancing synthesis methodologies for microporous titanosilicates, this work provides mechanistic insights into a crystallization route that enables templating of the crystal morphology through particle self-assembly.
Crystalline microporous silicates typically develop from a gel growth medium, where two crystallization models are possible: (1) classical crystal growth including a stepwise atom-by-atom attachment process, as described by the nucleation and growth theory,1,2 and (2) non-classical crystal growth involving particle-based assembly, where amorphous or crystalline nanoparticles merge to form larger crystalline structures.3 As part of the non-classical model, reversed crystal growth is a sub-model where the crystallization starts from the surface to the center of crystalline4 or amorphous aggregates.5 This type of crystallization has been described for zeolites,4–11 metal–organic frameworks,12 perovskites,13 metal oxides14,15 and hydroxides.16,17 In the family of zeolites, reversed crystallization generally includes the formation of nanoparticles, their aggregation, surface recrystallization and the formation of single crystals as described for the ANA type of zeolite.4 In other instances, as for the LTA zeolite, the recrystallization may occur only on the surface forming a crystalline shell and an amorphous core.7 Prolongation of the time for crystallization leads to nucleation and growth of clusters of sodalite nanoplates, which starts from the amorphous core and expands to the surface consuming the zeolite A crystals.6 Similar to zeolites, microporous mixed polyhedral silicates composed of SiO4 tetrahedra and MOx (M – transition or other metal ions with coordination higher than four) polyhedra crystallize from gel growth media but their mechanism of crystallization is less explored. ETS-4 is one of the most studied members of the family of microporous titanosilicates known for its thermal foaming,18 biomedical,19 ion exchange,20 and gas separation properties.21 ETS-4 is a stoichiometric metastable phase and in some systems at longer crystallization times transforms into another microporous titanosilicate-1,22 an analogue of the rare mineral ivanyukite.23 Although interzeolite transformation (IZT), in which a parent phase is transformed into a new framework, has been extensively studied in tetrahedral silicate systems,24–26 the transformation of pre-formed heteropolyhedral silicates has remained unexplored until now.
This study aims to demonstrate the transformation of ETS-4 into GTS-1, the first example of interstructural transformation in the class of microporous titanosilicates. The specific transformation uncovers a distinct mechanistic process in which pre-existing spherulitic ETS-4 particles are progressively reconstructed into self-assembled single crystals of the microporous titanosilicate GTS-1.
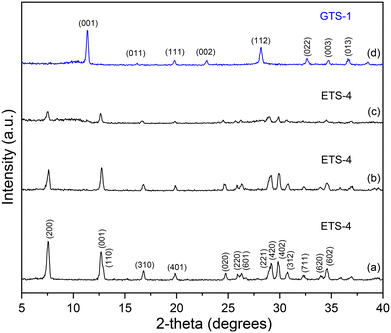
Fig. 1a shows the powder XRD patterns following the transformation of ETS-4 into GTS-1. ETS-4 was indexed in the orthorhombic space group Cmmm with lattice parameters a = 23.2272, b = 7.1751, c = 6.9727 Å27 using PDF: 96-400-2325, while GTS-1 was indexed in the cubic space group P![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) 3 m with lattice parameter a = 7.7647 Å (PDF: 96-153-3728).28 As the synthesis time increased to 60 and 120 minutes, the intensity of the ETS-4 diffraction peaks progressively decreased by 20% and 43%, respectively, indicating a gradual loss of crystallinity. By 150 minutes, ETS-4 was fully transformed into GTS-1. The intensity of the powder XRD peaks of GTS-1 continued to increase with longer synthesis times (1440 and 10080 min), showing improved crystallinity without phase change (Fig. S1). Following its formation, GTS-1 remains structurally stable, although longer reaction times enhance its crystallinity (Fig. S1), reflecting that time governs crystal maturation rather than phase transformation. Additional experiments (Table S1) with varying KOH concentration and temperature revealed a coupled dependence of the transformation on both variables. Higher temperatures (230 °C) enable the transformation at lower KOH concentration (1.72 M), whereas achieving the same transformation at lower temperature (150 °C) requires substantially higher alkalinity (6.88 M). However, below a critical KOH concentration (0.86 M), the transformation does not occur within the selected reaction time (150 min), even at elevated temperature (Fig. S7).
3 m with lattice parameter a = 7.7647 Å (PDF: 96-153-3728).28 As the synthesis time increased to 60 and 120 minutes, the intensity of the ETS-4 diffraction peaks progressively decreased by 20% and 43%, respectively, indicating a gradual loss of crystallinity. By 150 minutes, ETS-4 was fully transformed into GTS-1. The intensity of the powder XRD peaks of GTS-1 continued to increase with longer synthesis times (1440 and 10080 min), showing improved crystallinity without phase change (Fig. S1). Following its formation, GTS-1 remains structurally stable, although longer reaction times enhance its crystallinity (Fig. S1), reflecting that time governs crystal maturation rather than phase transformation. Additional experiments (Table S1) with varying KOH concentration and temperature revealed a coupled dependence of the transformation on both variables. Higher temperatures (230 °C) enable the transformation at lower KOH concentration (1.72 M), whereas achieving the same transformation at lower temperature (150 °C) requires substantially higher alkalinity (6.88 M). However, below a critical KOH concentration (0.86 M), the transformation does not occur within the selected reaction time (150 min), even at elevated temperature (Fig. S7).
Fig. 2a presents the SEM images of the as-synthesized ETS-4, showing particles approximately 40 µm in size. A characteristic radial intergrowth of lamellae-like crystals gives rise to a distinct multilayered, spherulitic morphology, consistent with previous reports on ETS-4.22 After 60 minutes of treatment in KOH solution, sporadic nucleation of layer-like nanoparticles (≈200 nm) (Fig. 2b) with an individual layer thickness of about 20 nm was observed on the ETS-4 surface (Fig. S2). At this stage, the Si/Ti ratio in both the nanoparticles and the parent crystal surface was 1.1 (Fig. S3), which deviated substantially from the measured value of 2.2 for the as-synthesized ETS-4. In the absence of XRD peaks corresponding to any secondary phase, this deviation may indicate surface-initiated amorphization, with both the newly formed particles and the underlying surface showing chemical compositions inconsistent with the original ETS-4 framework. The observed etched morphology (Fig. S2) is consistent with partial dissolution of the ETS-4 surface, which could create localized supersaturation of silicate and titanate species near the solid–liquid interface and thereby favor nanoparticle precipitation.29 After 120 minutes of synthesis, the continued dissolution of ETS-4 generates a higher concentration of dissolved silica and titania species. This abundance promotes a uniform deposition of layer-like nanoparticles across the entire parent particle (Fig. 2c). Interestingly, the layer thickness of the branched nanoparticles is maintained at approximately 20 nm (Fig. S4). The ongoing dissolution–precipitation cycle is followed by a significant loss of long-range order, as indicated by the substantial decrease in peak intensity of the XRD pattern (Fig. 1c). The spherulitic ETS-4 particles covered with layer-like nanoparticles exhibit more than a 40% loss in crystallinity compared to the parent ETS-4 sample, indicating significant structural degradation. Importantly, no well-defined diffraction peaks corresponding to any secondary crystalline phase are observed. Moreover, the Si/Ti ratio (1.8) of the nanoparticles (Fig. S5) deviates markedly from the measured value of 2.2 for ETS-4. This compositional mismatch suggests that the nanoparticles do not belong to the ETS-4 framework. While a detailed understanding of their structural role lies beyond the scope of this work, future studies could further clarify their involvement in the degradation process. Fig. 2d shows the SEM images of a replica of ETS-4 composed of self-organized cube-like single crystals of GTS-1 obtained after 150 minutes of hydrothermal treatment. These submicron crystals (600–900 nm) follow the curvatures of the parent spherulitic particle and are attached by their surfaces, showing the templating effect of the ETS-4 morphology. The self-organization of GTS-1 crystals may be driven by a combination of thermodynamic factors – such as the minimization of interfacial energy along the basal pinacoid surfaces – and kinetic constraints related to the localized supply of dissolved species,30 which together promote nearly oriented attachment and relatively uniform growth across the templating ETS-4 surface.
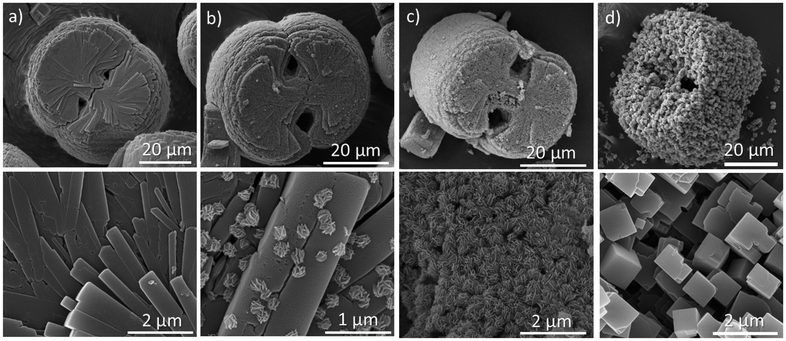 | ||
| Fig. 2 SEM images showing the evolution of the (a) as-synthesized ETS-4 particles after (b) 60 min, (c) 120 min and (d) 150 min of hydrothermal treatment in KOH solution. | ||
Considering previous studies, the appearance of nanoparticles on the surface of the dissolving crystals can be attributed to a local dissolution–reprecipitation process, a mechanism well documented in natural and other solid–fluid systems.31–34 As the parent crystal dissolves, the interfacial solution becomes locally supersaturated with respect to the new phase, promoting heterogeneous nucleation directly on the crystal surface. To capture the very early stages of nucleation of the layered nanoparticles, a synthesis lasting 30 min was performed. The SEM image (Fig. S8) shows approximately 20 nm thick vertically oriented particles with minimal branching. This observation indicates that the upward growth is faster than lateral growth, but it stops quickly because each burst of local supersaturation produces a new nucleation event rather than thickening the existing sheet (Fig. 2b and S9). As synthesis time increases, the degree of branching becomes progressively more pronounced and assembled on the particle surface as shown in Fig. 2c and S10. This surface evolution reflects the transition from primary nucleation to secondary growth and hierarchical organization.
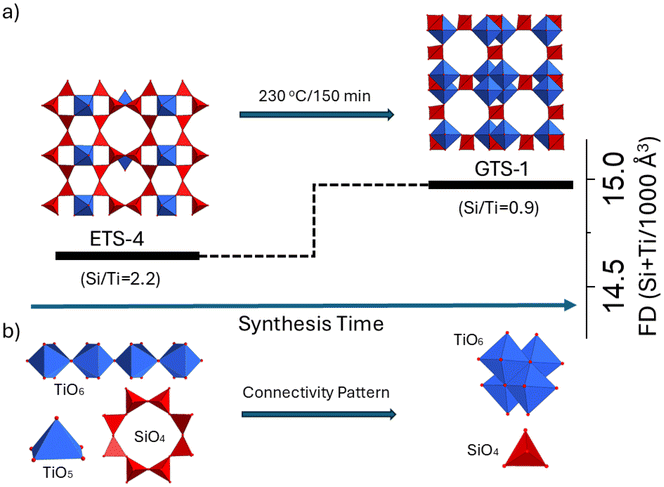
Fig. 3a provides a direct comparison of the framework characteristics of the parent and daughter phases. As in most zeolites, the transformation proceeds from a lower (ETS-4: 14.63 Ti + Si/1000 Å3) to a higher (GTS-1: 14.95 Ti + Si/1000 Å3) framework density, accompanied by a decrease in the silicon-to-metal ratio as determined by EDS analysis (ETS-4: Si/Ti = 2.2 → GTS-1: Si/Ti = 0.9; Fig. S6). However, unlike zeolites – where the distribution of the two chemically distinct units (SiO4 and AlO4) lacks an ordered pattern and involves only tetrahedral coordination – in the parent ETS-4 phase the metal atoms (Ti) are five- and six-coordinated, forming a regular arrangement of isolated TiO5 pyramids and chains of corner-sharing TiO6 octahedra in addition to 8-rings of SiO4 tetrahedra. Upon transformation to GTS-1, the TiO5 units disappear, and the chains of TiO6 octahedra reorganize into clusters of four edge-sharing TiO6 units and the 8-rings are reduced to single SiO4 tetrahedra (Fig. 3b).
Table 1 shows the comparison of the symmetry of the parent (ETS-4) and daughter (GTS-1) phases with that of zeolites exhibiting a similar tendency upon IZT. The transformations generally progress from lower to higher symmetry (e.g., orthorhombic → cubic, tetragonal → trigonal, orthorhombic → tetragonal, hexagonal → cubic), meaning that the daughter frameworks adopt more symmetric structures than their parents. In synthetic zeolites, however, such low-to-high symmetry transitions appear to be rare, with reported examples including *BEA → CHA,35 MOR → *BEA36 and AFI → RHO.37 Framework rearrangements more typically proceed in the opposite direction, from high to low symmetry, since distortions and local strain tend to break symmetry rather than enhance it. Unlike zeolite systems, where symmetry increases have only been realized through the use of organic structure-directing agents (OSDAs), titanosilicates exhibit such transformations without OSDA assistance, highlighting their intrinsic structural adaptability.
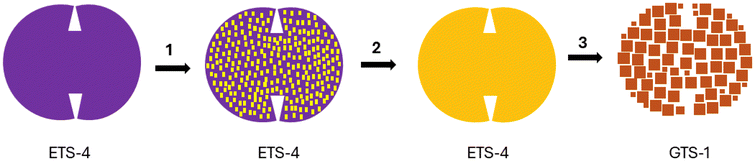
Based on powder XRD data, SEM images, and chemical EDS analyses of samples collected at different synthesis times, Fig. 4 outlines a plausible crystallization pathway for the transformation of ETS-4 particles into self-assemblies of GTS-1 single crystals. (1) In the earliest stage, partial dissolution of ETS-4, accompanied by sporadic precipitation of chemically distinct layer-like nanoparticles on the parent crystal surface, appears to initiate the structural transformation. At this point, a slight reduction in crystallinity is observed, all XRD reflections remain assignable to ETS-4, while the surface chemical composition deviates from the stoichiometric value. (2) In the subsequent stage, the layer-like nanoparticles progressively cover the entire ETS-4 surface, correlating with a pronounced decrease in overall crystallinity. These nanoparticles lack long-range order and exhibit a significantly lower Si/Ti ratio than the parent ETS-4 phase. (3) In the final stage, the layer-like nanoparticles reorganize into cube-like single crystals of GTS-1.
Compared to previously reported examples of non-classical crystal growth via reversed crystallization, the case presented here exhibits two key distinctions: (1) crystallization both initiates in and remains confined to a pre-existing crystalline particle and (2) the nanoparticle building blocks both originate from and reattach to this parent phase. In contrast, other reported systems typically initiate crystallization from an amorphous precursor that evolves to a crystalline phase by the well-known pathways to crystallization by particle/nanocrystal attachment.3 A mechanism most closely resembling the one presented here is the FAU-to-ANA transformation, which also begins with a crystalline phase – FAU zeolite seeds that aggregate into spheroidal structures – followed by surface-to-interior recrystallization and the formation of faceted ANA crystals.38 These parallels and distinctions underscore the unique advantage of initiating reversed crystallization from an ordered parent phase: it enables greater control over the crystal morphology, allowing preservation or templating of features from the original crystalline source. In this context, starting from crystalline seeds facilitates replication of the external morphology self-assembly of numerous smaller crystals. From the perspective of interzeolite transformation, previous studies have shown that the FAU zeolite can be converted to MFI via a pseudomorphic transformation using MFI seeds in NaOH solution.39 This happens when FAU starts to lose its structure, and MFI seeds help rebuild it into the MFI framework. In another example, hollow spheres of the amorphized FAU zeolite were transformed into the EDI zeolite.11 These spheres are composed of amorphous particles that transform over time into the FAU structure. Similarly, recrystallization of FAU in the presence of surfactants generates mesopores while preserving the external morphology and the overall structure of the parent zeolite.40,41 The key difference between the FAU and ETS-4 transformations lies in how morphology is preserved. In the FAU examples, the transformations occur largely within the original crystal, preserving the external shape while the internal structure reorganizes or recrystallizes. In contrast, the ETS-4 to GTS-1 transformation follows a templated, reconstructive pathway. This process fully replaces the parent structure via successive self-assembly of different particles, effectively replicating the original morphology using entirely new building blocks – akin to demolishing a building and reconstructing it with different bricks in the same shape. Such diversity in mechanistic behavior underscores why theoretical and simulation studies have long emphasized that interzeolite transformations cannot be explained by any single structural or energetic descriptor.42,43 These studies highlight the limitations of using shared building units or framework similarity to predict parent–daughter relationships, and energetic models, while useful, remain incomplete because they depend strongly on composition, solvent effects, and interfacial energetics.42 Recent molecular simulations further demonstrate that intact parent surfaces, rather than isolated structural fragments, can direct transformation through interface-mediated pathways such as cross-nucleation.44 Within this broader context, the findings of the present work provide a mechanistic perspective that complements and extends existing theoretical frameworks. Whereas prior models primarily distinguish between dissolution–reprecipitation and diffusionless or topotactic rearrangements, the results highlight a surface-initiated reconstructive pathway that operates through sequential structural reorganization rather than a direct framework rearrangement. This behavior illustrates how interzeolite-type transformations can proceed through intermediate states that couple surface processes with particle-level assembly, revealing a route that is neither conventionally pseudomorphic nor classically topotactic. Such a feature broadens the mechanistic scenarios of interstructural transformations and shows how surface-driven processes can direct the emergence of new microporous architectures.
In conclusion, this study demonstrates that microporous titanosilicates can be obtained through transformation of a preformed microporous phase. ETS-4 was converted into GTS-1 via a reversed crystallization pathway involving successive pseudomorphic substitutions and structural reorganization. Unlike conventional pseudomorphism, which typically entails internal restructuring, this process is characterized by the nucleation of chemically distinct nanoparticles on the surface of ETS-4 crystals, followed by their transformation into single crystals of GTS-1 while preserving the external morphology. This mechanism demonstrates that a pre-existing crystal can serve as an effective template for crystal engineering through self-assembly, offering a rational route for designing titanosilicate microstructures via controlled interstructural transformation.
Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included as part of the supplementary information (SI). The supplementary information provides details on the synthesis conditions of the parent phase, the parameters used for the interstructural transformation, and characterization data, including XRD patterns, SEM micrographs, and EDS analyses. See DOI: https://doi.org/10.1039/d5dt02300a.Acknowledgements
This work was supported by the Portuguese Foundation for Science and Technology (FCT) in the framework of the Strategic Funding UID/04650/2025 (https://doi.org/10.54499/UID/04650/2025) and the SEMAT (University of Minho).References
- J. D. H. Donnay and D. Harker, Am. Mineral., 1937, 22, 446–467 CAS.
- P. Hartman and W. Perdok, Acta Crystallogr., 1955, 8, 49–52 Search PubMed.
- J. J. De Yoreo, P. U. P. A. Gilbert, N. A. J. M. Sommerdijk, R. L. Penn, S. Whitelam, D. Joester, H. Zhang, J. D. Rimer, A. Navrotsky, J. F. Banfield, A. F. Wallace, F. M. Michel, F. C. Meldrum, H. Cölfen and P. M. Dove, Science, 2015, 349, aaa6760 CrossRef PubMed.
- X. Chen, M. Qiao, S. Xie, K. Fan, W. Zhou and H. He, J. Am. Chem. Soc., 2007, 129, 13305–13312 Search PubMed.
- Q. Yue, K. Kutukova, A. Li, J. Čejka, E. Zschech and M. Opanasenko, Chem. – Eur. J., 2022, 28, e202201468 Search PubMed.
- H. Greer, P. S. Wheatley, S. E. Ashbrook, R. E. Morris and W. Zhou, J. Am. Chem. Soc., 2009, 131, 17986–17992 CrossRef CAS PubMed.
- J. Yao, D. Li, X. Zhang, C.-H. Kong, W. Yue, W. Zhou and H. Wang, Angew. Chem., Int. Ed., 2008, 47, 8397–8399 CrossRef CAS PubMed.
- Q. Wang, G. Chen and S. Xu, Microporous Mesoporous Mater., 2009, 119, 315–321 CrossRef CAS.
- L. Han, J. Yao, D. Li, J. Ho, X. Zhang, C.-H. Kong, Z.-M. Zong, X.-Y. Wei and H. Wang, J. Mater. Chem., 2008, 18, 3337–3341 RSC.
- S. Ferdov, Microporous Mesoporous Mater., 2020, 303, 110263 CrossRef CAS.
- S. Ferdov, Molecules, 2024, 29, 1744 Search PubMed.
- C. Zheng, H. F. Greer, C.-Y. Chiang and W. Zhou, CrystEngComm, 2014, 16, 1064–1070 RSC.
- X. Yang, J. Fu, C. Jin, J. Chen, C. Liang, M. Wu and W. Zhou, J. Am. Chem. Soc., 2010, 132, 14279–14287 CrossRef CAS PubMed.
- K. Self, H. Zhou, H. F. Greer, Z. R. Tian and W. Zhou, Chem. Commun., 2013, 49, 5411–5413 Search PubMed.
- B. Zhang, S. Cao, M. Du, X. Ye, Y. Wang and J. Ye, Catalysts, 2019, 9, 91 CrossRef.
- L.-Y. Chen and Z.-D. Zhang, J. Phys. Chem. C, 2008, 112, 18798–18803 CrossRef CAS.
- M. T. Buscaglia, V. Buscaglia, C. Bottino, M. Viviani, R. Fournier, M. Sennour, S. Presto, R. Marazza and P. Nanni, Cryst. Growth Des., 2008, 8, 3847–3855 CrossRef CAS.
- S. Ferdov, Angew. Chem., Int. Ed., 2013, 52, 12135–12138 CrossRef CAS PubMed.
- S. Ferdov, E. Shikova, Z. Ivanova, L. T. Dimowa, R. P. Nikolova, Z. Lin and B. L. Shivachev, RSC Adv., 2013, 3, 8843–8848 RSC.
- R. P. Nikolova, B. L. Shivachev and S. Ferdov, Microporous Mesoporous Mater., 2013, 165, 121–126 CrossRef CAS.
- S. M. Kuznicki, V. A. Bell, S. Nair, H. W. Hillhouse, R. M. Jacubinas, C. M. Braunbarth, B. H. Toby and M. Tsapatsis, Nature, 2001, 412, 720–724 CrossRef CAS PubMed.
- V. Kostov-Kytin, S. Ferdov, Y. Kalvachev, B. Mihailova and O. Petrov, Microporous Mesoporous Mater., 2007, 105, 232–238 CrossRef CAS.
- V. N. Yakovenchuk, A. P. Nikolaev, E. A. Selivanova, Y. A. Pakhomovsky, J. A. Korchak, D. y. V. Spiridonova, O. A. Zalkind and S. V. Krivovichev, Am. Mineral., 2009, 94, 1450–1458 CrossRef CAS.
- S. Ferdov and R. Gonçalves, Dalton Trans., 2025, 54, 7621–7626 RSC.
- Z. Liu, A. Chokkalingam, S. Miyagi, M. Yoshioka, T. Ishikawa, H. Yamada, K. Ohara, N. Tsunoji, Y. Naraki, T. Sano, T. Okubo and T. Wakihara, Phys. Chem. Chem. Phys., 2022, 24, 4136–4146 RSC.
- L. Van Tendeloo, E. Gobechiya, E. Breynaert, J. A. Martens and C. E. A. Kirschhock, Chem. Commun., 2013, 49, 11737–11739 RSC.
- S. Nair, H.-K. Jeong, A. Chandrasekaran, C. M. Braunbarth, M. Tsapatsis and S. M. Kuznicki, Chem. Mater., 2001, 13, 4247–4254 Search PubMed.
- A. Tripathi, D. G. Medvedev, J. Delgado and A. Clearfield, J. Solid State Chem., 2004, 177, 2903–2915 CrossRef CAS.
- J. J. De Yoreo, E. Nakouzi, B. Jin, J. Chun and C. J. Mundy, Faraday Discuss., 2022, 235, 9–35 RSC.
- X. Xue, R. L. Penn, E. R. Leite, F. Huang and Z. Lin, CrystEngComm, 2014, 16, 1419–1429 Search PubMed.
- E. Ruiz-Agudo, C. V. Putnis and A. Putnis, Chem. Geol., 2014, 383, 132–146 CrossRef CAS.
- A. Putnis and C. V. Putnis, J. Solid State Chem., 2007, 180, 1783–1786 CrossRef CAS.
- A. Putnis, Rev. Mineral. Geochem., 2009, 70, 87–124 Search PubMed.
- R. L. Hartman and H. S. Fogler, Langmuir, 2007, 23, 5477–5484 CrossRef CAS PubMed.
- H. Wu, Y. Lv, J. Zhang, Y. Han and C. Guo, Microporous Mesoporous Mater., 2021, 328, 111469 CrossRef CAS.
- B. Wang, L. Ren, J. Zhang, R. Peng, S. Jin, Y. Guan, H. Xu and P. Wu, Microporous Mesoporous Mater., 2021, 314, 110894 CrossRef CAS.
- P. Tian, X. Su, Y. Wang, Q. Xia, Y. Zhang, D. Fan, S. Meng and Z. Liu, Chem. Mater., 2011, 23, 1406–1413 CrossRef CAS.
- Y. Wang, X. Li, Z. Xue, L. Dai, S. Xie and Q. Li, J. Phys. Chem. B, 2010, 114, 5747–5754 CrossRef CAS PubMed.
- S. Goel, S. I. Zones and E. Iglesia, Chem. Mater., 2015, 27, 2056–2066 CrossRef CAS.
- R. Chal, T. Cacciaguerra, S. van Donk and C. Gérardin, Chem. Commun., 2010, 46, 7840–7842 Search PubMed.
- J. Garcia-Martinez, C. Xiao, K. A. Cychosz, K. Li, W. Wan, X. Zou and M. Thommes, ChemCatChem, 2014, 6, 3110–3115 CrossRef CAS.
- R. Jain, A. J. Mallette and J. D. Rimer, J. Am. Chem. Soc., 2021, 143, 21446–21460 CrossRef CAS PubMed.
- A. Navrotsky, O. Trofymluk and A. A. Levchenko, Chem. Rev., 2009, 109, 3885–3902 CrossRef CAS PubMed.
- C. Chu-Jon, E. Martinez, A. A. Bertolazzo, S. Banik, J. D. Rimer, S. K. R. S. Sankaranarayanan and V. Molinero, J. Am. Chem. Soc., 2024, 146, 33204–33213 Search PubMed.
| This journal is © The Royal Society of Chemistry 2026 |