Open Access Article
Open Access ArticleFlux crystal growth of potassium lanthanide double vanadates with lanthanide-dependent coordination number variance
Hunter B. Tisdale ab and
Hans-Conrad zur Loye
ab and
Hans-Conrad zur Loye *ab
*ab
aCenter for Hierarchical Waste form Materials, Columbia, South Carolina 29208, USA. E-mail: zurloye@mailbox.sc.edu
bDepartment of Chemistry and Biochemistry, University of South Carolina, Columbia, South Carolina 29208, USA
First published on 2nd January 2026
Abstract
A series of potassium rare earth double vanadates is presented. Single crystals of double vanadates with stoichiometry K3Ln(VO4)2 (Ln = La, Pr, Nd, and Sm) and crystallizing in the glaserite structure type were synthesized via a molten alkali chloride/fluoride flux reaction. Different structures were observed depending on the constituent rare earth: K3La(VO4)2 crystallizes in an as-of-yet unreported modification of the typical glaserite structure in the space group P21/c, likely due to its larger ionic size that results in an increase in the lanthanide coordination number (seven to eight). The Pr, Nd, and Sm compositions are isostructural with their phosphate analogues and crystallize in the space group P21/m. A discussion of the synthetic details, structure determination, structure descriptions, and coordination number variance is presented.
Introduction
Alkali rare earth double phosphates with stoichiometry A3Ln(PO4)2 (A = Na, K, Rb, Cs) have demonstrated wide ranging properties and structural diversity that have made them attractive targets for ongoing research.1–16 For example, both doped (dopants include Pr3+, Gd3+, Eu3+, and Tb3+) and undoped compositions have been synthesized and evaluated as potential photoluminescent materials.2,4,17,18 Also, it has been shown that by varying the size of the constituent alkali and lanthanide elements, different structures may be obtained that vary in lanthanide content, coordination numbers, and symmetries.5,10,16,18 Recently, the lanthanide double phosphates containing K+ or Rb+ have shown promise as potential nuclear waste forms for the sequestration of transuranic elements, such as americium,10,19 as have other lanthanide phosphates, such as monazites.20 Lanthanide phosphates, in general, (not just the double phosphates) have been of interest for their radiation tolerance, which make them ideal for nuclear waste forms requiring persistent materials.10One of the most common structure types that the double phosphates crystallize in is the glaserite, K3Na(SO4)2, structure.12 The full lanthanide(III) series (excluding Pm) of K3Ln(PO4)2 has been synthesized and published by Farmer et al.,16 and we recently continued this work by synthesizing and publishing the full lanthanide(III) series (excluding Ce and Pm) of Rb3Ln(PO4)2.10 The majority of these phases crystallize in the monoclinic glaserite structure, however, for the smaller (heavier) lanthanides, a reduction in coordination number (seven to six coordination) for the constituent lanthanide with a change from to monoclinic to trigonal symmetry is observed. For the potassium analogues, this drop in coordination number occurs between Yb and Lu, while for the rubidium analogues, this change occurs between Tb and Dy. In addition, some double phosphates in the glaserite structure can by thermally induced to transition into this lower coordination number structure.10,15 While this helps elucidate the influence of varying the alkali and lanthanide elements on the formation of specific structure types, significantly less work has been performed on varying the phosphate unit.
A structural unit with very similar chemistry to the phosphate, PO43−, unit is the VO43−, or vanadate, unit. Both the phosphorus and vanadium exist in their +5 oxidation state, have noble gas electron configurations, and form tetrahedral XO4 units with 4 equal X–O bonds of order 1.25. This makes the vanadate unit an excellent candidate for substituting the phosphate unit in known compositions.9,11,21–23 For example, the Kolis group has previously reported a series of potassium lanthanide double vanadates with the glaserite structure and stoichiometry K3Ln(VO4)2.22 They successfully synthesized the Sc, Y, and Dy–Lu analogues via both a supercritical hydrothermal and molten flux routes. However, the La–Tb analogues have yet to be reported in the literature. In this paper, we report the synthesis of the La, Pr, Nd, and Sm analogues of K3Ln(VO4)2 via a molten flux route. The synthetic details, determination of their structures via single crystal X-ray diffraction, and structural details will be discussed.
Experimental
Reagents
La2O3 (Alfa Aesar, 99.9%), Nd2O3 (Acros Organics, 99.9%), Sm2O3 (Alfa Aesar, 99.99%), V2O5 (Alfa Aesar, 99.6%), KCl (Sigma Aldritch, 99.98%), and KF (Alfa Aesar, 99%) were used as received. Pr6O11 (Alfa Aesar, 99.9%) was heated at 1000 °C for 12 h in an alumina crucible under H2/N2 gas flow to reduce it to the sesquioxide. The produced Pr2O3 was then used as is.Synthesis
Single crystals of K3Ln(VO4)2 were synthesized via a molten flux method. First, 1 mmol of Ln2O3, 2 mmol of V2O5, 11 mmol of KCl, and 9 mmol of KF were mixed thoroughly and added to a bowl-shaped platinum crucible. The crucible was then covered with a platinum lid and loaded into a programmable furnace. The furnace was then programmed to heat to 875 °C, dwell at 875 °C for 12 h, cool to 500 °C at 10 °C h−1 and finally shut off. The crucible was then allowed to cool to room temperature before removing it from the furnace. The crystals were extracted from the flux by sonicating the crucible in deionized water for 1 h and finally removing the dissolved flux via suction filtration.Powder X-ray diffraction (PXRD)
A Bruker D2 Phaser equipped with a LYNXEYE XE-T silicon strip detector and a sealed-tube Cu Kα anode was used to collect PXRD data over a 2θ range of 5° to 65° on all products to determine phase identities and purities. The high energy resolution of the LYNXEYE XE-T detector allowed for energy cut-off of the Kβ X-rays.Single crystal X-ray diffraction (SXRD)
Single crystal X-ray diffraction data were collected on all K3Ln(VO4)2 products at ambient temperature using a Bruker D8 QUEST diffractometer equipped with a microfocus IµS 3.0 sealed-tube X-ray source (Mo Kα1, λ = 0.71073 Å) and a PHOTON II CPAD detector. Collected frames were integrated using SAINT+ and were corrected for absorption effects using SADABS in the Bruker APEX 3 software suite.24 Initial structure models were obtained with SHELXT using intrinsic phasing25 and refined with SHELXL using least-squares full-matrix methods26 in OLEX2. Space groups were determined using a combination of XPREP systematic absences analysis24 and ADDSYM (PLATON software suite) analysis.27 Crystallographic information can be found in Table 1.| Formula | K3La(VO4)2 | K3Pr(VO4)2 | K3Nd(VO4)2 | K3Sm(VO4)2 |
| System | Monoclinic | Monoclinic | Monoclinic | Monoclinic |
| Space group | P21/c | P21/m | P21/m | P21/m |
| a (Å) | 7.6047(1) | 7.5663(1) | 7.5519(2) | 7.5342(2) |
| b (Å) | 5.9188(1) | 5.9128(1) | 5.9169(1) | 5.9090(2) |
| c (Å) | 20.0090(4) | 9.9436(2) | 9.9126(2) | 9.8642(2) |
| β (°) | 90.914(1) | 90.895(1) | 90.872(1) | 90.809(1) |
| V (Å3) | 900.50(3) | 444.80(1) | 442.88(2) | 439.11(2) |
| Z | 4 | 2 | 2 | 2 |
| Crystal size (mm3) | 0.1 × 0.1 × 0.05 | 0.08 × 0.08 × 0.04 | 0.38 × 0.35 × 0.15 | 0.1 × 0.1 × 0.1 |
| Temperature (K) | 297 | 299 | 301 | 299 |
| Dx (g cm−3) | 3.585 | 3.644 | 3.685 | 3.763 |
| θ range (°) | 2.9–36.3 | 2.7–36.3 | 2.7–36.3 | 3.4–36.3 |
| Abs. coef. (mm−1) | 8.09 | 8.86 | 9.26 | 10.12 |
| Reflections collected | 101![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 214 214 |
54![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 234 234 |
60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 332 332 |
59![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 529 529 |
| Independent reflections | 4320 | 2316 | 2303 | 2260 |
| Rint | 0.026 | 0.035 | 0.043 | 0.028 |
| h | −12 ≤ h ≤ 12 | −12 ≤ h ≤ 12 | −12 ≤ h ≤ 12 | −11 ≤ h ≤ 12 |
| k | −9 ≤ k ≤ 9 | −9 ≤ k ≤ 9 | −9 ≤ k ≤ 9 | −9 ≤ k ≤ 9 |
| l | −33 ≤ l ≤ 33 | −16 ≤ l ≤ 16 | −16 ≤ l ≤ 16 | −16 ≤ l ≤ 16 |
| Δρmax (e− Å−3) | 0.94 | 0.83 | 1.67 | 1.05 |
| Δρmin (e− Å−3) | −0.55 | −0.66 | −3.09 | −1.13 |
| Goodness-of-fit on F2 | 1.31 | 1.16 | 1.42 | 1.41 |
| R1(F) for Fo2 > 2σ(Fo2) | 0.013 | 0.011 | 0.022 | 0.013 |
| wR2(Fo2) | 0.034 | 0.029 | 0.058 | 0.035 |
| R1(all) | 0.014 | 0.012 | 0.022 | 0.013 |
| wR2(all) | 0.035 | 0.029 | 0.058 | 0.035 |
| F(000) | 896 | 452 | 454 | 458 |
| Extinction coefficient | 0.00819(18) | 0.0112(5) | 0.300(6) | 0.229(3) |
| Restraints/parameters | 0/128 | 0/80 | 0/80 | 0/80 |
Results and discussion
Synthesis
A eutectic mixture of KCl and KF was utilized as a flux for the crystallization of K3Ln(VO4)2. A ratio of 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 of KCl
9 of KCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KF corresponds to the approximate ratio at which their eutectic is at its minimum melting temperature: about 600 °C. By taking advantage of this lower melting point, the range of temperatures during which crystallization may occur is extended. Also, the use of an alkali halide flux provides both a source of K+ for incorporation into products and a source of halides that may facilitate the solvation of the starting reagents. The Ln2O3 and V2O5 are unlikely to dissolve without the presence of such halides due to their high stabilities. Many fluxes, such as hydroxide salts, are prone to oxidizing constituents of the melt that have stable, lower oxidation states. In contrast, the alkali halide fluxes tend to be redox neutral which is necessary to maintain the correct oxidation states of the Ln(III) and V(V). As a result, high-quality, large, prismatic crystals were formed.28
KF corresponds to the approximate ratio at which their eutectic is at its minimum melting temperature: about 600 °C. By taking advantage of this lower melting point, the range of temperatures during which crystallization may occur is extended. Also, the use of an alkali halide flux provides both a source of K+ for incorporation into products and a source of halides that may facilitate the solvation of the starting reagents. The Ln2O3 and V2O5 are unlikely to dissolve without the presence of such halides due to their high stabilities. Many fluxes, such as hydroxide salts, are prone to oxidizing constituents of the melt that have stable, lower oxidation states. In contrast, the alkali halide fluxes tend to be redox neutral which is necessary to maintain the correct oxidation states of the Ln(III) and V(V). As a result, high-quality, large, prismatic crystals were formed.28
Kolis et al. have previously reported difficulties in synthesizing the La through Tb analogues of K3Ln(VO4)2 via molten flux and supercritical hydrothermal techniques.22 However, they have reported using 1000 °C as their dwelling temperature for their flux reactions, while we have utilized a temperature of 875 °C to successfully synthesize the La through Sm analogues. Thus, there is likely a strong dependence of double vanadate crystallization on reaction temperature with a preference of lower temperatures for the lighter lanthanides. However, the Ce, Eu, Gd, and Tb analogues have not yet been successfully synthesized. For Ce, the reason is likely due to the instability of Ce3+ in open air at high temperatures. For Eu, Gd, and Tb, on the other hand, the reason for failure is less clear.
Unfortunately, all but the La reaction yielded impurities despite attempts at adjusting reaction parameters to select for K3Ln(VO4)2 (Fig. 1 and 2). The amount of impurity formed increases across the series, suggesting that the smaller ionic size for later lanthanide ions leads to a mix of products. The impurity has been identified as a potassium lanthanide vanadate fluoride which will be discussed in a future publication.
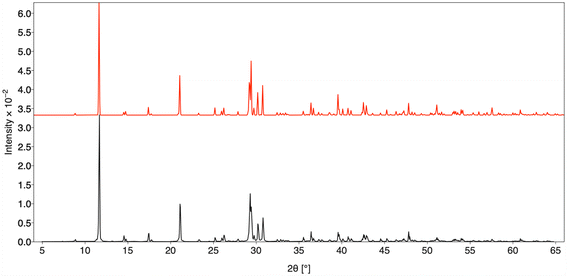 | ||
| Fig. 1 PXRD of K3La(VO4)2 (black) with simulated PXRD pattern calculated from SXRD model of K3La(VO4)2 (red). | ||
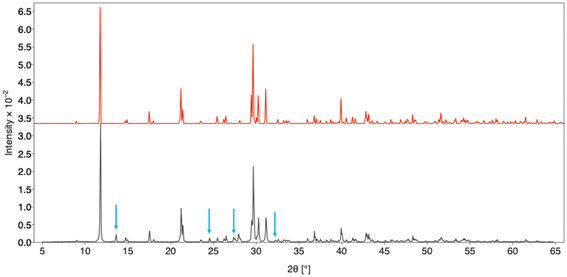 | ||
| Fig. 2 PXRD of K3Sm(VO4)2 (black) with simulated PXRD pattern calculated from SXRD model of K3Sm(VO4)2 (red). Peak corresponding to impurities are indicated with blue arrows. | ||
Structure description
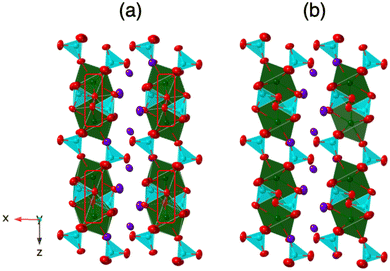
For Ln = Pr, Nd, and Sm, K3Ln(VO4)2 crystallizes in the space group P21/m and is isostructural with its phosphate analogues.9 This structure is analogous to the glaserite structure and consists of Ln(VO4)2 2D layers separated by K3 layers in an ABAB… repeating sequence (Fig. 3). The Ln(VO4)2 layers consist of LnO7 trigonal antiprisms and VO4 tetrahedra. Each Ln(III) cation is bound to six VO4 tetrahedra: five via corner sharing (monodentate) and one via edge sharing (bidentate). The Ln polyhedra do not share any anions with each other and the VO4 tetrahedra do not interconnect. All O atoms are part of a VO4 tetrahedron (Fig. 4). The K+ layer, on the other hand, consists of three sublayers of K+ atoms in a ccp arrangement. For Ln = La, however, K3Ln(VO4)2 crystallizes in the space group P21/c and is a modification of the typical glaserite structure (Fig. 3).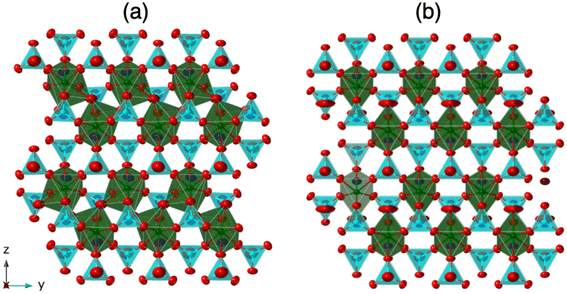 | ||
| Fig. 4 View down the a-axis of K3Ln(VO4)2 for (a) Ln = La and (b) Ln = Pr, Nd, and Sm. Ellipsoids are shown at 99% probabilities. | ||
In the K3Ln(VO4)2 models for Ln = Pr, Nd, and Sm, the thermal ellipsoid for O6 is elongated perpendicularly to the bond it creates with the Ln and extends towards two adjacent Ln atoms (Fig. 4 and 5). This phenomenon has been observed previously in the glaserite-type K+ and Rb+ alkali phosphates.10,16
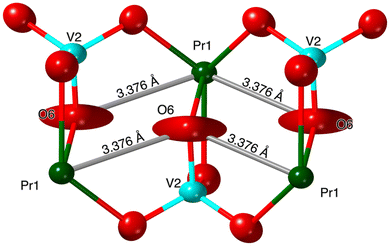 | ||
| Fig. 5 Structure of typical monoclinic glaserite Ln–O6 chains showcasing the elongated O6 thermal ellipsoids and their distances to neighboring Ln atoms (K3Pr(VO4)2 structure model shown). | ||
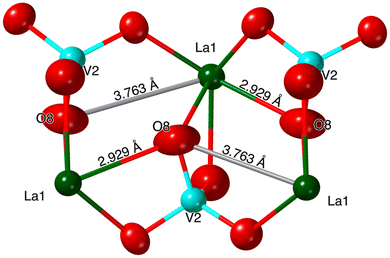
In the K3La(VO4)2 structure, this O atom (O8 in the La structure) no longer has an elongated ellipsoid but instead coordinates fully to one of the adjacent Ln atoms (Fig. 4). This changes the O atom from being bound to one V atom and one Ln atom to being bound to one V atom and two Ln atoms (Fig. 6 and 7). Consequently, the coordination number of La is eight instead of the typical seven in the glaserite structure. The elongated O ellipsoid in the K3Ln(VO4)2 models for Ln = Pr, Nd, and Sm implies the possible presence of an attractive force between the adjacent Ln atoms and the elongated O that only becomes strong enough to lock into one position upon the incorporation of La. Due to the lanthanide contraction, shielding of 4f-electrons, and equivalent Ln charges (+3), this is likely to be a consequence of the larger size of La compared to Pr, Nd, and Sm. What is especially striking, however, is that O8 is shifted in a symmetrical fashion throughout the structure (Fig. 7). Looking down the b direction, the direction of the O8 shift from its standard glaserite position is constant for a single line of O8 atoms extending down the b direction. However, looking down the c direction, the O8 atoms alternate between shifting in the positive and negative b directions (Fig. 3). This symmetry doubles the unit cell size relative to the glaserite unit cell. Despite this doubling, no superstructure peaks at low 2θ are visible on the observed PXRD patterns.
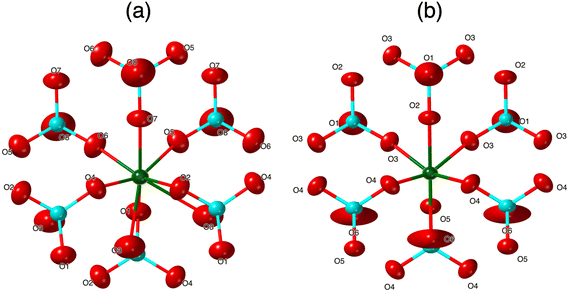 | ||
| Fig. 6 Coordination environment of Ln in K3Ln(VO4)2 looking down the b-axis for (a) Ln = La and (b) Ln = Pr, Nd, and Sm. Ellipsoids are shown at 99% probabilities. | ||
Summary
Single crystals of K3Ln(VO4)2 (Ln = La, Pr, Nd, and Sm) were synthesized via a molten KCl/KF eutectic flux. Challenges still exist in the synthesis of the full lanthanide(III) series with no successful attempts made at synthesizing high-quality single crystals of the Ce, Eu, Gd, and Tb analogues. The La analogue crystallizes in a novel modification of the glaserite structure with an increase in coordination number of the lanthanide from seven to eight. The Pr, Nd, and Sm products are all isostructural with their phosphate analogues. This change in coordination number is likely due to the significant difference in sizes of the lanthanides with La being the largest. Future work will focus on identifying new synthetic methods for targeting the Ce, Eu, Gd, and Tb analogues.Conflicts of interest
There are no conflicts to declare.Data availability
Raw data for other measurements are available upon request.Supplementary information (SI) is available. See DOI: https://doi.org/10.1039/d5dt02027a.
CCDC 2480797–2480800 contain the supplementary crystallographic data for this paper.29a–d
Acknowledgements
Research was conducted by the Center for Hierarchical Waste Form Materials (CHWM), an Energy Frontier Research Center (EFRC). Research was supported by the U.S. Department of Energy, Office of Basic Energy Sciences, Division of Materials Sciences and Engineering under award DE-SC0016574.References
- A. J. Pelczarska and I. Szczygieł, Characteristics of hydrothermally obtained Na3RE(PO4)2 phosphates, where RE = La, Ce, Nd, Gd or Er, J. Therm. Anal. Calorim., 2022, 147, 9913–9922 CrossRef.
- R. Salmon, C. Parent, M. Vlasse and G. Le Flem, The crystal structure of a new high Nd concentration laser material: Na3Nd(PO4)2, Mater. Res. Bull., 1978, 13, 439–444 CrossRef.
- Q. Jing, M. Hu, M. Zhu, J. Wang, H. Duan and Z. Chen, Structure and optical properties of alkali rare-earth double phosphates of M3RE(PO4)2 (M = K, Rb; RE = Y, La, and Lu), Opt. Mater., 2024, 157, 116278 CrossRef.
- H. B. Tisdale, R. L. Conner, L. G. Jacobsohn and H.-C. zur Loye, Alkali rare earth double phosphates: Promising new high temperature scintillators, Solid State Sci., 2024, 154, 107612 CrossRef.
- Y. Wang, Z. Lian, X. Su, Z. Yang, S. Pan, Q. Yan and F. Zhang, Cs6RE2(PO4)4(RE = Y and Gd): two new members of the alkali rare-earth double phosphates, New J. Chem., 2015, 39, 4328–4333 RSC.
- L. Schwarz, B. Finke, M. Kloss, A. Rohmann, U. Sasum and D. Haberland, Investigations on the electronic structure of double phosphates of the type M3RE(PO4)2 (RE = rare earths, lanthanides), J. Lumin., 1997, 72–74, 257–259 CrossRef.
- J. Legendziewicz, M. Guzik and J. Cybińska, VUV spectroscopy of double phosphates doped with rare earth ions, Opt. Mater., 2009, 31, 567–574 CrossRef.
- S. H. Smith and B. M. Wanklyn, Flux growth of rare earth vanadates and phosphates, J. Cryst. Growth, 1974, 21, 23–28 CrossRef.
- M. Vlasse, C. Parent, R. Salmon and G. Le Flem, in The Structures of Na3Ln(XO4)2 Phases (Ln = Rare-Earth, X = P, V, As), Springer US, 1980, pp. 195–201 Search PubMed.
- H. B. Tisdale, M. S. Christian, G. Morrison, T. M. Besmann, K. Sun, G. S. Was and H.-C. zur Loye, Investigation of Rare Earth-Containing Double Phosphates of the Type A3Ln(PO4)2 (Ln = Y, La, Pr, Nd, and Sm–Lu) as Potential Nuclear Waste Forms, Chem. Mater., 2022, 34, 3819–3830 Search PubMed.
- P. P. Mel’nikov and L. H. Komissarova, Double phosphates, arsenates, and vanadates of rare earth elements, scandium, and yttrium with alkali metals, Dokl. Akad. Nauk SSSR, 1981, 256, 878–881 Search PubMed.
- V. A. Eframov, P. P. Mel’nikov and L. N. Komissarova, New compounds of the glaserite type, Rev. Chim. Miner., 1985, 22, 666–675 Search PubMed.
- P. P. Mel'nikov, V. B. Kalinin, V. A. Efremov and L. N. Komissarova, Double orthophosphates of rare earths (Gd-Lu), yttrium and scandium with rubidium, Izv. Akad. Nauk SSSR, Neorg. Mater., 1981, 17, 1452–1455 Search PubMed.
- J. M. Farmer, L. A. Boatner, B. C. Chakoumakos, C. J. Rawn, D. Mandrus, R. Jin and J. C. Bryan, Polymorphism, phase transitions, and thermal expansion of K3Lu(PO4)2, J. Alloys Compd., 2014, 588, 182–189 CrossRef.
- S. V. Ushakov, A. Navrotsky, J. M. Farmer and L. A. Boatner, Thermochemistry of the alkali rare-earth double phosphates, A3RE(PO4)2, J. Mater. Res., 2004, 19, 2165–2175 CrossRef CAS.
- J. M. Farmer, L. A. Boatner, B. C. Chakoumakos, C. J. Rawn and J. Richardson, Structural and crystal chemical properties of alkali rare-earth double phosphates, J. Alloys Compd., 2016, 655, 253–265 CrossRef CAS.
- M. Trevisani, K. V. Ivanovskikh, F. Piccinelli and M. Bettinelli, Fast 5d-4f luminescence in Pr3+-doped K3Lu(PO4)2, J. Lumin., 2014, 152, 2–6 CrossRef CAS.
- D. Zhao, F.-X. Ma, P.-G. Duan, C.-K. Nie, J.-F. Guo and R.-J. Zhang, Commensurately modulated structure and luminescent properties of Na3Pr(PO4)2, J. Lumin., 2017, 192, 129–135 CrossRef CAS.
- T. K. Deason, H. B. Tisdale, A. T. Hines, G. Morrison, Y. Zhoujin, M. D. Smith, T. M. Besmann, A. M. Mofrad, S. L. Estes, J. W. Amoroso, D. P. DiPrete and H.-C. zur Loye, Investigation of Americium-Containing Phosphates, Silicates, Borates, Molybdates, and Fluorides Synthesized via High-Temperature Flux Crystal Growth, J. Am. Chem. Soc., 2025, 147, 21659–21671 CrossRef CAS PubMed.
- Y. Zhoujin, H. B. Tisdale, N. Keerthisinghe, G. Morrison, M. D. Smith, J. Hadermann, T. M. Besmann, J. Amoroso and H.-C. Z. Loye, A low-temperature, one-step synthesis for monazite can transform monazite into a readily usable advanced nuclear waste form, Sci. Adv., 2025, 11, eadt3518 CrossRef CAS PubMed.
- K. Byrappa, C. K. Chandrashekar, B. Basavalingu, K. M. LokanathaRai, S. Ananda and M. Yoshimura, Growth, morphology and mechanism of rare earth vanadate crystals under mild hydrothermal conditions, J. Cryst. Growth, 2007, 306, 94–101 CrossRef CAS.
- M. M. Kimani, L. Thompson, W. Snider, C. D. McMillen and J. W. Kolis, Hydrothermal synthesis and spectroscopic properties of a new glaserite material, K3RE(VO4)2 (RE = Sc, Y, Dy, Ho, Er, Yb, Lu, or Tm) with potential lasing and optical properties, Inorg. Chem., 2012, 51, 13271–13280 CrossRef CAS PubMed.
- M. M. Kimani, C. D. McMillen and J. W. Kolis, Synthetic and spectroscopic studies of vanadate glaserites II: Photoluminescence studies of Ln:K3Y(VO4)2 (Ln=Eu, Er, Sm, Ho, or Tm), J. Solid State Chem., 2015, 226, 320–325 CrossRef CAS.
- APEXIII Version 2016.5–0, SAINT Version 8.37A, SADABS Version 2016/2, 2016 Search PubMed.
- G. M. Sheldrick, SHELXT - Integrated space-group and crystal-structure determination, Acta Crystallogr., Sect. A: Found. Adv., 2015, 71, 3–8 Search PubMed.
- G. M. Sheldrick, Crystal structure refinement with SHELXL, Acta Crystallogr., Sect. C: Struct. Chem., 2015, 71, 3–8 Search PubMed.
- A. L. Spek, Single-crystal structure validation with the program PLATON, J. Appl. Crystallogr., 2003, 36, 7–13 CrossRef CAS.
- D. E. Bugaris and H.-C. zur Loye, Materials Discovery by Flux Crystal Growth: Quaternary and Higher Oxides, Angew. Chem., Int. Ed., 2012, 51, 3780–3811, DOI:10.1002/anie.201102676.
- (a) CCDC 2480797: Experimental Crystal Structure Determination, 2026, DOI:10.25505/fiz.icsd.cc2p8gq5; (b) CCDC 2480798: Experimental Crystal Structure Determination, 2026, DOI:10.25505/fiz.icsd.cc2p8gr6; (c) CCDC 2480799: Experimental Crystal Structure Determination, 2026, DOI:10.25505/fiz.icsd.cc2p8gs7; (d) CCDC 2480800: Experimental Crystal Structure Determination, 2026, DOI:10.25505/fiz.icsd.cc2p8gt8.
| This journal is © The Royal Society of Chemistry 2026 |