Open Access Article
Open Access ArticleFrom discrete to polymeric assemblies: exploring supramolecular structural diversity in transition metal phosphinates with N-donor ligands
Archana Kumari
Pattnaik
 *a and
Gobinda Chandra
Behera
b
*a and
Gobinda Chandra
Behera
b
aSupramolecular and Solid State Structural Chemistry Laboratory, School of Basic Sciences, Indian Institute of Technology Bhubaneswar, Bhubaneswar 752 050, India. E-mail: akp18@iitbbs.ac.in; arcpattnaik@gmail.com
bDepartment of Chemistry, Maharaja Purna Chandra Autonomous College, Takhatpur, Baripada 757003, Odisha, India
First published on 15th December 2025
Abstract
A series of coordination complexes derived from diphenylphosphinic acid (DPP) and transition metal ions (Mn(II), Co(II), Ni(II), Cu(II), and Cd(II)) were developed in the presence of various N-donor ligands, including 4,4′-bipyridine (bpy), 1,2-bis(4-pyridyl)ethane (bpyea), 1,2-bis(4-pyridyl)ethene (bpyee), and 1,10-phenanthroline (phen). The resulting structures unveil monomeric, dimeric, and polymeric frameworks, demonstrating the rich structural diversity achievable with phosphinate coordination chemistry. Single-crystal X-ray diffraction analysis revealed variable metal coordination environments—ranging from distorted octahedral to square pyramidal geometries—and highlighted the versatile and intriguing binding modes of DPP, particularly the formation of M–(O–P–O)2–M eight-membered rings and pseudo-chelating motifs. Notably, rigid co-ligands such as phen result in discrete or dimeric units, while linear spacers like bpy and bpyea facilitate polymeric chain and extended framework development. Solvothermal and ambient crystallisation conditions produced distinct structural outcomes, with cases of polymorphism and framework interconversion observed. Non-covalent interactions, especially hydrogen bonding and π–π stacking, significantly influence the crystal packing pattern and are responsible for the stability of the network solids. These findings highlight the potential of phosphinate-based ligands in constructing coordination networks with tunable dimensionality and topology, thereby expanding the utility of organophosphorus ligands in secondary building unit (SBU) formation and advancing the rational design of supramolecular architectures.
1. Introduction
Organophosphorus acids are an important class of organic substrates for the formation of organic–inorganic hybrid complexes and discrete species to clusters, along with extended networks, showing promising applications in the fields of ion exchange,1 sorption,2–4 catalysts,5–7 photoluminescence,8 magnetism,9,10etc. Organophosphonates, derived from phosphonic acids, show promising applications with variable coordination modes; however, their utility is limited, due to the presence of several strong and potential binding sites that form coordination bonds and non-covalent interactions, leading to the formation of random assemblies rather than targeted structures. However, alkoxy/phenoxy derivatives limit the number of such binding sites to yield targeted ensembles.11,12 But, as ester bonds are prone to hydrolysis, the synthetic conditions need to be modulated carefully, such as the pH of the solution, reaction environment, etc., to facilitate complexation without hydrolysis.13 Replacing the –OH group with direct P–C bonds—thus avoiding labile alkoxy or phenoxy moieties—enhances the supramolecular features of organophosphorus acids. The resulting phosphines or phosphinites are not only more resistant to hydrolysis, but their tunable functional groups also allow precise control over binding sites, enabling the formation of tailored hybrid structures under more flexible reaction conditions.14,15 This strategy opens up adequate ways to achieve complexes with active metal sites within the framework to exhibit substantial applications.16 In fact, diaryl and dialkyl phosphinic acids represent a relatively small fraction of known MOF structures compared to carboxylates, although they readily form coordination complexes through diverse metal–oxygen binding modes, typically resulting in non-porous and layered architectures.14 Among the possible coordination modes (Scheme 1), the common mode seen in phosphinate complexes is II (Scheme 1), which generally forms an 8-membered ring (M–O–P–O–M–O–P–O), as also noted in phosphates and phosphonates.Apart from these modes of coordination, pseudo-chelation is also observed in many heteroleptic complexes, due to strong H-bond donors/acceptors in the ligands. When both phosphinates and phosphinic acids are coordinated to a single metal atom, then pseudo-chelation (O–PR2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯H–O–PR2
O⋯H–O–PR2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) is established. These distinctive features, indeed, exhibit a significant role in the metal extraction process mechanism using phosphinate ligands (Fig. 1).17,18
O) is established. These distinctive features, indeed, exhibit a significant role in the metal extraction process mechanism using phosphinate ligands (Fig. 1).17,18
 | ||
| Fig. 1 Monomeric and dimeric forms of phosphinic acid showing retention of interligand H-bonding upon the formation of a tetrahedral complex.19 | ||
From a CSD search, it is apparent that among the eighteen possible modes, the most common binding mode is the dinucleating form 2.11 (Harris notation for mode II in Scheme 1), which is frequently observed in discrete, oligomeric and polymeric structures reported. For example, [Ni2(µ2-L1)2(2,2′-dipy)4]2+ and [(py)3Co2(µ2-L2)3py]+ are two complexes as illustrated in Fig. 2, in which the phosphinate group (O–P–O) bridges metal atoms.20,21 The Ni complex [Fig. 2(a)] which contains –PH group as a bridging unit, is of high significance because it enables direct functionalisation to tailor the structure and the properties of materials, such as catalysts and magnetoactive systems.22 Ni–(O–P–O)2–Ni with the eight membered heterocycle in a chair form involves a distorted octahedral environment around Ni(II), with a cis arrangement of oxygen atoms [Fig. 2(a)]. However, the complex shown in Fig. 2(b) is a dinuclear complex with mixed geometry of cobalt (both octahedral and tetrahedral sites), with the dibenzyl phosphinate ligand bridging the metal nodes, showing antiferromagnetic activity.
 | ||
| Fig. 2 Examples of dinuclear structures containing (a) two and (b) three bridging motifs (see Scheme 1(II)). | ||
Apart from this, these versatile phosphinate ligands also form linear polymeric chains bridging metal ions and show trinucleating,23,24 tetranucleating,25 and pentanucleating26–28 motifs through different coordination modes (Scheme 1). Similarly, polyphosphinic acids with non-chelating auxiliary ligands like bpy and its derivatives generate polymeric complexes with 2D and 3D frameworks acquiring permanent porosity. In fact, by using these strategies, a series of MOFs (ICR 2, 4, 6 and 7) have been reported in the literature.28 As the most common bridging is bidentate bridging, the obtained geometry, in general, is 1D polymeric chains, which show magnetic activity. Also, it is observed that phosphinates have more propensity for the formation of a large number of high-density polynuclear clusters than MOFs. In this study, we have used diphenyl phosphinic acid as a phosphorus containing ligand along with different N-donor coligands, incorporating transition metal ions to investigate herein the interactions of ligands with metal ions. Thus, the intriguing structural landscape with diverse architectures depends on novel arrangements and directions of building blocks to form discrete, polymeric and cluster structures that disperse ligand coordination features.
Possible coordination modes (Scheme 1) of phosphinic acids are able to develop interesting building blocks for coordination complex assemblage, as their coordination modes are similar to those of widely studied carboxylates, but their complexation process follows a distinct way. According to the HSAB principle, the acidity of phosphinic acids also falls between those of carboxylic and phosphonic acids.
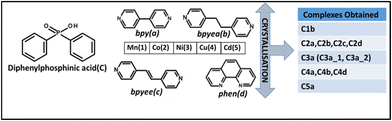
To achieve the higher dimensionality of the polymer network containing metal phosphinates, auxiliary ligands also play a pivotal role, which ultimately influence the topology and the physico-chemical behaviour of the complexes. Therefore, diphenylphosphinic acid (DPP), which is a monophosphinic acid along with different N-donor compounds such as 4,4′-bipyridine (bpy), 1,2-bis(4-pyridyl)ethane (bpyea), 1,2-bis(4-pyridyl)ethene (bpyee) and 1,10-phenanthroline (110phen), in the presence of transition metal species (Mn(II), Co(II), Ni(II), Cu(II) and Cd(II)) is crystallised, as listed in Scheme 2 through various crystallisation procedures. The structural features in terms of bonding and spatial arrangement of molecules in the lattice for the crystals obtained as listed in Scheme 2 are discussed in the following sections.
2. Experimental section
All reagents (metal salts – Mn(NO3)·4H2O, Co(NO3)2·6H2O, Ni(NO3)2·6H2O, Cu(ClO4)2·6H2O and Cd(NO3)2·6H2O) and organic ligands – diphenyl phosphinic acid (DPP), 4,4′-bipyridine (bpy), 1,2-bis (4-pyridyl)ethane (bpyea), 1,2-bis(4-pyridyl)ethene (bpyee) and 1,10-phenanthroline (110phen) of utmost purity – were purchased from commercial suppliers and used without any further purification. For the crystallisation experiments, spectroscopy-grade solvents and distilled water were used.2.1. Preparation of molecular complexes
All complexes were prepared by dissolving DPP (0.05 mmol) in 3 mL of CH3OH by gentle heating on a water bath and adding an aqueous solution of the corresponding metal ion (0.1 mmol in 2 mL of H2O), followed by dropwise addition of methanolic solution of ligands (a–d) (0.05 mmol in 2 mL of CH3OH), thus maintaining a molar ratio for DPP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) metal salt
metal salt![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) N-donor ligands of 1
N-donor ligands of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in all complexes. The reaction mixture was allowed to slowly evaporate at room temperature to afford the crystals of C2d, C3a_1, and C5a. Within 24–72 h, crystals appeared in the solution. All other complexes except C4a were crystallised using a solvothermal method, keeping the reaction mixture solution in a 25 mL Teflon vessel inside a stainless steel chamber maintained at 130 °C for two days, followed by cooling at a rate of 5 °C h−1. After that, the solution was allowed to slowly evaporate at room temperature. The C4a reaction mixture solution was kept in a vacuum for crystallisation through vacuum evaporation. In these processes, crystals appeared within a week, which were separated for the characterization using the X-ray diffraction technique.
1 in all complexes. The reaction mixture was allowed to slowly evaporate at room temperature to afford the crystals of C2d, C3a_1, and C5a. Within 24–72 h, crystals appeared in the solution. All other complexes except C4a were crystallised using a solvothermal method, keeping the reaction mixture solution in a 25 mL Teflon vessel inside a stainless steel chamber maintained at 130 °C for two days, followed by cooling at a rate of 5 °C h−1. After that, the solution was allowed to slowly evaporate at room temperature. The C4a reaction mixture solution was kept in a vacuum for crystallisation through vacuum evaporation. In these processes, crystals appeared within a week, which were separated for the characterization using the X-ray diffraction technique.
2.2. Characterization
3. Results and discussion
3.1. Structural description of the metal phosphinate ensembles
| C1b | C2a | C2b | C2c | C2d | |
|---|---|---|---|---|---|
| Formula | C36H32N2O4P2Mn | C34H34N2O7P2Co | C36H34N2O5P2Co | C84H98N6O22P4Co2 | C48H36N6O10P2Co2 |
| F w | 673.51 | 703.50 | 695.52 | 1785.42 | 1036.63 |
| Crystal colour | Colourless | Pink | Pink | Pink | Pink |
| Crystal shape | Plate | Plate | Block | Block | Block |
| T/K | 293(2) | 100(2) | 293(2) | 100(2) | 100(2) |
| Mo kα/Å | 0.71073 | 0.71073 | 0.71073 | 0.71073 | 0.71073 |
| Crystal system | Monoclinic | Monoclinic | Triclinic | Triclinic | Monoclinic |
| Space group | P21/n | P21/c |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
P21/n |
| a/Å | 13.7783(14) | 11.4798(13) | 11.9816(17) | 13.5255(16) | 12.5496(15) |
| b/Å | 5.6132(7) | 27.409(4) | 12.7736(17) | 13.5524(15) | 12.9240(17) |
| c/Å | 20.880(3) | 11.5762(14) | 13.4767(17) | 14.8154(16) | 14.449(2) |
| α/° | 90 | 90 | 112.507(4) | 98.577(4) | 90 |
| β/° | 94.922(3) | 119.306(4) | 91.947(5) | 104.184(4) | 104.083(4) |
| γ/° | 90 | 90 | 115.772(4) | 119.379(3) | 90 |
| V/Å3 | 1608.9(3) | 3176.3(7) | 1665.4(4) | 2174.6(4) | 2273.1(5) |
| Z | 2 | 4 | 2 | 1 | 2 |
| D c/g cm−3 | 1.390 | 1.471 | 1.387 | 1.363 | 1.515 |
| µ/mm−1 | 0.552 | 0.694 | 0.657 | 0.530 | 0.867 |
| F(000) | 698 | 1460 | 722 | 934 | 1060 |
| Crystal size/mm3 | 0.32 × 0.25 × 0.20 | 0.29 × 0.23 × 0.18 | 0.32 × 0.23 × 0.12 | 0.25 × 0.11 × 0.11 | 0.29 × 0.20 × 0.15 |
| 2θmax | 56.76 | 56.786 | 56.864 | 57.024 | 56.99 |
| Total reflections | 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 972 972 |
59![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 751 751 |
34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 937 937 |
32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 061 061 |
36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 889 889 |
| Unique reflections | 4002 | 7896 | 8282 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 882 882 |
5718 |
| Completeness (%) | 99.3% | 99.8% | 99.9% | 99.0% | 99.7% |
| No. of parameters | 205 | 433 | 421 | 577 | 307 |
| Goodness-of-fit on F2 | 1.038 | 1.112 | 1.021 | 1.025 | 1.054 |
| R 1, [I > 2σ] | 0.0328 | 0.0500 | 0.0434 | 0.0340 | 0.0393 |
| wR2, [I > 2σ] | 0.0944 | 0.0966 | 0.1053 | 0.0943 | 0.1023 |
| CCDC number | 2290893 | 2290901 | 2290894 | 2290898 | 2290896 |
| C3a_1 | C3a_2 | C4a | C4b | C4d | C5a | |
|---|---|---|---|---|---|---|
| Formula | C34H32N2O6P2Ni | C44H40N5O12P2Ni | C34H29ClN2O9P2Cu2 | C24H22ClN2O6PCu | C24H20ClN2O7PCu | C46H38N2O6P3Cd |
| F w | 685.26 | 958.51 | 834.06 | 564.39 | 578.38 | 920.09 |
| Crystal colour | Pale green | Pale green | Blue | Blue | Blue | Colourless |
| Crystal shape | Rod | Block | Rod | Rod | Rod | Block |
| T/K | 293(2) | 100(2) | 293(2) | 293(2) | 293(2) | 293(2) |
| Mo kα/Å | 0.71073 | 0.71073 | 0.71073 | 0.71073 | 0.71073 | 0.71073 |
| Crystal system | Monoclinic | Tetragonal | Monoclinic | Monoclinic | Orthorhombic | Monoclinic |
| Space group | C2/c | P4322 | C2/c | P21/c | Pbca | C2/c |
| a/Å | 26.447(5) | 11.3016(11) | 25.424(3) | 13.2632(14) | 21.733(3) | 22.865(2) |
| b/Å | 11.211(2) | 11.3016(11) | 11.3058(14) | 16.5624(18) | 9.2303(10) | 11.7446(8) |
| c/Å | 11.2987(16) | 33.869(5) | 25.572(3) | 11.6242(12) | 23.419(3) | 32.081(2) |
| α/° | 90 | 90 | 90 | 90 | 90 | 90 |
| β/° | 92.771(9) | 90 | 113.557(4) | 104.254(3) | 90 | 100.258(4) |
| γ/° | 90 | 90 | 90 | 90 | 90 | 90 |
| V/Å3 | 3346.0(10) | 4325.9(11) | 6737.8(14) | 2474.9(5) | 4697.8(10) | 8477.1(11) |
| Z | 4 | 4 | 8 | 4 | 8 | 8 |
| D c/g cm−3 | 1.360 | 1.472 | 1.644 | 1.515 | 1.636 | 1.442 |
| µ/mm−1 | 0.721 | 0.593 | 1.496 | 1.098 | 1.162 | 0.679 |
| F(000) | 1424 | 2000 | 3392 | 1156 | 2360 | 3752 |
| Crystal size/mm3 | 0.35 × 0.31 × 0.21 | 0.31 × 0.23 × 0.19 | 0.25 × 0.20 × 0.14 | 0.33 × 0.25 × 0.20 | 0.25 × 0.2 × 0.12 | 0.35 × 0.23 × 0.11 |
| 2θmax | 56.806 | 56.768 | 57.512 | 57.068 | 56.982 | 56.558 |
| Total reflections | 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 581 581 |
86![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 218 218 |
56![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 626 626 |
38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 255 255 |
75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 215 215 |
43![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 929 929 |
| Unique reflections | 4174 | 5419 | 8575 | 6227 | 5928 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 488 488 |
| Completeness (%) | 99.8% | 99.6% | 99.9% | 99.9% | 99.9% | 99.9% |
| No. of parameters | 212 | 311 | 455 | 316 | 331 | 523 |
| Goodness-of-fit on F2 | 1.039 | 1.044 | 1.036 | 1.036 | 1.060 | 0.993 |
| R 1, [I > 2σ] | 0.0390 | 0.0462 | 0.0335 | 0.0443 | 0.0370 | 0.0428 |
| wR2, [I > 2σ] | 0.0974 | 0.1142 | 0.0793 | 0.1129 | 0.0903 | 0.1197 |
| CCDC number | 2290897 | 2290903 | 2290902 | 2290895 | 2290900 | 2290899 |
In such coordination bonding, Mn–N distances lie in the range of 2.141–2.171 Å and the Mn–O distance is 2.331 Å. The pertinent bond characteristics are listed in Table 2. In particular, the bonding pattern between DPP and Mn(II) is in the most common bidentate bridging form, mode II (Scheme 1), thus yielding a M–(O–P–O)2–M ring motif. Packing analysis of molecules in C1b reveals that the coordination moieties also form polymeric chains, as observed in C1a and C1c (reported in the literature). In the three-dimensional arrangement, the units in C1b are held together by C–H⋯π interactions as denoted in Fig. 3, representing an arrangement of polymeric chains, stabilized by appropriate metal–organic bonds (Mn–O and Mn–N). Apart from two weak intermolecular C–H⋯O contacts (D⋯A = 3.620 and 3.954 Å), no other significant hydrogen-bonding interactions are present; the packing is thus dominated by van der Waals contacts (see SI(II)).
| Complex | M–O | M–N | P–O |
|---|---|---|---|
| C1b | 2.141(1) | 2.331(1) | 1.493(1) |
| 2.171(1) | 1.502(1) | ||
| C2a | 2.051(2) | 2.159(3) | 1.507(2) |
| 2.056(2) | 2.205(3) | 1.510(3) | |
| 2.130(2) | 1.511(3) | ||
| 2.183(3) | 1.515(3) | ||
| C2b | 2.056(1) | 2.148(2) | 1.482(1) |
| 2.059(1) | 2.152(2) | 1.492(1) | |
| 2.097(1) | 1.498(1) | ||
| 2.197(1) | 1.501(1) | ||
| C2c | 2.094(1) | 2.107(1) | 1.505(1) |
| 2.104(1) | 2.166(1) | 1.506(1) | |
| 2.121(1) | 1.515(1) | ||
| 2.129(1) | |||
| C2d | 1.997(1) | 2.119(2) | 1.489(1) |
| 2.005(1) | 2.122(2) | 1.501(1) | |
| 2.154(1) | |||
| 2.267(1) | |||
| C3a_1 | 2.060(1) | 2.086(3) | 1.498(2) |
| 2.124(1) | 2.095(2) | 1.499(2) | |
| C3a_2 | 2.089(5) | 2.107(4) | 1.501(5) |
| 2.095(5) | 2.110(4) | 1.508(5) | |
| 2.115(4) | 1.550(5) | ||
| 2.124(4) | 1.551(5) | ||
| C4a | 1.924(1) | 2.013(1) | 1.504(1) |
| 1.939(1) | 2.016(1) | 1.509(1) | |
| 1.962(1) | 1.512(1) | ||
| 1.963(1) | 1.519(1) | ||
| 1.984(1) | |||
| 2.029(1) | |||
| 2.320(1) | |||
| 2.339(1) | |||
| C4b | 1.929(1) | 1.995(2) | 1.509(1) |
| 1.934(1) | 2.004(2) | 1.511(1) | |
| 2.561(1) | |||
| C4d | 1.939(1) | 1.997(2) | 1.498(1) |
| 1.955(2) | 2.009(2) | 1.514(1) | |
| 2.325(2) | |||
| C5a | 2.250(2) | 2.365(3) | 1.487(3) |
| 2.274(3) | 2.377(3) | 1.495(3) | |
| 2.275(2) | 1.523(3) | ||
| 2.276(2) | 1.535(3) |
3.2. Structural description of Co(II) complexes (C2a–C2d)
DPP, along with metal ion species Co(II), successfully forms complexes, C2a–C2d, with N-donors, as listed in Scheme 2. These complexes show some similarities despite each one showing its specific molecular arrangement. All complexes contain the metal ion Co(II), in a distorted octahedral geometry by coordinating with four oxygen atoms and two nitrogen atoms, which are from organic ligands and solvent molecules. Among the structures, C2a:C34H34N2O7P2Co and C2d:C48H36N6O10P2Co2 crystallise in the monoclinic space groups P21/c and P21/n respectively, whereas C2b:C36H34N2O5P2Co and C2c:C84H98N6O22P4Co2 crystallise in the triclinic space group, P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) . The complete structure determination parameters are listed in Table 1. The asymmetric unit contents in each complex are presented in the SI. Thus, apart from coordination spheres, in crystals of C2a and C2c, uncoordinated water molecules are also present, while in the C2d counter anion, nitrate is also present in the asymmetric unit, coordinating to Co(II), apart from the other bonding features mentioned above. Furthermore, interestingly, the coordination of solvent molecules (H2O), indeed, leads to a pseudo-chelation form, instead of metal phosphinate 8-membered ring formation via bidentate bridging of DPP, in some complexes. Furthermore, in the structure of C2c, surprisingly, DPP molecules lie in the asymmetric unit without coordinating to Co(II) at all. Complete details of each complex with pertinent details of interactions between molecules, bonding patterns, coordination features, etc., are discussed below.
. The complete structure determination parameters are listed in Table 1. The asymmetric unit contents in each complex are presented in the SI. Thus, apart from coordination spheres, in crystals of C2a and C2c, uncoordinated water molecules are also present, while in the C2d counter anion, nitrate is also present in the asymmetric unit, coordinating to Co(II), apart from the other bonding features mentioned above. Furthermore, interestingly, the coordination of solvent molecules (H2O), indeed, leads to a pseudo-chelation form, instead of metal phosphinate 8-membered ring formation via bidentate bridging of DPP, in some complexes. Furthermore, in the structure of C2c, surprisingly, DPP molecules lie in the asymmetric unit without coordinating to Co(II) at all. Complete details of each complex with pertinent details of interactions between molecules, bonding patterns, coordination features, etc., are discussed below.
In C2a, as shown in Fig. 4, two bpy and DPP molecules along with a similar number of water molecules coordinate to Co(II). Co1 (general position) binds two DPP O atoms, O3/O5 (H2O), and N1(bpy); the sixth site is occupied by N2(phen) from a symmetry mate at 1 + x, y, z. Apart from this, lattice water O7 also occupies a general position as shown in the ORTEP (SI). All H atoms—including those on O3, O5 and O7—are sensibly located and refined. In such bonding, the observed Co–O and Co–N distances are in the ranges of 2.051–2.183 Å and 2.159–2.205 Å, respectively. Complete details of the bonding parameters are given in Table 2. The coordination spheres further extend through bpy molecules bridging adjacent metal centres, in the form of a 1D polymeric structure.
 | ||
| Fig. 4 Coordination sphere of C2a, with two water molecules coordinating and one in the lattice site (oxygen atom in purple). | ||
In two-dimensional assembly, the adjacent chains while interact with each other through C–H⋯O hydrogen bonds, with a H⋯O distance of 2.47 Å, formed between coordinated water (O) and bpy (–CH) molecules. In addition, the occluded water molecules between the chains also interact through similar interactions but with a H⋯O distance of 2.51 Å. Besides, though DPP possesses bidentate features, in complex C2a, the coordination reflects a monodentate feature, ensuring O–H⋯O (O⋯O, 2.673 and 2.778 Å) and C–H⋯O (H⋯O, 2.73 Å) hydrogen bonds with water molecules by connecting primary coordination spheres (inset in Fig. 5). Besides these, lattice water molecules (O7W) are also attributed to strong O⋯O interactions, as shown in Fig. 5.
 | ||
| Fig. 5 Packing diagram of C2a; phenyl rings are omitted from DPP for clarity and water molecules are presented in purple (left). Pseudo-chelation through H-bonding (right). | ||
Complex C2b upon structure determination by X-ray diffraction reveals that in its crystal lattice, the asymmetric unit shows a Co(II) ion along with three DPP and two bpyea molecules as well as a water molecule. The asymmetric unit as shown in ORTEP (see the SI(I)) has Co1 in a general position directly bonded to N1 of a (bpyea) ligand, to O2 and O4 of two DPP ligands, and to water oxygen O3. The Co1 coordination is completed by a bond to N2 of the (bpyea) ligand at (x, y, −1 + z). All H atoms including those on the water molecule O3 appear to be correctly placed. The salient crystallographic data are listed in Table 1.
The observed coordination bonds are in the ranges of 2.056–2.197 Å and 2.148–2.152 Å, respectively. In this process, each Co(II) center exists in an octahedral geometry (see Fig. 6). The coordination bond features are listed in Table 2. Furthermore, such coordination spheres extend into a polymeric form (see Fig. 7).
 | ||
| Fig. 6 (a) Octahedral coordination sphere of C2b, with one-water molecule coordinating (oxygen atom in purple). (b) Dimeric complex of C2b. | ||
Among three DPP ligands, two follow bidentate bridging, as shown in Fig. 6(b) in the form of a typical 8-membered ring, involving the M–(O–P–O)2–M moiety. The remaining DPP molecule also indeed forms a similar (pseudo) chelating bonding pattern, with the aid of hydrogen bonds along with coordination bonds (Fig. 7). The hydrogen bonds [O⋯O, 2.747 Å (intermolecular) and 2.760 Å (intramolecular)], that constitute this motif are annotated in Fig. 7, where the corresponding hetero atom distances are indicated. Thus, herein, the flexibility of phosphinate ligands further demonstrates the efficacy of H-bonds in accordance with coordination bonds, towards establishing specific topological arrangements.
In coordination polymers, C2c, DPP and Co(II), in the presence of a linear spacer, bpyee, under solvothermal conditions, crystallise as rod like crystals, in the triclinic P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space group. Complete crystallographic parameters are given in Table 1 and the ORTEP is illustrated in SI(I). The asymmetric unit has two Co atoms, Co1 and Co2, lying on inversion centres. The half-occupancy Co1 atom is directly bonded to N2 of a complete bpyee ligand and to two water oxygens O1 and O2. The half-occupancy Co2 atom is directly bonded to two water oxygens O3 and O4 and to the N1 atom of half of a bpyee ligand, which lies about another inversion centre. There are also three water molecules, O9, O10, and O11, in general positions in lattice sites. All H atoms, including those on the water oxygen atoms, appear to be correctly placed. Thus, in the crystal lattice, apart from coordinated motifs, two uncoordinated molecules of DPP and three molecules of water are also present. In crystals C2c, Co(II) forms two different coordination environments, labelled as A and B, as shown in Fig. 8, with the environments shown in different colors (A in grey and B in yellow). In both the environments, Co(II) is present in an octahedral geometry, with the coordination of two bpyee and four water molecules, with the corresponding Co–O and Co–N distances in the ranges of 2.094–2.129 Å and 2.107–2.166 Å, respectively.
space group. Complete crystallographic parameters are given in Table 1 and the ORTEP is illustrated in SI(I). The asymmetric unit has two Co atoms, Co1 and Co2, lying on inversion centres. The half-occupancy Co1 atom is directly bonded to N2 of a complete bpyee ligand and to two water oxygens O1 and O2. The half-occupancy Co2 atom is directly bonded to two water oxygens O3 and O4 and to the N1 atom of half of a bpyee ligand, which lies about another inversion centre. There are also three water molecules, O9, O10, and O11, in general positions in lattice sites. All H atoms, including those on the water oxygen atoms, appear to be correctly placed. Thus, in the crystal lattice, apart from coordinated motifs, two uncoordinated molecules of DPP and three molecules of water are also present. In crystals C2c, Co(II) forms two different coordination environments, labelled as A and B, as shown in Fig. 8, with the environments shown in different colors (A in grey and B in yellow). In both the environments, Co(II) is present in an octahedral geometry, with the coordination of two bpyee and four water molecules, with the corresponding Co–O and Co–N distances in the ranges of 2.094–2.129 Å and 2.107–2.166 Å, respectively.
In the crystal lattice, the two independent coordination units, A and B, aggregate through O–H⋯O, O–H⋯N and C–H⋯O hydrogen bonds. All hydrogen bond parameters are listed in SI(II). The corresponding H⋯O (O–H⋯O) distances lie in the range from 1.66 to 1.91 Å, whereas H⋯O (C–H⋯O) distances are observed to be between 2.37 and 2.55 Å. O11–H11A⋯N3 with a H⋯N distance of 1.78 Å is also attributed to the consolidated packing of the coordination assembly (see Fig. 9). Furthermore, the coordination sphere A polymer is found to be a linear chain extended to a polymeric form through the N-terminal of bpyee ligands coordinating with Co(II), while B is a discrete unit arranged in such a manner that it forms a host-kind of assemblage such that unit A is fit within the voids thus created. The arrangement of such a topology is shown in Fig. 9. Thus, it is clearly reflective that unlike in C2a and C2b, DPP molecules do not form coordination bonds with Co(II) and only participate in the creation and stabilization of secondary spheres.
 | ||
| Fig. 9 Grid arrangement of A and B in C2c with a host–guest topology (left) and enlarged picture showing the O–H⋯O bonds between coordinated and uncoordinated motifs (right). | ||
 | ||
| Fig. 10 Asymmetric unit of C2d illustrating the chelated coordination environment (left) and the dimeric complex (right). | ||
Coordination assembly of C2d is obtained upon crystallization of Co(NO3)2·6H2O in the presence of DPP along with the rigid linker 110phen by slow evaporation of a CH3OH solution, under ambient conditions. A peculiar observation during the formation of crystals is that block shaped crystals were obtained at the neck of the conical flask rather than in the bulk of the solution. Structure determination by X-ray diffraction reveals that the asymmetric unit consists of a nitrate anion (counter ion in the metal salt) along with other organic ligands and Co(II). C2d crystallizes in a monoclinic space group, P21/n. Structure determination parameters are listed in Table 1 and contents of the asymmetric unit (ORTEP) are presented in SI(I). Co(II) is coordinated with DPP and 110phen molecules and also the nitrate anion. Another interesting feature in the coordination aspects is that the coordination sphere remains in the form of discrete units as 110phen molecules do not modulate the propagation and in fact, it binds to Co(II) in a bidentate mode with Co–N distances being 2.119 and 2.122 Å. Also, the coordinated nitrate anion does not facilitate the realization of an infinite network in any direction, due to its bidentate mode of bonding. With such a coordination network around Co(II), it forms an octahedral geometry. Thus, the molecule is a centrosymmetric dimer; Co1 (general) binds N2/N3(phen), O3/O5(NO2), and O2(DPP), with the sixth site occupied by O1 from the inversion-related position at (1 − x, 1 − y, 1 − z).
The nitrate ion, however, participates in noncovalent interactions (C–H⋯O hydrogen bonds) connecting adjacent coordinated fragments, as shown in the inset of Fig. 11. Such interactions are through both DPP (H⋯O, 2.19 Å) and 110phen (H⋯O, 2.29 and 2.58 Å). In addition, 110phen ligands present in adjacent coordinated fragments contribute to structure stabilization through π–π interaction (3.55 Å). Such interactions are presented in the inset of Fig. 11 and other hydrogen bond parameters are listed in SI(II).
 | ||
| Fig. 11 2D packing arrangement of fragments in the crystal lattice of C2d. C–H⋯O and π–π interaction distances are shown in the inset. | ||
3.3. Structural elucidation of Ni(II) complexes, C3a_1 and C3a_2, with DPP in association with 4,4′-bipyridine (bpy)
Ni(II) nitrate along with DPP and bpy upon crystallisation forms different types of crystals, depending upon the methods followed and also the solvent employed in the crystallization process, although the same molecular composition is maintained. The crystals obtained from a slow evaporation of a CH3OH solution are labelled as C3a_1 (C34H32N2O6P2Ni), while the crystals obtained from a solvothermal process are labelled as C3a_2 (C44H47N5O12P2Ni). The solvothermal process involves keeping the solution in a Teflon container inside the stainless steel chamber maintained at 130 °C for three days followed by cooling at 5 °C per hour. X-ray diffraction analysis reveals that both types of crystals show different compositions and cell parameters. C3a_1 crystallises in a monoclinic C2/c space group, whereas C3a_2 crystallises in a tetragonal P4322 space group. Complete crystallographic parameters are presented in Table 1. In both cases, metal centres, Ni(II), adopt distorted octahedral but asymmetric units and accordingly the coordination environment shows wide differences. The asymmetric unit (ORTEP) contents are presented in SI(1), which clearly indicate the presence of uncoordinated DPP molecules, a nitrate anion and two water molecules in C3a_2, as also observed in C2c, except for the nitrate anion, while all ligands are involved in the coordination sphere in C3a_1. Thus, two molecules each of DPP, bpy and water coordinate with Ni(II) in C3a_1, while the same is observed in C3a_2 with four bpy and two water molecules.The asymmetric unit in C3a_1 has Ni1 in a twofold axis and directly bonded to O2 of a DPP ligand, to O3 of a water oxygen and to N1 of the bpy ligand, which also lies about the twofold axis. The corresponding Ni–O distance range is 2.060–2.124 Å in C3a_1 and 2.086–2.095 Å in C3a_2, respectively. Similarly, Ni–N distances are in the range of 2.086–2.095 Å for C3a_1 and the same are 2.107–2.124 Å in C3a_2 (see Table 2). The different coordination spheres present in both structures (C3a_1 and C3a_2) are shown in Fig. 12.
 | ||
| Fig. 12 View of octahedral coordination spheres of complexes (a) C3a_1 and (b) C3a_2. Water molecules are shown in purple color. | ||
Furthermore, in C3a_1, the coordination spheres extend in the form of a 1D linear chain [Fig. 13(a)] through the N-terminal of bpy only. However, DPP, being mononucleating (Scheme 1 mode I) in this structure, is involved in the formation of strong O–H⋯O hydrogen bonds (O⋯O, 2.737(2) Å, intermolecular and 2.785(2) Å), establishing connectivity with coordinated water molecules in the adjacent chains, as well as C–H⋯π interactions (3.148 and 3.448 Å) between adjacent DPP molecules, as shown in Fig. 13, thereby effectively contributing to the stabilisation of chains and also crystal lattice.
 | ||
| Fig. 13 (a) 1D linear chain of C3a_1. (b) Packing diagram of C3a_1 showing O⋯O distances and C–H⋯π interactions. | ||
The asymmetric unit contains Ni1 on a twofold axis coordinated to O1 (H2O, general position) and to N1 (twofold axis) and N4 (general position) from two bpy ligands. A half nitro group (N3/O3) lies on a twofold axis. One DPP ligand and a second water O6 occupy general positions, and another water O7 resides on a twofold axis (half occupancy). Hydrogen atoms attached to O6 and O7 were not modelled due to weak/ambiguous difference map density (ORTEP in the SI). Contacts involving these sites are given as O⋯O distances only. Crystal structure analysis of complex C3a_2 shows that the uncoordinated DPP molecules remain in the crystal lattice as the structure stabilization moiety through the formation of appropriate hydrogen bonds [SI(II)] (C–H⋯O distances are in the range of 2.33 to 2.89 Å and O⋯O distances accompanying strong hydrogen bonds are in the range of 2.64 to 3.02 Å.) with further uncoordinated species nitrate and water molecules, as presented in Fig. 14. The coordinated water molecules participate in O–H⋯O hydrogen bonding, with O⋯O distances in the range of 2.63–3.67 Å. As four bpy molecules are coordinated to Ni(II) in C3a_2, they construct a 2D framework with a square grid, which leads to the formation of channels along the represented axes, and the uncoordinated DPP, nitrate and water molecules occupy these channels, as shown in Fig. 15. The corresponding hydrogen bonds [SI(II)] between coordinated and uncoordinated fragments extend the structure through a channel like topology.
 | ||
| Fig. 14 Nitrate ions and uncoordinated DPP connected by H-bonds formed through uncoordinated fragments in C3a_2. | ||
 | ||
| Fig. 15 (a) 2D square grid framework of C3a_2. (b) Channel structure of C3a_2 showing DPP (maroon), nitrate (space filling) and water molecules (purple) inside the channel. | ||
3.4. Structural elucidation of Cu(II) complexes (C4a, C4b and C4d)
Crystallisation experiments of Cu(ClO4)2·6H2O with DPP in the presence of different N-heterocyclic ligands (a–d, as listed in Scheme 2) are carried out as mentioned in section 1.2. Three complexes, labelled C4a (C34H29ClN2O9P2Cu2), C4b (C24H22ClN2O6PCu) and C4d (C24H20ClN2O7PCu), with good quality mountable crystals, are formed with bpy, bpyee and 110phen, respectively. Their structural descriptions with a view of the different coordination modes of DPP and the counter-ion in the crystal lattices are given in the following paragraphs.The cage-type moieties thus formed (made up of metal, oxygen and phosphorous atoms) further expand into the polymeric form through bridging by bpy ligands, as presented in Fig. 17. Furthermore, ClO4− ions contribute to the stabilization of the structure with the formation of appropriate C–H⋯O (H⋯O, 2.43–2.79 Å) hydrogen bonds with both DPP and bpy ligands, as depicted in the inset of Fig. 17. Hydrogen bond parameters are listed in SI(II).
In this complex, DPP molecules follow a bidentate bridging mode, expanding the chain infinitely in the a direction. Similarly, bpyea, coordinated with Cu(II), also contributes to the extension of the molecular arrangement in the other directions, thus leading to the formation of a 2D framework (see Fig. 19). As noted in the structure of C4a, herein ClO4− anions also contribute to the crystal stabilization by participating in the formation of C–H⋯O (H⋯O, 2.40 and 2.41 Å) and hydrogen bonds with DPP, as shown in the inset of Fig. 19. Apart from this, bpyea is also involved in strong C–H⋯O bonds, as shown in SI(II).
 | ||
| Fig. 20 (a) View of the distorted square pyramidal environment of C4d. (b) Non-covalent interactions between adjacent discrete molecules. Water molecules are shown as purple spheres. | ||
Fig. 20(b), however, shows the interconnection between adjacent discrete molecules via C–H⋯O (H⋯O, 2.33–2.83 Å) and O–H⋯O (H⋯O, 1.64 Å) hydrogen bonds, as detailed in SI(2). The two-dimensional packing arrangement of the complex along the a-axis is illustrated in Fig. 21, with an inset of π–π interaction (3.52 Å) between 110phen ligands.
3.5. Structural elucidation of Cd(II) complex (C5a)
C5a (C46H38N2O6P3Cd), a coordination complex of DPP and bpy coordinated to Cd(II), is the lone structure obtained upon crystallization of DPP with Cd(II) and different N-donor ligands (a–d), following a slow evaporation method at room temperature. The asymmetric unit contains Cd1 at a general position, directly bonded to one 4,4′-bipyridine (bpy), and three crystallographically independent DPP ligands (all in general positions). X-ray diffraction analysis confirms the monoclinic C2/c space group. All the characteristic crystallographic parameters are listed in Table 1 and the contents of asymmetric units, in the form of ORTEP, are presented in section SI(1).Packing analysis further reveals that Cd(II) is octahedrally coordinated by four DPP and two bpy molecules as shown in Fig. 22(a), with typical Cd–O and Cd–N distances in the ranges of 2.250–2.276 Å and 2.365–2.377 Å, respectively (Table 2).
 | ||
| Fig. 22 (a) View of the octahedral environment of C5a. (b) Non-covalent interactions (C–H⋯O shown in Å) between adjacent dimeric fragments. | ||
The packing arrangement of C5a is similar to that of C2b. Because both DPP ligands are involved in bidentate bridging, they form the eight-membered ring M–(O–P–O)2–M, as well as pseudo-chelation through C–H⋯O hydrogen bonding (H⋯O, 2.55 and 2.87 Å), as projected in Fig. 22(b). In this process, water and DPP molecules in C2b and only DPP molecules in C5a are involved in pseudo-chelation. These dimeric complexes are extended through bpy molecules to form linear polymeric chains (Fig. 23).
5. Conclusion
In summary, different coordination assemblies are developed by varying the metal ions with a specific organophosphorus ligand and by varying N-donor species to form various supramolecular motifs with discrete to dimeric and polymeric structures including MOF and cage architectures. All complexes are thoroughly characterized and analysed by single crystal X-ray diffraction, unravelling diverse coordination geometries like square pyramidal and octahedral depending on central metal ions and the ligand environment. C1b is a 1D coordination polymer with dinucleating behaviour of DPP. Generally, when similar ligands are employed with the same metal ion species, isostructural/isomorphous structures are predicted, as is the case with Co(II), DPP and N-donors (a–c). However, this is not the case among C2a–C2c complexes; though N-donor ligands act in a similar way, DPP's coordination modes are different. Consequently, C2a does not have any metal phosphinate 8-membered ring structure like C2b; meanwhile, in the case of C2c, a completely different host–guest topology is found, without the formation of any coordination bonds by DPP.Mixed ligand strategies proved crucial in directing the assemblage of these architectures, particularly in the MOF structure of Ni(II) complexes. Also, different reaction conditions (solvothermal and slow evaporation at room temperature) result in different types of crystals; for example, C3a_2 (tetragonal) is found to exhibit a higher symmetry lattice in comparison with C3a_1 (monoclinic). Copper complexes adopt square pyramidal geometries, yet the 2D networks of C4a and C4b differ considerably, with C4a displaying a distinctive cage structure. The use of rigid N-donors like 1,10-phenanthroline (phen) results in both dimeric (C2d) and discrete (C4d) structures, even though they show similar coordination behavior. The only Cd(II) complex, C5a, displays a packing pattern analogous to C2b with alternating M–(O–P–O)2–M ring motifs. Noncovalent interactions, especially O–H⋯O and C–H⋯O hydrogen bonds and π–π interactions, formed by organic ligands contribute extensively to the aggregation of the complexes. This study emphasizes the importance of mixed ligands and highlights relatively less explored phosphinates in supramolecular coordination chemistry. Their versatile coordination modes and compatibility with diverse N-donors enable the construction of structurally tunable and functionally diverse architectures, highlighting their role as flexible building blocks for both discrete and polymeric assemblies with potential applications.
Conflicts of interest
There are no conflicts to declare.Data availability
Data supporting the findings of this study are available within the article and its electronic supplementary information files, and from publicly accessible repositories. Crystallographic data for all compounds have been deposited at the Cambridge Crystallographic Data Centre under deposition numbers CCDC 2290893–2290903.Supplementary information (SI) is available. Supplementary Information file includes all structural studies including ORTEP and Hydrogen bond parameter of the complexes. See DOI: https://doi.org/10.1039/d5dt01622c.
CCDC 2290893–2290903 contain the supplementary crystallographic data for this paper.30a–k
Acknowledgements
We acknowledge IIT Bhubaneswar for infrastructure facilities and support.References
- R. Silbernagel, C. H. Martin and A. Clearfield, Zirconium(IV) phosphonate–phosphates as efficient ion-exchange materials, Inorg. Chem., 2016, 55, 1651–1656 CrossRef CAS PubMed.
- S. A. Ondrušová, M. Kloda, J. Rohlíček, M. Taddei, J. K. Zaręba and J. Demel, Exploring the Isoreticular Continuum between Phosphonate-and Phosphinate-Based Metal–Organic Frameworks, Inorg. Chem., 2022, 61, 18990–18997 CrossRef.
- N. Hermer and N. Stock, The new triazine-based porous copper phosphonate [Cu 3 (PPT)(H 2 O) 3]·10H 2 O, Dalton Trans., 2015, 44, 3720–3723 RSC.
- M. Taddei, F. Costantino, A. Ienco, A. Comotti, P. V. Dau and S. M. Cohen, Synthesis, breathing, and gas sorption study of the first isoreticular mixed-linker phosphonate based metal–organic frameworks, Chem. Commun., 2013, 49, 1315–1317 RSC.
- C.-Y. Gao, J. Ai, H.-R. Tian, D. Wu and Z.-M. Sun, An ultrastable zirconium-phosphonate framework as bifunctional catalyst for highly active CO2 chemical transformation, Chem. Commun., 2017, 53, 1293–1296 RSC.
- X. Chen, Y. Peng, X. Han, Y. Liu, X. Lin and Y. Cui, Sixteen isostructural phosphonate metal-organic frameworks with controlled Lewis acidity and chemical stability for asymmetric catalysis, Nat. Commun., 2017, 8, 2171 CrossRef PubMed.
- P. Salcedo-Abraira, R. Serrano-Nieto, C. Biglione, M. A. Cabrero-Antonino, S. M. Vilela, A. A. Babaryk, D. Tilve-Martínez, A. Rodriguez-Diéguez, S. Navalón, H. García and P. Horcajada, Two Cu-Based Phosphonate Metal–Organic Frameworks as Efficient Water-Splitting Photocatalysts, Chem. Mater., 2023, 35, 4211–4219 CrossRef CAS.
- A. D. Firmino, R. F. Mendes, D. Ananias, F. Figueira, J. P. Tome, J. Rocha and F. A. Almeida Paz, Pyrene Tetraphosphonate–Based Metal–Organic Framework: Structure and Photoluminescence, Eur. J. Inorg. Chem., 2020, 2020, 3565–3572 CrossRef CAS.
- Z.-X. Wang, L.-F. Wu, H.-P. Xiao, X.-H. Luo and M.-X. Li, Structural diversity and magnetic properties of seven coordination polymers based on the 2, 2′-phosphinico-dibenzoate ligand, Cryst. Growth Des., 2016, 16, 5184–5193 CrossRef CAS.
- A. Bulut, Y. Zorlu, R. Topkaya, B. Aktaş, S. Doğan, H. Kurt and G. Yücesan, Macrocyclic Cu(II)-organophosphonate building block with room temperature magnetic ordering, Dalton Trans., 2015, 44, 12526–12529 RSC.
- S. J. Shearan, N. Stock, F. Emmerling, J. Demel, P. A. Wright, K. D. Demadis, M. Vassaki, F. Costantino, R. Vivani and S. Sallard, New directions in metal phosphonate and phosphinate chemistry, Crystals, 2019, 9, 270 CrossRef CAS.
- K. J. Gagnon, H. P. Perry and A. Clearfield, Conventional and unconventional metal-organic frameworks based on phosphonate ligands: MOFs and UMOFs, Chem. Rev., 2012, 112, 1034–1054 CrossRef CAS PubMed.
- M. M. Ayhan, C. Bayraktar, K. B. Yu, G. Hanna, A. O. Yazaydin, Y. Zorlu and G. Yücesan, A Nanotubular Metal–Organic Framework with a Narrow Bandgap from Extended Conjugation, Chem. – Eur. J., 2020, 26, 14813–14816 CrossRef CAS PubMed.
- M. Kloda, S. Ondrušová, K. Lang and J. Demel, Phosphinic acids as building units in materials chemistry, Coord. Chem. Rev., 2021, 433, 213748 CrossRef CAS.
- I. Carson, M. R. Healy, E. D. Doidge, J. B. Love, C. A. Morrison and P. A. Tasker, Metal-binding motifs of alkyl and aryl phosphinates; versatile mono and polynucleating ligands, Coord. Chem. Rev., 2017, 335, 150–171 CrossRef CAS.
- E. S. Wiedner, A. M. Appel, S. Raugei, W. J. Shaw and R. M. Bullock, Molecular Catalysts with Diphosphine Ligands Containing Pendant Amines, Chem. Rev., 2022, 122, 12427–12474 CrossRef CAS PubMed.
- D. S. Flett, Solvent extraction in hydrometallurgy: the role of organophosphorus extractants, J. Organomet. Chem., 2005, 690, 2426–2438 CrossRef CAS.
- A. M. Wilson, P. J. Bailey, P. A. Tasker, J. R. Turkington, R. A. Grant and J. B. Love, Solvent extraction: the coordination chemistry behind extractive metallurgy, Chem. Soc. Rev., 2014, 43, 123–134 RSC.
- J. R. Turkington, P. J. Bailey, J. B. Love, A. M. Wilson and P. A. Tasker, Exploiting outer-sphere interactions to enhance metal recovery by solvent extraction, Chem. Commun., 2013, 49, 1891–1899 RSC.
- D. Yakhvarov, E. Trofimova, O. Sinyashin, O. Kataeva, Y. Budnikova, P. Lönnecke, E. Hey-Hawkins, A. Petr, Y. Krupskaya and V. Kataev, New dinuclear nickel(II) complexes: synthesis, structure, electrochemical, and magnetic properties, Inorg. Chem., 2011, 50, 4553–4558 CrossRef CAS.
- J. S. Maass, M. Zeller, T. M. Breault, B. M. Bartlett, H. Sakiyama and R. L. Luck, Syntheses and Structures of Three Complexes of Formulas [L3Co (μ2-O2P (Bn) 2) 3CoL′][L ″], Featuring Octahedral and Tetrahedral Cobalt(II) Geometries; Variable-Temperature Magnetic Susceptibility Measurement and Analysis on [(py) 3Co (μ2-O2PBn2) 3Co (py)][ClO4], Inorg. Chem., 2012, 51, 4903–4905 CrossRef CAS PubMed.
- K. K. Tanabe and S. M. Cohen, Engineering a metal–organic framework catalyst by using postsynthetic modification, Angew. Chem., Int. Ed., 2009, 48, 7424–7427 CrossRef CAS PubMed.
- K. W. Oliver, S. J. Rettig, R. C. Thompson, J. Trotter and S. Xia, Crystal Structure and Magnetic Behavior of Copper(II) Dimethylphosphinate: A Chain Polymer Containing Triangular Trimetallic Bis (μ-dimethylphosphinato) copper(II) Units, Inorg. Chem., 1997, 36, 2465–2468 CrossRef CAS PubMed.
- C. M. Barnes, D. S. Bohle, R. E. Dinnebier, S. K. Madsen and P. W. Stephens, Structural and Spectroscopic Studies of Two Phases of the Organometallic Chain Polymer [Ru2 {μ2: μ2: η2-O2PMe2} 2 (CO) 4] n, Inorg. Chem., 1997, 36, 5793–5798 CrossRef CAS PubMed.
- A. Renz, M. Penney, R. Feazell and K. K. Klausmeyer, X-Ray Crystal Structures of the Silver Complexes of 3-Pyridyldiphenylphosphinite and Its Hydrolysis Product, J. Chem. Crystallogr., 2012, 42, 1129–1137 CrossRef CAS.
- R. Nassar, B. C. Noll and K. W. Henderson, Solid-state studies of the Horner–Wittig reagent [{{Ph2P (O)} 2CHLi·(THF)} 2·THF] and its mixed-anion intermediate [{{Ph2P (O)} 2CHLi} 4·{Ph2P (O) 2Li} 2·8C6H6] formed by reaction with dioxygen, Polyhedron, 2004, 23, 2499–2506 CrossRef CAS.
- V. Moodley, L. Mthethwa, M. N. Pillay, B. Omondi and W. E. van Zyl, The silver(I) coordination polymer [AgO2PPh2] n and unsupported Ag⋯Ag interactions derived from aminophosphinate and phosphinic acid, Polyhedron, 2015, 99, 87–95 CrossRef CAS.
- A. V. Anyushin, D. A. Mainichev, N. K. Moroz, P. A. Abramov, D. Y. Naumov, M. N. Sokolov and V. P. Fedin, Cd2+ Complexation with P (CH2OH) 3, OP (CH2OH) 3, and (HOCH2) 2PO2−: Coordination in Solution and Coordination Polymers, Inorg. Chem., 2012, 51, 9995–10003 CrossRef CAS PubMed.
- Y. Zhang, A.-Q. Jia, J.-J. Zhang, Z. Xin and Q.-F. Zhang, Construction of {Mn [Ph2P (O) NP (O) Ph2] 2} units with mono-and bi-pyridines: Syntheses, molecular structures, and spectroscopic properties of manganese(II) complexes with tetraphenylimidodiphosphinates, Polyhedron, 2018, 155, 50–58 CrossRef CAS.
- (a) CCDC 2290893: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2gwvs0; (b) CCDC 2290894: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2gwvt1; (c) CCDC 2290895: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2gwvv2; (d) CCDC 2290896: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2gwvw3; (e) CCDC 2290897: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2gwvx4; (f) CCDC 2290898: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2gwvy5; (g) CCDC 2290899: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2gwvz6; (h) CCDC 2290900: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2gww08; (i) CCDC 2290901: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2gww19; (j) CCDC 2290902: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2gww2b; (k) CCDC 2290903: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2gww3c.
| This journal is © The Royal Society of Chemistry 2026 |