CuNiO2 nano catalyst for efficient Csp2–S bond formation in water: toward green synthesis of bioactive molecules
Amlan
Swain
a,
Bedabyas
Behera
 a,
Saroj Lochan
Samal
a,
Saroj Lochan
Samal
 b,
Laxmidhar
Rout
b,
Laxmidhar
Rout
 *a and
Debendra K.
Mohapatra
*a and
Debendra K.
Mohapatra
 *cd
*cd
aPost Graduate Department of Chemistry, Berhampur University, Bhanjabihar, Ganjam, Odisha-760007, India. E-mail: ldr.chem@buodisha.edu.in
bDepartment of Chemistry, NIT Rourkela, Sundargarh, Odisha-769008, India
cDepartment of Chemical Sciences, Indian Institute of Science Education and Research Berhampur, Odisha-760003, India. E-mail: mohapatradk@iiserbpr.ac.in
dDepartment of Organic Synthesis and Process Chemistry, CSIR-Indian Institute of Chemical Technology Tarnaka, Hyderabad-500007, India. E-mail: mohapatra@iict.res.in
First published on 16th December 2025
Abstract
A green and efficient method for Migita-type Csp2–S cross-coupling of aryl halides with thiols has been developed using a heterogeneous CuNiO2 bimetallic nanocatalyst. This low-cost, easily synthesized catalyst operates under ligand-free conditions in water, enabling the coupling of heterocyclic thiols such as benzothiazole-2-thiol, benzooxa-zole-2-thiol, 1,3,4-thiadiazole-2-thiol, and thiazole-2-thiol with challenging electrophiles like 3-bromopyridine, 2-bromoquinoline, and 5-bromo-1H-indole. The methodology demonstrates broad scope and practicality, including gram-scale synthesis of diverse aryl, heteroaryl, and alkyl thioethers bearing bioactive motifs. Compared to existing systems, this catalyst exhibits greater sustainability and catalytic performance. Control experiments confirm a synergistic effect between Cu and Ni, while DFT calculations indicate that the reaction preferentially occurs at the Ni center, with an overall Gibbs free energy change of −102.7 kcal mol−1.
Introduction
In organic synthesis, transition metal catalysts have significantly advanced carbon–heteroatom cross-coupling reactions. Among these, C–S bond formation remains particularly challenging due to the strong coordinating nature of sulfur, which can deactivate metal centers and reduce catalytic efficiency.1 Despite this, C–S cross-coupling has become a highly attractive strategy, driven by the widespread presence of thioethers, sulfoxides, and sulfones in drug molecules and biologically active compounds.2 Thioether derivatives, in particular, are found in approximately 20% of FDA-approved pharmaceuticals.3 Beyond medicinal chemistry, thioethers also play a crucial role in materials science as functional fragments and polymerization precursors in soft materials, in semiconductor fabrication, and across various scientific and industrial applications.4–6The first successful C–S cross-coupling reaction was reported by Japanese chemists Kosugi and Migita between 1978 and 1980.7,8 Since then, this field has witnessed remarkable evolution from the use of homogeneous palladium catalysts9a to more sustainable and earth-abundant transition metals such as copper,9b cobalt,9c iron,9d and nickel,9e–j often employed in combination with bulky phosphorus- or nitrogen-based ligands. In the early 21st century, the development of nanoparticle-based and heterogeneous catalysts further advanced the field by offering recyclability, facile catalyst separation, and reduced metal contamination.10
To minimize bulk chemical waste generated from toxic and flammable organic solvents, water has emerged as an ideal green solvent for organic synthesis due to its abundance, non-toxicity, non-flammability, and simple work-up procedures.11 Consequently, C–S cross-coupling reactions have been extensively explored in aqueous media.12 Only a few examples of bimetallic systems such as Ni–Zr,13a Cu–Fe,13b Pd–Fe,13c Ni–Fe,13d and Co–Fe13e have been reported for C–S cross-coupling in water. However, these studies often lacked detailed mechanistic understanding, exhibited limited substrate scope, and offered minimal synthetic applicability for broader practical use.
Recently, Lipshutz and co-workers demonstrated the use of recyclable water as a reaction medium for C–S cross-coupling (Fig. 1a).14 Our group has previously developed a CuMoO4 bimetallic nano catalyst for efficient C–S cross-coupling at room temperature in DMSO, highlighting the concept of bi-metallic synergy where one metal enhances the catalytic activity of the other (Fig. 1b).15,16
 | ||
| Fig. 1 Representative literature reports on C–S cross-coupling by using metal catalysis (a and b) and this work (c). | ||
However, the poor reactivity of aliphatic and heteroaromatic thiols, the absence of significant synthetic applications toward bioactive molecules, and the lack of mechanistic understanding remain major limitations of the previous report. To overcome these challenges and advance sustainable methods for thioether synthesis, we have developed an oxo-bridged bimetallic CuNiO2 nano catalyst for C–S cross-coupling reactions in water (Fig. 1c). This methodology enables the direct synthesis of a wide range of bioactive molecules on a gram scale. A broad substrate scope was achieved, encompassing diverse heteroaryl halides and thiols (both alkyl and heteroaryl), affording good to excellent isolated yields. Furthermore, the reaction mechanism was elucidated through density functional theory (DFT) calculations.
Results and discussion
The catalyst was prepared via hydrothermal decomposition of Cu(OAc)2·H2O and NiCl2·6H2O (both purchased from Sigma-Aldrich) in water at 150 °C using a hydrothermal autoclave (Fig. 2). The resulting CuNiO2 catalyst was obtained as a smoky black solid and thoroughly characterized by IR, powder XRD, XPS, EDX, and SEM analyses. XPS spectra exhibited characteristic Cu2+ peaks at binding energies of 932.78 eV (2p3/2) and 953.05 eV (2p1/2), along with Ni2+ peaks at 855 eV (2p3/2) and 873 eV (2p1/2). The IR band observed at 681 cm−1 indicates the presence of a [Cu–O–Ni] linkage in the catalyst (for details, see SI).17,18The reaction optimization was carried out using 4-methoxyiodobenzene and adamantane-1-thiol (AdSH) as model substrates (Table 1). Gratifyingly, the desired thioether 1 [(adamantan-1-yl)(4-methoxyphenyl)sulfane] was obtained in 93% yield when the substrates were stirred with 5 mol% of CuNiO2, 1 equivalent of tetrabutylammonium bromide (TBAB), and 1.5 equivalents of K2CO3 in water at 80 °C (Table 1, entry 8). Among the solvents tested—THF, DMSO, toluene, acetonitrile, ethanol, DMF, and DMA—water proved to be the most efficient medium for this transformation (Table 1, entries 1–7).
| Sl. no. | Catalyst (5 mol%) | Base (1.5 equiv.) | Solvent (1 mL) | Yielda,b (%) |
|---|---|---|---|---|
a Reaction condition: 4-iodoanisole (1 mmol), adamantane-1-thiol (1.2 equiv.), base (1.5 equiv.), solvent (1 mL) were stirred at 80 °C for 10 h.
b Isolated yield.
c <5% disulfide observed.
d TBAB (1 equiv.).
e TBAF.
f PEG.
g H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMSO (4 DMSO (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) at 50 °C, AdSH = adamantane-1-thiol. 1) at 50 °C, AdSH = adamantane-1-thiol.
|
||||
| 1 | CuNiO2 | K2CO3 | THF | 13 |
| 2 | CuNiO2 | K2CO3 | DMSO | 21 |
| 3 | CuNiO2 | K2CO3 | Toluene | 14 |
| 4 | CuNiO2 | K2CO3 | CH3CN | 32 |
| 5 | CuNiO2 | K2CO3 | EtOH | nd |
| 6 | CuNiO2 | K2CO3 | DMF | 50 |
| 7 | CuNiO2 | K2CO3 | H2O | 77c |
| 8 | CuNiO2 | K2CO3 | H2O | 93d |
| 9 | CuNiO2 | KOH | H2O | 91d |
| 10 | CuNiO2 | Na2CO3 | H2O | 72d |
| 11 | CuNiO2 | K2CO3 | H2O | 79e |
| 12 | CuNiO2 | K2CO3 | H2O | 25f |
| 13 | CuNiO2 | K2CO3 | H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMSO DMSO |
25g |
| 14 | Cu(OAc)2·H2O | K2CO3 | H2O | 62d |
| 15 | NiCl2·6H2O | K2CO3 | H2O | 10d |
| 16 | NiCl2·6H2O + Cu(OAc)2·H2O | K2CO3 | H2O | 75d |
| 17 | None | K2CO3 | H2O | 0d |
| 18 | CuNiO2 | — | H2O | 0d |
Among the various bases screened, KOH, NaOH, Cs2CO3, KOtBu, KOAc, NaH, K2CO3, and Na2CO3, K2CO3 provided the best result (for details, see SI). Replacing TBAB with TBAF (tetrabutylammonium fluoride) or PEG (polyethylene glycol) resulted in a significant decrease in yield to 79% and 25%, respectively (Table 1, entries 12–13). The optimal reaction temperature was found to be 80 °C, giving the highest yield. Interestingly, even at 50 °C, the reaction afforded 25% of the thioether product 1 when a 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of H2O and DMSO was used as the solvent (for details, see SI).
1 mixture of H2O and DMSO was used as the solvent (for details, see SI).
To gain insight into the catalytic activity, control experiments were performed (Table 1, entries 15–18). A reaction carried out with 5 mol% of Cu(OAc)2·2H2O alone afforded 60% of the product, while NiCl2·5H2O alone provided only 10%. Interestingly, when a combination of Cu(OAc)2·2H2O (5 mol%) and NiCl2·5H2O (5 mol%) was used, the yield increased to 75%, indicating a synergistic catalytic effect between copper and nickel in CuNiO2.19 It is noteworthy that aliphatic and heterocyclic substrates are compatible in RC-1 condition including gram-scale synthesis of bioactive molecules in water at 80 °C (Schemes 1, 2 and 4–6); whereas aromatic thiols work well in RC-2 conditions in 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of water and DMSO at 50 °C (for details see SI). Subsequently, we examined the reactivity of various substrates using the pre-synthesized CuNiO2 catalyst. It is well known that long-chain thiols often exhibit poor reactivity in such transformations due to their low solubility.15 Aliphatic thiols are more nucleophilic to form strong metal–sulphur bond in comparison to acidic nature of Aromatic thiols. So, aliphatic thiols can deactivate the catalyst by chelation easily.14,20b,21
1 ratio of water and DMSO at 50 °C (for details see SI). Subsequently, we examined the reactivity of various substrates using the pre-synthesized CuNiO2 catalyst. It is well known that long-chain thiols often exhibit poor reactivity in such transformations due to their low solubility.15 Aliphatic thiols are more nucleophilic to form strong metal–sulphur bond in comparison to acidic nature of Aromatic thiols. So, aliphatic thiols can deactivate the catalyst by chelation easily.14,20b,21
Moreover, alkyl thiols have traditionally posed challenges in cross-coupling reactions because of their high nucleophilicity that can form a metal–ligand complex and deactivate the catalyst.21 Interestingly, our reaction was not affected by metal–ligand chelation for aliphatic thioethers even if in water solvent (Scheme 1).
Dodecyl and octyl thiols reacted efficiently with 3-bromopyridine, 5-bromo-1H-indole, and substituted iodo-benzenes bearing 4-OMe and 4-CHO groups, affording the corresponding thioethers (2–6) in high yields without any chelation or solubility issues. Notably, reactions of dodecylthiol with 3-bromopyridine and 5-iodoindole furnished the desired products in 88% (2) and 95% (3) yields, respectively, demonstrating the absence of catalyst chelation. Similarly, the cyclohexylthiol underwent smooth coupling with 4-acetyliodobenzene to afford the thioether 1-(4-(cyclohexylthio)phenyl)ethan-1-one (7) in 85% yield. Furthermore, the sterically demanding and less reactive ter-tiary alkyl 1-adamantanethiols successfully delivered the corresponding thioethers (1, 8–10) in excellent yields ranging from 87% to 93%.
The structure of ((1S,3S)-adamantan-1-yl)(4-nitrophenyl)sulfane (9) was confirmed by single-crystal X-ray analysis (CCDC no. 2388312). 2-Mercaptoethan-1-ol derivatives 11 and 12 were synthesized in 55–95% yields from 2-bromoquinoline and 4-methoxyiodobenzene, respectively. Benzyl thioethers 13–14 were obtained in 88–92% yields using a carbonate base in water. Remarkably, heterocyclic furan-2-ylmethane thiols coupled efficiently with aryl iodides bearing various functional groups (CHO, CN, OAc, OMe) to afford the corresponding thioethers 15–19 in 70–92% yields, without metal chelation by oxygen, nitrogen, or sulfur.
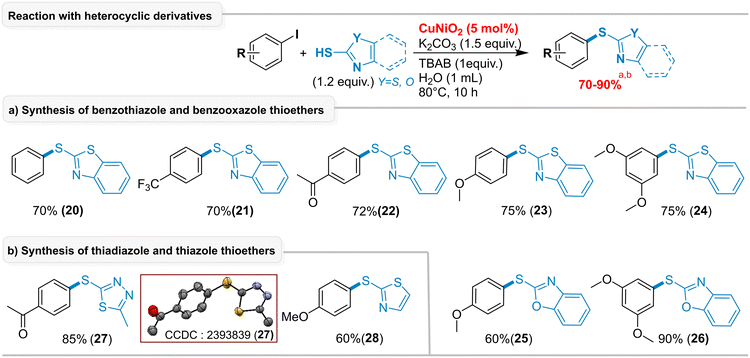
Heterocyclic thiols containing N, S, and O-atoms are prone to coordination, which can deactivate catalysts during coupling reactions. Previous studies have shown that nickel catalysts lose activity due to chelation with heteroatoms in the reaction medium.21c We explored the reactivity of heterocyclic thiazoles using our CuNiO2 catalyst with both electron-donating and electron-withdrawing aryl iodides (Scheme 2). Benzothiazole-2-thiols (20–24), benzooxazole-2-thiols (25, 26), 1,3,4-thiadiazole-2-thiol (27), and thiazole-2-thiol (28) were obtained in 60–90% yields. Remarkably, our CuNiO2 catalyst enabled the synthesis of these heterocyclic thioethers (20–28) in high yields without N/O/S chelation or catalyst deactivation, which represents a major advantage of this protocol.
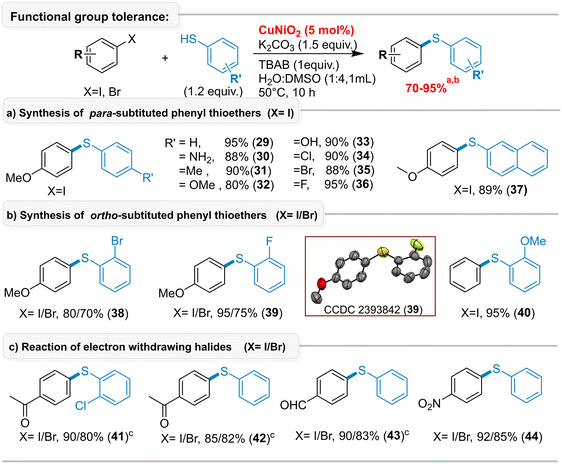
Under the optimized reaction conditions RC-2, the functional group tolerance and substrate scope were evaluated by coupling various functionalized aromatic thiols (alkyl, amine, ether, hydroxy, halide, acetyl) with aryl iodides and bromides, affording the desired thioethers (29–44) in good-to-excellent yields without metal–sulfur chelation (Scheme 3). Both electron-donating (29–40) and electron-withdrawing (41–44) aryl halides gave the corresponding products in 70–95% yields. Notably, aryl halides bearing reducible functional groups, such as carbonyls (41–43) and nitro (44), were well tolerated, even in the presence of nickel. Electron-donating thiols (29–33) and electron-withdrawing thiols (34–36, 38–39, 41) also provided excellent yields, and products 41–43 were compatible with KOH as the base. The reactions were performed at 50 °C in a H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMSO (4
DMSO (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) solvent system. These conditions are suitable for general aromatic thiols (RC-1 also gives good results), with reactivity following I ≫ Br; aryl chlorides and fluorides remained intact. The structure of (2-fluorophenyl)(4-methoxyphenyl)sulfane (39) was confirmed by single-crystal X-ray analysis (CCDC no. 2393842).
1) solvent system. These conditions are suitable for general aromatic thiols (RC-1 also gives good results), with reactivity following I ≫ Br; aryl chlorides and fluorides remained intact. The structure of (2-fluorophenyl)(4-methoxyphenyl)sulfane (39) was confirmed by single-crystal X-ray analysis (CCDC no. 2393842).
Application of the protocol for synthesis of bioactive molecules
Finally, we looked for meaningful application of this sustainable methodology (Schemes 4–6). All these applications confirmed for gram scale synthesis by using reaction condition (RC-1). Anti-cancer agents 45 and 46 (ref. 22) were synthesized from oxidation of bis(4-methoxy phenyl)sulfane (32) and 1-chloro-4-((4-methoxyphenyl)sulfonyl)benzene (34) with 30% H2O2 in glacial acetic acid respectively (Scheme 4).23 The precursors 32 and 34 were synthesized by standard reaction condition (RC-1) in gram scale. Anti-breast cancer molecule 47 (ref. 24) was synthesized in 47% by cross-coupling of benzo-oxazole-2-thiol and 5-iodo-1,2,3-trimethoxybenzene using 5 mol% of CuNiO2 catalyst under optimized conditions RC-1 (Scheme 4). Cathepsin D inhibitor analogue2548 was synthesized from coupling of 4-nitrobromobenzene and benzothiazole-2-thiol with 90% yield under reaction condition RC-1 at 80 °C in water solvent, whereas the attempt to synthesize the same analogue was failed when we tried to couple 4-bromoaniline with benzoxazole-2-thiol and benzothiazole-2-thiol, instead afforded symmetrical dithioacetals 49a and 49b respectively (the structure is confirmed by XRD, CCDC 2377843, 2377844) in 42–45% yield (Scheme 5). K2CO3 is believed to be the source of methylene (CH2) unit for dithioacetal.26 Further, structure of product 1-chloro-4-((4-methoxyphenyl)sulfonyl)benzene (46) is confirmed by single-X-ray (CCDC no. 2388314). | ||
| Scheme 4 Synthesis of bioactive molecules (anti-cancer and cathepsin D inhibitor) using NiCuO2 catalyst. | ||
 | ||
| Scheme 6 Synthesis of precursor of anti-depressant, anti-bacterial scaffold, precursor of CB2 receptor, and HSD-1 inhibitor using NiCuO2 catalyst. | ||
Moreover, the synthesis of antibacterial precursor dapsone 50,27 antimycobacterial analogue 51 (ref. 28) and anti-depressant agent 52 (analogue of vortioxetine)29 were synthesized in good to excellent yield (90–95%) using reaction condition RC-1 at 80 °C (Scheme 6). Similarly, CB2 receptor agonist3053 and 54 which are quinoline derivatives of thiol were synthesized by the reaction of corresponding aryl halides with thiols using reaction condition RC-1 at 80 °C. It is noteworthy that 2-bromoquinolines derivatives are difficult for coupling due to different electronic environment.24 Finally, precursor of HSD-1 inhibitor 55 (ref. 31) was synthesized in 97% yield from the oxidation of 1-(4-((2-chlorophenyl) thio) phenyl) ethan-1-one (41) (Scheme 6).
DFT mechanistic studies
Based on optimized reaction conditions and control experiments, a plausible mechanism for C–S cross-coupling was proposed using enthalpy and energy profile by DFT calculation32 for the different intermediates formed during the reaction of 4-methoxybromobenzene and 2-fluorobenzene thiol under reaction condition 1 (see SI) the overall reaction is highly exergonic, with a calculated Gibbs free energy change (ΔG) of −102.7 kcal (Fig. 3). The active centre of the catalyst is more likely to be nickel instead of copper as evident from the enthalpy and energy profile. The mechanistic investigation involves five consecutive steps. In the first step, 2-fluorobenzene thiol reacts with K2CO3 to form the intermediate potassium 2-fluorobenzenethiolate (a3) along with the by-product H2CO3. In this acidic condition, CuNiO2 catalyst (a0) releases its ring strain to give a2 and a2* (ΔG = −63.9 and −79.4 kcal mol−1), which involves oxidative addition with aryl bromide (a1) to form the intermediate a4 with Cu and a4* with Ni. This step is endergonic with a free energy of −10.8 and −14.8 kcal mol−1 for Cu and Ni, respectively. However, interaction of complex a4 and a4* with a3 affords thiol-bound intermediate (a5 and a5*), accompanied by the release of KBr (ΔG = −73.9 and −72.4 kcal mol−1). Subsequently, complex a5 and a5* rearrange to give a6 with strong S–Cu interaction and weak S–Ni interaction. This step is exergonic with a free energy of −14.9 kcal mol−1 for Cu–S complex, whereas it is −25.8 kcal mol−1 for Ni–S complex a6*. The final step involves the release of C–S cross-coupled product (a7) and catalyst a2, a2*. This step is exothermic. Cu–S complex a6 releases −1.6 kcal mol−1 energy for releasing the product and catalyst. However, Ni–S complex a6* releases product easily with loss of −4.4 kcal mol−1 energy along with catalyst a6*. From the energy profile diagram, it is clear that the reaction at the Nickel centre is more preferred in comparison to copper. It is noteworthy that Cu alone is substantially more active than Ni (Table 1, entry 14–15). However, in the oxygen-bridged [Cu–O–Ni] catalyst, complex a6* may be stabilised by electron transfer from Cu2+ to Ni2+ through the oxygen bridge stabilising Ni(II) during reductive elimination; [Ni2+/Ni (E° = −0.25 V); Cu2+/Cu (E° = +0.34 V)].33 A plausible mechanism is shown in Fig. 4.Recyclability
The recyclability of the catalyst was evaluated using the coupling of 4-methoxyiodobenzene with adamantane thiol under RC-1 conditions. Upon completion of the reaction, 10 mL of ethyl acetate was added, and the organic layer was separated by centrifugation. The organic phase was then evaporated to isolate the desired product. The aqueous layer containing the catalyst was heated at 50 °C for 20 minutes, and the pH was adjusted to 11 using 0.5 M KOH, regenerating the catalyst as a black powder. Powder XRD analysis confirmed that the regenerated catalyst maintained its original structure (see SI for details). The catalyst was successfully reused for three consecutive cycles with negligible loss in yield: 93% (1st cycle), 92% (2nd cycle), and 91% (3rd cycle).Conclusion
In summary, we have developed a novel oxygen-bridged bimetallic nano-catalyst, CuNiO2, from inexpensive copper and nickel sources via an autoclave method. This catalyst efficiently promotes Csp2–S cross-coupling of thiols with aryl halides in water. It exhibits broad substrate tolerance, accommodating aryl, heteroaryl, and alkyl thiols with aryl and heteroaryl iodides under ligand-free conditions. The protocol enables the high-yield synthesis of complex hetero thioethers, providing a pathway for the preparation of bioactive compounds. Its applicability in aqueous medium has been demonstrated for the synthesis of motifs relevant to antimycobacterial, anticancer, and antidepressant agents, CB2 receptor agonists, as well as precursors of cathepsin-D inhibitors, HSD1 inhibitors, and dapsone. This approach offers a practical, environmentally benign, scalable, and reliable route to alkyl and aryl (hetero) sulfides with potential applications in the development of therapeutics. The crucial role of synergistic Cu–Ni catalysis has been supported by control experiments and DFT studies. DFT study revealed that the catalyst is more likely to be nickel instead of copper, as evident from the enthalpy and energy profile.Experimental section
Procedure for synthesis of CuNiO2 catalyst
Cu(OAc)2·H2O (1 mmol, 199 mg) is dissolved in 50 mL of warm distilled water. In another beaker NiCl2·6H2O (1 mmol, 236 mg) is dissolved in 50 mL of warm distilled water. The later solution was added dropwise to first beaker dropwise. The solution was heated at 50 °C for 10 minutes to get a sky colour gummy solution. Next, 25 mL of 2 N KOH solution was added drop wise till the pH of solution is 11. The colour of solution gradually changed to black colour. The whole solution transferred into a Teflon lined hydrothermal autoclave and that kept in an oven at 150 °C for 24 h. The catalyst was washed repeatedly with 100 mL of distilled water and then 50 mL of ethanol by using REMI C-24 centrifuge instrument and then dried in an oven at 70 °C for 12 h. The catalyst was characterized in TEM, SEM, EDX, IR, Powder XRD and XPS.Procedure for C–S cross-coupling reaction
To an oven dry glass vial (5 mL) equipped with a magnetic bead, CuNiO2 (5 mol%), water 1 mL for RC-1 and water (0.8 mL) + DMSO (0.2 mL) for RC-2, TBAB (1 equiv.), haloarenes (1 mmol), corresponding thiols (1.2 equiv.) and base K2CO3 (1.5 equiv.) in air was added (adding sequence should be followed strictly). The reaction mixture was placed in a pre-heated oil bath maintained at 80 °C (for RC-1), and 50 °C (for RC-2). The reaction was monitored with TLC and KMnO4 stain. After the required time, the reaction mixture was quenched with NH4Cl solution (5 mL) and extracted with EtOAc (3 × 10 mL). The organic layer was dried over sodium sulfate, filtered and evaporated by rotor. The residue was purified via flash column chromatography (n-hexane and ethyl acetate).Author contributions
A. Swain conceptualized and synthesized the catalyst and investigated all reactions. Dr. L. Rout and Prof. Debendra K. Mohapatra written the manuscript. B. Behera helped in the DFT calculation. S. L. Samal measured provided XRD of molecules and characterised the catalyst.Conflicts of interest
The authors declare no conflicts of interest.Data availability
The data supporting this article have been included as part of the supplementary information (SI). Supplementary information is available. See DOI: https://doi.org/10.1039/d5cy01377a.CCDC 2377843, 2377844, 2388312, 2388314, 2393842, and 2393839 contain the supplementary crystallographic data for this paper.34a–f
Acknowledgements
Prof. Laxmidhar Rout acknowledges Anusandhan National Research Foundation (ANRF) SERB India, CRG/2022/001544 New Delhi, Council of Scientific and Industrial Research, CSIR/02/0393/21/EMR-II New Delhi, India for generous funding. Thanks to UGC and Berhampur University for the infrastructure facilities. Prof. Debendra K. Mohapatra gratefully acknowledges the ANRF, New Delhi (SERB) [SERB/CRG/2022/002490], for financial support and the Director, CSIR-IICT as well as the Director, IISER Berhampur for providing a conducive research environment and state-of-the-art infrastructural facilities.References
- (a) J. Oudar, Rev. Catal., 1980, 22, 171–195 CrossRef CAS; (b) A. Kolpin, G. Jones, S. Jones, W. Zheng, J. Cookson, A. P. E. York, P. J. Collier and S. C. E. Tsang, ACS Catal., 2017, 7, 592–605 CrossRef CAS.
- (a) S. V. Ley and A. W. Thomas, Angew. Chem., Int. Ed., 2003, 42, 5400–5449 CrossRef CAS PubMed; (b) P. Chauhan, S. Mahajan and D. Enders, Chem. Rev., 2014, 114, 8807–8864 CrossRef CAS PubMed; (c) I. P. Beletskaya and V. P. Ananikov, Chem. Rev., 2022, 122, 16110–16293 CrossRef CAS PubMed.
- K. A. Scott and J. T. Njardarson, Top. Curr. Chem., 2018, 22, 376 Search PubMed.
- L. Wang, L. Liu and H. Zhao, Angew. Chem., Int. Ed., 2023, 62, e202304073 CrossRef CAS PubMed.
- K. Takimiya, I. Osaka, T. Mori and M. Nakano, Acc. Chem. Res., 2014, 47, 1493–1502 CrossRef CAS PubMed.
- P. Kumar Behera, P. Choudhury, P. Behera, A. Swain, A. K. Pradhan and L. Rout, ChemistrySelect, 2022, 7, e202202919 CrossRef.
- M. Kosugi, T. Shimizu and T. Migita, Chem. Lett., 1978, 7, 13–14 CrossRef.
- T. Migita, T. Shimizu, Y. Asami, J.-i. Shiobara, Y. Kato and M. Kosugi, Bull. Chem. Soc. Jpn., 1980, 53, 1385–1389 CrossRef CAS.
- (a) M. A. Fernandez-Rodriguez, Q. Shen and J. F. Hartwig, J. Am. Chem. Soc., 2006, 128, 2180–2181 CrossRef CAS PubMed; (b) R. Xu, J.-P. Wan, H. Mao and Y. Pan, J. Am. Chem. Soc., 2010, 132, 15531–15533 CrossRef CAS PubMed; (c) Y.-C. Wong, T. T. Jayanth and C.-H. Cheng, Org. Lett., 2006, 8, 5613–5616 CrossRef CAS PubMed; (d) A. Correa, M. Carril and C. Bolm, Angew. Chem., Int. Ed., 2008, 47, 2880–2883 CrossRef CAS PubMed; (e) Y. Qin, R. Sun, N. P. Gianoulis and D. G. Nocera, J. Am. Chem. Soc., 2021, 143, 2005–2015 CrossRef CAS PubMed; (f) R. Martin, D. J. Nelson, S. Meiries, A. M. Z. Slawin and S. P. Nolan, Eur. J. Org. Chem., 2014, 2014, 3127–3131 CrossRef; (g) S.-C. Lee, H.-H. Liao, A. Chatupheeraphat and M. Rueping, Chem. – Eur. J., 2018, 24, 3608–3612 CrossRef CAS PubMed; (h) R. Sikari, S. Sinha, S. Das, A. Saha, G. Chakraborty, R. Mondal and N. D. Paul, J. Org. Chem., 2019, 84, 4072–4085 CrossRef CAS PubMed; (i) M. Nikitin, F. Babawale, S. Tastekin, M. Antonietti, I. Ghosh and B. König, Green Chem., 2024, 26, 5845–5851 RSC; (j) I. Ghosh, N. Shlapakov, T. A. Karl, J. Düker, M. Nikitin, J. V. Burykina, V. P. Ananikov and B. König, Nature, 2023, 619, 87–93 CAS.
- (a) L. Rout, T. K. Sen and T. Punniyamurthy, Angew. Chem., Int. Ed., 2007, 46, 5583–5586 CrossRef CAS PubMed; (b) T. A. Gazis, S. Palit, L. A. Cipriano, N. Allasia, S. M. Collins, Q. M. Ramasse, I. S. Kwon, M. Sterrer, G. D. Liberto and G. Vile, Angew. Chem., 2025, 64, e202510632 CrossRef CAS PubMed; (c) V. B. Saptal, V. Ruta, M. A. Bajada and G. Vile, Angew. Chem., Int. Ed., 2023, 62, e202219306 CrossRef CAS PubMed.
- (a) M.-O. Simona and C.-J. Li, Chem. Soc. Rev., 2012, 41, 1415–1427 RSC; (b) T. Kitanosono, K. Masuda, P. Xu and S. Kobayashi, Chem. Rev., 2018, 118, 679–746 CrossRef CAS PubMed; (c) D. K. Romney, F. H. Arnold, B. H. Lipshutz and C.-J. Li, J. Org. Chem., 2018, 83, 7319–7322 CrossRef CAS PubMed.
- (a) E. Vessally, K. Didehban, R. Mohammadi, A. Hosseinian and M. Babazadeh, J. Sulfur Chem., 2018, 39, 332–349 CrossRef CAS; (b) X. Ren, S. Tang, L. Li, J. Li, H. Liang, G. Li, G. Yang, H. Li and B. Yuan, J. Org. Chem., 2019, 84, 8683–8690 CrossRef CAS PubMed.
- (a) N. Pala and A. Bhaumik, Dalton Trans., 2012, 41, 9161–9169 RSC; (b) D. Kundu, T. Chatterjee and B. C. Ranu, Adv. Synth. Catal., 2013, 355, 2285–2296 CrossRef CAS; (c) P. G.-S. Abadi, E. Rafiee and M. Joshaghani, Inorg. Chim. Acta, 2016, 451, 162–170 CrossRef; (d) Md. A. Iqubal, Sk. S. Islam, K. Ghosh, R. A. Molla, Kamaluddin and Sk. M. Islam, J. Inorg. Organomet. Polym., 2017, 27, 1730–1739 CrossRef CAS; (e) S. Iraqui and Md. H. Rashid, New J. Chem., 2022, 46, 22766–22777 RSC.
- T.-Y. Yu, H. Pang, Y. Cao, F. Gallou and B. H. Lipshutz, Angew. Chem., Int. Ed., 2021, 60, 3708–3713 CrossRef CAS PubMed.
- R. Panigrahi, S. K. Sahu, P. K. Behera, S. Panda and L. Rout, Chem. – Eur. J., 2020, 26, 620–624 CrossRef CAS PubMed.
- (a) U. B. Kim, D. J. Jung, H. J. Jeon, K. Rathwell and S.-g. Lee, Chem. Rev., 2020, 120, 13382–13433 CrossRef CAS PubMed; (b) L. Liu and A. Corma, Chem. Rev., 2023, 123, 4855–4933 CrossRef CAS PubMed.
- M. P. Kumar, G. M. Murugadoss and M. R. Kumar, J. Mater. Sci.: Mater. Electron., 2020, 31, 11286–11294 CrossRef CAS.
- L. Fan, P. F. Liu, X. Yan, L. Gu, Z. Z. Yang, H. G. Yang, S. Qiu and X. Yao, Nat. Commun., 2016, 7, 10667 CrossRef CAS PubMed.
- U. B. Kim, D. J. Jung, H. J. Jeon, K. Rathwell and S.-G. Lee, Chem. Rev., 2020, 120, 13382–13433 CrossRef CAS PubMed.
- (a) S. Ranjit, R. Lee, D. Heryadi, C. Shen, J. Wu, P. Zhang, K.-W. Huang and X. Liu, J. Org. Chem., 2011, 76, 8999–9007 CrossRef CAS PubMed; (b) K. D. Jones, D. J. Power, D. Bierer, K. M. Gericke and S. G. Stewart, Org. Lett., 2018, 20, 208–211 CrossRef CAS PubMed; (c) I. M. Yonova, C. A. Osborne, N. S. Morrissette and E. R. Jarvo, J. Org. Chem., 2014, 79, 1947–1953 CrossRef CAS PubMed.
- (a) S. Biswas and D. J. Weix, J. Am. Chem. Soc., 2013, 135, 16192 CrossRef CAS PubMed; (b) L. Rout, P. Saha, S. Jammi and T. Punniyamurthy, Eur. J. Org. Chem., 2008, 640–643 CrossRef CAS; (c) R. Zhang, C. Ni, Q. Xie and J. Hu, Tetrahedron, 2020, 76, 131676 CrossRef CAS.
- A. Das and N. T. Patil, ACS Catal., 2023, 13, 3847–3853 CrossRef CAS.
- R. S. Varma and K. P. Naicker, Org. Lett., 1999, 1, 189–191 CrossRef CAS.
- I. M. Yonova, C. A. Osborne, N. S. Morrissette and E. R. Jarvo, J. Org. Chem., 2014, 79, 1947–1953 CrossRef CAS PubMed.
- S. Ranjit, R. Lee, D. Heryadi, C. Shen, J. Wu, P. Zhang, K.-W. Huang and X. Liu, J. Org. Chem., 2011, 76, 8999–9007 CrossRef CAS PubMed.
- (a) D. H. Dethe, M. Shukla and B. D. Dherange, Org. Lett., 2020, 22, 5778–5782 CrossRef CAS PubMed; (b) Z. Guo, B. Zhang, X. Wei and C. Xi, Org. Lett., 2018, 20, 6678–6681 CrossRef CAS PubMed.
- (a) A. Bhowmik, M. Yadav and R. A. Fernandes, Org. Biomol. Chem., 2020, 18, 2447–2458 RSC; (b) R. L. Lopez de Compadre, R. A. Pearlstein, A. J. Hopfinger and J. K. Seydel, J. Med. Chem., 1987, 30, 900–906 CrossRef PubMed.
- A. Almasirad, S. Samiee-sadr and A. Shafiee, Iran. J. Pharm. Res., 2011, 10, 727–731 CAS.
- Z. Boros, L. Nagy-Gyõr, K. Kátai-Fadgyas, I. Kõhegyi, I. Ling, T. Nagy, Z. Iványi, M. Oláh, G. Ruzsics, O. Temesi and B. Volk, J. Flow Chem., 2019, 9, 101–113 CrossRef CAS.
- A. Nandy, I. Kazi, G. Guha and G. Sekar, J. Org. Chem., 2021, 86, 2570–2581 CrossRef CAS PubMed.
- X. Yan, Z. Wang, A. Sudom, M. Cardozo, M. DeGraffenreid, P. Fan, X. He, J. C. Jaen, M. Labelle, J. Liu, J. Ma, D. McMinn, S. Miao, D. Sun, L. Tang, H. Tu, S. Ursu, N. Walker, Q. Ye and J. P. Powers, Bioorg. Med. Chem. Lett., 2010, 20, 7071–7075 CrossRef CAS PubMed.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery Jr., J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, N. J. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, Ö. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, Gaussian 09, Revision C.01, Gaussian, Inc., Wallingford CT, 2009 Search PubMed.
- A. J. Bard and L. R. Faulkner, Electrochemical Methods: Fundamentals and Applications, Wiley, New York, 2nd edn, 2001 Search PubMed.
- (a) CCDC 2377843: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2ktbmc; (b) CCDC 2377844: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2ktbnd; (c) CCDC 2388312: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2l57bc; (d) CCDC 2388314: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2l57df; (e) CCDC 2393842: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2lbzqn; (f) CCDC 2393839: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2lbzmk.
| This journal is © The Royal Society of Chemistry 2026 |