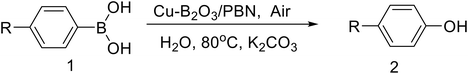
Solvoselective effect of Cu–B2O3/porous boron nitride on catalytic conversion of arylboric acids to phenols and biphenyls
Yumei
Zhang
a,
Yue
Wang
a,
Dongle
Wang
a,
Yating
Liu
a,
Tong
Gao
a,
Qingqiang
Zhou
a,
Qingzhi
Luo
a,
Jing
An
a,
Xueyan
Li
a,
Yandong
Duan
*a and
Desong
Wang
 *ab
*ab
aHebei Key Laboratory of Photoelectric Control on Surface and Interface, School of Sciences, Hebei University of Science and Technology, Shijiazhuang 050018, People's Republic of China. E-mail: ydduan@iccas.ac.cn; dswang06@126.com
bState Key Laboratory of Metastable Materials Science and Technology (MMST), Hebei Key Laboratory of Applied Chemistry, Yanshan University, Qinhuangdao 066004, People's Republic of China
First published on 10th December 2025
Abstract
A novel Cu–B2O3/porous boron nitride (PBN) composite was developed as a highly efficient and reusable heterogeneous catalyst for the solvent-controlled conversion of arylboronic acids. In water, the catalyst promotes ipso-hydroxylation to phenols, while in methanol, it facilitates aerobic homocoupling to biphenyls, using air as a green oxidant. The synergistic interaction between Cu nanoparticles and B2O3 sites enhances both catalytic activity and stability. This work offers a sustainable and selective approach for synthesizing phenolic and biphenyl compounds under mild conditions.
1. Introduction
Arylboronic acids are versatile reagents widely used in cross-coupling reactions for constructing C–C, C–N, and C-heteroatom bonds, with broad applications in pharmaceuticals, materials science, and fine chemicals.1–5 Among their transformations, the synthesis of phenols and biphenyls is of particular interest due to the utility of these structures in drug intermediates, agrochemicals, and polymers.6–10Traditional methods for phenol synthesis, such as diazonium salt hydrolysis11 and hydroxylation of aryl halides,12–14 often suffer from harsh conditions or poor selectivity. In contrast, the ipso-hydroxylation of arylboronic acids represents a greener and more atom-economical route. Various catalytic systems have been explored,15–20 including BN–OH,21 graphene oxide,22 N-oxide,23 hypervalent iodine, and supported metal nanoparticles (Pd, Au, Ag, Cu, etc.).24–33 However, many of these systems rely on H2O2 as an oxidant, which increases cost and limits sustainability. Molecular oxygen, especially from air, offers an ideal alternative, though efficient and stable catalysts for aerobic hydroxylation remain scarce.
Two-dimensional materials such as graphene and boron nitride have emerged as promising catalyst supports due to their high surface area and tunable surface chemistry. However, the weak interaction between metals and the basal plane of hexagonal boron nitride (h-BN) often leads to poor metal dispersion.34 To address this, we developed a Cu–B2O3/PBN composite with enhanced metal–support interaction and catalytic performance.
In this work, we report the solvoselective behavior of Cu–B2O3/PBN: in water, it catalyzes the ipso-hydroxylation of arylboronic acids to phenols, while in methanol, it promotes their homocoupling to biphenyls, using air as the sole oxidant. The catalyst exhibits excellent stability and reusability, underscoring its potential for sustainable chemical synthesis.
2. Experimental section
2.1 Materials
Cu(NO3)2·3H2O was purchased from Beijing InnoChem Science and Technology Co., Ltd. All substrates, including phenylboronic acid and its derivatives, were obtained from Shanghai Macklin Biochemical Co., Ltd. Melamine (C3H6N6) and boric acid (H3BO3) were acquired from Shanghai Aladdin Biochemical Technology Co., LTD (Shanghai, China). All materials were commercially available and were used without further purification. PBN was prepared according to the procedure reported by our group.352.2 Preparation of Cu–B2O3/PBN nanocatalyst
To prepare the Cu–B2O3/PBN nanocatalyst, Cu(NO3)2·3H2O (0.046 g) was dissolved in 15 mL of deionized water. Subsequently, 0.2 g of the as-obtained PBN powder was dispersed into the above solution, and then the mixture was stirred at room temperature for 5 h. After that, the mixed solution was stirred at 60 °C until the solvent had evaporated, followed by drying at 60 °C for 8 h. The reddish-brown Cu–B2O3/PBN powder was obtained by a reduction process conducted at a temperature of 400 °C in Ar/H2 (5%) for a duration of 2 h (Fig. S1).2.3 Cu–B2O3/PBN catalyzed for ipso-hydroxylation of phenylboronic acids in water
In a typical reaction, 0.2 mmol of phenylboric acid and 5 mg of Cu–B2O3/PBN were dispersed in 5 mL of water and stirred continuously at 80 °C. HPLC was used to evaluate the reaction's performance, while the yield was determined by using the standard curve. Once the reaction was finished, the product was subjected to extraction using methylene chloride. The purification of all phenol synthesis products was achieved using column chromatography, resulting in the production of white acicular crystals. The acquired compounds were further characterized using 1H-NMR spectra. After completion of the reaction, the reaction mixture was centrifuged to separate the catalyst and reused in further reactions. Following the completion of the reaction, the reaction mixture underwent centrifugation to isolate the catalyst, which was then used in subsequent processes.2.4 Cu–B2O3/PBN catalyzed for homocoupling of phenylboronic acids in methanol
A solution was prepared by dissolving 0.2 mmol of phenylboric acid (1a) in 5 mL of methanol at room temperature. Following this, 5 mg of Cu–B2O3/PBN catalyst was introduced under continuous stirring. The reaction progress was tracked using high-performance liquid chromatography (HPLC), and the yield was quantified using a standard calibration curve (Fig. S2 and S3). For the substrate scope experiments, 0.3 mmol of the corresponding derivatives was used. After the reaction, the catalyst was removed by filtration, and methanol was evaporated under reduced pressure. The product, white crystals of 1(b–m), was obtained through separation and purification via column chromatography (silica gel, eluent: petroleum ether and ethyl acetate). After each cycle, the catalyst was recovered from the solution, washed three times with water, dried under vacuum to remove residual solvents, and then reused for subsequent reaction cycles.2.5 Characterization
The morphology of the catalysts was observed by transmission electron microscopy (TEM, JEM-2100F). The X-ray diffraction (XRD) patterns of the synthesized samples were analyzed using an X-ray diffractometer (D/MAX-2500, Rigaku, Japan), set to operate at 40 kV and 150 mA with Cu Kα radiation (λ = 0.15406 nm) and a scanning range of 2θ from 5° to 100°. X-ray photoelectron spectroscopy (XPS) was performed on a Thermo Scientific K-Alpha instrument with 72 W Al Kα X-ray as the excitation source. The binding energies were calibrated by contaminant C at 284.8 eV. Fourier transform infrared (FT-IR) spectra were performed on a Thermo-Fisher scientific Prestige-21 FT-IR instrument using KBr as dilute agent. UV-vis absorption spectra were measured at room temperature with a UV-vis spectrophotometer (TU-1901, Persee, China). The actual Cu content in the Cu–B2O3/PBN catalyst was determined by inductively coupled plasma optical emission spectroscopy (ICP-OES, Agilent 5110). The morphology and elemental composition of the catalysts were observed using field emission scanning electron microscopy (FESEM) (TESCAN MIRA LMS, Czech Republic) with energy dispersive X-ray spectrometer (EDX). Thermal stability was evaluated using thermogravimetric-differential scanning calorimetry (TG-DSC) (PerkinElmer STA 6000, USA). The N2 adsorption–desorption isotherms were recorded at 77 K using a Micromeritics ASAP 2460 (USA), and the surface area was calculated using the BET method.3. Results and discussion
3.1 Characteristics of the Cu–B2O3/PBN nanocatalysts
The structure of the Cu–B2O3/PBN sample was analyzed using XRD (Fig. 1a). The XRD pattern of Cu–B2O3/PBN displays two distinct diffraction peaks at 2θ = 26.7° and 43.6°, which are closely aligned with the characteristic diffraction peaks observed in PBN (BN, PDF #34-0421). The crystal structure of boron oxide on the Cu–B2O3/PBN sample was validated by comparing the two prominent and distinct diffraction peaks at 2θ = 14.6° and 27.8° with the standard pattern of B2O3 (PDF #06-0297). The distinct peaks seen at 2θ = 43.19°, 50.29°, 73.88°, and 89.61° correspond to the Cu crystal planes (111), (200), (220), and (311), respectively (PDF #70-3038). These peaks confirm the successful incorporation of Cu. The actual Cu loading determined by ICP was 6.21 wt%, which is in good agreement with the nominal value based on the precursor amount used during synthesis. In the FT-IR spectra (Fig. 1b), the prominent peaks at 1439 and 782 cm−1 are associated with the in-plane stretching and out-of-plane bending vibrations of the B–N bond, similar to those observed in the PBN structure. The shift of the absorption band from 806 cm−1 in PBN to 782 cm−1 in Cu–B2O3/PBN is indicative of a strong interaction between Cu and the hydroxyl groups on the PBN support. The absorption peak at 1195 cm−1 corresponds to the stretching vibration of the B–O bond, which is formed due to the oxidation of the N–B bond.35,36 The peaks at 926 and 883 cm−1 are attributed to the out-of-plane bending vibrations of N–B–O bonds, providing further evidence for the formation of B2O3 within the Cu–B2O3/PBN composite. The morphology of Cu–B2O3/PBN samples was examined using TEM (Fig. 1c). The TEM images reveal a homogeneous distribution of Cu nanoparticles, measuring around 7.9 nm in size, on the PBN matrix (Fig. S4). In Fig. 1d, the HRTEM picture was magnified to a resolution of 5 nm, and the lattice fringes of copper nanoparticles on the Cu–B2O3/PBN sample were identified. The measurement of the lattice spacing yielded a value of 0.21 nm, which matches the lattice spacing of the (111) crystal plane in the face-centered cubic phase copper crystal. Simultaneously, the presence of lattice fringes in B2O3 was observed in the vicinity of the Cu nanoparticles. The lattice spacing was determined to be 0.32 nm, indicating the lattice spacing is specifically associated with the face-centered cubic B2O3 (310) crystal plane. The XPS analysis (Fig. S5) was used to describe the composition and chemical condition of the material. A blue shift was seen on the B1s orbital (Fig. 1e) in comparison to the B–N bond of PBN at 190.6 eV. This shift may be attributed to the replacement of some nitrogen atoms with oxygen atoms.37 The observed peak at 193.1 eV may be ascribed to the presence of the B–O bond, which aligns with the findings from XRD and FTIR analysis. This observation suggests that the introduction of B2O3 occurs during the synthesis of Cu–B2O3/PBN samples. A B–O bond is seen at 533.0 eV on the O 1s orbital (Fig. S6). The high-resolution X-ray photoelectron spectroscopy (XPS) analysis of Cu 2p (Fig. 1f) reveals a complex chemical state for the Cu component in Cu–B2O3/PBN. Deconvolution of the peaks at 933.9 and 952.7 eV suggests the presence of Cu+/Cu0 states. Due to the close binding energies, which make it difficult for XPS to distinguish between Cu+ and Cu0, Auger electron spectroscopy (AES) was employed for further analysis of Cu–B2O3/PBN. The Auger peak at 914.6 eV, as shown in Fig. 1g, is attributed to Cu+. After Ar ion etching, the Cu 2p XPS spectrum of the Cu–B2O3/PBN sample is shown in Fig. 1h. The Cu LMM spectrum reveals two distinct peaks corresponding to Cu0 at 918.8 eV and Cu+ at 914.6 eV. This study indicates that the copper nanoparticles within the Cu–B2O3/PBN structure possess a core–shell configuration (Fig. 1i). The porous structure of Cu–B2O3/PBN was characterized by N2 adsorption–desorption measurements (Fig. S7). The material exhibits a type IV isotherm with a BET surface area of 20 m2 g−1, pore volume of 0.023 cm3 g−1. This porous structure facilitates mass transfer and provides abundant active sites for catalytic reactions. The thermal stability of the Cu–B2O3/PBN catalyst, a crucial factor for its application, was assessed by TGA-DSC (Fig. S8). The minimal weight loss and absence of thermal events below 80 °C confirm the structural integrity of the material under the reaction conditions employed (≤80 °C).3.2 The ipso-hydroxylation of arylboronic acid over Cu–B2O3/PBN
The reaction progress of the ipso-hydroxylation of phenylboronic acid in water was closely monitored using HPLC (Fig. 2a), with comprehensive optimization results presented in Table 1. Initially, we dissolved 0.2 mmol of phenylboronic acid in 5.0 mL of water within a 25 mL round-bottom flask, maintaining constant stirring in open air, which yielded a 37.6% phenol production (Table 1, entries 1). Recognizing that bases often enhance yields in various catalytic systems, we experimented with different bases. Our findings indicated that 10 mol% K2CO3 notably accelerated the reaction, achieving a 99.0% yield within 300 minutes (Fig. 2b). We further optimized the reaction conditions by adjusting variables such as temperature, catalyst concentration, and solvent type (Table 1). Notably, increasing the reaction temperature to 80 °C in a water solvent significantly enhanced the product yield to 99% (Fig. 2c). A temperature of 80 °C was thus identified as the optimal condition for this ipso-hydroxylation reaction in open air.| Entry | Different catalysts | Catalyst amount (mg) | Reaction condition | Different base | Amount of base (%) | Reaction temperature (°C) | Solvent | Yieldb (%) |
|---|---|---|---|---|---|---|---|---|
| a Typical reaction conditions: 0.2 mmol of substrate 1a, 2.5–10 mg of catalyst, 5 mL of solvent, at room temperature for 5 h. b Based on HPLC yield. | ||||||||
| 1 | Cu–B2O3/PBN | 5 | Open-air | — | — | 30 | H2O | 37.6 |
| 2 | Cu–B2O3/PBN | 5 | Open-air | K2CO3 | 10 mol | 80 | H2O | 99 |
| 3 | Cu–B2O3/PBN | 5 | Open-air | TEA | 10 mol | 80 | H2O | 90 |
| 4 | Cu–B2O3/PBN | 5 | Open-air | NaOH | 10 mol | 80 | H2O | 36 |
| 5 | Cu–B2O3/PBN | 5 | Open-air | Py | 10 mol | 80 | H2O | 35 |
| 6 | Cu–B2O3/PBN | 5 | Open-air | Na2CO3 | 10 mol | 80 | H2O | 35 |
| 7 | Cu–B2O3/PBN | 5 | Open-air | K2CO3 | 5 mol | 80 | H2O | 85 |
| 8 | Cu–B2O3/PBN | 5 | Open-air | K2CO3 | 15 mol | 80 | H2O | 92 |
| 9 | Cu–B2O3/PBN | 5 | Open-air | K2CO3 | 20 mol | 80 | H2O | 86 |
| 10 | Cu–B2O3/PBN | 5 | Open-air | K2CO3 | 10 mol | 30 | H2O | 20 |
| 11 | Cu–B2O3/PBN | 5 | Open-air | K2CO3 | 10 mol | 50 | H2O | 80 |
| 12 | Cu–B2O3/PBN | 2.5 | Open-air | K2CO3 | 10 mol | 80 | H2O | 23 |
| 13 | Cu–B2O3/PBN | 7.5 | Open-air | K2CO3 | 10 mol | 80 | H2O | 80 |
| 14 | Cu–B2O3/PBN | 10 | Open-air | K2CO3 | 10 mol | 80 | H2O | 76 |
| 15 | Cu–B2O3/PBN | 5 | Open-air | K2CO3 | 10 mol | 80 | EtOH | 4 |
| 16 | Cu–B2O3/PBN | 5 | Open-air | K2CO3 | 10 mol | 80 | DMF | 3 |
| 17 | Cu–B2O3/PBN | 5 | Open-air | K2CO3 | 10 mol | 80 | MeCN | 0 |
| 18 | Cu–B2O3/PBN | 5 | Open-air | K2CO3 | 10 mol | 80 | EtOAc | 0 |
| 19 | Cu–B2O3/PBN | 0 | Open-air | K2CO3 | 10 mol | 80 | H2O | 0 |
| 20 | Cu–B2O3/PBN | 5 | Nitrogen | K2CO3 | 10 mol | 80 | H2O | 17 |
| 21 | Cu–B2O3/PBN | 5 | Open-air | — | — | 80 | H2O | 59.6 |
To determine the optimal conditions, we conducted the reaction using varying amounts of the Cu–B2O3/PBN catalyst, finding that 5 mg of catalyst was sufficient to complete the reaction in 300 minutes at 80 °C (Table 1, entries 2, 12–14; Fig. 2d). We also explored the solvent's influence on reaction reactivity, identifying water as the most suitable solvent for this system (Table 1, entry 2, 15–18). Notably, in the absence of the catalyst, no product formation occurred (Table 1, entry 19). Conducting the ipso-hydroxylation reaction under N2 conditions yielded only a 17% product (Table 1, entry 20), suggesting the critical role of oxygen from air in phenol formation.
Furthermore, we extended our study to include the ipso-hydroxylation of phenylboronic acid using other copper-supported catalysts and commercially available copper compounds under optimized conditions. These variations produced phenol in moderate to low yields (Table 2, entry 1–8). The influence of substituents on Cu–B2O3/PBN's catalytic activity was investigated using various phenylboronic acid derivatives (Table 3). Conducted under optimized conditions, this study revealed that phenolic products with electron-donating groups exhibit a decreasing yield over time, likely due to increased susceptibility to oxidation. Conversely, the yield for phenolic products with electron-withdrawing groups remained relatively stable (Table S1). Notably, the catalyst also demonstrated excellent compatibility with strong electron-withdrawing groups. As shown in Table 3, 4-cyanophenylboronic acid and 4-nitrophenylboronic acid were smoothly converted to the corresponding phenols 2i and 2j in high yields of 85% and 88% within 3 h, respectively. This result highlights the remarkable functional group tolerance and high reactivity of our Cu–B2O3/PBN catalytic system. After 90 minutes, we detected a 10% yield of 1-naphthol (2g), but no product was observed after 3 h. Additionally, 2-hydroxythiophene (2h) was not detected (Table S1).
| Entry | Different catalysts | Catalyst amount (mg) | Reaction condition | Different base | Amount of base (%) | Reaction temperature (°C) | Solvent | Yieldb (%) |
|---|---|---|---|---|---|---|---|---|
| a Typical reaction conditions: 0.2 mmol of substrate 1a, 2.5–10 mg of catalyst, 5 mL of solvent, at room temperature for 5 h. b Based on HPLC yield. | ||||||||
| 1 | PBN | 5 | Open-air | K2CO3 | 10 mol | 80 | H2O | 14 |
| 2 | B2O3–PBN | 5 | Open-air | K2CO3 | 10 mol | 80 | H2O | 17.8 |
| 3 | Cu/PBN | 5 | Open-air | K2CO3 | 10 mol | 80 | H2O | 51.2 |
| 4 | Cu(NO3)2·3H2O | 5 | Open-air | K2CO3 | 10 mol | 80 | H2O | 90.4 |
| 5 | CuCl | 5 | Open-air | K2CO3 | 10 mol | 80 | H2O | 85.4 |
| 6 | CuCl2 | 5 | Open-air | K2CO3 | 10 mol | 80 | H2O | 83.5 |
| 7 | Cu2O | 5 | Open-air | K2CO3 | 10 mol | 80 | H2O | 64 |
| 8 | CuO | 5 | Open-air | K2CO3 | 10 mol | 80 | H2O | 49 |
3.3 The C–C homocoupling of aryl boric acid over Cu–B2O3/PBN
The C–C homocoupling reactions of arylboronic acids were selected as the benchmark to further investigate the catalytic performance and selectivity of the synthesized Cu–B2O3/PBN catalyst. For initial experiments, 0.2 mmol of phenylboronic acid, serving as a model substrate, was mixed with 5 mg of the Cu–B2O3/PBN catalyst and 5 mL of methanol, and the reaction was conducted at room temperature in open air. The results demonstrated the high reactivity of the catalyst, achieving a biphenyl yield of 94% (Fig. 3a). It was observed that increasing the catalyst amount to 5 mg maximized the yield of biphenyl, with additional catalyst not significantly altering the outcome (Table 4, entries 1–5; Fig. 3b).| Entry | Different catalysts | Catalyst amount (mg) | Reaction condition | Different base | Reaction time | Solvent | Yieldb (%) | Yieldb (%) |
|---|---|---|---|---|---|---|---|---|
| a Typical reaction conditions: 0.2 mmol of substrate 1a, 2.5–10 mg of catalyst, 5 mL of solvent, at room temperature for 5 h. b Based on HPLC yield. | ||||||||
| 1 | Cu–B2O3/PBN | 5 | Open-air | None | 300 min | MeOH | 94 | 3 |
| 2 | Cu–B2O3/PBN | 0 | Open-air | None | 300 min | MeOH | 0 | 0 |
| 3 | Cu–B2O3/PBN | 2.5 | Open-air | None | 300 min | MeOH | 79 | 2 |
| 4 | Cu–B2O3/PBN | 7.5 | Open-air | None | 300 min | MeOH | 94 | 3 |
| 5 | Cu–B2O3/PBN | 10 | Open-air | None | 300 min | MeOH | 85 | 2 |
| 6 | Cu–B2O3/PBN | 5 | Open-air | None | 300 min | EtOH | 11 | 0.3 |
| 7 | Cu–B2O3/PBN | 5 | Open-air | None | 300 min | DMF | 8 | 0.24 |
| 8 | Cu–B2O3/PBN | 5 | Open-air | None | 300 min | MeCN | 0 | 0 |
| 9 | Cu–B2O3/PBN | 5 | Open-air | None | 300 min | EtOAc | 0 | 0 |
| 10 | Cu–B2O3/PBN | 5 | Open-air | None | 300 min | H2O | 0 | 99 |
| 11 | Cu–B2O3/PBN | 5 | Open-air | K2CO3 | 120 min | MeOH | 93 | 2.8 |
| 12 | Cu–B2O3/PBN | 5 | Open-air | Py | 120 min | MeOH | 58 | 1.7 |
| 13 | Cu–B2O3/PBN | 5 | Open-air | Na2CO3 | 120 min | MeOH | 46 | 1.4 |
| 14 | Cu–B2O3/PBN | 5 | Open-air | TEA | 120 min | MeOH | 19 | 0.58 |
| 15 | Cu–B2O3/PBN | 5 | Open-air | NaOH | 120 min | MeOH | 0 | 0 |
| 16 | Cu–B2O3/PBN | 5 | Open-air | KOH | 120 min | MeOH | 0 | 0 |
To optimize the reaction parameters, we explored the effect of various solvents on the yield (Table 4, entries 6–10; Fig. 3c), testing both protic and aprotic solvents. Methanol emerged as the most effective solvent for the C–C homocoupling reaction. Notably, conducting the reaction in water did not yield any biphenyl; instead, a considerable amount of phenol was produced. Thus, the optimal conditions for the arylboronic acid homocoupling reaction were identified as using Cu–B2O3/PBN as a catalyst in methanol under open air at room temperature for 5 h.
The catalyst Cu–B2O3/PBN is essential to this reaction since it would not be able to continue without it. Cu–B2O3/PBN is significantly more active and selective than those copper supported catalysts and commercially available copper compounds. We further compared the catalytic activity of this catalyst with other copper supported catalysts and the commercially available CuO, Cu2O, and copper salts like CuCl2 and Cu(NO3)2 for the model reaction under the standard conditions (Table 5, entries 1–5).
| Entry | Different catalysts | Catalyst amount (mg) | Reaction condition | Different base | Reaction time | Solvent | Yieldb (%) | Yieldb (%) |
|---|---|---|---|---|---|---|---|---|
| a Typical reaction conditions: 0.2 mmol of substrate 1a, 2.5–10 mg of catalyst, 5 mL of solvent, at room temperature for 5 h. b Based on HPLC yield. | ||||||||
| 1 | CuCl2 | 5 | Open-air | None | 360 min | MeOH | 13 | 0.39 |
| 2 | Cu(NO3)2·3H2O | 5 | Open-air | None | 360 min | MeOH | 23 | 0.69 |
| 3 | Cu2O | 5 | Open-air | None | 360 min | MeOH | 41 | 1.23 |
| 4 | CuO | 5 | Open-air | None | 360 min | MeOH | 60 | 1.8 |
| 5 | PBN | 5 | Open-air | None | 360 min | MeOH | 0 | 0 |
The Cu–B2O3/PBN catalyst also demonstrated broad applicability in the aerobic homocoupling of various arylboronic acids. As summarized in Table 6, uniformly high yields were obtained regardless of whether electron-donating or electron-withdrawing substituents were present. For sterically demanding substrates such as naphthalene-1-boronic acid, an 85% yield was achieved by extending the reaction time. In contrast, heteroarylboronic acids gave moderate yields, around 50%.
Notably, electron-deficient substrates bearing strong electron-withdrawing groups (–CN and –NO2) were also successfully transformed into the corresponding biphenyls 3i and 3j, with yields of 60% and 65%, respectively. The somewhat lower yields compared to electron-rich substrates can be attributed to the electron-withdrawing effect, which likely decelerates the transmetalation step in the catalytic cycle. Despite this, the results clearly demonstrate the versatility of the Cu–B2O3/PBN system in promoting C–C bond formation across substrates with diverse electronic properties.
3.4 Reusability of Cu–B2O3/PBN catalyst
The stability and reusability of a heterogeneous catalyst are critical factors for its commercial application. In evaluating these properties for the Cu–B2O3/PBN catalyst across both ipso-hydroxylation and C–C homocoupling reactions, we recovered the catalyst post-reaction via centrifugation and subjected it to three ethanol wash cycles. Subsequently, the catalyst was dried under vacuum at 60 °C overnight before being redeployed in new rounds of both reaction types. Remarkably, the Cu–B2O3/PBN catalyst demonstrated consistent performance, maintaining its catalytic efficiency through up to six cycles of reuse with negligible activity loss in both reactions, as illustrated in Fig. 3d. The excellent stability and reusability were further confirmed by characterization of the spent catalyst. FESEM images (Fig. S9–S11) show maintained morphology without significant aggregation after six cycles. EDAX analysis confirms minimal copper leaching (∼0.24–0.92 wt% loss). XRD patterns of the reused catalyst (Fig. S12) display identical diffraction peaks to the fresh catalyst, indicating preserved crystal structure of both Cu nanoparticles and B2O3 phases.3.5 The proposed catalytic mechanism of Cu–B2O3/PBN
The reaction mechanism was presented through a set of experiments conducted step by step. When conducting the control experiment, a nitrogen atmosphere surrounding was available, and a significance of low yield of the desired product (17%) was attained (Table 1, entry 20). We furthered the reaction experiment without the Cu–B2O3/PBN catalyst in air, while no desired product could be detected (Table 1, entry 19). All these can indicate that both the catalyst and the air are indispensable for the transformation.N,N-diethyl-p-phenylenediamine sulfate (DPD) and horseradish peroxidase (POD) solutions were used to monitor the generation of H2O2 in water. With no presence of the catalyst, H2O2 was not detected in the reaction solution, as shown in Fig. S13. Whenever the catalyst was added, the ultraviolet-visible spectrum of the DPD-POD mixed solution became pink from the past colorless. Hence, it can indicate that there existed the production of H2O2 during the reaction. It can be speculated that superoxide radicals are primarily responsible for the oxidative dehydrogenation of hydrogenated azobenzene. In order to investigate the chemical state of Cu nanoparticles, XPS and AES were adopted to analyze the used catalysts. The results signified no significant difference in the surface chemical state of the used catalyst, compared to the fresh catalyst (Fig. S14 and S15). Further control experiments were carried out to explore the reaction pathway and the role of the catalyst support. The findings demonstrated that B2O3–PBN can serve as an effective catalyst support compared to commercial PBN (Fig. S16–S18), and that copper ions played a crucial role in catalysis.
Based on our observations and previous studies,38,39 we hypothesize that the mechanism for phenol formation involves the generation of radicals, leading to a proposed reaction pathway (see Scheme 1). Initially, oxygen in the air is activated by Cu nanoparticles, resulting in the generation of superoxide radicals and H2O2. Subsequently, the H2O2 molecules on the surface of Cu–B2O3/PBN react with phenylboronic acid to form an adduct. This adduct undergoes rearrangement and subsequent hydrolysis, ultimately yielding phenol. The proposed mechanism for biphenyl formation involves a Cu(I)/Cu(III) catalytic cycle.40–42 Initially, Cu(I) undergoes double transmetallation with two molecules of phenylboronic acid to form a Ph–Cu(I)–Ph complex. This complex is then oxidized by air to generate a Cu(III) intermediate, which undergoes reductive elimination to release the homocoupled product Ph–Ph. Furthermore, a comparative analysis with other reported catalytic systems (Table S2) reveals that our Cu–B2O3/PBN catalyst not only achieves high yields in both ipso-hydroxylation and homocoupling reactions but also operates under milder and greener conditions (e.g., air as oxidant, low catalyst loading, and reusable heterogeneous nature). This positions our catalyst as a competitive and environmentally benign option for the synthesis of phenols and biphenyls.
 | ||
| Scheme 1 The possible mechanism of the solvent selectivity effect of Cu–B2O3/PBN on the catalytic conversion of arylboronic acid to phenol and biphenyl. | ||
4. Conclusion
In this study, we successfully synthesized a Cu–B2O3/PBN composite material through a straightforward impregnation process. The integration of Cu not only promoted the crystallization of B2O3 but also ensured its stable attachment to the PBN framework. This composite material proved to be an efficient and selective heterogeneous catalyst for two distinct reactions: the ipso-hydroxylation of arylboronic acids to phenols in an aqueous medium and the aerobic homocoupling of arylboronic acids to biphenyls in methanol. Both reactions utilized environmentally benign air as the oxidant under mild conditions, highlighting the sustainability of this catalytic system. Moreover, the Cu–B2O3/PBN catalyst exhibited excellent stability and reusability, maintaining its catalytic performance over multiple reaction cycles without significant loss of activity. This work underscores the potential of Cu–B2O3/PBN as a versatile and robust catalyst for green chemistry applications.Author contributions
Yumei Zhang: conceptualization, formal analysis, funding acquisition, methodology, resources, supervision, writing – original draft, writing – review & editing. Yue Wang: data curation, formal analysis, investigation, writing – original draft. Dongle Wang: investigation, data curation. Yating Liu: investigation, data curation. Tong Gao: investigation, data curation. Qingqiang Zhou: investigation, data curation. Qingzhi Luo: investigation, resources. Jing An: methodology. Xueyan Li: methodology. Yandong Duan: formal analysis, methodology, funding acquisition, writing – original draft. Desong Wang: conceptualization, formal analysis, resources, supervision.Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included as part of the supplementary information (SI).Supplementary information: supplementary data to this article can be found online. See DOI: https://doi.org/10.1039/d5cy00986c.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 22278349) and Natural Science Foundation of Hebei Province (No. B2025208009). The authors extend their gratitude to Mr. Li Cai (from Scientific Compass https://www.shiyanjia.com) for providing invaluable assistance with the XRD analysis.References
- N. Miyaura and A. Suzuki, Palladium-catalyzed cross-coupling reactions of organoboron compounds, Chem. Rev., 1995, 95, 2457–2483 CrossRef.
- J. D. Cope, P. E. Sheridan, C. J. Galloway, R. F. Awoyemi, S. L. Stokes and J. P. Emerson, Synthesis and characterization of a tetradentate, N-heterocyclic carbene copper(II) complex and its use as a Chan-Evans-Lam coupling catalyst, Organometallics, 2020, 39, 4457–4464 CrossRef.
- F. Monnier and M. Taillefer, Catalytic C-C, C-N, and C-O Ullmann-type coupling reactions: copper makes a difference, Angew. Chem., Int. Ed., 2008, 47, 3096–3099 CrossRef PubMed.
- F. Monnier and M. Taillefer, Katalytische C-C, C-N und C-O Ullmann-Kupplungen, Angew. Chem., 2009, 121, 7088–7105 CrossRef.
- C. Zhu and J. R. Falck, Transition metal-free ipso-functionalization of arylboronic acids and derivatives, Adv. Synth. Catal., 2014, 356, 2395–2410 CrossRef PubMed.
- J. C. Vantourout, H. N. Miras, A. Isidro-Llobet, S. Sproules and A. J. B. Watson, Spectroscopic studies of the Chan-Lam Amination: a mechanism-inspired solution to boronic ester reactivity, J. Am. Chem. Soc., 2017, 139, 4769–4779 CrossRef PubMed.
- M. J. West, J. W. B. Fyfe, J. C. Vantourout and A. J. B. Watson, Mechanistic development and recent applications of the Chan-Lam amination, Chem. Rev., 2019, 119, 12491–12523 CrossRef PubMed.
- N. Akatyev, M. Il'in, M. Il'in, S. Peregudova, A. Peregudov, A. Buyanovskaya, K. Kudryavtsev, A. Dubovik, V. Grinberg, V. Orlov, A. Pavlov, V. Novikov, I. Volkov and Y. Belokon, Chan-Evans-Lam C-N coupling promoted by a dinuclear positively charged Cu(II) complex. Catalytic performance and some evidence for the mechanism of CEL reaction obviating Cu(III)/Cu(I) catalytic cycle, ChemCatChem, 2020, 12, 3010–3021 CrossRef.
- R. Upadhyay, D. Singh and S. K. Maurya, Highly efficient heterogeneous V2O5@TiO2 catalyzed the rapid transformation of boronic acids to phenols, Eur. J. Org. Chem., 2021, 2021, 3925–3931 CrossRef.
- R. I. Khan and K. Pitchumani, A pyridinium modified β-cyclodextrin: an ionic supramolecular ligand for palladium acetate in C-C coupling reactions in water, Green Chem., 2016, 18, 5518–5528 RSC.
- G. Chen, A. S. C. Chan and F. Y. Kwong, Palladium-catalyzed C-O bond formation: direct synthesis of phenols and aryl/alkyl ethers from activated aryl halides, Tetrahedron Lett., 2007, 48, 473–476 CrossRef.
- W. D. Castro-Godoy, L. C. Schmidt and J. E. Argüello, A green alternative for the conversion of arylboronic acids/esters into phenols promoted by a reducing agent, Sodium Sulfite, Eur. J. Org. Chem., 2019, 2019, 3035–3039 CrossRef.
- A. Tlili, N. Xia, F. Monnier and M. Taillefer, A very simple copper-catalyzed synthesis of phenols employing hydroxide salts, Angew. Chem., Int. Ed., 2009, 48, 8725–8728 CrossRef PubMed.
- L. Jing, J. Wei, L. Zhou, Z. Huang, Z. Li and X. Zhou, Lithium pipecolinate as a facile and efficient ligand for copper-catalyzed hydroxylation of aryl halides in water, Chem. Commun., 2010, 46, 4767–4769 RSC.
- S. J. Choi, Y.-G. Lee, U. S. Shin and S.-H. Kim, Carbocatalyst-promoted oxidative hydroxylation of arylboronic acids, Tetrahedron Lett., 2022, 100, 153856 CrossRef.
- N. S. Bhat, S. L. Hegde, S. Dutta and P. Sudarsanam, Efficient Synthesis of 5-(hydroxymethyl)furfural esters from polymeric carbohydrates using 5-(chloromethyl)furfural as a reactive intermediate, ACS Sustainable Chem. Eng., 2022, 10, 5803–5809 CrossRef.
- R. Das, K. R. Rohit and G. Anilkumar, Recent trends in non-noble metal-catalyzed hydroxylation reactions, J. Organomet. Chem., 2022, 977, 122456 CrossRef.
- Y.-Q. Zou, J.-R. Chen, X.-P. Liu, L.-Q. Lu, R. L. Davis, K. A. Jørgensen and W.-J. Xiao, Highly efficient aerobic oxidative hydroxylation of arylboronic acids: photoredox catalysis using visible light, Angew. Chem., Int. Ed., 2012, 51, 784–788 CrossRef.
- A. Dandia, R. Sharma, P. Saini, R. S. Badgoti, K. S. Rathore and V. Parewa, The graphite-catalyzed ipso-functionalization of arylboronic acids in an aqueous medium: metal-free access to phenols, anilines, nitroarenes, and haloarenes, RSC Adv., 2021, 11, 18040–18049 RSC.
- M. Sharma, B. Adhikari, R. F. Awoyemi, A. M. Perkins, A. K. Duckworth, B. Donnadieu, D. O. Wipf, S. L. Stokes and J. P. Emerson, Copper(II) NHC catalyst for the formation of phenol from arylboronic acid, Chemistry, 2022, 4, 560–575 CrossRef PubMed.
- P. Choudhary, K. Kumari, D. Sharma, S. Kumar and V. Krishnan, Surface Nanoarchitectonics of boron nitride nanosheets for highly efficient and sustainable ipso-hydroxylation of arylboronic acids, ACS Appl. Mater. Interfaces, 2023, 15, 9412–9420 CrossRef PubMed.
- M. Karthik and P. Suresh, Graphene oxide as a carbocatalyst for sustainable ipso-hydroxylation of arylboronic acids: a simple and straightforward strategy to access phenols, ACS Sustainable Chem. Eng., 2019, 7, 9028–9034 CrossRef.
- C. Zhu, R. Wang and J. R. Falck, Mild and rapid hydroxylation of aryl/heteroaryl boronic acids and boronate esters with N-oxides, Org. Lett., 2012, 14, 3494–3497 CrossRef PubMed.
- M. Khazaei, A. Khazaei, M. Nasrollahzadeh and M. R. Tahsili, Highly efficient reusable Pd nanoparticles based on eggshell: green synthesis, characterization and their application in catalytic reduction of variety of organic dyes and ligand-free oxidative hydroxylation of phenylboronic acid at room temperature, Tetrahedron, 2017, 73, 5613–5623 CrossRef CAS.
- J. Zheng, S. Lin, X. Zhu, B. Jiang, Z. Yang and Z. Pan, Reductant-directed formation of PS-PAMAM-supported gold nanoparticles for use as highly active and recyclable catalysts for the aerobic oxidation of alcohols and the homocoupling of phenylboronic acids, Chem. Commun., 2012, 48, 6235–6237 RSC.
- A. Hatamifard, M. Nasrollahzadeh and S. M. Sajadi, Biosynthesis, characterization and catalytic activity of an Ag/zeolite nanocomposite for base- and ligand-free oxidative hydroxylation of phenylboronic acid and reduction of a variety of dyes at room temperature, New J. Chem., 2016, 40, 2501–2513 RSC.
- V. Kandula, U. Nagababu, M. Behera, S. Yennam and A. Chatterjee, A facile green synthesis of silver nanoparticles: An investigation on catalytic hydroxylation studies for efficient conversion of aryl boronic acids to phenol, J. Saudi Chem. Soc., 2019, 23, 711–717 CrossRef.
- R. Borah, E. Saikia, S. J. Bora and B. Chetia, On-water synthesis of phenols using biogenic Cu2O nanoparticles without using H2O2, RSC Adv., 2016, 6, 100443–100447 RSC.
- T. Chandra Saikia, X. Borgohain, S. Iraqui and M. H. Rashid, Template-less and surfactant-less synthesis of CeO2 nanostructures for catalytic application in ipso-hydroxylation of aryl boronic acids and the aza-michael reaction, ACS Omega, 2022, 7, 42126–42137 CrossRef CAS PubMed.
- T. C. Saikia, S. Iraqui and M. H. Rashid, Synergistic effect of PEG-coated ZnO nanoparticles and ultrasonic irradiation on the C-B bond cleavage of aryl boronic acids, Sustainable Chem. Pharm., 2022, 25, 100613 CrossRef CAS.
- P. Rani, W. Heena, N. Pundir, W. Gauri, A. Husain, K. K. Bhasin and G. Kumar, A doubly interpenetrated Cu(II)-based metal-organic framework as a heterogeneous catalyst for the ipso-hydroxylation of arylboronic acids, Eur. J. Org. Chem., 2023, 26, e202200654 CrossRef CAS.
- I. Saikia, M. Hazarika, N. Hussian, M. R. Das and C. Tamuly, Biogenic synthesis of Fe2O3@SiO2 nanoparticles for ipso-hydroxylation of boronic acid in water, Tetrahedron Lett., 2017, 58, 4255–4259 CrossRef CAS.
- M. H. Muhammad, X.-L. Chen, Y. Liu, T. Shi, Y. Peng, L. Qu and B. Yu, Recyclable Cu@C3N4-catalyzed hydroxylation of aryl boronic acids in water under visible light: synthesis of phenols under ambient conditions and room temperature, ACS Sustainable Chem. Eng., 2020, 8, 2682–2687 CrossRef.
- J. Dong, Q. Fu, H. Li, J. Xiao, B. Yang, B. Zhang, Y. Bai, T. Song, R. Zhang, L. Gao, J. Cai, H. Zhang, Z. Liu and X. Bao, Reaction-induced strong metal-support interactions between metals and inert boron nitride nanosheets, J. Am. Chem. Soc., 2020, 142, 17167–17174 CrossRef PubMed.
- Y. Zhang, X. Liu, Y. Duan, Y. Wang, J. Song, Q. Luo, J. An, X. Li, H. Mu and D. Wang, Practical mutual transformation of azobenzene and hydrazobenzene using porous BN supported Cu nanoparticles as a redox catalyst, J. Catal., 2023, 428, 115167 CrossRef.
- P. Li, X. Zhang, J. Wang, Y. Xue, Y. Yao, S. Chai, B. Zhou, X. Wang, N. Zheng and J. Yao, Engineering O-O Species in boron nitrous nanotubes increases olefins for propane oxidative dehydrogenation, J. Am. Chem. Soc., 2022, 144, 5930–5936 CrossRef PubMed.
- Z. Liu, B. Yan, S. Meng, R. Liu, W.-D. Lu, J. Sheng, Y. Yi and A.-H. Lu, Plasma tuning local environment of hexagonal boron nitride for oxidative dehydrogenation of propane, Angew. Chem., 2021, 60, 19691–19695 CrossRef PubMed.
- R. Borah, E. Saikia, S. J. Bora and B. Chetia, Banana pulp extract mediated synthesis of Cu2O nanoparticles: an efficient heterogeneous catalyst for the ipso-hydroxylation of arylboronic acids, Tetrahedron Lett., 2017, 58, 1211–1215 CrossRef.
- S. Phukan, A. Mahanta and M. H. Rashid, Size-tunable ZnO nanotapes as an efficient catalyst for oxidative chemoselective CB bond cleavage of arylboronic acids, Appl. Catal., A, 2018, 562, 58–66 CrossRef.
- P. Puthiaraj, P. Suresh and K. Pitchumani, Aerobic homocoupling of arylboronic acids catalysed by copper terephthalate metal-organic frameworks, Green Chem., 2014, 16, 2865–2875 RSC.
- S. K. Das, B. Krishna Chandra, R. A. Molla, M. Sengupta, S. M. Islam, A. Majee and A. Bhaumik, CuO grafted triazine functionalized covalent organic framework as an efficient catalyst for C-C homo coupling reaction, Mol. Catal., 2020, 480, 110650 Search PubMed.
- Y.-N. Cao, X.-C. Tian, X.-X. Chen, Y.-X. Yao, F. Gao and X.-L. J. S. Zhou, Rapid ligand-free base-accelerated copper-catalyzed homocoupling reaction of arylboronic acids, Synlett, 2016, 28, 601–606 Search PubMed.
| This journal is © The Royal Society of Chemistry 2026 |