Hierarchical CaO catalyst derived from rape pollen for high-efficiency glycerol-free biodiesel production via tri-component coupling transesterification
Kefan
Wang
ab,
Ying
Yang
*a,
Xiaoli
Wang
c,
Yang
Song
d,
Hua
Song
 e and
Ying
Tang
e and
Ying
Tang
 *ab
*ab
aEngineering Research Center of Oil and Gas Field Chemistry, Universities of Shaanxi Province, Xi'an Shiyou University, Xi'an, 710065, China. E-mail: tangying78@xsyu.dedu.cn
bXi'an Key Laboratory of Low-carbon Utilization for High-carbon Resources, Xi'an Shiyou University, Xi'an, 710065, China
cShaanxi Yanchang Petroleum Energy Technology Co., LTD, Xi'an, 710086, China
dXi'an Sino-Green Hi-Tech Co. LTD, Xi'an, 710060, China
eDepartment of Chemical and Petroleum Engineering, University of Calgary, Calgary, Alberta T2N1N4, Canada
First published on 24th November 2025
Abstract
This study develops a hierarchical calcium oxide (CaO) catalyst, designated as CaO(I), synthesized via the impregnation of a calcium acetate precursor onto porous hollow rape pollen templates. The catalyst demonstrates exceptional efficacy in glycerol-free biodiesel production through the tri-component transesterification of rapeseed oil with methyl acetate and methanol. Under optimized conditions (60 °C, 2 h reaction time, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 oil/methyl acetate/methanol molar ratio), CaO(I) achieves a near-quantitative fatty acid methyl ester (FAME) yield of 99.72%. Comprehensive characterization (SEM, XRD, N2-physisorption, FT-IR, TG, and CO2-TPD) confirms that CaO(I) possesses a well-defined hierarchical pore structure with superior textural properties—including enhanced basic site density (14.6161 mmol g−1) and BET surface area (34.8607 m2 g−1, versus 4.8 m2 g−1 for commercial CaO)—attributed to high active-phase dispersion and favorable pore architecture. Parameter optimization reveals 700 °C as the optimal calcination temperature, and it is observed that the precursor concentration critically influences the catalytic performance. The catalyst maintains robust reusability over multiple cycles with negligible activity loss. This novel templating approach leverages sustainable biomass resources to simultaneously address glycerol surplus issues in conventional biodiesel synthesis and enhance catalytic efficiency, establishing an environmentally conscious pathway for high-performance biofuel production.
8 oil/methyl acetate/methanol molar ratio), CaO(I) achieves a near-quantitative fatty acid methyl ester (FAME) yield of 99.72%. Comprehensive characterization (SEM, XRD, N2-physisorption, FT-IR, TG, and CO2-TPD) confirms that CaO(I) possesses a well-defined hierarchical pore structure with superior textural properties—including enhanced basic site density (14.6161 mmol g−1) and BET surface area (34.8607 m2 g−1, versus 4.8 m2 g−1 for commercial CaO)—attributed to high active-phase dispersion and favorable pore architecture. Parameter optimization reveals 700 °C as the optimal calcination temperature, and it is observed that the precursor concentration critically influences the catalytic performance. The catalyst maintains robust reusability over multiple cycles with negligible activity loss. This novel templating approach leverages sustainable biomass resources to simultaneously address glycerol surplus issues in conventional biodiesel synthesis and enhance catalytic efficiency, establishing an environmentally conscious pathway for high-performance biofuel production.
1. Introduction
Since air pollution is generally considered to be caused by diesel vehicle emissions, vehicle fuels need to be converted to renewable biomass fuels with unique biodegradability and non-toxicity, such as biodiesel.1,2 Currently, in addition to the dwindling depletion of conventional finite resources, a series of toxic chemicals with significant environmental and public health risks have emerged, including sulfur dioxide, carbon dioxide, and aromatic compounds,3,4 which are primarily attributed to the combustion of common fossil fuels. Consequently, the commissioning of biodiesel as fuel oil for machinery is urgent. Currently, transesterification is a prevailing and promising synthetic technique for biodiesel production. However, there are knotty troubles to its commercialization in many countries, mainly including the design of the reactors for biodiesel based on efficient processes, the evolution of heterogeneous solid catalyst and the selection of raw material. Considering the environmental pollution and additional economic costs associated with traditional processes that utilize homogeneous catalysts such as bases (e.g., NaOH, KOH, Na(CH3O)2) or acid (e.g., H2SO4, HCl), a tri-component coupling transesterification approach involves of a mixture comprising glycerol triglyceride (TG), methyl acetate (MeOAc), and short-chain methanol (MeOH) has been developed. The process yields glycerol-free biodiesel production by producing glyceryl triacetate as a by-product, which mitigates the adverse effects of industrial wastewater and alleviates market pressures caused by the oversupply of low-value-added glycerine.5 In general, conventional rapeseed oil is alcoholyzed, leading to the generation of glycerol and the relatively low-effective production of biodiesel. However, the second transesterification of methyl acetate with glycerol can be easily carried out under a mild temperature using alkaline earth metal oxides. In recent years, the supercritical process (SCP) and superheated vapour (SHV) technologies, as two major promising methods for a highly efficient heterogeneous reaction, have attracted extensive recognition.6 According to the report by Sawangkeaw et al.7 on the catalytic behaviour of coconut and palm kernel oil (under supercritical methanolysis), it was found that the FAME yield could reach 95% and 96%, respectively, at a reaction temperature of 350 °C within 6 min. An exception was depicted in the report by Farobie et al.8 who studied the transesterification of canola oil under supercritical conditions. The report presents the complete conversion of canola oil in 10 min at a high temperature (350 °C) and pressure (20 MPa) with the participation of methanol (oil![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol = 1
methanol = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40). However, the harsh reaction conditions and the high cost hinder its further exploration. Interestingly, the dilemma for the glycerol-free biodiesel production can be solved via the development of the tri-component coupling transesterification using heterogeneous catalysts.9 In the transesterification, methanol and methyl acetate first adsorb onto the catalyst surface. The adsorbed methanol then dissociates to form a methoxy group, while methyl acetate participates in the reaction to eliminate glycerol byproducts, ultimately yielding biodiesel as shown in eqn (1):
40). However, the harsh reaction conditions and the high cost hinder its further exploration. Interestingly, the dilemma for the glycerol-free biodiesel production can be solved via the development of the tri-component coupling transesterification using heterogeneous catalysts.9 In the transesterification, methanol and methyl acetate first adsorb onto the catalyst surface. The adsorbed methanol then dissociates to form a methoxy group, while methyl acetate participates in the reaction to eliminate glycerol byproducts, ultimately yielding biodiesel as shown in eqn (1):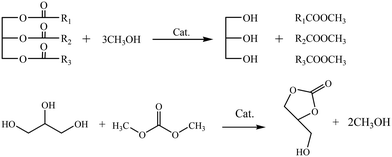 | (1) |
Researchers have endeavored to explore solid-base catalysts by a variety of methods in order to pursue the desirable yield of glycerol-free biodiesel by a greener method. To date, the rape pollen, as a renewable and cost-effective by-product in the edible oil industry, has been employed to produce oxide solid bases with a hierarchical meso/macroporous structure, which is attributed to the net-like morphology of the pollen grain exine. For instance, it was reported that a type of efficient nanostructure electrode material, P-NiO, was obtained for high-efficiency energy storage via chemical bath deposition.13 Generally, a variety of techniques can be employed to achieve the diversification of materials, such as sol–gel, spray pyrolysis, the citrate method, and molecular and surfactant templating techniques.18 Compared with other synthetic techniques, the impregnation method is attractive because of its simple synthesis operation. However, up to now, the reports focused on solid-base CaO derived from pollen for glycerol-free biodiesel production are absent.
Here, the rape pollen was inspirational for the preparation of CaO-based catalysts because of its exceptional structure and three-dimensional open pores.19 More importantly, on the basis of the manifold characterization techniques of BET, CO2-TPD, TG, FT-IR, SEM, and XRD, the effects of calcination temperature and the concentration of calcium sources (Ca(Ac)2·H2O) on CaO(I) samples were investigated to elucidate the relationship between the structure and performance of the catalysts. Thereafter, tri-component coupling transesterification was conducted to produce glycerol-free biodiesel. To the best of our knowledge, such a comprehensive study has not been performed yet.
2. Materials and methods
2.1 Materials
Calcium acetate monohydrate (Ca(Ac)2·H2O, ≥98.0%), high-purity deionized water and rape pollen broken at an ultra-low temperature were purchased from Damao Chemical Reagent Factory (Tianjin, China), Xi'an Laka Equipment Co., Ltd (Shanxi, China) and Changge Yanyuan Bee Products Co., Ltd. (Henan, China), respectively. They were used in the preparation of calcium oxide with high dispersibility. Rapeseed oil (TG) was supplied by Shanxi Jianxing Co., Ltd (Shanxi, China). Methyl acetate (MeOAc, ≥99.0%) and methanol (MeOH, ≥99.5%) were provided by Chengdu Kelon Co., Ltd (Sichuan, China) and Fuyu Co., Ltd (Tianjin, China), respectively. Methyl heptadecanoate (C18H36O, ≥97.0%), as the internal standard, was acquired from TCI Development Co., Ltd (Shanghai, China). Additionally, sodium hydroxide (Na(OH)2, ≥96.0%) and cyclohexane (C6H12, ≥99.5%) were produced by Tianli Co., Ltd (Tianjin, China). All reagents were of analytical grade and used directly as received.2.2 Preparation of the catalyst
Initially, natural rape pollen (60 g) was modified via immersion in dehydrated ethanol (90 g), and ultrasonic processing was carried out for 4 h for degreasing. Then, 1.0 mol L−1 of calcium acetate monohydrate Ca(Ac)2·H2O was dispersed into 300 mL of deionized water for the formation of a clarifying solution. Subsequently, 20 g of the preprocessed pollen was decanted into the above solution with intense magnetic stirring for 18 h at ambient temperature, followed by the centrifugation of the resulting suspension at 4000 rpm for 30 min. After a series of such manipulations, the precipitate was dried in an oven at 80 °C for 12 h and then calcinated in a muffle furnace under an O2 stream at a specific calcination temperature for 3 h to obtain CaO(I) particles. The as-synthesized samples were denoted as x-CaO(I)-y, where “x” was the concentration of calcium acetate and “y” represented the calcination temperature.2.3 Characterization of CaO(I)
The BET specific surface areas (SSA) and pore size distribution (PSD) of the CaO(I) samples were acquired using nitrogen adsorption–desorption isotherms measured using a physical adsorption instrument, Micromeritics ASAP 2020 HD88 (Norcross, Georgia, USA), at 77 K based on the BET equation and BJH model, respectively.20 CaO(I) was degassed in a liquid nitrogen atmosphere at 573 K for 4 h before the test.The alkalinity of the samples was determined using a multifunctional automatic chemical adsorption instrument, ChemiSorb 2750 (Norcross, Georgia, USA), applying the temperature-programmed desorption of CO2.21 The samples were pretreated in flowing argon (50 mL min−1) for 1.5 h at the required temperature and then in carbon dioxide gas for 3 h at cooled 50 °C prior to the operation in helium gas for 1.5 h at 50 °C. The resulting samples were further heated to 800 °C in helium gas (10 °C min−1).5
The thermal properties of the samples were collected using a TGA-SDTA851 analyzer (Shanghai, China) under a heating order of 10 °C min−1 in the range from 25 °C to 800 °C and nitrogen flow at 20 mL min−1. The FT-IR spectra of the samples were recorded on a Fourier infrared spectrometer, Nicolet 5700 (Madison, Wisconsin, USA).22 The IR source, KBr beam splitter and DTGS detector were used for the mid-IR measurements (4000–400 cm−1). MIR transmission spectra were collected using the KBr pellet technique (1 mg of a sample homogenized with 200 mg of KBr). For each sample, 64 scans with a resolution of 4 cm−1 were recorded. Spectra manipulations were performed using the Thermo Scientific OMNIC™ software package. The second derivatives were obtained using the Savitsky-Golay derivative filter for mid-IR (MIR). A scanning electron microscope observation was performed using JSM-6390A (Japan) to examine the CaO particle morphology.
The XRD measurements of the samples were carried out using an X-ray powder diffractometer, D8 ADVAHCL (Germany), with Cu-Kα radiation of 40 kV and 30 mA at a 2θ range of 10–80°, keeping CaO(I) packed into a 1 mm-deep cavity in a zero-background quartz sample holder.
2.4 Preparation of the biodiesel
A typical synthesis of the glycerol-free biodiesel catalyzed by CaO(I) of the test series was performed in a 250 mL three-necked round-bottom flask equipped with a reflux condenser; it involved the addition of methanol and methyl acetate to about 20 g of rapeseed oil to keep the molar ratio in proportion. Then, as-prepared CaO(I) was added to the three components, and the mixture was heated to 65 °C in a water bath under magnetic stirring using a thermostatic heating magnetic stirrer, DF-101S. Employing a standard Teflon-coated magnet, the catalyst was uniformly dispersed in the mixed solution at a stirring rate of 300 rpm. Subsequently, the samples taken out every 20 or 30 min were processed using a TG-16 centrifuge and RA-2000A rotary evaporator. The centrifugal operations aimed to separate the catalyst, and the rotary evaporator was employed to remove excess methanol and methyl acetate. The evaporated yellow liquid was biodiesel, and its yield was determined using a GC-7860 gas chromatograph (Yiyou Electronic Technology Co., Shanghai, China) with a KB-Wax capillary column (30 m × 0.32 mm × 0.25 μm) and a flame ionization detector (FID).4 To clearly and accurately calibrate the content of biodiesel, an appropriate portion of methyl heptadecanoate as the standard was diluted with cyclohexane to a final concentration of 1 mol L−1, and the temperature parameters of the detector, injector and oven were set as 280 °C, 250 °C and 100 °C, respectively. In addition, there were three stages of temperature programming. The first stage was to increase the temperature from 100 °C to 200 °C at a heating rate of 20 °C min−1, hold for 3 min, then increase it to 220 °C at a heating rate of 10 °C min−1 and maintain it for 3 min. Finally, the temperature was increased to 240 °C at a heating rate of 10 °C min−1 and maintained for 3 min, and the total analysis process was carried out in 20 min. The percentages of fatty acid conversion to methyl esters were determined by gas chromatography (GC), as shown in Fig. 1. The first set of peaks represents methyl esters, followed by those of monoglycerides, diglycerides, and triglycerides. The FAME yield was calculated according to eqn (2):23 | (2) |
3. Results and discussion
3.1 Preparation of the catalyst
As shown in Fig. 2, a simple and convenient impregnation approach for CaO with high dispersion was employed. It involved the hydroxylation of the template surface by the ultrasonic treatment of anhydrous ethanol (step 1),24 the absorption of pollen template in calcium acetate solution (step 2) and the combustion of the organic material to form the corresponding CaO material (step 3). In particular, the Ca2+ from the calcium salt solution was attracted by the –OH on the surface of the pollen during the impregnation, and the Ca2+ adsorbed on the template was hydrolyzed to generate Ca(OH)2, followed by aggregation on the pollen surface. Ultimately, Ca(OH)2 was decomposed to form CaO after high-temperature calcination.The photographs of the obtained CaO(I) calcinated at 600 °C, 700 °C and 800 °C are shown in Fig. 2. In view of the wide distribution of the germinating pores and the existence of groups such as hydroxyl and carboxyl on the pollen surface, it can be revealed that the easily adsorbed cations from the precursors are dispersed on the surface of those groups to form nucleation sites, and thereafter, assembly occur.25,26 Also, a great deal of work was done on the CaO-based catalysts produced from pollen for the preparation of glycerol-free biodiesel from rapeseed oil. Szkudlarka Ł et al. reported the synthesis of an alkaline earth metal oxide-based catalyst, comprising CaO supported on BEA zeolite, which was applied in the transesterification of rapeseed oil with methanol. The catalytic activity assessments revealed a notable enhancement in the performance of the prepared catalyst.27 According to the photographs of the CaO(I) catalysts, it was found that with an increase in the calcination temperature, the as-prepared samples gradually changed, from gray to white, and finally, the white powder was collected at the calcination temperature above 700 °C. This was due to the incomplete removal of biological organic matrix and the presence of a large number of residual impurities in the product at a low calcination temperature (600 °C), in accordance with the TG curve of pollen (Fig. 7(b)). It can be speculated that the favorable growth of the CaO crystal and the removal of the biotemplate were dependent on an appropriate calcination temperature.
3.2 Characterization of the catalyst
| Type of catalyst | BET surface area (m2 g−1) | Pore volume (cm2 g−1) | Average pore diameter (nm) |
|---|---|---|---|
| CaO(I)-600 | 14.5806 | 0.086757 | 23.80060 |
| CaO(I)-700 | 34.8607 | 0.108765 | 12.47991 |
| CaO(I)-800 | 18.6545 | 0.072543 | 15.55510 |
| Commercial CaO-700 | 11.4456 | 0.032367 | 11.31162 |
 | ||
| Fig. 3 N2 adsorption/desorption isotherms and BJH pore-size distributions (inset) of CaO(I) and commercial CaO. | ||
Similar to CaO directly calcined from commercial CaO under O2 atmosphere, the N2 adsorption isotherms of the as-prepared CaO(I)-600, CaO(I)-700 and CaO(I)-800 samples were of Langmuir type. This indicated that the pores of the samples were dominated by mesopores, which was attributed to their isotherms with a palpable H3-like hysteresis loop belonging to IV-type isotherms.30 Further, there was a relatively concentrated pore distribution near 3.5 nm (as shown in Fig. 3) formed by the interaction between the pollen and the supporting metal salt material. Meanwhile, it was worth noting that the nitrogen adsorption capacity of the CaO(I)-700 catalyst was stronger than that of the other catalysts. This was due to the accumulation of CaO crystals caused by the incomplete removal of the pollen template at a relatively low calcination temperature and the influence of the sintered CaO surface at a relatively high calcination temperature.
 | ||
| Fig. 6 CO2-TPD profiles of the prepared CaO(I) samples, at various calcination temperatures, and commercial CaO. | ||
| Type of catalyst | Total basicity (mmol g−1) | Desorption peaks (area%) |
|---|---|---|
| CaO(I)-600 | 14.5049 | 3.3524 |
| CaO(I)-700 | 14.6161 | 3.3991 |
| CaO(I)-800 | 14.3229 | 3.3309 |
| Commercial CaO-700 | 0.7903 | 0.1838 |
3.3 Catalytic behaviors of as-prepared CaO(I)
The calcium-based catalyst with a high catalytic activity, low price, and abundant raw material sources is a promising solid-base catalyst with industrial application prospects.35 In order to investigate the discrepancy between the catalytic activities of commercial CaO and as-obtained CaO(I), the glycerol-free biodiesel preparation via the tri-component coupling transesterification was explored using rapeseed oil/methyl acetate/methanol at a molar ratio of 1/1/8, reaction temperature of 65 °C and CaO(I) dosage of 10 wt% (as shown in Fig. 9). It was found that as-prepared CaO(I) had better performance, with 99.05% of FAME yield after 3 h reaction, than the commercial CaO, with only 72.49% yield. Therefore, the application of the CaO(I) catalyst supported on pollen was confirmed as the subsequent research objective.The effect of the calcination temperature of prepared CaO(I) on the FAME yield was recorded from 600 °C to 800 °C and is presented in Fig. 10(a). It was worth noting that a high FAME yield (99.05%) can be obtained over 10% CaO(I) at the calcination temperature of 700 °C with a rapeseed oil/methyl acetate/methanol molar ratio of 1/1/8 and reaction temperature of 65 °C. It should be due to the complete dislodgement of templates and the disappearance of a large number of impurities, leading to the formation of calcium oxide crystals. However, a subsequent decline in the yield was found when the calcination temperature was selected in the range from 700 °C to 800 °C, likely due to the low specific surface area caused by the large-area collapse and sintering of the catalyst at a high temperature, resulting in a decrease in the number of basic sites (as shown in Table 1 and Fig. 6).36 Another factor affecting the catalytic properties is the precursor concentration of prepared CaO(I). From the results shown in Fig. 10(b), it can be seen that the high FAME yield (99.05%) with the CaO(I) catalyst derived at a precursor concentration of 1.0 mol L−1 was obtained under the previous reaction condition (rapeseed oil/methyl acetate/methanol: 1/1/8, reaction temperature: 65 °C and CaO(I) weight: 10 wt%). However, the downward trend in yield was observed with the augmentation of the Ca2+ amount. This demonstrated that the heterogeneous basic materials calcined at a low temperature in a dilute solution have great potential to generate large pores, which allows reactant molecules to gather inside the catalyst, as certified by Shigapov's team.18–37 However, although the concentration of Ca2+ in the pollen template was higher after the long-term exposure to excess solution, the decrease in the catalytic effect of the final catalyst in the tri-component coupling transesterification may be mainly due to the relatively low pore parameters caused by the excessive expansion of pollen pores and capillaries.
 | ||
| Fig. 10 Effect of the preparation conditions of CaO(I) on the FAME yield: (a) calcination temperature and (b) precursor concentration. | ||
Fig. 11(a) indicates a disparity in the FAME yield when the as-prepared CaO(I) amount is in the range from 5 wt% to 15 wt%. From the results, it can be found that the dosage of CaO(I) had a conspicuous effect on the glycerol-free biodiesel production through the tri-component coupling transesterification. The yield of FAME can be enhanced to 99.05% under oil/methyl acetate/methanol of 1/1/8 for 3 h at 65 °C when CaO(I) consumption increases from 5 wt% to 10 wt%. With further increase in the quantity of CaO(I) to 15 wt%, the yield did not visibly improve with the increased number of active sites provided by the catalyst.38 However, a reduction in the FAME yield was observed as a result of increased viscosity (from 3.5 to 7.3 mm2 s−1) caused by excessive CaO(I), further leading to the enhancement of the mass transfer resistance and the generation of soap.39,40
The reaction temperature of the tri-component transesterification influenced the catalytic behavior in the biodiesel production. In order to determine the effect of the reaction temperature on the FAME yield, the results were obtained at various reaction temperatures between 55 °C and 70 °C under reaction conditions of oil/methyl acetate/methanol of 1/1/8, refluxing time of 3 h and CaO(I) quantity of 10 wt%, as shown in Fig. 11(b). Generally, transesterification is an endothermic reaction; the heightened reaction temperature was one of the achievable initiatives to improve the reaction rate and yield of the FAME, and the optimal yield (99.72%) of glycerol-free biodiesel can be obtained through the tri-component transesterification after 2 h. Further increasing the reaction temperature did not increase the FAME yield, which was attributed to the volatilization of methanol.41
Methanol, as a reaction reagent, has great potential to contribute to the tri-component transesterification.42 To examine the effect of methanol on the FAME yield, different proportions of the mixed solution consisting of rapeseed oil, methyl acetate and methanol were added to a three-necked flask for biodiesel production. The results are summarized in Fig. 11(c). From the results, it can be observed that the favorable yield of 99.72% occurred at a refluxing temperature of 60 °C and CaO(I) consumption of 10 wt% when the appropriate proportion of oil/methyl acetate/methanol was 1/1/8. However, at this ratio, a higher yield than those of other reactions with reactant ratios ranging from 1/1/6 to 1/1/10 cannot be obtained, indicating that excessive addition of methanol will reduce the FAME yield on account of the effect of dilution.43
In practice, the repeatability of high-efficiency solid bases played an important role in the tri-component coupling transesterification. Sample 1.0-CaO(I)-700 was regenerated through separation, drying and calcination of 700 °C for 3 h, followed by the transesterification (oil/methyl acetate/methanol = 1/1/8, reaction time = 2 h, reaction temperature = 60 °C and the CaO(I) quantity = 10 wt%), as shown in Fig. 12. A declining FAME yield from 99.72% to 85.29% was observed with the increase in the regeneration time, probably due to the decrease in the surface area after calcination. Nevertheless, its catalytic activity was still considerable (more than 10% of that of commercial CaO-700), indicating that CaO(I) could be applied as a better regenerative solid base for the glycerol-free biodiesel preparation.
Conclusion
In summary, we demonstrated an exotemplating method for the synthesis of a CaO(I) catalyst with high dispersion and pore distribution obtained using rape pollen as a bio-template and calcium acetate as a precursor. The calcination temperature and precursor concentration of the as-prepared CaO(I) samples were screened by the tri-component coupling transesterification of rapeseed oil, methyl acetate and methanol. Among them, CaO(I) derived at a calcination temperature of 700 °C with a Ca2+ salt concentration of 1 mol L−1 exhibited the best catalytic activity under the optimal conditions of oil/methyl acetate/methanol of 1/1/8, refluxing temperature of 60 °C, reaction time of 2 h and CaO(I) quantity of 10 wt% (mass ratio to oil). The FAME yield can effectively reach 99.72%. Moreover, it was found that the large BET surface area (34.8607 m2 g−1), high pore volume (0.1088 cm3 g−1) and increased number of basic sites of the obtained CaO(I) play an important role in the catalytic effect. Therefore, the as-prepared CaO(I) material is expected to be a high-performance heterogeneous catalyst with a wide application prospect in glycerol-free biodiesel preparation.Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this study's findings are available from the corresponding author upon reasonable request.Acknowledgements
This work was supported financially by the National Science Foundation of China (No. 21763030), Postgraduate Innovation Fund Project of Xi'an Shiyou University (YCS20211014) and Youth Innovation Team of Shaanxi University. We also thank the Center of Advanced Analysis and Testing at Xi'an Shiyou University for their work. One of the authors (Michal Slaný) acknowledges the financial support for this research by the Slovak Research and Development Agency (APVV-19-0490).References
- L. Y. Zhang, J. N. Lin and R. Z. Qiu, Characterizing the toxic gaseous emissions of gasoline and diesel vehicles based on a real-world on-road investigation, J. Cleaner Prod., 2021, 286, 124957 CrossRef CAS.
- L. M. Sun, M. M. Li and X. O. Ma, Study on obtaining high performance diesel/biodiesel fuel by using heterogeneous catalysts, Pet. Sci. Technol., 2019, 37(12), 1471–1477 CrossRef CAS.
- N. Mansir, S. H. Teo and N. A. Mijan, Efficient reaction for biodiesel manufacturing using bi-functional oxide catalyst, Catal. Commun., 2021, 149, 106201 CrossRef CAS.
- Y. Tang and H. Liu, Tri-component coupling transesterification for efficient no-glycerol biodiesel production using methyl acetate as methyl reagent, J. Chem. Technol. Biotechnol., 2020, 95, 1234–1242 CrossRef CAS.
- Y. Tang, H. Liu and H. M. Ren, Development KCl/CaO as a catalyst for biodiesel production by tri-component coupling transesterification, Environ. Prog. Sustainable Energy, 2018, 38, 647–653 CrossRef.
- K. Bisheswar and H. Gopinath, Progress and future of biodiesel synthesis: Advancements in oil extraction and conversion technologies, Energy Convers. Manage., 2019, 182, 307–339 CrossRef.
- R. Sawangkeaw, S. Teeravitud and K. Bunyakiat, Biofuel production from palm oil with supercritical alcohols: Effects of the alcohol to oil molar ratios on the biofuel chemical composition and properties, Bioresour. Technol., 2011, 102(22), 10704–10710 CrossRef CAS PubMed.
- O. Farobie and Y. Matsumura, A comparative study of biodiesel production using methanol, ethanol, and tert-butyl methyl ether (MTBE) under supercritical conditions, Bioresour. Technol., 2015, 191, 306–311 CrossRef CAS.
- Y. Tang, Q. Cheng and H. Cao, Coupling transesterifications for no-glycerol biodiesel production catalyzed by calcium oxide-ScienceDirect, C. R. Chim., 2015, 18(12), 1328–1334 CrossRef CAS.
- Y. Tang, S. Y. Li and J. L. Dong, An efficient CaO-based catalyst for rapid production of biodiesel without glycerol as a by-product using a tri-component reaction, J. Am. Oil Chem. Soc., 2018, 95(12), 1487–1496 CrossRef CAS.
- Y. Tang, Y. Y. Xue and Z. Y. Li, Heterogeneous synthesis of glycerol carbonate from glycerol and dimethyl carbonate catalyzed by LiCl/CaO, J. Saudi Chem. Soc., 2019, 23(4), 494–502 CrossRef CAS.
- C. Quan, N. Gao and H. Wang, Ethanol steam reforming on Ni/CaO catalysts for coproduction of hydrogen and carbon nanotubes, Int. J. Energy Res., 2019, 43(3), 1255–1271 CrossRef CAS.
- S. Wang, W. Li and L. Xin, Pollen-inspired synthesis of porous and hollow NiO elliptical microstructures assembled from nanosheets for high-performance electrochemical energy storage, Chem. Eng. J., 2017, 321, 546–553 CrossRef CAS.
- W. K. Zhu, X. G. Luo and C. Zhang, Regulation of Microstructure of Calcium Carbonate Crystals by Egg White Protein, Chem. Res. Chin. Univ., 2012, 2, 180–185 Search PubMed.
- L. J. Xie, W. Chu and Y. Y. Huang, Preparation and characterization of biomorphic nickel oxide microtubes templated from cotton, Mater. Lett., 2011, 65(2), 153–156 CrossRef CAS.
- Y. Zou, Y. Li and Y. Guo, Ultrasound-assisted synthesis of CuO nanostructures templated by cotton fibers, Mater. Res. Bull., 2012, 47(11), 3135–3140 CrossRef CAS.
- J. He, Q. Shen and S. Yang, Coupling effects in 3D plasmonic structures templated by Morpho butterfly wings, Nanoscale, 2018, 10(2), 533–537 RSC.
- M. T. Y. Paul, B. B. Yee and X. Zhang, Template assisted preparation of high surface area macroporous supports with uniform and tunable nanocrystal loadings, Nanoscale, 2019, 11(4), 1937–1948 RSC.
- X. Yao, S. Zhou and H. Zhou, Pollen-structured hierarchically meso/macroporous silica spheres with supported gold nanoparticles for high-performance catalytic CO oxidation, Mater. Res. Bull., 2017, 92, 129–137 CrossRef CAS.
- H. Wei, S. Qin and R. Zhang, Hierarchical porous transition metal oxide nanosheets templated from waste bagasse: General synthesis and Li/Na storage performance, Ceram. Int., 2022, 48(2), 2298–2305 CrossRef CAS.
- L. Ma, X. Y. Zhang and D. Lin, Preparation of shaped magnesium oxide/carbon catalysts using rice grains as an exotemplate and carbon precursor, Appl. Catal., A, 2013, 460, 26–35 CrossRef.
- M. Hayati-Ashtiani, Characterization of Nano-Porous Bentonite (Montmorillonite) Particles using FTIR and BET-BJH Analyses, Part. Part. Syst. Charact., 2012, 28(3–4), 71–76 CAS.
- N. A. M. Azam, Y. Uemura and K. Kusakabe, Biodiesel production from palm oil using micro tube reactors: Effects of catalyst concentration and residence time, Procedia Eng., 2016, 148, 354–360 CrossRef CAS.
- S. M. Zhu, D. Zhang and J. J. Gu, Biotemplate fabrication of SnO2 nanotubular materials by a sonochemical method for gas sensors, J. Nanopart. Res., 2010, 12(4), 1389–1400 CrossRef CAS.
- L. Qin, M. Liu and Y. Wu, Bioinspired hollow and hierarchically porous MOX (M =Ti, Si)/carbon microellipsoids supported with Fe2O3 for heterogenous photochemical oxidation, Appl. Catal., B, 2016, 194, 50–60 CrossRef CAS.
- J. Zhao, S. Ge and L. Liu, Microwave Solvothermal Fabrication of Zirconia Hollow Microspheres with Different Morphologies Using Pollen Templates and Their Dye Adsorption Removal, Ind. Eng. Chem. Res., 2017, 57(1), 231–241 CrossRef.
- Ł. Szkudlarek, K. Chałupka-Śpiewak and W. Maniukiewicz, Biodiesel production by methanolysis of rapeseed oil—influence of SiO2/Al2O3 Ratio in BEA zeolite structure on physicochemical and catalytic properties of zeolite systems with alkaline earth oxides (MgO, CaO, SrO), Int. J. Mol. Sci., 2024, 25(7), 3570 CrossRef PubMed.
- I. Riaz, I. Shafiq and F. Jamil, A review on catalysts of biodiesel (methyl esters) production, Catal. Rev.:Sci. Eng., 2024, 66(4), 1084–1136 CrossRef CAS.
- Y. Tang, T. L. Yan and B. Shen, Synthesis of no-glycerol biodiesel through transesterification catalyzed by CaO from different precursors, Can. J. Chem. Eng., 2016, 94(8), 1466–1471 CrossRef CAS.
- Y. C. Miao, Z. B. Zhai and J. He, Synthesis, characterizations and photocatalytic studies of mesoporous titania prepared by using four plant skins as templates, Mater. Sci. Eng., C, 2010, 30(6), 839–846 CrossRef CAS.
- W. W. Mar and E. Somsook, Methanolysis of soybean oil over KCl/CaO solid base catalyst for biodiesel production, ScienceAsia, 2012, 38(1), 90–94 CAS.
- Y. Tang, Y. Yang and H. Liu, Preparation of nano-CaO and catalyzing tri-component coupling transesterification to produce biodiesel, Inorg. Nano-Met. Chem., 2020, 50(7), 501–507 CrossRef CAS.
- K. Hu, H. Wang and Y. Liu, KNO3/CaO as cost-effective heterogeneous catalyst for the synthesis of glycerol carbonate from glycerol and dimethyl carbonate, J. Ind. Eng. Chem., 2015, 28, 334–343 CrossRef CAS.
- Q. T. Tien, G. Q. Guan and G. G. Liu, Direct synthesis of iso-paraffin fuel from palm oil on mixed heterogeneous acid and base catalysts, Monatsh. Chem., 2017, 148(7), 1235–1243 CrossRef.
- M. Sotenko, J. Fernández and G. Hu, Performance of novel CaO-based sorbents in high temperature CO2 capture under RF heating, Chem. Eng. Process.: Process Intesif., 2017, 122, 487–492 CrossRef CAS.
- Y. C. Wong, Y. P. Tan and Y. H. Taufiq-Yap, Effect of calcination temperatures of CaO/Nb2O5 Mixed Oxides Catalysts on Biodiesel Production, Sains Malays., 2014, 43(5), 783–790 CAS.
- N. Hassan, K. N. Ismail and K. H. Ku Hamid, CaO Nanocatalyst for Transesterification Reaction of Palm Oil to Biodiesel: Effect of Precursor’s Concentration on the Catalyst Behavior, IOP Conf. Ser.:Mater. Sci. Eng., 2018, 358, 1–12 Search PubMed.
- B. Sutrisno, A. D. Nafiah and I. S. Fauziah, CaO/Natural Dolomite as a Heterogeneous Catalyst for Biodiesel Production, Mater. Sci. Forum, 2020, 991, 117–122 Search PubMed.
- K. T. Tan, K. T. Lee and A. R. Nohamed, Optimization of supercritical dimethyl carbonate (SCDMC) technology for the production of biodiesel and value-added glycerol carbonate, Fuel, 2010, 89, 3833–3839 CrossRef CAS.
- A. P. Shi, J. M. Zhu and Q. Ma, Biodiesel Preparation with Solid Base Catalyst under Ultrasonic Assistant, Appl. Mech. Mater., 2014, 548, 158–163 Search PubMed.
- Dianursanti, M. Delaamira and S. Bismo, Effect of Reaction Temperature on Biodiesel Production from Chlorella vulgaris using CuO/Zeolite as Heterogeneous Catalyst, IOP Conf. Ser. Earth Environ. Sci., 2017, 55, 012033 CrossRef.
- R. Estevez, C. Luna and J. Calero, Optimization by response surface methodology of the reaction conditions in 1,3-selective transesterification of sunflower oil by using CaO as heterogeneous catalyst, Mol. Catal., 2020, 484, 110804 Search PubMed.
- Y. Kojima and S. Takai, Transesterification of vegetable oil with methanol using solid base catalyst of calcium oxide under ultrasonication, Chem. Eng. Process., 2019, 136, 101–106 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2026 |









