Open Access Article
Open Access ArticleRegulating the electrode potential offset in perovskite solar cells for boosting interfacial charge transfer
Qiong Wanga,
Mengfan Xueb,
Kaijian Zhuc,
Qiyu Qua,
Bo Wanga,
Shengyao Wang*d,
Bing Wang a,
Zhigang Zouae and
Wenjun Luo
a,
Zhigang Zouae and
Wenjun Luo *e
*e
aEco-materials and Renewable Energy Research Center (ERERC), Jiangsu Key Laboratory for Nano Technology, National Laboratory of Solid State Microstructures and Department of Physics, Nanjing University, Nanjing 210093, China
bInstitute of Scientific and Technical Information of China (ISTIC), China
cSchool of Energy and Environment, City University of Hong Kong, 83 Tat Chee Avenue, Kowloon, Hong Kong S.A.R., China
dSchool of Chemistry and Chemical Engineering, Shanghai Jiao Tong University, Shanghai 200240, P. R. China. E-mail: wangshengyao@sjtu.edu.cn
eNational Laboratory of Solid State Microstructures, College of Engineering and Applied Sciences, Nanjing University, Nanjing 210093, China. E-mail: wjluo@nju.edu.cn
First published on 3rd January 2026
Abstract
In perovskite solar cells (PSCs), the interfacial charge transfer is adjusted by a band offset to improve the device performance. However, the band offset cannot explain some abnormal phenomena of interfacial charge transfer. In this study, we adjusted the electrode potential offset (ΔEPO) at the perovskite/hole transport layer (HTL) interface by employing different side chain HTLs, which increased the device efficiency from 0.6% to 18.1%. We also investigated the relationship between chemical reaction kinetics at the perovskite/HTL interface and the ΔEPO by in situ FTIR, NMR, theoretical calculations and electrochemical measurements. Accordingly, we proposed ΔEPO as a driving force descriptor for interfacial charge transfer in PSCs for the first time. These results deepen the understanding of the interfacial charge transfer and offer a new design principle for the interface in the PSCs.
Introduction
Perovskite solar cells (PSCs) have emerged as a promising next-generation photovoltaic technology owing to their low cost and high power conversion efficiency (PCE).1–5 Since PSCs were first reported in 2009,6 the certified record efficiency has reached 26.9%.7 However, there is still a considerable gap compared with the theoretical Shockley–Queisser (S–Q) limit, primarily due to interfacial energy losses.8–12 The energy band alignment theory has been widely used to design heterojunctions to improve interfacial charge transfer.13–15 Generally, a smaller band offset between the perovskite and charge transport layer (CTL) leads to faster interfacial charge transfer.16–18 However, some recent studies suggest that the interfacial charge transfer rate is independent of the band offset at the interface.19–22 For example, some PSCs exhibit similar performance with large band offsets, whereas other PSCs exhibit very different performance with small band offset variations.23,24 These abnormal experimental phenomena suggest that interfacial charge transfer is beyond the classical semiconductor physics mechanism.Recently, interfacial charge transfer through chemical reactions has been observed at the perovskite/CTL interface under working conditions.25–27 Accordingly, PSCs are chemical faradaic junctions, which are intrinsically different from physical p–n junctions.28–32 The electrode potential in the chemical faradaic junction is similar to the energy band in the physical p–n junction. Therefore, regulating the electrode potential offset (ΔEPO) in the faradaic junction PSCs is important for the performance of the device.
In this study, we prepared FTO/SnO2/MAPbI3/hole transport layer (HTL)/Au solar cells. Taking the MAPbI3/HTL interface as the model, we regulated the ΔEPO at the interface by employing different side chain HTLs (Spiro-TAD, Spiro-TTB, and Spiro-OMeTAD). We found that the interfacial charge transfer process depends on ΔEPO. Accordingly, we proposed ΔEPO as a descriptor for the interface charge transfer driving force in PSCs, which is intrinsically different from the band offset. These results deepen our understanding of the interfacial charge transfer in PSCs and provide new guidance for designing efficient PSCs.
Results and discussion
Fig. 1a indicates the device structure diagram of the PSCs with three kinds of different side chain structures of HTLs (Fig. 1b). The current density–voltage (J–V) curves of three devices were measured and the results are shown in Fig. 1c. The performance of the three devices varies significantly. The device with Spiro-OMeTAD exhibits the highest efficiency (18.1%), followed by the device with Spiro-TTB (7.3%), while the device with Spiro-TAD exhibits the lowest efficiency (0.6%). The short-circuit current density (JSC) values of the devices with Spiro-OMeTAD and Spiro-TTB are 23.05 mA cm−2 and 21.35 mA cm−2, respectively, which are much higher than that of the device with Spiro-TAD (3.2 mA cm−2). The open-circuit voltage (VOC) of the devices with Spiro-OMeTAD and Spiro-TTB is 1.09 V and 1.01 V, respectively, which are higher than that of the device with Spiro-TAD (0.91 V). The fill factor (FF) of the three devices is 72.04% (Spiro-OMeTAD), 33.78% (Spiro-TTB) and 21.79% (Spiro-TAD). Generally, the efficiency variations in the devices with different HTLs possibly come from different bulk characteristics of these HTLs and/or band offsets at the interfaces between the HTLs and the perovskite material. Accordingly, we measured the electrical properties of the HTLs by space charge limited current (SCLC) and four-point probe methods, respectively. The results are shown in Fig. S2 and Table S1. Spiro-OMeTAD exhibits the highest hole mobility (8.10 × 10−3 cm2 V−1 s−1) and conductivity (7.35 × 10−5 S cm−1), while Spiro-TAD exhibits the lowest hole mobility (6.56 × 10−3 cm2 V−1 s−1) and conductivity (4.68 × 10−5 S cm−1). The differences in the electrical properties of the HTL bulk may lead to different JSC and the FF in the devices. However, the hole mobilities and conductivities of the three HTLs are all at the same order of magnitude, which is not sufficient to cause such a significant difference in efficiency of the device. Moreover, we also characterized the surface morphology of HTLs by atomic force microscopy (AFM) and the results are shown in Fig. S3. The morphologies of the three HTLs are similar, which suggest that the performance of different devices does not come from the different morphology of HTLs.To further investigate the differences in the VOC of the devices, we measured the highest occupied molecular orbital (HOMO) levels and lowest unoccupied molecular orbital (LUMO) levels of three HTLs, as well as the conduction band (CB) and valence band (VB) of MAPbI3, by ultraviolet photoelectron spectroscopy (UPS) (Fig. S4) and ultraviolet-visible absorption spectroscopy (UV-vis) (Fig. S5). The results are plotted in Fig. S6. The results show that the valence band offset (ΔVBO) at the perovskite/Spiro-OMeTAD interface is the smallest (0.22 eV), which is most conducive to interfacial charge transfer. The ΔVBO at the perovskite/Spiro-TTB interface is the largest (0.27 eV), which is most unfavorable for interfacial charge transfer. However, this does not conform to the VOC of the final device, because the device constructed with Spiro-TAD has the lowest VOC. Therefore, these results suggest that the VOC of these PSCs does not depend on the VBO at the perovskite/HTL interface. According to our previous studies, interfacial charge transfer in PSCs is the faradaic junction charge transfer process by chemical redox reactions. Therefore, we also investigated the interfacial charge transfer in the devices with three HTLs following the faradaic junction model.
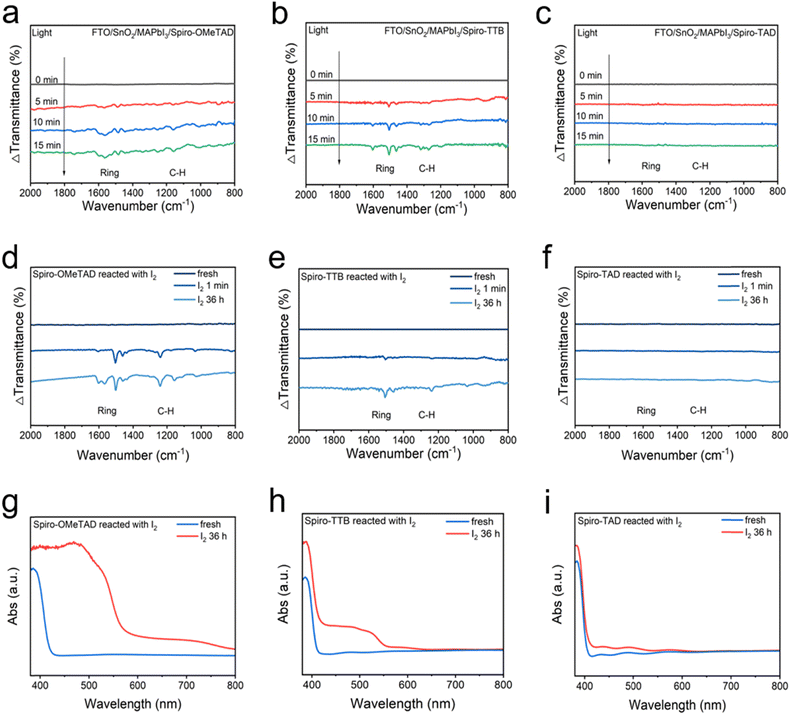
Fig. 2a–c show the in situ Fourier transform infrared spectroscopy (FTIR) spectra of FTO/SnO2/MAPbI3/HTLs (Spiro-OMeTAD, Spiro-TTB, and Spiro-TAD) under illumination for different time periods. We used the FTIR curves of the FTO/SnO2/MAPbI3/HTL in the dark as the baseline to investigate the spectral changes under illumination for different time periods. Fig. 2a shows the FTIR spectral changes of FTO/SnO2/MAPbI3/Spiro-OMeTAD under illumination from 0 to 15 minutes. Compared with the FTIR spectra in the dark, new peaks appear from 1450 cm−1 to 1650 cm−1 under illumination for 5 minutes, which are assigned to the vibration peaks of the benzene ring in Spiro-OMeTAD. Similarly, new peaks also appear from 1000 cm−1 to 1200 cm−1 under illumination, which correspond to the in-plane deformation of the C–H in Spiro-OMeTAD. According to a previous study, the FTIR spectrum changes originate from the interfacial oxidation of Spiro-OMeTAD by I2, which is generated from the faradaic reactions of 2I− + 2h+ ↔ I2 and I2 + Spiro-H+ ↔ I− + Spiro-I− + H+.27 The results suggest that charge transfer occurs at the MAPbI3/Spiro-OMeTAD interface under illumination. As the illumination time increases from 10 minutes to 15 minutes, no obvious changes of the vibration peaks are observed, indicating that the interfacial charge transfer at the MAPbI3/Spiro-OMeTAD has reached dynamic equilibrium. The FTIR spectral changes of FTO/SnO2/MAPbI3/Spiro-TTB under illumination from 0 to 15 minutes are shown in Fig. 2b. Both the benzene ring vibration peaks and C–H vibration peaks of Spiro-TTB exhibit a gradual increase as the illumination time increases from 0 to 15 minutes. The results suggest that similar charge transfer also occurs at the MAPbI3/Spiro-TTB interface, but the charge transfer rate is slower than that at the MAPbI3/Spiro-OMeTAD interface. In contrast, the benzene ring vibration peaks and C–H vibration peaks of Spiro-TAD show no obvious changes under illumination, which suggest that the interfacial charge transfer process at the MAPbI3/Spiro-TAD interface is negligible (Fig. 2c). These results are in contrast to the MAPbI3/Spiro-OMeTAD or MAPbI3/Spiro-TTB interfaces. Therefore, the interfacial charge transfer rates in the three heterojunctions follow the order: MAPbI3/Spiro-OMeTAD > MAPbI3/Spiro-TTB ≫ MAPbI3/Spiro-TAD under illumination.
We also measured the in situ FTIR spectra of the HTL layer and the perovskite layer separately under illumination and the results are shown in Fig. S13. No spectral changes can be observed. Therefore, the observed spectral changes originate from the interface rather than the bulk perovskite or HTL. In order to further confirm that the interfacial charge transfer of MAPbI3/HTL originates from the oxidation of the HTL by I2 generated via the faradaic reaction driven by photogenerated holes, we also measured the oxidation process of the HTL by I2 through the FTIR and UV-vis spectroscopy in the dark. Fig. 2d–f show the FTIR spectral changes of three HTLs oxidized by I2 for different time periods in the dark. Obvious changes of the benzene ring vibration peaks and C–H vibration peaks are observed in Spiro-OMeTAD (Fig. 2d) after oxidation by I2 in the dark, weaker changes of the benzene ring vibration peaks and C–H vibration peaks are observed in Spiro-TTB (Fig. 2e) and negligible changes are observed in Spiro-TAD (Fig. 2f). Fig. 2g–i show the UV-vis absorption spectra of three HTLs before and after I2 treatment for the same duration. A new absorption shoulder at ∼530 nm occurs in Spiro-OMeTAD after I2 treatment (Fig. 2g),33 which originates from the oxidation of Spiro-OMeTAD by I2.27 The intensity of the absorption shoulder decreases in Spiro-TTB (Fig. 2h) and is the lowest in Spiro-TAD (Fig. 2i). Both FTIR and UV-vis results indicate that the oxidation rates of HTLs by I2 follow the order: Spiro-OMeTAD > Spiro-TTB ≫ Spiro-TAD by I2 in the dark.
In order to investigate the relationship between the molecular structures of HTLs and their oxidation rates by I2, we conducted proton nuclear magnetic resonance spectroscopy (1H NMR) on Spiro-OMeTAD, Spiro-TTB, and Spiro-TAD before and after I2 treatment and the results are shown in Fig. 3a–c. The hydrogen atoms at different chemical shifts in the molecular structures of Spiro-OMeTAD, Spiro-TTB, and Spiro-TAD are labeled as 1 to 6. Before I2 treatment, chemical shifts of different hydrogen atoms (labeled 1–6) in Spiro-OMeTAD are observed in the 1H NMR spectra (Fig. 3a).34,35 After I2 treatment for 10 minutes, all hydrogen atoms labeled 1–6 in Spiro-OMeTAD exhibit significant peak broadening, which suggest that the hydrogen atoms at these chemical shifts are affected by I2 through substitution. In Spiro-TTB, only the hydrogen atom labeled 1 exhibits obvious peak broadening after I2 treatment for 10 minutes (Fig. 3b), while no peak broadening is observed in other hydrogen atoms. However, no peak broadening of any hydrogen atom is observed after I2 treatment in Spiro-TAD for the same time (Fig. 3c). Therefore, these results indicate that Spiro-OMeTAD is more readily oxidized by I2 than the other two HTLs. The enhanced oxidation of Spiro-OMeTAD can be attributed to the presence of methoxy side chains.
 | ||
| Fig. 3 1H NMR spectra of (a) Spiro-OMeTAD, (b) Spiro-TTB, and (c) Spiro-TAD powder before and after exposure to I2 vapor. | ||
Based on the faradaic junction charge transfer model,28–32 Fig. 4a–c show the interfacial charge transfer processes in the three perovskite/HTL heterojunctions under illumination. The I−/I2 redox couple serves as the hole transfer mediator at the perovskite/HTL interface. Efficient interfacial charge transfer requires rapid oxidation of I− to I2 by photogenerated holes at the surface faradaic layer of the perovskite, as well as rapid oxidation of the HTL by I2. In order to quantitatively understand the dependence of oxidation rates on the side chain structures of the three HTLs, we calculated the Gibbs free energy changes (ΔGs) of the three HTLs’ side chains oxidized by I2. The ΔGs of the chemical reactions between the three HTLs’ side chains and I2 are −3.05 eV (Spiro-OMeTAD), −2.66 eV (Spiro-TTB) and −2.45 eV (Spiro-TAD), respectively. Generally, the most negative ΔG value indicates the highest thermodynamic spontaneity. The ΔG of the reaction between Spiro-OMeTAD and I2 is the most negative, which suggests that the methoxy side chain reacts with I2 most easily. The Spiro-TAD without side chains is the most difficult to react with I2. The calculation results can be used to understand the different oxidation rates of the three HTLs by I2, as these ΔG values suggest that the methoxy group in the HTL play a critical role in boosting efficient charge transfer.
 | ||
| Fig. 4 Schematic diagram of charge transfer at the perovskite/HTL interface in faradaic junction PSCs under illumination: (a) MAPbI3/Spiro-OMeTAD, (b) MAPbI3/Spiro-TTB, and (c) MAPbI3/Spiro-TAD. The Gibbs free energy changes (ΔG) of the three HTLs’ side chains oxidized by I2 were calculated at the B3LYP-D3(BJ)/def2-TZVP level.36,37 | ||
Electrochemical measurement is a simple method to reflect the trend in free energy of the interfacial chemical reactions. The potentials were calibrated using ferrocene as the reference (Fig. S19).38 We tested the cyclic voltammetry (CV) curves of Spiro-OMeTAD, Spiro-TTB, and Spiro-TAD (Fig. 5a) as well as MAPbI3 (Fig. S20) in the dark. The onset oxidation potentials of Spiro-OMeTAD, Spiro-TTB, and Spiro-TAD are −0.22 V vs. Fc/Fc+, −0.02 V vs. Fc/Fc+, and 0.06 V vs. Fc/Fc+, respectively, while the oxidation potential of iodine on the surface faradaic layer of MAPbI3 is 0.18 V vs. Fc/Fc+. According to the electrochemical results, electrode potential alignments at the MAPbI3/HTLs (with three side chains) are plotted in Fig. 5b. The difference between the oxidation potential of iodine on the surface faradaic layer of MAPbI3 and the onset oxidation potential of the HTL is defined as the ΔEPO. The ΔEPOs at the interfaces between Spiro-OMeTAD, Spiro-TTB, Spiro-TAD and MAPbI3 are 0.40 V vs. Fc/Fc+, 0.20 V vs. Fc/Fc+, and 0.12 V vs. Fc/Fc+, respectively. The larger the ΔEPO, the faster the interfacial charge transfer rate. Therefore, the ΔEPO can be considered as a descriptor for the driving force of interfacial charge transfer in PSCs.
Conclusions
In conclusion, this study demonstrates that regulating the ΔEPO at the perovskite/HTL interface serves as a critical descriptor for interfacial charge transfer in PSCs, fundamentally differing from the conventional band offset approach. By employing HTLs with different side chains (Spiro-OMeTAD, Spiro-TTB, and Spiro-TAD), we systematically regulated ΔEPO, leading to a significant VOC enhancement from 0.91 V to 1.09 V, with larger ΔEPO values correlating to accelerated charge transfer kinetics as validated through in situ FTIR and electrochemical measurements. These findings reveal that ΔEPO governs the faradaic junction charge transfer process mediated by the I−/I2 redox couple, where thermodynamic spontaneity is indicated by theoretical calculations, underpins the oxidation rates of HTLs by I2 proved by UV-vis and 1H NMR. This work establishes ΔEPO as a novel design principle for optimizing PSC interfaces, providing deeper insights into charge transfer mechanisms beyond semiconductor physics.Author contributions
W. L. supervised the project, proposed the concept and designed the experiments. Q. W. carried out the sample preparation, characterization and electrochemistry measurement; W. L. and Q. W. analyzed the data and wrote the paper. All authors discussed the results and gave comments on the manuscript.Conflicts of interest
The authors declare no competing interests.Data availability
All data needed to support the conclusions in the paper are presented in the manuscript and/or the supplementary information (SI). Supplementary information is available. See DOI: https://doi.org/10.1039/d5cp04519c.Additional data related to this paper may be requested from the corresponding author upon request.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (22425202, 22279052, 22372067, and 22279053), the China Postdoctoral Science Foundation (2023M741613) and the Jiangsu Funding Program for Excellent Postdoctoral Talent (2023ZB067).References
- M. Saliba, S. Orlandi, T. Matsui, S. Aghazada, M. Cavazzini, J.-P. Correa-Baena, P. Gao, R. Scopelliti, E. Mosconi, K.-H. Dahmen, F. De Angelis, A. Abate, A. Hagfeldt, G. Pozzi, M. Graetzel and M. K. Nazeeruddin, Nat. Energy, 2016, 1(2), 15017 CrossRef CAS.
- X. Li, W. Zhang, X. Guo, C. Lu, J. Wei and J. Fang, Science, 2022, 375(6579), 434–437 CrossRef CAS.
- Q. Tan, Z. Li, G. Luo, X. Zhang, B. Che, G. Chen, H. Gao, D. He, G. Ma, J. Wang, J. Xiu, H. Yi, T. Chen and Z. He, Nature, 2023, 620(7974), 545–551 Search PubMed.
- K. Feng, G. Wang, Q. Lian, S. Gámez-Valenzuela, B. Li, R. Ding, W. Yang, K. Wang, J. Zeng, Y. Zhang, S. Y. Jeong, B. Xu, A. Ho-Baillie, H. Y. Woo, A. Facchetti and X. Guo, Nat. Mater., 2025, 24(5), 770–777 CrossRef CAS.
- X. Zhu, D. Yu, X. Zhou, N. Wang, H. Liu, Z. Liang, C. Wu, K. Wang, D. Jin, S. Liu and D. Yang, Joule, 2025, 101919 CrossRef CAS.
- A. Kojima, K. Teshima, Y. Shirai and T. Miyasaka, J. Am. Chem. Soc., 2009, 131(17), 6050–6051 Search PubMed.
- J. Du, J. Chen, B. Ouyang, A. Sun, C. Tian, R. Zhuang, C. Chen, S. Liu, Q. Chen, Z. Li, X. Wu, J. Cai, Y. Zhao, R. Li, T. Xue, T. Cen, K. Zhao and C.-C. Chen, Energy Environ. Sci., 2025, 18, 3196–3210 Search PubMed.
- C. Lin, L. Hu, X. Guan, J. Kim, C. Huang, J. Huang, S. Singh and T. Wu, Adv. Mater., 2022, 34(18), 2108616 CrossRef CAS.
- R. Azmi, D. S. Utomo, B. Vishal, S. Zhumagali, P. Dally, A. M. Risqi, A. Prasetio, E. Ugur, F. Cao, I. F. Imran, A. A. Said, A. R. Pininti, A. S. Subbiah, E. Aydin, C. Xiao, S. I. Seok and S. De Wolf, Nature, 2024, 628(8006), 93–98 CrossRef CAS PubMed.
- X. Li, W. Zhang, X. Guo, C. Lu, J. Wei and J. Fang, Science, 2022, 375(6579), 434–437 CrossRef CAS PubMed.
- S. Zhang, F. Ye, X. Wang, R. Chen, H. Zhang, L. Zhan, X. Jiang, Y. Li, X. Ji, S. Liu, M. Yu, F. Yu, Y. Zhang, R. Wu, Z. Liu, Z. Ning, D. Neher, L. Han, Y. Lin, H. Tian, W. Chen, M. Stolterfoht, L. Zhang, W.-H. Zhu and Y. Wu, Science, 2023, 380(6643), 404–409 CrossRef CAS PubMed.
- J. Qiu, X. Mei, M. Zhang, G. Wang, S. Zou, L. Wen, J. Huang, Y. Hua and X. Zhang, Angew. Chem., Int. Ed., 2024, 63(18), e202401751 CrossRef CAS PubMed.
- M. Stolterfoht, P. Caprioglio, C. M. Wolff, J. A. Márquez, J. Nordmann, S. Zhang, D. Rothhardt, U. Hörmann, Y. Amir, A. Redinger, L. Kegelmann, F. Zu, S. Albrecht, N. Koch, T. Kirchartz, M. Saliba, T. Unold and D. Neher, Energy Environ. Sci., 2019, 12(9), 2778–2788 RSC.
- X. Liu, B. Zheng, L. Shi, S. Zhou, J. Xu, Z. Liu, J. S. Yun, E. Choi, M. Zhang, Y. Lv, W.-H. Zhang, J. Huang, C. Li, K. Sun, J. Seidel, M. He, J. Peng, X. Hao and M. Green, Nat. Photonics, 2023, 17(1), 96–105 CrossRef CAS.
- S. Teale, M. Degani, B. Chen, E. H. Sargent and G. Grancini, Nat. Energy, 2024, 9(7), 779–792 Search PubMed.
- T. Wang, Y. Zhang, W. Kong, L. Qiao, B. Peng, Z. Shen, Q. Han, H. Chen, Z. Yuan, R. Zheng and X. Yang, Science, 2022, 377(6611), 1227–1232 Search PubMed.
- X. Liu, B. Ding, M. Han, Z. Yang, J. Chen, P. Shi, X. Xue, R. Ghadari, X. Zhang, R. Wang, K. Brooks, L. Tao, S. Kinge, S. Dai, J. Sheng, P. J. Dyson, M. K. Nazeeruddin and Y. Ding, Angew. Chem., Int. Ed., 2023, 62(29), e202304350 CrossRef CAS PubMed.
- M. Jeong, I. W. Choi, E. M. Go, Y. Cho, M. Kim, B. Lee, S. Jeong, Y. Jo, H. W. Choi, J. Lee, J.-H. Bae, S. K. Kwak, D. S. Kim and C. Yang, Science, 2020, 369(6511), 1615–1620 CrossRef CAS PubMed.
- Y. Zhou, X. Zhang, M. Han, N. Wu, J. Chen, G. Rahim, Y. Wu, S. Dai and X. Liu, Sol. Energy Mater. Sol. Cells, 2023, 257, 112375 CrossRef CAS.
- Q. Wang, F. Zu, P. Caprioglio, C. M. Wolff, M. Stolterfoht, M. Li, S.-H. Turren-Cruz, N. Koch, D. Neher and A. Abate, ACS Energy Lett., 2020, 5(7), 2343–2348 CrossRef CAS.
- L. E. Polander, P. Pahner, M. Schwarze, M. Saalfrank, C. Koerner and K. Leo, APL, Materials, 2014, 2(8), 081503 Search PubMed.
- T. Minemoto and M. Murata, Sol. Energy Mater. Sol. Cells, 2015, 133, 8–14 CrossRef CAS.
- R. A. Belisle, P. Jain, R. Prasanna, T. Leijtens and M. D. McGehee, ACS Energy Lett., 2016, 1(3), 556–560 CrossRef CAS.
- N. J. Jeon, H. Na, E. H. Jung, T.-Y. Yang, Y. G. Lee, G. Kim, H.-W. Shin, S. Il Seok, J. Lee and J. Seo, Nat. Energy, 2018, 3(8), 682–689 CrossRef CAS.
- R. A. Kerner, S. Heo, K. Roh, K. MacMillan, B. W. Larson and B. P. Rand, ACS Energy Lett., 2021, 6(2), 501–508 Search PubMed.
- Z. Xu, D. D. Astridge, R. A. Kerner, X. Zhong, J. Hu, J. Hong, J. A. Wisch, K. Zhu, J. J. Berry, A. Kahn, A. Sellinger and B. P. Rand, J. Am. Chem. Soc., 2023, 145(21), 11846–11858 CrossRef CAS PubMed.
- M. Xue, Z. Li, Q. Wang, Q. Qu, Y. Chen, J. Luo, R. Bao, D. Jiang, S. Wang, B. Wang, T. Yu, Y. Yao, Z. Zou and W. Luo, Sci. China: Chem., 2026, 69, 139–142 Search PubMed.
- X. Chen, K. Zhu, P. Wang, G. Sun, Y. Yao, W. Luo and Z. Zou, iScience, 2020, 23(3), 100949 Search PubMed.
- Z. Yin, X. Chen, C. Wang, Z. Guo, X. Wu, Z. Zhao, Y. Yao, W. Luo and Z. Zou, Chem. Sci., 2020, 11(24), 6297–6304 Search PubMed.
- M. Chen, H. Dong, M. Xue, C. Yang, P. Wang, Y. Yang, H. Zhu, C. Wu, Y. Yao, W. Luo and Z. Zou, Nat. Commun., 2021, 12(1), 6363 Search PubMed.
- P. Wang, M. Xue, D. Jiang, Y. Yang, J. Zhang, H. Dong, G. Sun, Y. Yao, W. Luo and Z. Zou, Nat. Commun., 2022, 13(1), 2544 Search PubMed.
- M. Xue, Z. Chu, D. Jiang, H. Dong, P. Wang, G. Sun, Y. Yao, W. Luo and Z. Zou, Natl. Sci. Rev., 2023, 10, nwac249 CrossRef CAS PubMed.
- R. S. Sanchez and E. Mas-Marza, Sol. Energy Mater. Sol. Cells, 2016, 158, 189–194 Search PubMed.
- Z. Li, J. Tinkham, P. Schulz, M. Yang, D. H. Kim, J. Berry, A. Sellinger and K. Zhu, Adv. Energy Mater., 2017, 7(4), 1601451 CrossRef.
- T. Zhang, F. Wang, H.-B. Kim, I.-W. Choi, C. Wang, E. Cho, R. Konefal, Y. Puttisong, K. Terado, L. Kobera, M. Chen, M. Yang, S. Bai, B. Yang, J. Suo, S.-C. Yang, X. Liu, F. Fu, H. Yoshida, W. M. Chen, J. Brus, V. Coropceanu, A. Hagfeldt, J.-L. Brédas, M. Fahlman, D. S. Kim, Z. Hu and F. Gao, Science, 2022, 377(6605), 495–501 CrossRef CAS PubMed.
- S. Hu, T. Li, M. Huang, J. Huang, W. Li, L. Wang, Z. Chen, Z. Fu, X. Li and Z. Liang, Adv. Mater., 2021, 33(7), 2005839 CrossRef CAS PubMed.
- Q. Zhang, Q. Zhao, H. Wang, Y. Yao, L. Li, Y. Wei, R. Xu, C. Zhang, E. O. Shalenov, Y. Tu, K. Wang and M. Xiao, Nano-Micro Lett., 2025, 17(1), 107 CrossRef CAS PubMed.
- C. M. Cardona, W. Li, A. E. Kaifer, D. Stockdale and G. C. Bazan, Adv. Mater., 2011, 23(20), 2367–2371 CrossRef CAS PubMed.
| This journal is © the Owner Societies 2026 |