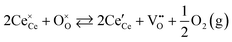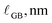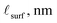Open Access Article
Open Access ArticleDirect in situ detection of grain boundary reduction in nanocrystalline ceria
Claire M.
Donahue
 a,
Qing
Ma
a,
Qing
Ma
 b and
Sossina M.
Haile
b and
Sossina M.
Haile
 *a
*a
aDepartment of Materials Science and Engineering, Northwestern University, Evanston, IL 60208, USA. E-mail: sossina.haile@northwestern.edu
bDow-Northwestern-Dupont Collaborative Access Team, Synchrotron Research Center, Argonne, USA
First published on 26th January 2026
Abstract
Enrichment of the reduced Ce3+ species near grain boundaries in ceria is a widely established phenomenon which has previously been observed in ex situ experiments. Here, in situ X-ray absorption near-edge spectroscopy (XANES) is employed to detect and quantify grain boundary reduction under device-relevant conditions. Single-crystal and dense nanocrystalline films of undoped ceria were characterized by Ce L3 XANES at high temperatures (615–845 °C) in humidified hydrogen. Nanocrystalline ceria (30–40 nm mean grain sizes) exhibited large enhancements in Ce3+ concentration, from 2.0× to 11× relative to bulk ceria. Implications for grain boundary reduction thermodynamics and anticipated conductivity enhancements are discussed.
Introduction
Ceria (CeO2 and doped derivatives) plays a crucial role in existing and emerging technologies in the areas of heterogeneous catalysis and energy storage and conversion. A key feature of the physical chemistry of ceria is the highly accessible and reversible Ce4+/Ce3+ redox couple, described according to the following reaction:where
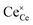 is the effectively neutral Ce4+ species and
is the effectively neutral Ce4+ species and 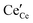 is the Ce3+ species with effective negative charge.1 Significantly, the extent of reduction of ceria is known to be higher at interfaces than within the bulk of the material. This includes gas-solid interfaces (surfaces),2–10 interfaces with metals11 or other oxides12 (heterointerfaces), and internal interfaces (grain boundaries).13–15 Interfacial reduction has important implications for the functional properties of ceria. When acceptor-doped ceria is employed as an electrolyte for its high ionic
is the Ce3+ species with effective negative charge.1 Significantly, the extent of reduction of ceria is known to be higher at interfaces than within the bulk of the material. This includes gas-solid interfaces (surfaces),2–10 interfaces with metals11 or other oxides12 (heterointerfaces), and internal interfaces (grain boundaries).13–15 Interfacial reduction has important implications for the functional properties of ceria. When acceptor-doped ceria is employed as an electrolyte for its high ionic 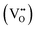 conductivity, enhanced Ce3+ at the grain boundaries is undesirable due to its association with space charge effects that deplete oxygen vacancies in the vicinity of the boundary.16–18 In undoped nanocrystalline ceria, grain boundary reduction results in enhanced electronic
conductivity, enhanced Ce3+ at the grain boundaries is undesirable due to its association with space charge effects that deplete oxygen vacancies in the vicinity of the boundary.16–18 In undoped nanocrystalline ceria, grain boundary reduction results in enhanced electronic 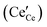 conductivity relative to bulk behavior.19–22 When employed as a catalyst or catalyst support, surface vacancies are desirable catalytic sites8,23 and thus surface reduction, where Ce3+ is presumed to be compensated by oxygen vacancies, is favorable. The detailed relationships between these various properties and defect chemistry remain murky because defect concentrations at interfaces, particularly under application-relevant conditions, are difficult to quantify. Moreover, at the very small length scales that define interface behavior, electroneutrality may be violated, and thus
conductivity relative to bulk behavior.19–22 When employed as a catalyst or catalyst support, surface vacancies are desirable catalytic sites8,23 and thus surface reduction, where Ce3+ is presumed to be compensated by oxygen vacancies, is favorable. The detailed relationships between these various properties and defect chemistry remain murky because defect concentrations at interfaces, particularly under application-relevant conditions, are difficult to quantify. Moreover, at the very small length scales that define interface behavior, electroneutrality may be violated, and thus 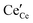 and
and 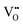 need not be present in direct proportion within the region of interest.
need not be present in direct proportion within the region of interest.
Enhanced reduction of ceria interfaces has been evidenced by several direct experimental studies. Both grain boundaries and surfaces in ceria have been characterized extensively by scanning transmission electron microscopy (STEM) methods, primarily by detection of Ce3+via electron energy loss spectroscopy (EELS).4–6,13–15 Surface reduction has furthermore been established by X-ray photoelectron spectroscopy (XPS).6 Both STEM and XPS are traditionally limited to high vacuum and ambient or low temperatures, but advances in differential pumping have enabled these methods to be utilized at conditions approaching those employed in operational devices. In particular, near-ambient XPS (NAXPS) has been exploited to quantify Ce3+ concentrations at surfaces.7–9 More recently, we have utilized grazing-incidence X-ray absorption near-edge spectroscopy (XANES)2,3 to gain access to the cerium oxidation state at surfaces under truly device-relevant conditions. Because XANES is entirely an X-ray method, differential pumping is not required, and the experimental configuration is readily integrated with environmental chambers for temperature and gas control, enabling true in situ studies. Moreover, by using thin-film samples and controlling the X-ray incidence angle α, one can achieve both full-film sensitivity and surface sensitivity (top 2–3 nm).
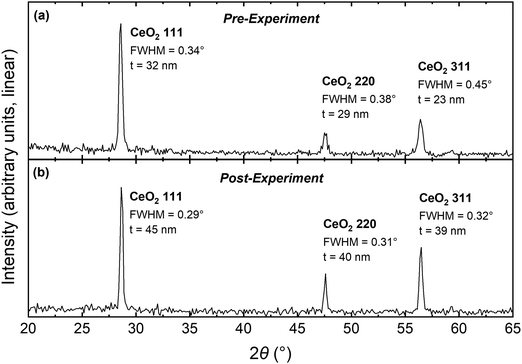
The present work is focused on in situ detection of Ce3+ at the grain boundaries of undoped ceria. Dense nanocrystalline ceria (grain size 25–45 nm) (Fig. 1 and Fig. S1, S2) is characterized using Ce L3 edge XANES, providing an aggregate measurement of grain boundary chemistry. The measurements are carried out at high temperatures and low oxygen partial pressures (pO2), conditions similar to those at which enhanced electronic conductivities have been reported.20,21 The behavior of the dense nanocrystalline CeO2 film is compared to that of a single-crystal CeO2(100) film. We observe significant excess reduction in the nanocrystalline film, the first direct in situ evidence of grain boundary reduction under device relevant conditions, and quantify the level of Ce3+ enrichment on a per-boundary-area basis.
Results and analysis
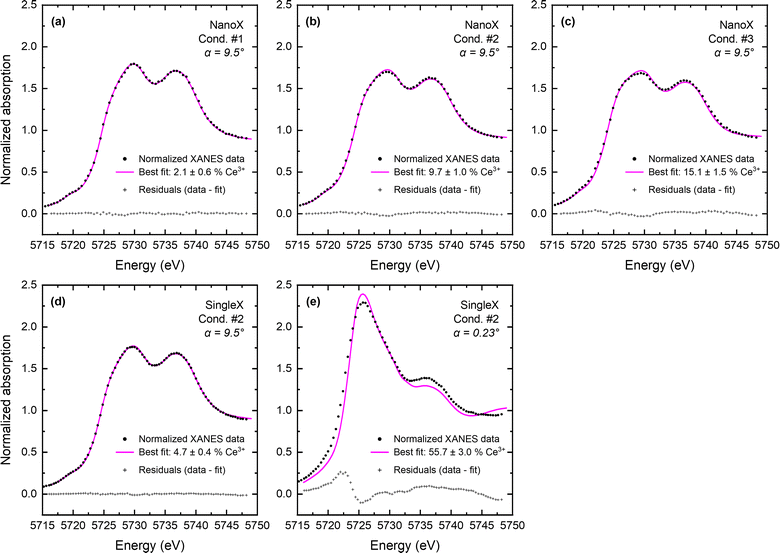
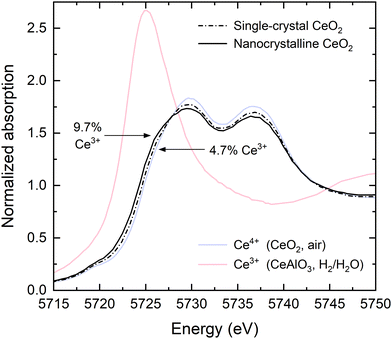
Three conditions were selected for XANES measurements: (1) T = 615 °C and pO2 = 1.0 × 10−24 atm, (2) T = 845 °C and pO2 = 9.9 × 10−19 atm, and (3) T = 845 °C and pO2 = 1.2 × 10−19 atm, where the low oxygen partial pressure is achieved through H2/H2O mixtures. At these conditions, moderate Ce reduction is expected in the bulk (from 0.2 to 7%), and thus the relative impact of grain boundary reduction can be expected to be sufficiently large for detection. Full-film-sensitive spectra were collected at an incidence angle of α = 9.5° for the nanocrystalline CeO2 film (Fig. 2a–c). The single-crystal CeO2 film was measured under condition #2 in both the full-film geometry and in a grazing-incidence geometry at α = 0.23°, below the critical angle of ∼0.45° (Fig. 2d and e). The latter was used to establish the extent of surface reduction. All spectra were analyzed using linear combination (LC) fitting against Ce4+ and Ce3+ reference spectra collected as part of this work. The fitting procedure extends beyond conventional LC fitting by including a treatment of self-absorption effects, as detailed in the supplemental information.Full-film XANES spectra at a temperature of 845 °C and an oxygen partial pressure of 9.9 × 10−19 atm (condition #2) directly reveal a higher concentration of Ce3+ in the nanocrystalline CeO2 than in the single-crystal CeO2 (Fig. 3). The enhanced reduction in the nanocrystalline film is evident in the shift in edge position towards lower energy. The spectra indicate that the presence of grain boundaries results in a two-fold enhancement of cerium reduction, with Ce3+ concentrations of 9.7% and 4.7%, respectively, in the nanocrystalline and single-crystal films.
Because the full-film measurements (α = 9.5°) are sensitive to surface reduction,2,3 in addition to reduction in the interior of the film, they cannot be directly used to quantify grain boundary reduction. To convert the cfilm![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) effective values obtained by LC fitting to the Ce3+ concentration in the bulk of the film, cfilm
effective values obtained by LC fitting to the Ce3+ concentration in the bulk of the film, cfilm![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) bulk, we make use of the surface measurement of the single-crystal film. Although the nanocrystalline film includes a variety of terminations, our prior work has established that, under an environment similar to those employed here, the {100}, {110}, and {111} terminations exhibit similar extents of surface reduction.3 Thus, the surface reduction of the (100)-terminated single-crystal film, which was sufficiently smooth to enable surface sensitivity in the grazing incidence geometry, is a suitable proxy for surface reduction in the nanocrystalline film. The nanocrystalline film surface was too rough for direct characterization.
bulk, we make use of the surface measurement of the single-crystal film. Although the nanocrystalline film includes a variety of terminations, our prior work has established that, under an environment similar to those employed here, the {100}, {110}, and {111} terminations exhibit similar extents of surface reduction.3 Thus, the surface reduction of the (100)-terminated single-crystal film, which was sufficiently smooth to enable surface sensitivity in the grazing incidence geometry, is a suitable proxy for surface reduction in the nanocrystalline film. The nanocrystalline film surface was too rough for direct characterization.
The conversion of cfilm![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) effective to cfilm
effective to cfilm![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) bulk is achieved by approximating the Ce3+ concentration profile of the single-crystal film as a step function (roughly similar to observed surface reduction profiles, such as that of Turner et al.4) in which the surface is fully reduced (100% Ce3+) to a depth of
bulk is achieved by approximating the Ce3+ concentration profile of the single-crystal film as a step function (roughly similar to observed surface reduction profiles, such as that of Turner et al.4) in which the surface is fully reduced (100% Ce3+) to a depth of 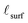 , while the Ce3+ level in the remainder of the film is cfilm
, while the Ce3+ level in the remainder of the film is cfilm![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) bulk. Our analysis (as detailed in the supplemental information) yields
bulk. Our analysis (as detailed in the supplemental information) yields 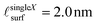 and csingleXfilm
and csingleXfilm![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) bulk = 3.6% in condition #2. While the true Ce3+ profile may differ from the step-function approximation, determination of cfilm
bulk = 3.6% in condition #2. While the true Ce3+ profile may differ from the step-function approximation, determination of cfilm![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) bulk was found to be relatively insensitive to the functional form of the profile. The derived csingleXfilm
bulk was found to be relatively insensitive to the functional form of the profile. The derived csingleXfilm![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) bulk value is in reasonable agreement with the expected Ce3+ concentration for bulk ceria at T = 845 °C and pO2 = 9.9 × 10−19 atm, 3.2%.24 Surface reduction in this 220 nm thick single-crystal film therefore accounts for a minor, but significant, portion of the full-film concentration, with a 23% difference between cfilm
bulk value is in reasonable agreement with the expected Ce3+ concentration for bulk ceria at T = 845 °C and pO2 = 9.9 × 10−19 atm, 3.2%.24 Surface reduction in this 220 nm thick single-crystal film therefore accounts for a minor, but significant, portion of the full-film concentration, with a 23% difference between cfilm![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) effective and cfilm
effective and cfilm![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) bulk. Assuming a reduced surface layer of the same thickness in the nanocrystalline CeO2 film (with a total thickness of 315 nm) under the same condition, we calculate the extent of reduction in the bulk of the film, cnanoXfilm
bulk. Assuming a reduced surface layer of the same thickness in the nanocrystalline CeO2 film (with a total thickness of 315 nm) under the same condition, we calculate the extent of reduction in the bulk of the film, cnanoXfilm![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) bulk, to be 9.0%, as compared to cnanoXfilm
bulk, to be 9.0%, as compared to cnanoXfilm![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) effective = 9.7%. In this case, surface reduction contributes less than 10% of the Ce3+ species in the entire film.
effective = 9.7%. In this case, surface reduction contributes less than 10% of the Ce3+ species in the entire film.
For treatment of the nanocrystalline film in the other two conditions, we make the plausible assumption that the fraction of the total Ce3+ concentration due to surface reduction is fixed. Because the correction to cnanoXfilm![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) effective to obtain cnanoXfilm
effective to obtain cnanoXfilm![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) bulk was found to be relatively small in condition #2, even moderate variations in the ratio of cfilm
bulk was found to be relatively small in condition #2, even moderate variations in the ratio of cfilm![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) effective to cfilm
effective to cfilm![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) bulk have only a small impact on the value of cfilm
bulk have only a small impact on the value of cfilm![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) bulk, introducing errors that lie within the overall uncertainty of the measurement.
bulk, introducing errors that lie within the overall uncertainty of the measurement.
The residuals from LC fitting to the XANES spectra, included in Fig. 2, are relatively small for the full-film measurements but notably large for the surface measurement (Fig. 2e) in which the Ce3+ concentration is large. The origin of this discrepancy is readily attributed to the difference between the coordination environment of Ce3+ within the fluorite crystal structure of ceria (coordination number 8) and that within the CeAlO3 reference (coordination number 12).26 This attribution is corroborated by the fact that the residuals across all five XANES spectra scale linearly with the fitted Ce3+ concentration (Fig. S6). The reported uncertainties in Ce3+ concentration account for this limitation of the LC fitting. We emphasize that the residuals (and hence uncertainties) are small for all nanocrystalline ceria measurements and note that the conversion of cnanoXfilm![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) effective to cnanoXfilm
effective to cnanoXfilm![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) bulk is largely insensitive to the precise value of the surface reduction within the uncertainty range obtained for this quantity.
bulk is largely insensitive to the precise value of the surface reduction within the uncertainty range obtained for this quantity.
The Ce3+ content in nanocrystalline ceria (cnanoXfilm![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) bulk), as compared to single-crystal or bulk ceria, is shown in Fig. 4 and Table 1 for all three conditions. In the conditions where no single-crystal measurement was performed, the expected bulk Ce3+ concentration for undoped ceria is taken from thermogravimetric analysis (TGA) data24 for the given T and pO2. In all three conditions, the results demonstrate a substantial enhancement of Ce3+ content in nanocrystalline ceria as compared to bulk ceria. Notably, the enhancement effect is not uniform for the three conditions. At 845 °C, grain boundary reduction increases roughly in proportion to bulk reduction by a factor between 2 and 2.5 in conditions #2–3, whereas at 615 °C (condition #1), at which little bulk reduction occurs (0.2% Ce3+), the enhancement factor is 11.
bulk), as compared to single-crystal or bulk ceria, is shown in Fig. 4 and Table 1 for all three conditions. In the conditions where no single-crystal measurement was performed, the expected bulk Ce3+ concentration for undoped ceria is taken from thermogravimetric analysis (TGA) data24 for the given T and pO2. In all three conditions, the results demonstrate a substantial enhancement of Ce3+ content in nanocrystalline ceria as compared to bulk ceria. Notably, the enhancement effect is not uniform for the three conditions. At 845 °C, grain boundary reduction increases roughly in proportion to bulk reduction by a factor between 2 and 2.5 in conditions #2–3, whereas at 615 °C (condition #1), at which little bulk reduction occurs (0.2% Ce3+), the enhancement factor is 11.
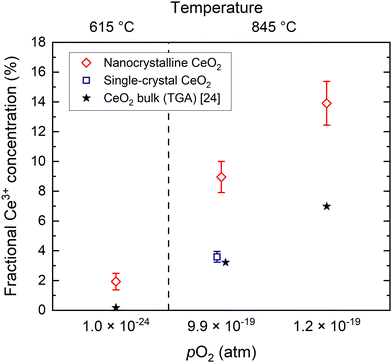 | ||
Fig. 4 Fractional Ce3+ concentrations (cfilm![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) bulk) determined by XANES in nanocrystalline and single-crystal CeO2 films under high-temperature reducing conditions. The expected equilibrium Ce3+ concentration for bulk CeO2 is calculated from (T, pO2) for each condition according to published TGA data.24 bulk) determined by XANES in nanocrystalline and single-crystal CeO2 films under high-temperature reducing conditions. The expected equilibrium Ce3+ concentration for bulk CeO2 is calculated from (T, pO2) for each condition according to published TGA data.24 | ||
To interpret grain boundary reduction in the nanocrystalline CeO2 film, we apply a form of the brick-layer model with fully reduced grain boundary regions, analogous to the treatment of surface reduction. The sample is represented by cubic grain interiors (side length 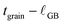 ), having the equilibrium bulk Ce3+ concentration (cCeO2
), having the equilibrium bulk Ce3+ concentration (cCeO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) bulk), that are separated by grain boundaries of width
bulk), that are separated by grain boundaries of width 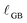 , with a Ce3+ concentration of 100%. The average crystallite size, tgrain, is obtained from XRD (Fig. 1). For condition #1 at 615 °C, where no grain growth is expected, we use the pre-experiment value, and for conditions #2 and #3 at 845 °C, where grain growth is assumed to be complete prior to the measurement, we use the post-experiment value. Based on the volume fraction of fully-reduced and bulk-like regions, we compute
, with a Ce3+ concentration of 100%. The average crystallite size, tgrain, is obtained from XRD (Fig. 1). For condition #1 at 615 °C, where no grain growth is expected, we use the pre-experiment value, and for conditions #2 and #3 at 845 °C, where grain growth is assumed to be complete prior to the measurement, we use the post-experiment value. Based on the volume fraction of fully-reduced and bulk-like regions, we compute 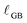 according to:
according to:
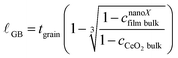 | (1) |
The value of cCeO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) bulk is taken from published thermogravimetric analysis (TGA) data24 for the given T and pO2, except in the case of condition #2, for which our measured csingleXfilm
bulk is taken from published thermogravimetric analysis (TGA) data24 for the given T and pO2, except in the case of condition #2, for which our measured csingleXfilm![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) bulk value is used. While the grain boundary reduction profile, as with the surface reduction profile, is unlikely to be a precise step function between partially and fully reduced ceria,
bulk value is used. While the grain boundary reduction profile, as with the surface reduction profile, is unlikely to be a precise step function between partially and fully reduced ceria, 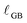 (and, analogously,
(and, analogously, 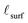 for surface reduction) serves to quantify area-normalized interfacial reduction regardless of the exact profile.
for surface reduction) serves to quantify area-normalized interfacial reduction regardless of the exact profile.
As summarized in Table 1, we find the width of grain boundary reduction 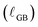 in nanocrystalline CeO2 to be 0.17 nm in condition #1, at which bulk reduction is lowest, 0.78 nm in condition #2, and 1.1 nm in condition #3, at which bulk reduction is greatest. Thus, while there are differences in grain size and hence grain boundary density, the grain boundary reduction trend follows the bulk reduction trend, though not with a uniform scaling factor. Furthermore, the results in condition #2 (845 °C, pO2 = 9.9 × 10−19 atm, cCeO2
in nanocrystalline CeO2 to be 0.17 nm in condition #1, at which bulk reduction is lowest, 0.78 nm in condition #2, and 1.1 nm in condition #3, at which bulk reduction is greatest. Thus, while there are differences in grain size and hence grain boundary density, the grain boundary reduction trend follows the bulk reduction trend, though not with a uniform scaling factor. Furthermore, the results in condition #2 (845 °C, pO2 = 9.9 × 10−19 atm, cCeO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) bulk = 3.6%) allow for direct comparison of grain boundary reduction to surface reduction. Ce3+ enrichment is observed at both types of interface, with the effect being approximately 2.5× as extensive at the (100) surface compared to the grain boundaries
bulk = 3.6%) allow for direct comparison of grain boundary reduction to surface reduction. Ce3+ enrichment is observed at both types of interface, with the effect being approximately 2.5× as extensive at the (100) surface compared to the grain boundaries 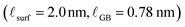 .
.
Discussion
The magnitude of grain boundary reduction in condition #1, while significantly lower than the values observed in conditions #2 and #3, is comparable to that reported under high vacuum at room temperature.13,14 EELS studies by Feng and Hojo et al. find that Ce3+ levels vary between grain boundary orientations, with some exhibiting zero reduction and others exhibiting roughly 10 to 40% Ce3+.13,14,25 Analysis of the three grain boundaries with nonzero Ce3+ content in ref. 13 indicates the spatial extent of Ce3+ reduction of these boundaries to be between approximately 0.1 and 0.3 nm.
with nonzero Ce3+ content in ref. 13 indicates the spatial extent of Ce3+ reduction of these boundaries to be between approximately 0.1 and 0.3 nm.
The fact that grain boundary reduction in condition #1 is not significantly higher than ex situ results, despite the much more strongly reducing environment in our measurement, is somewhat surprising. We speculate that Ce3+ enhancement at grain boundaries occurs at an approximately fixed baseline level over a wide range of conditions and becomes more extensive in conditions producing measurable bulk reduction, with an onset point spanned by the conditions of this study. In such a scenario, condition #1 produces only the baseline reduction. Analogous behavior appears to describe surface reduction in ceria, which occurs to an approximately fixed extent under a range of conditions—vacuum at room temperature,4–6 air at room temperature,2,3,6 and air at high temperature2—before increasing to a larger value at high temperature and low oxygen partial pressure.2,3 Under ex situ STEM conditions, Ce3+ enrichment near grain boundaries has been observed in conjunction with oxygen vacancies at low-coordination sites that are structurally necessary for certain grain boundary orientations.13 This behavior could account for the baseline level of grain boundary reduction. Generation of additional vacancies in the grain boundary region presumably requires removal of more stable oxygen species and thus corresponds to conditions which also drive measurable reduction in the bulk.
The results obtained here can also be considered in the context of the widely accepted space-charge model of ceria grain boundary behavior, in which a positively charged grain boundary core is balanced by a neighboring space charge region where oxygen vacancies are depleted and the Ce3+ concentration is enhanced. If a space charge model is applied to the present results, observation of such a large decrease in relative-to-bulk grain boundary reduction, despite the increase on an absolute basis, from condition #1 (615 °C) to conditions #2–3 (845 °C) would likely require a decrease in the space charge potential.
The community interest in ceria grain boundary reduction is largely driven by observations of high electronic conductivity in nanocrystalline ceria. In the absence of detailed knowledge of the Ce3+ concentration profile and the effective electronic mobility in the grain boundary region, it is impossible to predict with high confidence the conductivity expected for the nanoceria film studied here. Nevertheless, reasonable estimates of the conductivity enhancements in the three conditions can be made by the following approach. We first consider the measured Ce3+ concentration (cfilm![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) bulk) as if it is spatially uniform throughout the nanocrystalline film rather than concentrated near grain boundaries. From the measured cfilm
bulk) as if it is spatially uniform throughout the nanocrystalline film rather than concentrated near grain boundaries. From the measured cfilm![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) bulk values (Table 1) and the reported thermodynamic properties of ceria,24 we obtain equivalent oxygen partial pressures at which these cfilm
bulk values (Table 1) and the reported thermodynamic properties of ceria,24 we obtain equivalent oxygen partial pressures at which these cfilm![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) bulk values would be obtained: 2.9 × 10−29, 7.0 × 10−20, and 2.7 × 10−20 atm for conditions #1–3, respectively. We then estimate the conductivities at these implied conditions. The conductivity of undoped ceria in high-temperature reducing conditions follows a pO2−1/n power law behavior, where the value of n falls between 4 and 6 as explained by the defect chemistry.27 Taking n = 6, the experimentally observed value at the equivalent pO2 values, the increase in conductivity due to a decrease in oxygen partial pressure from the actual to equivalent values approaches 500% for condition #1. For conditions #2 and #3, however, it is much lower, approximately 50% and 25%, respectively. Thus, the greater differential between bulk and grain boundary reduction observed in condition #1 corresponds to a greater differential in conductivity as compared to the other two conditions. A more realistic spatial distribution of Ce3+, in which the Ce3+ concentration is enhanced only in the vicinity of grain boundaries, is treated in the SI and reveals that conductivity enhancement is expected to be even lower in such cases. This is due to the dependence of the electronic conductivity in ceria on Ce3+ concentration, which rises steeply at low Ce3+ concentrations before leveling out near the spatially-averaged concentrations in the film (cfilm
bulk values would be obtained: 2.9 × 10−29, 7.0 × 10−20, and 2.7 × 10−20 atm for conditions #1–3, respectively. We then estimate the conductivities at these implied conditions. The conductivity of undoped ceria in high-temperature reducing conditions follows a pO2−1/n power law behavior, where the value of n falls between 4 and 6 as explained by the defect chemistry.27 Taking n = 6, the experimentally observed value at the equivalent pO2 values, the increase in conductivity due to a decrease in oxygen partial pressure from the actual to equivalent values approaches 500% for condition #1. For conditions #2 and #3, however, it is much lower, approximately 50% and 25%, respectively. Thus, the greater differential between bulk and grain boundary reduction observed in condition #1 corresponds to a greater differential in conductivity as compared to the other two conditions. A more realistic spatial distribution of Ce3+, in which the Ce3+ concentration is enhanced only in the vicinity of grain boundaries, is treated in the SI and reveals that conductivity enhancement is expected to be even lower in such cases. This is due to the dependence of the electronic conductivity in ceria on Ce3+ concentration, which rises steeply at low Ce3+ concentrations before leveling out near the spatially-averaged concentrations in the film (cfilm![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) bulk) and ultimately decreasing with concentration above ∼25% Ce3+.28 Therefore, the enhancement factors reported above are taken as upper-bound estimates.
bulk) and ultimately decreasing with concentration above ∼25% Ce3+.28 Therefore, the enhancement factors reported above are taken as upper-bound estimates.
Comparison of these predictions to experimental studies of conductivity in nanocrystalline ceria are challenging due to differences in grain sizes, unknown impurity levels, and unknown substrate-film interfacial effects, which have led to a wide variation in the reported enhancement factors. Nevertheless, we note that Kosacki et al.20 report a 10× enhancement in thin films (several 100 nm in thickness, 30 nm grain size) at 600 °C and 10−24 atm pO2, whereas Göbel et al.22 report a ∼2× enhancement at 700 °C and 1 atm pO2 (films 50 to 200 nm in thickness, with mean grain sizes from 16 to 38 nm). These conductivities, with a very large enhancement under reducing conditions and a minimal enhancement under mild conditions, appear counter to the present analysis. On the other hand, Kogut et al.21 report a large enhancement (as measured from ∼2 µm thick films with mean grain size of 54 nm) under mild conditions, about a factor of 15 under air, which falls to effectively no enhancement at low pO2 for temperatures from 600 to 900 °C, a result that agrees with our predictions. While lateral conductivity measurements were precluded in our work due to the non-negligible conductivity of the Si substrate, the methodology developed here is well-suited for combined evaluation of Ce3+ concentration and conductivity using suitably insulating substrates.
Conclusions
By in situ XANES characterization of the cerium oxidation state, we report direct quantitative evidence of grain boundary reduction in ceria under high-temperature reducing conditions. Dense nanocrystalline CeO2 (with mean grain sizes in the 30 to 40 nm range) exhibits substantial enhancement in total concentration of the reduced Ce3+ species over that in single-crystal CeO2, where the enhancement factor, ranging from 2.0 to 11, decreases with increasing Ce3+ concentration in the bulk. The width of the reduced grain boundary region, using a simplified brick-layer model in which Ce near the boundary is entirely in the 3+ oxidation state, is calculated to be 0.17 nm, 0.78 nm, and 1.1 nm in each of the three conditions studied, from least to most bulk-reducing. Under the central condition, we also characterized the related phenomenon of surface reduction in single-crystal CeO2(100) and found it to be roughly 2.5× as extensive as grain boundary reduction. The diminishing enhancement factor for grain boundary reduction as bulk reduction increases is tentatively explained by the presence of a baseline concentration of structurally necessary oxygen vacancies at grain boundaries. Beyond this, additional vacancies in the grain boundary region are generated under redox conditions which also drive substantial bulk reduction. While electronic conductivity, generally observed to be enhanced by Ce3+ enrichment at grain boundaries, was not measured in this study, we predict that the conductivity enhancement factor decreases with increasing bulk reduction, in line with the factor of Ce3+ enhancement.Methods
The dense nanocrystalline CeO2 film, 315 nm in thickness as measured by ellipsometry, was grown on an Si(100) substrate by pulsed laser deposition (PLD) (KrF 248 nm excimer laser, 1.4 J cm−2 laser energy, 10 Hz repetition rate, room temperature, base pressure) and then annealed at 625 °C in air for 14 hours. The film is polycrystalline with a slight preferential orientation along (111) and an absence of (100)-oriented grains, as evidenced by specular X-ray diffraction (XRD) both before and after the XANES experiments (Fig. 1). Crystallite sizes, t, were determined from the full-width-half-maximum (FWHM) for each of the three observed XRD peaks, after subtracting instrumental broadening (FWHMinstrument = 0.22°, determined by XRD of 5 µm-sized CeO2 powder), by the Scherrer equation (K = 0.9).29 The effective grain size, tgrain, is calculated as the weighted average of the three t values, where the weights are determined by comparison of peak intensities to randomly-oriented CeO2. The analysis yields tgrain = 28.5 nm pre-experiment and tgrain = 41.4 nm post-experiment (grain growth due to elevated temperature in experiment). Following the XANES experiment, surface morphology was characterized by atomic force microscopy (AFM) (Fig. S1) and film density was assessed by X-ray reflectivity (XRR) (Fig. S2). The AFM root-mean-square roughness (1.6 nm) and the XRR density (99 ± 5% of theoretical) indicate that the CeO2 film is dense and non-porous, such that internal surfaces are not present in any consequential amount.The oriented single-crystal CeO2(100) film, 220 nm in thickness, was grown on a Y0.16Zr0.84O1.92 (yttria-stabilized zirconia, YSZ) (100) substrate by PLD (KrF 248 nm excimer laser, 1.7 J cm−2 laser energy, 10 Hz repetition rate, 625 °C, 15 mTorr O2) and then annealed at 950 °C in flowing oxygen for 2.5 hours. XRD shows the film to be fully (100)-oriented with high crystallinity (Fig. S3), and AFM shows the film surface to be sufficiently smooth (0.57 nm root-mean-square roughness) for the surface-sensitive XANES technique (Fig. S4).
XANES experiments were conducted at the DND-CAT 5-BM-D station at the Advanced Photon Source of Argonne National Laboratory. The incident X-ray beam energy was controlled by an Si(111) double-crystal monochromator with an energy resolution of ΔE/E = 1.4 × 10−4. Two Hitachi Vortex-ME4 silicon drift detectors, set at 90° relative to the beam path, were used to collect the Ce fluorescence signal which was divided by the incident beam intensity to yield the raw absorption data. Each Ce L3 XANES spectrum was normalized, by linear fitting to pre- and post-edge regions, to set the absorption edge height to unity. Gas and temperature conditions were controlled using an Anton Paar domed hot stage (DHS1100). Gas composition was 3.5% H2 (condition #1–2) or 10% H2 (condition #3), balance He, passed through liquid water held at 15 °C to establish light humidity (pH2O = 0.017). pO2 is determined by pH2O, pH2, and T according to the equilibrium of the water dissociation reaction at the sample surface.30
There is some subtlety in extracting Ce3+ concentrations from the XANES measurements. The fractional Ce3+ character of a given XANES spectrum, c′, is an average across all Ce absorption events in the sample and is inherently weighted toward low depths due to X-ray attenuation. In full-film XANES, at a relatively high incidence angle, the attenuation with depth is minimal compared to film thickness, and c′ is taken to equal the Ce3+ concentration across the entirety of the film, cfilm![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) effective. In surface XANES, the c′ value heavily reflects near-surface behavior and is interpreted using a more rigorous analysis to determine cfilm
effective. In surface XANES, the c′ value heavily reflects near-surface behavior and is interpreted using a more rigorous analysis to determine cfilm![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) bulk and
bulk and  (see SI). In either case, c′ values are determined by fitting experimental XANES data to a linear combination (LC) of reference spectra for Ce3+ (µCe3+(E)) and Ce4+ (µCe4+(E)):
(see SI). In either case, c′ values are determined by fitting experimental XANES data to a linear combination (LC) of reference spectra for Ce3+ (µCe3+(E)) and Ce4+ (µCe4+(E)):
| µLC(E) = (c′)µCe3+(E) + (1 − c′)µCe4+(E) | (2) |
To account for minor self-absorption effects, two additional fit parameters are included in the LC fitting (see SI). The complete expression (eqn (S4)) is fitted to the experimental XANES data in the energy range 5715 to 5750 eV by nonlinear least-squares regression. The resulting c′ values are obtained with uncertainty defined by their 95% confidence intervals. The reference spectra were collected at 800 °C using CeO2 in air (Ce4+ reference) and CeAlO3 in humidified hydrogen (Ce3+ reference).31–33
Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included as part of the supplementary information (SI). Supplementary information is available. See DOI: https://doi.org/10.1039/d5cp03733f. These data are also available at https://doi.org/10.17605/OSF.IO/JNKC3.Acknowledgements
This work was supported by the U.S. National Science Foundation (NSF) under award DMR-2130831. The XANES experiments were performed at the DuPont-Northwestern-Dow Collaborative Access Team (DND-CAT) located at Sector 5 of the Advanced Photon Source (APS). DND-CAT is supported by Northwestern University, The Dow Chemical Company, and DuPont de Nemours, Inc. This research used resources of the Advanced Photon Source, a U.S. Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02-06CH11357. This work made use of the Jerome B. Cohen X-ray Diffraction Core Facility (RRID:SCR_017866) and the Pulsed Laser Deposition Shared Facility (RRID:SCR_017889) at the Materials Research Center at Northwestern University, supported by the MRSEC program (NSF DMR-2308691) and the Soft and Hybrid Nanotechnology Experimental (SHyNE) Resource (NSF ECCS-2025633). This work made use of the SPID facility of Northwestern University's NUANCE Center, which has received support from the SHyNE Resource (NSF ECCS-2025633), the IIN, and Northwestern's MRSEC program (NSF DMR-2308691).References
- R. Schmitt, A. Nenning, O. Kraynis, R. Korobko, A. I. Frenkel, I. Lubomirsky, S. M. Haile and J. L. M. Rupp, Chem. Soc. Rev., 2019, 49, 554 RSC.
- W. Yuan, Q. Ma, Y. Liang, C. Sun, K. V. L. V. Narayanachari, M. J. Bedzyk, I. Takeuchi and S. M. Haile, J. Mater. Chem. A, 2020, 8, 9850 RSC.
- W. Yuan and S. M. Haile, MRS Commun., 2020, 1 Search PubMed.
- S. Turner, S. Lazar, B. Freitag, R. Egoavil, J. Verbeeck, S. Put, Y. Strauven and G. V. Tendeloo, Nanoscale, 2011, 3, 3385 RSC.
- X. Hao, A. Yoko, C. Chen, K. Inoue, M. Saito, G. Seong, S. Takami, T. Adschiri and Y. Ikuhara, Small, 2018, 14, 1802915 CrossRef PubMed.
- F. Zhang, P. Wang, J. Koberstein, S. Khalid and S.-W. Chan, Surf. Sci., 2004, 563, 74 CrossRef CAS.
- W. C. Chueh, A. H. McDaniel, M. E. Grass, Y. Hao, N. Jabeen, Z. Liu, S. M. Haile, K. F. McCarty, H. Bluhm and F. E. Gabaly, Chem. Mater., 2012, 24, 1876 CrossRef CAS.
- Z. A. Feng, F. E. Gabaly, X. Ye, Z.-X. Shen and W. C. Chueh, Nat. Commun., 2014, 5, 4374 CrossRef CAS PubMed.
- C. B. Gopal, F. E. Gabaly, A. H. McDaniel and W. C. Chueh, Adv. Mater., 2016, 28, 4692 CrossRef CAS PubMed.
- A. F. Zurhelle, X. Tong, A. Klein, D. S. Mebane and R. A. D. Souza, Angew. Chem., Int. Ed., 2017, 56, 14516 CrossRef CAS PubMed.
- P. Luches, L. Giordano, V. Grillo, G. C. Gazzadi, S. Prada, M. Campanini, G. Bertoni, C. Magen, F. Pagliuca, G. Pacchioni and S. Valeri, Adv. Mater. Interfaces, 2015, 2, 1500375 CrossRef.
- K. Song, H. Schmid, V. Srot, E. Gilardi, G. Gregori, K. Du, J. Maier and P. A. van Aken, APL Mater., 2014, 2, 032104 Search PubMed.
- B. Feng, I. Sugiyama, H. Hojo, H. Ohta, N. Shibata and Y. Ikuhara, Sci. Rep., 2016, 6, 20288 CrossRef CAS PubMed.
- H. Hojo, T. Mizoguchi, H. Ohta, S. D. Findlay, N. Shibata, T. Yamamoto and Y. Ikuhara, Nano Lett., 2010, 10, 4668 CrossRef CAS PubMed.
- Y. Lei, Y. Ito, N. D. Browning and T. J. Mazanec, J. Am. Ceram. Soc., 2002, 85, 2359 CrossRef CAS.
- S. Kim, J. Fleig and J. Maier, Phys. Chem. Chem. Phys., 2003, 5, 2268 RSC.
- H. J. Avila-Paredes, K. Choi, C.-T. Chen and S. Kim, J. Mater. Chem., 2009, 19, 4837 RSC.
- E. C. C. Souza, W. C. Chueh, W. Jung, E. N. S. Muccillo and S. M. Haile, J. Electrochem. Soc., 2012, 159, K127 CrossRef CAS.
- M. Chiang, E. B. Lavik, I. Kosacki, H. L. Tuller and J. Y. Ying, Appl. Phys. Lett., 1996, 69, 185 CrossRef.
- I. Kosacki, T. Suzuki, V. Petrovsky and H. U. Anderson, Solid State Ionics, 2000, 136, 1225 CrossRef.
- I. Kogut, C. Steiner, H. Wulfmeier, A. Wollbrink, G. Hagen, R. Moos and H. Fritze, J. Mater. Sci., 2021, 56, 17191 CrossRef CAS.
- M. C. Göbel, G. Gregori, X. Guo and J. Maier, Phys. Chem. Chem. Phys., 2010, 12, 14351 RSC.
- T. Montini, M. Melchionna, M. Monai and P. Fornasiero, Chem. Rev., 2016, 116, 5987 CrossRef CAS PubMed.
- R. J. Panlener, R. N. Blumenthal and J. E. Garnier, J. Phys. Chem. Solids, 1975, 36, 1213 CrossRef CAS.
- B. Feng, H. Hojo, T. Mizoguchi, H. Ohta, S. D. Findlay, Y. Sato, N. Shibata, T. Yamamoto and Y. Ikuhara, Appl. Phys. Lett., 2012, 100, 073109 CrossRef.
- Y. Takahashi, H. Sakami and M. Nomura, Anal. Chim. Acta, 2002, 468, 345 CrossRef CAS.
- Y.-P. Xiong, H. Kishimoto, K. Yamaji, M. Yoshinaga, T. Horita, M. E. Brito and H. Yokokawa, Electrochem. Solid-State Lett., 2010, 13, B21 CrossRef CAS.
- H. L. Tuller and A. S. Nowick, J. Phys. Chem. Solids, 1977, 38, 859 CrossRef CAS.
- U. Holzwarth and N. Gibson, Nat. Nanotechnol., 2011, 6, 534 CrossRef CAS PubMed.
- D. D. Wagman, J. E. Kilpatrick, W. J. Taylor, K. S. Pitzer and F. D. Rossini, J. Res. Natl. Bur. Stand., 1945, 34, 143 CrossRef CAS.
- S. L. Moffitt, Q. Ma, D. B. Buchholz, R. P. H. Chang, M. J. Bedzyk and T. O. Mason, J. Phys.: Conf. Ser., 2016, 712, 012116 CrossRef.
- P. Pfalzer, J.-P. Urbach, M. Klemm, S. Horn, M. L. DenBoer, A. I. Frenkel and J. P. Kirkland, Phys. Rev. B:Condens. Matter Mater. Phys., 1999, 60, 9335 CrossRef CAS.
- R. C. Lye, J. C. Phillips, D. Kaplan, S. Doniach and K. O. Hodgson, Proc. Natl. Acad. Sci. U. S. A., 1980, 77, 5884 CrossRef CAS PubMed.
| This journal is © the Owner Societies 2026 |