Systematic screening of transition metal dual-atom-doped phthalocyanine electrocatalysts for the oxygen reduction reaction
Xiaoyu
Li
 a,
Yongzhi
Wu
bc,
Qiuhe
Li
a,
Tingwei
Ma
a,
Xiaohua
Yu
a,
Yongzhi
Wu
bc,
Qiuhe
Li
a,
Tingwei
Ma
a,
Xiaohua
Yu
 *bc and
Guangyu
Li
*d
*bc and
Guangyu
Li
*d
aInstitution of Mechanical and Power Engineering, Yingkou Institute of Technology, Yingkou, Liaoning, China
bFaculty of Materials Science and Engineering, Kunming University of Science and Technology, Kunming, 115014, China. E-mail: xiaohua_y@163.com
cYunnan Key Laboratory of Integrated Com Materials Engineering for Advanced Light Metals, Kunming, 650093, China
dLiaoning Provincial Engineering Research Center for High-Value Utilization of Magnesite, Yingkou Institute of Technology, Yingkou, Liaoning, China. E-mail: 86046106@qq.com
First published on 1st December 2025
Abstract
Electrocatalysis is emerging as a promising technology for dealing with the energy crisis and environmental challenges. Phthalocyanines doped with two transition metal atoms (Tm2-Pc) show outstanding ORR catalytic activity and offer several catalytic sites. However, a systematic investigation of the ORR catalytic efficacy of Tm2-Pc monolayers with varying central metal atoms is lacking, hindering the establishment of design principles for this family of materials. Our research findings demonstrate that the d-band center, HOMO–LUMO, and adsorption energy are significant factors in the evaluation of catalysts. The ORR efficacy of Tm2-Pc is dependent on the interaction strength between the adsorbate and Tm2-Pc. With overpotentials of just 0.28 eV and 0.27 eV, respectively, Mn2-Pc and Ru2-Pc show good stability and outstanding catalytic activity. This work provides a theoretical foundation for the creation of high-performance catalysts by revealing the ORR catalytic mechanism of Tm2-Pc.
1. Introduction
The persistent increase in global energy demand, coupled with environmental damage caused by fossil fuels, has made the search for sustainable and clean energy alternatives a central focus of contemporary scientific research.1–3 Clean batteries are a significant method for mitigating environmental pollution.4,5 Nonetheless, identifying economical and consistently high-performing ORR catalytic materials for batteries continues to pose a considerable difficulty. While phthalocyanines doped with single transition metal atoms demonstrate commendable catalytic capability, their catalytic efficiency requires further examination.6,7 In contrast to single transition atom-doped Pc, Tm2-Pc offers a greater number of catalytic sites, thereby enhancing the ORR catalytic performance.8,9 The interactions and catalytic processes of Tm2-Pc in the ORR process are inadequately comprehended. Consequently, a methodical examination of the ORR catalysis of Tm2-Pc is crucial.To systematically elucidate the interactions and catalytic processes of Tm2-Pc in the ORR, one strategy is traditional experimental techniques.10–12 This approach initially synthesizes the Tm2-Pc materials and thereafter evaluates their ORR catalytic performance. Thoroughly evaluating the catalytic efficacy of many materials individually is time-consuming and yields data that are affected by experimental conditions, complicating the elucidation of the catalytic mechanism. An additional method is systematic screening that is performed using first principles.13–15 First-principles approaches provide microscopic insight into the interactions and catalytic processes in the oxygen reduction reaction. Simultaneously, systematic screening identifies the Tm2-Pc materials as exhibiting optimal ORR catalytic performance among a multitude of candidate materials. This approach offers a theoretical foundation for traditional tests while markedly reducing both time and cost.
Several pertinent studies have emerged about the catalytic efficacy of phthalocyanines doped with double transition metal center atoms.16–19 Wang et al.20 designed 18 varieties of heteronuclear diatomic Pc catalysts, of which FeTi@Pc has a limiting potential of −0.18 eV, enabling the reaction to proceed spontaneously. Mirshokraee et al.21 synthesized an iron-based Pc catalyst, revealing that the overpotential for the ORR is 0.93 eV. The findings by Ma et al.9 demonstrated that the Fe@Pc/Co@Pc substrates display superior catalytic performance for the ORR. Liao et al.8 synthesized Fe@Pc, Ni@Pc, and FeNi@Pc substrates, with the overpotential for the ORR reaction of FeNi@Pc measured at 0.85 eV. The aforementioned research results demonstrate that phthalocyanine doped with dual transition metal atoms displays superior electrocatalytic efficacy for the ORR. The transition state atoms have only been partially studied in the current studies, and it is as yet unknown how Tm2Pc interacts and catalyzes reactions.
This work utilizes systematic screening grounded in first principles to comprehensively evaluate prospective Tm2-Pcs, meticulously examining the interaction dynamics between Tm and Pc, and elucidating the catalytic processes of Tm2-Pc. The initial screening of the catalytic substrates was performed by evaluating the formation energy, adsorption energy, d-band center, density of states, and the ab initio molecular dynamics (AIMD) simulations. Subsequently, the catalytic mechanism of Tm2-Pc was examined through the examination of the reaction pathway and the volcano plot. Ultimately, the electronic interaction patterns of Tm2-Pc were revealed through Crystal Occupation Hamiltonian Population (COHP), differential charge density, molecular orbitals, and Bader charge analysis.
2. Computational method
The Vienna Ab initio Simulation Package (VASP), renowned for its efficacy in modeling catalytic materials, was utilized to conduct Density Functional Theory (DFT) calculations.22,23 The Perdew–Burke–Ernzerhof (PBE) variant of the generalized gradient approximation (GGA) was used to characterize the exchange–correlation function. Utilizing the projector-augmented wave method (PAW), the interaction between valence electrons and ions was described.24–27 Spin polarization was considered, and the van der Waals (vdW) interaction was corrected.28 The Brillouin region was sampled using a gamma-centered 3 × 3 × 1 k-point grid.29 For geometry optimizations, the convergence standard of energy and force was set to 10−6 eV and 0.01 eV Å−1 per atom, and a cutoff energy of 520 eV was applied for the plane-wave basis set.30 The thermal stability of the catalyst candidates was assessed using AIMD simulations conducted at 300 K for 5 ps, with a time step of 1 fs and temperature regulation implemented via the Nosé–Hoover technique.23,31 The COHP method, which is implemented by LOBSTER, was employed to analyze the electronic interaction mechanism.32,33 A Bader charge analysis was employed to quantitatively characterize the transfer of charge between the substrate and the adsorbates.34,35In acidic solution, the ORR occurs via a four-electron transfer mechanism, as illustrated below:35–37
| * + O2(g) + H+ + e− → OOH* | (1) |
| OOH* + H+ + e− → O* + H2O(l) | (2) |
| O* + H+ + e− → OH* | (3) |
| OH* + H+ + e− → H2O(l) + * | (4) |
The asterisk (*) denotes an active site on the catalyst surface, whereas O*, OH*, and OOH* represent the adsorbed intermediates. (l) and (g) imply the liquid and gas phases, respectively. The variation in free energy (ΔG) at each stage is quantified by the subsequent equation:38
| ΔG = ΔE + ΔEZPE − TΔS | (5) |
 | (6) |
The adsorption energy (Eads) of O2 on the transition metal atoms of the catalyst is a measure for assessing ability to adsorb. The Eads is represented by the subsequent formula:40
| Eads = E*O2 − EO2 − ETm2-Pc | (7) |
The formation energy (Eform) of Tm-Pc is displayed by the subsequent formula:41
| Eform = ETm2-Pc − n × ETm − EPc | (8) |
The dissolution potential (Udiss) of Tm-Pc is defined as follows:42,43
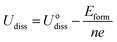 | (9) |
3. Results and discussion
3.1 High-throughput screening method
To identify high-performance and structurally stable catalysts, the catalytic effectiveness of 29 different Tm2-Pc structures was systematically evaluated. At the outset, Tm2-Pc compounds exhibiting superior ORR catalytic efficacy were identified, and their structural stability and O2 chemisorption were examined. Secondly, the electronic property of Tm2-Pc was assessed, followed by an examination of the catalytic performance, as illustrated in Fig. 1. Prior to screening Tm2-Pc, carcinogenic and radioactive transition elements, including Hg, Tc, Cd, and others, were first rejected.44,45 Furthermore, rare earth metals were omitted owing to the challenges associated with extraction and significant environmental contamination.46 Consequently, 26 transition elements were predominantly chosen as the atom center (Fig. 1). | ||
| Fig. 1 Screening process and candidate TM materials: (a) scheme of the workflow for high-throughput, and (b) TM candidate elements of TM-Pc. | ||
 | ||
| Fig. 2 Interaction rules of Tm2-Pc for the ORR: (a) adsorption energy of the candidate Tm2-Pc, and (b) d-band center and HOMO–LUMO of the candidate Tm2-Pc. | ||
A critical consideration is that when the d-band center exceeds zero, its effectiveness to donate electrons to the adsorbate diminishes, which is detrimental to the ORR catalytic process. Consequently, Tm2-Pc (where Tm represents Sc, Ti, V, Y, Zr, Hf, W) is ignored (Fig. 2(b)). A lower HOMO–LUMO band gap facilitates electron transfer, whereas a larger band gap hinders the reaction,52–54 thereby excluding Tm (Tm = Ni, Rh, Pd, Os, Ir, Pt)-Pc. The primary focus is on four candidate catalysts, namely Tm (Tm = Mn, Fe, Co, Ru)-Pc, which are excluded due to their unfavorable performance in promoting the ORR, as determined from the adsorption energy, d-band center, and HOMO–LUMO analysis of the Tm2-Pc.
 | ||
| Fig. 3 The stability evaluation of Tm2-Pc: (a)–(d) PDOS and AIMD of Mn2-Pc, Fe2-Pc, Co2-Pc and Ru2-Pc. | ||
Electron transfer and orbital hybridization are the primary mechanisms that control the binding stability between O2 and Tm2-Pc. The PDOS of the chosen Tm2-Pc was computed to examine the orbital hybridization and electron transport of the catalyst. Additionally, the PDOS of Tm2-Pc is displayed in Fig. 3. A pronounced peak is observed at the Fermi level (Ef) for Co2-Pc, signifying metallic characteristics. At this location, the Co 3d peak is stronger than the N 2p peak, indicating that the Co is the main contributor to the conductivity of Co2-Pc. Mn2-Pc, Fe2-Pc, and Ru2-Pc display tight spacing at the Ef, with the 3d peaks of the transition metals (Tm = Mn, Fe, and Ru) being more pronounced than the 2p peaks of nitrogen, indicating that the conductivity of Tm2-Pc is predominantly attributed to the transition metals. The Tm central atoms improve the conductivity of Tm2-Pc, which facilitates charge transfer for the ORR catalytic reaction, as evidenced by the density of states of Mn2-Pc, Co2-Pc, Fe2-Pc, and Ru2-Pc. The investigation of the structural and binding stability of Tm2-Pc established a basis for subsequent research on ORR catalysis.
3.2 Analysis of ORR catalytic performance
Following the examination of the structural stability of Tm2-Pc, the catalytic performance for the ORR was further evaluated. In the four-step ORR reaction, the catalytic efficacy of Tm2-Pc is contingent upon variations in the free energy of the intermediates (*OOH, *O, *OH).55 The free energy of the pathways for Tm2 (Tm = Mn, Co, Fe, and Ru)-Pc is shown in Fig. 4. Under 0 eV conditions, the free energy diminishes incrementally as the ORR reaction advances. Overpotential is a critical parameter for assessing the catalytic efficacy of the ORR and can be derived for Tm2-Pc utilizing formula (6). The *O2 to *OOH transition for Mn2-Pc has a free energy change of 0.95 eV. Thus, 0.28 eV is the overpotential for the ORR reaction on Mn2-Pc. In the cases of Fe2-Pc, Co2-Pc, and Ru2-Pc, the rate-limiting step transpires during the conversion of *O2 to *OOH, exhibiting overpotentials of 0.78 eV, 0.88 eV, and 0.27 eV, respectively. The precious metal Pt demonstrates exceptional ORR catalytic capability, characterized by an overpotential of 0.79 eV.36 The overpotentials of Fe2-Pc and Co2-Pc approximate that of Pt; however, the overpotentials of Mn2-Pc and Ru2-Pc are markedly lower than that of Pt, rendering them excellent catalysts for the ORR. | ||
| Fig. 4 Free energy diagram for the ORR and HER pathways: (a)–(d) Mn2-Pc, Fe2-Pc, Co2-Pc, and Ru2-Pc. | ||
At an applied potential of 1.23 V, the ORR continues to proceed, without a progressive decline in free energy. The free energy landscape shows simultaneous energy-increasing and energy-decreasing steps. This indicates that energy is necessary to surpass the positive free energy. The free energy for Co2-Pc gradually rises from *O2 to *O, whereas the other free energies initially rise and then progressively fall. The d-band center, HOMO–LUMO gap, and density of states are compared. The upward shift of the d-band centre, which increases the Co 3d states at the Fermi level, together with the enlarged HOMO–LUMO gap that limits electron transfer, results in a net decrease in ORR activity for Co2-Pc.
The hydrogen adsorption energy of Co2-Pc in the hydrogen evolution reaction (HER) is markedly superior to that of the other Tm2-Pc, influenced by the d-band center and the HOMO–LUMO gap (Fig. 4). Notably, the ORR and the HER are competing processes in aqueous electrochemical environments.56 The performance trends for both reactions, as shown in Fig. 4, can be rationalized by a unified electronic structure perspective. Catalysts like Fe2-Pc and Co2-Pc, with their d-band centers positioned closer to the Fermi level, exhibit excessively strong adsorption energies for various intermediates. This is evidenced by their high hydrogen adsorption free energies (ΔGH) of 0.40 eV and 0.54 eV, respectively. This strong H adsorption not only leads to moderate HER activity but, more importantly, reduces the availability of active sites for O2 adsorption and the subsequent ORR steps, thereby compromising the overall ORR efficiency. In contrast, Mn2-Pc and Ru2-Pc, identified as our top ORR performers, possess lower ΔGH values (0.25 eV and 0.35 eV, respectively). This indicates weaker H adsorption, which minimizes site-blocking and favors the ORR pathway. This interplay confirms that an optimal electronic structure—one that affords moderate adsorption of ORR intermediates while suppressing competitive H* binding—is key to achieving high ORR selectivity and activity. In conclusion, Mn2-Pc and Ru2-Pc exhibit optimal electronic characteristics and appropriate adsorption energies, exhibiting superior electrocatalytic efficacy.
In order to conduct a more thorough examination of the catalytic mechanism of Tm2-Pc, a volcano plot analysis was implemented. Volcano plots are commonly employed as a method for screening high-performance ORR catalysts. The Tm2-Pc situated near the summit of the volcano demonstrates superior catalytic performance, but the catalytic performance at the base of the volcano is somewhat worse. Fig. 5(a) illustrates the volcano plot of ΔGOH* in relation to the overpotential ηORR. As can be seen, Mn2-Pc and Ru2-Pc are both found near the summit of the volcano plot, suggesting low overpotential and superior electrocatalytic activity. In contrast, Co2-Pc and Fe2-Pc are found at the middle of the slope, where the ORR catalysis is weaker and the overpotential rises. At the base of the mountain, Pd2-Pc and Pt2-Pc exhibit extremely low catalytic activity.
 | ||
| Fig. 5 Volcano curve and 2D volcano diagram: (a) the relationship of −ηORRvs ΔGOH*, and (b) the function of the limiting overpotentials and the descriptors of ΔGO* and ΔGOH*. | ||
The limiting overpotential analysis is a critical parameter due to the fact that the overall catalytic rate of the reaction is influenced by the stability and catalytic activity of the ORR intermediate (*O). The 2D colored contour volcano plots of ΔGO* and ΔGOH* were produced based on the correlation between ΔGOOH* and ΔGOH* (Fig. S3). Mn2-Pc and Ru2-Pc exhibit the lowest limiting overpotentials, signifying a diminished adsorption strength of O*, therefore promoting the spontaneous transformation of the intermediate from O* to OH*, in accordance with Fig. 5(b). The integration of the pathways and contour volcano plots elucidates that the rate-determining step for Mn2-Pc and Ru2-Pc is the initial step. For Tm-Pc ORR catalysts, Mn exhibits catalytic efficiency comparable to that of the precious metal Ru, reducing reliance on precious metals and minimizing economic expenditures.
3.3 Analysis of electronic characteristics
The volcano plot examines the catalytic efficiency of Tm2-Pc by correlating adsorption energy with overpotential. The alteration in electrical structure works as the intrinsic impetus that triggers the response. Consequently, a further thorough study of the electrical characteristics of Tm2-Pc was undertaken. The projected density of the free O2 molecule and the Mn atom in Mn2-Pc was examined (Fig. S4). At the Fermi level, there exists substantial hybridization between the d orbitals of Mn atoms and the p orbitals of O2. Additionally, the projected density of states for Mn2-Pc and the schematic representation of the occupancy of the Mn 3d orbitals were investigated (Fig. 6(a)). d–π* orbitals that are partially occupied are the result of the coupling of Mn's d orbitals with O2's π* orbitals in the β spin. α spin and β spin have similar patterns. The results demonstrate that O2 can adsorb onto the Mn2-Pc substrate, hence promoting the efficient advancement of the ORR reaction. In comparison to Ru2-Pc, Fe2-Pc, and Co2-Pc, the orbital occupancy of Mn2-Pc and Ru2-Pc is markedly greater than that of Fe2-Pc and Co2-Pc, rendering it more advantageous for the adsorption of adsorbates.The adsorption ability of Tm2-Pc on O2 was investigated by analyzing the COHP between Mn and O atoms. COHP disaggregates the electronic state energy into atomic interactions, measuring bond strength. A negative integrated COHP value (ICOHP) signifies bonding, whereas a positive value denotes antibonding. The magnitude of the number indicates the intensity of the bonding or antibonding interaction. The ICOHP of O2 adsorbed on Mn2-Pc is −0.327 eV, signifying that the O2 molecule is activated and adsorbed by Mn2-Pc (Fig. 6(b)). The results are consistent with those reported by other researchers,39,57 further indicating a weakening of the bonding interactions within the O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond upon O2 adsorption. This observation suggests the formation of chemical bonds between O2 and the Tm2-Pc substrates. A diminished value enables the release of O atoms from the Mn2-Pc substrate, improving the sequential stages of the four-step ORR reaction. The intermediates of the four-step ORR (*OOH, *O, *OH) demand charge transfer, and the differential charge quantifies the magnitude of this charge transfer. During the formation of the *OOH, *O, and *OH intermediates, the Mn transfers 0.96 eV, 0.61 eV, and 0.42 eV to the adsorbates, respectively (Fig. 6(c)). Significant charge transfer occurs during the ORR process, indicating an “acceptance–donation” mechanism.58,59 This mechanism is characterized by charge accumulation on the Tm2-Pc substrate and concurrent charge depletion on the O atoms. The transfer of charge supplies the energy required for the ORR reaction to take place. The research elucidates the electronic properties of Tm2-Pc, offering theoretical justification for the synthesis of Tm2-Pc with superior ORR catalytic performance.
O bond upon O2 adsorption. This observation suggests the formation of chemical bonds between O2 and the Tm2-Pc substrates. A diminished value enables the release of O atoms from the Mn2-Pc substrate, improving the sequential stages of the four-step ORR reaction. The intermediates of the four-step ORR (*OOH, *O, *OH) demand charge transfer, and the differential charge quantifies the magnitude of this charge transfer. During the formation of the *OOH, *O, and *OH intermediates, the Mn transfers 0.96 eV, 0.61 eV, and 0.42 eV to the adsorbates, respectively (Fig. 6(c)). Significant charge transfer occurs during the ORR process, indicating an “acceptance–donation” mechanism.58,59 This mechanism is characterized by charge accumulation on the Tm2-Pc substrate and concurrent charge depletion on the O atoms. The transfer of charge supplies the energy required for the ORR reaction to take place. The research elucidates the electronic properties of Tm2-Pc, offering theoretical justification for the synthesis of Tm2-Pc with superior ORR catalytic performance.
4. Conclusion
Significant challenges remain in the pursuit of clean energy to mitigate the energy crisis and environmental damage. Due to the variety of catalytic sites, Tm2-Pc offers considerable advantages in ORR electrocatalysis. Nonetheless, the fundamental principles and catalytic processes of Tm2-Pc remain to be thoroughly investigated. This work utilizes a first-principles approach based on systematic screening to methodically investigate the ORR catalytic processes of Tm2-Pc. The results indicate that among several Tm2-Pcs, Mn2-Pc and Ru2-Pc demonstrate both structural and binding stability, as well as outstanding ORR catalytic performance, with overpotentials of merely 0.27 eV and 0.28 eV, respectively. This work offers a theoretical foundation and assistance for the development of highly efficient catalysts.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Data availability
All data have been included in the supplementary information (SI). See DOI: https://doi.org/10.1039/d5cp03639a.Acknowledgements
This research was supported by Yingkou Institute of Technology (YJRC202401), the National Natural Science Foundation of China (52462009, 52402077), and the Funds Project of Liaoning Provincial Engineering Research Center for High-Value Utilization of Magnesite (LMNKY202306).References
- J. H. Li, Z. X. Li and Q. H. Sun, Adv. Energy Mater., 2024, 14, 2402441 CrossRef CAS
.
- M. H. Ning, S. N. Wang and J. Wan, Angew. Chem., Int. Ed., 2024, 63, e202415794 CrossRef CAS PubMed
.
- M. V. Jyothirmai, R. Dantuluri and P. Sinha, ACS Appl. Mater. Interfaces, 2024, 16, 12437–12445 CrossRef CAS PubMed
.
- L. Li, M. Han and P. G. Zhang, ChemSusChem, 2025, 18, e202401186 CrossRef CAS PubMed
.
- M. Tamtaji, M. G. Kim and Z. M. Li, Nano Energy, 2024, 126, 109634 CrossRef CAS
.
- A. Kumar, G. Zhang and W. Liu, J. Electroanal. Chem., 2022, 922, 116799 CrossRef CAS
.
- C. X. Huang, G. Li and L. M. Yang, ACS Appl. Mater. Interfaces, 2021, 13, 608–621 CrossRef CAS PubMed
.
- Z. J. Liao, Y. L. Wang and Q. L. Wang, Appl. Catal., B, 2019, 243, 204–211 CrossRef CAS
.
- Y. Ma, J. T. Li and X. B. Liao, Adv. Funct. Mater., 2020, 30, 2005000 CrossRef CAS
.
- N. Komba, G. X. Zhang and Q. Wei, Int. J. Hydrogen Energy, 2019, 44, 18103–18114 CrossRef CAS
.
- S. Shi, J. Yang and L. Chen, J. Electrochem. Soc., 2024, 171, 046504 CrossRef CAS
.
- X. Meng, P. Yu and M. Zhang, Molecules, 2024, 29, 4272 CrossRef CAS PubMed
.
- F. Rehman, S. Kwon and C. B. Musgrave, Nano Energy, 2022, 103, 107866 CrossRef CAS
.
- G. Hai, X. Xue, Z. Wu and C. Zhang, J. Energy Chem., 2024, 91, 194–202 CrossRef CAS
.
- Q. Zhou, F. Gong and Y. Xie, Green. Energy. Environ., 2023, 8, 1753–1763 CrossRef CAS
.
- Q. Q. An, X. P. Qin and Q. H. Liu, JACS, 2025, 147(13), 11465–11476 CrossRef CAS PubMed
.
- Y. Che, J. Shang and X. Chen, At. Force Microsc., 2025, 2507544 CAS
.
- S. W. Bo, X. X. Zhang and X. Chen, Nat. Commun., 2025, 16, 2483 CrossRef CAS PubMed
.
- S. Yang, Y. Yu and X. Gao, Chem. Soc. Rev., 2021, 50, 12985–13011 RSC
.
- Z. Wang, A. Ma and Z. Liu, Appl. Surf. Sci., 2024, 669, 160532 CrossRef CAS
.
- S. A. Mirshokraee, M. Muhyuddin and J. Orsilli, Nanoscale, 2024, 16, 6531–6547 RSC
.
- G. Kresse and J. Furthmüller, Comput. Mater. Sci., 1996, 6, 15–50 CrossRef CAS
.
- G. Kresse and J. Hafner, Phys. Rev. B: Condens. Matter Mater. Phys., 1993, 47, 558–561 CrossRef CAS PubMed
.
- P. E. Blöchl, Phys. Rev. B: Condens. Matter Mater. Phys., 1994, 50, 17953–17979 CrossRef PubMed
.
- G. Kresse and J. Furthmüller, Phys. Rev. B: Condens. Matter Mater. Phys., 1996, 54, 11169–11186 CrossRef CAS PubMed
.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865–3868 CrossRef CAS PubMed
.
- G. Kresse and D. Joubert, Phys. Rev. B: Condens. Matter Mater. Phys., 1999, 59, 1758–1775 CrossRef CAS
.
- S. Grimme, J. Comput. Chem., 2006, 27, 1787–1799 CrossRef CAS PubMed
.
- H. J. Monkhorst and J. D. Pack, Phys. Rev. B, 1976, 13, 5188–5192 CrossRef
.
- X. Li, Z. Wang and Z. Su, ChemPhysMater, 2022, 1, 237–245 CrossRef CAS
.
- S. Nosé, J. Chem. Phys., 1984, 81, 511–519 CrossRef
.
- R. Dronskowski and P. E. Bloechl, J. Phys. Chem., 1993, 97, 8617–8624 CrossRef CAS
.
- S. Maintz, V. L. Deringer and A. L. Tchougréeff, J. Comput. Chem., 2016, 37, 1030–1035 CrossRef CAS PubMed
.
- G. Henkelman, A. Arnaldsson and H. Jónsson, Comput. Mater. Sci., 2006, 36, 354–360 CrossRef
.
- J. K. Nørskov, T. Bligaard and A. Logadottir, J. Electrochem. Soc., 2005, 152, J23 CrossRef
.
- Y. Li, J. Tan and X. Zhao, Mater. Today Commun., 2023, 37, 107157 CrossRef CAS
.
- M. D. Cueto, P. Ocón and J. M. L. Poyato, J. Phys. Chem. C, 2015, 119, 2004–2009 CrossRef
.
- J. K. Nørskov, J. Rossmeisl and A. Logadottir, J. Phys. Chem. B, 2004, 108, 17886–17892 CrossRef PubMed
.
- S. Wang, K. Meng and L. Qin, Int. J. Hydrogen Energy, 2024, 88, 850–857 CrossRef CAS
.
- X. Chen, J. Tan and M. Leng, Int. J. Hydrogen Energy, 2022, 47, 17611–17620 CrossRef CAS
.
- Y. Chen, P. Zhang and X. Liu, Int. J. Hydrogen Energy, 2024, 62, 673–683 CrossRef CAS
.
- X. Y. Guo, J. X. Gu and S. R. Lin, JACS, 2020, 142, 5709–5721 CrossRef CAS PubMed
.
- K. H. Yeoh, Y. H. Chang and H. W. Yu, ACS Appl. Electron. Mater., 2025, 7, 3521–3528 CrossRef CAS
.
-
D. Watson, Food chemical safety: volume 1: contaminants, Woodhead Publishing, 2001 Search PubMed
.
- S. J. Mulware, 3 Biotech, 2013, 3, 85–96 CrossRef PubMed
.
- V. Balaram, Geosci. Front., 2019, 10, 1285–1303 CrossRef CAS
.
- L. Lin, Y. Sun and K. Xie, Phys. Chem. Chem. Phys., 2023, 25, 15441–15451 RSC
.
- N. Ma, Y. Wang and Y. Zhang, Appl. Surf. Sci., 2022, 596, 153574 CrossRef CAS
.
- L. Ou, F. Yang and Y. Liu, J. Phys. Chem. C, 2009, 113, 20657–20665 CrossRef CAS
.
- S. Jiao, X. Fu and H. Huang, Adv. Funct. Mater., 2022, 32, 2107651 CrossRef CAS
.
- Q. Zhu, W. Huang and C. Huang, Int. J. Hydrogen Energy, 2022, 47, 38445–38454 CrossRef CAS
.
- N. Blankevoort, P. Bastante and R. J. Davidson, ACS Omega, 2024, 9, 8471–8477 CAS
.
- Y. Gao, X. Xu and Y. Niu, Nano Res., 2023, 16, 12936–12941 CrossRef CAS
.
- Y. Li, Y. Feng and D. Zheng, Chem. Eng. J., 2023, 476, 146753 CrossRef CAS
.
- I. C. Man, H. Y. Su and V. F. Calle, ChemCatChem, 2011, 3, 1159–1165 CrossRef CAS
.
- P. F. Liu, Z. R. Zhang and X. H. Liu, Int. J. Hydrogen Energy, 2025, 101, 212–221 CrossRef CAS
.
- Y. Yao, S. Y. Lv and G. Li, Phys. Chem. Chem. Phys., 2023, 25, 27131–27141 CAS
.
- S. Cao, H. Chen and X. Wei, Carbon, 2024, 225, 119094 CAS
.
- P. Xue, X. Zhao and D. Zheng, Comput. Mater. Sci., 2024, 234, 112778 CAS
.
| This journal is © the Owner Societies 2026 |

