Open Access Article
Open Access ArticleN-Aryl substituents have an influence on the photophysics of tetraaryl-pyrrolo[3,2-b]pyrroles
Wojciech D. Petrykowski a,
Marzena Banasiewicz
a,
Marzena Banasiewicz b,
Olaf Morawski
b,
Olaf Morawski b,
Zbigniew Kałużaa,
Cristina A. Barboza
b,
Zbigniew Kałużaa,
Cristina A. Barboza *b and
Daniel T. Gryko
*b and
Daniel T. Gryko *a
*a
aInstitute of Organic Chemistry, Polish Academy of Sciences, Kasprzaka 44-52, 01-224 Warsaw, Poland. E-mail: dtgryko@icho.edu.pl
bInstitute of Physics, Polish Academy of Sciences, Al. Lotników 32/46, 02-668 Warsaw, Poland. E-mail: crissetubal@ifpan.edu.pl
First published on 10th December 2025
Abstract
The photophysics of two series of 1,4-dihydro-tetraaryl-pyrrolo[3,2-b]pyrroles possessing N-aryl substituents with various electronic characteristics was investigated systematically. The molecular structure of these compounds was designed so that their solubility enabled us to study their absorption and emission in a broad range of solvents. The presence of N-4-nitrophenyl substituents is responsible for a weak charge-transfer absorption band and shifts the emission band hypsochromically. At the same time, their presence quenches fluorescence; although if electron-withdrawing substituents are present at positions 2 and 5, this effect is reduced by an order of magnitude. In the case of less electron-withdrawing N-4-cyanophenyl and N-3-cyanophenyl groups, strong emission is present only if the electron-withdrawing groups are located at positions 2 and 5. The combined experimental and computational study points out the existence of a barrier between au (bright) and ag (dark) CT states, the height of which is the key factor governing the fate of these molecules in the excited state. Weaker electronic communication at positions 1 and 4 of the DHPP core is responsible for strong charge separation. Polar solvents favor the formation of transient dipole moments due to excited-state symmetry-breaking, which amplifies the nonradiative deactivation of nitro-TAPPs. A large increase in fluorescence intensity at 77 K suggests that internal conversion is a key channel for non-radiative electronic relaxation. Conversely, moderate to weak electron-donating groups favor strong LE emission.
Introduction
The potential of 1,4-dihydropyrrolo[3,2-b]pyrroles (DHPPs), originally discovered by Hemetsberger and Knittel in 1972,1 was negatively impacted by their poor synthetic accessibility. This situation abruptly changed in 2012, when tetraaryl-pyrrolo[3,2-b]pyrroles (TAPPs) became easily available.2 Given the broad scope of substrates tolerated by the discovered multicomponent reaction,3 the TAPPs became a favorite building block to assemble N-doped non-planar nanographenes,4,5 and to study excited-state symmetry breaking (ES-SB),6,7 as well as the perplexing fluorescence of nitroaromatics.8,9 These studies predominantly focused on the impact of substitution at the carbon atoms of centrosymmetric TAPPs, due to their acceptor–donor–acceptor (A–D–A) configuration, which promotes the population of charge-separated states. These are crucial for applications in organic electronics.9–12 Numerous studies have explored the photophysics of TAPPs and their π-expanded analogs through both experimental and theoretical approaches.8–17Despite their ground-state quadrupolar nature, DHPPs are susceptible to ES-SB, inducing transient dipole moments that explain the solvatochromism experimentally observed in the absorption spectra.13,14 The photophysical versatility of DHPP derivatives is further evidenced by their ability to form charge-transfer states sensitive to solvent polarity. This effect is observed when the chromophore is bonded to polycyclic heteroaromatic molecules forming double helical systems, even in the absence of strong donor or acceptor groups bonded to the main dye.15
Interestingly, the presence of 4-NO2C6H4 at positions 2 and 5 highlights how the interplay between intersystem crossing (ISC) and IC affects the fluorescence efficiency of bis-nitro-DHPPs. These molecules show exceptionally large fluorescence quantum yields in non-polar solvents due to slow ISC and weak electronic coupling between nitrophenyl substituents and the main chromophore.8 Analogous studies reveal that these systems exhibit complex deactivation dynamics, governed by D–A electronic coupling, intra/intermolecular interactions, solvent effects, and molecular symmetry – all of which affect ISC and competing decay pathways.9,14,16,18,19 For instance, electronic coupling induced by electron-withdrawing groups in DHPP is highly sensitive to positional isomerism (e.g., 4-NO2 and 3-NO2) and to the presence of aryl spacers between D–A subunits, whose fine tuning can enhance the fluorescence of nitro-TAPPs.8,20–22 Because these compounds are susceptible to ES-SB, their deactivation channel strongly depends on solvent polarity/proticity.6–8,23 Interestingly, the inverse correlation between fluorescence quantum yields and the N-aryl torsion angle in bis-nitro-DHPPs arises from the increased charge separated state, which quenches their fluorescence.8 In addition, structural distortion controls the ISC/IC non-radiative decay interplay, with planar geometries promoting IC through the suppression of S1–T1 spin–orbit coupling (SOC).9
In the broad portfolio of structural effects affecting the photophysics of these dyes, one aspect has so far been neglected, i.e. the influence of the electronic character of the substituent linked to the DHPP core through the pyrrolic nitrogen atom. Scattered observations have revealed that, for example, the presence of an N-4-nitrophenyl substituent causes quenching of emission as well as a strong bathochromic shift in absorption.2 However, these random findings lack any systematic effect in the structure, which hampers the analysis of data. The goal of this paper is to design, synthesize and study the systematic series of TAPPs possessing various substituents at positions 1, 2, 4, and 5 and to attempt to elucidate the effect of these structural changes on absorption and emission. More specifically, we would like to investigate the influence of N-aryl substituents on the photophysics of TAPPs.
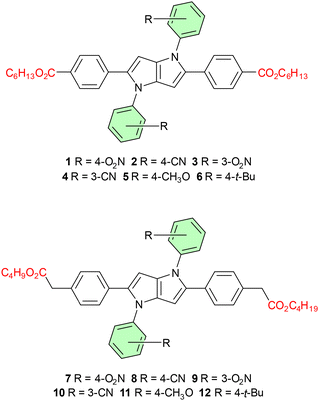
Design and synthesis
To rationalize the influence of different motifs showing distinct polarized effects at positions 1 and 4, we designed two sets of TAPPs varied at positions 2 and 5 (Fig. 1). In both sets, the N-aryl substituents are the same, and they provide a wide range of electronic effects influencing the DHPP core, i.e. 4-nitrophenyl, 3-nitrophenyl, 4-cyanophenyl, 3-cyanophenyl, 4-methoxyphenyl and 4-tert-butylphenyl. Compounds 1–6 and 7–12 differ in the electronic character of substituents at positions 2 and 5. Dyes 1–6 bear 4-(CO2C6H13)C6H4 substituents as the electron-acceptors forming a clear quadrupolar A–D–A architecture, while in the second series (dyes 7–12), 2,5-substituents possess the electron-neutral character. These substituents possess however the ester group electronically separated from the benzene ring, in order to secure suitable solubility in a broad range of solvents. All the products are easily accessible by the multicomponent reaction between an aromatic amine, an aromatic aldehyde and butanedione, discovered in our group, giving the desired TAPPs in 5–41% yields.2,24 Long alkyl chains attached to the aldehyde substrates are necessary to ensure sufficient solubility of the final products in organic solvents and can be easily introduced by carboxylate alkylation. This approach, so far unprecedented in the field of TAPPs,25 allows adjusting the solubility by using alkyl halides of various lengths.Photophysical properties
We measured the absorption and emission spectra and fluorescence quantum yields for dyes 1–12 in several solvents with a wide range of polarity to further examine this effect (Table 1, Fig. 2–4). What first catches the eye is that generally, electron-withdrawing groups in positions 1 and 4 shift the main absorption band hypsochromically, while, as we have shown previously, analogous substituents at positions 2 and 5 have the opposite effect.2,8| TAPP | Solvent | λabs/nm | ε × 103 (cm−1 M−1) | λem/nm | Δ ![[small nu, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0ce.gif) (cm−1) (cm−1) |
Φf |
|---|---|---|---|---|---|---|
| Quinine sulphate as a standard. | ||||||
| 1 | Hexane | 353 | — | 514 | 8900 | 0.031 |
| Toluene | 364 | 57.8 | 450, 640 | 5300 | 0.0017 | |
| Propyl butyrate | 363 | 49.9 | 428, 700 | 4200 | 0.0005 | |
| THF | 367 | 62.9 | — | — | 0.00017 | |
| CH2Cl2 | 368 | 68.1 | 461 | 5500 | 0.00011 | |
| Acetonitrile | 365 | 14.1 | 434, 550 | 4400 | 0.00003 | |
| DMF | 373 | 61.6 | — | — | — | |
| DMSO | 376 | 20.4 | 489 | 6100 | 0.00012 | |
| 2 | Hexane | — | — | — | — | |
| Toluene | 393 | 24.6 | 447 | 3100 | 0.77 | |
| Propyl butyrate | 391 | — | 448 | 3300 | 0.79 | |
| THF | 394 | — | 454 | 3400 | 0.83 | |
| CH2Cl2 | 393 | — | 455 | 3500 | 0.85 | |
| Acetonitrile | 389 | — | 469 | 4400 | 0.41 | |
| DMF | 398 | — | 473 | 4000 | 0.84 | |
| DMSO | 400 | — | 478 | 4100 | 0.77 | |
| 3 | Hexane | 376 | — | 552 | 8500 | 0.004 |
| Toluene | 386 | 45.6 | 450, 700 | 3700 | 0.00012 | |
| Propyl butyrate | 386 | — | 448, 780 | 3600 | 0.0002 | |
| THF | 388 | — | 450 | 3600 | 0.00012 | |
| CH2Cl2 | 387 | — | 445 | 3400 | 0.00019 | |
| Acetonitrile | 385 | — | 445 | 3500 | 0.00003 | |
| DMF | 393 | — | — | — | — | |
| DMSO | 395 | — | — | — | — | |
| 4 | Hexane | 380 | — | 428 | 3000 | 0.61 |
| Toluene | 391 | 43.3 | 445 | 3100 | 0.78 | |
| Propyl butyrate | 389 | — | 447 | 3300 | 0.74 | |
| THF | 392 | — | 453 | 3400 | 0.8 | |
| CH2Cl2 | 392 | — | 453 | 3400 | 0.8 | |
| Acetonitrile | 389 | — | 470 | 4400 | 0.5 | |
| DMF | 398 | — | 472 | 3900 | 0.75 | |
| DMSO | 400 | — | 479 | 4100 | 0.73 | |
| 5 | Hexane | 396 | 438 | 2400 | 0.72 | |
| Toluene | 404 | 51.8 | 456 | 2800 | 0.82 | |
| Propyl butyrate | 402 | — | 456 | 2900 | 0.77 | |
| THF | 404 | — | 462 | 3100 | 0.79 | |
| CH2Cl2 | 404 | — | 473 | 3600 | 0.85 | |
| Acetonitrile | 399 | — | 483 | 4400 | 0.74 | |
| DMF | 406 | — | 484 | 4000 | 0.84 | |
| DMSO | 408 | — | 492 | 4200 | 0.84 | |
| 6 | Hexane | 394 | — | 438 | 2500 | 0.61 |
| Toluene | 404 | 53.0 | 455 | 2800 | 0.77 | |
| Propyl butyrate | 400 | — | 455 | 3000 | 0.77 | |
| THF | 403 | — | 460 | 3100 | 0.75 | |
| CH2Cl2 | 403 | — | 472 | 3600 | 0.81 | |
| Acetonitrile | 397 | — | 481 | 4400 | 0.7 | |
| DMF | 404 | — | 482 | 4000 | 0.81 | |
| DMSO | 406 | — | 491 | 4300 | 0.83 | |
| 7 | Hexane | 341 | — | 573 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
0.0075 |
| Toluene | 344 | 52.0 | 380, 720 | 2800 | 0.0003 | |
| Propyl butyrate | 342 | — | 403 | 4400 | 0.0001 | |
| THF | 343 | — | — | — | 0.000001 | |
| CH2Cl2 | 342 | 47.2 | 606 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
0.00007 | |
| Acetonitrile | 339 | 56.9 | 382 | 3300 | 0.000005 | |
| DMF | 343 | — | 407 | 4600 | 0.0001 | |
| DMSO | 344 | 53.3 | — | — | — | |
| 8 | Hexane | 316 | — | 426 | 8200 | 0.4 |
| Toluene | 321 | 54.9 | 455 | 9200 | 0.24 | |
| Propyl butyrate | 319 | — | 485 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
0.082 | |
| THF | 320 | — | 509 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
0.044 | |
| CH2Cl2 | 321 | — | 538 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
0.042 | |
| Acetonitrile | 318 | — | 581 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
0.0075 | |
| DMF | 321 | — | 571 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
0.013 | |
| DMSO | 323 | — | 586 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
0.007 | |
| 9 | Hexane | 345 | — | — | — | — |
| Toluene | 351 | 36.7 | — | — | — | |
| Propyl butyrate | 349 | — | — | — | — | |
| THF | 349 | — | — | — | — | |
| CH2Cl2 | 350 | — | — | — | — | |
| Acetonitrile | 346 | — | — | — | — | |
| DMF | 352 | — | — | — | — | |
| DMSO | 352 | — | — | — | — | |
| 10 | Hexane | 347 | — | 425 | 5300 | 0.16 |
| Toluene | 352 | 30.6 | 480 | 7600 | 0.043 | |
| Propyl butyrate | 348 | — | 509 | 9100 | 0.02 | |
| THF | 352 | — | 535 | 9700 | 0.012 | |
| CH2Cl2 | 350 | — | 551 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
0.0096 | |
| Acetonitrile | 348 | — | 590 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
0.0019 | |
| DMF | 351 | — | 594 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
0.0031 | |
| DMSO | 354 | — | 605 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
0.0019 | |
| 11 | Hexane | 355 | — | 403 | 3400 | 0.63 |
| Toluene | 359 | 45.5 | 410 | 3500 | 0.65 | |
| Propyl butyrate | 356 | — | 407 | 3500 | 0.67 | |
| THF | 357 | — | 409 | 3600 | 0.69 | |
| CH2Cl2 | 356 | — | 413 | 3900 | 0.63 | |
| Acetonitrile | 351 | — | 411 | 4200 | 0.68 | |
| DMF | 356 | — | 414 | 3900 | 0.72 | |
| DMSO | 356 | — | 417 | 4100 | 0.75 | |
| 12 | Hexane | 355 | — | 405 | 3500 | 0.6 |
| Toluene | 359 | 42.0 | 412 | 3600 | 0.65 | |
| Propyl butyrate | 356 | — | 409 | 3600 | 0.63 | |
| THF | 358 | — | 411 | 3600 | 0.63 | |
| CH2Cl2 | 357 | — | 415 | 3900 | 0.61 | |
| Acetonitrile | 352 | — | 414 | 4300 | 0.64 | |
| DMF | 356 | — | 416 | 4100 | 0.67 | |
| DMSO | 358 | — | 418 | 4000 | 0.72 | |
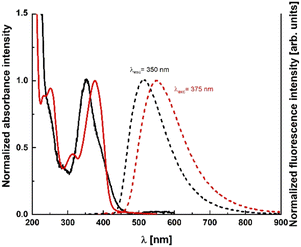 | ||
| Fig. 2 Absorption (solid line) and emission (dotted line) of dyes 1 (black) and 3 (red) in n-hexane. | ||
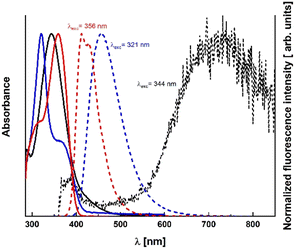 | ||
| Fig. 3 Absorption (solid line) and emission (dotted line) of dyes 7 (black), 8 (blue) and 12 (red) in toluene. | ||
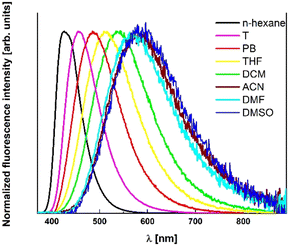
Absorption maxima of the main band of TAPP 1 in all the solvents are shifted by the presence of the nitro group outside the visible region, whereas λabs of 2–6 is above 380 nm in most of the cases (Fig. 2). At the same time for TAPPs 1 and 7, there is a weak absorption shoulder in the range of 500–600 nm. Its characteristic features, i.e. broadness and very low intensity, prompted us to tentatively assign it to charge-transfer (CT) character.26–28 This charge-transfer band is partly responsible for the visible yellow color of the solutions of these dyes. Weaker effect of that type is also observed in the case of TAPPs 2 and 8 possessing 4-CNC6H4 substituents. In the case of dye 8, while λabs is hypsochromically shifted as far as 316–323 nm, depending on the solvent, an additional intriguing feature is a strong shoulder at approx. 370 nm (Fig. 3). Shifting the NO2 group from position 4 to position 3 (TAPPs 3 and 9 versus TAPPs 1 and 7) causes a small bathochromic shift of main λabs (Fig. 2 and Table 1).
The variation of spectral properties is broader in the case of TAPPs 7–12 than of 1–6, where the electron-withdrawing ester groups linked with the DHPP core through biaryl linkages at positions 2 and 5 dominate the photophysics by creating a strong quadrupolar A–D–A system with the DHPP. Emission of dyes 1–4, as compared to 5 and 6, is shifted hypsochromically by the N-electron-withdrawing groups, while in the case of the second series, there is a large bathochromic shift into deep green in the fluorescence of 8 and 10 (compared to TAPPs 11 and 12), as only in the presence of the cyano groups push–pull systems are created, however the fluorescence quantum yield (Φf) of this emission is low (Fig. 4 and Table 1).
Nitro groups almost entirely quench the fluorescence of the studied compounds, even in non-polar solvents, which is also different from what is observed for TAPPs possessing 4-nitro-phenyl substituents at positions 2 and 5, which can have nearly quantitative emission in hexane.8 Compound 9 does not show detectable fluorescence in any solvent. The emission of DHPPs 1, 3 and 7 is also unmeasurable in some polar solvents, while in the others it is only slightly higher than the noise level (Fig. 3).
McRae plots were computed, and the results can be seen in Fig. S4 and Table S6. The data suggest that the most polarized molecules are those containing nitrophenyl and cyanophenyl substituents.
The very low fluorescence quantum yields of some compounds point to the existence of an efficient radiationless channel of electronic relaxation. Among the weakly emissive molecular structures, most possess the nitro group (1, 3, 7 and 9) and two CN substituents (8 and 10). This may suggest that nitro groups introduce a low-lying singlet state of nπ* character that enhances the intersystem crossing process, as was discussed already in the Introduction. To investigate this supposition, emission spectra have been recorded at low temperature (77 K). Comparison of fluorescence spectra recorded at room temperature and 77 K is presented in Fig. S99. The overlap of the RT absorption spectrum with the 77 K fluorescence excitation spectrum proves that emission originates from monomers and not from aggregates. The most striking difference between the RT and 77 K emission spectra is the huge increase in fluorescence intensity, of the 100–1000 order. Lack of any structured phosphorescence spectrum is another observation. Thus, we conclude that in frozen solvent internal conversion is stopped (most likely due to restriction of twists/torsions) and the ISC process is not very efficient. The same conclusion holds for dyes 1, 3 and 7 bearing NO2 groups as well as for dye 10 possessing CN groups. This points to internal conversion as the very efficient channel of electronic relaxation that effectively quenches fluorescence at elevated temperatures.
Computational studies
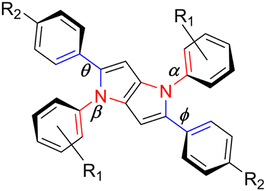
In order to gain in-depth insight into the factors governing the fate of TAPP molecules in the excited state, we performed theoretical exploration at the ab initio level of theory. Most studies in the literature on DHPPs use density functional theory (DFT), typically using exchange–correlation functionals such as M06-2X,10–14,29 B3LYP8,15 and CAM-B3LYP.8 However, wavefunction-based methods like ADC(2) outperform DFT because they avoid functional bias and provide a more accurate description of CT states.16,29–32 In this work, computations were performed for all selected molecules in both fully relaxed and constrained to Ci point group conformations using structures with truncated alkyl chains.This symmetry was imposed to prevent overestimating π-stacking between aromatic groups of TAPP branches, which may arise from the inherent limitations of MP2/ADC(2) in modelling dispersion forces.33,34 Within the Ci framework, it is possible to optimize molecular structures of populated intramolecular charge transfer (ICT) excited states ag (dark) and au (bright) separately, enabling the estimation of barriers to non-radiative decays of these systems. Ground state (S0) structures optimized at the MP2/cc-pVDZ level of theory (without symmetry constraints) correspond to near-centrosymmetric geometries. Computed dihedral angles between the core and subunits range from 35 to 55° (Table 2). Remarkably, S0 geometries remain non-polarized even with strong electron-accepting substituents, consistent with previous observations.20
| TAPP | S0 | S1(au) | ||||||
|---|---|---|---|---|---|---|---|---|
| C1 | Ci | C1 | Ci | |||||
| θ(φ) | α(β) | θ = φ | α = β | θ(φ) | α(β) | θ = φ | α = β | |
| a Dihedral angles computed for the ag optimized structure. | ||||||||
| 1 | 37 | 47 | 38 | 49 | 35(24) | 87(55) | 23(29)a | 48(66)a |
| 2 | 38 | 49 | 44 | 59 | 34(25) | 91(55) | 22 | 49 |
| 3 | 38 | 47 | 38 | 47 | 23(23) | 46(46) | 23 | 45 |
| 4 | 38 | 50 | 37 | 49 | 23(22) | 50 | 22 | 50 |
| 5 | 35 | 53 | 36 | 54 | 26 | 56 | 24 | 57 |
| 6 | 36 | 53 | 36 | 52 | 24 | 55 | 24 | 55 |
| 7 | 48 | 55 | 40 | 48 | 34(22) | 55(88) | 28 | 52 |
| 8 | 40 | 48 | 40 | 48 | 45(34) | 101(55) | 36 | 45 |
| 9 | 39(46) | 44 | 40 | 46 | 35(29) | 64(53) | 29 | 53 |
| 10 | 39 | 48 | 40 | 48 | 33(21) | 59(55) | 21 | 47 |
| 11 | 39 | 50 | 39 | 52 | 23(22) | 53 | 20 | 52 |
| 12 | 37 | 52 | 38 | 51 | 20 | 52 | 20 | 52 |
Three vertical excited states were computed at the ADC(2)/cc-pVDZ level of theory for the MP2 ground state equilibrium structures of 1–12 (Table S1a) without symmetry constraints. The bright Franck–Condon (FC) state shows evenly distributed electronic density across TAPPs subunits, followed by higher-energy dark states.7,20 Natural transition orbitals (Table S1b) analysis suggests that low-lying states correspond to ICT for dyes 1–3 and 7–10, and to locally excited (LE) in the case of TAPPs 4–6, 11 and 12. The weak solvatochromism in 7–12 reflects suppressed D–A, whereas dyes 1–6 exhibit pronounced bathochromic shifts from direct ester-DHPP conjugation.35
To further characterize these FC states, excitation energies were also computed imposing Ci symmetry for the most representative systems, i.e. DHPPs 7 and 12 (Table S3). In both these cases, the ag/au mixing is forbidden, preventing stabilization of the au bright state that would otherwise occur upon symmetry breaking. Compound 7 exhibits ICT with small overlap molecular orbitals of FCS1 (Fig. 5), resulting in low oscillator strength. Vertical excitation of compound 12 leads to intense LE emission, with frontier molecular orbitals localized on the DHPP core. Thus, the ratio of populating LE/ICT in non-polar solvents from FCS1 in 1–12 is modulated by the electron-accepting strength of 1,4-substituents.
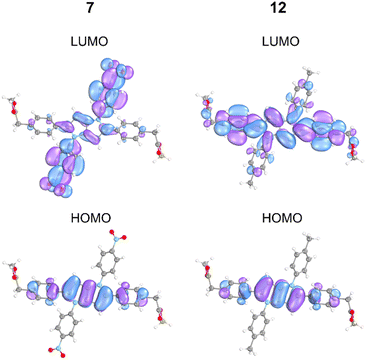 | ||
| Fig. 5 Frontier molecular orbitals for selected compounds 7 and 12 obtained at the ADC(2)/cc-pVDZ level of theory for molecules computed with Ci symmetry. | ||
While S0 structures maintain near-Ci symmetry across all TAPPs, the S1 minima are susceptible to ES-SB in dyes with strong N-electron-accepting groups (e.g., 4-NO2 and 4-CN substituents).5,7,20 The symmetry breaking induced upon photoexcitation promotes stabilization of charge-separated twisted intramolecular charge transfer (TICT), evidenced by a ≈40° increase in N-aryl dihedral angles in the S1 optimized structures for unconstrained conformation (C1 point group) with respect to structures computed imposing Ci symmetry (Table 2). The weak inter-branch dispersion forces that promote the formation of transient dipoles in compounds 1–3, and 7–10 (Table S4) stabilize dark TICT states, which, with the exception of 2 and 8, effectively quench their fluorescence, are consistent with experimental observations. Conversely, this behavior is not observed for TAPPs with dominant LE character (4–6 and 11, 12).
Examining Scheme S1 revealed that the strong A–D–A electronic coupling in 1–6 decreases electron density rearrangement upon excitation, promoting the population of a LE state, leading to larger fluorescence quantum yields compared to dyes 7–12. Overall, regarding DHPPs 1–6, the N-substituent's electron-donating/accepting strength controls their main deactivation channel, either via ES-SB or LE emission.
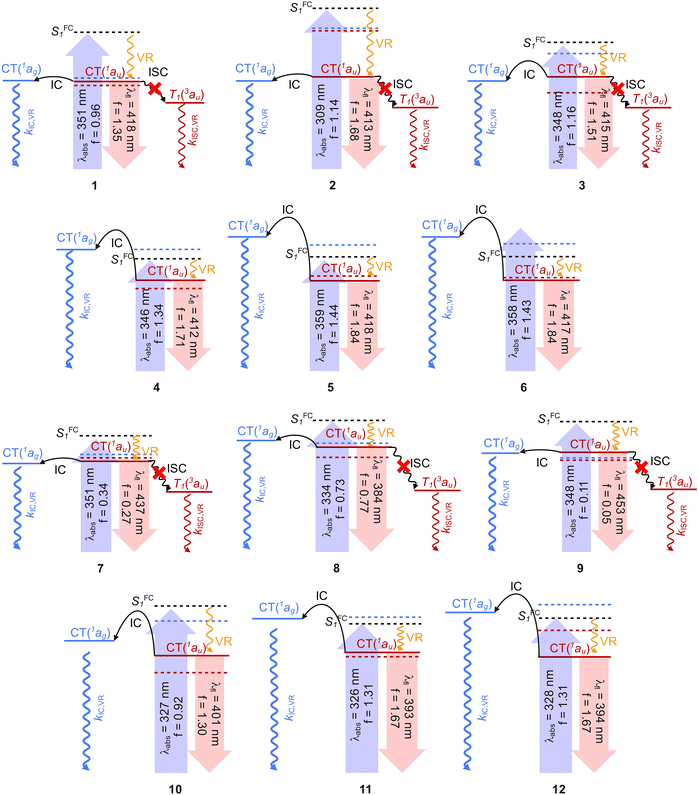
Upon photoexcitation in centrosymmetric TAPPs corresponding to the Ci point group, the SFC1 state relaxes into two ICT states with distinct symmetries: a bright au state and a dark ag state, with the latter decaying non-radiatively to S0. The energy barrier between these states controls the IC rate and consequently the fluorescence quantum yields (Table 1).36 To estimate the au/ag population barrier, geometry optimizations were performed for both excited states for all molecules, and the results were summarized in the simplified Jablonski diagrams (Fig. 6). Compounds 1 and 7, which possess small energy gaps between their ag and au states, are expected to access the conical intersection (CI) to the ground state in a barrierless fashion, efficiently quenching emission from the 1au. Ci symmetry constraints limit large amplitude movements between TAPP subunits. However, 1ag exhibits increased TICT state character compared to au, as suggested by an 18° increase in the twist along σ-bonds between the DHPP core and the 4-NO2C6H4 substituents. Thus, the low fluorescence quantum yields observed for these compounds arise from their strong ICT character and symmetry breaking, which promotes seamless access to the S1/S0 conical intersection.8,27 This conclusion corresponds well with results by Vullev and co-workers who noticed that in TAPPs strengthening D–A electronic coupling decreases CT and propensity for ES-SB.20
Conversely, 6 and 12 exhibit a significant barrier for IC (i.e. the au → ag) (≈0.6 eV), promoting strong emission from the au state. Thus, the barrier height governs the emission efficiency of TAPPs in non-polar solvents such as hexane, with fluorescence quantum yields equal to 0.031, 0.61, 0.0003, and 0.65 for 1, 6, 7, and 12, respectively. However, while this approach can reasonably correlate the measured fluorescence intensity with the height of the au/ag barrier, in both series, there are a few notable exceptions: compound 2 displays intense fluorescence while 3 and 10 are barely fluorescent, despite exhibiting small and large barriers for IC, respectively. The strong fluorescence observed for compound 2 is attributed to weaker electronic coupling between the N-4-cyanophenyl group and the DHPP core, in contrast to compound 1, which promotes the population of the LE excited state (Fig. S3) and enhances its emission. In contrast, compounds 3 and 10 exhibit weak fluorescence despite a predicted high barrier for accessing their non-emissive ag state. Unlike para-NO2,8,20 the meta-NO2 group disrupts conjugation between DHPP and nitroaryl groups, resulting in an asymmetric electronic distribution which makes these dyes susceptible to ES-SB, promoting the population of a non-emissive species, particularly in polar solvents.6,13,18,20,37
Nitro groups bonded to DHPPs act as pseudo-heavy atoms and enhance spin–orbit coupling (SOC), modulating the ISC/IC branching ratio in non-radiative deactivation pathways, as observed in 2,5-nitro-pyrrolo[3,2-b]pyrroles.8 In contrast, as can be seen in Fig. 4, no significant triplet-mediated deactivation is predicted due to the large singlet–triplet energy gap (ΔEST ≈ 1 eV). Thus, despite having nitroaryl groups, IC remains the main dissipation channel for the 1, 3, 7 and 9 gap (ΔEST ≈ 1 eV).
Solvation effects were evaluated using the COSMO continuum approximation (DMSO was chosen as the solvent) to compute excitation/emission energies. Computed FC states for selected compounds 1, 3, and 8 suggest minimal solvatochromism (approximately 0.1 eV between vacuum and solution), consistent with the small redshift observed in their absorption bands (Table 1). Emission bands for compounds 1, 3, 7, 8 and 10 were too weak to estimate their solvatochromism.
Interestingly, despite both having 4-CN substituents, compounds 2 and 8 exhibit markedly distinct emission profiles. The quadrupolar A–D–A architecture present in 2 favors strong LE emission, as also observed for dyes 4, 5, 6, 11 and 12, which is redshifted by 0.1–0.3 eV with increasing solvent polarity. On the other hand, the disruption of D–A electronic coupling by the addition of a CH2 spacer promotes ICT for 8, effectively suppressing its fluorescence, particularly in polar solvents.10,18 The absence of fluorescence in 9, regardless of solvent polarity, is associated with the disruption of the conjugation between DHPP and 3-nitrophenyl moieties, which promotes the near degeneracy between the π-system and the nitrogen lone-pair orbitals. As a result, this compound is susceptible to strong vibronic mixing (pseudo-Jahn–Teller effect), resulting in the population of a non-emissive chiral hybrid S1 state, as can be seen in Fig. S3.
This study extends beyond excited-state symmetry breaking, which was investigated for DHPPs.6,7,35 The influence of substituents linked to the DHPP core via a nitrogen atom has a remarkably different character compared to the influence of C2- and C3-substituents. The strong influence of electron-withdrawing substituents at positions 1 and 4 is reduced if electron-withdrawing substituents are present at positions 2 and 5. The disruption of the electronic coupling increases charge separation between subunits. This effect is particularly strong for N-4-nitrophenyl substituents, which are more susceptible to the formation of transient dipole moments and the population of dark states, effectively suppressing their fluorescence. This effect is amplified in polar solvents, which further stabilizes charge-separated states. While the height of the dark/bright state barriers has a direct correlation with the measured quantum yields, there are a few exceptions. The most interesting case is TAPP 9, for which no fluorescence is observed, regardless of the solvent polarity. Based on our computations, the nπ*–ππ* near degeneracy would make this molecule susceptible to strong vibronic mixing, quenching its emission. It is worth noting that intersystem crossing is not operating efficiently for N-4-nitrophenyl derivatives, as indicated by the absence of phosphorescence observed at cryogenic temperatures. Thus, internal conversion drives the radiationless deactivation of both series of compounds.
The above-described results will play a vital role in the design of TAPPs in the future. The current results clearly point out that the 4-methoxyphenyl substituent does not interfere markedly with strongly emissive characteristics of tetraarylpyrrolo[3,2-b]pyrroles possessing either electron-withdrawing substituents or neutral substituents at positions 2 and 5. This is of paramount importance since this type of dye is often investigated and substituting methyl with a longer and branched alkyl chain can enable the increase of the solubility. The latter one is critical since tetraarylpyrrolo[3,2-b]pyrroles are notoriously poorly soluble. At the same time, TAPPs bearing the 4-cyanophenyl or 3-cyanophenyl substituents at positions 1 and 4 have a strong fluorescence only of electron-withdrawing groups present at positions 2 and 5. This is a critical observation since especially 4-cyanophenyl groups help to regulate the photostability of TAPPs.
Conclusions
The comprehensive study of photophysical properties of 1,4-dihydropyrrolo[3,2-b]pyrroles enabled us to draw the following conclusions. (1) If electron-withdrawing 4-(CO2R)C6H4 substituents are present at positions 2 and 5, they dominate the photophysics of these DHPPs (fluorescence is strong across the scale of solvents’ polarity), unless nitrophenyl substituents are present at position 1 and 4. (2) If 4-nitrophenyl substituents are located at positions 1 and 4, non-radiative decay is dominated by the population of a TICT excited state (ag), which is predicted to occur seamlessly from the bright state au. (3) If, however, the substituents at positions 2 and 5 are electron-neutral and N-substituents are electron-withdrawing, strong quenching of fluorescence is observed in polar solvents. We attribute this behavior to ES-SB and the formation of transient dipole moments. This, in turn, leads to the stabilization of charge-separated states and favoring of non-radiative decay pathways. This study provides a foundation for the design of DHPP with fine-tuned, tailored photophysical properties.Author contributions
Conceptualization: D. T. G. and W. D. P.; investigation: W. D. P., Z. K., C. A. B., O. M. and M. B.; supervision: D. T. G.; visualization: W. D. P., C. A. B., and M. B.; writing – original draft: W. D. P., D. T. G., C. A. B., O. M. and M. B.; writing – review and editing: W. D. P., D. T. G., and C. A. B. All the authors discussed the results and commented on the manuscript.Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included as part of the supplementary information (SI). Supplementary information: detailed synthetic protocols, characterization data; 1H, 13C{1H} NMR and HRMS spectra, computational details (including Cartesian coordinates), photophysical measurement and additional figures as mentioned in the text. See DOI: https://doi.org/10.1039/d5cp03474d.Acknowledgements
This project has received funding from the European Union's Horizon 2020 Research and Innovation Program under the Marie Skłodowska-Curie grant agreement no. 101007804 and from the European Research Council (ARCHIMEDES, 101097337). Views and opinions expressed are, however, those of the authors only and do not necessarily reflect those of the European Union or the European Research Council Executive Agency. Neither the European Union nor the granting authority can be held responsible for them. The work was also financially supported by the Polish National Science Centre, Poland (OPUS 2020/37/B/ST4/00017). We gratefully acknowledge the Polish high-performance computing infrastructure PLGrid (HPC Center: ACK Cyfronet AGH) for providing computer facilities and support within the computational grant no. PLG/2024/017465.Notes and references
- H. Hemetsberger and D. Knittel, Monatsh. Chem., 1972, 103, 194–204 CrossRef.
- A. Janiga, E. Glodkowska-Mrowka, T. Stoklosa and D. T. Gryko, Asian J. Org. Chem., 2013, 2, 411–415 CrossRef.
- M. Krzeszewski, O. Vakuliuk, M. Tasior, A. Wołos, R. Roszak, K. Molga, M. B. Teimouri, B. A. Grzybowski and D. T. Gryko, J. Am. Chem. Soc., 2025, 147, 15636–15644 CrossRef.
- M. Krzeszewski, Ł. Dobrzycki, A. L. Sobolewski, M. K. Cyrański and D. T. Gryko, Chem. Sci., 2023, 14, 2353–2360 RSC.
- G. Sanil, M. Krzeszewski, W. Chaładaj, W. Danikiewicz, I. Knysh, Ł. Dobrzycki, O. Staszewska-Krajewska, M. K. Cyrański, D. Jacquemin and D. T. Gryko, Angew. Chem., Int. Ed., 2023, 62, e202311123 CrossRef.
- B. Dereka, A. Rosspeintner, M. Krzeszewski, D. T. Gryko and E. Vauthey, Angew. Chem., Int. Ed., 2016, 55, 15624–15628 CrossRef.
- C. Govind, E. Balanikas, G. Sanil, D. T. Gryko and E. Vauthey, Chem. Sci., 2024, 15, 17362–17371 RSC.
- Y. M. Poronik, G. V. Baryshnikov, I. Deperasińska, E. M. Espinoza, J. A. Clark, H. Ågren, D. T. Gryko and V. I. Vullev, Commun. Chem., 2020, 3, 190 CrossRef PubMed.
- K. Górski, D. Kusy, S. Ozaki, M. Banasiewicz, R. Valiev, S. R. Sahoo, K. Kamada, G. Baryshnikov and D. T. Gryko, J. Mater. Chem. C, 2024, 12, 1980–1987 RSC.
- M. Banasiewicz, R. Stężycki, G. D. Kumar, M. Krzeszewski, M. Tasior, B. Koszarna, A. Janiga, O. Vakuliuk, B. Sadowski, D. T. Gryko and D. Jacquemin, Eur. J. Org. Chem., 2019, 5247–5253 CrossRef CAS.
- M. Tasior, G. Clermont, M. Blanchard-Desce, D. Jacquemin and D. T. Gryko, Chem. – Eur. J., 2019, 25, 598–608 CrossRef PubMed.
- Y. M. Poronik, L. M. Mazur, M. Samoć, D. Jacquemin and D. T. Gryko, J. Mater. Chem. C, 2017, 5, 2620–2628 RSC.
- Ł. G. Łukasiewicz, M. Rammo, C. Stark, M. Krzeszewski, D. Jacquemin, A. Rebane and D. T. Gryko, ChemPhotoChem, 2020, 4, 508–519 CrossRef.
- R. Orłowski, M. Banasiewicz, G. Clermont, F. Castet, R. Nazir, M. Blanchard-Desce and D. T. Gryko, Phys. Chem. Chem. Phys., 2015, 17, 23724–23731 RSC.
- M. Krzeszewski, T. Kodama, E. M. Espinoza, V. I. Vullev, T. Kubo and D. T. Gryko, Chem. – Eur. J., 2016, 22, 16478–16488 CrossRef PubMed.
- S. Hatanaka, T. Ono, Y. Yano, D. T. Gryko and Y. Hisaeda, ChemPhotoChem, 2020, 4, 138–143 CrossRef.
- B. Szymański, S. R. Sahoo, O. Vakuliuk, R. Valiev, R. Ramazanov, P. Łaski, K. N. Jarzembska, R. Kamiński, M. B. Teimouri, G. Baryshnikov and D. T. Gryko, Chem. Sci., 2024, 16, 2170–2179 RSC.
- Ł. G. Łukasiewicz, H. G. Ryu, A. Mikhaylov, C. Azarias, M. Banasiewicz, B. Kozankiewicz, K. H. Ahn, D. Jacquemin, A. Rebane and D. T. Gryko, Chem. – Asian J., 2017, 12, 1736–1748 CrossRef PubMed.
- D. H. Friese, A. Mikhaylov, M. Krzeszewski, Y. M. Poronik, A. Rebane, K. Ruud and D. T. Gryko, Chem. – Eur. J., 2015, 21, 18364–18374 CrossRef PubMed.
- J. A. Clark, D. Kusy, O. Vakuliuk, M. Krzeszewski, K. J. Kochanowski, B. Koszarna, O. O’Mari, D. Jacquemin, D. T. Gryko and V. I. Vullev, Chem. Sci., 2023, 14, 13537–13550 RSC.
- A. M. Hawks, D. Altman, R. Faddis, E. M. Wagner, K. J. J. Bell, A. Charland-Martin and G. S. Collier, J. Phys. Chem. B, 2023, 127, 7352–7360 CrossRef PubMed.
- A. M. Hawks, L. M. Daniel, V. S. Sorto, J. Mauro, P. Skiouris and G. S. Collier, ACS Appl. Opt. Mater., 2024, 2, 1235–1244 CrossRef PubMed.
- F. Terenziani, A. Painelli, C. Katan, M. Charlot and M. Blanchard-Desce, J. Am. Chem. Soc., 2006, 128, 15742–15755 CrossRef PubMed.
- M. Tasior, O. Vakuliuk, D. Koga, B. Koszarna, K. Górski, M. Grzybowski, Ł. Kielesiński, M. Krzeszewski and D. T. Gryko, J. Org. Chem., 2020, 85, 13529–13543 CrossRef PubMed.
- G. Sanil, B. Koszarna, Y. M. Poronik, O. Vakuliuk, B. Szymański, D. Kusy and D. T. Gryko, Adv. Heterocycl. Chem., 2022, 138, 335–409 Search PubMed.
- J. B. Derr, J. Tamayo, J. A. Clark, M. Morales, M. F. Mayther, E. M. Espinoza, K. Rybicka-Jasińska and V. I. Vullev, Phys. Chem. Chem. Phys., 2020, 22, 21583–21629 Search PubMed.
- A. Neubauer, J. Bendig and W. Rettig, Chem. Phys., 2009, 358, 235–244 Search PubMed.
- S. Lee, M. Jen, T. Jang, G. Lee and Y. Pang, Sci. Rep., 2022, 12, 1–11 CrossRef PubMed.
- D. Mester and M. Kállay, J. Chem. Theory Comput., 2022, 18, 1646–1662 CrossRef PubMed.
- C. R. Zhang, J. S. Sears, B. Yang, S. G. Aziz, V. Coropceanu and J. L. Brédas, J. Chem. Theory Comput., 2014, 10, 2379–2388 CrossRef PubMed.
- Z. L. Cai, M. J. Crossley, J. R. Reimers, R. Kobayashi and R. D. Amos, J. Phys. Chem. B, 2006, 110, 15624–15632 CrossRef PubMed.
- A. Dreuw and M. Head-Gordon, J. Am. Chem. Soc., 2004, 126, 4007–4016 Search PubMed.
- N. M. P. Rosa and I. Borges, J. Comput. Chem., 2024, 45, 2885–2898 CrossRef PubMed.
- M. O. Sinnokrot and C. D. Sherrill, J. Phys. Chem. A, 2006, 110, 10656–10668 Search PubMed.
- A. H. Balawi, S. Stappert, J. Gorenflot, C. Li, K. Müllen, D. Andrienko and F. Laquai, J. Phys. Chem. C, 2019, 123, 16602–16613 Search PubMed.
- G. W. Robinson and R. P. Frosch, J. Chem. Phys., 1963, 38, 1187–1203 Search PubMed.
- B. Dereka, A. Rosspeintner, R. Stȩżycki, C. Ruckebusch, D. T. Gryko and E. Vauthey, J. Phys. Chem. Lett., 2017, 8, 6029–6034 CrossRef PubMed.
| This journal is © the Owner Societies 2026 |