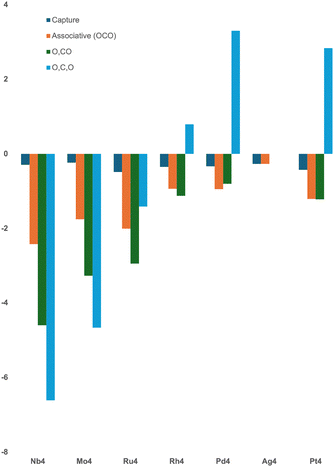Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Associative vs. dissociative binding of CO2 on M4 transition metal clusters
Sherfi Sherif,
Bala Aakash Velmurugan,
Naeem Abbas,
Muskanbanu Shaikh and
Matthew A. Addicoat *
*
School of Science and Technology, Nottingham Trent University, Clifton Lane, Nottingham, NG11 8NS, UK. E-mail: matthew.addicoat@ntu.ac.uk
First published on 9th December 2025
Abstract
Density functional theory calculations were performed to determine reaction paths for the reaction of CO2 with M4 transition metal clusters (M = Nb, Mo, Ru, Rh, Pd, Ag, Pt). Geometries incorporating associatively bound (CO2), partly dissociated (O + CO) and fully dissociated (O + C + O) carbon dioxide were identified for all clusters except Ag4. Nb4 and Mo4 are likely to dissociate CO2 fully. For Ru4, both partly and fully dissociative geometries were competitive, while Rh4, Pd4 and Pt4 activate CO2 without breaking either CO bond. Ag4 was found to interact only minimally with CO2. The change in νbend, the energy of the CO2 πu orbital in the physisorbed M4CO2 capture species and the charge transfer to the CO2 molecule, q(CO2), in the first transition state were found to correlate with the eventual fate of the CO2 molecule.
1 Introduction
Carbon dioxide CO2, is one of the key pollutant gasses contributing to global warming and ocean acidification.1 In recognition of the urgency of this problem, in recent years there have been increasing efforts to develop and deploy carbon capture and storage systems. To go one step further, rather than storing carbon captured from the atmosphere (as CO2), valorizing it as a C1 feedstock. Captured CO2 could be used to produce common feedstock chemicals and fuels such as formaldehyde, methanol and formic acid.2,3 Reduction of CO2 via the reverse Water Gas Shift (rWGS) reaction produces carbon monoxide, CO, that can be employed in a variety of industrially relevant reactions.4,5Somewhat frustrating the aim of employing captured CO2 as a reactant in various chemical processes, is its exceptional thermodynamic and kinetic stability: the Gibbs free energy of formation, ΔfG at 298 K is −394.36 kJ mol−1.6 Therefore, activating the CO2 molecule, by partly or fully breaking one of the two C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds requires one or more of; a careful choice of reactants that can donate to the CO2 molecule, a similarly careful choice of product, shifting the equilibrium – e.g. by removing product, and a large energy input.7
O bonds requires one or more of; a careful choice of reactants that can donate to the CO2 molecule, a similarly careful choice of product, shifting the equilibrium – e.g. by removing product, and a large energy input.7
Considering the mechanism of how a catalytic system can activate CO2, upon CO2 adsorption or coordination to the system, often at the site of a metal centre, electron density is introduced into the antibonding π* orbitals of CO2. The C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds are thereby weakened and the molecule subsequently adopts a bent geometry (θOCO ≈ 120–140°), creating a dipole and increasing the reactivity of CO2.7–11
O bonds are thereby weakened and the molecule subsequently adopts a bent geometry (θOCO ≈ 120–140°), creating a dipole and increasing the reactivity of CO2.7–11
A variety of novel catalysts for the activation of CO2 have been proposed, including MOFs,12–14 ceria15,16 and a variety of metal (oxide) surfaces including Fe17, Ti18, Cu19 and Pd/Mo.20 A key feature of all of these proposed catalysts is that they involve one or more metal atoms at the active site as the source of the electron density donated to the CO2 molecule.
Transition metal clusters have been studied both experimentally and computationally for many years for their capacity to adsorb and activate various small molecules including CO2.21,22 Transition metal clusters were initially studied as models of bulk surfaces.23 However, it was rapidly identified that the properties of these clusters is both size-24 and geometry-dependent,25,26 and therefore, that by modifying the size and composition of the (nano)clusters, that the properties, especially the reactivity, of the clusters could be tuned.23,27,28 Most recently, transition metal clusters have been confined within the pockets of porous framework materials, with the goal of protecting the active metal clusters from sintering, whilst the porous framework still allows mass transfer.29,30
Computational studies, normally employing density functional theory (DFT), are key in determining the geometric structures and reaction mechanisms in both gas-phase31–33 and surface studies,34 especially identifying where barriers may prevent thermodynamic products from being observed.35 A recent DFT-based mechanistic study from Mondal et al. showed that beyond simply the static cluster geometry, the fluxionality of the cluster was important in determining the reduction of CO2 on supported copper tetramers.36 Da Silva and coworkers studied a the reverse water gas shift reaction on series of M13 clusters, M = Fe–Cu and observed that increasing the d-state occupation favoured COOH formation.5 Recently, Mohanta and Jena attempted to address the poor selectivity of the Cu13 cluster by investigating a series of XCu12 clusters, where X was a variety of first and second row transition metal atoms.
With a similar aim of understanding cluster behaviour across the periodic table, in this work, we present DFT calculated reaction paths for CO2 addition to second row M4 transition metal clusters from Nb4 to Ag4, we exclude technetium, due to its radioactivity, but we include the third-row Pt4 cluster due to the popularity of platinum as a catalyst.
2 Computational method
Structures of M4 clusters, for M = Nb–Ag (excluding Tc) and Pt were generated using the Kick stochastic structure search procedure with four individual M atoms supplied.37,38 Full searches were undertaken on the lowest possible multiplicity (singlet for all M4 clusters) and all minima identified were re-optimized at higher multiplicities. For all species, the lowest four multiplicities were calculated, but for rhodium, palladium and platinum, the search was extended up to the nonet. Ruthenium is known to have low lying minima with high numbers of unpaired electrons and so the search was further extended up to the 15-tet. No symmetry was imposed at any point in the search, nevertheless, several clusters adopted clear point group symmetry, as evidenced by geometric parameters and frequencies. Where point group symmetry was observed in either bare M4 or M4CO2 clusters, we refer to this apparent symmetry, but we did not confirm the symmetry by further constrained calculation, as the addition of CO2, or further steps on the reaction path would immediately break symmetry.The lowest energy structure of each M4 cluster was then adopted as a fragment in a further stochastic search process. Kick runs were undertaken searching for with the following configurations: M4 + CO2 (i.e. intact CO2); M4 + CO + O; M4 + C + O + O. As activation of the CO2 molecule is expected to proceed via electron donation into the CO2 πu orbitals, thus bending the CO2 molecule,10 two Kick runs were also undertaken explicitly searching for minima and transition states, employing the M4 cluster and a bent CO2 molecule as fragments. Additional starting geometries were generated by hand (e.g. CO2 bound to different symmetry-distinct metal atoms, end-on/side-on, linear/bent, μ1/μ2/μ3-bound). From these calculations, the physisorbed “capture” species and the M4CO2 global minimum were identified, and the reaction pathway was then filled in and confirmed by a series of Quasi-Synchronous Transit (QST) and Intrinsic Reaction Coordinate (IRC) calculations. Where the global minimum was not a dissociated structure (i.e. M4 + O + C + O), the lowest energy dissociated structure was also identified and a pathway to that structure was calculated using QST and IRC calculations as above.
The zero energy for each M4 + CO2 system is defined as the sum of the energies of the M4 metal cluster in the singlet multiplicity and the CO2 molecule. Thus structures with a negative relative energy (below zero energy) are more stable than the separated reactants; structures with a positive relative energy (energy higher than zero energy) are unstable with respect to the infinitely separated reactants. As in a previous study, basis set superposition error (BSSE) was disregarded, as were zero-point energies and entropic contributions.39 All structure searches (Kick runs) were undertaken with the B3P86 density functional40 and Stuttgart Relativistic Small Core (SRSC) basis set,41–43 as previous studies39,44 have shown this to be an accurate and computationally efficient combination. The final pathways were re-optimized at all relevant multiplicities using the TPSS functional45 with the Def2TZVP basis set46,47 and employing the D3-BJ empirical dispersion term.48 This latter combination, while more expensive, has also been shown to reproduce energetic ordering and vibrational data for reactions of small molecules on gas phase transition metal clusters including Rhn49 and Ptn.50 Gaussian 16 was used for all calculations.51 Absolute and relative energies for all structures calculated with both functionals are presented in the SI (xlsx), structures are included in xyz format (zipfile).
3 Results and discussion
All M4 tetramers are even-electron species, and thus we calculate the singlet – septet surfaces by default.3.1 Nb4
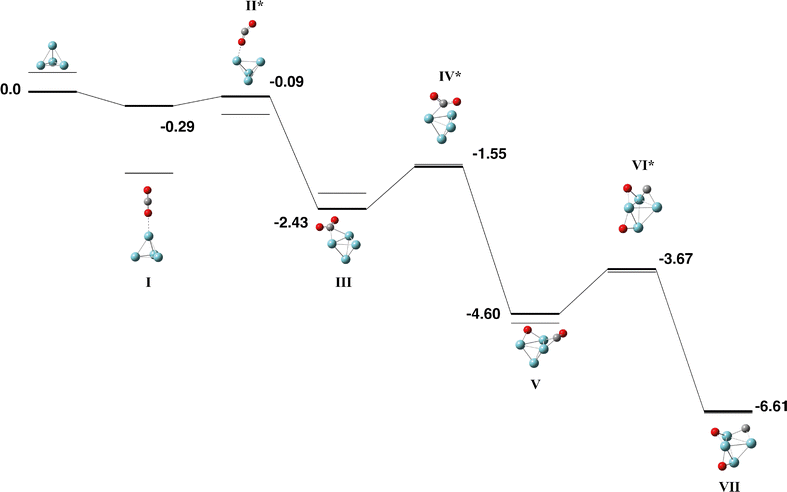
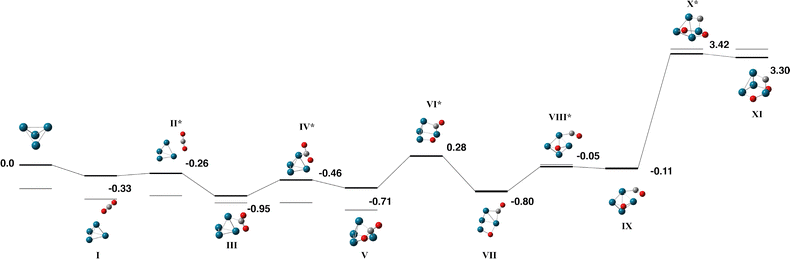
For the Nb4 cluster we find a tetrahedral structure of singlet multiplicity to be the lowest in energy, in line with previous calculations using several DFT methods,52–55 and confirmed by coupled cluster56 and multireference singles and doubles configuration interaction (MRSDCI).57 This cluster was used for the CO2 pathway without constraint.The Nb4 + CO2 pathway is shown in Fig. 1 and the corresponding geometric data is tabulated in Table S1. Only the singlet and triplet surfaces are shown as the quintet and septet were high in energy. The capture species (Fig. 1-I) is bound by −0.29 eV and consists of the CO2 molecule approaching one vertex of the Nb4 tetrahedron. The CO2 molecule bends in the first transition state and the central carbon atom is μ2 bound to a Nb–Nb edge, rotation of the CO2 molecule over a Nb3 face allows the first oxygen atom to dissociate (Fig. 1-V) and the remaining CO molecule dissociates the same way over the adjacent face resulting in a fully dissociated global minimum with a relative energy −6.61 eV below zero energy.
3.2 Mo4
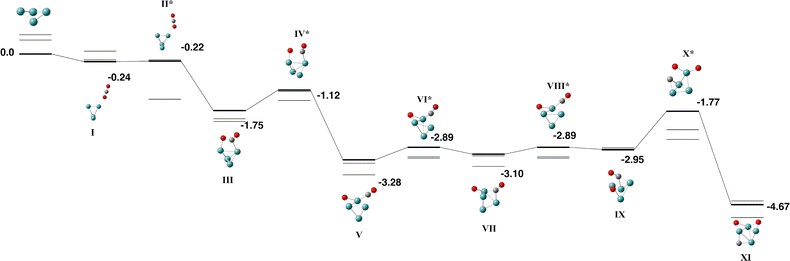
Several groups have undertaken calculations on the Mo4 cluster: a recent DFT study by Kantorovich and coworkers predicted seven isomers within 1 eV of the global minimum, and two of those structures were separated by only 0.034 eV per atom – both structures were “tetrahedron-like” and possessed D2d symmetry, but differed in the hinge angle.58 Liebing et al. predict a stretched tetrahedral structure,59 while Yin et al. predict a tetrahedral global minimum with an open “butterfly” structure 0.34 eV higher in energy.60 Sumer and Jellinek reverse this order, predicting an open butterfly structure with μB = 4 being 0.01 eV per atom lower in energy than a tetrahedral structure with μB = 2.61 Pis Diez undertook a detailed study of the symmetry of the Mo4 structure and identified four distorted tetrahedra of D2d symmetry, a D2 structure and a C3v triangular pyramid.62 Our structure search on the singlet surface identified the open butterfly (ϕ = 130°), stretched tetrahedron (ϕ = 50°) and tetrahedral structures and the open butterfly was employed as a fragment in the reaction path with CO2. It is to be noted, that despite starting from the open butterfly structure, upon interaction with CO2, the Mo4 cluster became more compact, resembling either the tetrahedron or stretched tetrahedral structures.The reaction path for Mo4 + CO2 is very similar to that of Nb4 and is shown in Fig. 2 with the corresponding geometric data in Table S2. The singlet–quintet surfaces are shown as the septet surface is high in energy. The CO2 molecule initially approaches a single Mo atom in the capture species before binding in a η2 fashion across a Mo–Mo bond (Fig. 2-III). The first O–CO bond breaks to yield a μ2-bound oxygen atom and a μ1-bound CO molecule (Fig. 2-V), which rotates to become μ2-bound (Fig. 2-VII) before dissociating. The lowest energy structure has CO2 fully dissociated and is −4.67 eV below the energy of the separated Mo4 (singlet) and CO2. The equivalent triplet structure is −5.07 eV below zero energy.
3.3 Ru4
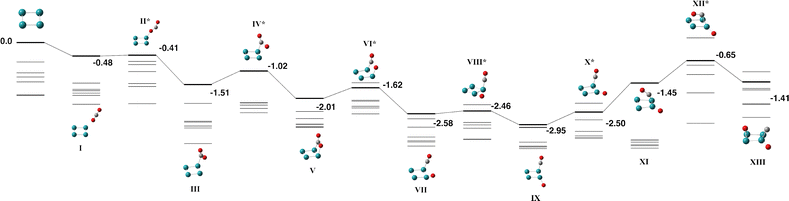
As ruthenium is known to have low-lying states with a high number of unpaired electrons, multipliticities up to 15-tet are investigated for the Ru4 + CO2 reaction. The lowest energy Ru4 structure was determined to be a singlet rectangle with side lengths of 2.21 and 2.24 Å which is in approximate agreement with previous calculations on the Ru4 cluster that predict a D4h singlet square structure.63–65Fig. 3 and Table S3 show the reaction path for Ru4 + CO2. Multiplicities up to the 13-tet are shown, the 15-tet was approximately 1 eV higher in energy throughout the entire pathway. The CO2 molecule interacts first with a single ruthenium atom in the capture species (Fig. 3-I), before bending and rotating to form a Ru–C covalent bond (Fig. 3-III). On the singlet surface this step requires surmounting a barrier at +0.57 eV, but for all other multiplicities, this transition state is below zero energy. The CO2 molecule rotates to locate one oxygen atom over the adjacent Ru atom, forming a μ2η2 structure. The lowest energy structure is Fig. 3-IX, where the CO2 molecule has partly dissociated to a μ1-bound oxygen atom in the Ru4 plane and a μ1-bound CO molecule approximately perpendicular to the plane. The remaining CO molecule can then fall across the Ru4 face and dissociate (Fig. 3-XIII), however, the dissociation of the second CO bond, while still below zero energy, is less favoured than the partly dissociated CO2 structure (Fig. 3-VII).
3.4 Rh4
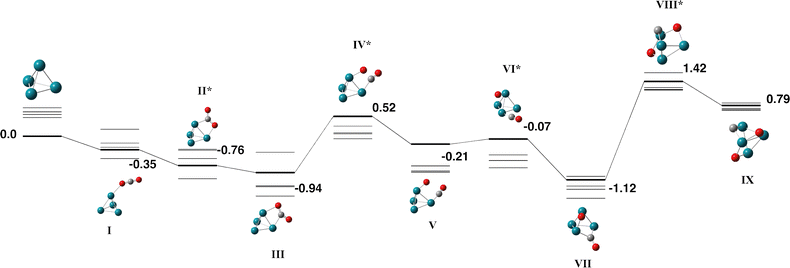
As with ruthenium, we consider additional multiplicities for the Rh4 cluster reaction, from singlet–nonet. The lowest energy Rh4 cluster was identified to be a singlet tetrahedron of Td symmetry and a side length of 2.45 Å. This result is consistent with that of many authors66–70 and we employ this structure, without constraint, as a fragment for reaction with CO2.Fig. 4 shows the reaction path for Rh4 + CO2, the corresponding geometric data is tabulated in Table S4. From the η1-O bound capture species, the CO2 molecule falls across a Rh–Rh bond, binding in a μ2η2 fashion, with a O–C–O angle, θOCO = 140°, but only marginally lengthening the CO bond (rCO = 1.26 and 1.27 Å). The transition state stretching the coordinated CO bond (νimag = 464 cm−1) is +0.52 eV higher than zero energy. After the first CO bond breaks, both the intact CO molecule and the dissociated oxygen atom rotate around the cluster, shifting from μ1 to μ2 binding in the lowest energy Rh4CO2 cluster (Fig. 4-VII). To reach the lowest energy fully dissociated structure, Fig. 4-IX, +0.79 eV above zero energy, the second CO bond needs to be stretched over an adjacent Rh3 face, surmounting a transition state of +1.42 eV. Given that all transition states involving stretching of a CO bond have energy above that of the separated reactants, it is likely that CO2 would remain intact on the Rh4 cluster.
3.5 Pd4
Pd4 has also been extensively calculated. Futschek et al. calculated square, rhombus and tetrahedral structures with different multiplicities and point group symmetries, concluding that the triplet tetrahedron was the global minimum.67 This assignment is in agreement with Kawazoe and coworkers71 Moc et al. also employ a tetrahedral triplet (3A″) Pd4 structure in their reaction with H2 molecules. Our structure search also identified a triplet global minimum, with a tetrahedral structure, though with a slightly expanded dihedral angle of 74° (viz. 80° in the singlet and 70.5° in Td symmetry). We use this fragment going forward.In addition to calculations determining the structure of the Pd4 cluster, there have been many DFT studies on reactions of Pd4. Borbolla et al. in their B3LYP/6-31G(d,p) study, determined that the adsorption of formic acid on Pd4 would favourably produce CO2.72 Lian and coworkers showed that Pd4− had a lower barrier to dissociating N2O than the neutral or cationic tetramers73 and Dutta et al. compared the bare and ZSM-5-supported Pd4 cluster for the same reaction.74 Kalita and Deka calculated reaction profiles for CO oxidation on bare and oxidized Pd4 clusters.75
The reaction path calculated for Pd4 + CO2 is shown in Fig. 5 with geometric data in Table S5. The lowest energy species for Pd4CO2 is Fig. 5-III, just after the capture species (Fig. 5-I). In structure Fig. 5-III, the CO2 molecule is bent over a Pd–Pd bond, with θOCO = 136° and rCO = 1.24 and 1.30 Å. From this lowest energy structure, the CO2 molecule can rotate over the Pd3 face to form a μ3η2 geometry, which possesses approximate Cs symmetry, and a highly activated CO bond, rCO = 1.36 Å. In transition state Fig. 5-VI, the activated CO bond breaks (νimag = 261 cm−1), but this barrier lies above zero energy at +0.28 eV. Structure Fig. 5-VII is very similar to the μ2-O and μ2-CO structure calculated by Kalita and Deka for the coadsorption of O2 and CO on Pd4.75 Continuing the reaction path in order to dissociate the CO molecule is highly disfavoured, crossing a barrier of +3.42 eV in order to reach a minimum +3.30 eV higher in energy than the separated Pd4 + CO2 reactants.
3.6 Pt4
Our structure search identified a singlet open butterfly structure (ϕ = 135°) as the global minimum structure. In contrast, Sebetci predicted a distorted tetrahedron ground state, followed by a butterfly structure, though these differed by only 0.1 eV per atom in binding energy.76 Grönbeck and Andreoni reversed this prediction for the neutral Pd4 cluster, and additionally predicted a Y-shape cluster as being low in energy, further noting that the singlet and triplet states were quasi-degenerate.77 The butterfly structure was also predicted by Singh et al.78 and by Kawazoe and coworkers.79 We employ our singlet butterfly as a fragment with CO2.Platinum is of long-standing interest and use as a catalyst. Of particular note, Mafuné and coworkers used DFT to show that small Ptn (n = 4–12) clusters could undergo oxygen transfer reactions with N2O the but did not catalyse the oxidation of CO, instead co-adsorbing O + CO.80 Mass spectra generated in the same joint experimental – computational study were unable to identify CO2 desorption from the Ptn clusters. In a later work, Green et al. predicted a dissociative (O + CO) global minimum for the reaction of the anionic Pt4− cluster, but infrared multiphoton dissociation (IR-MPD) showed that the CO2 molecule remained intact.35
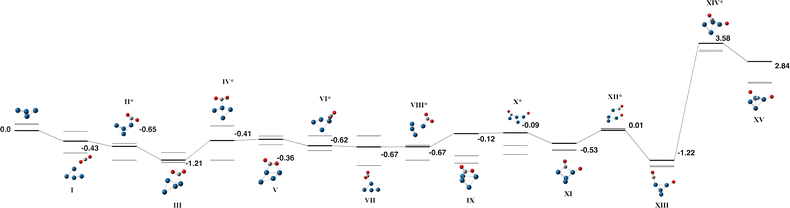
The calculated reaction path for neutral Pt4 + CO2 is shown in Fig. 6 with the corresponding data in Table S6. Green et al. noted that the anionic Pt4− cluster is a fluxional species,35 and similar fluxionality is observed here for the neutral Pt4 cluster, which contracts from an open butterfly structure, ϕ = 135° for Pt4 to a tetrahedral structure, ϕ = 76° for the lowest energy structure Fig. 6-XIII. The key features on the reaction path are similar to those identified for the anionic cluster; after the capture species (Fig. 6-I), which is bound by −0.43 eV, a C-bound η1 structure is formed (Fig. 6-III). The CO2 molecule may be η1-bound in this way to either an apex (Fig. 6-III) or spinal atom (Fig. 6-VII) of the Pt4 butterfly, converting via an η2 CO binding across the Pt–Pt bond (Fig. 6-V). From Fig. 6-IX, the CO2 molecule could dissociate via either a opening/closing of the Pt4 cluster (Fig. 6-X) or via stretching of the OC bond (Fig. 6-XII). The lowest energy Pt4CO2 structure has a μ1-bound CO molecule with the dissociated oxygen atom μ1 bound to an adjacent Pt atom. Searching for a pathway to dissociate the intact CO molecule identified a transition state +3.58 eV above zero energy and a minimum +2.84 eV above zero energy, indicating that full dissociation of the CO2 molecule to O + C + O is not thermodynamically feasible on the Pt4 cluster.
3.7 Ag4
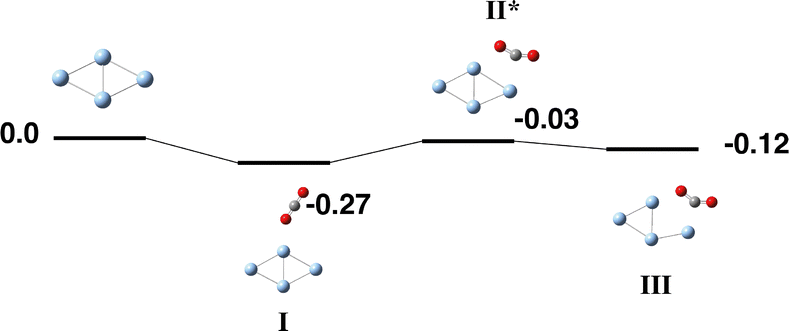
Silver has an electronic configuration of 4d105s1, and we study the singlet–septet multiplicities. The global minimum Ag4 structure is predicted to be a planar singlet rhombus structure of D2h symmetry, in agreement with several previous calculations.81–84 Attempts to converge a tetrahedral structure, similar to the Ag42+ structure calculated by Shimizu and coworkers, resulted in a stretched tetrahedron 0.95 eV higher in energy.85 A recent study indicated that the diamond/rhombus structure of Ag4 contributed strongly to the activation of O2.86The global minimum planar rhombus Ag4 structure was used as the basis for the reaction path finding and the resulting Ag4 +CO2 reaction path is shown in Fig. 7 and Table S7. Only the singlet surface is shown as all other multiplicities were significantly above zero energy. CO2 interacts only weakly with the Ag4 cluster, the capture species (Fig. 7-I) has CO2 μ1-bound to a spinal Ag atom. The CO2 molecule could then rotate in the Ag4 plane and bend to form a μ2η2 structure, but at the expense of the Ag–Ag bond, which is broken (3.79 Å vs. 2.76 Å). In Fig. 7-III, r(Ag–C) and r(Ag–O) are 2.23 and 2.24 Å respectively. No structures with CO2 either partly (O + CO) or fully (O + C + O) dissociated were obtained.
3.8 Periodic trends
For each M4 + CO2 reaction path, several key species are identified, these are: (1) the capture species, which is always the first structure shown on each reaction path and represents the first (physisorption) interaction between CO2 with the M4 cluster, the CO2 molecule is therefore still linear in the capture species. (2) The lowest energy species with associatively-bound CO2, the CO2 molecule is, by definition, intact, but may not be linear. This species represents an activated CO2 molecule. If a stable activated CO2 molecule could be thermodynamically or kinetically prevented from further activation → dissociation (to O + CO), the activated CO2 might thus be available for reaction with e.g. H2 to form formaldehyde. (3) The lowest energy structure with one CO bond broken, i.e. M4O·CO. (4) The lowest energy structure with the CO2 fully dissociated to atoms. In the context of CO2 activation, this would represent a catalyst poisoning process. The relative energies of these four geometric motifs are shown in Fig. 8.The energy of the capture species is consistent across the periodic table, ranging from −0.23 eV (Mo4) up to −0.48 eV for the square planar Ru4 cluster. The consistency of this interaction is expected, given the definition of the capture species as the minimally interacting species and has been observed previously.39 The relative energies of the associative (OCO), partly dissociated (O + CO) and fully dissociated (O + C + O) structures steadily increasing as one moves left to right across the periodic table, from Nb4 to Ag4. Note that no stuctures with dissociatively bound CO2 were identified for Ag4. The apparent ‘slope’ of energy increase, is mild for associative structures, moderate in the case of partly dissociated structures (O + CO) and strong for the fully dissociated structures. Fully dissociated structures are disfavoured (higher than zero energy) for Rh4, Pd4 and Pt4. The energies for the third row Pt4 cluster are similar, but slightly lower than the equivalent Pd4CO2 structures, suggesting that small clusters to the right of (and including) rhodium, would not fully dissociate CO2.
The initial adsorption step is considered key to the activation of CO2,87 it is the first step where charge transfer from the cluster to the CO2 molecule, typically via the electrophilic carbon atom occurs, weakening the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds, bending θOCO and thereby leading to a variety of stable intermediate structures.87 We therefore consider the properties of the M4CO2 capture species for each reaction pathway: Table 1 shows the calculated vibrational frequencies of CO2 in the capture species for each M4 cluster. The two bending frequencies, now non-degenerate, are strongly red-shifted on interaction with the M4 cluster, by ≈60 cm−1, consistent with the CO2 πu antibonding orbitals receiving electron density from the cluster. Considering the first of the two formerly degenerate frequencies (the one with the larger redshift), there is an apparent, though approximate, correlation between the degree of redshift and the energy of the fully dissociative species, with clusters that possess a fully dissociated CO2 below zero energy, regardless of whether this structure is the global minimum or not, show a redshift >60 cm−1 (viz. Nb4, Mo4 and Ru4). Clusters to the right of and including Rh4 yield a smaller redshift.
O bonds, bending θOCO and thereby leading to a variety of stable intermediate structures.87 We therefore consider the properties of the M4CO2 capture species for each reaction pathway: Table 1 shows the calculated vibrational frequencies of CO2 in the capture species for each M4 cluster. The two bending frequencies, now non-degenerate, are strongly red-shifted on interaction with the M4 cluster, by ≈60 cm−1, consistent with the CO2 πu antibonding orbitals receiving electron density from the cluster. Considering the first of the two formerly degenerate frequencies (the one with the larger redshift), there is an apparent, though approximate, correlation between the degree of redshift and the energy of the fully dissociative species, with clusters that possess a fully dissociated CO2 below zero energy, regardless of whether this structure is the global minimum or not, show a redshift >60 cm−1 (viz. Nb4, Mo4 and Ru4). Clusters to the right of and including Rh4 yield a smaller redshift.
| System | Bend 1 | Bend 2 | Symm stretch | Asym stretch | E(M4,HOMO) | E(M4,LUMO) | E(πu) | ΔE(πu) | Eads | Eint |
|---|---|---|---|---|---|---|---|---|---|---|
| (cm−1) | (eV) | |||||||||
| CO2 | 622 | 622 | 1283 | 2319 | −0.893 | 0.0 | ||||
| Nb4 | 546 | 547 | 1256 | 2317 | −4.237 | −2.304 | −2.304 | −1.411 | −0.291 | −0.292 |
| Mo4 | 471 | 540 | 1251 | 2333 | −3.920 | −2.399 | −2.399 | −1.507 | −0.237 | −0.516 |
| Ru4 | 527 | 579 | 1268 | 2324 | −4.905 | −3.470 | −2.140 | −1.248 | −0.481 | 0.514 |
| Rh4 | 572 | 587 | 1275 | 2322 | −5.416 | −3.000 | −1.393 | −0.501 | −0.349 | −0.351 |
| Pd4 | 581 | 585 | 1277 | 2322 | −5.495 | −4.245 | −1.498 | −0.605 | −0.333 | −0.341 |
| Ag4 | 599 | 606 | 1292 | 2331 | −5.232 | −3.130 | −1.822 | −0.930 | −0.269 | −0.269 |
| Pt4 | 567 | 588 | 1280 | 2330 | −6.060 | −4.227 | −1.797 | −0.904 | −0.430 | −0.433 |
Table 1 also shows the energy of the CO2 πu orbital and the ΔE, with respect to the calculated value for free CO2 (−0.03280 a.u.). Clusters that thermodynamically dissociate CO2 lower the energy of the πu orbital by ≈1.2–1.5 eV, while clusters without a fully dissociated CO2 below zero energy, lower the orbital energy by only ≈0.5–0.9 eV. Both of these correlations are indicative only, and provide no information on the relative stability of intermediate species (e.g. M4O·CO) but do act as a barometer for the thermodynamic stability (below zero energy) of the fully dissociative structure.
Table 1 shows the properties of the capture species for each reaction pathway, defined previously as the initial contact of CO2 with the metal cluster. As this species possesses CO2 physisorbed to the M4 cluster, the interaction of the two species is minimal, and it is perhaps unsurprising that consideration of this species alone is insufficient to predict the eventual fate of CO2. Table 2 shows the Hirshfeld charges on CO2 molecule in the capture species and the first transition state for each of the reaction pathways. q(CO2) for the capture species is consistent and positive (≈0.1) for all M4 clusters, and clearly represents the initial donation of charge from the CO2 molecule to the cluster. The charge transfer observed in the first transition state (i.e. the back-donation from the M4 cluster to the CO2 molecule upon chemisorption) is diagnostic, with the three species where full CO2 dissociation is thermodynamically possible (viz. Nb4, Mo4 and Ru4) having q(CO2) < −0.35e−. Clusters that do not dissociate either CO2 bond, (Pd4 and Ag4) have low back-donation to CO2, < 0.2e− and clusters that are likely to activate CO2 without fully dissociating it, Rh4, Pt4, having intermediate q(CO2) values.
| System | Capture species | TS 1 |
|---|---|---|
| q(CO2) | q(CO2) | |
| Nb4 | 0.098 | −0.577 |
| Mo4 | 0.097 | −0.488 |
| Ru4 | 0.125 | −0.354 |
| Rh4 | 0.097 | −0.299 |
| Pd4 | 0.080 | −0.006 |
| Ag4 | 0.087 | −0.151 |
| Pt4 | 0.114 | −0.250 |
Fig. 9 shows the barrier heights (transition state energies) for the key transition states in each pathway. The chosen barriers correspond to each CO bond breaking. Accordingly, no barriers are plotted for Ag4. Two trends can be clearly observed. Firstly, there is a clear left-right divide with all Nb4–Ru4 barriers being below zero energy, whereas Rh4–Pd4 barriers are above zero energy. The barrier to breaking the first CO bond on Pt4 is slightly below zero energy at −0.12 eV, reflecting the softer surface presented by the 5d metal compared to its 4d counterpart. Secondly, the barriers to the first CO bond breaking are relatively consistent either side of the left-right divide defined by Ru/Rh, whereas the barrier to the second CO bond breaking rises when moving from left to right (Nb4–Ag4), suggesting that activated CO2 or O + CO are likely outcomes of reaction on the Rh4 and Pd4 clusters.
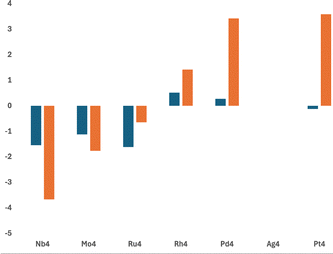 | ||
| Fig. 9 Plot of the relative energies of barriers to dissociation of the first (blue) and second (orange) C⋯O bonds for M4CO2 reaction pathways on the singlet surface. | ||
The general trends in adsorption energies shown in Fig. 8 are consistent with other materials proposed for CO2 adsorption. Peng and coworkers studied the adsorption of molecular CO2 on M2N MXenes, and found that end-on CO2 binding had a consistent binding energy, of ≈−0.25 eV, corresponding to a physisorbed geometry, but the adsorption energy of side-on CO2 rose from −2.5 eV for Y2N to −1.5 eV for Mo2N, and MXenes to the right of Ru2N bound CO2 only weakly, ≈−0.42 eV. Jurado et al. also studied a range of M2X MXenes, finding similar binding energies and trends with CO2 adsorption energies of −2.83, −1.73 and −1.34 eV for M = Zr, Nb and Mo respectively. In addition, they also noted the non-negligible role of the X atom, with carbides having lower adsorption energies than equivalent nitrides, and M3N2 MXenes having adsorption energies ≈1 eV greater than the equivalent M2N species. A study on single-atom doped Ti2CO2 showed that CO2 remains intact on these species, which is consistent with the CO2 adsorption energy on Y-doped Ti2CO2 of −0.808 eV,88 compared to ≈2 eV for the M4 species that dissociate CO2 in this work.
4 Conclusions
We have calculated reaction pathways for the reaction of CO2 on a series of neutral M4 transition metal clusters. Moving from left to right across the periodic table, the energies of the capture species remained relatively constant. The energies of the lowest energy M4CO2 species with CO2 fully intact (associatively bound CO2), lowest energy M4O·CO species and M4O·C·O (fully dissociated CO2) species all rose moving from Nb4 to Ag4, with the rate of increase M4O·C·O > M4O·CO > M4CO2. Therefore it is predicted that Nb4 and Mo4 will fully dissociate CO2. Ru4 may also fully dissociate CO2, but would be more likely to only break the first CO bond, resulting in a Ru4O·CO product. Rh4, Pd4 and Pt4 are good candidates to observe an activated CO2 molecule. The Ag4 cluster interacts only weakly with CO2. The energy of the CO2 πu orbital in the M4CO2 capture species and the related νbend was found to indicate those structures that dissociate CO2 fully (Nb4, Mo4 and Ru4) from those that do not, but did not distinguish between partly dissociated (O + CO) and fully intact CO2, however the degree of charge transfer in the first transition state was found to indicate all three possible fates of CO2.Author contributions
SS, BAV, NA, MS: investigation, writing – original draft MAA: conceptualization of this study, methodology, writing – review and editing.Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included as part of the supplementary information (SI). Supplementary information: geometric parameters (pdf), structures (xyz) and energies, vibrational data (xlsx) for all pathways. See DOI: https://doi.org/10.1039/d5cp03419a.Acknowledgements
MAA is grateful for HPC time via the UK Materials and Molecular Modelling Hub via grant no. (EP/T022213).Notes and references
- G. A. Olah, G. K. S. Prakash and A. Goeppert, J. Am. Chem. Soc., 2011, 133, 12881–12898 CrossRef CAS PubMed.
- E. V. Kondratenko, G. Mul, J. Baltrusaitis, G. O. Larrazábal and J. Pérez-Ramírez, Energy Environ. Sci., 2013, 6, 3112–3135 RSC.
- R. W. Dorner, D. R. Hardy, F. W. Williams and H. D. Willauer, Energy Environ. Sci., 2010, 3, 884–890 RSC.
- A. G. Saputro, A. L. Maulana, F. D. Aprilyanti and H. K. Dipojono, J. Eng. Technol. Sci., 2021, 53, 210402 CrossRef CAS.
- V. K. Ocampo-Restrepo, L. G. Verga and J. L. F. Da Silva, J. Phys. Chem. C, 2021, 125, 26296–26306 CrossRef CAS.
- X. Li, J. Yu, M. Jaroniec and X. Chen, Chem. Rev., 2019, 119, 3962–4179 CrossRef CAS PubMed.
- T. Sakakura, J.-C. Choi and H. Yasuda, Chem. Rev., 2007, 107, 2365–2387 CrossRef CAS.
- V. K. Ocampo-Restrepo, L. G. Verga and J. L. F. Da Silva, J. Phys. Chem. C, 2021, 125, 26296–26306 CrossRef CAS.
- V. K. Ocampo-Restrepo, L. Zibordi-Besse and J. L. F. Da Silva, J. Chem. Phys., 2019, 151, 214301 CrossRef PubMed.
- J. M. Weber, Int. Rev. Phys. Chem., 2014, 33, 489–519 Search PubMed.
- M. C. Thompson, J. Ramsay and J. M. Weber, J. Phys. Chem. A, 2017, 121, 7534–7542 CrossRef CAS PubMed.
- C. Wu, M. Luo, Y. Zhao, S. Wang, A. Zavabeti, P. Xiao and G. K. Li, Chem. Eng. J., 2023, 475, 146411 CrossRef CAS.
- Q. Sun, S. Huang, Z. Li, D. Su and J. Sun, J. Environ. Chem. Eng., 2023, 11, 109065 CrossRef CAS.
- M. Zhang, D. Zhang, X. Jing, B. Xu and C. Duan, Angew. Chem., Int. Ed., 2024, 63, e202402755 CrossRef CAS PubMed.
- P. Du, G. Deng, Z. Li, J. Sun, L. Wang, Y. Yang, J. Wang, Y. Li, X. Xu, Y. Zhang, W. Liu, G. Liu, Z. Zou and Z. Li, J. Mater. Sci. Technol., 2024, 189, 203–210 CrossRef CAS.
- Y. Wang, Y. S. Chan, R. Zhang and B. Yan, Chem. Eng. J., 2024, 481, 148360 CrossRef CAS.
- O. Hurtado-Aular, R. M. Ferullo and P. G. Belelli, Comput. Mater. Sci., 2024, 233, 112741 CrossRef CAS.
- Y. Shao, S. Zhang, X. Ou, F. Zeng, R. Cai, X. Fan and H. Chen, Chem. Eng. J., 2025, 518, 164589 CrossRef CAS.
- D. Y. Shin, J. H. Jo, J.-Y. Lee and D.-H. Lim, Comput. Theor. Chem., 2016, 1083, 31–37 CrossRef CAS.
- H. Sugiyama, M. Miyazaki, M. Sasase, M. Kitano and H. Hosono, J. Am. Chem. Soc., 2023, 145, 9410–9416 CrossRef CAS PubMed.
- V. Muman, A. Tennyson-Davies, O. Allegret and M. A. Addicoat, Phys. Chem. Chem. Phys., 2024, 26, 2218–2227 RSC.
- M. A. Addicoat, K. F. Lim and G. F. Metha, J. Chem. Phys., 2012, 137, 034301 CrossRef PubMed.
- A. Fielicke, Chem. Soc. Rev., 2023, 52, 3778–3841 RSC.
- A. Yamada, K. Miyajima and F. Mafune, Phys. Chem. Chem. Phys., 2012, 14, 4188–4195 RSC.
- D. Harding, M. S. Ford, T. R. Walsh and S. R. Mackenzie, Phys. Chem. Chem. Phys., 2007, 9, 2130–2136 RSC.
- S. Nayak, B. Rao, S. Khanna and P. Jena, Chem. Phys. Lett., 1996, 259, 588–592 CrossRef CAS.
- G. Kwon, G. A. Ferguson, C. J. Heard, E. C. Tyo, C. Yin, J. DeBartolo, S. Seifert, R. E. Winans, A. J. Kropf, J. Greeley, R. L. Johnston, L. A. Curtiss, M. J. Pellin and S. Vajda, ACS Nano, 2013, 7, 5808–5817 CrossRef CAS PubMed.
- R. K. Raju, P. Rodriguez and E. N. Brothers, Phys. Chem. Chem. Phys., 2023, 25, 11630–11652 RSC.
- K. Mori, J. Matsuo, Y. Kondo, H. Hata and H. Yamashita, ACS Appl. Energy Mater., 2021, 4, 11634–11642 CrossRef CAS.
- S. H. Pulumati, D. K. Sannes, C. R. Jabbour, L. D. B. Mandemaker, B. M. Weckhuysen, U. Olsbye, A. Nova and E. Skulason, ACS Catal., 2023, 14, 382–394 CrossRef.
- M. N. Collacique, V. K. Ocampo-Restrepo and J. L. F. Da Silva, J. Chem. Phys., 2022, 156, 124106 CrossRef CAS PubMed.
- D. Guo, J. Liu, X. Zhao, X. Yang and X. Chen, Sep. Purif. Technol., 2023, 313, 123462 CrossRef CAS.
- J. Li, P. Gonzalez-Navarrete, M. Schlangen and H. Schwarz, Chem. – Eur. J., 2015, 21, 7780–7789 CrossRef CAS PubMed.
- M. A. Nolen, S. A. Tacey, S. Kwon and C. A. Farberow, Appl. Surf. Sci., 2023, 637, 157873 CrossRef CAS.
- A. E. Green, J. Justen, W. Schöllkopf, A. S. Gentleman, A. Fielicke and S. R. Mackenzie, Angew. Chem., Int. Ed., 2018, 57, 14822–14826 CrossRef CAS PubMed.
- U. Mondal and P. Ghosh, Catal. Today, 2021, 370, 93–103 CrossRef CAS.
- M. A. Addicoat and G. F. Metha, J. Comput. Chem., 2009, 30, 57–64 CrossRef CAS PubMed.
- M. A. Addicoat, S. Fukuoka, A. J. Page and S. Irle, J. Comput. Chem., 2013, 34, 2591–2600 CrossRef CAS PubMed.
- M. A. Addicoat, M. A. Buntine, B. Yates and G. F. Metha, J. Comput. Chem., 2008, 29, 1497–1506 CrossRef CAS PubMed.
- A. D. Becke, J. Chem. Phys., 1993, 98, 5648–5652 CrossRef CAS.
- P. J. Hay and W. R. Wadt, J. Chem. Phys., 1985, 82, 270–283 CrossRef CAS.
- W. R. Wadt and P. J. Hay, J. Chem. Phys., 1985, 82, 284–298 CrossRef CAS.
- P. J. Hay and W. R. Wadt, J. Chem. Phys., 1985, 82, 299–310 CrossRef CAS.
- M. A. Addicoat, K. F. Lim and G. F. Metha, J. Chem. Phys., 2012, 137, 034301 CrossRef PubMed.
- J. Tao, J. P. Perdew, V. N. Staroverov and G. E. Scuseria, Phys. Rev. Lett., 2003, 91, 146401 CrossRef PubMed.
- F. Weigend and R. Ahlrichs, Phys. Chem. Chem. Phys., 2005, 7, 3297–3305 RSC.
- F. Weigend, Phys. Chem. Chem. Phys., 2006, 8, 1057–1065 RSC.
- S. Grimme, S. Ehrlich and L. Goerigk, J. Comput. Chem., 2011, 32, 1456–1465 CrossRef CAS PubMed.
- E. M. Cunningham, A. S. Gentleman, P. W. Beardsmore and S. R. Mackenzie, Phys. Chem. Chem. Phys., 2019, 21, 13959–13967 RSC.
- G. Meizyte, A. E. Green, A. S. Gentleman, S. Schaller, W. Schöllkopf, A. Fielicke and S. R. Mackenzie, Phys. Chem. Chem. Phys., 2020, 22, 18606–18613 RSC.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, G. A. Petersson, H. Nakatsuji, X. Li, M. Caricato, A. V. Marenich, J. Bloino, B. G. Janesko, R. Gomperts, B. Mennucci, H. P. Hratchian, J. V. Ortiz, A. F. Izmaylov, J. L. Sonnenberg, D. Williams-Young, F. Ding, F. Lipparini, F. Egidi, J. Goings, B. Peng, A. Petrone, T. Henderson, D. Ranasinghe, V. G. Zakrzewski, J. Gao, N. Rega, G. Zheng, W. Liang, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, K. Throssell, J. A. Montgomery, Jr., J. E. Peralta, F. Ogliaro, M. J. Bearpark, J. J. Heyd, E. N. Brothers, K. N. Kudin, V. N. Staroverov, T. A. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. P. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, J. M. Millam, M. Klene, C. Adamo, R. Cammi, J. W. Ochterski, R. L. Martin, K. Morokuma, O. Farkas, J. B. Foresman and D. J. Fox, Gaussian∼16 Revision B.01, Gaussian Inc., Wallingford CT, 2016 Search PubMed.
- X. X. Jin, J. G. Du, G. Jiang, X. Luo and X. W. Wang, Eur. Phys. J. D, 2011, 64, 323–329 CrossRef CAS.
- P. V. Nhat, V. T. Ngan and M. T. Nguyen, J. Phys. Chem. C, 2010, 114, 13210–13218 CrossRef CAS.
- P. Calaminici and R. Mejia-Olvera, J. Phys. Chem. C, 2011, 115, 11891–11897 CrossRef CAS.
- X. Wang, M. Lin and Q. Zhang, Acta Chim. Sin., 2004, 62, 1689–1694 CAS.
- C. V. S. Costa, L. d S. Barbosa, R. Gargano and D. L. Azevedo, J. Mol. Mod., 2024, 30, 406 CrossRef CAS PubMed.
- D. Majumdar and K. Balasubramanian, J. Chem. Phys., 2004, 121, 4014–4032 CrossRef CAS PubMed.
- Y. Wei, V. Veryazov and L. Kantorovich, APL Mater., 2024, 12, 031127 CrossRef CAS.
- S. Liebing, C. Martin, K. Trepte and J. Kortus, Phys. Rev. B: Condens. Matter Mater. Phys., 2015, 91, 155421 CrossRef.
- Y.-H. Yin and J. Chen, Comput. Theor. Chem., 2022, 1212, 113720 CrossRef CAS.
- A. Sumer and J. Jellinek, J. Chem. Phys., 2022, 157, 034301 CrossRef CAS PubMed.
- R. Pis Diez, Int. J. Quantum Chem., 2000, 76, 105–112 CrossRef CAS.
- G.-X. Ge, H.-X. Yan, Q. Jing and Y.-H. Luo, J. Cluster Sci., 2011, 22, 473–489 CrossRef CAS.
- Y.-C. Bae, H. Osanai, V. Kumar and Y. Kawazoe, Mater. Trans., 2005, 46, 159–162 CrossRef CAS.
- F. Aguilera-Granja, L. C. Balbás and A. Vega, J. Phys. Chem. A, 2009, 113, 13483–13491 CrossRef CAS PubMed.
- B. V. Reddy, S. K. Nayak, S. N. Khanna, B. K. Rao and P. Jena, Phys. Rev. B: Condens. Matter Mater. Phys., 1999, 59, 5214–5222 CrossRef CAS.
- T. Futschek, M. Marsman and J. Hafner, J. Phys.: Condens. Matter, 2005, 17, 5927 CrossRef CAS.
- C.-H. Chien, E. Blaisten-Barojas and M. R. Pederson, Phys. Rev. A: At., Mol., Opt. Phys., 1998, 58, 2196–2202 CrossRef CAS.
- S. K. Nayak, S. E. Weber, P. Jena, K. Wildberger, R. Zeller, P. H. Dederichs, V. S. Stepanyuk and W. Hergert, Phys. Rev. B: Condens. Matter Mater. Phys., 1997, 56, 8849–8854 CrossRef CAS.
- Y. Jinlong, F. Toigo and W. Kelin, Phys. Rev. B: Condens. Matter Mater. Phys., 1994, 50, 7915–7924 CrossRef CAS PubMed.
- V. Kumar and Y. Kawazoe, Phys. Rev. B: Condens. Matter Mater. Phys., 2002, 66, 144413 CrossRef.
- R. L. Camacho-Mendoza and J. Cruz-Borbolla, Chem. Phys. Lett., 2020, 755, 137794 CrossRef CAS.
- X. Tang, W. Zeng, H. Duan, S. Chen and X. Lian, React. Kinet., Mech. Catal., 2023, 136, 1933–1943 CrossRef CAS.
- P. Dutta, N. Biswakarma, D. Dowerah, S. Neog, A. Phonglo, P. J. Sarma, N. K. Gour and R. C. Deka, J. Phys. Chem. C, 2024, 128, 21263–21279 CrossRef CAS.
- B. Kalita and R. C. Deka, J. Am. Chem. Soc., 2009, 131, 13252–13254 CrossRef CAS PubMed.
- A. Sebetci, J. Chem. Phys., 2006, 331, 9–18 CAS.
- H. Grönbeck and W. Andreoni, Chem. Phys., 2000, 262, 1–14 CrossRef.
- N. B. Singh and U. Sarkar, J. Mol. Mod., 2014, 20, 2537 CrossRef PubMed.
- V. Kumar and Y. Kawazoe, Phys. Rev. B: Condens. Matter Mater. Phys., 2008, 77, 205418 CrossRef.
- H. Yamamoto, K. Miyajima, T. Yasuike and F. Mafuné, J. Phys. Chem. A, 2013, 117, 12175–12183 CrossRef CAS PubMed.
- N. Hartmann, R. Mitric, B. Stanca and V. Bonacic-Koutecky, Eur. Phys. J. D, 2001, 16, 151–155 CrossRef.
- V. Bonačić-Koutecky, L. Češpiva, P. Fantucci and J. Koutecky, J. Chem. Phys., 1993, 98, 7981–7994 CrossRef.
- Y. Wang and X. G. Gong, Eur. Phys. J. D, 2005, 34, 19–22 CrossRef.
- R. Fournier, J. Chem. Phys., 2001, 115, 2165–2177 CrossRef CAS.
- S. Yasumura, T. Kato, T. Toyao, Z. Maeno and K.-I. Shimizu, Phys. Chem. Chem. Phys., 2023, 25, 8524–8531 RSC.
- W. Wang, X. Liu and J. Pérez-Ros, J. Phys. Chem. A, 2021, 125, 5670–5680 CrossRef CAS PubMed.
- M. N. Collacique, V. K. Ocampo-Restrepo and J. L. F. Da Silva, J. Chem. Phys., 2022, 156, 124106 CrossRef CAS PubMed.
- Y.-X. Yu, J. Colloid Interface Sci., 2025, 695, 137799 CrossRef CAS PubMed.
| This journal is © the Owner Societies 2026 |