Open Access Article
Open Access ArticleA dynamic pcu MOF(Zn) containing a ditopic T-shaped [2]rotaxane linker
Hazem Amarnea,
Alexander J. Stirk b,
Christopher A. O'Keefeb,
Robert W. Schurko
b,
Christopher A. O'Keefeb,
Robert W. Schurko cd and
Stephen J. Loeb
cd and
Stephen J. Loeb *b
*b
aDepartment of Chemistry, The University of Jordan, Amman, 11942, Jordan
bDepartment of Chemistry and Biochemistry, University of Windsor, Windsor, ON, Canada N9B 3P4. E-mail: loeb@uwindsor.ca
cDepartment of Chemistry and Biochemistry, Florida State University, Tallahassee, FL, 32306 USA
dNational High Magnetic Field Laboratory, Tallahassee, FL 32310, USA
First published on 5th January 2026
Abstract
A 3D metal–organic framework, designated UWDM-16, formula [Zn6(μ3-OH)2(μ2-RCO2)6(η1-RCO2)4(η1-RCO2H)2(H2O)], with a (12)-c hexanuclear SBU was synthesized using a T-shaped [2]rotaxane linker and Zn(NO3)2·6H2O under solvothermal conditions in DMF.
Previous efforts to prepare MOFs that incorporate [2]rotaxanes by utilising reticular design strategies1–12 focused on derived nets containing two geometry dictating branch points with a transitivity, p q, of 2 2 (fof and lil specifically).13–17 This led to the utilization of diisophthalic and extended diisophthalic linkers that yielded MOF structures related to the well-known NOTT series,18–23 but also some new and quite complex arrangements of linkers and SBUs.1,24–26 In order to simplify the resulting MOF structures, it was decided to explore edge transitive (p q of 1 1) nets27 that utilise linear linkers without branch points. In particular, the reticular design of Yaghi's iconic IRMOF series was targeted for inclusion of a T-shaped, [2]rotaxane linker.28
Yaghi and coworker's IRMOF-15 and -16 use the linear ditopic linker, 1,1′:4′,1″-terphenyl-4,4″-dicarboxylic acid (TPDC), to form pcu nets. The former has a two-fold interpenetrated lattice and a void space of 80%, while the latter is not interpenetrated and has a larger void space of 91%.28 It is our contention that these void spaces, can be utilised as “free volume” to be filled when utilising bulkier linkers. This approach was successful for IRMOF-77,29 which contains the T-shaped ditopic linker [4,7-bis(4-carboxylphenyl)-1,3-dimethylbenzimidazol-2-ylid-ene](pyridyl) palladium(II)iodide (TPDC-Benz-PdI), allowing the PdII moieties to protrude into the available pores of the structure without obvious steric issues.13,30–34
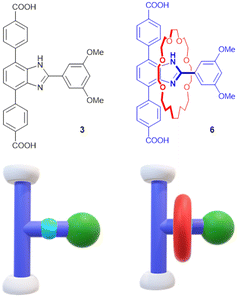
Herein, analogous reticular synthesis is applied to T-shaped ditopic linkers and octahedral Zn SBUs to target an edge transitive pcu net that allows incorporation of a compact [2]rotaxane. The [2]rotaxane 6 and naked axle analogue 3 used in this study are shown in Fig. 1. Although an edge-transitive pcu MOF, UWDM-16 (UWDM = University of Windsor Dynamic Material) was formed it contained an unexpected (12)-c hexanuclear SBU, rather than the ubiquitous tetra-zinc oxide cluster of the IRMOF series.35–37
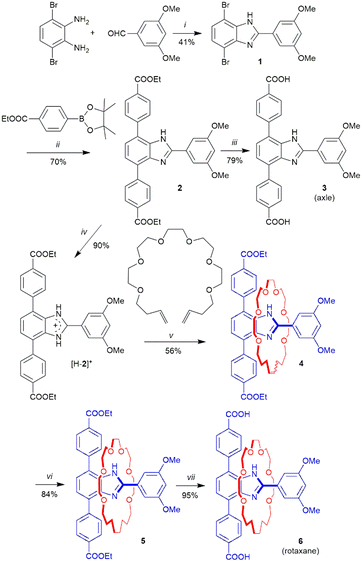
Scheme 1 outlines the synthetic routes to linkers axle 3 and [2]rotaxane 6. The benzimidazole group of the linkers was produced from the condensation of 1,2-diamino,3,6-dibromobenzene with 3,5-dimethoxybenzaldehyde. This was followed by a double Suzuki cross-coupling reaction to produce the diester 2. Upon protonation with HBF4, the ester [H-2]+ is capable of acting as a template for the Grubb's G1 catalyzed ring closure of a diolefin polyether to create a [2]rotaxane 4. Reduction of this olefinic precursor using H2 (or D2) over Pd/C yielded the diester 5. Diesters 2 and 5 were then hydrolyzed in basic solution to yield the diacid linkers 3 and 6 respectively. Full characterization (1H and 13C NMR, ESI-MS) of 3 and 6 are described in the ESI, including the SCXRD structure of the diester rotaxane 5 which clearly shows the mechanically interlocked nature of the product (see SI Fig. S4).
UWDM-16 were prepared via a solvothermal reaction at 85 °C using Zn(NO3)2·6H2O and linker 6 in DMF/EtOH/H2O in a 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 v/v ratio. Large, colourless rhomboid crystals were isolated after 12 h. Attempts to grow single crystals of analogous UWCM-16 (UWCM = University of Windsor Crystalline Material) employing linker 3 under the same solvothermal conditions resulted only in the isolation of a white powder. PXRD data for activated UWCM-16 indicated that this is a robust material, perhaps similar to UWDM-16, but no conclusion as to the exact nature of the structure was possible. Although several MOF models were investigated based on related penetrated and non-penetrated IRMOFs, no match between simulated and experimental PXRD could be achieved (see SI Fig. S8).
2 v/v ratio. Large, colourless rhomboid crystals were isolated after 12 h. Attempts to grow single crystals of analogous UWCM-16 (UWCM = University of Windsor Crystalline Material) employing linker 3 under the same solvothermal conditions resulted only in the isolation of a white powder. PXRD data for activated UWCM-16 indicated that this is a robust material, perhaps similar to UWDM-16, but no conclusion as to the exact nature of the structure was possible. Although several MOF models were investigated based on related penetrated and non-penetrated IRMOFs, no match between simulated and experimental PXRD could be achieved (see SI Fig. S8).
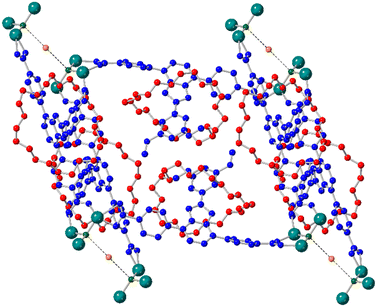
Crystals obtained directly from the synthesis of UWDM-16 were suitable for SCXRD. The structure was shown to have formula [Zn6(μ3-OH)2(μ2-RCO2)6(η1-RCO2)4(η1-RCO2H)2(H2O)] and crystallized in the space group R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) .Each face of the created cubes are filled with the [2]rotaxane such that each pore contains two [2]rotaxanes facing each other (Fig. 2).
.Each face of the created cubes are filled with the [2]rotaxane such that each pore contains two [2]rotaxanes facing each other (Fig. 2).
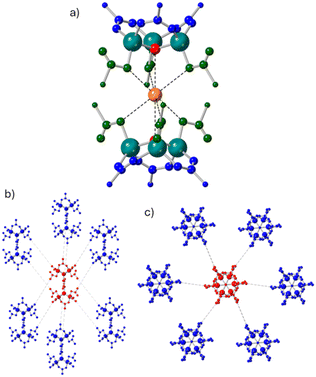
The SBU created during synthesis was different from the standard octahedral cluster (6)-c [Zn4(μ4-O)(μ2-O2CR)6] usually observed in IRMOFs. Instead, a (12)-c cluster with the overall formula [Zn6(μ3-OH)2(μ2-RCO2)6(η1-RCO2)2(η1-RCO2H)4(μ-H2O)] was observed (Fig. 3).
Six of the carboxylate groups form expected interactions with Zn, μ2-bridging pairs of Zn atoms to form distorted tetrahedrons containing apical μ3-hydroxides. The other six carboxylates interact with Zn ions through a single C–O–Zn bond and four of these carboxylates remain protonated. A single H2O molecule occupies the centre of the cluster hydrogen bonding to both HOOCR and hydroxide groups. This hexanuclear cluster has not previously been reported, though the bonding arrangement of the Zn(II) ions is quite similar to the heptanuclear, bimetallic (12)-c cluster, [NaZn6(μ3-OH)2(O2C–)12], reported by Ng and coworkers.38 (see SI Fig. S7).
The reason for formation of this unique cluster may be due to the purposeful presence of H2O during the synthesis. As a general rule, Zn containing MOFs avoid the use of excess H2O during synthesis; however, multiple attempts at forming a Zn MOF with 6 under anhydrous conditions produced only amorphous or semi-crystalline material that could not be characterised by X-ray diffraction. However, the addition of a small amount of water produced large single crystals, so it is reasonable to surmise that water plays a favourable part in the self-healing process required for MOF synthesis.39
It should be noted that since the central water molecule of the SBU only participates via non-covalent interactions, UWDM-16 can be reduced to a (412·63) uninodal edge-transitive (6)-c pcu net14,40,41 with a two-fold interpenetration (MOFkey: Zn.JJRWMIIYYSPHFA.MOFkey-v1.pcu).42
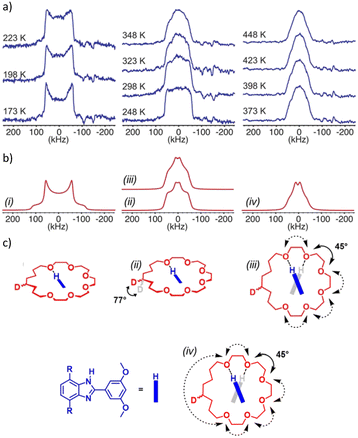
Most importantly, it is clear that the embedded [2]rotaxane has enough room to fit within the pores created by the framework. 2H VT-SSNMR verified that this positioning of the [2]rotaxane linkers allows for dynamic motion of the macrocycle inside the MOF (Fig. 4).
Conclusions
A T-shaped ditopic linker is a simple and useful design for creating MOFs with mechanically interlocked components and the resulting pcu framework allows the interlocked macrocycle to undergo dynamic motion in the solid-state. In the case of UWDM-16, reticular synthesis produced a somewhat unique SBU, but this did not fundamentally change the topological connectivity or the transitivity of the linker and still resulted in the targeted pcu net. As evidenced by VT-PXRD, this does not appear to greatly affect the thermal stability of the MOF. Further studies are needed to ascertain the overall chemical stability and reactivity of MOFs with this new SBU and elucidate the exact structure of UWCM-16.
Author contributions
Conceptualization, S. J. L. and A. J. S.; synthesis, H. A. and A. J. S.; materials characterisation, H. A. and A. J. S.; investigation – SCXRD; A. J. S. and S. J. L. SSNMR; C. A. O.; formal analysis – SSNMR, C. A. O. and R. W. S.; writing – original draft, A. J. S.; writing – review & editing, S. J. L., H. A. and A. J. S.; funding acquisition, S. J. L. and R. W. S.Conflicts of interest
There are no conflicts to declare.Data availability
The synthetic protocols, NMR and MS data supporting this article have been included as part of the supplementary information (SI).Supplementary information: synthetic details, 1H, 13C NMR spectra and MS data for all new compounds; PXRD data for UWCM16 and UWDM-16. See DOI: https://doi.org/10.1039/d5ce01108f.
CCDC 2500692 (2), 2500693 (5) and 2500694 (UWDM-16) contain the supplementary crystallographic data for this paper.43a–c
References
- B. H. Wilson and S. J. Loeb, in Mechanically Interlocked Materials, 2024, pp. 147–176 Search PubMed.
- P. Martinez-Bulit, A. J. Stirk and S. J. Loeb, Trends in Chemistry, 2019, 1, 588–600 Search PubMed.
- N. Farahani, K. Zhu, C. A. O'Keefe, R. W. Schurko and S. J. Loeb, ChemPlusChem, 2016, 81, 836–841 CrossRef PubMed.
- P. Martinez-Bulit, C. A. O'Keefe, K. Zhu, R. W. Schurko and S. J. Loeb, Cryst. Growth Des., 2019, 19, 5679–5685 CrossRef.
- D. J. Mercer, J. Yacoub, K. Zhu, S. K. Loeb and S. J. Loeb, Org. Biomol. Chem., 2012, 10, 6094–6104 RSC.
- V. N. Vukotic, K. J. Harris, K. Zhu, R. W. Schurko and S. J. Loeb, Nat. Chem., 2012, 4, 456–460 CrossRef PubMed.
- V. N. Vukotic and S. J. Loeb, Chem. Soc. Rev., 2012, 41, 5896–5906 RSC.
- V. N. Vukotic, C. A. O'Keefe, K. Zhu, K. J. Harris, C. To, R. W. Schurko and S. J. Loeb, J. Am. Chem. Soc., 2015, 137, 9643–9651 CrossRef.
- K. Zhu, C. A. O'keefe, V. N. Vukotic, R. W. Schurko and S. J. Loeb, Nat. Chem., 2015, 7, 514–519 CrossRef PubMed.
- K. Zhu, V. N. Vukotic, C. A. O'Keefe, R. W. Schurko and S. J. Loeb, J. Am. Chem. Soc., 2014, 136, 7403–7409 CrossRef.
- P. R. McGonigal, P. Deria, I. Hod, P. Z. Moghadam, A.-J. Avestro, N. E. Horwitz, I. C. Gibbs-Hall, A. K. Blackburn, D. Chen and Y. Y. Botros, Proc. Natl. Acad. Sci. U. S. A., 2015, 112, 11161–11168 CrossRef.
- G. Gholami, B. H. Wilson, K. Zhu, C. A. O'Keefe, R. W. Schurko and S. J. Loeb, Faraday Discuss., 2021, 225, 358–370 RSC.
- A. J. Stirk, B. H. Wilson, C. A. O'Keefe, H. Amarne, K. Zhu, R. W. Schurko and S. J. Loeb, Nano Res., 2021, 14, 417–422 CrossRef.
- M. Li, D. Li, M. O'Keeffe and O. M. Yaghi, Chem. Rev., 2014, 114, 1343–1370 CrossRef.
- Y. Zhou and L. Han, Coord. Chem. Rev., 2021, 430, 213665 CrossRef.
- J. A. Perman, A. J. Cairns, Ł. Wojtas, M. Eddaoudi and M. J. Zaworotko, CrystEngComm, 2011, 13, 3130–3133 RSC.
- H.-L. Xu, X.-S. Zeng, J. Li, Y.-C. Xu, H.-J. Qiu and D.-R. Xiao, CrystEngComm, 2018, 20, 2430–2439 RSC.
- X. Lin, I. Telepeni, A. J. Blake, A. Dailly, C. M. Brown, J. M. Simmons, M. Zoppi, G. S. Walker, K. M. Thomas and T. J. Mays, J. Am. Chem. Soc., 2009, 131, 2159–2171 CrossRef PubMed.
- J. Jiao, H. Liu, D. Bai and Y. He, Inorg. Chem., 2016, 55, 3974–3979 CrossRef PubMed.
- Z. Fan, J. Wang, W. Wang, S. Burger, Z. Wang, Y. Wang, C. Wöll, M. Cokoja and R. A. Fischer, ACS Appl. Mater. Interfaces, 2020, 12, 37993–38002 CrossRef.
- J. J. Perry IV, S. L. Teich-McGoldrick, S. T. Meek, J. A. Greathouse, M. Haranczyk and M. D. Allendorf, J. Phys. Chem. C, 2014, 118, 11685–11698 CrossRef.
- V. B. López-Cervantes, J. L. Obeso, A. Yañez-Aulestia, A. Islas-Jácome, C. Leyva, E. González-Zamora, E. Sánchez-González and I. A. Ibarra, Chem. Commun., 2023, 59, 10343–10359 RSC.
- I. A. Ibarra, S. Yang, X. Lin, A. J. Blake, P. J. Rizkallah, H. Nowell, D. R. Allan, N. R. Champness, P. Hubberstey and M. Schröder, Chem. Commun., 2011, 47, 8304–8306 RSC.
- H.-M. Wen, H. Wang, B. Li, Y. Cui, H. Wang, G. Qian and B. Chen, Inorg. Chem., 2016, 55, 7214–7218 CrossRef.
- J. Liu, B. Lukose, O. Shekhah, H. K. Arslan, P. Weidler, H. Gliemann, S. Bräse, S. Grosjean, A. Godt and X. Feng, Sci. Rep., 2012, 2, 921 CrossRef.
- X. Lin, J. Jia, X. Zhao, K. M. Thomas, A. J. Blake, G. S. Walker, N. R. Champness, P. Hubberstey and M. Schroder, Angew. Chem., Int. Ed., 2006, 45, 7358 CrossRef PubMed.
- O. Delgado-Friedrichs, M. O'Keeffe and O. M. Yaghi, Phys. Chem. Chem. Phys., 2007, 9, 1035–1043 RSC.
- M. Eddaoudi, J. Kim, N. Rosi, D. Vodak, J. Wachter, M. O'Keeffe and O. M. Yaghi, Science, 2002, 295, 469–472 CrossRef.
- K. Oisaki, Q. Li, H. Furukawa, A. U. Czaja and O. M. Yaghi, J. Am. Chem. Soc., 2010, 132, 9262–9264 CrossRef PubMed.
- X. Li, J. Xie, Z. Du, L. Jiang, G. Li, S. Ling and K. Zhu, Chem. Sci., 2022, 13, 6291–6296 RSC.
- S. Millan, B. Gil-Hernández, E. Milles, S. Gökpinar, G. Makhloufi, A. Schmitz, C. Schlüsener and C. Janiak, Dalton Trans., 2019, 48, 8057–8067 RSC.
- X. Jing, H. Meng, G. Li, Y. Yu, Q. Huo, M. Eddaoudi and Y. Liu, Cryst. Growth Des., 2010, 10, 3489–3495 CrossRef.
- T. He, Y.-Z. Zhang, X.-J. Kong, J. Yu, X.-L. Lv, Y. Wu, Z.-J. Guo and J.-R. Li, ACS Appl. Mater. Interfaces, 2018, 10, 16650–16659 CrossRef.
- B. Li, W. Jiang, Y. Xu, Z. Xu, Q. Yan and G. Yong, Dyes Pigm., 2020, 174, 108017 CrossRef.
- H. Li, M. Eddaoudi, M. O'Keeffe and O. M. Yaghi, Nature, 1999, 402, 276–279 CrossRef.
- J. L. Rowsell and O. M. Yaghi, J. Am. Chem. Soc., 2006, 128, 1304–1315 CrossRef.
- J. Yang, A. Grzech, F. M. Mulder and T. J. Dingemans, Microporous Mesoporous Mater., 2013, 171, 65–71 CrossRef.
- L. Hou, J. P. Zhang, X. M. Chen and S. W. Ng, Chem. Commun., 2008, 4019–4021 RSC.
- E. K. Berdichevsky, V. A. Downing, R. W. Hooper, N. W. Butt, D. T. McGrath, L. J. Donnelly, V. K. Michaelis and M. J. Katz, Inorg. Chem., 2022, 61, 7970–7979 CrossRef PubMed.
- M. O'Keeffe, M. A. Peskov, S. J. Ramsden and O. M. Yaghi, Acc. Chem. Res., 2008, 41, 1782–1789 Search PubMed.
- V. A. Blatov and D. M. Proserpio, in Modern Methods of Crystal Structure Prediction, Wiley Online Library, 2010, pp. 1–28 Search PubMed.
- B. J. Bucior, A. S. Rosen, M. Haranczyk, Z. Yao, M. E. Ziebel, O. K. Farha, J. T. Hupp, J. I. Siepmann, A. Aspuru-Guzik and R. Q. Snurr, Cryst. Growth Des., 2019, 19, 6682–6697 CrossRef.
- (a) CCDC 2500692: Experimental Crystal Structure Determination, 2025, DOI:10.2550/fix.icsd.cc2py5hb; (b) CCDC 2500693: Experimental Crystal Structure Determination, 2025, DOI:10.2550/fix.icsd.cc2py5jc; (c) CCDC 2500694: Experimental Crystal Structure Determination, 2025, DOI:10.2550/fix.icsd.cc2py5kd.
| This journal is © The Royal Society of Chemistry 2026 |